UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-Q
(Mark One)
[X] | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2018
or
[ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to __________
Commission file number: 001-15543
________________________
PALATIN TECHNOLOGIES, INC.
(Exact name of registrant as specified in its charter)
Delaware |
| 95-4078884 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification No.) |
|
|
|
4B Cedar Brook DriveCranbury, New Jersey |
| 08512 |
(Address of principal executive offices) |
| (Zip Code) |
(609) 495-2200
(Registrant's telephone number, including area code)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☒ |
|
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) for the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 11, 2018, 198,677,039 shares of the registrant's common stock, par value $0.01 per share, were outstanding.
PALATIN TECHNOLOGIES, INC .
Table of Contents
| Page |
PART I – FINANCIAL INFORMATION | |
Item 1. Financial Statements (Unaudited) |
|
Consolidated Balance Sheets as of March 31, 2018 and June 30, 2017 | 3 |
Consolidated Statements of Operations for the Three and Nine Months Ended March 31, 2018 and 2017 | 4 |
Consolidated Statements of Comprehensive (Loss) Income for the Three and Nine Months Ended March 31, 2018 and 2017 | 5 |
Consolidated Statements of Cash Flows for the Three and Nine Months Ended March 31, 2018 and 2017 | 6 |
Notes to Consolidated Financial Statements | 7 |
Item 2. Management's Discussion and Analysis of Financial Condition and Results of Operations | 19 |
Item 3. Quantitative and Qualitative Disclosures About Market Risk | 22 |
Item 4. Controls and Procedures | 22 |
PART II – OTHER INFORMATION | |
|
|
Item 1. Legal Proceedings | 23 |
Item 1A. Risk Factors | 23 |
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds | 42 |
Item 3. Defaults Upon Senior Securities | 42 |
Item 4. Mine Safety Disclosures | 42 |
Item 5. Other Information | 42 |
Item 6. Exhibits | 43 |
Signatures | 44 |
1
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
In this Quarterly Report on Form 10-Q, references to "we", "our", "us" or "Palatin" means Palatin Technologies, Inc. and its subsidiary.
Statements in this Quarterly Report on Form 10-Q, as well as oral statements that may be made by us or by our officers, directors, or employees acting on our behalf, that are not historical facts constitute "forward-looking statements", which are made pursuant to the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). The forward-looking statements in this Quarterly Report on Form 10-Q do not constitute guarantees of future performance. Investors are cautioned that statements that are not strictly historical statements contained in this Quarterly Report on Form 10-Q, including, without limitation, the following are forward looking statements:
●
estimates of our expenses, future revenue and capital requirements;
●
our ability to obtain additional financing on terms acceptable to us, or at all;
●
our ability to advance product candidates into, and successfully complete, clinical trials;
●
the initiation, timing, progress and results of future preclinical studies and clinical trials, and our research and development programs;
●
the timing or likelihood of regulatory filings and approvals;
●
our expectations regarding completion of required clinical trials and studies and validation of methods and controls used to manufacture bremelanotide for the treatment of premenopausal women with hypoactive sexual desire disorder ("HSDD"), which is a type of female sexual dysfunction ("FSD");
●
our expectation regarding the timing of our regulatory submissions for approval of bremelanotide for HSDD in the United States and in certain other jurisdictions outside the United States;
●
our expectation regarding performance of our exclusive licensees of bremelanotide, including;
o
AMAG Pharmaceuticals, Inc. ("AMAG") for North America,
o
Shanghai Fosun Pharmaceutical Industrial Development Co. Ltd. ("Fosun"), a subsidiary of Shanghai Fosun Pharmaceutical (Group) Co., Ltd., for the territories of the People's Republic of China, Taiwan, Hong Kong S.A.R. and Macau S.A.R. (collectively, "China"), and
o
Kwangdong Pharmaceutical Co., Ltd. ("Kwangdong") for the Republic of Korea ("Korea");
●
the potential for commercialization of bremelanotide for HSDD in North America by AMAG and other product candidates, if approved, by us;
●
our expectations regarding the potential market size and market acceptance for bremelanotide for HSDD and our other product candidates, if approved for commercial use;
●
our ability to compete with other products and technologies similar to our product candidates;
●
the ability of our third-party collaborators to timely carry out their duties under their agreements with us;
●
the ability of our contract manufacturers to perform their manufacturing activities for us in compliance with applicable regulations;
●
our ability to recognize the potential value of our licensing arrangements with third parties;
●
the potential to achieve revenues from the sale of our product candidates;
●
our ability to obtain adequate reimbursement from Medicare, Medicaid, private insurers and other healthcare payers;
●
our ability to maintain product liability insurance at a reasonable cost or in sufficient amounts, if at all;
●
the retention of key management, employees and third-party contractors;
●
the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and technology;
●
our compliance with federal and state laws and regulations;
●
the timing and costs associated with obtaining regulatory approval for our product candidates;
●
the impact of fluctuations in foreign exchange rates;
●
the impact of legislative or regulatory healthcare reforms in the United States;
●
our ability to adapt to changes in global economic conditions; and
●
our ability to remain listed on the NYSE American stock exchange.
Such forward-looking statements involve risks, uncertainties and other factors that could cause our actual results to be materially different from historical results or from any results expressed or implied by such forward-looking statements. Our future operating results are subject to risks and uncertainties and are dependent upon many factors, including, without limitation, the risks identified in this report, in our Annual Report on Form 10-K for the year ended June 30, 2017, and in our other Securities and Exchange Commission ("SEC") filings.
We expect to incur losses in the future as a result of spending on our planned development programs and results may fluctuate significantly from quarter to quarter.
Palatin Technologies® is a registered trademark of Palatin Technologies, Inc.
2
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements
PALATIN TECHNOLOGIES, INC . | ||
and Subsidiary | ||
Consolidated Balance Sheets | ||
(unaudited) | ||
|
|
|
|
March 31, 2018 |
June 30, 2017 |
ASSETS |
|
|
Current assets: |
|
|
Cash and cash equivalents | $ 25,736,158 | $ 40,200,324 |
Available-for-sale investments | - | 249,837 |
Accounts receivable | - | 15,116,822 |
Prepaid expenses and other current assets | 701,456 | 1,011,221 |
Total current assets | 26,437,614 | 56,578,204 |
|
|
|
Property and equipment, net | 165,080 | 198,153 |
Other assets | 556,915 | 56,916 |
Total assets | $ 27,159,609 | $ 56,833,273 |
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIENCY) |
|
|
Current liabilities: |
|
|
Accounts payable | $ 793,642 | $ 1,551,367 |
Accrued expenses | 5,894,877 | 10,521,098 |
Notes payable, net of discount and debt issuance costs | 6,921,032 | 7,824,935 |
Capital lease obligations | - | 14,324 |
Deferred revenue | 585,519 | 35,050,572 |
Total current liabilities | 14,195,070 | 54,962,296 |
|
|
|
Notes payable, net of discount and debt issuance costs | 1,328,973 | 6,281,660 |
Deferred revenue | 500,000 | - |
Other non-current liabilities | 909,179 | 753,961 |
Total liabilities | 16,933,222 | 61,997,917 |
|
|
|
Stockholders' equity (deficiency): |
|
|
Preferred stock of $0.01 par value – authorized 10,000,000 shares: |
|
|
Series A Convertible: issued and outstanding 4,030 shares as of March 31, 2018 and June 30, 2017 | 40 | 40 |
Common stock of $0.01 par value – authorized 300,000,000 shares: |
|
|
issued and outstanding 195,477,332 shares as of March 31, 2018 and 160,515,361 shares as of June 30, 2017, respectively | 1,954,773 | 1,605,153 |
Additional paid-in capital | 352,125,554 | 349,979,373 |
Accumulated other comprehensive loss | - | (590 ) |
Accumulated deficit | (343,853,980 ) | (356,748,620 ) |
Total stockholders' equity (deficiency) | 10,226,387 | (5,164,644 ) |
Total liabilities and stockholders' equity (deficiency) | $ 27,159,609 | $ 56,833,273 |
|
|
|
The accompanying notes are an integral part of these consolidated financial statements.
3
PALATIN TECHNOLOGIES, INC. | ||||
and Subsidiary | ||||
Consolidated Statements of Operations | ||||
(unaudited) | ||||
|
|
|
|
|
|
Three Months Ended March 31, |
Nine Months Ended March 31, | ||
|
2018 |
2017 |
2018 |
2017 |
|
|
|
|
|
REVENUES: |
|
|
|
|
License and contract revenue | $ 8,962,709 | $ 10,823,748 | $ 46,516,370 | $ 10,823,748 |
|
|
|
|
|
OPERATING EXPENSES: |
|
|
|
|
Research and development | 7,068,849 | 9,062,316 | 27,277,830 | 28,422,975 |
General and administrative | 2,411,302 | 4,773,696 | 5,581,066 | 7,289,342 |
Total operating expenses | 9,480,151 | 13,836,012 | 32,858,896 | 35,712,317 |
|
|
|
|
|
(Loss) Income from operations | (517,442 ) | (3,012,264 ) | 13,657,474 | (24,888,569 ) |
|
|
|
|
|
OTHER INCOME (EXPENSE): |
|
|
|
|
Interest income | 86,496 | 6,304 | 219,578 | 18,940 |
Interest expense | (326,983 ) | (558,702 ) | (1,175,023 ) | (1,777,222 ) |
Total other expense, net | (240,487 ) | (552,398 ) | (955,445 ) | (1,758,282 ) |
|
|
|
|
|
(Loss) Income before income taxes | (757,929 ) | (3,564,662 ) | 12,702,029 | (26,646,851 ) |
Income tax benefit, net | 18,746 | - | 192,611 | - |
|
|
|
|
|
NET (LOSS) INCOME | $ (739,183 ) | $ (3,564,662 ) | $ 12,894,640 | $ (26,646,851 ) |
|
|
|
|
|
Basic net (loss) income per common share | $ (0.00 ) | $ (0.02 ) | $ 0.07 | $ (0.15 ) |
|
|
|
|
|
Diluted net (loss) income per common share | $ (0.00 ) | $ (0.02 ) | $ 0.06 | $ (0.15 ) |
|
|
|
|
|
Weighted average number of common shares outstanding used in computing basic net (loss) income per common share | 197,485,758 | 196,580,519 | 197,277,286 | 179,841,133 |
|
|
|
|
|
Weighted average number of common shares outstanding used in computing diluted net (loss) income per common share | 197,485,758 | 196,580,519 | 202,712,963 | 179,841,133 |
The accompanying notes are an integral part of these consolidated financial statements.
4
PALATIN TECHNOLOGIES, INC. | ||||
and Subsidiary | ||||
Consolidated Statements of Comprehensive (Loss) Income | ||||
(unaudited) | ||||
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
Nine Months Ended March 31, | ||
|
2018 |
2017 |
2018 |
2017 |
|
|
|
|
|
Net (loss) income | $ (739,183 ) | $ (3,564,662 ) | $ 12,894,640 | $ (26,646,851 ) |
|
|
|
|
|
Other comprehensive (loss) income : |
|
|
|
|
Unrealized gain on available-for-sale investments | - | 587 | 590 | 525 |
|
|
|
|
|
Total comprehensive (loss) income | $ (739,183 ) | $ (3,564,075 ) | $ 12,895,230 | $ (26,646,326 ) |
The accompanying notes are an integral part of these consolidated financial statements.
5
PALATIN TECHNOLOGIES, INC. | ||
and Subsidiary | ||
Consolidated Statements of Cash Flows | ||
(unaudited) | ||
|
|
|
|
Nine Months Ended March 31, | |
|
2018 |
2017 |
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
Net income (loss) | $ 12,894,640 | $ (26,646,851 ) |
Adjustments to reconcile net income (loss) to net cash |
|
|
(used in) provided by operating activities: |
|
|
Depreciation and amortization | 42,573 | 22,891 |
Non-cash interest expense | 143,837 | 234,056 |
Stock-based compensation | 2,370,972 | 1,404,721 |
Deferred income tax benefit | (500,000 ) | - |
Changes in operating assets and liabilities: |
|
|
Accounts receivable | 15,116,822 | (4,657,577 ) |
Prepaid expenses and other assets | 309,766 | 256,026 |
Accounts payable | (757,725 ) | 529,254 |
Accrued expenses | (4,601,842 ) | 546,474 |
Deferred revenue | (33,965,053 ) | 53,833,828 |
Other non-current liabilities | 155,218 | 245,701 |
Net cash (used in) provided by operating activities | (8,790,792 ) | 25,768,523 |
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
Proceeds from matured investments | 250,000 | 450,000 |
Purchases of property and equipment | (9,500 ) | - |
Net cash provided by investing activities | 240,500 | 450,000 |
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
Payments on capital lease obligations | (14,324 ) | (20,418 ) |
Payment of withholding taxes related to restricted |
|
|
stock units | (45,165 ) | (222 ) |
Payment on notes payable obligations | (6,000,000 ) | (3,666,666 ) |
Proceeds from the exercise of options and warrants | 145,615 | 114,358 |
Proceeds from the sale of common stock and |
|
|
warrants, net of costs | - | 23,856,972 |
Net cash (used in) provided by financing activities | (5,913,874 ) | 20,284,024 |
|
|
|
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | (14,464,166 ) | 46,502,547 |
|
|
|
CASH AND CASH EQUIVALENTS, beginning of period | 40,200,324 | 8,002,668 |
|
|
|
CASH AND CASH EQUIVALENTS, end of period | $ 25,736,158 | $ 54,505,215 |
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
Cash paid for interest | $ 876,394 | $ 1,299,731 |
Unrealized gain on available-for-sale investments | 590 | 525 |
The accompanying notes are an integral part of these consolidated financial statements.
6
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
(1)
ORGANIZATION:
Nature of Business – Palatin Technologies, Inc. ("Palatin" or the "Company") is a biopharmaceutical company developing targeted, receptor-specific peptide therapeutics for the treatment of diseases with significant unmet medical need and commercial potential. Palatin's programs are based on molecules that modulate the activity of the melanocortin and natriuretic peptide receptor systems. The melanocortin system is involved in a large and diverse number of physiologic functions, and therapeutic agents modulating this system may have the potential to treat a variety of conditions and diseases, including sexual dysfunction and inflammation-related diseases. The natriuretic peptide receptor system has numerous cardiovascular functions, and therapeutic agents modulating this system may be useful in treatment of heart failure and other cardiovascular diseases.
The Company's primary product in development is bremelanotide for the treatment of hypoactive sexual desire disorder ("HSDD"), which is a type of female sexual dysfunction ("FSD"). The Company also has drug candidates or development programs for cardiovascular diseases, including heart failure and fibrosis, and inflammatory diseases, including inflammatory bowel disease and ocular indications.
Key elements of the Company's business strategy include using its technology and expertise to develop and commercialize therapeutic products; entering into alliances and partnerships with pharmaceutical companies to facilitate the development, manufacture, marketing, sale and distribution of product candidates that the Company is developing; and partially funding its product candidate development programs with the cash flow generated from its relationships with third parties.
Business Risk and Liquidity – Since inception, the Company has incurred negative cash flows from operations, and has expended, and expects to continue to expend, substantial funds to complete its planned product development efforts. As shown in the accompanying consolidated financial statements, the Company had an accumulated deficit as of March 31, 2018 of $343,853,980 and had net losses and income for the three and nine months ended March 31, 2018 of $739,183 and $12,894,640, respectively. The Company anticipates incurring losses in the future as a result of spending on its development programs and will require substantial additional financing to continue to fund its planned developmental activities. To achieve sustained profitability, if ever, the Company, alone or with others, must successfully develop and commercialize its technologies and proposed products, conduct successful preclinical studies and clinical trials, obtain required regulatory approvals and successfully manufacture and market such technologies and proposed products. The time required to reach sustained profitability is highly uncertain, and the Company may never be able to achieve profitability on a sustained basis, if at all.
As of March 31, 2018, the Company's cash and cash equivalents were $25,736,158 and current liabilities were $13,609,551, net of deferred revenue of $585,519. The Company intends to utilize existing capital resources for general corporate purposes and working capital, and preclinical and clinical development of the Company's other product candidates and programs, including natriuretic peptide receptor and melanocortin receptor programs.
Management believes that the Company's existing capital resources, together with proceeds received from sales of common stock in the Company's "at-the-market" program (if any) (Note 14), will be adequate to fund the Company's planned operations through at least June 30, 2019. The Company will need additional funding to complete required clinical trials for its other product candidates and, assuming those clinical trials are successful, as to which there can be no assurance, to complete submission of required applications to the U.S. Food and Drug Administration ("FDA"). If the Company is unable to obtain approval or otherwise advance in the FDA approval process, the Company's ability to sustain its operations would be materially adversely affected.
The Company may seek the additional capital necessary to fund its operations through public or private equity offerings, collaboration agreements, debt financings or licensing arrangements. Additional capital that is required by the Company may not be available on reasonable terms, or at all.
Concentrations – Concentrations in the Company's assets and operations subject it to certain related risks. Financial instruments that subject the Company to concentrations of credit risk primarily consist of cash and cash equivalents, accounts receivable and investments. The Company's cash and cash equivalents are primarily invested in one money market account sponsored by a large financial institution. For the three and nine months ended March 31, 2018, the Company reported $8,962,709 and $41,516,370, respectively, in license and contract revenue related to a license agreement with AMAG for bremelanotide for North America ("License Agreement with AMAG") (Note 5). In addition, for the nine months ended March 31, 2018, the Company reported $5,000,000 in license revenue related to a license agreement with Fosun for bremelanotide for China and certain other Asian territories ("License Agreement with Fosun") (Note 6). For the three and nine months ended March 31, 2017, the Company reported $10,823,748 in contract revenue related to the License Agreement with AMAG.
7
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
(2)
BASIS OF PRESENTATION:
The accompanying unaudited consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America ("U.S. GAAP") for interim financial information and with the instructions to Form 10-Q. Accordingly, they do not include all of the information and footnote disclosures required to be presented for complete financial statements. In the opinion of management, these consolidated financial statements contain all adjustments (consisting of normal recurring adjustments) considered necessary for fair presentation. The results of operations for the three and nine months ended March 31, 2018 may not necessarily be indicative of the results of operations expected for the full year.
The accompanying unaudited consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto included in the Company's Annual Report on Form 10-K for the year ended June 30, 2017, filed with the SEC, which includes consolidated financial statements as of June 30, 2017 and 2016 and for each of the fiscal years in the three-year period ended June 30, 2017.
(3)
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES:
Principles of Consolidation – The consolidated financial statements include the accounts of Palatin and its wholly-owned inactive subsidiary. All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates – The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents – Cash and cash equivalents include cash on hand, cash in banks and all highly liquid investments with a purchased maturity of less than three months. Cash equivalents consist of $25,461,335 and $40,019,336 in a money market account as of March 31,2018 and June 30, 2017, respectively.
Investments – The Company determines the appropriate classification of its investments in debt and equity securities at the time of purchase and reevaluates such determinations at each balance sheet date. Debt securities are classified as held-to-maturity when the Company has the intent and ability to hold the securities to maturity. Debt securities for which the Company does not have the intent or ability to hold to maturity are classified as available-for-sale. Held-to-maturity securities are recorded as either short-term or long-term on the balance sheet, based on the contractual maturity date and are stated at amortized cost. Marketable securities that are bought and held principally for the purpose of selling them in the near term are classified as trading securities and are reported at fair value, with unrealized gains and losses recognized in earnings. Debt and marketable equity securities not classified as held-to-maturity or as trading are classified as available-for-sale and are carried at fair market value, with the unrealized gains and losses, net of tax, included in the determination of other comprehensive (loss) income.
The fair value of substantially all securities is determined by quoted market prices. The estimated fair value of securities for which there are no quoted market prices is based on similar types of securities that are traded in the market.
Fair Value of Financial Instruments – The Company's financial instruments consist primarily of cash equivalents, accounts receivable, accounts payable and notes payable. Management believes that the carrying values of cash equivalents, accounts receivable, available-for-sale investments and accounts payable are representative of their respective fair values based on the short-term nature of these instruments. Management believes that the carrying amount of its notes payable approximates fair value based on the terms of the notes.
Credit Risk – Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents. Total cash and cash equivalent balances have exceeded balances insured by the Federal Depository Insurance Company.
Property and Equipment – Property and equipment consists of office and laboratory equipment, office furniture and leasehold improvements and includes assets acquired under capital leases. Property and equipment are recorded at cost. Depreciation is recognized using the straight-line method over the estimated useful lives of the related assets, generally five years for laboratory and computer equipment, seven years for office furniture and equipment and the lesser of the term of the lease or the useful life for leasehold improvements. Amortization of assets acquired under capital leases is included in depreciation expense. Maintenance and repairs are expensed as incurred while expenditures that extend the useful life of an asset are capitalized. Accumulated depreciation and amortization was $2,324,562 and $2,281,989 as of March 31, 2018 and June 30, 2017, respectively.
8
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
Impairment of Long-Lived Assets – The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. To determine recoverability of a long-lived asset, management evaluates whether the estimated future undiscounted net cash flows from the asset are less than its carrying amount. If impairment is indicated, the long-lived asset would be written down to fair value. Fair value is determined by an evaluation of available price information at which assets could be bought or sold, including quoted market prices, if available, or the present value of the estimated future cash flows based on reasonable and supportable assumptions.
Revenue Recognition – The Company has generated revenue solely through license and collaboration agreements. The Company recognizes revenue in accordance with Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") Topic 605-25, Revenue Recognition for Arrangements with Multiple Elements , which addresses the determination of whether an arrangement involving multiple deliverables contains more than one unit of accounting. A delivered item within an arrangement is considered a separate unit of accounting only if both of the following criteria are met:
●
the delivered item has value to the customer on a stand-alone basis; and
●
if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in control of the vendor.
Under FASB ASC Topic 605-25, if both of the criteria above are not met, then separate accounting for the individual deliverables is not appropriate.
The Company has determined that it is appropriate to recognize the consideration received under its License Agreement with AMAG as revenue using the input-based proportional method during the period of the Palatin Development Obligation as defined in the License Agreement with AMAG. Refer to Note 5 for additional information on this topic.
Under its License Agreement with Fosun (Note 6), the Company received consideration in the form of an upfront license fee payment and determined that it was appropriate to recognize such consideration as revenue in the first quarter of 2018, which was the quarter in which the license was granted, since the license has stand-alone value and the upfront payment received by the Company is non-refundable.
Under its License Agreement with Kwangdong (Note 7), the Company received consideration in the form of an upfront license fee payment and has currently determined that it is appropriate to record such consideration as non-current deferred revenue because the upfront payment received by the Company is subject to certain refund provisions.
Revenue resulting from the achievement of development milestones is recorded in accordance with the accounting guidance for the milestone method of revenue recognition.
Amounts received prior to satisfying the revenue recognition criteria are recorded as deferred revenue on the Company's consolidated balance sheet. Amounts expected to be recognized as revenue in the next 12 months following the balance sheet date are classified as current liabilities.
Research and Development Costs – The costs of research and development activities are charged to expense as incurred, including the cost of equipment for which there is no alternative future use.
Accrued Expenses – Third parties perform a significant portion of the Company's development activities. The Company reviews the activities performed under all contracts each quarter and accrues expenses and the amount of any reimbursement to be received from collaborators based upon the estimated amount of work completed. Estimating the value or stage of completion of certain services requires judgment based on available information. If the Company does not identify services performed but not billed by the service-provider, or if the Company underestimates or overestimates the value of services performed as of a given date, reported expenses will be understated or overstated.
Stock-Based Compensation – The Company charges to expense the fair value of stock options and other equity awards granted. The Company determines the value of stock options utilizing the Black-Scholes option pricing model. Compensation costs for share-based awards with pro-rata vesting are determined using the quoted market price of the Company's common stock on the date of grant and allocated to periods on a straight-line basis, while awards containing a market condition and performance conditions are valued using multifactor Monte Carlo simulations.
9
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
Income Taxes – The Company and its subsidiary file consolidated federal and separate-company state income tax returns. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of assets and liabilities and their respective tax basis and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences or operating loss and tax credit carryforwards are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that includes the enactment date. The Company has recorded a valuation allowance against its deferred tax assets based on the history of losses incurred.
Pursuant to the License Agreement with Fosun (Note 6) and the License Agreement with Kwangdong (Note 7), $500,000 and $82,500, respectively, was withheld in accordance with tax withholding requirements in China and the Republic of Korea, respectively, and will be recorded as an expense during the fiscal year ending June 30, 2018. For the three and nine months ended March 31, 2018, the Company recorded an income tax benefit of $18,746 and an income tax expense of $307,389, respectively, related to these transactions and the remaining balance of $275,111 was included in prepaid expenses and other current assets at March 31, 2018. Any potential credit to be received by the Company on its United States tax returns is currently offset by the Company's valuation allowance.
On December 22, 2017, the U.S. government enacted wide-ranging tax legislation, the Tax Cuts and Jobs Act (the "2017 Tax Act"). The 2017 Tax Act significantly revises U.S. tax law by, among other provisions, (a) lowering the applicable U.S. federal statutory corporate income tax rate from 35% to 21%, (b) eliminating or reducing certain income tax deductions, such as deductions for interest expense, executive compensation expenses and certain employee expenses, and (c) repealing the federal alternative minimum tax ("AMT") and providing for the refund of existing AMT credits.
As a result of the 2017 Tax Act, during the quarter ended December 31, 2017, the Company recorded a tax benefit of $500,000 related to the release of a valuation allowance against an AMT credit; accordingly, $500,000 is included in Other long-term assets at March 31, 2018. In addition, as a result of the enactment of the new corporate income tax rate, the Company remeasured certain deferred tax assets and liabilities based on the rates at which they are expected to reverse and with the exception of the AMT credit, the Company continues to maintain a full valuation allowance against its net deferred tax assets.
The June 30,
2017 tax return was filed during the quarter ended March 31, 2018.
As a result, the Company did not incur AMT liability. The Company
has an income tax receivable of $500,000 from previously paying tax
that can be refunded in the future.
Net (Loss) Income per Common Share – Basic and diluted earnings per common share ("EPS") are calculated in accordance with the provisions of FASB ASC Topic 260, " Earnings per Share ," which includes guidance pertaining to the warrants issued in connection with the July 3, 2012, December 23, 2014, and July 2, 2015 private placement offerings and the August 4, 2016 underwritten offering, that were exercisable for nominal consideration and, therefore, to the extent not yet exercised are considered in the computation of basic and diluted net loss per common share. As of March 31, 2018, all warrants exercisable for nominal value have been converted into common stock.
The following table is a reconciliation of net (loss) income and the shares used in calculating basic and diluted net (loss) income per common share for the three and nine months ended March 31, 2018 and 2017:
|
Three Months Ended March 31, |
Nine Months Ended March 31, | ||
|
2018 |
2017 |
2018 |
2017 |
|
|
|
|
|
Net (loss) income | $ (739,183 ) | $ (3,564,662 ) | $ 12,894,640 | $ (26,646,851 ) |
|
|
|
|
|
Denominator: |
|
|
|
|
Weighted average common shares outstanding - Basic | 197,485,758 | 196,580,519 | 197,277,286 | 179,841,133 |
|
|
|
|
|
Effect of dilutive shares: |
|
|
|
|
Common stock equivalents arising from stock options, |
|
|
|
|
warrants and conversion of preferred stock | - | - | 3,610,611 | - |
Restricted stock units | - | - | 1,825,066 | - |
Weighted average common shares outstanding - Diluted | 197,485,758 | 196,580,519 | 202,712,963 | 179,841,133 |
|
|
|
|
|
Net (loss) income per common share: |
|
|
|
|
Basic | $ (0.00 ) | $ (0.02 ) | $ 0.07 | $ (0.15 ) |
Diluted | $ (0.00 ) | $ (0.02 ) | $ 0.06 | $ (0.15 ) |
10
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
As of March 31, 2018 and 2017, common shares issuable upon conversion of Series A Convertible Preferred Stock, the exercise of outstanding options and warrants (excluding the Series A 2012, Series B 2012, Series C 2014, Series E 2015 and Series I 2016 warrants issued in connection with the July 3, 2012, December 23, 2014, and July 2, 2015 private placement offerings and the August 4, 2016 underwritten offering as such warrants, to the extent not yet exercised, are already included in the weighted average number of common shares outstanding used in computing basic net (loss) income per common share since they are exercisable for nominal consideration), and the vesting of restricted stock units amounted to an aggregate of 46,773,540 and 36,222,569 shares, respectively, and are excluded from the weighted average number of common shares outstanding used in computing basic net (loss) income per common share. For the nine months ended March 31, 2018, an additional 5,435,677 common shares are included in the computation of diluted EPS using the treasury stock and if-converted methods. However, for the three months ended March 31, 2018 and the three and nine months ended March 31, 2017, no additional common shares were added in the computation of diluted EPS because to do so would have been anti-dilutive.
(4)
NEW AND RECENTLY ADOPTED ACCOUNTING PRONOUNCEMENTS:
In May 2017, the FASB issued ASU No. 2017-09, Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting , which clarifies when to account for a change to the terms or conditions of a share-based payment award as a modification. Under the new guidance, modification accounting is required only if the fair value, the vesting conditions, or the classification of the award (as equity or liability) changes as a result of the change in terms or conditions. It is effective prospectively for the annual period ending June 30, 2019 and interim periods within that annual period. Early adoption is permitted. The Company is currently evaluating the effect that ASU No. 2017-09 will have on its consolidated financial statements and related disclosures.
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments Credit Losses: Measurement of Credit Losses on Financial Instruments, which requires measurement and recognition of expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. This is different from the current guidance as this will require immediate recognition of estimated credit losses expected to occur over the remaining life of many financial assets. The new guidance will be effective for the Company on July 1, 2020. Early adoption will be available on July 1, 2019. The Company is currently evaluating the effect that ASU No. 2016-13 will have on its consolidated financial statements and related disclosures.
In March 2016, the FASB issued ASU No. 2016-09, Compensation – Improvements to Employee Share-Based Payment Accounting , which amends the current guidance related to stock compensation. The updated guidance changes how companies account for certain aspects of share-based payment awards to employees, including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as classification in the statement of cash flows. Under this guidance, on a prospective basis, companies will no longer be able to record excess tax benefits and certain tax deficiencies as additional paid-in capital. Instead, companies will record all excess tax benefits and tax deficiencies as income tax expense or benefit in the income statement. In addition, the guidance eliminates the requirement that excess tax benefits be realized before companies can recognize them. The ASU requires a cumulative-effect adjustment for previously unrecognized excess tax benefits in opening retained earnings in the period of adoption. Effective July 1, 2017, the Company adopted this updated guidance and elected to recognize forfeitures when they occur using a modified retrospective approach. The adoption of ASU No. 2016-09 did not have a material impact on the Company's consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases , related to the recognition of lease assets and lease liabilities. The new guidance requires lessees to recognize almost all leases on their balance sheet as a right-of-use asset and a lease liability, other than leases that meet the definition of a short-term lease and requires expanded disclosures about leasing arrangements. The recognition, measurement, and presentation of expenses and cash flows arising from a lease have not significantly changed from the current guidance. Lessor accounting is similar to the current guidance but updated to align with certain changes to the lessee model and the new revenue recognition standard. The new guidance is effective for the Company on July 1, 2019, with early adoption permitted. The Company is evaluating the impact that ASU No. 2016-02 will have on its consolidated financial statements and related disclosures.
In January 2016, the FASB issued ASU No. 2016-01, Financial Instruments: Recognition and Measurement of Financial Assets and Financial Liabilities . The new guidance relates to the recognition and measurement of financial assets and liabilities. The new guidance makes targeted improvements to U.S. GAAP impacting equity investments (other than those accounted for under the equity method or consolidated), financial liabilities accounted for under the fair value election, and presentation and disclosure requirements for financial instruments, among other changes. The new guidance is effective for the Company on July 1, 2018, with early adoption prohibited other than for certain provisions. The Company is evaluating the impact that ASU No. 2016-01 will have on its consolidated financial statements and related disclosures.
11
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
In November 2015, the FASB issued ASU No. 2015-17, Income Taxes: Balance Sheet Classification of Deferred Taxes, which simplifies the balance sheet classification of deferred taxes. The new guidance requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. The current requirement that deferred tax liabilities and assets of a tax-paying component of an entity be offset and presented as a single amount is not affected by the new guidance. Effective July 1, 2017, the Company adopted this updated guidance, which did not have a material impact on the Company's financial position or results of operations because its net deferred tax assets were fully offset by a valuation allowance based on the history of losses incurred.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers , which requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. In July 2015, the FASB voted to defer the effective date of the new standard until fiscal years beginning after December 15, 2017 with early application permitted for fiscal years beginning after December 15, 2016. With the deferral, the new standard is effective for the Company on July 1, 2018. In addition, in April 2016 the FASB issued ASU No. 2016-10, Identifying Performance Obligations and Licensing , which addresses various issues associated with identifying performance obligations, licensing of intellectual property, royalty considerations, and other matters. ASU No. 2016-10 is effective in connection with ASU No. 2014-09. The two permitted transition methods under ASU 2014-09 are the full retrospective method, in which case the new standard would be applied to each prior period presented and the cumulative effect of applying the standard would be recognized as of the earliest period reported, or the modified retrospective method, in which case the cumulative effect of applying the new standard would be recognized as of the date of initial application. The Company is engaged in an ongoing review to assess the impact to its financial statements, including an assessment of the Company's three key contracts, and is evaluating the effect that these standards will have on its consolidated financial statements and related disclosures.
(5)
AGREEMENT WITH AMAG:
On January 8, 2017, the Company entered into the License Agreement with AMAG. Under the terms of the License Agreement with AMAG, the Company granted to AMAG (i) an exclusive license in all countries of North America (the "Territory"), with the right to grant sub-licenses, to research, develop and commercialize products containing bremelanotide (each a "Product," and collectively, "Products"), (ii) a non-exclusive license in the Territory, with the right to grant sub-licenses, to manufacture Products, and (iii) a non-exclusive license in all countries outside the Territory, with the right to grant sub-licenses, to research, develop and manufacture (but not commercialize) the Products.
Following the satisfaction of certain conditions to closing, the License Agreement with AMAG became effective on February 2, 2017. On that date, AMAG paid the Company $60,000,000 as a one-time initial payment. Pursuant to the terms of and subject to the conditions in the License Agreement with AMAG, AMAG is required to reimburse the Company up to an aggregate amount of $25,000,000 for reasonable, documented, direct out-of-pocket expenses incurred by the Company following February 2, 2017, in connection with the development and regulatory activities necessary to file an NDA for bremelanotide for HSDD in the United States related to Palatin's development obligations.
The Company has determined there is no stand-alone value for the license, and that the license and the reimbursable direct out-of-pocket expenses, pursuant to the terms of the License Agreement with AMAG, represent a combined unit of accounting which totals $85,000,000. The Company is recognizing revenue of the combined unit of accounting over the arrangement using the input-based proportional method as the Company completes its development obligations. For the three and nine months ended March 31, 2018, the Company recognized $8,962,709 and $41,516,370, respectively, as license and contract revenue related to this transaction. As of March 31, 2018, and June 30, 2017, there was $585,519 and $35,050,572, respectively, of current deferred revenue on the consolidated balance sheet related to this transaction.
In addition, pursuant to the terms of and subject to the conditions in the License Agreement with AMAG, the Company is eligible to receive from AMAG: (i) up to $80,000,000 in specified regulatory payments upon achievement of certain regulatory milestones, and (ii) up to $300,000,000 in sales milestone payments based on achievement of annual net sales amounts for all Products in the Territory.
12
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
AMAG is also obligated to pay the Company tiered royalties on annual net sales of Products, on a product-by-product basis, in the Territory ranging from the high single-digits to the low double-digits. The royalties will expire on a product-by-product and country-by-country basis until the latest to occur of (i) the earliest date on which there are no valid claims of the Company's patent rights covering such Product in such country, (ii) the expiration of the regulatory exclusivity period for such Product in such country and (iii) ten years following the first commercial sale of such Product in such country. Such royalties are subject to reductions in the event that: (a) AMAG must license additional third party intellectual property in order to develop, manufacture or commercialize a Product, or (b) generic competition occurs with respect to a Product in a given country, subject to an aggregate cap on such deductions of royalties otherwise payable to the Company. After the expiration of the applicable royalties for any Product in a given country, the license for such Product in such country will become a fully paid-up, royalty-free, perpetual and irrevocable license.
The Company engaged Greenhill & Co. LLC ("Greenhill") as the Company's sole financial advisor in connection with a potential transaction with respect to bremelanotide. Under the engagement agreement with Greenhill, the Company was obligated to pay Greenhill a fee equal to 2% of all proceeds and consideration paid to the Company by AMAG in connection with the License Agreement with AMAG, subject to a minimum fee of $2,500,000. The minimum fee of $2,500,000, less credit of $50,000 for an advisory fee previously paid by the Company, was paid to Greenhill upon the closing of the licensing transaction. This amount will be credited toward amounts that become due to Greenhill in the future, provided that the aggregate fee payable to Greenhill will not be less than 2% of all proceeds and consideration paid to the Company by AMAG in connection with the License Agreement with AMAG. The Company will pay Greenhill an aggregate total of 2% of all proceeds and consideration paid to the Company by AMAG in connection with the License Agreement with AMAG, including future milestone and royalty payments, after crediting the $2,500,000 that was paid to Greenhill upon entering into the License Agreement with AMAG. The Company also reimbursed Greenhill $7,263 for certain expenses incurred in connection with its advisory services.
Pursuant to the License Agreement with AMAG, the Company has assigned to AMAG the Company's manufacturing and supply agreements with Catalent Belgium S.A. to perform fill, finish and packaging of bremelanotide.
(6)
AGREEMENT WITH FOSUN:
On September 6, 2017, the Company entered into the License Agreement with Fosun for exclusive rights to commercialize bremelanotide in the territories of the People's Republic of China, Taiwan, Hong Kong S.A.R. and Macau S.A.R.
Under the terms of the agreement, the Company received $4,500,000 in October 2017, which consisted of an upfront payment of $5,000,000 less $500,000 that was withheld in accordance with tax withholding requirements in China and will be recorded as an expense during the fiscal year ending June 30, 2018. For the three and nine months ended March 31, 2018, the Company recorded an income tax benefit of $15,765 and an income tax expense of $264,202, respectively, utilizing an estimated effective annual income tax rate applied to (loss) income for the three and nine months ended March 31, 2018 and the remaining balance of $235,798 was included in prepaid expenses and other current assets at March 31, 2018. The Company will receive a $7,500,000 milestone payment when regulatory approval in China is obtained, provided that a commercial supply agreement for bremelanotide has been entered into. Palatin has the potential to receive up to $92,500,000 in additional sales related milestone payments and high single-digit to low double-digit royalties on net sales in the licensed territory. All development, regulatory, sales, marketing, and commercial activities and associated costs in the licensed territory will be the sole responsibility of Fosun.
(7)
AGREEMENT WITH KWANGDONG:
On November 21, 2017, the Company entered into the License Agreement with Kwangdong for exclusive rights to commercialize bremelanotide in the Republic of Korea.
Under the terms of the agreement, the Company received $417,500 in December 2017, consisting of an upfront payment of $500,000, less $82,500, which was withheld in accordance with tax withholding requirements in Korea and will be recorded as an expense during the fiscal year ending June 30, 2018. Based upon certain refund provisions, the upfront payment has been recorded as non-current deferred revenue at March 31, 2018. For the three and nine months ended March 31, 2018, the Company recorded an income tax benefit of $2,981 and an income tax expense of $43,187, respectively, utilizing an estimated effective annual income tax rate applied to (loss) income for the three and nine months ended March 31, 2018 and the remaining balance of $39,313 was included in prepaid expenses and other current assets at March 31, 2018. The Company will receive a $3,000,000 milestone payment based on the first commercial sale in Korea. Palatin has the potential to receive up to $37,500,000 in additional sales related milestone payments and mid-single-digit to low double-digit royalties on net sales in the licensed territory. All development, regulatory, sales, marketing, and commercial activities and associated costs in the licensed territory will be the sole responsibility of Kwangdong.
13
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
(8)
PREPAID EXPENSES AND OTHER CURRENT ASSETS:
Prepaid expenses and other current assets consist of the following:
|
March 31, 2018 |
June 30, 2017 |
Clinical study costs | $ 232,508 | $ 657,069 |
Insurance premiums | 5,426 | 182,966 |
Foreign withholding tax (Notes 6 & 7) | 275,111 | - |
Other | 188,411 | 171,186 |
| $ 701,456 | $ 1,011,221 |
(9)
INVESTMENTS:
The following summarizes the carrying value of the Company's available-for-sale investments, which consist of corporate debt securities:
|
March 31, 2018 |
June 30, 2017 |
Cost | $ - | $ 262,023 |
Amortization of premium | - | (11,596 ) |
Gross unrealized loss | - | (590 ) |
Fair value | $ - | $ 249,837 |
(10)
FAIR VALUE MEASUREMENTS:
The fair value of cash equivalents and investments is classified using a hierarchy prioritized based on inputs. Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level 2 inputs are quoted prices for similar assets and liabilities in active markets or inputs that are observable for the asset or liability, either directly or indirectly through market corroboration, for substantially the full term of the financial instrument. Level 3 inputs are unobservable inputs based on management's own assumptions used to measure assets and liabilities at fair value. A financial asset or liability's classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
The following table provides the assets carried at fair value measured on a recurring basis:
| Carrying Value | Quoted prices in active markets (Level 1) | Other quoted/observable inputs (Level 2) | Significant unobservable inputs (Level 3) |
March 31, 2018: |
|
|
|
|
Money market account | $ 25,461,335 | $ 25,461,335 | $ - | $ - |
June 30, 2017: |
|
|
|
|
Money market account | $ 40,019,336 | $ 40,019,336 | $ - | $ - |
Corporate debt securities | 249,837 | 249,837 | - | - |
TOTAL | $ 40,269,173 | $ 40,269,173 | $ - | $ - |
|
|
|
|
|
14
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
(11)
ACCRUED EXPENSES:
Accrued expenses consist of the following:
|
March 31, 2018 |
June 30, 2017 |
Clinical study costs | $ 4,428,593 | $ 9,138,827 |
Other research related expenses | 393,411 | 217,307 |
Professional services | 45,269 | 434,768 |
Other | 1,027,604 | 730,196 |
| $ 5,894,877 | $ 10,521,098 |
(12)
NOTES PAYABLE:
Notes payable consist of the following:
|
March 31, 2018 |
June 30, 2017 |
Notes payable under venture loan | $ 8,333,333 | $ 14,333,334 |
Unamortized related debt discount | (53,558 ) | (143,524 ) |
Unamortized debt issuance costs | (29,770 ) | (83,215 ) |
Notes payable | 8,250,005 | 14,106,595 |
|
|
|
Less: current portion | 6,921,032 | 7,824,935 |
|
|
|
Long-term portion | $ 1,328,973 | $ 6,281,660 |
On December 23, 2014, the Company closed on a $10,000,000 venture loan which was led by Horizon Technology Finance Corporation ("Horizon"). The debt facility is a four year senior secured term loan that bears interest at a floating coupon rate of one-month LIBOR (floor of 0.50%) plus 8.50%, and provides for interest-only payments for the first eighteen months followed by monthly payments of principal of $333,333 plus accrued interest through January 1, 2019. The lenders also received five-year immediately exercisable Series D 2014 warrants to purchase 666,666 shares of common stock exercisable at an exercise price of $0.75 per share. The Company recorded a debt discount of $267,820 equal to the fair value of these warrants at issuance, which is being amortized to interest expense over the term of the related debt. This debt discount is offset against the note payable balance and included in additional paid-in capital on the Company's balance sheet at March 31,2018 and June 30, 2017. In addition, a final incremental payment of $500,000 is due on January 1, 2019, or upon early repayment of the loan. This final incremental payment is being accreted to interest expense over the term of the related debt. The Company incurred $209,367 of costs in connection with the loan. These costs were capitalized as deferred financing costs and are offset against the note payable balance. These debt issuance costs are being amortized to interest expense over the term of the related debt.
On July 2, 2015, the Company closed on a $10,000,000 venture loan led by Horizon. The debt facility is a four-year senior secured term loan that bears interest at a floating coupon rate of one-month LIBOR (floor of 0.50%) plus 8.50% and provides for interest-only payments for the first eighteen months followed by monthly payments of principal of $333,333 plus accrued interest through August 1, 2019. The lenders also received five-year immediately exercisable Series G warrants to purchase 549,450 shares of the Company's common stock exercisable at an exercise price of $0.91 per share. The Company has recorded a debt discount of $305,196 equal to the fair value of these warrants at issuance, which is being amortized to interest expense over the term of the related debt. This debt discount is offset against the note payable balance and is included in additional paid-in capital on the Company's balance sheet at March 31, 2018 and June 30, 2017. In addition, a final incremental payment of $500,000 is due on August 1, 2019, or upon early repayment of the loan. This final incremental payment is being accreted to interest expense over the term of the related debt. The Company incurred $146,115 of costs in connection with the loan agreement. These costs were capitalized as deferred financing costs and are offset against the note payable balance. These debt issuance costs are being amortized to interest expense over the term of the related debt.
15
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
The Company's obligations under the 2015 amended and restated loan agreement, which includes both the 2014 venture loan and the 2015 venture loan, are secured by a first priority security interest in substantially all of its assets other than its intellectual property. The Company also has agreed to specified limitations on pledging or otherwise encumbering its intellectual property assets. The 2015 amended and restated loan agreement include customary affirmative and restrictive covenants, but does not include any covenants to attain or maintain specified financial metrics. The loan agreement includes customary events of default, including payment defaults, breaches of covenants, change of control and a material adverse change default. Upon the occurrence of an event of default and following any applicable cure periods, a default interest rate of an additional 5% may be applied to the outstanding loan balances, and the lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the loan agreement. As of March 31, 2018, the Company was in compliance with all of its loan covenants.
(13)
STOCKHOLDERS' EQUITY (DEFICIENCY):
Financing Transactions – On December 6, 2016, the Company closed on an underwritten public offering of units, with each unit consisting of a share of common stock and a Series J warrant to purchase 0.50 of a share of common stock. Gross proceeds were $16,500,000, with net proceeds to the Company, after deducting underwriting discounts and commissions and offering expenses, of $15,386,075. The Company issued 25,384,616 shares of common stock and Series J warrants to purchase 12,692,310 shares of common stock at an initial exercise price of $0.80 per share, which warrants are exercisable immediately upon issuance and expire on the fifth anniversary of the date of issuance. The Series J warrants are subject to limitation on exercise if the holder and its affiliates would beneficially own more than 9.99%, or 4.99% for certain holders, of the total number of the Company's shares of common stock outstanding following such exercise.
On August 4, 2016, the Company closed on an underwritten offering of units, with each unit consisting of a share of common stock and a Series H warrant to purchase 0.75 of a share of common stock. Investors whose purchase of units in the offering would result in them beneficially owning more than 9.99% of the Company's outstanding common stock following the completion of the offering had the option to acquire units with Series I prefunded warrants substituted for any common stock they would have otherwise acquired. Gross proceeds were $9,225,000, with net proceeds to the Company, after deducting offering expenses, of $8,470,897. The Company issued 11,481,481 shares of common stock and ten-year prefunded Series I warrants to purchase 2,218,045 shares of common stock at an exercise price of $0.01, together with Series H warrants to purchase 10,274,646 shares of common stock at an exercise price of $0.70 per share.
The Series I warrants were exercised during the fiscal year ended June 30, 2017. The Series H warrants are exercisable at an initial exercise price of $0.70 per share, are exercisable commencing six months following the date of issuance and expire on the fifth anniversary of the date of issuance. The Series H warrants are subject to a limitation on their exercise if the holder and its affiliates would beneficially own more than 9.99% of the total number of the Company's shares of common stock outstanding following such exercise.
On July 2, 2015, the Company closed on a private placement of Series E warrants to purchase 21,917,808 shares of Palatin common stock and Series F warrants to purchase 2,191,781 shares of the Company's common stock. Certain funds managed by QVT Financial LP ("QVT") invested $5,000,000 and another accredited investment fund invested $15,000,000. The funds paid $0.90 for each Series E warrant and $0.125 for each Series F warrant, resulting in gross proceeds to the Company of $20,000,000, with net proceeds, after deducting offering expenses, of $19,834,278.
The Series E warrants were exercisable immediately upon issuance at an initial exercise price of $0.01 per share. As of December 31, 2017, all of the Series E warrants were exercised. The Series F warrants are exercisable at an initial exercise price of $0.91 per share, are exercisable immediately upon issuance and expire on the fifth anniversary of the date of issuance. The Series F warrants are subject to limitation on exercise if QVT and its affiliates would beneficially own more than 9.99% (4.99% for the other accredited investment fund holder) of the total number of the Company's shares of common stock outstanding following such exercise.
During the nine months ended March 31, 2018 and 2017, the Company issued 23,344,451 and 27,989,685 shares, respectively of common stock pursuant to the cashless exercise provisions of warrants at an exercise price of $0.01 per share, and during the nine months ended March 31, 2018, the Company received $114,384 and issued 11,438,356 shares of common stock pursuant to the exercise of warrants at an exercise price of $0.01 per share. As of March 31, 2018, all warrants with an exercise price of $0.01 per share had been exercised.
Stock Options – In December 2017, the Company granted 1,200,000 options to its executive officers and 225,000 options to its non-employee directors under the Company's 2011 Stock Incentive Plan. The fair value of these options is $691,171 and $126,130, respectively. The Company is amortizing the fair value of these options over a 48-month vesting period for its executive officers and over a 36-month vesting period for its non-employee directors. The Company recognized $53,709 and $71,613 of stock-based compensation expense related to these options during the three and nine months ended March 31, 2018.
16
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
Also, in December 2017, the Company granted 1,075,000 and 125,000 performance-based options to its executive officers and employees, respectively, which vest during a performance period ending on December 31, 2020, if and upon i) as to 100% of the target number of shares upon achievement of a closing price for the Company's common stock equal to or greater than $1.50 per share for 20 consecutive trading days, which is considered a market condition; ii) as to thirty percent (30%) of the target number of shares, upon the acceptance for filing by the FDA of an NDA for bremelanotide for HSDD in premenopausal women during the performance period, which is considered a performance condition; iii) as to fifty percent (50%) of the target number of shares, upon the approval by the FDA of an NDA for bremelanotide for HSDD in premenopausal women during the performance period, which is also considered a performance condition; iv) as to twenty percent (20%) of the target number of shares, upon entry into a licensing agreement during the performance period for the commercialization of bremelanotide for FSD in at least two of the following geographic areas (a) four or more countries in Europe, (b) Japan, (c) two or more countries in Central and/or South America, (d) two or more countries in Asia, excluding Japan and China, and (e) Australia, which is also considered a performance condition. The fair value of these options, as calculated under a multifactor Monte Carlo simulation, is $602,760. The Company is amortizing the fair value over the derived service period of 1.1 years. The Company recognized $205,743 and $247,777 of stock-based compensation expense related to these options during the three and nine months ended March 31, 2018.
In September 2017, the Company granted 54,000 options to a newly appointed non-employee director under the Company's 2011 Stock Incentive Plan. The Company is amortizing the fair value of these options of $18,176 over a 48-month vesting period. The Company recognized $1,136 and $2,651, respectively, of stock-based compensation expense related to these options during the three and nine months ended March 31, 2018.
In June 2017, the Company granted 1,797,000 options to its executive officers, 780,000 options to its employees and 378,000 options to its non-employee directors under the Company's 2011 Stock Incentive Plan. The Company is amortizing the fair value of these options of $445,533, $194,689 and $89,220, respectively, over the vesting period of the options. The Company recognized $62,096 and $187,109, respectively, of stock-based compensation expense related to these options during the three and nine months ended March 31, 2018.
In September 2016, the Company granted 828,000 options to its executive officers and 336,000 options to its employees under the Company's 2011 Stock Incentive Plan. The Company is amortizing the fair value of the options vesting over a 48-month period, consisting of 595,000 options granted to its executive officers and all options granted to its employees, of $188,245 and $106,303, respectively, over the vesting period. The Company recognized $17,537 and $52,943, respectively, of stock-based compensation expense related to these options during the three and nine months ended March 31, 2018 and $16,426 and $38,210, respectively, during the three and nine months ended March 31, 2017. The remaining 233,000 options granted to the Company's executive officers vested 12 months from the date of grant, and the Company amortized the fair value of these options of $67,160 over this vesting period. The Company recognized $11,193 of stock-based compensation expense related to these options during the nine months ended March 31, 2018 and $16,370 and $36,238, respectively, during the three and nine months ended March 31, 2017.
Unless otherwise stated, stock options granted to the Company's executive officers and employees vest over a 48-month period, while stock options granted to its non-employee directors vest over a 12-month period.
During the three and nine months ended March 31, 2018, the Company received $31,231 and issued 56,400 shares of common stock pursuant to the exercise of options.
Restricted Stock Units – In December 2017, the Company granted 1,200,000 restricted stock units to its executive officers, 225,000 restricted stock units to its non-employee directors and 545,000 restricted stock units to its employees under the Company's 2011 Stock Incentive Plan. The fair value of these restricted stock units is $1,020,000, $191,250 and $463,250, respectively. For executive officers and employees, the restricted stock units vest 25% on the first, second, third and fourth anniversary dates from the date of grant. For non-employee directors, the restricted stock units vest 33 1/3% on the first, second and third anniversary dates from the date of grant. The Company recognized $222,350 and $269,290 of stock-based compensation expense related to these restricted stock units during the three and nine months ended March 31, 2018.
Also, in December 2017, the Company granted 1,075,000 performance-based restricted stock units to its executive officers and 670,000 performance-based restricted stock units to other employees which vest during a performance period, ending on December 31, 2020, if and upon i) as to 100% of the target number of shares upon achievement of a closing price for the Company's common stock equal to or greater than $1.50 per share for 20 consecutive trading days, which is considered a market condition; ii) as to thirty percent (30%) of the target number of shares, upon the acceptance for filing by the FDA of an NDA for bremelanotide for HSDD in premenopausal women during the performance period, which is considered a performance condition; iii) as to fifty percent (50%) of the target number of shares, upon the approval by the FDA of an NDA for bremelanotide for HSDD in premenopausal women during the performance period, which is also considered a performance condition; iv) as to twenty percent (20%) of the target number of shares, upon entry into a licensing agreement during the performance period for the commercialization of bremelanotide for FSD in at least two of the following geographic areas (a) four or more countries in Europe, (b) Japan, (c) two or more countries in Central and/or South America, (d) two or more countries in Asia, excluding Japan and China, and (e) Australia, which is also considered a performance condition. The fair value of these awards, as calculated under a multifactor Monte Carlo simulation, is $913,750 and $569,500, respectively. The Company is amortizing the fair value over the derived service period of 1.1 years. The Company recognized $506,132 and $612,982 of stock-based compensation expense related to these restricted stock units during the three and nine months ended March 31, 2018.
17
PALATIN TECHNOLOGIES, INC.
and Subsidiary
Notes to Consolidated Financial Statements
(unaudited)
In September 2017, the Company granted 54,000 restricted stock units to a newly appointed non-employee director under the Company's 2011 Stock Incentive Plan. The Company is amortizing the fair value of these restricted stock units of $27,000 over a 48- month vesting period. The Company recognized $3,516 and $7,930, respectively, of stock-based compensation expense related to these restricted stock units during the three and nine months ended March 31, 2018.
In June 2017, the Company granted 1,140,000 restricted stock units to its executive officers, 780,000 restricted stock units to its employees and 378,000 restricted stock units to its non-employee directors under the Company's 2011 Stock Incentive Plan. The Company is amortizing the fair value of these restricted stock units of $421,800, $288,600, and $139,860, respectively, over the vesting period. The Company recognized $150,364 and $473,568, respectively, of stock-based compensation expense related to these restricted stock units during the three and nine months ended March 31, 2018.
In September 2016, the Company granted 558,000 restricted stock units to its executive officers, 350,500 of which vest over 24 months and 207,500 of which vested over 12 months, and 336,000 restricted stock units to its employees under the Company's 2011 Stock Incentive Plan. The Company is amortizing the fair value of the restricted stock units of $284,580 and $171,360, respectively, over the vesting periods. The Company recognized $13,228 and $104,711, respectively, of stock-based compensation expense related to these restricted stock units during the three and nine months ended March 31, 2018 and $76,822 and $177,554, respectively, during the three and nine months ended March 31, 2017.
In December 2015, the Company granted 625,000 performance-based restricted stock units to its executive officers and 200,000 performance-based restricted stock units to its employees under the Company's 2011 Stock Incentive Plan, which vest during the performance period, ending December 31, 2017, if and upon the earlier of: i) achievement of a closing price for the Company's common stock equal to or greater than $1.20 per share for 20 consecutive trading days, which is considered a market condition, or ii) entering into a collaboration agreement (U.S. or global) of bremelanotide for FSD, which is considered a performance condition. This performance condition was deemed met as of February 2, 2017, the effective date of the License Agreement with AMAG. Prior to meeting the performance condition, the Company determined that it was not probable of achievement on the date of grant since meeting the condition was outside the control of the Company. The fair value of these awards, as calculated under a multifactor Monte Carlo simulation, was $338,250 and was recognized over the derived service period which was through December 2016. The Company recognized $55,410 and $142,289, respectively, of stock-based compensation expense related to these restricted stock units during the three and six months ended December 31, 2016. Upon the achievement of the performance condition, which occurred in the three-month period ended March 31, 2017, the grant date fair value was utilized and an incremental $222,075 was recognized as stock-based compensation expense during the three months ended March 31, 2017. The Company recognized $364,364 of stock-based compensation expense related to these restricted stock units during the nine months ended March 31, 2017.
Also, in December 2015, the Company granted 625,000 restricted stock units to its executive officers, 340,000 restricted stock units to its non-employee directors and 200,000 restricted stock units to its employees under the Company's 2011 Stock Incentive Plan. For executive officers and employees, the restricted stock units vest 25% on the date of grant and 25% on the first, second and third anniversary dates from the date of grant. For non-employee directors, the restricted stock units vest 50% on the first and second anniversary dates from the date of grant. The Company is amortizing the fair value of these restricted stock units of $425,000, $231,200 and $136,000, respectively, over the vesting period of the restricted stock units. The Company recognized $11,404 and $66,914, respectively, of stock-based compensation expense related to these restricted stock units during the three and nine months ended March 31, 2018 and $41,079 and $228,331, respectively, during the three and nine months ended March 31, 2017.
Unless otherwise stated, restricted stock units granted to the Company's executive officers, employees and non-employee directors vest over 24 months, 48 months and 12 months, respectively.
Stock-based compensation expense for the three and nine months ended March 31, 2018 for equity-based instruments issued other than the stock options and restricted stock units described above was $81,857 and $262,291, respectively, and $178,708 and $560,024, respectively, for the three and nine months ended March 31, 2017.
(14)
SUBSEQUENT EVENTS:
At-the-Market Offering – On April 20, 2018, the Company entered into an equity distribution agreement (the "Equity Distribution Agreement") with Canaccord Genuity LLC ("Canaccord"), pursuant to which the Company may, from time to time, sell shares of the Company's common stock at market prices by methods deemed to be an "at-the-market offering" as defined in Rule 415 promulgated under the Securities Act of 1933, as amended. The Company will pay Canaccord 3.0% of the gross proceeds as a commission. Between April 20, 2018 and May 11, 2018, a total of 203,900 shares of common stock were sold through Canaccord under the Equity Distribution Agreement for net proceeds of $243,696, after payment of commission fees of $7,537. The Company has no obligation to sell any shares under the Equity Distribution Agreement and may at any time suspend solicitation and offers under the Equity Distribution Agreement.
Outstanding Common Stock – Between April 1, 2018 and May 11, 2018, the Company issued 2,995,807 shares of common stock pursuant to the exercise of warrants at an exercise price of $0.80 and received proceeds of $2,396,646 upon exercise.
18
Item 2. Management's Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis should be read in conjunction with the consolidated financial statements and notes to the consolidated financial statements filed as part of this report and the audited consolidated financial statements and notes thereto included in our Annual Report on Form 10-K for the year ended June 30, 2017.
Critical Accounting Policies and Estimates
Our significant accounting policies, which are described in the notes to our consolidated financial statements included in this report and in our Annual Report on Form 10-K for the year ended June 30, 2017, have not changed as of March 31, 2018. We believe that our accounting policies and estimates relating to revenue recognition, accrued expenses and stock-based compensation are the most critical.
Overview
We are a biopharmaceutical company developing targeted, receptor-specific peptide therapeutics for the treatment of diseases with significant unmet medical need and commercial potential. Our programs are based on molecules that modulate the activity of the melanocortin and natriuretic peptide receptor systems. Our lead product in clinical development is bremelanotide for the treatment of premenopausal women with hypoactive sexual desire disorder ("HSDD"), which is a type of female sexual dysfunction ("FSD"), defined as low desire with associated distress. In addition, we have drug candidates and development programs for cardiovascular diseases and inflammatory diseases.
The following drug development programs are actively under development:
●
Bremelanotide, an as-needed subcutaneous injectable product for the treatment of HSDD in premenopausal women. Bremelanotide is a synthetic peptide analog of the naturally occurring hormone alpha-MSH (melanocyte-stimulating hormone). In two pivotal Phase 3 clinical studies of bremelanotide for HSDD in premenopausal women, bremelanotide met the pre-specified co-primary efficacy endpoints of improvement in desire and decrease in distress associated with low sexual desire as measured using validated patient-reported outcome instruments. In March 2018, our exclusive North American licensee for bremelanotide, AMAG, submitted a New Drug Application ("NDA") to the FDA for bremelanotide for the treatment of HSDD in premenopausal women. We have also licensed rights to bremelanotide in China to Fosun and in Korea to Kwangdong.
●
Melanocortin peptide system program, focused on development of treatments for a variety of inflammatory disease indications. PL-8177 is a selective melanocortin receptor 1 ("MC1r") agonist peptide we have designated as our lead clinical development candidate for inflammatory bowel diseases. We have filed an Investigational New Drug ("IND") application and announced dosing of human subjects in a Phase 1 clinical safety study in February 2018. A dual melanocortin receptor 1 and 5 peptide we developed, PL-8331, is a preclinical development candidate for treating ocular inflammation. We anticipate completing IND preclinical enabling activities on PL-8331 later this calendar year; and
●
Natriuretic peptide system program, including PL3994, a natriuretic peptide receptor-A ("NPR-A") agonist, for treatment of cardiovascular indications. PL3994, a synthetic mimetic of the neuropeptide hormone atrial natriuretic peptide ("ANP"), is in development for treatment of heart failure, and is scheduled to start Phase 2A clinical trials later this calendar year. A dual natriuretic peptide receptor A and C agonist we developed, PL-5028, is in preclinical development for cardiovascular diseases, including reducing cardiac hypertrophy and fibrosis. We may file an IND application in the first half of calendar year 2019, and thereafter initiate a Phase 1 clinical safety study.
The following chart illustrates the status of our drug development programs.
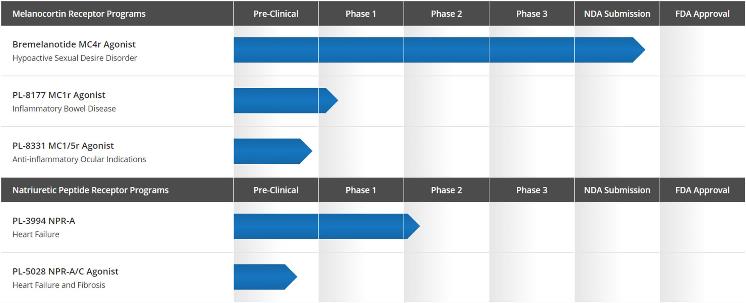
19
Our Phase 3 studies for HSDD in premenopausal women, called the RECONNECT studies, consisted of two double-blind placebo-controlled, randomized parallel group studies comparing the as desired use of 1.75 mg of bremelanotide versus placebo, in each case, delivered via a subcutaneous auto-injector. Each trial consisted of more than 600 patients randomized in a 1:1 ratio to either the treatment arm or placebo with a 24-week evaluation period. In both clinical trials, bremelanotide met the pre-specified co-primary efficacy endpoints of median improvement in desire and decrease in distress associated with low sexual desire as measured using validated patient-reported outcome instruments.
After completion of the trial, women in the trials had the option to continue in an open-label safety extension study for an additional 52 weeks. Nearly 80% of patients who completed the randomized portion of the study elected to remain in the open-label portion of the study.
In the Phase 3 clinical trials, the most frequent adverse events were nausea, flushing, and headache, which were generally mild-to-moderate in intensity and were transient.
We retain worldwide rights for bremelanotide for HSDD and all other indications outside North America, Korea and China. We are actively seeking potential partners for marketing and commercialization rights for bremelanotide for HSDD outside the licensed territories. However, we may not be able to enter into suitable agreements with potential partners on acceptable terms, if at all.
Our Strategy
Key elements of our business strategy include:
●
Using our technology and expertise to develop and commercialize products in our active drug development programs;
●
Entering into strategic alliances and partnerships with pharmaceutical companies to facilitate the development, manufacturing, marketing, sale and distribution of our product candidates;
●
Partially funding our product development programs with the cash flow generated from existing license agreements, as well as any future research, collaboration or license agreements with third parties; and
●
Completing development and seeking regulatory approval of certain of our product candidates.
We incorporated in Delaware in 1986 and commenced operations in the biopharmaceutical area in 1996. Our corporate offices are located at 4B Cedar Brook Drive, Cranbury, New Jersey 08512 and our telephone number is (609) 495-2200. We maintain an Internet site at http://www.palatin.com, where among other things, we make available free of charge on and through this website our Forms 3, 4 and 5, proxy statements, Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d), Section 14A and Section 16 of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Our website and the information contained in it or connected to it are not incorporated into this Quarterly Report on Form 10-Q.
Results of Operations
Three and Nine Months Ended March 31, 2018 Compared to the Three and Nine Months Ended March 31, 2017
Revenue – For the three and nine months ended March 31, 2018, we recognized $8,962,709 and $46,516,370, respectively, in revenue, of which $8,962,709 and $41,516,370, respectively, was attributable to our License Agreement with AMAG. For the nine months ended March 31, 2018, $5,000,000 in revenue was attributable to our License Agreement with Fosun. For the three and nine months ended March 31, 2017 we recognized $10,823,748 in revenue, all of which was attributable to our License Agreement with AMAG.
On January 8, 2017, we entered into the License Agreement with AMAG which provided for $60,000,000 as a one-time initial payment. Pursuant to the terms of and subject to the conditions in the License Agreement with AMAG, AMAG reimbursed us $25,000,000 for reasonable, documented, direct out-of-pocket expenses we incurred following the Effective Date of the License Agreement with AMAG in connection with the development and regulatory activities necessary to file an NDA for bremelanotide for HSDD in the United States less certain other expenses directly paid or to be paid by AMAG.
On September 6, 2017, we entered into the License Agreement with Fosun, for exclusive rights to commercialize bremelanotide in the territories of the People's Republic of China, Taiwan, Hong Kong S.A.R. and Macau S.A.R., which provided for $5,000,000 as a one-time non-refundable upfront payment. Pursuant to the License Agreement with Fosun, $500,000 was withheld in accordance with tax withholding requirements in China and will be recorded as an expense during the fiscal year ending June 30, 2018. For the three and nine months ended March 31, 2018, the Company recorded an income tax benefit of $15,765 and an income tax expense of $264,202, respectively, related to this transaction.
On November 21, 2017, we entered into the License Agreement with Kwangdong, for exclusive rights to commercialize bremelanotide in the Republic of Korea, which provided for a $500,000 as a one-time refundable upfront payment, which has been recorded as non-current deferred revenue as of March 31, 2018. Pursuant to the License Agreement with Kwangdong, $82,500 was withheld in accordance with tax withholding requirements in South Korea and will be recorded as an expense during the fiscal year ending June 30, 2018. For the three and nine months ended March 31, 2018, the Company recorded an income tax benefit of $2,981 and an income tax expense of $43,187, respectively, related to this transaction.
20
Research and Development – Research and development expenses were $7,068,849 and $27,277,830, respectively for the three and nine months ended March 31, 2018, compared to $9,062,316 and $28,422,975, respectively, for the three and nine months ended March 31, 2017.
Research and development expenses related to our bremelanotide, PL-3994, MC1r, MC4r and other preclinical programs were $5,342,113 and $23,377,721, respectively, for the three and nine months ended March 31, 2018, compared to $7,904,690 and $25,206,757, respectively, for the three and nine months ended March 31, 2017. Spending to date has been primarily related to our bremelanotide for the treatment of HSDD program. The fluctuations in research and development expenses for the periods presented are mainly attributable to the progression of the Phase 3 clinical trial and development of bremelanotide for HSDD program. The amount of such spending and the nature of future development activities are dependent on a number of factors, including primarily the availability of funds to support future development activities, success of our clinical trials and preclinical and discovery programs, and our ability to progress compounds in addition to bremelanotide and PL-3994 into human clinical trials.
The amounts of project spending noted above exclude general research and development spending, which was $1,726,736 and $3,900,109, respectively, for the three and nine months ended March 31, 2018 compared to $1,157,626 and $3,216,218, respectively, for the three and nine months ended March 31, 2017. The increase in general research and development spending is primarily attributable to employee-related expenses.
Cumulative spending from inception to March 31, 2018 is approximately $303,100,000 on our bremelanotide program and approximately $128,600,000 on all our other programs (which include PL3994, PL8177, other melanocortin receptor agonists, other discovery programs and terminated programs). Due to various risk factors described herein and in our Annual Report on Form 10-K for the year ended June 30, 2017, under "Risk Factors," including the difficulty in estimating the costs and timing of future Phase 1 clinical trials and larger-scale Phase 2 and Phase 3 clinical trials for any product under development, we cannot predict with reasonable certainty when, if ever, a program will advance to the next stage of development, be successfully completed, or generate net cash inflows.
General and Administrative – General and administrative expenses, which consist mainly of compensation and related costs, were $2,411,302 and $5,581,066, respectively, for the three and nine months ended March 31, 2018 compared to $4,773,696 and $7,289,342, respectively, for the three and nine months ended March 31, 2017. The decrease in general and administrative expenses is primarily attributable to professional services rendered in connection with our License Agreement with AMAG in 2017.
Other Income (Expense) – Other income (expense) was $(240,487) and $(955,445), respectively, for the three and nine months ended March 31, 2018 compared to $(552,398) and $(1,758,282), respectively, for the three and nine months ended March 31, 2017. For the three and nine months ended March 31, 2018, we recognized $86,496 and $219,578, respectively, of investment income offset by $(326,983) and $(1,175,023), respectively, of interest expense primarily related to our venture debt. For the three and nine months ended March 31, 2017, we recognized $6,304 and $18,940, respectively, of investment income offset by $(558,702) and $(1,777,222), respectively, of interest expense primarily related to our venture debt.
Income Tax Benefit – Net income tax benefit was $18,746 and $192,611, respectively, for the three and nine months ended March 31, 2018. Pursuant to the 2017 Tax Act, during the quarter ended December 31, 2017, we recorded a tax benefit of $500,000 related to the release of a valuation allowance against an AMT credit. We filed the June 30, 2017 tax return during the quarter ended March 31, 2018 and as a result was not subject to AMT liability. This benefit is offset by $307,389 in income tax expense for the nine months ended March 31, 2018. We recorded a benefit of $18,746 during the three months ended March 31, 2018 based on its estimated effective tax rate. No income tax expense or benefit was recognized for the three and nine months ended March 31, 2017.
Liquidity and Capital Resources
Since inception, we have incurred net operating losses, primarily related to spending on our research and development programs. We have financed our net operating losses primarily through debt and equity financings and amounts received under collaboration and license agreements.
Our product candidates are at various stages of development and will require significant further research, development and testing and some may never be successfully developed or commercialized. We may experience uncertainties, delays, difficulties and expenses commonly experienced by early stage biopharmaceutical companies, which may include unanticipated problems and additional costs relating to:
●
the development and testing of products in animals and humans;
●
product approval or clearance;
●
regulatory compliance;
●
good manufacturing practices ("GMP") compliance;
●
intellectual property or technology rights;
●
product introduction;
●
marketing, sales and competition; and
●
obtaining sufficient capital.
21
Failure to enter into or successfully perform under collaboration agreements and obtain timely regulatory approval for our product candidates and indications would impact our ability to increase revenues and could make it more difficult to attract investment capital for funding our operations. Any of these possibilities could materially and adversely affect our operations and require us to curtail or cease certain programs.
During the nine months ended March 31, 2018, cash used in operating activities was $8,790,792, compared to cash provided by operating activities of $25,768,523 for the nine months ended March 31, 2017. Higher net cash outflows from operations in the nine months ended March 31, 2018 was primarily the result of the cash payments received in the prior period relating to our license agreement with AMAG. Our periodic prepaid expenses, accounts payable and accrued expenses balances will continue to be highly dependent on the timing of our operating costs.
During the nine months ended March 31, 2018, cash provided by investing activities was $240,500, consisting of $250,000 in proceeds from the maturity of investments offset by $9,500, which was used for the purchase of equipment. During the nine months ended March 31, 2017, cash provided by investing activities was $450,000 relating to proceeds from the maturity of investments.
During the nine months ended March 31, 2018, net cash used in financing activities was $5,913,874, which consisted of $6,000,000 for the payment on notes payable, and $59,489 for capital lease payments and the payment of withholding taxes related to restricted stock units, offset by proceeds from the exercise of options and warrants of $145,615. During the nine months ended March 31, 2017 net cash provided by financing activities was $20,284,024, which consisted of net proceeds from our underwritten offerings in August and December 2016 of $23,856,972 and proceeds from the exercise of warrants of $114,358 offset by $3,687,306 for the payment on notes payable, capital lease payments and payment of withholding taxes.
We have incurred cumulative negative cash flows from operations since our inception, and have expended, and expect to continue to expend in the future, substantial funds to complete our planned product development efforts. Continued operations are dependent upon our ability to complete equity or debt financing activities, entering into licensing agreements or collaboration arrangements. As of March 31, 2018, our cash and cash equivalents were $25,736,158 and our current liabilities were $13,609,551, net of deferred revenue of $585,519.
We intend to utilize existing capital resources and sales of stock under the equity distribution agreement for general corporate purposes and working capital, and preclinical and clinical development of our MC1r and MC4r peptide programs and PL-3994 natriuretic peptide, and development of other portfolio products.
We believe that our existing capital resources, together with proceeds received from sales of common stock in our "at-the-market" program (if any), will be adequate to fund our planned operations through at least June 30, 2019. We will need additional funding to complete required clinical trials for our product candidates and development programs other than bremelanotide and, if those clinical trials are successful (which we cannot predict), to complete submission of required regulatory applications to the FDA.
We anticipate incurring additional losses over at least the next several years. To achieve or maintain profitability, if ever, we, alone or with others, must successfully develop and commercialize our technologies and proposed products, conduct preclinical studies and clinical trials, obtain required regulatory approvals and successfully manufacture and market our technologies and proposed products. The time required to reach sustained profitability is highly uncertain, and we do not know whether we will be able to achieve profitability on a sustained basis, if at all.
Off-Balance Sheet Arrangements
None.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not required to be provided by smaller reporting companies.
Item 4. Controls and Procedures.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures, as defined in Exchange Act Rules 13a-15(e) and 15d-15(e), as of the end of the period covered by this report. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of March 31, 2018. There were no changes in our internal control over financial reporting that occurred during our most recent fiscal quarter that materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
22
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
We may be involved, from time to time, in various claims and legal proceedings arising in the ordinary course of our business. We are not currently a party to any claim or legal proceeding.
Item 1A. Risk Factors.
This report and other documents we file with the SEC contain forward-looking statements that are based on current expectations, estimates, forecasts and projections about us, our future performance, our business, our beliefs and our management's assumptions. These statements are not guarantees of future performance, and they involve certain risks, uncertainties and assumptions that are difficult to predict. You should carefully consider the risks and uncertainties described below and the other information in this Quarterly Report on Form 10-Q, including our unaudited condensed financial statements and the related notes thereto and "Management's Discussion and Analysis of Financial Condition and Results of Operation."
Risks Related to Our Financial Results and Need for Financing
We have a history of substantial net losses, may continue to incur substantial net losses over the next few years, and we may never achieve or maintain profitability.
As of March 31, 2018, we had an accumulated deficit of $343.9 million, with net income for the nine months ended March 31, 2018 of $12.9 million. We may not achieve or sustain profitability in future years, which is dependent on numerous factors, including whether and when development and sales milestones are met, regulatory actions by the FDA and other regulatory bodies, the performance of our licensees, and market acceptance of our products. If we attain sustained profitability, it will not be until the fiscal year ending June 30, 2019 at the earliest, and then only if the FDA approves the NDA on bremelanotide for HSDD.
We expect to incur additional losses as we continue our development of natriuretic peptide and MC1r products. These losses, among other things, have had and will continue to have an adverse effect on our stockholders' equity, total assets and working capital.
Since 2005 we have not had any products available for commercial sale and have not received any revenues from the sale of our product candidates. For the foreseeable future, we will have to fund all of our operations and capital expenditures from license and contract revenue under collaborative development agreements, existing cash balances and outside sources of financing, which may not be available on acceptable terms, if at all. Unless and until we receive approval from the FDA or other equivalent regulatory authorities outside the United States, we cannot sell our products and will not have product revenues from them. We have devoted substantially all of our efforts to research and development, including preclinical and clinical trials. Because of the numerous risks associated with developing drugs, we are unable to predict the extent of future losses, whether or when any of our product candidates will become commercially available, or when we will become profitable, if at all.
We will need additional funding, including funding to complete clinical trials for our product candidates other than bremelanotide, which may not be available on acceptable terms, if at all.
We intend to focus future efforts on our product candidates other than bremelanotide, including our MC1r and natriuretic peptide programs. As of March 31, 2018, we had cash and cash equivalents of $25.7 million, with current liabilities of $13.6 million, net of deferred revenue of $0.6 million. We believe we currently have sufficient existing capital resources, together with proceeds received from sales of common stock in our "at-the-market" program (if any), to fund our planned operations through at least June 30, 2019. We will need additional funding to complete development activities and required clinical trials for our other product candidates and development programs and, if those clinical trials are successful (which we cannot predict), to complete submission of required regulatory applications to the FDA.
Until the FDA approves bremelanotide for HSDD and marketing commences, if at all, we will not have any recurring revenue. Even if bremelanotide is approved and marketing commences, we cannot predict product sales or our resulting royalties, so we may not have any source of significant recurring revenue and may need to depend on financing or partnering to sustain our operations. We may raise additional funds through public or private equity or debt financings, collaborative arrangements on our product candidates, or other sources. However, such financing arrangements may not be available on acceptable terms, or at all. To obtain additional funding, we may need to enter into arrangements that require us to develop only certain of our product candidates or relinquish rights to certain technologies, product candidates and/or potential markets.
If we are unable to raise sufficient additional funds when needed, we may be required to curtail operations significantly, cease clinical trials and decrease staffing levels. We may seek to license, sell or otherwise dispose of our product candidates, technologies and contractual rights on the best possible terms available. Even if we are able to license, sell or otherwise dispose of our product candidates, technologies and contractual rights, it is likely to be on unfavorable terms and for less value than if we had the financial resources to develop or otherwise advance our product candidates, technologies and contractual rights ourselves.
23
Our future capital requirements depend on many factors, including:
●
our ability to enter into one or more licensing or similar agreements for bremelanotide outside of North America, Korea and China;
●
the timing of, and the costs involved in, obtaining regulatory approvals for bremelanotide for HSDD and our other product candidates;
●
the number and characteristics of any additional product candidates we develop or acquire;
●
the scope, progress, results and costs of researching and developing our future product candidates, and conducting preclinical and clinical trials;
●
the cost of commercialization activities if any future product candidates are approved for sale, including marketing, sales and distribution costs;
●
the cost of manufacturing any future product candidates and any products we successfully commercialize;
●
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the terms and timing of such arrangements;
●
the degree and rate of market acceptance of any future approved products;
●
the emergence, approval, availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing products or treatments;
●
any product liability or other lawsuits related to our products;
●
the expenses needed to attract and retain skilled personnel;
●
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and
●
the timing, receipt and amount of sales of, or royalties on, future approved products, if any.
We have a limited operating history upon which to base an investment decision.
Our operations are primarily focused on acquiring, developing and securing our proprietary technology, conducting preclinical and clinical studies and formulating and manufacturing, through contract manufacturers, our principal product candidates on a small-scale basis. These operations provide a limited basis for stockholders to assess our ability to commercialize our product candidates.
While we have completed Phase 3 clinical trials on bremelanotide for HSDD in premenopausal women, and with AMAG have filed an NDA on bremelanotide for HSDD with the FDA, we have not yet demonstrated our ability to perform the functions necessary for the successful commercialization of any of our current product candidates. The successful commercialization of our product candidates will require us to perform a variety of functions, including:
●
continuing to conduct preclinical development and clinical trials;
●
participating in regulatory approval processes;
●
formulating and manufacturing products, or having third parties formulate and manufacture products;
●
post-approval monitoring and surveillance of our products;
●
conducting sales and marketing activities, either alone or with a partner; and
●
obtaining additional capital.
If we are unable to obtain regulatory approval of any of our product candidates, to successfully commercialize any products for which we receive regulatory approval or to obtain additional capital, we may not be able to recover our investment in our development efforts.
The clinical and commercial success of our product candidates will depend on a number of factors, including the following:
●
the ability to raise additional capital on acceptable terms, or at all;
●
timely completion of our clinical trials, which may be significantly slower or cost more than we currently anticipate and will depend substantially upon the performance of third-party contractors;
●
whether we are required by the FDA or similar foreign regulatory agencies to conduct additional clinical trials beyond those planned to support the approval and commercialization of our product candidates or any future product candidates;
●
acceptance of our proposed indications and primary endpoint assessments relating to the proposed indications of our product candidates by the FDA and similar foreign regulatory authorities;
●
our ability to demonstrate to the satisfaction of the FDA and similar foreign regulatory authorities, the safety and efficacy of our product candidates or any future product candidates;
●
the prevalence, duration and severity of potential side effects experienced with our product candidates or future approved products, if any;
24
●
the timely receipt of necessary marketing approvals from the FDA and similar foreign regulatory authorities;
●
achieving and maintaining, and, where applicable, ensuring that our third-party contractors achieve and maintain, compliance with our contractual obligations and with all regulatory requirements applicable to our product candidates or any future product candidates or approved products, if any;
●
the ability of third parties with whom we contract to manufacture clinical trial and commercial supplies of our product candidates or any future product candidates, remain in good standing with regulatory agencies and develop, validate and maintain commercially viable manufacturing processes that are compliant the FDA's current Good Manufacturing Practices ("GMP") regulations;
●
a continued acceptable safety profile and efficacy during clinical development and following approval of our product candidates or any future product candidates;
●
our ability to successfully commercialize our product candidates or any future product candidates in the United States and internationally, if approved for marketing, sale and distribution in such countries and territories, whether alone or in collaboration with others;
●
acceptance by physicians and patients of the benefits, safety and efficacy of our product candidates or any future product candidates, if approved, including relative to alternative and competing treatments;
●
our and our partners' ability to establish and enforce intellectual property rights in and to our product candidates or any future product candidates;
●
our and our partners' ability to avoid third-party patent interference or intellectual property infringement claims; and
●
our ability to in-license or acquire additional product candidates or commercial-stage products that we believe can be successfully developed and commercialized.
If we do not achieve one or more of these factors, many of which are beyond our control, in a timely manner or at all, we could experience significant delays or an inability to obtain regulatory approvals or commercialize our product candidates. Even if regulatory approvals are obtained, we may never be able to successfully commercialize any of our product candidates. Accordingly, we cannot assure you that we will be able to generate sufficient revenue through the sale of our product candidates or any future product candidates to continue our business.
Raising additional capital may cause dilution to existing shareholders, restrict our operations or require us to relinquish rights.
We may seek the additional capital necessary to fund our operations through public or private equity offerings, collaboration agreements, debt financings or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing shareholders' ownership interests will be diluted and the terms may include liquidation or other preferences that adversely affect their rights as a shareholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
Risks Related to Our Business, Strategy and Industry
We are substantially dependent on the clinical and commercial success of our product candidates, primarily our lead product candidate, bremelanotide for HSDD, but we and our licensees may never obtain regulatory approval for or successfully commercialize bremelanotide for HSDD or any of our product candidates.
To date, we have invested most of our efforts and financial resources in the research and development of bremelanotide for HSDD, which is currently our lead product candidate. We licensed all rights to bremelanotide in North America to AMAG, and were contractually obligated to complete development and regulatory activities necessary to file an NDA for bremelanotide for HSDD in the United States, for which AMAG reimbursed us for $25 million of expenses we incurred less certain other expenses directly paid or to be paid by AMAG. We licensed rights to bremelanotide for China to Fosun and licensed rights to bremelanotide for Korea to Kwangdong, but depending on the regulatory approval pathway utilized in China or Korea, approval in China or Korea may be contingent on approval of an NDA for bremelanotide for HSDD in the United States.
Our near-term prospects, including our ability to finance our company and generate revenue, will depend heavily on the successful development, regulatory approval and commercialization of bremelanotide for HSDD, as well as any future product candidates. The clinical and commercial success of our product candidates will depend on a number of factors, including the following:
25
●
timely completion of, or need to conduct additional clinical trials and studies, for our product candidates, which may be significantly slower or cost more than we currently anticipate and will depend substantially upon the accurate and satisfactory performance of third-party contractors;
●
the ability to demonstrate to the satisfaction of the FDA the safety and efficacy of bremelanotide for HSDD or any future product candidates through clinical trials;
●
whether we or our licensees are required by the FDA or other similar foreign regulatory agencies to conduct additional clinical trials to support the approval of bremelanotide for HSDD or any future product candidates;
●
the acceptance of parameters for regulatory approval, including our proposed indication, primary endpoint assessment and primary endpoint measurement, relating to our lead indications of bremelanotide for HSDD;
●
the success of our licensees in educating physicians and patients about the benefits, administration and use of bremelanotide for HSDD, if approved;
●
the prevalence and severity of adverse events experienced with bremelanotide for HSDD or any future product candidates or approved products;
●
the adequacy and regulatory compliance of the autoinjector device, supplied by an unaffiliated third party, to be used as part of the bremelanotide combination product;
●
the timely receipt of necessary marketing approvals from the FDA and similar foreign regulatory authorities;
●
our ability to raise additional capital on acceptable terms to achieve our goals;
●
achieving and maintaining compliance with all regulatory requirements applicable to bremelanotide for HSDD or any future product candidates or approved products;
●
the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments;
●
the effectiveness of our own or our future potential strategic collaborators' marketing, sales and distribution strategy and operations;
●
the ability to manufacture clinical trial supplies of bremelanotide for HSDD or any future product candidates and to develop, validate and maintain a commercially viable manufacturing process that is compliant with current GMP;
●
the ability of AMAG to successfully commercialize bremelanotide for HSDD;
●
our ability to successfully commercialize any future product candidates, if approved for marketing and sale, whether alone or in collaboration with others;
●
our ability to enforce our intellectual property rights in and to bremelanotide for HSDD or any future product candidates;
●
our ability to avoid third-party patent interference or intellectual property infringement claims;
●
acceptance of bremelanotide for HSDD or any future product candidates, if approved, as safe and effective by patients and the medical community; and
●
a continued acceptable safety profile and efficacy of bremelanotide for HSDD or any future product candidates following approval.
If we fail to satisfy any one of these prerequisites to our commercial success, many of which are beyond our control, in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates. Accordingly, we cannot assure you that we will be able to generate sufficient revenue through the sale of bremelanotide for HSDD by AMAG, Fosun and Kwangdong or through the sale of any future product candidate to continue our business. In addition to preventing us from executing our current business plan, any delays in our clinical trials, or inability to successfully commercialize our products could impair our reputation in the industry and the investment community and could hinder our ability to fulfill our existing contractual commitments. As a result, our share price would likely decline significantly, and we would have difficulty raising necessary capital for future projects.
We do not control the development or commercialization of bremelanotide in North America, which is licensed to AMAG, and as a result we may not realize a significant portion of the potential value of the license arrangement with AMAG.
Although we conducted development work to support an NDA for bremelanotide in HSDD, the license agreement with AMAG for bremelanotide in North America limits our control over development activities, including regulatory approvals, and we do not have any direct control over commercialization efforts. AMAG may abandon further development of bremelanotide in its licensed territory, including terminating the agreement, for any reason, including a change of priorities within AMAG or lack of success in ancillary clinical trials necessary to obtain regulatory approvals. Because the potential value of the license arrangement with AMAG is contingent upon the successful development and commercialization of bremelanotide in the United States and other countries in the licensed territory, the ultimate value of this license will depend on the efforts of AMAG. If AMAG does not succeed in obtaining regulatory approval of bremelanotide in the United States for any reason, does not succeed in securing market acceptance of bremelanotide in the United States, or elects for any reason to discontinue marketing of bremelanotide, we will be unable to realize the potential value of this arrangement and would experience significant delays or an inability to successfully commercialize bremelanotide.
26
Production and supply of bremelanotide depend on contract manufacturers over whom neither we nor AMAG have any control, and we may not have adequate supplies of bremelanotide.
We do not have the facilities to manufacture the bremelanotide active drug ingredient or the autoinjector pen component of the bremelanotide combination product, or to fill, assemble and package the bremelanotide combination product. AMAG, our exclusive licensee for North America for bremelanotide, has assumed responsibility for contract manufacturing. The contract manufacturers must perform these manufacturing activities in a manner that complies with FDA regulations. AMAG's ability to control third-party compliance with FDA requirements is limited to contractual remedies and rights of inspection. The manufacturers of approved products and their manufacturing facilities will be subject to ongoing review and periodic inspections by the FDA and other authorities where applicable, and must comply with regulatory requirements, including FDA regulations concerning GMP. Failure of third-party manufacturers to comply with GMP, medical device quality system regulations, or other FDA requirements may result in enforcement action by the FDA. Failure to conduct their activities in compliance with FDA regulations could delay bremelanotide development programs or negatively impact AMAG's ability to receive FDA approval of the bremelanotide potential products or continue marketing bremelanotide products if they are approved. Establishing relationships with new suppliers, who must be FDA-approved, is a time-consuming and costly process. If AMAG is not able to obtain adequate supplies of bremelanotide, it will be difficult for AMAG to develop bremelanotide and compete effectively.
Our product candidates other than bremelanotide are still in the early stages of development and remain subject to clinical testing and regulatory approval. If we are unable to successfully develop and test our product candidates, we will not be successful.
Our product candidates are at various stages of research and development, will require regulatory approval, and may never be successfully developed or commercialized. Our product candidates will require significant further research, development and testing before we can seek regulatory approval to market and sell them. We must demonstrate that our product candidates are safe and effective for use in patients in order to receive regulatory approval for commercial sale. Preclinical studies in animals, using various doses and formulations, must be performed before we can begin human clinical trials. Even if we obtain favorable results in the preclinical studies, the results in humans may be different. Numerous small-scale human clinical trials may be necessary to obtain initial data on a product candidate's safety and efficacy in humans before advancing to large scale human clinical trials. We face the risk that the results of our trials in later phases of clinical trials may be inconsistent with those obtained in earlier phases. Adverse or inconclusive results could delay the progress of our development programs and may prevent us from filing for regulatory approval of our product candidates. Additional factors that could inhibit the successful development of our product candidates include:
●
lack of effectiveness of any product candidate during clinical trials or the failure of our product candidates to meet specified endpoints;
●
failure to design appropriate clinical trial protocols;
●
uncertainty regarding proper dosing;
●
inability to develop or obtain a supplier for an autoinjector device that meets the FDA's medical device requirements;
●
insufficient data to support regulatory approval;
●
inability or unwillingness of medical investigators to follow our clinical protocols;
●
inability to add a sufficient number of clinical trial sites; or
●
the availability of sufficient capital to sustain operations and clinical trials.
You should evaluate us in light of these uncertainties, difficulties and expenses commonly experienced by early stage biopharmaceutical companies, as well as unanticipated problems and additional costs relating to:
●
product approval or clearance;
●
regulatory compliance;
●
good manufacturing practices;
●
intellectual property rights;
●
product introduction; and
●
marketing and competition.
27
If clinical trials for our product candidates are prolonged or delayed, we may be unable to commercialize our product candidates on a timely basis, which would require us to incur additional costs and delay our receipt of any revenue from potential product sales.
We may be unable to commercialize our product candidates on a timely basis due to unexpected delays in our human clinical trials. Potential delaying events include:
●
discovery of serious or unexpected toxicities or side effects experienced by study participants or other safety issues;
●
slower than expected rates of subject recruitment and enrollment rates in clinical trials resulting from numerous factors, including the prevalence of other companies' clinical trials for their product candidates for the same indication, or clinical trials for indications for which patients do not as commonly seek treatment;
●
difficulty in retaining subjects who have initiated a clinical trial but may withdraw at any time due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or for any other reason;
●
difficulty in obtaining institutional review board ("IRB") approval for studies to be conducted at each site;
●
delays in manufacturing or obtaining, or inability to manufacture or obtain, sufficient quantities of materials for use in clinical trials;
●
inadequacy of or changes in our manufacturing process or the product formulation or method of delivery;
●
changes in applicable laws, regulations and regulatory policies;
●
delays or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective contract research organizations ("CROs"), clinical trial sites and other third-party contractors;
●
failure of our CROs or other third-party contractors to comply with contractual and regulatory requirements or to perform their services in a timely or acceptable manner;
●
failure by us, our employees, our CROs or their employees or any partner with which we may collaborate or their employees to comply with applicable FDA or other regulatory requirements relating to the conduct of clinical trials or the handling, storage, security and recordkeeping for drug, medical device and biologic products;
●
delays in the scheduling and performance by the FDA of required inspections of us, our CROs, our suppliers, or our clinical trial sites, and violations of law or regulations by discovered in the course of FDA inspections;
●
scheduling conflicts with participating clinicians and clinical institutions; or
●
difficulty in maintaining contact with subjects during or after treatment, which may result in incomplete data.
Any of these events or other delaying events, individually or in the aggregate, could delay the commercialization of our product candidates and have a material adverse effect on our business, results of operations and financial condition.
We may not be able to secure and maintain relationships with research institutions and organizations to conduct our clinical trials.
We rely on research institutions and organizations to conduct our clinical trials, and we therefore have limited control over the timing and cost of clinical trials and our ability to recruit subjects. If we are unable to reach agreements with suitable research institutions or organizations on acceptable terms, or if any such agreement is terminated, we may be unable to quickly replace the research institution or organization with another qualified institution or organization on acceptable terms. We may not be able to secure and maintain suitable research institutions or organizations to conduct our clinical trials.
Even if our product candidates receive regulatory approval, they may never achieve market acceptance, in which case our business, financial condition and results of operation will be materially adversely affected.
Regulatory approval for the marketing and sale of any of our product candidates does not assure the product's commercial success. Any approved product will compete with other products manufactured and marketed by major pharmaceutical and other biotechnology companies. If any of our product candidates are approved by the FDA and do not achieve adequate market acceptance, our business, financial condition and results of operations will be materially adversely affected. The degree of market acceptance of any such product will depend on a number of factors, including:
●
perceptions by members of the healthcare community, including physicians, about the safety and effectiveness of any such product;
●
cost-effectiveness relative to competing products and technologies;
●
availability of reimbursement for our products from third-party payers such as health insurers, health maintenance organizations ("HMOs") and government programs such as Medicare and Medicaid; and
●
advantages over alternative treatment methods.
There is one FDA approved product for treatment of HSDD, flibanserin, which is sold under the trade name Addyi®, and started marketing in October 2015. The actual market size and market dynamics for HSDD are unknown, and there is significant uncertainty concerning the extent and scope of third-party reimbursement for products treating HSDD. While we believe that an on-demand drug for HSDD has competitive advantages compared to chronic or daily use hormones and other drugs, we may not be able to realize this perceived advantage in the market. Bremelanotide is administered by subcutaneous injection. While the single-use, disposable autoinjector pen format is designed to maximize market acceptability, bremelanotide as a subcutaneous injectable drug for HSDD may never achieve significant market acceptance. In addition, we believe reimbursement of bremelanotide from third-party payers such as health insurers, HMOs or other third-party payers of healthcare costs will be similar to reimbursement for erectile dysfunction ("ED") drugs, and that the ultimate user may pay a substantial part of the cost of bremelanotide for HSDD. If the market opportunity for bremelanotide is smaller than we anticipate, it may also be difficult for us to find marketing partners and, as a result, we may be unable to generate revenue and business from bremelanotide. If bremelanotide for HSDD does not achieve adequate market acceptance at an acceptable price point, our business, financial condition and results of operations will be materially adversely affected.
28
Even if our product candidates receive regulatory approval in the United States, we may never receive approval or commercialize our products outside of the United States.
In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. Failure to obtain regulatory approval in other countries or any delay or setbacks in obtaining such approval would impair our ability to develop foreign markets for our product candidates and may have a material adverse effect on our results of operations and financial condition.
If side effects emerge that can be linked to our product candidates (either while they are in development or after they are approved and on the market), we may be required to perform lengthy additional clinical trials, change the labeling of any such products, or withdraw such products from the market, any of which would hinder or preclude our ability to generate revenues.
If we identify side effects or other problems occur in future clinical trials, we may be required to terminate or delay clinical development of the product candidate. Furthermore, even if any of our product candidates receive marketing approval, as greater numbers of patients use a drug following its approval, if the incidence of side effects increases or if other problems are observed after approval that were not seen or anticipated during pre-approval clinical trials, a number of potentially significant negative consequences could result, including:
●
regulatory authorities may withdraw their approval of the product;
●
we may be required to reformulate such products or change the way the product is manufactured;
●
we may become the target of lawsuits, including class action suits; and
●
our reputation in the market place may suffer resulting in a significant drop in the sales of such products.
Any of these events could substantially increase the costs and expenses of developing, commercializing and marketing any such product candidates or could harm or prevent sales of any approved products.
The number of subjects in our study pools in our clinical trials may be deemed by regulators to be too small, which could cause unanticipated delays or higher than anticipated costs.
Our clinical trials have been conducted on a pool of subjects that is structured for such research. Nevertheless, there is the possibility that for statistical reasons, the pool of subjects may be determined by the FDA or another regulatory body to be too small to verify statistical significance. In such a case, the conclusions from the previous trials will need to be established with at least another set of clinical trials testing the relevant issue. Due to the need to find new subjects for any additional clinical trials and the limited pool from which such subjects can be selected, any such determination by the FDA could result in a delay in obtaining FDA approval or require additional financial expenditures.
We may not be able to keep up with the rapid technological change in the biotechnology and pharmaceutical industries, which could make any future approved products obsolete and reduce our revenue.
Biotechnology and related pharmaceutical technologies have undergone and continue to be subject to rapid and significant change. Our future will depend in large part on our ability to maintain a competitive position with respect to these technologies. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive from our research approach and proprietary technologies. In addition, any future products that we develop, including our clinical product candidates, may become obsolete before we recover expenses incurred in developing those products, which may require that we raise additional funds to continue our operations.
Competing products and technologies may make our proposed products noncompetitive.
Flibanserin, a daily-use oral drug sold under the trade name Addyi®, has been approved by the FDA for HSDD in premenopausal women. There are other products being developed for HSDD and other FSD indications, including a number of oral combination drugs, some of which incorporate testosterone, antidepressants or PDE-5 inhibitors. There is competition to develop drugs for treatment of HSDD and FSD in both premenopausal and postmenopausal patients. Our bremelanotide drug product is intended to be administered by subcutaneous injection, and an as desired drug product for the same indication which utilizes another route of administration, such as a conventional oral drug product, may make subcutaneous bremelanotide noncompetitive.
29
There are several products approved for use in treatment of obesity and related indications, and a number of other products being developed for treatment of obesity, including products in clinical trials. There is intense competition to develop drugs for treatment of obesity and related indications.
There are a number of products approved for use in treating inflammatory diseases and indications, and other products are being developed, including products in clinical trials.
We are aware of one recombinant natriuretic peptide product for acutely decompensated congestive heart failure approved and marketed in the United States, and another recombinant natriuretic peptide product approved and marketed in Japan. Clinical trials on other natriuretic peptide products are being conducted in the United States. In addition, other products for treatment of heart failure are either currently being marketed or in development, including a combination drug which increases active levels of ANP.
As discussed above, the biopharmaceutical industry is highly competitive. We are likely to encounter significant competition with respect to bremelanotide, other melanocortin receptor agonist compounds and PL-3994. Most of our competitors have substantially greater financial and technological resources than we do. Many of them also have significantly greater experience in research and development, marketing, distribution and sales than we do. Accordingly, our competitors may succeed in developing, marketing, distributing and selling products and underlying technologies more rapidly than we can. These competitive products or technologies may be more effective and useful or less costly than bremelanotide, other melanocortin receptor agonist compounds or PL-3994. In addition, academic institutions, hospitals, governmental agencies and other public and private research organizations are also conducting research and may develop competing products or technologies on their own or through strategic alliances or collaborative arrangements.
We rely on third parties over whom we have no control to conduct preclinical studies, clinical trials and other research for our product candidates and their failure to timely perform their obligations could significantly harm our product development.
We have limited research and development staff and do not have dedicated research or development facilities. We rely on third parties and independent contractors, such as researchers at CROs and universities, in certain areas that are particularly relevant to our research and product development plans. We engage such researchers to conduct our preclinical studies, clinical trials and associated tests. These outside contractors are not our employees and may terminate their engagements with us at any time. In addition, we have limited control over the resources that these contractors devote to our programs and they may not assign as great a priority to our programs or pursue them as diligently as we would if we were undertaking such programs ourselves. There is also competition for these relationships, and we may not be able to maintain our relationships with our contractors on acceptable terms. If our third-party contractors do not carry out their duties under their agreements with us, fail to meet expected deadlines or fail to comply with appropriate standards for preclinical or clinical research, our ability to develop our product candidates and obtain regulatory approval on a timely basis, if at all, may be materially adversely affected.
Production and supply of our product candidates depend on contract manufacturers over whom we have no control, with the risk that we may not have adequate supplies of our product candidates or products.
We do not have the facilities to manufacture our early stage potential products such as PL-3994, PL-8177 and other melanocortin receptor agonist compounds for use in preclinical studies and clinical trials. Contract manufacturers must perform these manufacturing activities in a manner that complies with FDA regulations. Our ability to control third-party compliance with FDA requirements is limited to contractual remedies and rights of inspection. The manufacturers of our potential products and their manufacturing facilities will be subject to continual review and periodic inspections by the FDA and other authorities where applicable, and must comply with ongoing regulatory requirements, including FDA regulations concerning GMP. Failure of third-party manufacturers to comply with GMP, medical device quality system regulations, or other FDA requirements may result in enforcement action by the FDA. Failure to conduct their activities in compliance with FDA regulations could delay our development programs or negatively impact our ability to receive FDA approval of our potential products. Establishing relationships with new suppliers, who must be FDA-approved, is a time-consuming and costly process.
If we are unable to establish sales and marketing capabilities within our organization or enter into and maintain agreements with third parties to market and sell our product candidates, we may be unable to generate product revenue.
We do not currently have any experience in sales, marketing and distribution of pharmaceutical products. We will need to establish sales and marketing capabilities within our organization or establish and maintain agreements with third parties to market and sell our product candidates. We do not have marketing partners for any of our products, including bremelanotide and PL-3994. If any of these products are approved by the FDA or other regulatory authorities, we must either develop marketing, distribution and selling capacity and expertise, which will be costly and time consuming, or enter into agreements with other companies to provide these capabilities. We may not be able to enter into suitable agreements on acceptable terms, if at all. Engaging a third party to perform these services could delay the commercialization of any of our product candidates, if approved for commercial sale. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and our business would suffer. In addition, if we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues are likely to be lower than if we could market and sell any products that we develop ourselves.
30
We will need to hire additional employees in order to commercialize our product candidates in the future. Any inability to manage future growth could harm our ability to commercialize our product candidates, increase our costs and adversely impact our ability to compete effectively.
In order to commercialize our product candidates, we will need to hire experienced sales and marketing personnel to sell and market those product candidates we decide to commercialize, and we will need to expand the number of our managerial, operational, financial and other employees to support commercialization. Competition exists for qualified personnel in the biopharmaceutical field.
Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively.
Our ability to achieve revenues from the sale of our products in development will depend, in part, on our ability to obtain adequate reimbursement from Medicare, Medicaid, private insurers and other healthcare payers.
Our ability to successfully commercialize our products in development will depend, in significant part, on the extent to which we or our marketing partners can obtain reimbursement for our products and also reimbursement at appropriate levels for the cost of our products. Obtaining reimbursement from governmental payers, insurance companies, HMOs and other third-party payers of healthcare costs is a time-consuming and expensive process. There is no guarantee that our products will ultimately be reimbursed. There is significant uncertainty concerning third-party reimbursement for the use of any pharmaceutical product incorporating new technology and third-party reimbursement might not be available for our proposed products once approved, or if obtained, might not be adequate.
There is significant uncertainty concerning the extent and scope of third-party reimbursement for products treating HSDD and other forms of FSD. Based on third-party reimbursement for approved products treating ED, we believe bremelanotide for HSDD will be classified as a Tier 3 drug, so that reimbursement will be limited for bremelanotide for treatment of premenopausal women with HSDD, assuming the product is approved by the FDA. If we are able to obtain reimbursement, continuing efforts by governmental and third-party payers to contain or reduce costs of healthcare may adversely affect our future revenues and ability to achieve profitability. Third-party payers are increasingly challenging the prices charged for diagnostic and therapeutic products and related services. Reimbursement from governmental payers is subject to statutory and regulatory changes, retroactive rate adjustments, administrative rulings and other policy changes, all of which could materially decrease the range of products for which we are reimbursed or the rates of reimbursement by government payers. In addition, recent legislation reforming the healthcare system may result in lower prices or the actual inability of prospective customers to purchase our products in development. The cost containment measures that healthcare payers and providers are instituting and the effect of any healthcare reform could materially and adversely affect our ability to operate profitably. Furthermore, even if reimbursement is available, it may not be available at price levels sufficient for us to realize a positive return on our investment, which would have a material adverse effect on our business, financial condition and results of operations.
Even if we receive regulatory approval for our products in Europe, we may not be able to secure adequate pricing and reimbursement in Europe for us or any strategic partner to achieve profitability.
Even if one or more of our products are approved in Europe, we may be unable to obtain appropriate pricing and reimbursement for such products. In most European markets, demand levels for healthcare in general and for pharmaceuticals in particular are principally regulated by national governments. Therefore, pricing and reimbursement for our products will have to be negotiated on a "Member State by Member State" basis according to national rules, as there does not exist a centralized European process. As each Member State has its own national rules governing pricing control and reimbursement policy for pharmaceuticals, there are likely to be uncertainties attaching to the review process, and the level of reimbursement that national governments are prepared to accept. In the current economic environment, governments and private payers or insurers are increasingly looking to contain healthcare costs, including costs on drug therapies. If we are unable to obtain adequate pricing and reimbursement for our products in Europe, we or a potential strategic partner or collaborator may not be able to cover the costs necessary to manufacture, market and sell the product, limiting or preventing our ability to achieve profitability.
We may incur substantial liabilities and may be required to limit commercialization of our products in response to product liability lawsuits.
The testing and marketing of medical products entails an inherent risk of product liability. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products or cease clinical trials. Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of pharmaceutical products we develop, alone or with corporate collaborators. We currently carry $10 million liability insurance in the aggregate as to certain clinical trial risks, but we do not have and have not obtained quotations for commercial product liability insurance. We, or any corporate collaborators, may not in the future be able to obtain insurance at a reasonable cost or in sufficient amounts, if at all. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
31
Our internal computer systems, or those of our third-party contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
In the ordinary course of our business, we collect, store and transmit confidential information. Despite the implementation of security measures, our internal computer systems and those of our third-party contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. We rely on industry accepted measures and technology to secure confidential and proprietary information maintained on our computer systems. However, these measures and technology may not adequately prevent security breaches. While we do not believe that we have experienced any such system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a loss of clinical trial data for our product candidates that could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Cyberattacks are increasing in their frequency, sophistication and intensity. Cyberattacks could include the deployment of harmful malware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. Significant disruptions of our information technology systems or security breaches could adversely affect our business operations and/or result in the loss, misappropriation, and/or unauthorized access, use or disclosure of, or the prevention of access to, confidential information (including trade secrets or other intellectual property, proprietary business information and personal information), and could result in financial, legal, business and reputational harm to us. To the extent that any disruption or security breach results in a loss of or damage to our data or applications or other data or applications relating to our technology, intellectual property, research and development or product candidates, or inappropriate disclosure of confidential or proprietary information, we could incur liabilities and the further development of our product candidates could be delayed.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We may in the future employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee's former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, and health information privacy and security laws. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
If we begin commercializing any of our products in the United States, our operations may be directly, or indirectly through our customers, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, the federal False Claims Act, and physician sunshine laws and regulations. These laws may impact, among other things, our proposed sales, marketing, and education programs. In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
●
the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from soliciting, receiving, offering or providing remuneration, directly or indirectly, in return for or to induce either the referral of an individual for, or the purchase order or recommendation of, any item or services for which payment may be made under a federal health care program such as the Medicare and Medicaid programs;
●
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent;
●
the federal Health Insurance Portability and Accountability Act of 1996 ("HIPAA"), which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
●
HIPPA, as amended by the Health Information Technology and Clinical Health Act, and its implementing regulations, which imposes certain requirements relating to the privacy, security, and transmission of individually identifiable health information;
32
●
The federal physician sunshine requirements under the Affordable Care Act, which require manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; and
●
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, state laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. In addition, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amends the intent requirement of the federal anti-kickback and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. Moreover, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in government health care programs, such as Medicare and Medicaid, imprisonment, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations
We are highly dependent on our management team, senior staff professionals and third-party contractors and consultants, and the loss of their services could materially adversely affect our business.
We rely on our relatively small management team and staff as well as various contractors and consultants to provide critical services. Our ability to execute our clinical programs for bremelanotide, PL-3994 and PL-8177, and our preclinical programs for melanocortin and natriuretic peptide drug candidates, depends on our continued retention and motivation of our management and senior staff professionals, including executive officers and senior members of product development and management, including commercialization, who possess significant technical expertise and experience and oversee our development and commercialization programs. If we lose the services of existing key personnel, our development programs could be adversely affected if suitable replacement personnel are not recruited quickly. Our success also depends on our ability to develop and maintain relationships with contractors, consultants and scientific advisors.
There is competition for qualified personnel, contractors and consultants in the pharmaceutical industry, which makes it difficult to attract and retain the qualified personnel, contractors and consultants necessary for the development and growth of our business. Our failure to attract and retain such personnel, contractors and consultants could have a material adverse effect on our business, results of operations and financial condition.
Because we expect bremelanotide for the treatment of HSDD to be classified as a Tier 3 drug with reimbursement by third-party payers similar to approved products for treating ED, demand for this product will be tied to discretionary spending levels of our targeted patient population and particularly affected by unfavorable economic conditions.
The market for HSDD may be particularly vulnerable to unfavorable economic conditions. We expect bremelanotide for the treatment of HSDD to have significant copay or deductible requirements and to be only partially reimbursed by third-party payers and, as a result, demand for this product may be tied to discretionary spending levels of our targeted patient population. The recent global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn could result in a variety of risks to our business, including weakened demand for bremelanotide for HSDD or any future product candidates, if approved, and our ability to raise additional capital when needed on acceptable terms, if at all. This is particularly true in Europe, which is undergoing a continued severe economic crisis. A weak or declining economy could also strain our suppliers, possibly resulting in supply disruption, or cause our customers to delay making payments for our services. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which future economic climates and financial market conditions could adversely impact our business.
33
Risks Related to Government Regulation
Both before and after marketing approval, our product candidates are subject to ongoing regulatory requirements and, if we fail to comply with these continuing requirements, we could be subject to a variety of sanctions and the sale of any approved commercial products could be suspended.
Both before and after regulatory approval to market a particular product candidate, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising and promotion and record keeping related to the product candidates are subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, we could be subject to administrative or judicially imposed sanctions, including:
●
restrictions on the products or manufacturing process;
●
warning letters;
●
civil or criminal penalties;
●
fines;
●
injunctions;
●
imposition of a Corporate Integrity Agreement requiring heightened monitoring of our compliance functions, overseen by outside monitors, and enhanced reporting requirements to, and oversight by, the FDA and other government agencies;
●
product seizures or detentions and related publicity requirements;
●
suspension or withdrawal of regulatory approvals;
●
regulators or IRBs may not authorize us or any potential future collaborators to commence a clinical trial or conduct a clinical trial at a prospective trial site;
●
total or partial suspension of production; and
●
refusal to approve pending applications for marketing approval of new product candidates.
Changes in the regulatory approval policy during the development period, changes in or the enactment of additional regulations or statutes, or changes in the regulatory review for each submitted product application may cause delays in the approval or rejection of an application. Even if the FDA approves a product candidate, the approval may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, marketing and/or production of such product, and may impose ongoing requirements for post-approval studies, including additional research and development and clinical trials. The approval may also impose a risk evaluation and mitigation strategy ("REMS") on a product if the FDA believes there is a reason to monitor the safety of the drug in the marketplace. REMS may include requirements for additional training for health care professionals, safety communication efforts and limits on channels of distribution, among other things. The sponsor would be required to evaluate and monitor the various REMS activities and adjust them if need be. The FDA also may impose various civil or criminal sanctions for failure to comply with regulatory requirements, including withdrawal of product approval.
Furthermore, the approval procedure and the time required to obtain approval varies among countries and can involve additional testing beyond that required by the FDA. Approval by one regulatory authority does not ensure approval by regulatory authorities in other jurisdictions. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies.
In addition, varying interpretations of the data obtained for preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Even if we submit an application to the FDA for marketing approval of a product candidate, it may not result in marketing approval from the FDA.
We do not expect to receive regulatory approval for the commercial sale of any of our product candidates that are in development in the near future, if at all. The inability to obtain FDA approval or approval from comparable authorities in other countries for our product candidates would prevent us or any potential future collaborators from commercializing these product candidates in the United States or other countries.
We may not be able to obtain regulatory approval of bremelanotide for HSDD even if the product is effective in treating HSDD.
Clinical drug development programs for our product candidates are very expensive, time-consuming, difficult to design and implement and their outcome is inherently uncertain. Approval of bremelanotide for treatment of HSDD in premenopausal women requires a determination by the FDA that the product is both safe and effective. Our Phase 3 clinical trials for HSDD demonstrated what we believe to be an acceptable safety profile and statistically significant efficacy. However, the FDA may ultimately disagree with our safety analysis, definition of efficacy in HSDD, our clinical trial designs, or our interpretation of our clinical trial results. It is not possible to predict, with any assurance, whether the FDA will approve bremelanotide for any indications. The FDA may deny or delay approval of any application for bremelanotide if the FDA determines that the clinical data do not adequately establish the safety of the drug even if efficacy is established. If FDA approves bremelanotide, the approved labeling of the product may be limited or restricted in such ways as to inhibit or prevent the successful market acceptance and profitability of the product. Bremelanotide could take a significantly longer time to obtain approval than we expect and it may never gain approval. If regulatory approval of bremelanotide is delayed, limited or never obtained, our business, financial condition and results of operations would be materially adversely affected.
34
The regulatory approval process is lengthy, expensive and uncertain, and may prevent us from obtaining the approvals that we require.
Government authorities in the United States and other countries extensively regulate the advertising, labeling, storage, record-keeping, safety, efficacy, research, development, testing, manufacture, promotion, marketing and distribution of drug products. Drugs are subject to rigorous regulation in the United States by the FDA and similar regulatory bodies in other countries. The steps ordinarily required by the FDA before a new drug may be marketed in the United States include:
●
completion of non-clinical tests including preclinical laboratory and formulation studies and animal testing and toxicology;
●
submission to the FDA of an IND application, which must become effective before clinical trials may begin, and which may be placed on "clinical hold" by the FDA, meaning the trial may not commence, or must be suspended or terminated prior to completion;
●
performance of adequate and well-controlled Phase 1, 2 and 3 human clinical trials to establish the safety and efficacy of the drug for each proposed indication, and potentially post-approval or Phase 4 studies to further define the drug's efficacy and safety, generally or in specific patient populations;
●
submission to the FDA of an NDA that must be accompanied by a substantial "user fee" payment;
●
FDA review and approval of the NDA before any commercial marketing or sale; and
●
compliance with post-approval commitments and requirements.
Satisfaction of FDA pre-market approval requirements for new drugs typically takes a number of years and the actual time required for approval may vary substantially based upon the type, complexity and novelty of the product or disease to be treated by the drug. The results of product development, preclinical studies and clinical trials are submitted to the FDA as part of an NDA. The NDA also must contain extensive manufacturing information, demonstrating compliance with applicable GMP requirements. Once the submission has been accepted for filing, the FDA generally has twelve months to review the application and respond to the applicant. Such response may be an approval or may be a "complete response letter" outlining additional data or steps that must be completed prior to further FDA review of the NDA. The review process is often significantly extended by FDA requests for additional information or clarification. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical trials is not always conclusive and may be susceptible to varying interpretations that could delay, limit or prevent regulatory approval. The FDA may refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved, but the FDA is not bound by the recommendation of the advisory committee. The FDA may deny or delay approval of applications that do not meet applicable regulatory criteria or if the FDA determines that the clinical data do not adequately establish the safety and efficacy of the drug. Therefore, our proposed products could take a significantly longer time than we expect or may never gain approval. If regulatory approval is delayed or never obtained, our business, financial condition and results of operations would be materially adversely affected.
Some of our products or product candidates, including bremelanotide, may be used in combination with a drug delivery device, such as an injector or other delivery system. Medical products containing a combination of new drugs, biological products or medical devices are regulated as "combination products" in the United States. A combination product generally is defined as a product comprised of components from two or more regulatory categories (e.g., drug/device, device/biologic, drug/biologic). Each component of a combination product is subject to the requirements established by the FDA for that type of component, whether a new drug, biologic or device. In order to facilitate pre-market review of combination products, the FDA designates one of its centers to have primary jurisdiction for the pre-market review and regulation of the overall product based upon a determination by the FDA of the primary mode of action of the combination product. The determination whether a product is a combination product or two separate products is made by the FDA on a case-by-case basis. Our product candidates intended for use with such devices, or expanded indications that we may seek for our products used with such devices, may not be approved or may be substantially delayed in receiving approval if the devices do not gain and/or maintain their own regulatory approvals or clearances. Where approval of the drug product and device is sought under a single application, the increased complexity of the review process may delay approval. In addition, because these drug delivery devices are provided by single source unaffiliated third-party companies, we are dependent on the sustained cooperation and effort of those third-party companies both to supply the devices, maintain their own regulatory compliance, and, in some cases, to conduct the studies required for approval or other regulatory clearance of the devices. We are also dependent on those third-party companies continuing to maintain such approvals or clearances once they have been received. Failure of third-party companies to supply the devices, to successfully complete studies on the devices in a timely manner, or to obtain or maintain required approvals or clearances of the devices, and maintain compliance with all regulatory requirements, could result in increased development costs, delays in or failure to obtain regulatory approval and delays in product candidates reaching the market or in gaining approval or clearance for expanded labels for new indications.
35
Upon approval, a product candidate may be marketed only in those dosage forms and for those indications approved by the FDA. Once approved, the FDA may withdraw the product approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the marketplace. In addition, the FDA may require post-marketing studies, referred to as Phase 4 studies, to monitor the approved products in a specific subset of patients or a larger number of patients than were required for product approval and may limit further marketing of the product based on the results of these post-market studies. The FDA has broad post-market regulatory and enforcement powers, including the ability to seek injunctions, levy fines and civil penalties, criminal prosecution, withdraw approvals and seize products or request recalls.
If regulatory approval of any of our product candidates is granted, it will be limited to certain disease states or conditions, patient populations, duration or frequency of use, and will be subject to other conditions as set forth in the FDA-approved labeling. Adverse experiences with the product must be reported to the FDA and could result in the imposition of market restriction through labeling changes or in product removal. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval.
Outside the United States, our ability to market our product candidates will also depend on receiving marketing authorizations from the appropriate regulatory authorities. The foreign regulatory approval process generally includes all of the risks associated with FDA approval described above. The requirements governing the conduct of clinical trials and marketing authorization vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Community ("EC"), registration procedures are available to companies wishing to market a product to more than one EC member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficiency has been presented, a marketing authorization will be granted. If we do not obtain, or experience difficulties in obtaining, such marketing authorizations, our business, financial condition and results of operations may be materially adversely affected.
Legislative or regulatory healthcare reforms in the United States may make it more difficult and costly for us to obtain regulatory clearance or approval of bremelanotide for HSDD or any future product candidates and to produce, market and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress, and court decisions are issued, that could significantly change the statutory provisions governing the regulatory clearance or approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of bremelanotide for HSDD or any future product candidates. We cannot determine what effect changes in regulations, statutes, court decisions, legal interpretation or policies, when and if promulgated, enacted, issued or adopted may have on our business in the future. Such changes could, among other things:
●
require changes to manufacturing methods;
●
require recall, replacement or discontinuance of one or more of our products;
●
require additional recordkeeping;
●
limit or restrict our ability to engage in certain types of marketing or promotional activities;
●
alter or eliminate the scope or terms of any currently available regulatory exclusivities; and
●
restrict or eliminate our ability to settle any patent litigation we may bring against potential generic competitors.
Each of these would likely entail substantial time and cost and could materially harm our business and our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products would harm our business, financial condition and results of operations.
Changes in healthcare policy could adversely affect our business.
U.S. and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. For example, the Medicare Prescription Drug Improvement and Modernization Act of 2003 ("MMA"), expanded Medicare coverage for drugs purchased by Medicare beneficiaries and introduced new reimbursement methodologies. In addition, this law provided authority for limiting the number of drugs that will be covered in any therapeutic class. Until our products are approved and drug plan formulary listing decisions are made, we do not know what impact the MMA and similar laws will have on the availability of coverage for and the price that we receive for any approved products. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payers often follow Medicare policies in setting their own reimbursement policies, and any reduction in reimbursement that results from the MMA may result in similar reductions by private payers.
The Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (together the "ACA"), was adopted in 2010. This law has resulted in an increase in the number of people who are covered by both public and private insurance and has changed the way health care is financed by both government health program and private insurers, with significant impacts on the pharmaceutical industry. The ACA contains a number of provisions that may impact our business and operations in ways that may negatively affect our potential revenues in the future. For example, the ACA imposes a non-deductible excise tax on pharmaceutical manufacturers or importers that sell branded prescription drugs to U.S. government programs that we believe will increase the cost of any products that we develop. In addition, as part of the ACA's provisions closing a funding gap that currently exists in the Medicare Part D prescription drug program (commonly known as the "donut hole"), we will be required to provide a 50% discount on any branded prescription drugs that we develop sold to beneficiaries who fall within the donut hole. We cannot predict all of the specific effects the ACA or any future healthcare reform legislation will have on our business, but they could have a material adverse effect on our business and financial condition.
36
Some of the provisions of the ACA have yet to be fully implemented, while certain provisions have been subject to judicial and Congressional challenges, as well as efforts by the Trump administration to repeal or replace certain aspects of the ACA . Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed repeal legislation, the 2017 Tax Act includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the "individual mandate". Congress may consider other legislation to repeal or replace elements of the ACA. Because of the continued uncertainty about the implementation of the ACA, including the potential for further legal challenges or repeal of the ACA, we cannot quantify or predict with any certainty the likely impact of the ACA or its repeal on our business, prospects, financial condition or results of operations.
The availability of government reimbursement for prescription drugs will be impacted by the Budget Control Act of 2011, which was signed into law on August 2, 2011. This law is expected to result in federal spending cuts totaling between $1.2 trillion and $1.5 trillion over the next decade, over half of which will include cuts in Medicare and other health-related spending.
Risks Related to Our Intellectual Property
If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish.
Our success, competitive position and future revenues will depend in part on our ability and the abilities of our licensors to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties. We cannot predict:
●
the degree and range of protection any patents will afford us against competitors, including whether third parties will find ways to invalidate or otherwise circumvent our patents;
●
if and when patents will be issued;
●
whether or not others will obtain patents claiming aspects similar to those covered by our patents and patent applications; and
●
whether we will need to initiate litigation or administrative proceedings, which may be costly whether we win or lose.
If our products, methods, processes and other technologies infringe the proprietary rights of other parties we could incur substantial costs and we may have to:
●
obtain licenses, which may not be available on commercially reasonable terms, if at all;
●
redesign our products or processes to avoid infringement;
●
stop using the subject matter claimed in the patents held by others;
●
pay damages; or
●
defend litigation or administrative proceedings, which may be costly whether we win or lose, and which could result in a substantial diversion of our management resources.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property or the patents of our licensors, which could be expensive and time consuming.
Competitors may infringe our intellectual property, including our patents or the patents of our licensors. As a result, we may be required to file infringement claims to stop third-party infringement or unauthorized use. This can be expensive, particularly for a company of our size, and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patent claims do not cover its technology or that the factors necessary to grant an injunction against an infringer are not satisfied.
An adverse determination of any litigation or other proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference, derivation or other proceedings brought at the United States Patent and Trademark Office ("USPTO") may be necessary to determine the priority or patentability of inventions with respect to our patent applications or those of our licensors or collaborators. Litigation or USPTO proceedings brought by us may fail or may be invoked against us by third parties. Even if we are successful, domestic or foreign litigation or USPTO or foreign patent office proceedings may result in substantial costs and distraction to our management. We may not be able, alone or with our licensors or collaborators, to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the United States.
37
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or other proceedings, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation or proceedings. In addition, during the course of this kind of litigation or proceedings, there could be public announcements of the results of hearings, motions or other interim proceedings or developments or public access to related documents. If investors perceive these results to be negative, the market price for our common stock could be significantly harmed.
If we infringe or are alleged to infringe intellectual property rights of third parties, our business could be harmed.
Our research, development and commercialization activities may infringe or otherwise violate or be claimed to infringe or otherwise violate patents owned or controlled by other parties. There may also be patent applications that have been filed but not published that, when issued as patents, could be asserted against us. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
As a result of patent infringement claims, or to avoid potential claims, we may choose or be required to seek licenses from third parties. These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms, if at all.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical industry. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference, derivation or post-grant proceedings declared or granted by the USPTO and similar proceedings in foreign countries, regarding intellectual property rights with respect to our current or future products. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could impair our ability to compete in the marketplace. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition or results of operations.
Our patent applications and the enforcement or defense of our issued patents may be impacted by the application of or changes in U.S. and foreign standards.
The standards that the USPTO and foreign patent offices use to grant patents are not always applied predictably or uniformly and can change. Consequently, our pending patent applications may not be allowed and, if allowed, may not contain the type and extent of patent claims that will be adequate to conduct our business as planned. Additionally, any issued patents we currently own or obtain in the future may have a shorter patent term than expected or may not contain claims that will permit us to stop competitors from using our technology or similar technology or from copying our product candidates. Similarly, the standards that courts use to interpret patents are not always applied predictably or uniformly and may evolve, particularly as new technologies develop. In addition, changes to patent laws in the United States or other countries may be applied retroactively to affect the validation enforceability, or term of our patent. For example, the U.S. Supreme Court has recently modified some legal standards applied by the USPTO in examination of U.S. patent applications, which may decrease the likelihood that we will be able to obtain patents and may increase the likelihood of challenges to patents we obtain or license. In addition, changes to the U.S. patent system have come into force under the Leahy-Smith America Invents Act, or the Leahy-Smith Act, which was signed into law in September 2011. The Leahy-Smith Act included a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a "first to file" system in which the first inventor to file a patent application will be entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO, and may become involved in opposition, derivation, reexamination, inter-parties review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, which could adversely affect our competitive position.
While we cannot predict with certainty the impact the Leahy-Smith Act or any potential future changes to the U.S. or foreign patent systems will have on the operation of our business, the Leahy-Smith Act and such future changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, results of operations, financial condition and cash flows and future prospects.
38
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States and in some cases may even force us to grant a compulsory license to competitors or other third parties. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
In addition, our ability to protect and enforce our intellectual property rights may be adversely affected by unforeseen changes in domestic and foreign intellectual property laws.
If we are unable to keep our trade secrets confidential, our technologies and other proprietary information may be used by others to compete against us.
In addition to our reliance on patents, we attempt to protect our proprietary technologies and processes by relying on trade secret laws and agreements with our employees and other persons who have access to our proprietary information. These agreements and arrangements may not provide meaningful protection for our proprietary technologies and processes in the event of unauthorized use or disclosure of such information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, our competitors may independently develop substantially equivalent technologies and processes or gain access to our trade secrets or technology, either of which could materially and adversely affect our competitive position.
Risks Related to the Ownership of Our Common Stock
Our stock price is volatile and may fluctuate in a way that is disproportionate to our operating performance and we expect it to remain volatile, which could limit investors' ability to sell stock at a profit.
The volatile price of our stock makes it difficult for investors to predict the value of their investment, to sell shares at a profit at any given time or to plan purchases and sales in advance. A variety of factors may affect the market price of our common stock. These include, but are not limited to:
●
publicity regarding actual or potential clinical results relating to products under development by our competitors or us;
●
delay or failure in initiating, completing or analyzing preclinical or clinical trials or unsatisfactory designs or results of these trials;
●
interim decisions by regulatory agencies, including the FDA, as to clinical trial designs, acceptable safety profiles and the benefit/risk ratio of products under development;
●
achievement or rejection of regulatory approvals by our competitors or by us;
●
announcements of technological innovations or new commercial products by our competitors or by us;
●
developments concerning proprietary rights, including patents;
●
developments concerning our collaborations;
●
regulatory developments in the United States and foreign countries;
●
economic or other crises and other external factors;
●
period-to-period fluctuations in our revenue and other results of operations;
●
changes in the structure of healthcare payment systems or other actions that affect the effective reimbursement rates for treatment regimens containing our products;
●
changes in financial estimates and recommendations by securities analysts following our business or our industry;
●
sales of our common stock (or the perception that such sales could occur); and
●
the other factors described in this "Risk Factors" section.
39
We will not be able to control many of these factors, and we believe that period-to-period comparisons of our financial results will not necessarily be indicative of our future performance. If our revenues, if any, in any particular period do not meet expectations, we may not be able to adjust our expenditures in that period, which could cause our operating results to suffer further. If our operating results in any future period fall below the expectations of securities analysts or investors, our stock price may fall by a significant amount.
For the 12-month period ended March 31, 2018, the price of our stock has been volatile, ranging from a high of $1.20 per share to a low of $0.29 per share. In addition, the stock market in general, and the market for biotechnology companies in particular, has experienced extreme price and volume fluctuations that may have been unrelated or disproportionate to the operating performance of individual companies. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance.
As a public company in the United States, we are subject to the Sarbanes-Oxley Act of 2002 ("Sarbanes-Oxley"). We can provide no assurance that we will, at all times, in the future be able to report that our internal controls over financial reporting are effective.
Companies that file reports with the SEC, including us, are subject to the requirements of Section 404 of Sarbanes-Oxley. Section 404 requires management to establish and maintain a system of internal control over financial reporting, and annual reports on Form 10-K filed under the Exchange Act, must contain a report from management assessing the effectiveness of a company's internal control over financial reporting. Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis will be a costly and time-consuming effort that needs to be re-evaluated frequently. Failure on our part to have effective internal financial and accounting controls would cause our financial reporting to be unreliable, could have a material adverse effect on our business, operating results, and financial condition, and could cause the trading price of our common stock to fall dramatically.
If securities or industry analysts do not publish research or publish unfavorable research about our business, our stock price and trading volume could decline.
As a smaller company, it may be difficult for us to attract or retain the interest of equity research analysts. A lack of research coverage may adversely affect the liquidity of and market price of our common stock. We do not have any control of the equity research analysts or the content and opinions included in their reports. The price of our stock could decline if one or more equity research analysts downgrade our stock or issue other unfavorable commentary or research. If one or more equity research analysts ceases coverage of our company, or fails to publish reports on us regularly, demand for our stock could decrease, which in turn could cause our stock price or trading volume to decline.
Holders of our preferred stock may have interests different from our common stockholders.
We are permitted under our certificate of incorporation to issue up to 10,000,000 shares of preferred stock. We can issue shares of our preferred stock in one or more series and can set the terms of the preferred stock without seeking any further approval from our common stockholders. 4,030 shares of our Series A Preferred Stock remain outstanding as of March 31, 2018. Each share of Series A Preferred Stock is convertible at any time, at the option of the holder, and such conversion could dilute the value of our common stock to current stockholders and could adversely affect the market price of our common stock. The conversion price decreases if we sell common stock (or equivalents) for a price per share less than the conversion price or less than the market price of the common stock and is also subject to adjustment upon the occurrence of a merger, reorganization, consolidation, reclassification, stock dividend or stock split which results in an increase or decrease in the number of shares of common stock outstanding. Upon (i) liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, (ii) sale or other disposition of all or substantially all of the assets of the Company, or (iii) any consolidation, merger, combination, reorganization or other transaction in which the Company is not the surviving entity or in which the shares of common stock constituting in excess of 50% of the voting power of the Company are exchanged for or changed into other stock or securities, cash and/or any other property, after payment or provision for payment of the debts and other liabilities of the Company, the holders of Series A Preferred Stock will be entitled to receive, pro rata and in preference to the holders of any other capital stock, an amount per share equal to $100 plus accrued but unpaid dividends, if any. Any preferred stock that we issue may rank ahead of our common stock in terms of dividend priority or liquidation premiums and may have greater voting rights than our common stock.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be your sole source of gains.
We do not anticipate paying any cash dividends in the foreseeable future and intend to retain future earnings, if any, for the development and expansion of our business. Our outstanding Series A Preferred Stock, consisting of 4,030 shares on March 31, 2018, provides that we may not pay a dividend or make any distribution to holders of any class of stock unless we first pay a special dividend or distribution of $100 per share to the holders of the Series A Preferred Stock. In addition, the terms of existing or future agreements may limit our ability to pay dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
40
Anti-takeover provisions of Delaware law and our charter documents may make potential acquisitions more difficult and could result in the entrenchment of management.
We are incorporated in Delaware. Anti-takeover provisions of Delaware law and our charter documents may make a change in control or efforts to remove management more difficult. Also, under Delaware law, our board of directors may adopt additional anti-takeover measures. Under Section 203 of the Delaware General Corporation Law, a corporation may not engage in a business combination with an "interested stockholder" for a period of three years after the date of the transaction in which the person first becomes an "interested stockholder," unless the business combination is approved in a prescribed manner.
We are authorized to issue up to 300,000,000 shares of common stock. To the extent that we sell or otherwise issue authorized but currently unissued shares, this could have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock.
Our charter authorizes us to issue up to 10,000,000 shares of preferred stock and to determine the terms of those shares of stock without any further action by our stockholders. If we exercise this right, it could be more difficult for a third party to acquire a majority of our outstanding voting stock.
In addition, our equity incentive plans generally permit us to accelerate the vesting of options and other stock rights granted under these plans in the event of a change of control. If we accelerate the vesting of options or other stock rights, this action could make an acquisition more costly.
The application of these provisions could have the effect of delaying or preventing a change of control, which could adversely affect the market price of our common stock.
We are a smaller reporting company and the reduced disclosure requirements applicable to smaller reporting companies may make our Common Stock less attractive to investors.
We are currently a "smaller reporting company" as defined in the Exchange Act. Smaller reporting companies are able to provide simplified executive compensation disclosures in their filings, are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that an independent registered public accounting firm provide an attestation report on the effectiveness of internal control over financial reporting and have certain other decreased disclosure obligations in their SEC filings. We cannot predict whether investors will find our common stock less attractive because of our reliance on any of these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
As of March 31, 2018, there were 46,773,540 shares of common stock underlying outstanding convertible preferred stock, options, restricted stock units and warrants. Stockholders may experience dilution from the conversion of preferred stock, exercise of outstanding options and warrants and vesting of restricted stock units.
As of March 31, 2018, holders of our outstanding dilutive securities had the right to acquire the following amounts of underlying common stock:
●
60,592 shares issuable on the conversion of our immediately convertible Series A Convertible Preferred Stock, subject to adjustment, for no further consideration;
●
11,481,412 shares issuable upon the exercise of stock options at a weighted-average exercise price of $0.73 per share;
●
8,831,683 shares issuable under restricted stock units which vest on dates between June 11, 2018 and December 12, 2021, subject to the fulfillment of service or performance conditions, and some of which are subject to delivery restrictions; and
●
26,399,853 shares issuable upon the exercise of warrants at a weighted-average exercise price of $0.77 per share.
If the holders convert, exercise or receive these securities, or similar dilutive securities we may issue in the future, stockholders may experience dilution in the net book value of their common stock. In addition, the sale or availability for sale of the underlying shares in the marketplace could depress our stock price. We have registered or agreed to register for resale substantially all of the underlying shares listed above. Holders of registered underlying shares could resell the shares immediately upon issuance, which could result in significant downward pressure on our stock price.
41
Our failure to meet the continued listing requirements of the NYSE American could result in a de-listing of our common stock.
Our common shares are listed on the NYSE American, a national securities exchange, under the symbol "PTN". Although we currently meet the NYSE American's listing standards, which generally mandate that we meet certain requirements relating to stockholders' equity, market capitalization, aggregate market value of publicly held shares and distribution requirements, we cannot assure you that we will be able to continue to meet the NYSE American's listing requirements. If we fail to satisfy the continued listing requirements of the NYSE American, such as the corporate governance requirements or the minimum closing bid price requirement, the NYSE American may take steps to de-list our common stock. If the NYSE American delists our securities for trading on its exchange, we could face significant material adverse consequences, including:
●
a limited availability of market quotations for our securities;
●
reduced liquidity with respect to our securities;
●
a determination that our shares of common stock are "penny stock" which will require brokers trading in our shares of common stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our shares of common stock;
●
a limited amount of news and analyst coverage for our company; and
●
a decreased ability to issue additional securities or obtain additional financing in the future.
Such a de-listing would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a de-listing, we may take actions to restore our compliance with the NYSE American's listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the NYSE American minimum bid price requirement or prevent future non-compliance with the NYSE American's listing requirements.
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as "covered securities." Our common shares are considered to be covered securities because they are listed on the NYSE American. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Further, if we were no longer listed on the NYSE American, our common stock would not be covered securities and we would be subject to regulation in each state in which we offer our securities.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
Issuer purchases of equity securities. We have not and do not currently intend to retire or repurchase any of our capital securities other than providing our employees in certain grants with the option to withhold shares to satisfy tax withholding amounts due upon the vesting of restricted stock units granted under our 2011 Stock Incentive Plan. The following 24,170 shares were withheld during the three-month period ended March 31, 2018 at the direction of the employees as permitted in specific grants under the 2011 Stock Incentive Plan in order to pay the minimum amount of tax liability owed by the employee from the vesting of those units:
Period |
Total Number of Shares Purchased (1) |
Weighted Average Price Paid per Share |
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs |
Maximum Number of Shares that May Yet be Purchased Under Announced Plans or Programs |
January 1-31, 2018 | - | $ - | - | - |
February 1-28, 2018 | - | - | - | - |
March 1-31, 2018 | 24,170 | 0.86 | - | - |
Total | 24,170 | $ 0.86 | - | - |
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
None.
42
Item 6. Exhibits.
Exhibits filed or furnished with this report:
Exhibit Number | Description | Filed Herewith | Form | Filing Date | SEC File No. |
31.1 | Certification of Chief Executive Officer. | X |
|
|
|
31.2 | Certification of Chief Financial Officer. | X |
|
|
|
32.1 | Certification of principal executive officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X |
|
|
|
32.2
| Certification of principal financial officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X |
|
|
|
101.INS | XBRL Instance Document. | X |
|
|
|
101.SCH | XBRL Taxonomy Extension Schema Document. | X |
|
|
|
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | X |
|
|
|
101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | X |
|
|
|
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | X |
|
|
|
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | X |
|
|
|
43
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
| Palatin Technologies, Inc. |
|
|
| (Registrant) |
|
|
|
| |
| |||
| |||
|
| /s/ Carl Spana |
|
Date: May 14, 2018 |
| Carl Spana, Ph.D. President and Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| /s/ Stephen T. Wills |
|
Date: May 14, 2018 |
| Stephen T. Wills, CPA, MST Executive Vice President, Chief Financial Officer and Chief Operating Officer (Principal Financial and Accounting Officer) |
|
44
EXHIBIT INDEX
Exhibit Number | Description | Filed Herewith | Form | Filing Date | SEC File No. |
31.1 | Certification of Chief Executive Officer. | X |
|
|
|
31.2 | Certification of Chief Financial Officer. | X |
|
|
|
32.1 | Certification of principal executive officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X |
|
|
|
32.2
| Certification of principal financial officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X |
|
|
|
101.INS | XBRL Instance Document. | X |
|
|
|
101.SCH | XBRL Taxonomy Extension Schema Document. | X |
|
|
|
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | X |
|
|
|
101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | X |
|
|
|
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | X |
|
|
|
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | X |
|
|
|
45
