UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-33708
PHILIP MORRIS INTERNATIONAL INC.
(Exact name of registrant as specified in its charter)
Virginia |
| 13-3435103 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification No.) |
|
| |
120 Park Avenue, New York, New York |
| 10017 |
(Address of principal executive offices) |
| (Zip Code) |
917-663-2000
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Name of each exchange on which registered |
Common Stock, no par value |
| New York Stock Exchange |
2.500% Notes due 2016 |
| New York Stock Exchange |
1.625% Notes due 2017 |
| New York Stock Exchange |
1.250% Notes due 2017 |
| New York Stock Exchange |
1.125% Notes due 2017 |
| New York Stock Exchange |
1.250% Notes due 2017 |
| New York Stock Exchange |
5.650% Notes due 2018 |
| New York Stock Exchange |
1.875% Notes due 2019 |
| New York Stock Exchange |
2.125% Notes due 2019 |
| New York Stock Exchange |
1.750% Notes due 2020 |
| New York Stock Exchange |
4.500% Notes due 2020 |
| New York Stock Exchange |
1.875% Notes due 2021 |
| New York Stock Exchange |
4.125% Notes due 2021 |
| New York Stock Exchange |
2.900% Notes due 2021 |
| New York Stock Exchange |
2.500% Notes due 2022 |
| New York Stock Exchange |
2.625% Notes due 2023 |
| New York Stock Exchange |
3.600% Notes due 2023 |
| New York Stock Exchange |
2.875% Notes due 2024 |
| New York Stock Exchange |
3.250% Notes due 2024 |
| New York Stock Exchange |
2.750% Notes due 2025 |
| New York Stock Exchange |
3.375% Notes due 2025 |
| New York Stock Exchange |
2.875% Notes due 2026 |
| New York Stock Exchange |
Title of each class |
| Name of each exchange on which registered |
2.875% Notes due 2029 |
| New York Stock Exchange |
3.125% Notes due 2033 |
| New York Stock Exchange |
6.375% Notes due 2038 |
| New York Stock Exchange |
4.375% Notes due 2041 |
| New York Stock Exchange |
4.500% Notes due 2042 |
| New York Stock Exchange |
3.875% Notes due 2042 |
| New York Stock Exchange |
4.125% Notes due 2043 |
| New York Stock Exchange |
4.875% Notes due 2043 |
| New York Stock Exchange |
4.250% Notes due 2044 |
| New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☑ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company ¨ |
|
| (Do not check if a smaller reporting company) | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No ☑
As of June 30, 2015 , the aggregate market value of the registrant's common stock held by non-affiliates of the registrant was approximately $124 billion based on the closing sale price of the common stock as reported on the New York Stock Exchange.
Class |
| Outstanding at | | January 29, 2016 |
Common Stock, no par value |
| 1,549,347,456 | | shares |
DOCUMENTS INCORPORATED BY REFERENCE
Document | Parts Into Which Incorporated |
Portions of the registrant's definitive proxy statement for use in connection with its annual meeting of shareholders to be held on May 4, 2016, to be filed with the Securities and Exchange Commission ("SEC") on or about March 24, 2016. | Part III |
TABLE OF CONTENTS
|
|
| Page |
PART I |
| ||
Item 1. |
| Business | 1 |
Item 1A. |
| Risk Factors | 7 |
Item 1B. |
| Unresolved Staff Comments | 11 |
Item 2. |
| Properties | 12 |
Item 3. |
| Legal Proceedings | 12 |
Item 4. |
| Mine Safety Disclosures | 22 |
|
|
|
|
PART II |
| ||
Item 5. |
| Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 22 |
Item 6. |
| Selected Financial Data | 25 |
Item 7. |
| Management's Discussion and Analysis of Financial Condition and Results of Operations | 26 |
Item 7A. |
| Quantitative and Qualitative Disclosures About Market Risk | 80 |
Item 8. |
| Financial Statements and Supplementary Data | 81 |
Item 9. |
| Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 135 |
Item 9A. |
| Controls and Procedures | 135 |
Item 9B. |
| Other Information | 135 |
|
|
|
|
PART III |
| ||
Item 10. |
| Directors, Executive Officers and Corporate Governance | 135 |
Item 11. |
| Executive Compensation | 136 |
Item 12. |
| Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 136 |
Item 13. |
| Certain Relationships and Related Transactions, and Director Independence | 136 |
Item 14. |
| Principal Accounting Fees and Services | 136 |
|
|
|
|
PART IV | |||
Item 15. |
| Exhibits and Financial Statement Schedules | 137 |
|
|
|
|
Signatures | 142 | ||
In this report, "PMI," "we," "us" and "our" refers to Philip Morris International Inc. and its subsidiaries.
PART I
Item 1. | Business. |
(a) General Development of Business
General
Philip Morris International Inc. is a Virginia holding company incorporated in 1987. Our subsidiaries and affiliates and their licensees are engaged in the manufacture and sale of cigarettes, other tobacco products and other nicotine-containing products in markets outside of the United States of America. Our products are sold in more than 180 markets, and in many of these markets they hold the number one or number two market share position. We have a wide range of premium, mid-price and low-price brands. Our portfolio comprises both international and local brands.
Our portfolio of international and local brands is led by Marlboro , the world's best-selling international cigarette, which accounted for approximately 34% of our total 2015 shipment volume. Marlboro is complemented in the premium-price category by Merit , Parliament and Virginia S . Our leading mid-price brands are L&M and Philip Morris . Other leading international brands include Bond Street , Chesterfield, Lark , Muratti , Next and Red & White .
We also own a number of important local cigarette brands, such as Dji Sam Soe , Sampoerna and U Mild in Indonesia; Champion, Fortune and Hope in the Philippines; Apollo-Soyuz and Optima in Russia; Morven Gold in Pakistan; Boston in Colombia, Belmont, Canadian Classics and Number 7 in Canada; Best in Serbia; f6 in Germany; Delicados in Mexico; Assos in Greece, and Petra in the Czech Republic and Slovakia. While there are a number of markets where local brands remain important, international brands are expanding their share in numerous markets. With international brands contributing approximately 73% of our shipment volume in 2015, we are well positioned to continue to benefit from this trend.
Separation from Altria Group, Inc.
We were a wholly owned subsidiary of Altria Group, Inc. ("Altria") until the distribution of all of our shares owned by Altria (the "Spin-off") was made on March 28, 2008 (the "Distribution Date").
Acquisitions and Other Business Arrangements
We enhanced our business with the following transactions:
In July 2015, we dissolved our exclusive joint venture agreement with Swedish Match AB ("SWMA") to commercialize Swedish snus and other smoke-free tobacco products worldwide, outside of Scandinavia and the United States. The dissolution, mutually agreed with SWMA, means that both companies will now focus on independent strategies for the commercialization of these products, and the trademarks and intellectual property licensed to the joint venture by the companies will revert to their original owners. The dissolution of this agreement was not material to our consolidated financial position, results of operations or cash flows in any of the periods presented.
On January 30, 2014, the Indonesian Stock Exchange ("IDX") adopted a regulation requiring all listed public companies to have at least a 7.5% public shareholding by January 30, 2016. In order to comply with this requirement, our subsidiary PT HM Sampoerna Tbk. ("Sampoerna"), of which we held a 98.18% interest, conducted a rights issue. The exercise price for the rights was set at Rp. 77,000 per share, a 1.349% premium to the closing price on the IDX as of September 30, 2015. In connection with the rights issue, PT Philip Morris Indonesia ("PMID"), a fully consolidated subsidiary of PMI, sold 264,209,711 of the rights to third-party investors. Delivery of the rights sold took place on October 26, 2015. The total net proceeds from the rights issue were $1.5 billion at prevailing exchange rates on the closing date. The sale of the rights resulted in an increase to our additional paid-in capital of $1.1 billion.
In June 2014, we acquired 100% of Nicocigs Limited, a leading U.K.-based e-vapor company, for the final purchase price of $103 million, net of cash acquired. For additional information, see Note 6. Acquisitions and Other Business Arrangements to our consolidated financial statements in Item 8. Financial Statements and Supplementary Data of this Annual Report on Form 10-K ("Item 8").
In the fourth quarter of 2013, as part of our initiative to enhance profitability and growth in North African and Middle Eastern markets, we decided to restructure our business in Egypt. The new business model entails a new contract manufacturing agreement with our long-standing, strategic business partner, Eastern Company S.A.E., the creation of a new PMI affiliate in Egypt and a new distribution agreement with Trans Business for Trading and Distribution LLC. To accomplish this restructuring and to ensure a smooth transition to the new model, we recorded, in the fourth quarter of 2013, a charge to our 2013 full-year reported diluted EPS of approximately $0.10 to reflect the discontinuation of existing contractual arrangements.
1
On December 20, 2013, we established a strategic framework with Altria under which Altria will make available its e-cigarette products exclusively to us for commercialization outside the United States, and we will make available two of our candidate reduced-risk tobacco products exclusively to Altria for commercialization in the United States. The agreements also provide for cooperation on the scientific assessment of these products and for the sharing of improvements to the existing generation of reduced-risk products.
On December 12, 2013, we acquired from Megapolis Investment BV a 20% equity interest in Megapolis Distribution BV, the holding company of CJSC TK Megapolis ("Megapolis"), PMI's distributor in Russia. The purchase price of $760 million excludes an additional payment of up to $100 million, which is contingent on Megapolis's operational performance over the four fiscal years following the closing of the transaction.
On September 30, 2013, we acquired a 49% equity interest in United Arab Emirates-based Emirati Investors-TA (FZC) ("EITA"), formerly Arab Investors-TA (FZC), for approximately $625 million. As a result of this transaction, we hold an approximate 25% economic interest in Société des Tabacs Algéro-Emiratie ("STAEM"), an Algerian joint venture which is 51% owned by EITA and 49% by the Algerian state-owned enterprise Société Nationale des Tabacs et Allumettes SpA. STAEM manufactures and distributes under license some of PMI's brands.
In September 2013, Grupo Carso, S.A.B. de C.V. ("Grupo Carso") sold to us its remaining 20% interest in our Mexican tobacco business for $703 million. As a result, we now own 100% of our Mexican tobacco business. A former director of PMI, whose term expired at the Annual Meeting of Shareholders in May 2015, had an affiliation with Grupo Carso. The final purchase price was subject to an adjustment based on the actual performance of the Mexican tobacco business over the three-year period ending two fiscal years after the closing of the purchase. In May 2015, we received a payment of $113 million from Grupo Carso as the final purchase price adjustment. This resulted in a total net purchase price of $590 million.
Source of Funds - Dividends
We are a legal entity separate and distinct from our direct and indirect subsidiaries. Accordingly, our right, and thus the right of our creditors and stockholders, to participate in any distribution of the assets or earnings of any subsidiary is subject to the prior rights of creditors of such subsidiary, except to the extent that claims of our company itself as a creditor may be recognized. As a holding company, our principal sources of funds, including funds to make payment on our debt securities, are from the receipt of dividends and repayment of debt from our subsidiaries. Our principal wholly owned and majority-owned subsidiaries currently are not limited by long-term debt or other agreements in their ability to pay cash dividends or to make other distributions with respect to their common stock.
(b) Financial Information About Segments
We divide our markets into four geographic regions, which constitute our segments for financial reporting purposes:
• | The European Union ("EU") Region is headquartered in Lausanne, Switzerland, and covers all the EU countries and also comprises Switzerland, Norway and Iceland, which are linked to the EU through trade agreements; |
• | The Eastern Europe, Middle East & Africa ("EEMA") Region is also headquartered in Lausanne and includes Eastern Europe, certain Balkan countries, Turkey, the Middle East and Africa and our international duty free business; |
• | The Asia Region is headquartered in Hong Kong and covers all other Asian markets as well as Australia, New Zealand and the Pacific Islands; and |
• | The Latin America & Canada Region is headquartered in New York and covers the South American continent, Central America, Mexico, the Caribbean and Canada. |
In the fourth quarter of 2015, to further align with the Member State composition of the European Union, PMI transferred the management of its operations in Bulgaria, Croatia, Romania and Slovenia from its EEMA Region to its European Union Region, resulting in the reclassification of prior year amounts between the two segments. The changes did not have an impact on our consolidated financial position, results of operations or cash flows in any of the periods presented.
Net revenues and operating companies income * (together with a reconciliation to operating income) attributable to each segment for each of the last three years are set forth in Note 12. Segment Reporting to the consolidated financial statements in Item 8. See Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations of this Annual Report on Form 10-K ("Item 7") for a discussion of our operating results by business segment.
2
The relative percentages of operating companies income attributable to each reportable segment were as follows:
| 2015 |
| 2014 |
| 2013 | |||
European Union | 32.6 | % |
| 31.6 | % |
| 31.3 | % |
Eastern Europe, Middle East & Africa | 31.2 | |
| 33.5 | |
| 26.9 | |
Asia | 26.3 | |
| 26.4 | |
| 33.6 | |
Latin America & Canada | 9.9 | |
| 8.5 | |
| 8.2 | |
| 100.0 | % |
| 100.0 | % |
| 100.0 | % |
______________________________
* | Our management evaluates segment performance and allocates resources based on operating companies income, which we define as operating income, excluding general corporate expenses and amortization of intangibles, plus equity (income)/loss in unconsolidated subsidiaries, net. The accounting policies of the segments are the same as those described in Note 2. Summary of Significant Accounting Policies to the consolidated financial statements in Item 8. |
We use the term net revenues to refer to our operating revenues from the sale of our products, net of sales and promotion incentives. Our net revenues and operating income are affected by various factors, including the volume of products we sell, the price of our products, changes in currency exchange rates and the mix of products we sell. Mix is a term used to refer to the proportionate value of premium-price brands to mid-price or low-price brands in any given market (product mix). Mix can also refer to the proportion of shipment volume in more profitable markets versus shipment volume in less profitable markets (geographic mix). We often collect excise taxes from our customers and then remit them to local governments, and, in those circumstances, we include excise taxes in our net revenues and excise taxes on products. Our cost of sales consists principally of tobacco leaf, non-tobacco raw materials, labor and manufacturing costs.
Our marketing, administration and research costs include the costs of marketing and selling our products, other costs generally not related to the manufacture of our products (including general corporate expenses), and costs incurred to develop new products. The most significant components of our marketing, administration and research costs are marketing and sales expenses and general and administrative expenses.
(c) Narrative Description of Business
Our subsidiaries and affiliates and their licensees are engaged in the manufacture, market and sale of cigarettes, other tobacco products and other nicotine-containing products in markets outside the United States of America.
Our total cigarette shipments decreased by 1.0% in 2015 to 847.3 billion units. We estimate that international cigarette market shipments were approximately 5.4 trillion units in 2015 , a 2.6% decrease over 2014 . We estimate that our reported share of the international cigarette market (which is defined as worldwide cigarette volume, excluding the United States of America) was approximately 15.6% in 2015 , 15.5% in 2014 and 15.7% in 2013 . Excluding the People's Republic of China ("PRC"), we estimate that our reported share of the international cigarette market was approximately 28.7%, 28.5%, and 28.3% in 2015 , 2014 and 2013 , respectively.
Shipments of our principal cigarette brand, Marlboro , increased by 0.9% in 2015 and represented approximately 9.6% of the international cigarette market, excluding the PRC, in 2015 , 9.4% in 2014 and 9.3% in 2013 .
We have a cigarette market share of at least 15% and, in a number of instances, substantially more than 15%, in 103 markets, including Algeria, Argentina, Australia, Austria, Belgium, Brazil, Canada, Colombia, the Czech Republic, Egypt, Finland, France, Germany, Greece, Hungary, Indonesia, Italy, Japan, Kazakhstan, Korea, Mexico, the Netherlands, the Philippines, Poland, Portugal, Romania, Russia, Saudi Arabia, Serbia, Singapore, Spain, Sweden, Switzerland, Thailand, Turkey and Ukraine.
References to total international cigarette market, total cigarette market, total market and market shares in this Form 10-K reflect our best estimates of tax-paid volumes based on a number of internal and external sources.
3
Consumer Focused Marketing & Sales
In 2015, we continued to deploy our new strategic framework that combines our marketing and sales expertise with our in-depth knowledge of various sales territories. This framework allows us not only to engage more effectively with our adult smokers but also to enhance the success of our direct and indirect trade partners. The main benefits are:
• | Improved effectiveness of direct adult smoker engagement activities; |
• | More effective communication with our retailers about our brands; |
• | Increased speed, efficiency and widespread availability of our products; and |
• | Distribution and Sales Strategies and Trade Engagement Programs tailored to the individual characteristics of each market (namely, according to the needs and capabilities of trade layers like retailers, wholesalers and distributors and, depending on our competitive position, operating costs and the regulatory framework). |
The four main types of distribution that we use globally, often simultaneously in a given market, are:
• | Direct Sales and Distribution, where we have set up our own distribution selling directly to the retailers; |
• | Distribution through Independent Distributors who also are distributing other fast-moving consumer goods and are responsible for distribution in a single market; |
• | Exclusive Zonified Distribution, where the distributors are dedicated to us in tobacco products distribution and assigned to exclusive territories within a market, enabling them to get an appropriate return on their investment; and |
• | Distribution through national or regional wholesalers that then supply the retail trade. |
In many markets we also directly supply key accounts, including gas stations, retail chains and supermarkets.
Our distribution and sales systems are supported by sales forces that total approximately 19,900 employees worldwide. Our sales forces are well trained and recognized by trade surveys for their professionalism.
Our products are marketed and promoted through various media and channels, including, where permitted by law, point of sale communications, brand events, access-restricted Websites and printed and direct communication to verified adult smokers. Our direct communication with verified adult smokers utilizes mail, e-mail and other electronic communication tools. Promotional activities include, where permitted by law, competitions, invitations to the events, interactive programs, consumer premiums and price promotions. To support advertising and promotional activities in the markets, we have a dedicated consumer engagement group that develops innovative engagement tools for adult smokers based on the latest technologies and adult smoker trends.
Competition
We are subject to highly competitive conditions in all aspects of our business. We compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, innovation, packaging, service, marketing, advertising and retail price. Our competitors include three large international tobacco companies and several regional and local tobacco companies and, in some instances, state-owned tobacco enterprises, principally in Algeria, Egypt, the PRC, Taiwan, Thailand and Vietnam. Industry consolidation and privatizations of state-owned enterprises have led to an overall increase in competitive pressures. Some competitors have different profit and volume objectives, and some international competitors are susceptible to changes in different currency exchange rates. We compete predominantly with American blend cigarette brands, such as Marlboro , L&M , Parliament and Chesterfield , which are the most popular across many of our markets. We seek to compete in all profitable retail price categories, although our brand portfolio is weighted towards the premium-price category.
Procurement and Raw Materials
We purchase tobacco leaf of various types, grades and styles throughout the world, the majority through independent tobacco suppliers. We also contract directly with farmers in several countries, including Argentina, Brazil, Colombia, the Dominican Republic, Ecuador, Italy, Kazakhstan, Mexico, Pakistan, the Philippines and Poland. Direct sourcing from farmers represents approximately 29% of PMI's global leaf requirements. The largest supplies of tobacco leaf are sourced from Brazil, the United States, China, Malawi, Indonesia (mostly for domestic use in kretek products), Argentina, Mozambique, India, Tanzania, Philippines and Turkey.
We believe that there is an adequate supply of tobacco leaf in the world markets to satisfy our current and anticipated production requirements.
4
In addition to tobacco leaf, we purchase a wide variety of direct materials from a total of approximately 420 suppliers. Our top ten suppliers of direct materials combined represent approximately 57% of our total direct materials purchases. The three most significant direct materials that we purchase are printed paper board used in packaging, acetate tow used in filter making and fine paper used in cigarette manufacturing. In addition, the adequate supply and procurement of cloves are of particular importance to our Indonesian business.
Business Environment
Information called for by this Item is hereby incorporated by reference to the paragraphs in Item 7, Management's Discussion and Analysis of Financial Condition and Results of Operations-Operating Results by Business Segment-Business Environment .
Other Matters
Customers
None of our business segments is dependent upon a single customer or a few customers, the loss of which would have a material adverse effect on our consolidated results of operations.
Employees
At December 31, 2015 , we employed approximately 80,200 people worldwide, including employees under temporary contracts and hourly paid part-time staff. Our businesses are subject to a number of laws and regulations relating to our relationship with our employees. Generally, these laws and regulations are specific to the location of each business. In addition, in accordance with European Union requirements, we have established a European Works Council composed of management and elected members of our workforce. We believe that our relations with our employees and their representative organizations are excellent.
Executive Officers of the Registrant
The disclosure regarding executive officers is set forth under the heading "Executive Officers as of February 17, 2016" in Item 10. Directors, Executive Officers and Corporate Governance of this Annual Report on Form 10-K ("Item 10").
Research and Development
Reduced-Risk Products . One of our strategic priorities is to develop, assess and commercialize a portfolio of innovative products with the potential to reduce individual risk and population harm in comparison to smoking cigarettes. We refer to these as reduced-risk products, or RRPs. The use of this term applies to tobacco-containing products and other nicotine-containing products that have the potential to reduce individual risk and population harm in comparison to smoking cigarettes. Our RRPs are in various stages of development, and we already launched iQOS in Japan, Switzerland and in various pilot cities including Milan, Moscow, Lisbon and Bucharest; and Solaris , an e-vapor product licensed from Altria, in Spain and Israel. We are conducting extensive and rigorous scientific studies to determine whether we can support claims for such products of reduced exposure to harmful and potentially harmful constituents in smoke, and ultimately claims of reduced disease risk, when compared to smoking cigarettes. Before making any such claims, we will need to rigorously evaluate the full set of data from the relevant scientific studies to determine whether they substantiate reduced risk. Any such claims may also be subject to government review and approval, as is the case in the U.S. today.
We draw upon a team of world-class scientists from a broad spectrum of scientific disciplines, whose efforts are guided by the following three key objectives:
• | to develop RRPs that provide adult smokers the taste, sensory experience, nicotine delivery profile and ritual characteristics that are similar to those currently provided by cigarettes; |
• | to substantiate the reduction of risk for the individual adult smoker and the reduction of harm to the population as a whole, based on robust scientific evidence derived from well-established assessment processes; and |
• | to advocate for the development of science-based regulatory frameworks for the approval and commercialization of RRPs, including the communication of substantiated health benefits to adult smokers. |
In addition to iQOS , we are developing three RRP platforms that are in various stages of commercialization readiness. We are commercializing an e-vapor product under the Nicolites and Vivid brand names in the U.K., are also developing other potential platforms and are working on developing the next generation of e-vapor technology.
5
Further information about our RRPs is set forth in Item 7, Business Environment - Taxes, Legislation, Regulation and Other Matters Regarding the Manufacture, Marketing, Sale and Use of Tobacco Products - Reduced-Risk Products.
Cigarette Products . We conduct research to support and reinforce our cigarette product business. We seek to be at the forefront of innovation for product enhancements and launches of innovative new products. We have also increased support for the cigarette business because compliance with applicable laws and regulations is requiring additional capacity for analysis and testing.
Other. Finally, working through biotechnology partners, we conduct research and development activities on technology platforms that can potentially lead to the development of alternative uses of tobacco, such as for the production of therapeutic molecules.
The research and development expense for the years ended December 31, 2015 , 2014 and 2013 , is set forth in Item 8, Note 14. Additional Information to the consolidated financial statements.
Intellectual Property
Our trademarks are valuable assets, and their protection and reputation are essential to us. We own the trademark rights to all of our principal brands, including Marlboro , or have the right to use them in all countries where we use them.
In addition, we have more than 5,500 granted patents worldwide and approximately 5,400 pending patent applications. Our patent portfolio, as a whole, is material to our business. However, no one patent, or group of related patents, is material to us. We also have registered industrial designs and proprietary secrets, technology, know-how, processes and other intellectual property rights that are not registered.
Effective January 1, 2008, PMI entered into an Intellectual Property Agreement with Philip Morris USA Inc. ("PM USA"). The Intellectual Property Agreement governs the ownership of intellectual property between PMI and PM USA. Ownership of the jointly funded intellectual property has been allocated as follows:
• | PMI owns all rights to the jointly funded intellectual property outside the United States, its territories and possessions; and |
• | PM USA owns all rights to the jointly funded intellectual property in the United States, its territories and possessions. |
Ownership of intellectual property related to patent applications and resulting patents based solely on the jointly funded intellectual property, regardless of when filed or issued, will be exclusive to PM USA in the United States, its territories and possessions and exclusive to PMI everywhere else.
The Intellectual Property Agreement contains provisions concerning intellectual property that is independently developed by us or PM USA following the Distribution Date. For ten years following the Distribution Date, independently developed intellectual property may be subject to rights under certain circumstances that would allow either us or PM USA a priority position to obtain the rights to the new intellectual property from the other party, with the price and other commercial terms to be negotiated.
In the event of a dispute between us and PM USA under the Intellectual Property Agreement, we have agreed with PM USA to submit the dispute first to negotiation between our and PM USA's senior executives and then to binding arbitration.
Seasonality
Our business segments are not significantly affected by seasonality, although in certain markets cigarette consumption trends rise during the summer months due to longer daylight time and tourism.
Environmental Regulation
We are subject to applicable international, national and local environmental laws and regulations in the countries in which we do business. We have specific programs across our business units designed to meet applicable environmental compliance requirements and reduce our carbon footprint and wastage as well as water and energy consumption. We report externally about our climate change mitigation strategy, together with associated targets and results in reducing our carbon footprint, through CDP (formerly, the Carbon Disclosure Project), the leading international non-governmental organization assessing the work of thousands of companies worldwide in the area of climate change. We have developed and implemented a consistent environmental and occupational health, safety and security management system ("EHSS"), which involves policies, standard practices and procedures at all our manufacturing centers. We also conduct regular safety assessments at our offices, warehouses and car fleet organizations. Furthermore, we have engaged an external certification body to validate the effectiveness of our EHSS management system at our manufacturing centers around the world, in
6
accordance with internationally recognized standards for safety and environmental management. The environmental performance data we report externally is also verified by a qualified third party. Our subsidiaries expect to continue to make investments in order to drive improved performance and maintain compliance with environmental laws and regulations. We assess and report the compliance status of all our legal entities on a regular basis. Based on the management and controls we have in place and our review of climate change risks (both physical and regulatory), environmental expenditures have not had, and are not expected to have, a material adverse effect on our consolidated results of operations, capital expenditures, financial position, earnings or competitive position.
(d) Financial Information About Geographic Areas
The amounts of net revenues and long-lived assets attributable to each of our geographic segments for each of the last three fiscal years are set forth in Item 8, Note 12. Segment Reporting to the consolidated financial statements.
(e) Available Information
We are required to file with the SEC annual, quarterly and current reports, proxy statements and other information required by the Securities Exchange Act of 1934, as amended (the "Exchange Act"). Investors may read and copy any document that we file, including this Annual Report on Form 10-K, at the SEC's Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Investors may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet Web site at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, from which investors can electronically access our SEC filings.
We make available free of charge on, or through, our Web site at www.pmi.com our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Investors can access our filings with the SEC by visiting www.pmi.com.
The information on our Web site is not, and shall not be deemed to be, a part of this report or incorporated into any other filings we make with the SEC.
Item 1A.
Risk Factors.
The following risk factors should be read carefully in connection with evaluating our business and the forward-looking statements contained in this Annual Report on Form 10-K. Any of the following risks could materially adversely affect our business, our operating results, our financial condition and the actual outcome of matters as to which forward-looking statements are made in this Annual Report on Form 10-K.
Forward-Looking and Cautionary Statements
We may from time to time make written or oral forward-looking statements, including statements contained in this Annual Report on Form 10-K and other filings with the SEC, in reports to stockholders and in press releases and investor webcasts. You can identify these forward-looking statements by use of words such as "strategy," "expects," "continues," "plans," "anticipates," "believes," "will," "estimates," "intends," "projects," "goals," "targets" and other words of similar meaning. You can also identify them by the fact that they do not relate strictly to historical or current facts.
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Achievement of future results is subject to risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements and whether to invest in or remain invested in our securities. In connection with the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results and outcomes to differ materially from those contained in any forward-looking statements made by us; any such statement is qualified by reference to the following cautionary statements. We elaborate on these and other risks we face throughout this document, particularly in Item 7, Business Environment . You should understand that it is not possible to predict or identify all risk factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. We do not undertake to update any forward-looking statement that we may make from time to time, except in the normal course of our public disclosure obligations.
7
Risks Related to Our Business and Industry
Ÿ Consumption of tax-paid cigarettes continues to decline in many of our markets.
This decline is due to multiple factors, including increased taxes and pricing, governmental actions, the diminishing social acceptance of smoking, continuing economic and geopolitical uncertainty, and the continuing prevalence of illicit products. These factors and their potential consequences are discussed more fully below and in Item 7, Business Environment .
Ÿ Cigarettes are subject to substantial taxes. Significant increases in cigarette-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions. These tax increases may disproportionately affect our profitability and make us less competitive versus certain of our competitors.
Tax regimes, including excise taxes, sales taxes and import duties, can disproportionately affect the retail price of cigarettes versus other tobacco products, or disproportionately affect the relative retail price of our cigarette brands versus cigarette brands manufactured by certain of our competitors. Because our portfolio is weighted toward the premium-price cigarette category, tax regimes based on sales price can place us at a competitive disadvantage in certain markets. As a result, our volume and profitability may be adversely affected in these markets.
Increases in cigarette taxes are expected to continue to have an adverse impact on our sales of cigarettes, due to resulting lower consumption levels, a shift in sales from manufactured cigarettes to other tobacco products and from the premium-price to the mid-price or low-price cigarette categories, where we may be under-represented, from local sales to legal cross-border purchases of lower price products, or to illicit products such as contraband, counterfeit and "illicit whites."
Ÿ Our business faces significant governmental action aimed at increasing regulatory requirements with the goal of reducing or preventing the use of tobacco products.
Governmental actions, combined with the diminishing social acceptance of smoking and private actions to restrict smoking, have resulted in reduced industry volume in many of our markets, and we expect that such factors will continue to reduce consumption levels and will increase down-trading and the risk of counterfeiting, contraband, "illicit whites" and legal cross-border purchases. Significant regulatory developments will take place over the next few years in most of our markets, driven principally by the World Health Organization's Framework Convention on Tobacco Control ("FCTC"). The FCTC is the first international public health treaty on tobacco, and its objective is to establish a global agenda for tobacco regulation. The FCTC has led to increased efforts by tobacco control advocates and public health organizations to reduce the palatability and attractiveness of tobacco products to adult smokers. Regulatory initiatives that have been proposed, introduced or enacted include:
• | restrictions on or licensing of outlets permitted to sell cigarettes; |
• | the levying of substantial and increasing tax and duty charges; |
• | restrictions or bans on advertising, marketing and sponsorship; |
• | the display of larger health warnings, graphic health warnings and other labeling requirements; |
• | restrictions on packaging design, including the use of colors, and plain packaging; |
• | restrictions on packaging and cigarette formats and dimensions; |
• | restrictions or bans on the display of tobacco product packaging at the point of sale and restrictions or bans on cigarette vending machines; |
• | requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and other smoke constituents; |
• | disclosure, restrictions, or bans of tobacco product ingredients; |
• | increased restrictions on smoking in public and work places and, in some instances, in private places and outdoors; |
• | restrictions on the sale of potentially reduced-risk tobacco products and other nicotine-containing products; |
• | elimination of duty free sales and duty free allowances for travelers; and |
• | encouraging litigation against tobacco companies. |
Our operating income could be significantly affected by regulatory initiatives resulting in a significant decrease in demand for our brands, in particular requirements that lead to a commoditization of tobacco products, as well as any significant increase in the cost of complying with new regulatory requirements.
8
Ÿ Litigation related to tobacco use and exposure to environmental tobacco smoke could substantially reduce our profitability and could severely impair our liquidity.
There is litigation related to tobacco products pending in certain jurisdictions. Damages claimed in some tobacco-related litigation are significant and, in certain cases in Brazil, Canada and Nigeria, range into the billions of U.S. dollars. We anticipate that new cases will continue to be filed. The FCTC encourages litigation against tobacco product manufacturers. It is possible that our consolidated results of operations, cash flows or financial position could be materially affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. See Item 3. Legal Proceedings ("Item 3") and Item 8, Note 21. Contingencies for a discussion of pending litigation.
Ÿ We face intense competition, and our failure to compete effectively could have a material adverse effect on our profitability and results of operations.
We compete primarily on the basis of product quality, brand recognition, brand loyalty, taste, innovation, packaging, service, marketing, advertising and price. We are subject to highly competitive conditions in all aspects of our business. The competitive environment and our competitive position can be significantly influenced by weak economic conditions, erosion of consumer confidence, competitors' introduction of lower-price products or innovative products, higher tobacco product taxes, higher absolute prices and larger gaps between retail price categories, and product regulation that diminishes the ability to differentiate tobacco products. Competitors include three large international tobacco companies and several regional and local tobacco companies and, in some instances, state-owned tobacco enterprises, principally in Algeria, the PRC, Egypt, Taiwan, Thailand and Vietnam. Industry consolidation and privatizations of state-owned enterprises have led to an overall increase in competitive pressures. Some competitors have different profit and volume objectives, and some international competitors are susceptible to changes in different currency exchange rates.
Ÿ Because we have operations in numerous countries, our results may be influenced by economic, regulatory and political developments, natural disasters or conflicts.
Some of the countries in which we operate face the threat of civil unrest and can be subject to regime changes. In others, nationalization, terrorism, conflict and the threat of war may have a significant impact on the business environment. Economic, political, regulatory or other developments or natural disasters could disrupt our supply chain, manufacturing capabilities or distribution capabilities. In addition, such developments could lead to loss of property or equipment that are critical to our business in certain markets and difficulty in staffing and managing our operations, which could reduce our volumes, revenues and net earnings.
There is an increasing number of conflicts, including in the Middle East and Ukraine. Political uncertainty, including potential effects from economic sanctions by the U.S. or other governments, could lead to significant disruptions to our business.
In certain markets, we are dependent on governmental approvals of various actions such as price changes, and failure to obtain such approvals could impair growth in our profitability.
In addition, despite our high ethical standards and rigorous control and compliance procedures aimed at preventing and detecting unlawful conduct, given the breadth and scope of our international operations, we may not be able to detect all potential improper or unlawful conduct by our employees and international partners.
Ÿ We may be unable to anticipate changes in consumer preferences or to respond to consumer behavior influenced by economic downturns.
Our tobacco business is subject to changes in consumer preferences, which may be influenced by local economic conditions. To be successful, we must:
• | promote brand equity successfully; |
• | anticipate and respond to new consumer trends; |
• | develop new products and markets and broaden brand portfolios; |
• | improve productivity; and |
• | be able to protect or enhance margins through price increases. |
In periods of economic uncertainty, consumers may tend to purchase lower-price brands, and the volume of our premium-price and mid-price brands and our profitability could suffer accordingly. Such down-trading trends may be reinforced by regulation that limits branding, communication and product differentiation.
9
Ÿ We lose revenues as a result of counterfeiting, contraband, cross-border purchases and non-tax-paid volume produced by local manufacturers.
Large quantities of counterfeit cigarettes are sold in the international market. We believe that Marlboro is the most heavily counterfeited international cigarette brand, although we cannot quantify the revenues we lose as a result of this activity. In addition, our revenues are reduced by contraband, legal cross-border purchases and non-tax-paid volume produced by local manufacturers.
Ÿ From time to time, we are subject to governmental investigations on a range of matters.
Investigations include allegations of contraband shipments of cigarettes, allegations of unlawful pricing activities within certain markets, allegations of underpayment of customs duties and/or excise taxes, allegations of false and misleading usage of descriptors and allegations of unlawful advertising. We cannot predict the outcome of those investigations or whether additional investigations may be commenced, and it is possible that our business could be materially affected by an unfavorable outcome of pending or future investigations. See Management's Discussion and Analysis of Financial Condition and Results of Operations-Operating Results by Business Segment-Business Environment-Governmental Investigations for a description of certain governmental investigations to which we are subject.
Ÿ We may be unsuccessful in our attempts to introduce Reduced-Risk Products, and regulators may not permit reduced exposure or risk claims or the commercialization of these products.
We continue to seek ways to develop commercially viable new product technologies with the potential to reduce exposure to harmful constituents in smoke and individual risk and population harm, all in comparison to smoking cigarettes. Our goal is to develop products whose potential to reduce exposure, individual risk and population harm can be substantiated by rigorous scientific studies and that provide adult smokers the taste, sensory experience, nicotine delivery profile and ritual characteristics that are similar to those currently provided by cigarettes. We may not succeed in these efforts. If we do not succeed, but others do, we may be at a competitive disadvantage. Furthermore, we cannot predict whether regulators will permit the marketing of tobacco products or other nicotine-containing products with claims of reduced exposure or risk as compared with cigarettes. A prohibition on any such claims could significantly undermine the commercial viability of these products.
Ÿ Our reported results could be adversely affected by unfavorable currency exchange rates, and currency devaluations could impair our competitiveness.
We conduct our business primarily in local currency, and for purposes of financial reporting the local currency results are translated into U.S. dollars based on average exchange rates prevailing during a reporting period. During times of a strengthening U.S. dollar, our reported net revenues and operating income will be reduced because the local currency translates into fewer U.S. dollars. During periods of local economic crises, foreign currencies may be devalued significantly against the U.S. dollar, reducing our margins. Actions to recover margins may result in lower volume and a weaker competitive position.
Ÿ The repatriation of our foreign earnings, changes in the earnings mix, and changes in U.S. tax laws may increase our effective tax rate. Our ability to receive payments from foreign subsidiaries or to repatriate royalties and dividends could be restricted by local country currency exchange controls.
Because we are a U.S. holding company, our most significant source of funds is distributions from our non-U.S. subsidiaries. Under current U.S. tax law, in general we do not pay U.S. taxes on our foreign earnings until they are repatriated to the U.S. as distributions from our non-U.S. subsidiaries. These distributions may result in a residual U.S. tax cost. It may be advantageous to us in certain circumstances to significantly increase the amount of such distributions, which could result in a material increase in our overall effective tax rate. Additionally, the Obama Administration has indicated that it favors changes in U.S. tax law that would fundamentally change how our earnings are taxed in the U.S. If enacted and depending upon its precise terms, such legislation could increase our overall effective tax rate. Certain countries in which we operate have adopted or could institute currency exchange controls that limit or prohibit our local subsidiaries' ability to convert local currency into U.S. dollars or to make payments outside the country. This could subject us to the risk of local currency devaluation.
Ÿ Our ability to grow profitability may be limited by our inability to introduce new products, enter new markets or improve our margins through higher pricing and improvements in our brand and geographic mix.
Our profit growth may suffer if we are unable to introduce new products or enter new markets successfully, to raise prices or to improve the proportion of our sales of higher margin products and in higher margin geographies.
10
Ÿ We may be unable to expand our brand portfolio through successful acquisitions or the development of strategic business relationships.
One element of our growth strategy is to strengthen our brand portfolio and market positions through selective acquisitions and the development of strategic business relationships. Acquisition and strategic business development opportunities are limited and present risks of failing to achieve efficient and effective integration, strategic objectives and anticipated revenue improvements and cost savings. There is no assurance that we will be able to acquire attractive businesses on favorable terms, or that future acquisitions or strategic business developments will be accretive to earnings.
Ÿ Government mandated prices, production control programs, shifts in crops driven by economic conditions and the impact of climate change may increase the cost or reduce the quality of the tobacco and other agricultural products used to manufacture our products.
As with other agricultural commodities, the price of tobacco leaf and cloves can be influenced by imbalances in supply and demand, and crop quality can be influenced by variations in weather patterns, including those caused by climate change. Tobacco production in certain countries is subject to a variety of controls, including government mandated prices and production control programs. Changes in the patterns of demand for agricultural products could cause farmers to plant less tobacco. Any significant change in tobacco leaf and clove prices, quality and quantity could affect our profitability and our business.
Ÿ Our ability to implement our strategy of attracting and retaining the best global talent may be impaired by the decreasing social acceptance of cigarette smoking.
The tobacco industry competes for talent with consumer products and other companies that enjoy greater societal acceptance. As a result, we may be unable to attract and retain the best global talent.
Ÿ The failure of our information systems to function as intended or their penetration by outside parties with the intent to corrupt them could result in business disruption, litigation and regulatory action, and loss of revenue, assets or personal or other sensitive data.
We use information systems to help manage business processes, collect and interpret business data and communicate internally and externally with employees, suppliers, customers and others. Some of these information systems are managed by third-party service providers. We have backup systems and business continuity plans in place, and we take care to protect our systems and data from unauthorized access. Nevertheless, failure of our systems to function as intended, or penetration of our systems by outside parties intent on extracting or corrupting information or otherwise disrupting business processes, could result in loss of revenue, assets or personal or other sensitive data, litigation and regulatory action, cause damage to our reputation and that of our brands and result in significant remediation and other costs to us.
Ÿ We may be required to replace third-party contract manufacturers or service providers with our own resources.
In certain instances, we contract with third parties to manufacture some of our products or product parts or to provide other services. We may be unable to renew these agreements on satisfactory terms for numerous reasons, including government regulations. Accordingly, our costs may increase significantly if we must replace such third parties with our own resources.
Item 1B. | Unresolved Staff Comments. |
None.
11
Item 2
. Properties.
At December 31, 2015 , we operated and owned 48 manufacturing facilities and maintained contract manufacturing relationships with 22 third-party manufacturers across 21 markets. In addition, we work with 38 third-party operators in Indonesia who manufacture our hand-rolled cigarettes.
PMI-Owned Manufacturing Facilities
| EU |
| EEMA |
| Asia |
| Latin America & Canada |
| TOTAL | |||||
Fully integrated | 9 | |
| 8 | |
| 9 | |
| 8 | |
| 34 | |
Make-pack | - | |
| - | |
| 1 | |
| 2 | |
| 3 | |
Other | 4 | |
| 1 | |
| 3 | |
| 3 | |
| 11 | |
Total | 13 | |
| 9 | |
| 13 | |
| 13 | |
| 48 | |
In 2015 , 24 of our facilities each manufactured over 10 billion cigarettes, of which ten facilities each produced over 30 billion units. Our largest factories are in St. Petersburg and Krasnodar (Russia), Marikina and Batangas (Philippines), Berlin (Germany), Izmir (Turkey), Krakow (Poland), Sukorejo and Karawang (Indonesia), Merlo (Argentina), Klaipeda (Lithuania), Bucharest (Romania) and Kutna Hora (Czech Republic). Our smallest factories are mostly in Latin America and Asia, where due to tariff and other constraints we have established small manufacturing units in individual markets. We will continue to optimize our manufacturing base, taking into consideration the evolution of trade blocks.
The plants and properties owned or leased and operated by our subsidiaries are maintained in good condition and are believed to be suitable and adequate for our present needs.
In 2012, we announced that we are working on all aspects that will lead to the commercialization of RRPs in the 2016 to 2017 period. On January 10, 2014, we announced an investment of up to €500 million to develop our first manufacturing facility in the European Union and an associated pilot plant near Bologna, Italy, to produce RRPs. On October 10, 2014, the pilot plant officially opened for production. Once fully operational in 2016, the factory and pilot plant combined annual production capacity is expected to reach up to 30 billion units ( HeatSticks ).
Item 3. | Legal Proceedings. |
Tobacco-Related Litigation
Legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. Our indemnitees include distributors, licensees and others that have been named as parties in certain cases and that we have agreed to defend, as well as to pay costs and some or all of judgments, if any, that may be entered against them. Pursuant to the terms of the Distribution Agreement between Altria and PMI, PMI will indemnify Altria and Philip Morris USA Inc. ("PM USA"), a U.S. tobacco subsidiary of Altria, for tobacco product claims based in substantial part on products manufactured by PMI or contract manufactured for PMI by PM USA, and PM USA will indemnify PMI for tobacco product claims based in substantial part on products manufactured by PM USA, excluding tobacco products contract manufactured for PMI.
It is possible that there could be adverse developments in pending cases against us and our subsidiaries. An unfavorable outcome or settlement of pending tobacco-related litigation could encourage the commencement of additional litigation.
Damages claimed in some of the tobacco-related litigation are significant and, in certain cases in Brazil, Canada and Nigeria, range into the billions of U.S. dollars. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. Much of the tobacco-related litigation is in its early stages, and litigation is subject to uncertainty. However, as discussed below, we have to date been largely successful in defending tobacco-related litigation.
We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. At the present time, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available to it (i) management has not concluded that it is probable that a loss has been incurred in any of the pending tobacco-related cases; (ii) management is unable to estimate the
12
possible loss or range of loss for any of the pending tobacco-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any. Legal defense costs are expensed as incurred.
It is possible that our consolidated results of operations, cash flows or financial position could be materially affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. Nevertheless, although litigation is subject to uncertainty, we and each of our subsidiaries named as a defendant believe, and each has been so advised by counsel handling the respective cases, that we have valid defenses to the litigation pending against us, as well as valid bases for appeal of adverse verdicts. All such cases are, and will continue to be, vigorously defended. However, we and our subsidiaries may enter into settlement discussions in particular cases if we believe it is in our best interests to do so.
To date, we have paid one judgment in a tobacco-related case. That judgment, including costs, was approximately €1,400 (approximately $1,500), and that payment was made in order to appeal an Italian small claims case, which was subsequently reversed on appeal. To date, no tobacco-related case has been finally resolved in favor of a plaintiff against us, our subsidiaries or indemnitees.
The table below lists the number of tobacco-related cases pending against us and/or our subsidiaries or indemnitees as of February 12, 2016, December 31, 2014 and December 31, 2013 :
| |||||||||
Type of Case |
| Number of |
| Number of Cases Pending as of |
| Number of Cases Pending as of | |||
Individual Smoking and Health Cases |
| 68 | |
| 63 | |
| 62 | |
Smoking and Health Class Actions |
| 11 | |
| 11 | |
| 11 | |
Health Care Cost Recovery Actions |
| 16 | |
| 15 | |
| 15 | |
Lights Class Actions |
| - | |
| - | |
| 1 | |
Individual Lights Cases |
| 3 | |
| 2 | |
| 2 | |
Public Civil Actions |
| 3 | |
| 2 | |
| 3 | |
Since 1995, when the first tobacco-related litigation was filed against a PMI entity, 442 Smoking and Health, Lights, Health Care Cost Recovery, and Public Civil Actions in which we and/or one of our subsidiaries and/or indemnitees were a defendant have been terminated in our favor. Twelve cases have had decisions in favor of plaintiffs. Nine of these cases have subsequently reached final resolution in our favor and three remain on appeal.
13
The table below lists the verdict and significant post-trial developments in the three pending cases where a verdict was returned in favor of the plaintiff:
Date |
| Location of |
| Type of |
| Verdict |
| Post-Trial |
February 2004 |
| Brazil/The Smoker Health Defense Association |
| Class Action |
| The Civil Court of São Paulo found defendants liable without hearing evidence. In April 2004, the court awarded "moral damages" of R$1,000 (approximately $250) per smoker per full year of smoking plus interest at the rate of 1% per month, as of the date of the ruling. The court did not assess actual damages, which were to be assessed in a second phase of the case. The size of the class was not defined in the ruling. |
| Defendants appealed to the São Paulo Court of Appeals, which annulled the ruling in November 2008, finding that the trial court had inappropriately ruled without hearing evidence and returned the case to the trial court for further proceedings. In May 2011, the trial court dismissed the claim. Plaintiff appealed the decision. In February 2015, the appellate court unanimously dismissed plaintiff's appeal. In September 2015, plaintiff appealed to the Superior Court of Justice. In addition, the defendants filed a constitutional appeal to the Federal Supreme Tribunal on the basis that plaintiff did not have standing to bring the lawsuit. This appeal is still pending. |
Date |
| Location of |
| Type of |
| Verdict |
| Post-Trial |
May 27, 2015 |
| Canada/Cecilia Letourneau |
| Class Action |
| On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec ruled in favor of the Letourneau class on liability and awarded a total of CAD 131 million (approximately $94.7 million) in punitive damages, allocating CAD 46 million (approximately $33.2 million) to our subsidiary. The trial court ordered defendants to pay the full punitive damage award into a trust within 60 days. The court did not order the payment of compensatory damages. |
| In June 2015, our subsidiary commenced the appellate process with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make the payment into a trust. In August 2015, plaintiffs filed a motion for security with the Court of Appeal covering both the Letourneau case and the Blais case described below. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling CAD 226 million (approximately $163 million) to cover both the Letourneau and Blais cases. A hearing for the merits appeal is scheduled in November 2016. (See below for further detail.) |
14
Date |
| Location of |
| Type of |
| Verdict |
| Post-Trial |
May 27, 2015 |
| Canada/Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais |
| Class Action |
| On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec ruled in favor of the Blais class on liability and found the class members' compensatory damages totaled approximately CAD 15.5 billion (approximately $11.2 billion), including pre-judgment interest. The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion including pre-judgment interest (approximately $2.2 billion)). The trial court awarded CAD 90,000 (approximately $65,000) in punitive damages, allocating CAD 30,000 (approximately $21,700) to our subsidiary. The trial court ordered defendants to pay CAD 1 billion (approximately $723 million) of the compensatory damage award, CAD 200 million (approximately $145 million) of which is our subsidiary's portion, into a trust within 60 days. |
| In June 2015, our subsidiary commenced the appellate process with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make the payment into a trust. In August 2015, plaintiffs filed a motion for security with the Court of Appeal. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling, together with the Letourneau case, CAD 226 million (approximately $163 million). A hearing for the merits appeal is scheduled in November 2016. (See below for further detail.) |
Pending claims related to tobacco products generally fall within the following categories:
Smoking and Health Litigation: These cases primarily allege personal injury and are brought by individual plaintiffs or on behalf of a class or purported class of individual plaintiffs. Plaintiffs' allegations of liability in these cases are based on various theories of recovery, including negligence, gross negligence, strict liability, fraud, misrepresentation, design defect, failure to warn, breach of express and implied warranties, violations of deceptive trade practice laws and consumer protection statutes. Plaintiffs in these cases seek various forms of relief, including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include licit activity, failure to state a claim, lack of defect, lack of proximate cause, assumption of the risk, contributory negligence, and statute of limitations.
15
As of February 12, 2016, there were a number of smoking and health cases pending against us, our subsidiaries or indemnitees, as follows:
• | 68 cases brought by individual plaintiffs in Argentina (32), Brazil (21), Canada (2), Chile (8), Costa Rica (2), Italy (1), the Philippines (1) and Scotland (1), compared with 63 such cases on December 31, 2014 , and 62 cases on December 31, 2013 ; and |
• | 11 cases brought on behalf of classes of individual plaintiffs in Brazil (2) and Canada (9), compared with 11 such cases on December 31, 2014 and December 31, 2013 . |
In the first class action pending in Brazil, The Smoker Health Defense Association (ADESF) v. Souza Cruz, S.A. and Philip Morris Marketing, S.A., Nineteenth Lower Civil Court of the Central Courts of the Judiciary District of São Paulo, Brazil , filed July 25, 1995, our subsidiary and another member of the industry are defendants. The plaintiff, a consumer organization, is seeking damages for all addicted smokers and former smokers, and injunctive relief. In 2004, the trial court found defendants liable without hearing evidence and awarded "moral damages" of R$1,000 (approximately $250) per smoker per full year of smoking plus interest at the rate of 1% per month, as of the date of the ruling. The court did not award actual damages, which were to be assessed in the second phase of the case. The size of the class was not estimated. Defendants appealed to the São Paulo Court of Appeals, which annulled the ruling in November 2008, finding that the trial court had inappropriately ruled without hearing evidence and returned the case to the trial court for further proceedings. In May 2011, the trial court dismissed the claim. Plaintiff appealed the decision. In February 2015, the appellate court unanimously dismissed plaintiff's appeal. In September 2015, plaintiff appealed to the Superior Court of Justice. In addition, the defendants filed a constitutional appeal to the Federal Supreme Tribunal on the basis that plaintiff did not have standing to bring the lawsuit. This appeal is still pending.
In the second class action pending in Brazil , Public Prosecutor of São Paulo v. Philip Morris Brasil Industria e Comercio Ltda., Civil Court of the City of São Paulo, Brazil, filed August 6, 2007, our subsidiary is a defendant. The plaintiff, the Public Prosecutor of the State of São Paulo, is seeking (i) damages on behalf of all smokers nationwide, former smokers, and their relatives; (ii) damages on behalf of people exposed to environmental tobacco smoke nationwide, and their relatives; and (iii) reimbursement of the health care costs allegedly incurred for the treatment of tobacco-related diseases by all Brazilian States and Municipalities, and the Federal District. In an interim ruling issued in December 2007, the trial court limited the scope of this claim to the State of São Paulo only. In December 2008, the Seventh Civil Court of São Paulo issued a decision declaring that it lacked jurisdiction because the case involved issues similar to the ADESF case discussed above and should be transferred to the Nineteenth Lower Civil Court in São Paulo where the ADESF case is pending. The court further stated that these cases should be consolidated for the purposes of judgment. In April 2010, the São Paulo Court of Appeals reversed the Seventh Civil Court's decision that consolidated the cases, finding that they are based on different legal claims and are progressing at different stages of proceedings. This case was returned to the Seventh Civil Court of São Paulo, and our subsidiary filed its closing arguments in December 2010. In March 2012, the trial court dismissed the case on the merits. In January 2014, the São Paulo Court of Appeals rejected plaintiff's appeal and affirmed the trial court decision. In July 2014, plaintiff appealed to the Superior Court of Justice.
In the first class action pending in Canada, Cecilia Letourneau v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI Macdonald Corp., Quebec Superior Court, Canada, filed in September 1998, our subsidiary and other Canadian manufacturers (Imperial Tobacco Canada Ltd. and JTI-MacDonald Corp.) are defendants. The plaintiff, an individual smoker, sought compensatory and punitive damages for each member of the class who is deemed addicted to smoking. The class was certified in 2005. Trial began in March 2012 and concluded in December 2014. The trial court issued its judgment on May 27, 2015. The trial court found our subsidiary and two other Canadian manufacturers liable and awarded a total of CAD 131 million (approximately $94.7 million) in punitive damages, allocating CAD 46 million (approximately $33 million) to our subsidiary. The trial court found that defendants violated the Civil Code of Quebec, the Quebec Charter of Human Rights and Freedoms, and the Quebec Consumer Protection Act by failing to warn adequately of the dangers of smoking. The trial court also found that defendants conspired to prevent consumers from learning the dangers of smoking. The trial court further held that these civil faults were a cause of the class members' addiction. The trial court rejected other grounds of fault advanced by the class, holding that: (i) the evidence was insufficient to show that defendants marketed to youth, (ii) defendants' advertising did not convey false information about the characteristics of cigarettes, and (iii) defendants did not commit a fault by using the descriptors light or mild for cigarettes with a lower tar delivery. The trial court estimated the size of the addiction class at 918,000 members but declined to award compensatory damages to the addiction class because the evidence did not establish the claims with sufficient accuracy. The trial court ordered defendants to pay the full punitive damage award into a trust within 60 days and found that a claims process to allocate the awarded damages to individual class members would be too expensive and difficult to administer. The trial court ordered a briefing on the proposed process for the distribution of sums remaining from the punitive damage award after payment of attorneys' fees and legal costs. In June 2015, our subsidiary commenced the appellate process by filing its inscription of appeal of the trial court's judgment with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust within 60 days notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make the payment into a trust within 60 days . In August 2015, plaintiffs filed a motion with the
16
Court of Appeal seeking security in both the Letourneau case and the Blais case described below. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling CAD 226 million (approximately $163 million), in the form of cash into a court trust or letters of credit, in six equal consecutive quarterly installments of approximately CAD 37.6 million (approximately $27 million) beginning in December 2015 through March 2017. See the Blais description for further detail concerning the security order. The Court of Appeal has scheduled a hearing for the merits appeal in November 2016. Our subsidiary and PMI believe that the findings of liability and damages were incorrect and should ultimately be set aside on any one of many grounds, including the following: (i) holding that defendants violated Quebec law by failing to warn class members of the risks of smoking even after the court found that class members knew, or should have known, of the risks, (ii) finding that plaintiffs were not required to prove that defendants' alleged misconduct caused injury to each class member in direct contravention of binding precedent, (iii) creating a factual presumption, without any evidence from class members or otherwise, that defendants' alleged misconduct caused all smoking by all class members, (iv) holding that the addiction class members' claims for punitive damages were not time-barred even though the case was filed more than three years after a prominent addiction warning appeared on all packages, and (v) awarding punitive damages to punish defendants without proper consideration as to whether punitive damages were necessary to deter future misconduct.
In the second class action pending in Canada, Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI Macdonald Corp., Quebec Superior Court, Canada , filed in November 1998, our subsidiary and other Canadian manufacturers (Imperial Tobacco Canada Ltd. and JTI-MacDonald Corp.) are defendants. The plaintiffs, an anti-smoking organization and an individual smoker, sought compensatory and punitive damages for each member of the class who allegedly suffers from certain smoking-related diseases. The class was certified in 2005. Trial began in March 2012 and concluded in December 2014. The trial court issued its judgment on May 27, 2015. The trial court found our subsidiary and two other Canadian manufacturers liable and found that the class members' compensatory damages totaled approximately CAD 15.5 billion , including pre-judgment interest (approximately $11.2 billion). The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion , including pre-judgment interest (approximately $2.2 billion)). In addition, the trial court awarded CAD 90,000 (approximately $65,000) in punitive damages, allocating CAD 30,000 (approximately $21,700) to our subsidiary and found that defendants violated the Civil Code of Quebec, the Quebec Charter of Human Rights and Freedoms, and the Quebec Consumer Protection Act by failing to warn adequately of the dangers of smoking. The trial court also found that defendants conspired to prevent consumers from learning the dangers of smoking. The trial court further held that these civil faults were a cause of the class members' diseases. The trial court rejected other grounds of fault advanced by the class, holding that: (i) the evidence was insufficient to show that defendants marketed to youth, (ii) defendants' advertising did not convey false information about the characteristics of cigarettes, and (iii) defendants did not commit a fault by using the descriptors light or mild for cigarettes with a lower tar delivery. The trial court estimated the disease class at 99,957 members. The trial court ordered defendants to pay CAD 1 billion (approximately $723 million) of the compensatory damage award into a trust within 60 days , CAD 200 million (approximately $145 million) of which is our subsidiary's portion and ordered briefing on a proposed claims process for the distribution of damages to individual class members and for payment of attorneys' fees and legal costs. In June 2015, our subsidiary commenced the appellate process by filing its inscription of appeal of the trial court's judgment with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust within 60 days notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make an initial payment within 60 days . In August 2015, plaintiffs filed a motion with the Court of Appeal seeking an order that defendants place irrevocable letters of credit totaling CAD 5 billion (approximately $3.6 billion) into trust, to secure the judgments in both the Letourneau and Blais cases. Plaintiffs subsequently withdrew their motion for security against JTI-MacDonald Corp. and proceeded only against our subsidiary and Imperial Tobacco Canada Ltd. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling CAD 226 million (approximately $163 million) to cover both the Letourneau and Blais cases. Such security may take the form of cash into a court trust or letters of credit, in six equal consecutive quarterly installments of approximately CAD 37.6 million (approximately $27 million) beginning in December 2015 through March 2017. The Court of Appeal ordered Imperial Tobacco Canada Ltd. to furnish security totaling CAD 758 million (approximately $548 million) in seven equal consecutive quarterly installments of approximately CAD 108 million (approximately $78 million) beginning in December 2015 through June 2017. In December 2015, our subsidiary made its first quarterly installment of security for approximately CAD 37.6 million (approximately $27 million) into a court trust. This payment is included in other assets on the consolidated balance sheets and in cash used in operating activities in the consolidated statements of cash flows. The Court of Appeal ordered that the security is payable upon a final judgment of the Court of Appeal affirming the trial court's judgment or upon further order of the Court of Appeal. The Court of Appeal has scheduled a hearing for the merits appeal in November 2016. Our subsidiary and PMI believe that the findings of liability and damages were incorrect and should ultimately be set aside on any one of many grounds, including the following: (i) holding that defendants violated Quebec law by failing to warn class members of the risks of smoking even after the court found that class members knew, or should have known, of the risks, (ii) finding that plaintiffs were not required to prove that defendants' alleged misconduct caused injury to each class member in direct contravention of binding precedent, (iii) creating a factual presumption, without any evidence from class members or otherwise, that defendants' alleged misconduct caused all smoking by all class members, (iv) relying on epidemiological evidence that did not meet recognized scientific standards, and (v) awarding punitive damages to punish defendants without proper consideration as to whether punitive damages were necessary to deter future misconduct.
17
In the third class action pending in Canada, Kunta v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Winnipeg, Canada , filed June 12, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic obstructive pulmonary disease ("COPD"), severe asthma, and mild reversible lung disease resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. In September 2009, plaintiff's counsel informed defendants that he did not anticipate taking any action in this case while he pursues the class action filed in Saskatchewan (see description of Adams , below).
In the fourth class action pending in Canada, Adams v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Saskatchewan, Canada , filed July 10, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, emphysema, heart disease, or cancer, as well as restitution of profits. Preliminary motions are pending.
In the fifth class action pending in Canada, Semple v. Canadian Tobacco Manufacturers' Council, et al., The Supreme Court (trial court), Nova Scotia, Canada , filed June 18, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and COPD resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. No activity in this case is anticipated while plaintiff's counsel pursues the class action filed in Saskatchewan (see description of Adams , above).
In the sixth class action pending in Canada, Dorion v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Alberta, Canada, filed June 15, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic bronchitis and severe sinus infections resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. To date, we, our subsidiaries, and our indemnitees have not been properly served with the complaint. No activity in this case is anticipated while plaintiff's counsel pursues the class action filed in Saskatchewan (see description of Adams , above).
In the seventh class action pending in Canada, McDermid v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada , filed June 25, 2010, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and heart disease resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from heart disease allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed.
In the eighth class action pending in Canada, Bourassa v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada , filed June 25, 2010, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, the heir to a deceased smoker, alleges that the decedent was addicted to tobacco products and suffered from emphysema resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from chronic respiratory diseases allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed. In December 2014, the plaintiff filed an amended statement of claim.
In the ninth class action pending in Canada, Suzanne Jacklin v. Canadian Tobacco Manufacturers' Council, et al., Ontario Superior Court of Justice, filed June 20, 2012, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, heart disease, or cancer, as well as restitution of profits. Plaintiff's counsel has indicated that he does not intend to take any action in this case in the near future.
Health Care Cost Recovery Litigation: These cases, brought by governmental and non-governmental plaintiffs, seek reimbursement of health care cost expenditures allegedly caused by tobacco products. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including unjust enrichment, negligence, negligent design, strict liability, breach of express and implied warranties,
18
violation of a voluntary undertaking or special duty, fraud, negligent misrepresentation, conspiracy, public nuisance, defective product, failure to warn, sale of cigarettes to minors, and claims under statutes governing competition and deceptive trade practices. Plaintiffs in these cases seek various forms of relief including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include lack of proximate cause, remoteness of injury, failure to state a claim, adequate remedy at law, "unclean hands" (namely, that plaintiffs cannot obtain equitable relief because they participated in, and benefited from, the sale of cigarettes), and statute of limitations.
As of February 12, 2016, there were 16 health care cost recovery cases pending against us, our subsidiaries or indemnitees in Canada (10), Korea (1) and Nigeria (5), compared with 15 such cases on December 31, 2014 and December 31, 2013 .
In the first health care cost recovery case pending in Canada, Her Majesty the Queen in Right of British Columbia v. Imperial Tobacco Limited, et al., Supreme Court, British Columbia, Vancouver Registry, Canada, filed January 24, 2001, we, our subsidiaries, our indemnitee (PM USA), and other members of the industry are defendants. The plaintiff, the government of the province of British Columbia, brought a claim based upon legislation enacted by the province authorizing the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, resulting from a "tobacco related wrong." The Supreme Court of Canada has held that the statute is constitutional. We and certain other non-Canadian defendants challenged the jurisdiction of the court. The court rejected the jurisdictional challenge. Pre-trial discovery is ongoing.
In the second health care cost recovery case filed in Canada, Her Majesty the Queen in Right of New Brunswick v. Rothmans Inc., et al., Court of Queen's Bench of New Brunswick, Trial Court, New Brunswick, Fredericton, Canada, filed March 13, 2008, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of New Brunswick based on legislation enacted in the province. This legislation is similar to the law introduced in British Columbia that authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Pre-trial discovery is ongoing.
In the third health care cost recovery case filed in Canada, Her Majesty the Queen in Right of Ontario v. Rothmans Inc., et al., Ontario Superior Court of Justice, Toronto, Canada , filed September 29, 2009, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Ontario based on legislation enacted in the province. This legislation is similar to the laws introduced in British Columbia and New Brunswick that authorize the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants are scheduled to file their defenses in April 2016.
In the fourth health care cost recovery case filed in Canada, Attorney General of Newfoundland and Labrador v. Rothmans Inc., et al., Supreme Court of Newfoundland and Labrador, St. Johns, Canada , filed February 8, 2011, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Newfoundland and Labrador based on legislation enacted in the province that is similar to the laws introduced in British Columbia, New Brunswick and Ontario. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Preliminary motions are pending.
In the fifth health care cost recovery case filed in Canada, Attorney General of Quebec v. Imperial Tobacco Limited, et al., Superior Court of Quebec, Canada , filed June 8, 2012, we, our subsidiary, our indemnitee (PM USA), and other members of the industry are defendants. The claim was filed by the government of the province of Quebec based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in December 2014 and July 2015. Pre-trial discovery is ongoing.
In the sixth health care cost recovery case filed in Canada, Her Majesty in Right of Alberta v. Altria Group, Inc., et al., Supreme Court of Queen's Bench Alberta, Canada , filed June 8, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Alberta based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants are scheduled to file their defenses in March 2016.
In the seventh health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Manitoba v. Rothmans, Benson & Hedges, Inc., et al., The Queen's Bench, Winnipeg Judicial Centre, Canada , filed May 31, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Manitoba based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has
19
incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in September 2014. Discovery is scheduled to begin in 2017.
In the eighth health care cost recovery case filed in Canada, The Government of Saskatchewan v. Rothmans, Benson & Hedges Inc., et al., Queen's Bench, Judicial Centre of Saskatchewan, Canada , filed June 8, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Saskatchewan based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in February 2015. Discovery is scheduled to begin in 2017.
In the ninth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Prince Edward Island v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Prince Edward Island (General Section), Canada , filed September 10, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Prince Edward Island based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in February 2015. Discovery is scheduled to begin in 2017.
In the tenth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Nova Scotia v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Nova Scotia, Canada , filed January 2, 2015, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Nova Scotia based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in July 2015. Discovery is scheduled to begin in 2017.
In the first health care cost recovery case in Nigeria, The Attorney General of Lagos State v. British American Tobacco (Nigeria) Limited, et al., High Court of Lagos State, Lagos, Nigeria, filed March 13, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of making challenges to service and the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the second health care cost recovery case in Nigeria, The Attorney General of Kano State v. British American Tobacco (Nigeria) Limited, et al., High Court of Kano State, Kano, Nigeria, filed May 9, 2007, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We are in the process of making challenges to service and the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the third health care cost recovery case in Nigeria, The Attorney General of Gombe State v. British American Tobacco (Nigeria) Limited, et al., High Court of Gombe State, Gombe, Nigeria, filed October 17, 2008, we and other members of the industry are defendants . Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In February 2011, the court ruled that the plaintiff had not complied with the procedural steps necessary to serve us. As a result of this ruling, plaintiff must re-serve its claim. We have not yet been re-served.
In the fourth health care cost recovery case in Nigeria, The Attorney General of Oyo State, et al., v. British American Tobacco (Nigeria) Limited, et al., High Court of Oyo State, Ibadan, Nigeria, filed May 25, 2007, we and other members of the industry are defendants . Plaintiffs seek reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. We challenged service as improper. In June 2010, the court ruled that plaintiffs did not have leave to serve the writ of summons on the defendants and that they must re-serve the writ. We have not yet been re-served.
In the fifth health care cost recovery case in Nigeria, The Attorney General of Ogun State v. British American Tobacco (Nigeria) Limited, et al., High Court of Ogun State, Abeokuta, Nigeria , filed February 26, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years, payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years, various forms of injunctive relief, plus punitive damages. In May 2010, the trial court rejected our service objections. We have appealed.
20
In the health care cost recovery case in Korea, the National Health Insurance Service v. KT&G, et. al., filed April 14, 2014, our subsidiary and other Korean manufacturers are defendants. Plaintiff alleges that defendants concealed the health hazards of smoking, marketed to youth, added ingredients to make their products more harmful and addictive, and misled consumers into believing that Lights cigarettes are safer than regular cigarettes. The National Health Insurance Service seeks to recover approximately $53.7 million allegedly incurred in treating 3,484 patients with small cell lung cancer, squamous cell lung cancer, and squamous cell laryngeal cancer from 2003 to 2012. The case is now in the evidentiary phase.
Lights Cases: These cases, brought by individual plaintiffs, allege that the use of the term "lights" constitutes fraudulent and misleading conduct. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including misrepresentation, deception, and breach of consumer protection laws. Plaintiffs seek various forms of relief including restitution, injunctive relief, and compensatory and other damages. Defenses raised include lack of causation, lack of reliance, assumption of the risk, and statute of limitations.
As of February 12, 2016, there were 3 lights cases brought by individual plaintiffs pending against our subsidiaries or indemnitees in Chile ( 2 ) and Italy ( 1 ), compared with 2 such cases on December 31, 2014 , and 2 such cases on December 31, 2013 .
Public Civil Actions: Claims have been filed either by an individual, or a public or private entity, seeking to protect collective or individual rights, such as the right to health, the right to information or the right to safety. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including product defect, concealment, and misrepresentation. Plaintiffs in these cases seek various forms of relief including injunctive relief such as banning cigarettes, descriptors, smoking in certain places and advertising, as well as implementing communication campaigns and reimbursement of medical expenses incurred by public or private institutions.
As of February 12, 2016, there were 3 public civil actions pending against our subsidiaries in Argentina (1), Romania (1) and Venezuela (1), compared with 2 such cases on December 31, 2014 , and 3 such cases on December 31, 2013 .
In the public civil action in Argentina, Asociación Argentina de Derecho de Danos v. Massalin Particulares S.A., et al., Civil Court of Buenos Aires, Argentina, filed February 26, 2007, our subsidiary and another member of the industry are defendants. The plaintiff, a consumer association, seeks the establishment of a relief fund for reimbursement of medical costs associated with diseases allegedly caused by smoking. Our subsidiary filed its answer in September 2007. In March 2010, the case file was transferred to the Federal Court on Administrative Matters after the Civil Court granted the plaintiff's request to add the national government as a co-plaintiff in the case. The case is currently in the evidentiary stage.
In a newly filed action in Romania, Foundation for the Defense of Citizens against Abuses of the State (FACIAS) v. the State of Romania, Philip Morris România (PMR) and Philip Morris Trading SLR (PMTR), et al., Administrative and Fiscal Litigation Section of the Bucharest Tribunal , filed November 20, 2015, our subsidiaries, several other members of the industry, and the State of Romania through various of its institutions are defendants. The plaintiff, a non-governmental organization, asks the court to compel the government to enact legislation as directed by the 2014 EU Tobacco Product Directive and to establish a fund for the treatment of smoking-related diseases and promotion of tobacco control efforts. The plaintiff also seeks an order directing that 1% of the excise taxes collected from tobacco manufacturers, "as well as an amount representing 1% of the turnover" of tobacco manufacturers and distributors, be used to finance the fund. It is unclear whether the "1% of turnover" is sought from the tobacco company defendants or the government. Our subsidiaries answered the complaint in December 2015. In January 2016, the Tribunal ruled that it lacked jurisdiction. The case was transferred to the Bucharest Court of Appeals, which has jurisdiction to hear administrative cases involving the central government.
In the public civil action in Venezuela, Federation of Consumers and Users Associations ("FEVACU"), et al. v. National Assembly of Venezuela and the Venezuelan Ministry of Health, Constitutional Chamber of the Venezuelan Supreme Court , filed April 29, 2008, we were not named as a defendant, but the plaintiffs published a notice pursuant to court order, notifying all interested parties to appear in the case. In January 2009, our subsidiary appeared in the case in response to this notice. The plaintiffs purport to represent the right to health of the citizens of Venezuela and claim that the government failed to protect adequately its citizens' right to health. The claim asks the court to order the government to enact stricter regulations on the manufacture and sale of tobacco products. In addition, the plaintiffs ask the court to order companies involved in the tobacco industry to allocate a percentage of their "sales or benefits" to establish a fund to pay for the health care costs of treating smoking-related diseases. In October 2008, the court ruled that plaintiffs have standing to file the claim and that the claim meets the threshold admissibility requirements. In December 2012, the court admitted our subsidiary and BAT's subsidiary as interested third parties. In February 2013, our subsidiary answered the complaint.
Other Litigation
The Department of Special Investigations of the government of Thailand has been conducting an investigation into alleged underpayment by our subsidiary, Philip Morris (Thailand) Limited ("PM Thailand"), of customs duties and excise taxes relating to imports from the Philippines covering the period 2003-2007. On January 18, 2016, the Public Prosecutor filed charges against our subsidiary and seven
21
former and current employees in the Bangkok Criminal Court alleging that PM Thailand and the individual defendants jointly and with the intention to defraud the Thai government, under declared import prices of cigarettes to avoid full payment of taxes and duties in connection with 272 import entries of cigarettes from the Philippines during the period of July 2003 to June 2006. The government is seeking a fine of approximately THB 80.8 billion (approximately $2.26 billion). The first hearing, which will focus on preliminary procedural matters, is scheduled for April 2016. PM Thailand contends that its declared import prices are in compliance with the Customs Valuation Agreement of the World Trade Organization and Thai law and that the allegations of the Public Prosecutor are inconsistent with several decisions already taken by Thai Customs and other Thai governmental agencies.
We are also involved in additional litigation arising in the ordinary course of our business. While the outcomes of these proceedings are uncertain, management does not expect that the ultimate outcomes of other litigation, including any reasonably possible losses in excess of current accruals, will have a material adverse effect on our consolidated results of operations, cash flows or financial position.
Item 4. | Mine Safety Disclosures . |
Not applicable.
PART II
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
The principal stock exchange on which our common stock (no par value) is listed is the New York Stock Exchange. At January 29, 2016 , there were approximately 64,400 holders of record of our common stock.
Our common stock is also listed on the NYSE Euronext in Paris and the SIX Swiss Exchange.
22
Performance Graph
The graph below compares the cumulative total shareholder return on PMI's common stock with the cumulative total return for the same period of PMI's Compensation Survey Group and the S&P 500 Index. The graph assumes the investment of $100 as of December 31, 2010, in PMI common stock (at prices quoted on the New York Stock Exchange) and each of the indices as of the market close and reinvestment of dividends on a quarterly basis.
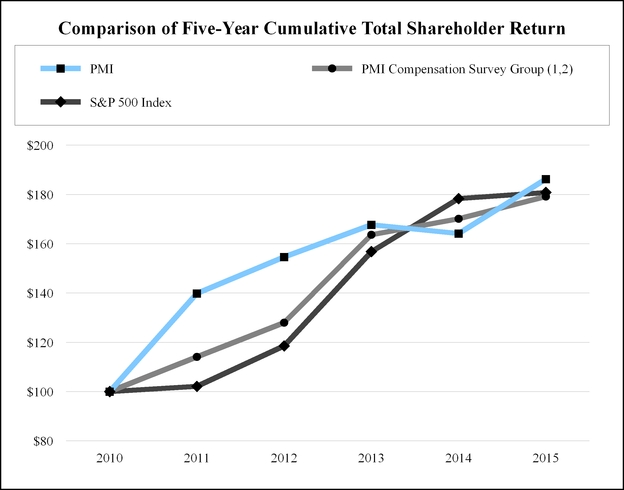
Date |
| PMI |
| PMI Compensation Survey Group (1,2) |
| S&P 500 Index |
December 31, 2010 |
| $100.00 |
| $100.00 |
| $100.00 |
December 31, 2011 |
| $139.80 |
| $114.10 |
| $102.10 |
December 31, 2012 |
| $154.60 |
| $128.00 |
| $118.50 |
December 31, 2013 |
| $167.70 |
| $163.60 |
| $156.80 |
December 31, 2014 |
| $164.20 |
| $170.10 |
| $178.30 |
December 31, 2015 |
| $186.20 |
| $179.20 |
| $180.80 |
(1) The PMI Compensation Survey Group consists of the following companies with substantial global sales that are direct competitors; or have similar market capitalization; or are primarily focused on consumer products (excluding high technology and financial services); and are companies for which comparative executive compensation data are readily available: Bayer AG, British American Tobacco p.l.c., The Coca-Cola Company, Diageo plc, GlaxoSmithKline, Heineken N.V., Imperial Brands PLC (formerly, Imperial Tobacco Group PLC), Johnson & Johnson, McDonald's Corp., Mondelēz International, Inc., Nestlé S.A., Novartis AG, PepsiCo, Inc., Pfizer Inc., Roche Holding AG, Unilever NV and PLC and Vodafone Group Plc.
(2) On October 1, 2012, Mondelēz International, Inc. (NASDAQ: MDLZ), formerly Kraft Foods Inc., announced that it had completed the spin-off of its North American grocery business, Kraft Foods Group, Inc. (NASDAQ: KRFT). Mondelēz International, Inc. was retained in the PMI Compensation Survey Group index because of its global footprint. The PMI Compensation Survey Group index total cumulative return calculation weights Mondelēz International, Inc.'s total shareholder return at 65% of historical Kraft Foods Inc.'s market capitalization on December 31, 2010, based on Mondelēz International, Inc.'s initial market capitalization relative to the combined market capitalization of Mondelēz International, Inc. and Kraft Foods Group, Inc. on October 2, 2012.
Note: Figures are rounded to the nearest $0.10.
23
Issuer Purchases of Equity Securities During the Quarter Ended December 31, 2015
Our share repurchase activity for each of the three months in the quarter ended December 31, 2015 , was as follows:
Period |
| Total Number of Shares Repurchased |
| Average Price Paid per Share |
| Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (2) |
| Approximate Dollar Value of Shares that May Yet be Purchased Under the Plans or Programs | ||||||
October 1, 2015 – |
| - | |
| $ | - | |
| - | |
| $ | - | |
November 1, 2015 – |
| - | |
| $ | - | |
| - | |
| $ | - | |
December 1, 2015 – |
| - | |
| $ | - | |
| - | |
| $ | - | |
Pursuant to Publicly Announced |
| - | |
| $ | - | |
|
|
|
| |||
October 1, 2015 – |
| 67 | |
| $ | 79.19 | |
|
|
|
| |||
November 1, 2015 – |
| 3,746 | |
| $ | 88.02 | |
|
|
|
| |||
December 1, 2015 – |
| 450 | |
| $ | 87.76 | |
|
|
|
| |||
For the Quarter Ended |
| 4,263 | |
| $ | 87.86 | |
|
|
|
| |||
(1) | Our authorized three-year share repurchase program of $18 billion expired in August 2015. During this reporting period, we did not have an authorized share repurchase program. |
(2) | Aggregate number of shares repurchased under the above-mentioned share repurchase program as of the end of the period presented. |
(3) | Shares repurchased represent shares tendered to us by employees who vested in deferred stock awards and used shares to pay all, or a portion of, the related taxes. |
The other information called for by this Item is included in Item 8, Note 26. Quarterly Financial Data (Unaudited) to the consolidated financial statements.
24
Item 6.
Selected Financial Data
(in millions of dollars, except per share data)
| 2015 |
| 2014 |
| 2013 |
| 2012 |
| 2011 | ||||||||||
Summary of Operations: |
|
|
|
|
|
|
|
|
| ||||||||||
Net revenues | $ | 73,908 | |
| $ | 80,106 | |
| $ | 80,029 | |
| $ | 77,393 | |
| $ | 76,346 | |
Cost of sales | 9,365 | |
| 10,436 | |
| 10,410 | |
| 10,373 | |
| 10,678 | | |||||
Excise taxes on products | 47,114 | |
| 50,339 | |
| 48,812 | |
| 46,016 | |
| 45,249 | | |||||
Gross profit | 17,429 | |
| 19,331 | |
| 20,807 | |
| 21,004 | |
| 20,419 | | |||||
Operating income | 10,623 | |
| 11,702 | |
| 13,515 | |
| 13,863 | |
| 13,342 | | |||||
Interest expense, net | 1,008 | |
| 1,052 | |
| 973 | |
| 859 | |
| 800 | | |||||
Earnings before income taxes | 9,615 | |
| 10,650 | |
| 12,542 | |
| 13,004 | |
| 12,542 | | |||||
Pre-tax profit margin | 13.0 | % |
| 13.3 | % |
| 15.7 | % |
| 16.8 | % |
| 16.4 | % | |||||
Provision for income taxes | 2,688 | |
| 3,097 | |
| 3,670 | |
| 3,833 | |
| 3,653 | | |||||
Net earnings | 7,032 | |
| 7,658 | |
| 8,850 | |
| 9,154 | |
| 8,879 | | |||||
Net earnings attributable to noncontrolling interests | 159 | |
| 165 | |
| 274 | |
| 354 | |
| 288 | | |||||
Net earnings attributable to PMI | 6,873 | |
| 7,493 | |
| 8,576 | |
| 8,800 | |
| 8,591 | | |||||
Basic earnings per share | 4.42 | |
| 4.76 | |
| 5.26 | |
| 5.17 | |
| 4.85 | | |||||
Diluted earnings per share | 4.42 | |
| 4.76 | |
| 5.26 | |
| 5.17 | |
| 4.85 | | |||||
Dividends declared per share | 4.04 | |
| 3.88 | |
| 3.58 | |
| 3.24 | |
| 2.82 | | |||||
Capital expenditures | 960 | |
| 1,153 | |
| 1,200 | |
| 1,056 | |
| 897 | | |||||
Depreciation and amortization | 754 | |
| 889 | |
| 882 | |
| 898 | |
| 993 | | |||||
Property, plant and equipment, net | 5,721 | |
| 6,071 | |
| 6,755 | |
| 6,645 | |
| 6,250 | | |||||
Inventories | 8,473 | |
| 8,592 | |
| 9,846 | |
| 8,949 | |
| 8,120 | | |||||
Total assets | 33,956 | |
| 35,187 | |
| 38,168 | |
| 37,670 | |
| 35,488 | | |||||
Long-term debt | 25,250 | |
| 26,929 | |
| 24,023 | |
| 17,639 | |
| 14,828 | | |||||
Total debt | 28,480 | |
| 29,455 | |
| 27,678 | |
| 22,839 | |
| 18,545 | | |||||
Stockholders' (deficit) equity | (11,476 | ) |
| (11,203 | ) |
| (6,274 | ) |
| (3,154 | ) |
| 551 | | |||||
Common dividends declared as a % of Diluted EPS | 91.4 | % |
| 81.5 | % |
| 68.1 | % |
| 62.7 | % |
| 58.1 | % | |||||
Market price per common share - high/low | 90.27-75.27 | |
| 91.63-75.28 | |
| 96.73-82.86 | |
| 94.13-72.85 | |
| 79.42-55.85 | | |||||
Closing price of common share at year end | 87.91 | |
| 81.45 | |
| 87.13 | |
| 83.64 | |
| 78.48 | | |||||
Price/earnings ratio at year end - Diluted | 20 | |
| 17 | |
| 17 | |
| 16 | |
| 16 | | |||||
Number of common shares outstanding at year end (millions) | 1,549 | |
| 1,547 | |
| 1,589 | |
| 1,654 | |
| 1,726 | | |||||
Number of employees | 80,200 | |
| 82,500 | |
| 91,100 | |
| 87,100 | |
| 78,100 | | |||||
This Selected Financial Data should be read in conjunction with Item 7 and Item 8.
25
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations. |
The following discussion should be read in conjunction with the other sections of this Annual Report on Form 10-K, including the consolidated financial statements and related notes contained in Item 8, and the discussion of risks and cautionary factors that may affect future results in Item 1A. Risk Factors .
|
|
|
|
|
|
|
Description of Our Company
We are a holding company whose subsidiaries and affiliates, and their licensees, are engaged in the manufacture and sale of cigarettes, other tobacco products and other nicotine-containing products in markets outside the United States of America. We manage our business in four segments:
• | European Union; |
• | Eastern Europe, Middle East & Africa ("EEMA"); |
• | Asia; and |
• | Latin America & Canada. |
Our products are sold in more than 180 markets, and in many of these markets they hold the number one or number two market share position. We have a wide range of premium, mid-price and low-price brands. Our portfolio comprises both international and local brands.
We use the term net revenues to refer to our operating revenues from the sale of our products, net of sales and promotion incentives. Our net revenues and operating income are affected by various factors, including the volume of products we sell, the price of our products, changes in currency exchange rates and the mix of products we sell. Mix is a term used to refer to the proportionate value of premium-price brands to mid-price or low-price brands in any given market (product mix). Mix can also refer to the proportion of shipment volume in more profitable markets versus shipment volume in less profitable markets (geographic mix). We often collect excise taxes from our customers and then remit them to governments, and, in those circumstances, we include the excise taxes in our net revenues and in excise taxes on products. Our cost of sales consists principally of tobacco leaf, non-tobacco raw materials, labor and manufacturing costs.
Our marketing, administration and research costs include the costs of marketing and selling our products, other costs generally not related to the manufacture of our products (including general corporate expenses), and costs incurred to develop new products. The most significant components of our marketing, administration and research costs are marketing and sales expenses and general and administrative expenses.
Philip Morris International Inc. is a legal entity separate and distinct from our direct and indirect subsidiaries. Accordingly, our right, and thus the right of our creditors and stockholders, to participate in any distribution of the assets or earnings of any subsidiary is subject to the prior rights of creditors of such subsidiary, except to the extent that claims of our company itself as a creditor may be recognized. As a holding company, our principal sources of funds, including funds to make payment on our debt securities, are from the receipt of dividends and repayment of debt from our subsidiaries. Our principal wholly owned and majority-owned subsidiaries currently are not limited by long-term debt or other agreements in their ability to pay cash dividends or to make other distributions with respect to their common stock.
Certain prior years' amounts have been reclassified to conform with the current year's presentation. In the fourth quarter of 2015, to further align with the Member State composition of the European Union, PMI transferred the management of its operations in Bulgaria, Croatia, Romania and Slovenia from its EEMA segment to its European Union segment, resulting in the reclassification of prior year amounts between the two segments. The changes did not have an impact on our consolidated financial position, results of operations or cash flows in any of the periods presented.
26
Executive Summary
The following executive summary provides significant highlights from the Discussion and Analysis that follows.
• | Consolidated Operating Results – The changes in our reported diluted earnings per share ("diluted EPS") for the year ended December 31, 2015 , from the comparable 2014 amounts, were as follows: |
| Diluted EPS | % Growth | |||
For the year ended December 31, 2014 | $ | 4.76 | |
| |
|
|
| |||
2014 Asset impairment and exit costs | 0.26 | |
| ||
2014 Tax items | - | |
| ||
Subtotal of 2014 items | 0.26 | |
| ||
|
|
| |||
2015 Asset impairment and exit costs | (0.03 | ) |
| ||
2015 Tax items | 0.03 | |
| ||
Subtotal of 2015 items | - | |
| ||
|
|
| |||
Currency | (1.20 | ) |
| ||
Interest | (0.01 | ) |
| ||
Change in tax rate | 0.04 | |
| ||
Impact of lower shares outstanding and share-based payments | 0.04 | |
| ||
Operations | 0.53 | |
| ||
For the year ended December 31, 2015 | $ | 4.42 | | (7.1 | )% |
See the discussion of events affecting the comparability of statement of earnings amounts in the Consolidated Operating Results section of the following Discussion and Analysis.
• | Asset Impairment and Exit Costs – During 2015, we recorded pre-tax asset impairment and exit costs of $68 million ($52 million after tax or $0.03 per share) related to severance costs for the organizational restructuring in the European Union segment. During 2014, we recorded pre-tax asset impairment and exit costs of $535 million ($409 million after tax or $0.26 per share) related to the factory closures in the Netherlands, Australia and Canada and the restructuring of the U.S. leaf purchasing model. |
On April 4, 2014, we announced the initiation by our affiliate Philip Morris Holland B.V. ("PMH") of consultations with employee representatives on a proposal to discontinue cigarette production at its factory located in Bergen op Zoom, the Netherlands. PMH reached an agreement with the trade unions and their members on a social plan and ceased cigarette production on September 1, 2014. During 2014, we recorded pre-tax asset impairment and exit costs of $489 million. For further details, see the Asset Impairment and Exit Costs section of the following Discussion and Analysis .
• | Income Taxes – Our effective income tax rate for 2015 decreased by 1.1 percentage points to 28.0% . The effective tax rate for 2014 was unfavorably impacted by the above asset impairment and exit costs related to the factory closures. The 2015 tax items that increased our diluted EPS by $0.03 per share in the table above represents a reduction in unrecognized tax benefits of $41 million following the conclusion of the IRS examinations of Altria Group, Inc.'s ("Altria") consolidated tax returns for the years 2007 and 2008 and PMI's consolidated tax returns for the years 2009 through 2011. Prior to March 28, 2008, PMI was a wholly owned subsidiary of Altria. The change in tax rate that increased our diluted EPS by $0.04 per share in the table above was primarily due to earnings mix by taxing jurisdiction and repatriation cost differences. |
• | Currency – The unfavorable currency impact during 2015 results from the strengthening of the U.S. dollar, especially against the Argentine peso, Australian dollar, Canadian dollar, Euro, Indonesian rupiah, Japanese yen, Mexican peso, Russian ruble, Turkish lira and the Ukrainian hryvnia. This unfavorable currency movement has impacted our profitability across our primary revenue markets and local currency cost bases. |
27
• | Interest – The unfavorable impact of interest was due primarily to higher average debt levels, partially offset by lower average interest rates on debt. |
• | Lower Shares Outstanding and Share-Based Payments – The favorable diluted EPS impact was due to the repurchase of our common stock in 2014 pursuant to our share repurchase program. |
• | Operations – The increase in diluted EPS of $0.53 from our operations in the table above was due to the following segments: |
• | EEMA: Higher pricing, partially offset by higher marketing, administration and research costs, unfavorable volume/mix and higher manufacturing costs; |
• | Latin America & Canada: Higher pricing, partially offset by unfavorable volume/mix, higher manufacturing costs and higher marketing, administration and research costs; |
• | European Union: Higher pricing and lower manufacturing costs, partially offset by higher marketing, administration and research costs and unfavorable volume/mix; and |
• | Asia: Higher pricing, partially offset by higher marketing, administration and research costs, unfavorable volume/mix and higher manufacturing costs. |
For further details, see the Consolidated Operating Results and Operating Results by Business Segment sections of the following Discussion and Analysis .
• 2016 Forecasted Results – On February 4, 2016, we announced our forecast for 2016 full-year reported diluted EPS to be in a range of $4.25 to $4.35, at prevailing exchange rates at that time, versus $4.42 in 2015. Excluding an unfavorable currency impact, at then-prevailing rates, of approximately $0.60 per share for the full-year 2016, the reported diluted earnings per share range represents an increase of approximately 10% to 12% versus adjusted diluted earnings per share of $4.42 in 2015. This forecast does not include any share repurchases in 2016. The company will revisit the potential for repurchases as the year unfolds, depending on the currency environment. We estimate 2016 international cigarette volume, excluding the People's Republic of China and the U.S., to decline by approximately 2.0%-2.5%, in line with the estimated decline of 2.4% in 2015.
We calculated 2015 adjusted diluted EPS as reported diluted EPS of $4.42 , plus the $0.03 per share charge related to asset impairment and exit costs, less the $0.03 per share benefit related to discrete tax items.
Adjusted diluted EPS is not a measure under accounting principles generally accepted in the United States of America ("U.S. GAAP"). We define adjusted diluted EPS as reported diluted EPS adjusted for asset impairment and exit costs, discrete tax items and unusual items. We believe it is appropriate to disclose this measure as it represents core earnings, improves comparability and helps investors analyze business performance and trends. Adjusted diluted EPS should be considered neither in isolation nor as a substitute for reported diluted EPS prepared in accordance with U.S. GAAP.
This 2016 guidance excludes the impact of future acquisitions, unanticipated asset impairment and exit cost charges, future changes in currency exchange rates and any unusual events. The factors described in Item 1A. Risk Factors represent continuing risks to this forecast.
Discussion and Analysis
Critical Accounting Estimates
Item 8, Note 2. Summary of Significant Accounting Policies to our consolidated financial statements includes a summary of the significant accounting policies and methods used in the preparation of our consolidated financial statements. In most instances, we must use a particular accounting policy or method because it is the only one that is permitted under U.S. GAAP.
The preparation of financial statements requires that we use estimates and assumptions that affect the reported amounts of our assets, liabilities, net revenues and expenses, as well as our disclosure of contingencies. If actual amounts differ from previous estimates, we include the revisions in our consolidated results of operations in the period during which we know the actual amounts. Historically, aggregate differences, if any, between our estimates and actual amounts in any year have not had a significant impact on our consolidated financial statements.
28
The selection and disclosure of our critical accounting estimates have been discussed with our Audit Committee. The following is a discussion of the more significant assumptions, estimates, accounting policies and methods used in the preparation of our consolidated financial statements:
• Revenue Recognition - We recognize revenue when persuasive evidence of an arrangement exists, delivery of product has occurred, the sales price is fixed or determinable and collectability is reasonably assured. For our company, this means that revenue is recognized when title and risk of loss is transferred to our customers. Title transfers to our customers upon shipment or upon receipt at the customer's location as determined by the sales terms for each transaction. The company estimates the cost of sales returns based on historical experience, and these estimates are normally immaterial.
• Goodwill and Non-Amortizable Intangible Assets Valuation - We test goodwill and non-amortizable intangible assets for impairment annually or more frequently if events occur that would warrant such review. We perform our annual impairment analysis in the first quarter of each year. While the company has the option to perform a qualitative assessment for both goodwill and non-amortizable intangible assets to determine if it is more likely than not that an impairment exists, the company elects to perform the quantitative assessment for our annual impairment analysis. The impairment analysis involves comparing the fair value of each reporting unit or non-amortizable intangible asset to the carrying value. If the carrying value exceeds the fair value, goodwill or a non-amortizable intangible asset is considered impaired. To determine the fair value of goodwill, we primarily use a discounted cash flow model, supported by the market approach using earnings multiples of comparable global and local companies within the tobacco industry. At December 31, 2015 , the carrying value of our goodwill was $7.4 billion, which is related to ten reporting units, each of which is comprised of a group of markets with similar economic characteristics. The estimated fair value of our ten reporting units exceeded the carrying value as of December 31, 2015 . To determine the fair value of non-amortizable intangible assets, we primarily use a discounted cash flow model applying the relief-from-royalty method. We concluded that the fair value of our non-amortizable intangible assets exceeded the carrying value, and any reasonable movement in the assumptions would not result in an impairment. These discounted cash flow models include management assumptions relevant for forecasting operating cash flows, which are subject to changes in business conditions, such as volumes and prices, costs to produce, discount rates and estimated capital needs. Management considers historical experience and all available information at the time the fair values are estimated, and we believe these assumptions are consistent with the assumptions a hypothetical marketplace participant would use. Since the March 28, 2008, spin-off from Altria, we have not recorded a charge to earnings for an impairment of goodwill or non-amortizable intangible assets.
• Marketing and Advertising Costs - We incur certain costs to support our products through programs which include advertising, marketing, consumer engagement and trade promotions. The costs of our advertising and marketing programs are expensed in accordance with U.S. GAAP. Recognition of the cost related to our consumer engagement and trade promotion programs contain uncertainties due to the judgment required in estimating the potential performance and compliance for each program. For volume-based incentives provided to customers, management continually assesses and estimates, by customer, the likelihood of the customer achieving the specified targets and records the reduction of revenue as the sales are made. For other trade promotions, management relies on estimated utilization rates that have been developed from historical experience. Changes in the assumptions used in estimating the cost of any individual marketing program would not result in a material change in our financial position, results of operations or operating cash flows. We have not made any material changes in the accounting methodology used to estimate our marketing programs during the past three years.
• Employee Benefit Plans - As discussed in Item 8, Note 13. Benefit Plans to our consolidated financial statements, we provide a range of benefits to our employees and retired employees, including pensions, postretirement health care and postemployment benefits (primarily severance). We record annual amounts relating to these plans based on calculations specified by U.S. GAAP. These calculations include various actuarial assumptions, such as discount rates, assumed rates of return on plan assets, compensation increases, mortality, turnover rates and health care cost trend rates. We review actuarial assumptions on an annual basis and make modifications to the assumptions based on current rates and trends when it is deemed appropriate to do so. As permitted by U.S. GAAP, any effect of the modifications is generally amortized over future periods. We believe that the assumptions utilized in calculating our obligations under these plans are reasonable based upon our historical experience and advice from our actuaries.
Weighted-average discount rate assumptions for pensions and postretirement plans are as follows:
| 2015 | 2014 |
U.S. pension plans | 4.30% | 3.95% |
Non-U.S. pension plans | 1.68% | 1.92% |
Postretirement plans | 4.45% | 4.20% |
We anticipate that assumption changes, coupled with decreased amortization of deferred losses, will decrease 2016 pre-tax U.S. and non-U.S. pension and postretirement expense to approximately $209 million as compared with approximately $240 million in 2015, excluding
29
amounts related to early retirement programs. The anticipated decrease is primarily due to lower amortization of deferred losses in the Netherlands due to a change in the amortization period.
Weighted-average expected rate of return and discount rate assumptions have a significant effect on the amount of expense reported for the employee benefit plans. A fifty-basis-point decrease in our discount rate would increase our 2016 pension and postretirement expense by approximately $52 million, and a fifty-basis-point increase in our discount rate would decrease our 2016 pension and postretirement expense by approximately $44 million. Similarly, a fifty-basis-point decrease (increase) in the expected return on plan assets would increase (decrease) our 2016 pension expense by approximately $30 million. See Item 8, Note 13. Benefit Plans to our consolidated financial statements for a sensitivity discussion of the assumed health care cost trend rates.
• Income Taxes - Income tax provisions for jurisdictions outside the United States, as well as state and local income tax provisions, are determined on a separate company basis, and the related assets and liabilities are recorded in our consolidated balance sheets.
The extent of our operations involves dealing with uncertainties and judgments in the application of complex tax regulations in a multitude of jurisdictions. The final taxes paid are dependent upon many factors, including negotiations with taxing authorities in various jurisdictions and resolution of disputes arising from federal, state, and international tax audits. In accordance with the authoritative guidance for income taxes, we evaluate potential tax exposures and record tax liabilities for anticipated tax audit issues based on our estimate of whether, and the extent to which, additional taxes will be due. We adjust these reserves in light of changing facts and circumstances; however, due to the complexity of some of these uncertainties, the ultimate resolution may result in a payment that is materially different from our current estimate of the tax liabilities. If our estimate of tax liabilities proves to be less than the ultimate assessment, an additional charge to expense would result. If payment of these amounts ultimately proves to be less than the recorded amounts, the reversal of the liabilities would result in tax benefits being recognized in the period when we determine the liabilities are no longer necessary.
The effective tax rates used for interim reporting are based on our full-year geographic earnings mix projections and cash repatriation plans. Changes in currency exchange rates, earnings mix by taxing jurisdiction or in cash repatriation plans could have an impact on the effective tax rates, which we monitor each quarter. Significant judgment is required in determining income tax provisions and in evaluating tax positions.
Prior to the spin-off of PMI by Altria, we were a wholly owned subsidiary of Altria. We participated in a tax-sharing agreement with Altria for U.S. tax liabilities, and our accounts were included with those of Altria for purposes of its U.S. federal income tax return. Under the terms of the agreement, taxes were computed on a separate company basis. To the extent that we generated foreign tax credits, capital losses and other credits that could not be utilized on a separate company basis, but were utilized in Altria's consolidated U.S. federal income tax return, we would recognize the resulting benefit in the calculation of our provision for income taxes. We made payments to, or were reimbursed by, Altria for the tax effects resulting from our inclusion in Altria's consolidated United States federal income tax return. On the date of the spin-off of PMI by Altria, we entered into a Tax Sharing Agreement with Altria. The Tax Sharing Agreement generally governs Altria's and our respective rights, responsibilities and obligations for pre-distribution periods and for potential taxes on the spin-off of PMI by Altria. With respect to any potential tax resulting from the spin-off of PMI by Altria, responsibility for the tax will be allocated to the party that acted (or failed to act) in a manner that resulted in the tax. Beginning March 31, 2008, we were no longer a member of the Altria consolidated tax return group, and we filed our own U.S. federal consolidated income tax return. In the third quarter of 2015, the IRS examination of Altria's consolidated tax returns for the years 2007-2008 was concluded with no tax adjustments to PMI.
For further details, see Item 8, Note 11. Income Taxes to our consolidated financial statements.
• Hedging - As discussed below in "Market Risk," we use derivative financial instruments principally to reduce exposures to market risks resulting from fluctuations in foreign currency exchange and interest rates by creating offsetting exposures. For derivatives to which we have elected to apply hedge accounting, gains and losses on these derivatives are initially deferred in accumulated other comprehensive losses on the consolidated balance sheet and recognized in the consolidated statement of earnings in the periods when the related hedged transactions are also recognized in operating results. If we had elected not to use the hedge accounting provisions, gains (losses) deferred in stockholders' (deficit) equity would have been recorded in our net earnings for these derivatives.
• Contingencies - As discussed in Item 8, Note 21. Contingencies to our consolidated financial statements, legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. Much of the tobacco-related litigation is in its early stages, and litigation is subject to uncertainty. At the present time, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the
30
information available to it: (i) management has not concluded that it is probable that a loss has been incurred in any of the pending tobacco-related cases; (ii) management is unable to estimate the possible loss or range of loss for any of the pending tobacco-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any. Legal defense costs are expensed as incurred.
Consolidated Operating Results
Our cigarette volume, net revenues, excise taxes on products and operating companies income by segment were as follows:
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Cigarette Volume |
|
|
|
|
| ||||||
European Union | 194,589 | |
| 194,746 | |
| 194,464 | | |||
Eastern Europe, Middle East & Africa | 279,411 | |
| 278,374 | |
| 287,094 | | |||
Asia | 281,350 | |
| 288,128 | |
| 301,324 | | |||
Latin America & Canada | 91,920 | |
| 94,706 | |
| 97,287 | | |||
Total cigarette volume | 847,270 | |
| 855,954 | |
| 880,169 | | |||
| | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Net Revenues |
|
|
|
|
| ||||||
European Union | $ | 26,563 | |
| $ | 30,517 | |
| $ | 29,656 | |
Eastern Europe, Middle East & Africa | 18,328 | |
| 20,469 | |
| 19,342 | | |||
Asia | 19,469 | |
| 19,255 | |
| 20,987 | | |||
Latin America & Canada | 9,548 | |
| 9,865 | |
| 10,044 | | |||
Net revenues | $ | 73,908 | |
| $ | 80,106 | |
| $ | 80,029 | |
| | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Excise Taxes on Products |
|
|
|
|
| ||||||
European Union | $ | 18,495 | |
| $ | 21,370 | |
| $ | 20,770 | |
Eastern Europe, Middle East & Africa | 10,964 | |
| 11,855 | |
| 10,866 | | |||
Asia | 11,266 | |
| 10,527 | |
| 10,486 | | |||
Latin America & Canada | 6,389 | |
| 6,587 | |
| 6,690 | | |||
Excise taxes on products | $ | 47,114 | |
| $ | 50,339 | |
| $ | 48,812 | |
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Operating Income |
|
|
|
|
| ||||||
Operating companies income: |
|
|
|
|
| ||||||
European Union | $ | 3,576 | |
| $ | 3,815 | |
| $ | 4,309 | |
Eastern Europe, Middle East & Africa | 3,425 | |
| 4,033 | |
| 3,708 | | |||
Asia | 2,886 | |
| 3,187 | |
| 4,622 | | |||
Latin America & Canada | 1,085 | |
| 1,030 | |
| 1,134 | | |||
Amortization of intangibles | (82 | ) |
| (93 | ) |
| (93 | ) | |||
General corporate expenses | (162 | ) |
| (165 | ) |
| (187 | ) | |||
Less: |
|
|
|
|
| ||||||
Equity (income)/loss in unconsolidated subsidiaries, net | (105 | ) |
| (105 | ) |
| 22 | | |||
Operating income | $ | 10,623 | |
| $ | 11,702 | |
| $ | 13,515 | |
31
As discussed in Item 8, Note 12. Segment Reporting to our consolidated financial statements, we evaluate segment performance and allocate resources based on operating companies income, which we define as operating income, excluding general corporate expenses and amortization of intangibles, plus equity (income)/loss in unconsolidated subsidiaries, net. We believe it is appropriate to disclose this measure to help investors analyze the business performance and trends of our various business segments.
References to total international cigarette market, total cigarette market, total market and market shares throughout this Discussion and Analysis reflect our best estimates of tax-paid volumes based on a number of internal and external sources.
The following events that occurred during 2015 , 2014 and 2013 affected the comparability of our statement of earnings amounts:
• Asset Impairment and Exit Costs – For the years ended December 31, 2015 , 2014 and 2013 , pre-tax asset impairment and exit costs by segment were as follows:
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Separation programs: |
|
|
|
|
| ||||||
European Union | $ | 68 | |
| $ | 351 | |
| $ | 13 | |
Eastern Europe, Middle East & Africa | - | |
| 2 | |
| 14 | | |||
Asia | - | |
| 35 | |
| 19 | | |||
Latin America & Canada | - | |
| 3 | |
| 5 | | |||
Total separation programs | 68 | |
| 391 | |
| 51 | | |||
Contract termination charges: |
|
|
|
|
| ||||||
Eastern Europe, Middle East & Africa | - | |
| - | |
| 250 | | |||
Asia | - | |
| - | |
| 8 | | |||
Total contract termination charges | - | |
| - | |
| 258 | | |||
Asset impairment charges: |
|
|
|
|
| ||||||
European Union | - | |
| 139 | |
| - | | |||
Latin America & Canada | - | |
| 5 | |
| - | | |||
Total asset impairment charges | - | |
| 144 | |
| - | | |||
Asset impairment and exit costs | $ | 68 | |
| $ | 535 | |
| $ | 309 | |
For further details, see Item 8, Note 5. Asset Impairment and Exit Costs to our consolidated financial statements.
• Acquisitions and Other Business Arrangements – For further details, see Item 8, Note 6. Acquisitions and Other Business Arrangements to our consolidated financial statements.
2015 compared with 2014
The following discussion compares our consolidated operating results for the year ended December 31, 2015 , with the year ended December 31, 2014 .
Our cigarette shipment volume was down by 1.0% , excluding acquisitions, reflected declines in:
• | Asia, mainly due to Korea, Pakistan and the Philippines; and |
• | Latin America & Canada, mainly due to Argentina, Brazil, Ecuador and Mexico; |
partially offset by growth in:
• | EEMA, notably Egypt, Saudi Arabia and Turkey, partially offset by Kazakhstan and Ukraine. |
Total cigarette volume in the European Union was essentially flat, with declines in Greece, Italy and the United Kingdom largely offset by growth in France, Germany and Spain.
32
For the year ended December 31, 2015 , estimated inventory movements were favorable, driven principally by a favorable comparison in Japan as a result of the 2014 correction of distributor inventory movements partly related to the VAT increase of April 2014. Excluding these estimated inventory movements, our total cigarette shipment volume decreased by 1.6% , excluding acquisitions.
Our cigarette market share increased in a number of key markets, including Argentina, Austria, Belgium, Egypt, France, Germany, Indonesia, Korea, the Netherlands, the Philippines, Poland, Russia, Saudi Arabia, Spain and Switzerland.
Our cigarette shipment volume by brand is shown in the table below:
PMI Cigarette Shipment Volume by Brand (Million Units) | |||||||
| Full-Year | ||||||
| 2015 | |
| 2014 | | Change | |
Marlboro | 285,583 | |
| 282,997 | | 0.9 | % |
L&M | 97,884 | |
| 94,168 | | 3.9 | % |
Parliament | 44,879 | |
| 47,199 | | (4.9 | )% |
Bond Street | 43,608 | |
| 43,585 | | 0.1 | % |
Chesterfield | 41,397 | |
| 42,144 | | (1.8 | )% |
Philip Morris | 35,815 | |
| 31,948 | | 12.1 | % |
Lark | 28,828 | |
| 28,473 | | 1.2 | % |
Others | 269,276 | |
| 285,440 | | (5.7 | )% |
Total PMI | 847,270 | |
| 855,954 | | (1.0 | )% |
The increase in cigarette shipment volume of Marlboro reflected growth in: the European Union, notably France, Germany and Spain, partly offset by Italy and the United Kingdom; EEMA, notably Saudi Arabia and Turkey, partly offset by North Africa and Ukraine; and Asia, notably the Philippines and Vietnam, partly offset by Japan and Korea. Cigarette shipment volume of Marlboro decreased in Latin America & Canada, mainly due to Argentina, Brazil and Mexico, partly offset by Colombia.
The increase in cigarette shipment volume of L&M was predominantly driven by growth in EEMA, notably Egypt, Turkey and Ukraine, partly offset by Russia. The decrease in cigarette shipment volume of Parliament was primarily due to Kazakhstan, Korea, Russia and Ukraine, partly offset by Japan and Turkey. Cigarette shipment volume of Bond Street was essentially flat, with growth, notably driven by Australia, Russia and Serbia, largely offset by declines in the European Union, Kazakhstan and Ukraine. The decrease in cigarette shipment volume of Chesterfield was due to EEMA, mainly Russia, Turkey and Ukraine, partly offset by the European Union, mainly the Czech Republic, Italy and Poland, and by Latin America & Canada, mainly Mexico. The increase in cigarette shipment volume of Philip Morris primarily reflects the morphing of Diana in Italy. The increase in cigarette shipment volume of Lark was principally driven by Japan, partly offset by Korea.
Our other tobacco products ("OTP") primarily include tobacco for roll-your-own and make-your-own cigarettes, pipe tobacco, cigars and cigarillos. Total shipment volume of OTP, in cigarette equivalent units, increased by 1.0% .
Total shipment volume for cigarettes and OTP, in cigarette equivalent units, decreased by 1.0% , excluding acquisitions.
Our net revenues and excise taxes on products were as follows:
|
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2015 |
| 2014 |
| Variance |
| % | |||||||
Net revenues |
| $ | 73,908 | |
| $ | 80,106 | |
| $ | (6,198 | ) |
| (7.7 | )% |
Excise taxes on products |
| 47,114 | |
| 50,339 | |
| (3,225 | ) |
| (6.4 | )% | |||
Net revenues, excluding excise taxes on products |
| $ | 26,794 | |
| $ | 29,767 | |
| $ | (2,973 | ) |
| (10.0 | )% |
33
Net revenues, which include excise taxes billed to customers, decreased by $6.2 billion ( 7.7% ). Excluding excise taxes, net revenues decreased by $3.0 billion ( 10.0% ) to $26.8 billion . This decrease was due primarily to:
• | unfavorable currency ( $4.7 billion ) and |
• | unfavorable volume/mix ( $325 million ), partly offset by |
• | price increases ( $2.1 billion ). |
Currency movements decreased net revenues by $13.5 billion and net revenues, excluding excise taxes on products, by $4.7 billion . The $4.7 billion decrease was due primarily to the Argentine peso, Australian dollar, Canadian dollar, Euro, Indonesian rupiah, Japanese yen, Mexican peso, Russian ruble, Turkish lira and the Ukrainian hryvnia.
Net revenues include $1.8 billion in 2015 and $2.0 billion in 2014 related to sales of OTP. These net revenue amounts include excise taxes billed to customers. Excluding excises taxes, net revenues for OTP were $673 million in 2015 and $753 million in 2014 .
Excise taxes on products decreased by $3.2 billion ( 6.4% ), due primarily to:
• | favorable currency ( $8.8 billion ), partly offset by |
• | higher excise taxes resulting from changes in retail prices and tax rates ( $5.4 billion ) and |
• | higher excise taxes resulting from volume/mix ( $142 million ). |
Governments have consistently increased excise taxes in most of the markets in which we operate. As discussed in Business Environment , we expect excise taxes to continue to increase.
Our cost of sales; marketing, administration and research costs; and operating income were as follows:
|
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2015 |
| 2014 |
| Variance |
| % | |||||||
Cost of sales |
| $ | 9,365 | |
| $ | 10,436 | |
| $ | (1,071 | ) |
| (10.3 | )% |
Marketing, administration and research costs |
| 6,656 | |
| 7,001 | |
| (345 | ) |
| (4.9 | )% | |||
Operating income |
| 10,623 | |
| 11,702 | |
| (1,079 | ) |
| (9.2 | )% | |||
Cost of sales decreased by $1.1 billion ( 10.3% ), due primarily to:
• | favorable currency ( $1.4 billion ), partly offset by |
• | higher manufacturing costs ( $166 million ) and |
• | higher cost of sales resulting from volume/mix ( $148 million ). |
Marketing, administration and research costs decreased by $345 million ( 4.9% ), due primarily to:
• | favorable currency ( $979 million ), partly offset by |
• | higher expenses ( $628 million , primarily higher marketing and selling expenses). |
Operating income decreased by $1.1 billion ( 9.2% ). This decrease was due primarily to:
• | unfavorable currency ( $2.3 billion ), |
• | higher marketing, administration and research costs ( $628 million ), |
• | unfavorable volume/mix ( $473 million ) and |
34
• | higher manufacturing costs ( $166 million ), partly offset by |
• | price increases ( $2.1 billion ) and |
• | lower pre-tax charges for asset impairment and exit costs ( $467 million ). |
On February 5, 2015, we announced that our productivity and cost savings initiatives would include, but were not limited to, the continued enhancement of production processes, the harmonization of tobacco blends, the streamlining of product specifications and number of brand variants, supply chain improvements and overall spending efficiency across the company. We anticipated that these initiatives, combined with savings associated with the manufacturing footprint restructuring implemented in 2014, notably in Australia and the Netherlands, should result in a total company cost-base increase, excluding RRPs and currency, of approximately 1%. In 2015, we decided to deploy additional investments, some of which will not recur in 2016, to support the strong momentum of our cigarette brand portfolio and accelerate the geographic expansion of iQOS. This resulted in a constant currency total cost-base increase of 3.6% excluding RRPs, or 5.3% including RRPs.
In 2016, we expect our total cost base including RRPs to increase by approximately 1%, excluding currency, reflecting productivity and cost-savings programs, coupled with moderating prices for key inputs, such as tobacco leaf, clove and non-tobacco materials.
Our effective tax rate decreased by 1.1 percentage points to 28.0% . The 2015 effective tax rate was unfavorably impacted by changes to repatriation assertions on certain foreign subsidiary historical earnings ($58 million), partially offset by a reduction in unrecognized tax benefits of $41 million following the conclusion of the IRS examinations of Altria's consolidated tax returns for the years 2007 and 2008 and PMI's consolidated tax returns for the years 2009 through 2011. Prior to March 28, 2008, PMI was a wholly owned subsidiary of Altria. The 2014 effective tax rate was unfavorably impacted by the asset impairment and exit costs related to the factory closures. The effective tax rate is based on our full-year earnings mix by taxing jurisdiction and cash repatriation plans. Changes in our cash repatriation plans could have an impact on the effective tax rate, which we monitor each quarter. Significant judgment is required in determining income tax provisions and in evaluating tax positions. Based upon tax regulations in existence at December 31, 2015 , and our cash repatriation plans, we estimate that our 2016 effective tax rate will be approximately 28%.
We are regularly examined by tax authorities around the world, and we are currently under examination in a number of jurisdictions. It is reasonably possible that within the next twelve months certain tax examinations will close, which could result in a change in unrecognized tax benefits along with related interest and penalties. An estimate of any possible charge cannot be made at this time.
Net earnings attributable to PMI of $6.9 billion decreased by $620 million ( 8.3% ). This decrease was due primarily to lower operating income as discussed above, partially offset by a lower effective tax rate. Diluted and basic EPS of $4.42 decreased by 7.1% . Excluding an unfavorable currency impact of $1.20 , diluted EPS increased by 18.1% .
2014 compared with 2013
The following discussion compares our consolidated operating results for the year ended December 31, 2014, with the year ended December 31, 2013.
Our cigarette shipment volume of 856.0 billion units decreased by 2.8%, excluding acquisitions, or 24.3 billion units. The decline in our cigarette shipment volume was due primarily to:
• | EEMA, principally Kazakhstan, Russia and Ukraine, partially offset by Algeria and Turkey; |
• | Asia, predominantly Japan, reflecting a lower total market, lower market share and the unfavorable impact of an adjustment in distributor inventories, as well as Australia, Indonesia and Pakistan; and |
• | Latin America & Canada, principally Canada and Mexico. |
The overall declines were partially offset by:
• | the positive impact of market share growth in the European Union, EEMA and Latin America & Canada Regions; and |
• | cigarette shipment volume in the European Union, which was slightly positive. |
Our market share increased, or was flat in a number of key markets, including Algeria, Argentina, Austria, Canada, France, Germany, Italy, Korea, the Netherlands, Poland, Russia, Saudi Arabia, Spain, Switzerland and the United Kingdom.
35
Our cigarette shipment volume by brand is shown in the table below:
PMI Cigarette Shipment Volume by Brand (Million Units) | |||||||
| Full-Year | ||||||
| 2014 |
| 2013 | Change | |||
Marlboro | 282,997 | |
| 291,090 | | (2.8 | )% |
L&M | 94,168 | |
| 95,004 | | (0.9 | )% |
Parliament | 47,199 | |
| 44,684 | | 5.6 | % |
Bond Street | 43,585 | |
| 44,869 | | (2.9 | )% |
Chesterfield | 42,144 | |
| 34,377 | | 22.6 | % |
Philip Morris | 31,948 | |
| 34,996 | | (8.7 | )% |
Lark | 28,473 | |
| 28,842 | | (1.3 | )% |
Others | 285,440 | |
| 306,307 | | (6.8 | )% |
Total PMI | 855,954 | |
| 880,169 | | (2.8 | )% |
The decrease in cigarette shipment volume of Marlboro reflected declines in: the European Union, notably France, Italy and Poland, partly offset by the Czech Republic and Spain; EEMA, notably in Egypt, Russia and Ukraine, partly offset by Algeria and Saudi Arabia; Asia, due almost entirely to Japan, partly offset by the Philippines; and Latin America & Canada, due predominantly to Mexico. The overall decline was partially offset by the positive impact of market share growth in the European Union and EEMA Regions. Market share of Marlboro in Asia and Latin America & Canada was essentially flat.
The decrease in cigarette shipment volume of L&M was due primarily to EEMA, notably Saudi Arabia and Turkey, partially offset by slightly increased or essentially flat shipments in the three other Regions. The increase in cigarette shipment volume of Parliament was driven by growth in all Regions and notably in Turkey. The decrease in cigarette shipment volume of Bond Street was due predominantly to Kazakhstan, Serbia and Ukraine, partially offset by Australia and Russia. The increase in cigarette shipment volume of Chesterfield was driven by growth in all Regions and notably in Italy, Poland and Turkey, partly offset by Russia and Ukraine. The decrease in cigarette shipment volume of Philip Morris was due almost entirely to Japan, principally reflecting the morphing to Lark , partly offset by growth in the three other Regions. The decrease in cigarette shipment volume of Lark was due predominantly to Turkey, partly offset by Japan (including the impact of the morphing of Philip Morris ).
Total shipment volume of OTP, in cigarette equivalent units, increased by 3.4% to 33.8 billion cigarette equivalent units, mainly due to growth in the fine cut category, notably in Belgium, the Czech Republic, Hungary and Poland, partially offset by France and Germany.
Total shipment volume for cigarettes and OTP, in cigarette equivalent units, was down by 2.5%.
Our net revenues and excise taxes on products were as follows:
|
| For the Years Ended December 31, |
|
|
|
| |||||||||
(in millions) |
| 2014 |
| 2013 |
| Variance |
| % | |||||||
Net revenues |
| $ | 80,106 | |
| $ | 80,029 | |
| $ | 77 | |
| 0.1 | % |
Excise taxes on products |
| 50,339 | |
| 48,812 | |
| 1,527 | |
| 3.1 | % | |||
Net revenues, excluding excise taxes on products |
| $ | 29,767 | |
| $ | 31,217 | |
| $ | (1,450 | ) |
| (4.6 | )% |
Net revenues, which include excise taxes billed to customers, increased by $77 million (0.1%). Excluding excise taxes, net revenues decreased by $1,450 million (4.6%) to $29.8 billion. This decrease was due to:
• | unfavorable currency ($2.1 billion) and |
• | unfavorable volume/mix ($1.3 billion), partly offset by |
• | price increases ($1.9 billion) and |
• | the impact of acquisitions ($13 million). |
36
Currency movements decreased net revenues by $5.3 billion and net revenues, excluding excise taxes on products, by $2.1 billion, due primarily to the Argentine peso, Indonesian rupiah, Japanese yen, Russian ruble, Turkish lira and the Ukrainian hryvnia, partially offset by the Euro.
Net revenues include $2.0 billion in 2014 and $1.9 billion in 2013 related to sales of OTP. These net revenue amounts include excise taxes billed to customers. Excluding excises taxes, net revenues for OTP were $753 million in 2014 and $739 million in 2013.
Excise taxes on products increased by $1.5 billion (3.1%), due primarily to:
• higher excise taxes resulting from changes in retail prices and tax rates ($5.5 billion), partly offset by
• favorable currency ($3.3 billion) and
• volume/mix ($755 million).
Our cost of sales; marketing, administration and research costs; and operating income were as follows:
|
| For the Years Ended December 31, |
|
|
|
| |||||||||
(in millions) |
| 2014 |
| 2013 |
| Variance |
| % | |||||||
Cost of sales |
| $ | 10,436 | |
| $ | 10,410 | |
| $ | 26 | |
| 0.2 | % |
Marketing, administration and research costs |
| 7,001 | |
| 6,890 |
| 111 | |
| 1.6 | % | ||||
Operating income |
| 11,702 | |
| 13,515 | |
| (1,813 | ) |
| (13.4 | )% | |||
Cost of sales increased by $26 million (0.2%), due to:
• | higher manufacturing costs ($545 million, principally in Egypt, due to the impact of the change to our new business structure; in Indonesia, due to higher distribution and manufacturing costs; investments related to the launch and commercialization of the company's Reduced-Risk Product, iQOS; and ongoing costs related to the factory closure in Australia and the decision to discontinue cigarette production in the Netherlands). For further details on our change in business structure in Egypt, see the Acquisitions and Other Business Arrangements section of this Discussion and Analysis and |
• | the impact of acquisitions ($8 million), partially offset by |
• | favorable currency ($380 million) and |
• | volume/mix ($147 million). |
Marketing, administration and research costs increased by $111 million (1.6%), due to:
• | higher expenses ($340 million, primarily higher marketing and selling expenses) and |
• | the impact of acquisitions ($15 million), partly offset by |
• | favorable currency ($244 million). |
Operating income decreased by $1.8 billion (13.4%). This decrease was due primarily to:
• | unfavorable currency ($1.5 billion), |
• | unfavorable volume/mix ($1.1 billion), |
• | higher manufacturing costs ($545 million), |
• | higher marketing, administration and research costs ($340 million) and |
• | higher pre-tax charges for asset impairment and exit costs ($226 million, primarily related to the decision to discontinue cigarette production in the Netherlands), partly offset by |
• | price increases ($1.9 billion). |
Interest expense, net, of $1.1 billion increased by $79 million, due primarily to higher average debt levels, partially offset by lower average interest rates on debt.
37
Our effective tax rate decreased by 0.2 percentage points to 29.1%. The 2014 effective tax rate was unfavorably impacted by the asset impairment and exit costs related to the factory closures. The 2013 effective tax rate was unfavorably impacted by the additional expense associated with the American Taxpayer Relief Act of 2012 ($17 million) and the enactment of tax law changes in Mexico ($14 million).
Equity (income)/loss in unconsolidated subsidiaries, net, of $(105) million increased by $127 million, due primarily to higher earnings from our investments in North Africa and Russia, which are reflected in the EEMA segment.
Net earnings attributable to PMI of $7.5 billion decreased by $1.1 billion (12.6%). This decrease was due primarily to an unfavorable currency impact on operating income and higher interest expense, net. Diluted and basic EPS of $4.76 decreased by 9.5%. Excluding an unfavorable currency impact of $0.80, diluted EPS increased by 5.7%.
Operating Results by Business Segment
Business Environment
Taxes, Legislation, Regulation and Other Matters Regarding the Manufacture, Marketing, Sale and Use of Tobacco Products
The tobacco industry and our business face a number of challenges that may adversely affect our business, volume, results of operations, cash flows and financial position. These challenges, which are discussed below and in Item 1A. Risk Factors , include:
• | fiscal challenges, such as excise tax increases and discriminatory tax structures; |
• | actual and proposed extreme regulatory requirements, including regulation of the packaging, marketing and sale of tobacco products, as well as the products themselves, that may reduce our competitiveness, eliminate our ability to communicate with adult smokers, ban certain of our products, limit our ability to differentiate our products from those of our competitors, and interfere with our intellectual property rights; |
• | illicit trade in cigarettes and other tobacco products, including counterfeit, contraband and so-called "illicit whites"; |
• | intense competition, including from non-tax paid volume by certain local manufacturers; |
• | pending and threatened litigation as discussed in Item 3 and Item 8, Note 21. Contingencies ; and |
• | governmental investigations. |
Ÿ FCTC: The World Health Organization's ("WHO") Framework Convention on Tobacco Control ("FCTC"), an international public health treaty with the objective of reducing tobacco use, drives much of the regulation that shapes the business environment in which we operate. The treaty, to which 179 countries and the European Union are Parties, requires Parties to have in place various tobacco control measures and recommends others.
We support many of the regulatory policies required by the FCTC, including measures that strictly prohibit the sale of tobacco products to minors, limit public smoking, require health warnings on tobacco packaging, and regulate product content to prevent increased adverse health effects of smoking. We advocate measures that establish a regulatory framework for Reduced-Risk Products. We also support the use of tax and price policies to achieve public health objectives, as long as such policies are not discriminatory or excessive, and do not result in increased illicit trade.
However, the FCTC governing body, the Conference of the Parties ("CoP"), has adopted non-binding guidelines and policy recommendations related to certain articles of the FCTC, some of which we strongly oppose, including extreme measures such as point-of-sale display bans, plain packaging, bans on all forms of communications with adult smokers, ingredient restrictions or bans based on the concepts of palatability or attractiveness and excessive taxation. Among other things, these measures would limit our ability to differentiate our products and disrupt competition, are not based on sound evidence of a public health benefit, are likely to lead to adverse consequences, such as increased illicit trade and, in some cases, result in the expropriation of our trademarks and violate international treaties.
It is not possible to predict whether or to what extent measures recommended in the FCTC guidelines will be implemented. In some instances where these extreme measures have been adopted by national governments, we have commenced legal proceedings challenging them.
Ÿ Excise, Sales and Other Taxes: Excessive and disruptive tax increases and discriminatory tax structures are expected to continue to have an adverse impact on our profitability, due to lower consumption and consumer down-trading from premium to non-premium, discount, other low-price or low-taxed tobacco products, such as fine cut tobacco and illicit products. In addition, in certain jurisdictions,
38
our products are subject to tax structures that discriminate against premium-price products and manufactured cigarettes. We believe that such tax structures undermine public health by encouraging consumers to turn to the illicit trade for cheaper tobacco products and ultimately undercut government revenue objectives, disrupt the competitive environment, and encourage criminal activity. Other jurisdictions have imposed, or are seeking to impose, levies or other taxes on tobacco companies. We oppose such extreme and discriminatory tax measures.
Ÿ EU Tobacco Products Directive: In April 2014, the EU adopted the text of a significantly revised EU Tobacco Products Directive that, among other things, provides for:
• | health warnings covering 65% of the front and back panels of packs, with specific health warning dimensions that will in effect prohibit various pack formats, such as certain packs for slim cigarettes, even though the agreed text does not ban slim cigarettes. Member States would also have the option to further standardize tobacco packaging, including, under certain conditions, by introducing plain packaging; |
• | a ban on packs of fewer than 20 cigarettes; |
• | a ban on characterizing flavors in some tobacco products, with a transition period for menthol expiring in May 2020; |
• | security features and tracking and tracing measures, including tracking at pack level down to retail as from May 2019, which we believe will most likely not provide any incremental benefit in the fight against illicit trade, but have the potential to increase operational expenses if excessive implementing regulation is enacted; and |
• | a framework for the regulation of novel tobacco products and e-cigarettes (except for those found to be medicines or medical devices), including requirements for health warnings and information leaflets, prohibiting product packaging text related to reduced risk, and introducing notification requirements in advance of commercialization. |
The revised Directive entered into force in May 2014. Member States are required to implement the Directive by May 2016.
In June 2014, two of our subsidiaries filed papers in the English High Court seeking judicial review of whether the Directive complies with existing EU Treaties. In November 2014, the English High Court referred the case to the Court of Justice of the European Union ("CJEU") and requested that the CJEU issue a judgment by May 2016. In July 2014, the government of Poland filed a complaint with the CJEU challenging the validity of various provisions in the Directive that ban menthol cigarettes. The CJEU conducted hearings in both proceedings in September and October 2015. In December 2015, the Advocate General of the CJEU issued opinions in both proceedings advising that the Directive complies with EU law. These opinions are not binding on the CJEU, which is expected to issue its judgment by May 2016. It is not possible to predict the outcome of these legal proceedings.
Ÿ Plain Packaging: Plain packaging regulation bans the use of branding, logos and colors on packaging of tobacco products other than the brand name and variant, which may be printed only in specified locations and in a uniform font. Similarly, the brand name and variant may be printed on individual cigarettes only in specified locations and in a uniform font or not at all.
To date, only Australia has implemented plain packaging.
France, Ireland and the U.K. have adopted plain packaging legislation, with implementation scheduled to begin no later than May 2016 and full compliance at retail required as of November 2016 in France and as of May 2017 in Ireland and the U.K. In Ireland, implementation is subject to a Ministerial Commencement Order, which has yet to be issued.
In May 2015, three of our subsidiaries filed papers in the English High Court seeking judicial review of the U.K.'s plain packaging legislation. The English High Court held a hearing in December 2015 and indicated that it would issue its judgment in February or March 2016. It is not possible to predict the outcome of these legal proceedings.
In other countries, including Hungary, New Zealand and Norway, proposals to implement plain packaging are in various stages of the legislative process. Additionally, several countries, including Canada, Finland, Singapore and Turkey, are considering plain packaging, but no legislative proposals have been published. It is not possible to predict whether any of these countries will implement plain packaging.
Australia's plain packaging legislation triggered three legal challenges. First, major tobacco manufacturers, including our Australian subsidiary, challenged the legislation's constitutionality in the High Court of Australia. Although the High Court found the legislation constitutional, a majority of the Justices concluded that plain packaging deprives tobacco manufacturers of their property, raising serious questions about the legality of similar proposals in other jurisdictions. Second, our Hong Kong subsidiary initiated arbitration proceedings against the Australian government pursuant to the Hong Kong-Australia Bilateral Investment Treaty and was seeking substantial compensation for the deprivation of its investments in Australia. In December 2015, the tribunal hearing the case declined jurisdiction
39
to hear the merits of our subsidiary's claim. The tribunal's decision, which is final, does not address the legality or effectiveness of Australia's plain packaging legislation. Third, several countries have initiated World Trade Organization ("WTO") dispute settlement proceedings against Australia. It is not possible to predict the outcome of these legal proceedings.
We oppose plain packaging because it expropriates our valuable intellectual property by taking away our trademarks and moves the industry much closer to a commodity business where there is no distinction among brands, and, therefore, the ability to compete for adult smoker market share is greatly reduced. Several studies, including industry-commissioned studies as well as data released by Australian state governments, show that there is no sound basis to conclude that the implementation of plain packaging in Australia has had any impact on smoking prevalence among adults or youth. Data from Australia also appear to confirm that, since the implementation of plain packaging, down-trading to lower price and lower margin brands has accelerated and illicit trade has increased.
In the event any particular jurisdiction adopts plain packaging regulation, we will consider all available options, including litigation, to ensure the protection of our intellectual property.
Ÿ Restrictions and Bans on the Use of Ingredients: Currently, the WHO and others in the public health community recommend restrictions or total bans on the use of some or all ingredients in tobacco products, including menthol. Some regulators have considered and rejected such proposals, while others have proposed and, in a few cases, adopted restrictions or bans. In particular, as mentioned above, the European Union has banned characterizing flavors in tobacco products, subject to an exemption until May 2020 for menthol. Other countries may follow the EU's approach. For instance, Turkey has banned menthol as of May 2020. More sweeping ingredient bans have been adopted by Canada and Brazil.
While the Canadian ingredient ban exempts menthol on the national level, some Canadian provinces have adopted or are in the process of adopting menthol bans.
The Brazil ingredients ban, which, as originally drafted, would prohibit the use of virtually all ingredients with flavoring or aromatic properties, is not in force due to a legal challenge by a tobacco industry union, of which our Brazilian subsidiary is a member. It is not possible to predict the outcome of this legal proceeding.
Broad restrictions and ingredient bans would require us to reformulate our American Blend tobacco products and could reduce our ability to differentiate these products in the market in the long term. Menthol bans would eliminate the entire category of mentholated tobacco products. We oppose broad bans or sweeping restrictions on the use of ingredients, as they are often based on the subjective and scientifically unsupported notion that ingredients make tobacco products more "attractive" or "palatable" and therefore could encourage tobacco consumption, and also because prohibiting entire categories of cigarettes, such as menthol, is likely to lead to a massive increase in illicit trade.
Many countries have enacted or proposed legislation or regulations that require cigarette manufacturers to disclose to governments and to the public the ingredients used in the manufacture of tobacco products and, in certain cases, to provide toxicological information about those ingredients. We have made, and will continue to make, full disclosures where adequate assurances of trade secret protection are provided.
Ÿ Bans on Display of Tobacco Products at Retail: In a few of our markets, governments have banned or propose to ban the display of tobacco products at the point of retail sale. Other countries have rejected display ban proposals. We oppose display bans because they restrict competition by favoring established brands and encourage illicit trade, while not reducing smoking or otherwise benefiting public health. In some markets, our subsidiaries and, in some cases, individual retailers have commenced legal proceedings to overturn display bans.
Ÿ Health Warning Requirements: In most countries, governments require large and often graphic health warnings covering at least 30% of the front and back of cigarette packs (the size mandated by the FCTC). A growing number of countries require warnings covering 50% of the front and back of the pack, and a small number of countries require larger warnings, such as Australia (75% front and 90% back), Mexico (30% front and 100% back), Uruguay (80% front and back) and Canada (75% front and back).
In March 2013, the Ministry of Public Health in Thailand issued a regulation mandating health warnings covering 85% of the front and back of cigarette packs. While a lower court suspended this requirement pending the outcome of legal challenges by two of our affiliates, Thailand's Supreme Administrative Court subsequently overturned this order and allowed the regulation to be implemented during the pendency of our affiliates' claims. The legal challenges by our affiliates are still pending. It is not possible to predict the outcome of these proceedings.
40
We support health warning requirements designed to inform consumers of the risks of smoking. Where health warnings are not required, we place them on packaging voluntarily in the official language or languages of the country. We defer to governments on the content of warnings except for content that vilifies tobacco companies or does not fairly represent the actual effects of smoking. However, we oppose excessively large health warnings, i.e., larger than 50%. The data show that disproportionately increasing the size of health warnings does not effectively reduce tobacco consumption. Yet, such health warnings impede our ability to compete in the market by leaving insufficient space for our distinctive trademarks and pack designs.
Ÿ Other Packaging Restrictions: Some governments have passed, or are seeking to pass, restrictions on packaging and labeling, including standardizing the shape, format and layout of packaging, as well as imposing broad restrictions on how the space left for branding and product descriptions can be used. Examples include prohibitions on: (1) the use of colors that are alleged to suggest that one brand is less harmful than others, (2) specific descriptive phrases deemed to be misleading, including, for example, "premium," "full flavor," "international," "gold," "silver," and "menthol" and (3) in one country, all but one variant per brand. We oppose broad packaging restrictions because they unnecessarily limit brand and product differentiation, are anticompetitive, prevent us from providing consumers with information about our products, unduly restrict our intellectual property rights, and violate international trade agreements. In some instances, we have commenced litigation challenging such regulations. It is not possible to predict the outcome of these proceedings.
Ÿ Bans and Restrictions on Advertising, Marketing, Promotions and Sponsorships: For many years, the FCTC has called for, and countries have imposed, partial or total bans on tobacco advertising, marketing, promotions and sponsorships, including bans and restrictions on advertising on radio and television, in print and on the Internet. The FCTC also requires disclosure of expenditures on advertising, promotion and sponsorship where such activities are not prohibited. The FCTC guidelines recommend that governments adopt extreme and sweeping prohibitions, including all forms of communication to adult smokers. Where restrictions on advertising prevent us from communicating directly and effectively with adult smokers, they impede our ability to compete in the market. For this reason and because we believe that the available evidence does not show that marketing restrictions effectively reduce smoking, we oppose complete bans on advertising and communication that do not allow manufacturers to communicate directly and effectively with adult smokers.
Ÿ Restrictions on Product Design: Anti-tobacco organizations and some regulators are calling for the further standardization of tobacco products by requiring, for example, that cigarettes have a certain minimum diameter, which amounts to a ban on slim cigarettes, or requiring the use of standardized filter and cigarette paper designs. We oppose such restrictions because they limit our ability to differentiate our products and because we believe that there is no correlation, let alone a causal link, between product design variations and smoking rates, nor is there any scientific evidence that these restrictions would improve public health.
Reduced cigarette ignition propensity ("RCIP") standards recommended by the FCTC guidelines, have been adopted in several of our markets (e.g., Australia, Canada, South Africa, South Korea, and the EU), and are being considered in several others. While the available evidence (namely, from two provinces in Canada, the State of New York and Sweden) so far suggests that the implementation of RCIP standards did not result in the predicted reduction of smoking-related fires, RCIP standards do increase production costs.
Ÿ Restrictions on Public Smoking: The pace and scope of public smoking restrictions have increased significantly in most of our markets. Many countries around the world have adopted, or are likely to adopt, regulations that restrict or ban smoking in public and/or work places, restaurants, bars and nightclubs. Some public health groups have called for, and some countries, regional governments and municipalities have adopted or proposed, bans on smoking in outdoor places, as well as bans on smoking in cars (typically, when minors are present) and private homes. The FCTC requires Parties to adopt restrictions on public smoking, and the guidelines call for broad bans in all indoor public places but limit their recommendations on private-place smoking, such as in cars and homes, to increased education on the risk of exposure to environmental tobacco smoke.
While we believe outright bans are appropriate in many public places, such as schools, playgrounds, youth facilities, and many indoor public places, governments can and should seek a balance between the desire to protect non-smokers from environmental tobacco smoke and allowing adults who choose to smoke to do so. Owners of restaurants, bars, cafes, and other entertainment establishments should have the flexibility to permit, restrict, or prohibit smoking, and workplaces should be permitted to provide designated smoking rooms for adult smokers. Finally, we oppose bans on smoking outdoors (beyond places and facilities for children) and in private places.
• Restrictions on the Sale of Innovative Tobacco Products : Some governments have passed, or are seeking to pass, regulations that ban the sale of e-cigarettes or "emerging" tobacco products, including novel tobacco or nicotine products, such as smokeless tobacco - where no combustion takes place and no smoke is produced - dissolvable tobacco products or nicotine, and nicotine delivery systems (i.e., e-vapor products). These regulations might foreclose consumer access even to products that might be shown to present significantly less risk of harm than existing products. We oppose such blanket bans of products that may have the potential to reduce the harm of smoking. By contrast, we support regulation that sets strict standards and propels innovation to benefit consumer and public health.
41
Ÿ Other Regulatory Issues: Some regulators are considering, or in some cases have adopted, regulatory measures designed to reduce the supply of tobacco products. These include regulations intended to reduce the number of retailers selling tobacco by, for example, reducing the overall number of tobacco retail licenses available or banning the sale of tobacco within arbitrary distances of certain public facilities. We oppose such measures because they stimulate illicit trade and could arbitrarily deprive business owners and their employees of their livelihood with no indication that such restrictions would improve public health.
Regulators in some countries have also called for the exclusion of tobacco from certain basic provisions of trade and investment agreements. The Trans-Pacific Partnership Agreement ("TPP") includes a provision that denies "investors," as defined in the TPP, access to the Investor State Dispute Settlement Mechanism to challenge "tobacco control measures." None of the 12 parties to this agreement has yet ratified it. If this carve-out enters into force, we believe it would constitute unfair discrimination against a legal industry, be at odds with fundamental principles of international investment protection, and constitute a dangerous precedent for many other sectors.
In a limited number of markets, most notably Argentina and Japan, we are dependent on governmental approvals that may limit our pricing flexibility.
Ÿ Illicit Trade: The illicit tobacco trade creates a cheap and unregulated supply of tobacco products, undermines efforts to reduce smoking, especially among youth, damages legitimate businesses, stimulates organized crime, increases corruption and reduces government tax revenue. Illicit trade may account for as much as 10% of global cigarette consumption; this includes counterfeit, contraband and the growing problem of "illicit whites," which are cigarettes legally produced in one jurisdiction for the sole purpose of being exported and illegally sold in another jurisdiction where they have no legitimate market. We estimate that illicit trade in the European Union accounted for more than 10% of total cigarette consumption in 2015.
A number of jurisdictions are considering regulatory measures and government action to prevent illicit trade. In November 2012, the FCTC adopted the Protocol to Eliminate Illicit Trade in Tobacco Products (the "Protocol"), which includes supply chain control measures, such as licensing of manufacturers and distributors, enforcement in free trade zones, controls on duty free and Internet sales and the implementation of tracking and tracing technologies. To date, 54 countries have signed the Protocol and 13 countries have ratified it. The Protocol will come into force once the fortieth country ratifies it, after which countries must implement its measures via national legislation. It is not possible to predict whether other countries will sign or ratify the Protocol.
Additionally, we and our subsidiaries have entered into cooperation agreements with governments and authorities to support their anti-illicit trade efforts. For example, in 2004, we entered into a 12-year cooperation agreement with the EU and its member states that provides for cooperation with European law enforcement agencies on anti-contraband and on anti-counterfeit efforts. Under the terms of this agreement we make financial contributions of approximately $75 million per year (recorded as an expense in cost of sales when product is shipped) to support these efforts. We are also required to pay the excise taxes, VAT and customs duties on qualifying seizures of up to 450 million genuine PMI products in the EU in a given year, and five times the applicable taxes and duties if seizures exceed this threshold in a given year. To date, our payments for product seizures have been immaterial.
In 2009, our Colombian subsidiaries entered into an Investment and Cooperation Agreement with the national and regional governments of Colombia to promote investment in, and cooperation on, anti-contraband and anti-counterfeit efforts. The agreement provides $200 million in funding over a 20-year period to address issues such as combating the illegal cigarette trade and increasing the quality and quantity of locally grown tobacco.
Ÿ Reduced-Risk Products: We use the term Reduced-Risk Products ("RRPs") to refer to products with the potential to reduce individual risk and population harm in comparison to smoking cigarettes. Our RRPs are in various stages of development and commercialization, and we are conducting extensive and rigorous scientific studies to determine whether we can support claims for such products of reduced exposure to harmful and potentially harmful constituents in smoke and, ultimately, claims of reduced disease risk when compared to smoking cigarettes. Before making any such claims, we will rigorously evaluate the full set of data from the relevant scientific studies to determine whether they substantiate reduced exposure or risk. Any such claims may also be subject to government review and approval, as is the case in the United States today. We draw upon a team of world-class scientists and engineers from a broad spectrum of scientific disciplines, and our efforts are guided by the following three key objectives:
• | to develop RRPs that provide adult smokers the taste, sensory experience, nicotine delivery profile and ritual characteristics that are similar to those currently provided by cigarettes; |
• | to substantiate the reduction of risk for the individual adult smoker and the reduction of harm to the population as a whole, based on robust scientific evidence derived from well-established assessment processes; and |
42
• | to advocate for the development of science-based regulatory frameworks for the development and commercialization of RRPs, including the communication to adult smokers of scientifically substantiated reduced exposure or reduced risk claims. |
Our product development is based on the elimination of combustion via tobacco heating and other innovative systems for aerosol generation, which we believe is the most promising path to reduce risk.
Our approach to individual risk assessment is to use cessation as the benchmark, because the short-term and long-term effects of smoking cessation on risk reduction are well known.
Four RRP platforms are in various stages of development and commercialization readiness:
• | Platform 1 , as discussed below, uses a precisely controlled heating device that we are commercializing under the iQOS brand name, into which a specially designed tobacco product under the Marlboro , Parliament and HeatSticks brand names is inserted and heated to generate an aerosol. Six short-term clinical studies have been completed. The study results show a substantial reduction in relevant biomarkers of exposure to harmful or potentially harmful constituents ("HPHCs") in adult consumers who switched to iQOS compared to adult consumers who continued to smoke cigarettes over a five-day period. The conduct phase of two three-month clinical reduced-exposure studies conducted in Japan and the United States of America has also been completed, and the final reports are expected shortly. In these studies we observed reduction in 15 HPHCs in those who switched to iQOS compared to those who either continued to smoke cigarettes or quit smoking for the duration of the study. The reductions measured in those who switched to iQOS approached those that were observed in study participants who quit smoking for the duration of the study. We also initiated a 6+6 month exposure response study in December 2014, and anticipate the results regarding the first six-month term in the first quarter of 2017. |
• | Platform 2 uses a pressed carbon heat source to generate an aerosol by heating tobacco. Clinical testing of Platform 2 started in the second quarter of 2015. |
• | Platform 3 is based on technology we acquired from Professor Jed Rose of Duke University and his co-inventors in May 2011. This product creates an aerosol of nicotine salt formed by the chemical reaction of nicotine with a weak organic acid and replicates the feel and ritual of smoking. We are exploring two routes for this platform, one with electronics and one without. We have begun pre-clinical and clinical testing of this product. |
• | Platform 4 covers e-vapor products, which are battery-powered devices that produce an aerosol by vaporizing a liquid nicotine solution. Our e-vapor products comprise devices using current generation technology, and we are working on developing the next generation of e-vapor technologies to address the challenges presented by the e-vapor products currently on the market, ranging from consumer satisfaction to manufacturing processes and product consistency. |
We are also developing other potential product platforms.
We are proceeding with the commercialization of RRPs. In January 2014, we announced an investment of up to €500 million over three years in our first manufacturing facility in the European Union and an associated pilot plant near Bologna, Italy, to produce our RRPs. The factory is designed to produce up to 30 billion units and is expected to become operational by the end of the first quarter of 2016. It will initially manufacture Platform 1 tobacco sticks ( HeatSticks ) .
In the United States of America, an established regulatory framework for assessing "Modified Risk Tobacco Products" exists under the jurisdiction of the Food and Drug Administration ("FDA"). We expect that future FDA actions are likely to influence the regulatory approach of other interested governments. Our assessment approach and the studies conducted to date reflect the rigorous evidentiary package contemplated in the FDA's Draft Guidance for Modified Risk Tobacco Product Applications (2012). We have shared our approach and studies with the FDA's Center for Tobacco Products. In parallel, we are engaging with regulators in several EU member states, as well as in a number of other countries. We plan to submit a Modified Risk Tobacco Product application for Platform 1 late in 2016.
As we work to develop evidence to substantiate the risk reduction potential of our products, we will review our ability to make claims of reduced exposure or risk based on applicable laws and regulations and, as we are already doing, engage with regulators and share the evidence with them. We are also engaging with the scientific community, sharing our assessment approach and the results we have generated. There can be no assurance that we will succeed in our efforts or that regulators will permit the marketing of our RRPs with substantiated claims of reduced formation, exposure, individual risk or population harm.
In 2014, we introduced the iQOS system in pilot city launches in Nagoya, Japan, and in Milan, Italy. We commenced national expansion in Japan in September 2015. We launched iQOS in Switzerland in August 2015 and started pilot city launches in Moscow, Lisbon and
43
Bucharest in November 2015. We also started our gradual expansion in Italy, beginning with Rome and Turin. To date, the product has not been marketed with claims of reduced risk.
In December 2013, we established a strategic framework with Altria under which Altria will make available its e-vapor products exclusively to us for commercialization outside the United States of America, and we will make available two of our RRPs exclusively to Altria for commercialization in the United States of America. In March 2015, we launched Solaris , a Platform 4 e-vapor product licensed from Altria, in Spain. In December 2015, we introduced Solaris in Israel .
In July 2015, we extended the strategic framework with Altria to include a Joint Research, Development and Technology Sharing Agreement. The additional agreement provides the framework under which PMI and Altria will collaborate to develop the next generation of e-vapor products for commercialization in the United States of America by Altria and in markets outside the United States of America by PMI. The collaboration between PMI and Altria in this endeavor is enabled by exclusive technology cross licenses and technical information sharing. The agreements also provide for cooperation on the scientific assessment of, and for the sharing of improvements to, the existing generation of licensed products.
In June 2014, we acquired 100% of Nicocigs Limited, a leading U.K.-based e-vapor company whose principal brand is Nicolites . This acquisition provided PMI with immediate access to, and a significant presence in, the U.K. e-vapor market.
Ÿ Other Legislation, Regulation or Governmental Action: In Argentina, the National Commission for the Defense of Competition issued a resolution in May 2010 in which it found that our affiliate's establishment in 1997 of a system of exclusive zonified distributors ("EZDs") in Buenos Aires city and region was anticompetitive, despite having issued two prior decisions (in 1997 and 2000) in which it had found the establishment of the EZD system was not anticompetitive. In February 2016, the Commission closed the investigation without finding any fault on the part of our affiliate. This decision might be appealed.
In Germany, in October 2013, the Administrative District Office Munich, acting under the policy supervision of the Bavarian Ministry of Health and Environment, sent our German affiliate an order alleging that certain components of its Marlboro advertising campaign do not comply with the applicable tobacco advertising law, and requiring our affiliate to stop this particular campaign throughout Germany. Our affiliate filed a challenge in the Munich Administrative Court, which was granted in part and denied in part. At an appeals hearing in April 2014, before the Bavarian Higher Administrative Court, the parties agreed that our affiliate could continue the campaign with certain limitations on image visuals and text slogans for the duration of the court proceedings. In April 2015, the Administrative District Office Munich issued a revised order, which again required our affiliate to stop using core elements of this particular campaign within one to three months from the effective date of the order. Our affiliate again challenged the order in the Munich Administrative Court, and, in October 2015, the first instance court nullified the order. The Administrative District Office Munich can appeal this decision.
It is not possible to predict what, if any, additional legislation, regulation or other governmental action will be enacted or implemented relating to the manufacturing, advertising, sale or use of tobacco products, or the tobacco industry generally. It is possible, however, that legislation, regulation or other governmental action could be enacted or implemented that might materially affect our business, volume, results of operations, cash flows and financial position.
Governmental Investigations
From time to time, we are subject to governmental investigations on a range of matters. The Department of Special Investigations ("DSI") of the government of Thailand has been conducting an investigation into alleged underpayment by Philip Morris (Thailand) Limited ("PM Thailand") of customs duties and excise taxes of approximately $1.8 billion, relating to imports from Indonesia covering the period 2000-2003. PM Thailand has been cooperating with the Thai authorities and believes that its declared import prices are in compliance with the Customs Valuation Agreement of the WTO and Thai law.
Additionally, in November 2010, a WTO panel issued its decision in a dispute relating to facts that arose from August 2006 between the Philippines and Thailand concerning a series of Thai customs and tax measures affecting cigarettes imported by PM Thailand into Thailand from the Philippines (see Item 3, Legal Proceedings, Other Litigation for additional information). The WTO panel decision, which was upheld by the WTO Appellate Body, concluded that Thailand had no basis to find that PM Thailand's declared customs values and taxes paid were too low, as alleged by the DSI in 2009. The decision also created obligations for Thailand to revise its laws, regulations, or practices affecting the customs valuation and tax treatment of future cigarette imports. Thailand agreed in September 2011 to fully comply with the decision by October 2012. The Philippines contends that to date Thailand has not fully complied and is pursuing bilateral discussions with Thailand to address the outstanding issues. The Philippines has repeatedly expressed concerns with ongoing investigations by Thailand of PM Thailand, including those that led to the criminal charges described in Item 3, Legal Proceedings, Other Litigation , noting that these investigations appear to be based on grounds not supported by WTO customs valuation rules and inconsistent with several decisions already taken by Thai Customs and other Thai governmental agencies.
44
Acquisitions and Other Business Arrangements
In July 2015, we dissolved our exclusive joint venture agreement with Swedish Match AB ("SWMA") to commercialize Swedish snus and other smoke-free tobacco products worldwide, outside of Scandinavia and the United States. The dissolution, mutually agreed with SWMA, means that both companies will now focus on independent strategies for the commercialization of these products, and the trademarks and intellectual property licensed to the joint venture by the companies will revert to their original owners. The dissolution of this agreement was not material to our consolidated financial position, results of operations or cash flows in any of the periods presented.
On January 30, 2014, the Indonesian Stock Exchange ("IDX") adopted a regulation requiring all listed public companies to have at least a 7.5% public shareholding by January 30, 2016. In order to comply with this requirement, our subsidiary PT HM Sampoerna Tbk. ("Sampoerna"), of which we held a 98.18% interest, conducted a rights issue. The exercise price for the rights was set at Rp. 77,000 per share, a 1.349% premium to the closing price on the IDX as of September 30, 2015. In connection with the rights issue, PT Philip Morris Indonesia ("PMID"), a fully consolidated subsidiary of PMI, sold 264,209,711 of the rights to third-party investors. Delivery of the rights sold took place on October 26, 2015. The total net proceeds from the rights issue were $1.5 billion at prevailing exchange rates on the closing date. The sale of the rights resulted in an increase to our additional paid-in capital of $1.1 billion.
In June 2014, we acquired 100% of Nicocigs Limited, a leading U.K.-based e-vapor company, for the final purchase price of $103 million, net of cash acquired, with additional contingent payments of up to $77 million, primarily relating to performance targets over a three-year period. As of December 31, 2015, PMI does not anticipate that the performance targets will be met. For additional information, see Item 8, Note 16. Fair Value Measurements to our consolidated financial statements. The effect of this acquisition was not material to our consolidated financial position, results of operations or cash flows in any of the periods presented.
In the fourth quarter of 2013, as part of our initiative to enhance profitability and growth in North African and Middle Eastern markets, we decided to restructure our business in Egypt. The new business model entails a new contract manufacturing agreement with our long-standing, strategic business partner, Eastern Company S.A.E., the creation of a new PMI affiliate in Egypt and a new distribution agreement with Trans Business for Trading and Distribution LLC. To accomplish this restructuring and to ensure a smooth transition to the new model, we recorded, in the fourth quarter of 2013, a charge to our 2013 full-year reported diluted EPS of approximately $0.10 to reflect the discontinuation of existing contractual arrangements.
In September 2013, Grupo Carso, S.A.B. de C.V. ("Grupo Carso") sold to us its remaining 20% interest in our Mexican tobacco business for $703 million. As a result, we now own 100% of the Mexican tobacco business. A former director of PMI, whose term expired at the Annual Meeting of Shareholders in May 2015, had an affiliation with Grupo Carso. The final purchase price was subject to an adjustment based on the actual performance of the Mexican tobacco business over the three-year period ending two fiscal years after the closing of the purchase. In May 2015, we received a payment of $113 million from Grupo Carso as the final purchase price adjustment. This resulted in a total net purchase price of $590 million. In addition, we agreed to pay a dividend of approximately $38 million to Grupo Carso related to the earnings of the Mexican tobacco business for the nine months ended September 30, 2013. In March 2014, the dividend was declared and paid. The purchase of the remaining 20% interest resulted in a net decrease to our additional paid-in capital of $559 million.
See Item 8, Note 6. Acquisitions and Other Business Arrangements to our consolidated financial statements for additional information.
Investments in Unconsolidated Subsidiaries
On September 30, 2013, we acquired a 49% equity interest in United Arab Emirates-based Emirati Investors-TA (FZC) ("EITA"), formerly Arab Investors-TA (FZC), for approximately $625 million. As a result of this transaction, we hold an approximate 25% economic interest in Société des Tabacs Algéro-Emiratie ("STAEM"), an Algerian joint venture which is 51% owned by EITA and 49% by the Algerian state-owned enterprise Société Nationale des Tabacs et Allumettes SpA. STAEM manufactures and distributes under license some of our brands. The initial investment in EITA was recorded at cost and is included in investments in unconsolidated subsidiaries on the consolidated balance sheets.
On December 12, 2013, we acquired from Megapolis Investment BV a 20% equity interest in Megapolis Distribution BV, the holding company of CJSC TK Megapolis ("Megapolis"), our distributor in Russia, for a purchase price of $760 million . An additional payment of up to $100 million , which is contingent on Megapolis's operational performance over the four fiscal years following the closing of the transaction, will also be made by us if the performance criteria are satisfied. We have also agreed to provide Megapolis Investment BV with a $100 million interest-bearing loan. We and Megapolis Investment BV have agreed to set off any future contingent payments owed by us against the future repayments due under the loan agreement. Any loan repayments in excess of the contingent consideration earned by the performance of Megapolis are due to be repaid, in cash, to us on March 31, 2017. At December 31, 2013, we recorded a $100
45
million asset related to the loan receivable and a discounted liability of $86 million related to the contingent consideration. The initial investment in Megapolis was recorded at cost and is included in investments in unconsolidated subsidiaries on the consolidated balance sheets.
See Item 8, Note 4. Investments in Unconsolidated Subsidiaries to our consolidated financial statements for additional information.
Asset Impairment and Exit Costs
In November 2015, we commenced the implementation of a restructuring program within our European Union segment. The program is expected to be completed by the end of 2017. In total, we expect to incur a total pre-tax charge of approximately $93 million for the program. During 2015, we recorded pre-tax exit costs of $68 million related to employee separation costs. In addition, as part of the total program, up to $25 million of pre-tax implementation costs, primarily related to costs for the project team and notice period payments, will be reflected in cost of sales and marketing, administration and research costs in our consolidated statement of earnings.
On April 4, 2014, we announced the initiation by our affiliate, Philip Morris Holland B.V. ("PMH"), of consultations with employee representatives on a proposal to discontinue cigarette production at its factory located in Bergen op Zoom, the Netherlands. PMH reached an agreement with the trade unions and their members on a social plan, and ceased cigarette production on September 1, 2014. In total, we have incurred a total pre-tax charge of approximately $549 million for the program. During 2014, we recorded pre-tax asset impairment and exit costs of $489 million. This amount included employee separation costs of $343 million, asset impairment costs of $139 million and other separation costs of $7 million. In addition, as part of the total program, approximately $60 million of pre-tax implementation costs, primarily related to notice period payments, have been reflected in cost of sales and marketing, administration and research costs in our consolidated statement of earnings, of which $50 million were recognized during 2014. Excluding asset impairment costs, substantially all of these charges have resulted in cash expenditures. The program has been substantially completed as of December 31, 2015.
Trade Policy
We are subject to various trade restrictions imposed by the United States of America and countries in which we do business ("Trade Sanctions"), including the trade and economic sanctions administered by the U.S. Department of the Treasury's Office of Foreign Assets Control and the U.S. Department of State. It is our policy to comply fully with these Trade Sanctions.
Tobacco products are agricultural products under U.S. law and are not technological or strategic in nature. From time to time we make sales in countries subject to Trade Sanctions, either where they do not apply to our business or pursuant to either exemptions or licenses granted under the applicable Trade Sanctions.
A subsidiary sells products to distributors that in turn sell those products to duty free customers that supply U.N. peacekeeping forces around the world, including those in the Republic of the Sudan. We do not believe that these exempt sales of our products for ultimate resale in the Republic of the Sudan, which are de minimis in volume and value, present a material risk to our shareholders, our reputation or the value of our shares. We have no employees, operations or assets in the Republic of the Sudan.
To our knowledge, none of our commercial arrangements results in the governments of any country identified by the U.S. government as a state sponsor of terrorism, nor entities controlled by those governments, receiving cash or acting as intermediaries in violation of U.S. laws.
We do not sell products in Cuba, Iran, North Korea and Syria.
Certain states within the U.S. have enacted legislation permitting state pension funds to divest or abstain from future investment in stocks of companies that do business with certain countries that are sanctioned by the U.S. We do not believe such legislation has had a material effect on the price of our shares.
46
2015 compared with 2014
The following discussion compares operating results within each of our reportable segments for 2015 with 2014 .
European Union:
European Union |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2015 |
| 2014 |
| Variance |
| % | |||||||
Net revenues |
| $ | 26,563 | |
| $ | 30,517 | |
| $ | (3,954 | ) |
| (13.0 | )% |
Excise taxes on products |
| 18,495 | |
| 21,370 | |
| (2,875 | ) |
| (13.5 | )% | |||
Net revenues, excluding excise taxes on products |
| 8,068 | |
| 9,147 | |
| (1,079 | ) |
| (11.8 | )% | |||
Operating companies income |
| 3,576 | |
| 3,815 | |
| (239 | ) |
| (6.3 | )% | |||
Net revenues, which include excise taxes billed to customers, decreased by $4.0 billion . Excluding excise taxes, net revenues decreased by $1.1 billion , due primarily to:
• | unfavorable currency ( $1.5 billion ) and |
• | unfavorable volume/mix ( $29 million ), partly offset by |
• | price increases ( $442 million ). |
The net revenues of the European Union segment include $1.5 billion in 2015 and $1.6 billion in 2014 related to sales of OTP. Excluding excise taxes, OTP net revenues for the European Union segment were $509 million in 2015 and $573 million in 2014 .
Operating companies income decreased by $239 million during 2015 . This decrease was due primarily to:
• | unfavorable currency ( $857 million ), |
• | higher marketing, administration and research costs ( $242 million ) and |
• | unfavorable volume/mix ( $47 million ), partly offset by |
• | price increases ( $442 million ), |
• | lower pre-tax charges for asset impairment and exit costs ( $422 million , primarily due to the non-recurrence of the 2014 pre-tax charge related to the decision to discontinue cigarette production in the Netherlands) and |
• | lower manufacturing costs ( $46 million ). |
European Union - Industry Volume
The estimated total cigarette market in the European Union of 507.9 billion units decreased by 0.9% . The net impact of estimated trade inventory movements was neutral. The moderate decline of the estimated total cigarette market reflected, in certain key geographies, improving economies, a decrease in the prevalence of illicit trade, lower out-switching to the fine cut category and a lower prevalence of e-vapor products.
The estimated total OTP market in the European Union of 164.9 billion cigarette equivalent units decreased by 0.3% . The total fine cut market was flat at 143.9 billion cigarette equivalent units.
47
European Union - Shipment Volume and Market Share
Cigarette shipment volume and market share performance by brand are shown in the tables below:
European Union Cigarette Shipment Volume by Brand (Million Units) | ||||||||
|
| Full-Year | ||||||
|
| 2015 | |
| 2014 | | Change | |
Marlboro |
| 95,588 | |
| 94,537 | | 1.1 | % |
L&M |
| 35,010 | |
| 34,943 | | 0.2 | % |
Chesterfield |
| 28,278 | |
| 27,100 | | 4.3 | % |
Philip Morris |
| 14,205 | |
| 10,224 | | 38.9 | % |
Others |
| 21,508 | |
| 27,942 | | (23.0 | )% |
Total EU |
| 194,589 | |
| 194,746 | | (0.1 | )% |
European Union Cigarette Market Shares by Brand | ||||||||
|
| Full-Year | ||||||
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | | p.p. | |
Marlboro |
| 18.9 | % |
| 18.7 | % | 0.2 | |
L&M |
| 6.9 | % |
| 6.8 | % | 0.1 | |
Chesterfield |
| 5.8 | % |
| 5.6 | % | 0.2 | |
Philip Morris |
| 3.2 | % |
| 3.2 | % | - | |
Others |
| 3.5 | % |
| 3.9 | % | (0.4 | ) |
Total EU |
| 38.3 | % |
| 38.2 | % | 0.1 | |
Our cigarette shipment volume of 194.6 billion units decreased by 0.1% , or by 0.4% excluding favorable net trade inventory movements, mainly in Italy. Market share increased by 0.1 point to 38.3% , with gains notably in France, Germany, Poland and Spain largely offset by the Czech Republic, Greece, Italy and Portugal.
Our shipments of OTP of 23.4 billion cigarette equivalent units increased by 2.2% . Our total OTP market share increased by 0.2 points to 14.2% .
48
European Union - Market Discussions
In France , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| France Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 45.5 | |
| 45.0 | |
| 1.0 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 18,943 | |
| 18,563 | |
| 2.0 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 25.9 | % |
| 25.1 | % |
| 0.8 | |
Philip Morris |
| 9.5 | % |
| 9.4 | % |
| 0.1 | |
Chesterfield |
| 3.3 | % |
| 3.4 | % |
| (0.1 | ) |
Others |
| 2.9 | % |
| 3.1 | % |
| (0.2 | ) |
Total |
| 41.6 | % |
| 41.0 | % |
| 0.6 | |
The increase in the estimated total cigarette market reflected its general recovery since the second half of 2014 and a lower prevalence of e-vapor products and illicit trade. The increase in our cigarette shipment volume mainly reflected market share growth, notably of premium brands Marlboro , benefiting from a round retail price point of €7.00 per pack and the launch of Marlboro 25s in the first quarter of 2015 , and Philip Morris . The estimated total industry fine cut category of 14.5 billion cigarette equivalent units increased by 6.9% . Our market share of the category decreased by 1.2 points to 25.0% .
In Germany , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Germany Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 80.0 | |
| 80.4 | |
| (0.4 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 29,778 | |
| 29,411 | |
| 1.2 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 22.1 | % |
| 21.7 | % |
| 0.4 | |
L&M |
| 11.9 | % |
| 11.8 | % |
| 0.1 | |
Chesterfield |
| 1.7 | % |
| 1.7 | % |
| - | |
Others |
| 1.5 | % |
| 1.4 | % |
| 0.1 | |
Total |
| 37.2 | % |
| 36.6 | % |
| 0.6 | |
The decline of the estimated total cigarette market was partly due to the impact of price increases, partially offset by a lower prevalence of illicit trade. The increase in our cigarette shipment volume principally reflected market share growth, driven by Marlboro , mainly reflecting the positive impact of the new Architecture 2.0, and L&M , benefiting from a rounded retail price point of €5.00 per pack of 19s. The estimated total industry fine cut category of 41.0 billion cigarette equivalent units decreased by 0.5% . Our market share of the category decreased by 0.2 points to 12.7% .
49
In Italy , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Italy Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 73.8 | |
| 74.4 | |
| (0.8 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 39,717 | |
| 40,439 | |
| (1.8 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 24.6 | % |
| 25.7 | % |
| (1.1 | ) |
Chesterfield |
| 11.0 | % |
| 9.2 | % |
| 1.8 | |
Philip Morris |
| 9.2 | % |
| 10.4 | % |
| (1.2 | ) |
Others |
| 8.9 | % |
| 9.6 | % |
| (0.7 | ) |
Total |
| 53.7 | % |
| 54.9 | % |
| (1.2 | ) |
The moderate decrease in the estimated total cigarette industry was driven by an improved macro-economic environment and a lower prevalence of illicit trade and e-vapor products. Excluding the favorable net impact of estimated trade inventory movements, our cigarette shipment volume declined by 2.9%, mainly reflecting market share loss, notably of: Marlboro , largely due to its price increase in the first quarter of 2015 to €5.20 per pack from its round retail price point of €5.00 per pack; and Philip Morris , including the morphed Diana that had been impacted by the growth of the super-low price segment; partly offset by super-low price Chesterfield . The estimated total industry fine cut category of 6.4 billion cigarette equivalent units increased by 5.1% . Our market share of the category decreased by 0.4 points to 41.1% .
In Poland , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Poland Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 41.1 | |
| 42.1 | |
| (2.3 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 16,763 | |
| 16,630 | |
| 0.8 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 11.4 | % |
| 11.2 | % |
| 0.2 | |
L&M |
| 18.1 | % |
| 18.2 | % |
| (0.1 | ) |
Chesterfield |
| 8.6 | % |
| 7.6 | % |
| 1.0 | |
Others |
| 2.7 | % |
| 3.1 | % |
| (0.4 | ) |
Total |
| 40.8 | % |
| 40.1 | % |
| 0.7 | |
The decrease in the estimated total cigarette market reflected the impact of price increases and an increase in the prevalence of illicit products, partly offset by a lower prevalence of e-vapor products. The increase in our cigarette shipment volume reflected higher market share, driven by Marlboro , partly reflecting the positive impact of the new Architecture 2.0, and Chesterfield , benefiting from its super-slims variants, partly offset by declines from super-low price brands. The estimated total industry fine cut category of 4.0 billion cigarette equivalent units increased by 11.0% , mainly reflecting the retail price impact of excise tax restructuring on the cigar and cigarillo categories that drove higher in-switching to the fine cut category, as well as a lower prevalence of illicit OTP. Our market share of the category decreased by 3.3 points to 31.4% , mainly due to increased price competition at the bottom end of the market.
50
In Spain , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Spain Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 46.7 | |
| 47.0 | |
| (0.6 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 15,435 | |
| 14,879 | |
| 3.7 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 17.0 | % |
| 15.9 | % |
| 1.1 | |
Chesterfield |
| 9.1 | % |
| 9.2 | % |
| (0.1 | ) |
L&M |
| 5.8 | % |
| 6.1 | % |
| (0.3 | ) |
Others |
| 1.5 | % |
| 0.9 | % |
| 0.6 | |
Total |
| 33.4 | % |
| 32.1 | % |
| 1.3 | |
The decrease in the total cigarette market mainly reflected the impact of price increases, partly offset by an improving economy, and a lower prevalence of illicit trade and e-vapor products. The increase in our cigarette shipment volume principally reflected higher market share, driven mainly by Marlboro , benefiting from a round price point in the vending channel, the new Architecture 2.0, and an improving economy. The estimated total industry fine cut category of 9.5 billion cigarette equivalent units decreased by 2.1% . Our market share of the fine cut category decreased by 1.3 points to 13.5% .
Eastern Europe, Middle East & Africa:
Eastern Europe, Middle East & Africa |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2015 |
| 2014 |
| Variance |
| % | |||||||
Net revenues |
| $ | 18,328 | |
| $ | 20,469 | |
| $ | (2,141 | ) |
| (10.5 | )% |
Excise taxes on products |
| 10,964 | |
| 11,855 | |
| (891 | ) |
| (7.5 | )% | |||
Net revenues, excluding excise taxes on products |
| 7,364 | |
| 8,614 | |
| (1,250 | ) |
| (14.5 | )% | |||
Operating companies income |
| 3,425 | |
| 4,033 | |
| (608 | ) |
| (15.1 | )% | |||
Net revenues, which include excise taxes billed to customers, decreased by $2.1 billion . Excluding excise taxes, net revenues decreased by $1.3 billion , due primarily to:
• | unfavorable currency ( $1.8 billion ) and |
• | unfavorable volume/mix ( $53 million ), partly offset by |
• | price increases ( $637 million ). |
51
Operating companies income decreased by $608 million during 2015 . This decrease was due primarily to:
• | unfavorable currency ( $938 million ), |
• | higher marketing, administration and research costs ( $175 million ), |
• | unfavorable volume/mix ( $123 million ) and |
• | higher manufacturing costs ( $54 million ), partially offset by |
• | price increases ( $637 million ) and |
• | higher equity income from unconsolidated subsidiaries ( $44 million ). |
Eastern Europe Middle East & Africa - PMI Cigarette Shipment Volume
Our cigarette shipment volume of 279.4 billion units increased by 0.4% , driven notably by Egypt, Saudi Arabia and Turkey, partially offset by Kazakhstan and Ukraine. Excluding favorable net estimated trade inventory movements, our cigarette shipment volume was essentially flat. Our cigarette shipment volume of premium brands decreased by 0.6% , mainly due to Parliament , down by 3.2% to 33.6 billion units, mainly due to Kazakhstan, Russia and Ukraine, partly offset by Turkey, partly offset by growth from Marlboro , up by 0.7% to 80.7 billion units, driven by Saudi Arabia and Turkey, partly offset by North Africa and Ukraine. Our cigarette shipment volume of L&M increased by 8.4% to 51.2 billion units, driven notably by Egypt, Turkey and Ukraine, partly offset by Russia.
Eastern Europe Middle East & Africa - Market Discussions
In North Africa (defined as Algeria, Egypt, Libya, Morocco and Tunisia), estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| North Africa Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 138.5 | |
| 143.3 | |
| (3.4 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 38,111 | |
| 37,782 | |
| 0.9 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 13.7 | % |
| 15.3 | % |
| (1.6 | ) |
L&M |
| 11.9 | % |
| 8.9 | % |
| 3.0 | |
Others |
| 2.3 | % |
| 1.9 | % |
| 0.4 | |
Total |
| 27.9 | % |
| 26.1 | % |
| 1.8 | |
The decline of the estimated total market was principally due to Egypt, reflecting the impact of excise tax-driven price increases. The increase in our cigarette shipment volume was primarily driven by Egypt, reflecting higher market share, mainly of L&M , resulting from improved territorial coverage and brand building activities, partly offset by Algeria and Tunisia.
52
In Russia , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below.
| Russia Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 294.5 | |
| 314.1 | |
| (6.2 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 84,422 | |
| 84,948 | |
| (0.6 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 1.4 | % |
| 1.6 | % |
| (0.2 | ) |
Parliament |
| 3.9 | % |
| 3.7 | % |
| 0.2 | |
Bond Street |
| 8.4 | % |
| 7.7 | % |
| 0.7 | |
Others |
| 14.7 | % |
| 14.5 | % |
| 0.2 | |
Total |
| 28.4 | % |
| 27.5 | % |
| 0.9 | |
The decline of the estimated total cigarette market was mainly due to the unfavorable impact of excise tax-driven price increases and lower consumer purchasing power as a result of a weak economy. The decrease in our cigarette shipment volume mainly reflected the lower total market, largely offset by market share gains, primarily by premium Parliament , low-price Bond Street , notably its Compact 7.0 variant, and super-low price Next in "Others."
In Turkey , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below.
| Turkey Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 103.2 | |
| 94.7 | |
| 9.0 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 49,014 | |
| 46,309 | |
| 5.8 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 9.5 | % |
| 8.6 | % |
| 0.9 | |
Parliament |
| 11.6 | % |
| 11.2 | % |
| 0.4 | |
Lark |
| 7.6 | % |
| 9.0 | % |
| (1.4 | ) |
Others |
| 15.1 | % |
| 15.2 | % |
| (0.1 | ) |
Total |
| 43.8 | % |
| 44.0 | % |
| (0.2 | ) |
The increase in the estimated total cigarette market mainly reflected a significantly lower prevalence of illicit trade. The increase in our cigarette shipment volume was driven by a higher total market. The decline in our market share was mainly due to low-price Lark , reflecting the impact of price repositioning by our principal competitor in May 2014, partly offset by Marlboro , notably its Touch 7.0 variants, and Parliament , benefiting from the growth of Parliament Night Blue KS , the leading SKU sold on the market, and from up-trading from the mid-price segment.
53
In Ukraine , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below.
| Ukraine Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 70.6 | |
| 69.7 | |
| 1.3 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 19,195 | |
| 23,273 | |
| (17.5 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 3.8 | % |
| 4.9 | % |
| (1.1 | ) |
Parliament |
| 2.9 | % |
| 3.1 | % |
| (0.2 | ) |
Bond Street |
| 8.3 | % |
| 8.9 | % |
| (0.6 | ) |
Others |
| 15.1 | % |
| 15.8 | % |
| (0.7 | ) |
Total |
| 30.1 | % |
| 32.7 | % |
| (2.6 | ) |
The increase in the estimated total market was mainly driven by a lower prevalence of illicit trade. The decrease in our cigarette shipment volume largely reflected lower market share, primarily due to Marlboro , reflecting the impact of widened price gaps, and Bond Street , mainly resulting from competitive price pressure in the low-price segment.
Asia:
Asia |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2015 |
| 2014 |
| Variance |
| % | |||||||
Net revenues |
| $ | 19,469 | |
| $ | 19,255 | |
| $ | 214 | |
| 1.1 | % |
Excise taxes on products |
| 11,266 | |
| 10,527 | |
| 739 | |
| 7.0 | % | |||
Net revenues, excluding excise taxes on products |
| 8,203 | |
| 8,728 | |
| (525 | ) |
| (6.0 | )% | |||
Operating companies income |
| 2,886 | |
| 3,187 | |
| (301 | ) |
| (9.4 | )% | |||
Net revenues, which include excise taxes billed to customers, increased by $214 million . Excluding excise taxes, net revenues decreased by $525 million , due to:
• | unfavorable currency ( $875 million ) and |
• | unfavorable volume/mix ( $100 million ), partly offset by |
• | price increases ( $450 million ). |
54
Operating companies income decreased by $301 million during 2015 . This decrease was due primarily to:
• | unfavorable currency ( $388 million ), |
• | higher marketing, administration and research costs ( $165 million ), |
• | unfavorable volume/mix ( $162 million ) and |
• | higher manufacturing costs ( $70 million ), partly offset by |
• | price increases ( $450 million ) and |
• | the non-recurrence of the 2014 pre-tax charges for asset impairment and exit costs ( $35 million ) due to the factory closure in Australia. |
Asia - PMI Cigarette Shipment Volume
Our cigarette shipment volume of 281.4 billion units decreased by 2.4% , mainly due to: Korea; Pakistan, reflecting a lower total estimated market resulting from the June and December 2015 excise tax-driven price increases, coupled with an increase in the prevalence of illicit trade and lower market share; and the Philippines. Excluding distributor inventory movements in Japan, reflecting a favorable comparison in 2015 resulting from the correction in 2014 of distributor inventory movements related to the VAT increase of April 2014, our cigarette shipment volume decreased by 3.1%.
Our cigarette shipment volume of Marlboro of 73.5 billion units increased by 3.0% , mainly driven by the Philippines and Vietnam, partly offset by Japan and Korea. Cigarette shipment volume of Parliament of 9.4 billion units decreased by 11.5% , primarily due to Korea, partly offset by Japan. Cigarette shipment volume of Lark of 18.3 billion units increased by 3.3% , principally driven by Japan, partly offset by Korea.
Asia - Market Discussions
In Indonesia , estimated industry size, our cigarette shipment volume, market share and segmentation performance are shown in the tables below.
| Indonesia Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 314.0 | |
| 314.0 | |
| - | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 109,840 | |
| 109,694 | |
| 0.1 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Sampoerna A |
| 14.9 | % |
| 14.4 | % |
| 0.5 | |
Dji Sam Soe |
| 7.0 | % |
| 6.3 | % |
| 0.7 | |
U Mild |
| 4.8 | % |
| 5.4 | % |
| (0.6 | ) |
Others |
| 8.3 | % |
| 8.8 | % |
| (0.5 | ) |
Total |
| 35.0 | % |
| 34.9 | % |
| 0.1 | |
55
|
| Indonesia Segmentation Data | |||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| p.p. | |
Segment % of Total Market |
|
|
|
|
|
| |||
Hand-Rolled Kretek (SKT) |
| 18.7 | % |
| 20.1 | % |
| (1.4 | ) |
Machine-Made Kretek (SKM) |
| 75.1 | % |
| 73.5 | % |
| 1.6 | |
Whites (SPM) |
| 6.2 | % |
| 6.4 | % |
| (0.2 | ) |
Total |
| 100.0 | % |
| 100.0 | % |
| - | |
|
|
|
|
|
|
| |||
PMI % Share of Segment |
|
|
|
|
|
| |||
Hand-Rolled Kretek (SKT) |
| 39.2 | % |
| 39.0 | % |
| 0.2 | |
Machine-Made Kretek (SKM) |
| 30.1 | % |
| 29.9 | % |
| 0.2 | |
Whites (SPM) |
| 80.9 | % |
| 79.7 | % |
| 1.2 | |
The estimated total cigarette market was essentially flat, reflecting a soft economic environment. The slight increase in our market share reflected a strong performance from our machine-made kretek brands, notably Sampoerna A , Dji Sam Soe Magnum and Dji Sam Soe Magnum Blue , largely offset by U Mild , and a decline in our hand-rolled kretek portfolio, notably due to Sampoerna Hijau in "Others," down by 0.4 points to 3.0% .
In Japan , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Japan Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 182.3 | |
| 186.2 | |
| (2.1 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 45,690 | |
| 45,556 | |
| 0.3 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 11.3 | % |
| 11.6 | % |
| (0.3 | ) |
Parliament |
| 2.3 | % |
| 2.2 | % |
| 0.1 | |
Lark |
| 9.9 | % |
| 10.0 | % |
| (0.1 | ) |
Others |
| 1.8 | % |
| 2.1 | % |
| (0.3 | ) |
Total |
| 25.3 | % |
| 25.9 | % |
| (0.6 | ) |
The decrease of the estimated total cigarette market moderated to 2.1%. Excluding estimated inventory movements, driven principally by a favorable comparison as a result of the 2014 correction of distributor inventory movements partly related to the VAT increase of April 2014, our cigarette shipment volume decreased by 4.3%. The decline was mainly due to a lower total market, and lower market share principally reflecting the impact of competitive retail price and new menthol taste product offerings.
56
In Korea , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Korea Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 67.3 | |
| 88.1 | |
| (23.6 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 14,201 | |
| 17,346 | |
| (18.1 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 9.6 | % |
| 7.8 | % |
| 1.8 | |
Parliament |
| 7.2 | % |
| 7.1 | % |
| 0.1 | |
Virginia S. |
| 3.8 | % |
| 4.1 | % |
| (0.3 | ) |
Others |
| 0.6 | % |
| 0.7 | % |
| (0.1 | ) |
Total |
| 21.2 | % |
| 19.7 | % |
| 1.5 | |
The decline of the estimated total cigarette market reflected the impact of the January 2015 excise tax increase and related retail price increases. Excluding the impact of estimated inventory movements associated with the timing of the excise tax increase, the total cigarette market declined by approximately 17.3%. The decline in our cigarette shipment volume reflected the lower estimated total market, partly offset by share growth, driven by Marlboro , benefiting from the positive impact of pricing for our principal domestic competitor's main brands.
In the Philippines , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below. Data for the total cigarette market have been restated to reflect estimated total market consumption compared to the previous methodology of reporting only estimated tax-paid industry volumes.
| Philippines Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 90.2 | |
| 94.9 | |
| (4.9 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 66,236 | |
| 68,358 | |
| (3.1 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 21.1 | % |
| 18.4 | % |
| 2.7 | |
Fortune |
| 31.1 | % |
| 30.4 | % |
| 0.7 | |
Jackpot |
| 9.9 | % |
| 10.7 | % |
| (0.8 | ) |
Others |
| 11.3 | % |
| 12.5 | % |
| (1.2 | ) |
Total |
| 73.4 | % |
| 72.0 | % |
| 1.4 | |
Estimated total consumption decreased by 4.9%, mainly due to the impact of price increases. The decline in our cigarette shipment volume reflected the lower total market combined with lower consumption of our low and super-low price brands, following price increases in late 2014 and early 2015, partly offset by higher market share, driven by adult smoker uptrading to Marlboro , combined with market share growth of Fortune , reflecting the narrowing of retail price gaps with brands at the bottom end of the market.
57
Latin America & Canada:
Latin America & Canada |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2015 |
| 2014 |
| Variance |
| % | |||||||
Net revenues |
| $ | 9,548 | |
| $ | 9,865 | |
| $ | (317 | ) |
| (3.2 | )% |
Excise taxes on products |
| 6,389 | |
| 6,587 | |
| (198 | ) |
| (3.0 | )% | |||
Net revenues, excluding excise taxes on products |
| 3,159 | |
| 3,278 | |
| (119 | ) |
| (3.6 | )% | |||
Operating companies income |
| 1,085 | |
| 1,030 | |
| 55 | |
| 5.3 | % | |||
Net revenues, which include excise taxes billed to customers, decreased by $317 million . Excluding excise taxes, net revenues decreased by $119 million , due primarily to:
• | unfavorable currency ( $505 million ) and |
• | unfavorable volume/mix ( $143 million ), partly offset by |
• | price increases ( $525 million ). |
Operating companies income increased by $55 million during 2015 . This increase was due primarily to:
• | price increases ( $525 million ), partly offset by |
• | unfavorable currency ( $210 million ), |
• | unfavorable volume/mix ( $141 million ), |
• | higher manufacturing costs ( $88 million ) and |
• | higher marketing, administration and research costs ( $42 million ). |
Latin America & Canada - PMI Cigarette Shipment Volume and Market Share
Our cigarette shipment volume of 91.9 billion units decreased by 2.9% , mainly due to Argentina, Brazil, Canada and Mexico. Although shipment volume of Marlboro of 35.8 billion units decreased by 3.2%, our Regional market share increased by 0.2 points to an estimated 15.2% . Market share of Marlboro increased notably in Brazil and Colombia, by 0.3 and 1.1 points to 9.5% and 9.0% , respectively. Shipment volume of Philip Morris of 19.4 billion units increased by 1.7% , driven mainly by Canada.
58
Latin America & Canada - Market Discussions
In Argentina , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Argentina Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 | |
| 2014 | |
| % / p.p. | |
Total Cigarette Market (billion units) |
| 40.8 | |
| 41.9 | |
| (2.5 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 31,910 | |
| 32,323 | |
| (1.3 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 24.3 | % |
| 24.3 | % |
| - | |
Parliament |
| 2.1 | % |
| 2.2 | % |
| (0.1 | ) |
Philip Morris |
| 44.7 | % |
| 43.4 | % |
| 1.3 | |
Others |
| 7.1 | % |
| 7.3 | % |
| (0.2 | ) |
Total |
| 78.2 | % |
| 77.2 | % |
| 1.0 | |
The decline of the estimated total cigarette market was mainly due to the impact of price increases and a challenging economic environment. The decrease in our shipment volume was mainly due to a lower estimated total market, partly offset by market share growth, driven primarily by Philip Morris , reflecting the positive impact of the brand's capsule variants. Our share of the growing capsule segment, representing 16.4% of the total market, grew by 4.4 points to 73.5%.
In Canada , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Canada Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 |
| 2014 |
| % / p.p. | | ||
Total Cigarette Market (billion units) |
| 26.7 | |
| 27.3 | |
| (2.3 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 9,926 | |
| 10,275 | |
| (3.4 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Belmont |
| 3.3 | % |
| 3.0 | % |
| 0.3 | |
Canadian Classics |
| 10.3 | % |
| 10.4 | % |
| (0.1 | ) |
Next |
| 10.6 | % |
| 10.6 | % |
| - | |
Others |
| 13.1 | % |
| 13.6 | % |
| (0.5 | ) |
Total |
| 37.3 | % |
| 37.6 | % |
| (0.3 | ) |
The estimated total cigarette market decreased by 2.3%. Excluding the favorable impact of estimated competitors' trade inventory movements, the total market declined by 4.6%, mainly due to the impact of tax-driven price increases. The decrease in our cigarette shipment volume was principally due to a lower estimated total market. Our market share was also negatively impacted by the above-mentioned estimated competitors' trade inventory movements.
59
In Mexico , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Mexico Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | | ||
|
| 2015 |
| 2014 |
| % / p.p. | | ||
Total Cigarette Market (billion units) |
| 33.6 | |
| 33.5 | |
| 0.4 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 23,246 | |
| 23,861 | |
| (2.6 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 48.1 | % |
| 49.7 | % |
| (1.6 | ) |
Delicados |
| 10.7 | % |
| 11.1 | % |
| (0.4 | ) |
Benson & Hedges |
| 4.6 | % |
| 5.2 | % |
| (0.6 | ) |
Others |
| 5.8 | % |
| 5.3 | % |
| 0.5 | |
Total |
| 69.2 | % |
| 71.3 | % |
| (2.1 | ) |
The estimated total cigarette market increased by 0.4%. Excluding the unfavorable impact of estimated trade inventory movements, the total market increased by 2.8%, primarily reflecting a lower prevalence of illicit trade. The decrease in our cigarette shipment volume was mainly driven by: lower market share, mainly due to Marlboro, reflecting adult smoker down-trading; and the timing of price increases by our principal competitor in the first quarter of 2015; partly offset by gains for certain low-price local trademark brands.
2014 compared with 2013
The following discussion compares operating results within each of our reportable segments for 2014 with 2013.
European Union:
European Union |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2014 |
| 2013 |
| Variance |
| % | |||||||
Net revenues |
| $ | 30,517 | |
| $ | 29,656 | |
| $ | 861 | |
| 2.9 | % |
Excise taxes on products |
| 21,370 | |
| 20,770 | |
| 600 | |
| 2.9 | % | |||
Net revenues, excluding excise taxes on products |
| 9,147 | |
| 8,886 | |
| 261 | |
| 2.9 | % | |||
Operating companies income |
| 3,815 | |
| 4,309 | |
| (494 | ) |
| (11.5 | )% | |||
Net revenues, which include excise taxes billed to customers, increased by $861 million . Excluding excise taxes, net revenues increased by $261 million , due to:
• | price increases ($134 million), |
• | favorable currency ($126 million) and |
• | the impact of acquisitions ($11 million), partly offset by |
• | unfavorable volume/mix ($10 million). |
The net revenues of the European Union segment include $1.7 billion in 2014 and $1.5 billion in 2013 related to sales of OTP. Excluding excise taxes, OTP net revenues for the European Union segment were $574 million in 2014 and $544 million in 2013.
60
Operating companies income decreased by $494 million during 2014. This decrease was due primarily to:
• | higher pre-tax charge for asset impairment and exit costs ($477 million, primarily related to the decision to discontinue cigarette production in the Netherlands in 2014), |
• | higher marketing, administration and research costs ($101 million), |
• | higher manufacturing costs ($44 million) and |
• | unfavorable volume/mix ($42 million), partly offset by |
• | price increases ($134 million) and |
• | favorable currency ($39 million). |
European Union - Industry Volume
The total estimated cigarette market in the European Union of 512.5 billion units decreased by 3.1% , due primarily to the impact of tax-driven price increases and the unfavorable economic and employment environment, partly offset by: the subdued performance of the e-vapor category; less out-switching to fine cut products; a reduction in the consumption of illicit products in several markets; and lower than historical average pricing, mainly in Italy.
The total OTP market in the European Union of 165.4 billion cigarette equivalent units increased by 1.3% , reflecting a larger total fine cut market, up by 1.2% to 143.9 billion cigarette equivalent units.
European Union - Shipment Volume and Market Share
Cigarette shipment volume and market share performance by brand are shown in the tables below:
European Union Cigarette Shipment Volume by Brand (Million Units) | ||||||||
|
| Full-Year | ||||||
|
| 2014 |
| 2013 | Change | |||
Marlboro |
| 94,537 | |
| 96,069 | | (1.6 | )% |
L&M |
| 34,943 | |
| 34,985 | | (0.1 | )% |
Chesterfield |
| 27,100 | |
| 19,707 | | 37.5 | % |
Philip Morris |
| 10,224 | |
| 9,768 | | 4.7 | % |
Others |
| 27,942 | |
| 33,935 | | (17.7 | )% |
Total EU |
| 194,746 | |
| 194,464 | | 0.1 | % |
European Union Cigarette Market Shares by Brand | ||||||||
|
| Full-Year | ||||||
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 | p.p. | | ||
Marlboro |
| 18.7 | % |
| 18.3 | % | 0.4 | |
L&M |
| 6.8 | % |
| 6.7 | % | 0.1 | |
Chesterfield |
| 5.6 | % |
| 4.5 | % | 1.1 | |
Philip Morris |
| 3.2 | % |
| 3.5 | % | (0.3 | ) |
Others |
| 3.9 | % |
| 4.2 | % | (0.3 | ) |
Total EU |
| 38.2 | % |
| 37.2 | % | 1.0 | |
Our cigarette shipment volume of 194.7 billion units increased by 0.1% , predominantly reflecting improved market share that increased by 1.0 share point to 38.2% .
61
While shipment volume of Marlboro decreased, mainly due to a lower total market, market share increased driven notably by the Czech Republic, Germany, Italy and Spain, partly offset by France and Poland. While cigarette shipment volume of L&M was essentially flat, market share increased slightly, driven notably by Germany, partly offset by Poland. Cigarette shipment volume of Chesterfield increased, and market share increased, driven notably by Italy and Poland. Cigarette shipment volume of Philip Morris increased, driven notably by Latvia, Lithuania, the Slovak Republic and Spain.
Our shipments of OTP of 22.9 billion cigarette equivalent units increased by 6.4%, driven principally by higher share. Our OTP total market share was 14.0% , up by 0.6 share points, reflecting gains in the fine cut category: notably in the Czech Republic, up by 7.8 share points to 26.5% ; Hungary, up by 6.4 share points to 18.3% ; Italy, up by 3.9 share points to 41.5% ; and Poland, up by 11.2 share points to 34.7% ; partly offset by France, down by 0.7 share points to 26.2% ; Germany down by 1.3 share points to 12.9% , and Portugal, down by 5.4 share points to 26.5% .
European Union - Market Discussions
In France , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| France Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 45.0 | |
| 47.5 | |
| (5.3 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 18,563 | |
| 19,123 | |
| (2.9 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 25.1 | % |
| 24.7 | % |
| 0.4 | |
Philip Morris |
| 9.4 | % |
| 9.1 | % |
| 0.3 | |
Chesterfield |
| 3.4 | % |
| 3.4 | % |
| - | |
Others |
| 3.1 | % |
| 3.0 | % |
| 0.1 | |
Total |
| 41.0 | % |
| 40.2 | % |
| 0.8 | |
The total cigarette market decreased , mainly reflecting the impact of price increases in January 2014, the increased incidence of e-vapor products and a weak economy. The decrease in our cigarette shipment volume was mainly driven by the lower total market, partially offset by our market share increase, mainly driven by the growth of Marlboro , L&M ( up by 0.1 share point to 2.6% ) and premium Philip Morris . The estimated total industry fine cut category of 13.6 billion cigarette equivalent units decreased by 2.2% . Our market share of the category decreased by 0.7 share points to 26.2% .
62
In Germany , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Germany Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 80.4 | |
| 79.6 | |
| 0.9 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 29,411 | |
| 28,838 | |
| 2.0 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 21.7 | % |
| 22.0 | % |
| (0.3 | ) |
L&M |
| 11.8 | % |
| 10.9 | % |
| 0.9 | |
Chesterfield |
| 1.7 | % |
| 1.7 | % |
| - | |
Others |
| 1.4 | % |
| 1.6 | % |
| (0.2 | ) |
Total |
| 36.6 | % |
| 36.2 | % |
| 0.4 | |
The total cigarette market increased , mainly reflecting the net favorable impact of estimated trade purchases and a lower incidence of illicit trade. Excluding the impact of these estimated inventory movements, the total cigarette market was essentially flat. The increase in our cigarette shipment volume mainly reflected market share growth, driven by L&M . The estimated total industry fine cut category of 41.2 billion cigarette equivalent units decreased by 1.0% . Our market share of the category decreased by 1.3 share points to 12.9% .
In Italy , estimated industry size, PMI cigarette shipment volume and market share performance are shown in the table below.
| Italy Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 74.4 | |
| 74.0 | |
| 0.5 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 40,439 | |
| 38,920 | |
| 3.9 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 25.7 | % |
| 26.4 | % |
| (0.7 | ) |
Chesterfield |
| 9.2 | % |
| 3.5 | % |
| 5.7 | |
Philip Morris |
| 10.4 | % |
| 13.2 | % |
| (2.8 | ) |
Others |
| 9.6 | % |
| 10.0 | % |
| (0.4 | ) |
Total |
| 54.9 | % |
| 53.1 | % |
| 1.8 | |
The total cigarette market increased , partly reflecting a lower incidence of e-vapor products. The increase in our cigarette shipment volume was driven by our market share increase, notably Chesterfield, partly offset by Marlboro, and Philip Morris (including the morphed Diana in the low-price segment) that had been impacted by the growth of the super-low price segment. The estimated total industry fine cut category of 6.1 billion cigarette equivalent units increased by 1.6% . Our market share of the category increased by 3.9 share points to 41.5% .
63
In Poland , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Poland Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 42.1 | |
| 46.6 | |
| (9.8 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 16,630 | |
| 17,079 | |
| (2.6 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 11.2 | % |
| 11.5 | % |
| (0.3 | ) |
L&M |
| 18.2 | % |
| 17.8 | % |
| 0.4 | |
Chesterfield |
| 7.6 | % |
| 5.6 | % |
| 2.0 | |
Others |
| 3.1 | % |
| 3.3 | % |
| (0.2 | ) |
Total |
| 40.1 | % |
| 38.2 | % |
| 1.9 | |
In Poland, the total estimated cigarette market decreased , reflecting the prevalence of e-cigarettes, illicit trade and non-duty paid OTP products. The decrease in our cigarette shipment volume reflected the lower total market, partially offset by our market share increase, driven by L&M and Chesterfield . The estimated total industry fine cut category of 3.6 billion cigarette equivalent units increased by 7.7% , and our market share of the category increased by 11.2 share points to 34.7% .
In Spain , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Spain Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 47.0 | |
| 47.7 | |
| (1.5 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 14,879 | |
| 14,606 | |
| 1.9 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 15.9 | % |
| 14.8 | % |
| 1.1 | |
Chesterfield |
| 9.2 | % |
| 9.3 | % |
| (0.1 | ) |
L&M |
| 6.1 | % |
| 6.3 | % |
| (0.2 | ) |
Others |
| 0.9 | % |
| 0.8 | % |
| 0.1 | |
Total |
| 32.1 | % |
| 31.2 | % |
| 0.9 | |
The total cigarette market decreased , mainly due to a deceleration in adult smoker down-trading to fine cut, e-vapor and illicit products. Our cigarette shipment volume increased , reflecting our market share growth, notably Marlboro and Philip Morris in "Others" ( up by 0.3 share points to 0.9% ). The estimated total industry fine cut category of 9.7 billion cigarette equivalent units decreased by 9.8% , partly reflecting lower consumption resulting from further tax harmonization with cigarettes following the July 2013 and July 2014 price increases. Our market share of the fine cut category increased by 1.0 share point to 14.8% .
64
Eastern Europe, Middle East & Africa:
Eastern Europe, Middle East & Africa |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2014 |
| 2013 |
| Variance |
| % | |||||||
Net revenues |
| $ | 20,469 | |
| $ | 19,342 | |
| $ | 1,127 | |
| 5.8 | % |
Excise taxes on products |
| 11,855 | |
| 10,866 | |
| 989 | |
| 9.1 | % | |||
Net revenues, excluding excise taxes on products |
| 8,614 | |
| 8,476 | |
| 138 | |
| 1.6 | % | |||
Operating companies income |
| 4,033 | |
| 3,708 | |
| 325 | |
| 8.8 | % | |||
Net revenues, which include excise taxes billed to customers, increased by $1.1 billion . Excluding excise taxes, net revenues increased by $138 million , due primarily to:
• | price increases ($1.1 billion), partly offset by |
• | unfavorable currency ($765 million) and |
• | unfavorable volume/mix ($231 million). |
Operating companies income increased by $325 million during 2014. This increase was due primarily to:
• | price increases ($1.1 billion), |
• | lower pre-tax charges for asset impairment and exit costs ($262 million) and |
• | higher equity income in unconsolidated subsidiaries ($135 million), partly offset by |
• | unfavorable currency ($613 million), |
• | higher manufacturing costs ($250 million, principally related to the impact of the change to our new business structure in Egypt), |
• | unfavorable volume/mix ($206 million) and |
• | higher marketing, administration and research costs ($128 million). |
Eastern Europe Middle East & Africa - PMI Cigarette Shipment Volume
Our cigarette shipment volume in EEMA decreased by 3.0% to 278.4 billion units, mainly due to Kazakhstan, Russia, Serbia and Ukraine, partly offset by Algeria, Saudi Arabia and Turkey. Our cigarette shipment volume of premium brands increased by 1.0%, driven by Parliament , up by 6.9% to 34.7 billion units, partly offset by Marlboro , down by 1.1% to 80.1 billion units.
65
Eastern Europe Middle East & Africa - Market Discussions
In North Africa , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| North Africa Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 143.3 | |
| 138.7 | |
| 3.4 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 37,782 | |
| 36,849 | |
| 2.5 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 15.3 | % |
| 15.3 | % |
| - | |
L&M |
| 8.9 | % |
| 9.1 | % |
| (0.2 | ) |
Others |
| 1.9 | % |
| 2.1 | % |
| (0.2 | ) |
Total |
| 26.1 | % |
| 26.5 | % |
| (0.4 | ) |
The estimated total cigarette market increased , driven by Algeria, Egypt and Tunisia, partially offset by Libya and Morocco. Our cigarette shipment volume increased , driven largely by Marlboro in Algeria and L&M in Egypt.
In Russia , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below.
| Russia Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 314.1 | |
| 346.4 | |
| (9.3 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 84,948 | |
| 88,021 | |
| (3.5 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 1.6 | % |
| 1.7 | % |
| (0.1 | ) |
Parliament |
| 3.7 | % |
| 3.4 | % |
| 0.3 | |
Bond Street |
| 7.7 | % |
| 6.5 | % |
| 1.2 | |
Others |
| 14.5 | % |
| 14.6 | % |
| (0.1 | ) |
Total |
| 27.5 | % |
| 26.2 | % |
| 1.3 | |
The total cigarette market decreased , mainly due to the unfavorable impact of tax-driven price increases and a weak economy. Our cigarette shipment volume decrease mainly reflected the lower total market, partially offset by market share growth. Shipment volume of our premium portfolio decreased by 2.5% , mainly due to Marlboro, down by 13.6% , partially offset by Parliament , up by 1.6% . In the mid-price segment, shipment volume decreased by 9.1% , mainly due to Chesterfield , down by 18.6% . In the low-price segment, shipment volume decreased by 1.4% , mainly due to Optima and Apollo Soyuz , down by 16.3% and 8.5% , respectively, partly offset by Bond Street , up by 2.5% . Our market share, as measured by Nielsen, was up , mainly driven by Bond Street and L&M (up by 0.3 share points to 3.1%), partially offset by Chesterfield (down by 0.2 share points to 2.8%).
66
In Turkey , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below.
| Turkey Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 94.7 | |
| 91.7 | |
| 3.3 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 46,309 | |
| 45,247 | |
| 2.3 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 8.6 | % |
| 8.9 | % |
| (0.3 | ) |
Parliament |
| 11.2 | % |
| 10.0 | % |
| 1.2 | |
Lark |
| 9.0 | % |
| 11.4 | % |
| (2.4 | ) |
Others |
| 15.2 | % |
| 15.2 | % |
| - | |
Total |
| 44.0 | % |
| 45.5 | % |
| (1.5 | ) |
The total cigarette market increased , primarily reflecting an increase in the adult population. Our market share, as measured by Nielsen, decreased , mainly due to: Marlboro, mid-price Muratti ( down by 1.4 share points to 5.5% ), low-price L&M ( down by 0.9 share points to 6.4% ) and low-price Lark , partly offset by premium Parliament , and low-price Chesterfield ( up by 2.3 share points to 3.1% ).
In Ukraine , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below.
| Ukraine Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 69.7 | |
| 70.7 | |
| (1.4 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 23,273 | |
| 25,526 | |
| (8.8 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 4.9 | % |
| 5.5 | % |
| (0.6 | ) |
Parliament |
| 3.1 | % |
| 3.3 | % |
| (0.2 | ) |
Bond Street |
| 8.9 | % |
| 8.8 | % |
| 0.1 | |
Others |
| 15.8 | % |
| 15.9 | % |
| (0.1 | ) |
Total |
| 32.7 | % |
| 33.5 | % |
| (0.8 | ) |
The total cigarette market decreased , mainly reflecting the impact of price increases in 2014 and business disruption due to the political instability in the east of the country, partially offset by a lower prevalence of illicit trade. Our market share, as measured by Nielsen, decreased , mainly due to: Marlboro, Parliament, Chesterfield ( down by 0.9 share points to 5.0% ) and Optima ( down by 0.8 share points to 1.0% ), partly offset by growth from low-price President ( up by 2.2 share points to 5.0% ).
67
Asia:
Asia |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2014 |
| 2013 |
| Variance |
| % | |||||||
Net revenues |
| $ | 19,255 | |
| $ | 20,987 | |
| $ | (1,732 | ) |
| (8.3 | )% |
Excise taxes on products |
| 10,527 | |
| 10,486 | |
| 41 | |
| 0.4 | % | |||
Net revenues, excluding excise taxes on products |
| 8,728 | |
| 10,501 | |
| (1,773 | ) |
| (16.9 | )% | |||
Operating companies income |
| 3,187 | |
| 4,622 | |
| (1,435 | ) |
| (31.0 | )% | |||
Net revenues, which include excise taxes billed to customers, decreased by $1.7 billion. Excluding excise taxes, net revenues decreased by $1.8 billion due to:
• | unfavorable currency ($1.0 billion) and |
• | unfavorable volume/mix ($906 million), partly offset by |
• | price increases ($155 million). |
Operating companies income decreased by $1.4 billion during 2014. This decrease was due primarily to:
• | unfavorable volume/mix ($746 million), |
• | unfavorable currency ($656 million), |
• | higher manufacturing costs ($181 million, principally in Indonesia driven mainly by higher clove prices and cost related to the transition from hand-rolled to machine-made kretek cigarette production) and |
• | higher pre-tax charges for asset impairment and exit costs ($8 million, principally due to the factory closure in Australia), partly offset by |
• | price increases ($155 million). |
Asia - PMI Cigarette Shipment Volume
Our cigarette shipment volume of 288.1 billion units decreased by 4.4%, due primarily to: the unfavorable impact of an adjustment in distributor inventories in Japan; lower total market and share in Australia, mainly reflecting the impact of excise tax-driven price increases and competitive pricing in the deep discount segment, Japan and Pakistan, and lower share in Indonesia.
Shipment volume of Marlboro of 71.4 billion units decreased by 5.3%, due almost entirely to Japan, partly offset by the Philippines. Shipment volume of Parliament of 10.7 billion units increased by 1.8%, driven by Korea. Shipment volume of Lark of 17.7 billion units increased by 7.4%, driven mainly by Japan (including the morphed Philip Morris ).
68
Asia - Market Discussions
In Indonesia , estimated industry size, our cigarette shipment volume, market share and segmentation performance are shown in the tables below.
| Indonesia Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 314.0 | |
| 308.0 | |
| 1.9 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 109,694 | |
| 111,332 | |
| (1.5 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Sampoerna A |
| 14.4 | % |
| 14.4 | % |
| - | |
Dji Sam Soe |
| 6.3 | % |
| 6.8 | % |
| (0.5 | ) |
U Mild |
| 5.4 | % |
| 4.4 | % |
| 1.0 | |
Others |
| 8.8 | % |
| 10.6 | % |
| (1.8 | ) |
Total |
| 34.9 | % |
| 36.2 | % |
| (1.3 | ) |
|
| Indonesia Segmentation Data | |||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| p.p. | |||
Segment % of Total Market |
|
|
|
|
|
| |||
Hand-Rolled Kretek (SKT) |
| 20.1 | % |
| 23.6 | % |
| (3.5 | ) |
Machine-Made Kretek (SKM) |
| 73.5 | % |
| 69.7 | % |
| 3.8 | |
Whites (SPM) |
| 6.4 | % |
| 6.7 | % |
| (0.3 | ) |
Total |
| 100.0 | % |
| 100.0 | % |
| - | |
|
|
|
|
|
|
| |||
PMI % Share of Segment |
|
|
|
|
|
| |||
Hand-Rolled Kretek (SKT) |
| 39.0 | % |
| 43.9 | % |
| (4.9 | ) |
Machine-Made Kretek (SKM) |
| 29.9 | % |
| 29.5 | % |
| 0.4 | |
Whites (SPM) |
| 79.7 | % |
| 77.7 | % |
| 2.0 | |
Our market share decreased , predominantly due to Sampoerna Hijau in "Others" ( down by 0.9 share points to 3.4% ), mainly reflecting the decline of the total hand-rolled kretek segment, and the hand-rolled, full-flavor variants of Dji Sam Soe in the premium segment, which decreased by 1.5 share points to 4.2%, mainly due to a retail price change ahead of competition . The decline in our market share was partly offset by machine-made mid-price U Mild , and machine-made Dji Sam Soe Magnum and Dji Sam Soe Magnum Blue , up by a combined 1.0 share point to 2.1% . While market share of Marlboro decreased by 0.1 share point to 5.1% (in "Others"), its share of the "white" cigarettes segment increased by 2.0 share points to 79.7%.
69
In Japan , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Japan Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 186.2 | |
| 192.6 | |
| (3.4 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 45,556 | |
| 52,997 | |
| (14.0 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 11.6 | % |
| 12.1 | % |
| (0.5 | ) |
Parliament |
| 2.2 | % |
| 2.2 | % |
| - | |
Lark |
| 10.0 | % |
| 10.0 | % |
| - | |
Others |
| 2.1 | % |
| 2.4 | % |
| (0.3 | ) |
Total |
| 25.9 | % |
| 26.7 | % |
| (0.8 | ) |
The total cigarette market decreased , partly reflecting the unfavorable impact of the consumption tax-driven retail price increases of April 1, 2014. Our cigarette shipment volume decreased , principally due to the unfavorable impact of an adjustment in distributor inventories and a lower total market and share. Excluding the impact of these inventory movements, our cigarette shipment volume decreased by 5.8%.
In Korea , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Korea Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 88.1 | |
| 88.4 | |
| (0.4 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 17,346 | |
| 17,160 | |
| 1.1 | % |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 7.8 | % |
| 7.7 | % |
| 0.1 | |
Parliament |
| 7.1 | % |
| 6.9 | % |
| 0.2 | |
Virginia S. |
| 4.1 | % |
| 4.1 | % |
| - | |
Others |
| 0.7 | % |
| 0.7 | % |
| - | |
Total |
| 19.7 | % |
| 19.4 | % |
| 0.3 | |
The estimated total cigarette market slightly decreased by 0.4%. Excluding favorable estimated trade inventory movements, the total cigarette market decreased by approximately 3.8%. The increase in our cigarette shipment volume was mainly driven by higher market share, notably Parliament .
70
In the Philippines , estimated industry size, our cigarette shipment volume and market share performance, as measured by Nielsen, are shown in the table below. Data for the total cigarette market have been restated to reflect estimated total market consumption compared to the previous methodology of reporting only estimated tax-paid industry volumes.
| Philippines Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 94.9 | |
| 91.0 | |
| 4.4 | % |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 68,358 | |
| 68,479 | |
| (0.2 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 18.4 | % |
| 20.3 | % |
| (1.9 | ) |
Fortune |
| 30.4 | % |
| 31.3 | % |
| (0.9 | ) |
Jackpot |
| 10.7 | % |
| 6.6 | % |
| 4.1 | |
Others |
| 12.5 | % |
| 17.0 | % |
| (4.5 | ) |
Total |
| 72.0 | % |
| 75.2 | % |
| (3.2 | ) |
The estimated total consumption increased , driven by the growth of the low and super-low price segments reflecting the prevalence of domestic non-duty-paid products. Our cigarette shipment volume decreased , mainly due to a lower market share.
Latin America & Canada:
Latin America & Canada |
| For the Years Ended December 31, |
|
| |||||||||||
(in millions) |
| 2014 |
| 2013 |
| Variance |
| % | |||||||
Net revenues |
| $ | 9,865 | |
| $ | 10,044 | |
| $ | (179 | ) |
| (1.8 | )% |
Excise taxes on products |
| 6,587 | |
| 6,690 | |
| (103 | ) |
| (1.5 | )% | |||
Net revenues, excluding excise taxes on products |
| 3,278 | |
| 3,354 | |
| (76 | ) |
| (2.3 | )% | |||
Operating companies income |
| 1,030 | |
| 1,134 | |
| (104 | ) |
| (9.2 | )% | |||
Net revenues, which include excise taxes billed to customers, decreased by $179 million . Excluding excise taxes, net revenues decreased by $76 million, due primarily to:
• | unfavorable currency ($431 million) and |
• | unfavorable volume/mix ($127 million), partly offset by |
• | price increases ($481 million). |
Operating companies income of $1.0 billion decreased by $104 million during 2014. This decrease was due primarily to:
• | unfavorable currency ($243 million), |
• | unfavorable volume/mix ($133 million), |
• | higher marketing, administration and research costs ($135 million) and |
• | higher manufacturing costs ($70 million), partly offset by |
• | price increases ($481 million). |
71
Latin America & Canada - PMI Cigarette Shipment Volume and Market share
Our cigarette shipment volume of 94.7 billion units decreased by 2.7%, principally due to a lower total market, predominantly in Canada and Mexico. While shipment volume of Marlboro of 37.0 billion units decreased by 4.3%, due predominantly to Mexico, its market share was up in Argentina, Brazil and Colombia by 0.2 , 0.5 and 1.0 share points to 24.3% %, 9.2% and 7.9% , respectively. Shipment volume of Philip Morris of 19.1 billion units increased by 2.1%, driven mainly by Argentina.
Latin America & Canada - Market Discussions
In Argentina , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Argentina Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 41.9 | |
| 42.5 | |
| (1.6 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 32,323 | |
| 32,384 | |
| (0.2 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 24.3 | % |
| 24.1 | % |
| 0.2 | |
Parliament |
| 2.2 | % |
| 2.1 | % |
| 0.1 | |
Philip Morris |
| 43.4 | % |
| 41.7 | % |
| 1.7 | |
Others |
| 7.3 | % |
| 8.3 | % |
| (1.0 | ) |
Total |
| 77.2 | % |
| 76.2 | % |
| 1.0 | |
The decrease in our cigarette shipment volume was primarily driven by a lower total market, largely offset by market share growth. Our market share growth was driven by Marlboro and mid-price Philip Morris , reflecting the positive impact of its capsule variants, partly offset by low-price Next in "Others" (down by 0.6 share points to 2.0%).
In Canada , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Canada Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 27.3 | |
| 28.9 | |
| (5.5 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 10,275 | |
| 10,769 | |
| (4.6 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Belmont |
| 3.0 | % |
| 2.6 | % |
| 0.4 | |
Canadian Classics |
| 10.4 | % |
| 10.1 | % |
| 0.3 | |
Next |
| 10.6 | % |
| 9.9 | % |
| 0.7 | |
Others |
| 13.6 | % |
| 14.6 | % |
| (1.0 | ) |
Total |
| 37.6 | % |
| 37.2 | % |
| 0.4 | |
The total cigarette market decreased , mainly due to the impact of both federal and provincial tax-driven price increases during the first half of the year. The decrease in our cigarette shipment volume was driven by the lower total market, partially offset by market share growth, notably Belmont, Canadian Classics and Next , partially offset by Number 7 (down by 0.2 share points to 4.0% ) and Accord (down by 0.5 share points to 2.4% ).
72
In Mexico , estimated industry size, our cigarette shipment volume and market share performance are shown in the table below.
| Mexico Key Market Data | ||||||||
|
| Full-Year | |||||||
|
|
|
|
|
| Change | |||
|
| 2014 |
| 2013 |
| % / p.p. | |||
Total Cigarette Market (billion units) |
| 33.5 | |
| 34.6 | |
| (3.2 | )% |
|
|
|
|
|
|
| |||
PMI Shipments (million units) |
| 23,861 | |
| 25,423 | |
| (6.1 | )% |
|
|
|
|
|
|
| |||
PMI Cigarette Market Share |
|
|
|
|
|
| |||
Marlboro |
| 49.7 | % |
| 52.3 | % |
| (2.6 | ) |
Delicados |
| 11.1 | % |
| 11.2 | % |
| (0.1 | ) |
Benson & Hedges |
| 5.2 | % |
| 5.5 | % |
| (0.3 | ) |
Others |
| 5.3 | % |
| 4.5 | % |
| 0.8 | |
Total |
| 71.3 | % |
| 73.5 | % |
| (2.2 | ) |
The total cigarette market decreased , primarily reflecting unfavorable estimated trade inventory movements compared to 2013. Excluding the impact of these inventory movements, the total cigarette market is estimated to have declined by approximately 0.5%. Our cigarette shipment volume decreased , driven by the lower total market and market share decline, notably due to Marlboro and Benson & Hedges, reflecting consumer down-trading. Our share of the premium price segment was up by 1.3 share points to 92.0% .
Financial Review
Ÿ Net Cash Provided by Operating Activities
Net cash provided by operating activities of $7.9 billion for the year ended December 31, 2015 , increased by $126 million from the comparable 2014 period. The change was due primarily to net earnings growth (excluding unfavorable currency of $1.9 billion) and working capital initiatives.
Excluding currency, the favorable movements in working capital were due primarily to the following:
• | more cash provided by accounts receivable, primarily due to the timing of sales and cash collections (including the sale of accounts receivable in 2015 to unaffiliated financial institutions as disclosed in Item 8. Note 23. Sale of Accounts Receivable ); and |
• | less cash used for accrued liabilities and other current assets, primarily due to the timing of payments for excise taxes; partially offset by |
• | more cash used for inventories, primarily related to higher finished goods inventories. |
Net cash provided by operating activities of $7.7 billion for the year ended December 31, 2014 , decreased by $2.4 billion from the comparable 2013 period. The decrease was due primarily to lower net earnings (primarily related to unfavorable currency movements), an increase in our working capital requirements, and higher cash payments related to exit costs.
The unfavorable movements in working capital were due primarily to the following:
• | more cash used for accrued liabilities and other current assets, largely due to the timing of payments for excise taxes, partially offset by |
• | more cash provided by inventories, primarily related to lower leaf tobacco and finished goods inventories. |
73
Ÿ Net Cash Used in Investing Activities
Net cash used in investing activities of $708 million for the year ended December 31, 2015 , decreased by $288 million from the comparable 2014 period, due primarily to lower capital expenditures and purchases of businesses in 2014.
Net cash used in investing activities of $996 million for the year ended December 31, 2014 , decreased by $1.7 billion from the comparable 2013 period, due primarily to less cash spent on investments in unconsolidated subsidiaries and higher cash collateral received from derivatives designated as net investment hedges, partially offset primarily by the purchase of Nicocigs Limited.
In June 2014, we acquired 100% of Nicocigs Limited, a leading U.K.-based e-vapor company, for the final purchase price of $103 million, net of cash acquired. For further details, see Item 8, Note 6. Acquisitions and Other Business Arrangements to our consolidated financial statements.
As previously discussed, on September 30, 2013, we acquired a 49% equity interest in United Arab Emirates-based Arab Investors-TA (FZC) for approximately $625 million. On December 12, 2013, we acquired from Megapolis Investment BV a 20% equity interest in Megapolis Distribution BV, the holding company of CJSC TK Megapolis, our distributor in Russia, for a purchase price of $760 million. For further details, see Item 8, Note 4. Investments in Unconsolidated Subsidiaries to our consolidated financial statements.
Our capital expenditures were $960 million in 2015 , $1.2 billion in 2014 and $1.2 billion in 2013 . The 2015 expenditures were primarily related to investments in RRPs, productivity-enhancing programs, and equipment for new products. We expect total capital expenditures in 2016 of approximately $1.1 billion (including additional capital expenditures related to our ongoing investment in RRPs), to be funded by operating cash flows.
Ÿ Net Cash Used in Financing Activities
During 2015 , net cash used in financing activities was $4.7 billion , compared with net cash used in financing activities of $6.8 billion during 2014 and $8.2 billion in 2013 .
The 2015 change was due primarily to the cash used in 2014 to repurchase our common stock pursuant to our share repurchase program, as well as the 2015 net proceeds received from the sale of subsidiary shares to noncontrolling interests, partially offset by lower net cash proceeds in 2015 from long-term debt.
On January 30, 2014, the Indonesian Stock Exchange ("IDX") adopted a regulation requiring all listed public companies to have at least a 7.5% public shareholding by January 30, 2016. In order to comply with this requirement, our subsidiary PT HM Sampoerna Tbk. ("Sampoerna"), of which we held a 98.18% interest, conducted a rights issue. In connection with the rights issue, PT Philip Morris Indonesia ("PMID"), a fully consolidated subsidiary of PMI, sold 264,209,711 of the rights to third party investors. Delivery of the rights sold took place on October 26, 2015. The total net proceeds from the rights issue were $1.5 billion at prevailing exchange rates on the closing date. For further details, see Item 8, Note 6. Acquisitions and Other Business Arrangements to our consolidated financial statements.
During 2014 , we used a total of $13.2 billion to repurchase our common stock, pay dividends and repay debt. These uses were partially offset by proceeds from our debt offerings and short-term borrowings in 2014 of $6.6 billion. During 2013 , we used a total of $17.1 billion to repurchase our common stock, pay dividends, repay debt and purchase subsidiary shares from noncontrolling interests. These uses were partially offset by proceeds from our debt offerings and short-term borrowings in 2013 of $9.2 billion.
In September 2013, Grupo Carso sold us its remaining 20% interest in our Mexican tobacco business for $703 million. As a result, we own 100% of our Mexican tobacco business. The final purchase price was subject to an adjustment based on the actual performance of the Mexican tobacco business over the three-year period ending two fiscal years after the closing of the purchase. In May 2015, PMI received a payment of $113 million from Grupo Carso as the final purchase price adjustment. This resulted in a total net purchase price of $590 million. For further details, see Item 8, Note 6. Acquisitions and Other Business Arrangements to our consolidated financial statements.
Dividends paid in 2015 , 2014 and 2013 were $6.3 billion , $6.0 billion and $5.7 billion , respectively.
74
Ÿ Debt and Liquidity
We define cash and cash equivalents as short-term, highly liquid investments, readily convertible to known amounts of cash that mature within a maximum of three months and have an insignificant risk of change in value due to interest rate or credit risk changes. As a policy, we do not hold any investments in structured or equity-linked products. Our cash and cash equivalents are predominantly held in short-term bank deposits with institutions having a long-term rating of A- or better.
Credit Ratings – The cost and terms of our financing arrangements, as well as our access to commercial paper markets, may be affected by applicable credit ratings. On July 10, 2015, Fitch affirmed our long-term credit rating at "A" and short-term at "F1," but it revised our outlook to "Negative" from "Stable." We do not expect the Fitch negative outlook to have an impact on our borrowing costs. On July 17, 2015, Standard & Poor's affirmed our long-term credit rating at "A" and short-term at "A-1," as well as our "Stable" outlook. On August 19, 2015, Moody's affirmed our long-term credit rating at "A2" and short-term at "P-1," as well as our "Stable" outlook. At February 16, 2016, our credit ratings and outlook by major credit rating agencies were as follows:
| Short-term | Long-term | Outlook |
Moody's | P-1 | A2 | Stable |
Standard & Poor's | A-1 | A | Stable |
Fitch | F1 | A | Negative |
Credit Facilities – On October 1, 2015, PMI replaced its $3.5 billion multi-year revolving credit facility, expiring October 25, 2016, with a new $3.5 billion multi-year revolving credit facility, expiring October 1, 2020. On January 27, 2016, PMI entered into an agreement to amend and extend its existing $2.0 billion 364-day revolving credit facility, effective February 9, 2016, from February 9, 2016, to February 7, 2017. On January 27, 2016, PMI also entered into an agreement to extend the term of its existing $2.5 billion multi-year revolving credit facility, effective February 28, 2016, from February 28, 2020, to February 28, 2021.
At February 16, 2016, our committed credit facilities were as follows:
(in billions)
Type |
| Committed Credit Facilities | ||
364-day revolving credit, expiring February 7, 2017 |
| $ | 2.0 | |
Multi-year revolving credit, expiring February 28, 2020 (1) |
| 2.5 | | |
Multi-year revolving credit, expiring October 1, 2020 |
| 3.5 | | |
Total facilities |
| $ | 8.0 | |
(1) Effective February 28, 2016, the term of our $2.5 billion multi-year revolving credit facility was
extended from February 28, 2020, to February 28, 2021.
At February 16, 2016, there were no borrowings under the committed credit facilities, and the entire $8.0 billion of committed amounts were available for borrowing.
All banks participating in our committed credit facilities have an investment-grade long-term credit rating from the credit rating agencies. We continuously monitor the credit quality of our banking group, and at this time we are not aware of any potential non-performing credit provider.
Each of these facilities requires us to maintain a ratio of consolidated earnings before interest, taxes, depreciation and amortization ("consolidated EBITDA") to consolidated interest expense of not less than 3.5 to 1.0 on a rolling four-quarter basis. At December 31, 2015 , our ratio calculated in accordance with the agreements was 10.5 to 1.0. These facilities do not include any credit rating triggers, material adverse change clauses or any provisions that could require us to post collateral. We expect to continue to meet our covenants. The terms "consolidated EBITDA" and "consolidated interest expense," both of which include certain adjustments, are defined in the facility agreements previously filed with the U.S. Securities and Exchange Commission.
75
In addition to the committed credit facilities discussed above, certain of our subsidiaries maintain short-term credit arrangements to meet their respective working capital needs. These credit arrangements, which amounted to approximately $2.9 billion at December 31, 2015 , and $3.2 billion at December 31, 2014 , are for the sole use of our subsidiaries. Borrowings under these arrangements amounted to $825 million at December 31, 2015 , and $1.2 billion at December 31, 2014 .
Commercial Paper Program – We have commercial paper programs in place in the U.S. and in Europe. At December 31, 2015 and December 31, 2014 , we had no commercial paper outstanding.
Effective April 19, 2013, our commercial paper program in the U.S. was increased by $2.0 billion. As a result, our commercial paper programs in place in the U.S. and in Europe currently have an aggregate issuance capacity of $8.0 billion.
We expect that the existence of the commercial paper program and the committed credit facilities, coupled with our operating cash flows, will enable us to meet our liquidity requirements.
Sale of Accounts Receivable – To mitigate credit risk and enhance cash and liquidity management we sell trade receivables to unaffiliated financial institutions. These arrangements allow us to sell, on an ongoing basis, certain trade receivables without recourse. The trade receivables sold are generally short-term in nature and are removed from the consolidated balance sheets. We sell trade receivables under two types of arrangements, servicing and non-servicing.
PMI's operating cash flows were positively impacted by the amount of the trade receivables sold and derecognized from the consolidated balance sheets, which remained outstanding with the unaffiliated financial institutions. The trade receivables sold that remained outstanding under these arrangements as of December 31, 2015 , 2014 and 2013 were $888 million , $120 million and $146 million , respectively. The net proceeds received are included in cash provided by operating activities in the consolidated statements of cash flows.
For further details, see Item 8, Note 23. Sale of Accounts Receivable to our consolidated financial statements.
Debt – Our total debt was $28.5 billion at December 31, 2015 , and $29.5 billion at December 31, 2014 . Our total debt is primarily fixed rate in nature. For further details, see Item 8, Note 7. Indebtedness . The weighted-average all-in financing cost of our total debt was 3.0% in 2015 , compared to 3.2% in 2014 . See Item 8, Note 16. Fair Value Measurements to our consolidated financial statements for a discussion of our disclosures related to the fair value of debt. The amount of debt that we can issue is subject to approval by our Board of Directors.
On February 21, 2014, we filed a shelf registration statement with the U.S. Securities and Exchange Commission, under which we may from time to time sell debt securities and/or warrants to purchase debt securities over a three-year period.
Our debt issuances in 2015 were as follows:
(in millions) |
|
|
|
|
|
|
|
| |
Type |
| Face Value |
| Interest Rate |
| Issuance |
| Maturity | |
|
|
|
|
|
|
|
|
| |
U.S. dollar notes | (a) | $500 |
| 1.250 | % |
| August 2015 |
| August 2017 |
U.S. dollar notes | (a) | $750 |
| 3.375 | % |
| August 2015 |
| August 2025 |
|
|
|
|
|
|
|
|
| |
(a) Interest on these notes is payable annually in arrears beginning in February 2016.
The net proceeds from the sale of the securities listed in the table above will be used for general corporate purposes.
The weighted-average time to maturity of our long-term debt was 10.8 years at the end of 2014 and 10.5 years at the end of 2015 .
• | Off-Balance Sheet Arrangements and Aggregate Contractual Obligations |
We have no off-balance sheet arrangements, including special purpose entities, other than guarantees and contractual obligations discussed below.
76
Guarantees – At December 31, 2015 , we were contingently liable for $0.7 billion of guarantees of our own performance, which were primarily related to excise taxes on the shipment of our products. There is no liability in the consolidated financial statements associated with these guarantees. At December 31, 2015, our third-party guarantees were insignificant.
Aggregate Contractual Obligations – The following table summarizes our contractual obligations at December 31, 2015 :
|
| Payments Due | |||||||||||||
| Total | 2016 | 2017-2018 | 2019-2020 | 2021 and | ||||||||||
(in millions) |
| ||||||||||||||
Long-term debt (1) | | $27,922 | | | $2,405 | | | $5,097 | | | $4,745 | | | $15,675 | |
RBH Legal Settlement (2) | 78 | | 32 | | 46 | | - | | - | | |||||
Colombian Investment and Cooperation Agreement (3) | 107 | | 15 | | 12 | | 12 | | 68 | | |||||
Interest on borrowings (4) | 10,786 | | 883 | | 1,566 | | 1,236 | | 7,101 | | |||||
Operating leases (5) | 682 | | 177 | | 207 | | 103 | | 195 | | |||||
Purchase obligations (6) : |
|
|
|
|
| ||||||||||
Inventory and production costs | 5,094 | | 2,007 | | 1,586 | | 852 | | 649 | | |||||
Other | 1,568 | | 1,049 | | 467 | | 50 | | 2 | | |||||
| 6,662 | | 3,056 | | 2,053 | | 902 | | 651 | | |||||
Other long-term liabilities (7) | 336 | | 27 | | 75 | | 25 | | 209 | | |||||
| | $46,573 | | | $6,595 | | | $9,056 | | | $7,023 | | | $23,899 | |
(1) Amounts represent the expected cash payments of our long-term debt and capital lease obligations.
(2) Amounts represent the estimated future payments due under the terms of the settlement agreement. See Item 8, Note 19. RBH Legal Settlement , to our consolidated financial statements for more details regarding this settlement.
(3) Amounts represent the expected cash payments under the terms of the Colombian Investment and Cooperation Agreement. See Item 8, Note 18. Colombian Investment and Cooperation Agreement to our consolidated financial statements for more details regarding this agreement.
(4) Amounts represent the expected cash payments of our interest expense on our long-term debt, including the current portion of long-term debt. Interest on our fixed-rate debt is presented using the stated interest rate. Interest on our variable rate debt is estimated using the rate in effect at December 31, 2015. Amounts exclude the amortization of debt discounts, the amortization of loan fees and fees for lines of credit that would be included in interest expense in the consolidated statements of earnings.
(5) Amounts represent the minimum rental commitments under non-cancelable operating leases.
(6) Purchase obligations for inventory and production costs (such as raw materials, indirect materials and supplies, packaging, co-manufacturing arrangements, storage and distribution) are commitments for projected needs to be utilized in the normal course of business. Other purchase obligations include commitments for marketing, advertising, capital expenditures, information technology and professional services. Arrangements are considered purchase obligations if a contract specifies all significant terms, including fixed or minimum quantities to be purchased, a pricing structure and approximate timing of the transaction. Amounts represent the minimum commitments under non-cancelable contracts. Any amounts reflected on the consolidated balance sheet as accounts payable and accrued liabilities are excluded from the table above.
(7) Other long-term liabilities consist primarily of postretirement health care costs and accruals established for employment costs. The following long-term liabilities included on the consolidated balance sheet are excluded from the table above: accrued pension and postemployment costs, tax contingencies, insurance accruals and other accruals. We are unable to estimate the timing of payments (or contributions in the case of accrued pension costs) for these items. Currently, we anticipate making pension contributions of approximately $113 million in 2016, based on current tax and benefit laws (as discussed in Item 8, Note 13. Benefit Plans to our consolidated financial statements).
The E.C. agreement payments discussed below are excluded from the table above, as the payments are subject to adjustment based on certain variables, including our market share in the EU.
E.C. Agreement – As discussed in Item 8, Note 20. E.C. Agreement , in 2004, we entered into an agreement with the European Commission (acting on behalf of the European Community) that provides for broad cooperation with European law enforcement agencies on anti-contraband and anti-counterfeit efforts. This agreement has been signed by all 27 Member States. This agreement calls for payments that are to be adjusted based on certain variables, including our market share in the European Union in the year preceding payment. Because future additional payments are subject to these variables, we record these payments as an expense in cost of sales when product is shipped. In addition, we are also responsible to pay the excise taxes, VAT and customs duties on qualifying product seizures of up to 90 million cigarettes and are subject to payments of five times the applicable taxes and duties if qualifying product seizures exceed 90 million cigarettes in a given year. In October 2014, this agreement was amended and the threshold was increased to 450 million cigarettes in a given year. This modification was effective as of July 2012. To date, our annual payments related to product seizures have been immaterial. Total charges related to the E.C. Agreement of $79 million , $71 million and $81 million were recorded in cost of sales in 2015 , 2014 and 2013 , respectively.
77
Ÿ Equity and Dividends
As discussed in Item 8, Note 9. Stock Plans to our consolidated financial statements, during 2015 , we granted 1.5 million shares of deferred stock awards to eligible employees at a weighted-average grant date fair value of $82.28 per share. Equity awards generally vest three or more years after the date of the award, subject to earlier vesting on death or disability or normal retirement, or separation from employment by mutual agreement after reaching age 58.
In May 2012, our stockholders approved the Philip Morris International Inc. 2012 Performance Incentive Plan (the "2012 Plan"). Under the 2012 Plan, we may grant to eligible employees restricted stock, restricted stock units and deferred stock units, performance-based cash incentive awards and performance-based equity awards. Up to 30 million shares of our common stock may be issued under the 2012 Plan. At December 31, 2015 , shares available for grant under the 2012 plan were 23,249,430 .
On August 1, 2012, we began repurchasing shares under a new three-year $18.0 billion share repurchase program that was authorized by our Board of Directors in June 2012. From August 1, 2012, through December 31, 2014, we repurchased 144.6 million shares of our common stock at a cost of $12.7 billion under this repurchase program. During 2015 , we did not repurchase any shares under this program.
On February 4, 2016, we announced that we do not plan any share repurchases in 2016. We will revisit the potential for repurchases as the year unfolds, depending on the currency environment.
Dividends paid in 2015 were $6.3 billion . During the third quarter of 2015, our Board of Directors approved a 2.0% increase in the quarterly dividend to $1.02 per common share. As a result, the present annualized dividend rate is $4.08 per common share.
Market Risk
Ÿ Counterparty Risk - We predominantly work with financial institutions with strong short- and long-term credit ratings as assigned by Standard & Poor's and Moody's. These banks are also part of a defined group of relationship banks. Non-investment grade institutions are only used in certain emerging markets to the extent required by local business needs. We have a conservative approach when it comes to choosing financial counterparties and financial instruments. As such we do not invest or hold investments in any structured or equity-linked products. The majority of our cash and cash equivalents is currently invested in bank deposits maturing within less than 30 days.
We continuously monitor and assess the credit worthiness of all our counterparties.
Ÿ Derivative Financial Instruments - We operate in markets outside of the U.S., with manufacturing and sales facilities in various locations throughout the world. Consequently, we use certain financial instruments to manage our foreign currency and interest rate exposure. We use derivative financial instruments principally to reduce our exposure to market risks resulting from fluctuations in foreign exchange rates by creating offsetting exposures. We are not a party to leveraged derivatives and, by policy, do not use derivative financial instruments for speculative purposes.
See Item 8, Note 15. Financial Instruments, Item 8, Note 16. Fair Value Measurements and Item 8, Note 22. Balance Sheet Offsetting to our consolidated financial statements for further details on our derivative financial instruments and the related collateral arrangements.
Ÿ Value at Risk - We use a value at risk computation to estimate the potential one-day loss in the fair value of our interest-rate-sensitive financial instruments and to estimate the potential one-day loss in pre-tax earnings of our foreign currency price-sensitive derivative financial instruments. This computation includes our debt, short-term investments, and foreign currency forwards, swaps and options. Anticipated transactions, foreign currency trade payables and receivables, and net investments in foreign subsidiaries, which the foregoing instruments are intended to hedge, were excluded from the computation.
The computation estimates were made assuming normal market conditions, using a 95% confidence interval. We use a "variance/co-variance" model to determine the observed interrelationships between movements in interest rates and various currencies. These interrelationships were determined by observing interest rate and forward currency rate movements over the preceding quarter for determining value at risk at December 31, 2015 and 2014 , and over each of the four preceding quarters for the calculation of average value at risk amounts during each year. The values of foreign currency options do not change on a one-to-one basis with the underlying currency and were valued accordingly in the computation.
78
The estimated potential one-day loss in fair value of our interest-rate-sensitive instruments, primarily debt, under normal market conditions and the estimated potential one-day loss in pre-tax earnings from foreign currency instruments under normal market conditions, as calculated in the value at risk model, were as follows:
| Pre-Tax Earnings Impact | ||||||
(in millions) | At 12/31/15 |
| Average |
| High |
| Low |
Instruments sensitive to: |
|
|
|
|
|
|
|
Foreign currency rates | $65 |
| $74 |
| $96 |
| $62 |
|
| ||||||
| Fair Value Impact | ||||||
(in millions) | At 12/31/15 |
| Average |
| High |
| Low |
Instruments sensitive to: |
|
|
|
|
|
|
|
Interest rates | $102 |
| $120 |
| $147 |
| $102 |
|
|
|
|
|
|
|
|
| Pre-Tax Earnings Impact | ||||||
(in millions) | At 12/31/14 |
| Average |
| High |
| Low |
Instruments sensitive to: |
|
|
|
|
|
|
|
Foreign currency rates | $39 |
| $25 |
| $39 |
| $13 |
|
|
|
|
|
|
|
|
| Fair Value Impact | ||||||
(in millions) | At 12/31/14 |
| Average |
| High |
| Low |
Instruments sensitive to: |
|
|
|
|
|
|
|
Interest rates | $95 |
| $69 |
| $95 |
| $55 |
The value at risk computation is a risk analysis tool designed to statistically estimate the maximum probable daily loss from adverse movements in interest and foreign currency rates under normal market conditions. The computation does not purport to represent actual losses in fair value or earnings to be incurred by us, nor does it consider the effect of favorable changes in market rates. We cannot predict actual future movements in such market rates and do not present these results to be indicative of future movements in market rates or to be representative of any actual impact that future changes in market rates may have on our future results of operations or financial position.
Contingencies
See Item 3 and Item 8, Note 21. Contingencies to our consolidated financial statements for a discussion of contingencies.
Cautionary Factors That May Affect Future Results
Forward-Looking and Cautionary Statements
We may from time to time make written or oral forward-looking statements, including statements contained in filings with the SEC, in reports to stockholders and in press releases and investor webcasts. You can identify these forward-looking statements by use of words such as "strategy," "expects," "continues," "plans," "anticipates," "believes," "will," "estimates," "intends," "projects," "goals," "targets" and other words of similar meaning. You can also identify them by the fact that they do not relate strictly to historical or current facts.
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and assumptions. Achievement of future results is subject to risks, uncertainties and inaccurate assumptions. Should known or unknown risks or uncertainties materialize, or should underlying assumptions prove inaccurate, actual results could vary materially from those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements and whether to invest in or remain invested in our securities. In connection with the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results and outcomes to differ materially from
79
those contained in any forward-looking statements made by us; any such statement is qualified by reference to the following cautionary statements. We elaborate on these and other risks we face throughout this document, particularly in Item 1A. Risk Factors, and Business Environment of this section. You should understand that it is not possible to predict or identify all risk factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties. We do not undertake to update any forward-looking statement that we may make from time to time, except in the normal course of our public disclosure obligations.
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. |
The information called for by this Item is included in Item 7, Market Risk .
80
Item 8. | Financial Statements and Supplementary Data. |
Consolidated Balance Sheets
(in millions of dollars, except share data)
at December 31, | 2015 |
| 2014 | ||||
Assets |
|
|
| ||||
Cash and cash equivalents | $ | 3,417 | |
| $ | 1,682 | |
Receivables (less allowances of $58 in 2015 and $50 in 2014) | 2,778 | |
| 4,004 | | ||
Inventories: |
|
|
| ||||
Leaf tobacco | 2,640 | |
| 3,135 | | ||
Other raw materials | 1,613 | |
| 1,696 | | ||
Finished product | 4,220 | |
| 3,761 | | ||
| 8,473 | |
| 8,592 | | ||
Deferred income taxes | 488 | |
| 533 | | ||
Other current assets | 648 | |
| 673 | | ||
Total current assets | 15,804 | |
| 15,484 | | ||
Property, plant and equipment, at cost: |
|
|
| ||||
Land and land improvements | 583 | |
| 639 | | ||
Buildings and building equipment | 3,361 | |
| 3,620 | | ||
Machinery and equipment | 6,978 | |
| 7,664 | | ||
Construction in progress | 845 | |
| 836 | | ||
| 11,767 | |
| 12,759 | | ||
Less: accumulated depreciation | 6,046 | |
| 6,688 | | ||
| 5,721 | |
| 6,071 | | ||
Goodwill (Note 3) | 7,415 | |
| 8,388 | | ||
Other intangible assets, net (Note 3) | 2,623 | |
| 2,985 | | ||
Investments in unconsolidated subsidiaries (Note 4) | 890 | |
| 1,083 | | ||
Other assets | 1,503 | |
| 1,176 | | ||
Total Assets | $ | 33,956 | |
| $ | 35,187 | |
See notes to consolidated financial statements.
81
at December 31, | 2015 |
| 2014 | ||||
Liabilities |
|
|
| ||||
Short-term borrowings (Note 7) | $ | 825 | |
| $ | 1,208 | |
Current portion of long-term debt (Note 7) | 2,405 | |
| 1,318 | | ||
Accounts payable | 1,289 | |
| 1,242 | | ||
Accrued liabilities: |
|
|
| ||||
Marketing and selling | 640 | |
| 549 | | ||
Taxes, except income taxes | 5,121 | |
| 5,490 | | ||
Employment costs | 903 | |
| 1,135 | | ||
Dividends payable | 1,589 | |
| 1,559 | | ||
Other | 1,438 | |
| 1,375 | | ||
Income taxes | 970 | |
| 1,078 | | ||
Deferred income taxes | 206 | |
| 158 | | ||
Total current liabilities | 15,386 | |
| 15,112 | | ||
Long-term debt (Note 7) | 25,250 | |
| 26,929 | | ||
Deferred income taxes | 1,543 | |
| 1,549 | | ||
Employment costs | 2,566 | |
| 2,202 | | ||
Other liabilities | 687 | |
| 598 | | ||
Total liabilities | 45,432 | |
| 46,390 | | ||
Contingencies (Note 21) | |
| | ||||
Stockholders' (Deficit) Equity |
|
|
| ||||
Common stock, no par value (2,109,316,331 shares issued in 2015 and 2014) | - | |
| - | | ||
Additional paid-in capital | 1,929 | |
| 710 | | ||
Earnings reinvested in the business | 29,842 | |
| 29,249 | | ||
Accumulated other comprehensive losses | (9,402 | ) |
| (6,826 | ) | ||
| 22,369 | |
| 23,133 | | ||
Less: cost of repurchased stock (559,972,262 and 562,416,635 shares in 2015 and 2014, respectively) | 35,613 | |
| 35,762 | | ||
Total PMI stockholders' deficit | (13,244 | ) |
| (12,629 | ) | ||
Noncontrolling interests | 1,768 | |
| 1,426 | | ||
Total stockholders' deficit | (11,476 | ) |
| (11,203 | ) | ||
Total Liabilities and Stockholders' (Deficit) Equity | $ | 33,956 | |
| $ | 35,187 | |
See notes to consolidated financial statements.
82
Consolidated Statements of Earnings
(in millions of dollars, except per share data)
for the years ended December 31, | 2015 |
| 2014 |
| 2013 | ||||||
Net revenues | $ | 73,908 | |
| $ | 80,106 | |
| $ | 80,029 | |
Cost of sales | 9,365 | |
| 10,436 | |
| 10,410 | | |||
Excise taxes on products | 47,114 | |
| 50,339 | |
| 48,812 | | |||
Gross profit | 17,429 | |
| 19,331 | |
| 20,807 | | |||
Marketing, administration and research costs | 6,656 | |
| 7,001 | |
| 6,890 | | |||
Asset impairment and exit costs (Note 5) | 68 | |
| 535 | |
| 309 | | |||
Amortization of intangibles | 82 | |
| 93 | |
| 93 | | |||
Operating income | 10,623 | |
| 11,702 | |
| 13,515 | | |||
Interest expense, net (Note 14) | 1,008 | |
| 1,052 | |
| 973 | | |||
Earnings before income taxes | 9,615 | |
| 10,650 | |
| 12,542 | | |||
Provision for income taxes | 2,688 | |
| 3,097 | |
| 3,670 | | |||
Equity (income)/loss in unconsolidated subsidiaries, net | (105 | ) |
| (105 | ) |
| 22 | | |||
Net earnings | 7,032 | |
| 7,658 | |
| 8,850 | | |||
Net earnings attributable to noncontrolling interests | 159 | |
| 165 | |
| 274 | | |||
Net earnings attributable to PMI | $ | 6,873 | |
| $ | 7,493 | |
| $ | 8,576 | |
Per share data (Note 10): |
|
|
|
|
| ||||||
Basic earnings per share | $ | 4.42 | |
| $ | 4.76 | |
| $ | 5.26 | |
Diluted earnings per share | $ | 4.42 | |
| $ | 4.76 | |
| $ | 5.26 | |
See notes to consolidated financial statements.
83
Consolidated Statements of Comprehensive Earnings
(in millions of dollars)
for the years ended December 31, | 2015 |
| 2014 |
| 2013 | ||||||
Net earnings | $ | 7,032 | |
| $ | 7,658 | |
| $ | 8,850 | |
Other comprehensive earnings (losses), net of income taxes: |
|
|
|
|
| ||||||
Change in currency translation adjustments: |
|
|
|
|
| ||||||
Unrealized gains (losses), net of income taxes of ($143) in 2015, ($161) in 2014 and $227 in 2013 | (2,248 | ) |
| (1,746 | ) |
| (1,876 | ) | |||
(Gains)/losses transferred to earnings, net of income taxes of $- in 2015, $- in 2014 and $- in 2013 | (1 | ) |
| (5 | ) |
| (12 | ) | |||
Change in net loss and prior service cost: |
|
|
|
|
| ||||||
Net gains (losses) and prior service costs, net of income taxes of $17 in 2015, $167 in 2014 and ($81) in 2013 | (536 | ) |
| (1,148 | ) |
| 1,079 | | |||
Amortization of net losses, prior service costs and net transition costs, net of income taxes of ($48) in 2015, ($42) in 2014 and ($49) in 2013 | 227 | |
| 173 | |
| 243 | | |||
Change in fair value of derivatives accounted for as hedges: |
|
|
|
|
| ||||||
Gains (losses) recognized, net of income taxes of ($5) in 2015, ($13) in 2014 and ($30) in 2013 | 38 | |
| 98 | |
| 206 | | |||
(Gains) losses transferred to earnings, net of income taxes of $14 in 2015, $10 in 2014 and $34 in 2013 | (102 | ) |
| (38 | ) |
| (235 | ) | |||
Total other comprehensive losses | (2,622 | ) |
| (2,666 | ) |
| (595 | ) | |||
Total comprehensive earnings | 4,410 | |
| 4,992 | |
| 8,255 | | |||
Less comprehensive earnings attributable to: |
|
|
|
|
| ||||||
Noncontrolling interests | 113 | |
| 135 | |
| 197 | | |||
Redeemable noncontrolling interest (Note 24) | - | |
| - | |
| 68 | | |||
Comprehensive earnings attributable to PMI | $ | 4,297 | |
| $ | 4,857 | |
| $ | 7,990 | |
See notes to consolidated financial statements.
84
Consolidated Statements of Stockholders' (Deficit) Equity
(in millions of dollars, except per share data)
| PMI Stockholders' (Deficit) Equity |
|
|
|
|
|
| ||||||||||||||||||||||
| Common |
| Additional |
| Earnings Reinvested |
| Accumulated Other |
| Cost of |
| Noncontrolling |
|
| Total |
| ||||||||||||||
Balances, January 1, 2013 | $ | - | |
| $ | 1,334 | |
| $ | 25,076 | |
| $ | (3,604 | ) |
| $ | (26,282 | ) |
| $ | 322 | |
|
| $ | (3,154 | ) |
|
Net earnings |
|
|
|
| 8,576 | |
|
|
|
|
| 175 | | (1) |
| 8,751 | | (1) | |||||||||||
Other comprehensive earnings (losses), net of income taxes |
|
|
|
|
|
| (535 | ) |
|
|
| (29 | ) | (1) |
| (564 | ) | (1) | |||||||||||
Issuance of stock awards and exercise of stock options |
|
| 61 | |
|
|
|
|
| 140 | |
|
|
|
| 201 | |
| |||||||||||
Dividends declared ($3.58 per share) |
|
|
|
| (5,809 | ) |
|
|
|
|
|
|
|
| (5,809 | ) |
| ||||||||||||
Payments to noncontrolling interests |
|
|
|
|
|
|
|
|
|
| (210 | ) |
|
| (210 | ) |
| ||||||||||||
Purchase of subsidiary shares from noncontrolling interests |
|
| (672 | ) |
|
|
| (51 | ) |
|
|
| (41 | ) |
|
| (764 | ) |
| ||||||||||
Transfer of redeemable noncontrolling interest |
|
|
|
|
|
|
|
|
|
| 1,275 | | (1) |
| 1,275 | | (1) | ||||||||||||
Common stock repurchased |
|
|
|
|
|
|
|
| (6,000 | ) |
|
|
|
| (6,000 | ) |
| ||||||||||||
Balances, December 31, 2013 | - | |
| 723 | |
| 27,843 | |
| (4,190 | ) |
| (32,142 | ) |
| 1,492 | |
|
| (6,274 | ) |
| |||||||
Net earnings |
|
|
|
| 7,493 | |
|
|
|
|
| 165 | |
|
| 7,658 | |
| |||||||||||
Other comprehensive earnings (losses), net of income taxes |
|
|
|
|
|
| (2,636 | ) |
|
|
| (30 | ) | |
| (2,666 | ) |
| |||||||||||
Issuance of stock awards and exercise of stock options |
|
| (13 | ) |
|
|
|
|
| 180 | |
|
|
|
| 167 | |
| |||||||||||
Dividends declared ($3.88 per share) |
|
|
|
| (6,087 | ) |
|
|
|
|
|
|
|
| (6,087 | ) |
| ||||||||||||
Payments to noncontrolling interests |
|
|
|
|
|
|
|
|
|
| (207 | ) |
|
| (207 | ) |
| ||||||||||||
Common stock repurchased |
|
|
|
|
|
|
|
| (3,800 | ) |
|
|
|
| (3,800 | ) |
| ||||||||||||
Other |
|
|
|
|
|
|
|
|
|
| 6 | |
|
| 6 | |
| ||||||||||||
Balances, December 31, 2014 | - | |
| 710 | |
| 29,249 | |
| (6,826 | ) |
| (35,762 | ) |
| 1,426 | |
|
| (11,203 | ) |
| |||||||
Net earnings |
|
|
|
| 6,873 | |
|
|
|
|
| 159 | | |
| 7,032 | |
| |||||||||||
Other comprehensive earnings (losses), net of income taxes |
|
|
|
|
|
| (2,576 | ) |
|
|
| (46 | ) | |
| (2,622 | ) |
| |||||||||||
Issuance of stock awards |
|
| (3 | ) |
|
|
|
|
| 149 | |
|
|
|
| 146 | |
| |||||||||||
Dividends declared ($4.04 per share) |
|
|
|
| (6,280 | ) |
|
|
|
|
|
|
|
| (6,280 | ) |
| ||||||||||||
Payments to noncontrolling interests |
|
|
|
|
|
|
|
|
|
| (171 | ) |
|
| (171 | ) |
| ||||||||||||
Sale (purchase) of subsidiary shares to/(from) noncontrolling interests (Note 6) |
|
| 1,222 | |
|
|
|
|
|
|
| 400 | |
|
| 1,622 | |
| |||||||||||
Balances, December 31, 2015 | $ | - | |
| $ | 1,929 | |
| $ | 29,842 | |
| $ | (9,402 | ) |
| $ | (35,613 | ) |
| $ | 1,768 | |
|
| $ | (11,476 | ) |
|
(1) Net earnings attributable to noncontrolling interests exclude $99 million of earnings related to the redeemable noncontrolling interest, which was originally reported outside of the equity section and was included in the redeemable noncontrolling interest amount transferred to equity during 2013. Other comprehensive earnings (losses), net of income taxes, also exclude $33 million of net currency translation adjustment losses and a $2 million reduction of net loss and prior service costs related to the redeemable noncontrolling interest prior to the date of transfer. In December 2013, the redeemable noncontrolling interest balance of $1,275 million was reclassified to noncontrolling interests due to the termination of an exit rights agreement. For further details, see Note 24. Redeemable Noncontrolling Interest .
See notes to consolidated financial statements.
85
Consolidated Statements of Cash Flows
(in millions of dollars)
for the years ended December 31, | 2015 |
| 2014 |
| 2013 | ||||||
CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES |
|
|
|
|
| ||||||
Net earnings | $ | 7,032 | |
| $ | 7,658 | |
| $ | 8,850 | |
Adjustments to reconcile net earnings to operating cash flows: |
|
|
|
|
| ||||||
Depreciation and amortization | 754 | |
| 889 | |
| 882 | | |||
Deferred income tax benefit | (18 | ) |
| (62 | ) |
| (28 | ) | |||
Asset impairment and exit costs, net of cash paid | (164 | ) |
| 175 | |
| 288 | | |||
Cash effects of changes, net of the effects from acquired companies: |
|
|
|
|
| ||||||
Receivables, net | 647 | |
| (463 | ) |
| (449 | ) | |||
Inventories | (841 | ) |
| 105 | |
| (1,413 | ) | |||
Accounts payable | 310 | |
| 177 | |
| 103 | | |||
Income taxes | (42 | ) |
| (230 | ) |
| (331 | ) | |||
Accrued liabilities and other current assets | (8 | ) |
| (507 | ) |
| 1,880 | | |||
Pension plan contributions | (154 | ) |
| (191 | ) |
| (150 | ) | |||
Other | 349 | |
| 188 | |
| 503 | | |||
Net cash provided by operating activities | 7,865 | |
| 7,739 | |
| 10,135 | | |||
CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES |
|
|
|
|
| ||||||
Capital expenditures | (960 | ) |
| (1,153 | ) |
| (1,200 | ) | |||
Investments in unconsolidated subsidiaries | (55 | ) |
| (29 | ) |
| (1,418 | ) | |||
Purchase of businesses, net of acquired cash | - | |
| (110 | ) |
| - | | |||
Other | 307 | |
| 296 | |
| (62 | ) | |||
Net cash used in investing activities | (708 | ) |
| (996 | ) |
| (2,680 | ) | |||
See notes to consolidated financial statements.
86
for the years ended December 31, | 2015 |
| 2014 |
| 2013 | ||||||
CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES |
|
|
|
|
| ||||||
Short-term borrowing activity by original maturity: |
|
|
|
|
| ||||||
Net repayments - maturities of 90 days or less | $ | (266 | ) |
| $ | (516 | ) |
| $ | (1,099 | ) |
Issuances - maturities longer than 90 days | - | |
| 1,007 | |
| 2,000 | | |||
Repayments - maturities longer than 90 days | - | |
| (1,571 | ) |
| (849 | ) | |||
Long-term debt proceeds | 1,539 | |
| 5,591 | |
| 7,181 | | |||
Long-term debt repaid | (1,229 | ) |
| (1,240 | ) |
| (2,738 | ) | |||
Repurchases of common stock | (48 | ) |
| (3,833 | ) |
| (5,963 | ) | |||
Dividends paid | (6,250 | ) |
| (6,035 | ) |
| (5,720 | ) | |||
Sale (purchase) of subsidiary shares to/(from) noncontrolling interests (Note 6) | 1,622 | |
| - | |
| (703 | ) | |||
Other | (104 | ) |
| (242 | ) |
| (324 | ) | |||
Net cash used in financing activities | (4,736 | ) |
| (6,839 | ) |
| (8,215 | ) | |||
Effect of exchange rate changes on cash and cash equivalents | (686 | ) |
| (376 | ) |
| (69 | ) | |||
Cash and cash equivalents: |
|
|
|
|
| ||||||
Increase (Decrease) | 1,735 | |
| (472 | ) |
| (829 | ) | |||
Balance at beginning of year | 1,682 | |
| 2,154 | |
| 2,983 | | |||
Balance at end of year | $ | 3,417 | |
| $ | 1,682 | |
| $ | 2,154 | |
|
|
|
|
|
| ||||||
Cash Paid: |
|
|
|
|
| ||||||
Interest | $ | 1,045 | |
| $ | 1,068 | |
| $ | 978 | |
Income taxes | $ | 2,771 | |
| $ | 3,577 | |
| $ | 3,999 | |
See notes to consolidated financial statements.
87
Notes to Consolidated Financial Statements
Note 1. | ||||
Background and Basis of Presentation:
Background
Philip Morris International Inc. is a holding company incorporated in Virginia, U.S.A., whose subsidiaries and affiliates and their licensees are engaged in the manufacture and sale of cigarettes, other tobacco products and other nicotine-containing products in markets outside of the United States of America. Throughout these financial statements, the term "PMI" refers to Philip Morris International Inc. and its subsidiaries.
Basis of presentation
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the dates of the financial statements and the reported amounts of net revenues and expenses during the reporting periods. Significant estimates and assumptions include, among other things: pension and benefit plan assumptions; useful lives and valuation assumptions of goodwill and other intangible assets; marketing programs, and income taxes. Actual results could differ from those estimates.
The consolidated financial statements include PMI, as well as its wholly owned and majority-owned subsidiaries. Investments in which PMI exercises significant influence (generally 20%-50% ownership interest) are accounted for under the equity method of accounting. Investments in which PMI has an ownership interest of less than 20%, or does not exercise significant influence, are accounted for under the cost method of accounting. All intercompany transactions and balances have been eliminated.
Certain prior years' amounts have been reclassified to conform with the current year's presentation, as reflected in Note 3. Goodwill and Other Intangible Assets, net , Note 12. Segment Reporting and Note 13. Benefit Plans . The changes did not have an impact on PMI's consolidated financial position, results of operations or cash flows in any of the periods presented.
Note 2. | |
Summary of Significant Accounting Policies:
Cash and cash equivalents
Cash equivalents include demand deposits with banks and all highly liquid investments with original maturities of three months or less.
Depreciation
Property, plant and equipment are stated at historical cost and depreciated by the straight-line method over the estimated useful lives of the assets. Machinery and equipment are depreciated over periods ranging from 3 to 15 years, and buildings and building improvements over periods up to 40 years.
Employee benefit plans
PMI provides a range of benefits to its employees and retired employees, including pensions, postretirement health care and postemployment benefits (primarily severance). PMI records annual amounts relating to these plans based on calculations specified under U.S. GAAP. PMI recognizes the funded status of its defined pension and postretirement plans on the consolidated balance sheets. The funded status is measured as the difference between the fair value of the plans assets and the benefit obligation. PMI measures the plan assets and liabilities at the end of the fiscal year. For defined benefit pension plans, the benefit obligation is the projected benefit obligation. For the postretirement health care plans, the benefit obligation is the accumulated postretirement benefit obligation. Any
88
plan with an overfunded status is recognized as an asset, and any plan with an underfunded status is recognized as a liability. Any gains or losses and prior service costs or credits that have not been recognized as a component of net periodic benefit costs are recorded as a component of other comprehensive earnings (losses), net of deferred taxes. PMI elects to recognize actuarial gains/(losses) using the corridor approach.
Foreign currency translation
PMI translates the results of operations of its subsidiaries and affiliates using average exchange rates during each period, whereas balance sheet accounts are translated using exchange rates at the end of each period. Currency translation adjustments are recorded as a component of stockholders' (deficit) equity. In addition, some of PMI's subsidiaries have assets and liabilities denominated in currencies other than their functional currencies, and to the extent those are not designated as net investment hedges, these assets and liabilities generate transaction gains and losses when translated into their respective functional currencies.
Goodwill and non-amortizable intangible assets valuation
PMI tests goodwill and non-amortizable intangible assets for impairment annually or more frequently if events occur that would warrant such review. PMI performs its annual impairment analysis in the first quarter of each year. The impairment analysis involves comparing the fair value of each reporting unit or non-amortizable intangible asset to the carrying value. If the carrying value exceeds the fair value, goodwill or a non-amortizable intangible asset is considered impaired.
Hedging instruments
Derivative financial instruments are recorded at fair value on the consolidated balance sheets as either assets or liabilities. Changes in the fair value of derivatives are recorded each period either in accumulated other comprehensive losses on the consolidated balance sheet, or in earnings, depending on whether a derivative is designated and effective as part of a hedge transaction and, if it is, the type of hedge transaction. Gains and losses on derivative instruments reported in accumulated other comprehensive losses are reclassified to the consolidated statements of earnings in the periods in which operating results are affected by the hedged item. Cash flows from hedging instruments are classified in the same manner as the affected hedged item in the consolidated statements of cash flows.
Impairment of long-lived assets
PMI reviews long-lived assets, including amortizable intangible assets, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. PMI performs undiscounted operating cash flow analyses to determine if an impairment exists. For purposes of recognition and measurement of an impairment for assets held for use, PMI groups assets and liabilities at the lowest level for which cash flows are separately identifiable. If an impairment is determined to exist, any related impairment loss is calculated based on fair value. Impairment losses on assets to be disposed of, if any, are based on the estimated proceeds to be received, less costs of disposal.
Impairment of investments in unconsolidated subsidiaries
Investments in unconsolidated subsidiaries are evaluated for impairment whenever events or changes in circumstances indicate that the carrying amount of the investments may not be recoverable. An impairment loss would be recorded whenever a decline in value of an equity investment below its carrying amount is determined to be other than temporary. PMI determines whether a loss is other than temporary by considering the length of time and extent to which the fair value of the equity investment has been less than the carrying amount, the financial condition of the equity investment, and the intent to retain the investment for a period of time is sufficient to allow for any anticipated recovery in market value.
Income taxes
Income tax provisions for jurisdictions outside the United States, as well as state and local income tax provisions, are determined on a separate company basis, and the related assets and liabilities are recorded in PMI's consolidated balance sheets. Significant judgment is required in determining income tax provisions and in evaluating tax positions. PMI recognizes accrued interest and penalties associated with uncertain tax positions as part of the provision for income taxes on the consolidated statements of earnings.
Inventories
Inventories are stated at the lower of cost or market. The first-in, first-out and average cost methods are used to cost substantially all inventories. It is a generally recognized industry practice to classify leaf tobacco inventory as a current asset, although part of such inventory, because of the duration of the aging process, ordinarily would not be utilized within one year.
89
Marketing costs
PMI supports its products with advertising, consumer engagement and trade promotions. Such programs include, but are not limited to, discounts, rebates, in-store display incentives, digital platforms and volume-based incentives. Advertising, as well as certain consumer engagement and trade activities costs, are expensed as incurred. Trade promotions are recorded as a reduction of revenues based on amounts estimated as being due to customers at the end of a period, based principally on historical utilization. For interim reporting purposes, advertising and certain consumer engagement expenses are charged to earnings based on estimated sales and related expenses for the full year.
Revenue recognition
PMI recognizes revenues, net of sales incentives and including shipping and handling charges billed to customers, either upon shipment or delivery of goods when title and risk of loss pass to customers. Excise taxes billed by PMI to customers are reported in net revenues. Shipping and handling costs are classified as part of cost of sales.
On May 28, 2014, the Financial Accounting Standards Board issued Accounting Standards Update ASU 2014-09, "Revenue from Contracts with Customers." For further details, see Note 25. New Accounting Standards .
Stock-based compensation
PMI measures compensation cost for all stock-based awards at fair value on date of grant and recognizes the compensation costs over the service periods for awards expected to vest. The fair value of restricted stock and deferred stock is determined based on the number of shares granted and the market value at date of grant.
Note 3. |
| |
Goodwill and Other Intangible Assets, net:
Goodwill and other intangible assets, net, by segment were as follows:
| Goodwill |
| Other Intangible Assets, net | ||||||||||||
(in millions) | December 31, 2015 |
| December 31, 2014 |
| December 31, 2015 |
| December 31, 2014 | ||||||||
European Union | $ | 1,310 | |
| $ | 1,439 | |
| $ | 516 | |
| $ | 582 | |
Eastern Europe, Middle East & Africa | 374 | |
| 476 | |
| 201 | |
| 215 | | ||||
Asia | 3,581 | |
| 3,904 | |
| 1,087 | |
| 1,207 | | ||||
Latin America & Canada | 2,150 | |
| 2,569 | |
| 819 | |
| 981 | | ||||
Total | $ | 7,415 | |
| $ | 8,388 | |
| $ | 2,623 | |
| $ | 2,985 | |
90
Goodwill primarily reflects PMI's acquisitions in Canada, Colombia, Greece, Indonesia, Mexico, Pakistan and Serbia, as well as the business combination in the Philippines.
In the fourth quarter of 2015, to further align with the Member State composition of the European Union, PMI transferred the management of its operations in Bulgaria, Croatia, Romania and Slovenia from its Eastern Europe, Middle East & Africa segment to its European Union segment, resulting in the reclassification of prior year amounts between the two segments.
The movements in goodwill were as follows:
(in millions) |
| European |
| Eastern Europe, & |
| Asia |
| Latin |
| Total | ||||||||||
Balance at January 1, 2014 |
| $ | 1,522 | |
| $ | 567 | |
| $ | 3,960 | |
| $ | 2,844 | |
| $ | 8,893 | |
Changes due to: |
|
|
|
|
|
|
|
|
|
| ||||||||||
Acquisitions |
| 118 | |
| - | |
| - | |
| 2 | |
| 120 | | |||||
Currency |
| (201 | ) |
| (91 | ) |
| (56 | ) |
| (277 | ) |
| (625 | ) | |||||
Balance at December 31, 2014 |
| 1,439 | |
| 476 | |
| 3,904 | |
| 2,569 | |
| 8,388 | | |||||
Changes due to: |
|
|
|
|
|
|
|
|
|
| ||||||||||
Currency |
| (129 | ) |
| (102 | ) |
| (323 | ) |
| (419 | ) |
| (973 | ) | |||||
Balance at December 31, 2015 |
| $ | 1,310 | |
| $ | 374 | |
| $ | 3,581 | |
| $ | 2,150 | |
| $ | 7,415 | |
The increase in goodwill from acquisitions in 2014 was due primarily to the purchase price allocation for PMI's June 2014 purchase of Nicocigs Limited, a U.K.-based e-vapor company. For further details, see Note 6. Acquisitions and Other Business Arrangements.
Additional details of other intangible assets were as follows:
| December 31, 2015 |
| December 31, 2014 | ||||||||||||
(in millions) | Gross | |
| Accumulated | |
| Gross | |
| Accumulated | | ||||
Non-amortizable intangible assets | $ | 1,527 | |
|
|
| $ | 1,704 | |
|
| ||||
Amortizable intangible assets | 1,609 | |
| $ | 513 | |
| 1,877 | |
| $ | 596 | | ||
Total other intangible assets | $ | 3,136 | |
| $ | 513 | |
| $ | 3,581 | |
| $ | 596 | |
Non-amortizable intangible assets substantially consist of trademarks from PMI's acquisitions in Indonesia in 2005 and Mexico in 2007. Amortizable intangible assets primarily consist of certain trademarks and distribution networks associated with business combinations. The gross carrying amount, the range of useful lives as well as the weighted-average remaining useful life of amortizable intangible assets at December 31, 2015 , were as follows:
Description (dollars in millions) | Gross | Initial Estimated |
| Weighted-Average | ||
Trademarks | $ | 1,374 | | 2 - 40 years |
| 21 years |
Distribution networks | 149 | | 5 - 30 years |
| 11 years | |
Other (including farmer contracts and intellectual property rights) | 86 | | 4 - 17 years |
| 11 years | |
| $ | 1,609 | |
|
|
|
91
Pre-tax amortization expense for intangible assets during the years ended December 31, 2015 , 2014 and 2013 , was $82 million , $93 million and $93 million , respectively. Amortization expense for each of the next five years is estimated to be $73 million or less, assuming no additional transactions occur that require the amortization of intangible assets.
The decrease in the gross carrying amount of other intangible assets from December 31, 2014 , was due to currency movements and the retirement of fully amortized intangible assets.
Note 4. | ||||
Investments in Unconsolidated Subsidiaries:
At December 31, 2015 and 2014 , PMI had total investments in unconsolidated subsidiaries of $890 million and $1,083 million , respectively, which were accounted for under the equity method of accounting. Equity method investments are initially recorded at cost. Under the equity method of accounting, the investment is adjusted for PMI's proportionate share of earnings or losses and movements in currency translation adjustments. The carrying value of our equity method investments at the acquisition date exceeded our share of the unconsolidated subsidiaries' book value by $1,417 million , including $1,264 million attributable to goodwill. The difference between the investment carrying value and the amount of underlying equity in net assets, excluding the $1,264 million attributable to goodwill, is being amortized on a straight-line basis over the underlying assets' estimated useful lives of 3 to 20 years . At December 31, 2015 and 2014 , PMI received year-to-date dividends from unconsolidated subsidiaries of $127 million and $107 million , respectively.
On September 30, 2013, PMI acquired a 49% equity interest in United Arab Emirates-based Emirati Investors-TA (FZC) ("EITA"), formerly Arab Investors-TA (FZC), for approximately $625 million . As a result of this transaction, PMI holds an approximate 25% economic interest in Société des Tabacs Algéro-Emiratie ("STAEM"), an Algerian joint venture that is 51% owned by EITA and 49% by the Algerian state-owned enterprise Société Nationale des Tabacs et Allumettes SpA. STAEM manufactures and distributes under license some of PMI's brands. The initial investment in EITA was recorded at cost and is included in investments in unconsolidated subsidiaries on the consolidated balance sheets.
On December 12, 2013, PMI acquired from Megapolis Investment BV a 20% equity interest in Megapolis Distribution BV, the holding company of CJSC TK Megapolis ("Megapolis"), PMI's distributor in Russia, for a purchase price of $760 million . An additional payment of up to $100 million , which is contingent on Megapolis's operational performance over the four fiscal years following the closing of the transaction, will also be made by PMI if the performance criteria are satisfied. PMI has also agreed to provide Megapolis Investment BV with a $100 million interest-bearing loan. PMI and Megapolis Investment BV have agreed to set off any future contingent payments owed by PMI against the future repayments due under the loan agreement. Any loan repayments in excess of the contingent consideration earned by the performance of Megapolis are due to be repaid, in cash, to PMI on March 31, 2017. At December 31, 2013, PMI had recorded a $100 million asset related to the loan receivable and a discounted liability of $86 million related to the contingent consideration. The initial investment in Megapolis was recorded at cost and is included in investments in unconsolidated subsidiaries on the consolidated balance sheets.
At December 31, 2015 and 2014, PMI's investments in other unconsolidated subsidiaries were $69 million and $38 million , respectively, with ownership percentages ranging from 14% to 50% .
PMI's earnings activity from unconsolidated subsidiaries was as follows:
|
| For the Years Ended December 31, | |||||
(in millions) |
| 2015 | 2014 | ||||
|
|
|
| ||||
Net revenues |
| $ | 4,172 | | $ | 5,508 | |
92
PMI's balance sheet activity related to unconsolidated subsidiaries was as follows:
|
|
| |||||
|
| At December 31, | |||||
(in millions) |
| 2015 | 2014 | ||||
|
|
|
| ||||
Receivables |
| $ | 64 | | $ | 407 | |
Notes receivable |
| $ | 100 | | $ | 100 | |
Other liabilities |
| $ | 100 | | $ | 93 | |
The activity primarily related to agreements with PMI's unconsolidated subsidiaries within the Eastern Europe, Middle East & Africa segment. These agreements, which are in the ordinary course of business, are primarily for distribution, contract manufacturing and licenses. PMI eliminated its respective share of all significant intercompany transactions with the equity method investees.
Note 5. | ||||
Asset Impairment and Exit Costs:
During 2015 , 2014 and 2013 , pre-tax asset impairment and exit costs consisted of the following:
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Separation programs: |
|
|
|
|
| ||||||
European Union | $ | 68 | |
| $ | 351 | |
| $ | 13 | |
Eastern Europe, Middle East & Africa | - | |
| 2 | |
| 14 | | |||
Asia | - | |
| 35 | |
| 19 | | |||
Latin America & Canada | - | |
| 3 | |
| 5 | | |||
Total separation programs | 68 | |
| 391 | |
| 51 | | |||
Contract termination charges: |
|
|
|
|
| ||||||
Eastern Europe, Middle East & Africa | - | |
| - | |
| 250 | | |||
Asia | - | |
| - | |
| 8 | | |||
Total contract termination charges | - | |
| - | |
| 258 | | |||
Asset impairment charges: |
|
|
|
|
| ||||||
European Union | - | |
| 139 | |
| - | | |||
Latin America & Canada | - | |
| 5 | |
| - | | |||
Total asset impairment charges | - | |
| 144 | |
| - | | |||
Asset impairment and exit costs | $ | 68 | |
| $ | 535 | |
| $ | 309 | |
93
Movement in Exit Cost Liabilities
The movement in exit cost liabilities for PMI was as follows:
(in millions) |
| ||
Liability balance, January 1, 2014 | $ | 308 | |
Charges, net | 391 | | |
Cash spent | (360 | ) | |
Currency/other | (69 | ) | |
Liability balance, December 31, 2014 | $ | 270 | |
Charges, net | 68 | | |
Cash spent | (232 | ) | |
Currency/other | (52 | ) | |
Liability balance, December 31, 2015 | $ | 54 | |
Cash payments related to exit costs at PMI were $232 million , $360 million and $21 million for the years ended December 31, 2015 , 2014 and 2013 , respectively. Future cash payments for exit costs incurred to date are expected to be approximately $54 million , and will be substantially paid by the end of 2017.
The pre-tax asset impairment and exit costs shown above are primarily a result of the following:
The Netherlands
On April 4, 2014, PMI announced the initiation by its affiliate, Philip Morris Holland B.V. ("PMH"), of consultations with employee representatives on a proposal to discontinue cigarette production at its factory located in Bergen op Zoom, the Netherlands. PMH reached an agreement with the trade unions and their members on a social plan and ceased cigarette production on September 1, 2014. During 2014 , total pre-tax asset impairment and exit costs of $489 million were recorded for this program in the European Union segment. This amount includes employee separation costs of $343 million , asset impairment costs of $139 million and other separation costs of $7 million .
Other
Separation Program Charges
PMI recorded other pre-tax separation program charges of $68 million , $41 million and $51 million for the years ended December 31, 2015, 2014 and 2013, respectively. The 2015 other pre-tax separation program charges primarily related to severance costs for the organizational restructuring in the European Union segment. The 2014 other pre-tax separation program charges primarily related to severance costs for factory closures in Australia and Canada and the restructuring of the U.S. leaf purchasing model. The 2013 pre-tax separation program charges primarily related to the restructuring of global and regional functions based in Switzerland and Australia.
Contract Termination Charges
During 2013, PMI recorded exit costs of $258 million related to the termination of distribution agreements in Eastern Europe, Middle East & Africa (due to a new business model in Egypt) and Asia.
Asset Impairment Charges
During 2014, PMI recorded other pre-tax asset impairment charges of $5 million related to a factory closure in Canada.
94
Note 6. | ||||
Acquisitions and Other Business Arrangements:
As announced in June 2015, PMI's subsidiary PT HM Sampoerna Tbk. ("Sampoerna"), of which PMI held a 98.18% interest, was required to comply with the January 30, 2014, Indonesian Stock Exchange ("IDX") regulation requiring all listed public companies to have at least a 7.5% public shareholding by January 30, 2016. In order to comply with this requirement, Sampoerna conducted a rights issue (the "Rights Issue"). The exercise price for the rights was set at Rp. 77,000 per share, a 1.349% premium to the closing price on the IDX as of September 30, 2015. In connection with the Rights Issue, PT Philip Morris Indonesia ("PMID"), a fully consolidated subsidiary of PMI, sold 264,209,711 of the rights to third-party investors. Delivery of the rights sold took place on October 26, 2015. The total net proceeds from the Rights Issue were approximately $1.5 billion at prevailing exchange rates on the closing date. The sale of the rights resulted in an increase to PMI's additional paid-in capital of $1.1 billion .
In June 2014, PMI acquired 100% of Nicocigs Limited, a leading U.K.-based e-vapor company, for the final purchase price of $103 million , net of cash acquired, with additional contingent payments of up to $77 million , primarily relating to performance targets over a three -year period. As of December 31, 2015, PMI does not anticipate that the performance targets will be met. For additional information regarding this contingent consideration, see Note 16. Fair Value Measurements .
In September 2013 , Grupo Carso, S.A.B. de C.V. ("Grupo Carso") sold to PMI its remaining 20% interest in PMI's Mexican tobacco business for $703 million . As a result, PMI now owns 100% of its Mexican tobacco business. A former director of PMI, whose term expired at the Annual Meeting of Shareholders in May 2015, had an affiliation with Grupo Carso. The final purchase price was subject to an adjustment based on the actual performance of the Mexican tobacco business over the three -year period ending two fiscal years after the closing of the purchase. In May 2015, PMI received a payment of $113 million from Grupo Carso as the final purchase price adjustment. This resulted in a total net purchase price of $590 million . In addition, PMI agreed to pay a dividend of approximately $38 million to Grupo Carso related to the earnings of the Mexican tobacco business for the nine months ended September 30, 2013. In March 2014, the dividend was declared and paid. The purchase of the remaining 20% interest resulted in a net decrease to PMI's additional paid-in capital of $559 million .
The effects of these and other smaller acquisitions were not material to PMI's consolidated financial position, results of operations or operating cash flows in any of the periods presented.
95
Note 7. | ||||
Indebtedness:
Short-Term Borrowings
At December 31, 2015 and 2014 , PMI's short-term borrowings and related average interest rates consisted of the following:
| December 31, 2015 |
| December 31, 2014 | ||||||||||
(in millions) | Amount Outstanding | |
| Average Year-End Rate | |
| Amount Outstanding | |
| Average Year-End Rate | | ||
Commercial paper | $ | - | |
| - | % |
| $ | - | |
| - | % |
Bank loans | 825 | |
| 6.1 | |
| 1,208 | |
| 4.9 | | ||
| $ | 825 | |
|
|
| $ | 1,208 | |
|
| ||
Given the mix of subsidiaries and their respective local economic environments, the average interest rate for bank loans above can vary significantly from day to day and country to country.
The fair values of PMI's short-term borrowings at December 31, 2015 and 2014 , based upon current market interest rates, approximate the amounts disclosed above.
Long-Term Debt
At December 31, 2015 and 2014 , PMI's long-term debt consisted of the following:
| December 31, | ||||||
(in millions) | 2015 |
| 2014 | ||||
U.S. dollar notes, 1.125% to 6.375% (average interest rate 3.780%), due through 2044 | $ | 18,091 | |
| $ | 17,229 | |
Foreign currency obligations: |
|
|
| ||||
Euro notes, 1.750% to 5.750% (average interest rate 2.799%), due through 2033 | 7,423 | |
| 9,161 | | ||
Swiss franc notes, 0.750% to 2.000% (average interest rate 1.217%), due through 2024 | 1,690 | |
| 1,690 | | ||
Other (average interest rate 3.124%), due through 2024 | 451 | |
| 167 | | ||
| 27,655 | |
| 28,247 | | ||
Less current portion of long-term debt | 2,405 | |
| 1,318 | | ||
| $ | 25,250 | |
| $ | 26,929 | |
Other debt:
Other foreign currency debt above includes mortgage debt in Switzerland and capital lease obligations at December 31, 2015 and 2014 . Other foreign currency debt above also includes a bank loan in the Philippines at December 31, 2015 .
96
Debt Issuances Outstanding:
PMI's debt issuances outstanding at December 31, 2015 , were as follows:
(in millions) |
|
|
|
|
|
|
|
|
Type |
| Face Value |
| Interest |
| Issuance |
| Maturity |
U.S. dollar notes |
| $650 |
| 2.500% |
| May 2011 |
| May 2016 |
U.S. dollar notes |
| $600 |
| 2.500% |
| August 2011 (a) |
| May 2016 |
U.S. dollar notes |
| $550 |
| 1.625% |
| March 2012 |
| March 2017 |
U.S. dollar notes |
| $750 |
| 1.125% |
| August 2012 |
| August 2017 |
U.S. dollar notes |
| $500 |
| 1.250% |
| August 2015 |
| August 2017 |
U.S. dollar notes |
| $500 |
| 1.250% |
| November 2014 |
| November 2017 |
U.S. dollar notes |
| $2,500 |
| 5.650% |
| May 2008 |
| May 2018 |
U.S. dollar notes |
| $750 |
| 1.875% |
| November 2013 |
| January 2019 |
U.S. dollar notes |
| $1,000 |
| 4.500% |
| March 2010 |
| March 2020 |
U.S. dollar notes |
| $350 |
| 4.125% |
| May 2011 |
| May 2021 |
U.S. dollar notes |
| $750 |
| 2.900% |
| November 2011 |
| November 2021 |
U.S. dollar notes |
| $750 |
| 2.500% |
| August 2012 |
| August 2022 |
U.S. dollar notes |
| $600 |
| 2.625% |
| March 2013 |
| March 2023 |
U.S. dollar notes |
| $500 |
| 3.600% |
| November 2013 |
| November 2023 |
U.S. dollar notes |
| $750 |
| 3.250% |
| November 2014 |
| November 2024 |
U.S. dollar notes |
| $750 |
| 3.375% |
| August 2015 |
| August 2025 |
U.S. dollar notes |
| $1,500 |
| 6.375% |
| May 2008 |
| May 2038 |
U.S. dollar notes |
| $750 |
| 4.375% |
| November 2011 |
| November 2041 |
U.S. dollar notes |
| $700 |
| 4.500% |
| March 2012 |
| March 2042 |
U.S. dollar notes |
| $750 |
| 3.875% |
| August 2012 |
| August 2042 |
U.S. dollar notes |
| $850 |
| 4.125% |
| March 2013 |
| March 2043 |
U.S. dollar notes |
| $750 |
| 4.875% |
| November 2013 |
| November 2043 |
U.S. dollar notes |
| $750 |
| 4.250% |
| November 2014 |
| November 2044 |
EURO notes | (b) | €750 (approximately $976) |
| 5.750% |
| March 2009 |
| March 2016 |
EURO notes | (b) | €750 (approximately $951) |
| 2.125% |
| May 2012 |
| May 2019 |
EURO notes | (b) | €1,250 (approximately $1,621) |
| 1.750% |
| March 2013 |
| March 2020 |
EURO notes | (b) | €750 (approximately $1,029) |
| 1.875% |
| March 2014 |
| March 2021 |
EURO notes | (b) | €600 (approximately $761) |
| 2.875% |
| May 2012 |
| May 2024 |
EURO notes | (b) | €750 (approximately $972) |
| 2.750% |
| March 2013 |
| March 2025 |
EURO notes | (b) | €1,000 (approximately $1,372) |
| 2.875% |
| March 2014 |
| March 2026 |
EURO notes | (b) | €500 (approximately $697) |
| 2.875% |
| May 2014 |
| May 2029 |
EURO notes | (b) | €500 (approximately $648) |
| 3.125% |
| June 2013 |
| June 2033 |
Swiss franc notes | (b) | CHF325 (approximately $362) |
| 1.000% |
| December 2011 |
| December 2016 |
Swiss franc notes | (b) | CHF200 (approximately $217) |
| 0.875% |
| March 2013 |
| March 2019 |
Swiss franc notes | (b) | CHF275 (approximately $311) |
| 0.750% |
| May 2014 |
| December 2019 |
Swiss franc notes | (b) | CHF325 (approximately $334) |
| 1.000% |
| September 2012 |
| September 2020 |
Swiss franc notes | (b) | CHF300 (approximately $335) |
| 2.000% |
| December 2011 |
| December 2021 |
Swiss franc notes | (b) | CHF250 (approximately $283) |
| 1.625% |
| May 2014 |
| May 2024 |
|
|
|
|
|
|
|
|
|
(a) These notes are a further issuance of the 2.500% notes issued by PMI in May 2011.
(b) USD equivalents for foreign currency notes were calculated based on exchange rates on the date of issuance.
The net proceeds from the sale of the securities listed in the table above were used to meet PMI's working capital requirements, to repurchase PMI's common stock, to refinance debt and for general corporate purposes.
97
Aggregate maturities:
Aggregate maturities of long-term debt are as follows:
(in millions) |
| ||
2016 | $ | 2,405 | |
2017 | 2,592 | | |
2018 | 2,505 | | |
2019 | 2,050 | | |
2020 | 2,695 | | |
2021-2025 | 7,441 | | |
2026-2030 | 1,638 | | |
Thereafter | 6,596 | | |
| 27,922 | | |
Debt discounts | (267 | ) | |
Total long-term debt | $ | 27,655 | |
See Note 16. Fair Value Measurements for additional disclosures related to the fair value of PMI's debt.
Credit Facilities
On January 23, 2015, PMI entered into an agreement to extend the term of its existing $2.0 billion 364-day revolving credit facility, effective February 10, 2015, from February 10, 2015, to February 9, 2016. On January 23, 2015, PMI also entered into an agreement to extend the term of its existing $2.5 billion multi-year revolving credit facility, effective February 28, 2015, from February 28, 2019, to February 28, 2020.
On October 1, 2015, PMI replaced its $3.5 billion multi-year revolving credit facility, expiring October 25, 2016, with a new $3.5 billion multi-year revolving credit facility, expiring October 1, 2020.
At December 31, 2015 , PMI's total committed credit facilities and commercial paper outstanding were as follows:
Type (in billions of dollars) | Committed |
| Commercial | ||||
364-day revolving credit, expiring February 9, 2016 | $ | 2.0 | |
|
| ||
Multi-year revolving credit, expiring February 28, 2020 | 2.5 | |
|
| |||
Multi-year revolving credit, expiring October 1, 2020 | 3.5 | |
|
| |||
Total facilities | $ | 8.0 | |
|
| ||
Commercial paper outstanding |
|
| $ | - | | ||
At December 31, 2015 , there were no borrowings under these committed credit facilities, and the entire committed amounts were available for borrowing.
On January 27, 2016, PMI entered into an agreement to amend and extend its existing $2.0 billion 364-day revolving credit facility, effective February 9, 2016, from February 9, 2016, to February 7, 2017. On January 27, 2016, PMI also entered into an agreement to extend the term of its existing $2.5 billion multi-year revolving credit facility, effective February 28, 2016, from February 28, 2020, to February 28, 2021.
98
Each of these facilities requires PMI to maintain a ratio of consolidated earnings before interest, taxes, depreciation and amortization ("consolidated EBITDA") to consolidated interest expense of not less than 3.5 to 1.0 on a rolling four-quarter basis. At December 31, 2015 , PMI's ratio calculated in accordance with the agreements was 10.5 to 1.0. These facilities do not include any credit rating triggers, material adverse change clauses or any provisions that could require PMI to post collateral. The terms "consolidated EBITDA" and "consolidated interest expense," both of which include certain adjustments, are defined in the facility agreements previously filed with the Securities and Exchange Commission.
In addition to the committed credit facilities discussed above, certain subsidiaries maintain short-term credit arrangements to meet their respective working capital needs. These credit arrangements, which amounted to approximately $2.9 billion at December 31, 2015 , and $3.2 billion at December 31, 2014 , are for the sole use of the subsidiaries. Borrowings under these arrangements amounted to $825 million at December 31, 2015 , and $1.2 billion at December 31, 2014 .
Note 8. | ||||
Capital Stock:
Shares of authorized common stock are 6.0 billion ; issued, repurchased and outstanding shares were as follows:
| Shares Issued |
| Shares |
| Shares | |||
Balances, January 1, 2013 | 2,109,316,331 | |
| (455,703,347 | ) |
| 1,653,612,984 | |
Repurchase of shares |
|
| (67,231,392 | ) |
| (67,231,392 | ) | |
Issuance of stock awards and exercise of stock options |
|
| 2,620,820 | |
| 2,620,820 | | |
Balances, December 31, 2013 | 2,109,316,331 | |
| (520,313,919 | ) |
| 1,589,002,412 | |
Repurchase of shares |
|
| (45,206,473 | ) |
| (45,206,473 | ) | |
Issuance of stock awards and exercise of stock options |
|
| 3,103,757 | |
| 3,103,757 | | |
Balances, December 31, 2014 | 2,109,316,331 | |
| (562,416,635 | ) |
| 1,546,899,696 | |
Issuance of stock awards |
|
| 2,444,373 | |
| 2,444,373 | | |
Balances, December 31, 2015 | 2,109,316,331 | |
| (559,972,262 | ) |
| 1,549,344,069 | |
On August 1, 2012 , PMI commenced a three -year $18 billion share repurchase program that was authorized by PMI's Board of Directors in June 2012. From August 1, 2012 , through December 31, 2014, PMI repurchased 144.6 million shares of its common stock at a cost of $12.7 billion , or $87.48 per share, under this repurchase program. During 2015 , PMI did not repurchase any shares of its common stock. During 2014 and 2013 , PMI repurchased $3.8 billion and $6.0 billion , respectively, of its common stock.
At December 31, 2015 , 29,642,862 shares of common stock were reserved for stock awards under PMI's stock plans, and 250 million shares of preferred stock, without par value, were authorized but unissued. PMI currently has no plans to issue any shares of preferred stock.
Note 9. | ||||
Stock Plans:
In May 2012, PMI's shareholders approved the Philip Morris International Inc. 2012 Performance Incentive Plan (the "2012 Plan"). Under the 2012 Plan, PMI may grant to eligible employees restricted stock, restricted stock units and deferred stock units, performance-based cash incentive awards and performance-based equity awards. Up to 30 million shares of PMI's common stock may be issued under the 2012 Plan. At December 31, 2015 , shares available for grant under the 2012 Plan were 23,249,430 .
99
In 2008, PMI adopted the Philip Morris International Inc. 2008 Stock Compensation Plan for Non-Employee Directors (the "Non-Employee Directors Plan"). A non-employee director is defined as a member of the PMI Board of Directors who is not a full-time employee of PMI or of any corporation in which PMI owns, directly or indirectly, stock possessing at least 50% of the total combined voting power of all classes of stock entitled to vote in the election of directors in such corporation. Up to 1 million shares of PMI common stock may be awarded under the Non-Employee Directors Plan. As of December 31, 2015 , shares available for grant under the plan were 691,432 .
Restricted and Deferred Stock Awards
PMI may grant restricted stock and deferred stock awards to eligible employees; recipients may not sell, assign, pledge or otherwise encumber such shares or awards. Such shares or awards are subject to forfeiture if certain employment conditions are not met. Restricted stock and deferred stock awards generally vest on the third anniversary of the grant date. Shares of restricted stock carry voting and dividend rights. Deferred stock awards carry no such rights, although they do earn dividend equivalents.
During 2015 , the activity for restricted stock and deferred stock awards was as follows:
| Number of |
| Weighted- | |||
Balance at January 1, 2015 | 7,039,377 | |
| $ | 81.94 | |
Granted | 1,535,830 | |
| 82.28 | | |
Vested | (2,711,974 | ) |
| 80.05 | | |
Forfeited | (161,233 | ) |
| 82.14 | | |
Balance at December 31, 2015 | 5,702,000 | |
| $ | 82.92 | |
The weighted-average grant date fair value of the restricted stock and deferred stock awards granted to PMI employees during the years ended December 31, 2015 , 2014 and 2013 , was $126 million , $189 million and $246 million , or $82.28 , $77.79 and $88.43 per restricted or deferred share, respectively. The fair value of the restricted stock and deferred stock awards at the date of grant is amortized to expense ratably over the restriction period. PMI recorded compensation expense related to stock awards of $166 million , $210 million and $220 million for the years ended December 31, 2015 , 2014 and 2013 , respectively. As of December 31, 2015 , PMI had $134 million of total unrecognized compensation costs related to non-vested deferred stock awards. These costs are expected to be recognized over a weighted-average period of two years , subject to earlier vesting on death or disability or normal retirement, or separation from employment by mutual agreement after reaching age 58 .
During the year ended December 31, 2015 , 2.7 million shares of PMI deferred stock awards vested. The grant date fair value of all the vested shares was approximately $217 million . The total fair value of the awards that vested in 2015 was approximately $224 million .
During the year ended December 31, 2014 , 4.0 million shares of PMI restricted and deferred stock awards vested. The grant date fair value of all the vested shares was approximately $255 million . The total fair value of the awards that vested in 2014 was approximately $320 million .
During the year ended December 31, 2013 , 3.3 million shares of PMI restricted and deferred stock awards vested. The grant date fair value of all the vested shares was approximately $164 million . The total fair value of the awards that vested in 2013 was approximately $296 million .
Note 10. | ||||
Earnings per Share:
Unvested share-based payment awards that contain non-forfeitable rights to dividends or dividend equivalents are participating securities and therefore are included in PMI's earnings per share calculation pursuant to the two-class method.
100
Basic and diluted earnings per share ("EPS") were calculated using the following:
| For the Years Ended December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Net earnings attributable to PMI | $ | 6,873 | |
| $ | 7,493 | |
| $ | 8,576 | |
Less distributed and undistributed earnings attributable to share-based payment awards | 24 | |
| 34 | |
| 45 | | |||
Net earnings for basic and diluted EPS | $ | 6,849 | |
| $ | 7,459 | |
| $ | 8,531 | |
Weighted-average shares for basic and diluted EPS | 1,549 | |
| 1,566 | |
| 1,622 | | |||
For the 2015 , 2014 and 2013 computations, there were no antidilutive stock options.
Note 11. | ||||
Income Taxes:
Earnings before income taxes and provision for income taxes consisted of the following for the years ended December 31, 2015 , 2014 and 2013 :
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Earnings before income taxes | $ | 9,615 | |
| $ | 10,650 | |
| $ | 12,542 | |
Provision for income taxes: |
|
|
|
|
| ||||||
United States federal and state: |
|
|
|
|
| ||||||
Current | $ | (56 | ) |
| $ | (56 | ) |
| $ | 247 | |
Deferred | 117 | |
| 162 | |
| (5 | ) | |||
Total United States | 61 | |
| 106 | |
| 242 | | |||
Outside United States: |
|
|
|
|
| ||||||
Current | 2,762 | |
| 3,215 | |
| 3,451 | | |||
Deferred | (135 | ) |
| (224 | ) |
| (23 | ) | |||
Total outside United States | 2,627 | |
| 2,991 | |
| 3,428 | | |||
Total provision for income taxes | $ | 2,688 | |
| $ | 3,097 | |
| $ | 3,670 | |
United States income tax is primarily attributable to repatriation costs.
At December 31, 2015 , applicable United States federal income taxes and foreign withholding taxes have not been provided on approximately $23 billion of accumulated earnings of foreign subsidiaries that are expected to be permanently reinvested. These earnings have been or will be invested to support the growth of PMI's international business. Further, PMI does not foresee a need to repatriate these earnings to the U.S. since its U.S. cash requirements are supported by distributions from foreign entities of earnings that have not been designated as permanently reinvested and existing credit facilities. Repatriation of earnings from foreign subsidiaries for which PMI has asserted that the earnings are permanently reinvested would result in additional U.S. income and foreign withholding taxes. The determination of the amount of deferred tax related to these earnings is not practicable due to the complexity of the U.S. foreign tax credit regime, as well as differences between earnings determined for book and tax purposes mainly resulting from intercompany transactions, purchase accounting and currency fluctuations.
On March 28, 2008, PMI entered into a Tax Sharing Agreement (the "Tax Sharing Agreement") with Altria. The Tax Sharing Agreement generally governed PMI's and Altria's respective rights, responsibilities and obligations for pre-distribution periods and for potential taxes on the spin-off of PMI by Altria. With respect to any potential tax resulting from the spin-off of PMI by Altria, responsibility for the tax would be allocated to the party that acted (or failed to act) in a manner that resulted in the tax. In the third quarter of 2015, the IRS examination of Altria's consolidated tax returns for the years 2007 and 2008 was concluded with no tax adjustments to PMI.
101
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Balance at January 1, | $ | 123 | |
| $ | 114 | |
| $ | 124 | |
Additions based on tax positions related to the current year | 17 | |
| 20 | |
| 15 | | |||
Additions for tax positions of previous years | 6 | |
| 11 | |
| 3 | | |||
Reductions for tax positions of prior years | (42 | ) |
| (3 | ) |
| (2 | ) | |||
Reductions due to lapse of statute of limitations | (7 | ) |
| (8 | ) |
| (16 | ) | |||
Settlements | (1 | ) |
| (3 | ) |
| (10 | ) | |||
Other | (8 | ) |
| (8 | ) |
| - | | |||
Balance at December 31, | $ | 88 | |
| $ | 123 | |
| $ | 114 | |
Unrecognized tax benefits and PMI's liability for contingent income taxes, interest and penalties were as follows:
(in millions) | December 31, 2015 | |
| December 31, 2014 | |
| December 31, 2013 | | |||
Unrecognized tax benefits | $ | 88 | |
| $ | 123 | |
| $ | 114 | |
Accrued interest and penalties | 28 | |
| 40 | |
| 24 | | |||
Tax credits and other indirect benefits | (40 | ) |
| (54 | ) |
| (56 | ) | |||
Liability for tax contingencies | $ | 76 | |
| $ | 109 | |
| $ | 82 | |
The amount of unrecognized tax benefits that, if recognized, would impact the effective tax rate was $47 million at December 31, 2015 . The remainder, if recognized, would principally affect deferred taxes.
For the years ended December 31, 2015 , 2014 and 2013 , PMI recognized income (expense) in its consolidated statements of earnings of $3 million , $(19) million and $10 million , respectively, related to interest and penalties.
PMI is regularly examined by tax authorities around the world and is currently under examination in a number of jurisdictions. The U.S. federal statute of limitations remains open for the years 2012 and onward. Foreign and U.S. state jurisdictions have statutes of limitations generally ranging from three to five years. Years still open to examination by foreign tax authorities in major jurisdictions include Germany ( 2011 onward), Indonesia ( 2008 onward), Russia ( 2012 onward) and Switzerland ( 2014 onward).
It is reasonably possible that within the next 12 months certain tax examinations will close, which could result in a change in unrecognized tax benefits, along with related interest and penalties. An estimate of any possible change cannot be made at this time.
The effective income tax rate on pre-tax earnings differed from the U.S. federal statutory rate for the following reasons for the years ended December 31, 2015 , 2014 and 2013 :
| 2015 |
| 2014 |
| 2013 | |||
U.S. federal statutory rate | 35.0 | % |
| 35.0 | % |
| 35.0 | % |
Increase (decrease) resulting from: |
|
|
|
|
| |||
Foreign rate differences | (12.3 | ) |
| (11.2 | ) |
| (12.2 | ) |
Dividend repatriation cost | 5.7 | |
| 5.0 | |
| 6.6 | |
Other | (0.4 | ) |
| 0.3 | |
| (0.1 | ) |
Effective tax rate | 28.0 | % |
| 29.1 | % |
| 29.3 | % |
The 2015 effective tax rate decreased 1.1 percentage points to 28.0% . The effective tax rate for 2015 was unfavorably impacted by changes to repatriation assertions on certain foreign subsidiary historical earnings ( $58 million ), partially offset by a reduction in unrecognized tax benefits of $41 million following the conclusion of the IRS examinations of Altria's consolidated tax returns for the years 2007 and 2008 and PMI's consolidated tax returns for the years 2009 through 2011. Prior to March 28, 2008, PMI was a wholly owned subsidiary of Altria. Excluding the effect of these items, the change in the effective tax rate for 2015 , as compared to 2014 , was primarily due to earnings mix by taxing jurisdiction and repatriation cost differences.
102
The 2014 effective tax rate decreased 0.2 percentage points to 29.1% . Excluding the 2013 special tax items described below, the change in the effective tax rate for the year ended December 31, 2014, was primarily due to earnings mix by taxing jurisdiction and repatriation cost differences.
The American Taxpayer Relief Act of 2012 (the "Act") was enacted on January 2, 2013. Included in the Act were extensions through 2013 of several expired or expiring temporary business tax provisions, commonly referred to as "extenders." The tax impact of new legislation is recognized in the reporting period in which it is enacted. Therefore, PMI recognized the impact of the Act, which was $17 million of expense, in the consolidated financial statements in the first quarter of 2013.
The 2013 effective tax rate decreased 0.2 percentage points to 29.3% . The 2013 effective tax rate was unfavorably impacted by the additional expense associated with the Act ( $17 million ) and the enactment of tax law changes in Mexico ( $14 million ). Excluding these special tax items, the change in the effective tax rate for the year ended December 31, 2013, was primarily due to earnings mix by taxing jurisdiction and repatriation cost differences.
The tax effects of temporary differences that gave rise to deferred income tax assets and liabilities consisted of the following:
| At December 31, | ||||||
(in millions) | 2015 |
| 2014 | ||||
Deferred income tax assets: |
|
|
| ||||
Accrued postretirement and postemployment benefits | $ | 275 | |
| $ | 274 | |
Accrued pension costs | 230 | |
| 247 | | ||
Inventory | 174 | |
| 198 | | ||
Accrued liabilities | 153 | |
| 147 | | ||
Other | 164 | |
| 162 | | ||
Total deferred income tax assets | 996 | |
| 1,028 | | ||
Deferred income tax liabilities: |
|
|
| ||||
Trade names | (593 | ) |
| (677 | ) | ||
Property, plant and equipment | (218 | ) |
| (260 | ) | ||
Unremitted earnings | (554 | ) |
| (559 | ) | ||
Foreign exchange | (532 | ) |
| (348 | ) | ||
Total deferred income tax liabilities | (1,897 | ) |
| (1,844 | ) | ||
Net deferred income tax liabilities | $ | (901 | ) |
| $ | (816 | ) |
Note 12. | ||||
Segment Reporting:
PMI's subsidiaries and affiliates are engaged in the manufacture and sale of cigarettes, other tobacco products and other nicotine-containing products in markets outside of the United States of America. Reportable segments for PMI are organized and managed by geographic region. PMI's reportable segments are European Union; Eastern Europe, Middle East & Africa; Asia; and Latin America & Canada. PMI records net revenues and operating companies income to its segments based upon the geographic area in which the customer resides.
PMI's management evaluates segment performance and allocates resources based on operating companies income, which PMI defines as operating income, excluding general corporate expenses and amortization of intangibles, plus equity (income)/loss in unconsolidated subsidiaries, net. Interest expense, net, and provision for income taxes are centrally managed; accordingly, such items are not presented by segment since they are excluded from the measure of segment profitability reviewed by management. Information about total assets by segment is not disclosed because such information is not reported to or used by PMI's chief operating decision maker. Segment goodwill and other intangible assets, net, are disclosed in Note 3. Goodwill and Other Intangible Assets, net. The accounting policies of the segments are the same as those described in Note 2. Summary of Significant Accounting Policies.
103
In the fourth quarter of 2015, to further align with the Member State composition of the European Union, PMI transferred the management of its operations in Bulgaria, Croatia, Romania and Slovenia from its Eastern Europe, Middle East & Africa segment to its European Union segment, resulting in the reclassification of prior year amounts between the two segments.
Segment data were as follows:
| For the Years Ended December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Net revenues: |
|
|
|
|
| ||||||
European Union | $ | 26,563 | |
| $ | 30,517 | |
| $ | 29,656 | |
Eastern Europe, Middle East & Africa | 18,328 | |
| 20,469 | |
| 19,342 | | |||
Asia | 19,469 | |
| 19,255 | |
| 20,987 | | |||
Latin America & Canada | 9,548 | |
| 9,865 | |
| 10,044 | | |||
Net revenues (1) | $ | 73,908 | |
| $ | 80,106 | |
| $ | 80,029 | |
(1) Total net revenues attributable to customers located in Germany, PMI's largest market in terms of net revenues, were $7.2 billion , $8.3 billion and $7.8 billion for the years ended December 31, 2015 , 2014 and 2013 , respectively. Total net revenues attributable to customers located in Indonesia were $7.1 billion for the year ended December 31, 2015 .
| For the Years Ended December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Earnings before income taxes: |
|
|
|
|
| ||||||
Operating companies income: |
|
|
|
|
| ||||||
European Union | $ | 3,576 | |
| $ | 3,815 | |
| $ | 4,309 | |
Eastern Europe, Middle East & Africa | 3,425 | |
| 4,033 | |
| 3,708 | | |||
Asia | 2,886 | |
| 3,187 | |
| 4,622 | | |||
Latin America & Canada | 1,085 | |
| 1,030 | |
| 1,134 | | |||
Amortization of intangibles | (82 | ) |
| (93 | ) |
| (93 | ) | |||
General corporate expenses | (162 | ) |
| (165 | ) |
| (187 | ) | |||
Less: |
|
|
|
|
| ||||||
Equity (income)/loss in unconsolidated subsidiaries, net | (105 | ) |
| (105 | ) |
| 22 | | |||
Operating income | 10,623 | |
| 11,702 | |
| 13,515 | | |||
Interest expense, net | (1,008 | ) |
| (1,052 | ) |
| (973 | ) | |||
Earnings before income taxes | $ | 9,615 | |
| $ | 10,650 | |
| $ | 12,542 | |
| For the Years Ended December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Depreciation expense: |
|
|
|
|
| ||||||
European Union | $ | 230 | |
| $ | 206 | |
| $ | 199 | |
Eastern Europe, Middle East & Africa | 163 | |
| 212 | |
| 218 | | |||
Asia | 184 | |
| 278 | |
| 277 | | |||
Latin America & Canada | 85 | |
| 90 | |
| 85 | | |||
| 662 | |
| 786 | |
| 779 | | |||
Other | 10 | |
| 10 | |
| 10 | | |||
Total depreciation expense | $ | 672 | |
| $ | 796 | |
| 789 | | |
104
| For the Years Ended December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Capital expenditures: |
|
|
|
|
| ||||||
European Union | $ | 497 | |
| $ | 537 | |
| $ | 493 | |
Eastern Europe, Middle East & Africa | 147 | |
| 216 | |
| 234 | | |||
Asia | 185 | |
| 272 | |
| 317 | | |||
Latin America & Canada | 130 | |
| 125 | |
| 156 | | |||
| 959 | |
| 1,150 | |
| 1,200 | | |||
Other | 1 | |
| 3 | |
| - | | |||
Total capital expenditures | $ | 960 | |
| $ | 1,153 | |
| $ | 1,200 | |
| At December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Long-lived assets: |
|
|
|
|
| ||||||
European Union | $ | 3,129 | |
| $ | 3,242 | |
| $ | 3,475 | |
Eastern Europe, Middle East & Africa | 743 | |
| 836 | |
| 1,193 | | |||
Asia | 1,743 | |
| 1,838 | |
| 1,758 | | |||
Latin America & Canada | 605 | |
| 704 | |
| 759 | | |||
| 6,220 | |
| 6,620 | |
| 7,185 | | |||
Other | 644 | |
| 269 | |
| 208 | | |||
Total long-lived assets | $ | 6,864 | |
| $ | 6,889 | |
| $ | 7,393 | |
Long-lived assets consist of non-current assets other than goodwill; other intangible assets, net; deferred tax assets, and investments in unconsolidated subsidiaries. PMI's largest market in terms of long-lived assets is Switzerland. Total long-lived assets located in Switzerland, which is reflected in the European Union segment above, were $0.9 billion , $1.0 billion and $1.1 billion at December 31, 2015 , 2014 and 2013 , respectively.
Items affecting the comparability of results from operations were as follows:
• | Asset Impairment and Exit Costs - See Note 5. Asset Impairment and Exit Costs for a breakdown of asset impairment and exit costs by segment. |
• | Acquisitions and Other Business Arrangements - For further details, see Note 6. Acquisitions and Other Business Arrangements. |
Note 13. | ||||
Benefit Plans:
Pension coverage for employees of PMI's subsidiaries is provided, to the extent deemed appropriate, through separate plans, many of which are governed by local statutory requirements. In addition, PMI provides health care and other benefits to substantially all U.S. retired employees and certain non-U.S. retired employees. In general, health care benefits for non-U.S. retired employees are covered through local government plans.
105
Pension and Postretirement Benefit Plans
Obligations and Funded Status
The postretirement health care plans are not funded. The projected benefit obligations, plan assets and funded status of PMI's pension plans, and the accumulated benefit obligation and net amount accrued for PMI's postretirement health care plans, at December 31, 2015 and 2014 , were as follows:
| Pension |
|
|
|
| ||||||||||||||||||
| U.S. Plans |
| Non-U.S. Plans |
| Postretirement | ||||||||||||||||||
(in millions) | 2015 |
| 2014 |
| 2015 |
| 2014 |
| 2015 |
| 2014 | ||||||||||||
Benefit obligation at January 1, | $ | 438 | |
| $ | 364 | |
| $ | 7,638 | |
| $ | 6,893 | |
| $ | 238 | |
| $ | 213 | |
Service cost | 5 | |
| 5 | |
| 200 | |
| 211 | |
| 4 | |
| 4 | | ||||||
Interest cost | 17 | |
| 17 | |
| 139 | |
| 205 | |
| 9 | |
| 10 | | ||||||
Benefits paid | (51 | ) |
| (23 | ) |
| (225 | ) |
| (245 | ) |
| (11 | ) |
| (10 | ) | ||||||
Settlement and curtailment | - | |
| (1 | ) |
| (16 | ) |
| (73 | ) |
| - | |
| (2 | ) | ||||||
Actuarial losses (gains) | (20 | ) |
| 76 | |
| 261 | |
| 1,384 | |
| (12 | ) |
| 35 | | ||||||
Currency | - | |
| - | |
| (365 | ) |
| (777 | ) |
| (17 | ) |
| (12 | ) | ||||||
Other | - | |
| - | |
| 65 | |
| 40 | |
| - | |
| - | | ||||||
Benefit obligation at December 31, | 389 | |
| 438 | |
| 7,697 | |
| 7,638 | |
| 211 | |
| 238 | | ||||||
Fair value of plan assets at January 1, | 312 | |
| 305 | |
| 6,410 | |
| 6,566 | |
|
|
|
| ||||||||
Actual return on plan assets | - | |
| 19 | |
| 56 | |
| 620 | |
|
|
|
| ||||||||
Employer contributions | 37 | |
| 11 | |
| 117 | |
| 180 | |
|
|
|
| ||||||||
Employee contributions | - | |
| - | |
| 37 | |
| 42 | |
|
|
|
| ||||||||
Benefits paid | (51 | ) |
| (23 | ) |
| (225 | ) |
| (245 | ) |
|
|
|
| ||||||||
Settlement and curtailment | - | |
| - | |
| (14 | ) |
| (37 | ) |
|
|
|
| ||||||||
Currency | - | |
| - | |
| (275 | ) |
| (716 | ) |
|
|
|
| ||||||||
Fair value of plan assets at December 31, | 298 | |
| 312 | |
| 6,106 | |
| 6,410 | |
|
|
|
| ||||||||
Net pension and postretirement liability recognized at December 31, | $ | (91 | ) |
| $ | (126 | ) |
| $ | (1,591 | ) |
| $ | (1,228 | ) |
| $ | (211 | ) |
| $ | (238 | ) |
At December 31, 2015 and 2014 , the Swiss pension plan represented 61% and 56% of the non-U.S. benefit obligation, respectively, and approximately 60% of the non-U.S. fair value of plan assets for each of the years.
At December 31, 2015 and 2014 , the amounts recognized on PMI's consolidated balance sheets for the combined U.S. and non-U.S. pension plans, and postretirement plans were as follows:
| Pension |
| Postretirement | ||||||||||||
(in millions) | 2015 |
| 2014 |
| 2015 |
| 2014 | ||||||||
Other assets | $ | 47 | |
| $ | 42 | |
|
|
|
| ||||
Accrued liabilities - employment costs | (23 | ) |
| (55 | ) |
| $ | (9 | ) |
| $ | (10 | ) | ||
Long-term employment costs | (1,706 | ) |
| (1,341 | ) |
| (202 | ) |
| (228 | ) | ||||
| $ | (1,682 | ) |
| $ | (1,354 | ) |
| $ | (211 | ) |
| $ | (238 | ) |
The accumulated benefit obligation, which represents benefits earned to date, for the U.S. pension plans was $360 million and $411 million at December 31, 2015 and 2014 , respectively. The accumulated benefit obligation for non-U.S. pension plans was $7,157 million and $7,082 million at December 31, 2015 and 2014 , respectively.
For U.S. pension plans with accumulated benefit obligations in excess of plan assets, the projected benefit obligation, accumulated benefit obligation and fair value of plan assets were $389 million , $360 million and $298 million , respectively, as of December 31, 2015 . The projected benefit obligation, accumulated benefit obligation and fair value of plan assets were $438 million , $411 million and $312
106
million , respectively, as of December 31, 2014 . The underfunding relates to plans for salaried employees that cannot be funded under IRS regulations. For non-U.S. plans with accumulated benefit obligations in excess of plan assets, the projected benefit obligation, accumulated benefit obligation and fair value of plan assets were $6,355 million , $5,961 million , and $4,766 million , respectively, as of December 31, 2015 , and $6,130 million , $5,745 million , and $4,974 million , respectively, as of December 31, 2014 .
The following weighted-average assumptions were used to determine PMI's pension and postretirement benefit obligations at December 31:
| Pension |
|
|
|
| ||||||||||||
| U.S. Plans |
| Non-U.S. Plans |
| Postretirement | ||||||||||||
| 2015 |
| 2014 |
| 2015 |
| 2014 |
| 2015 |
| 2014 | ||||||
Discount rate | 4.30 | % |
| 3.95 | % |
| 1.68 | % |
| 1.92 | % |
| 4.45 | % |
| 4.20 | % |
Rate of compensation increase | 3.00 | |
| 3.00 | |
| 1.98 | |
| 2.06 | |
|
|
|
| ||
Health care cost trend rate assumed for next year |
|
|
|
|
|
|
|
| 6.23 | |
| 6.62 | | ||||
Ultimate trend rate |
|
|
|
|
|
|
|
| 4.75 | |
| 4.99 | | ||||
Year that rate reaches the ultimate trend rate |
|
|
|
|
|
|
|
| 2029 | |
| 2029 | | ||||
The discount rate for the largest U.S. and non-U.S. pension plans is based on a yield curve constructed from a portfolio of high quality corporate bonds that produces a cash flow pattern equivalent to each plan's expected benefit payments. The discount rate for the remaining non-U.S. plans is developed from local bond indices that match local benefit obligations as closely as possible.
Components of Net Periodic Benefit Cost
Net periodic pension and postretirement health care costs consisted of the following for the years ended December 31, 2015 , 2014 and 2013 :
| Pension |
|
|
|
|
|
| ||||||||||||||||||||||||||||
| U.S. Plans |
| Non-U.S. Plans |
| Postretirement | ||||||||||||||||||||||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 |
| 2015 |
| 2014 |
| 2013 |
| 2015 |
| 2014 |
| 2013 | ||||||||||||||||||
Service cost | $ | 5 | |
| $ | 5 | |
| $ | 7 | |
| $ | 200 | |
| $ | 211 | |
| $ | 255 | |
| $ | 4 | |
| $ | 4 | |
| $ | 5 | |
Interest cost | 17 | |
| 17 | |
| 16 | |
| 139 | |
| 205 | |
| 169 | |
| 9 | |
| 10 | |
| 10 | | |||||||||
Expected return on plan assets | (15 | ) |
| (16 | ) |
| (16 | ) |
| (325 | ) |
| (357 | ) |
| (347 | ) |
| - | |
| - | |
| - | | |||||||||
Amortization: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||
Net losses | 14 | |
| 6 | |
| 11 | |
| 180 | |
| 115 | |
| 205 | |
| 4 | |
| 2 | |
| 5 | | |||||||||
Prior service cost | - | |
| 1 | |
| 1 | |
| 4 | |
| 5 | |
| 9 | |
| - | |
| (1 | ) |
| - | | |||||||||
Settlement and curtailment | 1 | |
| 5 | |
| - | |
| 2 | |
| 1 | |
| 1 | |
| - | |
| - | |
| - | | |||||||||
Net periodic pension and postretirement costs | $ | 22 | |
| $ | 18 | |
| $ | 19 | |
| $ | 200 | |
| $ | 180 | |
| $ | 292 | |
| $ | 17 | |
| $ | 15 | |
| $ | 20 | |
Settlement and curtailment charges were due primarily to early retirement programs.
For the combined U.S. and non-U.S. pension plans, the estimated net loss and prior service cost that are expected to be amortized from accumulated other comprehensive earnings into net periodic benefit cost during 2016 are $178 million and $10 million , respectively.
The following weighted-average assumptions were used to determine PMI's net pension and postretirement health care costs:
107
| Pension |
|
|
|
|
|
| |||||||||||||||||||
| U.S. Plans |
| Non-U.S. Plans |
| Postretirement | |||||||||||||||||||||
| 2015 |
| 2014 |
| 2013 |
| 2015 |
| 2014 |
| 2013 |
| 2015 |
| 2014 |
| 2013 | |||||||||
Discount rate | 3.95 | % |
| 4.80 | % |
| 4.05 | % |
| 1.92 | % |
| 3.09 | % |
| 2.38 | % |
| 4.20 | % |
| 5.01 | % |
| 4.29 | % |
Expected rate of return on plan assets | 5.10 | |
| 5.70 | |
| 5.70 | |
| 5.38 | |
| 5.63 | |
| 6.11 | |
|
|
|
|
|
| |||
Rate of compensation increase | 3.00 | |
| 3.00 | |
| 3.50 | |
| 2.06 | |
| 2.34 | |
| 2.61 | |
|
|
|
|
|
| |||
Health care cost trend rate |
|
|
|
|
|
|
|
|
|
|
|
| 6.62 | |
| 6.60 | |
| 7.01 | | ||||||
PMI's expected rate of return on pension plan assets is determined by the plan assets' historical long-term investment performance, current asset allocation and estimates of future long-term returns by asset class.
PMI and certain of its subsidiaries sponsor defined contribution plans. Amounts charged to expense for defined contribution plans totaled $52 million , $62 million and $69 million for the years ended December 31, 2015 , 2014 and 2013 , respectively.
Plan Assets
PMI's investment strategy for U.S. and non-U.S. pension plans is based on an expectation that equity securities will outperform debt securities over the long term. Accordingly, the target allocation of PMI's plan assets is broadly characterized as approximately a 60% / 40% split between equity and debt securities. The strategy primarily utilizes indexed U.S. equity securities, international equity securities and investment-grade debt securities. PMI's plans have no investments in hedge funds, private equity or derivatives. PMI attempts to mitigate investment risk by rebalancing between equity and debt asset classes once a year or as PMI's contributions and benefit payments are made.
The fair value of PMI's pension plan assets at December 31, 2015 and 2014 , by asset category was as follows:
Asset Category (in millions) | At December 31, 2015 |
| Quoted Prices |
| Significant |
| Significant (Level 3) | ||||||||
Cash and cash equivalents | $ | 225 | |
| $ | 225 | |
| | |
| | | ||
Equity securities: |
|
|
|
|
|
|
| ||||||||
U.S. securities | 120 | |
| 120 | |
| | |
| | | ||||
International securities | 409 | |
| 409 | |
| | |
| | | ||||
Investment funds (a) | 5,337 | |
| 3,446 | |
| 1,891 | |
| | | ||||
International government bonds | 289 | |
| 289 | |
| | |
| | | ||||
Other | 24 | |
| 24 | |
| | |
| | | ||||
Total | $ | 6,404 | |
| $ | 4,513 | |
| $ | 1,891 | |
| $ | - | |
(a) Investment funds whose objective seeks to replicate the returns and characteristics of specified market indices (primarily MSCI - Europe, Switzerland, North America, Asia Pacific, Japan; Russell 3000; S&P 500 for equities, and Citigroup EMU and Barclays Capital U.S. for bonds), primarily consist of mutual funds, common trust funds and commingled funds. Of these funds, 59% are invested in U.S. and international equities; 21% are invested in U.S. and international government bonds; 10% are invested in real estate and other money markets, and 10% are invested in corporate bonds.
108
Asset Category (in millions) | At December 31, 2014 |
| Quoted Prices |
| Significant |
| Significant | ||||||||
Cash and cash equivalents | $ | 286 | |
| $ | 286 | |
| $ | - | |
| $ | - | |
Equity securities: |
|
|
|
|
|
|
| ||||||||
U.S. securities | 136 | |
| 136 | |
| - | |
| - | | ||||
International securities | 418 | |
| 418 | |
| - | |
| - | | ||||
Investment funds (a) | 5,558 | |
| 3,689 | |
| 1,869 | |
| - | | ||||
International government bonds | 293 | |
| 293 | |
| - | |
| - | | ||||
Other | 31 | |
| 31 | |
| - | |
| - | | ||||
Total | $ | 6,722 | |
| $ | 4,853 | |
| $ | 1,869 | |
| $ | - | |
(a) Investment funds whose objective seeks to replicate the returns and characteristics of specified market indices (primarily MSCI - Europe, Switzerland, North America, Asia Pacific, Japan; Russell 3000; S&P 500 for equities, and Citigroup EMU and Barclays Capital U.S. for bonds), primarily consist of mutual funds, common trust funds and commingled funds. Of these funds, 61% were invested in U.S. and international equities; 22% were invested in U.S. and international government bonds; 9% were invested in real estate and other money markets, and 8% were invested in corporate bonds.
See Note 16. Fair Value Measurements for a discussion of the fair value of pension plan assets.
PMI makes, and plans to make, contributions to the extent that they are tax deductible and to meet specific funding requirements of its funded U.S. and non-U.S. pension plans. Currently, PMI anticipates making contributions of approximately $113 million in 2016 to its pension plans, based on current tax and benefit laws. However, this estimate is subject to change as a result of changes in tax and other benefit laws, as well as asset performance significantly above or below the assumed long-term rate of return on pension assets, or changes in interest rates.
The estimated future benefit payments from PMI pension plans at December 31, 2015 , are as follows:
(in millions) | U.S. Plans |
| Non-U.S. Plans | ||||
2016 | $ | 20 | |
| $ | 255 | |
2017 | 21 | |
| 255 | | ||
2018 | 20 | |
| 264 | | ||
2019 | 26 | |
| 265 | | ||
2020 | 23 | |
| 284 | | ||
2021 - 2025 | 125 | |
| 1,636 | | ||
PMI's expected future annual benefit payments for its postretirement health care plans are estimated to be not material through 2025 .
Assumed health care cost trend rates have a significant effect on the amounts reported for the health care plans. A one-percentage-point change in assumed health care trend rates would have the following effects as of December 31, 2015 :
| One-Percentage-Point Increase | |
| One-Percentage-Point Decrease | |
Effect on total service and interest cost | 18.8 | % |
| (14.4 | )% |
Effect on postretirement benefit obligation | 16.7 | |
| (13.3 | ) |
109
Postemployment Benefit Plans
PMI and certain of its subsidiaries sponsor postemployment benefit plans covering substantially all salaried and certain hourly employees. The cost of these plans is charged to expense over the working life of the covered employees. Net postemployment costs were $187 million , $167 million and $147 million for the years ended December 31, 2015 , 2014 and 2013 , respectively.
The estimated net loss for the postemployment benefit plans that will be amortized from accumulated other comprehensive losses into net postemployment costs during 2016 is approximately $62 million .
The amounts recognized in accrued postemployment costs on PMI's consolidated balance sheets at December 31, 2015 and 2014 , were $745 million and $734 million , respectively.
During 2015 , 2014 and 2013 , certain salaried employees left PMI under separation programs. These programs resulted in incremental postemployment costs and benefit obligations. For further details see Note 5. Asset Impairment and Exit Costs .
The accrued postemployment costs were determined using a weighted-average discount rate of 3.5% and 3.7% in 2015 and 2014 , respectively; an assumed ultimate annual weighted-average turnover rate of 2.7% and 2.2% in 2015 and 2014 , respectively; assumed compensation cost increases of 2.2% in 2015 and 2.2% in 2014 , and assumed benefits as defined in the respective plans. In accordance with local regulations, certain postemployment plans are funded. As a result, the accrued postemployment costs disclosed above are presented net of the related assets of $26 million and $28 million at December 31, 2015 and 2014 , respectively. Postemployment costs arising from actions that offer employees benefits in excess of those specified in the respective plans are charged to expense when incurred.
Comprehensive Earnings (Losses)
The amounts recorded in accumulated other comprehensive losses at December 31, 2015 , consisted of the following:
(in millions) | Pension |
| Post- |
| Post- |
| Total | ||||||||
Net losses | $ | (3,074 | ) |
| $ | (61 | ) |
| $ | (710 | ) |
| $ | (3,845 | ) |
Prior service cost | (40 | ) |
| 5 | |
| - | |
| (35 | ) | ||||
Net transition obligation | (5 | ) |
| - | |
| - | |
| (5 | ) | ||||
Deferred income taxes | 320 | |
| 20 | |
| 213 | |
| 553 | | ||||
Losses to be amortized | $ | (2,799 | ) |
| $ | (36 | ) |
| $ | (497 | ) |
| $ | (3,332 | ) |
110
The amounts recorded in accumulated other comprehensive losses at December 31, 2014 , consisted of the following:
(in millions) | Pension |
| Post- |
| Post- |
| Total | ||||||||
Net losses | $ | (2,760 | ) |
| $ | (77 | ) |
| $ | (721 | ) |
| $ | (3,558 | ) |
Prior service cost | (45 | ) |
| 6 | |
| - | |
| (39 | ) | ||||
Net transition obligation | (6 | ) |
| - | |
| - | |
| (6 | ) | ||||
Deferred income taxes | 342 | |
| 25 | |
| 216 | |
| 583 | | ||||
Losses to be amortized | $ | (2,469 | ) |
| $ | (46 | ) |
| $ | (505 | ) |
| $ | (3,020 | ) |
The amounts recorded in accumulated other comprehensive losses at December 31, 2013 , consisted of the following:
(in millions) | Pension |
| Post- |
| Post- |
| Total | ||||||||
Net losses | $ | (1,746 | ) |
| $ | (47 | ) |
| $ | (661 | ) |
| $ | (2,454 | ) |
Prior service cost | (51 | ) |
| 7 | |
| - | |
| (44 | ) | ||||
Net transition obligation | (6 | ) |
| - | |
| - | |
| (6 | ) | ||||
Deferred income taxes | 245 | |
| 14 | |
| 199 | |
| 458 | | ||||
Losses to be amortized | $ | (1,558 | ) |
| $ | (26 | ) |
| $ | (462 | ) |
| $ | (2,046 | ) |
The movements in other comprehensive earnings (losses) during the year ended December 31, 2015 , were as follows:
(in millions) | Pension |
| Post- |
| Post- |
| Total | ||||||||
Amounts transferred to earnings as components of net periodic benefit cost: |
|
|
|
|
|
|
| ||||||||
Amortization: |
|
|
|
|
|
|
| ||||||||
Net losses | $ | 194 | |
| $ | 4 | |
| $ | 69 | |
| $ | 267 | |
Prior service cost | 4 | |
| - | |
| - | |
| 4 | | ||||
Other income/expense: |
|
|
|
|
|
|
| ||||||||
Net losses | 3 | |
| - | |
| - | |
| 3 | | ||||
Prior service cost | 1 | |
| - | |
| - | |
| 1 | | ||||
Deferred income taxes | (26 | ) |
| (2 | ) |
| (20 | ) |
| (48 | ) | ||||
| 176 | |
| 2 | |
| 49 | |
| 227 | | ||||
Other movements during the year: |
|
|
|
|
|
|
| ||||||||
Net losses | (510 | ) |
| 12 | |
| (58 | ) |
| (556 | ) | ||||
Deferred income taxes | 4 | |
| (4 | ) |
| 17 | |
| 17 | | ||||
| (506 | ) |
| 8 | |
| (41 | ) |
| (539 | ) | ||||
Total movements in other comprehensive earnings (losses) | $ | (330 | ) |
| $ | 10 | |
| $ | 8 | |
| $ | (312 | ) |
111
The movements in other comprehensive earnings (losses) during the year ended December 31, 2014 , were as follows:
(in millions) | Pension |
| Post- |
| Post- |
| Total | ||||||||
Amounts transferred to earnings as components of net periodic benefit cost: |
|
|
|
|
|
|
| ||||||||
Amortization: |
|
|
|
|
|
|
| ||||||||
Net losses | $ | 121 | |
| $ | 2 | |
| $ | 66 | |
| $ | 189 | |
Prior service cost | 6 | |
| (1 | ) |
| - | |
| 5 | | ||||
Other income/expense: |
|
|
|
|
|
|
| ||||||||
Net losses | 14 | |
| 2 | |
| - | |
| 16 | | ||||
Prior service cost | 5 | |
| - | |
| - | |
| 5 | | ||||
Deferred income taxes | (21 | ) |
| (1 | ) |
| (20 | ) |
| (42 | ) | ||||
| 125 | |
| 2 | |
| 46 | |
| 173 | | ||||
Other movements during the year: |
|
|
|
|
|
|
| ||||||||
Net losses | (1,149 | ) |
| (34 | ) |
| (126 | ) |
| (1,309 | ) | ||||
Prior service cost | (5 | ) |
| - | |
| - | |
| (5 | ) | ||||
Deferred income taxes | 118 | |
| 12 | |
| 37 | |
| 167 | | ||||
| (1,036 | ) |
| (22 | ) |
| (89 | ) |
| (1,147 | ) | ||||
Total movements in other comprehensive earnings (losses) | $ | (911 | ) |
| $ | (20 | ) |
| $ | (43 | ) |
| $ | (974 | ) |
The movements in other comprehensive earnings (losses) during the year ended December 31, 2013 , were as follows:
(in millions) | Pension |
| Post- |
| Post- |
| Total | ||||||||
Amounts transferred to earnings as components of net periodic benefit cost: |
|
|
|
|
|
|
| ||||||||
Amortization: |
|
|
|
|
|
|
| ||||||||
Net losses | $ | 216 | |
| $ | 5 | |
| $ | 60 | |
| $ | 281 | |
Prior service cost | 10 | |
| - | |
| - | |
| 10 | | ||||
Other income/expense: |
|
|
|
|
|
|
| ||||||||
Net losses | 1 | |
| - | |
| - | |
| 1 | | ||||
Deferred income taxes | (29 | ) |
| (2 | ) |
| (18 | ) |
| (49 | ) | ||||
| 198 | |
| 3 | |
| 42 | |
| 243 | | ||||
Other movements during the year: |
|
|
|
|
|
|
| ||||||||
Net losses | 1,236 | |
| 30 | |
| (109 | ) |
| 1,157 | | ||||
Prior service cost | (1 | ) |
| - | |
| - | |
| (1 | ) | ||||
Net transition obligation | 1 | |
| - | |
| - | |
| 1 | | ||||
Deferred income taxes | (103 | ) |
| (10 | ) |
| 32 | |
| (81 | ) | ||||
| 1,133 | |
| 20 | |
| (77 | ) |
| 1,076 | | ||||
Total movements in other comprehensive earnings (losses) | $ | 1,331 | |
| $ | 23 | |
| $ | (35 | ) |
| $ | 1,319 | |
112
Note 14. | ||||
Additional Information:
| For the Years Ended December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Research and development expense | $ | 423 | |
| $ | 433 | |
| $ | 449 | |
Advertising expense | $ | 448 | |
| $ | 439 | |
| $ | 435 | |
Foreign currency net transaction losses | $ | 102 | |
| $ | 174 | |
| $ | 123 | |
Interest expense | $ | 1,132 | |
| $ | 1,170 | |
| $ | 1,104 | |
Interest income | (124 | ) |
| (118 | ) |
| (131 | ) | |||
Interest expense, net | $ | 1,008 | |
| $ | 1,052 | |
| $ | 973 | |
Rent expense | $ | 286 | |
| $ | 336 | |
| $ | 334 | |
Minimum rental commitments under non-cancelable operating leases in effect at December 31, 2015 , were as follows:
(in millions) |
| ||
2016 | $ | 177 | |
2017 | 125 | | |
2018 | 82 | | |
2019 | 61 | | |
2020 | 42 | | |
Thereafter | 195 | | |
| $ | 682 | |
Note 15. | ||||
Financial Instruments:
Overview
PMI operates in markets outside of the United States of America, with manufacturing and sales facilities in various locations around the world. PMI utilizes certain financial instruments to manage foreign currency and interest rate exposure. Derivative financial instruments are used by PMI principally to reduce exposures to market risks resulting from fluctuations in foreign currency exchange and interest rates by creating offsetting exposures. PMI is not a party to leveraged derivatives and, by policy, does not use derivative financial instruments for speculative purposes. Financial instruments qualifying for hedge accounting must maintain a specified level of effectiveness between the hedging instrument and the item being hedged, both at inception and throughout the hedged period. PMI formally documents the nature and relationships between the hedging instruments and hedged items, as well as its risk-management objectives, strategies for undertaking the various hedge transactions and method of assessing hedge effectiveness. Additionally, for hedges of forecasted transactions, the significant characteristics and expected terms of the forecasted transaction must be specifically identified, and it must be probable that each forecasted transaction will occur. If it were deemed probable that the forecasted transaction would not occur, the gain or loss would be recognized in earnings. PMI reports its net transaction gains or losses in marketing, administration and research costs on the consolidated statements of earnings.
PMI uses deliverable and non-deliverable forward foreign exchange contracts, foreign currency swaps and foreign currency options, collectively referred to as foreign exchange contracts ("foreign exchange contracts"), and interest rate contracts to mitigate its exposure to changes in exchange and interest rates from third-party and intercompany actual and forecasted transactions. The primary currencies
113
to which PMI is exposed include the Australian dollar, Euro, Indonesian rupiah, Japanese yen, Mexican peso, Russian ruble, Swiss franc and Turkish lira. At December 31, 2015 and 2014 , PMI had contracts with aggregate notional amounts of $24.9 billion and $21.9 billion , respectively. Of the $24.9 billion aggregate notional amount at December 31, 2015 , $3.2 billion related to cash flow hedges, $6.4 billion related to hedges of net investments in foreign operations and $15.3 billion related to other derivatives that primarily offset currency exposures on intercompany financing. Of the $21.9 billion aggregate notional amount at December 31, 2014 , $2.2 billion related to cash flow hedges, $4.3 billion related to hedges of net investments in foreign operations and $15.4 billion related to other derivatives that primarily offset currency exposures on intercompany financing.
The fair value of PMI's foreign exchange contracts included in the consolidated balance sheet as of December 31, 2015 and 2014 , were as follows:
| Asset Derivatives |
| Liability Derivatives | ||||||||||||||||
|
|
| Fair Value |
|
|
| Fair Value | ||||||||||||
(in millions) | Balance Sheet Classification |
| 2015 |
| 2014 |
| Balance Sheet Classification |
| 2015 |
| 2014 | ||||||||
Foreign exchange contracts designated as hedging instruments | Other current assets |
| $ | 301 | |
| $ | 248 | |
| Other accrued liabilities |
| $ | 26 | |
| $ | - | |
| Other assets |
| 181 | |
| 122 | |
| Other liabilities |
| 117 | |
| 25 | | ||||
Foreign exchange contracts not designated as hedging instruments | Other current assets |
| 7 | |
| 34 | |
| Other accrued liabilities |
| 29 | |
| 126 | | ||||
|
|
|
|
|
|
|
|
|
|
|
| ||||||||
| Other assets |
| 85 | |
| 2 | |
| Other liabilities |
| - | |
| - | | ||||
Total derivatives |
|
| $ | 574 | |
| $ | 406 | |
|
|
| $ | 172 | |
| $ | 151 | |
For the years ended December 31, 2015 , 2014 and 2013 , PMI's cash flow and net investment hedging instruments impacted the consolidated statements of earnings and comprehensive earnings as follows:
(pre-tax, millions) | For the Year Ended December 31, | ||||||||||||||||||||||||
| Amount of Gain/(Loss) Recognized in Other Comprehensive Earnings/(Losses) on Derivatives |
| Statement of Earnings |
| Amount of Gain/(Loss) Reclassified from Other Comprehensive Earnings/(Losses) into Earnings | ||||||||||||||||||||
| 2015 |
| 2014 |
| 2013 |
|
|
| 2015 |
| 2014 | 2013 |
| ||||||||||||
Derivatives in Cash Flow Hedging Relationship |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||
Foreign exchange contracts | $ | 43 | |
| $ | 111 | |
| $ | 236 | |
|
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
| Net revenues |
| $ | 149 | |
| $ | 115 | | $ | 319 | |
| ||||||
|
|
|
|
|
|
| Cost of sales |
| (3 | ) |
| - | | 6 | |
| |||||||||
|
|
|
|
|
|
| Marketing, administration and research costs |
| 1 | |
| (28 | ) | - | |
| |||||||||
|
|
|
|
|
|
| Interest expense, net |
| (31 | ) |
| (39 | ) | (56 | ) |
| |||||||||
Derivatives in Net Investment Hedging Relationship |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||
Foreign exchange contracts | 253 | |
| 269 | |
| (79 | ) |
|
|
|
|
|
|
|
| |||||||||
Total | $ | 296 | |
| $ | 380 | |
| $ | 157 | |
|
|
| $ | 116 | |
| $ | 48 | | $ | 269 | |
|
Cash Flow Hedges
PMI has entered into foreign exchange contracts to hedge foreign currency exchange risk related to certain forecasted transactions. The effective portion of gains and losses associated with qualifying cash flow hedge contracts is deferred as a component of accumulated other comprehensive losses until the underlying hedged transactions are reported in PMI's consolidated statements of earnings. During the years ended December 31, 2015 , 2014 and 2013 , ineffectiveness related to cash flow hedges was not material. As of December 31,
114
2015 , PMI has hedged forecasted transactions for periods not exceeding the next 12 months with the exception of one foreign exchange contract that expires in May 2024 . The impact of these hedges is primarily included in operating cash flows on PMI's consolidated statements of cash flows.
Hedges of Net Investments in Foreign Operations
PMI designates certain foreign currency denominated debt and foreign exchange contracts as net investment hedges of its foreign operations. For the years ended December 31, 2015 , 2014 and 2013 , these hedges of net investments resulted in gains/(losses), net of income taxes, of $761 million , $952 million and $(285) million , respectively. These gains/(losses) were reported as a component of accumulated other comprehensive losses within currency translation adjustments. For the years ended December 31, 2015 , 2014 and 2013 , ineffectiveness related to net investment hedges was not material. Other investing cash flows on PMI's consolidated statements of cash flows include the premiums paid for, and settlements of, net investment hedges.
Other Derivatives
PMI has entered into foreign exchange contracts to hedge the foreign currency exchange and interest rate risks related to intercompany loans between certain subsidiaries, and third-party loans. While effective as economic hedges, no hedge accounting is applied for these contracts; therefore, the unrealized gains (losses) relating to these contracts are reported in PMI's consolidated statements of earnings. For the years ended December 31, 2015 , 2014 and 2013 , the gains/(losses) from contracts for which PMI did not apply hedge accounting were $(587) million , $(481) million and $99 million , respectively. The gains/(losses) from these contracts substantially offset the losses and gains generated by the underlying intercompany and third-party loans being hedged.
For the years ended December 31, 2015 , 2014 and 2013 , the net impact of these contracts on the consolidated statements of earnings was not material.
|
|
|
|
|
|
|
|
Qualifying Hedging Activities Reported in Accumulated Other Comprehensive Losses
Derivative gains or losses reported in accumulated other comprehensive losses are a result of qualifying hedging activity. Transfers of these gains or losses to earnings are offset by the corresponding gains or losses on the underlying hedged item. Hedging activity affected accumulated other comprehensive losses, net of income taxes, as follows:
| For the Years Ended December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Gain as of January 1, | $ | 123 | |
| $ | 63 | |
| $ | 92 | |
Derivative gains transferred to earnings | (102 | ) |
| (38 | ) |
| (235 | ) | |||
Change in fair value | 38 | |
| 98 | |
| 206 | | |||
Gain as of December 31, | $ | 59 | |
| $ | 123 | |
| $ | 63 | |
At December 31, 2015 , PMI expects $49 million of derivative gains that are included in accumulated other comprehensive losses to be reclassified to the consolidated statement of earnings within the next 12 months . These gains are expected to be substantially offset by the statement of earnings impact of the respective hedged transactions.
Contingent Features
PMI's derivative instruments do not contain contingent features.
Credit Exposure and Credit Risk
PMI is exposed to credit loss in the event of non-performance by counterparties. While PMI does not anticipate non-performance, its risk is limited to the fair value of the financial instruments less any cash collateral received or pledged. PMI actively monitors its exposure to credit risk through the use of credit approvals and credit limits and by selecting and continuously monitoring a diverse group of major international banks and financial institutions as counterparties.
Fair Value
See Note 16. Fair Value Measurements and Note 22. Balance Sheet Offsetting for additional discussion of derivative financial instruments.
115
Note 16. | ||||
Fair Value Measurements:
The authoritative guidance defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The guidance also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The guidance describes three levels of input that may be used to measure fair value, which are as follows:
Level 1 | - | Quoted prices in active markets for identical assets or liabilities; |
Level 2 | - | Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and |
Level 3 | - | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
PMI's policy is to reflect transfers between hierarchy levels at the end of the reporting period.
Derivative Financial Instruments
PMI assesses the fair value of its foreign exchange contracts and interest rate contracts using standard valuation models that use, as their basis, readily observable market inputs. The fair value of PMI's foreign exchange forward contracts is determined by using the prevailing foreign exchange spot rates and interest rate differentials and the respective maturity dates of the instruments. The fair value of PMI's currency options is determined by using a Black-Scholes methodology based on foreign exchange spot rates and interest rate differentials, currency volatilities and maturity dates. PMI's derivative financial instruments have been classified within Level 2 at December 31, 2015 and 2014 . See Note 15. Financial Instruments for additional discussion of derivative financial instruments.
Pension Plan Assets
The fair value of pension plan assets, determined by using readily available quoted market prices in active markets, has been classified within Level 1 of the fair value hierarchy at December 31, 2015 and 2014 . The fair value of pension plan assets determined by using quoted prices in markets that are not active has been classified within Level 2 at December 31, 2015 and 2014 . See Note 13. Benefit Plans for additional discussion of pension plan assets.
Debt
The fair value of PMI's outstanding debt, which is utilized solely for disclosure purposes, is determined using quotes and market interest rates currently available to PMI for issuances of debt with similar terms and remaining maturities. The aggregate carrying value of PMI's debt, excluding short-term borrowings and $13 million of capital lease obligations, was $27,642 million at December 31, 2015 . The aggregate carrying value of PMI's debt, excluding short-term borrowings and $14 million of capital lease obligations, was $28,233 million at December 31, 2014 . The fair value of PMI's outstanding debt, excluding the aforementioned short-term borrowings and capital lease obligations, was classified within Level 1 and Level 2 at December 31, 2015 and 2014.
Contingent Consideration
The fair value of PMI's contingent consideration relating to acquisitions is determined utilizing a discounted cash flow approach using various probability-weighted scenarios. The significant unobservable inputs used in calculating the fair value of the contingent consideration includes financial performance scenarios, the probability of achieving those scenarios and the discount rate. PMI's contingent consideration has been classified within Level 3 in the table shown below. For additional information, see Note 6. Acquisitions and Other Business Arrangements .
116
The aggregate fair values of PMI's derivative financial instruments, pension plan assets, debt and contingent consideration as of December 31, 2015 and 2014 , were as follows:
(in millions) | Fair Value At |
| Quoted Prices in Active Markets for |
| Significant Other |
| Significant Unobservable Inputs | ||||||||
Assets: |
|
|
|
|
|
|
| ||||||||
Foreign exchange contracts | $ | 574 | |
| $ | - | |
| $ | 574 | |
| $ | - | |
Pension plan assets | 6,404 | |
| 4,513 | |
| 1,891 | |
| - | | ||||
Total assets | $ | 6,978 | |
| $ | 4,513 | |
| $ | 2,465 | |
| $ | - | |
Liabilities: |
|
|
|
|
|
|
| ||||||||
Debt | $ | 29,287 | |
| $ | 28,822 | |
| $ | 465 | |
| $ | - | |
Foreign exchange contracts | 172 | |
| - | |
| 172 | |
| - | | ||||
Total liabilities | $ | 29,459 | |
| $ | 28,822 | |
| $ | 637 | |
| $ | - | |
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
|
|
|
| ||||||||
|
|
|
|
| |||||||||||
(in millions) | Fair Value At |
| Quoted Prices in Active Markets for |
| Significant Other |
| Significant Unobservable Inputs | ||||||||
Assets: |
|
|
|
|
|
|
| ||||||||
Foreign exchange contracts | $ | 406 | |
| $ | - | |
| $ | 406 | |
| $ | - | |
Pension plan assets | 6,722 | |
| 4,853 | |
| 1,869 | |
| - | | ||||
Total assets | $ | 7,128 | |
| $ | 4,853 | |
| $ | 2,275 | |
| $ | - | |
Liabilities: |
|
|
|
|
|
|
| ||||||||
Debt | $ | 30,582 | |
| $ | 30,405 | |
| $ | 177 | |
| $ | - | |
Foreign exchange contracts | 151 | |
| - | |
| 151 | |
| - | | ||||
Contingent consideration | 22 | |
| - | |
| - | |
| 22 | | ||||
Total liabilities | $ | 30,755 | |
| $ | 30,405 | |
| $ | 328 | |
| $ | 22 | |
| |||||||||||||||
117
Note 17. | ||||
Accumulated Other Comprehensive Losses:
PMI's accumulated other comprehensive losses, net of taxes, consisted of the following:
(Losses) Earnings | At December 31, | ||||||||||
(in millions) | 2015 |
| 2014 |
| 2013 | ||||||
Currency translation adjustments | $ | (6,129 | ) |
| $ | (3,929 | ) |
| $ | (2,207 | ) |
Pension and other benefits | (3,332 | ) |
| (3,020 | ) |
| (2,046 | ) | |||
Derivatives accounted for as hedges | 59 | |
| 123 | |
| 63 | | |||
Total accumulated other comprehensive losses | $ | (9,402 | ) |
| $ | (6,826 | ) |
| $ | (4,190 | ) |
Reclassifications from Other Comprehensive Earnings
The movements in accumulated other comprehensive losses and the related tax impact, for each of the components above, that are due to current period activity and reclassifications to the income statement are shown on the consolidated statements of comprehensive earnings for the years ended December 31, 2015 , 2014 , and 2013 . The movement in currency translation adjustments for the year ended December 31, 2013, was also impacted by the purchase of the remaining shares of the Mexican tobacco business. In addition, $1 million , $5 million and $12 million of net currency translation adjustment gains were transferred from other comprehensive earnings to marketing, administration and research costs in the consolidated statements of earnings for the years ended December 31, 2015, 2014 and 2013, respectively, upon liquidation of subsidiaries. For additional information, see Note 13. Benefit Plans and Note 15. Financial Instruments for disclosures related to PMI's pension and other benefits and derivative financial instruments.
Note 18. | ||||
Colombian Investment and Cooperation Agreement:
On June 19, 2009, PMI announced that it had signed an agreement with the Republic of Colombia, together with the Departments of Colombia and the Capital District of Bogota, to promote investment and cooperation with respect to the Colombian tobacco market and to fight counterfeit and contraband tobacco products. The Investment and Cooperation Agreement provides $200 million in funding to the Colombian governments over a 20 -year period to address issues of mutual interest, such as combating the illegal cigarette trade, including the threat of counterfeit tobacco products, and increasing the quality and quantity of locally grown tobacco. As a result of the Investment and Cooperation Agreement, PMI recorded a pre-tax charge of $135 million in the operating results of the Latin America & Canada segment during the second quarter of 2009.
At December 31, 2015 and 2014 , PMI had $73 million and $71 million , respectively, of discounted liabilities associated with the Colombian Investment and Cooperation Agreement. These discounted liabilities are primarily reflected in other long-term liabilities on the consolidated balance sheets and are expected to be paid through 2028 .
Note 19. | ||||
RBH Legal Settlement:
On July 31, 2008 , Rothmans Inc. ("Rothmans") announced the finalization of a CAD 550 million settlement (or approximately $540 million , based on the prevailing exchange rate at that time) between itself and Rothmans, Benson & Hedges Inc. ("RBH"), on the one hand, and the Government of Canada and all 10 provinces, on the other hand. The settlement resolved the Royal Canadian Mounted Police's investigation relating to products exported from Canada by RBH during the 1989-1996 period. Rothmans' sole holding was a 60% interest in RBH. The remaining 40% interest in RBH was owned by PMI.
118
Subsequent to the finalization of the settlement, PMI announced that it had entered into an agreement with Rothmans to purchase, by way of a tender offer, all of the outstanding common shares of Rothmans. In October 2008, PMI completed the acquisition of all of Rothmans shares.
At December 31, 2015 and 2014 , PMI had $72 million and $114 million , respectively, of discounted accrued settlement charges associated with the RBH legal settlement. These accrued settlement charges are primarily reflected in other long-term liabilities on the consolidated balance sheets and are expected to be paid through 2019 .
Note 20. | ||||
E.C. Agreement:
In 2004, PMI entered into an agreement with the European Commission ("E.C.") and 10 Member States of the European Union that provides for broad cooperation with European law enforcement agencies on anti-contraband and anti-counterfeit efforts. This agreement has been signed by all 27 Member States. The agreement resolves all disputes between the parties relating to these issues. Under the terms of the agreement, PMI will make 13 payments over 12 years, including an initial payment of $250 million , which was recorded as a pre-tax charge against its earnings in 2004. The agreement calls for additional payments of approximately $150 million on the first anniversary of the agreement (this payment was made in July 2005), approximately $100 million on the second anniversary (this payment was made in July 2006) and approximately $75 million each year thereafter for 10 years, each of which is to be adjusted based on certain variables, including PMI's market share in the European Union in the year preceding payment. Because future additional payments are subject to these variables, PMI records charges for them as an expense in cost of sales when product is shipped. In addition, PMI was also responsible to pay the excise taxes, VAT and customs duties on qualifying product seizures of up to 90 million cigarettes and is subject to payments of five times the applicable taxes and duties if qualifying product seizures exceed 90 million cigarettes in a given year. In October 2014, this agreement was amended, and the threshold was increased to 450 million cigarettes in a given year. This modification was effective as of July 2012. To date, PMI's annual payments related to product seizures have been immaterial. Total charges related to the E.C. Agreement of $79 million , $71 million and $81 million were recorded in cost of sales in 2015 , 2014 and 2013 , respectively.
Note 21. | ||||
Contingencies:
Tobacco-Related Litigation
Legal proceedings covering a wide range of matters are pending or threatened against us, and/or our subsidiaries, and/or our indemnitees in various jurisdictions. Our indemnitees include distributors, licensees and others that have been named as parties in certain cases and that we have agreed to defend, as well as to pay costs and some or all of judgments, if any, that may be entered against them. Pursuant to the terms of the Distribution Agreement between Altria Group, Inc. ("Altria") and PMI, PMI will indemnify Altria and Philip Morris USA Inc. ("PM USA"), a U.S. tobacco subsidiary of Altria, for tobacco product claims based in substantial part on products manufactured by PMI or contract manufactured for PMI by PM USA, and PM USA will indemnify PMI for tobacco product claims based in substantial part on products manufactured by PM USA, excluding tobacco products contract manufactured for PMI.
It is possible that there could be adverse developments in pending cases against us and our subsidiaries. An unfavorable outcome or settlement of pending tobacco-related litigation could encourage the commencement of additional litigation.
Damages claimed in some of the tobacco-related litigation are significant and, in certain cases in Brazil, Canada and Nigeria, range into the billions of U.S. dollars. The variability in pleadings in multiple jurisdictions, together with the actual experience of management in litigating claims, demonstrate that the monetary relief that may be specified in a lawsuit bears little relevance to the ultimate outcome. Much of the tobacco-related litigation is in its early stages, and litigation is subject to uncertainty. However, as discussed below, we have to date been largely successful in defending tobacco-related litigation.
We and our subsidiaries record provisions in the consolidated financial statements for pending litigation when we determine that an unfavorable outcome is probable and the amount of the loss can be reasonably estimated. At the present time, while it is reasonably possible that an unfavorable outcome in a case may occur, after assessing the information available to it (i) management has not concluded that it is probable that a loss has been incurred in any of the pending tobacco-related cases; (ii) management is unable to estimate the
119
possible loss or range of loss for any of the pending tobacco-related cases; and (iii) accordingly, no estimated loss has been accrued in the consolidated financial statements for unfavorable outcomes in these cases, if any. Legal defense costs are expensed as incurred.
It is possible that our consolidated results of operations, cash flows or financial position could be materially affected in a particular fiscal quarter or fiscal year by an unfavorable outcome or settlement of certain pending litigation. Nevertheless, although litigation is subject to uncertainty, we and each of our subsidiaries named as a defendant believe, and each has been so advised by counsel handling the respective cases, that we have valid defenses to the litigation pending against us, as well as valid bases for appeal of adverse verdicts. All such cases are, and will continue to be, vigorously defended. However, we and our subsidiaries may enter into settlement discussions in particular cases if we believe it is in our best interests to do so.
To date, we have paid one judgment in a tobacco-related case. That judgment, including costs, was approximately €1,400 (approximately $1,500 ), and that payment was made in order to appeal an Italian small claims case, which was subsequently reversed on appeal. To date, no tobacco-related case has been finally resolved in favor of a plaintiff against us, our subsidiaries or indemnitees.
The table below lists the number of tobacco-related cases pending against us and/or our subsidiaries or indemnitees as of December 31, 2015 , December 31, 2014 and December 31, 2013 :
Type of Case |
| Number of Cases Pending as of December 31, 2015 |
| Number of Cases Pending as of |
| Number of Cases Pending as of | |||
Individual Smoking and Health Cases |
| 68 | |
| 63 | |
| 62 | |
Smoking and Health Class Actions |
| 11 | |
| 11 | |
| 11 | |
Health Care Cost Recovery Actions |
| 16 | |
| 15 | |
| 15 | |
Lights Class Actions |
| - | |
| - | |
| 1 | |
Individual Lights Cases |
| 3 | |
| 2 | |
| 2 | |
Public Civil Actions |
| 3 | |
| 2 | |
| 3 | |
Since 1995, when the first tobacco-related litigation was filed against a PMI entity, 442 Smoking and Health, Lights, Health Care Cost Recovery, and Public Civil Actions in which we and/or one of our subsidiaries and/or indemnitees were a defendant have been terminated in our favor. Twelve cases have had decisions in favor of plaintiffs. Nine of these cases have subsequently reached final resolution in our favor and three remain on appeal.
120
The table below lists the verdict and significant post-trial developments in the three pending cases where a verdict was returned in favor of the plaintiff:
|
|
|
|
|
|
|
|
|
Date |
| Location of |
| Type of |
| Verdict |
| Post-Trial |
February 2004 |
| Brazil/The Smoker Health Defense Association |
| Class Action |
| The Civil Court of São Paulo found defendants liable without hearing evidence. In April 2004, the court awarded "moral damages" of R$1,000 (approximately $240) per smoker per full year of smoking plus interest at the rate of 1% per month, as of the date of the ruling. The court did not assess actual damages, which were to be assessed in a second phase of the case. The size of the class was not defined in the ruling. |
| Defendants appealed to the São Paulo Court of Appeals, which annulled the ruling in November 2008, finding that the trial court had inappropriately ruled without hearing evidence and returned the case to the trial court for further proceedings. In May 2011, the trial court dismissed the claim. Plaintiff appealed the decision. In February 2015, the appellate court unanimously dismissed plaintiff's appeal. In September 2015, plaintiff appealed to the Superior Court of Justice. In addition, the defendants filed a constitutional appeal to the Federal Supreme Tribunal on the basis that plaintiff did not have standing to bring the lawsuit. This appeal is still pending. |
Date |
| Location of |
| Type of |
| Verdict |
| Post-Trial |
May 27, 2015 |
| Canada/Cecilia Letourneau |
| Class Action |
| On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec, ruled in favor of the Letourneau class on liability and awarded a total of CAD 131 million (approximately $93.7 million) in punitive damages, allocating CAD 46 million (approximately $33 million) to our subsidiary. The trial court ordered defendants to pay the full punitive damage award into a trust within 60 days. The court did not order the payment of compensatory damages. |
| In June 2015, our subsidiary commenced the appellate process with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make the payment into a trust. In August 2015, plaintiffs filed a motion for security with the Court of Appeal covering both the Letourneau case and the Blais case described below. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling CAD 226 million (approximately $162 million) to cover both the Letourneau and Blais cases. A hearing for the merits appeal is scheduled in November 2016. (See below for further detail.) |
121
Date |
| Location of |
| Type of |
| Verdict |
| Post-Trial |
May 27, 2015 |
| Canada/Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais |
| Class Action |
| On May 27, 2015, the Superior Court of the District of Montreal, Province of Quebec ruled in favor of the Blais class on liability and found the class members' compensatory damages totaled approximately CAD 15.5 billion (approximately $11.1 billion), including pre-judgment interest. The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion including pre-judgment interest (approximately $2.2 billion)). The trial court awarded CAD 90,000 (approximately $64,000) in punitive damages, allocating CAD 30,000 (approximately $21,500) to our subsidiary. The trial court ordered defendants to pay CAD 1 billion (approximately $715 million) of the compensatory damage award, CAD 200 million (approximately $143 million) of which is our subsidiary's portion, into a trust within 60 days. |
| In June 2015, our subsidiary commenced the appellate process with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make the payment into a trust. In August 2015, plaintiffs filed a motion for security with the Court of Appeal. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling, together with the Letourneau case, CAD 226 million (approximately $162 million). A hearing for the merits appeal is scheduled in November 2016. (See below for further detail.) |
Pending claims related to tobacco products generally fall within the following categories:
Smoking and Health Litigation: These cases primarily allege personal injury and are brought by individual plaintiffs or on behalf of a class or purported class of individual plaintiffs. Plaintiffs' allegations of liability in these cases are based on various theories of recovery, including negligence, gross negligence, strict liability, fraud, misrepresentation, design defect, failure to warn, breach of express and implied warranties, violations of deceptive trade practice laws and consumer protection statutes. Plaintiffs in these cases seek various forms of relief, including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include licit activity, failure to state a claim, lack of defect, lack of proximate cause, assumption of the risk, contributory negligence, and statute of limitations.
As of December 31, 2015 , there were a number of smoking and health cases pending against us, our subsidiaries or indemnitees, as follows:
• | 68 cases brought by individual plaintiffs in Argentina ( 32 ), Brazil ( 21 ), Canada ( 2 ), Chile ( 8 ), Costa Rica ( 2 ), Italy ( 1 ), the Philippines ( 1 ) and Scotland ( 1 ), compared with 63 such cases on December 31, 2014 , and 62 cases on December 31, 2013 ; and |
• | 11 cases brought on behalf of classes of individual plaintiffs in Brazil ( 2 ) and Canada ( 9 ), compared with 11 such cases on December 31, 2014 and December 31, 2013 . |
In the first class action pending in Brazil, The Smoker Health Defense Association (ADESF) v. Souza Cruz, S.A. and Philip Morris Marketing, S.A., Nineteenth Lower Civil Court of the Central Courts of the Judiciary District of São Paulo, Brazil , filed July 25, 1995,
122
our subsidiary and another member of the industry are defendants. The plaintiff, a consumer organization, is seeking damages for all addicted smokers and former smokers, and injunctive relief. In 2004, the trial court found defendants liable without hearing evidence and awarded "moral damages" of R$1,000 (approximately $240 ) per smoker per full year of smoking plus interest at the rate of 1% per month, as of the date of the ruling. The court did not award actual damages, which were to be assessed in the second phase of the case. The size of the class was not estimated. Defendants appealed to the São Paulo Court of Appeals, which annulled the ruling in November 2008, finding that the trial court had inappropriately ruled without hearing evidence and returned the case to the trial court for further proceedings. In May 2011, the trial court dismissed the claim. Plaintiff appealed the decision. In February 2015, the appellate court unanimously dismissed plaintiff's appeal. In September 2015, plaintiff appealed to the Superior Court of Justice. In addition, the defendants filed a constitutional appeal to the Federal Supreme Tribunal on the basis that plaintiff did not have standing to bring the lawsuit. This appeal is still pending.
In the second class action pending in Brazil , Public Prosecutor of São Paulo v. Philip Morris Brasil Industria e Comercio Ltda., Civil Court of the City of São Paulo, Brazil, filed August 6, 2007, our subsidiary is a defendant. The plaintiff, the Public Prosecutor of the State of São Paulo, is seeking (i) damages on behalf of all smokers nationwide, former smokers, and their relatives; (ii) damages on behalf of people exposed to environmental tobacco smoke nationwide, and their relatives; and (iii) reimbursement of the health care costs allegedly incurred for the treatment of tobacco-related diseases by all Brazilian States and Municipalities, and the Federal District. In an interim ruling issued in December 2007, the trial court limited the scope of this claim to the State of São Paulo only. In December 2008, the Seventh Civil Court of São Paulo issued a decision declaring that it lacked jurisdiction because the case involved issues similar to the ADESF case discussed above and should be transferred to the Nineteenth Lower Civil Court in São Paulo where the ADESF case is pending. The court further stated that these cases should be consolidated for the purposes of judgment. In April 2010, the São Paulo Court of Appeals reversed the Seventh Civil Court's decision that consolidated the cases, finding that they are based on different legal claims and are progressing at different stages of proceedings. This case was returned to the Seventh Civil Court of São Paulo, and our subsidiary filed its closing arguments in December 2010. In March 2012, the trial court dismissed the case on the merits. In January 2014, the São Paulo Court of Appeals rejected plaintiff's appeal and affirmed the trial court decision. In July 2014, plaintiff appealed to the Superior Court of Justice.
In the first class action pending in Canada, Cecilia Letourneau v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI Macdonald Corp., Quebec Superior Court, Canada, filed in September 1998, our subsidiary and other Canadian manufacturers (Imperial Tobacco Canada Ltd. and JTI-MacDonald Corp.) are defendants. The plaintiff, an individual smoker, sought compensatory and punitive damages for each member of the class who is deemed addicted to smoking. The class was certified in 2005. Trial began in March 2012 and concluded in December 2014. The trial court issued its judgment on May 27, 2015. The trial court found our subsidiary and two other Canadian manufacturers liable and awarded a total of CAD 131 million (approximately $93.7 million ) in punitive damages, allocating CAD 46 million (approximately $33 million ) to our subsidiary. The trial court found that defendants violated the Civil Code of Quebec, the Quebec Charter of Human Rights and Freedoms, and the Quebec Consumer Protection Act by failing to warn adequately of the dangers of smoking. The trial court also found that defendants conspired to prevent consumers from learning the dangers of smoking. The trial court further held that these civil faults were a cause of the class members' addiction. The trial court rejected other grounds of fault advanced by the class, holding that: (i) the evidence was insufficient to show that defendants marketed to youth, (ii) defendants' advertising did not convey false information about the characteristics of cigarettes, and (iii) defendants did not commit a fault by using the descriptors light or mild for cigarettes with a lower tar delivery. The trial court estimated the size of the addiction class at 918,000 members but declined to award compensatory damages to the addiction class because the evidence did not establish the claims with sufficient accuracy. The trial court ordered defendants to pay the full punitive damage award into a trust within 60 days and found that a claims process to allocate the awarded damages to individual class members would be too expensive and difficult to administer. The trial court ordered a briefing on the proposed process for the distribution of sums remaining from the punitive damage award after payment of attorneys' fees and legal costs. In June 2015, our subsidiary commenced the appellate process by filing its inscription of appeal of the trial court's judgment with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust within 60 days notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make the payment into a trust within 60 days . In August 2015, plaintiffs filed a motion with the Court of Appeal seeking security in both the Letourneau case and the Blais case described below. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling CAD 226 million (approximately $162 million ), in the form of cash into a court trust or letters of credit, in six equal consecutive quarterly installments of approximately CAD 37.6 million (approximately $27 million ) beginning in December 2015 through March 2017. See the Blais description for further detail concerning the security order. The Court of Appeal has scheduled a hearing for the merits appeal in November 2016. Our subsidiary and PMI believe that the findings of liability and damages were incorrect and should ultimately be set aside on any one of many grounds, including the following: (i) holding that defendants violated Quebec law by failing to warn class members of the risks of smoking even after the court found that class members knew, or should have known, of the risks, (ii) finding that plaintiffs were not required to prove that defendants' alleged misconduct caused injury to each class member in direct contravention of binding precedent, (iii) creating a factual presumption, without any evidence from class members or otherwise, that defendants' alleged misconduct caused all smoking by all class members, (iv) holding that the addiction class members' claims for punitive damages were not time-barred even though the case was filed more than three years after a prominent addiction warning appeared on all packages, and (v) awarding punitive damages to punish defendants without proper consideration as to whether punitive damages were necessary to deter future misconduct.
123
In the second class action pending in Canada, Conseil Québécois Sur Le Tabac Et La Santé and Jean-Yves Blais v. Imperial Tobacco Ltd., Rothmans, Benson & Hedges Inc. and JTI Macdonald Corp., Quebec Superior Court, Canada , filed in November 1998, our subsidiary and other Canadian manufacturers (Imperial Tobacco Canada Ltd. and JTI-MacDonald Corp.) are defendants. The plaintiffs, an anti-smoking organization and an individual smoker, sought compensatory and punitive damages for each member of the class who allegedly suffers from certain smoking-related diseases. The class was certified in 2005. Trial began in March 2012 and concluded in December 2014. The trial court issued its judgment on May 27, 2015. The trial court found our subsidiary and two other Canadian manufacturers liable and found that the class members' compensatory damages totaled approximately CAD 15.5 billion , including pre-judgment interest (approximately $11.1 billion ). The trial court awarded compensatory damages on a joint and several liability basis, allocating 20% to our subsidiary (approximately CAD 3.1 billion , including pre-judgment interest (approximately $2.2 billion )). In addition, the trial court awarded CAD 90,000 (approximately $64,000 ) in punitive damages, allocating CAD 30,000 (approximately $21,500 ) to our subsidiary and found that defendants violated the Civil Code of Quebec, the Quebec Charter of Human Rights and Freedoms, and the Quebec Consumer Protection Act by failing to warn adequately of the dangers of smoking. The trial court also found that defendants conspired to prevent consumers from learning the dangers of smoking. The trial court further held that these civil faults were a cause of the class members' diseases. The trial court rejected other grounds of fault advanced by the class, holding that: (i) the evidence was insufficient to show that defendants marketed to youth, (ii) defendants' advertising did not convey false information about the characteristics of cigarettes, and (iii) defendants did not commit a fault by using the descriptors light or mild for cigarettes with a lower tar delivery. The trial court estimated the disease class at 99,957 members. The trial court ordered defendants to pay CAD 1 billion (approximately $715 million ) of the compensatory damage award into a trust within 60 days , CAD 200 million (approximately $143 million ) of which is our subsidiary's portion and ordered briefing on a proposed claims process for the distribution of damages to individual class members and for payment of attorneys' fees and legal costs. In June 2015, our subsidiary commenced the appellate process by filing its inscription of appeal of the trial court's judgment with the Court of Appeal of Quebec. Our subsidiary also filed a motion to cancel the trial court's order for payment into a trust within 60 days notwithstanding appeal. In July 2015, the Court of Appeal granted the motion to cancel and overturned the trial court's ruling that our subsidiary make an initial payment within 60 days . In August 2015, plaintiffs filed a motion with the Court of Appeal seeking an order that defendants place irrevocable letters of credit totaling CAD 5 billion (approximately $3.6 billion ) into trust, to secure the judgments in both the Letourneau and Blais cases. Plaintiffs subsequently withdrew their motion for security against JTI-MacDonald Corp. and proceeded only against our subsidiary and Imperial Tobacco Canada Ltd. In October 2015, the Court of Appeal granted the motion and ordered our subsidiary to furnish security totaling CAD 226 million (approximately $162 million ) to cover both the Letourneau and Blais cases. Such security may take the form of cash into a court trust or letters of credit, in six equal consecutive quarterly installments of approximately CAD 37.6 million (approximately $27 million ) beginning in December 2015 through March 2017. The Court of Appeal ordered Imperial Tobacco Canada Ltd. to furnish security totaling CAD 758 million (approximately $542 million ) in seven equal consecutive quarterly installments of approximately CAD 108 million (approximately $77 million ) beginning in December 2015 through June 2017. In December 2015, our subsidiary made its first quarterly installment of security for approximately CAD 37.6 million (approximately $27 million ) into a court trust. This payment is included in other assets on the consolidated balance sheets and in cash used in operating activities in the consolidated statements of cash flows. The Court of Appeal ordered that the security is payable upon a final judgment of the Court of Appeal affirming the trial court's judgment or upon further order of the Court of Appeal. The Court of Appeal has scheduled a hearing for the merits appeal in November 2016. Our subsidiary and PMI believe that the findings of liability and damages were incorrect and should ultimately be set aside on any one of many grounds, including the following: (i) holding that defendants violated Quebec law by failing to warn class members of the risks of smoking even after the court found that class members knew, or should have known, of the risks, (ii) finding that plaintiffs were not required to prove that defendants' alleged misconduct caused injury to each class member in direct contravention of binding precedent, (iii) creating a factual presumption, without any evidence from class members or otherwise, that defendants' alleged misconduct caused all smoking by all class members, (iv) relying on epidemiological evidence that did not meet recognized scientific standards, and (v) awarding punitive damages to punish defendants without proper consideration as to whether punitive damages were necessary to deter future misconduct.
In the third class action pending in Canada, Kunta v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Winnipeg, Canada , filed June 12, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic obstructive pulmonary disease ("COPD"), severe asthma, and mild reversible lung disease resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. In September 2009, plaintiff's counsel informed defendants that he did not anticipate taking any action in this case while he pursues the class action filed in Saskatchewan (see description of Adams , below).
In the fourth class action pending in Canada, Adams v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Saskatchewan, Canada , filed July 10, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, emphysema, heart disease, or cancer, as well as restitution of profits. Preliminary motions are pending.
124
In the fifth class action pending in Canada, Semple v. Canadian Tobacco Manufacturers' Council, et al., The Supreme Court (trial court), Nova Scotia, Canada , filed June 18, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and COPD resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, as well as restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. No activity in this case is anticipated while plaintiff's counsel pursues the class action filed in Saskatchewan (see description of Adams , above).
In the sixth class action pending in Canada, Dorion v. Canadian Tobacco Manufacturers' Council, et al., The Queen's Bench, Alberta, Canada, filed June 15, 2009, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and chronic bronchitis and severe sinus infections resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers, their estates, dependents and family members, restitution of profits, and reimbursement of government health care costs allegedly caused by tobacco products. To date, we, our subsidiaries, and our indemnitees have not been properly served with the complaint. No activity in this case is anticipated while plaintiff's counsel pursues the class action filed in Saskatchewan (see description of Adams , above).
In the seventh class action pending in Canada, McDermid v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada , filed June 25, 2010, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges his own addiction to tobacco products and heart disease resulting from the use of tobacco products. He is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from heart disease allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed.
In the eighth class action pending in Canada, Bourassa v. Imperial Tobacco Canada Limited, et al., Supreme Court, British Columbia, Canada , filed June 25, 2010, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, the heir to a deceased smoker, alleges that the decedent was addicted to tobacco products and suffered from emphysema resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who were alive on June 12, 2007, and who suffered from chronic respiratory diseases allegedly caused by smoking, their estates, dependents and family members, plus disgorgement of revenues earned by the defendants from January 1, 1954, to the date the claim was filed. In December 2014, the plaintiff filed an amended statement of claim.
In the ninth class action pending in Canada, Suzanne Jacklin v. Canadian Tobacco Manufacturers' Council, et al., Ontario Superior Court of Justice, filed June 20, 2012, we, our subsidiaries, and our indemnitees (PM USA and Altria), and other members of the industry are defendants. The plaintiff, an individual smoker, alleges her own addiction to tobacco products and COPD resulting from the use of tobacco products. She is seeking compensatory and punitive damages on behalf of a proposed class comprised of all smokers who have smoked a minimum of 25,000 cigarettes and have allegedly suffered, or suffer, from COPD, heart disease, or cancer, as well as restitution of profits. Plaintiff's counsel has indicated that he does not intend to take any action in this case in the near future.
Health Care Cost Recovery Litigation: These cases, brought by governmental and non-governmental plaintiffs, seek reimbursement of health care cost expenditures allegedly caused by tobacco products. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including unjust enrichment, negligence, negligent design, strict liability, breach of express and implied warranties, violation of a voluntary undertaking or special duty, fraud, negligent misrepresentation, conspiracy, public nuisance, defective product, failure to warn, sale of cigarettes to minors, and claims under statutes governing competition and deceptive trade practices. Plaintiffs in these cases seek various forms of relief including compensatory and other damages, and injunctive and equitable relief. Defenses raised in these cases include lack of proximate cause, remoteness of injury, failure to state a claim, adequate remedy at law, "unclean hands" (namely, that plaintiffs cannot obtain equitable relief because they participated in, and benefited from, the sale of cigarettes), and statute of limitations.
As of December 31, 2015 , there were 16 health care cost recovery cases pending against us, our subsidiaries or indemnitees in Canada ( 10 ), Korea ( 1 ) and Nigeria ( 5 ), compared with 15 such cases on December 31, 2014 and December 31, 2013 .
In the first health care cost recovery case pending in Canada, Her Majesty the Queen in Right of British Columbia v. Imperial Tobacco Limited, et al., Supreme Court, British Columbia, Vancouver Registry, Canada, filed January 24, 2001, we, our subsidiaries, our indemnitee (PM USA), and other members of the industry are defendants. The plaintiff, the government of the province of British Columbia, brought a claim based upon legislation enacted by the province authorizing the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, resulting from a "tobacco related wrong." The Supreme Court of Canada has held that the statute is constitutional. We and certain other non-Canadian defendants challenged the jurisdiction of the court. The court rejected the jurisdictional challenge. Pre-trial discovery is ongoing.
125
In the second health care cost recovery case filed in Canada, Her Majesty the Queen in Right of New Brunswick v. Rothmans Inc., et al., Court of Queen's Bench of New Brunswick, Trial Court, New Brunswick, Fredericton, Canada, filed March 13, 2008, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of New Brunswick based on legislation enacted in the province. This legislation is similar to the law introduced in British Columbia that authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Pre-trial discovery is ongoing.
In the third health care cost recovery case filed in Canada, Her Majesty the Queen in Right of Ontario v. Rothmans Inc., et al., Ontario Superior Court of Justice, Toronto, Canada , filed September 29, 2009, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Ontario based on legislation enacted in the province. This legislation is similar to the laws introduced in British Columbia and New Brunswick that authorize the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants are scheduled to file their defenses in April 2016.
In the fourth health care cost recovery case filed in Canada, Attorney General of Newfoundland and Labrador v. Rothmans Inc., et al., Supreme Court of Newfoundland and Labrador, St. Johns, Canada , filed February 8, 2011, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Newfoundland and Labrador based on legislation enacted in the province that is similar to the laws introduced in British Columbia, New Brunswick and Ontario. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Preliminary motions are pending.
In the fifth health care cost recovery case filed in Canada, Attorney General of Quebec v. Imperial Tobacco Limited, et al., Superior Court of Quebec, Canada , filed June 8, 2012, we, our subsidiary, our indemnitee (PM USA), and other members of the industry are defendants. The claim was filed by the government of the province of Quebec based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in December 2014 and July 2015. Pre-trial discovery is ongoing.
In the sixth health care cost recovery case filed in Canada, Her Majesty in Right of Alberta v. Altria Group, Inc., et al., Supreme Court of Queen's Bench Alberta, Canada , filed June 8, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Alberta based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants are scheduled to file their defenses in March 2016.
In the seventh health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Manitoba v. Rothmans, Benson & Hedges, Inc., et al., The Queen's Bench, Winnipeg Judicial Centre, Canada , filed May 31, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Manitoba based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in September 2014. Discovery is scheduled to begin in 2017.
In the eighth health care cost recovery case filed in Canada, The Government of Saskatchewan v. Rothmans, Benson & Hedges Inc., et al., Queen's Bench, Judicial Centre of Saskatchewan, Canada , filed June 8, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Saskatchewan based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in February 2015. Discovery is scheduled to begin in 2017.
In the ninth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Prince Edward Island v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Prince Edward Island (General Section), Canada , filed September 10, 2012, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Prince Edward Island based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in February 2015. Discovery is scheduled to begin in 2017.
126
In the tenth health care cost recovery case filed in Canada, Her Majesty the Queen in Right of the Province of Nova Scotia v. Rothmans, Benson & Hedges Inc., et al., Supreme Court of Nova Scotia, Canada , filed January 2, 2015, we, our subsidiaries, our indemnitees (PM USA and Altria), and other members of the industry are defendants. The claim was filed by the government of the province of Nova Scotia based on legislation enacted in the province that is similar to the laws enacted in several other Canadian provinces. The legislation authorizes the government to file a direct action against cigarette manufacturers to recover the health care costs it has incurred, and will incur, as a result of a "tobacco related wrong." Defendants filed their defenses in July 2015. Discovery is scheduled to begin in 2017.
In the first health care cost recovery case in Nigeria, The Attorney General of Lagos State v. British American Tobacco (Nigeria) Limited, et al., High Court of Lagos State, Lagos, Nigeria, filed March 13, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years , payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years , various forms of injunctive relief, plus punitive damages. We are in the process of making challenges to service and the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the second health care cost recovery case in Nigeria, The Attorney General of Kano State v. British American Tobacco (Nigeria) Limited, et al., High Court of Kano State, Kano, Nigeria, filed May 9, 2007, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years , payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years , various forms of injunctive relief, plus punitive damages. We are in the process of making challenges to service and the court's jurisdiction. Currently, the case is stayed in the trial court pending the appeals of certain co-defendants relating to service objections.
In the third health care cost recovery case in Nigeria, The Attorney General of Gombe State v. British American Tobacco (Nigeria) Limited, et al., High Court of Gombe State, Gombe, Nigeria, filed October 17, 2008, we and other members of the industry are defendants . Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years , payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years , various forms of injunctive relief, plus punitive damages. In February 2011, the court ruled that the plaintiff had not complied with the procedural steps necessary to serve us. As a result of this ruling, plaintiff must re-serve its claim. We have not yet been re-served.
In the fourth health care cost recovery case in Nigeria, The Attorney General of Oyo State, et al., v. British American Tobacco (Nigeria) Limited, et al., High Court of Oyo State, Ibadan, Nigeria, filed May 25, 2007, we and other members of the industry are defendants . Plaintiffs seek reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years , payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years , various forms of injunctive relief, plus punitive damages. We challenged service as improper. In June 2010, the court ruled that plaintiffs did not have leave to serve the writ of summons on the defendants and that they must re-serve the writ. We have not yet been re-served.
In the fifth health care cost recovery case in Nigeria, The Attorney General of Ogun State v. British American Tobacco (Nigeria) Limited, et al., High Court of Ogun State, Abeokuta, Nigeria , filed February 26, 2008, we and other members of the industry are defendants. Plaintiff seeks reimbursement for the cost of treating alleged smoking-related diseases for the past 20 years , payment of anticipated costs of treating alleged smoking-related diseases for the next 20 years , various forms of injunctive relief, plus punitive damages. In May 2010, the trial court rejected our service objections. We have appealed.
In the health care cost recovery case in Korea, the National Health Insurance Service v. KT&G, et. al., filed April 14, 2014, our subsidiary and other Korean manufacturers are defendants. Plaintiff alleges that defendants concealed the health hazards of smoking, marketed to youth, added ingredients to make their products more harmful and addictive, and misled consumers into believing that Lights cigarettes are safer than regular cigarettes. The National Health Insurance Service seeks to recover approximately $53.7 million allegedly incurred in treating 3,484 patients with small cell lung cancer, squamous cell lung cancer, and squamous cell laryngeal cancer from 2003 to 2012. The case is now in the evidentiary phase.
Lights Cases: These cases, brought by individual plaintiffs, allege that the use of the term "lights" constitutes fraudulent and misleading conduct. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including misrepresentation, deception, and breach of consumer protection laws. Plaintiffs seek various forms of relief including restitution, injunctive relief, and compensatory and other damages. Defenses raised include lack of causation, lack of reliance, assumption of the risk, and statute of limitations.
As of December 31, 2015 , there were 3 lights cases brought by individual plaintiffs pending against our subsidiaries or indemnitees in Chile ( 2 ) and Italy ( 1 ), compared with 2 such cases on December 31, 2014 , and 2 such cases on December 31, 2013 .
Public Civil Actions: Claims have been filed either by an individual, or a public or private entity, seeking to protect collective or individual rights, such as the right to health, the right to information or the right to safety. Plaintiffs' allegations of liability in these cases are based on various theories of recovery including product defect, concealment, and misrepresentation. Plaintiffs in these cases seek various forms
127
of relief including injunctive relief such as banning cigarettes, descriptors, smoking in certain places and advertising, as well as implementing communication campaigns and reimbursement of medical expenses incurred by public or private institutions.
As of December 31, 2015 , there were 3 public civil actions pending against our subsidiaries in Argentina ( 1 ), Romania ( 1 ) and Venezuela ( 1 ), compared with 2 such cases on December 31, 2014 , and 3 such cases on December 31, 2013 .
In the public civil action in Argentina, Asociación Argentina de Derecho de Danos v. Massalin Particulares S.A., et al., Civil Court of Buenos Aires, Argentina, filed February 26, 2007, our subsidiary and another member of the industry are defendants. The plaintiff, a consumer association, seeks the establishment of a relief fund for reimbursement of medical costs associated with diseases allegedly caused by smoking. Our subsidiary filed its answer in September 2007. In March 2010, the case file was transferred to the Federal Court on Administrative Matters after the Civil Court granted the plaintiff's request to add the national government as a co-plaintiff in the case. The case is currently in the evidentiary stage.
In a newly filed actio n in Romania, Foundation for the Defense of Citizens against Abuses of the State (FACIAS) v. the State of Romania, Philip Morris România (PMR) and Philip Morris Trading SLR (PMTR), et al., Administrative and Fiscal Litigation Section of the Bucharest Tribunal , filed November 20, 2015, our subsidiaries, several other members of the industry, and the State of Romania through various of its institutions are defendants. The plaintiff, a non-governmental organization, asks the court to compel the government to enact legislation as directed by the 2014 EU Tobacco Product Directive and to establish a fund for the treatment of smoking-related diseases and promotion of tobacco control efforts. The plaintiff also seeks an order directing that 1% of the excise taxes collected from tobacco manufacturers, "as well as an amount representing 1% of the turnover" of tobacco manufacturers and distributors, be used to finance the fund. It is unclear whether the "1% of turnover" is sought from the tobacco company defendants or the government. Our subsidiaries answered the complaint in December 2015.
In the public civil action in Venezuela, Federation of Consumers and Users Associations ("FEVACU"), et al. v. National Assembly of Venezuela and the Venezuelan Ministry of Health, Constitutional Chamber of the Venezuelan Supreme Court , filed April 29, 2008, we were not named as a defendant, but the plaintiffs published a notice pursuant to court order, notifying all interested parties to appear in the case. In January 2009, our subsidiary appeared in the case in response to this notice. The plaintiffs purport to represent the right to health of the citizens of Venezuela and claim that the government failed to protect adequately its citizens' right to health. The claim asks the court to order the government to enact stricter regulations on the manufacture and sale of tobacco products. In addition, the plaintiffs ask the court to order companies involved in the tobacco industry to allocate a percentage of their "sales or benefits" to establish a fund to pay for the health care costs of treating smoking-related diseases. In October 2008, the court ruled that plaintiffs have standing to file the claim and that the claim meets the threshold admissibility requirements. In December 2012, the court admitted our subsidiary and BAT's subsidiary as interested third parties. In February 2013, our subsidiary answered the complaint.
Other Litigation
The Department of Special Investigations of the government of Thailand has been conducting an investigation into alleged underpayment by our subsidiary, Philip Morris (Thailand) Limited ("PM Thailand"), of customs duties and excise taxes relating to imports from the Philippines covering the period 2003-2007. On January 18, 2016, the Public Prosecutor filed charges against our subsidiary and seven former and current employees in the Bangkok Criminal Court alleging that PM Thailand and the individual defendants jointly and with the intention to defraud the Thai government, under declared import prices of cigarettes to avoid full payment of taxes and duties in connection with 272 import entries of cigarettes from the Philippines during the period of July 2003 to June 2006. The government is seeking a fine of approximately THB 80.8 billion (approximately $2.2 billion ). The first hearing, which will focus on preliminary procedural matters, is scheduled for April 2016. PM Thailand contends that its declared import prices are in compliance with the Customs Valuation Agreement of the World Trade Organization and Thai law and that the allegations of the Public Prosecutor are inconsistent with several decisions already taken by Thai Customs and other Thai governmental agencies.
We are also involved in additional litigation arising in the ordinary course of our business. While the outcomes of these proceedings are uncertain, management does not expect that the ultimate outcomes of other litigation, including any reasonably possible losses in excess of current accruals, will have a material adverse effect on our consolidated results of operations, cash flows or financial position.
128
Note 22. | |||||
Balance Sheet Offsetting:
Derivative Financial Instruments
PMI uses foreign exchange contracts and interest rate contracts to mitigate its exposure to changes in exchange and interest rates from third-party and intercompany actual and forecasted transactions. Substantially all of PMI's derivative financial instruments are subject to master netting arrangements, whereby the right to offset occurs in the event of default by a participating party. While these contracts contain the enforceable right to offset through close-out netting rights, PMI elects to present them on a gross basis in the consolidated balance sheets. Collateral associated with these arrangements is in the form of cash and is unrestricted. See Note 15. Financial Instruments for disclosures related to PMI's derivative financial instruments.
The effects of these derivative financial instrument assets and liabilities on PMI's consolidated balance sheets were as follows:
(in millions) | Gross Amounts Recognized | Gross Amount Offset in the Consolidated Balance Sheet | Net Amounts Presented in the Consolidated Balance Sheet | Gross Amounts Not Offset in the Consolidated Balance Sheet |
| |||||||||||||
Financial Instruments | Cash Collateral Received/Pledged |
| ||||||||||||||||
Net Amount | ||||||||||||||||||
|
|
|
|
|
|
| ||||||||||||
At December 31, 2015 |
|
|
|
|
|
| ||||||||||||
Assets |
|
|
|
|
|
| ||||||||||||
Foreign exchange contracts | $ | 574 | | $ | - | | $ | 574 | | $ | (131 | ) | $ | (432 | ) | $ | 11 | |
Liabilities |
|
|
|
|
|
| ||||||||||||
Foreign exchange contracts | $ | 172 | | $ | - | | $ | 172 | | $ | (131 | ) | $ | (30 | ) | $ | 11 | |
At December 31, 2014 |
|
|
|
|
|
| ||||||||||||
Assets |
|
|
|
|
|
| ||||||||||||
Foreign exchange contracts | $ | 406 | | $ | - | | $ | 406 | | $ | (77 | ) | $ | (306 | ) | $ | 23 | |
Liabilities |
|
|
|
|
|
| ||||||||||||
Foreign exchange contracts | $ | 151 | | $ | - | | $ | 151 | | $ | (77 | ) | $ | (63 | ) | $ | 11 | |
Note 23. | |||||
Sale of Accounts Receivable:
To mitigate credit risk and enhance cash and liquidity management PMI sells trade receivables to unaffiliated financial institutions. These arrangements allow PMI to sell, on an ongoing basis, certain trade receivables without recourse. The trade receivables sold are generally short-term in nature and are removed from the consolidated balance sheets. PMI sells trade receivables under two types of arrangements, servicing and non-servicing. For servicing arrangements, PMI continues to service the sold trade receivables on an administrative basis and does not act on behalf of the unaffiliated financial institutions. When applicable, a servicing liability is recorded for the estimated fair value of the servicing. The amounts associated with the servicing liability were not material for the years ended December 31, 2015 and 2014 . Under the non-servicing arrangements, PMI does not provide any administrative support or servicing after the trade receivables have been sold to the unaffiliated financial institutions.
Cumulative trade receivables sold for the years ended December 31, 2015 and 2014 , were $3,299 million and $1,569 million , respectively. PMI's operating cash flows were positively impacted by the amount of the trade receivables sold and derecognized from the consolidated balance sheets, which remained outstanding with the unaffiliated financial institutions. The trade receivables sold that remained outstanding under these arrangements as of December 31, 2015 , 2014 and 2013 , were $888 million , $120 million and $146 million , respectively. The net proceeds received are included in cash provided by operating activities in the consolidated statements of cash flows. The difference between the carrying amount of the trade receivables sold and the sum of the cash received is recorded as a loss on sale of trade receivables
129
within marketing, administration and research costs in the consolidated statements of earnings. For the years ended December 31, 2015 , 2014 and 2013 the loss on sale of trade receivables was immaterial.
Note 24. | |||||
Redeemable Noncontrolling Interest:
Philippines Business Combination:
On February 25, 2010 , PMI's affiliate, Philip Morris Philippines Manufacturing Inc. ("PMPMI"), and Fortune Tobacco Corporation ("FTC") combined their respective business activities by transferring selected assets and liabilities of PMPMI and FTC to a new company called PMFTC Inc. ("PMFTC"). PMPMI and FTC hold equal economic interests in PMFTC, while PMI manages the day-to-day operations of PMFTC and has a majority of its Board of Directors. Consequently, PMI accounted for the contributed assets and liabilities of FTC as a business combination.
The fair value of the assets and liabilities contributed by FTC in this non-cash transaction was determined to be $1.17 billion . At the time of the business combination, FTC was given the right to sell its interest in PMFTC to PMI, except in certain circumstances, during the period from February 25, 2015 , through February 24, 2018 , at an agreed-upon value of $1.17 billion , which was recorded on PMI's consolidated balance sheet as a redeemable noncontrolling interest at the date of the business combination. On December 10, 2013, FTC terminated the agreement related to this exit right. As a result, the amount included in the consolidated balance sheet as redeemable noncontrolling interest at that date was reclassified to noncontrolling interests within stockholders' deficit on the December 31, 2013, consolidated balance sheet.
The movement in redeemable noncontrolling interest during the year ended December 31, 2013, was as follows:
(in millions) |
| ||
Redeemable noncontrolling interest at January 1, 2013 | $ | 1,301 | |
Share of net earnings | 99 | | |
Dividend payments | (94 | ) | |
Currency translation losses | (33 | ) | |
Net loss and prior service cost | 2 | | |
Termination of rights agreement | (1,275 | ) | |
Redeemable noncontrolling interest at December 31, 2013 | $ | - | |
Note 25. | |||||
New Accounting Standards:
On January 5, 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standard Update ASU 2016-01, "Financial Instruments - Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities" ("ASU 2016-01"). ASU 2016-01 will require equity investments (except those accounted for under the equity method of accounting, or those that result in consolidation of the investee) to be measured at fair value with changes in fair value recognized in net income. Additionally, ASU 2016-01 also changes certain disclosure requirements and other aspects of current U.S. GAAP. ASU 2016-01 is effective for interim and annual reporting periods beginning on or after January 1, 2018. PMI is currently assessing the impact that the adoption of ASU 2016-01 will have on its financial position or results of operations.
On November 20, 2015, the FASB issued Accounting Standard Update ASU 2015-17, "Balance Sheet Classification of Deferred Taxes" ("ASU 2015-17"). ASU 2015-17 requires that all deferred tax assets and liabilities, along with any related valuation allowance, be classified as noncurrent on the balance sheet. ASU 2015-17 is effective for interim and annual reporting periods beginning on or after January 1, 2017. Early adoption is permitted; however, as of December 31, 2015, PMI has not elected to early adopt ASU 2015-17. Entities can apply the final standard either prospectively, for all deferred tax assets and liabilities, or retrospectively with disclosures providing qualitative information about the effects of the accounting change on prior periods. The adoption of ASU 2015-17 will not have a material impact on PMI's consolidated results of operations, financial position or cash flows.
130
On May 28, 2014, the FASB issued Accounting Standards Update ASU 2014-09, "Revenue from Contracts with Customers" ("ASU 2014-09"). ASU 2014-09 contains principles that an entity will need to apply to determine the measurement of revenue and timing of when it is recognized. The underlying principle is that an entity will recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services.
Entities can apply the final standard using one of the following two methods:
1. | retrospectively to each prior period presented; or |
2. | retrospectively, with the cumulative effect of initially applying ASU 2014-09 recognized at the date of initial application, with additional disclosures in reporting periods that include the date of initial application. |
ASU 2014-09 is effective for interim and annual reporting periods beginning on or after January 1, 2017. In July 2015, the FASB approved a proposal which allows for a deferral of the implementation until January 1, 2018, and permits early application, but not before the original effective date of January 1, 2017. PMI is currently assessing the impact that the adoption of ASU 2014-09 will have on its financial position or results of operations.
Note 26. | |||||||||
Quarterly Financial Data (Unaudited):
| 2015 Quarters | ||||||||||||||
(in millions, except per share data) | 1st |
| 2nd |
| 3rd |
| 4th | ||||||||
Net revenues | $ | 17,352 | |
| $ | 18,763 | |
| $ | 19,422 | |
| $ | 18,371 | |
Gross profit | $ | 4,387 | |
| $ | 4,481 | |
| $ | 4,544 | |
| $ | 4,017 | |
Net earnings attributable to PMI | $ | 1,795 | |
| $ | 1,887 | |
| $ | 1,942 | |
| $ | 1,249 | |
Per share data: |
|
|
|
|
|
|
| ||||||||
Basic EPS | $ | 1.16 | |
| $ | 1.21 | |
| $ | 1.25 | |
| $ | 0.80 | |
Diluted EPS | $ | 1.16 | |
| $ | 1.21 | |
| $ | 1.25 | |
| $ | 0.80 | |
Dividends declared | $ | 1.00 | |
| $ | 1.00 | |
| $ | 1.02 | |
| $ | 1.02 | |
Market price: |
|
|
|
|
|
|
| ||||||||
- High | $ | 85.29 | |
| $ | 86.91 | |
| $ | 86.51 | |
| $ | 90.27 | |
- Low | $ | 75.30 | |
| $ | 75.27 | |
| $ | 76.54 | |
| $ | 78.41 | |
|
| ||||||||||||||
| 2014 Quarters | ||||||||||||||
(in millions, except per share data) | 1st |
| 2nd |
| 3rd |
| 4th | ||||||||
Net revenues | $ | 17,779 | |
| $ | 21,051 | |
| $ | 21,335 | |
| $ | 19,941 | |
Gross profit | $ | 4,543 | |
| $ | 5,101 | |
| $ | 5,122 | |
| $ | 4,565 | |
Net earnings attributable to PMI | $ | 1,875 | |
| $ | 1,851 | |
| $ | 2,155 | |
| $ | 1,612 | |
Per share data: |
|
|
|
|
|
|
| ||||||||
Basic EPS | $ | 1.18 | |
| $ | 1.17 | |
| $ | 1.38 | |
| $ | 1.03 | |
Diluted EPS | $ | 1.18 | |
| $ | 1.17 | |
| $ | 1.38 | |
| $ | 1.03 | |
Dividends declared | $ | 0.94 | |
| $ | 0.94 | |
| $ | 1.00 | |
| $ | 1.00 | |
Market price: |
|
|
|
|
|
|
| ||||||||
- High | $ | 87.20 | |
| $ | 91.63 | |
| $ | 86.85 | |
| $ | 90.25 | |
- Low | $ | 75.28 | |
| $ | 81.70 | |
| $ | 81.19 | |
| $ | 81.16 | |
|
|
|
|
|
|
|
| ||||||||
Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
131
During 2015 and 2014 , PMI recorded the following pre-tax charges in earnings:
| 2015 Quarters | ||||||||||||||
(in millions) | 1st |
| 2nd |
| 3rd |
| 4th | ||||||||
Asset impairment and exit costs | $ | - | |
| $ | - | |
| $ | - | |
| $ | 68 | |
|
| ||||||||||||||
| 2014 Quarters | ||||||||||||||
(in millions) | 1st |
| 2nd |
| 3rd |
| 4th | ||||||||
Asset impairment and exit costs | $ | 23 | |
| $ | 489 | |
| $ | (9 | ) |
| $ | 32 | |
|
|
|
|
|
|
|
| ||||||||
See Note 5. Asset Impairment and Exit Costs for additional information on these pre-tax charges.
132
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Philip Morris International Inc. and Subsidiaries:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of earnings, comprehensive earnings, stockholders' (deficit) equity, and cash flows, present fairly, in all material respects, the financial position of Philip Morris International Inc. and its subsidiaries ("PMI") at December 31, 2015 and 2014 , and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2015 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, PMI maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015 , based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). PMI's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Report of Management on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements and on PMI's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
PricewaterhouseCoopers SA
/ S / BARRY J. MISTHAL |
| /S/ DR. MICHAEL ABRESCH |
Barry J. Misthal |
| Dr. Michael Abresch |
|
|
|
Lausanne, Switzerland |
|
|
February 4, 2016 |
|
|
133
Report of Management on Internal Control Over Financial Reporting
Management of Philip Morris International Inc. ("PMI") is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. PMI's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes those written policies and procedures that:
• | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of PMI; |
• | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America; |
• | provide reasonable assurance that receipts and expenditures of PMI are being made only in accordance with the authorization of management and directors of PMI; and |
• | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements. |
Internal control over financial reporting includes the controls themselves, monitoring and internal auditing practices and actions taken to correct deficiencies as identified.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of PMI's internal control over financial reporting as of December 31, 2015 . Management based this assessment on criteria for effective internal control over financial reporting described in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management's assessment included an evaluation of the design of PMI's internal control over financial reporting and testing of the operational effectiveness of its internal control over financial reporting. Management reviewed the results of its assessment with the Audit Committee of our Board of Directors.
Based on this assessment, management determined that, as of December 31, 2015 , PMI maintained effective internal control over financial reporting.
PricewaterhouseCoopers SA, an independent registered public accounting firm, who audited and reported on the consolidated financial statements of PMI included in this report, has audited the effectiveness of PMI's internal control over financial reporting as of December 31, 2015 , as stated in their report herein.
February 4, 2016
134
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
Item 9A. | Controls and Procedures. |
PMI carried out an evaluation, with the participation of PMI's management, including PMI's Chief Executive Officer and Chief Financial Officer, of the effectiveness of PMI's disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this report. Based upon that evaluation, PMI's Chief Executive Officer and Chief Financial Officer concluded that PMI's disclosure controls and procedures are effective. There have been no changes in PMI's internal control over financial reporting during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, PMI's internal control over financial reporting.
The Report of Management on Internal Control over Financial Reporting and the Report of Independent Registered Public Accounting Firm are included in Item 8.
Item 9B. | Other Information. |
None
PART III
Except for the information relating to the executive officers set forth in Item 10 and the information relating to equity compensation plans set forth in Item 12, the information called for by Items 10-14 is hereby incorporated by reference to PMI's definitive proxy statement for use in connection with its annual meeting of stockholders to be held on May 4, 2016 , that will be filed with the SEC on or about March 24, 2016 (the "proxy statement"), and, except as indicated therein, made a part hereof.
Item 10. | Directors, Executive Officers and Corporate Governance . |
Name |
| Office |
| Age |
| |
André Calantzopoulos |
| Chief Executive Officer |
| 58 | |
|
Drago Azinovic |
| President, Eastern Europe, Middle East & Africa Region & PMI Duty Free |
| 53 | |
|
Werner Barth |
| Senior Vice President, Marketing & Sales |
| 51 | |
|
Patrick Brunel |
| Senior Vice President and Chief Information Officer |
| 50 | |
|
Frederic de Wilde |
| President, European Union Region |
| 48 | |
|
Marc S. Firestone |
| Senior Vice President and General Counsel |
| 56 | |
|
Martin King |
| President, Asia Region |
| 51 | |
|
Andreas Kurali |
| Vice President and Controller |
| 50 | |
|
Peter J. Luongo |
| Vice President, Treasury and Planning |
| 37 | |
|
Marco Mariotti |
| Senior Vice President, Corporate Affairs |
| 51 | |
|
Antonio Marques |
| Senior Vice President, Operations |
| 60 | |
|
James R. Mortensen |
| Senior Vice President, Human Resources |
| 58 | |
|
Jacek Olczak |
| Chief Financial Officer |
| 51 | |
|
Jeanne Pollès |
| President, Latin America & Canada Region |
| 51 | |
|
Jerry E. Whitson |
| Deputy General Counsel and Corporate Secretary |
| 60 | |
|
Miroslaw Zielinski |
| President, Reduced-Risk Products |
| 54 | |
|
All of the above-mentioned officers, except for Messrs. Firestone and Luongo, have been employed by us in various capacities during the past five years.
Before joining Philip Morris International Inc. in April 2012, Mr. Firestone was Executive Vice President, Corporate and Legal Affairs and General Counsel of Kraft Foods Inc., where he served since 2003. From 1988 to 2003, Mr. Firestone held numerous positions in the
135
law departments of Philip Morris Companies Inc. and Philip Morris International Inc., lastly as Senior Vice President & General Counsel of PMI.
Before joining Philip Morris International Inc. in June 2013, Mr. Luongo was a partner at the investment banking firm of Centerview Partners LLC, where he had served since 2004.
Codes of Conduct and Corporate Governance
We have adopted the Philip Morris International Code of Conduct, which complies with requirements set forth in Item 406 of Regulation S-K. This Code of Conduct applies to all of our employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, and persons performing similar functions. We have also adopted a code of business conduct and ethics that applies to the members of our Board of Directors. These documents are available free of charge on our Web site at www.pmi.com.
In addition, we have adopted corporate governance guidelines and charters for our Audit, Finance, Compensation and Leadership Development, Product Innovation and Regulatory Affairs and Nominating and Corporate Governance committees of the Board of Directors. All of these documents are available free of charge on our Web site at www.pmi.com. Any waiver granted by Philip Morris International Inc. to its principal executive officer, principal financial officer or controller or any person performing similar functions under the Code of Conduct, or certain amendments to the Code of Conduct, will be disclosed on our Web site at www.pmi.com.
The information on our Web site is not, and shall not be deemed to be, a part of this Report or incorporated into any other filings made with the SEC.
Also refer to Board Operations and Governance - Committees of the Board , Election of Directors - Process for Nominating Directors and Election of Directors - Director Nominees .
Item 11. | Executive Compensation. |
Refer to Compensation Discussion and Analysis and Compensation of Directors sections of the proxy statement.
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The number of shares to be issued upon exercise or vesting and the number of shares remaining available for future issuance under PMI's equity compensation plans at December 31, 2015 , were as follows:
| Number of Shares to be Issued upon Exercise of Outstanding Options and Vesting of Deferred Stock (a) |
| Weighted Average Exercise Price of Outstanding Options (b) |
| Number of Shares Remaining Available for Future Issuance Under Equity Compensation Plans (excluding Securities reflected in column (a)) (c) | ||||
Equity compensation plans approved by stockholders | 5,702,000 | | (1) | $ | - | |
| 23,940,862 | |
(1) Represents shares of deferred stock.
Refer to Ownership of Equity Securities section of the proxy statement.
Item 13. | Certain Relationships and Related Transactions, and Director Independence. |
Refer to Related Person Transactions and Code of Conduct and Independence of Nominees sections of the proxy statement.
Item 14. | Principal Accounting Fees and Services . |
Refer to Audit Committee Matters section of the proxy statement.
136
PART IV
Item 15. | Exhibits and Financial Statement Schedules . |
(a) Index to Consolidated Financial Statements and Schedules
| Page |
Consolidated Balance Sheets at December 31, 2015 and 2014 | 81 - 82 |
Consolidated Statements of Earnings for the years ended December 31, 2015, 2014 and 2013 | 83 |
Consolidated Statements of Comprehensive Earnings for the years ended December 31, 2015, 2014 and 2013 | 84 |
Consolidated Statements of Stockholders' (Deficit) Equity for the years ended December 31, 2015, 2014 and 2013 | 85 |
Consolidated Statements of Cash Flows for the years ended December 31, 2015, 2014 and 2013 | 86 - 87 |
Notes to Consolidated Financial Statements | 88 - 132 |
Report of Independent Registered Public Accounting Firm | 133 |
Report of Management on Internal Control Over Financial Reporting | 134 |
Schedules have been omitted either because such schedules are not required or are not applicable.
(b) The following exhibits are filed as part of this Report:
2.1 |
| - |
| Distribution Agreement between Altria Group, Inc. and Philip Morris International Inc. dated January 30, 2008 (incorporated by reference to Exhibit 2.1 to the Registration Statement on Form 10 filed February 7, 2008). |
3.1 |
| - |
| Amended and Restated Articles of Incorporation of Philip Morris International Inc. (incorporated by reference to Exhibit 3.1 to the Registration Statement on Form 10 filed February 7, 2008). |
3.2 |
| - |
| Amended and Restated By-laws of Philip Morris International Inc. (incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K filed September 18, 2015). |
4.1 |
| - |
| Specimen Stock Certificate of Philip Morris International Inc. (incorporated by reference to Exhibit 4.1 to the Registration Statement on Form 10 filed February 7, 2008). |
4.2 |
| - |
| Indenture dated as of April 25, 2008, between Philip Morris International Inc. and HSBC Bank USA, National Association, as Trustee (incorporated by reference to Exhibit 4.3 to the Registration Statement on Form S-3, dated April 25, 2008). |
4.3 |
| - |
| Issue and Paying Agency Agreement, dated March 13, 2009, by and among Philip Morris International Inc., HSBC Private Bank (C.I.) Limited, Jersey Branch, as registrar, HSBC Bank PLC, as principal paying agent and HSBC Corporate Trustee Company (UK) Limited, as trustee (incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K filed March 19, 2009). |
4.4 |
| - |
| Trust Deed relating to Euro Medium Term Note Programme, dated March 13, 2009, between Philip Morris International Inc., as issuer, and HSBC Corporate Trustee Company (UK) Limited, as trustee (incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K filed March 19, 2009). |
|
|
|
|
|
4.5 |
| - |
| The Registrant agrees to furnish copies of any instruments defining the rights of holders of long-term debt of the Registrant and its consolidated subsidiaries that does not exceed 10 percent of the total assets of the Registrant and its consolidated subsidiaries to the Commission upon request. |
10.1 |
| - |
| Tax Sharing Agreement between Altria Group, Inc. and Philip Morris International Inc., dated as of March 28, 2008 (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed March 31, 2008). |
137
10.2 |
| - |
| Employee Matters Agreement between Altria Group, Inc. and Philip Morris International Inc., dated as of March 28, 2008 (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed March 31, 2008). |
10.3 |
| - |
| Intellectual Property Agreement between Philip Morris International Inc. and Philip Morris USA Inc., dated as of January 1, 2008 (incorporated by reference to Exhibit 10.4 to the Registration Statement on Form 10 filed March 5, 2008). |
10.4 |
| - |
| Credit Agreement relating to a US$3,500,000,000 Revolving Credit Facility (including a US$800,000,000 swingline option) dated as of October 25, 2011, among Philip Morris International Inc. and the Initial Lenders named therein and Citibank International plc, as Facility Agent, and Citibank, N.A., as Swingline Agent, and Citigroup Global Markets Limited, Barclays Capital, BNP Paribas, Credit Suisse AG, Cayman Islands Branch, Deutsche Bank Securities Inc., Goldman Sachs International, HSBC Bank PLC, J.P. Morgan Limited, RBS Securities Inc. and Société Générale as Mandated Lead Arrangers and Bookrunners (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed October 26, 2011). |
10.5 |
| __ |
| Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein and Citibank Europe PLC, UK Branch (formerly, The Royal Bank of Scotland plc), as Administrative Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 15, 2013). |
10.6 |
| __ |
| Extension Agreement, effective as of February 10, 2015, to Credit Agreement dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein and Citibank Europe PLC, UK Branch (formerly, The Royal Bank of Scotland plc), as Administrative Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed January 29, 2015). |
10.7 |
| __ |
| Credit Agreement, dated as of February 28, 2014, among Philip Morris International Inc., the lenders named therein, J.P. Morgan Europe Limited, as Facility Agent, and JPMorgan Chase Bank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed March 3, 2014). |
10.8 |
| __ |
| Extension Agreement, effective as of February 28, 2015, to the Credit Agreement, dated as of February 28, 2014, among Philip Morris International Inc., the lenders named therein, J.P. Morgan Europe Limited, as Facility Agent, and JPMorgan Chase Bank, N.A. as Swingline Agent (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed January 29, 2015). |
10.9 |
| __ |
| Extension Agreement, dated as of January 31, 2014, to Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders party thereto and Citibank Europe PLC, UK Branch (formerly, The Royal Bank of Scotland plc), as Administrative Agent (incorporated by reference to Exhibit 10.3 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2014). |
10.10 |
| __ |
| Amendment No. 1, dated as of August 31, 2012, to the Credit Agreement, dated as of October 25, 2011, among Philip Morris International Inc., the lenders named therein and Citibank International plc, as Facility Agent (incorporated by reference to Exhibit 10.6 to the Quarterly Report on Form 10-Q for the quarter ended September 30, 2012). |
10.11 |
| - |
| Credit Agreement, dated as of October 1, 2015, among Philip Morris International Inc., the lenders named therein, Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as Facility Agent, and Citibank, N.A., as Swingline Agent (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed October 5, 2015). |
10.12 |
| - |
| Anti-Contraband and Anti-Counterfeit Agreement and General Release, dated as of July 9, 2004, and Appendices (Portions of this exhibit have been omitted pursuant to a request for confidential treatment filed with the Securities and Exchange Commission) (incorporated by reference to Exhibit 10.7 to the Registration Statement on Form 10 filed February 7, 2008). |
10.13 |
| - |
| Modification Agreement, dated as of October 14, 2014, to the Anti-Contraband and Anti-Counterfeit Agreement and General Release, dated as of July 9, 2004 (incorporated by reference to Exhibit 10.2 to the Quarterly Report on Form 10-Q for the quarter ended September 30, 2014). |
10.14 |
| - |
| Philip Morris International Inc. Automobile Policy (incorporated by reference to Exhibit 10.8 to the Registration Statement on Form 10 filed February 7, 2008).* |
138
10.15 |
| - |
| Philip Morris International Benefit Equalization Plan, as amended and in effect on August 6, 2012 (incorporated by reference to Exhibit 10.2 to the Quarterly Report on Form 10-Q for the quarter ended September 30, 2012).* |
10.16 |
| - |
| Philip Morris International Inc. 2012 Performance Incentive Plan, effective May 9, 2012 (incorporated by reference to Exhibit A to the Definitive Proxy Statement filed on March 30, 2012).* |
10.17 |
| - |
| Pension Fund of Philip Morris in Switzerland (IC) (incorporated by reference to Exhibit 10.2 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2015).* |
10.18 |
| - |
| Summary of Supplemental Pension Plan of Philip Morris in Switzerland (incorporated by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q for the quarter ended June 30, 2015).* |
10.19 |
| - |
| Form of Restated Employee Grantor Trust Enrollment Agreement (Executive Trust Arrangement) (incorporated by reference to Exhibit 10.18 to the Registration Statement on Form 10 filed February 7, 2008).* |
10.20 |
| - |
| Form of Restated Employee Grantor Trust Enrollment Agreement (Secular Trust Arrangement) (incorporated by reference to Exhibit 10.19 to the Registration Statement on Form 10 filed February 7, 2008).* |
10.21 |
| - |
| Philip Morris International Inc. 2008 Stock Compensation Plan for Non-Employee Directors (amended and restated as of January 1, 2015) (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed December 15, 2014).* |
10.22 |
| - |
| Philip Morris International Inc. 2008 Deferred Fee Plan for Non-Employee Directors (incorporated by reference to Exhibit 10.21 to the Registration Statement on Form 10 filed February 7, 2008).* |
10.23 |
| - |
| Supplemental Letter to the Employment Agreement with André Calantzopoulos (as amended). The Employment Agreement was previously filed as Exhibit 10.22 to the Registration Statement on Form 10 filed February 7, 2008 and is incorporated by reference to this Exhibit 10.23. The Amendment to the Employment Agreement was previously filed as Exhibit 10.1 to the Current Report on Form 8-K/A filed June 13, 2013, and is incorporated by reference to this Exhibit 10.23.* |
10.24 |
| - |
| Amendment to Employment Agreement with Marc S. Firestone (incorporated by reference to Exhibit 10.25 to the Annual Report on Form 10-K for the year ended December 31, 2013). The Employment Agreement was previously filed as Exhibit 10.1 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2013, and is incorporated by reference to this Exhibit 10.24.* |
10.25 |
| - |
| Amendment to Employment Agreement with Matteo Pellegrini (incorporated by reference to Exhibit 10.26 to the Annual Report on Form 10-K for the year ended December 31, 2013). The Employment Agreement was previously filed as Exhibit 10.4 to the Quarterly Report on Form 10-Q for the quarter ended June 30, 2011, and is incorporated by reference to this Exhibit 10.25.* |
10.26 |
| - |
| Agreement with Louis C. Camilleri (incorporated by reference to Exhibit 10.25 to the Registration Statement on Form 10 filed February 7, 2008).* |
10.27 |
| - |
| Amendment to Employment Agreement with Martin King (incorporated by reference to Exhibit 10.2 to the Quarterly Report on Form 10-Q for the quarter ended September 30, 2015). The Employment Agreement was previously filed as Exhibit 10.1 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, and is incorporated by reference to this Exhibit 10.27.* |
10.28 |
| - |
| Supplemental Letter to the Employment Agreement (as amended) with Miroslaw Zielinski (incorporated by reference to Exhibit 10.31 to the Quarterly Report on Form 10-Q for the quarter ended September 30, 2015). The Employment Agreement was previously filed as Exhibit 10.2 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2013 and is incorporated by reference to this Exhibit 10.28. The Amendment to the Employment Agreement was previously filed as Exhibit 10.28 to the Annual Report on Form 10-K for the year ended December 31, 2013, and is incorporated by reference to this Exhibit 10.28.* |
10.29 |
| - |
| Early Retirement and Release Agreement with Matteo Pellegrini (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed July 27, 2015).* |
10.30 |
| - |
| Time Sharing Agreement between PMI Global Services Inc. and Louis C. Camilleri dated August 18, 2010 (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed August 19, 2010).* |
10.31 |
| - |
| Amendment No. 1 to the Time Sharing Agreement between PMI Global Services Inc. and Louis C. Camilleri, dated August 22, 2012 (incorporated by reference to Exhibit 10.4 to the Quarterly Report on Form 10-Q for the quarter ended September 30, 2012).* |
139
10.32 |
| - |
| Amendment No. 2 to the Time Sharing Agreement between PMI Global Services Inc. and Louis C. Camilleri, dated October 23, 2012 (incorporated by reference to Exhibit 10.27 to the Annual Report on Form 10-K for the year ended December 31, 2012).* |
10.33 |
| - |
| Amendment No. 3 to the Time Sharing Agreement between PMI Global Services Inc. and Louis C. Camilleri, dated December 31, 2014 (incorporated by reference to Exhibit 10.34 to the Annual Report on Form 10-K for the year ended December 31, 2014).* |
10.34 |
| - |
| Time Sharing Agreement between PMI Global Services Inc. and André Calantzopoulos, dated May 8, 2013 (incorporated by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q for the quarter ended June 30, 2013).* |
10.35 |
| - |
| Amendment No. 1 to the Time Sharing Agreement between PMI Global Services Inc. and André Calantzopoulos, dated December 23, 2014 (incorporated by reference to Exhibit 10.36 to the Annual Report on Form 10-K for the year ended December 31, 2014).* |
10.36 |
| - |
| Amendment to the Employment Agreement with Jacek Olczak (incorporated by reference to Exhibit 10.33 to the Annual Report on Form 10-K for the year ended December 31, 2013). The Employment Agreement was previously filed as Exhibit 10.4 to the Quarterly Report on Form 10-Q for the quarter ended June 30, 2012, and is incorporated by reference to this Exhibit 10.36.* |
10.37 |
| - |
| Amended and Restated Supplemental Management Employees' Retirement Plan (incorporated by reference to Exhibit 10.27 to the Annual Report on Form 10-K for the year ended December 31, 2008).* |
10.38 |
| - |
| Supplemental Equalization Plan, amended and restated as of June 29, 2015 (incorporated by reference to Exhibit 10.2 to the Quarterly Report on Form 10-Q for the quarter ended June 30, 2015).* |
10.39 |
| - |
| Form of Supplemental Equalization Plan Employee Grantor Trust Enrollment Agreement (Secular Trust) (incorporated by reference to Exhibit 10.31 to the Annual Report on Form 10-K for the year ended December 31, 2008).* |
10.41 |
| - |
| Form of Supplemental Equalization Plan Employee Grantor Trust Enrollment Agreement (Executive Trust) (incorporated by reference to Exhibit 10.32 to the Annual Report on Form 10-K for the year ended December 31, 2008).* |
10.42 |
| - |
| Philip Morris International Inc. Form of Indemnification Agreement with Directors and Executive Officers (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed September 18, 2009).* |
10.43 |
| - |
| Form of Deferred Stock Agreement (April 16, 2012) (incorporated by reference to Exhibit 10.1 to the Quarterly Report on Form 10-Q for the quarter ended March 31, 2012).* |
10.44 |
| - |
| Philip Morris International Inc. 2008 Performance Incentive Plan, as amended and restated effective February 11, 2010 (incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K filed February 17, 2010).* |
10.45 |
| - |
| Form of Deferred Stock Agreement (2012 Grants) (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 13, 2012).* |
10.46 |
| - |
| Form of Deferred Stock Agreement (2013 Grants) (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 12, 2013).* |
10.47 |
| - |
| Form of Deferred Stock Agreement (2014 Grants) (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 7, 2014).* |
10.48 |
| - |
| Form of Deferred Stock Agreement (2015 Grants) (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 10, 2015).* |
10.49 |
| - |
| Philip Morris International Inc. Tax Return Preparation Services Policy (incorporated by reference to Exhibit 10.51 to the Annual Report on Form 10-K for the year ended December 31, 2014).* |
10.50 |
| - |
| Form of Restricted Stock Unit Agreement (2016 Grants) (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed February 9, 2016).* |
10.51 |
| - |
| Form of Performance Share Unit Agreement (2016 Grants) (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed February 9, 2016).* |
10.52 |
| - |
| Amendment No. 1, dated as of July 20, 2015, to the Credit Agreement, dated as of February 12, 2013, among Philip Morris International Inc., the lenders named therein, The Royal Bank of Scotland plc, as resigning administrative agent, and Citibank Europe PLC, UK Branch (formerly, Citibank International Limited), as successor administrative agent. |
140
12 |
| - |
| Statement regarding computation of ratios of earnings to fixed charges. |
21 |
| - |
| Subsidiaries of Philip Morris International Inc. |
23 |
| - |
| Consent of independent registered public accounting firm. |
24 |
| - |
| Powers of attorney. |
31.1 |
| - |
| Certification of the Registrant's Chief Executive Officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934, as amended, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
31.2 |
| - |
| Certification of the Registrant's Chief Financial Officer pursuant to Rule 13a-14(a)/15d-14(a) of the Securities Exchange Act of 1934, as amended, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
32.1 |
| - |
| Certification of the Registrant's Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
32.2 |
| - |
| Certification of the Registrant's Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
101.INS |
| - |
| XBRL Instance Document. |
101.SCH |
| - |
| XBRL Taxonomy Extension Schema. |
101.CAL |
| - |
| XBRL Taxonomy Extension Calculation Linkbase. |
101.DEF |
| - |
| XBRL Taxonomy Extension Definition Linkbase. |
101.LAB |
| - |
| XBRL Taxonomy Extension Label Linkbase. |
101.PRE |
| - |
| XBRL Taxonomy Extension Presentation Linkbase. |
* | Denotes management contract or compensatory plan or arrangement in which directors or executive officers are eligible to participate. |
141
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
PHILIP MORRIS INTERNATIONAL INC. | |
|
|
By: | /s/ A NDRÉ C ALANTZOPOULOS |
| (André Calantzopoulos Chief Executive Officer) |
Date: February 17, 2016
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the date indicated:
Signature | Title | Date |
|
|
|
/s/ ANDRÉ CALANTZOPOULOS | Chief Executive Officer | February 17, 2016 |
(André Calantzopoulos) | ||
/s/ JACEK OLCZAK | Chief Financial Officer | February 17, 2016 |
(Jacek Olczak) | ||
/s/ ANDREAS KURALI | Vice President and Controller | February 17, 2016 |
(Andreas Kurali) | ||
*HAROLD BROWN, LOUIS C. CAMILLERI, WERNER GEISSLER, JENNIFER LI, JUN MAKIHARA, SERGIO MARCHIONNE, KALPANA MORPARIA, LUCIO A. NOTO, FREDERIK PAULSEN, ROBERT B. POLET, STEPHEN M. WOLF | Directors |
|
*By: | /s/ ANDRÉ CALANTZOPOULOS |
| February 17, 2016 |
| (André Calantzopoulos Attorney-in-fact) |
| |
142
