Table of Contents
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fisc al year ended December 31, 20 1 7 | |
O r | |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to ___________ |
Commission File No. 0 00-23143
(Exact name of registrant as specified in its charter)
Delaware | 13-3379479 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
New York, NY 10007
(Address of principal executive offices, including zip code)
Registrant 's telephone number, including area code: (646) 975-2500
Securities registered pursuant to Section 12(b) of the Act: | |
Title of each class | Name of each exchange on which registered |
Common Stock, par value $0.0013 per share | The NASDAQ Stock Market LLC |
| Securities r egistered pursuant to Section 12( g ) of the Act: | None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant 's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definition of "large accelerated filer," "accelerated filer," "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Act:
Large accelerated filer ☐ | Accelerated filer ☒ |
Non-accelerated filer ☐ (Do not check if a smaller reporting company) | Smaller reporting company ☐ |
| Emerging Growth Company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act ). Yes ☐ No ☒
The aggregate market value of the voting and non-voting stock held by non-affiliates of the registrant on June 30, 2017, based upon the closing price of the Common Stock on The NASDAQ Stock Market LLC on that date of $6.79 per share, was $204,694,687 (1) .
(1) | Calculated by excluding all shares that may be deemed to be beneficially owned by executive officers, directors and five percent stockholders of the registrant, without conceding that any such person is an "affiliate" of the registrant for purposes of the federal securities laws. |
As of March 5, 2018, a total of 72,661,983 shares of Common Stock, par value $.0013 per share, were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant's definitive proxy statement to be filed in connection with solicitation of proxies for its 2018 Annual Meeting of Shareholders are hereby incorporated by reference into Part III of this Form 10-K where such portions are referenced.
Table of Contents
Table of Contents
Page
PART I |
| 1 |
| Item 1. Business | 2 |
| Item 1A. Risk Factors | 13 |
| Item 1B. Unresolved Staff Comments | 31 |
| Item 2. Properties | 31 |
| Item 3. Legal Proceedings | 31 |
| Item 4. Not Applicable | 32 |
PART II |
| 32 |
| Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 32 |
| Item 6. Selected Financial Data | 34 |
| Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations (MD&A) | 35 |
| Item 7A. Quantitative and Qualitative Disclosures About Market Risk | 41 |
| Item 8. Financial Statements and Supplementary Data | 42 |
| Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 42 |
| Item 9A. Controls and Procedures | 42 |
| Item 9B. Other Information | 44 |
PART III |
| 45 |
| Item 10. Directors, Executive Officers and Corporate Governance | 45 |
| Item 11. Executive Compensation | 45 |
| Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 45 |
| Item 13. Certain Relationships and Related Transactions, and Director Independence | 45 |
| Item 14. Principal Accounting Fees and Services | 45 |
PART IV |
| 46 |
| Item 15. Exhibits, Financial Statement Schedules | 46 |
| INDEX TO CONSOLIDATED FINANCIAL STATEMENTS | F-1 |
| SIGNATURES | S-1 |
| EXHIBIT INDEX | E-1 |
-i-
Table of Contents
PART I
This document and other public statements we make may contain statements that do not relate strictly to historical fact, any of which may be forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Statements contained in this communication that refer to our estimated or anticipated future results or other non-historical facts are forward-looking statements that reflect our current perception of existing trends and information as of the date of this communication. Forward looking statements generally will be accompanied by words such as "anticipate," "believe," "plan," "could," "should," "estimate," "expect," "forecast," "outlook," "guidance," "intend," "may," "might," "will," "possible," "potential," "predict," "project," or other similar words, phrases or expressions. Such statements are predictions only, and are subject to risks and uncertainties that could cause actual events or results to differ materially. Forward-looking statements involve known and unknown risks and uncertainties which may cause our actual results, performance or achievements to be materially different from those expressed or implied by forward-looking statements. While it is im possible to identify or predict all such matters, these differences between forward-looking statements and our actual results, performance or achievement may result from, among other things, the inherent uncertainty of the timing and success of, and expense associated with, research, development, regulatory approval and commercialization of our products and product candidates, including the risks that clinical trials will not commence or proceed as planned; products which appear to be promising in early trials will not demonstrate efficacy or safety in larger-scale trials; clinical trial data on our products and product candidates will be unfavorable; our products will not receive marketing approval from regulators or, if approved, do not gain sufficient market acceptance to justify development and commercialization costs; the sales of RELISTOR ® and other products by our partners and the revenue and income generated for us thereby may not meet expectations; competing products currently on the market or in development might reduce the commercial potential of our products; we, our collaborators or others might identify side effects after the product is on the market; or efficacy or safety concerns regarding marketed products, whether or not originating from subsequent testing or other activities by us, governmental regulators, other entities or organizations or otherwise, and whether or not scientifically justified, may lead to product recalls, withdrawals of marketing approval, reformulation of the product, additional pre-clinica l testing or clinical trials, changes in labeling of the product, the need for additional marketing applications, declining sales , or other adverse events.
We are also subject to risks and uncertainties associated with the actions of our corporate, academic and other collaborators and government regulatory agencies, including risks from market forces and trends; potential product liability; intellectual property, litigation and other dispute resolution, environmental and other risks; the risk that we may not be able to obtain sufficient capital, recruit and retain employees, enter into favorable collaborations or transactions , or other relationships or that existing or future relationships or transactions may not proceed as planned; the risk that current and pending patent protection for our products may be invalid, unenforceable or challenged, or fail to provide adequate market exclusivity, or that our rights to in-licensed intellectual property may be terminated for our failure to satisfy performance milestones; the risk of difficulties in, and regulatory compliance relating to, manufacturing products; and the uncertainty of our future profitability.
Risks and uncertainties to which we are subject also include general economic conditions, including interest and currency exchange-rate fluctuations and the availability of capital; changes in generally accepted accounting principles; the impact of legislation and regulatory compliance; the highly regulated nature of our business, including government cost-containment initiatives and restrictions on third-party payments for our products; trade buying patterns; the competitive climate of our industry; and other factors set forth in this document and other reports filed with the U.S. Securities and Exchange Commission ( " SEC " ). In particular, we cannot assure you that RELISTOR will be commercially successful or be approved in the future in other formulations, indications or jurisdictions, that any of our other programs , including AZEDRA ® , will result in a commercial product.
We do not have a policy of updating or revising forward-looking statements and, except as expressly required by law, we disclaim any intent or obligation to update or revise any statements as a result of new information or future events or developments. It should not be assumed that our silence over time means that actual events are bearing out as expressed or implied in forward-looking statements.
Available Information
We file annual, quarterly and current reports, proxy statements and other documents with the SEC under the Securities Exchange Act of 1934. The SEC maintains an Internet website that contains reports, proxy and information statements and other information regarding issuers, including Progenics, which file electronically with the SEC. You may obtain documents that we file with the SEC at www.sec.gov , and read and copy them at the SEC's Public Reference Room at 100 F Street NE, Washington, DC 20549. You may obtain information on operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. We also make available free of charge our annual, quarterly and current reports and proxy materials on www.progenics.com .
1
Table of Contents
Additional information concerning Progenics and its business may be available in press releases or other public announcements and quarterly and current reports and documents filed with the SEC. Information on or accessed through our website is not included in Progenics ' SEC filings.
In this document, RELISTOR ® , a registered trademark, refers to methylnaltrexone – the active ingredient of RELISTOR – as it has been and is being developed and commercialized by or in collaboration with Salix Pharmaceuticals, Inc., a subsidiary of Valeant Pharmaceuticals International, Inc. ("Valeant"), under a license agreement (the "RELISTOR License Agreement"). RELISTOR has received regulatory marketing approval for specific indications, and references to RELISTOR do not imply that any other form or possible use of the drug has received approval. RELISTOR's approved U.S. label and full U.S. prescribing information is available at www.RELISTOR.com . Other approved labels for RELISTOR apply in ex-U.S. markets. AZEDRA ® (iobenguane I 131) is a registered trademark.
Item 1. Business
Overview
Progenics Pharmaceuticals, Inc. and its subsidiaries (collectively the "Company," "Progenics", "we", or "us") develop innovative medicines and other technologies to target and treat cancer. Our pipeline includes: (1) therapeutic agents designed to precisely target cancer (AZEDRA, 1095, and PSMA TTC); (2) prostate-specific membrane antigen ("PSMA") targeted imaging agents for prostate cancer (1404 and PyL TM ); and (3) imaging analysis technology. Our first commercial product, RELISTOR (methylnaltrexone bromide) for opioid-induced constipation, is partnered with Valeant. Progenics Pharmaceuticals, Inc. was incorporated in the State of Delaware in 1986.
Pipeline
Our goal is to become a preeminent, patient-centric oncology company and we intend to make a difference in how patients with prostate cancer, pheochromocytoma and paraganglioma are diagnosed and treated. Our pipeline includes the following products and product candidates:
Product / Candidate | Description | Status | ||
Ultra-Orphan Theranostic |
|
|
|
|
AZEDRA |
| Treatment of malignant and/or recurrent and/or unresectable pheochromocytoma and paraganglioma |
| New Drug Application ("NDA") submitted and accepted by the U.S. Food and Drug Administration ("FDA"); target action date of April 30, 2018 under the Prescription Drug User Fee Act ("PDUFA") |
Expanded Access Program in progress | ||||
|
|
|
|
|
Prostate Cancer Theranostics |
|
| ||
1404 |
| Technetium-99m labeled PSMA targeted SPECT/CT imaging agent for prostate cancer |
| Completed enrollment in Phase 3 trial |
PyL |
| Fluorinated PSMA-targeted PET/CT imaging agent for prostate cancer |
| Phase 2/3 trial in progress |
1095 |
| Iodine-131 labeled PSMA-targeted small molecule therapeutic for treatment of metastatic prostate cancer |
| Phase 1 trial in progress |
PSMA TTC (Targeted Thorium Conjugate) [antibody licensed to Bayer] |
| Thorium-227 labeled PSMA-targeted antibody therapeutic for treatment of metastatic prostate cancer |
| Preclinical development in progress |
| automated bone scan index ("aBSI") [licensed to Fuji] | Software that quantifies the hotspots on bone scans and automatically calculates the bone scan index value | Sold in Japan | ||
|
|
|
|
|
Opioid-Induced Constipation ("OIC") Treatment |
|
| ||
RELISTOR Subcutaneous Injection [licensed to Valeant] |
| Treatment of OIC in adults with chronic non-cancer pain and treatment of OIC in advanced-illness adult patients receiving palliative care when laxative therapy has not been sufficient |
| Sold in the U.S., European Union, and Canada |
RELISTOR Tablets [licensed to Valeant] |
| Treatment of OIC in adults with chronic non-cancer pain |
| Sold in the U.S. (commercialization commenced in third quarter of 2016) |
2
Table of Contents
Our principal clinical-stage product candidates are:
● | AZEDRA is a radiotherapeutic product candidate in development as a treatment for malignant, recurrent, and/or unresectable pheochromocytoma and paraganglioma, rare tumors found in the adrenal glands and outside of the adrenal glands, respectively. AZEDRA has been granted Breakthrough Therapy and Orphan Drug designations, as well as Fast Track status in the U.S. Under a Special Protocol Assessment ("SPA") agreement with the FDA, we completed a Phase 2 registrational trial in patients with malignant, recurrent, and/or unresectable pheochromocytoma and paraganglioma. The FDA granted Priority Review of our NDA and has set an action date of April 30, 2018 under the Prescription Drug User Fee Act ("PDUFA"). There are currently no FDA-approved therapies for the treatment of these ultra-rare diseases. |
● | 1404 is a technetium-99m labeled small molecule which binds to PSMA and is used as an imaging agent to diagnose and detect localized prostate cancer as well as soft tissue and bone metastases. In December 2017, we completed enrollment in a Phase 3 trial assessing the diagnostic accuracy of 1404 imaging in men with newly-diagnosed or low-grade prostate cancer, whose biopsy indicates a histopathologic Gleason grade of ≤3+4 severity and/or are candidates for active surveillance. The study was designed to evaluate the (i) specificity of 1404 imaging to identify patients without clinically significant prostate cancer and (ii) sensitivity of 1404 to identify patients with clinically significant disease. The Phase 3 study enrolled approximately 450 patients in the U.S. and Canada. We are developing, based on data from our 1404 clinical trials, PSMA computer-assisted diagnostic tools ("PSMA CADx") that will automate reading of PSMA-targeted SPECT images based on deep learning. |
● | PyL (also known as [18F]DCFPyL) is a fluorinated PSMA-targeted Positron Emission Topography ("PET") imaging agent that enables visualization of both bone and soft tissue metastases to determine the presence or absence of recurrent and/or metastatic prostate cancer. A Phase 2/3 trial is ongoing to assess the diagnostic performance of PyL PET/CT imaging to detect prostate cancer in patients with recurrent and/or metastatic disease. In 2016, we launched PyL Research Access Program TM making limited doses of PyL available to researchers. |
● | 1095 is a PSMA-targeted Iodine-131 labeled small molecule that is designed to deliver a dose of beta radiation directly to prostate cancer cells with minimal impact on the surrounding healthy tissues. In collaboration with Memorial Sloan-Kettering Cancer Center, a Phase 1 trial is ongoing to assess the safety, tolerability, and preliminary efficacy of 1095, as well as define an optimal dose for a Phase 2 trial, in patients with metastatic castration-resistant prostate cancer ("mCRPC") who have demonstrated tumor avidity to 1095. |
● | PSMA TTC is a thorium-227 labeled PSMA-targeted antibody therapeutic. The PSMA TTC is designed to deliver a dose of alpha radiation directly to prostate cancer cells with minimal impact on the surrounding healthy tissues. We granted Bayer AS ("Bayer") exclusive worldwide rights to develop and commercialize products using our PSMA antibody technology in combination with Bayer's alpha-emitting radionuclides. |
● | aBSI quantifies the hotspots on bone scans of prostate cancer patients and automatically calculates the bone scan index value, representing the disease burden of prostate cancer shown on the bone scan. This quantifiable and reproducible calculation of the bone scan index value is intended to aid in the diagnosis and treatment of prostate cancer and may have utility in monitoring the course of the disease. aBSI is sold as a standalone software program to FUJIFILM RI Pharma Co., Ltd. ("Fuji") in Japan. |
● | RELISTOR is a treatment for OIC that addresses its underlying mechanism of OIC and decreases the constipating side effects induced by opioid pain medications such as morphine and codeine without diminishing their ability to relieve pain. RELISTOR is approved in two forms a subcutaneous injection and an oral tablet (450 mg once daily). RELISTOR subcutaneous injection is being sold in the U.S., European Union ("E.U."), and Canada, and RELISTOR tablets are being sold in the U.S. Under the RELISTOR License Agreement, Valeant is responsible for developing and commercializing RELISTOR. |
3
Table of Contents
RELISTOR net sales and related royalty income during the years 201 5 – 2017 are set forth below. Our recognition of royalty income for financial reporting purposes is explained in Management's Discussion and Analysis of Financial Condition and Results of Operations ( " MD&A " ) and our consolidated financial statements included elsewhere in this document.
First | Second | Third | Fourth | Full | ||||||||||||||||
Quarter | Quarter | Quarter | Quarter | Year | ||||||||||||||||
(in thousands) | ||||||||||||||||||||
2017 | ||||||||||||||||||||
Net sales | $ | 14,100 | $ | 17,300 | $ | 17,100 | $ | 24,600 | $ | 73,100 | ||||||||||
Royalty income | 2,119 | 2,601 | 2,562 | 3,683 | 10,965 | |||||||||||||||
2016 | ||||||||||||||||||||
Net sales | $ | 16,600 | $ | 15,900 | $ | 22,100 | $ | 16,000 | $ | 70,600 | ||||||||||
Royalty income | 2,189 | 2,380 | 3,319 | 2,407 | 10,295 | |||||||||||||||
2015 | ||||||||||||||||||||
Net sales | $ | 900 | $ | 11,900 | $ | 8,000 | $ | 23,000 | $ | 43,800 | ||||||||||
Royalty income | 140 | 1,773 | 1,208 | 3,452 | 6,573 | |||||||||||||||
We continue to consider opportunities for strategic collaborations, out-licenses, and other arrangements with biopharmaceutical companies involvin g proprietary research, development, clinical and commercialization programs, and may in the future also in-license or acquire additional oncology compounds and/or programs.
Clinical Trial Activities
For purposes of this report, in general Phase 1 trials are initial evaluations of safety in humans which study mechanism of action and metabolism; Phase 2 trials evaluate safety, dosing and activity or efficacy, and continue safety evaluation; and Phase 3 trials involve larger scale evaluations of safety, efficacy and dosing.
Our practice is and has been to announce commencement and results of all our significant clinical trials in press releases, medical and scientific meetings, and other venues. The following is a summary of current clinical trial activities involving our principal product candidates.
AZEDRA. In 2006, a Phase 1 trial was commenced with AZEDRA in 11 patients with malignant, recurrent and/or unresectable pheochromocytoma and paraganglioma and malignant carcinoid tumors to assess the safety, radiation dosimetry, and distribution metabolism of a single imaging dose of this compound. Following completion of this study, two dose-finding studies were conducted to determine a maximum tolerated therapeutic dose, and to assess safety, dosimetry and preliminary efficacy of AZEDRA in 21 patients with malignant, recurrent, and/or unresectable pheochromocytoma and paraganglioma and 15 with high-risk neuroblastoma, respectively.
Subsequently, a Phase 2 trial of AZEDRA was commenced under an SPA agreement with the FDA regarding the design of this trial to evaluate the efficacy and safety of the administration of two therapeutic doses of the compound in patients with malignant, recurrent, and/or unresectable pheochromocytoma and paraganglioma. These data supported the filing of an NDA, which was accepted by the FDA on December 29, 2017, and granted Priority Review with an action date of April 30, 2018 under the PDUFA. The final results showed that 17 (25%) of the 68 evaluable patients experienced a 50% or greater reduction of all antihypertensive medication for at least 6 months, achieving the primary endpoint specified in the SPA. Favorable results were observed from a key secondary endpoint, the proportion of patients with overall tumor response as measured by Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Of the patients who received at least one AZEDRA therapeutic dose 92.2% achieved partial response or stable disease. Median survival time as of March 10, 2017 was 36.7 months (95% CI 29.9 – 49.1) from first AZEDRA therapeutic dosing in the overall study population, and 48.73 months among patients who received two therapeutic doses, compared to 17.42 months among patients who received only one therapeutic dose. The Kaplan-Meier estimates of survival were 91.0%, 66.8%, and 51.5% at 1, 2, and 3 years, respectively, following initial therapeutic dosing. Long term follow-up continues.
Of the 74 patients who receive d any dose of AZEDRA, including an imaging dose, 43.2% of patients reported serious treatment-emergent adverse events ("SAEs"), the most frequently reported of which were hematologic (17.6%) treatment-related events. Other treatment-related SAEs reported by more than one subject included pulmonary embolism (2.7%) and myelodysplastic syndrome ("MDS") (4.1%). There were two additional related malignancies, one case of acute myeloid leukemia ("AML") (1.4%) and one case of acute lymphocytic leukemia (1.4%). There were two treatment related deaths during long term follow up, one due to MDS and the other due to AML.
1404. We conducted five Phase 1 trials with 1404 in healthy volunteers as well as men with prostate cancer, to establish proof-of-concept and dosimetry, and to assess a simplified kit preparation as compared to multi-step preparation. We then conducted a Phase 2 trial in the U.S. and Europe to assess the safety and ability of 1404 to detect prostate cancer within the prostate gland. Analysis of 1404 SPECT/CT images from this study showed that uptake of 1404 in the lobes of the prostate gland correlated significantly with Gleason score (p<0.0001). No deaths, SAEs, or adverse events ("AEs") leading to discontinuations occurred during the study. Of the 105 subjects who received a 1404 injection, 4 subjects reported a total of 10 treatment-emergent adverse events ("TEAEs") with 1 related to 1404 (infusion site extravasation). No discernible trends in hematology, clinical chemistries, vital signs, or physical findings were observed during the study.
4
Table of Contents
Based on results from these studies, a multi-center, open-label Phase 3 trial was initiated in December 2015 to evaluate the (i) specificity of 1404 to detect clinically insignificant prostate cancer and (ii) sensitivity of 1404 to detect clinically significant disease in patients with newly-diagnosed or low-grade prostate cancer, whose biopsy indicates a histopathologic Gleason grade of ≤3+4 severity and/or were candidates for active surveillance (N=approximately 450 patients). In December 2017, we completed enrollment in the Phase 3 trial.
PyL. In July 2015, we and the Johns Hopkins University entered into an exclusive worldwide licensing agreement for PyL, pursuant to which we obtained exclusive, worldwide (excluding Australia and New Zealand) rights to develop and commercialize PyL in PET imaging applications. PyL is a clinical-stage fluorinated PSMA-targeted PET imaging agent for prostate cancer. PyL has shown potential for use in identifying metastatic prostate cancer.
In December 2016, we initiated a Phase 2/3 trial to assess the safety and efficacy of PyL in the detection of prostate cancer. In addition to our sponsored studies and clinical trial collaborations we anticipate that PyL's potential activity will also be explored in investigator sponsored studies at various academic institutions.
1095. In February 2017, a Phase 1 clinical trial with 1095 began at Memorial Sloan-Kettering Cancer Center. This Phase 1 open-label, dose-escalation study plans to enroll approximately 30 patients with mCRPC who have demonstrated tumor avidity to 1095. The primary objectives of this study include determination of maximum tolerated dose, safety and tolerability, biodistribution, and efficacy.
EXINI Acquisition Update
In November 2015, we concluded a public tender offer (the "Offer") conducted pursuant to Swedish law to acquire EXINI, which develops advanced imaging analysis tools. As of the end of the offer acceptance period on that date, the Offer had been accepted by shareholders representing a total of 17,794,850 shares, corresponding to 96.81% of the total shares in EXINI. In December 2015, we commenced a judicial process in Sweden for acquiring the remaining shares of EXINI and EXINI was delisted and ceased to be publicly traded effective as of the close of trading on December 4, 2015. On December 8, 2016, a Swedish arbitral tribunal awarded us advanced title to the remaining shares of EXINI and, as of that date, EXINI became a wholly-owned subsidiary with 100% of the voting shares owned by us. In connection with the acquisition of the remaining shares of EXINI, in December 2016, we paid $368 thousand to the minority interest shareholders for the original purchase price and estimated interest, of which a net amount of $15 thousand was returned in 2017.
Research and Development Expenses
Research and development is essential to our business. During each of the years ended December 31, 201 7, 2016, and 2015, we incurred research and development costs of $42.5 million, $37.6 million, and $28.2 million, respectively. For additional information relating to our research and development expenses, see Management's Discussion and An alysis of Financial Condition and Results of Operations – Results of Operations – Research and Development Expenses .
License and Other Agreements
Oncology
In January 2013, w e acquired Molecular Insight by purchasing all of its outstanding capital stock for 4,452,593 shares of our common stock in a private transaction. Under the agreement, we also agreed to pay to the former stockholders potential milestones, in cash or our stock at our option, of up to $23.0 million contingent upon achieving specified commercialization events ($8.0 million for the first commercial sale of AZEDRA) and up to $70.0 million contingent upon achieving specified sales targets relating to the acquired company's products. The timing of any such payments, if any, is highly uncertain. In addition to utilizing our own proprietary technology, we have a number of agreements with owners of intellectual property which we use or believe may be useful in the research, development and commercialization of product candidates, including:
• | A 2012 co-exclusive license agreement with the University of Zurich and the Paul Scherrer Institute for worldwide sublicensable rights to certain intellectual property related to production methodologies relevant to 1404. Under this agreement, we maintain related patent rights and are obligated to pay low single-digit royalties on products using the licensed technology, license maintenance fees creditable against royalties, an annual fee for an option to expand the license's field of use, and clinical and regulatory milestone payments aggregating to approximately $1.3 million. The agreement may be terminated by the licensors upon certain material defaults by, and automatically terminates upon certain bankruptcy events relating to Molecular Insight, and may be terminated by us on prior written notice. |
5
Table of Contents
• | A 2012 out-license agreement with Fuji for the development and commercialization of 1404 in Japan. Under this agreement, we received upfront and milestone payments, of $3.0 million and $1.0 million, respectively, and we have the right to receive additional potential future milestone and royalty payments. |
• | A 2000 exclusive license agreement with The University of Western Ontario for worldwide sublicensable rights to certain intellectual property related to production methodologies relevant to AZEDRA. Under this agreement, we maintain related patent rights and are obligated to pay low single-digit royalties on products using the licensed technology, minimum annual royalties creditable against royalties and clinical and regulatory milestone payments aggregating to approximately $0.3 million. The agreement, which either party may terminate upon certain bankruptcy events or material defaults, continues through the last to expire of the related patent rights. |
I n August 2015, we entered into an exclusive worldwide licensing agreement for PyL with Johns Hopkins University. PyL, when used in conjunction with high-resolution PET imaging, has shown potential for use in identifying prostate cancer and sites of distant metastasis. Progenics intends to focus the development of PyL with high resolution PET imaging to detect and localize recurrent disease in patients who have experienced a biochemical relapse. Under this agreement, we are obligated to pay milestone payments, low single-digit royalties, patent costs and minimum annual royalties which are creditable against royalties, aggregating to approximately $2.9 million.
In April 2016, we entered into an agreement with a Bayer subsidiary granting Bayer exclusive worldwide rights to develop and commercialize products using our PSMA antibody technology in combination with Bayer's alpha-emitting radionuclides. Under this agreement, we received an upfront payment of $4.0 million and two milestone payments totaling $3.0 million, and we have the right to receive up to an additional $46.0 million in potential future development milestones and up to $130.0 million in commercialization milestones as well as royalty payments.
RELISTOR
Under the RELISTOR License Agreement, Valeant is responsible for developing and commercializing RELISTOR worldwide, including completing clinical development necessary to support regulatory marketing approvals for potential new indications and formulations, and marketing and selling the product. Valeant is marketing RELISTOR directly through its specialty sales force in the U.S., and outside the U.S. directly through distribution and marketing partners. Under our Agreement with Valeant, we recognized a development milestone payment of $40.0 million upon U.S. marketing approval for subcutaneous RELISTOR in non-cancer pain patients in 2014, and a development milestone payment of $50.0 million for the U.S. marketing approval of an oral formulation of RELISTOR in July 2016. We are also eligible to receive up to $200.0 million of commercialization milestone payments upon achievement of specified U.S. sales targets, including:
Net Sales Level in any Single Calendar Year | Payment | |||
In excess of $100 million | $ | 10,000 | ||
In excess of $150 million | 15,000 | |||
In excess of $200 million | 20,000 | |||
In excess of $300 million | 30,000 | |||
In excess of $750 million | 50,000 | |||
In excess of $1 billion | 75,000 | |||
| $ | 200,000 | |||
Each commercialization milestone payment is payable one time only, regardless of the number of times the condition is satisfied, and all six payments could be made within the same calendar year. We are also eligible to receive royalties from Valeant and its affiliates based on the following royalty scale: 15% on worldwide net sales up to $100 .0 million, 17% on the next $400.0 million in worldwide net sales, and 19% on worldwide net sales over $500.0 million each calendar year, and 60% of any upfront, milestone, reimbursement or other revenue (net of costs of goods sold, as defined, and territory-specific research and development expense reimbursement) Valeant receives from sublicensees outside the U.S.
The RELISTOR License Agreement may be terminated by either party upon an uncured material breach or specified bankruptcy events. In addition, Valeant may terminate the RELISTOR License Agreement for unresolved safety or efficacy issues or at its discretion upon specified prior notice at any time, subject to our one-time right to postpone such termination for a specified period of time if we have not successfully transitioned the development and commercialization of the drug despite good faith and diligent efforts. See Risk Factors.
6
Table of Contents
W e have licensed to Valeant our exclusive rights to develop and commercialize methylnaltrexone, the active ingredient of RELISTOR, which we in-licensed from the University of Chicago ("UC"). Our agreement with UC provides for an exclusive license to intellectual property in exchange for development and potential commercialization obligations, low single-digit royalties on commercial sales of resulting products and single-digit percentages of milestone and sublicensing revenues, and shared patent policing responsibilities. Under the UC agreement, as amended in connection with our RELISTOR collaborations, all of our royalty payment obligations in the U.S. expired at the end of 2017 and will expire at the end of 2018 outside the U.S. on the approved indications.
Valeant has also entered into license and distribution agreements to expand its sales channels outside of the U.S. for RELISTOR. In January 2016, Valeant entered into a distribution agreement with Swedish Orphan Biovitrum AB, also known as Sobi, for RELISTOR in Western Europe, Russia and Greece. In 2016, we recognized license revenue of $720 thousand for our share of the upfront payment Valeant received from Lupin Limited pursuant to a distribution agreement for RELISTOR in Canada.
Patents and Proprietary Technology
P rotection of our intellectual property rights is important to our business. We seek U.S. patent protection for many of our inventions, and generally file patent applications in Canada, Japan, European countries that are party to the European Patent Convention, and other countries on a selective basis in order to protect inventions we consider to be important to the development of business in those areas. Generally, patents issued in the U.S. are effective for either (i) 20 years from the earliest asserted filing date, if the application was filed on or after June 8, 1995, or (ii) the longer of 17 years from the date of issue or 20 years from the earliest asserted filing date, if the application was filed prior to that date.
In certain instances, the U.S. patent term can be extended up to a maximum of five years to recapture a portion of the term during which FDA regulatory review was being conducted. The duration of foreign patents varies in accordance with the provisions of applicable local law, although most countries provide for patent terms of 20 years from the earliest asserted filing date and allow patent extensions similar to those permitted in the U.S.
Patents may not enable us to preclude competitors from commercializing drugs in direct competition with our products, and consequently may not provide us with any meaningful competitive advantage. See Risk Factors. We also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a competitive position in our product areas. We require our employees, consultants and corporate partners who have access to our proprietary information to sign confidentiality agreements.
Information with respect to our current patent portfolio is set forth below.
The original patents surrounding the A ZEDRA program were licensed from the University of Western Ontario ("UWO"). The patent family directed to processes for making polymer precursors, as well as processes for making the final product, expire in 2018 in the U.S. and Canada. Other licensed patent families from UWO relate to alternative approaches for preparing AZEDRA, which if implemented, would expire in 2024 worldwide. We have pending applications worldwide directed to manufacturing improvements and the resulting compositions which, if issued, would expire in 2035.
Owned and in-licensed patents relating to 1404 have expiration ranges of 2020 to 2029; we view as most significant the composition-of-matter patent on the compound, as well as technetium-99 labeled forms, which expire in 2029 worldwide. Patent applications directed to methods of use are pending worldwide, which if issued would expire in 2034.
The PyL patent family was licensed from Johns Hopkins University. Patent protection for the composition-of-matter patents on the compound, radiolabeled forms of the compound, as well as methods of use, expire in 2030 in the U.S. Corresponding patent family members are pending or issued worldwide, all expiring in 2029.
Company-o wned patents relating to 1095 have expiration ranges of 2027 to 2031 in the U.S. We view as most significant the composition-of-matter patent on this compound, as well as radiolabeled forms, which expires in 2027 in the U.S., as well as Europe. Additional U.S. patents are directed to stable compositions and radiolabeling processes which expire in 2030 and 2031, respectively.
We own patents relating to automated detection of bone cancer metastases. The patents on this technology expire in 2028.
The intellectual property directed to PSMA antibody comprises co-owned and in-licensed patents. Composition-of-matter patents have expirations of 2022 and 2023 in the U.S. Corresponding foreign counterpart patents will expire 2022. We view all of these patents as significant.
7
Table of Contents
With regar d to our RELISTOR-related intellectual property, the composition-of-matter patent for the active ingredient of RELISTOR, methylnaltrexone, has expired. UC, as well as we and our collaborators, have extended the methylnaltrexone patent estate with additional patents and pending patent applications covering various inventions relating to the product. Valeant has listed in the FDA Orange Book eight U.S. patents relating to subcutaneous RELISTOR, which have expiration dates ranging from 2017 to 2030, and eight U.S. patents relating to RELISTOR tablet, which have expiration dates ranging from 2017 to 2031. Four Canadian patents (expiring in 2024 and 2029) have been listed with Health Canada relating to subcutaneous RELISTOR.
We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example , we are aware of others investigating and developing technologies, imaging agents and drug candidates directed toward PSMA or related compounds as well as in the case of methylnaltrexone other peripheral opioid antagonists, and of patents and applications held or filed by others in those areas. The validity of issued patents, patentability of claimed inventions in pending applications and applicability of any of them to our programs are uncertain and subject to change, and patent rights asserted against us could adversely affect our ability to commercialize or collaborate with others on specific products.
R esearch, development, and commercialization of a biopharmaceutical product often require choosing between alternative development and optimization routes at various stages in the development process. Preferred routes depend upon current – and may be affected by subsequent – discoveries and test results and cannot be identified with certainty at the outset. There are numerous third-party patents in fields in which we work, and we may need to obtain license under patents of others in order to pursue a preferred development route of one or more of our product candidates. The need to obtain a license would decrease the ultimate value and profitability of an affected product. If we cannot negotiate such a license, we might have to pursue a less desirable development route or terminate the entire program altogether.
Government Regulation
We and our product candidates are subject to comprehensive regulation by the FDA and comparable authorities in other countries. Pharmaceutical regulation currently is a topic of substantial interest in lawmaking and regulatory bodies in the U.S. and internationally, and numerous proposals exist for changes in FDA and non-U.S. regulation of pre-clinical and clinical testing, approval, safety, effectiveness, manufacturing, storage, recordkeeping, labeling, marketing, export, advertising, promotion and other aspects of biologics, small molecule drugs and medical devices, many of which, if adopted, could significantly alter our business and the current regulatory structure described below. See Risk Factors.
FDA Regulation
FDA approval, which involves review of scientific, clinical and commercial data, manufacturing processes and facilities, is required before a product candidate may be marketed in the U.S. This process is costly, time consuming and subject to unanticipated delays, and a drug candidate may fail to progress at any point.
None of our product candidates other than RELISTOR has received marketing approval from the FDA or any other regulatory authority. The process required by the FDA before product candidates may be approved for marketing in the U.S. generally involves:
| ● | pre-clinical laboratory and animal tests; | |
| ● | submission to and favorable review by the FDA of an investigational new drug application before clinical trials may begin; | |
| ● | adequate and well-controlled human clinical trials to establish the safety and efficacy of the product for its intended indication (animal and other nonclinical studies also are typically conducted during each phase of human clinical trials); | |
| ● | submission to the FDA of a marketing application; and | |
| ● | FDA review of the marketing application in order to determine, among other things, whether the product is safe and effective for its intended uses. |
Pre-clinical tests include laboratory evaluation of product chemistry and animal studies to gain preliminary information about a compound's pharmacology and toxicology and to identify safety problems that would preclude testing in humans. Since product candidates must generally be manufactured according to current Good Manufacturing Practices ("cGMP"), pre-clinical safety tests must be conducted by laboratories that comply with FDA good laboratory practices regulations. Pre-clinical testing is preceded by initial research related to specific molecular targets, synthesis of new chemical entities, assay development and screening for identification and optimization of lead compound(s).
8
Table of Contents
R esults of pre-clinical tests are submitted to the FDA as part of an IND which must become effective before clinical trials may commence. The IND submission must include, among other things, a description of the sponsor's investigational plan; protocols for each planned study; chemistry, manufacturing and control information; pharmacology and toxicology information and a summary of previous human experience with the investigational drug. Unless the FDA objects to, makes comments or raises questions concerning an IND, it becomes effective 30 days following submission, and initial clinical studies may begin. Companies often obtain affirmative FDA approval, however, before beginning such studies.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or to individuals under the supervision of a qualified principal investigator. Clinical trials must be conducted in accordance with the FDA's Good Clinical Practice requirements under protocols submitted to the FDA that detail, among other things, the objectives of the study, parameters used to monitor safety and effectiveness criteria to be evaluated. Each clinical study must be conducted under the auspices of an Institutional Review Board, which considers, among other things, ethical factors, safety of human subjects, possible liability of the institution and informed consent disclosure which must be made to participants in the trial.
Clinical trials are typically conducted in three sequential phases, which may overlap. During Phase 1, when the drug is initially administered to human subjects, the product is tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. Phase 2 involves studies in a limited population to evaluate preliminarily the efficacy of the product for specific, targeted indications, determine dosage tolerance and optimal dosage and identify possible adverse effects and safety risks.
When a product candidate is found in Phase 2 evaluation to have an effect and an acceptable safety profile, Phase 3 trials are undertaken in order to further evaluate clinical efficacy and test for safety within an expanded population. Safety studies are conducted in accordance with the FDA's International Conference on Harmonization Guidelines. Phase 2 results do not guarantee a similar outcome in Phase 3 trials. The FDA may suspend clinical trials at any point in this process if it concludes that clinical subjects are being exposed to an unacceptable health risk.
A n NDA is an application to the FDA to market a new drug. A Biologic License Application ("BLA") is an application to market a biological product. The new drug or biological product may not be marketed in the U.S. until the FDA has approved the NDA or issued a biologic license. The NDA must contain, among other things, information on chemistry, manufacturing and controls; non-clinical pharmacology and toxicology; human pharmacokinetics and bioavailability; and clinical data. The BLA must contain, among other things, data derived from nonclinical laboratory and clinical studies which demonstrate that the product meets prescribed standards of safety, purity and potency, and a full description of manufacturing methods. Supplemental NDAs ("sNDAs") are submitted to obtain regulatory approval for additional indications for a previously approved drug, and are reviewed by the FDA in a similar manner.
The results of the pre-clinical studies and clinical studies, the chemistry and manufacturing data, and the proposed labeling, among other things, are submitted to the FDA in the form of an NDA or BLA. The FDA may refuse to accept the application for filing if certain administrative and content criteria are not satisfied, and even after accepting the application for review, the FDA may require additional testing or information before approval of the application, in either case based upon changes in applicable law or FDA policy during the period of product development and FDA regulatory review. The applicant's analysis of the results of clinical studies is subject to review and interpretation by the FDA, which may differ from the applicant's analysis, and in any event, the FDA must deny an NDA or BLA if applicable regulatory requirements are not ultimately satisfied. If regulatory approval of a product is granted, such approval may be made subject to various conditions, including post-marketing testing and surveillance to monitor the safety of the product, or may entail limitations on the indicated uses for which it may be marketed. Product approvals may also be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
Orphan Drug, Fast Track , Breakthrough Therapy Designations , and Priority Review
Other FDA regulations and policies relating to drug approval have implications for certain of our current or future product candidates, particularly A ZEDRA. Designation as an Orphan Drug is available under U.S., E.U., and other laws for drug candidates intended to treat rare diseases or conditions, and which if approved are granted a period of market exclusivity, subject to various conditions. Orphan Drug designation does not shorten or otherwise convey any advantage in the regulatory approval process. Under the Orphan Drug Act, the FDA may designate a product as an Orphan Drug if it is intended to treat a rare disease or condition, generally defined as a patient population of fewer than 200,000 in the U.S. AZEDRA is designated as an Orphan Drug.
In the U.S., Orphan Drug designation entitles a party to certain financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. In addition, if a product receives the first FDA approval for the indication for which it has orphan designation, the product is entitled to Orphan Drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity or where the manufacturer is unable to assure sufficient product quantity.
9
Table of Contents
In cases where the extent and scope of patent protection for a product is limited, the exclusivity period resulting from Orphan Drug designation may be important in helping products maintain a competitive position. Even if a product obtains Orphan Drug exclusivity, however, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties may be approved for the same condition. Even after an Orphan Drug is approved, the FDA may subsequently approve the same drug with the same active moiety for the same condition if the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care.
The FDA is also authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These mechanisms for expedited review include fast track designation, breakthrough therapy designation and priority review designation. AZEDRA has received both fast track and breakthrough therapy designations.
T he FDA may designate a product for fast track review if it is intended, whether alone or in combination with one or more other drugs, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For fast track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a fast track product's NDA before the application is complete. This rolling review may be available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a fast track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA's time period goal for reviewing a fast track application does not begin until the last section of the NDA is submitted. In addition, the fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
In 2012, as part of the Food and Drug Administration Safety and Improvement Act, a new regulatory scheme was established allowing expedited review of products designated as "breakthrough therapies." A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
Finally , the FDA may designate a product for priority review if it is a drug that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case-by-case basis, whether the proposed drug represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting drug reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA's goal for taking action on a marketing application from ten months to six months.
Both before and after approval is obtained, a product, its manufacturer and the sponsor of the marketing application for the product are subject to comprehensive regulatory oversight. Violations of existing or newly-adopted regulatory requirements at any stage, including the pre-clinical and clinical testing process, the approval process, or thereafter, may result in various adverse consequences, including FDA delay in approving or refusal to approve a product, withdrawal of an approved product from the market or the imposition of criminal penalties against the manufacturer or sponsor. Later discovery of previously unknown problems may result in restrictions on the product, manufacturer or sponsor, including withdrawal of the product from the market.
Regulation Outside the U.S.
Whether or not FDA approval has been obtained, approval of a pharmaceutical product by comparable government regulatory authorities abroad must be obtained prior to marketing the product there. The approval procedure varies from country to country, and the time required may be longer or shorter than that required for FDA approval. The requirements for regulatory approval by governmental agencies in other countries prior to commercialization of products there can be rigorous, costly and uncertain, and approvals may not be granted on a timely basis or at all.
10
Table of Contents
In E.U. countries, Canada, Australia, and Japan, regulatory requirements and approval processes are similar in principle to those in the U.S. Regulatory approval in Japan requires that clinical trials of new drugs be conducted in Japanese patients. Depending on the type of drug for which approval is sought, there are currently two potential tracks for marketing approval in E.U. countries: mutual recognition and the centralized procedure. These review mechanisms may ultimately lead to approval in all E.U. countries, but each method grants all participating countries some decision-making authority in product approval. The centralized procedure, which is mandatory for biotechnology derived products, results in a recommendation in all member states, while the E.U. mutual recognition process involves country-by-country approval.
In other countries, regulatory requirements may require additional pre-clinical or clinical testing regardless of whether FDA or European approval has been obtained. This is the case in Japan, where trials are required to involve patient populations which we and our other collaborators have not examined in detail. If a product is manufactured in the U.S., it is also subject to FDA and other U.S. export provisions. In most countries outside the U.S., coverage, pricing and reimbursement approvals are also required, which may affect the profitability of the affected product.
Other Regulation
In addition to regulations enforced by the FDA, we are also subject to regulation under the U.S. Occupational Safety and Health Act, Environmental Protection Act, Toxic Substances Control Act, Resource Conservation and Recovery Act, and various other current and potential future U.S. federal, state or local regulations. In addition, our research is dependent on maintenance of licenses from various authorities permitting the acquisition, use and storage of quantities of radioactive isotopes that are critical for its manufacture and testing of research products. Biopharmaceutical research and development generally involves the controlled use of hazardous materials, chemicals, viruses, and various radioactive compounds. Even strict compliance with safety procedures for storing, handling, using and disposing of such materials prescribed by applicable regulations cannot completely eliminate the risk of accidental contaminations or injury from these materials, which may result in liability for resulting legal and regulatory violations as well as damages.
Manufacturing
Under the RELISTOR License Agreement, Valeant is responsible for the manufacture and supply, at its expense, of all active pharmaceutical ingredient ("API") and finished and packaged products for its RELISTOR commercialization efforts, including contracting with contract manufacturing organizations ("CMOs") for supply of RELISTOR API and subcutaneous and oral finished drug product.
As to our other product candidates, the manufacture of biopharmaceuticals and radiopharmaceuticals is relatively complex and requires significant capital expenditures. As part of our ongoing efforts to manage our development costs and timely execute on our development plans, we rely on third parties for clinical manufacturing. We have engaged third-party CMOs to manufacture API and finished drug products for clinical trial supplies of all of our product candidates, including AZEDRA, 1404, PyL, and 1095. We are in the final stages of establishing manufacturing capacity that we believe will be sufficient to deliver commercial supplies of AZEDRA. We have partnered with third-parties to produce clinical trial supplies of current clinical-stage product candidates, and may in the future undertake such efforts with respect to other assets and programs. As a result, we depend significantly on the availability of high quality CMO services. If we are unable to arrange for satisfactory CMO services, we would need to undertake such responsibilities on our own, resulting in our having to incur additional expenses and potentially delaying the development of our product candidates. See Risk Factors .
Commercial Organization
We plan to commercialize AZEDRA in the U.S. ourselves, if regulatory approval is obtained, and to seek strategic partnerships to commercialize AZEDRA in other countries. We are building a small commercial organization for our U.S. efforts in preparation of the potential AZEDRA launch.
11
Table of Contents
Competition
Competition in the biopharmaceutical industry is intense and characterized by ongoing research and development and technological change. We face competition from many for-profit companies and major universities and research institutions in the U.S. and abroad. We face competition from companies marketing existing products or developing new products for diseases targeted by our technologies. Many of our competitors have substantially greater resources, experience in conducting pre-clinical studies and clinical trials and obtaining regulatory approvals for their products, operating experience, research and development and marketing capabilities and production capabilities than we do. Our products and product candidates under development may not compete successfully with existing products or products under development by other companies, universities and other institutions. Drug manufacturers that are first in the market with a therapeutic for a specific indication generally obtain and maintain a significant competitive advantage over later entrants and therefore, the speed with which industry participants move to develop products, complete clinical trials, approve processes and commercialize products is an important competitive factor.
RELISTOR was the first FDA-approved product for any indication involving OIC. We are, however, aware of other approved and marketed products, as well as candidates in pre-clinical or clinical development, that target the side effects of opioid pain therapy. Our principal competitors in the field of OIC include Nektar Therapeutics, in collaboration with AstraZeneca PLC; Cubist Pharmaceuticals, a subsidiary of Merck & Co., Inc.; Mallinckrodt plc, in collaboration with Takeda Pharmaceutical Company Limited; Shionogi & Co., in collaboration with Purdue Pharma L.P.; and Mundipharma International Limited. Other prescription, as well as over-the-counter, laxatives are also used as first line for OIC.
As to our oncology pipeline, radiation and surgery are two traditional forms of treatment for prostate cancer. If the disease spreads, hormone (androgen) suppression therapy is often used to slow the cancer's progression, but this form of treatment can eventually become ineffective. We are aware of several competitors who are developing or have received approval for treatments for castration-resistant prostate cancer. Our principal competitors in the field of mCRPC include Johnson & Johnson subsidiary Janssen Biotech, Inc.; Pfizer, Inc. in collaboration with Astellas Pharma US, Inc.; and Bayer HealthCare Pharmaceuticals Inc. Our principal competitors in the field of PSMA-targeted imaging agents include Aytu Bioscience Inc., Blue Earth Diagnostic, Inc. and Novartis AG.
There are currently no approved anticancer treatments in the U .S. for malignant, recurrent, and/or unresectable pheochromocytoma and paraganglioma.
A significant amount of research in the biopharmaceutical field is carried out at academic and government institutions. An element of our research and development strategy has been to in-license technology and product candidates from academic and government institutions. These institutions are sensitive to the commercial value of their findings and pursue patent protection and negotiate licensing arrangements to collect royalties for use of technology they develop. They may also market competitive commercial products on their own or in collaboration with competitors and compete with us in recruiting highly qualified scientific personnel, which may result in increased costs or decreased availability of technology or product candidates from these institutions to other industry participants.
Competition with respect to our technologies and products is based on, among other things, product efficacy, safety, reliability, method of administration, availability, price and clinical benefit relative to cost; timing and scope of regulatory approval; sales, marketing and manufacturing capabilities; collaborator capabilities; insurance and other reimbursement coverage; and patent protection. Competitive position in our industry also depends on a participant's ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary products or processes, and secure sufficient capital resources for the typically substantial period between technological conception and commercial sales.
Product Liability
The testing, manufacturing and marketing of our product candidates and products involves an inherent risk of product liability attributable to unwanted and potentially serious health effects. To the extent we elect to test, manufacture or market product candidates and products independently, we bear the risk of product liability directly. We maintain product liability insurance coverage in amounts and pursuant to terms and conditions customary for our industry, scale, and the nature of our activities. Where local statutory requirements exceed the limits of our existing insurance or local policies of insurance are required, we maintain additional clinical trial liability insurance to meet these requirements. This insurance is subject to deductibles and coverage limitations. The availability and cost of maintaining insurance may change over time.
Human Resources
At December 31, 2017, we had 64 full-time employees, 12 of whom hold Ph.D./PharmD degrees and 3 of whom hold M.D. degrees. At that date, 43 employees were engaged in research and development, medical, regulatory affairs, and manufacturing related activities and 21 were engaged in finance, legal, administration, commercial, and business development. We consider our relations with our employees to be good. None of our employees is covered by a collective bargaining agreement.
12
Table of Contents
Item 1A. R isk Factors
General Risks Related to our Business
Drug development is a long and inherently uncertain process with a high risk of failure at every stage of development.
Drug development is a highly uncertain scientific and medical endeavor, and failure can unexpectedly occur at any stage of clinical development. Typically, there is a high rate of attrition for product candidates in preclinical and clinical trials due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. The risk of failure increases for our product candidates that are based on new technologies, as well as technologies with which we have limited prior experience. Pre-clinical studies and clinical trials are long, expensive and highly uncertain processes that can take many years. It will take us several years to complete all pre-clinical work and clinical trials and the time required for completing testing and obtaining approvals is uncertain. The start or end of a clinical trial is often delayed or halted due to changing regulatory requirements, manufacturing challenges, required clinical trial administrative actions, slower than anticipated patient enrollment, changing standards of care, availability or prevalence of use of a comparator drug or required prior therapy, clinical outcomes, or financial constraints. The FDA and other U.S. and foreign regulatory agencies have substantial discretion, at any phase of development, to terminate clinical trials, require additional clinical development or other testing, delay, condition or withhold registration and marketing approval and mandate product withdrawals, including recalls. Additionally, we may also amend, suspend or terminate clinical trials at any time if we believe that the participating patients are being exposed to unacceptable health risks. Results attained in early human clinical trials may not be indicative of results in later clinical trials. In addition, some of our investigational or experimental drugs are at an early stage of development, and successful commercialization of early stage product candidates requires significant research, development, testing and approvals by regulators, and additional investment. Our failure to demonstrate adequately the safety and efficacy of a product under development would delay or prevent marketing approval, which could adversely affect our operating results and credibility. The failure of one or more of our product candidates could have a material adverse effect on our business, financial condition and results of operations.
The future of our business and operations depends on the success of our development and commercialization programs.
Our business and operations entail a variety of serious risks and uncertainties and are inherently risky. The development programs on which we focus involve novel approaches to human therapeutics and diagnostics. Our product candidates are in clinical development, and in some respects , involve technologies with which we have limited prior experience. We are subject to the risks of failure inherent in the development and commercialization of product candidates based on new technologies. There is little precedent for the successful commercialization of products based on our technologies, and there are a number of technological challenges that we must overcome to complete most of our development efforts. We may not be able to successfully further develop any of our product candidates. We must successfully complete clinical trials and obtain regulatory approvals for potential commercial products. Once approved, if at all, commercial product sales are subject to general and industry-specific local and international economic, regulatory, technological and policy developments and trends. Delays, higher costs or other weaknesses in the manufacturing process of our CMOs could hinder the development and commercialization of our product pipeline. The oncology space in which we operate presents numerous significant risks and uncertainties that may be expected to increase to the extent it becomes more competitive or less favored in the commercial healthcare marketplace.
Failure to realize the anticipated benefits of any strategic acquisition and/or licensing transaction could adversely affect our business, operations and financial condition.
A part of our business strategy has been to identify and advance a pipeline of product candidates by identifying product candidates, technologies and businesses for acquisition and in-licensing that we believe are a strategic fit with our existing business. The ultimate success of any strategic transactions entails numerous operational and financial risks, including:
● | higher than expected development and integration costs; |
● | difficulty in combining the technologies, operations and personnel of acquired businesses with our technologies, operations and personnel; |
● | exposure to unknown liabilities; |
● | difficulty or inability to form a unified corporate culture across multiple office sites both nationally and internationally; |
● | inability to retain key employees of acquired businesses; |
13
Table of Contents
● | disruption of our business and diversion of our management 's time and attention; and |
● | difficulty or inability to secure financing to fund development activities for such acquired or in-licensed product candidates, technologies or businesses. |
We have limited resources to integrate acquired and in-licensed product candidates, technologies and businesses into our current infrastructure, and we may fail to realize the anticipated benefits of any strategic transactions. Any such failure could have an adverse effect on our business, operations and financial condition.
If we do not obtain regulatory approval for our product candidates on a timely basis, or at all, or if the terms of any approval impose significant restrictions or limitations on use, our business, results of operations and financial condition will be adversely affected. Setbacks in clinical development programs could have a material adverse effect on our business.
Regulatory approvals are necessary to market product candidates and require demonstration of a product 's safety and efficacy through extensive pre-clinical and clinical trials. We may not obtain regulatory approval for product candidates on a timely basis, or at all, and the terms of any approval (which in some countries includes pricing and reimbursement approval) may impose significant restrictions, limitations on use or other commercially unattractive conditions. The process of obtaining FDA and foreign regulatory approvals often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. We have had only limited experience in filing and pursuing applications and other submissions necessary to gain marketing approvals. Products under development may never obtain marketing approval from the FDA or other regulatory authorities necessary for commercialization.
We or our regulators may also amend, suspend or terminate clinical trials if we or they believe that the participating patients are being exposed to unacceptable health risks, and after reviewing trial results, we may abandon projects which we previously believed to be promising for commercial or other reasons unrelated to patient risks. During this process, we may find, for example, that results of pre-clinical studies are inconclusive or not indicative of results in human clinical trials, clinical investigators or contract research organizations do not comply with protocols or applicable regulatory requirements, or that product candidates do not have the desired efficacy or have undesirable side effects or other characteristics that preclude marketing approval or limit their potential commercial use if approved. In such circumstances, the entire development program for that product candidate could be adversely affected, resulting in delays in trials or regulatory filings for further marketing approval and a possible need to reconfigure our clinical trial programs to conduct additional trials or abandon the program involved. Conducting additional clinical trials or making significant revisions to a clinical development plan would lead to delays in regulatory filings. If clinical trials indicate, or regulatory bodies are concerned about, actual or possible serious problems with the safety or efficacy of a product candidate, we may stop or significantly slow development or commercialization of affected products. As a result of such concerns, the development programs for our product candidates may be significantly delayed or terminated altogether.
If the results of any of our clinical trials are not satisfactory or we encounter problems and/or delays enrolling patients, clinical trial supply issues, setbacks in developing drug formulations, including raw material supply, manufacturing, stability or other difficulties, or issues complying with protocols or applicable regulatory requirements, the entire development program for our product candidates could be adversely affected in a material manner.
We must design and conduct successful clinical trials for our product candidates to obtain regulatory approval. We rely on third parties for conduct of clinical trials, which reduces our control over their timing, conduct and expense and may expose us to conflicts of interest. Clinical trial results may be unfavorable or inconclusive, and often take longer and cost more than expected.
We have limited internal resources with conducting clinical trials, and we rely on or obtain the assistance of others to design, conduct, supervise, or monitor some or all aspects of some of our clinical trials. In relying on these third parties, we have less control over the timing and other aspects of clinical trials than if we conducted them entirely on our own. Problems with the timeliness or quality of the work of a contract research organization or clinical data management organization may lead us to seek to terminate the relationship and use an alternative service provider. However, making this change may be costly and may delay our trials and contractual restrictions may make such a change difficult or impossible. These third parties may also have relationships with other entities, some of which may be our competitors. In all events, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. The FDA and other foreign regulatory authorities require us to comply with good clinical practices for conducting and recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements.
14
Table of Contents
To obtain regulatory approval of our product candidates, we must demonstrate through preclinical studies and clinical trials that they are safe and effective. Adverse or inconclusive clinical trial results concerning any of our product candidates that regulators find deficient in scope, design or one or more other material respects, could require additional trials, resulting in increased costs, significant delays in submissions of approval applications, approvals in narrower indications than originally sought, or denials of approval, none of which we can predict. As a result, any projections that we publicly announce of commencement and duration of clinical trials are not certain. We have experienced clinical trial delays in the past as a result of slower than anticipated enrollment and such delays may recur. Delays can be caused by, among other things, deaths or other adverse medical events; regulatory or patent issues; interim or final results of ongoing clinical trials; failure to enroll clinical sites as expected; competition for enrollment from other clinical trials; scheduling conflicts with participating clinicians and institutions; disagreements, disputes or other matters arising from collaborations; our inability to obtain necessary funding; or manufacturing problems.
Risks related to RELISTOR
We have been and expect to continue to be dependent on Valeant to develop and commercialize RELISTOR, exposing us to significant risks.
We rely on Valeant to pursue and complete further development and obtain regulatory approvals for RELISTOR worldwide. At present, our revenue is almost exclusively derived from royalty and milestone payments from our RELISTOR collaboration with Valeant, which can result in significant fluctuation in our revenue from period to period, and our past revenue is therefore not necessarily indicative of our future revenue. We are and will be dependent upon Valeant and any other business partners with which we may collaborate in the future to perform and fund development, including clinical testing of RELISTOR, making related regulatory filings and manufacturing and marketing products, including for new indications and in new formulations, in their respective territories. Revenue from the sale of RELISTOR depends entirely upon the efforts of Valeant and its sublicensees, which have significant discretion in determining the efforts and resources they apply to sales of RELISTOR. Valeant may not be effective in obtaining approvals for new indications or formulations, marketing existing or future products or arranging for necessary sublicense or distribution relationships. Our business relationships with Valeant and other partners may not be scientifically, clinically or commercially successful. For example, Valeant has a variety of marketed products and its own corporate objectives, which may not be consistent with our best interests, and may change its strategic focus or pursue alternative technologies in a manner that results in reduced or delayed revenue to us. Valeant may also have commercial and financial interests that are not fully aligned with ours in a given territory or territories - which may make it more difficult for us to fully realize the value of RELISTOR. We may have future disagreements with Valeant, which has significantly greater financial and managerial resources which it could draw upon in the event of a dispute. Such disagreements could lead to lengthy and expensive litigation or other dispute-resolution proceedings as well as extensive financial and operational consequences to us and have a material adverse effect on our business, results of operations and financial condition. In addition, independent actions may be taken by Valeant concerning product development, marketing strategies, manufacturing and supply issues, and rights relating to intellectual property.
Under our agreements with Valeant relating to RELISTOR, we rely on Valeant to, among other things, effectively commercialize the product and manage pricing, sales and marketing practices and inventory levels in the distribution channel. Assessing and reporting on these and other activities and metrics in connection with RELISTOR has been difficult as a result of financial reporting and internal control issues that have surfaced both at Valeant and its predecessor licensee, Salix. Our already limited visibility into the internal operations of Valeant and reliance on Valeant to accurately report information concerning the commercialization of RELISTOR has been further obscured by certain recent events at Valeant. As a result of certain incorrectly recognized revenues, both Valeant 's Form 10-K for 2015 and its Form 10-Q for the first quarter of 2016 were filed late, resulting in Valeant receiving notices of default from certain of its noteholders, in each instance. We remain exposed to Valeant's credit risk and the possibility of default under the RELISTOR License Agreement in the event that Valeant were to terminate the agreement at its discretion or to become insolvent or bankrupt. In the event of a Valeant bankruptcy, Valeant may be able to reject our agreement with it related to RELISTOR such that it would no longer be obligated to commercialize the product or provide related services, or to assign that agreement without our consent to third parties with an unknown capacity to commercialize and market the product, which could expose us to greater counterparty risk of breaches under such agreement. Valeant announced that it continued to work with its independent advisors in its ongoing assessment and remediation efforts with respect to financial reporting and internal controls in the second quarter of 2016. However, there is no assurance as to the adequacy of such efforts in averting future losses incurred in connection with any failures of Valeant's internal controls.
15
Table of Contents
We are also dependent on Valeant for compliance with regulatory requirements as they apply to RELISTOR. Valeant 's subsidiary, Salix is currently the subject of an SEC investigation, for which Valeant has indicated that as of the filing of its report on Form 10-Q for the quarterly period ended March 31, 2017, the SEC staff had substantially completed its investigation and will be making recommendations to the Commission in the near future and that it cannot predict the outcome of the SEC investigation or any other legal proceedings or any enforcement actions or other remedies that may be imposed on Salix or Valeant arising out of the SEC investigation. Additionally, Salix, beginning on November 7, 2014, became the target of three putative class action lawsuits filed by its shareholders. Valeant has indicated that as of the filing of its report on Form 10-Q for the quarterly period ended March 31, 2017, the parties reached an agreement in principle to settle the consolidated action, for which the court granted preliminary approval, but that there can be no assurance that the settling parties will ultimately enter a stipulation of settlement that the court will approve. Accordingly, no assurance can be given as to Valeant's financial condition or results of operations, or ability to meet its obligations to Progenics.
The RELISTOR commercialization program continues to be subject to risk.
Future developments in the commercialization of RELISTOR may result in Valeant or any other business partner with which we may collaborate in the future taking independent actions concerning product development, marketing strategies or other matters, including termination of its efforts to develop and commercialize the drug.
Under our license agreement with Valeant, Valeant is responsible for obtaining supplies of RELISTOR, including contracting with contract manufacturing organizations for supply of RELISTOR active pharmaceutical ingredient and subcutaneous and oral finished drug product. These arrangements may not be on terms that are advantageous and, as a result of our royalty and other interests in RELISTOR 's commercial success, will subject us to risks that the counterparties may not perform optimally in terms of quality or reliability.
Valeant 's ability to optimally commercialize either oral or subcutaneous RELISTOR in a given jurisdiction may be impacted by applicable labeling and other regulatory requirements. If clinical trials indicate, or regulatory bodies are concerned about, actual or possible serious problems with the safety or efficacy of RELISTOR, Valeant may stop or significantly slow further development or commercialization of RELISTOR. In such an event, we could be faced with either further developing and commercializing the drug on our own or with one or more substitute collaborators, either of which paths would subject us to the development, commercialization, collaboration and/or financing risks discussed in these risk factors.
We are also aware of other approved and marketed products, as well as product candidates in pre-clinical or clinical development that are intended to target the side effects of opioid pain therapy and are direct competitors to RELISTOR. For instance, there are three approved products that target opioid-induced constipation: MOVANTIK ® (naloxegol), AMITIZA ® (lubiprostone), and Symproic ® (naldemedine) which could compete with RELISTOR. The competitors who have developed these products and product candidates may have superior resources that allow them to implement more effective approaches to sales and marketing. There is no guarantee that RELISTOR will be able to compete commercially with these products. Additionally, there has been growing public concern regarding the use of opioid drugs. Any efforts by the FDA or other governmental authorities to restrict or limit the use of opioids may negatively impact the market for RELISTOR.
Any such significant action adverse to the further development and commercialization of RELISTOR could have a material adverse impact on our business and on the price of our stock.
RELISTOR patents are subject to generic challenge, and the validity, enforceability and commercial value of these patents are highly uncertain.
Third parties have challenged and are likely to continue challenging the patents that have been issued or licensed to us. In October 2015, we received notifications of Paragraph IV certifications with respect to certain patents for RELISTOR subcutaneous injection, which are listed in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations (the "Orange Book"). The certifications accompanied the filing by Actavis Inc. and Mylan Pharmaceuticals, Inc. of Abbreviated New Drug Applications (ANDAs) challenging such patents for RELISTOR subcutaneous injection.
We and our licensee for RELISTOR, Valeant, have timely filed suit and commenced litigation against Actavis and Mylan. FDA approval of the ANDA has been automatically stayed until the earlier of (i) 30 months from receipt of the notice or (ii) a District Court decision finding that the identified patents are invalid, unenforceable or not infringed.
In October 2016, we received a notification of a Paragraph IV certification with respect to certain patents for RELISTOR Tablets, which are listed in the Orange Book. The certification accompanied the filing by Actavis LLC of an ANDA challenging such patents for RELISTOR Tablets. Valeant has timely filed suit for patent infringement against Actavis to vigorously enforce RELISTOR intellectual property rights.
16
Table of Contents
In July 2017, we received notification of a Paragraph IV certification from Par Sterile Products, LLC with respect to Orange Book listed patents for RELISTOR subcutaneous injection. Valeant timely filed suit for patent infringement against Par.
In litigation relating to RELISTOR subcutaneous injection, a Motion And Brief For Partial Summary Judgment on the validity of U.S. Patent 8,552,025 was filed February 16, 2018 with the United States District Court of New Jersey. (See Item 3. Legal Proceedings for additional information relating to the parties and substance of this litigation).
In addition to the above described ANDA notifications, in October 2015, we also received notices of opposition to three European patents (EP 1615646, EP 2368554 and EP 2368553) relating to pharmaceutical compositions of methylnaltrexone. The oppositions were filed separately b y each of Actavis Group PTC ehf and Fresenius Kabi Deutschland GmbH. Decisions of revocation of the patents by the EU Opposition Division are under appeal.
Although we and Valeant are cooperating to defend against both the ANDA challenges and the European oppositions, and intend to continue vigorously enforcing RELISTOR intellectual property rights, such litigation is inherently subject to significant risks and uncertainties, and there can be no assurance that the outcome of these litigations will be favorable to Progenics or Valeant. An unfavorable outcome in these cases could result in the rapid genericization of RELISTOR products, or could result in the shortening of available patent life. Any such outcome could have a material impact on our financial performance and stock price.
Pursuant to the RELISTOR license agreement between us and Valeant, Valeant has the first right to enforce the intellectual property rights at issue and is responsible for the costs of such enforcement. At the same time, we may incur substantial further costs in supporting the effort to uphold the validity of patents or to prevent infringement.
The composition-of-matter patent for the active ingredient of RELISTOR, methylnaltrexone, was invented in the 1970 's and has expired. The University of Chicago, as well as we and our collaborators, have extended the methylnaltrexone patent estate with additional patents and pending patent applications covering various inventions relating to the product. Valeant has listed in the FDA Orange Book eight U.S. patents relating to subcutaneous RELISTOR, which have expiration dates ranging from 2017 to 2030. Eight Orange Book listed U.S. patents relating to RELISTOR tablets, have expiration dates ranging from 2017 to 2031. Four Canadian patents (expiring 2024 and 2029) have been listed with Health Canada relating to subcutaneous RELISTOR.
Risks Related to our Product Candidates
Even if our product candidates obtain marketing approval, our ability to generate revenue will be diminished if our products are not accepted in the marketplace, or if we select pricing strategies for our products that are less competitive than those of our competitors, or fail to obtain acceptable prices or an adequate level of reimbursement for products from third-party payers or government agencies.
The commercial success of our products will depend upon their acceptance by the medical community and third-party payers as clinically useful, cost effective and safe. Market acceptance of approved products, is affected by a wide range of factors, including the timing of regulatory approvals, product launches and the presence of generic, over-the-counter or other competitors; the pricing of the product and relative prices of competing products; product development efforts for new indications; the availability of reimbursement for the product; our ability to obtain sufficient commercial quantities of the product; success in arranging for necessary sublicense or distribution relationships; and general and industry-specific local and international economic pressures. If health care providers believe that patients can be managed adequately with alternative, currently available therapies, they may not prescribe our products, especially if the alternative therapies are viewed as more effective, as having a better safety or tolerability profile, as being more convenient to the patient or health care providers or as being less expensive. Third-party insurance coverage may not be available to patients for any products we develop. For pharmaceuticals administered in an institutional setting, the ability of the institution to be adequately reimbursed from government and health administration authorities, private health insurers and other third-party payers could also play a significant role in demand for our products. Significant uncertainty exists as to the reimbursement status of newly-approved pharmaceuticals. Government and other third-party payers increasingly are attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for indications for which the FDA has not granted labeling approval. In most foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the U.S., we expect that there will continue to be a number of federal and state proposals to implement similar government control and that the emphasis on managed care in the U.S. will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we can receive for any products in the future and adversely affect our ability to successfully commercialize our products. If any of our product candidates do not achieve market acceptance, we will likely lose our entire investment in that product candidate.
17
Table of Contents
We are subject to extensive and ongoing regulation, which can be costly and time consuming, may interfere with marketing approval for our product candidates, and can subject us to unanticipated limitations, restrictions, delays and fines.
Our business, products and product candidates are subject to comprehensive regulation by the FDA and comparable authorities in other countries, and include the Sunshine Act under the Patient Protection and Affordable Care Act ("PPACA"). These agencies and other entities regulate the pre-clinical and clinical testing, safety, effectiveness, approval, manufacture, labeling, marketing, export, storage, recordkeeping, advertising, promotion and other aspects of our products and product candidates. We cannot guarantee that approvals of product candidates, processes or facilities will be granted on a timely basis, or at all. If we experience delays or failures in obtaining approvals, commercialization of our product candidates will be slowed or stopped. In addition to these uncertainties, the U.S. House of Representatives voted in May 2017 to pass a bill similar to that which had previously and unsuccessfully sought to repeal PPACA, replace it with a curtailed system of tax credits and dissolve an expansion of the Medicaid program. While the fate of this bill in the U.S. Senate is uncertain, there is considerable uncertainty regarding the future of the current PPACA framework, and any changes will likely take time to unfold. As such, we cannot predict what effect the PPACA or other healthcare reform initiatives that may be adopted in the future will have on our business.
Even if we obtain regulatory approval for a product candidate, the approval may include significant limitations on indicated uses for which the product could be marketed or other significant marketing restrictions.
If we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may be subject to forced removal of a product from the market, product seizure, civil and criminal penalties and other adverse consequences.
Our products may face regulatory, legal or commercial challenges even after approval.
Even if a product receives regulatory approval:
It might not obtain labeling claims necessary to make the product commercially viable (in general, labeling claims define the medical conditions for which a drug product may be marketed, and are therefore very important to the commercial success of a product), or may be required to carry Boxed or other warnings that adversely affect its commercial success.
Approval may be limited to uses of the product for treatment or prevention of diseases or conditions that are relatively less financially advantageous to us than approval of greater or different scope or subject to an FDA imposed Risk Evaluation and Mitigation Strategy ("REMS") that imposes limits on the distribution or use of the product. While we may develop a product candidate with the intention of addressing a large, unmet medical need, the FDA or other foreign regulatory authorities may only approve the use of the drug for indications affecting a relatively small number of patients, thus greatly reducing the market size and our potential revenues.
Side effects identified after the product is on the market might hurt sales or result in mandatory safety labeling changes, additional pre-clinical testing or clinical trials, imposition of a REMS, product recalls or withdrawals from the market, reputational harm to us, and lawsuits (including class-action suits).
Efficacy or safety concerns regarding a marketed product, or manufacturing or other problems, may lead to a recall, withdrawal of marketing approval, marketing restrictions, reformulation of the product, additional pre-clinical testing or clinical trials, changes in labeling, imposition of a REMS, warnings and contraindications, the need for additional marketing applications, declining sales or other adverse events. These potential consequences may occur whether or not the concerns originate from subsequent testing or other activities by us, governmental regulators, other entities or organizations or otherwise, and whether or not they are scientifically justified. If products lose previously received marketing and other approvals, our business, results of operations and financial condition would be materially adversely affected.
In certain foreign jurisdictions , it cannot be marketed until pricing and reimbursement for the product is also approved.
We will be subject to ongoing FDA obligations and continuous regulatory review, and might be required to undertake post-marketing trials to verify the product 's efficacy or safety or other regulatory obligations.
18
Table of Contents
Our NDA filing for AZEDRA has been accepted, but approval may be delayed, conditioned , or denied by the FDA.
Approval of a product candidate as safe and effective for use in humans is never certain. There remains significant unce rtainty that AZEDRA will eventually receive regulatory approval. Additional testing results or adverse market conditions may cause us to withdraw the NDA in a timely manner. In addition, data obtained from clinical trials are susceptible to varying interpretations, and the FDA may not agree with our interpretation of our AZEDRA clinical trial results. The FDA has substantial discretion in the approval process and may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. Further, the FDA may not meet, or may extend, the PDUFA date with respect to our AZEDRA NDA, which may delay the start of any AZEDRA commercialization effort.
Any AZEDRA commercialization program would expose us to significant risk.
It is very difficult to estimate the commercial potential of product candidates , due to factors such as safety and efficacy compared to other available treatments (including potential generic drug alternatives with similar efficacy profiles), changing standards of care, third party payer reimbursement, patient and physician preferences and the availability of competitive alternatives that may emerge either during the approval process or after commercial introduction. Frequently, products that have shown promising results in clinical trials suffer significant setbacks even after they are approved for commercial sale.
Even if approved, there is no guarantee that AZEDRA will be a commercial success. Future uses of AZEDRA commercially may reveal that AZEDRA is ineffective, unacceptably toxic, has other undesirable side effects, is difficult to manufacture on a large scale, is not cost-effective or economically viable, infringes on proprietary rights of another party or is otherwise not fit for further use.
AZEDRA, designated as an Orphan Drug is intended to treat a rare disease with a small patient population. If pricing for AZEDRA is not approved or accepted in the market at an appropriate level it may not generate enough revenue to make it economically viable. Based upon the complication of delivery and high costs associated with the development of radiopharmaceuticals, among other factors, we expect that the price of AZEDRA will be high. There have been recent examples of the market reacting poorly to the high cost of certain drugs. If the market reacts similarly to AZEDRA, it could result in negative publicity and reputational harm to us. Further, the Trump administration has indicated support for possible new measures related to drug pricing, which could increase the pricing pressures related to AZEDRA and further limit its economic viability.
Additionally, we have little experience as a company in commercializing products. Given this lack of experience, there is a heightened risk that we are able to adequately commercialize AZEDRA. If AZEDRA is determined to be unsafe or ineffective in humans, not economically viable or we are unable to successfully commercialize it, our business will be materially adversely affected.
Our relationships with customers and third-party payers are or may become subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Health care providers, physicians and third-party payers play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payers and customers will or already do require us and them to comply with broadly applicable fraud and abuse and other health care laws and regulations, including both federal and state anti-kickback and false claims laws, that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products that obtain marketing approval. Efforts to ensure that business arrangements comply with applicable health care laws and regulations involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If such operations are found to be in violation of any of these laws or other applicable governmental regulations, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of related operations. If physicians or other providers or entities involved with our products are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, which may adversely affect us.
19
Table of Contents
If we or our partners are unable to obtain sufficient quantities of the raw and bulk materials needed to make our products or product candidates, development of our products or product candidates or commercialization of our approved products could be slowed or stopped.
We or our partners may not be able to obtain the materials necessary to make a particular product or product candidate in adequate volume and quality. If any materials needed to make a product or product candidate is insufficient in quantity or quality, if a supplier fails to deliver in a timely fashion or at all or if these relationships terminate, we or our partners may not be able to fulfill manufacturing obligations for our products or product candidates, either on our own or through third-party suppliers. A delay or disruption of supplies of our products or product candidates would have a material adverse effect on our business as a whole. Our existing arrangements with suppliers may result in the supply of insufficient quantities of our product candidates needed to accomplish our clinical development programs or commercialization, and we may not have the right and in any event , do not currently have the capability to manufacture these products if our suppliers are unable or unwilling to do so. We currently arrange for supplies of critical raw materials used in production of our product candidates from single sources. We do not have long-term contracts with any of these suppliers. Any delay or disruption in the availability of raw materials would slow or stop product development and commercialization of the relevant product.
Manufacturing resources could limit or adversely affect our ability to commercialize products.
We or our partners may engage third parties to manufacture our product candidates. We or our partners may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturers at acceptable costs.
In order to commercialize our product candidates successfully, we need to be able to manufacture or arrange for the manufacture of products in commercial quantities, in compliance with regulatory requirements, at acceptable costs and in a timely manner. Manufacture of our product candidates, can be complex, difficult to accomplish even in small quantities, difficult to scale-up for large-scale production and subject to delays, inefficiencies and low yields of quality products. The manufacture of radiopharmaceuticals is relatively complex and requires significant capital expenditures. We rely on contract manufacturing organizations to manufacture active pharmaceutical ingredient and finished drug products for clinical trial supplies and for commercial supply of AZEDRA. The cost of manufacturing our product candidates may make them prohibitively expensive. If adequate supplies of any of our product candidates or related materials are not available on a timely basis or at all, our clinical trials or commercialization of our product candidates could be seriously delayed, since these materials are time consuming to manufacture and cannot be readily obtained from third-party sources. We continue to be dependent on a limited number of highly specialized manufacturing and development partners, including single source manufacturers for certain of our product candidates. If we were to lose one or more of these key relationships, it could materially adversely affect our business. Establishing new manufacturing relationships, or creating our own manufacturing capability, would require significant time, capital and management effort, and the transfer of product-related technology and know-how from one manufacturer to another is an inherently complex and uncertain process.
Failure of any manufacturer of our various product candidates to comply with applicable regulatory requirements could subject us to penalties and have a material adverse effect on supplies of our product candidates.
Third-party manufacturers are required to comply with cGMP or similar regulatory requirements outside of the U.S. If manufacturers of our product candidates cannot successfully manufacture material that conforms to the strict regulatory requirements of the FDA and any applicable foreign regulatory authority, they may not be able to supply us with our product candidates. If these facilities are not approved for commercial manufacture, we may need to find alternative manufacturing facilities, which could result in delays of several years in obtaining approval for a product candidate. We do not control the manufacturing operations and are completely dependent on our third-party manufacturing partners or contractors for compliance with the applicable regulatory requirements for the manufacture of some of our product candidates. Manufacturers are subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMP and similar regulatory requirements. Failure of any manufacturer of any of our product candidates to comply with applicable cGMP or other regulatory requirements could result in sanctions being imposed on our collaborators or us, including fines, injunctions, civil penalties, delays, suspensions or withdrawals of approvals, operating restrictions, interruptions in supply and criminal prosecutions, any of which could significantly and adversely affect supplies of our product candidates and have a material adverse impact on our business, financial condition and results of operations.
20
Table of Contents
The validity, enforceability and commercial value of our patents and other intellectual property rights are highly uncertain.
We own or license a number of issued patents. We must obtain, maintain and enforce patent and other rights to protect our intellectual property. The patent position of biotechnology and pharmaceutical firms is highly uncertain and involves many complex legal and technical issues. There are many laws, regulations and judicial decisions that dictate and otherwise influence the manner in which patent applications are filed and prosecuted and in which patents are granted and enforced, all of which are subject to change from time to time. There is no clear policy involving the breadth of claims allowed, or the degree of protection afforded, under patents in this area. In addition, we are aware of others who have patent applications or patents containing claims similar to or overlapping those in our patents and patent applications. Accordingly, patent applications owned by or licensed to us may not result in patents being issued. Even if we own or license a relevant issued patent, we may not be able to preclude competitors from commercializing drugs that may compete directly with one or more of our products or product candidates, in which event such rights may not provide us with any meaningful competitive advantage. In the absence or upon successful challenge of patent protection, drugs may be subject to generic competition, which could adversely affect pricing and sales volumes of the affected products.
It is generally difficult to determine the relative strength or scope of a biotechnology or pharmaceutical patent position in absolute terms at any given time. The issuance of a patent is not conclusive as to its validity or enforceability, which can be challenged in litigation or via administrative proceedings. The license agreements from which we derive or out-license intellectual property provide for various royalty, milestone and other payment, commercialization, sublicensing, patent prosecution and enforcement, insurance, indemnification and other obligations and rights, and are subject to certain reservations of rights. While we generally have the right to defend and enforce patents licensed to or by us, either in the first instance or if the licensor or licensee chooses not to do so, we must usually bear the cost of doing so.
Patents have a limited life and expire by law.
In addition to uncertainties as to scope, validity, enforceability and changes in law, patents by law have limited lives. Upon expiration of patent protection, our drug candidates and/or products may be subject to generic competition, which could adversely affect pricing and sales volumes of the affected products.
The original patents surrounding the AZEDRA program were licensed from the University of Western Ontario ("UWO"). The patent family directed to processes for making polymer precursors, as well as processes for making the final product, expire in 2018 in the U.S. and Canada. Other licensed patent families from UWO relate to alternative approaches for preparing AZEDRA, which if implemented would expire in 2024, worldwide. Progenics has pending applications worldwide directed to manufacturing improvements and the resulting compositions which, if issued, would expire in 2035.
Owned and in-licensed patents relating to the 1404 product candidate have expiration ranges of 2020 to 2029; we view as most significant the composition-of-matter patent on the compound, as well as technetium-99 labeled forms, which expires in 2029 worldwide. Patent applications directed to methods of use are pending worldwide, which if issued would expire in 2034.
Patent protection for the composition-of-matter patent on PyL compound, radiolabeled form of the compound, as well as methods of use expire in 2030 in the United States. Corresponding patent family members are pending or issued worldwide, all with expirations of 2029.
Company-owned patents relating to MIP-1095 have expiration ranges of 2027 to 2031 in the U.S. We view as most significant the composition-of-matter patent on this compound, as well as radiolabeled forms, which expires in 2027 in the U.S., as well as Europe. Additional U.S. patents are directed to stable compositions and radiolabeling processes which expire in 2030 and 2031, respectively.
We own patents relating to automated detection of bone cancer metastases through. The patents on this technology expire in 2028.
With respect to PSMA antibody, currently issued composition-of-matter patents comprising co-owned and in-licensed patents have expiration ranges of 2022 to 2023 in the U.S. Corresponding foreign counterpart patents will expire 2022. We view all of these patents as significant.
21
Table of Contents
We depend on intellectual property licensed from third parties and unpatented technology, trade secrets and confidential information. If we lose any of these rights, including by failing to achieve milestone requirements or to satisfy other conditions, our business, results of operations and financial condition could be harmed.
Most of our product candidates incorporate intellectual property licensed from third parties. For example, PyL utilizes technology licensed to us from Johns Hopkins University. We could lose the right to patents and other intellectual property licensed to us if the related license agreement is terminated due to a breach by us or otherwise. Our ability to commercialize products incorporating licensed intellectual property would be impaired if the related license agreements were terminated. In addition, we are required to make substantial cash payments, achieve milestones and satisfy other conditions, including filing for and obtaining marketing approvals and introducing products, to maintain rights under our intellectual property licenses. Due to the nature of these agreements and the uncertainties of development, we may not be able to achieve milestones or satisfy conditions to which we have contractually committed, and as a result may be unable to maintain our rights under these licenses. If we do not comply with our license agreements, the licensors may terminate them, which could result in our losing our rights to, and therefore being unable to commercialize, related products.
We also rely on unpatented technology, trade secrets and confidential information. Third parties may independently develop substantially equivalent information and techniques or otherwise gain access to our technology or disclose our technology, and we may be unable to effectively protect our rights in unpatented technology, trade secrets and confidential information. We require each of our employees, consultants and advisors to execute a confidentiality agreement at the commencement of an employment or consulting relationship with us. These agreements may, however, not provide effective protection in the event of unauthorized use or disclosure of confidential information. Any loss of trade secret protection or other unpatented technology rights could harm our business, results of operations and financial condition.
If we do not achieve milestones or satisfy conditions regarding some of our product candidates, we may not maintain our rights under related licenses.
We are required to make substantial cash payments, achieve milestones and satisfy other conditions, including filing for and obtaining marketing approvals and introducing products, to maintain rights under certain intellectual property licenses. Due to the nature of these agreements and the uncertainties of research and development, we may not be able to achieve milestones or satisfy conditions to which we have contractually committed, and as a result may be unable to maintain our rights under these licenses. If we do not comply with our license agreements, the licensors may terminate them, which could result in our losing our rights to, and therefore being unable to commercialize, related products.
If we infringe third-party patent or other intellectual property rights, we may need to alter or terminate a product development program.
There may be patent or other intellectual property rights belonging to others that require us to alter our products, pay licensing fees or cease certain activities. If our products infringe patent or other intellectual property rights of others, the owners of those rights could bring legal actions against us claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products. We may not prevail in any action brought against us, and any license required under any rights that we infringe may not be available on acceptable terms or at all. We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example, we are aware of other groups investigating PSMA or related compounds and monoclonal antibodies directed at PSMA, PSMA-targeted imaging agents and therapeutics, and methylnaltrexone and other peripheral opioid antagonists, and of patents held, and patent applications filed, by these groups in those areas. While the validity of these issued patents, the patentability of these pending patent applications and the applicability of any of them to our products and programs are uncertain, if asserted against us, any related patent or other intellectual property rights could adversely affect our ability to commercialize our products.
Research, development and commercialization of a biopharmaceutical product often require choosing between alternative development and optimization routes at various stages in the development process. Preferred routes may depend on subsequent discoveries and test results and cannot be predicted with certainty at the outset. There are numerous third-party patents in our field, and we may need to obtain a license under a patent in order to pursue the preferred development route of one or more of our products or product candidates. The need to obtain a license would decrease the ultimate profitability of the applicable product. If we cannot negotiate a license, we might have to pursue a less desirable development route or terminate the program altogether.
22
Table of Contents
We have been and expect to continue to be dependent on collaborators for the development, manufacturing and sales of certain products and product candidates, which expose us to the risk of reliance on these collaborators.
In conducting our operations, we currently depend, and expect to continue to depend, on numerous collaborators. Key among these new collaborations, are those with Bayer to develop and commercialize products using our PSMA antibody technology and with Fuji for the development and commercialization of 1404 and bone BSI in Japan. In addition, certain clinical trials for our product candidates may be conducted by government-sponsored agencies, and consequently will be dependent on governmental participation and funding. These arrangements expose us to the same considerations we face when contracting with third parties for our own trials.
If any of our collaborators breach or terminate its agreement with us or otherwise fail to conduct successfully and in a timely manner the collaborative activities for which they are responsible, the preclinical or clinical development or commercialization of the affected product candidate or research program could be delayed or terminated. We generally do not control the amount and timing of resources that our collaborators devote to our programs or product candidates. We also do not know whether current or future collaboration partners, if any, might pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases or conditions targeted by our collaborative arrangements. Our collaborators are also subject to similar development, regulatory, manufacturing, cyber-security and competitive risks as us, which may further impede their ability to successfully perform the collaborative activities for which they are responsible. Setbacks of these types to our collaborators could have a material adverse effect on our business, results of operations and financial condition.
We are dependent upon third parties for a variety of functions. These arrangements may not provide us with the benefits we expect.
We rely on third parties to perform a variety of functions. We are party to numerous agreements which place substantial responsibility on clinical research organizations, consultants and other service providers for the development of our product candidates. We also rely on medical and academic institutions to perform aspects of our clinical trials of product candidates. In addition, an element of our research and development strategy has been to in-license technology and product candidates from academic and government institutions in order to minimize or eliminate investments in early research. We may not be able to enter new arrangements without undue delays or expenditures, and these arrangements may not allow us to compete successfully. Moreover, if third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct clinical trials in accordance with regulatory requirements or applicable protocols, our product candidates may not be approved for marketing and commercialization or such approval may be delayed. If that occurs, we or our collaborators will not be able, or may be delayed in our efforts, to commercialize our product candidates.
Business and Operational Risks
We lack sales and marketing experience .
We have not had established sales, marketing or distribution infrastructure but are building out this capability in advance of the possible approval and launch of AZEDRA in the U.S. We may not be successful in developing an effective commercial infrastructure or in achieving sufficient market acceptance. We do plan to market and sell products through distribution, co-marketing, co-promotion or licensing arrangements with third parties for territories outside the U.S. We may consider contracting with a third-party professional pharmaceutical detailing and sales organization to perform marketing functions for one or more products. To the extent that we enter into distribution, co-marketing, co-promotion, detailing or licensing arrangements for the marketing and sale of product candidates, any revenues we receive will depend primarily on the efforts of third parties. We will not control the amount and timing of marketing resources these third parties devote to our products.
We are involved in various legal proceedings that are uncertain, costly and time-consuming and could have a material adverse impact on our business, financial condition and results of operations and could cause the market value of our common stock to decline.
From time to time we are involved in legal proceedings and disputes and may be involved in litigation in the future. These proceedings are complex and extended and occupy the resources of our management and employees. These proceedings are also costly to prosecute and defend and may involve substantial awards or damages payable by us if not found in our favor. We may also be required to pay substantial amounts or grant certain rights on unfavorable terms in order to settle such proceedings. Defending against or settling such claims and any unfavorable legal decisions, settlements or orders could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common stock to decline. For more information regarding legal proceedings, see Item 1 of Part II, and Note 8 in the notes to the consolidated financial statements in Part I, Item 1 of this Form 10-Q.
23
Table of Contents
In particular, the pharmaceutical and medical device industries historically have generated substantial litigation concerning the manufacture, use and sale of products and we expect this litigation activity to continue. As a result, we expect that patents related to our products will be routinely challenged, and our patents may not be upheld. In order to protect or enforce patent rights, we may initiate litigation against third parties. If we are not successful in defending an attack on our patents and maintaining exclusive rights to market one or more of our products still under patent protection, we could lose a significant portion of sales in a very short period. We may also become subject to infringement claims by third parties and may have to defend against charges that we violated patents or the proprietary rights of third parties. If we infringe the intellectual property rights of others, we could lose our right to develop, manufacture or sell products, or could be required to pay monetary damages or royalties to license proprietary rights from third parties.
In addition, in the U.S., it has become increasingly common for patent infringement actions to prompt claims that antitrust laws have been violated during the prosecution of the patent or during litigation involving the defense of that patent. Such claims by direct and indirect purchasers and other payers are typically filed as class actions. The relief sought may include treble damages and restitution claims. Similarly, antitrust claims may be brought by government entities or private parties following settlement of patent litigation, alleging that such settlements are anti-competitive and in violation of antitrust laws. In the U.S. and Europe, regulatory authorities have continued to challenge as anti-competitive so-called "reverse payment" settlements between branded and generic drug manufacturers. We may also be subject to other antitrust litigation involving competition claims unrelated to patent infringement and prosecution. A successful antitrust claim by a private party or government entity against us could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common stock to decline.
We are exposed to product liability claims, and in the future may not be able to obtain insurance against claims at a reasonable cost or at all.
Our business exposes us to product liability risks, which are inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. We may not be able to avoid product liability exposure. If a product liability claim is successfully brought against us, our financial position may be adversely affected. Product liability insurance for the biopharmaceutical industry is generally expensive, when available at all, and may not be available to us at a reasonable cost in the future. Our current insurance coverage and indemnification arrangements may not be adequate to cover claims brought against us, and are in any event subject to the insuring or indemnifying entity discharging its obligations to us.
We, our CMOs and our distributors handle hazardous materials and must comply with environmental laws and regulations, which can be expensive and restrict how we our CMOs or our distributors do business. If we our CMOs or our distributors are involved in a hazardous waste spill or other accident, we our CMOs or our distributors could be liable for damages, penalties or other forms of censure.
Research and development work and manufacturing processes with our pipeline products involve the use of hazardous, controlled and/or radioactive materials. We, our CMOs and our distributors are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials. In particular we, our CMOs and our distributors are subject to regulation by the U.S. Environmental Protection Agency, U.S. Department of Transportation, Occupational Safety and Health Administration and comparable state regulatory agencies. Despite procedures that we, our CMOs and our distributors implement for handling and disposing of these materials, the risk of accidental contamination or injury cannot be eliminated. In the event of a hazardous waste spill or other accident, we, our CMOs and our distributors could be liable for damages, penalties or other forms of censure. There may be significant costs to comply with applicable environmental laws and regulations in the future, and such costs may be incurred by us directly or passed through to us by our CMOs and our distributors. In the event of the damages, penalties, censures or higher costs outlined above, the efficiency and cost of our research, development and commercialization pipeline may be adversely impacted.
If we lose key personnel on whom we depend, our business could suffer.
We are dependent upon our key management, commercial and scientific personnel, the loss of whom could require us to identify and engage qualified replacements, and could cause our management and operations to suffer in the interim. Competition for qualified employees among companies in the biopharmaceutical industry is intense. Future success in our industry depends in significant part on the ability to attract, retain and motivate highly skilled employees, which we may not be successful in doing.
24
Table of Contents
Health care reform measures could adversely affect our operating results and our ability to obtain marketing approval of and to commercialize our product candidates.
In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the health care system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval. For example, the Trump administration has indicated support for possible new measures related to drug pricing. New government legislation or regulations related to pricing or government or third-party payer decisions not to approve pricing for, or provide adequate coverage and reimbursements of, our products, hold the potential to severely limit market opportunities of such products. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. In addition, increased scrutiny by the U.S. Congress of the FDA 's approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements. In the U.S., federal legislation has changed the way Medicare covers and pays for pharmaceutical products. Cost reduction initiatives and other provisions of legislation have decreased coverage and reimbursement. Though such legislation applies only to drug benefits for Medicare beneficiaries, private payers often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. More recent legislation is intended to broaden access to health insurance, further reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, and impose new taxes and fees on the health industry and additional health policy reforms. New laws impose significant annual fees on companies that manufacture or import branded prescription drug products, and contain substantial new compliance provisions, which in each case may affect our business practices with health care practitioners. Subject to federal and state agencies issuing regulations or guidance, it appears likely that new laws will continue to pressure pharmaceutical pricing, especially under the Medicare program, and may also increase regulatory burdens and operating costs. We cannot be sure whether additional legislative changes will be enacted, whether the FDA regulations, guidance or interpretations will be changed or what the impact of such changes on the marketing approvals of our product candidates, if any, may be.
Our future depends on the proper management of our current and future business operations, including the associated expenses.
Our business strategy requires us to manage our business to provide for the continued development and potential commercialization of our proprietary and partnered product candidates. Our strategy also calls for us to undertake increased research and development activities and to manage an increasing number of relationships with partners and other third parties, while simultaneously managing the capital necessary to support this strategy. If we are unable to manage effectively our current operations and any growth we may experience, our business, financial condition and results of operations may be adversely affected. If we are unable to effectively manage our expenses, we may find it necessary to reduce our personnel-related costs through reductions in our workforce, which could harm our operations, employee morale and impair our ability to retain and recruit talent. Furthermore, if adequate funds are not available, we may be required to obtain funds through arrangements with partners or other sources that may require us to relinquish rights to certain of our technologies, products or future economic rights that we would not otherwise relinquish or require us to enter into other financing arrangements on unfavorable terms.
Risks associated with our operations outside of the United States could adversely affect our business.
Although we currently conduct most of our business in the U.S., we also conduct business internationally, which exposes us to additional risks, including risks associated with foreign legal requirements, economic and political conditions and fluctuations in foreign currency exchange rates. We expect that we will continue to conduct business internationally. These business operations subject us to a number of risks and uncertainties, including but not limited to:
● | changes in international regulatory and compliance requirements that could restrict our ability to develop, market and sell our products; |
● | political and economic instability; |
● | the impact of any trade or international regulatory policy changes brought about by the new U.S. federal administration; |
● | diminished protection of intellectual property in some countries outside of the U.S.; |
● | trade protection measures and import or export licensing requirements; |
● | difficulty in staffing and managing international operations; |
● | differing labor regulations and business practices; |
● | heightened risk of a failure of our overseas employees to comply with U.S. and foreign laws, including export regulations, the FCPA and trade regulations; |
25
Table of Contents
● | potentially negative consequences from changes in or interpretations of tax laws; |
● | changes in international medical reimbursement policies and programs; |
● | financial risks such as longer payment cycles, difficulty collecting accounts receivable and exposure to fluctuations in foreign currency exchange rates; |
● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors ' and service providers' activities that may fall within the purview of the FCPA or similar foreign laws such as the U.K. Bribery Act; and |
● | regulatory and compliance risks that relate to data practices and privacy, including those resulting from the EU adopted General Data Protection Regulation, which becomes effective in May 2018 and imposes monetary penalties for non-compliance. |
Any of these factors may, individually or as a group, have a material adverse effect on our business and results of operations. These or other similar risks could adversely affect our revenue and profitability.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance of our future products. If there is not sufficient reimbursement for our future products, it is less likely that such products will be widely used.
Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on payments allowed for lower-cost products that are already reimbursed, may be incorporated into existing payments for other products or services, and may reflect budgetary constraints and/or imperfections in Medicare or Medicaid data used to calculate these rates. Net prices for drugs may be reduced by mandatory discounts or rebates required by government health care programs. Such legislation, or similar regulatory changes or relaxation of laws that restrict imports of drugs from other countries, could reduce the net price we receive for any future marketed drugs. As a result, our future drugs might not ultimately be considered cost-effective.
We cannot be certain that reimbursement will be available for AZEDRA or any other drug candidates that we develop. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for, any future drugs. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize AZEDRA or any other drug candidates that we develop.
In general, other factors that could affect the demand for and sales and profitability of our future products include, but are not limited to:
● | the timing of regulatory approval, if any, of competitive drugs; |
● | our or any other of our partners' pricing decisions, as applicable, including a decision to increase or decrease the price of a drug, and the pricing decisions of our competitors; |
● | government and third-party payor reimbursement and coverage decisions that affect the utilization of our future drugs and competing drugs; |
● | negative safety or efficacy data from new clinical studies conducted either in the U.S. or internationally by any party could cause the sales of our future drugs to decrease or a future drug to be recalled; |
● | the degree of patent protection afforded our future drugs by patents granted to or licensed by us and by the outcome of litigation involving our or any of our licensor's patents; |
● | the outcome of litigation involving patents of other companies concerning our future drugs or processes related to production and formulation of those drugs or uses of those drugs; the increasing use and development of alternate therapies; |
● | the rate of market penetration by competing drugs; and |
● | the termination of, or change in, existing arrangements with our partners. |
Any of these factors could have a material adverse effect on the sales of any drug candidates that we may commercialize in the future.
26
Table of Contents
A significant disruption in our information technology systems or a cyber-security breach could compromise our clinical/patient data or other data, trade secrets and confidential information and adversely affect our business.
We, our current and future contract research organizations, CMOs, licensees and partners are increasingly dependent on critical, complex and interdependent information technology systems to operate our businesses. Like other companies in our industry, we rely on such systems for many aspects of our business. The size and complexity of our information technology systems make them potentially vulnerable to breakdown, malicious cyber-attacks, intrusion, viruses and data security breaches by computer hackers, foreign governments, foreign companies or competitors, or by employee error or malfeasance. Such events may permit unauthorized persons to access, misappropriate and/or destroy sensitive data and result in the impairment or disruption of important business processes, loss or misuse of trade secrets, confidential information or other proprietary intellectual property or public exposure of personal information (including sensitive personal information) of employees, business partners, clinical trial patients, customers and others. Any compromise of our data security could also result in a violation of applicable privacy and other laws and a loss of confidence in our data security measures. Any of the foregoing could have a material adverse effect on our business, prospects, reputation, operating results and financial condition, including as a result of our being required to make significant investments to fix, fortify or replace our technology systems and/or being subject to lawsuits, fines, penalties or other government action. There can be no assurance that our efforts to protect our data and information technology systems will prevent breakdowns or breaches in our systems, or those of third parties with which we do business.
If our facility or those of our vendors incur damage or power is lost for a significant length of time, our business will suffer.
We store some of our preclinical and clinical data at our facilities as well as those of our vendors. Any significant degradation or failure of our or our vendors ' computer systems could cause us to inaccurately calculate or lose our data.
In addition, our stability samples are stored at our vendors ' facilities. If their facilities incur physical damage or have an extended power failure, it could result in a loss of these samples. Loss of our clinical data or stability samples could result in significant delays in our drug development process and could harm our business and operations.
Competitive Risks
Competing products in development may adversely affect acceptance of our future products.
We are aware of a number of products and product candidates which compete or may potentially compete with our future products. Any of these approved products or product candidates, or others which may be developed in the future, may achieve a significant competitive advantage relative to our future products and, in any event, the existing or future marketing and sales capabilities of these competitors may impair our or our collaborators ' ability to compete effectively in the market.
We are also aware of competitors, who are developing alternative treatments for disease targets to which our research and development programs are directed, any of which – or others which may be developed in the future – may achieve a significant competitive advantage relative to any future product we may develop.
Marketplace acceptance depends in part on competition in our industry, which is intense, and competing products in development may adversely affect acceptance of our products.
The extent to which any of our future products achieves market acceptance will depend on competitive factors. Competition in the biopharmaceutical industry is intense and characterized by ongoing research and development and technological change. We face competition from many for-profit companies and major universities and research institutions in the U.S. and abroad. We face competition from companies marketing existing products or developing new products for diseases and conditions targeted by our technologies. We are aware of a number of products and product candidates which compete or may potentially compete with PSMA-targeted imaging agents and therapeutics, or our other product candidates. We are aware of several competitors, such as Janssen Biotech, Inc., Aytu Bioscience, Inc. and Bayer HealthCare Pharmaceuticals Inc., which have received approval for or are developing treatments or diagnostics for prostate cancer. Any of these competing approved products or product candidates, or others which may be developed in the future, may achieve a significant competitive advantage relative to 1404, AZEDRA, PyL, 1095, or other product candidates.
Competition with respect to our technologies and product candidates is based on, among other things, product efficacy, safety, reliability, method of administration, availability, price and clinical benefit relative to cost; timing and scope of regulatory approval; sales, marketing and manufacturing capabilities; collaborator capabilities; insurance and other reimbursement coverage; and patent protection. Competitive disadvantages in any of these factors could materially harm our business and financial condition. Many of our competitors have substantially greater research and development capabilities and experience and greater manufacturing, marketing, financial and managerial resources than we do. These competitors may develop products that are superior to those we are developing and render our products or technologies non-competitive or obsolete. Our product candidates under development may not compete successfully with existing products or product candidates under development by other companies, universities and other institutions. Drug manufacturers that are first in the market with a therapeutic for a specific indication generally obtain and maintain a significant competitive advantage over later entrants and therefore, the speed with which industry participants move to develop products, complete clinical trials, approve processes and commercialize products is an important competitive factor. If our product candidates receive marketing approval but cannot compete effectively in the marketplace, our operating results and financial position would suffer.
27
Table of Contents
Financial Risks
We have outstanding debt - and failure by us or our royalty subsidiary to fulfill our obligations under the applicable loan agreements may cause the repayment obligations to accelerate.
In November 2016, our subsidiary, MNTX Royalties, entered into a loan agreement (the "Royalty-Backed Loan") with HealthCare Royalty Partners III, L.P. ("HCRP") pursuant to which MNTX Royalties borrowed $50 million and had the ability, subject to mutual agreement with HCRP, to borrow an additional $50 million up to twelve months after the initial closing date of the loan. The loan will be repaid from the royalty payments from the commercial sales of RELISTOR products owed under our agreement with Valeant.
The obligations of MNTX Royalties under the loan agreement to repay the Royalty-Backed Loan may be accelerated upon the occurrence of certain events of default, including but not limited to, if:
● | MNTX Royalties fails to pay any principal or interest (except as permitted) within three Business days of when such payment is due and payable or otherwise made in accordance with the terms of the Royalty-Backed Loan; |
● | MNTX Royalties fails to pay when due any indebtedness of $15 thousand or more; |
● | any representation or warranty made by MNTX Royalties in the loan agreement or any other transaction document proves to be incorrect or misleading in any material respect when made, and such failure is uncured on or before the 30th day following notice thereof; |
● | MNTX Royalties fails to perform or observe any covenant or agreement contained in the loan agreement or any other transaction document; |
● | any uninsured judgment, decree, or order in an amount in excess of $25 thousand is rendered against MNTX Royalties and enforcement proceedings have commenced upon such judgment, decree, or order or such judgment, decree, or order has not been stayed or bonded pending appeal, vacated, or discharged, within 30 days from entry; |
● | any of a set of defined insolvency events occurs; |
● | we default under the agreement pursuant to which we contributed the royalty and related rights under the RELISTOR license to MNTX Royalties, and such default is continuing; |
● | any of the loan transaction documents cease to be in full force and effect or valid and enforceable; |
● | MNTX Royalties fails to perform or observe any covenant or agreement contained in any material contract and such failure is not cured or waived within any applicable grace period; |
● | the agreement with Valeant is terminated or cancelled and is not replaced within 270 days after such termination or cancellation; and |
● | any security interest purported to be created by the loan agreement or the related agreement ceases to be in full force and effect, or any rights, powers, and privileges purported to be created and granted under the loan agreement or such security agreement ceases to be in full force and effect. |
In connection with the Royalty-Backed Loan, MNTX Royalties granted a first priority lien and security interest (subject only to certain defined permitted liens) in all of its assets and all real, intangible and personal property, including all of its right, title, and interest in and to the royalty payments under our agreement with Valeant. Under the terms of the loan agreement, HCRP has no recourse for non-payment of the Royalty-Backed Loan to Progenics Pharmaceuticals, Inc., or to any of our assets other than the RELISTOR royalty rights held by MNTX Royalties. However, Progenics Pharmaceuticals, Inc. does have certain obligations that run to the benefit of HCRP with respect to the representations, warranties and covenants it makes under the agreement pursuant to which we contributed the royalty and related rights under the RELISTOR License to MNTX Royalties. A breach of these obligations could lead to recourse against Progenics Pharmaceuticals, Inc. with respect to any losses suffered by HCRP as a result of such breach.
Our level of indebtedness could adversely affect our ability to raise additional capital to fund our operations, and limit our ability to react to changes in the economy or our industry.
As of December 31, 2017, our outstanding non-recourse long term debt amounted to $49.7 million. This level could have adverse consequences for us, including:
● | heightening our vulnerability to downturns in our business or our industry or the general economy and restricting us from making improvements or acquisitions, or exploring business opportunities; and |
● | limiting our ability to adjust to changing market conditions and placing us at a competitive disadvantage compared to our competitors who have greater capital resources. |
28
Table of Contents
Developing product candidates requires us to obtain additional financing from time to time. Our access to capital funding is uncertain.
We incur significant costs to develop our product candidates and to prepare for the possible launch of AZEDRA. We do not have committed external sources of funding for these projects. We fund our operations, to a significant extent, with the proceeds from capital-raising. We may do so via equity securities issuances in public offerings, through our three-year facility with an investment bank pursuant to which we have sold our stock in at-the-market transactions for net proceeds of approximately $14.5 million and may sell from time to time up to a remaining $60.0 million of our stock, or through further debt financing. We may also fund operations through collaboration, license, further royalty financings, private placement or other agreements with one or more pharmaceutical or other companies, or the receipt of milestone and other payments for out-licensed products. To the extent we raise additional capital by issuing equity securities, existing stockholders could experience substantial dilution, and if we issue securities other than common stock, new investors could have rights superior to existing stockholders. Any further debt financing that we may obtain may involve operating covenants that restrict our business and significant repayment obligations. To the extent we raise additional funds through new collaboration and licensing arrangements, we may be required to relinquish some rights to technologies or product candidates, or grant licenses on terms that are not favorable to us.
We cannot predict with certainty when we will need additional funds, how much we will need, the form a financing may take or whether additional funds will be available at all. The variability of conditions in global financial and credit markets may exacerbate the difficulty of timing capital raising or other financing, as a result of which we may seek to consummate such transactions substantially in advance of immediate need. Our need for future funding will depend on numerous factors, including the advancement of existing product development projects and the availability of new projects; the achievement of events, most of which are out of our control and depend entirely on the efforts of others, triggering milestone payments to us; the progress and success of clinical trials and pre-clinical activities (including studies and manufacturing) involving product candidates, whether conducted by collaborators or us; the progress of research programs carried out by us; changes in the breadth of our research and development programs; the progress of research and development efforts of collaborators; our ability to acquire or license necessary, useful or otherwise attractive technologies; competing technological and market developments; the costs and timing of obtaining, enforcing and defending patent and other intellectual property rights; the costs and timing of regulatory filings and approvals; our ability to manage Progenics ' growth or contraction; and unforeseen litigation. These factors may be more important with respect to product candidates and programs that involve technologies with which we have limited prior experience. Insufficient funds may require us to delay, scale back or eliminate some or all of our research and development or commercialization programs, cause us to lose rights under existing licenses or to relinquish greater or all rights to product candidates at an earlier stage of development or on less favorable terms than we would otherwise choose and may adversely affect our ability to operate as a going concern. We may not be able at a given necessary time to obtain additional funding on acceptable terms, or at all. Our inability to raise additional capital on terms reasonably acceptable to us would seriously jeopardize our business.
We have a history of operating losses.
We have incurred substantial losses throughout its history. A large portion of our revenue has historically consisted of upfront and milestone payments from licensing transactions. We have reported operating losses for 2017 and 2015, while we reported operating income for 2016, as a result of a milestone payment from Valeant. The timing and amount of any similar transactions in the future is highly unpredictable and uncertain. Without upfront or other such payments, we operate at a loss, due in large part to the significant research and development expenditures required to identify and validate new product candidates and pursue our development efforts. Moreover, we have derived no significant revenue from product sales and have only in the last several years derived revenue from royalties. We may not achieve significant product sales or royalty revenue for a number of years, if ever. We expect to incur net operating losses and negative cash flow from operations in the future, which could increase significantly if we expand our clinical trial programs and other product development efforts. Our ability to achieve and sustain profitability is dependent in part on obtaining regulatory approval for and then commercializing AZEDRA and other product candidates, either alone or with others. Our operations may not be profitable even if AZEDRA and any of our other product candidates under development are commercialized. Failure to become and remain profitable may adversely affect the market price of our common stock and our ability to raise capital and continue operations.
29
Table of Contents
Our ability to use net operating losses to offset future taxable income is subject to certain limitations.
We currently have significant net operating losses ("NOLs") that may be used to offset future taxable income. The U.S. Internal Revenue Code limits the amount of taxable income that may be offset annually by NOL carryforwards after a change in control (generally greater than 50% change in ownership) of a loss corporation, and our use of NOL carryforwards may be further limited as a result of any future equity transactions that result in an additional change of control.
Progenics ' stock price has a history of volatility and may be affected by selling pressure. You should consider an investment in Progenics stock as risky and invest only if you can withstand a significant loss.
Our stock price has a history of significant volatility. It has varied between a high of $11.72 and a low of $4.60 in 2017, between a high of $9.78 and a low of $3.61 in 2016 and between a high of $11.15 and a low of $4.86 in 2015. Factors that may have a significant impact on the market price of our common stock include the results of clinical trials and pre-clinical studies undertaken by us or our collaboration partners; delays, terminations or other changes in development programs; developments in marketing approval efforts; developments in collaborator or other business relationships, particularly regarding RELISTOR, AZEDRA or other significant products or programs; technological innovation or product announcements by us, our collaborators or our competitors; patent or other proprietary rights developments; governmental regulation; changes in reimbursement policies or health care legislation; safety and efficacy concerns about products developed by us, our collaborators or our competitors; our ability to fund ongoing operations; fluctuations in our operating results; general market conditions; and the reporting of or commentary on such matters by the press and others. At times, our stock price has been volatile even in the absence of significant news or developments. The stock prices of biotechnology companies and securities markets generally have been subject to dramatic price swings in recent years, and financial and market conditions during that period have resulted in widespread pressures on securities of issuers throughout the world economy.
Our stockholders may be diluted, and the price of our common stock may decrease, as a result of future issuances of securities, exercises of outstanding stock options, or sales of outstanding securities.
We expect to issue additional common stock in public offerings, private placements and/or through our January 2017 sales agreement with an investment bank, pursuant to which we have sold our stock in at-the-market transactions for net proceeds of approximately $14.5 million and may sell from time to time up to a remaining $60.0 million of our stock, and to issue options to purchase common stock for compensation purposes. We may issue preferred stock, restricted stock units or securities convertible into or exercisable or exchangeable for our common stock. All such issuances would dilute existing investors and could lower the price of our common stock. Sales of substantial numbers of outstanding shares of common stock could also cause a decline in the market price of our stock. We require substantial external funding to finance our research and development programs and may seek such funding through the issuance and sale of our common stock, which we have done in follow-on primary offerings in late 2012, mid-2013, and February 2014, and at-the-market transactions in the fourth quarter of 2017 and first quarter of 2018. We have a shelf registration statement which may be used to issue up to a remaining $235.0 million of common stock and other securities before any underwriter discounts, commissions, and offering expenses. We also have in place registration statements covering shares issuable pursuant to our equity compensation plans, and sales of our securities under them could cause the market price of our stock to decline. Sales by existing stockholders or holders of options or other rights may adversely affect the market price of our common stock.
We are exposed to potential risks from legislation requiring companies to evaluate controls under Section 404 of the Sarbanes-Oxley Act.
The Sarbanes-Oxley Act requires that we maintain effective internal controls over financial reporting and disclosure controls and procedures. Among other things, we must perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on, and our independent registered public accounting firm to attest to, our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act and related regulations ("Section 404"). Compliance with Section 404 requires substantial accounting expense and significant management efforts. Our testing, or the subsequent review by our independent registered public accounting firm, may reveal deficiencies in our internal controls that would require us to remediate in a timely manner so as to be able to comply with the requirements of Section 404 each year. Because of its inherent limitations, internal control over financial reporting may not prevent or detect all deficiencies or weaknesses in our financial reporting. If we are not able to comply with the requirements of Section 404 in a timely manner each year, we could be subject to sanctions or investigations by the SEC, the NASDAQ Stock Market or other regulatory authorities that would require additional financial and management resources and could adversely affect the market price of our common stock. No assurance is given that our procedures and processes for detecting weaknesses in our internal control over financial reporting will be effective.
30
Table of Contents
We do not intend to pay dividends on our common stock. Until such time as we pay cash dividends, our stockholders must rely on increases in our stock price for appreciation.
We have never declared or paid dividends on our common stock. We intend to retain future earnings to develop and commercialize our product candidates. Therefore , we do not intend to pay cash dividends in the foreseeable future. Until such time as we determine to pay cash dividends on our common stock, our stockholders must rely on increases in the market price of our common stock for appreciation of their respective investments.
Other Risks
Our principal stockholders are able to exert significant influence over matters submitted to stockholders for approval.
At December 31, 2017, our directors and executive officers together beneficially owned or controlled approximately 4.2% of our outstanding common shares, including shares currently issuable upon option exercises, and our five largest other stockholders held approximately 44.6%. Should these parties choose to act alone or together, they could exert significant influence in determining the outcome of corporate actions requiring stockholder approval and otherwise control our business. This control could, among other things, have the effect of delaying or preventing a change in control of the Company, adversely affecting our stock price.
Anti-takeover provisions may make removal of our Board and/or management more difficult, discouraging hostile bids for control that may be beneficial to our stockholders.
Our Board is authorized, without further stockholder action, to issue from time to time shares of preferred stock in one or more designated series or classes. The issuance of preferred stock, as well as provisions in some outstanding stock options that provide for acceleration of vesting upon a change of control, and Section 203 and other provisions of the Delaware General Corporation Law could make a takeover or the removal of our Board or management more difficult; discourage hostile bids for control in which stockholders may receive a premium for their shares; and otherwise dilute the rights of common stockholders and depress the market price of our stock.
Item 1B. Unresolved Staff Comments
There were no unresolved SEC staff comments regarding our periodic or current reports under the Exchange Act as of December 31, 201 7.
Item 2. Properties
A t December 31, 2017, we occupied approximately 26,000 square feet of corporate office space located in New York City, pursuant to lease agreements expiring in September 2030 (subject to an early termination right) under which we pay rent and facilities charges including utilities, taxes, and operating expenses.
We also lease approximately 4,000 square feet of office space in Lund, Sweden. The lease term expires on December 31, 2018, with an option to renew the term for an additional three years.
Item 3. Legal Proceedings
On October 25, 2016, Progenics, Valeant, and Wyeth LLC ("Wyeth", Valeant 's predecessor as licensee of RELISTOR) received a notification of a Paragraph IV certification with respect to certain patents for RELISTOR Tablets. The certification accompanied the filing by Actavis LLC of an Abbreviated New Drug Application ("ANDA") challenging such patents for RELISTOR Tablets and seeking to obtain approval to market a generic version of RELISTOR tablets before some or all of these patents expire.
On October 28, 2015, Progenics, Valeant, and Wyeth received a second notification of a Paragraph IV certification with respect to the same patents for RELISTOR subcutaneous injection from Actavis LLC as a result of Actavis LLC 's filing of an ANDA with the FDA, also challenging these patents and seeking to obtain approval to market a generic version of RELISTOR subcutaneous injection before some or all of these patents expire.
31
Table of Contents
On October 7, 2015 , Progenics, Valeant, and Wyeth received notification of a Paragraph IV certification for certain patents for RELISTOR (methylnaltrexone bromide) subcutaneous injection, which are listed in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, or the Orange Book. The certification resulted from the filing by Mylan Pharmaceuticals, Inc. of an ANDA with the FDA, challenging such patents for RELISTOR subcutaneous injection and seeking to obtain approval to market a generic version of RELISTOR subcutaneous injection before some or all of these patents expire.
On May 3, 2017 , ANDA filer, Mylan Pharmaceuticals received a tentative approval letter from the FDA for Methylnaltrexone Bromide Subcutaneous Injection, 12 mg/0.6 mL single-dose vial. In accordance with the Drug Price Competition and Patent Term Restoration Act (commonly referred to as the Hatch-Waxman Act), Progenics, Valeant, and Wyeth (collectively "Plaintiffs") timely commenced litigation against each of these ANDA filers (collectively "Defendants") in order to obtain an automatic stay of FDA approval of the ANDA until the earlier of (i) 30 months from receipt of the notice or (ii) a District Court decision finding that the identified patents are invalid, unenforceable or not infringed. The 30-month stays will begin expiring in the second quarter of 2018.
On February 9, 2018, Plaintiffs filed a Motion And Brief For Partial Summary Judgment on the validity of U.S. Patent 8,552,025 with the United States District Court of New Jersey. On February 16, 2018, the Court denied Defendant 's motion to strike the Summary Judgment motion, ordering Defendant to respond by February 20, 2018, with an extension available. The schedule for pretrial-order exchanges and a trial date have not been set.
In addition to the above described ANDA notifications, in October 2015 , we received notices of opposition to three European patents relating to methylnaltrexone. The oppositions were filed separately by each of Actavis Group PTC ehf. and Fresenius Kabi Deutschland GmbH. The matters are on appeal.
Each of the above-described proceedings is in its early stages and we and Valeant continue to cooperate closely to vigorously defend and enforce RELISTOR intellectual property rights. Pursuant to the RELISTOR License Agreement between us and Valeant, Valeant has the first right to enforce the intellectual property rights at issue and is responsible for the costs of such enforcement.
We are or may be from time to time involved in various other disputes, governmental and/or regulatory inspections, inquires, investigations and proceedings that could result in litigation, and other litigation matters that arise from time to time. The process of resolving matters through litigation or other means is inherently uncertain and it is possible that an unfavorable resolution of these matters will adversely affect us, our results of operations, financial condition, and cash flows.
Item 4. Not Applicable
PART II
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Price Range of Common Stock
Our common stock is quoted on The NASDAQ Stock Market LLC under the symbol PGNX. The following table sets forth, for the periods indicated, the high and low sales price per share of the common stock, as reported on NASDAQ.
High | Low | |||||||
2017 | ||||||||
Fourth quarter | $ | 7.81 | $ | 5.16 | ||||
Third quarter | 7.39 | 4.60 | ||||||
Second quarter | 9.56 | 6.10 | ||||||
First quarter | 11.72 | 8.15 | ||||||
2016 | ||||||||
Fourth quarter | $ | 9.78 | $ | 4.84 | ||||
Third quarter | 7.09 | 4.19 | ||||||
Second quarter | 5.75 | 4.00 | ||||||
First quarter | 6.13 | 3.61 | ||||||
On March 5, 2018, the last sale price for our common stock, as reported by The NASDAQ Stock Market LLC, was $7.08. There were approximately 64 holders of record of our common stock as of that date.
32
Table of Contents
Comparative Stock Performance Graph
The graph below compares , for the past five years, the cumulative stockholder returns on our common stock with the cumulative stockholder returns of (i) the NASDAQ U.S. Benchmark (TR) Index and (ii) the ICB: 4577 Pharmaceuticals (Subsector) Index, assuming an investment in each of $100 on December 30, 2012.
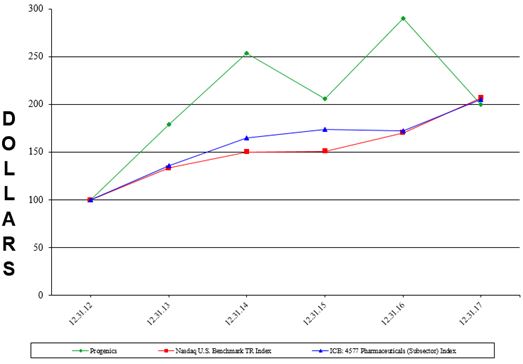 |
33
Table of Contents
Dividends
We have never paid any dividends, and we currently anticipate that all earnings, if any, will be retained for development of our business and no dividends will be declared in the foreseeable future.
Item 6. Selected Financial Data
The selected historical consolidated statement of operations data presented below for the years ended December 31, 2017, 2016, and 2015 and the historical consolidated balance sheet data as of December 31, 2017 and 2016 have been derived from our audited consolidated financial statements, which are included elsewhere in this Annual Report on Form 10-K. The historical consolidated statement of operations data presented below for the years ended December 31, 2014 and 2013 and the historical consolidated balance sheet data as of December 31, 2015, 2014, and 2013 have been derived from our audited consolidated financial statements that do not appear in this report. The data set forth below should be read in conjunction with Management's Discussion and Analysis of Financial Condition and Results of Operations and the consolidated financial statements and related notes included elsewhere herein. The selected historical financial information in this section is not intended to replace our financial statements and the related notes thereto.
Years Ended December 31, | ||||||||||||||||||||
2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
(In thousands, except per share data) | ||||||||||||||||||||
Consolidated Statements of Operations Data: | ||||||||||||||||||||
Revenue: | ||||||||||||||||||||
Royalty income | $ | 10,965 | $ | 10,295 | $ | 6,608 | $ | 3,101 | $ | 5,923 | ||||||||||
License revenue | 690 | 59,081 | 1,955 | 41,196 | 1,595 | |||||||||||||||
Research grants | - | - | - | - | 275 | |||||||||||||||
Other revenue | 43 | 53 | 113 | 80 | 69 | |||||||||||||||
Total revenue | 11,698 | 69,429 | 8,676 | 44,377 | 7,862 | |||||||||||||||
Expenses: | ||||||||||||||||||||
Research and development | 42,589 | 37,569 | 28,196 | 28,592 | 34,582 | |||||||||||||||
General and administrative | 24,909 | 23,356 | 18,184 | 15,489 | 15,541 | |||||||||||||||
Intangible impairment charges | - | - | - | 2,676 | 919 | |||||||||||||||
Change in contingent consideration liability | 2,600 | (4,600 | ) | 1,600 | 1,500 | (200 | ) | |||||||||||||
Total operating expenses | 70,098 | 56,325 | 47,980 | 48,257 | 50,842 | |||||||||||||||
Other operating income | - | - | - | 7,250 | - | |||||||||||||||
Operating (loss) income | (58,400 | ) | 13,104 | (39,304 | ) | 3,370 | (42,980 | ) | ||||||||||||
Other (expense) income: | ||||||||||||||||||||
Interest (expense) income, net | (4,038 | ) | (493 | ) | 52 | 51 | 46 | |||||||||||||
Other expense, net | (247 | ) | (34 | ) | - | - | - | |||||||||||||
Total other (expense) income | (4,285 | ) | (527 | ) | 52 | 51 | 46 | |||||||||||||
(Loss) income before income tax (expense) benefit | (62,685 | ) | 12,577 | (39,252 | ) | 3,421 | (42,934 | ) | ||||||||||||
Income tax benefit (expense) | 11,672 | (1,844 | ) | 133 | 989 | 362 | ||||||||||||||
Net (loss) income | (51,013 | ) | 10,733 | (39,119 | ) | 4,410 | (42,572 | ) | ||||||||||||
Net loss attributable to noncontrolling interests | - | (73 | ) | (7 | ) | - | - | |||||||||||||
Net (loss) income attributable to Progenics | $ | (51,013 | ) | $ | 10,806 | $ | (39,112 | ) | $ | 4,410 | $ | (42,572 | ) | |||||||
Per share amount on net (loss) income attributable to Progenics: | ||||||||||||||||||||
Basic | $ | (0.73 | ) | $ | 0.15 | $ | (0.56 | ) | $ | 0.06 | $ | (0.76 | ) | |||||||
Diluted | $ | (0.73 | ) | $ | 0.15 | $ | (0.56 | ) | $ | 0.06 | $ | (0.76 | ) | |||||||
December 31, | ||||||||||||||||||||
2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
(In thousands) | ||||||||||||||||||||
Consolidated Balance Sheets Data: | ||||||||||||||||||||
Cash and cash equivalents | $ | 90,642 | $ | 138,909 | $ | 74,103 | $ | 119,302 | $ | 65,860 | ||||||||||
Auction rate securities | - | - | - | - | 2,208 | |||||||||||||||
Working capital | 81,511 | 131,744 | 73,556 | 115,241 | 64,055 | |||||||||||||||
Total assets | 145,957 | 198,986 | 131,251 | 161,037 | 114,541 | |||||||||||||||
Other liabilities - long term | 67,145 | 77,867 | 30,861 | 29,443 | 28,935 | |||||||||||||||
Total stockholders' equity | 63,453 | 104,762 | 90,661 | 124,909 | 78,979 | |||||||||||||||
34
Table of Contents
Item 7. Management 's Discussion and Analysis of Financial Condition and Results of Operations (MD&A)
Overview
We develop innovative medicines and other technologies to target and treat cancer. Our pipeline includes: (1) therapeutic agents designed to precisely target cancer (AZEDRA , 1095, and PSMA TTC), (2) PSMA-targeted imaging agents for prostate cancer (1404 and PyL), and (3) imaging analysis technology. Our first commercial product, RELISTOR (methylnaltrexone bromide) for OIC, is partnered with Salix Pharmaceuticals, Inc. (a wholly-owned subsidiary of Valeant).
On October 31, 2017, we completed the rolling submission of our NDA for AZEDRA. The FDA has accepted our NDA for review, granted our request for Priority Review, and set an action date of April 30, 2018 under the PDUFA. We are developing AZEDRA as a treatment for patients with malignant, recurrent, and/or unresectable pheochromocytoma and paraganglioma, which are rare neuroendocrine tumors. There are currently no approved therapies in the U.S. for the treatment of these ultra-rare diseases. While AZEDRA has received Breakthrough Therapy, Orphan Drug, and Fast Track designations from the FDA, there can be no assurance that our NDA will be approved.
We have licensed RELISTOR to Valeant and our PSMA antibody technology to Bayer, and have partnered other internally-developed or acquired compounds and technologies with third parties. We continue to consider opportunities for strategic collaborations, out-licenses and other arrangements with biopharmaceutical companies involving proprietary research, development and clinical programs, and may in the future also in-license or acquire additional oncology compounds and/or programs.
Valeant Agreement
Under our agreement with Valeant, we received a development milestone of $40.0 million upon U.S. marketing approval for subcutaneous RELISTOR in non-cancer pain patients in 2014, and a development milestone of $50.0 million for the U.S. marketing approval of an oral formulation of RELISTOR in 2016. We are also eligible to receive up to $200.0 million of commercialization milestone payments upon first achievement of specified U.S. sales targets in any single calendar year. The following table summarizes the commercialization milestones (in thousands):
Net Sales Level in any Single Calendar Year | Payment | |||
In excess of $100 million | $ | 10,000 | ||
In excess of $150 million | 15,000 | |||
In excess of $200 million | 20,000 | |||
In excess of $300 million | 30,000 | |||
In excess of $750 million | 50,000 | |||
In excess of $1 billion | 75,000 | |||
| $ | 200,000 | |||
Each commercialization milestone payment is payable one time only, regardless of the number of times the condition is satisfied, and all six payments could be made within the same calendar year. We are also eligible to receive royalties from Valeant and its affiliates based on the following royalty scale: 15% on worldwide net sales up to $100 .0 million, 17% on the next $400.0 million in worldwide net sales, and 19% on worldwide net sales over $500.0 million each calendar year, and 60% of any upfront, milestone, reimbursement or other revenue (net of costs of goods sold, as defined, and territory-specific research and development expense reimbursement) Valeant receives from sublicensees outside the U.S.
Valeant has also entered into license and distribution agreements to expand its sales channels outside of the U.S. for RELISTOR.
Bayer Agreement
Under our agreement with Bayer, we received an upfront payment of $4.0 million and milestone payments totaling $3.0 million , and could receive up to an additional $46.0 million in potential clinical and regulatory development milestones. We are also entitled to single digit royalties on net sales, and potential net sales milestone payments up to an aggregate total of $130.0 million, as well as royalty payments.
35
Table of Contents
Results of Operations
The following table is an overview of our results of operations (in thousands, except percentages):
2017 | 2016 | 2015 | 2017 vs. 2016 | 2016 vs. 2015 | ||||||||||||||||
Total revenue | $ | 11,698 | $ | 69,429 | $ | 8,676 | (83 | %) | 700 | % | ||||||||||
Operating expenses | $ | 70,098 | $ | 56,325 | $ | 47,980 | 24 | % | 17 | % | ||||||||||
Operating income (loss) | $ | (58,400 | ) | $ | 13,104 | $ | (39,304 | ) | (546 | %) | (133 | %) | ||||||||
Net income (loss) | $ | (51,013 | ) | $ | 10,733 | $ | (39,119 | ) | (575 | %) | (127 | %) | ||||||||
Net income (loss) attributable to Progenics | $ | (51,013 | ) | $ | 10,806 | $ | (39,112 | ) | (572 | %) | (128 | %) | ||||||||
Revenue
Our s ources of revenue during the years indicated below include royalties and license fees from Valeant, Bayer, and other licensees and, to a small extent, sale of research reagents. The following table is a summary of our worldwide revenue (in thousands, except percentages):
Source | 2017 | 2016 | 2015 | 2017 vs. 2016 | 2016 vs. 2015 | |||||||||||||||
Royalty income | $ | 10,965 | $ | 10,295 | $ | 6,608 | 7 | % | 56 | % | ||||||||||
License revenue | 690 | 59,081 | 1,955 | (99 | %) | 2922 | % | |||||||||||||
Other revenue | 43 | 53 | 113 | (19 | %) | (53 | %) | |||||||||||||
Total revenue | $ | 11,698 | $ | 69,429 | $ | 8,676 | (83 | %) | 700 | % | ||||||||||
Royalty income. We recognized royalty income primarily based on the below net sales of RELISTOR as reported to us by Valeant (in thousands).
2017 | 2016 | 2015 | 2017 vs. 2016 | 2016 vs. 2015 | ||||||||||||||||
U.S. | $ | 71,100 | $ | 66,900 | $ | 40,700 | 6 | % | 64 | % | ||||||||||
Outside U.S. | 2,000 | 3,700 | 3,100 | (46 | %) | 19 | % | |||||||||||||
Worldwide net sales of RELISTOR | $ | 73,100 | $ | 70,600 | $ | 43,800 | 4 | % | 61 | % | ||||||||||
Royalty income increased by $0.7 million, or 7%, in 2017 compared to 2016, primarily attributable to higher sales of RELISTOR tablets. Valeant launched RELISTOR tablets in the U.S. in September 2016 following receipt of FDA approval in July 2016. Royalty income increased by $3.7 million, or 56%, in 2016 compared to 2015, primarily attributable to higher sales of RELISTOR subcutaneous injection. The 2016 period also benefited from the launch of RELISTOR tablets.
License revenue. The decrease in license revenue of $58.4 million, or 99%, in 2017 compared to 2016 was primarily attributable to the $50.0 million milestone payment under the Valeant license agreement and $7.0 million upfront and milestone payments under the Bayer license agreement, all of which were received in 2016. The increase in license revenue of $57.1 million, or 2,922%, in 2016 compared to 2015 was primarily attributable to the aforementioned milestone payments received from Valeant and Bayer ($57.0 million in aggregate).
Operating Expenses
The following table is a summary of our operating expenses (in thousands, except percentages):
Operating Expenses | 2017 | 2016 | 2015 | 2017 vs. 2016 | 2016 vs. 2015 | |||||||||||||||
Research and development | $ | 42,589 | $ | 37,569 | $ | 28,196 | 13 | % | 33 | % | ||||||||||
General and administrative | 24,909 | 23,356 | 18,184 | 7 | % | 28 | % | |||||||||||||
Change in contingent consideration liability | 2,600 | (4,600 | ) | 1,600 | (157 | %) | (388 | %) | ||||||||||||
Total operating expenses | $ | 70,098 | $ | 56,325 | $ | 47,980 | 24 | % | 17 | % | ||||||||||
Research and Development ("R&D")
R&D expenses increased by $5.0 million, or 13%, in 2017 compared to 2016, primarily attributable to higher clinical trial and contract manufacturing costs for PyL and higher consulting fees to support the NDA filing and pre-approval inspection readiness for AZEDRA. Partially offsetting the increase were lower clinical drug supply costs for AZEDRA (as the Phase 2 registrational trial was completed) and lower manufacturing scale-up costs for 1095. R&D expenses increased by $9.4 million, or 33%, in 2016 compared to 2015, primarily attributable to higher clinical trial expenses related to the start of the Phase 3 trial for 1404 and the Phase 2/3 trial for PyL. In addition, higher contract manufacturing expenses for the planned Phase 1 trial of 1095 and the Phase 2 registrational trial for AZEDRA contributed to the year-over-year increase in 2016.
36
Table of Contents
General and Administrative ( " G&A " )
G&A expenses increased by $1.6 million, or 7%, in 2017 compared to 2016, primarily attributable to higher costs associated with building our commercial capabilities in preparation for a potential AZEDRA launch if approved by the FDA, partially offset by depreciation expense. In addition, the 2016 period included an accrual for compensation related to litigation with a former employee, which was not repeated in 2017. G&A expenses increased by $5.2 million, or 28%, in 2016 compared to 2015, primarily attributable to higher depreciation expense because of a reduction in the remaining useful lives of the leasehold improvements at our former location in Tarrytown, New York, higher compensation costs related to increases in headcount, and higher market research expenses.
Change in Contingent Consideration Liability
The increase in the contingent consideration liability of $2.6 million in 2017 was primarily attributable to a higher estimated probability of success of AZEDRA used to calculate the potential milestone payments to former Molecular Insight stockholders. Our registrational Phase 2b trial of AZEDRA achieved the primary endpoint under a SPA agreement with the FDA. The reduction in the discount period used to calculate the present value of the contingent consideration liability also contributed to the increase in the contingent consideration liability.
The decrease in the contingent consideration liability of $ 4.6 million in 2016 resulted primarily from a change in the discount rate and sales projections used in calculating the contingent consideration liability for the potential sales-based milestone payments to former Molecular Insight stockholders.
Other (Expense) Income
The following table is a summary of our other (expense) income (in thousands, except percentages):
Other (Expense) Income | 2017 | 2016 | 2015 | 2017 vs. 2016 | 2016 vs. 2015 | |||||||||||||||
Interest (expense) income, net | (4,038 | ) | (493 | ) | 52 | 719 | % | (1048 | %) | |||||||||||
Other expense, net | (247 | ) | (34 | ) | - | 626 | % | N/A | ||||||||||||
Other (expense) income | $ | (4,285 | ) | $ | (527 | ) | $ | 52 | 713 | % | (1113 | %) | ||||||||
Total o ther (expense) income, net increased by $3.8 million, or 713%, in 2017 compared to 2016, primarily attributable to interest expense for the Royalty-Backed Loan, which was executed in November 2016. Other expense, net increased by $0.6 million, or 1,113%, in 2016 compared to 2015, primarily attributable to interest expense on the Royalty-Backed Loan.
Income Taxes
The following table is a summary of our income tax benefit (expense) and effective tax rate (in thousands, except percentages):
2017 | 2016 | 2015 | ||||||||||
Income tax benefit (expense) | $ | 11,672 | $ | (1,844 | ) | $ | 133 | |||||
Effective tax rate | 18.6 | % | 14.7 | % | 0.3 | % | ||||||
On December 22, 2017, the U.S. government enacted comprehensive tax legislation , commonly referred to as the Tax Cuts and Jobs Act ("Tax Act"). The Tax Act makes broad and complex changes to the U.S. tax code, including, but not limited to, (1) reducing the U.S. federal corporate tax rate from 35% to 21%; (2) requiring companies to pay a one-time transition tax on certain unrepatriated earnings of foreign subsidiaries; (3) generally eliminating U.S. federal income taxes on dividends from foreign subsidiaries; (4) requiring a current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations; (5) eliminating the corporate AMT and changing how existing AMT credits can be realized; (6) creating the base erosion anti-abuse tax ("BEAT"), a new minimum tax; (7) creating a new limitation on deductible interest expense; and (8) changing rules related to uses and limitations of net operating loss ("NOL") carryforwards created in tax years beginning after December 31, 2017.The indefinite carryforward period for NOLs may also mean that deferred tax liabilities related to indefinite-lived intangibles, commonly referred to as "naked credits," can be considered as support for realization of deferred tax assets, which can affect the need to record or maintain a valuation allowance for deferred tax assets.
37
Table of Contents
We are required to recognize the effect of the tax law changes in the period of enactment, including by re-measuring our U.S. deferred tax assets and liabilities as well as our valuation allowance against our net U.S. deferred tax assets. We are also required to assess our naked credit as it may provide an income source for our existing temporary differences that will reverse to generate indefinite lived NOLs. As a result, we recorded an income tax benefit of approximately $11.7 million in 2017, of which $6.6 million related to the reduction in the federal and state tax rates, $4.8 million related to the use of our naked credit as a source of income to release a portion of our valuation allowance and the remaining $0.2 million related to a refundable AMT credit. Our effective tax rate for 2017 was 18.6%.
In 2016, we recorded an income tax expense of approximately $1.8 million in 2016 as a result of an increase in our effective tax rate to 14.7%. Our effective tax rate in 2016 was impacted by our relocation to New York City, which has its own local tax rate and adds to the overall tax rate used for calculating the income tax provision. In 2015, we recorded an income tax benefit of approximately $133 thousand as a result of the change in the temporary difference between carrying amounts of in process research and development assets for financial reporting purposes and the amounts used for income tax purposes. Our effective tax rate for 2015 was 0.3%.
Net (Loss) Income
Our net loss was $51.0 million in 2017 and $39.1 million in 2015, compared to net income of $10.8 million in 2016. The $50.0 million milestone payment received from Valeant in 2016 for the approval of RELISTOR tablets was the primary driver for realizing net income in 2016.
Liquidity and Capital Resources
The following table is a s ummary of selected financial data (in thousands):
2017 | 2016 | 2015 | ||||||||||
Cash and cash equivalents | $ | 90,642 | $ | 138,909 | $ | 74,103 | ||||||
Accounts receivable, net | $ | 3,972 | $ | 4,864 | $ | 3,543 | ||||||
Total assets | $ | 145,957 | $ | 198,986 | $ | 131,251 | ||||||
Working capital | $ | 81,511 | $ | 131,744 | $ | 73,556 | ||||||
Our current principal sources of revenue from operations are royalt ies, development and commercial milestones, and sublicense revenue-sharing payments. Our principal sources of liquidity are our existing cash and cash equivalents. As of December 31, 2017, we had cash and cash equivalents of approximately $90.6 million, a decrease of $48.3 million from $138.9 million at December 31, 2016. We will continue to have significant cash requirements to support product development activities and the potential commercial launch of AZEDRA if approved by the FDA. The amount and timing of cash requirements will depend on the progress and success of our clinical development programs, regulatory and market acceptance, and the resources we devote to research and commercialization activities. The amount of cash on-hand will depend on the progress of various clinical programs, the timing of our commercialization effort scale-up, and the achievement of various milestones and royalties under our existing license agreements.
We believe that our current cash and cash equivalents, which includes $5.0 million of net proceeds received from the sale of our stock in at-the-market transactions under a controlled equity offering sales agreement (see Shelf Registration section below for additional details), together with the net proceeds of approximately $9.5 million received from additional at-the-market transactions in the first quarter of 2018, will be sufficient to fund our operations for at least the next twelve months. We expect to fund our operations going forward with existing cash resources, anticipated revenues from our existing license agreements, and cash that we may raise through future capital raising and other financing transactions.
I f we do not realize sufficient royalty or milestone revenue from our license agreements, or are unable to enter into favorable collaboration, license, asset sale, additional capital raising, or other financing transactions, we will have to reduce, delay, or eliminate spending on certain programs, and/or take other economic measures.
Shelf Registration
During the first quarter of 2017, we filed a $250.0 million replacement shelf registration statement, which was declared effective as of January 19, 2017. In addition, we also entered into a controlled equity offering sales agreement ("Sales Agreement") with Cantor Fitzgerald & Co. ("Cantor"), as sales agent, pursuant to which we may offer and sell through Cantor, from time to time, shares of our common stock up to an aggregate offering price of $75.0 million. This Sales Agreement may be terminated by Cantor or us at any time upon ten (10) days' notice, or by Cantor at any time in certain circumstances, including the occurrence of a material adverse change in our business or financial condition.
38
Table of Contents
During the fourth quarter of 2017, we sold a total of 854,606 shares of our common stock in at-the-market transactions under the Sales Agreement for net proceeds, after deducting commissions, of approximately $5.0 million at an average selling price of $6.06 per share. At December 31, 2017, we had 320,182 shares of our common stock subscribed in at-the-market transactions under the Sales Agreement for net proceeds, after deducting commissions, of approximately $2.1 million at an average selling price of $6.79 per share. Accordingly, we have recorded a subscription receivable of $2.1 million as a reduction of stockholders' equity in our consolidated balance sheet at December 31, 2017. Subsequent to the close of the quarter, in January 2018, we sold an additional 1,217,346 shares of our common stock in at-the-market transactions under the Sales Agreement for net proceeds, after deducting commissions, of approximately $7.4 million at an average selling price of $6.30 per share. Together with the subscription receivable, we received net proceeds of approximately $9.5 million in January 2018.
Cash Flows
The fol lowing table is a summary of our cash flow activities (in thousands):
2017 | 2016 | 2015 | ||||||||||
Net cash (used in) provided by operating activities | $ | (53,628 | ) | $ | 19,209 | $ | (40,137 | ) | ||||
Net cash used in investing activities | $ | (232 | ) | $ | (3,939 | ) | $ | (6,524 | ) | |||
Net cash provided by financing activities | $ | 5,510 | $ | 49,622 | $ | 1,445 | ||||||
Operating Activities
Net cash used in operating activities during 2017 and 2015 was primarily attributable to funding operating expenses, net of non-cash items. Net cash provided by operating activities during 2016 was primarily attributable to the milestone payment received from Valeant for the approval of RELISTOR tablets ($50.0 million in 2016), partially offset by operating expenses, net of non-cash items.
Investing Activities
Net cash used in investing activities was primarily related to capital expenditures in 2017 and 2016 and the acquisition of EXINI in 2015, partially offset by proceeds from the sale of fixed assets.
Financing Activities
Net cash provided by financing activities during 2017, 2016, and 2015 was primarily attributable to net proceeds from the sale of our common stock in at-the-market transactions, net proceeds from the royalty monetization, and proceeds from the exercise of stock options, respectively.
Contractual Obligations
Our funding requirements for the next 12 months and beyond will include required payments under operating leases and fixed payments under license agreements. The following table summarizes our contractual obligations as of December 31, 2017 for future payments under these agreements:
Payments Due by Period (1) | ||||||||||||||||||||
Total | Less than one year | 1 to 3 years | 3 to 5 years | Greater than 5 years | ||||||||||||||||
Operating leases | $ | 28.1 | $ | 1.9 | $ | 3.7 | $ | 4.0 | $ | 18.5 | ||||||||||
Fixed payments under license agreements | 0.8 | 0.1 | 0.1 | 0.2 | 0.4 | |||||||||||||||
Total | $ | 28.9 | $ | 2.0 | $ | 3.8 | $ | 4.2 | $ | 18.9 | ||||||||||
(1) Does not include milestone or contractual payment obligations contingent upon the achievement of certain milestones or events if the amount and timing of such obligations are unknown or uncertain. We may be required to pay additional amounts up to approximately: (i) $77.3 million in contingent milestone payments under our license agreements; (ii) $93.0 million in payments to the former stockholders of Molecular Insight, contingent upon achieving specified commercialization events or sales targets; and (iii) $71.6 million in future principal and interest, based upon estimated sales projections, under the Royalty-Backed Loan.
We periodically assess the scientific progress and merits of each of our programs to determine if continued research and development is commercially and economically viable. Certain of our programs have been terminated due to the lack of scientific progress and prospects for ultimate commercialization. Because of the uncertainties associated with research and development in these programs, the duration and completion costs of our research and development projects are difficult to estimate and are subject to considerable variation. Our inability to complete research and development projects in a timely manner or failure to enter into collaborative agreements could significantly increase capital requirements and adversely affect our liquidity.
39
Table of Contents
Our cash requirements may vary materially from those now planned because of results of research and development and product testing, changes in existing relationships or new relationships with licensees, licensors or other collaborators, changes in the focus and direction of our research and development programs, competitive and technological advances, the cost of filing, prosecuting, defending and enforcing patent claims, the regulatory approval process, manufacturing and marketing and other costs associated with the commercialization of products following receipt of regulatory approvals and other factors.
The above discussion contains forward-looking statements based on our current operating plan and the assumptions on which it relies. There could be deviations from that plan that would consume our assets earlier than planned.
Off-Balance Sheet Arrangements and Guarantees
We have no obligations under off-balance sheet arrangements and do not guarantee the obligations of any other unconsolidated entity.
Critical Accounting Policies
We prepare our financial statements in conformity with accounting principles generally accepted in the U.S. Our significant accounting policies are disclosed in Note 2 to our financial statements included in this Report. The selection and application of these accounting principles and methods requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, as well as certain financial statement disclosures. We evaluate these estimates on an ongoing basis. We base these estimates on historical experience and on various other assumptions that we believe reasonable under the circumstances. The results of these evaluations form the basis for making judgments about the carrying values of assets and liabilities that are not otherwise readily apparent. While we believe that the estimates and assumptions we use in preparing the financial statements are appropriate, they are subject to a number of factors and uncertainties regarding their ultimate outcome and, therefore, actual results could differ from these estimates.
The critical accounting policies we use and the estimates we make are described below. These are policies and estimates that we believe are the most important in portraying our financial condition and results of operations, and that require our most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. We have discussed the development, selection, and disclosure of these critical accounting policies and estimates with the Audit Committee of our Board of Directors.
Revenue Recognition . We recognize revenue from all sources based on the provisions of the SEC's Staff Accounting Bulletin ("SAB") No. 104 ("SAB 104") and the Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") 605 (Topic 605, Revenue Recognition ).
ASC 605 specifies how to separate deliverables in multiple-deliverable arrangements, and how to measure and allocate arrangement consideration to one or more units of accounting, and provides that the delivered item(s) are separate units of accounting, if (i) the delivered item(s) have value to a collaborator on a stand-alone basis, and (ii), if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item(s) is considered probable and substantially in our control.
Royalty revenue is recognized based upon net sales of related licensed products , and is recognized in the period the sales (losses) occur, provided that the royalty amounts are fixed or determinable, collection of the related receivable is reasonably assured and we have no remaining performance obligations under the arrangement providing for the royalty.
Share-Based Payment Arrangements . Our share-based compensation of employees includes non-qualified stock options and restricted stock, which are compensatory under ASC 718 (Topic 718, Compensation – Stock Compensation ). We account for share-based compensation to non-employees, including non-qualified stock options and restricted stock, in accordance with ASC 505 (Topic 505, Equity ).
The fair value of each non-qualified stock option award is estimated on the date of grant using the Black-Scholes option pricing model. The model requires input assumptions with respect to (i) expected volatility of our common stock, which is based upon the daily quoted market prices on The NASDAQ Stock Market LLC over a period equal to the expected term, (ii) the period of time over which employees, officers, directors and non-employee consultants are expected to hold their options prior to exercise, (iii) expected dividend yield (zero in our case due to never having paid dividends and not expecting to pay dividends in the future), and (iv) risk-free interest rates for periods within the expected term of the options, which are based on the U.S. Treasury yield curve in effect at the time of grant.
40
Table of Contents
Historical volatilities are based upon daily quoted market prices of our common stock on The NASDAQ Stock Market LLC over a period equal to the expected term of the related equity instruments. We rely only on historical volatility since we believe it is generally viewed as providing the most reliable indication of future volatility. In estimating expected future volatility, we assume it will be consistent with historical; we calculate historical volatility using a simple average calculation; we use available historical data for the length of the option's expected term, and we consistently use a sufficient number of price observations. Since our stock options are not traded on a public market, we do not use implied volatility.
The expected term of options granted represents the period of time that options granted are expected to be outstanding based upon historical data related to exercise and post-termination cancellation activity. The expected term of stock options granted to our Chief Executive Officer ("CEO") and non-employee directors, consultants and officers are calculated separately from stock options granted to other employees.
We apply a forfeiture rate to the number of unvested awards in each reporting period in order to estimate the number of awards that are expected to vest. Estimated forfeiture rates are based upon historical data on vesting behavior of employees. We adjust the total amount of compensation cost recognized for each award, in the period in which each award vests, to reflect the actual forfeitures related to that award. Changes in our estimated forfeiture rate will result in changes in the rate at which compensation cost for an award is recognized over its vesting period.
Changes in the assumptions us ed to compute the fair value of the option awards are likely to affect their fair value and the amount of compensation expense recognized in future periods. A higher volatility, longer expected term and higher risk-free rate increases the resulting compensation expense recognized in future periods. Conversely, a lower volatility, shorter expected term and lower risk-free rate decreases such expense recognized in future periods.
Clinical Trial and Other Research and Develo pment Expenses . Clinical trial expenses, which are included in research and development expenses, represent obligations resulting from contracts with various clinical investigators and clinical research organizations in connection with conducting clinical trials for our product candidates. Such costs are expensed as incurred, and are generally based on the total number of patients in the trial, the rate at which the patients enter the trial and the period over which the clinical investigators and clinical research organizations provide services. We believe that this method best aligns the efforts expended on a clinical trial with the expenses we record. We adjust our rate of clinical expense recognition if actual results differ from our estimates. In addition to clinical trial expenses, we estimate the amounts of other research and development expenses, for which invoices have not been received at the end of a period, based upon communication with third parties that have provided services or goods during the period. Such estimates are subject to change as additional information becomes available.
In –Process Research and Development , Intangible Assets -Technology , and Goodwill . We have a policy for accounting for intangible assets, under which in-process research and development ("IPR&D"), intangible assets-technology, and goodwill are initially measured at fair value and capitalized as intangible assets. Impairment tests for goodwill and IPR&D, which are indefinite-lived intangibles, are performed annually in the fourth quarter, unless impairment indicators require an earlier evaluation. Finite-lived intangible assets, including intangible assets-technology, are evaluated only when impairment indicators are present. IPR&D will be amortized upon and subject to commercialization of the underlying candidates and intangible assets-technology is amortized over the relevant estimated useful life.
Contingent Consideration Liability. The estimated fair value of the contingent consideration liability, initially measured and recorded on the acquisition date, is considered to be a Level 3 instrument and is reviewed quarterly, or whenever events or circumstances occur that indicate a change in fair value. The contingent consideration liability is recorded at fair value at the end of each reporting period.
Legal Proceedings. From time to time, we may be a party to legal proceedings in the course of our business. The outcome of any such proceedings, regardless of the merits, is inherently uncertain. The assessment of whether a loss is probable or reasonably possible, and whether the loss or a range of loss is estimable, often involves a series of complex judgments about future events. We record accruals for contingencies to the extent that the occurrence of the contingency is probable and the amount of liability is reasonably estimable. If the reasonable estimate of liability is within a range of amounts and some amount within the range appears to be a better estimate than any other, then we record that amount as an accrual. If no amount within the range is a reasonable estimate, then we record the lowest amount as an accrual. Loss contingencies that are assessed as remote are not reported in the financial statements, or in the notes to the consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our primary investment objective is to preserve principal. Our money market fun ds have interest rates that are variable and totaled $87.2 million at December 31, 2017. As a result, we do not believe that these investment balances have a material exposure to interest-rate risk.
41
Table of Contents
The majority of our business is conducted in U.S. dollars. However, we do conduct certain transactions in other currencies, including Euros, British Pounds, Swiss Francs, and Swedish Krona. Historically, fluctuations in foreign currency exchange rates have not materially affected our consolidated results of operations and during the years ended December 31, 201 7, 2016, and 2015, our consolidated results of operations were not materially affected by fluctuations in foreign currency exchange rates.
Item 8. Financial Statements and Supplementary Data
See page F-1, Index to Consolidated Financial Statements.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the timelines specified in the SEC 's rules and forms, and that such information is accumulated and communicated to our management, including our CEO and Chief Financial Officer ("CFO"), as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives, and in reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. We have a Disclosure Committee consisting of members of our senior management which monitors and implements our policy of disclosing material information concerning the Company in accordance with applicable law.
As required by SEC Rule 13a-15( e), we carried out an evaluation, under the supervision and with the participation of our management, including our CEO and CFO, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this report. Based on the foregoing, our CEO and CFO concluded that our current disclosure controls and procedures, as designed and implemented, were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting, as such term is defined in the Exchange Act Rules 13a-15(f) and 15d-15(f) during our fiscal quarter ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management 's Report on Internal Control Over Financial Reporting
Internal control over financial reporting is a process designed by, or under the supervision of, our CEO and CFO and effected by our Board, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our management is responsible for establishing and maintaining adequate internal control over financial reporting which includes policies and procedures that:
● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of assets; |
● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorization of management and directors; and |
42
Table of Contents
● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management has used the framework se t forth in the report entitled Internal Co ntrol – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), known as COSO, to evaluate the effectiveness of our internal control over financial reporting. Management has concluded that our internal control over financial reporting was effective as of December 31, 2017.
The effectiveness of our internal control over financial reporting has been audited by Ernst & Young LLP, an independent registered public accounting firm, as of December 31, 2017 as stated in their report which is provided below.
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Progenics Pharmaceuticals, Inc.
Opinion on the Internal Control over Financial Reporting
We have audited Progenics Pharmaceuticals, Inc. 's internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the "COSO criteria"). In our opinion, Progenics Pharmaceuticals, Inc. (the "Company") maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) ( "PCAOB"), the consolidated balance sheets of Progenics Pharmaceuticals, Inc. as of December 31, 2017 and 2016, the related consolidated statements of operations, comprehensive (loss) income, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes and financial statement schedule listed in the Index at Item 15(a) and our report dated March 8, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
The Company 's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company 's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
43
Table of Contents
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Stamford, Connecticut
March 8, 2018
Item 9B. Other Information
None .
44
Table of Contents
PART III
The information required by the Form 10-K Items listed in the following table will be included under the respective headings specified for such Items in our definitive proxy statement for our 2018 Annual Meeting of Stockholders to be filed with the SEC no later than 120 days after December 31, 2017, which proxy statement is incorporated herein by reference:
Item of Form 10-K | Location in 201 8 Proxy Statement |
Item 10. Directors, Executive Officers and Corporate Governance | Election of Directors. |
| Executive and Other Officers. | |
| Corporate Governance. | |
| Code of Bus iness Ethics and Conduct.* | |
| Section 16(a) Beneficial Ownership Reporting and Compliance. | |
| * The full text of our Code of Business Ethics and Conduct is available on our website ( www.progenics.com ). | |
Item 11. Executive Compensation | Executive Compensation . |
| Compensation Committee Report. | |
| Pay Ratio Disclosure. | |
| Compensation Committee Interlocks and Insider Participation. | |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | Equity Compensation Plan Information. Security Ownership of Certain Beneficial Owners and Management . |
Item 13. Certain Relationships and Related Transactions, and Director Independence | Certain Relationships and Related Transactions. Affirmative Determinations Regarding Director Independence and Other Matters. |
Item 14. Principal Accounting Fees and Services | Fees Billed for Services Rendered by our Independent Registered Public Accounting Firm. |
| Pre-approval of Audit and Non-Audit Services by the Audit Committee. |
45
Table of Contents
PART IV
Item 15. Exhibits , Financial Statement Schedules
The following documents or the portions thereof indicated are filed as a part of this Annual Report.
(a) | Documents filed as part of this Annual Report: |
(1) | Consolidated Financial Statements of Progenics Pharmaceuticals, Inc. : |
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets at December 31, 2017 and 2016
Consolidated Statements of Operations for the years ended December 31, 2017, 2016 and 2015
Consolidated Statements of Comprehensive (Loss) Income for the years ended December 31, 2017, 2016 and 2015
Consolidated Statements of Stockholders' Equity for the years ended December 31, 2017, 2016 and 2015
Consolidated Statements of Cash Flows for the years ended December 31, 2017, 2016 and 2015
Notes to Consolidated Financial Statements
( 2 ) | Financial Statement Schedules |
Schedule II – Valuation and Qualifying Accounts
F inancial statement schedules referred to in Item 12-01 of Regulation S-X and not listed above are inapplicable and therefore have been omitted.
( 3 ) | Item 601 Exhibits |
E xhibits required to be filed by Item 601 of Regulation S-K are listed in the Exhibit Index immediately following the signature page of this Report and incorporated herein by reference.
46
Table of Contents
PROGENICS PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Page | |
Report of Independent Registered Public Accounting Firm | F-2 |
Financial Statements: | |
Consolidated Balance Sheets | F- 3 |
Consolidated Statements of Operations | F- 4 |
Consolidated Statements of Comprehensive (Loss) Income | F-5 |
Consolidated Statements of Stockholders' Equity | F- 6 |
Consolidated Statements of Cash Flows | F- 7 |
Notes to Consolidated Financial Statements | F- 8 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Progenics Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Progenics Pharmaceuticals, Inc. (the "Company") as of December 31, 2017 and 2016, the related consolidated statements of operations, comprehensive (loss) income, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes and the financial statement schedule listed in the Index at Item 15(a) (collectively referred to as the "consolidated financial statements"). In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company at December 31, 2017 and 2016, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company 's internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 8, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company 's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company 's auditor since 2012.
Stamford, Connecticut
March 8, 2018
F-2
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
CONSOLIDATED BALANCE SHEETS
( I n thousands, except per share data )
December 31, | ||||||||
2017 | 2016 | |||||||
ASSETS | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 90,642 | $ | 138,909 | ||||
Accounts receivable, net | 3,972 | 4,864 | ||||||
Other current assets | 2,256 | 4,328 | ||||||
Total current assets | 96,870 | 148,101 | ||||||
Property and equipment, net | 4,122 | 4,760 | ||||||
Intangible assets, net | 30,369 | 30,581 | ||||||
Goodwill | 13,074 | 13,074 | ||||||
Other assets | 1,522 | 2,470 | ||||||
Total assets | $ | 145,957 | $ | 198,986 | ||||
LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 3,359 | $ | 567 | ||||
Accrued expenses | 9,555 | 15,790 | ||||||
Current portion of debt, net | 2,445 | - | ||||||
Total current liabilities | 15,359 | 16,357 | ||||||
Long-term debt, net | 47,242 | 49,453 | ||||||
Contingent consideration liability | 16,800 | 14,200 | ||||||
Deferred tax liability | 1,575 | 13,010 | ||||||
Other liabilities | 1,528 | 1,204 | ||||||
Total liabilities | 82,504 | 94,224 | ||||||
Commitments and Contingencies | ||||||||
Stockholders ' equity: | ||||||||
Preferred stock, $0.001 par value Authorized - 20,000 shares; issued and outstanding - none | - | - | ||||||
Common stock, $0.0013 par value Authorized - 160,000 shares; issued - 71,645 shares in 2017 and 70,390 shares in 2016 | 93 | 92 | ||||||
Additional paid-in capital | 609,829 | 598,069 | ||||||
Treasury stock at cost, 200 shares of common stock | (2,741 | ) | (2,741 | ) | ||||
Subscription receivable | (2,109 | ) | - | |||||
Accumulated other comprehensive loss | (33 | ) | (85 | ) | ||||
Accumulated deficit | (541,586 | ) | (490,573 | ) | ||||
Total stockholders ' equity | 63,453 | 104,762 | ||||||
Total liabilities and stockholders ' equity | $ | 145,957 | $ | 198,986 | ||||
The accompanying notes are an integral part of the financial statements.
F-3
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
CONSOLIDATED STATEMENTS OF OPERATIONS
( I n thousands, except per share data )
Years Ended December 31, | ||||||||||||
2017 | 2016 | 2015 | ||||||||||
Revenue: | ||||||||||||
Royalty income | $ | 10,965 | $ | 10,295 | $ | 6,608 | ||||||
License revenue | 690 | 59,081 | 1,955 | |||||||||
Other revenue | 43 | 53 | 113 | |||||||||
Total revenue | 11,698 | 69,429 | 8,676 | |||||||||
Operating expenses: | ||||||||||||
Research and development | 42,589 | 37,569 | 28,196 | |||||||||
General and administrative | 24,909 | 23,356 | 18,184 | |||||||||
Change in contingent consideration liability | 2,600 | (4,600 | ) | 1,600 | ||||||||
Total operating expenses | 70,098 | 56,325 | 47,980 | |||||||||
Operating (loss) income | (58,400 | ) | 13,104 | (39,304 | ) | |||||||
Other (expense) income: | ||||||||||||
Interest (expense) income, net | (4,285 | ) | (527 | ) | 52 | |||||||
Total other (expense) income | (4,285 | ) | (527 | ) | 52 | |||||||
(Loss) income before income tax (expense) benefit | (62,685 | ) | 12,577 | (39,252 | ) | |||||||
Income tax benefit (expense) | 11,672 | (1,844 | ) | 133 | ||||||||
Net (loss) income | (51,013 | ) | 10,733 | (39,119 | ) | |||||||
Net loss attributable to noncontrolling interests | - | (73 | ) | (7 | ) | |||||||
Net (loss) income attributable to Progenics | $ | (51,013 | ) | $ | 10,806 | $ | (39,112 | ) | ||||
Net (loss) income per share attributable to Progenics - basic | $ | (0.73 | ) | $ | 0.15 | $ | (0.56 | ) | ||||
Weighted-average shares - basic | 70,284 | 70,003 | 69,716 | |||||||||
Net (loss) income per share attributable to Progenics - diluted | $ | (0.73 | ) | $ | 0.15 | $ | (0.56 | ) | ||||
Weighted-average shares - diluted | 70,284 | 70,155 | 69,716 | |||||||||
The accompanying notes are an integral part of the financial statements.
F-4
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
( I n thousands)
Years Ended December 31, | ||||||||||||
2017 | 2016 | 2015 | ||||||||||
Net (loss) income | $ | (51,013 | ) | $ | 10,733 | $ | (39,119 | ) | ||||
Other comprehensive loss: | ||||||||||||
Foreign currency translation adjustments | 52 | (62 | ) | (26 | ) | |||||||
Comprehensive (loss) income | (50,961 | ) | 10,671 | (39,145 | ) | |||||||
Comprehensive loss attributable to noncontrolling interests | - | (73 | ) | (7 | ) | |||||||
Comprehensive (loss) income attributable to Progenics | $ | (50,961 | ) | $ | 10,744 | $ | (39,138 | ) | ||||
The accompanying notes are an integral part of the financial statements.
F-5
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
( I n thousands)
Common Stock | Common Stock Subscribed | Additional | Accumulated Other | Treasury Stock | Total | |||||||||||||||||||||||||||||||||||||||||||
Number | Number | Paid-in | Accumulated | Comprehensive | Subscription | Number | Noncontrolling | Stockholders' | ||||||||||||||||||||||||||||||||||||||||
of Shares | Par Value | of Shares | Par Value | Capital | Deficit | Loss | Receivable | of Shares | Cost | Interests | Equity | |||||||||||||||||||||||||||||||||||||
Balance at December 31, 2014 | 69,833 | $ | 91 | - | $ | - | $ | 589,826 | $ | (462,267 | ) | $ | - | $ | - | (200 | ) | $ | (2,741 | ) | $ | - | $ | 124,909 | ||||||||||||||||||||||||
Net loss | - | - | - | - | - | (39,112 | ) | - | - | - | - | (7 | ) | (39,119 | ) | |||||||||||||||||||||||||||||||||
Acquisition of subsidiary | - | - | - | - | - | - | - | - | - | - | 504 | 504 | ||||||||||||||||||||||||||||||||||||
Purchase of noncontrolling interests | - | - | - | - | - | - | - | - | - | - | (292 | ) | (292 | ) | ||||||||||||||||||||||||||||||||||
Other comprehensive loss | - | - | - | - | - | - | (26 | ) | - | - | - | - | (26 | ) | ||||||||||||||||||||||||||||||||||
Stock-based compensation expense | - | - | - | - | 2,948 | - | - | - | - | - | - | 2,948 | ||||||||||||||||||||||||||||||||||||
Exercise of stock options | 313 | - | - | - | 1,737 | - | - | - | - | - | - | 1,737 | ||||||||||||||||||||||||||||||||||||
Balance at December 31, 2015 | 70,146 | $ | 91 | - | $ | - | $ | 594,511 | $ | (501,379 | ) | $ | (26 | ) | $ | - | (200 | ) | $ | (2,741 | ) | $ | 205 | $ | 90,661 | |||||||||||||||||||||||
Net income | - | - | - | - | - | 10,806 | - | - | - | - | (73 | ) | 10,733 | |||||||||||||||||||||||||||||||||||
Purchase of noncontrolling interests | - | - | - | - | (239 | ) | - | - | - | - | - | (129 | ) | (368 | ) | |||||||||||||||||||||||||||||||||
Foreign currency translation adjustment | - | - | - | - | - | - | (59 | ) | - | - | - | (3 | ) | (62 | ) | |||||||||||||||||||||||||||||||||
Stock-based compensation expense | - | - | - | - | 2,457 | - | - | - | - | - | - | 2,457 | ||||||||||||||||||||||||||||||||||||
Exercise of stock options | 244 | 1 | - | - | 1,340 | - | - | - | - | - | - | 1,341 | ||||||||||||||||||||||||||||||||||||
Balance at December 31, 2016 | 70,390 | $ | 92 | - | $ | - | $ | 598,069 | $ | (490,573 | ) | $ | (85 | ) | $ | - | (200 | ) | $ | (2,741 | ) | $ | - | $ | 104,762 | |||||||||||||||||||||||
Net loss | - | - | - | - | - | (51,013 | ) | - | - | - | - | - | (51,013 | ) | ||||||||||||||||||||||||||||||||||
Foreign currency translation adjustment | - | - | - | - | - | - | 52 | - | - | - | - | 52 | ||||||||||||||||||||||||||||||||||||
Return of purchase premium for noncontrolling interests | - | - | - | - | 15 | - | - | - | - | - | - | 15 | ||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | - | - | - | - | 4,142 | - | - | - | - | - | - | 4,142 | ||||||||||||||||||||||||||||||||||||
Exercise of stock options | 80 | - | - | - | 469 | - | - | - | - | - | - | 469 | ||||||||||||||||||||||||||||||||||||
Issuance of common stock in connection with at-the-market offering, net of commissions and issuance costs | 855 | 1 | - | - | 5,025 | - | - | - | - | - | - | 5,026 | ||||||||||||||||||||||||||||||||||||
Subscription of common stock in connection with at-the-market offering, net of commissions | 320 | - | - | - | 2,109 | - | - | (2,109 | ) | - | - | - | - | |||||||||||||||||||||||||||||||||||
Balance at December 31, 2017 | 71,645 | $ | 93 | - | $ | - | $ | 609,829 | $ | (541,586 | ) | $ | (33 | ) | $ | (2,109 | ) | (200 | ) | $ | (2,741 | ) | $ | - | $ | 63,453 | ||||||||||||||||||||||
The accompanying notes are an integral part of the financial statements.
F-6
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
CONSOLIDATED STATEMENTS OF CASH FLOWS
( In thousands)
Years Ended December 31, | ||||||||||||
2017 | 2016 | 2015 | ||||||||||
Cash flows from operating activities: | ||||||||||||
Net (loss) income | $ | (51,013 | ) | $ | 10,733 | $ | (39,119 | ) | ||||
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: | ||||||||||||
Stock-based compensation expense | 4,142 | 2,457 | 2,948 | |||||||||
Depreciation and amortization | 1,121 | 2,078 | 565 | |||||||||
Gain on sale of fixed assets | 10 | (296 | ) | (2 | ) | |||||||
Paid in-kind interest | (13 | ) | 765 | - | ||||||||
Non-cash interest expense | 247 | 38 | - | |||||||||
Deferred income tax | (11,435 | ) | 1,811 | (133 | ) | |||||||
Change in fair value of contingent consideration liability | 2,600 | (4,600 | ) | 1,600 | ||||||||
Changes in assets and liabilities: | ||||||||||||
Accounts receivable | 892 | (1,322 | ) | (3,415 | ) | |||||||
Other current assets | 2,046 | 1,314 | (3,058 | ) | ||||||||
Other assets | 947 | (778 | ) | (1,535 | ) | |||||||
Accounts payable | 2,779 | 243 | (202 | ) | ||||||||
Accrued expenses | (6,274 | ) | 6,581 | 2,354 | ||||||||
Deferred tax and other current liabilities | - | (158 | ) | (60 | ) | |||||||
Other liabilities | 323 | 343 | (80 | ) | ||||||||
Net cash (used in) provided by operating activities | (53,628 | ) | 19,209 | (40,137 | ) | |||||||
Cash flows from investing activities: | ||||||||||||
Acquisition of subsidiary, net of cash acquired | - | - | (6,202 | ) | ||||||||
Purchases of property and equipment | (269 | ) | (4,286 | ) | (370 | ) | ||||||
Proceeds from sale of fixed assets | 37 | 347 | 48 | |||||||||
Net cash used in investing activities | (232 | ) | (3,939 | ) | (6,524 | ) | ||||||
Cash flows from financing activities: | ||||||||||||
Net proceeds from issuance of long-term debt | - | 48,650 | - | |||||||||
Net proceeds from issuance of common stock in connection with at-the-market offering | 5,026 | - | - | |||||||||
Proceeds from exercise of stock options | 469 | 1,340 | 1,737 | |||||||||
Return of estimated interest payment for noncontrolling interest | 15 | (368 | ) | (292 | ) | |||||||
Net cash provided by financing activities | 5,510 | 49,622 | 1,445 | |||||||||
Effect of currency rate changes on cash and cash equivalents | 83 | (86 | ) | 17 | ||||||||
Net (decrease) increase in cash and cash equivalents | (48,267 | ) | 64,806 | (45,199 | ) | |||||||
Cash and cash equivalents at beginning of period | 138,909 | 74,103 | 119,302 | |||||||||
Cash and cash equivalents at end of period | $ | 90,642 | $ | 138,909 | $ | 74,103 | ||||||
Noncash financing activity | ||||||||||||
Subscription receivable | $ | 2,109 | $ | - | $ | - | ||||||
The accompanying notes are an integral part of the financial statements.
F-7
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(amounts in thousands, except per share amounts or as otherwise noted)
1. Organization and Business
Business
Progenics Pharmaceuticals, Inc. and its subsidiaries ("the Company," "Progenics," "we" or "us") develop innovative medicines and other technologies to target and treat cancer. Our pipeline includes: 1 ) therapeutic agents designed to precisely target cancer (AZEDRA ® , 1095, and PSMA TTC), 2 ) PSMA-targeted imaging agents for prostate cancer ( 1404 and PyL TM ), and 3 ) imaging analysis technology.
W e licensed our first commercial drug, RELISTOR ® (methylnaltrexone bromide) subcutaneous injection for the treatment of opioid induced constipation ("OIC"), to Salix Pharmaceuticals, Inc. (a wholly-owned subsidiary of Valeant Pharmaceuticals International, Inc. ("Valeant")). RELISTOR received an expanded approval from the U.S. Food and Drug Administration ("FDA") for the treatment of OIC in patients taking opioids for chronic non-cancer pain, and in July 2016, RELISTOR Tablets were approved by the FDA for the treatment of OIC in adults with chronic non-cancer pain.
On October 31, 2017, we completed the rolling submission of our NDA for AZEDRA. The FDA has accepted our NDA for review, granted our request for Priority Review, and set an action date of April 30, 2018 under the Prescription Drug User Fee Act ("PDUFA"). We are developing AZEDRA as a treatment for patients with malignant, recurrent, and/or unresectable pheochromocytoma and paraganglioma, which are rare neuroendocrine tumors. There are currently no approved therapies in the U.S. for these ultra-rare diseases. While AZEDRA has received Breakthrough Therapy, Orphan Drug, and Fast Track designations from the FDA, there can be no assurance that our NDA will be approved.
We have in the past considered opportunities for strategic collaborations, out-licenses, and other arrangements with biopharmaceutical companies involving proprietary research, development and clinical programs, and we continue to do so. We may in the future also in-license or acquire additional oncology compounds and/or programs.
Our current principal sources of revenue from operations are royalty, development and commercial milestones, and sublicense revenue-sharing payments from Valeant and Bayer AG ("Bayer"). Royalty and further milestone payments from Valeant or Bayer depend on success in development and commercialization, which is dependent on many factors, such as Valeant or Bayer's respective efforts, decisions by the FDA and other regulatory bodies, competition from drugs for the same or similar indications, and the outcome of clinical and other testing of the licensed products.
We commenced principal operations in 1988, became publicly traded in 1997, and throughout have been engaged primarily in research and development efforts, establishing corporate collaborations , and related business activities. Certain of our intellectual property rights are held by wholly-owned subsidiaries. All of our U.S. operations are presently conducted at our headquarters in New York, and the operations of our wholly-owned foreign subsidiary, EXINI Diagnostics A.B. ("EXINI"), are conducted at our facility in Lund, Sweden. We operate under a single research and development operating segment.
Liquidity
At December 31, 201 7, we had $90.6 million in cash and cash equivalents, a decrease of $48.3 million from $138.9 million at December 31, 2016. We expect that this amount will be sufficient to fund operations as currently anticipated beyond one year from the filing date of this Annual Report on Form 10 -K. We have historically funded our operations to a significant extent from capital-raising and we expect to require additional funding in the future, the availability of which is never guaranteed and may be uncertain.
During the fourth quarter of 2017, we raised net proceeds of $5.0 million in at-the-market transactions under a controlled equity offering sales agreement ("Sales Agreement") with Cantor Fitzgerald & Co. ("Cantor"). (See Note 10 . Stockholders' Equity for additional information). During 2016, we raised net proceeds of $48.7 million through a royalty monetization transaction (See Note 9. Long-Term Debt, Net for additional information). We expect that we may continue to incur operating losses for the foreseeable future.
F-8
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
2. Summary of Significant Accounting Policies
Basis of Presentation
The consolidated financial statements have been prepared on the basis of accounting principles generally accepted in the U.S. ("GAAP"). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and the accompanying notes. On an ongoing basis, we evaluate our estimates, including but not limited to those related to collectability of receivables, intangible assets and contingencies. As additional information becomes available or actual amounts become determinable, the recorded estimates are revised and reflected in the operating results. Actual results could differ from those estimates. Certain expense amounts have been combined in prior periods' financial statements to conform to the current year presentation.
Principles of Consolidation
The condensed consolidated financial statements include the accounts of Progenics as well as its wholly-owned subsidiaries. All material intercompany transactions and balances have been eliminated in consolidation.
In December 2015, we commenced a judicial process in Sweden for acquiring the remaining shares of EXINI. On December 8, 2016, a Swedish arbitral tribunal awarded us advanced title to the remaining shares of EXINI and, as of that date, EXINI became a wholly-owned subsidiary with 100% of the voting shares owned by us. In connection with the acquisition of the remaining shares of EXINI, in December 2016, we paid $368 thousand to the minority interest shareholders for the original purchase price and estimated interest, of which a net amount of $15 thousand was returned in 2017.
Foreign Currency Translation
O ur international subsidiaries generally consider their respective local currency to be their functional currency. Assets and liabilities of these international subsidiaries are translated into U.S. dollars at quarter-end exchange rates and revenues and expenses are translated at average exchange rates during the quarter and year-to-date period. Foreign currency translation adjustments for the reported periods are included in accumulated other comprehensive loss ("AOCL") in our condensed consolidated statements of comprehensive loss, and the cumulative effect is included in the stockholders' equity section of our condensed consolidated balance sheets. Realized gains and losses denominated in foreign currencies are recorded in operating expenses in our condensed consolidated statements of operations and were not material to our consolidated results of operations for the years ended December 31, 2017, 2016, and 2015.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results may differ from those estimates.
Revenue Recognition
We recognize revenue from all sources based on the provisions of the U.S. Securities and Exchange Commission ("SEC") Staff Accounting Bulletin ("SAB") No. 104 ("SAB 104" ) and the Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") 605 (Topic 605, Revenue Recognition ). Under ASC 605, delivered items are separate units of accounting, provided (i) the delivered items have value to a collaborator on a stand-alone basis, and (ii) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered items is considered probable and substantially in our control. A separate update to ASC 605 provides guidance on the criteria that should be met when determining whether the milestone method of revenue recognition is appropriate.
If we are involved in a steering or other committee as part of a multiple-deliverable arrangement, we assess whether our involvement constitutes a performance obligation or a right to participate. For those committees that are deemed obligations, we will evaluate our participation along with other obligations in the arrangement and will attribute revenue to our participation through the period of our committee responsibilities. We recognize revenue for payments that are contingent upon performance solely by our collaborator immediately upon the achievement of the defined event if we have no related performance obligations. Reimbursement of costs is recognized as revenue provided the provisions of ASC 605 are met, the amounts are determinable and collection of the related receivable is reasonably assured.
F-9
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue. Amounts not expected to be recognized within one year of the balance sheet date are classified as long-term.
Royalty revenue is recognized in the period the sales occur, provided the royalty amounts are fixed or determinable, collection of the related receivable is reasonably assured and we have no remaining performance obligations under the arrangement providing for the royalty.
During the past three years, we also recognized revenue from sales of research reagents, which is reflected as other revenue in our consolidated statements of operations.
D uring 2016, we recognized license revenue of $50.7 million under the license agreement with Valeant, of which $50.0 million related to the achievement of a development milestone (FDA approval of RELISTOR tablets) and $0.7 million related to our share of the upfront payment Valeant received from a Canadian-based distributor of RELISTOR. In addition, during 2016, we recognized license revenue of $7.0 million under the license agreement with Bayer, of which $4.0 million related to the upfront payment and $3.0 million related to the achievement of a preclinical development milestones. We are eligible for future milestone and royalty payments under both license agreements with Valeant and Bayer.
During 2015 , we recognized $1.5 million milestone revenue under our agreement with CytoDyn Inc. as a result of CytoDyn's dosing of the first patient in its Phase 2B/3 clinical trial of PRO 140. We are eligible for future milestone and royalty payments.
Research and Development Expenses
Research and development expenses include costs directly attributable to the conduct of research and development programs, including the cost of salaries ; payroll taxes; employee benefits; materials; supplies; maintenance of research equipment; costs related to research collaboration and licensing agreements; the purchase of in-process research and development; the cost of services provided by outside contractors, including services related to our clinical trials; and the full cost of manufacturing drug for use in research, pre-clinical development, and clinical trials. All costs associated with research and development are expensed as incurred.
At each period end , we evaluate the accrued expense balance related to these activities based upon information received from the suppliers and estimated progress towards completion of the research or development objectives to ensure that the balance is reasonably stated. Such estimates are subject to change as additional information becomes available.
Patents
As a result of research and development efforts conducted by us, we have applied, or are applying, for a number of patents to protect proprietary inventions. All costs associated with patents are expensed as incurred.
Net (Loss) Income Per Share
We prepare earnings per share ("EPS") data in accordance with ASC 260 (Topic 260, Earnings Per Share ). Basic net (loss) income per share amounts have been computed by dividing net (loss) income attributable to Progenics by the weighted-average number of common shares outstanding during the period. For 2017 and 2015, we reported net losses and, therefore, potential common shares, and amounts of unrecognized compensation expense have been excluded from diluted net loss per share since their inclusion would be anti-dilutive. For 2016, we reported net income, and the computation of diluted earnings per share is based upon the weighted-average number of our common shares and dilutive effect, determined using the treasury stock method, of potential common shares outstanding including amounts of unrecognized compensation expense. Shares to be issued upon the assumed conversion of the contingent consideration liability are excluded from the diluted earnings per share calculation, if performance conditions have not been met.
Comprehensive (Loss) Income
Comprehensive (loss) income represents the change in net assets of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Our comprehensive (loss) income includes net (loss) income adjusted for the changes in foreign currency translation adjustment. The disclosures required by ASC 220 (Topic 220, Comprehensive Income ) for 2017, 2016, and 2015 have been included in the consolidated statements of comprehensive (loss) income. There was no income tax expense/benefit allocated to any component of other comprehensive (loss) income (see Note 10. Stockholders' Equity for additional information).
F-10
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Concentrations of Credit Risk
Financial instruments which potentially subject us to concentrations of risk consist principally of cash, cash equivalents, and receivables. We invest our excess cash in money market funds, which are classified as cash and cash equivalents. We have established guidelines that relate to credit quality, diversification and maturity and that limit exposure to any one issue of securities. We hold no collateral for these financial instruments.
Cash and Cash Equivalents
We consider all highly liquid investments which have maturities of three months or less, when acquired, to be cash equivalents. The carrying amount reported in the balance sheet for cash and cash equivalents approximates its fair value. Cash and cash equivalents subject us to concentrations of credit risk. At December 31, 2017 and 2016, we had invested approximately $87.2 million and $137.3 million, respectively, in cash equivalents in the form of money market funds with two investment companies and held approximately $3.4 million and $1.6 million, respectively, in two commercial banks.
Accounts Receivable
We estimate the level of accounts receivable which ultimately will be uncollectable based on a review of specific receivable balances, industry experience and the current economic environment. We reserve for affected accounts receivable an allowance for doubtful accounts. At December 31, 2017, we had no allowance for doubtful accounts.
In-Process Research and Development , Othe r Identified Intangible Assets and Goodwill
The fair values of in-process research and development ( "IPR&D") and other identified intangible assets acquired in business combinations are capitalized. The Company utilizes the "income method", which applies a probability weighting that considers the risk of development and commercialization to the estimated future net cash flows that are derived from projected sales revenues and estimated costs or "replacement costs", whichever is greater. These projections are based on factors such as relevant market size, patent protection, historical pricing of similar products and expected industry trends. The estimated future net cash flows are then discounted to the present value using an appropriate discount rate. This analysis is performed for each IPR&D project and other identified intangible assets independently. IPR&D assets are treated as indefinite-lived intangible assets until completion or abandonment of the projects, at which time the assets are amortized over the remaining useful life or written off, as appropriate. Other identified intangible assets are amortized over the relevant estimated useful life. The IPR&D assets are tested at least annually or when a triggering event occurs that could indicate a potential impairment and any impairment loss is recognized in our consolidated statements of operations.
Goodwill represents excess consideration in a business combination over the fair value of identifiable net assets acquired. Goodwill is not amortized, but is subject to impairment testing at least annually or when a triggering event occurs that could indicate a potential impairment. The Company determines whether goodwill may be impaired by comparing the fair value of the reporting unit (the Company has determined that it has only one reporting unit for this purpose), calculated as the product of shares outstanding and the share price as of the end of a period, to its carrying value (for this purpose, the Company's total stockholders' equity). No goodwill impairment has been recognized as of December 31, 2017 or 2016.
Fair Value Measurements
In accordance with ASC 820 (Topic 820, Fair Value Measurements and Disclosures ), we use a three -level hierarchy for fair value measurements of certain assets and liabilities for financial reporting purposes that distinguishes between market participant assumptions developed from market data obtained from outside sources (observable inputs) and our own assumptions about market participant assumptions developed from the best information available to us in the circumstances (unobservable inputs). We assign hierarchy levels to our contingent consideration liability arising from the Molecular Insight Pharmaceuticals, Inc. ("Molecular Insight") acquisition based on our assessment of the transparency and reliability of the inputs used in the valuation. ASC 820 defines the three hierarchy levels as:
● | Level 1 - Valuations based on unadjusted quoted market prices in active markets for identical securities . |
● | Level 2 - Valuations based on observable inputs other than Level 1 prices, such as quoted prices for similar assets at the measurement date, quoted prices in markets that are not active or other inputs that are observable, either directly or indirectly . |
● | Level 3 - Valuations based on unobservable inputs that are significant to the overall fair value measurement, which as noted above involve management judgment. |
F-11
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
To estimate the fair values of our financial assets and liabilities, we use valuation approaches within a hierarchy that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of us. Unobservable inputs are inputs that reflect our assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances. The fair value hierarchy is divided into three levels based on the source of inputs as follows:
The availability of observable inputs can vary among the various types of financial assets and liabilities. To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. In certain cases, the inputs used for measuring fair value may fall into different levels of the fair value hierarchy. In such cases, for financial statement disclosure purposes, the level in the fair value hierarchy within which the fair value measurement is categorized is based on the lowest level of input used that is significant to the overall fair value measurement.
Recurring Fair Value Measurements
We believe the carrying amounts of our cash equivalents, accounts receivable, other current assets, other assets, accounts payable, and accrued expenses approximated their fair values as of December 31, 2017 and 2016.
The fair value of the contingent consideration liability represents future potential milestone payments related to the Molecular Insight acquisition. The fair value of the contingent consideration liability is categorized as a Level 3 instrument, as displayed in Note 4. We record the contingent consideration liability at fair value with changes in estimated fair values recorded in the consolidated statements of operations. We reassess the fair value of the contingent consideration at each reporting period. The contingent consideration liability results from probability adjusted discounted cash flows and Monte Carlo simulation models which include estimates of milestone payments to former Molecular Insight stockholders under the acquisition agreement.
Nonrecurring Fair Value Measurements
Our non-financial assets, such as intangible assets and property and equipment, are measured and recorded at fair value on the acquisition date, and if indicators of impairment exist, we assess recoverability by measuring the amount of any impairment by comparing the carrying value of the asset to its then-current estimated fair value (for intangible assets) or to market prices for similar assets (for property and equipment). If the carrying value is not recoverable we record an impairment charge in our consolidated statements of operations. No impairments occurred for the year ended December 31, 2017.
O ther current assets are comprised of prepaid expenses, interest, other receivables, and, in the 2016 balance, amount held in escrow for former employee litigation, all of which are expected to be settled within one year. Restricted cash, included in other assets, represents collateral for letters of credit securing a lease obligation, and, in the 2016 balance, advanced title to the remaining shares held by noncontrolling minority interests of EXINI. We believe the carrying value of these assets approximates fair value and are considered Level 1 assets.
Fixed Assets
Leasehold improvements, furniture and fixtures, and equipment are stated at cost. Furniture, fixtures and equipment are depreciated on a straight-line basis over their estimated useful lives. Leasehold improvements are amortized on a straight-line basis over the life of the lease or of the improvement, whichever is shorter. Costs of construction of long-lived assets are capitalized but are not depreciated until the assets are placed in service.
F-12
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Expenditures for maintenance and repairs which do not materially extend the useful lives of the assets are charged to expense as incurred. The cost and accumulated depreciation of assets retired or sold are removed from the respective accounts and any gain or loss is recognized in operations. The estimated useful lives of fixed assets are as follows:
Computer equipment (in years) |
| 3 | |
Machinery and equipment (in years) | 5 | - | 7 |
Furniture and fixtures (in years) |
| 5 | |
Leasehold improvements (in years) | Earlier of life of improvement or lease | ||
Deferred L ease L iability and Incentive
Our lease agreements include fixed escalations of minimum annual lease paymen ts and we recognize rental expense on a straight-line basis over the lease terms and record the difference between rent expense and current rental payments as deferred lease liability. Deferred lease incentive includes a construction allowance from our landlord which is amortized as a reduction to rental expense on a straight-line basis over the lease term. As of December 31, 2017, and 2016, our consolidated balance sheets include the following:
2017 | 2016 | |||||||
Other current liabilities: | ||||||||
Deferred lease incentive | $ | 26 | $ | 26 | ||||
Other liabilities: | ||||||||
Deferred lease liability | $ | 1,225 | $ | 876 | ||||
Deferred lease incentive | $ | 303 | $ | 328 | ||||
Income Taxes
We account for income taxes in accordance with the provisions of ASC 740 (Topic 740, Income Taxes ), which requires that we recognize deferred tax liabilities and assets for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined on the basis of the difference between the tax basis of assets and liabilities and their respective financial reporting amounts (temporary differences) at enacted tax rates in effect for the years in which the temporary differences are expected to reverse. A valuation allowance is established for deferred tax assets for which realization is uncertain.
In accordance with ASC 718 (Topic 718, Compensation – Stock Compensation ) and ASC 505 (Topic 505, Equity ), we have made a policy decision related to intra-period tax allocation, to account for utilization of windfall tax benefits based on provisions in the tax law that identify the sequence in which amounts of tax benefits are used for tax purposes (i.e., tax law ordering). We adopted Accounting Standards Update ("ASU") No. 2016 - 09, Compensation – Stock Compensation (Topic 718 ) ("ASU 2016 - 09" ) on January 1, 2017, which requires that all excess tax benefits and tax deficiencies during the period be recognized in income (rather than in equity) on a prospective basis.
Uncertain tax positions are accounted for in accordance with ASC 740, which prescribes a comprehensive model for the manner in which a company should recognize, measure, present and disclose in its financial statements all material uncertain tax positions that we have taken or expect to take on a tax return. ASC 740 applies to income taxes and is not intended to be applied by analogy to other taxes, such as sales taxes, value-add taxes, or property taxes. We review our nexus in various tax jurisdictions and our tax positions related to all open tax years for events that could change the status of our ASC 740 liability, if any, or require an additional liability to be recorded. Such events may be the resolution of issues raised by a taxing authority, expiration of the statute of limitations for a prior open tax year or new transactions for which a tax position may be deemed to be uncertain. Those positions, for which management's assessment is that there is more than a 50% probability of sustaining the position upon challenge by a taxing authority based upon its technical merits, are subjected to the measurement criteria of ASC 740. We record the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement with a taxing authority having full knowledge of all relevant information. Any ASC 740 liabilities for which we expect to make cash payments within the next twelve months are classified as "short term." In the event that we conclude that we are subject to interest and/or penalties arising from uncertain tax positions, we will record interest and penalties as a component of income taxes (see Note 13. Income Taxes for additional information).
F-13
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Risks and Uncertainties
To date, w e have relied principally on external funding, license agreements with Valeant, Bayer and others, out-licensing and asset sale arrangements, and royalty and product revenue to finance our operations. There can be no assurance that our research and development will be successfully completed, that any products developed will obtain necessary marketing approval by regulatory authorities or that any approved products will be commercially viable. In addition, we operate in an environment of rapid change in technology, and we are dependent upon satisfactory relationships with our partners and the continued services of our current employees, consultants and subcontractors. We are also dependent upon Valeant and Fuji fulfilling their manufacturing obligations, either on their own or through third -party suppliers. For 2017, 2016, and 2015, the primary sources of our revenues were royalty and milestone payments. There can be no assurance that such revenues will continue. Substantially all of our accounts receivable at December 31, 2017 and 2016 were from the above-named sources.
Legal Proceedings
From time to time, we may be a party to legal proceedings in the course of our business. The outcome of any such proceedings, r egardless of the merits, is inherently uncertain. The assessment of whether a loss is probable or reasonably possible, and whether the loss or a range of loss is estimable, often involves a series of complex judgments about future events. We record accruals for contingencies to the extent that the occurrence of the contingency is probable and the amount of liability is reasonably estimable. If the reasonable estimate of liability is within a range of amounts and some amount within the range appears to be a better estimate than any other, then we record that amount as an accrual. If no amount within the range is a reasonable estimate, then we record the lowest amount within the range as an accrual. Loss contingencies that are assessed as remote are not reported in the financial statements, or in the notes to the consolidated financial statements.
Impact of Recently Issued and Adopted Accounting Standards
Recently Adopted
In March 2016, the Financial Accounting Standards Board (the "FASB") issued ASU 2016 - 09, Compensation – Stock Compensation (Topic 718 ) . The standard simplifies several aspects of accounting for stock-based payment transactions, including the accounting for income taxes, forfeitures, statutory tax withholding requirements, as well as classification in the statement of cash flows. We adopted this standard on January 1, 2017. For the year ended December 31, 2017, we recognized all excess tax benefits and tax deficiencies associated with exercise of stock options as income tax expense or benefit as a discrete event. The adoption of this ASU did not have a material impact on our consolidated financial statements.
Not Yet Adopted
In January 2017, the FASB issued ASU No. 2017 - 01 ("ASU 2017 - 01" ), Business Combinations (Topic 805 ): Clarifying the Definition of a Business. The standard narrows the application of when an integrated set of assets and activities is considered a business and provides a framework to assist entities in evaluating whether both an input and a substantive process are present to be considered a business. It is expected that this new guidance will reduce the number of transactions that would be accounted for as a business. ASU 2017 - 01 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017, with early adoption permitted. We do not expect the adoption of this new standard to have a material impact on our consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017 - 04 ("ASU 2017 - 04" ), Intangibles - Goodwill and Other (Topic 350 ): Simplifying the Test for Goodwill Impairment . The standard simplifies how an entity is required to test goodwill for impairment by eliminating Step 2 from the goodwill impairment test. Step 2 measures a goodwill impairment loss by comparing the implied fair value of a reporting unit's goodwill with the carrying amount. ASU 2017 - 04 will be effective for the annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. We do not expect the adoption of the new standard to have a material impact on our consolidated financial statements.
F-14
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
In November 2016, the FASB issued ASU No. 2016 - 18 ("ASU 2016 - 18" ), Statement of Cash Flows (Topic 230 ) – Restricte d Cash. For entities that have restricted cash and are required to present a statement of cash flows, ASU 2016 - 18 changes the cash flow presentation for restricted cash. ASU 2016 - 18 will be effective for fiscal years beginning after December 15, 2017, and interim periods within those annual periods. We do not expect the adoption of this new standard to have a material impact on our consolidated financial statements or statement of cash flows.
In August 2016, the FASB issued ASU No. 2016 - 15 ("ASU 2016 - 15" ), Statement of Cash Flows (Topic 230 ): Classification of Certain Cash Receipts and Cash Payments . The standard provides guidance on eight ( 8 ) cash flow issues: ( 1 ) debt prepayment or debt extinguishment costs; ( 2 ) settlement of zero -coupon bonds; ( 3 ) contingent consideration payments after a business combination; ( 4 ) proceeds from the settlement of insurance claims; ( 5 ) proceeds from the settlement of corporate-owned life insurance policies; ( 6 ) distributions received from equity method investees; ( 7 ) beneficial interests in securitization transactions; and ( 8 ) separately identifiable cash flows and application of the predominance principle. ASU 2016 - 15 addresses how certain cash receipts and cash payments are presented and classified in the statement of cash flows. ASU 2016 - 15 is effective for fiscal years, and interim periods within those years, beginning after December 15, 2017 with early adoption permitted. We do not expect the adoption of this new standard to have a material impact on our consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016 - 02, Leases (Topic 842 ) ("ASU 2016 - 02" ) . The standard requires lessees to recognize leases on their balance sheets, and leaves lessor accounting largely unchanged. Additionally, ASU 2016 - 02 requires a modified retrospective approach for all leases existing at, or entered into after, the date of initial application, with an option to elect to use certain transition relief. ASU 2016 - 02 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018, with early adoption permitted. We are currently evaluating the impact of this new standard on our consolidated financial statements.
In January 2016, the FASB issued ASU No. 2016 - 01, Recognition and Measurement of Financial Assets and Financial Liabilities ("ASU 2016 - 01" ). The standard requires equity investments (except those accounted for under the equity method of accounting or those that result in consolidation of the investee) to be measured at fair value with changes in fair value recognized in net income, and separate presentation of financial assets and financial liabilities by measurement category and form of financial asset. Additionally, ASU 2016 - 01 eliminates the requirement to disclose the methods and significant assumptions used to estimate the fair value that is required to be disclosed for financial instruments on the balance sheet. ASU 2016 - 01 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. Other than an amendment relating to presenting in comprehensive income the portion of the total change in the fair value of a liability resulting from a change in instrument-specific credit risk (if the entity has elected to measure the liability at fair value), early adoption is not permitted. We do not expect the adoption of this new standard to have a material impact on our consolidated financial statements.
In May 2014, the FASB issued ASU No. 2014 - 09, Revenue from Contracts with Customers (Topic 606 ) ("ASU 2014 - 09" ). The standard provides a single model for revenue arising from contracts with customers and supersedes current revenue recognition guidance. This ASU provides that an entity should recognize revenue to depict transfers of promised goods or services to customers in amounts reflecting the consideration to which the entity expects to be entitled in the transaction by: ( 1 ) identifying the contract; ( 2 ) identifying the contract's performance obligations; ( 3 ) determining the transaction price; ( 4 ) allocating the transaction price to the performance obligations; and ( 5 ) recognizing revenue when or as the entity satisfies the performance obligations. The guidance permits companies to apply the requirements either retrospectively to all prior periods presented or in the year of adoption through a cumulative adjustment by applying a modified retrospective transition method. ASU 2014 - 09 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. In 2016, the FASB issued several amendments to the standard, including principal versus agent considerations when another party is involved in providing goods or services to a customer and the process of identifying performance obligations.
We identified four primary revenue streams from contracts with customers as part of our assessment: ( 1 ) royalties, ( 2 ) licensing arrangements, ( 3 ) software licensing arrangements, and ( 4 ) product sales. We have completed our evaluation of the new standard and assessed the impacts of adoption on the consolidated financial statements and disclosures. Based on the evaluation of our current contracts and revenue streams, revenue recognition is mostly consistent under both the previous and new standard, except for contracts under the software licensing arrangements revenue stream. Under the previous accounting policy, revenue related to the bonus payments earned under the software license arrangements have been recognized when paid by the customer. Upon adoption of the new standard, revenue related to the bonus payments will be estimated and recognized quarterly as applicable. We will adopt the new standard effective January 1, 2018 using the modified retrospective transition method. We will record a cumulative credit adjustment of approximately $0.1 million to the opening balance of accumulated deficit, with a corresponding debit to accounts receivable, related to the change in accounting treatment for the bonus payments under the software license arrangements revenue stream. We do not anticipate implementing significant changes to our internal controls or systems due to the adoption of the new standard. The disclosures, which we are still evaluating, in our notes to consolidated financial statements related to revenue recognition will be significantly expanded under the new standard.
F-15
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
3. Goodwill and Acquired Intangible Assets
I ntangible assets and goodwill were initially measured at the acquisition date at estimated fair value and capitalized for the acquisitions of our wholly-owned subsidiaries EXINI and Molecular Insight.
The following table summarizes the activity related to goodwill and intangible assets:
Other | ||||||||||||
Intangible | ||||||||||||
Goodwill | IPR&D | Assets | ||||||||||
Balance at January 1, 2016 | $ | 13,074 | $ | 28,700 | $ | 2,093 | ||||||
Amortization expense | - | - | (212 | ) | ||||||||
Balance at December 31, 2016 | 13,074 | 28,700 | 1,881 | |||||||||
Amortization expense | - | - | (212 | ) | ||||||||
Balance at December 31, 2017 | $ | 13,074 | $ | 28,700 | $ | 1,669 | ||||||
The following table reflects the components of the finite-lived intangible assets as of December 31, 201 7:
Gross Amount | Accumulated Amortization | Net Carrying Value | ||||||||||
Finite lived intangible assets | $ | 2,120 | $ | 451 | $ | 1,669 | ||||||
Total | $ | 2,120 | $ | 451 | $ | 1,669 | ||||||
The weighted-average remaining life of the finite-lived intangible assets was approximately eight years at December 31, 2017.
Amortization expense was calculated on a straight-line basis over the estimated useful life of the asset and was $212 thousand per year for the years ended December 31, 2017 and 2016. Assuming no changes in the gross carrying amount of finite lived intangible assets, the future annual amortization expense related to finite lived intangible assets is expected to be $212 thousand in each of the next five years ( 2018 through 2022 ) .
4 . Fair Value Measurements
We record the contingent consideration liability resulting from our acquisition of Molecular Insight at fair value in accordance with ASC 820 (Topic 820, Fair Value Measurement ).
F-16
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
The following tables summarize each major class of our financial assets and liabilities measured at fair value on a recurring basis as of the dates indicated, classified by valuation hierarchy (in thousands):
Fair Value Measurements at December 31, 2017 | ||||||||||||||||
Quoted Prices in | Significant Other | Significant | ||||||||||||||
Active Markets for | Observable | Unobservable | ||||||||||||||
Balance at | Identical Assets | Inputs | Inputs | |||||||||||||
December 31, 2017 | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
Assets: | ||||||||||||||||
Money market funds | $ | 87,231 | $ | 87,231 | $ | - | $ | - | ||||||||
Total assets | $ | 87,231 | $ | 87,231 | $ | - | $ | - | ||||||||
Liabilities: | ||||||||||||||||
Contingent consideration liability | $ | 16,800 | $ | - | $ | - | $ | 16,800 | ||||||||
Total liabilities | $ | 16,800 | $ | - | $ | - | $ | 16,800 | ||||||||
Fair Value Measurements at December 31, 2016 | ||||||||||||||||
Quoted Prices in | Significant Other | Significant | ||||||||||||||
Active Markets for | Observable | Unobservable | ||||||||||||||
Balance at | Identical Assets | Inputs | Inputs | |||||||||||||
December 31, 2016 | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
Assets: | ||||||||||||||||
Money market funds | $ | 137,340 | $ | 137,340 | $ | - | $ | - | ||||||||
Total assets | $ | 137,340 | $ | 137,340 | $ | - | $ | - | ||||||||
Liabilities: | ||||||||||||||||
Contingent consideration liability | $ | 14,200 | $ | - | $ | - | $ | 14,200 | ||||||||
Total liabilities | $ | 14,200 | $ | - | $ | - | $ | 14,200 | ||||||||
The contingent consideration liability of $16.8 million and $ 14.2 million at December 31, 2017 and 2016, respectively, represents the estimated fair value of the future potential milestone payments to former Molecular Insight stockholders (shown in the tables below).
Milestone payments due upon first commercial sale (in thousands):
Program | Consideration | Form of Payment at Progenics' Option | ||||
AZEDRA | $ | 8,000 | Cash or Progenics common stock | |||
| 1404 | 10,000 | Cash or Progenics common stock | ||||
| 1095 | 5,000 | Cash or Progenics common stock | ||||
| $ | 23,000 | |||||
Net sales milestone payments due upon first achievement of specified net sales target in any single calendar year across all MIP-related programs (in thousands):
Calendar Year Net Sales Level (in millions) | Consideration | Form of Payment at Progenics' Option | |||||
| $ | 30 | $ | 5,000 | Cash or Progenics common stock | |||
| $ | 60 | 5,000 | Cash or Progenics common stock | ||||
| $ | 100 | 10,000 | Cash or Progenics common stock | ||||
| $ | 250 | 20,000 | Cash or Progenics common stock | ||||
| $ | 500 | 30,000 | Cash or Progenics common stock | ||||
| $ | 70,000 | ||||||
We consider this liability a Level 3 instrument ( one with significant unobservable inputs) in the fair value hierarchy. The estimated fair value was determined based on probability adjusted discounted cash flow and Monte Carlo simulation models that included significant estimates and assumptions pertaining to commercialization events and sales targets. The most significant unobservable inputs are the probabilities of achieving regulatory approval of the development projects and subsequent commercial success and discount rates.
The estimated fair value was determined based on probability adjusted discounted cash flow and Monte Carlo simulation models that included significant estimates and assumptions pertaining to commercialization events and sales targets. The most significant unobservable inputs were the probabilities of achieving regulatory approval of the development projects and subsequent commercial success, and discount rates.
F-17
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Significant changes in any of the probabilities of success would result in a significantly higher or lower fair value measurement, respectively. Significant changes in the probabilities as to the periods in which milestones will be achieved would result in a significantly lower or higher fair value measurement, respectively. We record the contingent consideration liability at fair value with changes in estimated fair values recorded in change in contingent consideration liability in our condensed consolidated statements of operations.
The following table s summarizes quantitative information and assumptions pertaining to the fair value measurement of the Level 3 inputs at December 31, 2017 and 2016 (in thousands). The 2017 increase in the contingent consideration liability of $2.6 million was primarily attributable to the higher probability of success of AZEDRA used to calculate the potential milestone payments to former Molecular Insight stockholders and a reduction in the discount period.
Fair Value at | ||||||||||||
December 31, | Range | |||||||||||
2017 | Valuation Technique | Unobservable Input | (Weighted-Average) | |||||||||
| Contingent Consideration Liability: | ||||||||||||
AZEDRA commercialization | $ | 5,500 | Probability adjusted | Probability of success | 72% | |||||||
| discounted cash flow model | Period of expected milestone achievement | 2018 | ||||||||||
Discount rate | 10% | |||||||||||
1404 commercialization | 4,500 | Probability adjusted | Probability of success | 59% | ||||||||
| discounted cash flow model | Period of expected milestone achievement | 2020 | ||||||||||
Discount rate | 10% | |||||||||||
1095 commercialization | 400 | Probability adjusted | Probability of success | 16% | ||||||||
| discounted cash flow model | Period of expected milestone achievement | 2025 | ||||||||||
Discount rate | 10% | |||||||||||
Net sales targets | 6,400 | Monte-Carlo simulation | Probability of success | 16% | - | 72% | ||||||
Discount rate | 10% | |||||||||||
Total | $ | 16,800 | ||||||||||
Fair Value at | ||||||||||||
December 31, | Range | |||||||||||
2016 | Valuation Technique | Unobservable Input | (Weighted-Average) | |||||||||
| Contingent Consideration Liability: | ||||||||||||
AZEDRA commercialization | $ | 3,800 | Probability adjusted | Probability of success | 54% | |||||||
| discounted cash flow model | Period of expected milestone achievement | 2018 | ||||||||||
Discount rate | 10% | |||||||||||
1404 commercialization | 4,700 | Probability adjusted | Probability of success | 59% | ||||||||
| discounted cash flow model | Period of expected milestone achievement | 2019 | ||||||||||
Discount rate | 10% | |||||||||||
1095 commercialization | 400 | Probability adjusted | Probability of success | 16% | ||||||||
| discounted cash flow model | Period of expected milestone achievement | 2024 | ||||||||||
Discount rate | 10% | |||||||||||
Net sales targets | 5,300 | Monte-Carlo simulation | Probability of success | 16% | - | 59% | ||||||
Discount rate | 10% | |||||||||||
Total | $ | 14,200 | ||||||||||
For those financial instruments with significant Level 3 inputs, the following table summarizes the activities for the periods indicated:
Liability - Contingent Consideration | ||||||||
Fair Value Measurements Using | ||||||||
Significant Unobservable Inputs | ||||||||
(Level 3) | ||||||||
2017 | 2016 | |||||||
Balance at beginning of period | $ | 14,200 | $ | 18,800 | ||||
Fair value change included in net income (loss) | 2,600 | (4,600 | ) | |||||
Balance at end of period | $ | 16,800 | $ | 14,200 | ||||
Changes in unrealized gains or losses for the period included in earnings (or changes in net assets) for liabilities held at the end of the reporting period | $ | 2,600 | $ | (4,600 | ) | |||
F-18
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
5 . Accounts Receivable
Our a ccounts receivable represent amounts due to us from collaborators, royalties, and sales of research reagents, and consisted of the following at December 31, 2017 and 2016:
2017 | 2016 | |||||||
Royalties | $ | 3,683 | $ | 2,407 | ||||
Collaborators | 13 | 2,000 | ||||||
Other | 276 | 457 | ||||||
Accounts receivable, net | $ | 3,972 | $ | 4,864 | ||||
6 . Fixed Assets
Fixed assets as of December 31, 201 7 and 2016 consisted of the following:
2017 | 2016 | |||||||
Machinery and equipment | $ | 2,516 | $ | 1,493 | ||||
Leasehold improvements | 1,734 | 1,640 | ||||||
Computer equipment | 714 | 695 | ||||||
Furniture and fixtures | 874 | 877 | ||||||
Construction in progress | - | 893 | ||||||
Property and equipment, gross | 5,838 | 5,598 | ||||||
Less - accumulated depreciation | (1,716 | ) | (838 | ) | ||||
Property and equipment, net | $ | 4,122 | $ | 4,760 | ||||
At December 31, 201 7 and 2016, $1.6 million of net leasehold improvements were being amortized over periods of 12.8 years and 13.8 years, respectively, under leases with terms through September 30, 2030. We recorded depreciation expense of $0.9 million, $1.9 million, and $0.5 million during 2017, 2016, and 2015, respectively.
7 . Accrued Expenses
The carrying value of our accrued expenses approximates fair value as it represents amounts that will be satisfied within one year. Accrued expenses consisted of the following at December 31, 2017 and 2016:
2017 | 2016 | |||||||
Accrued clinical trial costs | $ | 2,570 | $ | 4,885 | ||||
Accrued payroll and related costs | 2,400 | 1,957 | ||||||
Accrued consulting and service fee expenses | 1,860 | 1,026 | ||||||
Accrued legal and professional fees | 1,022 | 1,123 | ||||||
Accrued contract manufacturing costs | 666 | 2,048 | ||||||
Loss contingency for litigation | - | 4,000 | ||||||
Other | 1,037 | 751 | ||||||
Accrued expenses | $ | 9,555 | $ | 15,790 | ||||
8 . Commitments and Contingencies
Operating Leases
At December 31, 201 7, we leased corporate office space in New York City, pursuant to a lease agreement expiring in September 2030 ( subject to an early termination right), and additional office space in Lund, Sweden, pursuant to a lease agreement which expires in December 2018.
Rental payments are recognized as rent expense on a straight-line basis over the term of the lease. In addition to rents due under these agreements, we are obligated to pay additional facilities charges, including utilities, taxes and operating expenses.
F-19
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
As of December 31, 2017, future minimum annual payments under all operating lease agreements are as follows:
Years ending | Minimum Annual | |||
December 31, | Payments | |||
2018 | $ | 1,877 | ||
2019 | 1,818 | |||
2020 | 1,852 | |||
2021 | 1,886 | |||
2022 | 2,134 | |||
Thereafter | 18,549 | |||
Total | $ | 28,116 | ||
Rental ex pense totaled approximately $1.9 million, $1.2 million, and $1.9 million for 2017, 2016, and 2015, respectively. For 2017 and 2016, rent expense exceeded amounts paid by $323 thousand and $210 thousand, respectively and for 2015 amounts paid exceeded rent expense by $49 thousand. Additional facility charges, including utilities, taxes, and operating expenses, for 2017, 2016, and 2015 were approximately $0.1 million, $1.1 million, and $2.5 million, respectively.
Licensing , Service , and Supply Agreements
We have entered into intellectual property-based license and service agreements in connection with product development programs, and have incurred milestone, license and sublicense fees and supply costs, included in research and development expenses, totaling approximately $343 thousand, $324 thousand, and $388 thousand during the 2017, 2016, and 2015, respectively.
Paid from inception/acquisition to December 31, 2017 | Future Commitments (1) | Terms | |||||||
Amgen Fremont, Inc. (formerly Abgenix) | $ | 1,350 | $ | 5,750 | Milestones and royalties to use XenoMouse ® technology for generating fully human antibodies to PSMA LLC's PSMA antigen. | ||||
Former member of PSMA LLC | $ | 428 | $ | 52,188 | Annual minimum royalty payments and milestones to use technology related to PSMA. | ||||
University of Zurich and the Paul Scherrer Institute | $ | 500 | $ | 840 | Annual maintenance and license fee payments, milestones and royalties in respect of licensed technology related to 1404. | ||||
University of Western Ontario | $ | 78 | $ | 257 | Annual minimum royalty, administration and milestone payments in respect of licensed technology related to AZEDRA. | ||||
Johns Hopkins University Technology | $ | 150 | $ | 2,760 | Annual minimum royalty payments and milestones to use technology related to PyL. | ||||
Selexis Commercial License Agreement | $ | 59 | $ | 1,792 | Milestones and royalties to use Selexis technology related to PSMA Antibodies. | ||||
( 1 ) Amounts based on known contractual obligations as specified in the respective license agreements, which are dependent on the achievement or occurrence of future milestones or events and exclude amounts for royalties which are dependent on future sales and are unknown.
In addition, w e are planning to out-license or terminate non-germane licenses and service agreements, as to which we have paid $4.9 million through December 31, 2017, and have future commitments of $28.5 million, subject to occurrence of future milestones or events.
F-20
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Consulting Agreements
As part of our research and development efforts, we have from time to time entered into consulting agreements with external scientific specialists. These agreements contain various terms and provisions, including fees to be paid by us and royalties, in the event of future sales, and milestone payments, upon achievement of defined events, payable by us. Certain of these scientists are advisors to us, and some have purchased our common stock or received stock options which are subject to vesting provisions. We have recognized expenses with regard to the consulting agreements of $326 thousand, $164 thousand, and $54 thousand for 2017, 2016, and 2015, respectively.
Legal Proceedings
On January 4, 2017, we settled all claims against us under a federal action brought in 2010 by a former employee, where such former employee had complained that we had violated the anti-retaliation provisions of the federal Sarbanes-Oxley law by terminating him. In connection with this settlement, we and the former employee exchanged mutual releases and we paid an aggregate sum of $4.0 million to the former employee and his attorneys.
On October 7, 2015 Progenics, Valeant and Wyeth LLC (Valeant's predecessor as licensee of RELISTOR) received notification of a Paragraph IV certification for certain patents for RELISTOR ® (methylnaltrexone bromide) subcutaneous injection, which are listed in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, or the Orange Book. The certification resulted from the filing by Mylan Pharmaceuticals, Inc. of an Abbreviated New Drug Application ("ANDA") with the FDA, challenging such patents for RELISTOR subcutaneous injection and seeking to obtain approval to market a generic version of RELISTOR subcutaneous injection before some or all of these patents expire.
On October 28, 2015, Progenics, Valeant and Wyeth LLC received a secon d notification of a Paragraph IV certification with respect to the same patents for RELISTOR subcutaneous injection from Actavis LLC as a result of Actavis LLC's filing of an ANDA with the FDA, also challenging these patents and seeking to obtain approval to market a generic version of RELISTOR subcutaneous injection before some or all of these patents expire.
In October 2016 , Progenics, Valeant, and Wyeth LLC received a notification of a Paragraph IV certification with respect to certain patents for RELISTOR Tablets. The certification accompanied the filing by Actavis LLC of an ANDA challenging such patents for RELISTOR Tablets and seeking to obtain approval to market a generic version of RELISTOR tablets before some or all of these patents expire.
In accordance with the Drug Price Competition and Patent Term Restoration Act (commonly referred to as the Hatch-Waxman Act), Progenics and Valeant timely commenced litigation against each of these ANDA filers in order to obtain an automatic stay of FDA approval of the ANDA until the earlier of (i) 30 months from receipt of the notice or (ii) a District Court decision finding that the identified patents are invalid, unenforceable or not infringed.
In addition to the above described ANDA notifications, in October 2015 Progenics received notices of opposition to three European patents relating to methylnaltrexon e. The oppositions were filed separately by each of Actavis Group PTC ehf. and Fresenius Kabi Deutschland GmbH.
Each of the above-described proceedings is in its early stages and Progenics and Valeant continue to cooperate closely to vigorously defend an d enforce RELISTOR intellectual property rights. Pursuant to the RELISTOR license agreement between Progenics and Valeant, Valeant has the first right to enforce the intellectual property rights at issue and is responsible for the costs of such enforcement.
We are or may be from time to time involved in various other disputes, governmental and/or regulatory inspections, inquires, investigations and proceedings that could result in litigation, and other litigation matters that arise from time to time. The process of resolving matters through litigation or other means is inherently uncertain and it is possible that an unfavorable resolution of these matters will adversely affect us, our results of operations, financial condition, and cash flows.
9. No n -Recourse Long-Term Debt, Net
On November 4, 2016, through a new wholly-owned subsidiary MNTX Royalties Sub LLC ("MNTX Royalties"), we entered into a $50.0 million loan agreement (the "Royalty-Backed Loan") with a fund managed by HealthCare Royalty Partners III, L.P. ("HCRP"). Under the terms of the Royalty-Backed Loan, the lenders have no recourse to us or to any of our assets other than the right to receive royalty payments from the commercial sales of RELISTOR products owed under our agreement with Valeant. The RELISTOR royalty payments will be used to repay the principal and interest on the loan. The Royalty-Backed Loan bears interest at a per annum rate of 9.5%.
F-21
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Under the terms of the loan agreement, payments of interest and principal, if any, under the loan will be made on the last day of each calendar quarter out of RELISTOR royalty payments received since the immediately preceding payment date. On each payment date prior to March 31, 2018, RELISTOR royalty payments received since the immediately preceding payment date will be applied solely to the payment of interest on the loan, with any royalties in excess of the interest amount retained by us. Beginning on March 31, 2018, 50 percent of RELISTOR royalty payments received since the immediately preceding payment date in excess of accrued interest on the loan will be used to repay the principal of the loan, with the balance retained by us. Starting on September 30, 2021, all of the RELISTOR royalties received since the immediately preceding payment date will be used to repay the interest and outstanding principal balance until the balance is fully repaid. The loan has a maturity date of June 30, 2025. Upon the occurrence of certain triggers in the loan agreement, or if HCRP so elects on or after January 1, 2018, all of the RELISTOR royalty payments received after the immediately preceding payment date shall be applied to the payment of interest and repayment of principal until the principal of the loan is fully repaid. In the event of such an election by HCRP, we have the right to repay the loan without any prepayment penalty.
In connection with the Royalty-Backed Loan, the debt issuance costs have been recorded as a debt discount in our consolidated balance sheets and are being amortized and recorded as interest expense throughout the life of the loan using the effective interest method.
As of December 31, 201 7, we were in compliance with all material covenants under the Royalty-Backed Loan and there was no material adverse change in our business, operations, or financial conditions, as defined in the loan agreement.
Future principal , based upon estimated sales projections, under the Royalty-Backed Loan as of December 31, 2017, are as follows:
2018 | $ | 2,686 | ||
2019 | 3,738 | |||
2020 | 4,858 | |||
2021 | 8,156 | |||
2022 | 12,069 | |||
Thereafter | 19,245 | |||
Total payments | $ | 50,752 |
Interest expense, including amortization of debt discount, related to the Royalty-Backed Loan for the year ended December 31, 201 7 was approximately $5.1 million.
10 . Stockholders' Equity
Common Stock and Preferred Stock
We are authorized to issue 160 million shares of our common stock, par value $.0013, and 20 million shares of preferred stock, par value $.001. The Board of Directors (the "Board") has the authority to issue common and preferred shares, in series, with rights and privileges as determined by the Board.
Shelf Registration
During the first quarter of 2017, we established a $250 .0 million replacement shelf registration statement. All sales of shares have been and will continue to be made pursuant to an effective shelf registration statement on Form S- 3 filed with the U.S. Securities and Exchange Commission. In addition, in January 2017 we entered into a Sales Agreement with Cantor, as sales agent, pursuant to which we may offer and sell through Cantor, from time to time, shares of our common stock up to an aggregate offering price of $75.0 million.
During the fourth quarter of 2017, we sold a total of 854,606 shares of our common stock in at-the-market transactions under the Sales Agreement for net proceeds, after deducting commissions and other transaction costs, of approximately $5.0 million at an average selling price of $6.06 per share. At December 31, 2017, we had 320,182 shares of our common stock subscribed in at-the-market transactions under the Sales Agreement for net proceeds, after deducting commissions and other transaction costs, of approximately $2.1 million at an average selling price of $6.79 per share. Accordingly, we have recorded a subscription receivable of $2.1 million as a reduction of stockholders' equity in our consolidated balance sheet at December 31, 2017.
F-22
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Accumulated Other Comprehensive Loss ("AOCL")
The following table summarizes the components of AOCL at December 31, 2017:
Foreign Currency Translation | AOCL | |||||||
Balance at January 1, 2017 | $ | (85 | ) | $ | (85 | ) | ||
Foreign currency translation adjustment | 52 | 52 | ||||||
Balance at December 31, 2017 | $ | (33 | ) | $ | (33 | ) | ||
We did not have any reclassifications out of AOCL to losses during 2017.
11 . S tock -Based Compensation
Equity Incentive Plans
W e adopted the following stockholder-approved equity incentive plans:
● | The 1996 Amended Stock Incentive Plan (the "1996 Plan") authorized the issuance of up to 5,000,000 shares of our common stock covering several different types of awards, including stock options, restricted shares, stock appreciation rights, performance shares, and phantom stock. The 1996 Plan was terminated in 2006. Options granted before termination of the 1996 Plan will continue to remain outstanding until exercised, cancelled, or expired. |
● | The 2005 Stock Incentive Plan (the "2005 Plan"), pursuant to which we are authorized to issue up to 11,450,000 shares of common stock covering several different types of awards, including stock options, restricted shares, stock appreciation rights, performance shares, and phantom stock. The 2005 Plan will terminate on March 25, 2024. |
The stock option plans provide that options may be granted at an exercise price of 100% of fair market value of our common stock on the date of grant, may be exercised in full or in installments, at the discretion of the Board or its Compensation Committee, and must be exercised within ten years from date of grant. Stock options generally vest pro rata over three to five years. We recognize stock-based compensation expense on a straight-line basis over the requisite service (vesting) period based on fair values. We use historical data to estimate expected employee behaviors related to option exercises and forfeitures and included these expected forfeitures as a part of the estimate of stock-based compensation expense as of the grant date. We adjust the total amount of stock-based compensation expense recognized for each award, in the period in which each award vests, to reflect the actual forfeitures related to that award. Changes in our estimated forfeiture rate will result in changes in the rate at which compensation cost for an award is recognized over its vesting period.
F-23
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
Stock Options
The following table summarizes stock options activity for the year ended December 31, 201 7:
Weighted | ||||||||||||
Weighted | Average | |||||||||||
Number | Average | Remaining | ||||||||||
of Shares | Exercise Price | Contractual Life | ||||||||||
Outstanding at January 1, 2017 | 4,887 | $ | 7.57 | 5.81 | ||||||||
Granted | 1,232 | $ | 10.34 | |||||||||
Exercised | (80 | ) | $ | 5.89 | ||||||||
Cancelled | (159 | ) | $ | 8.96 | ||||||||
Expired | (345 | ) | $ | 22.42 | ||||||||
Outstanding at December 31, 2017 | 5,535 | $ | 7.25 | 6.04 | ||||||||
Exercisable at December 31, 2017 | 3,736 | $ | 6.71 | 4.77 | ||||||||
Vested and expected to vest at December 31, 2017 | 5,227 | $ | 7.15 | 5.87 | ||||||||
The weighted average fair value of options granted during 201 7, 2016, and 2015 was $6.96, $3.24, and $5.02 per share, respectively.
The total intrinsic value (the excess of the market price over the exercise price) was approximately $ 2.7 million for stock options outstanding, $2.0 million for stock options exercisable, and $2.6 million for stock options vested and expected to vest, as of December 31, 2017. The total intrinsic value for stock options exercised during 2017, 2016, and 2015 was $233 thousand, $476 thousand, and $465 thousand, respectively.
Stock-Based Compensa tion Expense
We account for stock-based awards issued to employees in accordance with the provisions of ASC 718 (Topic 718, Compensation – Stock Compensation ). We recognize stock-based compensation expense on a straight-line basis over the service period of the award, which is generally three to five years. Stock-based awards issued to consultants are accounted for in accordance with the provisions of ASC 718 and ASC 505 - 50 (Subtopic 50 "Equity-Based Payments to Non-Employees" of Topic 505, Equity ). Options granted to consultants are periodically revalued as the options vest, and are recognized as an expense over the related period of service or the vesting period, whichever is longer. Under the provisions of ASC 718, members of the Board are considered employees for calculation of stock-based compensation expense.
We estimated the fair value of the stock options granted on the date of grant using a Black-Scholes valuation model that used the weighted average assumptions noted in the following table. The risk-fr ee interest rate assumption we use is based upon U.S. Treasury interest rates appropriate for the expected life of the awards. The expected life (estimated period of time that we expect employees, directors, and consultants to hold their stock options) was estimated based on historical rates for three group classifications, (i) employees, (ii) outside directors and officers, and (iii) consultants. Expected volatility was based on historical volatility of our stock price for a period equal to the stock option's expected life and calculated on a daily basis. The expected dividend rate is zero since we do not currently pay cash dividends on our common stock and do not anticipate doing so in the foreseeable future.
The following table presents assumptions used in computing the fair value of option grants during 2017, 2016, and 2015:
2017 | 2016 | 2015 | |||||||
Risk-free interest rate | 2.17% | 1.53% | 2.00% | ||||||
Expected life (in years) | 6.76 | 6.77 | 6.97 | ||||||
Expected volatility | 72% | 74% | 81% | ||||||
Expected dividend yield | -- | -- | -- |
Stock-based compensation expense for the years ended December 31, 2017, 2016, and 2015 was recorded in our consolidated statement of operations as follows:
2017 | 2016 | 2015 | ||||||||||
Research and development expenses | $ | 1,371 | $ | 843 | $ | 1,099 | ||||||
General and administrative expenses | 2,771 | 1,614 | 1,849 | |||||||||
Total stock-based compensation expense | $ | 4,142 | $ | 2,457 | $ | 2,948 | ||||||
F-24
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
At December 31, 201 7, unrecognized stock-based compensation expense related to stock options was approximately $5.5 million and is expected to be recognized over a weighted average period of approximately 2.3 years.
1 2 . Employee Savings Plan
The terms of the amended and restated Progenics Pharmaceuticals 401 (k) Plan (the "401 (k) Plan"), among other things, allow eligible employees to participate in the Amended Plan by electing to contribute to the 401 (k) Plan a percentage of their compensation to be set aside to pay their future retirement benefits. We matched 50% of employee contributions equal to 1% - 10% of compensation during the three years ended December 31, 2017, made by eligible employees to the 401 (k) Plan (the "Matching Contribution"). In addition, we may also make a discretionary contribution each year on behalf of all participants who are non-highly compensated employees. We made Matching Contributions of approximately $302 thousand, $281 thousand, and $327 thousand to the 401 (k) Plan for the years ended December 31, 2017, 2016, and 2015, respectively. No discretionary contributions were made during those years.
1 3 . Income Taxes
We account for income taxes using the liability method in accordance with ASC 740 (Topic 740, Income Taxes ). Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
On December 22, 2017, the U.S. government enacted comprehensive tax legislation , commonly referred to as the Tax Cuts and Jobs Act ("Tax Act"). The Tax Act makes broad and complex changes to the U.S. tax code, including, but not limited to, ( 1 ) reducing the U.S. federal corporate tax rate from 35% to 21%; ( 2 ) requiring companies to pay a one -time transition tax on certain unrepatriated earnings of foreign subsidiaries; ( 3 ) generally eliminating U.S. federal income taxes on dividends from foreign subsidiaries; ( 4 ) requiring a current inclusion in U.S. federal taxable income of certain earnings of controlled foreign corporations; ( 5 ) eliminating the corporate AMT and changing how existing AMT credits can be realized; ( 6 ) creating the base erosion anti-abuse tax ("BEAT"), a new minimum tax; ( 7 ) creating a new limitation on deductible interest expense; and ( 8 ) changing rules related to uses and limitations of net operating loss carryforwards created in tax years beginning after December 31, 2017.
In 201 7, we recorded an income tax benefit of approximately $11.7 million, of which $6.6 million related to the reduction in the federal and state tax rates, $4.8 million related to the use of our naked credit as a source of income to release a portion of our valuation allowance and the remaining $0.2 million related to a refundable AMT credit. Our effective tax rate for 2017 was 18.6%. In 2016, we recorded an income tax expense of approximately $1.8 million as a result of an increase in our effective tax rate to 14.7%. Our effective tax rate in 2016 was impacted by our relocation to New York City, which has its own local tax rate and adds to the overall tax rate used for calculating the income tax provision. In 2015, we recorded an income tax benefit of approximately $133 thousand as a result of the change in the temporary difference between carrying amounts of in process research and development assets for financial reporting purposes and the amounts used for income tax purposes. Our effective tax rate for 2015 was 0.3%.
We have completed calculations through June 30, 2016, under Internal Revenue Code Section 382, the results of which indicate that past ownership changes will limit annual utilization of net operating losses ("NOLs") in the future. Ownership changes subsequent to June 30, 2016, may further limit the future utilization of net operating loss and tax credit carry-forwards as defined by the federal and state tax codes.
Our accounting for the following elements of the Tax Act is not yet complete as we are still in the process of analyzing its impact on us. Where we have been able to make reasonable estimates of the effects for which our analysis is not yet complete, we have recorded provisional amounts pursuant to the guidance within SEC Staff Accounting Bulletin No. 118 ("SAB 118" ). Our accounting treatment is expected to be complete when our 2017 U.S. corporate income tax return is filed in 2018 .
Reduction of U.S. federal corporate tax rate : The Tax Act reduces the corporate tax rate to 21%, effective January 1, 2018. Consequently, we have adjusted our deferred tax asset, liability and corresponding valuation allowance based on the enacted tax rate, which resulted in a provisional income tax benefit of approximately $3.7 million in 2017.
Deemed Repatriation Transition Tax : The Deemed Repatriation Transition Tax ("Transition Tax") is a tax on previously untaxed accumulated and current earnings and profits ("E&P") of certain of our foreign subsidiaries. To determine the amount of the Transition Tax, we must determine, in addition to other factors, the amount of post- 1986 E&P of the relevant subsidiaries, as well as the amount of non-U.S. income taxes paid on such earnings.
F-25
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
The components of the (benefit from) provision for income taxes during each of the three years ending December 31, 2017 consisted of the following:
2017 | 2016 | 2015 | ||||||||||
Current taxes: | ||||||||||||
United States | $ | 3 | $ | 11 | $ | - | ||||||
Foreign | - | - | - | |||||||||
State | (16 | ) | 22 | - | ||||||||
Total current taxes | $ | (13 | ) | $ | 33 | $ | - | |||||
Deferred taxes: | ||||||||||||
United States | $ | (8,777 | ) | $ | - | $ | - | |||||
Foreign | - | - | - | |||||||||
State | (2,882 | ) | 1,811 | (133 | ) | |||||||
Total deferred taxes | $ | (11,659 | ) | $ | 1,811 | $ | (133 | ) | ||||
(Benefit from) provision for income taxes | $ | (11,672 | ) | $ | 1,844 | $ | (133 | ) | ||||
Deferred tax assets and liabilities as of December 31, 2017 and 2016 consisted of the following:
2017 | 2016 | |||||||
Deferred tax assets: | ||||||||
Depreciation and amortization | $ | 100 | $ | 788 | ||||
Research & Experimental and Orphan Drug tax credit carry-forwards | 33,496 | 27,361 | ||||||
NYS investment tax credit carry-forwards | 1,284 | 933 | ||||||
AMT credit carry-forwards | - | 221 | ||||||
Net operation loss carry-forwards | 177,313 | 227,171 | ||||||
Capitalized research and development expenditures | 3,066 | 11,915 | ||||||
Stock compensation | 5,666 | 12,343 | ||||||
Other items | 1,279 | 4,513 | ||||||
Total gross deferred tax assets | 222,204 | 285,245 | ||||||
Less valuation allowance | (217,382 | ) | (285,245 | ) | ||||
Deferred tax assets | 4,822 | - | ||||||
Deferred tax liability | (6,397 | ) | (13,010 | ) | ||||
Net deferred tax liability | $ | (1,575 | ) | $ | (13,010 | ) | ||
We maintain a tax valuation allowance on substantially all deferred tax assets considering our history of taxable losses and the uncertainty regarding our ability to generate sufficient taxable income in the future to utilize these deferred tax assets. For 2017 and 2016, we incurred net losses for tax purposes. We recognized a tax valuation against deferred tax assets at December 31, 2017 and 2016. In 2017 and 2016, we recognized deferred tax liabilities of $1.6 million and $13.0 million, respectively, to reflect the net tax effects of temporary differences between the carrying amounts of in process research and development assets for financial reporting purposes and the amounts used for income tax purposes.
F-26
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
The following is a reconciliation of the U.S. statutory income tax rate to our effective tax rate for the years ended December 31, 2017, 2016, and 2015:
2017 | 2016 | 2015 | ||||||||||
U.S. Federal statutory rate | 34.0 | % | 34.0 | % | 34.0 | % | ||||||
State income taxes, net of Federal benefit | 1.5 | % | 10.8 | % | 4.8 | % | ||||||
Foreign rate differential | (0.6% | ) | 2.6 | % | (0.1% | ) | ||||||
Research and experimental and Orphan Drug tax credit | 9.4 | % | (63.0% | ) | 1.7 | % | ||||||
Effect of federal and state tax rate changes | (6.1% | ) | (43.0% | ) | (4.8% | ) | ||||||
| Tax reform impact | 13.9 | % | - | - | ||||||||
Permanent differences | (4.4% | ) | 0.1 | % | (1.4% | ) | ||||||
Stock option shortfalls | (3.1% | ) | 22.3 | % | (6.1% | ) | ||||||
Change in valuation allowance | (26.0% | ) | 50.9 | % | (27.8% | ) | ||||||
Effective tax rate | 18.6 | % | 14.7 | % | 0.3 | % | ||||||
As of December 31, 201 7, we had available, for tax return purposes, unused federal NOLs of approximately $664.8 million, which will expire in various years from 2018 to 2037. Also, we had available, for tax return purposes, unused state NOLs of approximately $569.0 million, which will expire in various years from 2030 to 2037.
We have reviewed our nexus in various tax jurisdictions and our tax positions related to all open tax years for events that could change the status of our tax liability under ASC 740, if any, or require an additional liability to be recorded. We have not, as of yet, conducted a study of our research and experimental ("R&E") credit carry-forwards. Such a study might result in an adjustment to our R&E credit carry-forwards, but until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position under ASC 740 - 10 except for uncertain tax positions acquired in connection with the Molecular Insight acquisition. A full valuation allowance has been provided against our R&E credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. Thus, there would be no impact to the statements of operations and comprehensive loss if an adjustment was required.
As of December 31, 2017, we are subject to federal, foreign, and state income tax. Open tax years relate to years in which unused net operating losses were generated or, if used, for which the statute of limitation for examination by taxing authorities has not expired. Our open tax years extend back to 1997. No amounts of interest or penalties were recognized in our consolidated statements of operations or consolidated balance sheets as of and for the years ended December 31, 2017, 2016, and 2015.
Our R&E and Orphan Drug tax credit carry-forwards of approximately $34.2 million at December 31, 2017 expire in various years from 2018 to 2037.
As of December 31, 2017, and 2016, we have not recognized any liability for uncertain tax positions, because of our full valuation allowance on net operating losses and R&E credits. We will recognize interest and penalties related to these positions, should such costs be assessed. The recognition of unrecognized tax benefits would not affect our effective tax rate because the tax benefit would be offset by an increase in our valuation allowance.
The following is a tabular reconciliation of the total amounts of unrecognized tax benefits for the years ended December 31, 201 7 and 2016.
2017 | 2016 | |||||||
Beginning uncertain tax benefits | $ | 2,661 | $ | 2,661 | ||||
Prior year - increases (decreases) | (710 | ) | - | |||||
Current year - increases (decreases) | - | - | ||||||
Settlements | - | - | ||||||
Expired statuses | - | - | ||||||
Ending uncertain tax benefits | $ | 1,951 | $ | 2,661 | ||||
F-27
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
1 4 . Net (Loss) Income Per Share
Our basic net (loss) income per share attributable to Progenics amounts have been computed by dividing net (loss) income attributable to Progenics by the weighted-average number of common shares outstanding during the period. For 2017 and 2015 we reported net losses and, accordingly, potential common shares were not included since such inclusion would have been anti-dilutive. As a result, basic and diluted EPS are the same for 2017 and 2015. For 2016, we reported net income, and the computation of diluted earnings per share is based upon the weighted-average number of our common shares and dilutive effect of stock options (determined using the treasury stock method).
In applying the treasury stock method for the calculation of diluted EPS, amounts of unrecognized compensation expense are required to be included in the assumed proceeds in the denominator of the diluted EPS calculation unless they are anti-dilutive. We have made an accounting policy decision to calculate windfall tax benefits/shortfalls, for purposes of diluted EPS calculation, excluding the impact of deferred tax assets. This policy decision will apply when we have net income and windfall tax benefits/shortfalls are realizable.
The calculations of net (loss) income per share, basic and diluted, are as follows:
Net (loss) income | Weighted-average | |||||||||||
attributable | shares | |||||||||||
to Progenics | outstanding | Per share | ||||||||||
(Numerator) | (Denominator) | amount | ||||||||||
2017 | ||||||||||||
Basic and diluted | $ | (51,013 | ) | 70,284 | $ | (0.73 | ) | |||||
2016 | ||||||||||||
Basic | $ | 10,806 | 70,003 | $ | 0.15 | |||||||
Dilutive effect of stock options | - | 152 | ||||||||||
Diluted | $ | 10,806 | 70,155 | $ | 0.15 | |||||||
2015 | ||||||||||||
Basic and diluted | $ | (39,112 | ) | 69,716 | $ | (0.56 | ) | |||||
The following table summarizes anti-dilutive common shares or common shares where performance conditions have not been met, that were excluded from the calculation of the diluted net (loss) income per share:
2017 | 2016 | 2015 | ||||||||||
Stock options | 2,395 | 3,577 | 6,381 | |||||||||
Contingent consideration liability (1) | 2,824 | 1,644 | 2,827 | |||||||||
Total securities excluded | 5,219 | 5,221 | 9,208 | |||||||||
___________________________
( 1 ) Calculated as follows: (a) the contingent consideration liability balance at December 31 divided by (b) the closing stock price of our common stock on the last day of trading of the fiscal year.
F-28
Table of Contents
PROGENICS PHARMACEUTICALS, INC .
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - continued
(amounts in thousands, except per share amounts or as otherwise noted)
1 5 . Unaudited Quarterly Results (unaudited)
Summarized quarterly financial data during 2017 and 2016 are as follows:
2017 Quarter Ended | ||||||||||||||||
March 31 | June 30 | September 30 | December 31 | |||||||||||||
Revenues | $ | 2,347 | $ | 2,765 | $ | 2,697 | $ | 3,889 | ||||||||
Net (loss) income | $ | (16,360 | ) | $ | (16,636 | ) | $ | (15,352 | ) | $ | (2,665 | ) | ||||
Net loss attributable to noncontrolling interests | $ | - | $ | - | $ | - | $ | - | ||||||||
Net (loss) income attributable to Progenics | $ | (16,360 | ) | $ | (16,636 | ) | $ | (15,352 | ) | $ | (2,665 | ) | ||||
Net (loss) income per share attributable to Progenics - basic | $ | (0.23 | ) | $ | (0.24 | ) | $ | (0.22 | ) | $ | (0.04 | ) | ||||
Net (loss) income per share attributable to Progenics - diluted | $ | (0.23 | ) | $ | (0.24 | ) | $ | (0.22 | ) | $ | (0.04 | ) | ||||
2016 Quarter Ended | ||||||||||||||||
March 31 | June 30 | September 30 | December 31 | |||||||||||||
Revenues (1) | $ | 2,450 | $ | 8,476 | $ | 53,850 | $ | 4,653 | ||||||||
Net (loss) income | $ | (12,673 | ) | $ | (5,657 | ) | $ | 36,282 | $ | (7,219 | ) | |||||
Net loss attributable to noncontrolling interests | $ | (18 | ) | $ | (19 | ) | $ | (21 | ) | $ | (15 | ) | ||||
Net (loss) income attributable to Progenics | $ | (12,655 | ) | $ | (5,638 | ) | $ | 36,303 | $ | (7,204 | ) | |||||
Net (loss) income per share attributable to Progenics - basic | $ | (0.18 | ) | $ | (0.08 | ) | $ | 0.52 | $ | (0.10 | ) | |||||
Net (loss) income per share attributable to Progenics - diluted | $ | (0.18 | ) | $ | (0.08 | ) | $ | 0.52 | $ | (0.10 | ) | |||||
( 1 ) Revenues in the second and fourth quarters of 2016 included $5.0 million and $2.0 million upfront and milestone payments received from Bayer, respectively, and revenues in the third quarter of 2016 included $50.0 million milestone payment received from Valeant.
F-29
Table of Contents
SCHEDULE II – VALUATION AND QUALIFYING ACCOUNT S
Allowance for Doubtful Accounts
Year ended December 31, | Beginning Balance | Additions Charged to General and Administrative Expenses | Deductions Accounts Written Off During Period | Ending Balance | ||||||||||||
(in thousands) | ||||||||||||||||
2017 | $ | - | $ | - | $ | - | $ | - | ||||||||
2016 | $ | 10 | $ | - | $ | (10 | ) | $ | - | |||||||
2015 | $ | 10 | $ | - | $ | - | $ | 10 | ||||||||
F-30
Table of Contents
S IGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
PROGENICS PHARMACEUTICALS, INC. | ||
By: | /s/ MARK R. BAKER | |
Mark R. Baker Chief Executive Officer and Director (Principal Executive Officer) | ||
Date: March 8, 2018
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
Signature | Capacity | Date | |
/s/ PETER J. CROWLEY | Chairman | March 8, 2018 | |
Peter J. Crowley | |||
/s/ MARK R. BAKER | Chief Executive Officer and Director (Principal Executive Officer) | March 8, 2018 | |
Mark R. Baker | |||
/s/ BRADLEY CAMPBELL | Director | March 8, 2018 | |
Bradley Campbell |
| ||
/s/ KAREN J. FERRANTE | Director | March 8, 2018 | |
Karen J. Ferrante, M.D. | |||
/s/ MICHAEL D. KISHBAUCH | Director | March 8, 2018 | |
Michael D. Kishbauch | |||
/s/ DAVID A. SCHEINBERG | Director | March 8, 2018 | |
David A. Scheinberg, M.D., Ph.D. | |||
/s/ NICOLE S. WILLIAMS | Director | March 8, 2018 | |
Nicole S. Williams | |||
/s/ PATRICK FABBIO | Senior Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) | March 8, 2018 | |
Patrick Fabbio |
|
S-1
Table of Contents
EXHIBIT INDEX
Exhibit |
| |
Number * | Description | |
3.1( 1) | Amended and Restated Certificate of Incorporation of the Registrant. | |
3.2( 2) | Amended and Restated By-laws of the Registrant. | |
4.1( 3) | Specimen Certificate for Common Stock, $0.0013 par value per share, of the Registrant. | |
10.5( 4) ‡ | Amended and Restated 1996 Stock Incentive Plan | |
10.6.3( 5) ‡ | Amended 2005 Stock Incentive Plan | |
10.6.4( 6) ‡ | Form of Non-Qualified Stock Option Award Agreement | |
10.6.5( 6) ‡ | Form of Restricted Stock Award Agreement | |
10.7( 7) ‡ | Form of Indemnification Agreement | |
10.21 .1(9) | Amended and Restated Agreement of Lease, dated October 28, 2009, between BMR-Landmark at Eastview LLC and the Registrant. | |
10.25( 10) † | Option and License Agreement, dated May 8, 1985, by and between the University of Chicago and UR Labs, Inc., as amended by (i) Amendment to Option and License Agreement, dated September 17, 1987, by and between the University of Chicago and UR Labs, Inc. and (ii) Second Amendment to Option and License Agreement, dated March 3, 1989, by and among the University of Chicago, ARCH Development Corporation and UR Labs, Inc. | |
10.26( 11) | Membership Interest Purchase Agreement, dated April 20, 2006, between the Registrant Inc. and Cytogen Corporation. | |
10.27( 11) † | Amended and Restated PSMA/PSMP License Agreement, dated April 20, 2006, by and among the Registrant, Cytogen Corporation and PSMA Development Company LLC. | |
10.34 (12) † | Collaboration Agreement, effective June 14, 2005, by and between Seattle Genetics, Inc. and PSMA Development Company, LLC. | |
10.3 7(13) † | License Agreement dated as of February 3, 2011, by and between Salix Pharmaceuticals, Inc., the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Excelsior Life Sciences Ireland Limited. | |
10.3 7.1(13) † | 2010 Agreement Related to Progenics ' MNTX In-License, dated February 3, 2011, by and among the University of Chicago, acting on behalf of itself and ARCH Development Corporation, the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Salix Pharmaceuticals, Inc. | |
10.38( 14) † | Stock Purchase and Sale Agreement, dated January 16, 2013, by and between Molecular Insight Pharmaceuticals, Inc., its Stockholders, the Registrant, and Highland Capital Management, L.P., as Stockholders Representative. | |
10.39( 14) † | License Agreement, dated September 1, 2012, by and between FUJIFILM RI Pharma Co., Ltd. and Molecular Insight Pharmaceuticals, Inc. | |
10.40 (15) † | License Agreement, dated May 4, 2012, between Molecular Insight Pharmaceuticals, Inc., the University of Zurich and the Paul Scherrer Institute. | |
10.4 1(16) | License Agreement, dated as of December 15, 2000, between Molecular Insight Pharmaceuticals, Inc. and The Board of Governors of the University of Western Ontario. | |
10.4 3(17) | Controlled Equity Offering SM Sales Agreement dated as of January 23, 2014, by and between the Registrant and Cantor Fitzgerald & Co. | |
10.45( 12) † | Collaboration Agreement, effective February 21, 2001, by and between Abgenix, Inc. and PSMA Development Company, LLC. | |
10.46 (1 8) † | Lease, dated December 31, 2015, between Progenics Pharmaceuticals, Inc. and WTC TOWER 1 LLC. | |
10.47 (19) † | License Agreement, dated as of 30 July, 2015 between the Registrant and The Johns Hopkins University. | |
10.48 (19) | Employment Offer Letter Agreement between the Registrant and Sheldon Hirt. | |
10.49 (19) | Employment Offer Letter Agreement between the Registrant and Patrick Fabbio . | |
10.50( 20) | Exclusive License Agreement, dated as of April 28, 2016, between PSMA Development Company LLC and Bayer AS. | |
10.51( 21) | Assignment and Assumption Agreement, dated as of May 6, 2016, between Progenics Pharmaceuticals, Inc., BMR-Landmark at Eastview LLC and Regeneron Pharmaceuticals, Inc. | |
10.52( 22) | Loan Agreement, dated as of November 4, 2016, between Progenics Pharmaceuticals, Inc. through its wholly-owned subsidiary MNTX Royalties Sub LLC, and Healthcare Royalty Partners. | |
10.53( 23) | Controlled Equity Offering SM Sales Agreement, dated January 6, 2017, between Progenics Pharmaceuticals, Inc. and Cantor Fitzgerald & Co. | |
21.1 | Subsidiaries of the Registrant. | |
23. 1 | Consent of Ernst & Young LLP. | |
31.1 | Certification of Mark R. Baker, Chief Executive Officer of the Registrant pursuant to 13a-14(a) and Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended. | |
31.2 | Certification of Patrick Fabbio, Senior Vice President and Chief Financial Officer of the Registrant pursuant to 13a-14(a) and Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended. |
E-1
Table of Contents
32.1 |
| Certification of Mark R. Baker, Chief Executive Officer of the Registrant pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
32.2 |
| Certification of Patrick Fabbio, Senior Vice President and Chief Financial Officer of the Registrant pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
101 | Interactive Data File | ||
101.INS | XBRL Instance Document | ||
101.SCH | XBRL Taxonomy Extension Schema | ||
101.CAL | XBRL Taxonomy Extension Calculation Linkbase | ||
101.LAB | XBRL Taxonomy Extension Label Linkbase | ||
101.PRE | XBRL Taxonomy Extension Presentation Linkbase | ||
101.DEF | XBRL Taxonomy Extension Definition Document | ||
* | Exhibits footnoted as previously filed have been filed as an exhibit to the document of the Registrant or other registrant referenced in the footnote below, and are incorporated by reference herein. | |
(1) | Previously filed in Current Report on Form 8-K filed on June 13, 2013. | |
(2) | Previously filed in Current Report on Form 8-K filed on January 30, 2017. | |
(3) |
| Previously filed in Registration Statement on Form S-1, Commission File No. 333-13627. |
(4) | Previously filed in Registration Statement on Form S-8, Commission File No. 333-120508. | |
(5) | Previously filed in Current Report on Form 8-K filed on June 18, 2014. | |
(6) | Previously filed in Current Report on Form 8-K filed on July 8, 2008. | |
(7) | Previously filed in Quarterly Report on Form 10-Q for the quarter ended March 31, 2007. | |
( 9) | Previously filed in Current Report on Form 8-K filed on November 28, 2012. | |
( 10) | Previously filed in Annual Report on Form 10-K for the year ended December 31, 2005. | |
( 11) | Previously filed in Quarterly Report on Form 10-Q for the quarter ended June 30, 2006 | |
( 12) | Previously filed in Amendment No. 2 to Annual Report on Form 10-K/A for the year ended December 31, 2009. | |
(1 3) | Previously filed in Quarterly Report on Form 10-Q for the quarter ended March 31, 2011. | |
( 14) | Previously filed in Quarterly Report on Form 10-Q for the quarter end ed March 31, 2013. | |
( 15) | Previously filed in Annual Report on Form 10-K for the year ended December 31, 2013. | |
( 16) | Previously filed in Registration Statement on Form S-1, Commission File No. 333-129570 filed by Molecular Insight Pharmaceuticals, Inc. | |
( 17) | Previously filed in Registration Statement on Form S-3, Commission File No. 333-193521. | |
(1 8) | Previously filed in Current Report on Form 8-K filed on January 5, 2016. | |
( 19) | Previously filed in Annual Report on Form 10-K for the year ended December 31, 2015. | |
( 20) | Previously filed in Current Report on Form 8-K filed on May 4, 2016. | |
(2 1) | Previously filed in Current Report on Form 8-K filed on May 10, 2016. | |
| (2 2) | Previously filed in Current Report on Form 8-K filed on November 7, 2016. | |
| (2 3) | Previously filed in Registration Statement on Form S-3, Commission File No. 333-215454 | |
† | Confidential treatment granted as to certain portions omitted and filed separately with the Commission. | |
‡ | Management contract or compensatory plan or arrangement. |
E-2
