UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended April 28, 2012
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 0-20572
PATTERSON COMPANIES, INC.
(Exact name of registrant as specified in its charter)
| Minnesota | 41-0886515 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
1031 Mendota Heights Road
St. Paul, Minnesota 55120
(Address of principal executive offices including Zip Code)
Registrant's telephone number, including area code: (651) 686-1600
Securities registered pursuant to Section 12(b) of the Act: Common Stock, par value $.01
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months, and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer x Accelerated filer ¨ Non-accelerated filer ¨ Smaller reporting company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of voting stock held by non-affiliates of the registrant, computed by reference to the closing sales price as quoted on the NASDAQ Global Select Market on October 29, 2011, was approximately $3,447,000,000. (For purposes of this calculation all of the registrant's officers, directors, presidents of operating units and 10% owners known to Patterson are deemed affiliates of the registrant.)
As of June 22, 2012, there were 110,248,829 shares of Common Stock of the registrant issued and outstanding.
Documents Incorporated By Reference
Portions of the registrant's definitive proxy statement to be filed pursuant to Regulation 14A within 120 days after the registrant's fiscal year-end of April 28, 2012 are incorporated by reference into Part III.
FORM 10-K INDEX
| Page | ||||||
PART I | 3 | |||||
Item 1. | BUSINESS | 3 | ||||
Item 1A. | RISK FACTORS | 26 | ||||
Item 1B. | UNRESOLVED STAFF COMMENTS | 30 | ||||
Item 2. | PROPERTIES | 31 | ||||
Item 3. | LEGAL PROCEEDINGS | 32 | ||||
Item 4. | MINE SAFETY DISCLOSURES | 32 | ||||
PART II | 33 | |||||
Item 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 33 | ||||
Item 6. | SELECTED FINANCIAL DATA | 35 | ||||
Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 36 | ||||
Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 46 | ||||
Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 47 | ||||
Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 76 | ||||
Item 9A. | CONTROLS AND PROCEDURES | 76 | ||||
Item 9B. | OTHER INFORMATION | 77 | ||||
PART III | 78 | |||||
Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 78 | ||||
Item 11. | EXECUTIVE COMPENSATION | 78 | ||||
Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 78 | ||||
Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 78 | ||||
Item 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES | 78 | ||||
PART IV | 79 | |||||
Item 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES | 79 | ||||
SIGNATURES | 83 | |||||
SCHEDULE II | 84 | |||||
INDEX TO EXHIBITS | 85 | |||||
2
PART I
| Item 1. | BUSINESS |
Certain information of a non-historical nature contained in Items 1, 2, 3 and 7 of this Form 10-K includes forward-looking statements. Reference is made to Item 7-Management's Discussion and Analysis of Financial Condition and Results of Operations-Factors that May Affect Future Operating Results, for a discussion of certain factors that could cause our Company's actual operating results to differ materially from those expressed in any forward-looking statements.
General
Patterson is a value-added distributor serving three major markets:
| • | North American dental supply; |
| • | U.S. companion animal (dogs, cats and other common household pets) and equine veterinary supply; and |
| • | The worldwide rehabilitation and assistive products supply market. |
Patterson began distributing dental supplies in 1877. The modern history of the business dates to May 1985, when management and certain investors purchased the dental supply business of a subsidiary of The Beatrice Companies, Inc. Patterson became a publicly traded company in October 1992 and is a corporation organized under the laws of the state of Minnesota.
We had historically reported one operating segment, dental supply. In July 2001, the veterinary supply assets of J.A. Webster, Inc., were purchased and became a reportable business segment. Then in September 2003, AbilityOne Products Corp. was acquired, creating a third business segment which serves the rehabilitation supply market.
In June 2004, we changed our corporate name from Patterson Dental Company to Patterson Companies, Inc. ("Patterson" or the "Company"). Patterson retained its existing NASDAQ stock symbol-PDCO. The corporate name change was adopted to reflect Patterson's expanded base of business in the veterinary and rehabilitation supply markets, as well as its traditional base of operations in the dental supply market. Patterson's operating units include Patterson Dental, Webster Veterinary and Patterson Medical.
Our three reportable segments, dental supply, veterinary supply and rehabilitation supply, are strategic business units that offer similar products and services to different customer bases. Each business is a market leader with a strong competitive position, serves a fragmented market that offers consolidation opportunities and offers relatively low-cost consumable supplies, making our value-added business proposition highly attractive to customers.
Dental Supply
Overview
As Patterson's largest business, Patterson Dental is one of the two largest distributors of dental products in North America. Our dental business has operations in the United States and Canada. Patterson Dental, a full-service, value-added supplier to dentists, dental laboratories, institutions, and other healthcare professionals, provides consumable products (including x-ray film, restorative materials, hand instruments and sterilization products); basic and advanced technology dental equipment; practice management and clinical software; patient education systems; and office forms and stationery. Patterson Dental offers our customers a broad selection of dental products including more than 96,000 stock keeping units ("SKUs") of which approximately 5,000 are private-label products sold under the Patterson brand. Patterson Dental also offers customers a full range of related services including dental equipment installation, maintenance and repair, dental office design and equipment financing. We market our dental products and services through more than 1,500 direct sales representatives, of which approximately 300 are equipment specialists.
3
Patterson Dental has over 130 years of experience providing quality products and services to dental professionals. Net sales of this segment have increased from $165.8 million in fiscal 1986 to approximately $2.3 billion in fiscal 2012 and profitability has increased from an operating loss in fiscal 1986 to operating income of $246.7 million in fiscal 2012.
We estimate the dental supply market we serve to be approximately $6.8 billion annually and that our share of this market is approximately 33%. The underlying structure of the dental supply market consists of a sizeable geographically dispersed number of fragmented dental practices and is attractive for our Company's role as a value-added, full-service distributor. According to the American Dental Association, there are over 186,000 dentists practicing in the United States. According to the Canadian Dental Association, there are approximately 20,000 licensed dentists in Canada. The average general practitioner generated approximately $728,000 in annual revenue in 2009, while the average specialty practitioner produces about $1,005,000. We believe that most dentists use between 5% and 7% of their annual revenue to purchase consumable supplies used in the daily operations of their practice. This translates into between $36,000 and $51,000 of supplies purchased by the average practice each year. We believe the average dental practitioner purchases about 40% of their supplies from their top supplier.
Total expenditures for dental services in the United States increased from $31 billion in 1990 to $104.8 billion in 2010. We believe that the demand for dental services, equipment and supplies will continue to be influenced by the following factors:
| • | Demographics. The U.S. population grew from 235 million in 1980 to 309 million in 2010, and is expected to reach 335 million by 2020. The median age of the population is also increasing, and we believe that older dental patients spend more on a per capita basis for dental services. |
| • | Dental products and techniques. Technological developments in dental products continue to contribute to advances in dental techniques and procedures, including cosmetic dentistry and dental implants. |
| • | Demand for certain dental procedures. Demand is growing for preventive dentistry and specialty services such as periodontic (the treatment of gums), endodontic (root canals), orthodontic (braces), and other dental procedures that enable patients to keep their natural teeth longer and improve their appearance. |
| • | Increased dental office productivity. The number of dentists per 100,000 persons in the U.S. is forecasted to decline over the next two decades. As a result, the number of patients per dental practice is expected to rise. For this reason, dentists are showing an increased willingness to invest in dental equipment and office infrastructure that can strengthen the productivity of their practices. |
| • | Demand for infection control products. Greater public awareness as well as regulations and guidelines instituted by OSHA, the American Dental Association and state regulatory authorities have resulted in increased use of infection control (asepsis) products such as protective clothing, gloves, facemasks, and sterilization equipment to prevent the spread of communicable diseases such as AIDS, hepatitis and herpes. |
| • | Coverage by dental plans. An increasing number of dental services are being funded by private dental insurance. In 2009, over 55% of the U.S. population had some form of dental coverage. |
Strategy
Our objective is to remain a leading national distributor of supplies, equipment and related services in the market while continuing to improve our profitability and enhance our value to customers. To achieve this objective, we have adopted a strategy of emphasizing our value-added, full-service capabilities, using technology to enhance customer service, continuing to improve our own operating efficiencies, and growing through internal expansion and acquisitions.
4
Emphasizing Value-Added, Full-Service Capabilities. Patterson Dental is positioned to meet virtually all of the needs of dental practitioners by providing a full range of consumable supplies, equipment and software, and value-added services. We believe that our customers value full service and responsive delivery of quality supplies and equipment. Customers also increasingly expect suppliers to be knowledgeable about products and services, and generally, a superior sales representative can create a special relationship with the practitioner by providing an informational link to the overall industry. Our knowledgeable sales representatives assist customers in the selection and purchase of supplies and equipment. In addition, our high quality sales force allows us to offer broader product lines. Because most dental practices lack a significant degree of back office support, the convenience of our full-service capabilities enables dentists to spend more time with patients and, thus, generate additional revenues.
We meet our customers' requirements by delivering frequent, small quantity orders rapidly and reliably from our strategically located distribution centers. Equipment specialists, service technicians and technology advisors also support our value-added strategy. Equipment specialists offer consultation on office design, equipment requirements and financing. Our experienced service technicians perform equipment installation, maintenance and repair services, including services on products purchased from others. Technology advisors from our sales branches provide guidance on integrating technology solutions, including practice management and clinical software, digital radiography, custom hardware and networking into the dental practice.
Using Technology to Enhance Customer Service. As part of Patterson Dental's commitment to providing superior customer service, we offer our customers easy order placement. We have offered electronic ordering capability to our dental supply segment since 1987, when we first introduced Remote Order Entry (REMO SM ). Over the years, Patterson Dental has continued to introduce new order entry systems designed to meet the varying needs of our customers. We believe that computerized order entry systems help establish relationships with new customers and increase loyalty among existing customers by permitting customers to place orders from their offices directly to Patterson Dental 24 hours a day, seven days a week.
Today we offer four convenient ordering systems to the dental supply segment, eMAGINE ® , REMO SM , PDXpress ® and www.pattersondental.com , which we launched in fiscal 2012 to provide our customers with better search capabilities, easier access to their order history, customized purchase reports, and fingertip access to Patterson Advantage, the segment's customer loyalty program. Customers, as well as our sales force, use these systems. Over the years, the number of orders transmitted electronically has grown steadily to approximately 74% of our consumable dental product volume or $960 million in fiscal year 2012. In addition to enhancing customer service by offering electronic order entry systems to our customers, these systems enable our sales representatives to spend more time with existing customers and to call on additional customers.
The goal of Patterson Dental's Internet strategy is to distribute information and service related products over the Internet to enhance customers' practices and to increase sales force productivity. Our Internet environment includes order entry, customer-loyalty program reports and services, access to " Patterson Today " articles and manufacturers' product information. Additionally, Patterson Dental utilizes a tool, InfoSource ® , to provide real time customer and sales information to our sales force, managers and vendors via the Internet.
Our proprietary practice management and clinical software, EAGLESOFT ® , is developed and maintained by our Patterson Technology Center (PTC). We believe the PTC differentiates Patterson Dental from our competition by positioning Patterson Dental as the only company providing a single-source solution for the high growth area of digital radiography. This technology, which we expect to install in most dental offices, has a current market penetration of approximately 50%. Among our many specialized capabilities, the PTC, in conjunction with specialists from the sales branch, provides system configuration, as well as the seamless integration of all digital operatory components with clinical software, including our EAGLESOFT ® products. This integration creates an electronic patient database that combines the patient's front office record with digital information from the clinical x-ray, intra-oral camera, CEREC ® system and other digital equipment. Patterson
5
Dental offers our EAGLESOFT ® practice management software at no cost to customers with a subscription to support services. The local sales branch, in conjunction with the PTC, will network the digital x-ray system throughout the entire office, offers all required custom computer hardware for the system, and provides installation and customer training. The PTC has a call center that troubleshoots customer problems and arranges for local service when needed.
Software and digital radiography customers also have access to the PTC's support capabilities. The PTC provides support for Patterson's proprietary products as well as select branded product from manufacturers, such as SIRONA and SCHICK. In addition to troubleshooting problems through the PTC's support center, customers can access various service capabilities offered by the PTC including electronic claims and statement processing and system back-up capabilities.
Continuing to Improve Operating Efficiencies. Patterson Dental continues to implement programs designed to improve our operating efficiencies and allow for continued sales growth over time. These programs include a wide variety of initiatives from our continuing investment in management information systems to consolidation of distribution centers and sales branches. More recent initiatives include upgrading our order entry and order management systems.
Patterson Dental has improved operating efficiencies by converting our communications architecture to faster, higher capacity data lines that combine voice and data transmissions. We have developed a new field service management tool for our technical service operations, improving our ability to coordinate the actions of service technicians and enhancing customer service while reducing the overall cost of operations.
An integral part of our shared services concept is the consolidation and leveraging of distribution centers between Patterson's segments. As of April 2012, eight distribution centers are shared between two or all three of our operating units. In addition, we have established shared sales branch offices in several locations between multiple segments. Because of these and other efforts, we expect to continue to improve our operating expense leverage and efficiencies going forward.
Growing Through Internal Expansion and Acquisitions. We intend to continue to grow by opening additional sales offices, hiring established sales representatives, hiring and training skilled sales professionals as territory sales representatives, and acquiring other distributors in order to enter new, or more deeply penetrate existing, geographic markets and expand our customer base. We believe that Patterson Dental is well positioned to take advantage of expected continued consolidation in the dental distribution market. Over the past 25 years Patterson has made a number of acquisitions, including the following:
Dental distribution acquisitions
| • | Since 1987, Patterson has acquired over 30 dental products distributors in the United States and Canada. These acquisitions have included the third largest dental dealer in the United States and the second largest dental dealer in Canada, as well as regional and local dental dealers throughout North America. As a result of these acquisitions, along with internal expansion, we are now one of the two largest full service dental products distributors in both the United States and Canada. |
Printed office products acquisitions
| • | In October 1996, we acquired the Colwell Systems division of Deluxe Corporation. Colwell Systems, now known as Patterson Office Supplies, produces and sells a variety of printed office products used in medical, dental and veterinary offices, as well as other clinical based settings. |
Software acquisitions
| • | In July 1997, Patterson Dental acquired Eaglesoft ® , Inc., a developer and marketer of Windows ® -based practice management and clinical software for dental offices. Eaglesoft ® 's operations evolved to |
6
become the Patterson Technology Center and are located in Effingham, Illinois. In December 2001, Patterson purchased Modern Practice Technologies, a company that provided custom computing solutions to the dental industry. This acquisition helped us to position ourselves to provide all of the custom hardware and networking required for interfacing the entire dental office. |
| • | In May 2004, Patterson Dental acquired CAESY Education Systems, Inc., the leading provider of electronic patient education services to dental practices in North America. Headquartered in Vancouver, Washington, CAESY provides dental practices with a range of communications media that educate patients about professional dental care, procedures and treatment alternatives with the goal of facilitating patient decisions about dental services and increasing the productivity of the dental professional. Educational materials are communicated through CD/DVD media, software-as-a-service, computer programs and the dentist's web site. These materials can be used within the dental waiting room, at chair side and in the patient's home. |
| • | In December 2008, Patterson Dental acquired Dolphin Imaging Systems, LLC and Dolphin Practice Management, LLC, the leading providers of 3D imaging and practice management software for specialized dental practitioners, including orthodontists, oral maxillofacial surgeons and dental radiologists. Dolphin's imaging software maximizes the benefit of cone beam and other digital photography and radiography systems. We believe there are no major competitors for Dolphin's full range of products. |
Products and Services
The following table sets forth the percentage of total sales by the principal categories of products and services offered to our dental segment customers:
| 2012 | 2011 | 2010 | ||||||||||
Consumable and printed products | 55 | % | 56 | % | 56 | % | ||||||
Equipment and software | 34 | 33 | 34 | |||||||||
Other (1) | 11 | 11 | 10 | |||||||||
|
|
|
|
|
| |||||||
Total | 100 | % | 100 | % | 100 | % | ||||||
|
|
|
|
|
| |||||||
| (1) | Consists of other value-added products and services including technical service and software maintenance. |
Consumable and Printed Products
Dental Supplies. We offer a broad product line of consumable dental supplies such as x-ray film and solutions; impression materials; restorative materials (composites and alloys); hand instruments; sterilization products; anesthetics; infection control products such as protective clothing, gloves and facemasks; paper, cotton and other disposable products; toothbrushes and a full line of dental accessories including hand instruments, burs, and diamonds. In addition to representing a wide array of branded products from numerous manufacturers, we also market our own private label line of dental supplies including anesthetics, instruments, preventive and restorative products, and cotton and paper products. Our private label line complements the branded products for customers seeking a lower cost alternative on products that have become a commodity in the market. Compared to most name brand supplies, the private label line provides lower prices for our customers and higher margins for Patterson.
Printed Office Products. Patterson Dental, through Patterson Office Supplies (POS) provides a variety of printed office products, office filing supplies, and practice management systems to office-based healthcare providers including dental, veterinary and medical offices. Products include custom printed products, insurance and billing forms, stationery, envelopes, business cards, labels, file folders, appointment books and other stock office supply products.
7
These office products are sold through our sales force as well as direct mail catalogs distributed to over 100,000 customers several times per year. A staff of telemarketing personnel located in Champaign, Illinois supports both channels, receiving orders by telephone, through the mail or electronically from the dental, veterinary and medical distribution order processing system.
Equipment and Software
Dental Equipment. Patterson Dental is the largest supplier of dental equipment in the U.S. and Canada. We offer a wide range of dental equipment products including radiography equipment, high and low-speed hand pieces, dental chairs, dental hand piece control units, diagnostic equipment, sterilizers, dental lights and compressors. We also distribute newer technology equipment that provides our customers with tools to improve productivity and patient satisfaction. Examples of such innovative and higher productivity products include the CEREC ® family of products, a chair-side restoration system; digital imaging products (including intra-oral, panoramic and 3-D or cone beam x-rays); and inter-oral cameras.
Software. Patterson Dental develops and markets our own proprietary line of practice management and clinical software for dental professionals, including software for scheduling, billing, charting and capture/storage/retrieval of digital images. Patterson Dental also sells software products developed by third parties, including SIDEXIS ® by Sirona and Dimax2 by Planmeca. These value-added products are designed to help achieve office productivity improvements, which translate into higher profitability for the customer.
Hardware. We offer our dental customers custom hardware and networking solutions required for integrating the entire dental office, which marks another step in our overall strategy of providing customers with the convenience and cost-effectiveness of a virtually complete range of products and value-added services.
Patient Education Services. The CAESY ® education systems line of products, now available as software-as-a-service in addition to DVD format, offers patient education products and services. These communication tools are designed to educate patients in an efficient, cost-effective manner.
Other
Software Services. Patterson offers a variety of services to complement our software products, such as service agreements, software training, electronic claims processing and billing statement processing. These services provide value to customers by allowing them to keep their software products current or receive payments more rapidly while obtaining greater productivity.
Equipment Installation, Repair and Maintenance. To keep their practices running efficiently, dentists require reliable performance from their equipment. All major equipment sold by Patterson includes installation and our 90-day labor warranty at no additional charge. Patterson also provides complete repair and maintenance services for all dental equipment, whether or not purchased from Patterson, including 24-hour hand piece repair service. In addition to service technicians, who provide installation and repair services on basic dental equipment, we have also invested in personnel who specialize in installing and troubleshooting issues with technology solutions such as practice management software, digital imaging products, hardware and networking. The goal of this group, which is comprised of both local service technicians and the PTC, is to help customers integrate newer technology into their dental practices. The PTC helps our customers minimize costly downtime by offering a single point of contact for post-sale technology related issues.
Dental Office Design. Patterson provides dental office layout and design services using a computer-aided design (CAD) program. Equipment specialists can create original or revise existing dental office designs in a fraction of the time required to produce conventional drawings. Customers purchasing major equipment items receive dental office design services at no additional charge.
8
Equipment Financing. Patterson Dental provides a variety of options to fulfill our customers' financing needs. For qualified purchasers of equipment, we will arrange customer financing through Patterson or a third party. For non-equipment related needs, such as for working capital or real estate, customers are also referred to a third party. These alternatives allow us to offer our customers convenience while still meeting their diverse financing needs. In fiscal 2012, we originated approximately $287.6 million of equipment finance contracts in the United States. Patterson Dental, or our third party vendor, financed approximately 42% of the equipment purchased by our customers during fiscal 2012.
Since November 1998, Patterson Dental has maintained one or more finance referral agreements with outside finance companies to provide a more extensive selection of finance opportunities to our customers. This might include financing for practice transition transactions, working capital, leasing, real estate and long-term capital. Currently this service is provided by Wells Fargo Practice Finance, a division of Wells Fargo Bank N.A. Patterson receives referral fees under this agreement, and Wells Fargo extends credit and services the accounts.
Patterson Dental has created a special purpose entity ("SPE"), PDC Funding Company, LLC, a wholly-owned and fully consolidated subsidiary, and entered into a Receivables Purchase Agreement in order to participate in a commercial paper conduit. We transfer finance contracts we originate to the SPE. In turn, the SPE sells the contracts to the commercial paper conduit. As of April 28, 2012, the maximum outstanding capacity of this arrangement at any one time is $500 million.
A second SPE, PDC Funding Company II, LLC, sells contracts through a Contract Purchase Agreement to a bank. This agreement operates similarly to the Receivables Purchase Agreement described above, except that the capacity is $75 million.
There is no recourse to Patterson for contracts purchased by either the commercial paper conduit or the bank, but there is a deferred purchase price equal to approximately 16% of the principal of these contracts. Patterson services the customer contracts under both of these arrangements in exchange for a fee that approximates our cost for providing the service.
Sales and Marketing
During fiscal 2012, Patterson Dental sold products or services to over 120,000 customers in the U.S. and Canada who made one or more purchases during the year. Patterson Dental's customers include dentists, laboratories, institutions and other healthcare professionals. No single customer accounted for more than 1% of sales during fiscal 2012, and Patterson Dental is not dependent on any single customer or geographic group of customers. Our sales and marketing efforts are designed to establish and improve customer relationships through personal interaction with our sales representatives and frequent direct marketing contact, which underscores our value-added approach.
Patterson Dental has over 90 local offices throughout the U.S. and Canada so that we can provide a presence in the market and decision making near the customer. These offices, or sales branches, are staffed with a complete complement of Patterson Dental capabilities, including sales, customer service and technical service personnel, as well as a local manager who has broad decision making authority with regard to customer related transactions and issues.
A primary component of Patterson Dental's value-added approach is our sales force. Due to the fragmented nature of the dental supply market, we believe that a large sales force is necessary to reach potential customers and to provide full service. Sales representatives provide an informational link to the overall industry; assist practitioners in selecting and purchasing products, and help customers efficiently manage their supply inventories. Each sales representative works within an assigned sales territory under the supervision of a location (branch) manager. Sales representatives are all Patterson employees and are generally compensated on a commission basis, with some, less experienced, representatives receiving a base salary and commission.
9
To assist our sales representatives, Patterson Dental publishes a variety of catalogs and fliers containing product and service information. Dental customers receive a full-line product catalog containing over 35,000 inventoried items. The catalog includes pictures of products, detailed descriptions and specifications of products and is used by practitioners as a reference source. Selected consumable supplies, new products, specially priced items and high demand items such as infection control products, are promoted through merchandise fliers printed and distributed bi-monthly. In addition, dental equipment sold by Patterson is featured in our tri-yearly publication, Patterson Today, which also includes articles on dental office design, trends in dental practice, products and services offered by Patterson Dental and information on equipment maintenance.
To enhance the total value we bring to our customers, Patterson Dental offers a value-added benefit program for our preferred customers. The PATTERSON ADVANTAGE SM program enables members to earn "Advantage Dollars" which can be applied toward future purchases of equipment and technology products. Patterson Advantage also entitles our best customers to priority technical services, automated supply management summary reports, educational materials and a variety of exclusive discount offers.
Distribution
Patterson Dental believes that responsive delivery of quality supplies and equipment is key to customer satisfaction. We ship dental consumable supplies from eight strategically located distribution centers in the U.S. and two in Canada. Orders for consumable dental supplies can be placed by telephone or electronically 24 hours a day, seven days a week. Printed office products are shipped from our manufacturing and distribution facility in central Illinois.
All orders are routed through our centralized computer ordering, shipping and inventory management systems, which are linked to each of our strategically located distribution centers. If an item is not available in the distribution center nearest to the customer, the computer system automatically directs fulfillment of the item from another center. Rapid and accurate order fulfillment is another principal component of our value-added approach. We estimate that 98% of Patterson Dental's consumable goods orders are shipped to the customer on time, which is generally within 24 hours.
In order to assure the availability of Patterson Dental's broad product lines for prompt delivery to customers, we must maintain sufficient inventories at our distribution centers. Purchasing of consumables and standard equipment is centralized, and our purchasing department uses a real-time perpetual inventory system to manage inventory levels. Our inventory consists mostly of consumable supply items. By utilizing computerized inventory management and ordering systems, we are able to accurately predict inventory turns in order to minimize inventory levels for each item.
Patterson Dental's more than 90 dental sales offices are generally configured with display areas where the latest dental equipment can be demonstrated. Dental equipment is generally custom ordered and is staged at our sales branches before delivery to dental offices for installation.
Sources of Supply
Effective purchasing is a key strategy Patterson Dental has adopted in order to achieve our objective of continuing to improve profitability. We have a program to effectuate electronic data interchange (EDI) with our major vendor partners. In fiscal 2012, we processed approximately 63% of our invoices from dental vendors using EDI capabilities. In addition, approximately 67% of our dental purchase order volume was conducted through EDI. Utilizing EDI allows us to improve efficiencies and reduce administrative costs.
Patterson Dental obtains products from more than 800 vendors. In June 2012 we entered into an exclusive distribution agreement with Sirona Dental Systems, Inc., the manufacturer of the CEREC ® dental restorative systems and Schick ® digital x-rays, in addition to sophisticated panoramic and cone beam radiography product. We are also the only national dealer for A-dec equipment, including chairs, units and cabinetry. A-dec Inc., is the largest manufacturer of dental equipment in the U.S.
10
While Patterson Dental makes purchases from many suppliers, and there is generally more than one source of supply for most of the categories of products we sell, the concentration of business with key suppliers is considerable. Our top ten supply vendors accounted for approximately 45% and 43% of the cost of dental products sold in both fiscal 2012 and 2011. Of these ten, the top two vendors accounted for 13% and 11% of both fiscal 2012 and fiscal 2011 cost of sales, respectively.
Competition
The highly competitive U.S. dental products distribution industry consists principally of national, regional and local full-service and mail-order distributors. The dental supply market is extremely fragmented. In addition to Patterson Dental and one other national, full-service firm, Sullivan-Schein Dental, a unit of Henry Schein, Inc., there are at least 15 full-service distributors that operate on a regional level, and hundreds of small local distributors. Also, some manufacturers sell directly to end users.
We approach our markets by emphasizing and delivering a value-added model to the practitioner. To differentiate ourselves from our competition, we deploy a strategy of premium customer service, a highly qualified and motivated sales force, experienced service technicians, an extensive breadth and mix of products and services, accurate and timely delivery of product, strategic location of sales offices and distribution centers, and competitive pricing.
Patterson Dental also experiences competition in Canada in the dental supply market. The principal competitor is a national, full-service dental distributor, Henry Schein Ash Arcona, a unit of Henry Schein, Inc. We believe we compete in Canada on essentially the same basis as in the United States.
Veterinary Supply
Overview
Webster Veterinary, or "Webster", is a leading distributor of veterinary supplies primarily to companion-pet (dogs, cats and other common household pets) and equine veterinary clinics across the United States. Webster provides products and services used for the diagnosis, treatment and/or prevention of diseases in companion animals and equine. Originally founded in 1946 and headquartered in Massachusetts, Webster has developed a strong brand identity as a value-added, full-service distributor with a comprehensive offering of pharmaceuticals, vaccines, parasiticides, diagnostics, veterinary diets, nutritionals, consumable supplies, equipment, and software. Webster's product offering, totaling more than 36,000 items, is sold by approximately 214 field sales representatives.
Net sales by Webster in fiscal 2012 were $734.4 million and operating income totaled $38.8 million. In addition to our core business of distributing veterinary products, Webster has a significant agency commission business with several pharmaceutical manufacturers. Under the agency relationships, Webster receives orders for products from customers, transmits these orders to vendors who then pick, pack, and ship the products. These vendors then invoice and collect payment from our customers. Webster receives a commission payment for soliciting the order and for providing other customer service activities. The agency commissions that Webster earns range from 3% to 6%, a portion of which is shared with the field sales and customer service representatives. Webster's agency commissions accounted for less than 1% of our net sales in fiscal 2012.
We estimate the market for pharmaceuticals and supplies sold to companion animal and equine veterinarians through distribution is approximately $3.2 billion on an annual basis. Based upon the estimated $3.2 billion market, we believe our share of this market is approximately 21%. Similar to the dental supply market, the veterinary supply market is fragmented and geographically diverse. As of December 31, 2011, according to the American Veterinary Medical Association, or AVMA, there were more than 63,000 veterinarians in private practice nationwide specializing in small animals, predominately companion pets. The average private veterinary practice generates approximately $1,000,000 of annual revenue. These practices purchase between $120,000 and
11
$140,000 of supplies each year, and similar to the dental practitioner, tend not to maintain a large supply of inventory on hand. The typical veterinary practice purchases approximately 80% of its supplies from its top two suppliers. The average purchase of consumables by the veterinary practice is noticeably higher than that of the dental practitioner due predominately to pharmaceutical products, which are administered and dispensed by veterinarians.
Historically, the demand for veterinary services has grown significantly faster than growth in the overall economy. More recently the market growth has slowed as a result of a decrease in consumer spending. However we anticipate increasing demand for veterinary services due to the following favorable factors:
| • | Number of households with companion animals. The number of households with companion animals is steadily expanding which increases the demand for veterinary services. Today, the percentages of U.S. households owning dogs, cats and horses are 46.3%, 38.9%, and 2.4%, respectively. |
| • | Veterinary expenditures per household. The amount pet owners are willing to spend caring for their pets is increasing substantially. The American Pet Products Manufacturers Association estimates that pet owners will spend $53 billion in 2012 to care for the American pet population, a significant increase compared to $41.2 billion spent in 2007. |
| • | Veterinary products and techniques. Many new therapeutic and preventive products are being developed for the companion animal market. Technological developments have resulted in new innovative veterinary products and advances in veterinary services. |
Strategy
Webster's objective is to build a leading national position in the companion animal veterinary market through internal expansion and acquisitions, while continuing to improve our profitability and enhance our value to customers. Our key strategies and priorities for accomplishing these objectives include growing through internal expansion and acquisitions, emphasizing our value-added full-service capabilities, continuing to improve operating efficiencies, and expanding our service offerings.
Growing Through Internal Expansion and Acquisitions. We intend to continue to grow by opening additional sales offices, hiring established sales representatives, hiring and training skilled sales professionals as territory sales representatives, and acquiring other distributors in order to enter new, or more deeply penetrate existing, geographic markets and expand our customer base. We believe that Webster is well positioned to take advantage of expected continued consolidation in the veterinary distribution market. Over the past 10 years Webster has made a number of acquisitions, including the following:
Veterinary distribution acquisitions
| • | Since 2004, Webster has acquired several veterinary distributors in the United States. These acquisitions include two regional distributors, ProVet, which was the companion animal veterinary supply division of Lextron, Inc., and Columbus Serum Company, as well as Milburn Distributions, Inc., the largest distributor specializing in the U.S. equine veterinary supply market, and selected local and specialty distributors. Most recently, in August 2011, Webster acquired American Veterinary Supply Corporation, a full service distributor on Long Island, New York with annualized sales of approximately $25 million that served approximately 2,000 veterinary practices and hospitals in the New York metropolitan area. |
| • | In April 2010, Webster made a minority equity investment in Strategic Pharmaceutical Solutions, Inc. dba VetSource, a leading North American provider of integrated specialty pharmacy distribution, including home delivery capabilities. |
Software Acquisitions
| • | In December 2005, Webster acquired Intra Corp., one of the nation's leading developers of veterinary practice management software that is marketed under the INTRAVET ® brand name. INTRAVET ® had more than 1,600 software installations nationwide. |
12
| • | In October 2008, Webster acquired Odyssey Veterinary Software, LLC, a developer and marketer of DIAGNOSTIC IMAGING ATLAS ® ("DIA ® ") software. At that time, DIA encompassed over 2,000 3D clinical animations and images, which enable the veterinarian to more fully explain and illustrate the pet's diagnosis and recommend treatment to their clients. |
| • | In January 2011, Webster acquired ePet Records, LLC. Rebranded as ePETHEALTH™, this innovative communication platform provides practitioners with a secure Internet portal for their clients to access 24/7 for their pets medical information plus descriptive, easy-to-understand resources and educational materials with 3D graphics. Through ePETHEALTH™, veterinarians can also send their clients automated electronic health service and appointment reminders, eNewsletters and health education articles and videos. |
Emphasizing Value-Added, Full-Service Capabilities. Webster believes that veterinary customers value full service and responsive delivery of product, in addition to competitive prices. Customers also increasingly expect suppliers to be knowledgeable about products and services, and, generally, a superior sales representative can create a special relationship with the practitioner by providing an informational link to the overall industry. Webster's knowledgeable sales representatives assist customers in the selection and purchasing of supplies. Most veterinarians are independent, or small unit based, practitioners who are unable to store and manage large volumes of supplies in their offices. We meet our customer's requirements by delivering frequent, small quantity orders rapidly and reliably from strategically located distribution centers.
Equipment specialists, technology specialists and service technicians also support our value-added strategy. Equipment specialists offer consultation on office design, equipment requirements and financing. Technology specialists provide guidance on integrating technology solutions including practice management software, client education and communication software, and our home delivery service offering. Our experienced service technicians perform equipment installation, maintenance and repair services, including service on products or equipment not purchased through Webster.
Continuing to Improve Operating Efficiencies . Webster continues to implement programs designed to improve our operating efficiencies and allow for continued sales growth over time. These programs include a wide variety of initiatives from our continuing investment in management information systems to consolidation of distribution centers and sales branches.
In June 2011, Webster implemented a centralized return processing center located in Columbus, Ohio. This initiative has allowed us to more efficiently handle customer product returns, improve the customer's experience as well as meet vendor return requirements. As a result of implementing the centralized return processing center, approximately 87% of returns are credited back to the customer within 24 hours of receipt. Centralizing this process has also lead to both operational efficiencies as well as a reduction in shipping costs associated with product returns.
Expanding our Services. Webster continuously seeks to broaden our service offerings to maximize sales opportunities within our existing customer base while strengthening and enhancing practice and pet health.
Technical Service. In October 2011, Webster launched onsite preventative maintenance and repair services in select markets covering a wide range of equipment. Our experienced service technicians perform equipment installation, maintenance and repair services including services on products not purchased through Webster.
ePetHealth. A leading suite of communication and educational tools designed to help the clinic increase compliance and pet-owner loyalty to the practice through: clinic eMarketing tools, health service reminders, online medical records, drug home delivery and award winning DIA videos and content.
DIA. Our premier client education software for companion animal and equine practices. Provides over 3,000 illustrations, animations, clinical images, radiographs and other diagnostic images cover a wide range of medical
13
conditions. Additionally, DIA Reception provides high definition footage and animations to better explain common pet health issues conditions to pet owners.
Webster VetSource. A leading professional pharmacy providing home delivery service of medications to pet owners which improves client compliance, retains drug revenues within the veterinary practice, and optimizes inventory investments.
Webster Veterinary University . Webster's exclusive training course for veterinary office managers and veterinarians provides practice management tools and solutions with four in-depth courses covering human resources, inventory, marketing, and finance. Additionally, a program has been developed for veterinary hospital receptionists.
Dental Wet Labs . Webster offers dental wet labs throughout the United States providing approved continuing education credits to attendees through lecture and hands-on labs for companion animal dental techniques.
National Handpiece Repair . In February 2012, we launched Webster's Veterinary dental handpiece repair initiative utilizing Patterson Dental's National Repair Center. Practitioners can now have both their high-speed and slow-speed handpieces cleaned, repaired, and returned within 24 hours from receipt.
Anesthesia Technical Support Hotline . Using our dedicated hotline, with one phone call customers can obtain answers and detail support for their anesthesia and monitoring equipment.
Products and Services
The following table sets forth the percentage of total sales by the principal categories of products and services offered to veterinary segment customers:
| 2012 | 2011 | 2010 | ||||||||||
Consumable and printed products | 93 | % | 94 | % | 94 | % | ||||||
Equipment and software | 5 | 5 | 5 | |||||||||
Other | 2 | 1 | 1 | |||||||||
|
|
|
|
|
| |||||||
Total | 100 | % | 100 | % | 100 | % | ||||||
|
|
|
|
|
| |||||||
Consumable and Printed Products
Webster offers our customers a broad selection of veterinary supply products including pharmaceuticals, vaccines, parasiticides, diagnostics, veterinary pet foods, nutritional products and consumable supplies. Our pharmaceutical products typically include anesthetics, analgesics, antibiotics, and ophthalmics. Our biological products are primarily comprised of vaccines and injectibles. Our parasiticides are used for control of external parasites (fleas, ticks, flies, mosquitoes) and internal parasites. Our diagnostics product category includes consumable in-clinic tests for detecting heartworm, lyme disease, feline leukemia and parvovirus, as well as consumable products for measuring blood chemistry, electrolyte balance and cell counts. Veterinary pet foods consist of both specialty diets and premium pet foods. Nutritional products are comprised of dietary supplements, vitamins, dental chews and specialty treats. Consumable supplies include lab supplies, various types and sizes of paper goods, needles and syringes, instruments, gauze and wound dressings, sutures, latex gloves, orthopedic and casting products. Many of the office supply products sold by Patterson Office Supplies are also offered to the veterinary supply market.
Equipment and Software
Veterinary Equipment. Webster sells equipment for hospital, laboratory and general surgical use within the veterinary practice. We also offer innovative, quality equipment that differentiates Webster from the competition
14
including anesthesia machines, surgical monitors, diagnostic equipment, ultrasound, dental power units, cages, lights, digital x-ray systems and x-ray machines. Approximately 50% of veterinary equipment orders are drop shipped directly to the customer from the manufacturer. The remaining half of veterinary equipment is distributed in a manner similar to consumable supplies.
Practice Management Software . Webster develops and markets our own proprietary line of practice management software, INTRAVET ® , for veterinary professionals, including software for scheduling, billing, charting and capture/storage/retrieval of digital images.
Client Education Software. Webster also develops and markets our own proprietary client education software, DIA ® , for veterinary professionals. DIA encompasses over 3000 3D clinical animations and images, enabling veterinarians to more fully explain and illustrate a pet's diagnosis and treatment recommendations to their clients.
Client Communication portal . Webster develops and markets our own innovative web-based client communication platform, ePetHealth, providing practitioners a suite of client offerings such as online medical records, eMarketing tools, client education resources, and a home delivery portal.
Other
Other products and services include commissions earned on agency sales, equipment repair revenues, software maintenance contract revenue and freight recovery on shipments to customers.
Sales and Marketing
During fiscal 2012, Webster sold products or services to over 22,000 customers in the U.S. who made one or more purchases during the year. Our customers include veterinarians, laboratories, institutions and other animal health professionals. No single customer accounted for more than 1% of sales during fiscal 2012, and Webster is not dependent on any single customer or geographic group of customers. Our sales and marketing efforts are designed to establish and improve customer relationships through personal interaction with our field sales and customer service representatives and frequent direct marketing contact, which underscores our value-added approach.
Webster currently has 18 local offices throughout the U.S. so that we can provide a presence in the market and decision making near the customer. These offices, or branches, are staffed with a complete complement of Webster's capabilities, including sales, customer service and technical service personnel, as well as a local manager who has broad decision making authority with regard to customer related transactions and issues.
Field Sales Representatives . A primary component of Webster's value-added approach is our sales force. Due to the fragmented nature of the veterinary supply market, we believe that a large sales force is necessary to reach potential customers and to provide full service. Sales representatives provide an informational link to the overall industry; assist practitioners in selecting and purchasing products and help customers efficiently manage their supply inventories. Each sales representative works within an assigned sales territory under the supervision of a location (branch) manager. Sales representatives are all Webster employees and are generally compensated on a commission basis, with some, less experienced, representatives receiving a base salary and commission.
Customer Service Representative. We support our field sales representatives and direct marketing efforts with customer service representatives in ten call centers covering the United States. As of April 28, 2012, we had 135 customer service representatives. Customer service representatives work in tandem with our field sales representatives, providing a dual coverage approach for individual customers. In addition to processing orders, customer service representatives are responsible for assisting customers with ordering, informing customers of monthly promotions, and responding to general inquiries. Customer service representatives utilize our
customized order entry system to process customer orders, access pricing, determine product availability, provide promotional information about products, research customer preferences, and review order histories.
15
Direct Marketing. To assist our sales representatives, Webster publishes a catalog containing product and service information. Webster customers receive a full-line product catalog containing over 11,000 inventoried items. The catalog includes pictures of products, detailed descriptions and specifications of products and is used by practitioners as a reference source. We also promote selected consumable products, our value-added program and services, new products, specially priced items and high demand items through our monthly magazine, Insight . Additional direct marketing tools that we utilize include equipment catalogs, customer loyalty programs, specific product and vendor programs, flyers, faxes, and other promotional materials. In order to extend our customer reach and enhance customer interaction, we also participate in national, regional and local trade shows.
E-Commerce Platform. Webster's website allows customers the ability to order items 24 hours a day, seven days a week. The website also incorporates value-added functions that permit customers to check their invoice, payment and credit history, build a "shopping list" of frequently purchased items and track their order status.
Distribution
Webster believes that responsive delivery of quality supplies and equipment is key to customer satisfaction. We ship veterinary consumable supplies from 11 strategically located distribution centers in the United States. Orders for veterinary consumable supplies can be placed through our field sales force, customer service representatives or electronically 24 hours a day, seven days a week. Printed office products are shipped from Patterson's manufacturing and distribution facility in central Illinois.
All orders are routed through our centralized computer ordering, shipping and inventory management systems, which are linked to each of our strategically located distribution centers. If an item is not available in the distribution center nearest to the customer, the computer system automatically directs fulfillment of the item from another center. Rapid and accurate order fulfillment is another principal component of our value-added approach. We estimate that 97% of Webster's consumable orders are received by the customer the next business day.
In order to assure the availability of Webster's broad product lines for prompt delivery to customers, we must maintain sufficient inventories at our distribution centers. Purchasing of consumables and standard equipment is centralized, and our purchasing department uses a real-time perpetual inventory system to manage inventory levels. Our inventory consists mostly of consumable supply items and pharmaceutical products. By utilizing computerized inventory management and ordering systems, we are able to accurately predict inventory turns in order to minimize inventory levels for each item.
Sources of Supply
Webster obtains products from nearly 600 vendors. While Webster makes purchases from many vendors and there is generally more than one source of supply for most of the categories of products, the concentration of business with key vendors is considerable. In fiscal 2012 and 2011, Webster's top 10 veterinary supply manufacturers comprised 67%, and the single largest supplier comprised 13%, of the total cost of veterinary supply sales.
There are two major types of arrangements that account for the flow of transactions between us and our customers. Traditional "buy/sell" transactions, which account for the vast majority of our business, involve direct purchases of products by us from vendors, which we manage and store in our distribution centers. A customer then places an order with us, and the order is then picked, packed, shipped and billed by us to our customer.
We also process orders from our customers under "agency agreements" with some of our vendors, primarily for certain seasonal pharmaceutical products. Under this model, when we receive orders for products from the customer, we transmit the order to the vendor who then picks, packs, and ships the products. The vendor then invoices and collects payment from our customer. We receive a commission payment for soliciting the order and for providing other customer service activities.
16
Competition
The distribution and manufacture of veterinary products is highly competitive. In addition to two other national, full-service firms, Butler Schein Animal Health, Inc. and MWI Veterinary Supply, Inc., Webster competes with several full-service distributors that operate on a regional level and numerous smaller local and specialty distributors and to lesser extent, mail order distributors or buying groups. Webster also competes directly with pharmaceutical companies who sell certain products directly to the customer.
Webster approaches its markets by emphasizing and delivering a value-added model to the practitioner. To differentiate our self from our competition we deploys a strategy of premium customer service, a highly qualified and motivated sales force, an extensive breadth and mix of products and services, accurate and timely delivery of product, strategic location of sales offices and competitive pricing.
Rehabilitation Supply
Overview
Patterson Medical is headquartered in Bolingbrook, Illinois, and is the world's leading value added distributor of rehabilitation medical supplies and equipment. We believe Patterson Medical offers the most comprehensive range of distributed and self-manufactured rehabilitation products to health care professionals globally. Our mission is to provide health care professionals and their patients with access to products that improve peoples' lives by helping them to attain their highest achievable level of independence, safety and comfort. We operate as Patterson Medical globally, and are transitioning our acquired catalog brands such as Sammons Preston, Homecraft, and Ausmedic into product brands. Patterson Medical still operates as Medco Sports Medicine in the North American sports medicine market.
Patterson Medical serves as the gateway through which over 30,000 rehabilitation products originating from more than 1,500 suppliers and manufacturers are sold to a diverse customer base with an emphasis on physical therapists ("PTs") and occupational therapists ("OTs"). We offer our customers a "one-stop shop" through what we believe to be the most comprehensive catalogs in the industry, the largest direct sales force and the category's most efficient customer service and distribution operations. Major channels of distribution are acute care hospitals, long-term care facilities, rehabilitation clinics, dealers and schools. In addition, we believe Patterson Medical's reputation, global market presence and highly transferable business model will facilitate entry into new markets.
Patterson Medical offers a wide range of differentiated, non-invasive products and expertise to users and their health care providers, while focusing on niches, worldwide, where our capabilities, reputation and customer partnerships can result in a competitive advantage. Patterson Medical's goal is to become our customers' first choice for rehabilitation supplies and equipment in each of our chosen markets.
Patterson Medical is highly diversified with no single product, customer or purchasing group representing a significant percentage of total revenue. In addition, given the relatively small average order size (approximately $225), our products often do not represent a major expense category for our customers.
Patterson Medical has been pursuing a strategy of organic growth, complemented by strategic acquisitions in its domestic and international markets. This has included building and buying branch offices in the U.S., and buying and integrating distributors in the U.K., France, and Australia. Patterson Medical also uses a robust international dealer network to service customers in countries where we do not have an established direct presence. Approximately 65% of Patterson Medical's revenue originates in North America.
Patterson Medical manufactures or has exclusively manufactured for us products representing approximately 35% of our total revenue. These products carry the Patterson Medical brand, or one of the many brands added through acquisition. Patterson Medical owns manufacturing facilities in the U.S., France, the U.K.
17
Australia, and Thailand, and has a China sourcing office. Patterson Medical is a distributor for the other 65% of our product offering, carrying the top brands in the rehabilitation industry. One of Patterson Medical's strengths is our trading relationship with the top manufacturers of rehabilitation products in the U.S. and abroad.
We believe the rehabilitation medical supply and assistive product industry is approximately $5.6 billion worldwide and is expected to grow faster than the overall economy over the next several years. Industry growth is driven by strong growth in the physical and occupational therapy markets and favorable demographic trends associated with the aging of the baby-boom generation. We do not participate in all product segments, so Patterson Medical's addressable market (defined as the collective market for products sold by Patterson Medical) is approximately $3.4 billion worldwide. We believe that Patterson Medical has an industry leading market share of approximately 15% in a highly fragmented rehabilitation and assistive products market.
We believe that the demand for rehabilitation products will continue to be influenced by the following factors:
| • | Favorable Demographics. Favorable demographic trends such as extended life expectancy, active lifestyles and a general willingness to spend discretionary income on health care and well being, are expected to contribute to increased demand for products distributed by Patterson Medical. Specifically, the aging baby-boomer population, together with their increased disposable income and desire for independence, will fuel product purchases to assist with the frailties associated with old age and provide sustained sales growth. |
The U.S. Census Bureau has projected the 85 and older population in the U.S. to increase significantly, from less than 6 million in 2011 to 14 million by 2040 and 19 million by 2050. The 65 to 84 year old population is expected to more than double between 2011 and 2040. Current trends indicate that these age groups represent the majority of home and community-based health care patients.
The aging of the population is a revenue growth driver because approximately 10% of people over the age of 65 and approximately 50% of people over the age of 85 need assistance with everyday activities. Patterson Medical believes we are well positioned to benefit from this trend by providing aids to daily living, namely dressing devices; toileting, dining, bathing aids; and grooming devices, all of which promote greater patient independence, improved patient responsibility and improved responsiveness to treatment.
| • | Increasing Number of PTs and OTs, Patterson Medical's Primary User Groups. According to the U.S. Department of Labor Bureau of Labor Statistics (BLS), there were approximately 199,000 PTs and 109,000 OTs in the U.S. in 2010. Approximately 60 percent of PTs were employed in either hospitals or offices of physical therapists. The remaining 40 percent of PTs was employed in home health agencies, outpatient rehabilitation clinics, physician offices and nursing homes. The majority of OTs work in hospitals, including many in rehabilitation and psychiatric hospitals. The remaining OTs work in outpatient occupational therapy offices and clinics, schools, home health agencies, nursing homes, community mental health centers, adult day care programs, job training services and residential care facilities. The demand for PTs and OTs is expected to remain strong. The BLS estimates a growth of 33.5% for OTs between 2010 and 2020, and a growth of 39% for PTs in that same time period. Both professions are expected to grow much faster than average. |
| • | Increasing Frequency of Reconstructive and Implant Surgery. Another important driver of the growth in the PT market is the growing need for rehabilitation products resulting from the increasing frequency of reconstructive implant procedures, including hip and knee replacements. The worldwide reconstructive implant market is currently in excess of $5.0 billion and expected to grow between 7% and 8% annually. This growth trajectory is largely driven by favorable demographics, as patient populations are expanding at both ends of the age spectrum. Among seniors, more active lifestyles and longer life expectancies are responsible for the increasing frequency of reconstructive implants. We believe Patterson Medical is well positioned to benefit from the growth in reconstructive implants, by providing physical therapy and exercise products that help the patients return to a normal level of function. |
18
| • | Volatility in Funding and Regulation. The demographic growth in aged population both in the U.S. and abroad will continue to pose funding challenges for governments. Whether private pay, Medicare, Medicaid or some other funding mechanism, the population need has been outpacing the available funds. This has resulted in governments changing funding methods, allocations, and rules to try and better match supply with demand. Although we do not directly participate in third party reimbursed (patient specific) product, the pressure on healthcare providers can have a pass through effect. Patterson Medical has pursued a diversified strategy so we do not rely too heavily on any one product category. |
International Operations
Patterson Medical's international operations are based in the United Kingdom ("U.K.") and are made up of Patterson Medical International (PMI) in the U.K., Kinetec and Sacedi in France, and Patterson Medical/Ausmedic in Australasia. Our international domestic operations broadly reflect the same business model as used in the North American market. In the U.K., France and Australia, operations include sales and marketing, customer service, distribution, purchasing and administration. France also includes a self contained manufacturing unit. Outside of these countries, PMI uses a network of over 200 distributors to reach its customers.
PMI is a leading supplier of aids to daily living ("ADL") and rehabilitation products in the U.K., and a significant player in the international markets. Having developed and designed many proprietary products, we are the prime source for numerous established and market leading ADL brands, including products sold under the names Homecraft, Days, and Physiomed. The PMI catalog offers a broad line of ADL, therapy, rehabilitation and pediatric products containing over 10,000 items and is circulated to PTs, OTs, loan equipment stores and private clinics, trade outlets and the general public.
PMI's central sales and marketing strategy is to provide a "one-stop shop" proposition to hospitals, local government and trade customers (Dealers) throughout the U.K. Customers are reached through a combination of mail order, a 54 person sales force, telemarketing and in-market promotional and exhibition activity.
In April 2009, PMI acquired Mobilis Healthcare, increasing our global presence. While the Homecraft catalog has historically been focused on occupational therapy, Mobilis was a complementary addition, given its strong position in the physiotherapy market.
In June 2010, PMI acquired the rehabilitation business of DCC Healthcare. The acquired DCC businesses, Days Healthcare, Physiomed and Ausmedic, rank among the leaders in their overseas markets, providing assistive living products and rehabilitation equipment and supplies to hospitals, physical and occupational therapists, long-term care facilities, dealers and consumers in the U.K., continental Europe, Australia, New Zealand and other international markets.
Kinetec consists of two operations, the manufacturing and distribution of Continuous Passive Motion machines ("CPMs") for sale on a worldwide basis and the sale and distribution of Patterson Medical products in France. Products are marketed to customers through product brochures, mailings, telemarketing and a sales force that covers the French rehabilitation market. Sacedi focuses on distribution in the Therapy and Sports Medicine market segments.
Strategy
Our objective is for Patterson Medical to be the customers' first choice for rehabilitation medical supplies and equipment in each of its chosen markets. We intend to continue Patterson Medical's growth through internal expansion and acquisitions in both rehabilitation and related products.
19
Emphasizing Value-Added, Full-Service Capabilities. Patterson Medical currently offers our customers a "one-stop shop" for products through our industry leading catalog with over 20,000 items, focused primarily on physical and occupational therapy products. Patterson Medical adds new products each year to our ever-expanding catalog and is committed to doing so long-term. Consistent with Patterson Medical's current product offering, some of these new products are branded, exclusive or self-manufactured.
We recognize that different customer groups have very different economic, product, distribution channel requirements and treatment goals. Patterson Medical proactively attempts to anticipate and flexibly respond to the diverse needs of our customers, while focusing on niches, worldwide, where our capabilities, reputation and customer partnerships can result in a competitive advantage. As such, we foresee an on-going evolution of our product offerings to meet the ever-increasing demands of our diverse customer segments.
Improving Operating Efficiencies. Patterson Medical's proprietary products, which consist of self-manufactured products, products manufactured for Patterson Medical and products sold through exclusive distribution arrangements, represent approximately 35% of total revenues. Over the past four years, we have made investments in new sales and marketing programs as a part of our accelerated adoption of Patterson's value-added strategy, including the expansion of our sales force and the establishment of a branch office structure. We believe these investments will result in additional sales and operating efficiencies into the future.
As of April 2012, Patterson Medical is distributing products from four shared distribution facilities that distribute products for two or all three of our operating units. As of April 2012, Patterson Medical now shares eight branch facilities with other business units, allowing the two business units to share in certain common expenses.
Growing Through Internal Expansion and Acquisitions. Patterson Medical believes we are well positioned to expand in our core markets. Our market presence, clinical understanding and close customer relationships allow us to anticipate and flexibly respond to the diverse needs of our customers. We believe our market knowledge, strong vendor relationships and manufacturing capabilities will continue to drive the delivery of value-added solutions through the continual enrichment of our product mix. Additionally, we believe our broad portfolio of national accounts and commitment to expand our sales force will enhance Patterson Medical's growth and penetration within our current and new customer base.
Patterson Medical acquired Homecraft, Mobilis, Physiomed, Days Medical, and other businesses to establish a platform for international expansion. Each business that was added has been integrated and has added to an ever expanding brand and product portfolio. The international business continues to accelerate, in terms of both product lines and geographic regions. Since the Homecraft/Kinetec transaction, Patterson Medical has added over 550 pages of new products to the catalog. The international acquisitions brought with them a proven capability to source products at very favorable costs and at high levels of quality from China, which has resulted in meaningful cost savings. We believe our business model is transferable to other countries, and are using PMI to cultivate new relationships through an enhanced product array, sales effort, distribution capabilities and catalog expertise.
In May 2004, Patterson Medical acquired the assets of Medco Supply Company, Inc., or "Medco," from NCH Corporation. With sales of approximately $60 million, Medco is one of the nation's leading sports medicine distributors and is based in Buffalo, New York. In addition to Medco's sports medicine business, Medco sells first aid, safety and medical consumable products to commercial and institutional customers, as well as consumable supplies and equipment to podiatrists. The complete product offering includes approximately 10,000 SKUs that are sold through direct mail catalogs and 11 territory sales people. Medco markets to athletic trainers, schools and school nurses, daycare providers and healthcare professionals including podiatrists, chiropractors and physical therapists.
Over the past five years, Patterson Medical has opened eighteen branch offices through acquisitions and internal start-ups in desirable locations throughout the U.S. In November 2007, Patterson Medical acquired PTOS software, a leading line of practice management software for physical therapists.
20
In April 2009, Patterson Medical acquired Mobilis Healthcare, a U.K.-based business with sales of $28 million. Mobilis serves 12,000 customers in the U.K. and France and owns several leading brands that are sold into its primary markets.
In June 2010, Patterson Medical acquired the rehabilitation business of Empi Therapy Solutions, with sales of approximately $30 million.
In June 2010, Patterson Medical acquired the rehabilitation business of DCC Healthcare, with combined sales of approximately $70 million.
Products and Services
The following table sets forth the percentage of total sales represented by the principal categories of products and services offered to rehabilitation segment customers:
| 2012 | 2011 | 2010 | ||||||||||
Consumables | 71 | % | 69 | % | 71 | % | ||||||
Equipment and software | 24 | 26 | 24 | |||||||||
Other | 5 | 5 | 5 | |||||||||
|
|
|
|
|
| |||||||
Total | 100 | % | 100 | % | 100 | % | ||||||
|
|
|
|
|
| |||||||
Consumables
Patterson Medical offers a large selection of supply products that can be categorized as follows:
| • | Aids to Daily Living-dressing devices, toileting, dining, bathing aids and grooming devices |
| • | Orthopedic Soft Goods / Splints-braces, splints and orthotics for protecting, supporting and positioning |
| • | Clinical-products that assist in the examination and treatment of patients, such as exercise bands, putty, weights, balls and mats |
| • | Mobility-walkers, canes and wheelchair accessories such as gloves, trays and carrying bags |
| • | Pediatric Seating and Positioning-rolls, wedges, specialty seating and standers, and mobility assistance products for special needs children; category also includes sensory motor stimulation products such as toys, crafts and devices to assist with balance. |
| • | Modalities-products for heating and cooling therapies, electrical stimulation, laser, ultrasound, paraffin, iontophoresis and therapeutic creams and lotions |
Equipment and Software
Rehabilitation equipment consists of exercise, examination, treatment and therapy equipment and furniture. These products include parallel bars, treatment tables, mat platforms, treadmills, stationary bicycles and CPMs. The November 2007 acquisition of PTOS ™ software added a line of practice management software to Patterson Medical's wide array of product offerings. In addition, certain acquisitions in the recent past have given us access to premium equipment lines that were previously unavailable to Patterson Medical.
Other
Other products and services include equipment repair revenues, software maintenance contract revenue and freight recovery on shipments to customers.
21
Sales and Marketing
Patterson Medical has been going through a process of establishing its brand globally to better leverage its international scope. This has led to a catalog branding transition of our acquired businesses (in various stages) including Sammons Preston in the U.S. and Canada, and Homecraft, Days, Physiomed, and Ausmedic in the remainder of the world.
A core element of Patterson Medical's strategy is to maintain the most comprehensive single catalog of rehabilitation products and supplies. The catalog, published for over 50 years, is considered the gold standard of the industry and features the most comprehensive product offering with longstanding industry leading positions and recognized brand names. Our product management group works closely with customers, suppliers and the sales force to evaluate new products for inclusion in Patterson Medical's product offering. We add approximately 2,000 new products to the catalog each year.
Patterson Medical has an experienced sales force, national account contracts with major customer groups, unmatched customer service within the industry and the proven ability to introduce new products each year, allowing us to compete across the entire spectrum of the rehabilitation medical supplies and non-wheelchair assistive products industry.
A key priority has been the expansion of our field sales force, which has grown by more than 100 sales representatives since late in fiscal 2006 and now totals nearly 280 worldwide. New sales representatives are generally hired from the ranks of physical and occupational therapists, manufacturer representatives and others with extensive industry knowledge.
Patterson Medical began developing a branch office structure in fiscal 2007 through a combination of internal start-ups and dealer/distributor acquisitions. Similar to Patterson Dental's branches, these offices have a showroom, commissioned sales staff and service department that provides equipment installation, repair and warranty service for equipment manufacturers. As of April 2012, eighteen branches had been established.
Patterson Medical's U.S. national accounts group collaborates with our sales force to meet the changing needs of our expanding account base. The national accounts program is staffed by seasoned professionals who have developed a comprehensive portfolio of contracts. Furthermore, the integrated Patterson Medical organization has national contracts with major purchasing groups within each submarket, including hospitals, nursing homes and dealers.
The rehabilitation medical supplies and equipment business is highly fragmented. No one manufacturer, distributor or customer represents a significant portion of Patterson Medical's revenue.
To enhance the total value we bring to our customers, Patterson Medical created a value-added benefit program for its preferred customers. The Patterson Advantage sm program entitles our best customers to discount pricing and cash rebates, priority service scheduling, supply management summary reports and continuing education course discounts.
Distribution
Patterson Medical's distribution process centers on our ability to efficiently fill small dollar amount orders. In the U.S., over 6,000 packages ship daily from six locations. A majority of products are shipped out of two full service, shared Patterson distribution centers, one in Mt. Joy, Pennsylvania, and the other in Dinuba, California. Patterson Medical also uses two additional shared facilities, in Jacksonville, Florida, and Ft. Worth, Texas, to provide local distribution of high volume items to those regions. Approximately 95% of the small packages in the U.S. ship via UPS.
Patterson Medical uses a branch structure to more effectively distribute equipment to our customers. Large equipment can be shipped to our branches, inspected for damage, and then put on Patterson Medical trucks for consolidated delivery and installation at customers' facilities.
22
Patterson Medical's U.S. call center operates from 7am – 7pm Monday through Friday, processing in excess of 5,000 calls per day. In addition, customers can order 24 hours a day through Patterson Medical's Internet websites. The combination of in-house staff and web ordering options provides customers with 24 hours a day, seven days a week ordering capabilities. Approximately 36% of customer orders are through the web or EDI, which has decreased call center activity, allowing Patterson Medical to provide more personalized service to customers.
Sources of Supply
Among Patterson Medical's core strengths is our ability to obtain premier products from vendors. Our products are purchased from over 1,500 suppliers and manufacturers. Although no single supplier accounted for more than 5% of Patterson Medical's total purchases in fiscal 2012, we frequently are the largest single customer of these manufacturers. Suppliers view the ability to distribute their products through our global network positively due to our reputation, longstanding industry leading position, comprehensive catalogs, national account contracts, sales force presence and distribution capabilities. We continually work at strengthening our supplier relationships through the introduction of supplier programs.
Competition
We operate in the highly fragmented rehabilitation medical supplies and equipment industry. Patterson Medical's competition is generally either locally or regionally focused. Patterson Medical intends to pursue expansion opportunities when prudent in order to add products, customers and capabilities, which will further differentiate Patterson Medical from our competition.
We believe Patterson Medical is the only national player to offer, "one-stop shopping" to our customers. Patterson Medical's national and international scale and purchasing power provides us with a favorable cost position and strong pricing trends relative to our competition.
For further information on our three operating segments, and operations by geographic area, see "Management's Discussion and Analysis of Financial Condition and Results of Operations" in Item 7 of this document and Note 11 to the Consolidated Financial Statements.
Shared Services Initiative
We have continued to consolidate our distribution infrastructure and business systems over the past several years. As of April 28, 2012, we have eight facilities that serve two or three of our business units. These strategically located facilities enable us to realize operating efficiencies and improve customer service.
Our business units also share a number of sales branch office locations, enabling multiple business units to operate at one physical location. As of April 28, 2012, we have 13 shared locations, and we plan to leverage additional branch sharing between two or three business units in select markets in fiscal 2013 and beyond.
The PTC has staff dedicated to support the technology offerings of each of our business units. Such technology product and service offerings have expanded in recent years and we will continue that focus. We support over 80,000 customers nationwide through the PTC, and strive to resolve any situation in one call, whether the question or concern involves hardware, software, computer networking or digital technology. Development of our proprietary practice management and certain of our patient education products takes place at the PTC. In addition to the PTC, technology support is provided to customers through our some of our business unit's sales branches, which provide network installation and customer training.
Patterson Companies, Inc.
Trademarks and Patents
Our products and services are sold under numerous trademarks including "PATTERSON ® ," "EAGLESOFT ® , " "CASEY ® ," "SAMMONS PRESTON ® ," "KINETEC ® ," "TUMBLE FORMS, ® "
23
"INTRAVET ® ," and "DIA ® ,". Because we believe our trademarks are well-recognized within their respective industries and valuable assets, we protect them against infringement. Some of our proprietary software in the orthodontia field is protected by patents, which have varying terms, generally of 17-20 years.
Employees
As of April 28, 2012, we had approximately 7,059 employees. We have not experienced a shortage of qualified personnel in the past and believe that we will be able to attract such employees in the future. None of our employees are subject to collective bargaining agreements or represented by a union. We believe our relations with employees to be good.
Website
Our Annual Report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports are made available on our website as soon as reasonably practicable after the material is electronically filed with or furnished to the Securities and Exchange Commissions. This material may be accessed by visiting the Investor Relations section of our website at www.pattersoncompanies.com .
Information relating to our corporate governance, including our Principles of Business Conduct and Code of Ethics, and information concerning executive officers, board of directors and board committees, and transactions in Patterson securities by directors and officers, is available on or through our website www.pattersoncompanies.com in the Investor Relations section.
Information maintained on the website is not being included as a part of our Annual Report on Form 10-K.
Governmental Regulation
The marketing, distribution and sale of certain products we sell are subject to the requirements of various federal, state, and local laws and regulations. We are subject to regulation by the Federal Food and Drug Administration, the Drug Enforcement Administration and the U.S. Department of Transportation. Among the federal laws which impact us are the Federal Food, Drug and Cosmetic Act, which regulates the advertising, recordkeeping, labeling, handling, storage and sale of drugs and medical devices which are distributed by us, and which further requires we are registered with the Federal Food and Drug Administration; the Safe Medical Devices Act, which imposes certain reporting requirements on us in the event of an incident involving serious illness, injury or death caused by a medical device we have distributed; and the Controlled Substance Act, which regulates the recordkeeping, handling, storage and sale of certain drugs sold by us and requires we are registered with the Drug Enforcement Administration. In addition, the transportation of certain products we distribute that are considered hazardous materials is subject to regulation by the U.S. Department of Transportation.
We also are required to be licensed as a distributor of drugs and medical devices by each state in which we conduct business. In addition, several state Boards of Pharmacy require we are licensed in their state for the sale of animal health products within their jurisdiction. Our Company is also subject to the requirements of foreign laws and regulations, which impact our operations in those foreign countries we conduct business.
While we believe we are in substantial compliance with the laws and regulations which regulate our business, and that we possess the licenses required in the conduct of our business, our failure to comply with any of applicable laws or regulations, or the imposition of new laws or regulations, could negatively impact our business.
24
Executive Officers of the Registrant
Set forth below is the name, age and position of the executive officers of Patterson as of June 27, 2012.
Scott P. Anderson | 45 | President and Chief Executive Officer-Patterson Companies, Inc. | ||||
Peter L. Frechette | 74 | Chairman of the Board-Patterson Companies, Inc. | ||||
R. Stephen Armstrong | 61 | Executive Vice President, Chief Financial Officer and Treasurer-Patterson Companies, Inc. | ||||
Ranell M. Hamm | 50 | Chief Information Officer-Patterson Companies, Inc. | ||||
Jerome E. Thygesen | 54 | Vice President, Human Resources-Patterson Companies, Inc. | ||||
Paul A. Guggenheim | 52 | President-Patterson Dental Supply, Inc. | ||||
George L. Henriques | 51 | President-Webster Veterinary Supply, Inc. | ||||
David P. Sproat | 45 | President-Patterson Medical Supply, Inc. | ||||
Our officers are elected annually and serve at the discretion of the Board of Directors.
Background of Executive Officers
Scott P. Anderson became our Chief Executive Officer in April 2010 and was appointed to our Board of Directors in June 2010. Mr. Anderson had held the position of President of Patterson Dental Supply, Inc., since June 2006 and, prior to that, the positions of Vice President, Sales and Vice President, Marketing of Patterson Dental Supply, Inc. Mr. Anderson joined Patterson in 1993 and currently serves on the board of directors of the Dental Trade Alliance, the trade association of dental manufacturers, distributors and laboratories. Mr. Anderson has been a director of C.H. Robinson since 2012.
Peter L. Frechette currently serves as the Chairman of the Board and has held that position since May of 1985. He served as Chief Executive Officer from September 1982 through May 2005 and as President from September 1982 to April 2003. He has been one of our directors since March 1983. Prior to joining our Company, Mr. Frechette was employed by American Hospital Supply Corporation for 18 years, the last seven of which he served as President of its Scientific Products Division.
R. Stephen Armstrong was elected Executive Vice President, Chief Financial Officer and Treasurer of Patterson effective July 1999. Before joining Patterson, Mr. Armstrong had been an Assurance Partner with Ernst & Young LLP. Ernst & Young LLP is currently Patterson's independent registered public accounting firm. Mr. Armstrong has been a director of Delphax Technologies, Inc. since 2000.
Ranell M. Hamm became Chief Information Officer, in April 2011. Prior to joining us, Ms. Hamm was Senior Director of Clinical Information Delivery for UnitedHealth Group. Prior to UnitedHealth Group she was employed by Assurant, Inc., where she was Senior Vice President of Finance Systems & Services, IT Security; Chief Information Officer/ Chief Operating Officer of Shared Business Services; and Senior Vice President of Shared Services Organization.
Jerome E. Thygesen became Vice President, Human Resources, in March 2006. Prior to joining Patterson, Mr. Thygesen was Vice President, Organizational Development for Fairview Red Wing Health Services from September 2001 to February 2006, and Director of Human Resources for Red Wing Shoe Company from March 1987 to June 2001.
Paul A. Guggenheim became President of Patterson Dental Supply, Inc. in April 2010. Mr. Guggenheim previously was the southwest region manager of Patterson Dental. Mr. Guggenheim joined us in 2000 following our acquisition of Guggenheim Brothers Dental Supply. Mr. Guggenheim has worked in the dental industry for over 25 years and is former chairman of the American Dental Trade Association (now the Dental Trade Alliance). He also is past president of the Dental Dealers of America and former chairman of the American Dental Cooperative.
25
George L. Henriques was named President of Webster Veterinary Supply, Inc. in August 2006. Mr. Henriques previously served as chief information officer of Webster since 2000 and is former chairman and board member of the American Veterinary Distributors Association.
David P. Sproat was named President of Patterson Medical Supply, Inc. in September 2005. Mr. Sproat joined our Company in 1997 and has served in various sales and marketing capacities, including Vice President, Sales of Patterson Dental Supply, Inc. from June 2004 through September 2005.
| Item 1A. | RISK FACTORS |
The statements in this section describe the major risks to our business and should be considered carefully, in connection with all of the other information set forth in this annual report on Form 10-K. The risks that follow, individually or in the aggregate, are those that we think could cause our actual results to differ materially from those stated or implied in forward-looking statements.
Continuing uncertain economic conditions and volatility in the financial markets could adversely affect our operating results and financial condition.
Continued recessionary trends in the U.S. or global economy, or an uncertain economic outlook, could materially adversely affect our operating results and financial condition. Unfavorable economic conditions may continue to cause customers to reduce, modify, delay, or cancel purchasing our products and services, and a prolonged period of economic instability could reduce their ability to make payments. Furthermore, such conditions could cause our suppliers to reduce their production, decrease their number of product offerings, or change their terms of sale to us. Increasing commodity prices would also increase our cost of operations, either directly through increased energy costs or indirectly through what we are charged by our suppliers. Unfavorable economic conditions could also cause changes in our product mix as our customers prioritize established, low-margin products rather than innovative, high-margin products, which could reduce our profit margin.
In addition, volatility and other disruptions in the financial markets could adversely affect the cost and availability of credit to us, as well as the cost of, and ability to sell finance contracts we receive from customers to outside financial institutions. Reduced access to capital for our customers limits the amount of investment that they can make in their practices, and with limited investment by the customer our revenues from equipment sales would be lower.
The dental supply, veterinary supply, and rehabilitation and assistive products supply markets are highly fragmented and competitive, and we may not be able to compete successfully.
Our competitors include national, regional and local full-service distributors, mail-order distributors and, increasingly, Internet-based businesses. Some of our competitors have greater resources than we do, or operate through different sales and distribution models that could allow them to compete more successfully. For example, many of our suppliers are manufacturers, some of whom compete with us by selling directly to customers. Furthermore, Internet-based businesses may be able to offer the same product at a lower cost.
Most of our products are available from multiple sources, and our customers tend to have relationships with several different distributors who can fulfill their orders. Our competitors could obtain exclusive rights to market particular products, which we would then be unable to market. Manufacturers also could increase their efforts to sell directly to end-users and thereby eliminate or reduce our role and that of other distributors. Industry consolidation among suppliers, price competition, the unavailability of products, whether due to our inability to gain access to products or to interruptions in supply from manufacturers, or the emergence of new competitors also could increase competition. Our failure to compete effectively may limit and/or reduce our revenue, profitability and cash flow.
26
Risks inherent in acquiring other businesses could offset the anticipated benefits of such acquisitions and we may face difficulty in efficiently and effectively integrating acquired businesses since we operate in three distinct segments.
As a part of our business strategy, we have acquired businesses in the ordinary course and expect to continue acquiring businesses in the future. These acquisitions can involve a number of risks and challenges, any of which could cause significant operating inefficiencies and adversely affect our growth and profitability. Such risks and challenges include underperformance relative to our expectations and the price paid for the acquisition; unanticipated demands on our management and operational resources; difficulty in integrating personnel, operations and systems; retention of customers of the combined businesses; assumption of contingent liabilities; and acquisition-related earnings charges.
Our ability to continue to make acquisitions will depend upon our success in identifying suitable targets, which requires substantial judgment in assessing their values, strengths, weaknesses, liabilities and potential profitability, as well as the availability of suitable candidates at acceptable prices, and whether restrictions are imposed by anti-trust or other regulations.
In addition, our acquisitions may not result in the benefits and revenue growth we expect because of difficulties integrating the acquired businesses, including their personnel, customers, operations and systems. As we operate in three distinct segments, we need to consolidate the distribution, information technology, human resources, financial and other administrative functions of those business units jointly to meet their needs while addressing distinctions in the individual markets of those segments. We may not be able to do so effectively and efficiently.
Our international operations are subject to inherent risks that could adversely affect our operating results.
There are a number of risks inherent in foreign operations, including complex regulatory requirements, staffing and management complexities, import and export costs, other economic factors and political considerations subject to unanticipated changes. Additionally, foreign operations expose us to foreign currency fluctuations. Furthermore, we generally do not hedge translation exposure with respect to foreign operations.
We depend on our relationships with our sales representatives and customers, as well as suppliers of the products that we distribute.
The inability to attract or retain qualified employees, particularly sales representatives who relate directly with our customers, or our inability to build or maintain relationships with suppliers of products that we distribute may have an adverse effect on our business.
We are dependent on our suppliers because we do not manufacture the majority of the products we sell.
Interruptions in supply could adversely affect our operating results. If a supplier is unable to deliver product in a timely and efficient manner, whether due to financial difficulties, natural disasters or other reasons, we could experience lost sales. We generally do not have long-term contracts with our suppliers that commit them to producing products for us.
While there is generally more than one source of supply for most of the categories of products we sell, there is considerable concentration within our veterinary and dental businesses with a few key suppliers. For example, in fiscal 2012 and 2011, Webster's top 10 veterinary supply manufacturers were both comprised of 67%, respectively, and the single largest supplier was comprised 13%, respectively, of the total cost of veterinary supply sales. In the event that any of our suppliers were to become unable or unwilling to continue to provide the products we sell in the amounts we require, we would need to identify and obtain acceptable replacement sources on a timely basis. There is no guarantee that we would be able to obtain such alternative sources of supply on a timely basis, if at all. An extended interruption in the supply of our products would have an adverse effect on our results of operations.
27
In addition, a portion of our products are sourced, directly or indirectly, from outside the United States. Political or financial instability, increased tariffs, restrictions on trade, currency exchange rates, labor unrest, outbreak of pandemics or other events could slow distribution activities and affect foreign trade beyond our control and adversely affect our results of operations.
The products we sell are subject to market and technological obsolescence.
We carry over 100,000 different product stock keeping units (SKUs). Some of these products are subject to technological obsolescence outside of our control, since we do not manufacture the majority of the products we sell. If our customers discontinue purchasing a given product, we might have to record expense related to the diminution in value of inventories we have in stock, and depending on the magnitude, that expense could adversely impact our operating results.
Audits by tax authorities could result in additional tax payments for prior periods.
The amount of income taxes we pay is subject to ongoing audits by U.S. federal, state and local tax authorities and by non-U.S. tax authorities. If these audits result in assessments different from our reserves, our future results may include unfavorable adjustments to our tax liabilities.
We are subject to a variety of litigation that could adversely affect our results of operations and financial condition.
We are subject to a variety of litigation incidental to our business, including product liability claims, intellectual property claims, and employment claims. We also may be subject to securities litigation. Defending these lawsuits may divert our management's attention, may be expensive, and may require that we pay damage awards or settlements or become subject to equitable remedies that could adversely affect our financial condition and results of operations. Any insurance or indemnification rights that we may have may be insufficient or unavailable to protect us against potential loss exposure. A successful claim brought against us in excess of available insurance or not covered by indemnification agreements, or any claim that results in significant adverse publicity against us, could have an adverse effect on our business and our reputation.
Our future success depends on our leadership development and succession planning.
Our success depends, in large part, on our ability to recruit skilled personnel, and then identify and train our personnel to transition into key roles to support the long-term growth of our business. While our board of directors and management actively monitor our succession plans and processes, our business could suffer if we lose key personnel unexpectedly. In addition, competition for senior management is intense and we may not be successful in attracting and retaining key personnel.
We may be required to record a significant charge to earnings if our goodwill or other intangible assets become impaired.
Our balance sheet includes goodwill and other identifiable intangible assets. If impairment of our goodwill or other identifiable intangible assets is determined, we may be required to record a significant charge to earnings in the period of such determination under U.S. generally accepted accounting principles (GAAP).
The healthcare industry is experiencing substantial changes, which are causing uncertainty in the market and may adversely affect our dental and rehabilitation and assistive products supply businesses.
The healthcare industry is highly regulated and subject to changing political, economic and regulatory influences. In recent years, the healthcare industry has undergone significant change driven by various efforts to reduce costs, including: trends toward managed care; consolidation of healthcare distribution companies;
28
consolidation of healthcare manufacturers; collective purchasing arrangements and consolidation among office-based healthcare practitioners that may enable purchasing at more favorable prices than we can obtain and may shift purchasing decisions to entities or persons with whom we do not have a historical relationship; and changes in reimbursements to customers. Our profit margins and the profit margins of our suppliers and our customers may be adversely affected by industry changes. If we are unable to react effectively to these and other changes in the healthcare industry, our operating results could be adversely affected.
In particular, recent healthcare related legislation and regulation in the U.S. may affect expenditures or reimbursements for rehabilitation and assistive products or expenditures or reimbursements for dental services by private dental insurance plans. Other new regulatory requirements could subject us to additional reporting and disclosure requirements, taxes, and/or restrictions. Regulations under healthcare reform legislation continue to be in flux, resulting in uncertainty surrounding their application and related enforcement, as well as consuming resources necessary to comply.
Healthcare markets are rapidly changing, as well. For example, our assumptions concerning future per capita expenditures for dental services, including assumptions as to population growth and the demand for preventive and specialty dental services such as periodontic, endodontic and orthodontic procedures, may be mistaken. Fluctuations in demand for infection control products currently used for prevention of the spread of communicable diseases such as AIDS, hepatitis and herpes may adversely affect our revenue.
Failure to comply with existing and future U.S. and foreign laws and regulatory requirements could subject us to claims or otherwise harm our business.
The marketing, distribution and sale of certain products we sell are subject to the requirements of various federal, state and local laws and regulations in the U.S. and abroad. Our failure to comply with applicable laws may subject us to claims, additional liabilities, or enforcement actions by an administrative agency, which could require us to make settlement payments, be subject to civil or criminal penalties (including fines or loss of licenses), or damage our reputation, any of which could adversely affect our business, financial condition and results of operations.
In the U.S., we are subject to regulation by the Federal Food and Drug Administration, the Drug Enforcement Administration and the U.S. Department of Transportation. Among the federal laws which impact our business are the Federal Food, Drug and Cosmetic Act, which regulates the advertising, record keeping, labeling, handling, storage and sale of drugs and medical devices we distribute, and which requires us to be registered with the Federal Food and Drug Administration; the Safe Medical Devices Act, which imposes certain reporting requirements on us in the event of an incident involving serious illness, injury or death caused by a medical device we distributed; and the Controlled Substance Act, which regulates the record keeping, handling, storage and sale of certain drugs we sell, and which requires us to be registered with the Drug Enforcement Administration. In addition, the transportation of certain products we distribute that are considered hazardous materials is subject to regulation by the U.S. Department of Transportation.
We are also required to be licensed as a distributor of drugs and medical devices by each state in which we conduct business. In addition, several state Boards of Pharmacy require us to be licensed for the sale of animal health products within their jurisdiction. We are also subject to the requirements of foreign laws and regulations, which impact our operations in those foreign countries where we conduct business.
Furthermore, as discussed above, the industries in which we operate have recently experienced an increase in new regulations, which makes compliance increasingly difficult. Costs and resources associated with complying with these increasing regulations can be considerable.
We are exposed to the risk of changes in interest rates.
Our balance sheet includes certain non-current assets that are sensitive to movements in short-term interest rates. The variable rates are comprised of both LIBOR and commercial paper rates plus a spread and reset on
29
certain dates, as set forth in the respective agreements. In addition, our balance sheet includes fixed rate long-term debt, whose fair value could be adversely affected by movements in interest rates. We finance purchases by our customers using finance contracts that are issued at fixed interest rates, and sell these contracts under various funding arrangements that are priced using variable interest rates. Sudden and dramatic changes in the interest rates within relevant markets could adversely affect our results of operations.
Risks generally associated with our information systems could adversely affect our results of operations.
We rely on information systems ("IS") in our business to obtain, rapidly process, analyze and manage data to, among other things:
| • | maintain and manage worldwide systems to facilitate the purchase and distribution of thousands of inventory items from numerous distribution centers; |
| • | receive, process and ship orders on a timely basis; |
| • | manage the accurate billing and collections for thousands of customers; and |
| • | process payments to suppliers. |
A cyber-attack that bypasses our IS security causing an IS security breach may lead to a material disruption of our IS and/or the loss of business information, which could adversely affect our business. These risks may include, among others, the following:
| • | future results could be adversely affected due to the theft, destruction, loss, misappropriation or release of confidential data or intellectual property; |
| • | operational or business delays resulting from the disruption of IS and subsequent clean-up and mitigation activities; and |
| • | negative publicity resulting in reputation or brand damage with our customers, suppliers or industry peers. |
Our results of operations could be adversely affected if our IS are interrupted, damaged by unforeseen events, cyber-attacks or fail for any extended period of time.
Our governing documents and Minnesota law may discourage takeovers and business combinations that our shareholders might consider to be in their best interests.
Anti-takeover provisions of our articles of incorporation, bylaws, and Minnesota law could diminish the opportunity for shareholders to participate in acquisition proposals at a price above the then current market price of our common stock. For example, while we have no present plans to issue any preferred stock, our Board of Directors, without further shareholder approval, may issue up to approximately 30 million shares of undesignated preferred stock and fix the powers, preferences, rights and limitations of such class or series, which could adversely affect the voting power of our common stock. In addition, our bylaws divide our Board of Directors into three classes serving staggered three-year terms. Further, as a Minnesota corporation, we are subject to provisions of the Minnesota Business Corporation Act, or MBCA, regarding "control share acquisitions" and "business combinations." We may, in the future, consider adopting additional anti-takeover measures. The authority of our Board of Directors to issue undesignated preferred stock and the anti-takeover provisions of the MBCA, as well as any future anti-takeover measures adopted by us, may, in certain circumstances, delay, deter or prevent takeover attempts and other changes in control of our company not approved by our Board of Directors.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
We have not received any written comments regarding our reports from the staff of the SEC issued 180 days or more preceding the end of the 2012 fiscal year that remain unresolved, nor have we received any written comments regarding our reports from the SEC within the past 180 days.
30
| Item 2. | PROPERTIES |
We own our principal executive offices in St. Paul, Minnesota, and the majority of our distribution and manufacturing facilities. Leases of other distribution, manufacturing and administrative facilities generally are on a long-term basis, expiring at various times, with options to renew for additional periods. Most sales offices are leased for varying and usually shorter periods, with or without renewal options. We believe our properties are in good operating condition and are suitable for the purposes for which they are being used.
Patterson Logistics Services
The majority of assets we use to distribute product are owned and operated by Patterson Logistics Services, Inc. ("PLSI"), a wholly-owned subsidiary, which operates the distribution function for the benefit of all three of our sales and marketing business segments.
As of April 28, 2012, PLSI operates 14 distribution centers totaling approximately 1,200,000 square feet of distribution space as follows:
| • | 1 dental distribution center is located in Hawaii. |
| • | 4 veterinary distribution centers are located in Alabama, Colorado and Texas. |
| • | 1 rehabilitation distribution center is located in New York State. |
| • | 1 distribution center is located in Texas, which stocks and distributes both dental and rehabilitation product. |
| • | 3 distribution centers are located in Iowa, South Carolina and Washington state, which stock and distribute dental and veterinary products; and, |
| • | 4 distribution centers are located in California, Florida, Indiana and Pennsylvania, which distribute product for all three of the business units. |
Approximately 90% of the PLSI distribution center space is owned.
Patterson Technology Center
The Patterson Technology Center is a state-of-the-art 100,000 square foot facility in Effingham, Illinois, which was completed in fiscal 2012.
Dental Supply
In addition to the locations operated by PLSI, Patterson Dental utilizes an owned location in Illinois to manufacture and ship printed office products. The dental sales operations in Canada are supported by distribution centers located in Quebec and Alberta, Canada.
The dental supply segment is headquartered in our principal executive offices, which is an owned facility. This segment also maintains sales and administrative offices inside the U.S. at approximately 75 locations in over 40 states and at 10 locations in Canada. The majority of these locations are leased.
Veterinary Supply
Webster's headquarters is a leased facility in Devens, Massachusetts. Our veterinary sales personnel generally reside within branch locations shared with the dental supply segment.
Rehabilitation Supply
Patterson Medical is headquartered in a leased facility in Bolingbrook, Illinois. Domestically, the rehabilitation supply segment maintains manufacturing facilities in Wisconsin, New York and South Carolina. Eighteen branch office locations include six that are shared with the dental supply segment.
31
Internationally, this segment has facilities located in the U.K., France, Canada, Australia, New Zealand, China and Thailand.
| Item 3. | LEGAL PROCEEDINGS |
We are involved in various product-related, employment-related and other legal proceedings arising in the ordinary course of our business. Some of these proceedings involve product liability claims arising out of the use of products we distribute. Product liability indemnification is generally obtained from suppliers. However, in the event a supplier of a defective product is unable to pay a judgment for which we may be jointly liable, we may be liable for the entire judgment.
We maintain product liability insurance coverage for any potential liability for claims arising out of products we sell. While we believe our insurance coverage is adequate, there can be no assurance that the insurance maintained is sufficient or will be available to us in adequate amounts or at reasonable costs in the future. Also, there can be no assurance that the indemnification agreements we have with our suppliers will provide us with adequate protection. In addition, future claims brought against us could involve claims not covered by insurance or indemnification agreements and could have a material adverse effect on our business or financial condition.
As of April 28, 2012, we had accrued our best estimate of potential losses relating to product liability and other claims likely to result in liability and for which it is possible to reasonably estimate a loss. This accrued amount, as well as related expenses, was not material to our financial position, results of operations or cash flows. Our method for determining estimated losses considers currently available facts, presently enacted laws and regulations and other external factors, including probable recoveries from third parties.
| Item 4. | MINE SAFETY DISCLOSURES |
32
PART II
| Item 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Patterson's common stock trades on the NASDAQ Global Select Market ® under the symbol "PDCO."
The following table sets forth the range of high and low sale prices for Patterson's common stock for each full quarterly period within the two most recent fiscal years. Such quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily represent actual transactions.
| High | Low | Dividends per share | ||||||||||
Fiscal 2012 | ||||||||||||
First Quarter | $ | 36.93 | $ | 30.43 | $ | 0.12 | ||||||
Second Quarter | $ | 32.66 | $ | 26.19 | $ | 0.12 | ||||||
Third Quarter | $ | 32.36 | $ | 27.48 | $ | 0.12 | ||||||
Fourth Quarter | $ | 34.03 | $ | 30.45 | $ | 0.14 | ||||||
| High | Low | |||||||||||
Fiscal 2011 | ||||||||||||
First Quarter | $ | 32.22 | $ | 24.13 | $ | 0.10 | ||||||
Second Quarter | $ | 29.02 | $ | 24.88 | $ | 0.10 | ||||||
Third Quarter | $ | 33.29 | $ | 27.54 | $ | 0.10 | ||||||
Fourth Quarter | $ | 34.80 | $ | 30.42 | $ | 0.12 | ||||||
On June 22, 2012, the number of holders on record of common stock was 2,494. The transfer agent for Patterson's common stock is Wells Fargo Bank, N.A., 161 North Concord Exchange, South St. Paul, Minnesota, 55075-0738, telephone: (651) 450-4064.
We did not pay any cash dividends on our common stock since its initial public offering in 1992 until the fourth quarter of fiscal 2010, at which time a $0.10 per share cash dividend was paid. In fiscal 2012 a quarterly cash dividend of $0.12 per share was paid throughout the year, except in the fourth quarter when the dividend was increased to $0.14 per share. We expect to continue to pay a quarterly cash dividend for the foreseeable future; however, the payment of dividends is within the discretion of our Board of Directors and will depend upon our earnings, capital requirements, operating results and financial condition among other factors.
For information relating to securities authorized for issuance under equity compensation plans, see Part III, Item 12.
In December 2007, Patterson's Board of Directors expanded a share repurchase program to allow for the purchase of up to 25 million shares of common stock in open market transactions. As of March 2011, approximately 20.5 million shares had been repurchased under this authorization. At that time, the Board of Directors replaced the existing share repurchase program with a new authorization to repurchase an additional 25 million shares of common stock. As of April 28, 2012, 11,136,742 shares remain available under the repurchase authorization, which expires on March 15, 2016.
33
The following table presents activity under the stock repurchase program during the fourth quarter of fiscal 2012 ended April 28, 2012.
| Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Number of Shares That May Be Purchased Under the Plan | |||||||||||||
January 29, 2012 to February 25, 2012 | - | - | 12,336,902 | |||||||||||||
February 26, 2012 to March 24, 2012 | - | - | 12,336,902 | |||||||||||||
March 25, 2012 to April 28, 2012 | 1,200,160 | $ | 32.98 | 1,200,160 | 11,136,742 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
| 1,200,160 | 32.98 | 1,200,160 | ||||||||||||||
The graph below compares the cumulative total shareholder return on $100 invested at the market close on April 28, 2006, the last trading day before the beginning of the 2007 fiscal year, through the end of fiscal 2012 with the cumulative return of the same time period on the same amount invested in the S&P 500 Index and a Peer Group Index, consisting of 9 companies (including Patterson) based on the same Standard Industrial Classification Code.* The chart below the graph sets forth the actual numbers depicted on the graph.
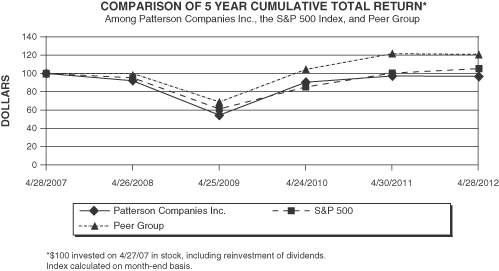
| Fiscal Year Ending | ||||||||||||||||||||||||
Company/Market/Peer Group | 4/28/07 | 4/25/08 | 4/25/09 | 4/24/10 | 4/30/11 | 4/28/12 | ||||||||||||||||||
Patterson Companies Inc. | 100.00 | 92.36 | 54.85 | 90.64 | 97.31 | 96.84 | ||||||||||||||||||
S&P 500 | 100.00 | 95.32 | 61.66 | 85.61 | 100.36 | 105.13 | ||||||||||||||||||
Peer Group | 100.00 | 100.18 | 69.22 | 104.53 | 121.67 | 120.55 | ||||||||||||||||||
| * | The current composition of SIC Code 5047-Medical, Dental & Hospital Equipment & Supplies-is as follows: |
Chindex International, Inc., Discount Dental Materials, Inc., Henry Schein, Inc., Modern Mobility Aids Inc., MWI Veterinary Supply, Inc., Owens & Minor, Inc., Patterson Companies, Inc., PSS World Medical, Inc., and Vertical Health Solutions, Inc.
34
| Item 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
| (In thousands, except per share amounts) |
| Fiscal Year Ended | ||||||||||||||||||||
| April 28, 2012 | April 30, 2011 | April 24, 2010 | April 25, 2009 | April 26, 2008 | ||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
Net sales | $ | 3,535,661 | $ | 3,415,670 | $ | 3,237,376 | $ | 3,094,227 | $ | 2,998,729 | ||||||||||
Cost of sales | 2,373,147 | 2,271,445 | 2,147,975 | 2,050,703 | 1,967,004 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Gross margin | 1,162,514 | 1,144,225 | 1,089,401 | 1,043,524 | 1,031,725 | |||||||||||||||
Operating expenses | 804,505 | 768,217 | 734,110 | 697,298 | 672,522 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Operating income | 358,009 | 376,008 | 355,291 | 346,226 | 359,203 | |||||||||||||||
Other income – net | (28,197 | ) | (20,121 | ) | (16,250 | ) | (26,575 | ) | (1,775 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Income before income taxes | 329,812 | 355,887 | 339,041 | 319,651 | 357,428 | |||||||||||||||
Income taxes | 116,997 | 130,502 | 126,787 | 120,016 | 132,570 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net Income | $ | 212,815 | $ | 225,385 | $ | 212,254 | $ | 199,635 | $ | 224,858 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Diluted earnings per share | $ | 1.92 | $ | 1.89 | $ | 1.78 | $ | 1.69 | $ | 1.69 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Weighted average shares and potentially dilutive shares outstanding | 110,846 | 119,066 | 119,202 | 118,355 | 132,910 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Dividends per common share | $ | 0.50 | $ | 0.42 | $ | 0.10 | 0 | 0 | ||||||||||||
Balance Sheet Data: | ||||||||||||||||||||
Working capital | $ | 873,865 | $ | 863,278 | $ | 785,407 | $ | 603,295 | $ | 518,974 | ||||||||||
Total assets | 2,739,368 | 2,564,968 | 2,422,969 | 2,133,620 | 2,076,373 | |||||||||||||||
Total long-term debt | 725,000 | 525,000 | 525,000 | 547,000 | 655,034 | |||||||||||||||
Stockholders' equity | 1,375,202 | 1,560,540 | 1,441,511 | 1,186,320 | 1,004,787 | |||||||||||||||
Note: See the Notes to the Consolidated Financial Statements included in Item 8. of this Annual Report on Form 10-K.
35
| Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
Our fiscal 2012 financial information is summarized in this Management's Discussion and Analysis, Consolidated Financial Statements, and related Notes. The following background is essential to more fully understand our Company's financial information.
Patterson operates a distribution business in three complementary markets: dental supply, veterinary supply and rehabilitation supply. Historically our strategy for growth focused on internal growth and the acquisition of smaller distributors and businesses offering related products and services to the dental market. In fiscal 2002, we expanded our strategy to take advantage of a parallel growth opportunity in the veterinary supply market by acquiring the assets of J. A. Webster, Inc. on July 9, 2001. Patterson added a third component to our business platform in fiscal 2004 when it entered the rehabilitation supply market with the acquisition of AbilityOne Products Corp. ("AbilityOne") on September 12, 2003. AbilityOne is now known as Patterson Medical.
Operating margins of the veterinary business are considerably lower than the dental and rehabilitation supply businesses. While operating expenses run at a lower rate in the veterinary business, their gross margin is substantially lower.
There are several important aspects of Patterson's business that are useful in analyzing it, including: (1) market growth in the various markets in which we operate; (2) internal growth; (3) growth through acquisition; and (4) continued focus on controlling costs and enhancing efficiency. Management defines "internal growth" as the increase in net sales from period to period, excluding the impact of changes in currency exchange rates, and excluding the net sales, for a period of twelve months following the transaction date, of businesses we have acquired.
There are two matters that have an overriding impact on our financial results for fiscal 2012. First, we operate with a 52-53 week accounting convention with our fiscal year ending on the last Saturday in April. Fiscal year 2011 included 53 weeks, with an additional, or fourteenth, week included in the first quarter ended July 31, 2010. Fiscal 2012 ending April 28, 2012 included 52 weeks, and the first quarter operations included thirteen weeks of activity compared to the prior year period which contained 14 weeks. It is difficult to precisely quantify the impact of the extra week, but estimates have been provided in those areas where it is possible to make reasonable approximations. We estimate that the impact of the extra week reduced sales growth by one or two percentage points in fiscal 2012 as compared to fiscal 2011.
The second matter involves the level of expense associated with our Employee Stock Ownership Plan ("ESOP"). For the past twenty years allocations of shares to employees have been made almost entirely from shares of Company stock acquired by the ESOP in 1990 ("the 1990 Shares"). Although the accounting standards in effect in 1990 were subsequently revised, the accounting for the 1990 shares was grandfathered under the revised standards and called for the expensing of the shares released for allocation to employees to be based on the original cost of the shares.
The revised standards require the expensing of shares released to be based on fair value of the shares at the time they are committed to be released and since the revision of the accounting standards, the ESOP has acquired approximately 4.5 million additional shares, nearly all of which are still held for future allocation to employees. As the final allocation of the 1990 shares was made at the end of fiscal 2011, the ESOP shares that have been subsequently acquired will be released and allocated to employees in fiscal 2012 and beyond. When these shares are committed to be released to employees, it will result in a non-cash expense equal to the average fair value of the shares committed to be released.
The ESOP expense increased our operating expenses by approximately $24 million and $0.13 per diluted share in fiscal 2012 as compared to fiscal 2011. This change from historical cost to fair value in recognizing ESOP expense creates a comparability discrepancy between our past and foreseeable future operating results.
36
The following table presents the ESOP expense reconciliation for comparability purposes:
| Three Months Ended | Twelve Months Ended | |||||||||||||||
| April 28, 2012 | April 30, 2011 | April 28, 2012 | April 30, 2011 | |||||||||||||
Net Income | $ | 62,143 | $ | 62,707 | $ | 212,815 | $ | 225,385 | ||||||||
Incremental ESOP expense | 3,496 | - | 13,867 | - | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Adjusted Net Income (non-GAAP) | $ | 65,639 | $ | 62,707 | $ | 226,682 | $ | 225,385 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Diluted Earnings Per Share | $ | 0.58 | $ | 0.53 | $ | 1.92 | $ | 1.89 | ||||||||
Incremental ESOP expense | 0.03 | - | 0.13 | - | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Adjusted Earnings Per Share (non-GAAP) | $ | 0.61 | $ | 0.53 | $ | 2.05 | $ | 1.89 | ||||||||
|
|
|
|
|
|
|
| |||||||||
In fiscal 2012, we made a cash contribution of approximately $24 million to the ESOP, which was then used by the ESOP to acquire shares through open market purchases. We instructed the ESOP trustee to hold these shares in suspense for allocation to employees at the end of the current ESOP fiscal year. Since the fiscal 2012 contribution to the ESOP was made in cash as opposed to using shares previously acquired by the ESOP, the expense for the current fiscal year will be a cash expense to us.
During fiscal 2012, we repurchased approximately 12 million shares of our common stock at a cost of approximately $361 million. Through these repurchases and the cash dividends, we returned nearly $400 million of value to our shareholders.
Results of Operations
The following table summarizes our consolidated results of operations over the past three fiscal years as a percent of sales:
| 2012 | 2011 | 2010 | ||||||||||
Net sales | 100.0 | % | 100.0 | % | 100.0 | % | ||||||
Cost of sales | 67.1 | % | 66.5 | % | 66.3 | % | ||||||
|
|
|
|
|
| |||||||
Gross margin | 32.9 | % | 33.5 | % | 33.7 | % | ||||||
Operating expenses | 22.8 | % | 22.5 | % | 22.7 | % | ||||||
|
|
|
|
|
| |||||||
Operating income | 10.1 | % | 11.0 | % | 11.0 | % | ||||||
Other income, net | 0.1 | % | 0.2 | % | 0.3 | % | ||||||
Interest expense | 0.9 | % | 0.8 | % | 0.8 | % | ||||||
|
|
|
|
|
| |||||||
Income before taxes | 9.3 | % | 10.4 | % | 10.5 | % | ||||||
Income taxes | 3.3 | % | 3.8 | % | 3.9 | % | ||||||
|
|
|
|
|
| |||||||
Net income | 6.0 | % | 6.6 | % | 6.6 | % | ||||||
|
|
|
|
|
| |||||||
Fiscal 2012 Compared to Fiscal 2011
As described in the Overview section above, the first quarter of fiscal 2011 included an extra week due to the Company's fiscal year convention. Accordingly, the fiscal year ended April 30, 2011 included 53 weeks while the fiscal year ended April 28, 2012 included 52 weeks. As noted above, we estimate that the impact of the extra week reduced sales growth by one to two percentage points in fiscal 2012 as compared to fiscal 2011.
37
Net Sales. Consolidated net sales in fiscal 2012 were $3,535.7 million, an increase of 3.5%, from $3,415.7 million in fiscal 2011. The growth in sales includes a 0.6% contribution from acquisitions and a 0.2% favorable impact of changes in foreign currency translation rates. Excluding the impact of the extra week, consolidated sales grew an estimated 5.1%.
Dental segment sales in fiscal 2012 rose 3.7% to $2,287.9 million from $2,236.1 million in fiscal 2011. The impact of currency translation rates was a favorable 0.1%. Consumable sales increased 2.6%, although they remained sluggish due to continuing weak general economic trends, low consumer confidence and high unemployment.
Dental equipment and software sales increased 5.3% in fiscal 2012 to $768.6 million. Sales of technology oriented equipment, including digital radiography and CAD/CAM products, accounted for the growth. Revenues from the sale of basic dental equipment infrastructure, primarily consisting of chairs, power units and cabinetry, continued to be soft in fiscal 2012, as practitioners focused purchases on products that they believed gave them higher returns in the near term.
Other dental sales, consisting primarily of technical service parts and labor, software support services and artificial teeth, increased 4.1% in fiscal 2012.
Webster sales grew 12.6% to $734.4 million. Sales of consumables were 10.9% higher in fiscal 2012 and equipment and software sales of $38.3 million represented an increase of 13.4% compared to fiscal 2011. Acquisitions added 1.5% to sales in fiscal 2012. Consumable sales in this segment have benefited from a higher percentage of pharmaceutical sales made under buy-sell versus agency distribution agreements, and an earlier and more severe flea and tick season. We have been investing in the Veterinary segment's equipment and technical service offering to expand this unit's full-service platform.
Patterson Medical sales of $513.3 million were 3.3% higher than fiscal 2011. Acquisitions, contributed 2.3% of sales growth. The positive impact from foreign currency translation rates was 0.6% in fiscal 2012. The capital equipment portion of this segment was negatively impacted by uncertainty in the market caused by regulatory changes in healthcare
Gross Margin. Consolidated gross margin was 32.9% in fiscal 2012 and 33.5% in fiscal 2011.
The Dental segment's gross margin decreased 30 basis points to 36.2% in fiscal 2012. This decrease is mainly due to sales mix as equipment growth outpaced consumable growth during the year.
Gross margin of the Veterinary unit was 18.3%, a decrease of 100 basis points from 19.3% in fiscal 2011. Sales mix was the primary factor in the decline as a higher percentage of revenue came from pharmaceutical sales, which have a lower margin.
Patterson Medical's gross margin declined 10 basis points to 39.0%. This was driven by a slight decrease in point of sale margin and an increase in freight rates, both domestically and internationally.
Operating Expenses . The consolidated operating expense ratio in fiscal 2012 was 22.8%, or 30 basis points higher than fiscal 2011. On a comparable basis, after adjusting for the increase in the ESOP expense discussed above, the operating expense ratio would have been 22.1%, or a decrease of nearly 40 basis points.
The Dental unit's operating expense ratio increased 110 basis points as this unit absorbs the majority of the ESOP expense. In addition, this segment was investing in training of sales personnel and deploying a new order entry system.
The ratio of the Veterinary unit's operating expenses as a percent of sales decreased 80 basis in the current year largely due to leverage of the higher sales levels.
Patterson Medical's operating expense ratio decreased 100 in fiscal 2012. The segment benefited from further integration of recent acquisitions and aggressive expense management.
38
Operating Income. Operating income was $358.0 million in fiscal 2012, compared to $376.0 million in fiscal 2011. Adjusting for the ESOP expense impact, operating income would have increased year-over-year.
Interest Expense. Interest expense was $30.3 million in fiscal 2012 compared to $25.8 million in fiscal 2011. This increase is due to the issuance of $325 million of debt in the third quarter of the current year. The Company made the decision to raise additional debt capital to take advantage of the favorable rate environment.
Other Income, net. Other income, net of other expenses, was $2.1 million in fiscal 2012 compared to $5.7 million in fiscal 2011. Interest income totaled $4.9 million in fiscal 2012, compared to $8.2 million in fiscal 2011. During fiscal 2011 the Company carried higher average balances of finance contracts while we modified agreements with our funding sources in the period.
Income Taxes. The effective income tax rate was 35.5% in fiscal 2012 as compared to 36.7% in fiscal 2011. The effective tax rate decreased in fiscal 2012 as compared to fiscal 2011 primarily due to an increase in the deductible dividends paid on shares held by our Employee Stock Ownership Plan and the release of reserves resulting from expiring statute of limitations.
Net Income and Earnings Per Share. Net income decreased 6.0% to $212.8 million in fiscal 2012 due primarily to the increase in operating expense as discussed above. Adjusting for the impact of the extra week on fiscal 2011 and the incremental ESOP expense in fiscal 2012, net income would have increased approximately 3%. Earnings per diluted share and dilutive shares outstanding were $1.92 and 110.8 million, respectively, in fiscal 2012 and $1.89 and 119.1 million, respectively, in fiscal 2011.
Fiscal 2011 Compared to Fiscal 2010
Net Sales. Consolidated net sales in fiscal 2011 were $3,415.7 million, an increase of 5.5% from $3,237.4 million in fiscal 2010. The growth in sales includes a 2.1% contribution from acquisitions and a 0.4% favorable impact of changes in foreign currency translation rates. Fiscal 2011 also included an additional or fifty-third week due to our fiscal year convention of ending on the last Saturday in April. It is difficult to quantify the exact impact of this additional week, but estimates have been provided in those areas where it is possible to make reasonable approximations. The impact of the extra week on consolidated sales is estimated to have been approximately one and a half percent.
Dental segment sales in fiscal 2011 rose 3.2% to $2,236.1 million from $2,167.5 million in fiscal 2010. Acquisitions added 0.1% to sales and the impact of currency translation rates was a favorable 0.7%. Consumable sales increased 3.2%, although they were affected by uneven patient demand for dental services as a result of the weak economy. While the economic recovery has been slow, it is believed the fundamentals of the North American dental market continued to strengthen as fiscal 2011 progressed.
Dental equipment and software sales increased 3.6% in fiscal 2011 to $734.7 million. Sales of basic dental equipment and software grew 5.4%, led by sales of digital sensors and cone beam and panoramic imaging systems. Sales of CEREC dental systems declined 2.4%.
As market fundamentals have started to improve, dentists are believed to have gradually become more confident about investing in their practices. In addition, additional marketing programs were implemented at the beginning of the fourth quarter of fiscal 2011. These two factors helped in reaching sales growth of 11.2% in the fourth quarter.
Other dental sales, consisting primarily of technical service parts and labor, software support services and artificial teeth, increased 2.0% in fiscal 2011.
Webster Veterinary sales grew 4.9% to $674.9 million. Sales of consumables were 4.0% higher in fiscal 2011 and equipment and software sales of $34.3 million represented an increase of 16.8% compared to fiscal 2010. The Veterinary equipment business has been growing at solid rates in recent quarters, and we intend to
39
continue investing in this relatively new portion of Webster's operation that has expanded the unit's full-service platform.
Patterson Medical sales of $504.7 million were 18.4% higher than fiscal 2010. Acquisitions, primarily that of the health care business of DCC plc, contributed 15.3% of sales growth. Internally generated sales growth, which excludes the contribution of acquisitions and a 0.4% negative impact from foreign currency translation rates, was 3.5% in fiscal 2011.
Gross Margin. Consolidated gross margin was 33.5% in fiscal 2011 and 33.7% in fiscal 2010.
The Dental segment's gross margin decreased 30 basis points to 36.5% in fiscal 2011. Lower vendor rebates and a higher level of promotional activities as compared to fiscal 2010 contributed to the decline.
Gross margin of the Veterinary unit was 19.3%, a decrease of 20 basis points from 19.5% in fiscal 2010. Price increases on a line of pharmaceutical products by a manufacturer and lower vendor rebates were primary factors in the decline in gross margin.
Patterson Medical's gross margin of its historical operations expanded during fiscal 2011; however, these improvements were offset by the lower gross margins of the DCC Healthcare acquisitions. For the year, gross margin declined 10 basis points to 39.1%.
Operating Expenses . The consolidated operating expense ratio in fiscal 2011 was 22.5%, or 20 basis points lower than fiscal 2010.
The Dental unit's operating expense ratio decreased 30 basis points, reflecting expense controls and leverage gained from higher sales on fixed costs.
The ratio of the Veterinary unit's operating expenses as a percent of sales decreased 80 basis points, due to leverage on higher sales and cost savings from the integration of the October, 2008 Columbus Serum acquisition. Those integration activities were completed in fiscal 2010.
Patterson Medical's operating expense ratio increased 80 basis points in fiscal 2011 due to the cost structure of the DCC acquisition. Integration of the DCC entities has progressed throughout the year and is largely complete.
Operating Income. Operating income was $376.0 million in fiscal 2011, or 5.8% higher compared to $355.3 million in fiscal 2010. Operating margin was 11.0% in fiscal years 2011 and 2010, respectively.
Interest Expense. Interest expense was $25.8 million in fiscal 2011 compared to $25.7 million in fiscal 2010.
Other Income, net. Other income, net of other expenses, was $5.7 million in fiscal 2011 compared to $9.4 million in fiscal 2010. The decrease was due to losses related to the Veterinary unit's equity interest in VetSource. Interest income totaled $8.2 million in fiscal 2011, compared to $8.6 million in fiscal 2010.
Income Taxes. The effective income tax rate was 36.7% in fiscal 2011 as compared to 37.4% in fiscal 2010. A portion of the dividends paid on shares held by our Employee Stock Ownership Plan are deductible on our income tax return. As there were more dividends paid in fiscal 2011 than in fiscal 2010, the deductible amount was larger, resulting in a lower effective income tax rate in fiscal 2011.
Net Income and Earnings Per Share. Net income increased 6.2% to $225.4 million in fiscal 2011 due primarily to the increase in operating income as discussed above. Earnings per diluted share and dilutive shares outstanding were $1.89 and 119.1, respectively, in fiscal 2011 and $1.78 and 119.2 million, respectively, in fiscal 2010.
40
Liquidity and Capital Resources
Patterson's operating cash flow has been our principal source of liquidity in the last three fiscal years. During fiscal 2012, we used our revolving credit facility periodically as a source of liquidity in addition to operating cash flow.
Operating activities generated cash of $321.2 million in fiscal 2012, compared to $262.6 million in fiscal 2011 and $265.5 million in fiscal 2010. In fiscal 2012, 2011 and 2010, we invested in financing programs to support marketing efforts directed at the equipment product lines, particularly in the Dental segment.
Capital expenditures were $29.7, $36.9 and $29.8 million in fiscal years 2012, 2011 and 2010, respectively. Significant expenditures in these years included the purchase and expansion of distribution facilities to accommodate multiple business units, the construction of a new facility for the Patterson Technology Center, the expansion of the general office building and continuing investments in information systems. In fiscal 2012, a project to build-out a purchased building in Indiana was completed, the building serves as a distribution facility used by all three business units. This facility is replacing several smaller distribution facilities. In addition, the Patterson Technology Center facility in Illinois was completed in fiscal 2012. This 100,000 square foot state-of-the-art facility replaced a nearby leased location and opened in the second quarter of fiscal 2012.
We expect to invest approximately $23 million in capital expenditures during fiscal 2013, our main investment is in information systems.
Cash used for acquisitions and equity investments totaled $22.6 million in fiscal 2012, $52.2 million in fiscal 2011 and $53.7 million in fiscal 2010. The majority of the cash used for acquisitions in fiscal 2012 related to the acquisitions of AVSC and Surgical Synergies. The Medical segment's acquisition of the healthcare assets of DCC plc. accounted for the majority of the cash used in fiscal 2011, while the acquisition of the rehabilitation business of Empi Therapy Solutions and the investment in Strategic Pharm-dba VetSource accounted for the majority of the cash used in fiscal 2010.
In fiscal 2012 we entered in to a new debt agreement for $325 million; see Note 7 of the Consolidated Financial Statements, "Long-term Debt." footnote for further information. There were neither issuances of, nor payments on, debt during fiscal 2011. In fiscal 2010, Patterson fully paid the $22 million that was outstanding under a revolving credit facility at the end of fiscal 2009. A maximum of $300 million is available under this facility, which expires in fiscal 2013.
In the fourth quarter of fiscal 2010, we declared and paid an initial quarterly cash dividend of $0.10 per share, which was increased to $0.12 per share in the fourth quarter of fiscal 2011 and the dividend continued at this rate through the third quarter of fiscal 2012. The dividend was increased to $0.14 per share in the fourth quarter of fiscal 2012. Total dividends paid in fiscal 2012 and fiscal 2011 were $54.7 million and $50.0 million, respectively. We expect to continue to pay a quarterly cash dividend for the foreseeable future. In addition, during fiscal 2012 we repurchased approximately 12.0 million shares of common stock for approximately $361 million. In fiscal 2011 we repurchased approximately 3.3 million shares of common stock for approximately $99 million. Under a share repurchase plan authorized by the board of directors, as of April 28, 2012, Patterson may repurchase up to an additional 11 million shares of its common stock. This authorization remains in effect through March 15, 2016.
Management expects funds generated from operations and existing cash to be sufficient to meet our working capital needs for the next fiscal year. We have approximately $189 million in foreign bank accounts. None of which are subject to any withdrawal restrictions. See Note 11, "Income Taxes" for further information regarding our intention to permanently reinvest these funds. We expect to continue to obtain liquidity from the sale of equipment finance contracts. Patterson's existing debt facilities are believed to be adequate as a supplement to internally generated cash flows to fund anticipated expansion plans and strategic initiatives, including acquisitions.
41
Patterson sells a significant portion of our finance contracts to a commercial paper funded conduit managed by a third party bank, and as a result, commercial paper is indirectly an important source of liquidity for Patterson. Patterson is allowed to participate in the conduit due to the quality of our finance contracts and our financial strength. Cash flow could be impaired if our financial strength diminishes to a level that precluded us from taking part in this facility or other similar facilities. Also, market conditions outside of our control could adversely affect the ability for us to sell the contracts.
Customer Financing Arrangements
Patterson is a party to two arrangements under which we have sold finance contracts received from our customers to outside financial institutions. These arrangements provide sources of liquidity for us that would have to be replaced should any of the current financial institutions be unable or unwilling to continue under them.
In December 2010, the Receivables Purchase Agreement was amended to make The Bank of Tokyo-Mitsubishi UFJ, Ltd. ("BTMU") the managing agent. As of April 28, 2012, the capacity under this agreement is $500 million, $300 million with BTMU and the remainder with Royal Bank of Canada [RBC]. In August 2011, Fifth Third Bank [FTB] replaced U.S. Bank National Association as the agent under the Contract Purchase Agreement, which has a capacity of $75 million as of April 28, 2012. Our financing business is described in further detail in Note 6 of the Notes to the Consolidated Financial Statements in Item 8 of this Form 10-K. Note 6, "Customer Financing", discusses the nature and business purpose of the arrangements and the activity under each arrangement during fiscal 2012, including the amount of finance contracts sold and the holdback receivable owed to us.
Contractual Obligations
A summary of Patterson's contractual obligations as of April 28, 2012 follows (in thousands):
| Payment due by year | ||||||||||||||||||||
Contractual Obligations | Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
Long-Term Debt | $ | 850,000 | $ | 125,000 | $ | 250,000 | 000,000 | 475,000 | ||||||||||||
Interest on Long-Term Debt | 210,639 | 36,592 | 65,967 | 40,117 | 67,963 | |||||||||||||||
Operating Leases | 71,119 | 18,392 | 25,392 | 16,511 | 10,824 | |||||||||||||||
Patterson is unable to determine its contractual obligations by year related to the provisions of ASC Topic 740, "Income Taxes", as the ultimate amount or timing of settlement of its reserves for income taxes cannot be reasonably estimated. The total liability for unrecognized tax benefits including interest and penalties at April 28, 2012, is $20.7 million.
For a more complete description of Patterson's contractual obligations, see Notes 7 and 10 to the Consolidated Financial Statements in Item 8 of this Form 10-K.
Outlook
Over the last ten years, we have been able to grow revenue and earnings through our strategy of emphasizing value-added, full-service capabilities, using technology to enhance customer service, continuing to improve operating efficiencies, and growing through internal expansion and acquisitions. While the weakness in the general economy that has existed during much of fiscal 2012 and 2011 is expected to continue to affect our performance for at least the near term, Patterson's strategy will continue to focus on these key elements. With strong operating cash flow and available credit capacity, we are confident that we will be able to financially support our future growth. We believe that the strategic initiatives that we have implemented in the past several years, as well as those that will be implemented in fiscal 2013 and beyond, will strengthen our operational
42
platform and contribute to future growth. Given these factors, we consider ourself well positioned to capitalize upon the growth opportunities in the dental supply, companion-animal veterinary supply and the worldwide rehabilitation supply markets.
Asset Management
The following table summarizes Patterson's days sales outstanding (DSO) and inventory turnover the past three fiscal years:
| 2012 | 2011 | 2010 | ||||||||||
Days sales outstanding (1) | 45 | 48 | 51 | |||||||||
Inventory turnover ( 2 ) | 6.7 | 6.9 | 7.1 | |||||||||
| (1) | Receivables as of April 28, 2012, April 30, 2011 and April 24, 2010 include approximately $20 million, $19 million and $63 million, respectively, of finance contracts received from customers related to certain financing promotions in fiscal 2012, 2011 and 2010. Patterson has sold contracts in fiscal 2012 and expects to sell the contracts held as of April 28, 2012 to outside institutions under an existing agreement during fiscal 2013. If these finance contracts are excluded from the calculation of DSO, the pro forma DSO would be 43, 46 and 44 as of April 28, 2012, April 30, 2011 and April 24, 2010. |
| (2) | The inventory values used in this calculation are the LIFO inventory values for all inventories except for manufactured inventories and foreign inventories, which are valued using FIFO inventory methods. |
Foreign Operations
Foreign sales derive primarily from Patterson Dental and Patterson Medical operations in Canada and from Patterson Medical operations in the U.K. and France. Fluctuations in currency exchange rates have not significantly impacted earnings. However, changes in exchange rates enhanced net sales in fiscal 2012 and 2011, and adversely affected net sales in fiscal 2010. Without foreign currency effects, net sales would have been $6.1 million lower, $12.4 million lower, and $11.5 million higher in fiscal years 2012, 2011 and 2010, respectively. Changes in currency exchange rates are a risk accompanying foreign operations, but this risk is not considered material with respect to our consolidated operations.
Critical Accounting Policies and Estimates
Patterson has adopted various accounting policies to prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States. Management believes that our policies are conservative and our philosophy is to adopt accounting policies that minimize the risk of adverse events having a material impact on recorded assets and liabilities. However, the preparation of financial statements requires the use of estimates and judgments regarding the realization of assets and the settlement of liabilities based on the information available to management at the time. Changes subsequent to the preparation of the financial statements in economic, technological and competitive conditions may materially impact the recorded values of Patterson's assets and liabilities. Therefore, the users of the financial statements should read all the notes to the Consolidated Financial Statements and be aware that conditions currently unknown to management may develop in the future. This may require a material adjustment to a recorded asset or liability to consistently apply to our significant accounting principles and policies that are discussed in Note 1 to the Consolidated Financial Statements. The financial performance and condition of Patterson may also be materially impacted by transactions and events that we have not previously experienced and for which we have not been required to establish an accounting policy or adopt a generally accepted accounting principle.
Revenue Recognition -Revenues are generated from the sale of consumable products, equipment, software products and services, technical service parts and labor, freight and delivery charges, and other sources. Revenues are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed and final, and there is reasonable assurance of collection of the sale. Estimates for returns, damaged goods, rebates, loyalty programs and other revenue allowances are made at the time the revenue is recognized based on the historical experience for such items.
43
Consumable product sales are recorded upon delivery, except in those circumstances where terms of the sale are FOB shipping point.
Equipment and software product revenues are recognized upon delivery and, if necessary, installation. In those circumstances where terms of the sale are FOB shipping point, revenues are recognized when products are transferred to the shipping carrier. Revenue derived from post contract customer support for software is deferred and recognized ratably over the period in which the support is provided.
Revenue for arrangements with multiple deliverables meeting the criteria for a separate unit of accounting use the relative fair value method and revenue is recognized for each deliverable in accordance with applicable revenue recognition criteria.
Other revenue including freight and delivery charges and technical service parts and labor is recognized when the related product revenue is recognized or when the product or services are provided to the customer.
Patterson Advantage Loyalty Program- Patterson Dental provides a point-based awards program to qualifying customers involving the issuance of "Patterson Advantage dollars" which can be used toward equipment and technology purchases. The program was initiated on January 1, 2009 and runs on a calendar year schedule. Patterson Advantage dollars earned during a program year expire one year after the end of the program year. The cost and corresponding liability associated with the program is recognized as contra-revenue in accordance with ASC Topic 605-50, "Revenue Recognition-Customer Payments and Incentives." As of April 28, 2012, we believe we have sufficient experience with the program to reasonably estimate the amount of Patterson Advantage dollars that will not be redeemed, thus have recorded a liability for 87% of the maximum potential amount that could be redeemed. We use the redemption recognition method and we recognize the estimated value of unused–Advantage dollars as a percentage of Patterson Advantage as redemptions occur. Breakage recognized was immaterial to all periods presented.
Inventory and Reserves -Inventory consists primarily of merchandise held for sale and is stated at the lower of cost or market. Cost is determined using the last-in, first-out (LIFO) method for all inventories, except for foreign inventories and manufactured inventories, which are valued using the first-in, first-out (FIFO) method. We continually assess the valuation of inventories and reduce the carrying value of those inventories that are obsolete or in excess of forecasted usage to estimated realizable value. Estimates are made of the net realizable value of such inventories based on analyses and assumptions including, but not limited to, historical usage, future demand and market requirements.
Goodwill and Other Indefinite-Lived Intangible Assets- Goodwill represents the excess of cost over the fair value of identifiable net assets of businesses acquired. Other indefinite-lived intangible assets include copyrights, trade names and trademarks.
Goodwill for each reporting unit is evaluated using a two-step impairment test at the reporting unit level. Patterson has three reporting units at April 28, 2012, which are the same as our operating units. The first step of the goodwill impairment test compares the book value of a reporting unit, including goodwill, with its fair value, as determined by its discounted cash flows. If the book value of a reporting unit exceeds its fair value, the second step of the impairment test is performed to determine the amount of goodwill impairment loss to be recorded. The determination of fair value involves uncertainties because it requires management to make assumptions and to apply judgment to estimate industry and economic factors and the profitability of future business strategies. Patterson conducts impairment testing based on current business strategy in light of present industry and economic conditions, as well as future expectations. Additionally, in assessing goodwill for impairment, the reasonableness of the implied control premium is considered based on market capitalizations and recent market transactions.
Other indefinite-lived intangible assets are assessed for impairment by comparing the carrying value of an asset with its fair value. If the carrying value exceeds fair value, an impairment loss is recognized in an amount equal to the excess. The determination of fair value involves assumptions, including projected revenues and gross profit levels, as well as consideration of any factors that may indicate potential impairment.
44
In the fourth quarter of fiscal 2012, management completed its annual goodwill and other indefinite-lived intangible asset impairment tests and determined there was no impairment. Although we believe estimates and assumptions used in estimating cash flows and determining fair value are reasonable, making material changes to such estimates and assumptions could materially affect such impairment analyses and financial results, including an impairment charge that could be material.
Long-Lived Assets -Long-lived assets, including definite-lived intangible assets, are evaluated for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable through the estimated undiscounted future cash flows derived from such assets. Our definite-lived intangible assets primarily consist of an exclusive distribution agreement and customer lists. When impairment exists, the related assets are written down to fair value.
Income Taxes- We are subject to income taxes in both the U.S. and numerous foreign jurisdictions. Significant judgments are required in determining the consolidated provision for income taxes.
During the ordinary course of business, there are many transactions and calculations for which the ultimate tax determination is uncertain. As a result, we recognize tax liabilities based on estimates of whether additional taxes and interest will be due. These tax liabilities are recognized when, despite our belief, our tax return positions are supportable; we believe that certain positions may not be fully sustained upon review by tax authorities. We believe that our accruals for tax liabilities are adequate for all open audit years based on our assessment of many factors including past experience and interpretations of tax law. This assessment relies on estimates and assumptions and may involve a series of complex judgments about future events. To the extent that the final tax outcome of these matters is different than the amounts recorded, such differences will impact income tax expense in the period in which such determination is made and could materially affect our financial results.
To the extent that the provision for income taxes would have increased/decreased by 1 percent of income before taxes, consolidated net income would have decreased/increased $3.3 million in fiscal 2012.
Valuation allowances are established for deferred tax assets if, after assessment of available positive and negative evidence, it is more likely than not that the deferred tax asset will not be fully realized. The valuation allowance reflected in the footnote disclosure relates to net operating loss carryforwards of Mobilis Healthcare Group, acquired during Fiscal 2009 and principally based in the United Kingdom.
Self-insurance -Patterson is self-insured for certain losses related to general liability, product liability, automobile, workers' compensation and medical claims. We estimate our liabilities based upon an analysis of historical data and actuarial estimates. While current estimates are believed reasonable based on information currently available, actual results could differ and affect financial results due to changes in the amount or frequency of claims, medical cost inflation or other factors. Historically, actual results related to these types of claims have not varied significantly from estimated amounts.
Stock-based Compensation -We recognize stock-based compensation based on certain assumptions including inputs within the Black-Scholes Model and estimated forfeitures. These assumptions require subjective judgment and changes in the assumptions can materially affect fair value estimates. Management assesses the assumptions and methodologies used to estimate forfeitures and to calculate estimated fair value of stock-based compensation on a regular basis. Circumstances may change, and additional data may become available over time, which could result in changes to these assumptions and methodologies and thereby materially impact the fair value determination or estimates of forfeitures. If factors change and we employ different assumptions, the amount of compensation expense associated with stock-based compensation may differ significantly from what was recorded in the current period.
45
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Market Risk
We are exposed to market risk consisting of foreign currency rate fluctuations and changes in interest rates.
Patterson is exposed to foreign currency exchange rate fluctuations in its operating statement due to transactions denominated primarily in Canadian Dollars, British Pounds, Euros, Australian Dollars and New Zealand Dollars. Although Patterson is not currently involved with foreign currency hedge contracts, it continually evaluates our foreign currency exchange rate risk and the different mechanisms for use in managing such risk. A hypothetical 10% change in the value of the U.S. dollar in relation to our most significant foreign currency exposures would have reduced fiscal 2012 net sales by approximately $30 million . This amount is not indicative of the hypothetical net earnings impact due to the partially offsetting impact of the currency exchange movements on cost of sales and operating expenses.
Patterson's earnings are also affected by fluctuations in short-term interest rates through the investment of cash balances, borrowings under LIBOR-based debt instruments and the practice of selling fixed rate equipment finance contracts under agreements with both a commercial paper conduit and a group of banks that provide for pricing based on variable interest rates.
As of April 28, 2012, we had a $75 million variable-rate term note outstanding. We view our variable interest rate debt position on a net basis that gives effect to our cash and cash equivalents balances.
When considering the exposure under the agreements whereby Patterson sells equipment finance contracts to both a commercial paper conduit and a group of banks, Patterson has the ability to select pricing based on interest rates ranging from 30 day LIBOR up to twelve month LIBOR. In addition, the majority of the portfolio of installment contracts generally turns over in less than 48 months and Patterson can adjust the rate we charge on new customer contracts at any time. Therefore, in times where the interest rate markets are not rapidly increasing or decreasing, the average interest rate in the portfolio generally moves with the interest rate markets and thus would parallel the underlying interest rate movement of the pricing built into the sale agreements. In calculating the gain on the contract sales, we use an interest rate curve that approximates the maturity period of the then-outstanding contracts. If increases in the interest rate markets occur, the average interest rate in our contract portfolio may not increase at the same rate, resulting in a reduction of gain on the contracts sales as compared to the gain that would be realized if the average interest rate in our portfolio were to increase at a more similar rate to the interest rate markets.
Patterson estimates that if interest rates had been 10% higher during the year, the annual impact would have been to reduce earnings before income tax by less than $1 million.
46
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Patterson Companies, Inc.
We have audited Patterson Companies, Inc. internal control over financial reporting as of April 28, 2012, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Patterson Companies, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control Over Financial Reporting appearing in Item 9A, Controls and Procedures, of this Annual Report on Form 10-K. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Patterson Companies, Inc. maintained, in all material respects, effective internal control over financial reporting as of April 28, 2012, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Patterson Companies, Inc. as of April 28, 2012 and April 30, 2011, and the related consolidated statements of income and other comprehensive income, changes in stockholders' equity, and cash flows for each of the three fiscal years in the period ended April 28, 2012, and our report dated June 27, 2012, expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Minneapolis, Minnesota
June 27, 2012
47
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Patterson Companies, Inc.
We have audited the accompanying consolidated balance sheets of Patterson Companies, Inc. as of April 28, 2012 and April 30, 2011, and the related consolidated statements of income and other comprehensive income, changes in stockholders' equity, and cash flows for each of the three fiscal years in the period ended April 28, 2012. Our audits also included the financial statement schedule listed in Item 15(a)(2). These financial statements and the schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Patterson Companies, Inc. at April 28, 2012 and April 30, 2011, and the consolidated results of their operations and their cash flows for each of the three fiscal years in the period ended April 28, 2012, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Patterson Companies, Inc.'s internal control over financial reporting as of April 28, 2012, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated June 27, 2012, expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Minneapolis, Minnesota
June 27, 2012
48
PATTERSON COMPANIES, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share amounts)
| April 28, 2012 | April 30, 2011 | |||||||
ASSETS | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 573,781 | $ | 388,665 | ||||
Receivables, net of allowance for doubtful accounts of $7,831 and $8,365 at April 28, 2012 and April 30, 2011, respectively | 464,869 | 465,170 | ||||||
Inventory | 319,952 | 336,094 | ||||||
Prepaid expenses and other current assets | 44,911 | 40,780 | ||||||
|
|
|
| |||||
Total current assets | 1,403,513 | 1,230,709 | ||||||
Property and equipment, net | 195,465 | 189,583 | ||||||
Long-term receivables, net | 92,049 | 90,285 | ||||||
Goodwill | 810,252 | 795,616 | ||||||
Identifiable intangibles, net | 212,557 | 227,216 | ||||||
Other | 25,532 | 31,559 | ||||||
|
|
|
| |||||
Total assets | $ | 2,739,368 | $ | 2,564,968 | ||||
|
|
|
| |||||
LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 207,915 | $ | 210,033 | ||||
Accrued payroll expense | 66,386 | 56,575 | ||||||
Other accrued expense | 130,347 | 100,823 | ||||||
Current maturities of long-term debt | 125,000 | - | ||||||
|
|
|
| |||||
Total current liabilities | 529,648 | 367,431 | ||||||
Long-term debt | 725,000 | 525,000 | ||||||
Deferred income taxes | 81,856 | 78,239 | ||||||
Other | 27,662 | 33,758 | ||||||
|
|
|
| |||||
Total liabilities | 1,364,166 | 1,004,428 | ||||||
Stockholders' equity: | ||||||||
Common Stock, $.01 par value: | ||||||||
Authorized shares-600,000 | ||||||||
Issued and outstanding shares-109,920 and 121,100 at April 28, 2012, and April 30, 2011, respectively | 1,099 | 1,211 | ||||||
Additional paid-in capital | 0 | 0 | ||||||
Accumulated other comprehensive income (loss) | 32,455 | 41,950 | ||||||
Retained earnings | 1,456,233 | 1,632,497 | ||||||
Unearned ESOP shares | (114,585 | ) | (115,118 | ) | ||||
|
|
|
| |||||
Total stockholders' equity | 1,375,202 | 1,560,540 | ||||||
|
|
|
| |||||
Total liabilities and stockholders' equity | $ | 2,739,368 | $ | 2,564,968 | ||||
|
|
|
| |||||
See accompanying notes
49
PATTERSON COMPANIES, INC.
CONSOLIDATED STATEMENTS OF INCOME
AND OTHER COMPREHENSIVE INCOME
(In thousands, except per share amounts)
| Twelve Months Ended | ||||||||||||
| April 28, 2012 | April 30, 2011 | April 24, 2010 | ||||||||||
Net sales | $ | 3,535,661 | $ | 3,415,670 | $ | 3,237,376 | ||||||
Cost of sales | 2,373,147 | 2,271,445 | 2,147,975 | |||||||||
|
|
|
|
|
| |||||||
Gross profit | 1,162,514 | 1,144,225 | 1,089,401 | |||||||||
Operating expenses | 804,505 | 768,217 | 734,110 | |||||||||
|
|
|
|
|
| |||||||
Operating income | 358,009 | 376,008 | 355,291 | |||||||||
Other income and expense: | ||||||||||||
Other income, net | 2,146 | 5,719 | 9,444 | |||||||||
Interest expense | (30,343 | ) | (25,840 | ) | (25,694 | ) | ||||||
|
|
|
|
|
| |||||||
Income before taxes | 329,812 | 355,887 | 339,041 | |||||||||
Income taxes | 116,997 | 130,502 | 126,787 | |||||||||
|
|
|
|
|
| |||||||
Net income | $ | 212,815 | $ | 225,385 | $ | 212,254 | ||||||
|
|
|
|
|
| |||||||
Earnings per share: | ||||||||||||
Basic | $ | 1.93 | $ | 1.91 | $ | 1.79 | ||||||
|
|
|
|
|
| |||||||
Diluted | $ | 1.92 | $ | 1.89 | $ | 1.78 | ||||||
|
|
|
|
|
| |||||||
Shares: | ||||||||||||
Basic | 110,121 | 118,290 | 118,443 | |||||||||
|
|
|
|
|
| |||||||
Diluted | 110,846 | 119,066 | 119,202 | |||||||||
|
|
|
|
|
| |||||||
Dividends declared per common share | $ | 0.50 | $ | 0.42 | $ | 0.10 | ||||||
|
|
|
|
|
| |||||||
Comprehensive Income | ||||||||||||
Net Income | 212,815 | 225,385 | 212,254 | |||||||||
Foreign currency translation (loss) gain | (9,372 | ) | 18,782 | 32,281 | ||||||||
Cash flow hedge | (123 | ) | (123 | ) | (123 | ) | ||||||
|
|
|
|
|
| |||||||
Comprehensive Income | 203,320 | 244,044 | 244,412 | |||||||||
|
|
|
|
|
| |||||||
See accompanying notes
50
PATTERSON COMPANIES, INC.
CONSOLIDATED STATEMENTS OF
CHANGES IN STOCKHOLDERS' EQUITY
(Dollars in thousands)
| Common Stock | Additional Paid-in Capital | Accumulated Other Comprehensive (Loss) Income | Retained Earnings | Unearned ESOP Shares | ||||||||||||||||||||||||
| Number | Amount | Total | ||||||||||||||||||||||||||
Balance at April 25, 2009 | 122,042,467 | $ | 1,220 | $ | 20,320 | $ | (8,867 | ) | $ | 1,293,609 | $ | (119,962 | ) | $ | 1,186,320 | |||||||||||||
Foreign currency translation | - | - | - | 32,281 | - | - | 32,281 | |||||||||||||||||||||
Cash flow hedge | - | - | - | (123 | ) | - | - | (123 | ) | |||||||||||||||||||
Net income | - | - | - | - | 212,254 | 212,254 | ||||||||||||||||||||||
|
| |||||||||||||||||||||||||||
Comprehensive income | 244,412 | |||||||||||||||||||||||||||
Dividends declared | - | - | - | - | (11,978 | ) | - | (11,978 | ) | |||||||||||||||||||
Common stock issued and related tax benefits | 1,394,599 | 14 | 12,557 | - | - | - | 12,571 | |||||||||||||||||||||
Stock-based compensation | - | - | 8,826 | - | - | - | 8,826 | |||||||||||||||||||||
ESOP activity | - | - | - | - | - | 1,360 | 1,360 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance at April 24, 2010 | 123,437,066 | $ | 1,234 | $ | 41,703 | $ | 23,291 | $ | 1,493,885 | $ | (118,602 | ) | $ | 1,441,511 | ||||||||||||||
Foreign currency translation | - | - | - | 18,782 | - | - | 18,782 | |||||||||||||||||||||
Cash flow hedge | - | - | - | (123 | ) | - | - | (123 | ) | |||||||||||||||||||
Net income | - | - | - | - | 225,385 | 225,385 | ||||||||||||||||||||||
|
| |||||||||||||||||||||||||||
Comprehensive income | 244,044 | |||||||||||||||||||||||||||
Dividends declared | - | - | - | - | (50,022 | ) | - | (50,022 | ) | |||||||||||||||||||
Common stock issued and related tax benefits | 920,158 | 9 | 9,993 | - | - | - | 10,002 | |||||||||||||||||||||
Repurchase of Common Stock | (3,257,374 | ) | (32 | ) | (62,177 | ) | - | (36,751 | ) | - | (98,960 | ) | ||||||||||||||||
Stock-based Compensation | - | - | 10,481 | - | - | - | 10,481 | |||||||||||||||||||||
ESOP activity | - | - | - | - | - | 3,484 | 3,484 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance at April 30, 2011 | 121,099,850 | $ | 1,211 | $ | 0 | $ | 41,950 | $ | 1,632,497 | $ | (115,118 | ) | $ | 1,560,540 | ||||||||||||||
Foreign currency translation | - | - | - | (9,372 | ) | - | - | (9,372 | ) | |||||||||||||||||||
Cash flow hedge | - | - | - | (123 | ) | - | - | (123 | ) | |||||||||||||||||||
Net income | - | - | - | - | 212,815 | 212,815 | ||||||||||||||||||||||
|
| |||||||||||||||||||||||||||
Comprehensive income | 203,320 | |||||||||||||||||||||||||||
Dividends declared | - | - | - | - | (55,319 | ) | - | (55,319 | ) | |||||||||||||||||||
Common stock issued and related tax benefits | 778,856 | 8 | 27,167 | - | - | - | 27,175 | |||||||||||||||||||||
Repurchase of Common Stock | (11,954,257 | ) | (120 | ) | (27,167 | ) | - | (333,760 | ) | - | (361,047 | ) | ||||||||||||||||
Stock-based Compensation | - | - | - | - | ||||||||||||||||||||||||
ESOP activity | 533 | 533 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance at April 28, 2012 | 109,924,449 | $ | 1,099 | $ | 0 | $ | 32,455 | $ | 1,456,233 | $ | (114,585 | ) | $ | 1,375,202 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
See accompanying notes
51
PATTERSON COMPANIES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Dollars in thousands)
| Fiscal Year Ended | ||||||||||||
| April 28, 2012 | April 30, 2011 | April 24, 2010 | ||||||||||
Operating activities: | ||||||||||||
Net income | $ | 212,815 | $ | 225,385 | $ | 212,254 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation | 25,254 | 24,613 | 25,692 | |||||||||
Amortization | 16,955 | 16,726 | 13,782 | |||||||||
Bad debt expense | 2,445 | 3,409 | 4,579 | |||||||||
Stock-based compensation | 12,615 | 10,481 | 8,826 | |||||||||
Excess tax benefits from stock-based compensation | (1,371 | ) | (1,276 | ) | (387 | ) | ||||||
ESOP compensation | 756 | 2,113 | 1,726 | |||||||||
Deferred income taxes | 89 | 17,170 | 251 | |||||||||
Change in assets and liabilities net of acquired: | ||||||||||||
Decrease in receivables | 468 | 3,784 | 24,185 | |||||||||
Decrease (increase) in inventory | 16,859 | (26,639 | ) | (12,090 | ) | |||||||
(Decrease) increase in accounts payable | (6,847 | ) | 9,664 | 9,215 | ||||||||
Increase (Decrease) in accrued liabilities | 37,948 | (5,754 | ) | 3,680 | ||||||||
Increase in long-term receivables | (1,766 | ) | (15,212 | ) | (23,501 | ) | ||||||
Other changes from operating activities, net | 4,938 | (1,852 | ) | (2,727 | ) | |||||||
|
|
|
|
|
| |||||||
Net cash provided by operating activities | 321,158 | 262,612 | 265,485 | |||||||||
Investing activities: | ||||||||||||
Additions to property and equipment, net of acquisitions | (29,650 | ) | (36,822 | ) | (29,804 | ) | ||||||
Acquisitions and equity investments, net of cash | (22,620 | ) | (52,187 | ) | (53,672 | ) | ||||||
|
|
|
|
|
| |||||||
Net cash used in investing activities | (52,270 | ) | (89,009 | ) | (83,476 | ) | ||||||
Financing activities: | ||||||||||||
Dividends paid | (54,741 | ) | (49,992 | ) | (11,886 | ) | ||||||
Payments on revolver | 0 | 0 | (22,000 | ) | ||||||||
Repurchases of common stock | (362,379 | ) | (97,153 | ) | 0 | |||||||
Other | (931 | ) | 707 | 1,360 | ||||||||
Common stock issued, net | 13,621 | 11,940 | 12,184 | |||||||||
Proceeds from issuance of long-term debt Excess tax benefits from stock-based compensation |
| 325,000 1,371 |
|
| 0 1,276 |
|
| 0 387 |
| |||
|
|
|
|
|
| |||||||
Net cash used in financing activities | (78,059 | ) | (133,222 | ) | (19,955 | ) | ||||||
Effect of exchange rate changes on cash | (5,713 | ) | 7,693 | 20,472 | ||||||||
|
|
|
|
|
| |||||||
Net increase in cash and cash equivalents | 185,116 | 48,074 | 182,526 | |||||||||
Cash and cash equivalents at beginning of period | 388,665 | 340,591 | 158,065 | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents at end of period | $ | 573,781 | $ | 388,665 | $ | 340,591 | ||||||
|
|
|
|
|
| |||||||
Supplemental disclosures: | ||||||||||||
Income taxes paid | $ | 81,959 | $ | 112,840 | $ | 139,504 | ||||||
Interest paid | 24,868 | 24,703 | 25,628 | |||||||||
Repurchases of common stock with liability due to broker | 475 | 1,807 | - | |||||||||
See accompanying notes
52
PATTERSON COMPANIES, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
APRIL 28, 2012
(Dollars in thousands, except share and per share amounts)
1. Summary of Significant Accounting Policies
Description of Business
Patterson Companies, Inc., (referred to herein as "Patterson" or in the first person notations "we," "our," and "us") is a value-added distributor serving the dental, companion-animal veterinarian and rehabilitation supply markets. Patterson Companies has three reportable segments: dental supply, veterinary supply and rehabilitation supply.
Basis of Presentation
The consolidated financial statements include the accounts of our wholly owned subsidiaries. Significant inter-company transactions and balances have been eliminated in consolidation. The respective assets of PDC Funding Company, LLC and PDC Funding Company II, LLC, would be available first and foremost to satisfy the claims of their respective creditors. There are no known creditors of PDC Funding Company, LLC or PDC Funding Company II, LLC.
Fiscal Year End
We utilize a fifty-two, fifty-three week fiscal year ending on the last Saturday in April. Accordingly, fiscal year 2011 included fifty-three weeks and fiscal years 2012 and 2010 included fifty-two weeks.
Use of Estimates in the Preparation of Financial Statements
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash equivalents consist primarily of investments in money market funds and government securities. The maturity of these securities at the time of purchase is 90 days or less. All cash and cash equivalents are classified as available-for-sale and carried at fair value, which approximates cost.
Inventory
Inventory consists of merchandise held for sale and is stated at the lower of cost or market. Cost is determined using the last-in, first-out (LIFO) method for all inventories, except for foreign inventories and manufactured inventories, which are valued using the first-in, first-out (FIFO) method. Inventories valued at LIFO represent 81% and 82% of total inventories at April 28, 2012 and April 30, 2011, respectively.
The accumulated LIFO reserve was $66,808 at April 28, 2012 and $61,385 at April 30, 2011. We believe that inventory replacement cost exceeds the inventory balance by an amount approximating the LIFO reserve.
Property and Equipment
Property and equipment are stated at cost. Our Company calculates depreciation on the straight-line method over estimated useful lives of up to 39 years for buildings or the expected remaining life of purchased buildings, the term of the lease for leasehold improvements, 3 years for laptops, 5 years for data processing equipment, and 5 to 10 years for office furniture and equipment.
53
Goodwill and Other Indefinite-Lived Intangible Assets
Goodwill represents the excess of cost over the fair value of identifiable net assets of businesses acquired. Other indefinite-lived intangible assets include copyrights, trade names and trademarks.
Goodwill for each reporting unit is evaluated using a two-step impairment test at the reporting unit level. We have three reporting units as of April 28, 2012, which are the same as our operating units. The first step of the goodwill impairment test compares the book value of a reporting unit, including goodwill, with its fair value, as determined by its discounted cash flows. If the book value of a reporting unit exceeds its fair value, the second step of the impairment test is performed to determine the amount of goodwill impairment loss to be recorded. The determination of fair value involves uncertainties because it requires management to make assumptions and to apply judgment to estimate industry and economic factors and the profitability of future business strategies. We conduct impairment testing based on our current business strategy in light of present industry and economic conditions, as well as future expectations. Additionally, in assessing goodwill for impairment, we consider the reasonableness of the implied control premium based on market capitalizations and recent market transactions.
We assess other indefinite-lived intangible assets for impairment by comparing the carrying value of an asset with its fair value. If the carrying value exceeds fair value, an impairment loss is recognized in an amount equal to the excess. The determination of fair value involves assumptions, including projected revenues and gross profit levels, as well as consideration of any factors that may indicate potential impairment.
In the fourth quarter of fiscal 2012, management completed its annual goodwill and other indefinite-lived intangible asset impairment tests and determined there was no impairment. Although we believe our estimates and assumptions used in estimating cash flows and determining fair value are reasonable, making material changes to such estimates and assumptions could materially affect such impairment analyses and our financial results, including an impairment charge that could be material.
Long-Lived Assets
Long-lived assets, including definite-lived intangible assets, are evaluated for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable through the estimated undiscounted future cash flows derived from such assets. Our definite-lived intangible assets primarily consist of an exclusive distribution agreement and customer lists. When impairment exists, the related assets are written down to fair value.
Financial Instruments
We account for derivative financial instruments under the provisions of ASC Topic 815, "Derivatives and Hedging." Our use of derivative financial instruments is generally limited to managing well-defined interest rate risks. Patterson does not use financial instruments or derivatives for any trading purposes.
Revenue Recognition
Revenues are generated from the sale of consumable products, equipment, software products and services, technical service parts and labor, freight and delivery charges, and other sources. Revenues are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed and final, and there is reasonable assurance of collection of the sale. Estimates for returns, damaged goods, rebates, loyalty programs and other revenue allowances are made at the time the revenue is recognized based on the historical experience for such items.
Consumable product sales are recorded upon delivery, except in those circumstances where terms of the sale are FOB shipping point.
54
Equipment and software product revenues are recognized upon delivery and, if necessary, installation. In those circumstances where terms of the sale are FOB shipping point, revenues are recognized when products are transferred to the shipping carrier. Revenue derived from post contract customer support for software is deferred and recognized ratably over the period in which the support is provided.
Other revenue including freight and delivery charges and technical service parts and labor is recognized when the related product revenue is recognized or when the product or services are provided to the customer.
The receivables that result from the recognition of revenue are reported net of the related allowances discussed above. We also maintain a valuation allowance based upon the expected collectability of receivables held. Estimates are used to determine the valuation allowance and are based on several factors, including historical collection data, economic trends and credit worthiness of customers. Receivables are written off when we determine the amounts to be uncollectible, typically upon customer bankruptcy or non-response to continuous collection efforts. See also Note 6, "Customer Financing". The portion of receivable amounts that are not expected to be collected during the next twelve months are classified as long-term.
We have a relatively dispersed customer base and no single customer accounts for more than 1% of consolidated net sales. In addition, the equipment sold to customers under finance contracts generally serves as collateral for the contract and the customer provides a personal guarantee as well.
Patterson Advantage Loyalty Program
Patterson Dental provides a point-based awards program to qualifying customers involving the issuance of "Patterson Advantage dollars" which can be used toward equipment and technology purchases. The program was initiated on January 1, 2009 and runs on a calendar year schedule. Patterson Advantage dollars earned during a program year expire one year after the end of the program year. The cost and corresponding liability associated with the program are recognized as contra-revenue in accordance with ASC Topic 605-50, "Revenue Recognition-Customer Payments and Incentives." As of April 28, 2012, we believe we have sufficient experience with the program to reasonably estimate the amount of Patterson Advantage dollars that will not be redeemed and thus have recorded a liability for 87% of the maximum potential amount that could be redeemed. We use the redemption recognition method and we recognize the estimated value of unused–Advantage dollars as a percentage of Patterson Advantage dollars earned. Breakage recognized was immaterial to all periods presented.
Freight and Delivery Charges
Freight and delivery charges we incur are included in cost of sales.
Advertising
We expense all advertising and promotional costs as incurred, except for direct marketing expenses, which are expensed over the shorter of the life of the asset or one year. Total advertising and promotional expenses were $18,811, $20,630 and $21,368 for fiscal years 2012, 2011 and 2010, respectively. Deferred direct-marketing expenses included in prepaid and other current assets on the consolidated balance sheet as of April 28, 2012, and April 30, 2011 were $3,312 and $3,045, respectively.
Income Taxes
The liability method is used to account for income tax expense. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
Valuation allowances are established for deferred tax assets if, after assessment of available positive and negative evidence, it is more likely than not that the deferred tax asset will not be fully realized.
55
Employee Stock Ownership Plan (ESOP)
Compensation expense related to our defined contribution ESOP is computed based on the shares allocated method.
Stock-based Compensation
We recognize stock-based compensation expense based on the grant-date fair value of awards estimated in accordance with ASC Topic 718, "Stock Compensation".
Comprehensive Income
Comprehensive income is computed as net income plus certain other items that are recorded directly to stockholders' equity. The only significant item included in comprehensive income is foreign currency translation adjustments. Foreign currency translation adjustments do not include a provision for income tax because earnings from foreign operations are considered to be indefinitely reinvested outside the U.S.
Earnings Per Share
The amount of basic earnings per share is computed by dividing net income by the weighted average number of outstanding common shares during the period. The amount of diluted earnings per share is computed by dividing net income by the weighted average number of outstanding common shares and common share equivalents, when dilutive, during the period.
The following table sets forth the denominator for the computation of basic and diluted earnings per share. There were no material adjustments to the numerator.
| Fiscal Year | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| (in thousands) | ||||||||||||
Denominator: | ||||||||||||
Denominator for basic earnings per share-weighted average shares | 110,121 | 118,290 | 118,443 | |||||||||
Effect of dilutive securities-stock options, restricted stock and stock purchase plans | 725 | 776 | 759 | |||||||||
|
|
|
|
|
| |||||||
Denominator for diluted earnings per share-adjusted weighted average shares | 110,846 | 119,066 | 119,202 | |||||||||
|
|
|
|
|
| |||||||
Options to purchase 520, 559 and 932 shares of common stock during fiscal years 2012, 2011 and 2010, respectively, were excluded from the calculation of diluted earnings per share because the effect would have been anti-dilutive. Unvested restricted stock awards outstanding which were excluded from the calculation of diluted earnings per share during fiscal years 2012, 2011 and 2010 were 140, 173, and 60, respectively, because the effect would have been anti-dilutive.
Recent Accounting Pronouncements
In December 2011, the Financial Accounting Standards Board ("FASB") issued ASU 2011-11 "Disclosures about Offsetting Assets and Liabilities." ASU 2011-11 enhances disclosures surrounding offsetting (netting) assets and liabilities. The standard applies to financial instruments and derivatives and requires companies to disclose both gross and net information about instruments and transactions eligible for offset in the statement of financial position and instruments and transactions subject to a master netting arrangement. ASU No. 2011-11 is effective for interim and annual periods beginning on or after January 1, 2013. We are still evaluating the effect this standard will have on our Consolidated Financial Statements, but do not believe that the adoption of this new pronouncement will have a material effect on our Consolidated Financial Statements.
56
In September 2011, the FASB issued Accounting Standards Update ("ASU") No. 2011-08, Testing Goodwill for Impairment ("ASU 2011-08") . Under ASU 2011-08, entities testing goodwill for impairment now have the option to perform a qualitative assessment before having to calculate the fair value of a reporting unit. If an entity determines, on the basis of qualitative factors, that the fair value of the reporting unit is more-likely-than-not less than the carrying amount, the existing quantitative impairment test is required. Otherwise, no further impairment testing is required. This update is effective for fiscal years beginning after December 15, 2011, with early adoption permitted. We do not anticipate that the adoption of this provision will have a material impact on our financial position, results of operations or cash flows.
In June 2011, the FASB issued ASU No. 2011-05, Presentation of Comprehensive Income ("ASU 2011-05"), which requires an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income, either in a single continuous statement of comprehensive income or in two separate but consecutive statements. ASU 2011-05, which should be applied retrospectively, is effective for annual or interim periods beginning after December 15, 2011, with early adoption permitted. We early adopted ASU 2011-05 effective at the beginning of fiscal 2012. This adoption did not have an impact on our financial position, results of operations or cash flows, as it only requires a change in the format of our current presentation. We have presented other comprehensive income in two consecutive statements in conjunction with our statement of income.
In May 2011, the FASB issued ASU No. 2011-04, Fair Value Measurement: Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs ("ASU 2011-04") . ASU 2011-04 clarifies guidance on how to measure fair value and is largely consistent with existing fair value measurement principles. This ASU also expands existing disclosure requirements for fair value measurements and makes other amendments. This ASU was effective prospectively beginning January 29, 2012, the first day of our fourth quarter of fiscal 2012. The adoption of this standard did not have a material impact on our financial position, results of operations or cash flows.
2. Cash and cash equivalents
At April 28, 2012 and April 30, 2011, cash and short-term investments consisted of the following:
| April 28, 2012 | April 30, 2011 | |||||||
Cash on hand | $ | 251,849 | $ | 200,443 | ||||
Cash equivalents: | ||||||||
Government securities | 0 | 76,137 | ||||||
Money market funds | 321,932 | 112,085 | ||||||
|
|
|
| |||||
| 321,932 | 188,222 | |||||||
|
|
|
| |||||
Total | $ | 573,781 | $ | 388,665 | ||||
|
|
|
| |||||
Cash on hand is generally in interest earning accounts.
3. Goodwill and Other Intangible Assets
The changes in the carrying value of goodwill for each of our reportable segments for the fiscal year ended April 28, 2012 are as follows:
| Balance
at April 30, 2011 | Acquisition Activity | Other Activity | Balance
at April 28, 2012 | |||||||||||||
Dental supply | $ | 132,670 | $ | - | $ | 7 | $ | 132,677 | ||||||||
Rehabilitation supply | 537,995 | 304 | 4,832 | 543,131 | ||||||||||||
Veterinary supply | 124,951 | 4,895 | 4,598 | 134,444 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 795,616 | $ | 5,199 | $ | 9,437 | $ | 810,252 | ||||||||
|
|
|
|
|
|
|
| |||||||||
57
The increase in the acquisition activity column during the twelve month period ended April 28, 2012 primarily reflects the preliminary purchase price allocations of the rehabilitation businesses of Surgical Synergies and the veterinary acquisition of AVSC, which were acquired in fiscal 2012. The other activity column is comprised primarily of earn-out payments made related to acquisitions completed prior to fiscal 2012 and foreign currency translation. The other activity column is comprised primarily of earn-out payments made related to acquisitions completed prior to the adoption of the guidance in ASC 805 and foreign currency translation.
Other intangibles acquired in the acquisitions in fiscal 2012 had a fair value of approximately $2.6 million and a weighted average useful life of 10 years.
Balances of other intangible assets excluding goodwill are as follows:
| April 28, 2012 | April 30, 2011 | |||||||
Unamortized-indefinite lived: | ||||||||
Copyrights, trade names and trademarks | $ | 76,464 | $ | 76,422 | ||||
Amortized: | ||||||||
Distribution agreement, customer lists and other | 231,739 | 229,649 | ||||||
Less: Accumulated amortization | (95,646 | ) | (78,855 | ) | ||||
|
|
|
| |||||
Net amortized intangible assets | 136,093 | 150,794 | ||||||
|
|
|
| |||||
Total identifiable intangible assets, net | $ | 212,557 | $ | 227,216 | ||||
|
|
|
| |||||
In 2006, we extended our exclusive North American distribution agreement with Sirona Dental Systems GmbH ("Sirona") for Sirona's CEREC 3D dental restorative system. We paid a $100 million distribution fee to extend the agreement for a 10-year period that began in October 2007, which is included in identifiable intangibles, net in the consolidated balance sheet. The amortization of the distribution agreement fee is recorded over the 10-year period based on estimates of the pattern in which the economic benefits of the fee are expected to be realized, consisting primarily of revenues generated from the sale of CEREC 3D dental restorative systems. Amortization expense in any year may differ significantly from other years. In early fiscal 2013, we expanded our exclusive distribution relationship with Sirona to add SIRONA imaging products to our exclusive offerings, as well as add mechanisms to adjust the exclusivity term depending on performance. No additional monies were exchanged as part of this expanded relationship.
With respect to the amortized intangible assets, future amortization expense is expected to approximate $19,915, $21,416, $22,647, $24,449 and $27,028 for fiscal years 2013, 2014, 2015, 2016 and 2017, respectively. The preceding expected amortization expense is an estimate. Actual amounts of amortization expense may differ from estimated amounts due to additional intangible asset acquisitions, actual revenues generated from the sale of CEREC 3D dental restorative systems, changes in foreign currency exchange rates, impairment of intangible assets, accelerated amortization of intangible assets and other events.
58
4. Acquisitions and Equity Investments
We completed smaller acquisitions during fiscal years 2012, 2011 and 2010. The operating results of each of these acquisitions are included in our consolidated statements of income from the date of each acquisition. Pro forma results of operations and details of the purchase price allocations have not been presented for these acquisitions since the effects of these business acquisitions were not material either individually or in the aggregate. A listing of acquisitions completed during the periods covered by these financial statements is presented below:
Entity | Segment | |
Fiscal 2012: | ||
American Veterinarian Supply Corp. | Veterinary supply | |
Surgical Synergies Pty Ltd. | Rehabilitation supply | |
Orthoplast | Rehabilitation supply | |
Fiscal 2011: | ||
DCC Healthcare | Rehabilitation supply | |
ePet Records LLC | Veterinary supply | |
Fiscal 2010: | ||
Therapeutic Technologies | Rehabilitation supply | |
Global Medical & Dental | Dental supply | |
Empi Therapy Solutions | Rehabilitation supply | |
Summit Sports Medical | Rehabilitation supply | |
In April 2010, we made a minority equity investment of 30.3% in VetSource, a leading North American provider of integrated specialty pharmacy distribution, including home delivery capabilities. The investment in VetSource is being accounted for under the equity method of accounting and is included in other long-term assets in the consolidated balance sheet.
5. Property and Equipment
| April 28, 2012 | April 30, 2011 | |||||||
Land | $ | 15,578 | $ | 14,103 | ||||
Buildings | 130,009 | 113,563 | ||||||
Leasehold improvements | 15,058 | 14,336 | ||||||
Furniture and equipment | 139,506 | 124,217 | ||||||
Data processing equipment | 87,780 | 76,990 | ||||||
Construction-in-progress | 3,385 | 19,487 | ||||||
|
|
|
| |||||
| 391,316 | 362,696 | |||||||
Accumulated depreciation | (195,851 | ) | (173,113 | ) | ||||
|
|
|
| |||||
Property and equipment, net | $ | 195,465 | $ | 189,583 | ||||
|
|
|
| |||||
The variance of construction in progress from last year to this year is due to the new Patterson Technology Center facility in Illinois. The facility was completed in the first half of fiscal 2012.
6. Customer Financing
As a convenience to our customers, we offer several different financing alternatives including both a Company sponsored program and a third party program. For the third party program, Patterson acts as a facilitator between the customer and the third party financing entity with no on-going involvement in the financing transaction. Under this sponsored program, equipment purchases by customers with strong credit are
59
financed to a maximum of $0.4 million for any one customer. We generally sell the customers' financing contracts to outside financial institutions in the normal course of its business. Patterson currently has two arrangements under which we have sold these contracts.
In fiscal 2003, Patterson initiated an agreement to sell our equipment finance contracts to a commercial paper conduit managed by JPMorgan Chase Bank N.A. To participate in the commercial paper conduit, Patterson was required to establish a special purpose entity ("SPE"), PDC Funding Company, LLC, a consolidated, wholly owned subsidiary. In December 2010, this agreement was amended and restated and The Bank of Tokyo-Mitsubishi UFJ, Ltd. became the managing agent under the agreement.
We transfer financing contracts to the SPE and in turn, the SPE sells the contracts to the commercial paper conduits. The SPE does not issue any debt. While there is no recourse to Patterson by the commercial paper conduits on the sale of contracts, we receive only approximately 84% of the principal amount of the contracts upon the sale. The remaining 16% of the proceeds is held by the conduit as security against the eventual performance of the portfolio. The deferred purchase price receivable from the conduit is recorded as a non-current asset, which is carried at its estimated fair market value. As of April 28, 2012, the maximum outstanding capacity of this arrangement with the conduits at any one time is $500 million.
Patterson also maintains an agreement with commercial banks whereby the banks purchase customers' financing contracts. Patterson has established another SPE, PDC Funding LLC II ("PDC II"), as a consolidated, wholly owned subsidiary, which sells financing contracts to the banks. We receive a portion of the principal amounts of the contracts upon sale with the remaining portion of the proceeds held by the banks as security against the eventual performance of the portfolio. The holdback receivable from the banks is recorded as a non-current asset, which is carried at its estimated fair market value. The capacity under the agreement at April 28, 2012 was $75 million. In the fourth quarter of fiscal 2010, this agreement was amended such that no additional contracts were sold, but the remaining contracts previously sold and outstanding under the agreement will continue under the agreement. On August 12, 2011, Fifth Third Bank replaced U.S. Bank National Association and the agreement was amended and restated. Under the restated agreement, Fifth Third Bank is the agent and contracts are again being sold. Approximately $75 million of such contracts were outstanding as of April 28, 2012.
These financing arrangements are accounted for as a sale of assets under the provisions of ASC Topic No. 860, "Transfers and Servicing." During fiscal 2012, 2011 and 2010, we sold approximately $287.6, $296.4 and $300.8 million, respectively, of contracts under these arrangements. Patterson retains servicing responsibilities under both agreements, for which we are paid a servicing fee. The servicing fees received by Patterson are considered adequate compensation for services rendered. Accordingly, no servicing asset or liability has been recorded. The agreements require us to maintain a minimum current ratio and maximum leverage ratio. Patterson was in compliance with the covenants at April 28, 2012.
Included in current receivables in the consolidated balance sheets are approximately $81.8 million, net of unearned income of $7.4 million, and $78.5 million, net of unearned income of $3.6 million, as of April 28, 2012 and April 30, 2011, respectively, of finance contracts not yet sold by Patterson. A total of $479.4 million of finance contracts receivable sold under the agreements were outstanding at April 28, 2012. The residual receivable under the arrangements was approximately $78.9 and $78.0 million as of April 28, 2012 and April 30, 2011, respectively. Since the internal financing program began in 1994, bad debt write-offs have amounted to less than one-percent of the loans originated.
7. Long-Term Debt
Expected future minimum principal payments under our debt obligations are as follows: $125.0 million in fiscal year 2013; $250.0 million in fiscal 2015; and $475.0 million in years thereafter.
60
In March 2008, Patterson issued fixed-rate senior notes with an aggregate principal amount of $450 million, consisting of (i) $50 million 4.63% senior notes, due fiscal 2013; (ii) $250 million 5.17% senior notes, due fiscal 2015; and (iii) $150 million 5.75% senior notes, due fiscal 2018.
Also in March 2008, we entered into a term loan agreement with a principal amount of $75 million, which matures in fiscal 2013. The term loan bears interest at a floating rate based on LIBOR plus a spread that can range from 0.50% to 1.25% based on our leverage ratio, as defined in the agreement. During the years ended April 28, 2012 and April 30, 2011, the weighted average interest rate of this term loan was 1.20% and 1.11%, respectively.
In December 2011, we issued fixed-rate senior notes with an aggregate principal amount of $325 million, consisting of (i) $60 million 2.95% senior notes, due fiscal 2018; (ii) $165 million 3.59% senior notes, due fiscal 2021; and (iii) $100 million 3.74% senior notes, due fiscal 2023.
A portion of the proceeds from the issuance of debt in December 2011 was used to repurchase shares of our common stock and to repay borrowings under our revolving line of credit. The remaining proceeds are intended to retire the senior note and term loan due fiscal 2013, or will be used for general corporate purposes. Debt issuance costs associated with the issuance of debt in March 2008 of $1.8 million and in December 2011 of $1.8 million are being amortized to interest expense over the life of the related debt.
In addition, in March 2008 we entered into two forward starting interest rate swap agreements, each with notional amounts of $100 million and accounted for as cash flow hedges, to hedge interest rate fluctuations in anticipation of the issuance of the 5.17% senior notes due fiscal 2015 and the 5.75% senior notes due fiscal 2018, respectively. Upon issuance of the hedged debt, Patterson settled the forward starting interest rate swap agreements and recorded a $1.0 million increase, net of income taxes, to other comprehensive income, which is being amortized against interest expense over the life of the related debt. The pre-tax amount reclassified into earnings during fiscal years 2012, 2011 and 2010 was $0.2 million. The amount expected to be reclassified into earnings during fiscal 2013 is also expected to be $0.2 million.
Patterson has available a $300 million revolving credit facility through December 2016. Interest on borrowings is based on LIBOR plus a spread which can range from 1.125% to 1.875%. This spread as well as a commitment fee on the unused portion of the facility are based on our leverage ratio, as defined in the agreement. There were no outstanding borrowings under the facility at April 28, 2012 or April 30, 2011.
The debt agreements contain various financial covenants including certain leverage and interest coverage ratios as defined in the agreements. Patterson met the financial and nonfinancial covenants under the debt agreements as of April 28, 2012.
Patterson's debt consists of the following (in thousands):
| April 28, 2012 | April 30, 2011 | |||||||
4.63% senior notes due fiscal 2013 | $ | 50,000 | $ | 50,000 | ||||
5.17% senior notes due fiscal 2015 | 250,000 | 250,000 | ||||||
5.75% senior notes due fiscal 2018 | 150,000 | 150,000 | ||||||
2.95% senior notes due fiscal 2018 | 60,000 | 0 | ||||||
3.59% senior notes due fiscal 2021 | 165,000 | 0 | ||||||
3.74% senior notes due fiscal 2023 | 100,000 | 0 | ||||||
Variable rate (LIBOR plus 1.25%) term loan due fiscal 2013 | 75,000 | 75,000 | ||||||
|
|
|
| |||||
Total debt | 850,000 | 525,000 | ||||||
Less: current debt obligations | 125,000 | 0 | ||||||
|
|
|
| |||||
Long-term debt | $ | 725,000 | $ | 525,000 | ||||
|
|
|
| |||||
61
8. Derivative Financial Instruments
Patterson is a party to certain offsetting and identical interest rate cap agreements. These cap agreements are not designated for hedge accounting treatment and were entered into to fulfill certain covenants of a sale agreement between a commercial paper conduit managed by The Bank of Tokyo-Mitsubishi UFJ, Ltd. and PDC Funding. On December 2, 2011 this agreement was amended on terms consistent with the expiring agreement. These agreements are structured to expire at the end of a 364-day term, so effectively are amended annually. The cap agreements provide a credit enhancement feature for the financing contracts sold by PDC Funding to the commercial paper conduit and replace a minimum interest rate margin previously required under the sale agreement.
The cap agreements are cancelled and new agreements entered into periodically to maintain consistency with the dollar maximum of the sale agreements and the maturity of the underlying financing contracts. As of April 28, 2012, PDC Funding had purchased two interest rate caps from a bank with combined notional amounts of $416 million and maturity dates of September 2018. Patterson Companies, Inc. sold two identical interest rate caps to the same bank.
Similar to the above agreements, PDC Funding II and Patterson Companies, Inc. had entered into offsetting and identical interest rate swap agreements with a notional amount of $110 million. During the second quarter of 2012, these agreements were terminated and replaced with offsetting and identical interest rate cap agreements. As of April 28, 2012 these agreements had notional amounts of $75 million and maturity dates of October 2017.
In addition to the identical purchased and sold interest rate contracts described above, we entered into two interest rate swap agreements with banks to economically hedge the interest rate risk associated with our finance contracts. One agreement ended in November 2011, and other matured in February 2012.
Our interest rate contracts do not qualify for hedge accounting treatment and, accordingly, we record the fair value of the agreements as an asset or liability and the change in any period as income or expense during the period in which the change occurs.
In the first quarter of fiscal 2011, we entered into a foreign currency forward contract that was settled in the same quarter. This contract served as an economic hedge and was not designated as a hedge for accounting purposes. The total gain on the contract was $0.1 million.
In the second quarter of fiscal 2011, we entered into a foreign currency forward contract that served to manage foreign exchange risk on a short-term intercompany loan. The forward contract and intercompany loan were both settled during the quarter. The loss on the contract was $2.0 million that effectively was offset by the gain on the intercompany loan.
The following presents the fair value of interest rate contracts included in the consolidated balance sheets:
Assets | Liabilities | |||||||||||||||||||
Classification | Fair Value | Classification | Fair Value | |||||||||||||||||
Derivative type | April 28, 2012 | April 30, 2011 | April 28, 2012 | April 30, 2011 | ||||||||||||||||
Interest rate contracts | Other noncurrent assets | $ | 0.2 | $ | 5.5 | Other noncurrent liabilities | $ | 0.2 | $ | 5.6 | ||||||||||
The following presents the effect of interest rate and foreign currency contracts on the consolidated statements of income:
Derivative type | Classification of gain (loss) recognized on derivative | Gain
(loss) recognized on derivative | ||||||||||||
| Fiscal Year | ||||||||||||||
| 2012 | 2011 | 2010 | ||||||||||||
Interest rate contracts | Other income (expense), net | $0.0 | ($ | 0.1 | ) | ($0.9 | ) | |||||||
Foreign currency contracts | Other income (expense), net | $0.0 | ($ | 1.9 | ) | $0.0 | ||||||||
62
9. Fair Value Measurements
Fair value is the price at which an asset could be exchanged in a current transaction between knowledgeable, willing parties. The fair value hierarchy of measurements is categorized into one of three levels based on the lowest level of significant input used:
Level 1 – | Quoted prices in active markets for identical assets and liabilities at the measurement date. | |
Level 2 – | Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data. | |
Level 3 – | Unobservable inputs for which there is little or no market data available. These inputs reflect management's assumptions of what market participants would use in pricing the asset or liability. | |
Our hierarchy for assets and liabilities measured at fair value on a recurring basis as of April 28, 2012 is as follows:
| Total | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
| (in millions) | ||||||||||||||||
Assets: | ||||||||||||||||
Cash equivalents | $ | 321.9 | $ | 321.9 | - | |||||||||||
Derivative instruments | $ | 0.2 | - | $ | 0.2 | - | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Total assets | $ | 322.1 | $ | 321.9 | $ | 0.2 | - | |||||||||
|
|
|
|
|
|
|
| |||||||||
Liabilities: | ||||||||||||||||
Derivative instruments | $ | 0.2 | - | $ | 0.2 | - | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Our hierarchy for assets and liabilities measured at fair value on a recurring basis as of April 30, 2011 is as follows:
| Total | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
| (in millions) | ||||||||||||||||
Assets: | ||||||||||||||||
Cash equivalents | $ | 188.2 | $ | 188.2 | - | - | ||||||||||
Derivative instruments | $ | 5.5 | - | $ | 5.5 | - | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Total assets | $ | 193.7 | $ | 188.2 | $ | 5.5 | - | |||||||||
|
|
|
|
|
|
|
| |||||||||
Liabilities: | ||||||||||||||||
Derivative instruments | $ | 5.6 | - | $ | 5.6 | - | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Cash equivalents -We value cash equivalents at their current market rates. The carrying value of cash equivalents approximates fair value and maturities are less than three months.
Derivative instruments -Patterson's derivative instruments consist of interest rate contracts. These instruments are valued using inputs such as interest rates and credit spreads.
63
Certain assets are measured at fair value on a nonrecurring basis. These assets are not measured at fair value on an ongoing basis, but are subject to fair value adjustments under certain circumstances, such as when there is evidence of impairment. There were no fair value adjustments to such assets in fiscal years 2012, 2011 and 2010.
Patterson's debt is not measured at fair value in the consolidated balance sheets. The estimated fair value of our debt as of April 28, 2012 and April 30, 2011 was $869.7 million and $527.0 million, respectively. The fair value of debt was measured using a discounted cash flow analysis based on expected market based yields. These are considered to be Level 2 inputs under the fair value measurements and disclosure guidance.
The carrying amounts of receivables, net of allowances, accounts payable, and certain accrued and other current liabilities approximated fair value at April 28, 2012 and April 30, 2011.
10. Lease Commitments
Patterson leases facilities for its branch office locations, a few small distribution facilities, and certain equipment. These leases are accounted for as operating leases. Future minimum rental payments under non-cancelable operating leases are as follows at April 28, 2012:
2013 | $ | 18,392 | ||
2014 | 14,116 | |||
2015 | 11,276 | |||
2016 | 9,226 | |||
2017 | 7,285 | |||
Thereafter | 10,824 | |||
|
| |||
Total minimum payments required | $ | 71,119 | ||
|
|
Rent expense was $20,819, $20,459 and $19,238 for the years ended April 28, 2012, April 30, 2011 and April 24, 2010, respectively.
11. Income Taxes
Significant components of the provision for income taxes are as follows:
| Fiscal Year | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
Current: | ||||||||||||
Federal | $ | 89,612 | $ | 87,315 | $ | 98,481 | ||||||
Foreign | 16,726 | 14,548 | 16,308 | |||||||||
State | 10,570 | 11,469 | 11,747 | |||||||||
|
|
|
|
|
| |||||||
Total current | 116,908 | 113,332 | 126,536 | |||||||||
Deferred: | ||||||||||||
Federal | 872 | 14,938 | 544 | |||||||||
Foreign | (895 | ) | 310 | (360 | ) | |||||||
State | 112 | 1,922 | 67 | |||||||||
|
|
|
|
|
| |||||||
Total deferred | 89 | 17,170 | 251 | |||||||||
|
|
|
|
|
| |||||||
Provision for income taxes | $ | 116,997 | $ | 130,502 | $ | 126,787 | ||||||
|
|
|
|
|
| |||||||
64
Deferred tax assets and liabilities are included in prepaid expenses and other current assets and in non-current liabilities on the balance sheet. Significant components of Patterson's deferred tax assets (liabilities) as of April 28, 2012 and April 30, 2011 are as follows:
| 2012 | 2011 | |||||||
Deferred current income tax asset (liability): | ||||||||
Capital Accumulation Plan | $ | 4,045 | $ | 4,048 | ||||
Inventory obsolescence | 2,124 | 3,455 | ||||||
Bad debt allowance | 2,022 | 2,424 | ||||||
LIFO reserve | (12,773 | ) | (14,755 | ) | ||||
Other | 17,544 | 14,229 | ||||||
|
|
|
| |||||
Deferred net current income tax asset | 12,962 | 9,401 | ||||||
Deferred long-term income tax (liability) asset: | ||||||||
Amortizable intangibles | (29,142 | ) | (30,033 | ) | ||||
Goodwill | (56,026 | ) | (49,089 | ) | ||||
Property, plant, equipment | (3,901 | ) | (3,737 | ) | ||||
Stock based compensation expense | 7,897 | 6,146 | ||||||
Net operating loss carryforwards | 7,656 | 9,431 | ||||||
Other | (3,568 | ) | (4,612 | ) | ||||
|
|
|
| |||||
| (77,084 | ) | (71,894 | ) | |||||
Valuation allowance | (4,772 | ) | (6,345 | ) | ||||
|
|
|
| |||||
Deferred net long-term income tax liability | (81,856 | ) | (78,239 | ) | ||||
|
|
|
| |||||
Net deferred income tax liability | $ | (68,894 | ) | $ | (68,838 | ) | ||
|
|
|
| |||||
At April 28, 2012, we had foreign net operating loss carryforwards ("NOLs") of $31.5 million attributable to the fiscal 2009 acquisition of Mobilis Healthcare Group. A valuation allowance of $4.8 million has been recorded since we believe it is more likely than not that the deferred tax asset of $7.7 million arising from the NOL's will not be fully utilized due to uncertainties relating to future taxable income from the acquired operations.
No provision has been made for U.S. federal income taxes on certain undistributed earnings of foreign subsidiaries that we intend to permanently invest or that may be remitted substantially tax-free. The total of undistributed earnings that would be subject to federal income tax if remitted under existing law is approximately $228.0 million as of April 28, 2012. Determination of the unrecognized deferred tax liability related to these earnings is not practicable because of the complexities with its hypothetical calculation. Upon distribution of these earnings, we will be subject to U.S. taxes and withholding taxes payable to various foreign governments. A credit for foreign taxes already paid would be available to reduce the U.S. tax liability.
Income tax expense varies from the amount computed using the U.S. statutory rate. The reasons for this difference and the related tax effects are shown below:
| Fiscal Year | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
Tax at U.S. statutory rate | $ | 115,434 | $ | 124,561 | $ | 118,664 | ||||||
State tax provision, net of federal benefit | 7,277 | 8,950 | 8,103 | |||||||||
Effect of foreign taxes | 530 | 2,398 | 1,411 | |||||||||
Other | (6,244 | ) | (5,407 | ) | (1,391 | ) | ||||||
|
|
|
|
|
| |||||||
| $ | 116,997 | $ | 130,502 | $ | 126,787 | |||||||
|
|
|
|
|
| |||||||
65
We have adopted ASC Topic 740, "Income Taxes" related to accounting for the uncertainty in income taxes recognized in the financial statements. These standards clarify the separate identification and reporting of estimated amounts that could be assessed upon audit. The potential assessments are considered unrecognized tax benefits, because, if it is ultimately determined they are unnecessary, the reversal of these previously recorded amounts will result in a beneficial impact to our financial statements.
As of April 28, 2012 and April 30 2011, Patterson's gross unrecognized tax benefits were $18.1 million and $19.0 million, respectively. If determined to be unnecessary, these amounts (net of deferred tax assets of $5.0 million and $4.9 million, respectively, related to the tax deductibility of the gross liabilities) would decrease our effective tax rate. The gross unrecognized tax benefits are included in other long-term liabilities on the consolidated balance sheet.
A summary of the changes in the gross amounts of unrecognized tax benefits for the years ended April 28, 2012 and April 30, 2011 are shown below:
| 2012 | 2011 | |||||||
Balances beginning of period | $ | 18,963 | $ | 18,685 | ||||
Additions for tax positions related to the current year | 2,288 | 2,737 | ||||||
Additions for tax positions of prior years | 474 | 468 | ||||||
Reductions for tax positions of prior years | (1,778 | ) | (1,309 | ) | ||||
Statute expirations | (1,618 | ) | (1,504 | ) | ||||
Settlements | (230 | ) | (114 | ) | ||||
|
|
|
| |||||
Balance, end of period | $ | 18,099 | $ | 18,963 | ||||
|
|
|
| |||||
We also recognize both interest and penalties with respect to unrecognized tax benefits as a component of income tax expense. As of April 28, 2012 and April 30, 2011, we had recorded $2.6 million and $2.9 million, respectively, for interest and penalties. These amounts are also included in other long-term liabilities on the consolidated balance sheet. These amounts, net of related deferred tax assets, if determined to be unnecessary, would decrease our effective tax rate. During the year ended April 28, 2012, we recorded as part of tax expense $.7 million related to an increase in our estimated liability for interest and penalties.
Patterson files income tax returns, including returns for our subsidiaries, with federal, state, local and foreign jurisdictions. During the year, our federal tax return for the year ended April 24, 2010 was audited by the Internal Revenue Service ("IRS"). The outcome of this audit did not have a material adverse impact on our financial statements. Besides this audit, the IRS has either examined or waived examination of all periods up to and including our fiscal year ended April 28, 2008. Periodically, state, local and foreign income tax returns are examined by various taxing authorities. We do not believe the outcome of these various examinations would have a material adverse impact on our financial statements.
12. Segment and Geographic Data
Patterson Companies, Inc. is comprised of three reportable segments: dental, veterinary, and rehabilitation supply. Our reportable business segments are strategic business units that offer similar products and services to different customer bases. The dental supply segment provides a virtually complete range of consumable dental products, clinical and laboratory equipment and value-added services to dentists, dental laboratories, institutions and other dental healthcare providers throughout North America. The veterinary supply segment provides consumable supplies, equipment, diagnostic products, biologicals (vaccines) and pharmaceuticals to companion-pet veterinary clinics in the majority of regions throughout the United States. The rehabilitation supply segment provides a comprehensive range of distributed and self-manufactured rehabilitation medical supplies and assistive products to acute care hospitals, long-term care facilities, rehabilitation clinics, dealers and schools.
66
We evaluate segment performance based on operating income. The corporate office general and administrative expenses are included in the dental supply segment and consist of home office support costs in areas such as informational technology, finance, human resources and facilities. If these corporate expenses were allocated to the segments, the results would not be materially different as the dental segment would absorb a significant portion of these expenses. The cost to operate the distribution centers are allocated to the operating units based on the through-put of the unit.
The following table presents information about Patterson's reportable segments:
| Fiscal Year | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
Net sales | ||||||||||||
Dental supply | $ | 2,287,875 | $ | 2,236,056 | $ | 2,167,495 | ||||||
Rehabilitation supply | 513,340 | 504,734 | 426,297 | |||||||||
Veterinary supply | 734,446 | 674,880 | 643,584 | |||||||||
|
|
|
|
|
| |||||||
Consolidated net sales | $ | 3,535,661 | $ | 3,415,670 | $ | 3,237,376 | ||||||
|
|
|
|
|
| |||||||
Operating income | ||||||||||||
Dental supply | $ | 246,755 | $ | 272,185 | $ | 263,750 | ||||||
Rehabilitation supply | 72,442 | 66,809 | 59,922 | |||||||||
Veterinary supply | 38,812 | 37,014 | 31,619 | |||||||||
|
|
|
|
|
| |||||||
Consolidated operating income | $ | 358,009 | $ | 376,008 | $ | 355,291 | ||||||
|
|
|
|
|
| |||||||
Depreciation and amortization | ||||||||||||
Dental supply | $ | 31,375 | $ | 27,410 | $ | 26,277 | ||||||
Rehabilitation supply | 7,472 | 7,833 | 5,909 | |||||||||
Veterinary supply | 3,362 | 6,096 | 7,288 | |||||||||
|
|
|
|
|
| |||||||
Consolidated depreciation and amortization | $ | 42,209 | $ | 41,339 | $ | 39,474 | ||||||
|
|
|
|
|
| |||||||
Total assets | ||||||||||||
Dental supply | $ | 1,502,672 | $ | 1,359,659 | $ | 1,331,510 | ||||||
Rehabilitation supply | 883,287 | 863,930 | 773,732 | |||||||||
Veterinary supply | 353,409 | 341,379 | 317,727 | |||||||||
|
|
|
|
|
| |||||||
Consolidated total assets | $ | 2,739,368 | $ | 2,564,968 | $ | 2,422,969 | ||||||
|
|
|
|
|
| |||||||
67
The following table presents sales information by product for Patterson and its reportable segments:
| Fiscal Year | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
Consolidated | ||||||||||||
Consumable and printed products | $ | 2,312,309 | $ | 2,232,876 | $ | 2,124,558 | ||||||
Equipment and software | 930,601 | 900,846 | 839,436 | |||||||||
Other | 292,751 | 281,948 | 273,382 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 3,535,661 | $ | 3,415,670 | $ | 3,237,376 | ||||||
|
|
|
|
|
| |||||||
Dental supply | ||||||||||||
Consumable and printed products | $ | 1,263,515 | $ | 1,253,224 | $ | 1,214,796 | ||||||
Equipment and software | 768,633 | 734,749 | 709,468 | |||||||||
Other | 255,727 | 248,083 | 243,231 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 2,287,875 | $ | 2,236,056 | $ | 2,167,495 | ||||||
|
|
|
|
|
| |||||||
Rehabilitation supply | ||||||||||||
Consumable and printed products | $ | 363,003 | $ | 348,641 | $ | 303,044 | ||||||
Equipment and software | 123,650 | 131,776 | 100,583 | |||||||||
Other | 26,687 | 24,317 | 22,670 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 513,340 | $ | 504,734 | $ | 426,297 | ||||||
|
|
|
|
|
| |||||||
Veterinary supply | ||||||||||||
Consumable and printed products | $ | 685,790 | $ | 631,011 | $ | 606,718 | ||||||
Equipment and software | 38,319 | 34,321 | 29,385 | |||||||||
Other | 10,337 | 9,548 | 7,481 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 734,446 | $ | 674,880 | $ | 643,584 | ||||||
|
|
|
|
|
| |||||||
The following table presents information about Patterson by geographic area. No individual country, except for the United States, generated sales greater than 10% of consolidated net sales. There were no material sales between geographic areas.
| Fiscal Year | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
Net sales | ||||||||||||
United States | $ | 3,104,047 | $ | 3,006,984 | $ | 2,903,407 | ||||||
International | 431,614 | 408,686 | 333,969 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 3,535,661 | $ | 3,415,670 | $ | 3,237,376 | ||||||
|
|
|
|
|
| |||||||
Income before tax | ||||||||||||
United States | $ | 286,215 | $ | 316,293 | $ | 298,061 | ||||||
International | 43,597 | 39,594 | 40,980 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 329,812 | $ | 355,887 | $ | 339,041 | ||||||
|
|
|
|
|
| |||||||
Long-lived assets | ||||||||||||
United States | $ | 1,192,437 | $ | 1,195,359 | $ | 1,179,361 | ||||||
International | 143,418 | 138,900 | 109,850 | |||||||||
|
|
|
|
|
| |||||||
Total | $ | 1,335,855 | $ | 1,334,259 | $ | 1,289,211 | ||||||
|
|
|
|
|
| |||||||
68
13. Stockholders' Equity
Dividends
We declared and paid cash dividends of
| Period | ||||||||||||||||
Fiscal year | 1 | 2 | 3 | 4 | ||||||||||||
2012 | $ | 0.12 | $ | 0.12 | $ | 0.12 | $ | 0.14 | ||||||||
2011 | $ | 0.10 | $ | 0.10 | $ | 0.10 | $ | 0.12 | ||||||||
2010 | $ | - | $ | - | $ | - | $ | 0.10 | ||||||||
Prior to March 2010, Patterson had not declared or paid any cash dividends on its common stock since our initial public offering in 1992. Patterson expects to continue paying a quarterly cash dividend into the foreseeable future.
Share Repurchases
During fiscal 2012, we repurchased and retired 11,954,257 shares of our common stock for $361,047, or an average of $30.20 per share. During fiscal 2011, Patterson repurchased and retired 3,257,374 shares of our common stock for $98,960, or an average of $30.38 per share. There were no share repurchases in fiscal 2010.
In December 2007, Patterson's Board of Directors expanded a share repurchase program to allow for the purchase of up to 25 million shares of common stock in open market transactions. As of March 2011, approximately 20.5 million shares had been repurchased under this authorization. At that time, the Board of Directors cancelled and replaced the existing share repurchase program with a new authorization to repurchase an additional 25 million shares of common stock. As of April 28, 2012, 11,136,742 shares remain available under the repurchase authorization, which expires on March 15, 2016.
Employee Stock Ownership Plan (ESOP)
During 1990, Patterson's Board of Directors adopted a leveraged ESOP. In fiscal 1991, under the provisions of the plan and related financing arrangements, Patterson loaned the ESOP $22,000 (the "1990 note") for the purpose of acquiring its then outstanding preferred stock which was subsequently converted to common stock. The Board of Directors determines the contribution from the Company to the ESOP annually. The contribution is used to retire a portion of the debt, which triggers a release of shares that are then allocated to the employee participants. Shares of stock acquired by the plan are allocated to each participant who has completed 1,000 hours of service during the plan year. The shares under the 1990 note were grandfathered from the accounting provisions of ASC Topic 718-40, "Employer Stock Ownership Plans" ("ASC 718-40") and therefore the provisions of the former SOP 76-3 apply. Accordingly, the expense recognized when these shares were released and allocated to participants was based on the original cost to acquire the shares. In fiscal 2011, the final payment on the 1990 note was made and all remaining shares were released for allocation to participants. During fiscal 2011 and 2010, shares secured by the 1990 note with an aggregate cost of $1,635 and $1,615, respectively, were committed for release and allocated to ESOP participants.
In fiscal 2002, Patterson's ESOP and an ESOP sponsored by the Thompson Dental Company ("Thompson") were used to facilitate the acquisition and merger of Thompson into Patterson. The net result of this transaction was an additional loan of $12,612 being made to the ESOP and the ESOP acquiring 665,978 shares of common stock. These shares are accounted for under ASC 718-40 and accordingly these shares are not considered outstanding for the computation of earnings per share until the shares are committed for release to the participants. When the shares are committed for release and allocated to the participants, the expense to Patterson is determined based on current fair value. The loan bears interest at current rates but principal did not begin to amortize until fiscal 2012. Beginning in fiscal 2012 and through fiscal 2020, an annual payment of $200 plus interest is due and in fiscal 2020, a final payment of any outstanding principal and interest balance is due.
69
Prepayments of principal can be made at any time without penalty. Of the 665,978 shares issued in the transaction 97,810 were previously allocated to Thompson employees. The remaining 568,168 shares began to be allocated in fiscal 2004. During fiscal 2012, 2011 and 2010, shares secured by the Thompson note with an aggregate fair value of $298, $81 and $111, respectively, were committed for release and allocated to ESOP participants.
On September 11, 2006, we entered into a third loan agreement with the ESOP and loaned $105 million (the "2006 note") for the sole purpose of enabling the ESOP to purchase shares of our common stock. The ESOP purchased 3,159,645 shares with the proceeds from the 2006 note. These shares are also accounted for under ASC 718-40. Interest on the unpaid principal balance accrues at a rate equal to six-month LIBOR, with the rate resetting semi-annually. Interest payments were not required during the period from and including September 11, 2006 through April 30, 2010. On April 30, 2010, accrued and unpaid interest was added to the outstanding principal balance under the note, with interest thereafter accruing on the increased principal amount. Unpaid interest accruing after April 30, 2010 is due and payable on each successive April 30 occurring through September 10, 2026. No principal payments are due until September 10, 2026; however, prepayments can be made without penalty. During fiscal 2012 and 2011, shares secured by the 2006 note with aggregate fair values of $458 and $397, respectively, were committed for release and allocated to ESOP participants. No shares secured by the 2006 note were released prior to fiscal 2011.
At April 28, 2012, a total of 14,432,446 shares of common stock that have been allocated to participants remained in the ESOP and had a fair market value of $490.6 million. Related to the shares from the Thompson transaction, committed-to-be-released shares were 9,569 and suspense shares were 473,669. Finally, with respect to the 2006 note, committed-to-be-released shares were 14,730 and suspense shares were 3,131,694.
We anticipate the allocation of the remaining suspense, or unearned, shares to occur over a period of approximately 10 to 15 years. As of April 28, 2012, the fair value of all unearned shares held by the ESOP was approximately $122.5 million. We will recognize an income tax deduction as the unearned ESOP shares are released. Such deductions will be limited to the ESOP's original cost to acquire the shares.
Dividends on allocated shares are passed through to the ESOP participants. Dividends on unallocated shares are used by the ESOP to make debt service payments on the notes due to Patterson.
14. Stock-based Compensation
The consolidated statements of income for fiscal years 2012, 2011 and 2010 include pre-tax stock-based compensation expense of $12.6 million ($8.4 million after-tax), $10.5 million ($7.2 million after-tax) and $8.8 million ($6.2 million after-tax), respectively, recorded in accordance with the provisions of ASC Topic 718 "Stock Compensation". All pre-tax expense is included in operating expenses within the consolidated statements of income. The consolidated statement of cash flows present the pre-tax stock-based compensation expense as an adjustment to reconcile net income to net cash provided by operating activities. In addition, benefits associated with tax deductions in excess of recognized compensation expense are presented as a cash inflow from financing activities. For fiscal years 2012, 2011 and 2010, these excess benefits totaled $1.4, $1.3 and $0.4, respectively.
As of April 28, 2012, the total compensation cost, before income taxes, related to non-vested awards yet to be recognized was $23.1 million, and it is expected to be recognized over a weighted average period of approximately 3.4 years.
Description of General Methods and Assumptions Used to Estimate Fair Value
Described below are certain methods and assumptions used to estimate the fair value of stock-based compensation awards. Further information is presented below within this Note that may be unique to a particular award or group of awards.
70
Expected dividend yield-Patterson's initial quarterly dividend occurred in the fourth quarter of fiscal 2010. Accordingly, the expected dividend yield used had been 0% for awards issued prior to that time. For awards issued since, Patterson has included an expected dividend yield based on estimates as of the grant date of awards.
Expected stock price volatility-We have considered historical volatility trends, implied future volatility based on certain traded options and other factors.
Risk-free interest rate-We base the risk-free interest rate on the U.S. Treasury yield curve in effect at the grant date with similar terms to the expected term of the award.
Expected term of stock options and restricted stock-We estimate the expected term, or life, of awards based on several factors, including grantee types, vesting schedules, contractual terms and various factors surrounding exercise behavior of different groups.
Director and Employee Stock Option Plans
In June 1992, we adopted a Director Stock Option Plan. Options were granted at the fair market value of the underlying stock on the date of grant, vest over one year, and are exercisable for a period of four years commencing one year after the date of grant. This plan terminated during fiscal 2002.
In September 2001, we adopted a new Director Stock Option Plan. A total of 800,000 shares of common stock have been reserved for issuance under this plan. Options are granted at fair market value of the underlying stock on the option grant date, vest over one year, and are exercisable for a period of nine years commencing one year after the grant date.
In June 1992, we adopted the Patterson Dental Company 1992 Stock Option Plan, a plan for employees. Due to the expiration of this plan in fiscal 2003, no options remain available for future issuance under this plan. In September 2002, we adopted a new employee equity award plan. A total of 6,000,000 shares of common stock were reserved for issuance under the plan. In September 2004, our shareholders voted to approve the Amended and Restated 2002 Stock Option Plan, a restatement of the 2002 plan. Upon approval, the Plan was renamed the "Patterson Companies, Inc. Equity Incentive Plan" ("Equity Incentive Plan").
The Equity Incentive Plan amendments did not change the number of shares reserved for awards under the plan. The Equity Incentive Plan authorizes various award types to be issued under the plan, including stock options, restricted stock and restricted stock units, stock bonuses, cash bonuses, stock appreciation rights, performance awards and dividend equivalents. Awards may have a term no longer than ten years and vesting terms are determined by the compensation committee of the Board of Directors. The minimum restriction period for restricted stock and restricted stock units is three years, or one year in the case of performance-based awards. Additionally, a plan amendment in September 2010 increased the maximum number of shares that may be issued pursuant to awards of restricted stock, restricted stock awards and stock bonuses from 2,000,000 shares to 6,000,000 shares. Prior to fiscal 2006, only stock option awards had been granted under the Equity Incentive Plan. During fiscal years 2012, 2011 and 2010, expense recognized related to stock options was $1.6 million, $1.7 million and $2.0 million, respectively.
The fair value of stock options granted was estimated as of the grant date using a Black-Scholes option-pricing model with the following weighted average assumptions during fiscal years 2012, 2011 and 2010:
| April 28, 2012 | April 30, 2011 | April 24, 2010 | ||||||||||
Expected dividend yield | 1.5 | % | 1.2 | % | - | |||||||
Expected stock price volatility | 33.0 | % | 38.0 | % | 32.7 | % | ||||||
Risk-free interest rate | 2.5 | % | 3.6 | % | 2.7 | % | ||||||
Expected life of options (years) | 7.4 | 8.8 | 8.3 | |||||||||
71
Following is a summary of stock option activity for all plans during fiscal years 2012, 2011 and 2010:
| Total Outstanding | ||||||||||||
| Number of Options | Exercise Price (a) | Intrinsic Value | ||||||||||
Balance as of April 25, 2009 | 1,898 | $ | 25.24 | |||||||||
Granted | 86 | 20.76 | ||||||||||
Exercised | (281 | ) | 12.36 | |||||||||
Canceled | (55 | ) | 33.15 | |||||||||
|
|
|
| |||||||||
Balance as of April 24, 2010 | 1,648 | $ | 26.94 | |||||||||
Granted | 51 | 32.08 | ||||||||||
Exercised | (334 | ) | 17.82 | |||||||||
Canceled | (92 | ) | 23.02 | |||||||||
|
|
|
| |||||||||
Balance as of April 30, 2011 | 1,273 | $ | 29.82 | |||||||||
Granted | 64 | 33.90 | ||||||||||
Exercised | (232 | ) | 24.11 | |||||||||
Canceled | (119 | ) | 29.64 | |||||||||
|
|
|
| |||||||||
Balance as of April 28, 2012 | 986 | $ | 31.45 | $ | 4,453 | |||||||
|
|
|
|
|
| |||||||
Vested or expected to vest as of April 28, 2012 | 863 | $ | 31.15 | $ | 4,161 | |||||||
|
|
|
|
|
| |||||||
Exercisable as of April 28, 2012 | 576 | $ | 29.94 | $ | 3,479 | |||||||
|
|
|
|
|
| |||||||
| (a) | Weighted-average exercise price |
The weighted average fair values of options granted during fiscal years 2012, 2011 and 2010 were $11.47, $14.07, and $8.95, respectively. The weighted average remaining contractual lives of options outstanding and options exercisable as of April 28, 2012 were 3.3 and 2.3 years, respectively. We settle stock option exercises with newly issued common shares.
Related to stock options exercised, the intrinsic value, cash received and tax benefits realized were $1.7, $5.6 and $0.4 million, respectively, in fiscal 2012; $4.4, $6.1 and $1.2 million, respectively, in fiscal 2011; and $3.4, $3.5 and $1.3 million, respectively, in fiscal 2010.
Restricted Stock and Performance Unit Awards
In fiscal 2006, we began to issue restricted stock and performance unit awards under the Equity Incentive Plan. The grant date fair value is based on the closing stock price on the day of the grant. Restricted stock awards to employees generally vest over a five, seven or nine-year period and are subject to forfeiture provisions. Certain restricted stock awards, which are held by line management, are subject to accelerated vesting provisions beginning three years after the grant date, based on certain operating goals. Restricted stock awards are also granted to non-employee directors on the date of each annual board meeting. These awards vest over three years. The performance unit awards, issued primarily to executive management, are earned at the end of a three-year period if certain operating goals are met, and are settled in an equivalent number of common shares or in cash as determined by the compensation committee of the Board of Directors. The satisfaction of operating goals is not finally determined until the end of a three-year period. Accordingly, Patterson recognizes expense related to performance unit awards over the requisite service period using the straight-line method based on the outcome that is probable. During fiscal years 2012, 2011 and 2010, expense recognized related to restricted stock and performance unit awards was $8.0 million, $5.5 million and $3.7 million, respectively. The total intrinsic value of restricted stock awards that vested in fiscal 2012, 2011 and 2010 was $4.6 million, $3.5 million and $0.8 million, respectively. No performance units were granted in fiscal 2011 or fiscal 2010 and none of the
72
performance units that had been awarded prior to fiscal 2010 were ultimately earned. Patterson granted performance units in fiscal 2012 which can be earned at the end of fiscal 2014, subject to the achievement of certain financial objectives.
The following tables summarize information concerning non-vested restricted stock awards and performance unit awards for fiscal years 2012, 2011 and 2010:
| Restricted Stock Awards | ||||||||
| Shares | Weighted
Average Grant-Date Fair Value | |||||||
Outstanding at April 25, 2009 | 537 | $ | 35.77 | |||||
Granted | 451 | 20.35 | ||||||
Vested | (34 | ) | (36.65 | ) | ||||
Forfeitures | (35 | ) | (29.21 | ) | ||||
|
|
|
| |||||
Outstanding at April 24, 2010 | 919 | $ | 28.42 | |||||
Granted | 406 | 31.91 | ||||||
Vested | (104 | ) | (33.07 | ) | ||||
Forfeitures | (110 | ) | (28.81 | ) | ||||
|
|
|
| |||||
Outstanding at April 30, 2011 | 1,111 | $ | 29.22 | |||||
Granted | 272 | 34.60 | ||||||
Vested | (138 | ) | (25.45 | ) | ||||
Forfeitures | (83 | ) | (29.25 | ) | ||||
|
|
|
| |||||
Outstanding at April 28, 2012 | 1,162 | $ | 30.92 | |||||
|
|
|
| |||||
| Performance Unit Awards | ||||||||
| Shares | Weighted
Average Grant-Date Fair Value | |||||||
Outstanding at April 25, 2009 | 63 | $ | 32.80 | |||||
Forfeitures and cancellations | (36 | ) | (34.24 | ) | ||||
|
|
|
| |||||
Outstanding at April 24, 2010 | 27 | $ | 30.88 | |||||
Forfeitures and cancellations | (27 | ) | (30.88 | ) | ||||
|
|
|
| |||||
Outstanding at April 30, 2011 | 0 | $ | 0 | |||||
Granted | 102 | 35.41 | ||||||
Forfeitures and cancellations | (6 | ) | (35.41 | ) | ||||
|
|
|
| |||||
Outstanding at April 28, 2012 | 96 | $ | 35.41 | |||||
|
|
|
| |||||
Employee Stock Purchase Plan
In June 1992, Company adopted an Employee Stock Purchase Plan (the "Stock Purchase Plan"). A total of 4,750,000 shares of common stock are reserved for issuance under the Stock Purchase Plan. The Stock Purchase Plan, which is intended to qualify under Section 423 of the Internal Revenue Code, is administered by the Board of Directors or by a committee appointed by the Board of Directors and follows a calendar plan year. Employees are eligible to participate after nine months of employment, if they are employed for at least 20 hours per week and more than five months per year. The Stock Purchase Plan permits eligible employees to purchase common stock through payroll deductions, which may not exceed 10 percent of an employee's compensation, at 85 percent of the lower of the fair market value of the common stock on the offering date or at the end of each three-month period following the offering date during the applicable offering period. Employees may end their participation in the offering at any time during the offering period, and participation ends automatically on termination of employment. At April 28, 2012, there were 271,886 shares available for purchase under the Stock Purchase Plan.
73
The Stock Purchase Plan includes a look-back option and accordingly there are several option elements for which the fair value is estimated on the grant date using the Black-Scholes option-pricing model. Total expense recognized related to the employee stock purchase plan was $1.8, $1.8 and $1.6 million during fiscal years 2012, 2011 and 2010, respectively. The following table summarizes the weighted-average assumptions relating to the Stock Purchase Plan for fiscal years 2012, 2011 and 2010:
| 2012 | 2011 | 2010 | ||||||||||
Expected dividend yield | 1.4 | % | 0.4 | % | 0.0 | % | ||||||
Expected stock price volatility | 31.3 | % | 31.3 | % | 29.0 | % | ||||||
Risk-free interest rate | 0.2 | % | 0.2 | % | 0.1 | % | ||||||
Expected life of options (years) | 0.5 | 0.5 | 0.5 | |||||||||
Capital Accumulation Plan
In 1996, we adopted an employee Capital Accumulation Plan (the "CAP Plan"). A total of 6,000,000 shares of common stock are reserved for issuance under the CAP Plan. Key employees of Patterson or its subsidiaries are eligible to participate by purchasing common stock through payroll deductions, which must be between 5% and 25% of an employee' compensation, at 75% of the price of the common stock at the beginning of or the end of the calendar year, whichever is lower. The shares issued are restricted stock and are held in the custody of Patterson until the restrictions lapse. The restriction period is three years from the beginning of the plan year, but restricted shares are subject to forfeiture provisions. At April 28, 2012, 2,345,792 shares were available for purchase under the CAP Plan.
Based on the provisions of the CAP Plan, there are option elements for which the fair value is estimated on the grant date using the Black-Scholes option-pricing model. Total expense recognized related to the CAP Plan was $1.3, $1.4 and $1.6 million during fiscal years 2012, 2011 and 2010, respectively. The following table summarizes the weighted-average assumptions relating to the CAP Plan for fiscal years 2012, 2011 and 2010:
| 2012 | 2011 | 2010 | ||||||||||
Expected dividend yield | 1.4 | % | 0.4 | % | 0.0 | |||||||
Expected stock price volatility | 31.3 | % | 31.3 | % | 29.0 | % | ||||||
Risk-free interest rate | 0.3 | % | 0.4 | % | 1.1 | % | ||||||
Expected life of options (years) | 1.0 | 1.0 | 1.0 | |||||||||
15. Litigation
We are involved in various product related, employment related and other legal proceedings arising in the ordinary course of business. Some of these proceedings involve product liability claims arising out of the use of products we distribute. Product liability indemnification is generally obtained from our suppliers. However, in the event a supplier of a defective product is unable to pay a judgment for which Patterson may be jointly liable, Patterson would have liability for the entire judgment.
We maintain product liability insurance coverage for any potential liability for claims arising out of products sold by us. While we believe our insurance coverage is adequate, there can be no assurance that our insurance coverage is sufficient or will be available to in adequate amounts or at reasonable costs in the future. Also, there can be no assurance that the indemnification agreements with our suppliers will provide adequate protection. In addition, future claims brought against us could involve claims not covered by insurance or indemnification agreements, and could have a material adverse effect on our business or financial condition.
As of April 28, 2012 and April 24, 2011, Patterson had accrued our best estimate of potential losses relating to product liability and other claims that were probable to result in a liability and for which it was possible to reasonably estimate a loss. These accrued amounts, as well as related expenses, have not been material to our
74
financial position, results of operations or cash flows. Our method for determining estimated losses considers currently available facts, presently enacted laws and regulations and other external factors, including probable recoveries from third parties.
16. Quarterly Results (unaudited)
Quarterly results are determined in accordance with the accounting policies used for annual data and include certain items based upon estimates for the entire year. All fiscal quarters include results for 13 weeks, except for the quarter ended July 31, 2011, which included 14 weeks. The following table summarizes results for fiscal 2012 and 2011.
| Quarter Ended | ||||||||||||||||
| Apr. 28, 2012 | Jan. 28, 2012 | Oct. 29, 2011 | July 30, 2011 | |||||||||||||
Net sales | $ | 936,334 | $ | 895,030 | $ | 856,875 | $ | 847,422 | ||||||||
Gross profit | 313,721 | 289,534 | 280,983 | 278,276 | ||||||||||||
Operating income | 102,851 | 89,906 | 83,259 | 81,993 | ||||||||||||
Net income | 62,143 | 53,108 | 48,954 | 48,610 | ||||||||||||
Earnings per share-basic | $ | 0.59 | $ | 0.50 | $ | 0.43 | $ | 0.42 | ||||||||
Earnings per share-diluted | $ | 0.58 | $ | 0.50 | $ | 0.43 | $ | 0.42 | ||||||||
| Quarter Ended | ||||||||||||||||
| Apr. 30, 2011 | Jan. 29, 2011 | Oct. 30, 2010 | July 31, 2010 | |||||||||||||
Net sales | $ | 883,819 | $ | 824,650 | $ | 857,414 | $ | 849,787 | ||||||||
Gross profit | 303,949 | 280,875 | 279,201 | 280,200 | ||||||||||||
Operating income | 104,125 | 92,707 | 90,152 | 89,024 | ||||||||||||
Net income | 62,707 | 55,396 | 53,357 | 53,925 | ||||||||||||
Earnings per share-basic | $ | 0.52 | $ | 0.47 | $ | 0.45 | $ | 0.45 | ||||||||
Earnings per share-diluted | $ | 0.52 | $ | 0.47 | $ | 0.45 | $ | 0.45 | ||||||||
75
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| Item 9A. | CONTROLS AND PROCEDURES |
Management's Annual Report on Internal Control Over Financial Reporting
The management of Patterson Companies, Inc. (the "Company") is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. Our internal control system is designed to provide reasonable assurance to our management and Board of Directors regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Patterson's internal control over financial reporting includes those policies and procedures that:
Pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of Patterson;
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of Patterson are being made only in accordance with authorizations of management and directors; and
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those internal control systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial and accounting officer, we assessed the effectiveness of our internal control over financial reporting as of April 28, 2012, using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework. Based on its assessment, management has concluded that our internal control over financial reporting was effective as of April 28, 2012. During its assessment, management did not identify any material weaknesses in our internal control over financial reporting. Ernst & Young LLP, the independent registered public accounting firm that audited our consolidated financial statements included in Item 8, Financial Statements and Supplementary Data, of this Annual Report on Form 10-K, has issued an unqualified report on our internal control over financial reporting.
/s/ S COTT P. A NDERSON |
| President and Chief Executive Officer |
/s/ R. S TEPHEN A RMSTRONG |
| Executive Vice President, Chief Financial |
| Officer and Treasurer |
76
The report of our independent registered public accounting firm on internal control over financial reporting is included in Item 8. of this Annual Report on Form 10-K.
Evaluation of Disclosure Controls and Procedures
As of April 28, 2012, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rules 13a-15 and 15d-15 of the Securities and Exchange Act of 1934 (the "Exchange Act"). Based on that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective. Disclosure controls and procedures are defined by Rules 13a-15(e) and 15d-15(e) of the Exchange Act as controls and other procedures that are designed to ensure that information required to be disclosed by Patterson in reports filed with the SEC under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in reports filed under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officers, or person performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting
During the fourth quarter of fiscal year 2012, there were no significant changes in our internal controls over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
| Item 9B. | OTHER INFORMATION |
None.
77
PART III
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information regarding the directors of Patterson is incorporated herein by reference to the descriptions set forth under the caption "Proposal No. 1 Election of Directors" in Patterson's Proxy Statement for its Annual Meeting of Shareholders to be held on September 10, 2012 (the "2012 Proxy Statement"). Information regarding executive officers of Patterson is incorporated herein by reference to Item 1 of Part I of this Form 10-K under the caption "Executive Officers of the Registrant." Information regarding compliance with Section 16(a) of the Securities Exchange Act of 1934 is incorporated herein by reference to the information set forth under the caption "Section 16(a) Beneficial Ownership Reporting Compliance" in the 2012 Proxy Statement. The information called for by Item 10, as to the audit committee and the audit committee financial expert, is set forth under the captions "Proposal No. 1 Election of Directors" and "Our Board of Directors and Committees" in the 2012 Proxy Statement and such information is incorporated by reference herein.
Code of Ethics
We have adopted Principles of Business Conduct and Code of Ethics for our Chief Executive Officer, Chief Financial Officer, Directors and all employees. Our Code of Ethics is available on our website (www.pattersoncompanies.com) under the section "Investor Relations-Governance." We intend to satisfy the disclosure requirement of Form 8-K regarding an amendment to, or waiver from, a provision of its Code of Ethics by posting such information on its website at the address and location specified above.
| Item 11. | EXECUTIVE COMPENSATION |
Information regarding executive compensation and director compensation is incorporated herein by reference to the information set forth under the captions "Non-Employee Director Compensation" and "Executive Compensation" in the 2012 Proxy Statement.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information regarding the security ownership of certain beneficial owners and management is incorporated herein by reference to the information set forth under the caption "Security Ownership of Certain Beneficial Owners and Management: and "Equity Compensation Plan Information" in the 2012 Proxy Statement.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Information called for by Item 13 is incorporated herein by reference to the information set forth under the captions "Certain Relationships and Related Transactions" and "Our Board of Directors and Committees" in the 2012 Proxy Statement.
| Item 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
Information relating to principal accounting fees and services and pre-approval policies and procedures is set forth under the captions "Proposal No. 6 Ratification of Selection of Independent Registered Public Accounting Firm-Principal Accountant Fees and Services" in the 2012 Proxy Statement and such information is incorporated by reference herein.
78
PART IV
| Item 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
| (a) | 1. Financial Statements. |
The following Consolidated Financial Statements and supplementary data of Patterson and its subsidiaries are included in Part II, Item 8:
Reports of Independent Registered Public Accounting Firm | 48 | |||
Consolidated Balance Sheets as of April 28, 2012 and April 30, 2011 | 49 | |||
Consolidated Statements of Income for the Years Ended April 28, 2012, April 30, 2011 and April 24, 2010 | 50 | |||
Consolidated Statement of Changes in Stockholders' Equity for the Years Ended April 28, 2012, April 30, 2011 and April 24, 2010 | 51 | |||
Consolidated Statements of Cash Flows for the Years Ended April 28, 2012, April 30, 2011 and April 24, 2010 | 52 | |||
Notes to Consolidated Financial Statements | 53 | |||
2. Financial Statement Schedules.
The following financial statement schedule is filed herewith: Schedule II-Valuation and Qualifying Accounts for the Years Ended April 28, 2012, April 30, 2011 and April 24, 2010.
Schedules other than that listed above have been omitted because they are not applicable or the required information is included in the financial statements or notes thereto.
3. Exhibits.
Exhibit | ||
| 2.1 | Article of Merger and Plan of Merger dated June 23, 2004 10 | |
| 3.1 | Patterson's Restated Articles of Incorporation 10 | |
| 3.2 | Patterson's Bylaws, as amended 1 | |
| 4.1 | Specimen form of Patterson's Common Stock Certificate 10 | |
| 4.2 | Pursuant to Item 601 (b)(4)(iii)(A) of Regulation S-K, the registrant has omitted to file certain unregistered convertible debentures. The total amount of securities authorized thereunder does not exceed 10 percent of the total assets of the registrant and its subsidiaries on a consolidated basis. The registrant hereby agrees to furnish a copy of such convertible debentures to the Commission upon request. 8 | |
| 4.3 | Credit Agreement dated as of November 25, 2003 among Patterson Dental Company, the Subsidiary Borrowers from time to time parties hereto, the Lenders from time to time parties hereto, Bank One, NA (main office Chicago), as Administrative Agent, Bank of America, N.A., as Syndication Agent and Suntrust Bank, the Northern Trust Company, and U.S. Bank National Association, as Documentation Agents 9 | |
| 4.4 | Note Purchase Agreement dated as of November 15, 2003 among Patterson Dental Company, AbilityOne Products Corp., AbilityOne Corporation, Patterson Dental Supply, Inc., Webster Veterinary Supply, Inc. and Webster Management, LP 9 | |
79
Exhibit | ||
| 10.1 | Patterson Dental Company Employee Stock Ownership Plan, as amended 1 | |
| 10.2 | Patterson Dental Company 1992 Stock Option Plan 1 | |
| 10.3 | Patterson Dental Company 1992 Director Stock Option Plan 1 | |
| 10.4 | Patterson Dental Company Employee Stock Purchase Plan 1 | |
| 10.5 | Patterson Dental Company Capital Accumulation Plan 2 | |
| 10.6 | Patterson Companies, Inc. fiscal 2012 Incentive Plan | |
| 10.7 | ESOP Loan Agreement dated June 15, 1990 as amended July 13, 1992 1 | |
| 10.8 | Amended and Restated Term Promissory Note dated July 13, 1992 1 | |
| 10.9 | Second Amended and Restated Contract Purchase Agreement dated April 28, 2000 between Patterson Dental Company and U.S. Bank National Association 3 | |
| 10.10 | Amended and Restated Credit Agreement dated April 28, 2000 between Patterson Dental Company and U.S. Bank National Association 3 | |
| 10.11 | Asset Purchase Agreement by and among Patterson Dental Company and J. A. Webster, Inc. 4 | |
| 10.12 | Third Amended and Restated Contract Purchase Agreement dated June 19, 2002 between Patterson Dental Company and U. S. Bank National Association 5 | |
| 10.13 | Third Amended and Restated Receivables Purchase Agreement dated December 3, 2011 between PDC Funding Company, LLC, Patterson Companies, Inc., The Bank of Tokyo-Mitsubishi UFJ, Ltd., New York Branch (the "Bank") and a commercial paper conduit managed by the Bank. 12 | |
| 10.14 | Receivables Sale Agreement dated May 10, 2002 among PDC Funding Company, LLC, Patterson Dental Supply, Inc., and Webster Veterinary Supply, Inc. 5 | |
| 10.15 | 2001 Non-Employee Director Stock Option Plan 5 | |
| 10.16 | Amendments to Restated Employee Stock Purchase Plan 5 | |
| 10.17 | Amended and Restated Employee Stock Ownership Plan 5 | |
| 10.18 | Stock Option Plan for Canadian Employees 6 | |
| 10.19 | Patterson Companies, Inc. Equity Incentive Plan 11 | |
| 10.20 | ESOP Loan Agreement dated April 1, 2002 7 | |
| 10.21 | Promissory Note dated April 1, 2002 between GreatBanc Trust Company, an Illinois corporation, not in its individual or corporate capacity, but solely as trustee of the Thompson Dental Company Employee Stock Ownership Plan and Trust and Thompson Dental Company 7 | |
| 10.22 | Bridge Credit Facility dated as of September 12, 2003 among Patterson Dental Company as the borrower and Banc One Mezzanine Corporation, as Administrative Agent and Bank of America, N.A., as Syndication Agent. 8 | |
| 10.23 | ESOP Loan Agreement dated September 11, 2006 13 | |
| 10.24 | ESOP Note dated September 11, 2006 13 | |
| 10.25 | Receivables Sale Agreement dated April 27, 2007 among PDC Funding Company II, LLC, Patterson Dental Supply, Inc., and Webster Veterinary Supply, Inc. 14 | |
| 10.26 | Contract Purchase Agreement dated April 27, 2007 among PDC Funding Company II, LLC, Patterson Companies, Inc., U.S. Bank National Association and The Northern Trust Company 14 | |
80
Exhibit | ||
| 10.27 | Amended and Restated Credit Agreement, dated as of November 28, 2007, among Patterson Companies, Inc., as Patterson, the Subsidiary Borrowers from time to time parties hereto, the Lenders from time to time parties hereto, JPmorgan Chase Bank, National Association (Successor by merger to Bank One, NA (Main Office Chicago)), as Administrative Agent, Bank of America, N.A., as Syndication Agent, and SunTrust Bank, the Northern Trust Company, and U.S. Bank National Association, as Documentation Agents. 15 | |
| 10.28 | Note Purchase Agreement dated March 19, 2008 among Patterson Companies, Inc., Patterson Medical Holdings, Inc., Patterson Medical Supply, Inc., Patterson Dental Holdings, Inc., Patterson Dental Supply, Inc., Webster Veterinary Supply, Inc. and Webster Management, LP 16 | |
| 10.29 | Term Loan Credit Agreement dated March 20, 2008 among Patterson Companies, Inc., as the Borrower, the Lenders from time to time parties hereto and JPMorgan Chase Bank, National Association (Successor by merger to Bank One, NA (Main Office Chicago)), as Administrative Agent 16 | |
| 10.30 | Accelerated Share Repurchase Agreement, dated March 19, 2008, by and between Patterson Companies, Inc. and JPMorgan Chase Bank, National Association 16 | |
| 21 | Subsidiaries | |
| 23 | Consent of Independent Registered Public Accounting Firm | |
| 31.1 | Certification of the Chief Executive Officer pursuant to Rules 13a-4(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2 | Certification of the Chief Financial Officer pursuant to Rule 13a-4(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1 | Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2 | Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101 | (Filed Electronically) The following financial information from our Annual Report on Form 10-K for fiscal 2012, filed with the SEC on June 29, 2012, formatted in Extensible Business Reporting Language (XBRL): (i) the consolidated balance sheets at April 28, 2012 and April 24, 2011, (ii) the consolidated statements of income for the year ended April 28, 2012, April 24, 2011 and April 25, 2010, (iii) the consolidated statements of cash flows for the years ended April 28, 2012, April 24, 2011 and April 25, 2010, (iv) the consolidated statements of changes in stockholders' equity for the years ended April 28, 2012, April 24, 2011 and April 25, 2010 and (v) the notes to the consolidated financial statements. (a) | |
| (a) | The XBRL related information in Exhibit 101 to this Annual Report on Form 10-K shall not be deemed "filed" for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to liability of that section and shall not be incorporated by reference into any filing or other document pursuant to the Securities Act of 1933, as amended, except as shall be expressly set forth by specific reference in such filing or document. |
| 1 | Incorporated by reference to the Registrant's Registration Statement on Form S-1 (No. 33-51304) filed with the Securities and Exchange Commission August 26, 1992. |
| 2 | Incorporated by reference to the Registrant's Form 10-K for the fiscal year ended April 27, 1996. |
| 3 | Incorporated by reference to the Registrant's Form 10-K for the fiscal year ended April 29, 2000. |
| 4 | Incorporated by reference to the Registrant's Form 10-K for the fiscal year ended April 28, 2001. |
| 5 | Incorporated by reference to the Registrant's Form 10-K for the fiscal year ended April 27, 2002. |
| 6 | Incorporated by reference to the Registrant's Form 10-Q for the quarterly period ended January 25, 2003. |
81
| 7 | Incorporated by reference to the Registrant's Form 10-K for the fiscal year ended April 26, 2003. |
| 8 | Incorporated by reference to the Registrant's Form 10-Q for the quarterly period ended October 25, 2003. |
| 9 | Incorporated by reference to the Registrant's Form 10-Q for the quarterly period ended January 24, 2004. |
| 10 | Incorporated by reference to the Registrant's Form 10-Q for the quarterly period ended July 31, 2004. |
| 11 | Incorporated by reference to the Registrant's Form 8-K/A dated September 14, 2004, filed on September 29, 2004. |
| 12 | Incorporated by reference to the Registrant's Form 8-K dated December 3, 2011, filed on December 8, 2011. |
| 13 | Incorporated by reference to the Registrant's Form 8-K dated September 11, 2006, filed on September 12, 2006. |
| 14 | Incorporated by reference to the Registrant's Form 8-K dated April 27, 2007, filed on May 3, 2007. |
| 15 | Incorporated by reference to the Registrant's Form 8-K dated November 28, 2007, filed on December 3, 2007. |
| 16 | Incorporated by reference to the Registrant's Form 8-K dated March 19, 2008, filed on March 24, 2008. |
| (b) | See Schedule II. |
| (c) | See Index to Exhibits. |
82
SIGNATURES
Pursuant to the requirements of section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| PATTERSON COMPANIES, INC. | ||||||
| Dated: June 27, 2012 | By | /s/ S COTT P. A NDERSON | ||||
| Scott P. Anderson, | ||||||
| President and Chief Executive Officer | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Date | ||||
/s/ S COTT P. A NDERSON Scott P. Anderson | President and Chief Executive Officer (Principal Executive Officer) | June 27, 2012 | ||
/s/ R. S TEPHEN A RMSTRONG R. Stephen Armstrong | Executive Vice President, Treasurer, and Chief Financial Officer (Principal Financial and Accounting Officer) | June 27, 2012 | ||
/s/ P ETER L. F RECHETTE Peter L. Frechette | Director (Chairman of the Board of Directors) | June 27, 2012 | ||
/s/ J OHN D. B UCK John D. Buck | Director | June 27, 2012 | ||
/s/ J ODY H. F ERAGEN Jody H. Feragen | Director | June 27, 2012 | ||
/s/ A NDRE B. L ACY Andre B. Lacy | Director | June 27, 2012 | ||
/s/ C HARLES R EICH Charles Reich | Director | June 27, 2012 | ||
/s/ E LLEN A. R UDNICK Ellen A. Rudnick | Director | June 27, 2012 | ||
/s/ H AROLD C. S LAVKIN Harold C. Slavkin | Director | June 27, 2012 | ||
/s/ B RIAN S. T YLER Brian S. Tyler | Director | June 27, 2012 | ||
/s/ L ES C. V INNEY Les C. Vinney | Director | June 27, 2012 | ||
/s/ J AMES W. W ILTZ James W. Wiltz | Director | June 27, 2012 | ||
83
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
PATTERSON COMPANIES, INC.
(Dollars in thousands)
| Balance at Beginning of Period | Charged to Costs and Expenses | Charged to Other Accounts | Deductions | Balance at End of Period | ||||||||||||||||
Year ended April 28, 2012: | ||||||||||||||||||||
Deducted from asset accounts: | ||||||||||||||||||||
Allowance for doubtful accounts | $ | 8,365 | $ | 2,445 | $ | - | $ | 2,979 | $ | 7,831 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
LIFO inventory adjustment | $ | 61,385 | $ | 5,422 | $ | - | $ | - | $ | 66,807 | ||||||||||
Inventory obsolescence reserve | 5,844 | 11,508 | - | 11,896 | 5,456 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total inventory reserve | $ | 67,229 | $ | 16,930 | $ | - | $ | 11,896 | $ | 72,263 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Year ended April 30, 2011: | ||||||||||||||||||||
Deducted from asset accounts: | ||||||||||||||||||||
Allowance for doubtful accounts | $ | 9,111 | $ | 3,409 | $ | - | $ | 4,155 | $ | 8,365 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
LIFO inventory adjustment | $ | 56,961 | $ | 4,424 | $ | - | $ | - | $ | 61,385 | ||||||||||
Inventory obsolescence reserve | 8,329 | 10,403 | - | 12,888 | 5,844 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total inventory reserve | $ | 65,290 | $ | 14,827 | $ | - | $ | 12,888 | $ | 67,229 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Year ended April 24, 2010: | ||||||||||||||||||||
Deducted from asset accounts: | ||||||||||||||||||||
Allowance for doubtful accounts | $ | 9,773 | $ | 4,579 | $ | - | $ | 5,241 | $ | 9,111 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
LIFO inventory adjustment | $ | 51,934 | $ | 5,027 | $ | - | $ | - | $ | 56,961 | ||||||||||
Inventory obsolescence reserve | 8,381 | 10,347 | - | 10,399 | 8,329 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total inventory reserve | $ | 60,315 | $ | 15,374 | $ | - | $ | 10,399 | $ | 65,290 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
84
INDEX TO EXHIBITS
| Exhibit 10.6 | Patterson Companies, Inc. Fiscal 2012 Incentive Compensation Plan | |
| Exhibit 21 | Subsidiaries | |
| Exhibit 23 | Consent of Independent Registered Public Accounting Firm | |
| Exhibit 31.1 | Certification of the Chief Executive Officer pursuant to Rules 13a-4(a) and 15d-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| Exhibit 31.2 | Certification of the Chief Financial Officer pursuant to Rules 13a-4(a) and 15d-14(a), under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| Exhibit 32.1 | Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| Exhibit 32.2 | Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101 | (Filed Electronically) The following financial information from our Annual Report on Form 10-K for fiscal 2012, filed with the SEC on June 29, 2012, formatted in Extensible Business Reporting Language (XBRL): (i) the consolidated balance sheets at April 28, 2012 and April 24, 2011, (ii) the consolidated statements of income for the year ended April 28, 2012, April 24, 2011 and April 25, 2010, (iii) the consolidated statements of cash flows for the years ended April 28, 2012, April 24, 2011 and April 25, 2010, (iv) the consolidated statements of changes in stockholders' equity for the years ended April 28, 2012, April 24, 2011 and April 25, 2010 and (v) the notes to the consolidated financial statements. (a) | |
85
