UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2013
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 0-26642
MYRIAD GENETICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 87-0494517 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
| 320 Wakara Way, Salt Lake City, UT | 84108 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant's telephone number, including area code: (801) 584-3600
Securities registered pursuant to Section 12(b) of the Exchange Act:
Title of each class | Name of each exchange on which registered | |
| Common Stock, $.01 Par Value Per Share | The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer", and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold on December 31, 2012, the last business day of the registrant's most recently completed second fiscal quarter, was $2,209,171,779.
As of August 1, 2013 the registrant had 80,446,692 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the Registrant's Proxy Statement for the Annual Meeting of Stockholders to be held on December 5, 2013.
TABLE OF CONTENTS
| Page | ||||||
| PART I | ||||||
Item 1. | Business | 3 | ||||
Item 1A. | Risk Factors | 20 | ||||
Item 1B. | Unresolved Staff Comments | 36 | ||||
Item 2. | Properties | 36 | ||||
Item 3. | Legal Proceedings | 37 | ||||
Item 4. | Mine Safety Disclosures | 38 | ||||
| PART II | ||||||
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 39 | ||||
Item 6. | Selected Financial Data | 42 | ||||
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | 45 | ||||
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 55 | ||||
Item 8. | Financial Statements and Supplementary Data | 55 | ||||
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 56 | ||||
Item 9A. | Controls and Procedures | 56 | ||||
Item 9B. | Other Information | 58 | ||||
| PART III | ||||||
Item 10. | Directors, Executive Officers and Corporate Governance | 59 | ||||
Item 11. | Executive Compensation | 59 | ||||
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 59 | ||||
Item 13. | Certain Relationships and Related Transactions, and Director Independence | 59 | ||||
Item 14. | Principal Accounting Fees and Services | 59 | ||||
| PART IV | ||||||
Item 15. | Exhibits, Financial Statement Schedules | 60 | ||||
| Signatures | 61 | |||||
"We," "us," "Myriad" and the "Company" as used in this Annual Report on Form 10-K refer to Myriad Genetics, Inc., a Delaware corporation, and its subsidiaries.
"Myriad," BRAC Analysis , COLARIS, COLARIS AP , MELARIS, PANEXIA, PREZEON, TheraGuide, Prolaris, TruCulture, DiscoveryMAP and RodentMap are registered trademarks or trademarks of Myriad.
2
PART I
| Item 1. | BUSINESS |
Overview
We are a leading molecular diagnostic company dedicated to making a difference in patients' lives through the discovery and commercialization of transformative tests which assess a person's risk of developing disease, guide treatment decisions and assess risk of disease progression and recurrence. We believe in improving healthcare for patients by providing physicians with critical information to solve unmet medical needs. By understanding the underlying genetic basis of disease, we believe that individuals who have a greater risk of developing disease can be identified and physicians may be able to use this information to improve patient outcomes and better manage patient healthcare. In addition, by understanding the RNA expression levels of certain genes, we believe that we can improve patient healthcare by providing information on the aggressiveness of their disease. Further, we believe that the analysis of the expression of groups of proteins may provide a physician with life-saving information to guide treatment decisions for their patients with cancer and other major diseases.
Our goal is to provide physicians with this critical information that may guide the healthcare management of their patients to prevent disease, diagnose the disease at an earlier stage, determine the most appropriate therapy, or assess the aggressiveness of their disease. We employ a number of proprietary technologies, including DNA, RNA and protein analysis, that help us to understand the genetic basis of human disease and the role that genes and their related proteins may play in the onset and progression of disease. We use this information to guide the development of new molecular diagnostic tests that are designed to assess an individual's risk for developing disease later in life (predictive medicine), identify a patient's likelihood of responding to drug therapy and guide a patient's dosing to ensure optimal treatment (personalized medicine), or assess a patient's risk of disease progression and disease recurrence (prognostic medicine).
Our business strategy for future growth is focused on three key initiatives. First, we are working to grow and expand our existing products and markets. Second, we are developing our business internationally and have recently established operations in Europe. Finally, we intend to launch new transformative products across a diverse set of disease indications, complementing our current businesses in oncology, women's health and urology.
We offer nine commercial molecular diagnostic tests, including six predictive medicine tests, two personalized medicine tests, and one prognostic medicine test. We market these tests through our own sales force of approximately 400 people in the United States. We also market our BRACAnalysis, COLARIS, COLARIS AP, and Prolaris tests through our own European sales force and have entered into marketing collaborations and distributor agreements with other organizations in selected Latin American, European, Asian and African countries. We also generate revenue by providing companion diagnostic services to the pharmaceutical and biotechnology industries and medical research institutions utilizing our multiplexed immunoassay technology. Total revenue was $613.2 million for the year ended June 30, 2013, an increase of 24% over the prior fiscal year.
During the fiscal year ended June 30, 2013, we devoted our resources to supporting (i) our predictive medicine, personalized medicine and prognostic medicine tests, (ii) our companion diagnostic business, and (iii) our research and development efforts on future molecular diagnostic candidate tests. For the year ended June 30, 2013, we had net income of $147.1 million. For the years ended June 30, 2013, 2012 and 2011, we had research and development expense of $53.7 million, $42.6 million and $27.8 million, respectively. Additional financial information about our three reportable segments is included in Note 10 to our audited financial statements for the fiscal year ended June 30, 2013 included with this Annual Report.
Our Business Strategy
Our business strategy is to understand the relationship between genes and their protein products and human diseases in order to develop the next generation of molecular diagnostic tests. Through our proprietary
3
technologies, we believe we are positioned to identify important disease genes, the proteins they produce, and the biological pathways in which they are involved to better understand the underlying molecular basis for the cause of human disease. We believe that identifying these genes, proteins, and pathways will enable us to develop novel molecular diagnostic tests. Our business strategy includes the following key elements:
| • | Discover important DNA, RNA and protein biomarkers, understand their function and determine their role in human disease. We plan to continue to use our proprietary DNA sequencing, RNA expression and protein analysis technologies, including our supporting bioinformatics and robotic technologies, in an effort to efficiently discover important genes and their proteins and to understand their role in human disease. We believe that our technologies provide us with a significant competitive advantage and the potential for numerous product opportunities. |
| • | Acquire promising biomarkers from other organizations. We intend to continue to take advantage of in-licensing or acquisition opportunities to augment our in-house tests development programs. For example, in September 2011, we obtained a three-year exclusive option to acquire Crescendo Bioscience, Inc., a company that is developing and marketing molecular diagnostic tests for patients suffering from autoimmune disorders, including rheumatoid arthritis, as described further in Note 13 to our financial statements for the fiscal year ended June 30, 2013 included in this Annual Report. We recognize that we cannot meet all of our research discovery goals internally and can benefit from the research performed by other organizations. We hope to leverage our financial strength, product development expertise, and sales and marketing presence to acquire new product opportunities in molecular diagnostic areas of focus. |
| • | Independently develop and commercialize new transformative molecular diagnostic tests. Our goal is to internally develop informative molecular diagnostic tests that can save lives and improve the quality of life of patients. Additionally, we plan to sell these tests through our own internal sales force and marketing efforts. In connection with any additional tests that we may launch, we plan to expand our existing oncology, urology, and women's health sales forces and build new sales forces to address other physician specialty groups. |
| • | Grow our molecular diagnostic business in the United States across multiple disease indications. We plan to continue to seek to expand our markets and increase the market penetration of our existing molecular diagnostic tests. Additionally, we plan to pursue new test opportunities in oncology, women's health, urology, dermatology, autoimmune and neuroscience diseases to capitalize on our leadership position in the molecular diagnostic industry. |
| • | Expand our molecular diagnostic business internationally. We believe that the market for our molecular diagnostic products in the major market countries in Europe, Latin America and Asia represents an attractive commercial opportunity. We have established sales offices in Canada, France, Spain, United Kingdom, Germany, Switzerland and Italy; laboratory operations in Germany; and international headquarters in Switzerland. We believe that our predictive medicine, personalized medicine, prognostic medicine and companion diagnostic products would benefit patients world-wide by assisting physicians in guiding their health care decisions. Our strategy is to continue to focus primarily on Europe and then expand to Latin America and Asia. |
Molecular Diagnostic Tests
Our molecular diagnostic tests are designed to analyze genes, their mutations, expression levels and proteins to assess an individual's risk for developing disease later in life, determine a patient's likelihood of responding to a particular drug, and assess a patient's risk of disease progression and disease recurrence. Armed with this valuable information, physicians may be able to more effectively manage their patient's healthcare to prevent or delay the onset of disease and ensure that patients receive the most appropriate treatment for their disease.
4
We offer nine primary commercial molecular diagnostic tests. Our current commercial molecular diagnostic tests are:
| • | BRACAnalysis ® : predictive medicine test for hereditary breast and ovarian cancer. Our BRAC Analysis test is an analysis of the BRCA1 and BRCA2 genes for assessing a woman's risk of developing hereditary breast and ovarian cancer. A woman who tests positive for a deleterious mutation with the BRAC Analysis test has up to an 87% risk of developing breast cancer and up to a 44% risk of developing ovarian cancer by age 70. As published in the Journal of the National Cancer Institute , researchers have shown that pre-symptomatic individuals who have a high risk of developing breast cancer can reduce their risk by approximately 50% with appropriate preventive therapies. Additionally, as published in the New England Journal of Medicine , researchers have shown that pre-symptomatic individuals who carry gene mutations can lower their risk of developing ovarian cancer by approximately 60% with appropriate preventive therapies. Additionally, BRAC Analysis may be used to assist patients already diagnosed with breast or ovarian cancer and their physicians in determining the most appropriate therapeutic interventions to address their disease. |
According to the American Cancer Society, in 2013 there will be approximately 255,000 women in the United States diagnosed with breast cancer or ovarian cancer. The test is currently priced at $3,340 and is covered by all major managed care organizations, or MCOs, and health insurance providers in the United States. We own or have exclusive rights to 24 U.S. patents covering BRAC Analysis testing. BRAC Analysis accounted for 75.1% of our total revenue during the year ended June 30, 2013.
| • | BART ® (BRAC Analysis Large Rearrangement Test): predictive medicine test for hereditary breast and ovarian cancer. Our BART test is a predictive medicine test for detecting large genomic rearrangements in the genes involved in hereditary breast and ovarian cancer patients. |
As published in the journal Cancer, researchers have shown that up to 10% of hereditary breast and ovarian cancer susceptibility is due to large rearrangement mutations that can't be detected using conventional sequencing technology. BART may be used to identify these mutation carriers. The test is currently priced at $700 and is covered by all major MCOs and health insurance providers in the United States. We own seven U.S. patents covering BART testing. BART accounted for 9.6% of our total revenue during the year ended June 30, 2013.
| • | COLARIS ® : predictive medicine test for hereditary colorectal cancer and uterine cancer. Our COLARIS test is an analysis of the MLH1, MSH2, MSH6, PMS2, EPCAM and MYH genes for assessing a person's risk of developing colorectal cancer or uterine cancer. Individuals who carry a deleterious mutation in one of the colon cancer genes in the COLARIS test have a greater than 80% lifetime risk of developing colon cancer and women have up to a 71% lifetime chance of developing uterine cancer. Highly effective preventive measures for colon cancer include colonoscopy and the removal of precancerous polyps and for uterine cancer includes hysterectomy. Through proper application of screening and polyp removal, colon cancer is a preventable disease. |
According to the American Cancer Society, approximately 192,000 new cases of colorectal or uterine cancer will be diagnosed in 2013. According to the American Society of Clinical Oncologists, familial forms of colorectal cancer are estimated to account for 10% to 30% of all cases. The test is currently priced at $4,480 and is covered by all major MCOs and health insurance providers in the United States. We own or have non-exclusive licensed rights to eight U.S. patents covering COLARIS testing.
| • | COLARIS AP ® : predictive medicine test for hereditary colorectal cancer. Our COLARIS AP test detects mutations in the APC and MYH genes, which cause a colon polyp-forming syndrome known as Familial Adenomatous Polyposis (FAP), a more common variation of the syndrome known as attenuated FAP, and the MYH-associated polyposis signature (MAP). Individuals who carry a deleterious mutation in the APC or MYH gene may have a greater than 90% lifetime risk of developing colon cancer. Effective preventive measures include colonoscopy and the removal of pre-cancerous polyps and prophylactic surgery. |
5
Our COLARIS AP test is currently priced at $2,050 and is covered by all major MCOs and health insurance providers in the United States. We own or have exclusive rights to ten U.S. patents covering COLARIS AP testing.
COLARIS and COLARIS AP accounted for 8.5% or our total revenue during the year ended June 30, 2013.
| • | MELARIS ® : predictive medicine test for hereditary melanoma. Our MELARIS test analyzes mutations in the p16 gene to determine genetic susceptibility to malignant melanoma. Individuals who test positive for a deleterious mutation in the p16 gene with the MELARIS test have a 75-fold increased risk of developing melanoma during their lifetimes as compared to the general population. Melanoma may be prevented through appropriate screening and a specific threshold of action for mutation carriers, in which pre-cancerous lesions are removed before cancer can develop. |
According to the American Cancer Society, approximately 77,000 new cases of melanoma will be diagnosed in the United States in 2013. Melanoma is lethal within five years in 86% of cases where it has spread to another site in the body. However, when melanoma is diagnosed at an early stage, fewer than 10% of patients die within five years. The MELARIS test is currently priced at $900 and is covered by most major MCOs and health insurance providers in the United States. We own or have license rights to five U.S. patents covering MELARIS testing.
| • | PANEXIA™: predictive medicine test for pancreatic cancer. Our PANEXIA test is a comprehensive analysis of the PALB2 and BRCA2 genes for assessing a person's risk of developing pancreatic cancer later in life. Individuals with a mutation detected by the PANEXIA test have up to an 8.6-fold higher risk than the general population of developing pancreatic cancer. If an individual with a family history of pancreatic cancer receives the PANEXIA test and is identified as having a deleterious mutation, increased surveillance and other predictive steps can be taken in an effort to detect the cancer at an early stage where it may be more treatable. |
According to the American Cancer Society, pancreatic cancer is estimated to affect approximately 45,000 men and women in the United States in 2013. Pancreatic cancer generally has a very poor prognosis for most patients because it is usually detected at a late stage after the cancer has already metastasized to other parts of the body. The PANEXIA test is currently priced at $3,025. We own or have exclusive patent rights to ten U.S. patent applications covering PANEXIA testing.
| • | PREZEON ® : personalized and prognostic medicine test for cancer. Our PREZEON test is an immunohistochemistry test that analyzes the PTEN gene and assesses loss of PTEN function in many cancer types. The PTEN gene is one of the most important tumor suppressor genes and its loss of function is associated with more aggressive disease progression and poorer survival. The PTEN gene plays a role in the disease progression of all four of the major cancers-breast, prostate, colon, and lung cancer. The PTEN gene also plays a critical role in cell signaling pathways that are the target of a number of cancer drugs such as EGFR, mTOR and PIK3CA inhibitors. Analysis of PTEN function may help oncologists in identifying patients who may not respond to these classes of cancer drugs. |
According to the American Cancer Society, approximately 844,000 new cases of these cancers will be diagnosed this year. The PREZEON test is currently priced at $500. We own or have exclusive patent rights to six U.S. patents covering PREZEON testing.
| • | Prolaris ® : prognostic medicine test for prostate cancer. Our Prolaris test is a 46-gene molecular diagnostic assay that assesses whether a patient is likely to have a slow growing, indolent form of prostate cancer that can be safely monitored through active surveillance, or a more aggressive form of the disease that would warrant aggressive intervention such as a radical prostatectomy or radiation therapy. The Prolaris test was developed to meet this significant need to improve physicians' ability to predict disease outcome and to thereby optimize treatment. The Prolaris test is based on the understanding of cell division and tumor growth and provides rigorous, quantitative measures of the expression levels of multiple genes related to progression of the cell cycle. As published in the British Journal of Cancer , researchers analyzed the Prolaris test scores of 352 men with prostate cancer who |
6
were managed through active surveillance and the Prolaris test was the strongest predictor of prostate cancer death and was highly statistically significant (p = 1.4 x 10 -10 ). The Prolaris test outperformed both the Gleason and PSA score in this study. |
According to the American Cancer Society, in the United States approximately 239,000 men are expected to be diagnosed with prostate cancer this year. The Prolaris test is currently priced at $3,400. We own or have exclusive patent rights to four U.S. patent applications covering Prolaris testing.
| • | TheraGuide ® 5-FU: personalized medicine test for drug toxicity. Our TheraGuide 5-FU test analyzes mutations in the DPYD gene and variations in the TYMS gene to assess patient risk of toxicity to 5-FU (fluorouracil) anti-cancer drug therapy. Cancer patients who test positive for a deleterious mutation in the DPYD gene and variations in the TYMS gene have an increased risk of suffering toxicity from 5-FU chemotherapy and up to 20% of patients will experience medically significant toxicity issues (grade 3 or 4 toxicity). These patients should be considered for either a reduced dose of 5-FU or other chemotherapy regimens. 5-FU is widely prescribed for the treatment of colorectal cancer, metastatic breast cancer, skin cancer, and head and neck cancers. |
According to IMS prescription data, there are approximately 425,000 prescriptions written for patients who receive 5-FU therapy each year in the United States. The TheraGuide 5-FU test is currently priced at $1,175 and is covered by many MCOs and health insurance providers in the United States. We own or have exclusive rights to two U.S. patent applications covering TheraGuide 5-FU testing.
We plan to launch three new molecular diagnostic tests in fiscal year 2014. These planned new diagnostic tests include:
| • | myRisk Hereditary Cancer™: predictive medicine test for hereditary cancer. Our myRisk Hereditary Cancer test represents the next generation of our existing hereditary cancer franchise and will eventually replace our current predictive medicine test offerings (BRAC Analysis , Colaris, Colaris AP, Melaris, and Panexia) with a single comprehensive test. myRisk Hereditary Cancer is designed to determine a patient's hereditary cancer risk for breast cancer, ovarian cancer, colon cancer, endometrial cancer, melanoma, and pancreatic cancer. The test analyzes 25 separate genes to look for deleterious mutations which would put a patient at a substantially higher risk than the general population for developing one or more of the above six cancers. All 25 genes in the panel are well documented in clinical literature for the role they play in hereditary cancer and have actionable clinical interventions for the patient to lower disease risk or risk of cancer recurrence. |
Based on current American Cancer Society data, in the United States there are over 600,000 new cancer diagnoses in the six cancers covered by myRisk Hereditary Cancer every year. myRisk Hereditary Cancer will have a list price between $4,000 and $4,500. We own or have exclusive license rights to 54 issued U.S. patents and 4 pending U.S. patent applications relating to myRisk Hereditary Cancer testing.
| • | myPath Melanoma™: diagnostic test for the identification of melanoma. Our myPath Melanoma test is a gene expression based profile that is performed on biopsy tissue for the purpose of aiding a dermatopathologist in the diagnosis of melanoma. Every year in the United States, there are approximately two million skin biopsies performed specifically for the diagnosis of melanoma. Approximately fourteen percent of these biopsies are indeterminate where a dermatopathologist cannot make a definitive call of whether the biopsy is benign or malignant. Outcomes for patients are poor if melanoma is not caught in early stages with five year survival rates dropping from 95% for stage 1 cancer to less than 20% for stage 4 cancer based upon data from the American Cancer Society. We believe myPath Melanoma may provide an accurate tool to assist physicians in correctly diagnosing indeterminate skin lesions. |
There are approximately 280,000 indeterminate diagnosis of melanoma every year. The pricing for myPath Melanoma has not yet been determined. We own or have exclusive license rights to one U.S. patent applications relating to myPath Melanoma testing.
7
| • | myPlan Lung Cancer™: prognostic medicine test for lung cancer. Our myPlan Lung Cancer test is a gene expression based profile that may aid a physician in making a determination as to the aggressiveness of a patient's lung cancer and based upon this determination more accurately guide patient therapy. Most early stage lung cancer patients do not see added benefit from chemotherapy. In a clinical study presented at the American Society of Clinical Oncology Meeting in 2012, 27% of patients with a myPlan Lung Cancer low-risk score died of lung cancer within five years of diagnosis compared to 89% of the patients with a high-risk score. We believe this test may be clinically applicable in the approximately 30,000 new lung cancer diagnoses every year that are early stage lung cancer. |
The pricing for myPlan Lung Cancer has not yet been determined. We own or have exclusive license rights to two U.S. patent applications relating to myPlan Lung Cancer testing.
Companion Diagnostic Services and Other Revenue
On May 31, 2011, we completed the acquisition of the privately-held molecular diagnostic company, Rules-Based Medicine, Inc. of Austin, Texas, for a cash purchase price of approximately $80.0 million. As of June 30, 2013, Rules-Based Medicine is operating as a wholly-owned subsidiary of Myriad under the name of Myriad RBM, Inc. or "Myriad RBM". The acquisition expanded our test pipeline into new disease states, including neuroscience disorders, infectious diseases and inflammatory diseases. We believe that the tests being developed by Myriad RBM will complement the tests that we are developing using our strong research capabilities in nucleic acid (DNA and RNA) analysis with proprietary multiplex immunoassay (protein) technology. Myriad RBM has strategic collaborations with over 20 major pharmaceutical and biotechnology companies, which coupled with our industry-leading position in PARP inhibitor and PI3K inhibitor companion diagnostics, creates a leading franchise in companion diagnostics. In addition, our acquisition of Myriad RBM provides us with access to samples from additional patient cohorts for new molecular diagnostic test development and clinical validation activities.
Through Myriad RBM, we provide biomarker discovery and companion diagnostic services to the pharmaceutical, biotechnology, and medical research industries utilizing our multiplexed immunoassay technology. Our technology enables us to efficiently screen large sets of well-characterized clinical samples from both diseased and non-diseased populations against our extensive menu of biomarkers. By analyzing the data generated from these tests, we attempt to discover biomarker patterns that indicate a particular disease or disorder with a high degree of accuracy. During the year ended June 30, 2013, Myriad RBM generated $30.8 million in revenue from providing its companion diagnostic services. In addition to the fees received from analyzing these samples, we also use this information to create and validate potential diagnostic test panels that can aid us in the development of potential new molecular diagnostic tests that could aid a physician in making diagnostic and treatment decisions.
Our companion diagnostic services consist of the following:
| • | Multi-Analyte Profile ("MAP"): We have compiled a library of over 550 individual human and rodent immunoassays for use in our multi-analyte profile (MAP) testing services and we are continuously adding new assays to this library. We have assembled what we believe are the most clinically relevant human immunoassays from this library into our DiscoveryMAP ® assay panel, which we typically employ with pharmaceutical collaborators in human clinical trials. We have also developed RodentMAP ® , a proprietary panel for use in pre-clinical animal studies and OncologyMAP ® , which measures cancer-related proteins to assist researchers accelerate the pace of discovery, validation and translation of cancer biomarkers for early detection, patient stratification and therapeutic monitoring. Our MAP services are designed to provide a comprehensive and cost-effective evaluation of the biomarker patterns critical to applications such as drug safety and efficacy, disease diagnosis, diseases modeling, patient stratification as well as personal health assessments. |
8
Importantly, the data generated through our companion diagnostic services business can provide new insights into biological systems and enable us to generate potential new molecular diagnostic tests. Under the terms of the agreements with many of our collaborators, we retain the rights to the companion diagnostic products. We have licensed rights to the Luminex platform used in our MAP testing services.
| • | Multiplexed Immunoassay Kits: Customers in all segments of the life sciences market often require both outsourced and in-house testing. Many of our pharmaceutical and biotechnology customers need bioassay kits for complimentary in-house testing. Therefore, we have developed multiplexed immunoassay kits that enable our customers to leverage our technology services with their in-house capabilities. Our internally developed multiplexed immunoassay kits include all of the components necessary for a customer to perform a test on their own Luminex instrument. We have licensed rights to the Luminex platform used in our multiplexed immunoassay kits. |
| • | TruCulture ® : TruCulture is a simple, self-contained whole blood culture that can be deployed to clinical sites around the world for acquiring cell culture data without specialized facilities or training. The TruCulture system may allow pharmaceutical and biotechnology companies to identify drug toxicity prior to human trials, potentially enabling a decision as to whether to continue a drug's development earlier in the development process and thereby save significant research and development costs. We have exclusive patent rights to one U.S. patent covering our TruCulture and other co-culture services. |
Patents and Proprietary Rights
We own or have license rights to 234 issued patents as well as numerous patent applications in the United States and foreign countries. These patents and patent applications cover a variety of subject matter including, diagnostic biomarkers, genes, proteins, gene expression signatures, antibodies, primers, probes, assays, disease-associated genetic mutations and single-nucleotide polymorphisms, methods for determining genetic predisposition, methods for disease diagnosis, methods for determining disease progression, methods for correlation claims, and methods for disease treatment, and general molecular diagnostic techniques.
The following is a summary of key U.S. patents covering our current molecular diagnostic tests and companion diagnostic services. Many of the issued U.S. patents relating to BRAC Analysis , COLARIS, COLARIS AP, MELARIS, PREZEON, PANEXIA and TruCulture also have related foreign issued patents in various countries, including in Europe, Canada, Japan, Australia and New Zealand, claiming similar subject matter and having similar expiration dates. For many of the patents, we hold rights through exclusive or non-exclusive license agreements, which are summarized in the following section under the caption "License Agreements." We also own additional patent applications and hold other non-exclusive license rights to patents which cover various aspects of our tests or processes.
BRACAnalysis. We own or have exclusive license rights to over 500 claims in 24 issued U.S. patents relating to BRAC Analysis testing. These U.S. patents have terms that are expected to expire commencing in 2014, with the last patent expected to expire in 2029. These patents contain multiple claims, including claims relating to compositions of matter on synthetic BRCA1 and BRCA2 nucleic acids, probes and primers, methods of detecting genetic mutations in the BRCA1 and BRCA2 genes and the use thereof for diagnosing predisposition to breast or ovarian cancer, and general molecular diagnostic technology relating to BRAC Analysis testing.
BART . We own or have exclusive license rights to seven issued U.S. patents relating to BART testing. These U.S. patents have terms that are expected to expire commencing in 2015, with the last patent expected to expire in 2025. These patents contain multiple claims, including but not limited to claims relating to composition of matter on synthetic BRAC1 and BRCA2 nucleic acids, composition of matter on probes and primers, methods of detecting genomic rearrangements and methods of determining BRCA1 and BRCA2 related predisposition to cancer.
9
COLARIS . We own or have exclusive or non-exclusive license rights to 19 issued U.S. patents relating to COLARIS testing. These U.S. patents have terms that are expected to expire commencing in 2013, with the last patent expected to expire in 2023. These patents contain multiple claims, including but not limited to claims relating to MLH1 , MSH2, PMS2 and MYH compositions of matter on synthetic MLH1 , MSH2, PMS2 and MYH nucleic acids, methods of detecting mutations in the MLH1, MSH2 and MYH genes, methods for determining MLH1- , MSH2-, PMS2-, and MYH- related predisposition to cancer, such as Lynch Syndrome cancers, and general molecular diagnostic technology applicable to COLARIS testing.
COLARIS AP. We own or have exclusive license rights to 11 issued U.S. patents relating to COLARIS AP testing. These U.S. patents have terms that are expected to expire commencing in 2017, with the last patent expected to expire in 2026. These patents contain multiple claims, including claims relating to compositions of matter on synthetic MYH nucleic acids, methods of detecting MYH mutations and methods of detecting a predisposition to colorectal cancer using MYH , and general molecular diagnostic technology applicable to COLARIS AP testing.
MELARIS. We own or have exclusive license rights to five issued U.S. patents relating to MELARIS testing. These U.S. patents have terms that are expected to expire commencing in 2014, with the last patent expected to expire in 2023. These patents contain multiple claims, including claims relating to methods of detecting mutations in the p16 gene and their use for diagnosing predisposition to melanoma, and general molecular diagnostic technology applicable to MELARIS testing.
PANEXIA. We own or have exclusive license rights to eight U.S. patents and two U.S. patent applications relating to PANEXIA testing. These U.S. patents have terms that are expected to expire commencing in 2015 with the last patent expected to expire in 2029. Subject to applicable extensions, we anticipate that the expiration dates of these patent applications, if issued, will commence in 2029. These patent applications disclose varied subject matter, including but not limited to composition of matter claims on PALB2 and BRCA2 gene mutations and methods of diagnosing a predisposition to pancreatic cancer based on PALB2 and BRCA2 gene mutations.
PREZEON. We have exclusive license rights to six issued U.S. patents relating to PREZEON testing. These U.S. patents have terms that are expected to expire commencing in 2017, with the last patent expected to expire in 2018. These patents contain multiple claims, including but not limited to claims relating to PTEN compositions of matter on antibodies, methods of detecting PTEN expression and PTEN mutations, and methods of detecting cancer or a predisposition to cancer using PTEN , and methods of guiding therapeutic treatment decisions based on PTEN status.
Prolaris. We own or have exclusive license rights to six U.S. patent applications relating to Prolaris testing. Subject to applicable extensions, we anticipate that the expiration dates of these patent applications, if issued, will commence in 2030. These patent applications disclose varied subject matter, including but not limited to compositions of matter claims on gene expression signatures and methods of determining aggressiveness of prostate cancer and the likelihood of progression risk of recurrence of prostate cancer based on gene expression signatures.
TheraGuide 5-FU. We own one U.S. patent and one U.S. patent application relating to TheraGuide 5-FU testing. The patent will expire in 2023. Subject to applicable extensions, we anticipate that the expiration date of the U.S. patent applications, if issued, will commence in 2027. The patent and application disclose varied subject matter, including but not limited to subject matter relating to compositions of matter on synthetic DPYD nucleic acids containing specific mutations, diagnostic methods relating to DPYD mutations, and general molecular diagnostic technology applicable to TheraGuide 5-FU.
TruCulture. We have exclusive license rights to commercialize technology covered by one issued U.S. patent for our TruCulture product. This U.S. patent is expected to expire in 2019. This patent contains multiple claims, including but not limited to claims relating to methods and kits for determining the immune defense activity of blood.
10
myRisk Hereditary Cancer. We own or have exclusive license rights to 54 issued U.S. patents and 4 pending U.S. patent applications relating to myRisk Hereditary Cancer TM testing. Subject to applicable extensions, we anticipate that the expiration dates of these patents will commence in 2014, with the last patent, if issued from the currently pending applications, expected to expire in 2029. These patents and patent applications disclose and claim varied subject matter, including claims relating to compositions of matter on synthetic nucleic acids, probes and primers, methods of detecting genetic mutations in the 25 genes that comprise the test (individually and in numerous combinations) and the use thereof for diagnosing predisposition to various cancers, and general molecular diagnostic technology relating to myRisk Hereditary.
myPath Melanoma. We own or have exclusive license rights to one U.S. patent applications relating to myPath Melanoma ™ testing. Subject to applicable extensions, we anticipate that the expiration date of this patent application, if issued, will commence in 2027. This patent application discloses varied subject matter, including but not limited to compositions of matter claims on gene expression signatures and methods of detecting melanoma, methods of determining aggressiveness, likelihood of progression, risk of recurrence and optimal therapy based on gene expression signatures.
myPlan Lung Cancer. We own or have exclusive license rights to two U.S. patent applications relating to myPlan Lung Cancer ™ testing. Subject to applicable extensions, we anticipate that the expiration dates of these patent applications, if issued, will commence in 2032. These patent applications disclose varied subject matter, including but not limited to compositions of matter claims on gene expression signatures and methods of determining cancer aggressiveness, likelihood of progression, risk of recurrence and optimal therapy for lung cancer based on gene expression signatures.
We intend to seek patent protection in the United States and major foreign jurisdictions for synthetic nucleic acids, proteins, antibodies, biomarker signatures, assays, probes, primers, technologies, methods, processes and other inventions which we believe are patentable and where we believe our interests would be best served by seeking patent protection. However, any patents issued to us or our licensors may not afford meaningful protection for our products or technology or may be subsequently circumvented, invalidated or narrowed or found unenforceable such as was the case in our recent Supreme Court case discussed in Item 3, Legal Proceedings. Any patent applications which we have filed or will file or to which we have licensed or will license rights may not issue, and patents that do issue may not contain commercially valuable claims. In addition, others may obtain patents having claims which cover aspects of our tests or processes which are necessary for or useful to the development, use or performance of our diagnostic products. Should any other group obtain patent protection with respect to our discoveries, our commercialization of our molecular diagnostic tests could be limited or prohibited.
Our tests and processes may also conflict with patents which have been or may be granted to competitors, academic institutions or others. As the molecular diagnostic industries expand and more patents are issued, the risk increases that our products and processes may give rise to interferences filed by others in the U.S. Patent and Trademark Office or foreign patent offices, or to claims of patent infringement by other companies, institutions or individuals. In addition, third parties could bring legal actions against us seeking to invalidate our owned or licensed patents, claiming damages, or seeking to enjoin clinical testing, developing and marketing of our tests or processes. If any of these actions are successful, in addition to any potential liability for damages, we could lose patent coverage for our tests, be required to cease the infringing activity or obtain a license in order to continue to develop or market the relevant test or process. We may not prevail in any such action, and any license required under any such patent may not be made available on acceptable terms, if at all. Our failure to maintain patent protection for our test and processes or to obtain a license to any technology that we may require to commercialize our tests and technologies could have a material adverse effect on our business.
We also rely upon unpatented proprietary technology, and in the future may determine in some cases that our interests would be better served by reliance on trade secrets or confidentiality agreements rather than patents or licenses. These include some of our genomic, proteomic, RNA expression, mutation analysis, IHC, robotic and
11
bioinformatic technologies which may be used in discovering and characterizing new genes and proteins and ultimately used in the development or analysis of molecular diagnostic tests. We also maintain a database of gene mutations and their status as either harmful or benign for all of our predictive medicine tests. To further protect our trade secrets and other proprietary information, we require that our employees and consultants enter into confidentiality and invention assignment agreements. However, those confidentiality and invention assignment agreements may not provide us with adequate protection. We may not be able to protect our rights to such unpatented proprietary technology and others may independently develop substantially equivalent technologies. If we are unable to obtain strong proprietary rights to our processes or tests, competitors may be able to market competing processes and tests.
License Agreements
We are a party to multiple license agreements which give us the rights to use certain technologies in the research, development, testing processes, and commercialization of our molecular diagnostic tests and companion diagnostic services. We may not be able to continue to license these technologies on commercially reasonable terms, if at all. Additionally, patents underlying our license agreements may not afford meaningful protection for our technology or tests or may be subsequently circumvented, invalidated or narrowed, or found unenforceable. Our failure to maintain rights to this technology could have a material adverse effect on our business.
In October 1991, we entered into a license agreement with the University of Utah Research Foundation (the ‘University"), for the exclusive rights to utilize certain intellectual property rights of the University, including issued patents that relate to the BRCA1 gene, on a world-wide basis. Under this license agreement we pay the University a royalty based on net sales of our BRAC Analysis test. This license agreement ends on the last to expire patent covered by the license agreement which presently is not anticipated to expire until April 2015. The University has the right to terminate the license agreement for the uncured breach of any material term of the license agreement.
We entered into separate license agreements with the University, Endorecherche, Inc., The Hospital for Sick Children and The Trustees of the University of Pennsylvania (collectively referred to as the "BRCA2 Licensors") in November 1994, January 1995, March 1995 and March 1996, respectively, for exclusive rights to utilize certain intellectual property rights of the respective BRCA2 Licensors, including issued patents that relate to the BRCA2 gene, on a world-wide basis. Under these license agreements we pay each of the BRCA2 Licensors a royalty based on net sales of our BRAC Analysis test. Each of these license agreements ends on the expiration date of the last to expire patent covered by the respective license agreements which presently is not anticipated to expire until December 2015. The BRCA2 Licensors have the right to terminate the license agreements for the uncured breach of any material term of the license agreements.
In April 2000, we entered into a license agreement with Dana-Farber Cancer Institute, Inc., Oregon Health Sciences University, University of Vermont and State Agricultural College and Yale University (collectively the "COLARIS Licensors") for the non-exclusive rights to utilize certain intellectual property rights of the COLARIS Licensors, including issued patents that relate to the MLH1, MLH2 and PMS2 genes, on a world-wide basis. Under this license agreement we pay the COLARIS Licensors a royalty based on net sales of our COLARIS test. This license agreement ends on the expiration date of the last to expire patent covered by the license agreement, which presently is not anticipated to expire until October 2023. The COLARIS Licensors have the right to terminate the license agreement for the uncured breach of any material term of the license agreement.
In April 2000, we entered into a license agreement with Genzyme Corporation ("Genzyme") for the non-exclusive rights to utilize certain intellectual property rights of Genzyme, including issued patents that relate to the MSH2 gene, on a world-wide basis. Under this license agreement we pay Genzyme a royalty based on net sales of our COLARIS test. This license agreement ends, on a country by country basis, on the expiration date of the last to expire patent covered by the license agreement, which presently is not anticipated to expire until
12
October 2023. Either party has the right to terminate the license agreement for the uncured breach of any material term of the license agreement.
In March 2004 and June 2007, we entered into separate license agreements with the University of Wales and Human Genome Sciences, Inc. ("HGSI") respectively (collectively referred to as the "COLARIS AP Licensors") for the exclusive rights to certain intellectual property rights of the respective licensors, including issued patents that relate to the MYH gene, on a world-wide basis. Under these license agreements we pay each of the COLARIS AP Licensors a royalty based on net sales of our COLARIS and COLARIS AP tests. Each of these license agreements ends on the expiration date of the last to expire patent covered by the respective license agreements which presently is not anticipated to expire until February of 2018 for the HGSI license and April 2023 for the University of Wales license. The COLARIS AP Licensors have the right to terminate the license agreements for the uncured breach of any material term of the license agreements. On July 1, 2013 and July 19, 2013 respectively, all rights of University of Wales and HGSI related to the MYH gene were assigned to Myriad.
In October 2009, we entered into a license agreement with Johns Hopkins University for the exclusive right to utilize certain intellectual property rights of Johns Hopkins, including patent applications that relate to the PALB2 gene, on a world-wide basis. Under this license agreement we pay John Hopkins University a royalty based on net sales of our PANEXIA test. This license agreement ends on the expiration date of the last to expire patent covered by the license agreement, which presently is not anticipated to expire until March 2030. Johns Hopkins University has the right to terminate the license agreement for the uncured breach of any material term of the license agreements.
Competition
Competition is intense in our existing and potential markets. Our competitors in the United States and abroad are numerous and include, other molecular diagnostic companies, diagnostic reference laboratories, large multi-national healthcare companies, and universities and other research institutions. For instance, some laboratories provide a test intended to predict the cancer's aggressiveness among patients with prostate cancer and other laboratories provide hereditary cancer testing for melanoma, colorectal and uterine cancer. Some of our potential competitors have considerably greater financial, technical, marketing and other resources than we do. We expect competition to intensify in our current fields as technical advances occur and become more widely known. For example, following our Supreme Court case discussed in Item 3, Legal Proceedings, Ambry Genetics Corporation and Gene By Gene commenced offering certain clinical diagnostic testing for hereditary breast and ovarian cancer that compete with our BRACAnalysis testing and future molecular diagnostic testing we plan to launch. We anticipate that others may also launch their own molecular diagnostic tests which may compete with our testing products and services.
The technologies for discovering the underlying cause of major diseases, patients' response to therapies, and disease progression, as well as the approaches for commercializing those discoveries are rapidly evolving. Rapid technological developments could result in our potential tests or processes becoming obsolete before we recover a significant portion of our related research and development costs and associated capital expenditures. If we do not discover biomarkers, develop molecular diagnostic tests and related information services based on such discoveries, obtain regulatory and other approvals, and launch such services before our competitors, we could be adversely affected. Moreover, any molecular diagnostic tests that we may develop could be made obsolete by less expensive or more effective tests or methods that may be developed in the future.
Governmental Regulation
The services that we provide are regulated by federal, state and foreign governmental authorities. Failure to comply with the applicable laws and regulations can subject us to repayment of amounts previously paid to us, significant civil and criminal penalties, loss of licensure, certification, or accreditation, or exclusion from government health care programs. The significant areas of regulation are summarized below.
13
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
Each of our clinical laboratories must hold certain federal, state and local licenses, certifications and permits to conduct our business. Laboratories that perform testing on human specimens for the purpose of providing information for the diagnosis, prevention, or treatment of disease are subject to the Clinical Laboratory Improvement Amendments of 1988, or CLIA. CLIA requires such laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification also is a prerequisite to be eligible to bill state and federal health care programs, as well as many private insurers, for laboratory testing services.
Standards for testing under CLIA vary based on the level of test complexity. Laboratories performing high complexity testing must comply with more stringent requirements than laboratories performing waived or moderate complexity testing. Our laboratories in Salt Lake City, Utah and Austin, Texas are CLIA certified to perform high complexity tests.
In addition, CLIA requires each certified laboratory to enroll in an approved proficiency testing program if it performs testing in any category for which proficiency testing is required. Such laboratories must periodically test specimens received from an outside proficiency testing organization and then must submit the results back to that organization for evaluation. A laboratory that fails to achieve a passing score on a proficiency test may lose its right to perform testing in the category at issue. Further, failure to comply with other proficiency testing regulations, such as the prohibition on referral of a proficiency testing specimen to another laboratory for analysis, can result in revocation of the referring laboratory's CLIA certification.
As a condition of CLIA certification, each of our laboratories is subject to survey and inspection every other year, in addition to being subject to additional random inspections. The biennial survey is conducted by the Centers for Medicare & Medicaid Services, or CMS, a CMS agent (typically a state agency), or, if the laboratory holds a CLIA Certificate of Accreditation, a CMS-approved accreditation organization. Our laboratories are accredited by the College of American Pathologists, or CAP, which is a CMS-approved accreditation organization. Those laboratories must comply with all CLIA requirements as well as with any additional requirements imposed by CAP.
CLIA provides that a state may adopt laboratory regulations that are more stringent than those under federal law. In some cases, state licensure programs actually substitute for the federal CLIA program. In other instances, the state's regulations may be in addition to the CLIA program. Our laboratories are licensed by the appropriate state agencies in the states in which they operate, if such licensure is required. In addition, our laboratories hold state licenses from California, Florida, and New York, to the extent that they accept specimens from one or more of these states, each of which require out-of-state laboratories to obtain licensure. If a laboratory is out of compliance with state laws or regulations governing licensed laboratories, penalties for violation vary from state to state but may include suspension, limitation, revocation or annulment of the license, assessment of financial penalties or fines, or imprisonment. We believe that we are in material compliance with all applicable licensing laws and regulations.
We may become aware from time to time of other states that require out-of-state laboratories to obtain licensure to accept specimens from the state, and other states may impose such requirements in the future. If we identify any other state with such requirements, or if we are contacted by any other state advising us of such requirements, we intend to follow all instructions from the state regulators regarding compliance with such requirements.
Food and Drug Administration
Although the Food and Drug Administration (FDA) has consistently claimed that it has the authority to regulate laboratory-developed tests, or LDTs, that are validated by the developing laboratory and performed only by that
14
laboratory, it has generally exercised enforcement discretion in not otherwise regulating most tests developed and performed by high complexity CLIA-certified laboratories. Nevertheless, the FDA indicated that it is reviewing the regulatory requirements that apply to LDTs. In July 2010, the FDA held a two-day public meeting to obtain input from stakeholders on how it should apply its authority to implement a reasonable, risk-based, and effective regulatory framework for LDTs, including genetic tests. However, the FDA has not yet issued the promised additional guidance but may do so in the future. Before any draft or final guidance is issued, however, the FDA will be required to provide Congress at least sixty days prior notice in accordance with the requirements of the Food and Drug Administration Safety and Innovation Act, or FDASIA. The notice must include anticipated details of the action. FDASIA was signed into law on July 9, 2012, and the notice requirement will sunset five years thereafter.
The FDA issued a Draft Guidance for Industry and Food and Drug Administrative Staff on In-Vitro Companion Diagnostic Devices on July 14, 2011, which, if finalized, is intended to assist companies developing in vitro companion diagnostics and companies developing therapeutic products that depend on the use of a specific in vitro companion diagnostic for the safe and effective use of the product. The FDA defined an in-vitro companion diagnostic device, or IVD Companion Diagnostic Device, as a device that provides information that is essential for the safe and effective use of a corresponding therapeutic product. This definition is much narrower than the commonly used term "companion diagnostic," which refers generally to tests that may be useful, but are not necessarily a determining factor in the safe and effective use of the therapeutic product. The FDA expects that the therapeutic sponsor will address the need for an approved or cleared IVD Companion Diagnostic Device in its therapeutic product development plan. The sponsor of the therapeutic product can decide to develop its own IVD Companion Diagnostic Device, partner with a diagnostic device sponsor to develop the appropriate IVD Companion Diagnostic Device, or explore modification of an existing IVD diagnostic device (its own or another sponsor's) to accommodate the appropriate intended use. The FDA has approved a number of drug/diagnostic device companions in accordance with the Draft Guidance. However, this guidance may not apply to the LDTs that are used as companion diagnostics that merely provide useful information.
HIPAA and other privacy laws
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, established for the first time comprehensive federal protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations, or "Covered Entities": health plans, healthcare clearing houses, and healthcare providers which conduct certain healthcare transactions electronically. Title II of HIPAA, the Administrative Simplification Act, contains provisions that address the privacy of health data, the security of health data, the standardization of identifying numbers used in the healthcare system and the standardization of certain healthcare transactions. The privacy regulations protect medical records and other protected health information by limiting their use and release, giving patients the right to access their medical records and limiting most disclosures of health information to the minimum amount necessary to accomplish an intended purpose. The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures. HIPAA requires Covered Entities to obtain a written assurance of compliance from individuals or organizations who provide services to Covered Entities involving the use or disclosure of protected health information ("Business Associates").
On February 17, 2009, Congress enacted Subtitle D of the Health Information Technology for Economic and Clinical Health Act, or HITECH, provisions of the American Recovery and Reinvestment Act of 2009. HITECH amends HIPAA and, among other things, expands and strengthens HIPAA, creates new targets for enforcement, imposes new penalties for noncompliance and establishes new breach notification requirements for Covered Entities and Business Associates. Regulations implementing major provisions of HITECH were finalized on January 25, 2013 through publication of the HIPAA Omnibus Rule (the "Omnibus Rule"). The Omnibus Rule contained significant changes for Covered Entities and Business Associates with respect to permitted uses and disclosures of Protected Health Information.
15
Under HITECH's new breach notification requirements, Covered Entities must report breaches of protected health information that has not been encrypted or otherwise secured in accordance with guidance from the Secretary of the U.S. Department of Health and Human Services (the "Secretary"). Required breach notices must be made as soon as is reasonably practicable, but no later than 60 days following discovery of the breach. Reports must be made to affected individuals and to the Secretary and in some cases, they must be reported through local and national media, depending on the size of the breach.
We are currently subject to the HIPAA regulations and maintain an active compliance program. We are subject to audit under HHS's HITECH-mandated audit program. We may also be audited in connection with a privacy complaint. We are subject to prosecution and/or administrative enforcement and increased civil and criminal penalties for non-compliance, including a new, four-tiered system of monetary penalties adopted under HITECH. We are also subject to enforcement by state attorneys general who were given authority to enforce HIPAA under HITECH. To avoid penalties under the HITECH breach notification provisions, we must ensure that breaches of protected health information are promptly detected and reported within the company, so that we can make all required notifications on a timely basis. However, even if we make required reports on a timely basis, we may still be subject to penalties for the underlying breach.
In addition to the federal privacy regulations, there are a number of state laws regarding the privacy and security of health information and personal data that are applicable to clinical laboratories. The compliance requirements of these laws, including additional breach reporting requirements, and the penalties for violation vary widely and new privacy and security laws in this area are evolving. Many states have also implemented genetic testing and privacy laws imposing specific patient consent requirements and protecting test results. In some cases, we are prohibited from conducting certain tests without a certification of patient consent by the physician ordering the test. Requirements of these laws and penalties for violations vary widely. We believe that we have taken the steps required of us to comply with health information privacy and security statutes and regulations in all jurisdictions, both state and federal. However, we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal laws regarding privacy or security, could result in civil and/or criminal penalties and could have a material adverse effect on our business.
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials. For example, the U.S. Occupational Safety and Health Administration, or OSHA, has established extensive requirements relating specifically to workplace safety for healthcare employers in the U.S. This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, such as HIV and hepatitis B and C, including preventing or minimizing any exposure through needle stick injuries. For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the United States Postal Service and the International Air Transport Association. We generally use third-party vendors to dispose of regulated medical waste, hazardous waste and radioactive materials and contractually require them to comply with applicable laws and regulations.
Foreign regulations
We market our tests outside of the United States and are subject to foreign regulatory requirements governing laboratory licensure, human clinical testing, use of tissue, privacy and data security, and marketing approval for our tests. These requirements vary by jurisdiction, differ from those in the United States and may require us to implement additional compliance measures or perform additional pre-clinical or clinical testing. On September 26, 2012, the European Commission ("EC") released the first drafts of the new EU regulations for medical devices and IVDs that if finalized will impose additional regulatory requirements on IVDs used in the EU. In many countries outside of the United States, coverage, pricing and reimbursement approvals are also required. We are also required to maintain accurate information and control over sales and distributors' activities that may fall within the purview of the Foreign Corrupt Practices Act, its books and records provisions and its anti-bribery provisions.
16
Reimbursement and Billing
Reimbursement and billing for diagnostic services is generally highly complex. Laboratories must bill various payors, such as private third-party payors, including MCOs and state and federal health care programs, such as Medicare and Medicaid, and each may have different billing requirements. Additionally, the audit requirements we must meet to ensure compliance with applicable laws and regulations, as well as our internal compliance policies and procedures, add further complexity to the billing process. Other factors that complicate billing include:
| • | variability in coverage and information requirements among various payors; |
| • | missing, incomplete or inaccurate billing information provided by ordering physicians; |
| • | billings to payors with whom we do not have contracts; |
| • | disputes with payors as to which party is responsible for payment; and |
| • | disputes with payors as to the appropriate level of reimbursement. |
Depending on the reimbursement arrangement and applicable law, the party that reimburses us for our services may be:
| • | a third party who provides coverage to the patient, such as an insurance company or MCO; |
| • | a governmental payor; or |
| • | the patient. |
Presently, approximately 85% of our revenue comes from third party payors.
In February 2011, the American Medical Association CPT Editorial Panel approved 101 new molecular pathology codes to describe molecular diagnostic tests that currently require multiple CPT codes for billing purposes. The new reimbursement rates for the new codes went into effect on January 1, 2013.
Federal and State Fraud and Abuse Laws
A variety of federal laws prohibit fraud and abuse involving state and federal health care programs, such as Medicare and Medicaid. These laws are interpreted broadly and enforced aggressively by various state and federal agencies, including CMS, the Department of Justice, or DOJ, the Office of Inspector General for the Department of Health and Human Services, or OIG, and various state agencies. In addition, the Medicare and Medicaid programs increasingly use a variety of contractors to review claims data and to identify improper payments as well as fraud and abuse. These contractors include Recovery Audit Contractors, or RACs, Medicaid Integrity Contractors, or MICs, and Zone Program Integrity Contractors, or ZPICs. In addition, CMS conducts CERT audits, the purpose of which is to detect improper Medicare payments. Any overpayments identified must be repaid to the Medicare program unless a favorable decision is obtained on appeal. In some cases, these overpayments can be used as the basis for an extrapolation, by which the error rate is applied to a larger universe of claims, and which can result in even higher repayments.
Anti-Kickback Laws
The Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program. "Remuneration" is broadly defined to include anything of value, such as, for example, cash payments, gifts or gift certificates, discounts, or the furnishing of services, supplies or equipment. The Anti-Kickback Statue is broad and prohibits many arrangements and practices that are lawful in businesses outside of the health care industry.
17
Recognizing the breadth of the Anti-Kickback Statute and the fact that it may technically prohibit many innocuous or beneficial arrangements within the health care industry, the OIG has issued a series of regulations, or safe harbors. Compliance with all requirements of a safe harbor immunizes the parties to the business arrangement from prosecution under the Anti-Kickback Statute. The failure of a business arrangement to fit within a safe harbor does not necessarily mean that the arrangement is illegal or that the OIG will pursue prosecution. Still, in the absence of an applicable safe harbor, a violation of the Anti-Kickback Statute may occur even if only one purpose of an arrangement is to induce referrals. The penalties for violating the Anti-Kickback Statute can be severe. These sanctions include criminal and civil penalties, imprisonment and possible exclusion from the federal health care programs. Many states have adopted laws similar to the Anti-Kickback Statute, and some apply to items and services reimbursable by any payor, including private third-party payors.
Physician Self-Referral Bans
The federal ban on physician self-referrals, commonly known as the Stark Law, prohibits, subject to certain exceptions, physician referrals of Medicare patients to an entity providing certain "designated health services" (which include laboratory services) if the physician or an immediate family member of the physician has any financial relationship with the entity. A "financial relationship" is created by an investment interest or a compensation arrangement. A laboratory cannot bill the Medicare Part B program for services furnished pursuant to a prohibited self-referral, and Medicaid reimbursement may be at risk as well. Several Stark Law exceptions are relevant to arrangements involving clinical laboratories, including: (1) fair market value compensation for the provision of items or services; (2) payments by physicians to a laboratory for clinical laboratory services; (3) certain space and equipment rental arrangements that satisfy certain requirements; and (4) personal services arrangements. Penalties for violating the Stark Law include the return of funds received for all prohibited referrals, fines, civil monetary penalties and possible exclusion from the federal health care programs. In addition to the Stark Law, many states have their own self-referral bans, which may extend to all self-referrals, regardless of the payor.
State and Federal Prohibitions on False Claims
The federal False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government. Under the False Claims Act, a person acts knowingly if he has actual knowledge of the information or acts in deliberate ignorance or in reckless disregard of the truth or falsity of the information. Specific intent to defraud is not required. The qui tam provisions of the False Claims Act allow a private individual to bring an action on behalf of the federal government and to share in any amounts paid by the defendant to the government in connection with the action. The number of filings of qui tam actions has increased significantly in recent years. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 and $11,000 for each false claim. Conduct that violates the False Claims Act may also lead to exclusion from the federal health care programs. Given the number of claims likely to be at issue, potential damages under the False Claims Act for even a single inappropriate billing arrangement could be significant. In addition, various states have enacted similar laws modeled after the False Claims Act that apply to items and services reimbursed under Medicaid and other state health care programs, and, in several states, such laws apply to claims submitted to all payors.
Federal Prohibitions on Health Care Fraud and False Statements Related to Health Care Matters
In addition to the administrative simplification regulations discussed above, HIPAA created two new federal crimes: health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including a private insurer. The false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the
18
delivery of or payment for health care benefits, items, or services. A violation of this statute is a felony and may result in fines, imprisonment, or exclusion from the federal health care programs.
Program Integrity Requirements
The Affordable Care Act included a number of provisions intended to strengthen the integrity of the Medicare and Medicaid programs as well as the Children's Health Insurance Program, or CHIP. These provisions are expected to bolster the ability of state and federal agencies to prevent and detect fraud and abuse. Such measures include enhanced background screening procedures for providers and suppliers participating or enrolling in Medicare, Medicaid, or CHIP; expansion of state and federal authority to suspend Medicare and Medicaid payments pending an investigation of a "credible allegation of fraud"; a grant of broad discretion to CMS to impose temporary moratoria on the enrollment of providers and suppliers by category; and a mandate that all Medicare, Medicaid and CHIP providers and suppliers implement an ethics and compliance program that contains the "core elements" to be established by CMS.
Human Resources
As of June 30, 2013, we had 1,325 full-time equivalent employees. Most of our employees are engaged directly in research, development, production, sales and marketing activities. We believe that the success of our business will depend, in part, on our ability to attract and retain qualified personnel. Our employees are not covered by a collective bargaining agreement, and we consider our relations with our employees to be good.
Available Information
We are a Delaware corporation with our principal executive offices located at 320 Wakara Way, Salt Lake City, Utah 84108. Our telephone number is (801) 584-3600 and our web site address is www.myriad.com. We make available free of charge through the Investor Relations section of our web site our Corporate Code of Conduct and Ethics, our Audit Committee and other committee charters and our other corporate governance policies, as well as our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission. We include our web site address in this Annual Report on Form 10-K only as an inactive textual reference and do not intend it to be an active link to our web site.
19
| Item 1A. | RISK FACTORS |
Risks Related to Our Business and Our Strategy
We may not be able to generate sufficient revenue from our existing tests or develop new tests to maintain profitability.
Although we have developed and marketed several molecular diagnostic tests to date, we believe our future success is dependent upon our ability to successfully market our existing molecular diagnostic tests to additional patients within the United States, to expand into new markets outside the United States, and to develop and commercialize new molecular diagnostic tests and companion diagnostic services. The demand for our existing molecular diagnostic tests may decrease or may not continue to increase at historical rates for a number of reasons. For example, because BRAC Analysis testing and most of our molecular diagnostic tests are only utilized once per patient, we will need to sell our services through physicians to new patients or develop new molecular diagnostic tests in order to continue to generate revenue. During fiscal 2012 we opened a reference laboratory in Germany and expanded sales efforts into selected countries in Europe but we may not be able to generate sufficient profits from European sales to recover the costs of our investment. Our pipeline of new molecular diagnostic and companion diagnostic candidates is in various stages of development and may take several more years to develop and must undergo extensive clinical validation. We may be unable to discover or develop any additional molecular diagnostic tests or companion diagnostic services through the utilization of our technologies or technologies we license or acquire from others. Even if we develop tests or services for commercial use, we may not be able to develop tests or services that:
| • | meet applicable regulatory standards, in a timely manner or at all; |
| • | successfully compete with other technologies and tests; |
| • | avoid infringing the proprietary rights of others; |
| • | are adequately reimbursed by third-party payors; |
| • | can be performed at commercial levels or at reasonable cost; or |
| • | can be successfully marketed. |
We must generate significant revenue to maintain profitability. Even if we succeed in marketing our existing molecular diagnostic tests and companion diagnostic services to physicians for use in new patients and in developing and commercializing any additional molecular diagnostic tests and companion diagnostic services, we may not be able to generate sufficient revenue and we may not be able to maintain profitability.
We may not be able to sustain or increase profitability on a quarterly or annual basis.
In order to develop and commercialize our molecular diagnostic test and companion diagnostic service candidates, we expect to incur significant expenses over the next several years as we increase our research and development activities, expand clinical validation trials for our molecular diagnostic tests and companion diagnostic services currently in development, potentially license or acquire additional companies or technologies and engage in commercialization activities in anticipation of the launch of additional molecular diagnostic tests and companion diagnostic services. Because of the numerous risks and uncertainties associated with developing our tests and their potential for commercialization, we are unable to predict the extent of any future profits. If we are unable to sustain or increase profitability, the market value of our common stock will likely decline. Our ability to maintain profitability will depend upon numerous factors, including:
| • | our ability to sell our existing molecular diagnostic tests and companion diagnostic services to new patients; |
| • | our ability to identify biomarkers that may lead to future molecular diagnostic tests and companion diagnostic services; |
| • | our ability to develop test candidates and receive required regulatory approvals; |
20
| • | our ability to successfully commercialize our tests in our existing markets and to extend into new markets outside the United States; |
| • | the approval and introduction of competitive tests; |
| • | the willingness of third-party payors to provide full or even partial reimbursement for our tests; |
| • | our ability to maintain and grow our sales force and marketing team to market our tests; |
| • | our ability to successfully integrate, develop and grow products and services and the business of any other companies or technologies that we may license or acquire; |
| • | our ability to increase commercial acceptance of our current molecular diagnostic tests and companion diagnostic services; and |
| • | our ability to maintain or grow our current revenues. |
Our Term Loan and Option Agreement with Crescendo Bioscience may result in a substantial loss.
On September 8, 2011, we issued a six-year term loan for $25.0 million to Crescendo Bioscience, Inc., or Crescendo, of South San Francisco, California, which is developing molecular diagnostic tests for patients suffering from autoimmune disorders, including rheumatoid arthritis. We made this loan under a Loan and Security Agreement, or Loan Agreement, and also secured an exclusive three-year option to acquire the company pursuant to a definitive merger agreement, which we refer to as the Option Agreement. As of June 30, 2013, we had recorded on our balance sheet a $21.7 million note receivable related to the Loan Agreement and an $8.0 million other asset related to the Option Agreement. Although we do not anticipate that Crescendo will default under the Loan Agreement or that the value of the Option Agreement will deteriorate over time, there can be no assurance that Crescendo will repay the loan or ultimately succeed in its business plan. In the event that Crescendo does not make the principal and accrued interest payments in accordance with the Loan Agreement and we do not exercise our option to purchase Crescendo, we would be required to record a loss up to $25.0 million and any unpaid interest.
If our current operating plan changes and we find that our existing capital resources will not meet our needs, we may find it necessary to raise additional funding, which may not be available.
We anticipate that our existing capital resources and expected net cash to be generated from sales of our molecular diagnostic tests will enable us to maintain our currently planned operations for the foreseeable future. However, we base this expectation on our current operating plan, which may change. We have incurred, and will continue to incur, significant costs in the discovery, development and marketing of current and prospective molecular diagnostic and companion diagnostic tests. Our ongoing efforts to develop tests and expand our business which may be through internally developed products, in licensing and mergers and acquisitions will require substantial cash resources. For example, if we exercise our option to acquire Crescendo, the purchase price will be paid in cash and will be based on a predetermined multiple of revenue based on Crescendo's growth rate at the time the option is exercised, or else a fixed purchase price, in accordance with the agreement. If we exercise the Crescendo option or another acquisition target is identified, we would require funds in addition to our current operating plan to acquire and integrate the target company. If, due to changes in our current operating plan, adequate funds are not available, we may be required to raise additional funds. Sources of potential additional capital resources may include, but are not limited to, public or private equity financings, establishing a credit facility, or selling convertible debt securities. This additional funding, if necessary, may not be available to us on reasonable terms, or at all. If we issue shares of stock or other securities to acquire new companies or technologies, the ownership interests of our existing stockholders may be significantly diluted.
Because of our potential long-term capital requirements, we may access the public or private equity or debt markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. Under SEC rules, we currently qualify as a well-known seasoned issuer, or WKSI, and can at any time file a registration statement registering securities to be sold to the public which would become effective upon filing. If additional funds are raised by issuing equity securities, existing shareholders may suffer significant
21
dilution. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or tests, or grant licenses on terms that are not favorable to us.
If we do not continue to generate sufficient revenue from sales of our molecular diagnostic tests and are unable to secure additional funding, we may have to reduce our operations.
As of June 30, 2013, we had $531.1 million in cash, cash equivalents and marketable securities. For the fiscal year ended June 30, 2013 our consolidated revenues were $613.2 million, and net cash from operating activities was $173.9 million. To develop and bring new molecular diagnostic tests and companion diagnostics to market, we must commit substantial resources to costly and time-consuming research, development testing and clinical testing.
While we anticipate that our existing cash, cash equivalents and marketable securities and expected net cash to be generated from sales of our molecular diagnostic tests and companion diagnostic services will be sufficient to fund our current operations for the foreseeable future, changes could occur that would consume available capital resources more quickly than we currently expect and we may need or want to raise additional financing. If we are unable to secure additional funding, we may be required to reduce research and development projects, limit sales and marketing activities, scale back our expansion efforts outside the United States, reduce headcount or potentially even discontinue operations. Our future capital requirements will depend on many factors that are currently unknown to us, including:
| • | our ability to maintain the existing licenses to our molecular diagnostic tests and enter into collaborations, licensing or other arrangements favorable to us; |
| • | the scope, progress, results and cost of development, clinical testing and pre-market studies of any new molecular diagnostic tests that we may discover or acquire; |
| • | the progress, results, and costs to develop additional molecular diagnostic tests; |
| • | the costs by us or our licensors of preparing, filing and prosecuting patent applications, maintaining and enforcing our current issued patents, and defending intellectual property-related claims; |
| • | the costs of acquiring technologies or businesses, and our ability to successfully integrate and achieve the expected benefits of our business development activities and acquisitions; |
| • | the progress, cost and results of our international expansion efforts; |
| • | the costs of expanding our sales and marketing functions and commercial operation facilities in the United States and in new markets; |
| • | the costs, timing and outcome of any litigation against us; and |
| • | the costs to satisfy our current and future obligations. |
We may acquire technologies, assets or other businesses that could cause us to incur significant expense and expose us to a number of unanticipated operational and financial risks.
In addition to organic growth, we intend to continue to pursue growth through the acquisition of technology, assets or other businesses that may enable us to enhance our technologies and capabilities, expand our geographic market, add experienced management personnel and increase our test offerings. For example, in May 2011, we completed the acquisition of Rules-Based Medicine, Inc., which we renamed Myriad RBM, and are now offering companion diagnostic services and developing additional product candidates using the acquired technology. Additionally, in September 2011, we acquired a three-year exclusive option to acquire Crescendo, a company that is developing molecular diagnostic tests for patients suffering from autoimmune disorders, including
22
rheumatoid arthritis. However, we may be unable to implement our growth strategy if we cannot identify suitable acquisition candidates, reach agreement on potential acquisitions on acceptable terms, successfully integrate personnel or assets that we acquire or for other reasons. Our acquisition efforts may involve certain risks, including:
| • | we may have difficulty integrating operations and systems; |
| • | key personnel and customers of the acquired company may terminate their relationships with the acquired company as a result of the acquisition; |
| • | we may not be successful in launching new molecular diagnostic tests or companion diagnostic services, or if those tests are launched they may not prove successful in the market place; |
| • | we may experience additional financial and accounting challenges and complexities in areas such as tax planning and financial reporting; |
| • | we may assume or be held liable for risks and liabilities, including for environmental-related costs, as a result of our acquisitions, some of which we may not discover during our due diligence; |
| • | we may incur significant additional operating expenses; |
| • | our ongoing business may be disrupted or receive insufficient management attention; and |
| • | we may not be able to realize synergies, the cost savings or other financial and operational benefits we anticipated, or such synergies, savings or benefits may take longer than we expected. |
The process of negotiating acquisitions and integrating acquired tests, services, technologies, personnel or businesses might result in operating difficulties and expenditures and might require significant management attention that would otherwise be available for ongoing development of our business, whether or not any such transaction is ever consummated. Moreover, we might never realize the anticipated benefits of any acquisition. Future acquisitions could result in the use of our available cash and marketable securities, potentially dilutive issuances of equity securities, the incurrence of debt, contingent liabilities, or impairment expenses related to goodwill, and impairment or amortization expenses related to other intangible assets, which could harm our financial condition. In addition, if we are unable to integrate any acquired businesses, tests or technologies effectively, our business, financial condition and results of operations may be materially adversely affected.
We may not be able to successfully integrate the operations of businesses that we acquire with our own or realize the anticipated benefits of the acquisitions, which could adversely affect our financial condition, results of operations and business prospects.
There can be no assurance that we will be able to successfully integrate our recent acquisitions or develop or commercialize products based on recently acquired technologies, or that we will be able to successfully integrate any other companies, products or technologies that we acquire and may not realize all or any of the expected benefits of any acquisitions as and when planned. Additionally, we may experience increased expenses, distraction of our management, personnel and customer uncertainty.
The difficulties and risks associated with the integration of any other businesses that we may acquire include:
| • | possible inconsistencies in the standards, controls, procedures, policies and compensation structures; |
| • | the increased scope and complexity of the acquired company's operations; |
| • | the potential loss of key employees and the costs associated to retain key employees; |
| • | risks and limitations on our ability to consolidate corporate and administrative infrastructures of the two companies; and |
| • | the possibility of unanticipated delays, costs or inefficiencies associated with the integration of our operations with the operations of any other companies that we may acquire. |
23
As a result of these difficulties and risks, we may not accomplish the integration of the business of any companies we may acquire smoothly, successfully or within our budgetary expectations and anticipated timetable. Accordingly, we may fail to realize some or all of the anticipated benefits of the acquisition, such as increase in our scale, diversification, cash flows and operational efficiency and meaningful accretion to our diluted earnings per share.
If we were successfully sued for product liability, we could face substantial liabilities that exceed our resources.
Our business exposes us to potential liability risks inherent in the testing, marketing and processing of molecular diagnostic products, including possible misdiagnoses. Although we are insured against such risks in amounts that we believe to be commercially reasonable, our present professional and product liability insurance may be inadequate. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on our business. Any successful product liability claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable or reasonable terms. An inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of our products.
We are dependent on our information technology and telecommunications systems, and any failure of these systems could harm our business.
We depend on information technology, or IT, and telecommunications systems for significant aspects of our business. These IT and telecommunications systems support a variety of functions, including sample processing, tracking, quality control, customer service and support, billing, research and development activities, and various general and administrative activities. Failures or significant downtime of our IT or telecommunications systems could prevent us from processing samples, providing test results to physicians, billing payors, addressing patient or physician inquiries, conducting research and development activities and conducting general and administrative elements of our business. Any disruption or loss of IT or telecommunications systems on which critical aspects of our operations depend could have an adverse effect on our business.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business or prevent us from accessing critical information and expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we collect and store sensitive data, including legally protected health information, credit card information, personally identifiable information about our employees, intellectual property, and our proprietary business information. We manage and maintain our applications and data utilizing on-site systems. These applications and data encompass a wide variety of business critical information including research and development information, commercial information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers, or viruses, breaches or interruptions due to employee error, malfeasance or other disruptions. Any such virus, breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the Health Insurance Portability and Accountability Act of 1996, and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to process samples, provide test results, bill payors or patients, provide customer support services, conduct research and development activities, process and prepare company financial information, manage various general and administrative aspects of our business and damage our reputation, any of which could adversely affect our business.
24
Our business involves environmental risks that may result in liability for us.
In connection with our research and development activities, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens, chemicals and wastes. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Although we believe that our safety procedures for handling and disposing of controlled materials comply with the standards prescribed by state and federal regulations, accidental contamination or injury from these materials may occur. In the event of such an occurrence, we could be held liable for any damages that result and any such liability could exceed our resources.
Changes in healthcare policy could increase our costs, decrease our revenues and impact sales of and reimbursement for our tests.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the ACA became law. This law substantially changes the way health care is financed by both governmental and private insurers, and significantly impacts our industry. The ACA contains a number of provisions that are expected to impact our business and operations, some of which in ways we cannot currently predict, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse, which will impact existing government healthcare programs and will result in the development of new programs.
In addition to the ACA, there will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payors to reduce costs while expanding individual healthcare benefits. Certain of these changes could impose additional limitations on the prices we will be able to charge for our tests or the amounts of reimbursement available for our tests from governmental agencies or third-party payors. While in general it is too early to predict specifically what effect the ACA and its implementation or any future healthcare reform legislation or policies will have on our business, current and future healthcare reform legislation and policies could have a material adverse effect on our business and financial condition.
We face risks associated with currency exchange rate fluctuations, which could adversely affect our operating results.
We receive a portion of our revenues and pay a portion of our expenses in currencies other than the United States dollar, such as the Euro, the Swiss franc, the British pound and the Canadian dollar. As a result, we are at risk for exchange rate fluctuations between such foreign currencies and the United States dollar, which could affect the results of our operations. If the U.S. dollar strengthens against foreign currencies, the translation of these foreign currency denominated transactions will result in decreased revenues, operating expenses and net income. We may not be able to offset adverse foreign currency impact with increased revenues. We do not currently utilize hedging strategies to mitigate foreign currency risk and even if we were to implement hedging strategies to mitigate foreign currency risk, these strategies might not eliminate our exposure to foreign exchange rate fluctuations and would involve costs and risks of their own, such as ongoing management time and expertise, external costs to implement the strategies and potential accounting implications.
Risks Related to Commercialization of Our Tests, Our Services and Test Candidates
We generate most of our revenues from a single product and we may not be able to maintain or increase revenue growth and profitability.
Even though we have experienced double-digit revenue growth in our molecular diagnostic business every year since the initial launch of our first test in 1996; we may not be able to continue this revenue growth or maintain existing revenue levels. Presently, our molecular diagnostic business operates profitably providing a cash contribution to our current funding and operational needs. We may not, however, be able to continue to operate
25
our molecular diagnostic business on a profitable basis. We launched our first molecular diagnostic test, BRAC Analysis , our test for hereditary breast and ovarian cancer, in November 1996. BRAC Analysis test sales accounted for 75% of our revenues for the year ended June 30, 2013. An interruption or cessation of BRAC Analysis sample flow would have a material impact on our revenues and future profitability. Other potential events or factors that may have a significant impact on our ability to sustain revenue growth and profitability for our molecular diagnostic business include the following:
| • | increased costs of reagents and other consumables required for molecular diagnostic testing; |
| • | increased licensing or royalty costs; |
| • | increased personnel and facility costs; |
| • | our inability to hire competent, trained staff, including laboratory directors required to review and approve all reports we issue in our molecular diagnostic business, and sales personnel; |
| • | our inability to obtain necessary equipment or reagents to perform molecular diagnostic testing; |
| • | our inability to increase production capacity as demand increases; |
| • | our inability to expand into new markets outside the United States; |
| • | the efforts of third party payors to limit or decrease the amounts that they are willing to pay for our tests; |
| • | changes in intellectual propriety laws of our patents or enforcement in the United States and foreign countries; |
| • | potential obsolescence of our tests; |
| • | our inability to increase commercial acceptance of our molecular diagnostic tests; |
| • | increased competition and loss of market share; and |
| • | increased regulatory requirements. |
The international expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States.
As part of our business strategy, we are expanding into international markets. We have established sales offices in Germany, Switzerland, France, Spain, the United Kingdom, Italy and Canada; laboratory operations in Germany; and international headquarters in Switzerland. Doing business internationally involves a number of risks, including:
| • | failure by us to obtain regulatory approvals or adequate reimbursement for the use of our tests in various countries; |
| • | difficulty in staffing and managing foreign operations; |
| • | managing multiple payor reimbursement and self-pay systems; |
| • | logistics and regulations associated with shipping patient samples, including infrastructure conditions and transportation delays; |
| • | limits in our ability to penetrate international markets if we are not able to process tests locally; |
| • | financial risks, such as longer payment cycles, difficulty collecting accounts receivable and exposure to foreign currency exchange rate fluctuations; |
| • | political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; |
| • | multiple, conflicting and changing laws and regulations such as tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; and |
26
| • | regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors' activities that may fall within the purview of the U.S. Foreign Corrupt Practice Act, anti-boycott and other laws. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenues and results of operations. In addition, any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments, and restrictions on certain business activities. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our distribution and sales activities.
Our expanding international operations could be affected by changes in laws, trade regulations, labor and employment regulations, and procedures and actions affecting approval, production, pricing, reimbursement and marketing of tests, as well as by inter-governmental disputes. Any of these changes could adversely affect our business.
Our success expanding internationally will depend, in part, on our ability to develop and implement policies and strategies that are effective in anticipating and managing these and other risks in the countries in which we do business. Failure to manage these and other risks may have a material adverse effect on our operations in any particular country and on our business as a whole.
Foreign governments may impose reimbursement standards, which may adversely affect our future profitability.
We market our tests in foreign jurisdictions and as such may be subject to rules and regulations in those jurisdictions relating to our testing. In some foreign countries, including countries in the European Union, the reimbursement of diagnostic tests is subject to governmental control. In these countries, reimbursement negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a test candidate. If reimbursement of our future tests is unavailable or limited in scope or amount, or if reimbursement rates are set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
Our pharmaceutical testing services customers may reduce the amount of testing they conduct through us.
If there is a change in the regulatory environment or intellectual property law, or our pharmaceutical testing services customers consolidate, our customers may divert resources from testing, resulting in a reduced demand for our laboratory testing services. Alternatively, customers may decide to perform their own laboratory testing services in-house.
We rely on a single laboratory facility to process our molecular diagnostic tests in the United States, a single laboratory facility to process our molecular diagnostic tests in Europe and a single laboratory facility to perform our companion diagnostic services.
We rely on a single CLIA-certified laboratory facility in Salt Lake City, Utah to perform our United States molecular diagnostic tests, a single laboratory facility in Munich, Germany to perform our European molecular diagnostic tests, and a single CLIA-certified laboratory facility in Austin, Texas to perform our companion diagnostic testing services. These facilities and certain pieces of laboratory equipment would be difficult to replace and may require significant replacement lead-time. In the event our clinical testing facilities were to lose their CLIA certification or other required certifications or licenses or were affected by man-made or natural disasters, we would be unable to continue our molecular diagnostic and companion diagnostic business at current levels to meet customer demands for a significant period of time. Although we maintain insurance on these facilities, including business interruption insurance, it may not be adequate to protect us from all potential losses
27
if these facilities were damaged or destroyed. In addition, any interruption in our molecular diagnostic or companion diagnostic business would result in a loss of goodwill, including damage to our reputation. If our molecular diagnostic or companion diagnostic business were interrupted, it would seriously harm our business.
Our molecular diagnostic and companion diagnostic tests in development may never achieve significant commercial market acceptance.
We may not succeed in achieving significant commercial market acceptance of our test and service offerings that we have launched in recent years or that we are currently developing. Our ability to successfully develop and commercialize our current molecular diagnostic and companion diagnostic tests, as well as any future molecular diagnostic and companion diagnostic tests that we may develop, will depend on several factors, including:
| • | our ability to convince the medical community of the clinical utility of our tests and their potential advantages over existing tests; |
| • | our ability to collaborate with biotechnology and pharmaceutical companies to develop and commercialize companion diagnostic tests for their therapeutic drugs and drug candidates; |
| • | the agreement by third-party payors to reimburse our tests, the scope and extent of which will affect patients' willingness or ability to pay for our tests and will likely heavily influence physicians' decisions to recommend our tests; and |
| • | the willingness of physicians to utilize our tests, which can be difficult to interpret. This difficulty is caused by a combination of factors, including the large number, sometimes thousands, of different mutations in the genes which our tests analyze, the need to characterize each specific mutation, and the ability of our tests to predict only as to a statistical probability, not certainty, that a tested individual will develop the disease that the test is intended to predict. |
These factors present obstacles to commercial acceptance of our tests, which we will have to spend substantial time and money to overcome, if we can do so at all. Our inability to successfully do so will harm our business.
If we do not compete effectively with scientific and commercial competitors, we may not be able to successfully commercialize our tests.
The biotechnology and genetics testing fields are intense and highly competitive. Tests that are developed are characterized by rapid technological change. Our competitors in the United States and abroad are numerous and include, among others, major diagnostic companies, reference laboratories, molecular diagnostic firms, universities and other research institutions. Many of our potential competitors have considerably greater financial, technical, marketing and other resources than we do, which may allow these competitors to discover important genes and determine their function before we do. We could be adversely affected if we do not discover genes, proteins or biomarkers and characterize their function, develop molecular diagnostic and companion diagnostic tests based on these discoveries, obtain required regulatory and other approvals and launch these tests and their related services before our competitors. We also expect to encounter significant competition with respect to any molecular diagnostic and companion diagnostic tests that we may develop or commercialize. Those companies that bring to market new molecular diagnostic and companion tests before we do may achieve a significant competitive advantage in marketing and commercializing their tests. We may not be able to develop additional molecular diagnostic tests successfully and we or our licensors may not obtain patents covering these tests that provide protection against our competitors. Moreover, our competitors may succeed in developing molecular diagnostic and companion diagnostic tests that circumvent our technologies or tests. Furthermore, our competitors may succeed in developing technologies or tests that are more effective than those developed by us or that would render our technologies or tests less competitive or obsolete. We expect competition to intensify in the fields in which we are involved as technical advances in these fields occur and become more widely known.
28
If our current research collaborators or scientific advisors terminate their relationships with us or develop relationships with a competitor, our ability to discover genes, proteins, and biomarkers, and to commercialize molecular diagnostic and companion diagnostic tests could be adversely affected.
We have relationships with research collaborators at academic and other institutions who conduct research at our request. These research collaborators are not our employees. As a result, we have limited control over their activities and, except as otherwise required by our collaboration agreements, can expect only limited amounts of their time to be dedicated to our activities. Our ability to discover genes, proteins, and biomarkers involved in human disease and commercialize molecular diagnostic and companion diagnostic tests will depend in part on the continuation of these collaborations. If any of these collaborations are terminated, we may not be able to enter into other acceptable collaborations. In addition, our existing collaborations may not be successful.
Our research collaborators and scientific advisors may have relationships with other commercial entities, some of which could compete with us. Our research collaborators and scientific advisors sign agreements which provide for the confidentiality of our proprietary information and the results of studies conducted at our request. We may not, however, be able to maintain the confidentiality of our technology and other confidential information related to all collaborations. The dissemination of our confidential information could have a material adverse effect on our business.
If we fail to retain our key personnel and hire, train and retain qualified employees and consultants, we may not be able to successfully continue our business.
Because of the specialized scientific nature of our business, we are highly dependent upon our ability to attract and retain qualified management, scientific and technical personnel. We are currently recruiting additional qualified management, scientific and technical personnel. Competition for such personnel is intense. Loss of the services of or failure to recruit additional key management, scientific and technical personnel would adversely affect our research and development programs and molecular diagnostic and companion diagnostic business and may have a material adverse effect on our business as a whole.
Our agreements with our employees generally provide for employment that can be terminated by either party without cause at any time, subject to specified notice requirements. Further, the non-competition provision to which each employee is subject expires for certain key employees on the applicable date of termination of employment.
As we expand our commercial tests we may be required to incur significant costs and devote significant efforts to expand our existing tests sales and marketing capabilities.
Our sales and marketing experience and capabilities consist primarily of our sales force that markets our cancer-related molecular diagnostic tests to oncologists, Ob/Gyns and urologists in the United States. We are currently expanding our sales efforts outside the United States, which will require us to hire additional personnel and engage in additional sales and marketing efforts. We have limited sales and marketing experience outside the Unites States. As we expand our business operations internationally, we expect to face a number of additional costs and risks, including the need to recruit a large number of additional experienced marketing and sales personnel.
We depend on a limited number of third parties for some of our supplies of equipment and reagents. If these supplies become unavailable, then we may not be able to successfully perform our research or operate our business at all or on a timely basis.
We currently rely on a small number of suppliers to provide our gene sequencing equipment, multiplex protein analysis equipment, robots, and specialty reagents required in connection with our research. We believe that currently there are limited alternative suppliers of gene sequencing and multiplex protein analysis equipment, robots, and reagents. The equipment, robots, or the reagents may not remain available in commercial quantities at
29
acceptable costs. If we are unable to obtain when needed additional equipment, robots, or an adequate supply of reagents or other ingredients at commercially reasonable rates, our ability to continue to identify genes and perform molecular diagnostic testing and companion diagnostic services would be adversely affected.
Risks Related to Our Intellectual Property
If we fail to comply with our obligations under license or technology agreements with third parties, we could lose license rights that are critical to our business.
We license intellectual property that is critical to our business, including licenses underlying the technology in our molecular diagnostic and companion diagnostic tests, and in the future we may enter into additional agreements that provide us with licenses to valuable intellectual property or technology. These licenses impose various royalty payments, milestones, and other obligations on us. If we fail to comply with any of these obligations, the licensor may have the right to terminate the license. Termination by the licensor would cause us to lose valuable rights, and could prevent us from distributing our current tests, or inhibit our ability to commercialize future test candidates. Our business would suffer if any current or future licenses terminate, if the licensors fail to abide by the terms of the license, if the licensors fail to prevent infringement by third parties, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms.
If we are not able to protect our proprietary technology, others could compete against us more directly, which would harm our business.
As of June 30, 2013, our patent portfolio included 234 issued patents owned or licensed by us and numerous patent applications in the United States and other countries with claims covering our intellectual property rights. Our commercial success will depend, in part, on our ability to obtain additional patents and licenses and protect our existing patent position, both in the United States and in other countries, for predisposing genes we identify and related technologies, processes, methods and other inventions that we believe are patentable. Our ability to preserve our trade secrets and other intellectual property is also critical to our long-term success. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to maintain profitability. Patents may also issue to third parties which could interfere with our ability to bring our molecular diagnostic tests to market. The laws of some foreign countries do not protect our proprietary rights to the same extent as U.S. laws, and we may encounter significant problems in protecting our proprietary rights in these countries.
The patent positions of diagnostic companies, including our patent position, are generally highly uncertain and involve complex legal and factual questions, and, therefore, any patents issued to us may be challenged, deemed unenforceable, invalidated or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies and any future tests are covered by valid and enforceable patents or are effectively maintained as trade secrets. Our patent applications may never issue as patents, and the claims of any issued patents may not afford meaningful protection for our technology or tests. In addition, any patents issued to us or our licensors may be challenged, and subsequently narrowed, invalidated or circumvented.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| • | we or our licensors were the first to make the inventions covered by each of our patent applications; |
| • | we or our licensors were the first to file patent applications for these inventions; |
| • | others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | any of our or our licensors' patent applications will result in issued patents; |
| • | any of our or our licensors' patents will be valid or enforceable; |
30
| • | any patents issued to us or our licensors and collaborators will provide a basis for commercially viable tests, will provide us with any competitive advantages or will not be challenged by third parties; |
| • | we will develop additional proprietary technologies or tests that are patentable; |
| • | the patents of others will not have an adverse effect on our business; or |
| • | our patents or patents that we license from others will survive legal challenges, and remain valid and enforceable. |
If a third party files a patent application with claims to a gene, protein, or biomarker we have discovered, the PTO may declare interference between competing patent applications. If an interference is declared, we may not prevail in the interference. If the other party prevails in the interference, we may be precluded from commercializing services or tests based on the gene, protein, or biomarker or may be required to seek a license. A license may not be available to us on commercially acceptable terms, if at all.
We also rely upon unpatented proprietary technologies. Although we require employees, consultants and collaborators to sign confidentiality agreements, we may not be able to adequately protect our rights in such unpatented proprietary technologies, which could have a material adverse effect on our business. For example, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to our proprietary technologies or disclose our technologies to our competitors.
If we were sued for patent infringement by third parties, we might incur significant costs and delays in test introduction.
Our tests may also conflict with patents that have been or may be granted to others. Our industry includes many organizations that have or are seeking to discern gene and protein biomarkers and develop genomic, proteomic and other technologies. To the extent any patents are issued or have been issued to those organizations, the risk increases that the sale of our molecular diagnostic and companion diagnostic tests currently being marketed or under development may give rise to claims of patent infringement. Others may have filed and in the future are likely to file patent applications covering genes or proteins that are similar or identical to our tests. Any of these patent applications may have priority over our patent applications and these entities or persons could bring legal proceedings against us seeking damages or seeking to enjoin us from testing or marketing our tests. Patent litigation is costly, and even if we prevail, the cost of such litigation could have a material adverse effect on us. If the other parties in any such actions are successful, in addition to any liability for damages, we could be required to cease the infringing activity or obtain a license. Any license required may not be available to us on commercially acceptable terms, if at all. Our failure to obtain a license to any technology that we may require to commercialize our tests could have a material adverse effect on our business. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights. If we become involved in this litigation, it could consume a substantial portion of our managerial and financial resources.
We may be unable to adequately prevent disclosure of trade secrets, proprietary databases, and other proprietary information.
We rely on trade secrets to protect our proprietary technologies and databases, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and others to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy if unauthorized disclosure of confidential information occurs. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive position.
31
We may be subject to claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is commonplace in our industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Government Regulation
If we fail to comply with the complex federal, state, local and foreign laws and regulations that apply to our business, we could suffer severe consequences that could materially and adversely affect our operating results and financial condition.
Our operations are subject to extensive federal, state, local and foreign laws and regulations, all of which are subject to change. These laws and regulations currently include, among other things:
| • | CLIA, which requires that laboratories obtain certification from the federal government; |
| • | FDA laws and regulations; |
| • | HIPAA, which established comprehensive federal standards with respect to the privacy and security of protected health information and requirements for the use of certain standardized electronic transactions; amendments to HIPAA under the Health Information Technology for Economic and Clinical Health Act, or HITECH, which strengthen and expand HIPAA privacy and security compliance requirements, increase penalties for violators, extend enforcement authority to state attorneys general and impose requirements for breach notification; |
| • | state laws regulating genetic testing and protecting the privacy of genetic test results, as well as state laws protecting the privacy and security of health information and personal data and mandating reporting of breaches to affected individuals and state regulators; |
| • | the federal anti-kickback law, or the Anti-Kickback Statute, which prohibits knowingly and willfully offering, paying, soliciting, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for, or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program; |
| • | the federal False Claims Act, which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government; |
| • | the federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or state health care program beneficiary if the person knows or should know it is likely to influence the beneficiary's selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies; |
| • | other federal and state fraud and abuse laws, such as anti-kickback laws, prohibitions on self-referral, and false claims acts, which may extend to services reimbursable by any third-party payor, including private insurers; and |
| • | similar foreign laws and regulations that apply to us in the countries in which we operate. |
These laws and regulations are complex and are subject to interpretation by the courts and by government agencies. Our failure to comply could lead to civil or criminal penalties, exclusion from participation in government health care programs, or prohibitions or restrictions on our laboratories' ability to provide services.
32
We believe that we are in material compliance with all statutory and regulatory requirements, but there is a risk that one or more government agencies could take a contrary position. Such occurrences, regardless of their outcome, could damage our reputation and adversely affect important business relationships with third parties, including managed care organizations, or MCOs, and other private third-party payors.
Failure to comply with government laws and regulations related to submission of claims for our services could result in significant monetary damages and penalties and exclusion from the Medicare and Medicaid programs and corresponding foreign reimbursement programs.
We are subject to laws and regulations governing the submission of claims for payment for our services, such as those relating to: coverage of our services under Medicare, Medicaid and other state, federal and foreign health care programs; the amounts that we may bill for our services; and the party to which we must submit claims. Our failure to comply with applicable laws and regulations could result in our inability to receive payment for our services or in attempts by government healthcare programs, such as Medicare and Medicaid, to recover payments already made. Submission of claims in violation of these laws and regulations can result in recoupment of payments already received, substantial civil monetary penalties, and exclusion from government health care programs, and can subject us to liability under the federal False Claims Act and similar laws. The failure to report and return an overpayment to the Medicare or Medicaid program within 60 days of identifying its existence can give rise to liability under the False Claims Act. Further, a government agency could attempt to hold us liable for causing the improper submission of claims by another entity for services that we performed if we were found to have knowingly participated in the arrangement at issue.
Our business could be harmed by the loss, suspension, or other restriction on a license, certification, or accreditation, or by the imposition of a fine or penalties, under CLIA, its implementing regulations, or other state, federal and foreign laws and regulations affecting licensure or certification, or by future changes in these laws or regulations.
The diagnostic testing industry is subject to extensive laws and regulations, many of which have not been interpreted by the courts. CLIA requires virtually all laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification is also a prerequisite to be eligible to bill state and federal health care programs, as well as many private third-party payors, for laboratory testing services. As a condition of CLIA certification, each of our laboratories is subject to survey and inspection every other year, in addition to being subject to additional random inspections. The biennial survey is conducted by the Centers for Medicare and Medicaid Services, or CMS; a CMS agent (typically a state agency); or, if the laboratory holds a CLIA certificate of accreditation, a CMS-approved accreditation organization. Sanction for failure to comply with CLIA requirements, including proficiency testing violations, may be suspension, revocation, or limitation of a laboratory's CLIA certificate, which is necessary to conduct business, as well as the imposition of significant fines or criminal penalties. In addition, we are subject to regulation under state laws and regulations governing laboratory licensure. Some states have enacted state licensure laws that are more stringent than CLIA. We are also subject to laws and regulations governing our reference laboratory in Germany. Changes in state or foreign licensure laws that affect our ability to offer and provide diagnostic services across state or foreign country lines could materially and adversely affect our business. In addition, state and foreign requirements for laboratory certification may be costly or difficult to meet and could affect our ability to receive specimens from certain states or foreign countries.
Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our failure to renew a CLIA certificate, a state or foreign license, or accreditation, could have a material adverse effect on our business. If the CLIA certificate of any one of our laboratories is revoked, CMS could seek revocation of the CLIA certificates of our other laboratories based on their common ownership or operation, even though they are separately certified.
33
Changes in the way that the FDA regulates tests performed by laboratories like ours could result in delay or additional expense in offering our tests and tests that we may develop in the future.
While the FDA does not currently regulate the activities or tests performed by laboratories like our clinical laboratories, the FDA has stated that it has the right to do so, and the FDA may seek to regulate or require clearance or approval of our molecular diagnostic or personalized medicine tests in the future. In July, 2010, the FDA's office of In-Vitro Diagnostics held a public meeting to discuss oversight of laboratory developed tests. The FDA highlighted the lack of standardized clinical validation at the assay level under current CLIA regulatory guidelines and noted that CLIA does not require post-market surveillance or monitoring of laboratory developed tests. The comment period for providing the FDA with written comments expired on August 15, 2010, but the FDA has not yet published additional guidance on the oversight of laboratory developed tests. We cannot provide any assurance that FDA regulation, including pre-market review, will not be required in the future for our molecular diagnostic tests. If pre-market review is required, our business could be negatively impacted if we are required to stop selling molecular diagnostic tests pending their clearance or approval or the launch of any new tests that we develop could be delayed by new requirements.
If the government and third-party payors fail to provide coverage and adequate payment for our tests and future tests, if any, our revenue and prospects for profitability will be harmed.
In both domestic and foreign markets, sales of our molecular diagnostic tests or any future diagnostic tests will depend in part, upon the availability of reimbursement from third-party payors. Such third-party payors include government healthcare programs such as Medicare, managed care providers, private health insurers and other organizations. These third-party payors are increasingly attempting to contain healthcare costs by demanding price discounts or rebates and limiting both coverage on which diagnostic tests they will pay for and the amounts that they will pay for new molecular diagnostic tests. The fact that a diagnostic test has been approved for reimbursement in the past, for any particular indication or in any particular jurisdiction, does not guarantee that such a diagnostic test will remain approved for reimbursement or that similar or additional diagnostic tests will be approved in the future. As a result, third-party payors may not cover or provide adequate payment for our current or future molecular diagnostic tests. Adequate third-party reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
U.S. and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. For example, in some foreign markets, the government controls the pricing of many healthcare products. We expect that there will continue to be federal and state proposals to implement governmental controls or impose healthcare requirements. In addition, the Medicare program and increasing emphasis on managed care in the United States will continue to put pressure on product pricing. Cost control initiatives could decrease the price that we would receive for any tests in the future, which would limit our revenue and profitability.
Our business could be adversely impacted by the adoption of the ICD-10-CM Code Set.
CMS has adopted a new coding set for diagnoses, commonly known as ICD-10-CM, which significantly expands the current coding set. ICD-10-CM is currently required to be used on all claims with dates of service on or after October 1, 2014. We may be required to incur significant expense in implementing ICD-10-CM, and, if we do not adequately implement it, our business could be adversely impacted. In addition, if as a result of the new coding set, physicians fail to provide appropriate codes for desired tests, we may not be reimbursed for tests we perform.
Risks Related to Our Common Stock
Our stock price is highly volatile, and our stock may lose all or a significant part of its value.
The market prices for securities of molecular diagnostic and other life science companies have been volatile. This volatility has significantly affected the market prices for these securities for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the
34
market price of our common stock. The market price for our common stock has fluctuated significantly since public trading commenced in October 1995, and it is likely that the market price will continue to fluctuate in the future. In the two years ended June 30, 2013, our stock price has ranged from $17.51 per share to $38.27 per share. In addition, the stock market has experienced extreme price and volume fluctuations. Events or factors that may have a significant impact on our business and on the market price of our common stock include the following:
| • | termination of the licenses underlying our molecular diagnostic and companion diagnostic tests; |
| • | delays or other problems with operating our laboratory facilities; |
| • | failure of any of our research and development programs; |
| • | changes in intellectual property laws of our patents or enforcement in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights involving us directly or otherwise affecting the industry as a whole; |
| • | introduction of technological innovations or new commercial tests by us or our competitors; |
| • | missing or changing the financial guidance we provide; |
| • | changes in estimates or recommendations by securities analysts relating to our common stock or the securities of our competitors; |
| • | changes in the governmental regulatory approved process for our existing and new tests: |
| • | failure to meet estimates or recommendations by securities analysts that cover our common stock; |
| • | public concern over our approved tests and any test candidates; |
| • | litigation; |
| • | future sales or anticipated sales of our common stock by us or our stockholders; |
| • | the timing and amount of repurchases of our common stock; |
| • | general market conditions; |
| • | changes in the structure of healthcare payment systems and changes in the governmental or private insurers reimbursement levels for our molecular diagnostic tests; |
| • | failure to sustain revenue growth or margins in our molecular diagnostic business; |
| • | failure of any of our test candidates to achieve commercial success; |
| • | seasonal slowness in sales, particularly in the quarters ending September 30 and March 31, the effects of which may be difficult to understand during periods of growth; |
| • | economic, healthcare and diagnostic trends, disasters or crises and other external factors; and |
| • | period-to-period fluctuations in our financial results. |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit regardless of the outcome. Such a lawsuit could also divert the time and attention of our management.
35
Anti-takeover provisions of Delaware law, provisions in our charter and bylaws and re-adoption of our stockholders' rights plan, or poison pill, could make a third-party acquisition of us difficult.
Because we are a Delaware corporation, the anti-takeover provisions of Delaware law could make it more difficult for a third party to acquire control of us, even if the change in control would be beneficial to stockholders. We are subject to the provisions of Section 203 of the General Corporation Law of Delaware, which prohibits us from engaging in certain business combinations, unless the business combination is approved in a prescribed manner. In addition, our restated certificate of incorporation and restated bylaws also contain certain provisions that may make a third-party acquisition of us difficult, including:
| • | a classified board of directors, with three classes of directors each serving a staggered three-year term; |
| • | the ability of the board of directors to issue preferred stock; |
| • | a 70% super-majority shareholder vote to amend our bylaws and certain provisions of our certificate of incorporation; and |
| • | the inability of our stockholders to call a special meeting or act by written consent. |
In the past, we also implemented a stockholders' rights plan, also called a poison pill, which could make it uneconomical for a third party to acquire our company on a hostile basis. Although the plan expired in July 2011, our Board of Directors could adopt a new plan at any time. The provisions in a stockholders' rights plan, as well as Section 203, may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over then current market price, and may limit the ability of our stockholders to approve transactions that they think may be in their best interests.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| Item 2. | PROPERTIES |
Our corporate headquarters and facilities are located in Salt Lake City, Utah. We currently lease a total of 307,000 square feet of building space in Salt Lake City dedicated to research and development, administration and laboratory space that has received federal certification under CLIA. Activity related to our molecular diagnostic business is performed at this location. The leases on our existing Salt Lake City facilities have terms of fifteen years, expiring from 2017 through 2025, and provide for renewal options for up to ten additional years.
In addition, we lease approximately 36,000 square feet in Austin, Texas under a lease that expires in June 2015. This space is dedicated to administration, research and development and laboratory space that has received federal certification under CLIA. We also lease approximately 8,300 square feet of laboratory and office space in Saranac Lake, New York under a lease that expires in August 2017 with the right to renew for two additional five-year periods. Our immunoassay development and manufacturing of immunoassay kits are performed at the Saranac Lake facility.
In May 2011, we entered into a lease agreement for approximately 3,600 square feet in Munich, Germany. This space is used as a laboratory for our molecular diagnostic business in Europe. The lease on our Munich Germany facility has a term of approximately 5 years expiring in October of 2016. We also lease approximately 6,000 square feet of laboratory and office space in Reutlingen, Germany under a lease that expires in March of 2015 with the option to extend for one year periods. Cell co-culture systems and TruCulture products are manufactured at this location. This facility is designed to comply with ISO standards, the European Union equivalent of Good Manufacturing, or GMP, in the United States.
In December 2012, we entered into a lease agreement for approximately 5,000 square feet in Zurich, Switzerland. This space is used for the administration of our international operations. We also maintain lease agreements for our administrative offices in Paris, France; Madrid, Spain; and Milan, Italy.
36
We believe that our existing facilities and equipment are well maintained and in good working condition. We believe our current facilities and those planned or under construction will provide adequate capacity for at least the next two years. We continue to make investments in capital equipment as needed to meet the anticipated demand for our molecular diagnostic tests.
| Item 3. | LEGAL PROCEEDINGS |
Association for Molecular Pathology
On June 13, 2013, the Supreme Court of the United States rendered its decision in the matter of Association for Molecular Pathology et al. v. Myriad Genetics, Inc. et al. In its decision, the Supreme Court ruled that a naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated. However, the Supreme Court went on to rule that complementary DNA, or cDNA, is patent eligible because it is not naturally occurring. The Supreme Court also noted that the case did not involve patents on new applications of knowledge about the BRCA1 and BRCA2 genes.
Following the Supreme Court case, we believe that Myriad, as the first party with knowledge of the BRCA1 and BRCA2 sequences, is in an excellent position to claim applications of that knowledge, and that many of our existing patent claims that were not challenged in this case are limited to such applications. In addition, we continue to have over 500 claims in 24 different patents which we believe confer strong patent protection for our BRACAnalysis testing and services.
New Litigation Matters
In June 2013, following the Supreme Court decision in Association for Molecular Pathology et al. v. Myriad Genetics, Inc. et al. , two companies, Ambry Genetics Corporation and Gene By Gene LTD, commenced offering clinical diagnostic and genomic laboratory services, including the testing and analysis of the BRCA1 and BRCA2 genes, that purport to compete with our BRACAnalysis testing and services. We believe that Ambry's and Gene by Gene's testing services infringe various patent claims that we own or have exclusively licensed from the University of Utah Research Foundation, HSC Research and Development Limited Partnership (and affiliate of Hospital For Sick Children), the Trustees of the University of Pennsylvania, and Endorecherche, Inc. (collectively, the "Patent Owners"). Under our license agreements with the Patent Owners, we are responsible for pursuing these patent infringement litigations, defending any counterclaims and paying related costs. Accordingly, we have commenced the two litigation matters described below against Ambry and Gene by Gene.
Ambry Genetics Corporation
On July 9, 2013, we and the other Patent Owners filed a complaint against Ambry Genetics Corporation in the United States District Court for the District of Utah, Central Division, alleging that Ambry's testing services infringe various patent claims owned by the Patent Owners, and seeking an injunction against Ambry from selling any product or service that infringes the claims of these patents. We also requested a preliminary injunction to prevent Ambry from selling these testing services pending a final decision on the merits of the case. On July 19, 2013, we amended the complaint to allege that Ambry is also infringing various patent claims owned by Myriad that relate to the MYH gene. On August 5, 2013, Ambry filed an answer to the complaint, including affirmative defenses and counterclaims for antitrust violations of the Sherman Act (only against Myriad) and seeking declaratory relief of invalidity and non-infringement. The Patent Owners intend to vigorously enforce their patent rights against Ambry and defend the counterclaims asserted against them.
Gene By Gene
On July 10, 2013, we and the other Patent Owners filed a complaint against Gene By Gene LTD in the United States District Court for the District of Utah, Central Division, alleging that Gene By Gene's testing
37
services infringe various patent claims owned by the Patent Owners, and seeking an injunction against Gene by Gene from selling any product or service that infringes the claims of these patents. We also requested a preliminary injunction to prevent Gene By Gene from selling these testing services pending a final decision on the merits of the case. The Patent Owners intend to vigorously enforce their patent rights against Gene by Gene.
* **
Other than as set forth above, we are not a party to any legal proceedings that we believe will have a material impact on our business, financial position or results of operations.
| Item 4. | MINE SAFETY DISCLOSURES |
None.
38
PART II
| Item 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on The NASDAQ Global Select Market under the symbol "MYGN." The following table sets forth the high and low sales prices for our common stock, as reported by The NASDAQ Global Select Market for the last two fiscal years:
| High | Low | |||||||
Fiscal Year Ended June 30, 2013: | ||||||||
Fourth Quarter | $ | 38.27 | $ | 24.12 | ||||
Third Quarter | $ | 27.89 | $ | 24.08 | ||||
Second Quarter | $ | 31.80 | $ | 24.81 | ||||
First Quarter | $ | 28.00 | $ | 23.07 | ||||
Fiscal Year Ended June 30, 2012: | ||||||||
Fourth Quarter | $ | 27.00 | $ | 22.02 | ||||
Third Quarter | $ | 25.75 | $ | 19.95 | ||||
Second Quarter | $ | 23.96 | $ | 17.90 | ||||
First Quarter | $ | 24.21 | $ | 17.51 | ||||
Stockholders
As of August 1, 2013, there were approximately 99 stockholders of record of our common stock and, according to our estimates, approximately 31,324 beneficial owners of our common stock.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
We have previously announced the following stock repurchase programs for repurchases of our common stock:
Date Authorized | Amount Authorized | Date Completed | ||||
May 2010 | $ | 100 million | August 2011 | |||
August 2010 | $ | 100 million | February 2011 | |||
March 2011 | $ | 100 million | September 2011 | |||
August 2011 | $ | 200 million | January 2013 | |||
February 2013 | $ | 200 million | ongoing | |||
|
| |||||
Total: | $ | 700 million | ||||
In connection with our most recent stock repurchase authorization, we have been authorized to complete the repurchase through open market transactions or through an accelerated share repurchase program, in each case to be executed at management's discretion based on market conditions. As of the date of this report, we have not entered into an accelerated share repurchase agreement under our most recent stock repurchase program.
39
The details of the activity under our stock repurchase programs during the fiscal quarter ended June 30, 2013, were as follows:
Issuer Purchases of Equity Securities
Period | (a) Total Number of Shares Purchased | (b) Average Price Paid per Share | (c) Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | (d) Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs | ||||||||||||
April 1, 2013 to April 30, 2013 | 199,694 | $ | 25.00 | 199,694 | $ | 169,951,899 | ||||||||||
May 1, 2013 to May 31, 2013 | - | $ | - | - | 169,951,899 | |||||||||||
June 1, 2013 to June 30, 2013 | 626,603 | $ | 26.45 | 626,603 | 153,379,727 | |||||||||||
Total | 826,297 | 826,297 | $ | 153,379,727 | ||||||||||||
Stock Performance Graph
The graph set forth below compares the annual percentage change in our cumulative total stockholder return on our common stock, as adjusted for a two-for-one stock split effected on March 25, 2009, during a period commencing on June 30, 2008 and ending on June 30, 2013 (as measured by dividing (A) the difference between our share price at the end and the beginning of the measurement period; by (B) our share price at the beginning of the measurement period) with the cumulative total return of The NASDAQ Stock Market, Inc. and the NASDAQ Health Services Stock Index during such period. We have not paid any cash dividends on our common stock, and we do not include cash dividends in the representation of our performance. The price of a share of common stock is based upon the closing price per share as quoted on The NASDAQ Global Select Market on the last trading day of the year shown. The graph lines merely connect year-end values and do not reflect fluctuations between those dates. The comparison assumes $100 was invested on June 30, 2008 in our common stock and in each of the foregoing indices. The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
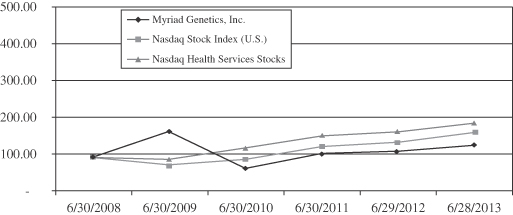
| 6/30/2008 | 6/30/2009 | 6/30/2010 | 6/30/2011 | 6/29/2012 | 6/28/2013 | |||||||||||||||||||
Myriad Genetics, Inc. | 100.00 | 164.24 | 72.43 | 110.03 | 115.16 | 130.18 | ||||||||||||||||||
NASDAQ Stock Index (U.S.) | 100.00 | 81.85 | 95.01 | 126.63 | 137.96 | 162.34 | ||||||||||||||||||
NASDAQ Health Services Stocks | 100.00 | 94.78 | 123.67 | 153.50 | 163.68 | 185.42 | ||||||||||||||||||
40
Note: Information used on the graph was obtained from the CRSP Total Return Indexes, a source believed to be a reliable, but we are not responsible for any errors or omission in such information.
The performance graph shall not be deemed to be incorporated by reference by means of any general statement incorporating by reference this Form 10-K into any filing under the Securities Act of 1933, as amended or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate such information by reference, and shall not otherwise be deemed filed under such acts.
41
| Item 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
The following table sets forth our selected consolidated financial data and has been derived from our audited consolidated financial statements. Consolidated balance sheets as of June 30, 2013 and 2012, as well as consolidated statements of comprehensive income for the years ended June 30, 2013, 2012 and 2011 and the reports thereon are included elsewhere in this Annual Report on Form 10-K. The information below should be read in conjunction with our audited consolidated financial statements (and notes thereon) and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included in Item 7.
| In thousands, except per share amounts | Years Ended June 30, | |||||||||||||||||||
| 2013 | 2012 | 2011 | 2010 | 2009 | ||||||||||||||||
Consolidated Statement of Comprehensive Income Data: | ||||||||||||||||||||
Molecular diagnostic testing | $ | 582,392 | $ | 472,390 | $ | 400,046 | $ | 362,648 | $ | 326,527 | ||||||||||
Companion diagnostic services | 30,773 | 23,615 | 2,038 | - | - | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total Revenue | 613,165 | 496,005 | 402,084 | 362,648 | 326,527 | |||||||||||||||
Costs and expenses: | ||||||||||||||||||||
Cost of molecular diagnostic testing | 64,376 | 51,452 | 45,637 | 44,286 | 43,267 | |||||||||||||||
Cost of companion diagnostic services | 15,242 | 13,207 | 1,077 | - | - | |||||||||||||||
Research and development expense | 53,706 | 42,645 | 27,751 | 21,873 | 17,914 | |||||||||||||||
Selling, general and administrative expense | 251,839 | 208,383 | 169,841 | 161,414 | 138,884 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total costs and expenses | 385,163 | 315,687 | 244,306 | 227,573 | 200,065 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Operating income | 228,002 | 180,318 | 157,778 | 135,075 | 126,462 | |||||||||||||||
Other income (expense): | ||||||||||||||||||||
Interest income | 5,497 | 4,629 | 2,226 | 5,660 | 12,478 | |||||||||||||||
Other | (223 | ) | (407 | ) | (353 | ) | 99 | (2,493 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Income from continuing operations before income taxes | 233,276 | 184,540 | 159,651 | 140,834 | 136,447 | |||||||||||||||
Income tax provision (benefit) | 86,137 | 72,389 | 58,941 | (11,469 | ) | 193 | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Income from continuing operations | 147,139 | 112,151 | 100,710 | 152,303 | 136,254 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Loss from discontinued operations | - | - | - | - | (51,639 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net income | $ | 147,139 | $ | 112,151 | $ | 100,710 | $ | 152,303 | $ | 84,615 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Earnings per basic share: | ||||||||||||||||||||
Continuing operations | $ | 1.82 | $ | 1.33 | $ | 1.12 | $ | 1.58 | $ | 1.46 | ||||||||||
Discontinued operations | - | - | - | - | (0.60 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Earnings per basic share | $ | 1.82 | $ | 1.33 | $ | 1.12 | $ | 1.58 | $ | 0.91 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Earnings per diluted share: | ||||||||||||||||||||
Continuing operations | $ | 1.77 | $ | 1.30 | $ | 1.10 | $ | 1.54 | $ | 1.38 | ||||||||||
Discontinued operations | - | - | - | - | (0.50 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Earnings per diluted share | $ | 1.77 | $ | 1.30 | $ | 1.10 | $ | 1.54 | $ | 0.86 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Weighted average shares outstanding: | ||||||||||||||||||||
Basic | 80,948 | 84,608 | 89,794 | 96,338 | 93,492 | |||||||||||||||
Diluted | 83,327 | 86,465 | 91,704 | 99,152 | 98,573 | |||||||||||||||
| As of June 30, | ||||||||||||||||||||
| 2013 | 2012 | 2011 | 2010 | 2009 | ||||||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||||||||||
Cash, cash equivalents and marketable investment securities | $ | 531,064 | $ | 454,224 | $ | 417,314 | $ | 488,382 | $ | 392,225 | ||||||||||
Working capital | 419,483 | 377,525 | 383,874 | 446,510 | 333,951 | |||||||||||||||
Total assets | 803,821 | 690,635 | 610,827 | 593,847 | 466,421 | |||||||||||||||
Stockholders' equity | 728,594 | 635,660 | 566,792 | 557,581 | 434,219 | |||||||||||||||
42
Quarterly Financial Data (Unaudited)
| In thousands, except per share amounts | Quarters Ended | |||||||||||||||
| Jun 30, 2013 | Mar 31, 2013 | Dec 31, 2012 | Sep 30, 2012 | |||||||||||||
Consolidated Statement of Comprehensive Income Data: | ||||||||||||||||
Molecular diagnostic testing | $ | 166,089 | $ | 148,384 | $ | 140,651 | $ | 127,268 | ||||||||
Companion diagnostic services | 8,027 | 8,088 | 8,489 | 6,169 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total Revenue | 174,116 | 156,472 | 149,140 | 133,437 | ||||||||||||
Costs and expenses: | ||||||||||||||||
Cost of molecular diagnostic testing | 18,416 | 16,462 | 15,566 | 13,932 | ||||||||||||
Cost of companion diagnostic services | 3,657 | 3,872 | 4,318 | 3,395 | ||||||||||||
Research and development expense | 14,581 | 13,618 | 14,107 | 11,400 | ||||||||||||
Selling, general and administrative expense | 71,546 | 64,602 | 59,563 | 56,128 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total costs and expenses | 108,200 | 98,554 | 93,554 | 84,855 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating income | 65,916 | 57,918 | 55,586 | 48,582 | ||||||||||||
Other income (expense): | ||||||||||||||||
Interest income | 1,310 | 1,434 | 1,385 | 1,368 | ||||||||||||
Other | 2 | (111 | ) | 14 | (128 | ) | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Total other income | 1,312 | 1,323 | 1,399 | 1,240 | ||||||||||||
Income before income taxes | 67,228 | 59,241 | 56,985 | 49,822 | ||||||||||||
Income tax provision | 23,153 | 21,349 | 21,949 | 19,686 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net income | $ | 44,075 | $ | 37,892 | $ | 35,036 | $ | 30,136 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Earnings per share: | ||||||||||||||||
Basic | $ | 0.55 | $ | 0.47 | $ | 0.43 | $ | 0.37 | ||||||||
Diluted | $ | 0.53 | $ | 0.46 | $ | 0.42 | $ | 0.36 | ||||||||
Weighted average shares outstanding: | ||||||||||||||||
Basic | 80,166 | 80,375 | 81,692 | 81,572 | ||||||||||||
Diluted | 82,639 | 82,434 | 84,240 | 83,914 | ||||||||||||
43
| In thousands, except per share amounts | Quarters Ended | |||||||||||||||
| Jun 30, 2012 | Mar 31, 2012 | Dec 31, 2011 | Sep 30, 2011 | |||||||||||||
Consolidated Statement of Comprehensive Income Data: | ||||||||||||||||
Molecular diagnostic testing revenue | $ | 127,499 | $ | 123,312 | $ | 117,610 | $ | 103,969 | ||||||||
Companion diagnostic services | 5,466 | 6,465 | 5,201 | 6,483 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total Revenue | 132,965 | 129,777 | 122,811 | 110,452 | ||||||||||||
Costs and expenses: | ||||||||||||||||
Cost of molecular diagnostic testing revenue | 13,872 | 13,465 | 12,815 | 11,300 | ||||||||||||
Cost of companion diagnostic services | 3,081 | 3,763 | 3,302 | 3,061 | ||||||||||||
Research and development expense | 12,144 | 11,753 | 10,243 | 8,505 | ||||||||||||
Selling, general and administrative expense | 56,583 | 54,700 | 50,986 | 46,114 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total costs and expenses | 85,680 | 83,681 | 77,346 | 68,980 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating income | 47,285 | 46,096 | 45,465 | 41,472 | ||||||||||||
Other income (expense): | ||||||||||||||||
Interest income | 1,395 | 1,379 | 1,382 | 473 | ||||||||||||
Other | (209 | ) | 6 | (64 | ) | (140 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||
Total other income | 1,186 | 1,385 | 1,318 | 333 | ||||||||||||
Income before income taxes | 48,471 | 47,481 | 46,783 | 41,805 | ||||||||||||
Income tax provision | 19,330 | 17,866 | 18,487 | 16,706 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net income | $ | 29,141 | $ | 29,615 | $ | 28,296 | $ | 25,099 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Earnings per share: | ||||||||||||||||
Basic | $ | 0.35 | $ | 0.35 | $ | 0.33 | $ | 0.29 | ||||||||
Diluted | $ | 0.34 | $ | 0.34 | $ | 0.33 | $ | 0.29 | ||||||||
Weighted average shares outstanding: | ||||||||||||||||
Basic | 84,285 | 84,403 | 84,498 | 85,241 | ||||||||||||
Diluted | 86,323 | 86,462 | 86,231 | 87,037 | ||||||||||||
44
| Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
We are a leading molecular diagnostic company dedicated to making a difference in patients' lives through the discovery and commercialization of transformative tests which assess a person's risk of developing disease, guide treatment decisions and assess risk of disease progression and recurrence. We believe in improving healthcare for patients by providing physicians with critical information to solve unmet medical needs. By understanding the underlying genetic basis of disease, we believe that individuals who have a greater risk of developing disease can be identified and physicians may be able to use this information to improve patient outcomes and better manage patient healthcare. In addition, by understanding the RNA expression levels of certain genes, we believe that we can improve patient healthcare by providing information on the aggressiveness of their disease. Further, we believe that the analysis of the expression of groups of proteins may provide a physician with life-saving information to guide treatment decisions for their patients with cancer and other major diseases.
Our goal is to provide physicians with this critical information that may guide the healthcare management of their patients to prevent disease, diagnose the disease at an earlier stage, determine the most appropriate therapy, or assess the aggressiveness of their disease. We employ a number of proprietary technologies, including DNA, RNA and protein analysis, that help us to understand the genetic basis of human disease and the role that genes and their related proteins may play in the onset and progression of disease. We use this information to guide the development of new molecular diagnostic tests that are designed to assess an individual's risk for developing disease later in life (predictive medicine), identify a patient's likelihood of responding to drug therapy and guide a patient's dosing to ensure optimal treatment (personalized medicine), or assess a patient's risk of disease progression and disease recurrence (prognostic medicine).
Our business strategy for future growth is focused on three key initiatives. First, we are working to grow and expand our existing products and markets. Second, we are developing our business internationally and have recently established operations in Europe. Finally, we intend to launch new transformative products across a diverse set of disease indications, complementing our current businesses in oncology, women's health and urology.
On May 31, 2011, we completed the acquisition of the privately-held molecular diagnostic company, Rules-Based Medicine, Inc. of Austin, Texas, for a cash purchase price of approximately $80.0 million. The acquired company has been consolidated into our operations as Myriad RBM. The acquisition expanded our product pipeline into new disease states, including neuroscience disorders, infectious diseases and inflammatory diseases, and added eight new molecular diagnostic test candidates to our current product pipeline.
During the fiscal year ended June 30, 2013, we devoted our resources to supporting and growing our transformative molecular diagnostic and companion diagnostic businesses, as well as to the research and development of future molecular diagnostic and companion diagnostic candidates. See Note 10 "Segment and Related Information" in the notes to our consolidated financial statements for information regarding our operating segments. Our consolidated revenues primarily consisted of sales of molecular diagnostic tests through our wholly-owned Myriad Genetic Laboratories subsidiary and companion diagnostic services through our wholly-owned Myriad RBM subsidiary. During the year ended June 30, 2013, we reported net income of $147.1 million and diluted earnings per share of $1.77 that included income tax expense of $86.1 million.
We incurred research and development expenses of $53.7 million, $42.6 million, and $27.8 million for the years ended June 30, 2013, 2012 and 2011, respectively. Our research and development expenses include costs incurred in maintaining and improving our nine current molecular diagnostic test offerings and costs incurred for the discovery, development and validation of our pipeline of molecular diagnostic and companion diagnostic test candidates.
45
Our selling, general and administrative expenses include costs associated with building our molecular diagnostic and companion diagnostic businesses domestically and internationally. Selling, general and administrative expenses consist primarily of salaries, commissions and related personnel costs for sales, marketing, customer service, billing and collection, executive, legal, finance and accounting, information technology, human resources, and allocated facilities expenses. We expect that our selling, general and administrative expenses will increase from quarter to quarter and that such increases may be substantial, depending on the number and scope of any new molecular diagnostic and companion diagnostic launches, our efforts in support of our existing molecular diagnostic tests and companion diagnostic services as well as our continued international expansion efforts.
Between May 2010 and January 2013, we repurchased $500 million of our outstanding common stock. In February 2013, our board of directors authorized us to repurchase an additional $200 million of our outstanding common stock. In connection with this latest stock repurchase authorization, we have been authorized to repurchase shares at management's discretion based on market conditions and have repurchased $46.6 million of our outstanding common stock as of June 30, 2013 under this authorization. See also "Part II, Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Repurchases of Equity Securities-Issuer Purchases of Equity Securities."
Critical Accounting Policies
Critical accounting policies are those policies which are both important to the portrayal of a company's financial condition and results and require management's most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies are as follows:
| • | revenue recognition; |
| • | allowance for doubtful accounts; |
| • | goodwill; and |
| • | income taxes |
Revenue Recognition . Revenue includes the sale of molecular diagnostic tests and of our companion diagnostic services. Revenue is recorded at the invoiced amount net of any discounts or allowances and is recognized when persuasive evidence of an agreement exists, delivery has occurred, the fee is fixed or determinable, and collection is reasonably assured. Revenue is recognized upon completion of the test or service, communication of results, and when collectability is reasonably assured.
Allowance for Doubtful Accounts. The preparation of our financial statements in accordance with U.S. GAAP requires us to make estimates and assumptions that affect the reported amount of assets at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Trade accounts receivable are comprised of amounts due from sales of our molecular diagnostic tests, which are recorded net of any discounts or contractual allowances. We analyze trade accounts receivable and consider historic experience, customer creditworthiness, facts and circumstances specific to outstanding balances, and payment terms when evaluating the adequacy of the allowance for doubtful accounts.
We periodically evaluate and adjust the allowance for doubtful accounts through a charge or credit to expense when trends or significant events indicate that a change in estimate is appropriate. Such changes in estimate could materially affect our results of operations or financial position; however, to date these changes have not been material. It is possible that we may need to adjust our estimates in future periods.
After a review of our allowance for doubtful accounts as of June 30, 2013 and 2012, we have determined that a hypothetical ten percent increase in our allowance for doubtful accounts would result in additional bad debt expense and an increase to our allowance for doubtful accounts of $750,000 and $460,000, respectively.
46
Goodwill. We test goodwill for impairment on an annual basis and in the interim by reporting unit if events and circumstances indicate that goodwill may be impaired. The events and circumstances that are considered include business climate and market conditions, legal factors, operating performance indicators and competition. Impairment of goodwill is evaluated on a qualitative basis to determine if using a two-step process is necessary. If the qualitative assessment suggests that impairment is more likely than not, a two-step impairment analysis is performed. The first step involves comparison of the fair value of a reporting unit with its carrying amount. The valuation of a reporting unit requires judgment in estimating future cash flows, discount rates and other factors. In making these judgments, we evaluate the financial health of our business, including such factors as industry performance, market saturation and opportunity, changes in technology and operating cash flows. Changes in our forecasts or decreases in the value of our common stock could cause book value of reporting units to exceed their fair values. If the carrying amount of a reporting unit exceeds its fair value, the second step of the process involves a comparison of the fair value and the carrying amount of the goodwill of that reporting unit. If the carrying amount of the goodwill of the reporting unit exceeds the fair value of that goodwill, an impairment loss would be recognized in an amount equal to the excess of carrying value over fair value. If an event occurs that would cause a revision to the estimates and assumptions used in analyzing the value of the goodwill, the revision could result in a non-cash impairment charge that could have a material impact on the financial results.
At June 30, 2013, the Company has recorded goodwill of $56.9 million related to the Companion Diagnostic segment, which we have concluded represents a reporting unit. We measured the fair value of the Companion Diagnostic reporting unit utilizing income and market approaches. The income approach considered management's business plans and projections as the basis for expected cash flows for the next nine years and a 3.0% residual growth rate thereafter. We also used a weighted average discount rate of 28% for the analysis. Other significant estimates used in the analysis include the profitability of the respective reporting unit and working capital effects. The market approach used values for comparable companies and market transactions. We noted the fair value of the Companion Diagnostic reporting unit exceeded its carrying value by slightly more than 10% using these assumptions mentioned. A hypothetical increase in the weighted average discount rate of 0.5% would decrease the calculated fair value as a percentage of book value for the Companion Diagnostic reporting unit by 2%. A hypothetical decrease in the residual growth rate of 0.5% would decrease the calculated fair value as a percent of book value for the Companion Diagnostic reporting unit by 1%.
Income taxes . Our income tax provision is based on income before taxes and is computed using the liability method in accordance with Accounting Standards Codification ("ASC") 740- Income Taxes . Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using tax rates projected to be in effect for the year in which the differences are expected to reverse. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations, or the expected results from any future tax examinations. Various internal and external factors may have favorable or unfavorable effects on our future provision for income taxes. Those factors include, but are not limited to, changes in tax laws, regulations and/or rates, the results of any future tax examinations, changing interpretations of existing tax laws or regulations, changes in estimates of prior years' items, past levels of R&D spending, acquisitions, changes in our corporate structure, and changes in overall levels of income before taxes all of which may result in periodic revisions to our provision for income taxes.
Developing our provision for income taxes, including our effective tax rate and analysis of potential uncertain tax positions, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowance we deem necessary to offset deferred tax assets. If we do not maintain taxable income from operations in future periods, we may increase the valuation allowance for our deferred tax assets and record material adjustments to our income tax expense. Our judgment and tax strategies are subject to audit by various taxing authorities. While we believe we have provided adequately for our uncertain income tax positions in our consolidated financial statements, adverse determination by these taxing authorities could have a material adverse effect on our consolidated financial condition, results of operations or cash flows. Interest and penalties on income tax items are included as a component of overall income tax expense.
47
Recent Accounting Pronouncements
In February 2013, the FASB issued an amendment to the accounting guidance for the reporting of amounts reclassified out of accumulated other comprehensive income ("AOCI"). The amendment expands the existing disclosure requirements by requiring entities to present information about significant items reclassified out of AOCI by component. In addition, an entity is required to provide information about the effects on net income of significant amounts reclassified out of each component of AOCI to net income either on the face of the statement where net income is presented or as a separate disclosure in the notes of the financial statements. The amendment is effective prospectively for annual or interim reporting periods beginning after December 15, 2012. The adoption of this accounting pronouncement did not have a material impact on our financial statements.
Results of Operations
Years ended June 30, 2013 and 2012
Revenue is comprised of sales of our molecular diagnostic tests and companion diagnostic services. Total revenue for the fiscal year ended June 30, 2013 was $613.2 million compared to $496.0 million for the prior fiscal year, an increase of 24%. This 24% increase in revenue is primarily due to increased molecular diagnostic testing volume for our BRACAnalysis, Colaris and Colaris AP, a significant increase in BART testing volume as a result of revised medical guidelines, and a significant increase in companion diagnostic services due to increased research collaborations, as disclosed in the table below. Sales of our BRAC Analysis test accounted for 75.1% of our total revenues in fiscal 2013 compared to 81.7% in the prior year. We believe that our increased sales, marketing, and education efforts resulted in wider acceptance of our molecular diagnostic tests by the medical community and increased patient testing volumes. However, there can be no assurance that our revenue will continue to increase or remain at current levels or that we will be successful in expanding the sale of our tests outside the United States.
Total revenue of our molecular diagnostic tests and companion diagnostic services and revenue by product as a percent of total revenue for the year ended June 30, 2013 and 2012 were as follows:
| June 30, | % of Total Revenue | |||||||||||||||||||
| (In thousands) | 2013 | 2012 | % Change | 2013 | 2012 | |||||||||||||||
Molecular diagnostic revenues: | ||||||||||||||||||||
BRACAnalysis | $ | 460,272 | $ | 405,478 | 14 | % | 75 | % | 82 | % | ||||||||||
COLARIS & COLARIS AP | 51,938 | 43,277 | 20 | % | 8 | % | 8 | % | ||||||||||||
BART | 59,140 | 13,587 | 335 | % | 10 | % | 3 | % | ||||||||||||
Other | 11,042 | 10,048 | 10 | % | 2 | % | 2 | % | ||||||||||||
|
|
|
|
|
| |||||||||||||||
Total molecular diagnostic revenues | 582,392 | 472,390 | 23 | % | ||||||||||||||||
|
|
|
|
|
| |||||||||||||||
Companion diagnostic service revenues | 30,773 | 23,615 | 30 | % | 5 | % | 5 | % | ||||||||||||
|
|
|
|
|
| |||||||||||||||
Total revenues | $ | 613,165 | $ | 496,005 | 24 | % | 100 | % | 100 | % | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Our molecular diagnostic sales force is focused on two major markets, oncology and women's health. Sales of molecular diagnostic tests in each market for the fiscal years ended June 30, 2013 and 2012 were as follows:
| June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | % Change | |||||||||
Molecular diagnostic revenues: | ||||||||||||
Oncology | $ | 370,257 | $ | 320,106 | 16 | % | ||||||
Women's Health | 212,135 | 152,284 | 39 | % | ||||||||
|
|
|
|
|
| |||||||
Total molecular diagnostic revenues | $ | 582,392 | $ | 472,390 | 23 | % | ||||||
|
|
|
|
|
| |||||||
48
Certain prior period reclassifications to oncology and women's health revenue have been made to conform to current period presentation.
Cost of revenue is comprised primarily of salaries and related personnel costs, laboratory supplies, royalty payments, equipment costs and facilities expense. Cost of molecular diagnostic testing revenue for the fiscal year ended June 30, 2013 was $64.4 million compared to $51.5 million for the prior fiscal year. This increase of 25% in molecular diagnostic cost of revenue is primarily due to the increase in molecular diagnostic testing volumes. Cost of companion diagnostic service revenue for the fiscal year ended June 30, 2013 was $15.2 million compared to $13.2 million for the prior fiscal year. Many of these costs associated with the performance of our companion diagnostic services are fixed; consequently, gross margins will vary as we experience fluctuations in our companion diagnostic service revenue.
Our cost of revenue may fluctuate based on the introduction of new molecular diagnostic tests, testing volumes, changes in companion diagnostic services, price changes of existing tests and services, changes in our costs associated with such tests and services, the adoption of new technologies and operating systems in our molecular diagnostic laboratories and costs associated with operating a molecular diagnostic laboratory outside the United States. There can be no assurance that gross profit margins will remain at current levels.
Our research and development expenses include costs incurred in maintaining and improving our current molecular diagnostic tests and costs incurred for the discovery, validation and development of our pipeline of molecular and companion diagnostic test candidates. Research and development expenses are comprised primarily of salaries and related personnel costs, laboratory supplies, molecular and companion diagnostic development, equipment and facility costs. Research and development expenses incurred during the fiscal year ended June 30, 2013 were $53.7 million compared to $42.6 million for the prior fiscal year. This increase of 26% was primarily due to the following:
| • | an increase of approximately $5.0 million due to the internal development of future molecular diagnostic product candidates; |
| • | an increase of approximately $3.0 million in internal development to support our companion diagnostic services business; |
| • | an increase of approximately $1.9 million in internal development activities and clinical studies to support our existing molecular diagnostic testing products; and |
| • | an increase of approximately $0.8 million in costs associated with the in-license of new molecular diagnostic product candidates. |
We expect our research and development expenses will increase over the next several years as we work to develop our product pipeline and introduce new molecular diagnostic and companion diagnostic tests.
Our selling, general and administrative expenses include costs associated with growing our molecular diagnostic and companion diagnostic businesses domestically and internationally. Selling, general and administrative expenses consist primarily of salaries, commissions and related personnel costs for sales, marketing, customer service, billing and collection, executive, legal, finance and accounting, information technology, human resources, and allocated facilities expenses. Selling, general and administrative expenses for the fiscal year ended June 30, 2013 were $251.8 million compared to $208.4 million for the prior fiscal year. The increase in selling, general and administrative expense of 21% was primarily to support the 24% increase in revenue and include:
| • | an increase in sales and marketing expense of approximately $29.3 million due to various marketing programs and initiatives, added headcount and increased sales commissions; |
| • | an increase of approximately $8.5 million in bad debt expense, a portion of which was associated with the 24% increase in revenues; and |
| • | an increase of approximately $5.2 million in costs from our international operations. |
49
We expect that our selling, general and administrative expenses will continue to increase and that such increases may be substantial, depending on the number and scope of any new molecular diagnostic and companion diagnostic product launches, our efforts in support of our existing molecular diagnostic tests and companion diagnostic services as well as our continued international expansion efforts.
Interest income for the fiscal year ended June 30, 2013 was $5.5 million, compared to $4.6 million for the prior fiscal year. Interest income consists primarily of interest income recorded from our note receivable from Crescendo Bioscience, Inc., or Crescendo.
Income tax expense for the fiscal year ended June 30, 2013 was $86.1 million, for an effective rate of approximately 37%, compared to income tax expense of $72.4 million and an effective rate of approximately 39% in the 2012 period. Our tax rate is a product of a U.S. federal effective rate of 35% and a blended state income tax rate of 2%. Certain significant or unusual items are separately recognized during the period in which they occur and can be a source of variability in the effective tax rates from period to period. For the year ended June 30, 2013 we realized $7.9 million of excess tax benefits from stock-based compensation as a reduction of taxes payable. Excess tax benefits from stock-based compensation are credited directly to additional paid-in-capital and are not included in income tax expense. Accordingly, they do not impact our effective income tax rate. Due to the realization of these excess tax benefits that offset our taxes payable, our current income tax expense in fiscal 2013 is higher than our actual cash paid. (See Note 8 in the fiscal 2013 Notes to Consolidated Financial Statements.)
Net income for the fiscal year ended June 30, 2013 was $147.1 million compared to $112.2 million in the prior fiscal year. This 31% increase was primarily due to an increase in revenues partially offset by higher research and development expenses and sales general and administrative expenses. Earnings per diluted share was $1.77 for the fiscal year ended June 30, 2013 as compared to $1.30 for the prior fiscal year, an increase of 36%. This increase was due to increased net income and a reduced number of weighted shares outstanding during the 2013 fiscal year from our share repurchase program.
Years ended June 30, 2012 and 2011
Revenue was comprised of sales of our molecular diagnostic tests and companion diagnostic services. Total revenue for the fiscal year ended June 30, 2012 was $496.0 million compared to $402.1 million for the prior fiscal year, an increase of 23%. Of this 23% increase in revenue, approximately 18% was attributable to increased molecular diagnostic testing volume and approximately 5% was due to companion diagnostic service revenue in connection with our acquisition of Myriad RBM on May 31, 2011. Sales of our BRAC Analysis test accounted for 81.7% of our total revenues in fiscal 2012 compared to 87.8% in the prior year. We believe that increased sales, marketing, and education efforts resulted in wider acceptance of our tests by the medical community and increased patient testing volumes.
Total revenue of our molecular diagnostic tests and companion diagnostic services and revenue by product as a percent of total revenue for the year ended June 30, 2012 and 2011 were as follows:
| June 30, | % of Total Revenue | |||||||||||||||||||
| (In thousands) | 2012 | 2011 | % Change | 2012 | 2011 | |||||||||||||||
Molecular diagnostic revenues: | ||||||||||||||||||||
BRACAnalysis | $ | 405,478 | $ | 352,964 | 15 | % | 82 | % | 88 | % | ||||||||||
COLARIS & COLARIS AP | 43,277 | 29,165 | 48 | % | 8 | % | 7 | % | ||||||||||||
Other | 23,635 | 17,917 | 32 | % | 5 | % | 4 | % | ||||||||||||
|
|
|
|
|
| |||||||||||||||
Total molecular diagnostic revenues | 472,390 | 400,046 | 18 | % | ||||||||||||||||
|
|
|
|
|
| |||||||||||||||
Companion diagnostic service revenues | 23,615 | 2,038 | 1059 | % | 5 | % | 1 | % | ||||||||||||
|
|
|
|
|
| |||||||||||||||
Total revenues | $ | 496,005 | $ | 402,084 | 23 | % | 100 | % | 100 | % | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
50
Our molecular diagnostic sales force was focused on two major markets, oncology and women's health. Sales of molecular diagnostic tests in each market for the fiscal years ended June 30, 2012 and 2011 were as follows:
| June 30, | ||||||||||||
| (In thousands) | 2012 | 2011 | % Change | |||||||||
Molecular diagnostic revenues: | ||||||||||||
Oncology | $ | 327,605 | $ | 283,323 | 16 | % | ||||||
Women's Health | 144,785 | 116,723 | 24 | % | ||||||||
|
|
|
|
|
| |||||||
Total molecular diagnostic revenues | $ | 472,390 | $ | 400,046 | 18 | % | ||||||
|
|
|
|
|
| |||||||
Cost of molecular diagnostic revenue was comprised primarily of salaries and related personnel costs, laboratory supplies, royalty payments, equipment costs and facilities expense. Cost of molecular diagnostic testing revenue for the fiscal year ended June 30, 2012 was $51.5 million compared to $45.6 million for the prior fiscal year. This increase of 13% in molecular diagnostic cost of revenue was primarily due to the increase in molecular diagnostic testing volumes. Our costs of companion diagnostic services include similar items. Cost of companion diagnostic services was $13.2 million for the fiscal year ended June 30, 2012. Many of these costs associated with the performance of our companion diagnostic services are fixed; consequently, gross margins vary as we experience fluctuations in our companion diagnostic service revenue.
Research and development expenses are comprised primarily of salaries and related personnel costs, laboratory supplies, molecular and companion diagnostic development, equipment and facility costs. Research and development expenses incurred during the fiscal year ended June 30, 2012 were $42.6 million compared to $27.8 million for the prior fiscal year. This increase of 54% was primarily due to the following:
| • | an additional $7.3 million in protein biomarker discovery costs for companion diagnostic development at our newly acquired Myriad RBM subsidiary; |
| • | an increase of approximately $5.3 million in internal development activities and clinical studies to support our existing molecular diagnostic testing products; |
| • | an increase of approximately $1.4 million in clinical studies to support future molecular diagnostic testing products; and |
| • | an increase of approximately $0.8 million in costs associated with the in-licensing of new molecular diagnostic product candidates. |
Selling, general and administrative expenses consist primarily of salaries, commissions and related personnel costs for sales, marketing, customer service, billing and collection, executive, legal, finance and accounting, information technology, human resources, and allocated facilities expenses. Selling, general and administrative expenses for the fiscal year ended June 30, 2012 were $208.4 million compared to $169.8 million for the prior fiscal year. The increase in selling, general and administrative expense of 23% was due primarily to support the 23% increase in revenue and include:
| • | an increase in sales and marketing expense of approximately $15.2 million due to various marketing programs and initiatives, added headcount and increased sales commissions; |
| • | the addition of $9.2 million of administrative costs from Myriad RBM; |
| • | an increase of approximately $7.9 million in bad debt expense, some of which was associated with the 23% increase in revenues; |
| • | an increase of approximately $4.4 million in international administrative costs from our European operations; and |
| • | an increase in share-based compensation expense of approximately $1.9 million. |
51
Interest income for the fiscal year ended June 30, 2012 was $4.6 million, compared to $2.2 million for the prior fiscal year. The increase was due primarily to interest income recorded from a $25 million note receivable with Crescendo Bioscience, Inc., or Crescendo.
Income tax expense for the fiscal year ended June 30, 2012 was $72.4 million, for an effective rate of approximately 39%, compared to income tax expense of $58.9 million and an effective rate of approximately 37% in the 2011 period. Income tax expense for the year ended June 30, 2012 was based on the application of an annual effective tax rate, adjusted by discrete items recognized during the period. Our annual effective tax rate is a product of the U.S. federal statutory rate of 35%, a blended state income tax rate of 3% and a 1% impact from our international operations. The effective rate was adjusted by alternative minimum income taxes and timing differences related to the recognition of the tax effect of equity compensation expense from incentive stock options and the deduction realized if those options were disqualified upon exercise. Certain significant or unusual items are separately recognized during the period in which they occur and can be a source of variability in the effective tax rates from period to period. For the year ended June 30, 2012 we realized $34.2 million of excess tax benefits from stock-based compensation as a reduction of taxes payable. Excess tax benefits from stock-based compensation were credited directly to additional paid-in-capital and were not included in income tax expense. Accordingly, they did not impact our effective income tax rate. Due to the realization of these excess tax benefits that offset our taxes payable, our current income tax expense in fiscal 2012 was significantly higher than our actual cash paid. (See Note 8 in the fiscal 2012 Notes to Consolidated Financial Statements.)
Net income for the fiscal year ended June 30, 2012 was $112.2 million compared to $100.7 million in the prior fiscal year. This 11% increase was primarily due to an increase in revenues partially offset by higher research and development expenses. Earnings per diluted share was $1.30 for the fiscal year ended June 30, 2012 as compared to $1.10 for the prior fiscal year, an increase of 18%. This increase was due to a larger net income and a reduced number of weighted shares outstanding during the 2012 fiscal year due to the our share repurchase program.
Liquidity and Capital Resources
Cash, cash equivalents, and marketable investment securities increased $76.9 million, or 17%, from $454.2 million at June 30, 2012 to $531.1 million at June 30, 2013. This increase in our balance was attributable to increased sales and $57.8 million in proceeds from issuance of common stock under share-based compensation plans; partially offset by the repurchase of $146.3 million of our common stock under our share repurchase programs, payments of $80.3 million in estimated income tax obligations, and operating expenditures during fiscal 2013.
Net cash provided by operating activities was $173.9 million, $141.8 million and $130.8 million during the fiscal years ended June 30, 2013, 2012 and 2011, respectively. During the year ended June 30, 2013, our net income was reduced by non-cash charges in the form of share-based compensation and depreciation and amortization, which totaled $26.6 million and $8.9 million, respectively. Net cash provided by operating activities for year ended June 30, 2013 was also impacted by changes in bad debt expense, trade accounts receivable, accounts payable, inventory, and accrued liabilities.
Our investing activities used cash of $75.6 million, $38.9 million and $54.4 million during the fiscal years ended June 30, 2013, 2012 and 2011, respectively. Cash used in investing activities for the fiscal year ended June 30, 2013 was primarily comprised of $443.8 million for the purchase of marketable investment securities offset by $384.6 million in proceeds from maturities and sales of marketable investment securities. Capital expenditures for equipment and facilities for the year ended June 30, 2013 were $11.4 million. Cash used in investing activities from the prior fiscal years ended June 30, 2012 and 2011 was primarily due to the use of $80.0 million for the acquisition of Myriad RBM in May of 2011, the purchase of marketable investment securities offset by proceeds from maturities and sales of marketable investment securities as well as capital expenditures for research and laboratory equipment.
52
Financing activities used cash of $80.6 million, $69.2 million, and $116.6 million during the fiscal years ended June 30, 2013, 2012 and 2011. Cash utilized from financing activities in 2013 and 2012 was primarily due to the purchase of $146.3 million and $128.5 million of our common stock through our share repurchase program. The cash used in the share purchase was partially offset by cash provided by the exercise of stock options and sales of our shares under our Employee Stock Purchase Plan.
We believe that with our existing capital resources and expected net cash to be generated from sales of our molecular diagnostic tests and companion diagnostic services, we will have adequate funds to maintain our current and planned operations for the foreseeable future, although no assurance can be given that changes will not occur that would consume available capital resources more quickly than we currently expect and that we may need or want to raise financing. Our future capital requirements, cash flows, and results of operations could be affected by and will depend on many factors that are currently unknown to us, including:
| • | failure to sustain revenue growth or margins in our molecular diagnostic testing and companion diagnostic services businesses; |
| • | termination of the licenses underlying our molecular diagnostic tests and companion diagnostic services or failure to enter into product or technology licensing or other arrangements favorable to us; |
| • | delays or other problems with operating our laboratory facilities; |
| • | the costs and expenses incurred in supporting our existing molecular diagnostic tests and companion diagnostic services; |
| • | the progress, results and cost of developing and launching additional molecular diagnostic tests and offering additional companion diagnostic services; |
| • | potential business development activities, in-licensing agreements and acquisitions, such as our acquisition of Myriad RBM and our strategic debt investment and option to acquire Crescendo Biosciences, Inc., and our ability to successfully integrate and achieve the expected benefits of our business development activities, in-licensing agreements and acquisitions; |
| • | changes in the government regulatory approval process for our tests; |
| • | the progress, costs and results of our international expansion efforts; |
| • | the timing and amount of repurchases of our common stock; |
| • | the costs, timing, outcome, and enforcement of any regulatory review of our existing or future molecular diagnostic tests and companion diagnostic services; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our issued patents and pursuing or defending intellectual property-related claims; |
| • | the costs, timing and outcome of any litigation against us or that we pursue; |
| • | the introduction of technological innovations or new commercial tests by our competitors; |
| • | changes in intellectual property laws covering our molecular diagnostic tests and companion diagnostic services and patents or enforcement in the United States and foreign countries, such as the Supreme Court decision in the lawsuit brought against us by the Association for Molecular Pathology et al; |
| • | changes in the governmental or private insurers reimbursement levels for our tests; and |
| • | changes in structure of the healthcare system or healthcare payment systems. |
Off-Balance Sheet Arrangements
None.
53
Contractual Obligations
The following table represents our consolidated contractual obligations as of June 30, 2013 (in thousands):
| Total | Less than one year | 1-3 Years | 4-5 Years | More than 5 years | ||||||||||||||||
Operating leases | $ | 73,180 | $ | 9,446 | $ | 18,636 | $ | 14,643 | $ | 30,455 | ||||||||||
Purchase obligations | 2,596 | 2,596 | - | - | - | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total | $ | 75,776 | $ | 12,042 | $ | 18,636 | $ | 14,643 | $ | 30,455 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
The expected timing of payment for the obligations listed above is estimated based on current information. Actual payment timing and amounts may differ depending on the timing of goods or services received or other changes. The table above only includes payment obligations that are fixed or determinable. The table excludes royalties to third parties based on future sales of any of our product candidates that are approved for sale, as the amounts, timing, and likelihood of any such payments are based on the level of future sales of tests and are unknown.
Effects of Inflation
We do not believe that inflation has had a material impact on our business, revenues, or operating results during the periods presented.
Certain Factors That May Affect Future Results of Operations
The Securities and Exchange Commission encourages companies to disclose forward-looking information so that investors can better understand a company's future prospects and make informed investment decisions. This Annual Report on Form 10-K contains such "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995.
Words such as "may," "anticipate," "estimate," "expects," "projects," "intends," "plans," "believes" and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements. All forward-looking statements are management's present expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to: the risk that sales and profit margins of our existing molecular diagnostic tests and companion diagnostic services may decline or will not continue to increase at historical rates; risks related to changes in the governmental or private insurers reimbursement levels for our tests; the risk that we may be unable to develop or achieve commercial success for additional molecular diagnostic tests and companion diagnostic services in a timely manner, or at all; the risk that we may not successfully develop new markets for our molecular diagnostic tests and companion diagnostic services, including our ability to successfully generate revenue outside the United States; the risk that licenses to the technology underlying our molecular diagnostic tests and companion diagnostic services tests and any future tests are terminated or cannot be maintained on satisfactory terms; risks related to delays or other problems with operating our laboratory testing facilities; risks related to public concern over our genetic testing in general or our tests in particular; risks related to regulatory requirements or enforcement in the United States and foreign countries and changes in the structure of the healthcare system or healthcare payment systems; risks related to our ability to obtain new corporate collaborations or licenses and acquire new technologies or businesses on satisfactory terms, if at all; risks related to our ability to successfully integrate and derive benefits from any technologies or businesses that we license or acquire; risks related to increased competition and the development of new competing tests and services; the risk that we or our licensors may be unable to protect or that third parties will infringe the proprietary technologies underlying our tests; the risk of patent-infringement claims or challenges to the validity of our patents; risks related to changes in intellectual property laws covering our molecular diagnostic tests and companion diagnostic
54
services and patents or enforcement in the United States and foreign countries, such as the Supreme Court decision in the lawsuit brought against us by the Association for Molecular Pathology et al; risks of new, changing and competitive technologies and regulations in the United States and internationally; and other factors discussed under the heading "Risk Factors" contained in Item 1A of this Annual Report.
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Annual Report or in any document incorporated by reference might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this Annual Report. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We maintain an investment portfolio in accordance with our written investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any single issue, issuer or type of investment.
Our investments consist of securities of various types and maturities of five years or less, with a maximum average maturity of three years. These securities are classified as available for sale. Available-for-sale securities are recorded on the balance sheet at fair market value with unrealized gains or losses reported as part of accumulated other comprehensive income/loss. Realized gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned. A decline in the market value of any available-for-sale security below cost that is deemed other-than-temporary results in a charge to earnings and establishes a new cost basis for the security.
Although our investment policy guidelines are intended to ensure the preservation of principal, current market conditions have resulted in high levels of uncertainty. Our ability to trade or redeem the marketable investment securities in which we invest, including certain corporate bonds, has become difficult. Valuation and pricing of these securities has also become variable and subject to uncertainty.
As of June 30, 2013 we have net unrealized losses of $433,000 in our investment portfolio. For the year ended June 30, 2013 we have experienced fluctuations in our portfolio value primarily from our investments in bonds of various municipalities. If interest rates rise, the market value of our investments may decline, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. A hypothetical increase in interest rates by 25 basis points would have resulted in a decrease in the fair value of our net investment position of approximately $1.3 million and $1.1 million as of June 30, 2013 and 2012, respectively. We do not utilize derivative financial instruments to manage our interest rate risks.
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
MYRIAD GENETICS, INC.
Index to Financial Statements | Number | |||
Report of Independent Registered Public Accounting Firm | F-1 | |||
Consolidated Balance Sheets as of June 30, 2013 and 2012 | F-2 | |||
Consolidated Statements of Comprehensive Income for the Years Ended June 30, 2013, 2012 and 2011 | F-3 | |||
Consolidated Statements of Stockholders' Equity for the Years Ended June 30, 2013, 2012 and 2011 | F-4 | |||
Consolidated Statements of Cash Flows for the Years Ended June 30, 2013, 2012 and 2011 | F-5 | |||
Notes to Consolidated Financial Statements | F-6 | |||
55
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| Item 9A. | CONTROLS AND PROCEDURES |
1. Disclosure Controls and Procedures
We maintain disclosure controls and procedures (Disclosure Controls) within the meaning of Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Our Disclosure Controls are designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act, such as this Annual Report on Form 10-K, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms. Our Disclosure Controls are also designed to ensure that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our Disclosure Controls, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily applied its judgment in evaluating and implementing possible controls and procedures.
As of the end of the period covered by this Annual Report on Form 10-K, we evaluated the effectiveness of the design and operation of the Company's Disclosure Controls, which was done under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer. Based on the evaluation of our Disclosure Controls, our Chief Executive Officer and Chief Financial Officer have concluded that, as of June 30, 2013, our Disclosure Controls were effective to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms and is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
2. Internal Control Over Financial Reporting
| a. | Management's Report on Internal Control over Financial Reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, a company's principal executive and principal financial officers and effected by the company's board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that:
| • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| • | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
56
Our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2013. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework . Based on our assessment, management believes that, as of June 30, 2013, our internal control over financial reporting is effective based on those criteria.
The effectiveness of Myriad Genetics, Inc.'s internal control over financial reporting as of June 30, 2013, has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report as follows:
| b. | Report of the Independent Registered Public Accounting Firm |
The Board of Directors and Stockholders
Myriad Genetics, Inc.
We have audited Myriad Genetics, Inc. and subsidiaries' internal control over financial reporting as of June 30, 2013, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 Framework) (the COSO criteria). Myriad Genetics Inc. and subsidiaries management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Myriad Genetics, Inc. maintained, in all material respects, effective internal control over financial reporting as of June 30, 2013, based on the COSO criteria .
57
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Myriad Genetics, Inc. and subsidiaries as of June 30, 2013 and 2012, and the related consolidated statements of comprehensive income, stockholders' equity, and cash flows for each of the three years in the period ended June 30, 2013 of Myriad Genetics, Inc. and subsidiaries, and our report dated August 14, 2013 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP |
Salt Lake City, Utah |
August 14, 2013 |
| c. | Change in Internal Control over Financial Reporting |
There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during the fourth quarter of our last fiscal year that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | OTHER INFORMATION |
None.
58
PART III
| Item 10. | DIRECTORS AND EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Management and Corporate Governance," "Section 16(a) Beneficial Ownership Reporting Compliance" and "Corporate Code of Conduct and Ethics" in our Proxy Statement for the 2013 Annual Meeting of Stockholders to be held on December 5, 2013.
| Item 11. | EXECUTIVE COMPENSATION |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Executive Compensation-Compensation Discussion and Analysis," "Executive Compensation," "Management and Corporate Governance-Committees of the Board of Directors and Meetings-Compensation Committee Interlocks and Insider Participation," "Executive Compensation-Director Compensation" "Compensation Committee Report" and "Management and Corporate Governance-Board's Role in the Oversight of Risk Management" in our Proxy Statement for the 2013 Annual Meeting of Stockholders to be held on December 5, 2013.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Security Ownership of Certain Beneficial Owners and Management" and "Executive Compensation-Equity Compensation Plan Information" in our Proxy Statement for the 2013 Annual Meeting of Stockholders to be held on December 5, 2013.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the caption "Certain Relationships and Related Person Transactions" and "Management and Corporate Governance-Director Independence" in our Proxy Statement for the 2013 Annual Meeting of Stockholders to be held on December 5, 2013.
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The response to this item is incorporated by reference from the discussion responsive thereto in the proposal entitled "Independent Public Accountants" in our Proxy Statement for the 2013 Annual Meeting of the Stockholders to be held on December 5, 2013.
59
PART IV
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are included as part of this Annual Report on Form 10-K.
| 1. | Financial Statements |
See "Index to Consolidated Financial Statements" at Item 8 to this Annual Report on Form 10-K.
| 2. | Financial Statement Schedule |
The following schedule is filed as part of this Form 10-K:
Schedule II-Schedule of Valuation and Qualifying Accounts for the Years Ended June 30, 2013, 2012 and 2011.
Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
| 3. | Exhibits |
The exhibits which are filed with or incorporated by reference into this Annual Report on Form 10-K are set forth in the Exhibit Index beginning on page A-1, which is incorporated herein by reference."
60
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on August 14, 2013.
| MYRIAD GENETICS, INC. | ||
| By: | /s/ P ETER D. M ELDRUM | |
| Peter D. Meldrum | ||
| President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
Signatures | Title | Date | ||||
| By: | /s/ P ETER D. M ELDRUM Peter D. Meldrum | President, Chief Executive Officer and Director (principal executive officer) | August 14, 2013 | |||
| By: | /s/ J AMES S. E VANS James S. Evans | Chief Financial Officer (principal financial and accounting officer) | August 14, 2013 | |||
| By: | /s/ J OHN T. H ENDERSON John T. Henderson, M.D. | Chairman of the Board | August 14, 2013 | |||
| By: | /s/ W ALTER G ILBERT Walter Gilbert, Ph.D. | Vice Chairman of the Board | August 14, 2013 | |||
| By: | /s/ L AWRENCE C. B EST Lawrence C. Best | Director | August 14, 2013 | |||
| By: | /s/ H EINER D REISMANN Heiner Dreismann, Ph.D. | Director | August 14, 2013 | |||
| By: | /s/ D ENNIS L ANGER Dennis Langer, M.D., J.D. | Director | August 14, 2013 | |||
| By: | /s/ S. L OUISE P HANSTIEL S. Louise Phanstiel | Director | August 14, 2013 | |||
61
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Myriad Genetics, Inc.
We have audited the accompanying consolidated balance sheets of Myriad Genetics, Inc. and subsidiaries as of June 30, 2013 and 2012, and the related consolidated statements of comprehensive income, stockholders' equity, and cash flows for each of the three years in the period ended June 30, 2013. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Myriad Genetics, Inc. and subsidiaries at June 30, 2013 and 2012, and the consolidated results of their operations and their cash flows for each of the three years in the period ended June 30, 2013, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Myriad Genetics, Inc. and subsidiaries' internal control over financial reporting as of June 30, 2013, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated August 14, 2013 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
| Salt Lake City, Utah |
| August 14, 2013 |
F-1
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Balance Sheets
June 30, 2013 and 2012
(In thousands, except per share amounts)
| 2013 | 2012 | |||||||
| Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 104,073 | $ | 86,352 | ||||
Marketable investment securities | 268,243 | 254,180 | ||||||
Prepaid expenses | 956 | 1,713 | ||||||
Inventory | 5,007 | 11,574 | ||||||
Trade accounts receivable, less allowance for doubtful accounts of $7,500 in 2013 and $4,600 in 2012 | 94,333 | 60,441 | ||||||
Deferred taxes | 8,007 | 5,572 | ||||||
Other receivables | 3,373 | 2,660 | ||||||
|
|
|
| |||||
Total current assets | 483,992 | 422,492 | ||||||
|
|
|
| |||||
Equipment and leasehold improvements: | ||||||||
Equipment | 65,903 | 54,728 | ||||||
Leasehold improvements | 18,294 | 17,800 | ||||||
|
|
|
| |||||
| 84,197 | 72,528 | |||||||
Less accumulated depreciation | 56,595 | 48,297 | ||||||
|
|
|
| |||||
Net equipment and leasehold improvements | 27,602 | 24,231 | ||||||
|
|
|
| |||||
Long-term marketable investment securities | 158,748 | 113,692 | ||||||
Long-term deferred taxes | 28,632 | 30,648 | ||||||
Note receivable | 21,667 | 19,000 | ||||||
Other assets | 13,000 | 8,000 | ||||||
Intangibles, net | 13,330 | 15,722 | ||||||
Goodwill | 56,850 | 56,850 | ||||||
|
|
|
| |||||
Total assets | $ | 803,821 | $ | 690,635 | ||||
|
|
|
| |||||
| Liabilities and Stockholders' Equity | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 18,132 | $ | 10,141 | ||||
Accrued liabilities | 44,334 | 32,772 | ||||||
Deferred revenue | 2,043 | 2,054 | ||||||
|
|
|
| |||||
Total current liabilities | 64,509 | 44,967 | ||||||
|
|
|
| |||||
Unrecognized tax benefits | 10,718 | 10,008 | ||||||
|
|
|
| |||||
Total liabilities | 75,227 | 54,975 | ||||||
|
|
|
| |||||
Commitments and contingencies | ||||||||
Stockholders' equity: | ||||||||
Preferred stock, $0.01 par value, authorized 5,000 shares; no shares issued and outstanding | - | - | ||||||
Common stock, $0.01 par value, authorized 150,000 shares; issued and outstanding 80,577 shares in 2013 and 82,569 shares in 2012 | 806 | 826 | ||||||
Additional paid-in capital | 697,346 | 647,680 | ||||||
Accumulated other comprehensive loss | (424 | ) | (162 | ) | ||||
Retained earnings (accumulated deficit) | 30,866 | (12,684 | ) | |||||
|
|
|
| |||||
Total stockholders' equity | 728,594 | 635,660 | ||||||
|
|
|
| |||||
Total liabilities and stockholders' equity | $ | 803,821 | $ | 690,635 | ||||
|
|
|
| |||||
See accompanying notes to consolidated financial statements.
F-2
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Comprehensive Income
Years ended June 30, 2013, 2012 and 2011
(In thousands, except per share amounts)
| 2013 | 2012 | 2011 | ||||||||||
Molecular diagnostic testing | $ | 582,392 | $ | 472,390 | $ | 400,046 | ||||||
Companion diagnostic services | 30,773 | 23,615 | 2,038 | |||||||||
|
|
|
|
|
| |||||||
Total revenue | 613,165 | 496,005 | 402,084 | |||||||||
Costs and expenses: | ||||||||||||
Cost of molecular diagnostic testing | 64,376 | 51,452 | 45,637 | |||||||||
Cost of companion diagnostic services | 15,242 | 13,207 | 1,077 | |||||||||
Research and development expense | 53,706 | 42,645 | 27,751 | |||||||||
Selling, general, and administrative expense | 251,839 | 208,383 | 169,841 | |||||||||
|
|
|
|
|
| |||||||
Total costs and expenses | 385,163 | 315,687 | 244,306 | |||||||||
|
|
|
|
|
| |||||||
Operating income | 228,002 | 180,318 | 157,778 | |||||||||
Other income (expense): | ||||||||||||
Interest income | 5,497 | 4,629 | 2,226 | |||||||||
Other | (223 | ) | (407 | ) | (353 | ) | ||||||
|
|
|
|
|
| |||||||
Total other income (expense): | 5,274 | 4,222 | 1,873 | |||||||||
Income before income taxes | 233,276 | 184,540 | 159,651 | |||||||||
Income tax provision | 86,137 | 72,389 | 58,941 | |||||||||
|
|
|
|
|
| |||||||
Net income | $ | 147,139 | $ | 112,151 | $ | 100,710 | ||||||
|
|
|
|
|
| |||||||
Earnings per share: | ||||||||||||
Basic | $ | 1.82 | $ | 1.33 | $ | 1.12 | ||||||
Diluted | 1.77 | 1.30 | 1.10 | |||||||||
Weighted average shares outstanding: | ||||||||||||
Basic | 80,948 | 84,608 | 89,794 | |||||||||
Diluted | 83,327 | 86,465 | 91,704 | |||||||||
Net income | $ | 147,139 | $ | 112,151 | $ | 100,710 | ||||||
Comprehensive income: | ||||||||||||
Unrealized gain (loss) on available-for-sale securities, net of tax | (257 | ) | (135 | ) | 6 | |||||||
Change in foreign currency translation adjustment, net of tax | (5 | ) | (178 | ) | 6 | |||||||
|
|
|
|
|
| |||||||
Comprehensive income | $ | 146,877 | $ | 111,838 | $ | 100,722 | ||||||
|
|
|
|
|
| |||||||
See accompanying notes to consolidated financial statements.
F-3
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Stockholders' Equity
Years ended June 30, 2013, 2012 and 2011
(In thousands)
| Common stock | Additional paid-in capital | Accumulated other comprehensive income (loss) | Retained Earnings (accumulated deficit) | Stockholders' equity | ||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
Balances at June 30, 2010 | 94,046 | $ | 940 | $ | 566,967 | $ | 139 | $ | (10,465 | ) | $ | 557,581 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Issuance of common stock for cash upon exercise of options and employee stock purchase plan | 2,022 | 20 | 25,040 | - | - | 25,060 | ||||||||||||||||||
Share-based payment expense | - | - | 25,088 | - | - | 25,088 | ||||||||||||||||||
Stock-based compensation tax benefits | - | - | 58,831 | 58,831 | ||||||||||||||||||||
Repurchase and retirement of common stock | (9,824 | ) | (98 | ) | (71,517 | ) | - | (128,875 | ) | (200,490 | ) | |||||||||||||
Net income | - | - | - | - | 100,710 | 100,710 | ||||||||||||||||||
Other comprehensive income, net of tax | - | - | - | 12 | - | 12 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balances at June 30, 2011 | 86,244 | $ | 862 | $ | 604,409 | $ | 151 | $ | (38,630 | ) | $ | 566,792 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Issuance of common stock for cash upon exercise of options and employee stock purchase plan | 2,013 | 21 | 25,008 | - | - | 25,029 | ||||||||||||||||||
Share-based payment expense | - | - | 26,275 | - | - | 26,275 | ||||||||||||||||||
Stock-based compensation tax benefits | - | - | 34,193 | 34,193 | ||||||||||||||||||||
Repurchase and retirement of common stock | (5,688 | ) | (57 | ) | (42,205 | ) | - | (86,205 | ) | (128,467 | ) | |||||||||||||
Net income | - | - | - | - | 112,151 | 112,151 | ||||||||||||||||||
Other comprehensive income, net of tax | - | - | - | (313 | ) | - | (313 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balances at June 30, 2012 | 82,569 | $ | 826 | $ | 647,680 | $ | (162 | ) | $ | (12,684 | ) | $ | 635,660 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Issuance of common stock for cash upon exercise of options and employee stock purchase plan | 3,640 | 36 | 57,789 | - | - | 57,825 | ||||||||||||||||||
Share-based payment expense | - | - | 26,612 | - | - | 26,612 | ||||||||||||||||||
Stock-based compensation tax benefits | - | - | 7,888 | 7,888 | ||||||||||||||||||||
Repurchase and retirement of common stock | (5,632 | ) | (56 | ) | (42,623 | ) | - | (103,589 | ) | (146,268 | ) | |||||||||||||
Net income | - | - | - | - | 147,139 | 147,139 | ||||||||||||||||||
Other comprehensive income, net of tax | - | - | - | (262 | ) | - | (262 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balances at June 30, 2013 | 80,577 | $ | 806 | $ | 697,346 | $ | (424 | ) | $ | 30,866 | $ | 728,594 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
See accompanying notes to consolidated financial statements.
F-4
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Years ended June 30, 2013, 2012 and 2011
(In thousands)
| 2013 | 2012 | 2011 | ||||||||||
Cash flows from operating activities: | ||||||||||||
Net income | $ | 147,139 | $ | 112,151 | $ | 100,710 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation and amortization | 8,889 | 9,069 | 7,219 | |||||||||
Loss on disposition of assets | 8 | 194 | - | |||||||||
Share-based compensation expense | 26,612 | 26,275 | 25,088 | |||||||||
Bad debt expense | 33,294 | 24,742 | 16,182 | |||||||||
Non-cash expense related to in-process research and development technology | - | 750 | 2,000 | |||||||||
Impairment of intangible assets | 1,490 | - | - | |||||||||
Deferred income taxes | 7,469 | 33,625 | 53,156 | |||||||||
Unrecognized tax benefits | 710 | 560 | (148 | ) | ||||||||
Accreted interest on note receivable | (2,667 | ) | (2,000 | ) | - | |||||||
Excess tax benefit from share-based compensation | (7,888 | ) | (34,193 | ) | (58,831 | ) | ||||||
Gain on sale of marketable investment securities | (165 | ) | (566 | ) | (3 | ) | ||||||
Changes in operating assets and liabilities: | ||||||||||||
Prepaid expenses | 757 | 1,204 | (412 | ) | ||||||||
Trade accounts receivable | (67,185 | ) | (34,986 | ) | (14,918 | ) | ||||||
Other receivables | (706 | ) | (2,312 | ) | (202 | ) | ||||||
Inventory | 6,567 | (3,426 | ) | 203 | ||||||||
Accounts payable | 7,991 | (1,249 | ) | 287 | ||||||||
Accrued liabilities | 11,562 | 11,218 | 924 | |||||||||
Deferred revenue | (11 | ) | 748 | (425 | ) | |||||||
|
|
|
|
|
| |||||||
Net cash provided by operating activities | 173,866 | 141,804 | 130,830 | |||||||||
|
|
|
|
|
| |||||||
Cash flows from investing activities: | ||||||||||||
Capital expenditures for equipment and leasehold improvements | (11,373 | ) | (9,408 | ) | (3,792 | ) | ||||||
Acquisition of Rules-Based Medicine, Inc. (see Note 12), net of cash acquired | - | (799 | ) | (79,417 | ) | |||||||
Purchase of in-process research and development technology | - | (750 | ) | (2,000 | ) | |||||||
Purchase of other assets | - | (100 | ) | (100 | ) | |||||||
Purchase of an acquisition option | - | (8,000 | ) | - | ||||||||
Issuance of note receivable (Crescendo) | - | (17,000 | ) | - | ||||||||
Equity investment (Rain Dance) | (5,000 | ) | ||||||||||
Purchases of marketable investment securities | (443,777 | ) | (388,067 | ) | (425,153 | ) | ||||||
Proceeds from maturities and sales marketable investment securities | 384,560 | 385,236 | 456,072 | |||||||||
|
|
|
|
|
| |||||||
Net cash used in investing activities | (75,590 | ) | (38,888 | ) | (54,390 | ) | ||||||
|
|
|
|
|
| |||||||
Cash flows from financing activities: | ||||||||||||
Net proceeds from common stock issued under share-based compensation plans | 57,825 | 25,029 | 25,060 | |||||||||
Excess tax benefit from share-based compensation | 7,888 | 34,193 | 58,831 | |||||||||
Repurchase and retirement of common stock | (146,268 | ) | (128,467 | ) | (200,490 | ) | ||||||
|
|
|
|
|
| |||||||
Net cash used in financing activities | (80,555 | ) | (69,245 | ) | (116,599 | ) | ||||||
|
|
|
|
|
| |||||||
Net increase (decrease) in cash and cash equivalents | 17,721 | 33,671 | (40,159 | ) | ||||||||
Cash and cash equivalents at beginning of year | 86,352 | 52,681 | 92,840 | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents at end of year | $ | 104,073 | $ | 86,352 | $ | 52,681 | ||||||
|
|
|
|
|
| |||||||
Supplemental cash flow information: | ||||||||||||
Cash paid during the year for income taxes | $ | 80,317 | $ | 33,382 | $ | 9,091 | ||||||
Non-cash investing and financing activities: | ||||||||||||
Fair value adjustment on marketable investment securities recorded to stockholders' equity | $ | 433 | $ | (14 | ) | $ | (4 | ) | ||||
See accompanying notes to consolidated financial statements.
F-5
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| (1) | Organization and Summary of Significant Accounting Policies |
| (a) | Business Description and Basis of Presentation |
Myriad Genetics, Inc. and subsidiaries (collectively, the Company) is a leading molecular diagnostic company focused on developing and marketing novel predictive medicine, personalized medicine and prognostic medicine tests. The Company employs a number of proprietary technologies, including DNA, RNA and protein analysis, that help it to understand the genetic basis of human disease and the role that genes and their related proteins may play in the onset and progression of disease. The Company uses this information to guide the development of new molecular diagnostic and companion diagnostic tests that are designed to assess an individual's risk for developing disease later in life (predictive medicine), identify a patient's likelihood of responding to drug therapy and guide a patient's dosing to ensure optimal treatment (personalized medicine), or assess a patient's risk of disease progression and disease recurrence (prognostic medicine). The Company currently offers nine commercial molecular diagnostic tests, including six predictive medicine tests, two personalized medicine tests, and one prognostic medicine test. The Company also generates revenue by providing companion diagnostic services to the pharmaceutical and biotechnology industries and medical research institutions utilizing its multiplexed immunoassay technology. The Company's corporate headquarters is located in Salt Lake City, Utah.
The consolidated financial statements of the Company are prepared in accordance with U.S. generally accepted accounting principles ("GAAP") and include the accounts of the Company and its wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated in consolidation. In the opinion of management, the accompanying financial statements contain all adjustments (consisting of normal and recurring accruals) necessary to present fairly all financial statements in accordance with U.S. GAAP. Certain reclassifications have been made to prior period amounts to conform to the current period presentation.
| (b) | Marketable Investment Securities |
The Company has classified its marketable investment securities as available-for-sale. Available-for-sale investment securities with remaining maturities of greater than one year are classified as long-term. Available-for-sale investment securities with remaining maturities of less than one year are classified as short-term. Available-for-sale investment securities with remaining maturities of less than three months at the time of purchase are classified as cash equivalents. Marketable securities are carried at estimated fair value with unrealized holding gains and losses, net of the related tax effect, included in accumulated other comprehensive (loss) in stockholders' equity until realized. Gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned.
A decline in the market value of any available-for-sale security below cost that is deemed other than temporary results in a charge to earnings and establishes a new cost basis for the security. Losses are charged against "Other income" when a decline in fair value is determined to be other than temporary. We review several factors to determine whether a loss is other than temporary. These factors include but are not limited to: (i) the extent to which the fair value is less than cost and the cause for the fair value decline, (ii) the financial condition and near term prospects of the issuer, (iii) the length of time a security is in an unrealized loss position and (iv) our ability to hold the security for a period of time sufficient to allow for any anticipated recovery in fair value. There were no other-than-temporary impairments recognized during the years ended June 30, 2013, 2012 and 2011.
F-6
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| (c) | Trade Accounts Receivable and Allowance for Doubtful Accounts |
Trade accounts receivable are comprised of amounts due from sales of the Company's molecular diagnostic tests and companion diagnostic services and are recorded at the invoiced amount, net of discounts and contractual allowances. The allowance for doubtful accounts is based on the Company's best estimate of the amount of probable losses in the Company's existing accounts receivable, which is based on historical write-off experience, customer creditworthiness, facts and circumstances specific to outstanding balances, and payment terms. Account balances are charged against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers and does not require collateral.
| (d) | Equipment and Leasehold Improvements |
Equipment and leasehold improvements are stated at cost less accumulated depreciation. Depreciation and amortization are computed using the straight-line method based on the lesser of estimated useful lives of the related assets or lease terms. Equipment items have depreciable lives of five to seven years. Leasehold improvements are depreciated over the shorter of the estimated useful lives or the associated lease terms, which range from three to fifteen years. Repairs and maintenance costs are charged to expense as incurred. For the years ended June 30, 2013, 2012 and 2011, the Company recorded the depreciation expense as follows:
| Years Ended June 30, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| (In thousands) | ||||||||||||
Depreciation expense | $ | 7,994 | $ | 7,969 | $ | 6,833 | ||||||
| (e) | Inventory |
Inventories consist of reagents and testing kits. Inventories are stated at the lower of cost or market on a first-in, first-out basis. In order to assess the ultimate realization of inventories, the Company is required to make judgments as to future demand requirements compared to current or committed inventory levels.
The Company evaluates its inventories for excess quantities and obsolescence. Inventories that are considered obsolete are expensed. The valuation of inventories requires the use of estimates as to the amounts of current inventories that will be sold. These estimates are dependent on management's assessment of current and expected orders from the Company's customers.
| (f) | Intangible Assets and Other Long-Lived Assets |
Intangible and other assets as of June 30, 2013 and 2012 are comprised of acquired patents and intellectual property and purchased in-process research and development. Acquired intangible assets are recorded at fair value and amortized over the shorter of the contractual life or the estimated useful life.
The Company continually reviews and monitors long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If the carrying
F-7
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
amount of an asset exceeds its estimated future undiscounted cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. In December 2012, the Company notified the licensor of the Company's OnDose product of the Company's intent to terminate the license agreement, and as a result, recorded an impairment charge of approximately $1,490,000 associated with the purchased license agreement. Other than this $1,490,000 impairment charge, the Company concluded there was no impairment of long-lived assets for the years ended June 30, 2013, 2012 and 2011.
| (g) | Goodwill |
The Company has recorded goodwill of $56,850,000 from the acquisition of Rules-Based Medicine, Inc. that was completed on May 31, 2011 (see Note 12). This goodwill relates solely to the Company's Companion Diagnostic segment. Goodwill is tested for impairment on an annual basis as of April 1 and in the interim by reporting unit if events and circumstances indicate that goodwill may be impaired. The events and circumstances that are considered include business climate and market conditions, legal factors, operating performance indicators and competition. Impairment of goodwill was evaluated using a two-step process. The first step involves a comparison of the fair value of the reporting unit with its carrying amount. If the carrying amount of the reporting unit exceeds its fair value, the second step of the process involves a comparison of the fair value and the carrying amount of the goodwill of that reporting unit. If the carrying amount of the goodwill of the reporting unit exceeds the fair value of that goodwill, an impairment loss would be recognized in an amount equal to the excess of carrying value over fair value. If an event occurs that would cause a revision to the estimates and assumptions used in analyzing the value of the goodwill, the revision could result in a non-cash impairment charge that could have a material impact on the financial results.
| (h) | Revenue Recognition |
Molecular diagnostic testing revenue is recognized when persuasive evidence of an agreement exists, delivery has occurred, the fee is fixed or determinable, and collection is probable. Revenue from the sale of molecular diagnostic tests and related marketing agreements is recorded at the invoiced amount net of any discounts or contractual allowances. Revenue is recognized upon completion of the test, communication of results to the patient, and when collectability is reasonably assured.
Companion diagnostic service revenue is recognized when the testing service has been completed and the results of the tests are transferred to the customer. TruCulture revenues are recorded upon shipment to customers. In addition, the Company's wholly owned subsidiary, Myriad RBM, has received national, state, foreign government and private foundation grants and contracts. Revenue associated with these grants and contracts are recognized in the period in which qualifying costs for the services by the grants and contracts are incurred and the related grant or contract fee is earned.
| (i) | Income Taxes |
The Company recognizes income taxes under the asset and liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities.
The provision for income taxes, including the effective tax rate and analysis of potential tax exposure items, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any
F-8
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
estimated valuation allowances deemed necessary to recognize deferred tax assets at an amount that is more likely than not to be realized. The Company's filings, including the positions taken therein, are subject to audit by various taxing authorities. While the Company believes it has provided adequately for its income tax liabilities in the consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on the consolidated financial condition, results of operations or cash flows.
| (j) | Earnings Per Share |
Basic earnings per share is computed based on the weighted-average number of shares of common stock outstanding. Diluted earnings per share is computed based on the weighted-average number of shares of common stock, including the dilutive effect of common stock equivalents outstanding.
The following is a reconciliation of the denominators of the basic and diluted earnings per share computations:
| (In thousands) | Years Ended June 30, | |||||||||||
| 2013 | 2012 | 2011 | ||||||||||
Denominator: | ||||||||||||
Weighted-average shares outstanding used to compute basic EPS | 80,948 | 84,608 | 89,794 | |||||||||
Effect of dilutive stock options | 2,379 | 1,857 | 1,910 | |||||||||
|
|
|
|
|
| |||||||
Weighted-average shares outstanding and dilutive securities used to compute diluted EPS | 83,327 | 86,465 | 91,704 | |||||||||
|
|
|
|
|
| |||||||
Certain outstanding stock options were excluded from the computation of diluted earnings per share because the effect would have been anti-dilutive. These potential dilutive common shares, which may be dilutive to future diluted earnings per share, are as follows:
| (In thousands) | Years Ended June 30, | |||||||||||
| 2013 | 2012 | 2011 | ||||||||||
Anti-dilutive options excluded from EPS computation | 5,136 | 8,585 | 8,666 | |||||||||
| (k) | Use of Estimates |
The preparation of the consolidated financial statements in accordance with U.S. GAAP requires Company management to make estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the period. Significant items subject to such estimates and assumptions include the carrying amount of fixed assets, valuation allowances for receivables and deferred income tax assets, certain accrued liabilities, share-based compensation and impairment analysis of goodwill and intangible assets. Actual results could differ from those estimates.
| (l) | Recent Accounting Pronouncements |
In February 2013, the FASB issued an amendment to the accounting guidance for the reporting of amounts reclassified out of accumulated other comprehensive income ("AOCI"). The amendment expands the existing disclosure requirements by requiring entities to present information about
F-9
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
significant items reclassified out of AOCI by component. In addition, an entity is required to provide information about the effects on net income of significant amounts reclassified out of each component of AOCI to net income either on the face of the statement where net income is presented or as a separate disclosure in the notes of the financial statements. The amendment is effective prospectively for annual or interim reporting periods beginning after December 15, 2012. The adoption of this accounting pronouncement did not have a material impact on our financial statements.
| (2) | Marketable Investment Securities |
The amortized cost, gross unrealized holding gains, gross unrealized holding losses, and fair value for available-for-sale securities by major security type and class of security at June 30, 2013 and 2012 were as follows:
| (In thousands) | Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Estimated fair value | ||||||||||||
At June 30, 2013: | ||||||||||||||||
Cash and cash equivalents: | ||||||||||||||||
Cash | $ | 40,412 | $ | - | $ | - | $ | 40,412 | ||||||||
Cash equivalents | 63,653 | 8 | - | 63,661 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total cash and cash equivalents | 104,065 | 8 | - | 104,073 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Available-for-sale: | ||||||||||||||||
Corporate bonds and notes | 71,626 | 13 | (15 | ) | 71,624 | |||||||||||
Municipal bonds | 251,513 | 109 | (537 | ) | 251,085 | |||||||||||
Federal agency issues | 104,293 | 24 | (35 | ) | 104,282 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 531,497 | $ | 154 | $ | (587 | ) | $ | 531,064 | |||||||
|
|
|
|
|
|
|
| |||||||||
| (In thousands) | Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Estimated fair value | ||||||||||||
At June 30, 2012: | ||||||||||||||||
Cash and cash equivalents: | ||||||||||||||||
Cash | $ | 34,217 | $ | - | $ | - | $ | 34,217 | ||||||||
Cash equivalents | 52,135 | - | - | 52,135 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total cash and cash equivalents | 86,352 | - | - | 86,352 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Available-for-sale: | ||||||||||||||||
Corporate bonds and notes | 116,581 | 112 | (18 | ) | 116,675 | |||||||||||
Municipal bonds | 141,299 | 85 | (20 | ) | 141,364 | |||||||||||
Federal agency issues | 108,478 | 33 | (28 | ) | 108,483 | |||||||||||
Auction rate securities | 1,500 | - | (150 | ) | 1,350 | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 454,210 | $ | 230 | $ | (216 | ) | $ | 454,224 | |||||||
|
|
|
|
|
|
|
| |||||||||
F-10
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
In April 2013, the Company sold its auction rate securities at par. Cash, cash equivalents, and maturities of debt securities classified as available-for-sale are as follows at June 30, 2013:
| (In thousands) | Amortized cost | Estimated fair value | ||||||
Cash | $ | 40,412 | $ | 40,412 | ||||
Cash equivalents | 63,653 | 63,661 | ||||||
Available-for-sale: | ||||||||
Due within one year | 268,184 | 268,243 | ||||||
Due after one year through five years | 159,248 | 158,748 | ||||||
Due after five years | - | - | ||||||
|
|
|
| |||||
Total | $ | 531,497 | $ | 531,064 | ||||
|
|
|
| |||||
Debt securities in an unrealized loss position as of June 30, 2013 were not impaired at acquisition and the declines in fair value are not attributed to declines in credit quality. Management believes that it is more likely than not that the securities will be held until a recovery of par value. All securities in an unrealized loss position as of June 30, 2013 are debt securities. Debt securities available-for-sale in a gross unrealized loss position as of June 30, 2013 and 2012 are summarized as follows:
| Less than 12 months | More than 12 months | Total | ||||||||||||||||||||||
| (In thousands) | Fair value | Unrealized losses | Fair value | Unrealized losses | Fair value | Unrealized losses | ||||||||||||||||||
At June 30, 2013: | ||||||||||||||||||||||||
Cash equivalents | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||
Debt securities: | ||||||||||||||||||||||||
Corporate bonds and notes | 30,309 | (15 | ) | - | - | 30,309 | (15 | ) | ||||||||||||||||
Municipal bonds | 93,992 | (538 | ) | - | - | 93,992 | (538 | ) | ||||||||||||||||
Federal agency issues | 45,528 | (34 | ) | - | - | 45,528 | (34 | ) | ||||||||||||||||
Auction rate securities | - | - | - | - | - | - | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| $ | 169,829 | $ | (587 | ) | $ | - | $ | - | $ | 169,829 | $ | (587 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| Less than 12 months | More than 12 months | Total | ||||||||||||||||||||||
| (In thousands) | Fair value | Unrealized losses | Fair value | Unrealized losses | Fair value | Unrealized losses | ||||||||||||||||||
At June 30, 2012: | ||||||||||||||||||||||||
Cash equivalents | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||
Debt securities: | ||||||||||||||||||||||||
Corporate bonds and notes | 24,933 | (18 | ) | - | - | 24,933 | (18 | ) | ||||||||||||||||
Municipal bonds | 25,346 | (20 | ) | - | - | 25,346 | (20 | ) | ||||||||||||||||
Federal agency issues | 74,966 | (28 | ) | - | - | 74,966 | (28 | ) | ||||||||||||||||
Auction rate securities | - | - | 1,350 | (150 | ) | 1,350 | (150 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| $ | 125,245 | $ | (66 | ) | $ | 1,350 | $ | (150 | ) | $ | 126,595 | $ | (216 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
F-11
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| (3) | Fair Value Measurements |
The fair value of the Company's financial instruments reflects the amounts that the Company estimates to receive in connection with the sale of an asset or paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The fair value hierarchy prioritizes the use of inputs used in valuation techniques into the following three levels:
Level 1-quoted prices in active markets for identical assets and liabilities.
Level 2-observable inputs other than quoted prices in active markets for identical assets and liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Some of the Company's marketable securities primarily utilize broker quotes in a non-active market for valuation of these securities.
Level 3-unobservable inputs.
The substantial majority of the Company's financial instruments are valued using quoted prices in active markets or based on other observable inputs. The following table sets forth the fair value of the Company's financial assets that are re-measured on a regular basis:
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
at June 30, 2013 | ||||||||||||||||
Money market funds (a) | $ | 12,691 | $ | - | $ | - | $ | 12,691 | ||||||||
Corporate bonds and notes | - | 71,624 | - | 71,624 | ||||||||||||
Municipal bonds | - | 302,055 | - | 302,055 | ||||||||||||
Federal agency issues | - | 104,282 | - | 104,282 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 12,691 | $ | 477,961 | $ | - | $ | 490,652 | ||||||||
|
|
|
|
|
|
|
| |||||||||
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
at June 30, 2012 | ||||||||||||||||
Money market funds (a) | $ | 38,835 | $ | - | $ | - | $ | 38,835 | ||||||||
Corporate bonds and notes | - | 129,975 | - | 129,975 | ||||||||||||
Municipal bonds | - | 141,364 | - | 141,364 | ||||||||||||
Federal agency issues | - | 108,483 | - | 108,483 | ||||||||||||
Auction rate securities | - | - | 1,350 | 1,350 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | $ | 38,835 | $ | 379,822 | $ | 1,350 | $ | 420,007 | ||||||||
|
|
|
|
|
|
|
| |||||||||
| (a) | Money market funds are primarily comprised of exchange traded funds and accrued interest |
The Company's Level 1 assets include money market instruments. Level 2 assets consist of marketable investment securities that include federal agency issues, commercial paper, corporate bonds, and municipal bonds. Level 2 securities are valued based upon observable inputs that may include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and reference data including market research publications. As of June 30, 2013 and 2012, the investments which were measured using unobservable (Level 3) inputs were limited to the Company's investment in auction rate securities. These investments are measured at an amount based on valuations which approximate fair value. In April 2013 the Company sold its auction rate securities at par. There was no change in the composition of the Company's Level 3 assets during the periods ended June 30, 2012 and 2011.
F-12
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| (4) | Goodwill and Other Intangible Assets |
Goodwill
At June 30, 2013, the Company had goodwill of $56,850,000 recorded as a result of the acquisition of Rules-Based Medicine, Inc. that was completed on May 31, 2011 (see Note 12). The Company assessed goodwill for impairment in accordance with the appropriate guidance (see Note 1( g )) and recorded no impairment of goodwill for the period ended June 30, 2013.
Intangible Assets
Intangible assets primarily consist of amortizable assets of purchased licenses and technologies, and customer relationships as well as non-amortizable intangible assets of in-process technologies, research and development and trademarks. Certain of these intangible assets were recorded as part of the Company's purchase of Rules-Based Medicine, Inc. ("RBM") on May 31, 2011 (see Note 12). As part of the Company's annual impairment analysis of intangible assets it was determined that the Company's trademarks acquired in conjunction with the purchase of RBM had an estimated remaining useful life of approximately 15 years. Accordingly, these trademarks were reclassified from indefinite lived or unamortizable intangible assets to finite lived or amortizable intangible assets as of June 30, 2013. The estimated useful life of acquired in-process research and development was also evaluated in conjunction with the annual impairment analysis of intangible assets and the classification of the acquired in-process research and development as an indefinite lived asset was deemed appropriate as the related research and development was not yet complete nor had it been abandoned.
In December 2012, the Company notified the licensor of the Company's OnDose product of the Company's intent to terminate the license agreement, and as a result, recorded an impairment charge of approximately $1,490,000 associated with the purchased license agreement. The fair value was estimated for the license agreement using the undiscounted future cash flows method, under which the Company determined that the fair value was less than the carrying value. The impairment is included in research and development in the condensed consolidated statement of comprehensive income and is part of the molecular diagnostic segment. The following summarizes the amounts reported as intangible assets:
| (In thousands) | Gross Carrying Amount | Accumulated Amortization | Net | |||||||||
at June 30, 2013 | ||||||||||||
Purchased licenses and technologies | $ | 4,500 | $ | (2,644 | ) | $ | 1,856 | |||||
Customer relationships | 4,650 | (976 | ) | 3,674 | ||||||||
Trademarks | 3,000 | - | 3,000 | |||||||||
|
|
|
|
|
| |||||||
Total amortized intangible assets | 12,150 | (3,620 | ) | 8,530 | ||||||||
|
|
|
|
|
| |||||||
In-process research and development | 4,800 | - | 4,800 | |||||||||
|
|
|
|
|
| |||||||
Total unamortized intangible assets | 4,800 | - | 4,800 | |||||||||
|
|
|
|
|
| |||||||
Total intangible assets | $ | 16,950 | $ | (3,620 | ) | $ | 13,330 | |||||
|
|
|
|
|
| |||||||
F-13
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
(In thousands) | Gross Carrying Amount | Accumulated Amortization | Net | |||||||||
at June 30, 2012 | ||||||||||||
Purchased licenses and technologies | $ | 6,500 | $ | (2,724 | ) | $ | 3,776 | |||||
Customer relationships | 4,650 | (504 | ) | 4,146 | ||||||||
|
|
|
|
|
| |||||||
Total amortized intangible assets | 11,150 | (3,228 | ) | 7,922 | ||||||||
|
|
|
|
|
| |||||||
Trademarks | 3,000 | - | 3,000 | |||||||||
In-process research and development | 4,800 | - | 4,800 | |||||||||
|
|
|
|
|
| |||||||
Total unamortized intangible assets | 7,800 | - | 7,800 | |||||||||
|
|
|
|
|
| |||||||
Total intangible assets | $ | 18,950 | $ | (3,228 | ) | $ | 15,722 | |||||
|
|
|
|
|
| |||||||
As of June 30, 2013 the weighted average remaining amortization period for purchased licenses and technologies and customer relationships is approximately 10 years.
The Company recorded amortization during the respective periods for these intangible assets as follows:
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Amortization on intangible assets | $ | 895 | $ | 1,100 | $ | 386 | ||||||
Future estimated amortization expense as of June 30, 2013 for the five succeeding fiscal years is as follows:
| (In thousands) | ||||
Fiscal year ending: | ||||
2014 | $ | 978 | ||
2015 | 978 | |||
2016 | 978 | |||
2017 | 978 | |||
2018 | 978 | |||
|
| |||
| $ | 4,890 | |||
|
| |||
| (5) | Leases |
The Company leases office and laboratory space under five non-cancelable operating leases, with terms that expire between 2017 and 2025 in Salt Lake City, Utah, one cancelable lease for office and laboratory space with a term of five years in Munich, Germany, and three non-cancelable operating leases for Myriad RBM for office and laboratory space that expire between 2015 and 2017 in Austin, Texas; Saranac Lake, New York; and Reutlingen, Germany. The Company also maintains lease agreements that expire between 2013 and 2018 for administrative offices in Zurich, Switzerland; Paris, France; Madrid, Spain; and Milan, Italy. Furthermore, the Company leases information technology equipment under two non-cancelable leases, with terms that expire in 2016.
F-14
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
The following is a summary of the Company's rental expense for the fiscal years reported:
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Rental expense | $ | 8,155 | $ | 6,819 | $ | 5,548 | ||||||
Future minimum lease payments under the Company's current leases as of June 30, 2013 are as follows:
| (In thousands) | ||||
Fiscal year ending: | ||||
2014 | $ | 9,446 | ||
2015 | 9,642 | |||
2016 | 8,994 | |||
2017 | 8,959 | |||
2018 | 5,684 | |||
Thereafter | 30,455 | |||
|
| |||
| $ | 73,180 | |||
|
| |||
| (6) | Share-Based Compensation |
The Company maintains a share-based compensation plan, the 2010 Employee, Director and Consultant Equity Incentive Plan, as amended (the "2010 Plan"), that has been approved by the Company's shareholders. The 2010 Plan allows the Company, under the direction of the Compensation Committee of the Board of Directors, to make grants of stock options, restricted and unrestricted stock awards and other stock-based awards to employees, consultants and directors. On December 5, 2012, the shareholders approved an amendment to the 2010 Plan to set the number of shares available for grant to 4,500,000. As of June 30, 2013, a total of 4,486,733 shares of common stock are reserved for issuance under the 2010 Plan. In addition, as of June 30, 2013, the Company may grant up to 7,286,907 additional shares under the 2010 Plan if options previously granted under the Company's terminated 2002 Amended and Restated Employee, Director and Consultant Stock Option Plan or 2003 Employee, Director and Consultant Option Plan are cancelled or expire in the future without the issuance of shares of common stock by the Company. The exercise price of options granted in 2013, 2012 and 2011 was equivalent to the fair market value of the stock at the date of grant. The number of shares, terms, and vesting periods are determined by the Company's board of directors or a committee thereof on an option-by-option basis. Options generally vest ratably over service periods of four years. Options granted after December 5, 2012 generally expire eight years from the date of grant, and options granted prior to that date generally expire ten years from the date of grant.
The fair value of each option grant is estimated on the date of the grant using the Black-Scholes option-pricing model with the following weighted-average assumptions used for grants for the fiscal year ended June 30:
| 2013 | 2012 | 2011 | ||||
Risk-free interest rate | 0.8% | 1.0% | 1.8% | |||
Expected dividend yield | 0% | 0% | 0% | |||
Expected lives (in years) | 4.2 - 4.7 | 4.2 - 4.6 | 4.2 - 4.4 | |||
Expected volatility | 44% | 44% | 43% |
F-15
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
Expected option lives and volatilities are based on historical data of the Company and other factors.
A summary of activity is as follows:
| 2013 | 2012 | 2011 | ||||||||||||||||||||||
| Number of shares | Weighted average exercise price | Number of shares | Weighted average exercise price | Number of shares | Weighted average exercise price | |||||||||||||||||||
Options outstanding at beginning of year | 15,233,281 | $ | 19.32 | 14,453,913 | $ | 18.22 | 14,116,938 | $ | 18.03 | |||||||||||||||
Options granted | 2,957,623 | 27.09 | 3,188,160 | 20.42 | 3,102,440 | 17.63 | ||||||||||||||||||
Less: | ||||||||||||||||||||||||
Options exercised | (3,490,495 | ) | 15.65 | (1,852,245 | ) | 11.94 | (1,876,303 | ) | 12.06 | |||||||||||||||
Options canceled or expired | (265,439 | ) | 22.26 | (556,547 | ) | 21.48 | (889,162 | ) | 26.09 | |||||||||||||||
|
|
|
|
|
| |||||||||||||||||||
Options outstanding at end of year | 14,434,970 | 21.75 | 15,233,281 | 19.32 | 14,453,913 | 18.22 | ||||||||||||||||||
|
|
|
|
|
| |||||||||||||||||||
Options exercisable at end of year | 7,480,472 | 20.51 | 8,397,678 | 18.01 | 7,794,782 | 15.53 | ||||||||||||||||||
Options vested and expected to vest | 13,543,852 | 21.58 | 14,514,637 | 19.28 | 13,662,687 | 18.10 | ||||||||||||||||||
Weighted average fair value of options granted during the year | 9.87 | 7.47 | 6.61 | |||||||||||||||||||||
The following table summarizes information about stock options outstanding at June 30, 2013:
| Options outstanding | Options exercisable | |||||||||||||||||||
Range of exercise prices | Number outstanding at June 30, 2013 | Weighted average remaining contractual life (years) | Weighted average exercise price | Number exercisable at June 30, 2013 | Weighted average exercise price | |||||||||||||||
$3.97 - 18.00 | 4,010,323 | 5.69 | $ | 14.48 | 2,705,177 | $ | 13.16 | |||||||||||||
18.06 - 22.93 | 3,814,080 | 7.05 | 20.15 | 2,056,757 | 20.71 | |||||||||||||||
22.97 - 27.07 | 4,487,807 | 8.40 | 25.71 | 1,048,628 | 23.63 | |||||||||||||||
27.61 - 32.17 | 2,122,760 | 6.24 | 29.98 | 1,669,910 | 30.20 | |||||||||||||||
|
|
|
| |||||||||||||||||
| 14,434,970 | 6.97 | 7,480,472 | 20.51 | |||||||||||||||||
|
|
|
| |||||||||||||||||
Options exercisable at June 30, 2013 had a weighted average remaining contractual life of 5.67 years.
Share-based compensation expense recognized and included in the consolidated statements of comprehensive income for the fiscal years ended June 30, 2013, 2012 and 2011 was as follows:
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Cost of molecular diagnostic testing | $ | 1,030 | $ | 1,158 | $ | 1,200 | ||||||
Cost of companion diagnostic services | 219 | 85 | - | |||||||||
Research and development expense | 3,246 | 3,350 | 3,902 | |||||||||
Selling, general, and administrative expense | 22,117 | 21,682 | 19,986 | |||||||||
|
|
|
|
|
| |||||||
Total share-based compensation expense | $ | 26,612 | $ | 26,275 | $ | 25,088 | ||||||
|
|
|
|
|
| |||||||
F-16
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
The Company has unrecognized share-based compensation cost related to share-based compensation granted under its current plans. The estimated unrecognized share-based compensation cost and related weighted average recognition period, aggregate intrinsic value of options outstanding, and aggregate intrinsic value of options that are fully vested for the year ended June 30, 2013 is as follows:
(In thousands) | As
of June 30, 2013 | |||
Unrecognized share-based compensation cost | $ | 34,445 | ||
Aggregate intrinsic value of options outstanding | $ | 81,063 | ||
Aggregate intrinsic value of options fully vested | $ | 53,154 | ||
The estimated unrecognized share-based compensation cost will be recognized over a weighted-average period of 2.3 years.
The total intrinsic value of options exercised during 2013, 2012 and 2011 was as follows:
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Total intrinsic value of options exercised | $ | 51,785 | $ | 21,575 | $ | 20,215 | ||||||
The Company also had an Employee Stock Purchase Plan that was approved by shareholders in 1995 (the "1995 Purchase Plan"), and subsequently amended, under which 2,000,000 shares of common stock had been authorized. On November 30, 2012, 82,000 shares were purchased under the 1995 Purchase Plan. As of December 5, 2012, a total of 1,990,000 shares of common stock had been issued under the 1995 Purchase Plan when it was terminated. On December 5, 2012, following the shareholder approval, the Company's adopted the 2012 Employee Stock Purchase Plan (the "2012 Purchase Plan"), under which 2,000,000 shares of common stock have been authorized. Shares are issued under the 2012 Purchase Plan twice yearly at the end of each offering period. At June 30, 2013, a total of 67,000 shares of common stock had been purchased under the 2012 Plan. Shares purchased under and compensation expense associated with the 1995 and 2012 Plans for the years reported are as follows:
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Shares purchased under the Plans | 149 | 161 | 145 | |||||||||
Plan compensation expense | $ | 885 | $ | 892 | $ | 807 | ||||||
The fair value of shares issued under the Plan that was in effect for each period reported was calculated using the Black-Scholes option-pricing model using the following weighted-average assumptions:
| 2013 | 2012 | 2011 | ||||||||||
Risk-free interest rate | 0.1 | % | 0.1 | % | 0.1 | % | ||||||
Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||
Expected lives (in years) | 0.5 | 0.5 | 0.5 | |||||||||
Expected volatility | 37 | % | 30 | % | 35 | % | ||||||
F-17
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| (7) | Stockholders' Equity |
Stock Repurchase Program
The Company previously announced the following stock repurchase programs for its common stock:
Date Authorized | Amount Authorized | Date Completed | ||||
May 2010 | $ | 100,000,000 | August 2010 | |||
August 2010 | $ | 100,000,000 | February 2011 | |||
March 2011 | $ | 100,000,000 | September 2011 | |||
August 2011 | $ | 200,000,000 | January 2013 | |||
February 2013 | $ | 200,000,000 | ongoing | |||
|
| |||||
Total: | $ | 700,000,000 | ||||
In January 2013, the Company completed its fourth share repurchase program, which authorized the repurchase of up to $200 million of the Company's common stock. From July 2012 through January 2013, the Company repurchased $99,648,000 worth of shares under this program. In February 2013, the Company's Board of Directors authorized a fifth share repurchase program of $200 million of the Company's outstanding common stock. The Company plans to repurchase the $200 million of its common stock from time to time or on an accelerated basis through open market transactions or privately negotiated transactions as determined by the Company's management. The amount and timing of stock repurchases under the program will depend on business and market conditions, stock price, trading restrictions, acquisition activity and other factors. As of June 30, 2013, the Company has repurchased $46,620,000 of shares under the current $200 million share repurchase authorization.
The Company uses the par value method of accounting for its stock repurchases. As a result of the stock repurchases, the Company reduced common stock and additional paid-in capital and recorded charges to accumulated deficit. The shares retired, aggregate common stock and additional paid-in capital reductions, and related charges to accumulated deficit for the repurchases for periods ended June 30, 2013, 2012 and 2011 were as follows:
| Year ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Shares purchased and retired | 5,632 | 5,688 | 9,824 | |||||||||
Common stock and additional paid-in-capital reductions | $ | 42,679 | $ | 42,262 | $ | 71,615 | ||||||
Charges to retained earnings | $ | 103,589 | $ | 86,205 | $ | 128,875 | ||||||
F-18
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| (8) | Income Taxes |
Income tax expense consists of the following:
| Year ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Current: | ||||||||||||
Federal | $ | 80,333 | $ | 67,492 | $ | 61,172 | ||||||
State | 6,021 | 4,647 | 3,444 | |||||||||
|
|
|
|
|
| |||||||
Total Current | 86,354 | 72,139 | 64,616 | |||||||||
|
|
|
|
|
| |||||||
Deferred: | ||||||||||||
Federal | 660 | (2,192 | ) | 27,370 | ||||||||
State | (431 | ) | 2,326 | 1,979 | ||||||||
Foreign | (2,051 | ) | (1,735 | ) | - | |||||||
Change in valuation allowance | 1,605 | 1,851 | (35,024 | ) | ||||||||
|
|
|
|
|
| |||||||
Total Deferred | (217 | ) | 250 | (5,675 | ) | |||||||
|
|
|
|
|
| |||||||
Total income tax expense | $ | 86,137 | $ | 72,389 | $ | 58,941 | ||||||
|
|
|
|
|
| |||||||
Income (loss) before income taxes consists of the following:
| Year ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
United States | $ | 243,556 | $ | 189,702 | $ | 159,973 | ||||||
Foreign | (10,280 | ) | (5,162 | ) | (322 | ) | ||||||
|
|
|
|
|
| |||||||
| 233,276 | 184,540 | 159,651 | ||||||||||
|
|
|
|
|
| |||||||
The differences between income taxes at the statutory federal income tax rate and income taxes reported in the consolidated statements of comprehensive income were as follows:
| Year ended June 30, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
Federal income tax expense at the statutory rate | 35.0 | % | 35.0 | % | 35.0 | % | ||||||
State income taxes, net of federal benefit | 1.7 | 2.7 | 2.7 | |||||||||
Research and development credits, net of the federal tax on state credits | (1.0 | ) | (1.3 | ) | (0.9 | ) | ||||||
Uncertain tax positions, net of federal benefit on state positions | 0.2 | 0.2 | 0.3 | |||||||||
Incentive stock option and employee stock purchase plan expense | (0.5 | ) | 1.0 | 2.1 | ||||||||
Change in valuation allowance | 0.7 | 1.0 | (2.1 | ) | ||||||||
Other, net | 0.8 | 0.6 | (0.1 | ) | ||||||||
|
|
|
|
|
| |||||||
| 36.9 | % | 39.2 | % | 37.0 | % | |||||||
|
|
|
|
|
| |||||||
F-19
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
The significant components of the Company's deferred tax assets and liabilities were comprised of the following at June 30, 2013 and 2012:
| Year ended June 30, | ||||||||
| (In thousands) | 2013 | 2012 | ||||||
Net operating loss carryforwards | $ | 8,336 | $ | 7,781 | ||||
Property, plant and equipment | 3,103 | 2,781 | ||||||
Accrued vacation | 1,269 | 979 | ||||||
Allowance for doubtful accounts | 2,771 | 1,725 | ||||||
Stock compensation expense | 21,135 | 21,353 | ||||||
Capital loss carryover | 1,424 | 1,553 | ||||||
Research and development credits | 5,367 | 5,365 | ||||||
Uncertain state tax positions | 1,247 | 1,216 | ||||||
Other, net | 205 | 80 | ||||||
|
|
|
| |||||
Total gross deferred tax assets | 44,857 | 42,833 | ||||||
Less valuation allowance | (8,218 | ) | (6,613 | ) | ||||
|
|
|
| |||||
Net deferred tax assets | $ | 36,639 | $ | 36,220 | ||||
|
|
|
| |||||
Due to sustained positive operating performance and the availability of expected future taxable income, the Company concluded that it is more likely than not that the benefits of certain of its deferred income tax assets will be realized. For the years ended June 30, 2013 and 2012, the Company's valuation allowance increased by $1,605,000 and $1,851,000, respectively, primarily due to foreign net operating losses, for which the Company concluded it was more likely than not that the benefits of the losses will not be realized. For the year ended June 30, 2011, the Company's valuation allowance decreased by $35,024,000, which was primarily attributable to the realization of net operating losses from excess tax benefits.
For the years ended June 30, 2013 and 2012, the Company realized $7,888,000 and $34,193,000, respectively, of excess tax benefits from stock-based compensation as a reduction of taxes payable. Excess tax benefits from stock based compensation are credited directly to additional paid-in-capital. The Company has adopted the with-and-without tax allocation approach for excess tax benefits, which results in the windfall tax benefits being utilized last after considering all other tax attributes available to the Company.
For the year ended June 30, 2011, the Company realized $58,831,000 of excess tax benefits from stock-based compensation as a reduction of taxes payable. Of this amount, $31,653,000 resulted from excess tax benefits incurred prior to the adoption of FASB Statement 123(R) (as codified in ASC 718). The remaining $27,178,000 resulted from excess tax benefits incurred subsequent to the adoption of Statement 123(R).
F-20
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
The Company adopted Statement 123(R) on July 1, 2005. Prior to the adoption of Statement 123(R), the Company recorded deferred tax assets for net operating losses attributable to stock-based compensation excess tax benefits and a corresponding valuation allowance. According to guidance, the valuation allowance attributable to these excess tax benefits is not reversed until the excess tax benefits are realized as a reduction of taxes payable. During the year ended June 30, 2011, the Company realized a significant portion of the excess tax benefits attributable to the periods prior to the adoption of Statement 123(R) and reversed the corresponding valuation allowance. The following table presents a reconciliation of income tax expense to reflect the realization of the deferred tax asset related to these excess tax benefits and the corresponding change in valuation allowance:
| Years ended June 30, | ||||||||||||
| (in thousands) | 2013 | 2012 | 2011 | |||||||||
Total current tax expense | $ | 86,354 | $ | 72,139 | $ | 64,616 | ||||||
Deferred tax expense (benefit): | ||||||||||||
Deferred tax expense attributable to realization of stock-based compensation excess tax benefits due to utilization of net operating loss carryforward deferred tax assets-credited to additional paid-in capital | - | - | 31,653 | |||||||||
Other deferred tax expense | (1,822 | ) | (1,601 | ) | (2,304 | ) | ||||||
|
|
|
|
|
| |||||||
Net deferred tax expense (benefit) before change in valuation allowance | (1,822 | ) | (1,601 | ) | 29,349 | |||||||
|
|
|
|
|
| |||||||
Decrease in valuation allowance attributable to stock-based compensation tax benefits-credited to additional paid-in capital | - | - | (31,653 | ) | ||||||||
Change in valuation allowance attributable to income tax expense (benefit) | 1,605 | 1,851 | (3,371 | ) | ||||||||
|
|
|
|
|
| |||||||
Net increase (decrease) in valuation allowance | 1,605 | 1,851 | (35,024 | ) | ||||||||
|
|
|
|
|
| |||||||
Total deferred tax expense (benefit) | (217 | ) | 250 | (5,675 | ) | |||||||
|
|
|
|
|
| |||||||
Total income tax expense | $ | 86,137 | $ | 72,389 | $ | 58,941 | ||||||
|
|
|
|
|
| |||||||
On May 31, 2011, the Company acquired 100% of the stock of Rules-Based Medicine, Inc. ("RBM".) Certain of RBM's assets and liabilities have tax bases that differ from the recorded bases for book purposes and RBM had certain net operating loss and credit carryforwards at acquisition. A net deferred tax asset of $2,104,000 was recorded in the purchase accounting and included in the Company's total deferred tax asset balance.
At June 30, 2013, the Company had total federal and alternative minimum tax net operating loss carryforwards of approximately $3,753,000. This federal net operating loss carryforward results from the acquisition of RBM at May 31, 2011 and is subject to the limitations imposed by Section 382 of the Internal Revenue Code. If not utilized, this net operating loss carryforward expires beginning in 2030 through 2031. The Company has foreign net operating loss carryforwards in various countries totaling $16,331,000, which carryforwards expire at various dates. A valuation allowance has been established on all foreign net operating loss carryforwards. The Company had Utah net operating loss carryforwards of approximately $229,900,000. If not utilized, these operating loss carryforwards expire beginning in 2015 through 2025.
F-21
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
None of the Utah net operating loss carryforwards are subject to the limitations imposed by Section 382 of the Internal Revenue Code. The Company had approximately $8,257,000 of Utah research and development tax credit carryforwards, which can be carried forward to reduce Utah income taxes. Upon utilization to reduce Utah income tax, there will be a corresponding federal tax due resulting in a net benefit from Utah credits of $5,367,000. If not utilized, the Utah research and development tax credit carryforwards expire beginning in 2022 through 2028.
All of the Utah net operating loss carryforwards are ‘excess tax benefits' as defined by ASC guidance and, if realized in future years, will be recognized as a credit to additional paid-in capital. Approximately $92,557,000 of the Utah net operating loss ‘excess tax benefits' are attributable to periods prior to adoption of guidance limiting recognition of the deferred tax asset and are included in deferred tax assets (prior to any offset by valuation allowance.) The remaining $137,343,000 of Utah net operating loss ‘excess tax benefits' are not included in deferred tax assets and will be recognized only upon realization of the tax benefit.
The Company's deferred tax asset for the Utah net operating loss ‘excess tax benefits' attributable to periods prior to the adoption of the standard is approximately $3,008,000 and is offset by a full valuation allowance at June 30, 2013. If the ‘excess tax benefits' are recognized as additional paid-in-capital in future years, the corresponding valuation allowance will be reversed. At June 30, 2013, the Company has a valuation allowance of $1,424,000 offsetting its capital loss carryforward. The capital loss carryforward expires in the year ended June 30, 2014 and the Company does not expect to have capital gains to offset the loss prior to expiration of the carryforward period. At June 30, 2013, the Company also has a valuation allowance of $3,786,000 offsetting its foreign net operating loss carryforwards.
In July 2006, the FASB issued ASC Topic 740 Subtopic 10 Section 05, which clarifies the accounting for uncertainty in tax positions. ASC guidance requires that the impact of a tax position be recognized in the financial statements if that position is more likely than not of being sustained on audit, based on the technical merits of the position. The Company adopted the guidance on July 1, 2007 and recorded $0 cumulative effect. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Year ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Unrecognized tax benefits at the beginning of year | $ | 10,208 | $ | 9,648 | $ | 9,797 | ||||||
Gross increases-current year tax positions | $ | 710 | 560 | - | ||||||||
Gross increases-prior year tax positions | - | - | 848 | |||||||||
Decreases related to settlements | - | - | (997 | ) | ||||||||
|
|
|
|
|
| |||||||
Unrecognized tax benefits at end of year | $ | 10,918 | $ | 10,208 | $ | 9,648 | ||||||
|
|
|
|
|
| |||||||
Interest and penalties in year-end balance | $ | 270 | $ | - | $ | - | ||||||
|
|
|
|
|
| |||||||
Interest and penalties related to uncertain tax positions are included as a component of income tax expense.
The Company files U.S., foreign and state income tax returns in jurisdictions with various statutes of limitations. The 2009 through 2012 tax years remain subject to examination at June 30, 2013. The Company's New York State income tax returns for the years ended June 30, 2007, 2008 and 2009 are currently under examination by the New York State Department of Taxation and Finance. Annual tax provisions include amounts considered necessary to pay assessments that may result from examination of
F-22
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
prior year tax returns; however, the amount ultimately paid upon resolution of issues may differ materially from the amount accrued. The Company's U.S. federal tax return, U.K. income tax return and all other state tax returns are not currently under examination.
| (9) | Employee Deferred Savings Plan |
The Company has a deferred savings plan which qualifies under Section 401(k) of the Internal Revenue Code. Substantially all of the Company's U.S. employees are covered by the plan. The Company makes matching contributions of 50% of each employee's contribution with the employer's contribution not to exceed 4% of the employee's compensation. The Company's recorded contributions to the plan as follows:
| Years ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Deferred savings plan Company contributions | $ | 3,450 | $ | 2,955 | $ | 2,283 | ||||||
| (10) | Segment and Related Information |
The Company's business units have been aggregated into three reportable segments: (i) research, (ii) molecular diagnostics and (iii) companion diagnostics. The research segment is focused on the discovery of genes related to major common diseases and includes corporate services such as finance, human resources, legal, and information technology. The molecular diagnostics segment provides testing that is designed to assess an individual's risk for developing disease later in life, identify a patient's likelihood of responding to drug therapy and guide a patient's dosing to ensure optimal treatment, or assess a patient's risk of disease progression and disease recurrence. The companion diagnostics segment provides testing products and services to the pharmaceutical, biotechnology and medical research industries.
The accounting policies of the segments are the same as those described in the summary of significant accounting policies (Note 1). The Company evaluates segment performance based on income (loss) before interest income and other income and expense.
| (In thousands) | Research | Molecular diagnostics | Companion diagnostics | Total | ||||||||||||
Year ended June 30, 2013: | ||||||||||||||||
Revenues | $ | - | $ | 582,392 | $ | 30,773 | $ | 613,165 | ||||||||
Depreciation and amortization | 2,182 | 4,974 | 1,733 | 8,889 | ||||||||||||
Segment operating income (loss) | (56,428 | ) | 291,509 | (7,079 | ) | 228,002 | ||||||||||
Year ended June 30, 2012: | ||||||||||||||||
Revenues | $ | - | $ | 472,390 | $ | 23,615 | $ | 496,005 | ||||||||
Depreciation and amortization | 2,021 | 5,395 | 1,653 | 9,069 | ||||||||||||
Segment operating income (loss) | (49,231 | ) | 237,737 | (8,188 | ) | 180,318 | ||||||||||
Year ended June 30, 2011: | ||||||||||||||||
Revenues | $ | - | $ | 400,046 | $ | 2,038 | $ | 402,084 | ||||||||
Depreciation and amortization | 1,976 | 5,105 | 138 | 7,219 | ||||||||||||
Segment operating income (loss) | (48,651 | ) | 206,840 | (411 | ) | 157,778 | ||||||||||
F-23
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2013 | 2012 | 2011 | |||||||||
Total operating income for reportable segments | $ | 228,002 | $ | 180,318 | $ | 157,778 | ||||||
Unallocated amounts: | ||||||||||||
Interest income | 5,497 | 4,629 | 2,226 | |||||||||
Other | (223 | ) | (407 | ) | (353 | ) | ||||||
|
|
|
|
|
| |||||||
Income from operations before income taxes | 233,276 | 184,540 | 159,651 | |||||||||
|
|
|
|
|
| |||||||
Income tax provision | 86,137 | 72,389 | 58,941 | |||||||||
|
|
|
|
|
| |||||||
Net income | $ | 147,139 | $ | 112,151 | $ | 100,710 | ||||||
|
|
|
|
|
| |||||||
The following table sets forth a comparison of balance sheet assets by operating segment:
| June 30, | ||||||||
| (In thousands) | 2013 | 2012 | ||||||
Net equipment and leasehold improvements: | ||||||||
Research | $ | 8,590 | $ | 8,924 | ||||
Molecular diagnostics | 15,769 | 12,852 | ||||||
Companion diagnostics | 3,243 | 2,455 | ||||||
|
|
|
| |||||
Total | $ | 27,602 | $ | 24,231 | ||||
|
|
|
| |||||
Total Assets: | ||||||||
Research | $ | 82,517 | $ | 77,221 | ||||
Molecular diagnostics | 110,329 | 72,777 | ||||||
Companion diagnostics | 79,911 | 86,413 | ||||||
|
|
|
| |||||
Total | $ | 272,757 | $ | 236,411 | ||||
|
|
|
| |||||
The following table reconciles assets by operating segment to total assets:
| June 30, | ||||||||
| (In thousands) | 2013 | 2012 | ||||||
Total assets by segment | $ | 272,757 | $ | 236,411 | ||||
Cash, cash equivalents, and marketable investment securities (1) | 531,064 | 454,224 | ||||||
|
|
|
| |||||
Total | $ | 803,821 | $ | 690,635 | ||||
|
|
|
| |||||
| (1) | The Company manages cash, cash equivalents, and marketable investment securities at the consolidated level for all segments |
The majority of the Company's revenues were derived from the sale of molecular diagnostic tests in the United States. There were no customers that accounted for greater than 10% of revenue in the years ended June 30, 2013, 2012 and 2011.
Additionally, the majority of the Company's long-lived assets are located in the United States.
F-24
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
| (11) | Commitments and Contingencies |
The Company is subject to various claims and legal proceedings covering matters that arise in the ordinary course of its business activities. As of June 30, 2013, management of the Company believes any liability that may ultimately result from the resolution of these matters will not have a material adverse effect on the Company's consolidated financial position, operating results, or cash flows.
| (12) | Acquisitions |
Rules-Based Medicine
On May 31, 2011, the Company completed the acquisition of all of the outstanding capital stock of Rules-Based Medicine, Inc. ("RBM"), a life sciences company focused on the development and commercialization of companion diagnostic tests on novel biomarker patterns for therapeutic drugs on the market and in development. This acquisition is consistent with the Company's strategic value creation through utilization of new and proprietary technology to assess a person's risk of disease.
The Company is required to allocate the purchase price to tangible and identifiable intangible assets acquired and liabilities assumed based on their fair values at the acquisition date. The excess of the purchase price over those fair values is recorded as goodwill. Management estimated the fair values in accordance with the applicable accounting guidance for business combinations and utilized the services of third-party valuation consultants. Income-based and cost-based approaches were utilized in determining the value of the intangible assets.
The following table summarizes the Company's allocation of the purchase price:
| (In thousands) | Estimated Fair Value | |||
Cash and cash equivalents | $ | 1,974 | ||
Accounts receivable | 3,667 | |||
Inventory | 5,865 | |||
Prepaid expenses and other assets | 1,185 | |||
Property, equipment and leasehold improvements | 2,883 | |||
Deferred tax asset | 2,014 | |||
Intangible assets | 14,950 | |||
Goodwill | 56,850 | |||
|
| |||
Total assets acquired | 89,388 | |||
Accounts payable and other liabilities | 5,431 | |||
Deferred revenue | 1,767 | |||
|
| |||
Liabilities assumed | 7,198 | |||
|
| |||
Total net assets acquired | $ | 82,190 | ||
|
| |||
At the time of acquisition, amortizable intangible assets consisted of customer relationships and technology with an estimated useful life of approximately 10 and 8 years, respectively. Indefinite life assets of consisted of trademarks and in-process research and development assets. As part of the Company's annual impairment analysis of intangible assets it was determined that the trademarks had an estimated useful life of approximately 15 years. Accordingly these trademarks were reclassified from indefinite lived intangible assets to amortizable intangible assets as of June 30, 2013 (see Note 4).
F-25
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
The goodwill recorded by the Company as part of this acquisition is not deductible for tax purposes.
| (13) | Term Loan and Option Agreement |
On September 8, 2011, the Company issued a $25,000,000 term loan to Crescendo Bioscience, Inc. ("Crescendo") of South San Francisco, CA under a Loan and Security Agreement ("Loan Agreement") and also secured an exclusive three-year option to acquire the company pursuant to a definitive merger agreement (the "Option Agreement"). Crescendo develops molecular diagnostic tests for patients suffering from autoimmune disorders, including rheumatoid arthritis.
Term Loan
Under the Loan Agreement, the Company loaned Crescendo $25,000,000 for a term of six years, with the principal due upon maturity. Interest accrues at 6% per year and is due annually. During the fiscal quarter ended September 30, 2012, the Loan Agreement was amended to increase the stated interest rate from 6% to 7% per year. In the event Crescendo defaults on the loan, additional interest will accrue at 5% per year. The loan will mature on the earlier of (i) September 8, 2017 or (ii) the third anniversary following the date that the Company's option to acquire Crescendo under the Option Agreement expires or; otherwise terminates under the terms of the agreement. The option can be accelerated by Crescendo as a result of (a) Crescendo's delivery of an early termination notice due to the achievement of triggering events under the Option Agreement or (b) Crescendo's delivery of an initial public offering notice under the Option Agreement. Crescendo has the right to prepay the entire loan amount plus accrued interest at any time without incurring a penalty.
Option Agreement
Under the Option Agreement, the Company has an exclusive three-year option, exercisable in the Company's sole discretion, to cause the closing of the merger if Crescendo attains a minimum revenue milestone during the three-year option term. If Crescendo attains the minimum revenue milestone, the purchase price to acquire Crescendo will be based on a predetermined multiple of revenue based on Crescendo's growth rate at the time the option is exercised. If Crescendo does not attain the minimum revenue milestone during the three-year option term, the Company will have a one-time right to exercise the option at the end of the option term and acquire Crescendo at a fixed purchase price. In either case, the purchase price would be all cash and would be subject to adjustment for Crescendo's cash, debt and other items at closing. If the Company exercises its option to purchase Crescendo, all amounts due under the term loan will be offset against the purchase price paid in the acquisition. The Option Agreement has received the requisite corporate approvals of both parties, including approval from Crescendo's stockholders.
Because the option to purchase Crescendo is contingently exercisable by the Company under the Option Agreement, and repayment of the term loan will be accelerated if the option is exercised, the Company has recorded the Option Agreement at fair value as of September 8, 2011 in other assets on the consolidated balance sheet. The fair value of the Option Agreement of $8,000,000 was determined utilizing valuation models, including the market and income based approaches, which utilize various inputs including projected income, volatility, risk free rates and projected terms. Under the applicable accounting guidance the Company has an initial policy decision to either re-value the Option Agreement each reporting period or carry the Option Agreement at the original recorded amount and periodically assess the Option Agreement for impairment. The Company has elected to periodically evaluate the Option Agreement for impairment. No impairment indicators were noted at June 30, 2013.
F-26
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2013, 2012 and 2011
The residual $17,000,000 value of the term loan has been classified as a note receivable on the consolidated balance sheets as of June 30, 2013 and 2012. The Company recorded interest income related to accretion of the note receivable and the stated interest rates for the years ended June 30, 2013 and 2012 of $4,411,000 and $3,250,000 in the consolidated comprehensive income statement. The Company is also utilizing the effective interest method to accrete the discount portion of the note receivable through interest income over the three-year term of the Company's option to acquire Crescendo under the Option Agreement. The note receivable is evaluated for collectability each reporting period based on qualitative and quantitative factors. If the Company determines that the note receivable and any accrued interest is not collectible, such amount will be written off in the period that such a determination is made. No amounts related to the note receivable or accrued interest were written off during the fiscal years ended June 30, 2013 and 2012.
| (14) | Cost Basis Investment |
In April 2013, the Company acquired approximately 28 million shares of Series E preferred stock of RainDance Technologies, Inc. ("RainDance") of Lexington, Massachusetts, for $5,000,000. RainDance provides high-throughput picodroplet-based technology that can encapsulate a single molecule, cell or reaction and be digitally analyzed and sorted one at a time. The Series E shares purchased by the Company represent less than 5% of the total shares outstanding of RainDance's capital stock. Subsequent to the investment the Company evaluated its relationship with RainDance and determined it did not have significant influence over the operations of RainDance. The Company's investment in RainDance has been recorded under the cost method as an "Other Asset" on the Company's consolidated balance sheet. The Company will periodically evaluate the investment for impairment. No impairment indicators were noted at June 30, 2013.
F-27
Schedule II
MYRIAD GENETICS, INC.
Schedule of Valuation and Qualifying Accounts
Years Ended June 30, 2013, 2012, and 2011
(In thousands)
| Balance at Beginning of Period | Addition Charged to Cost and Expenses | Deductions (1) | Balance at End of Period | |||||||||||||
Allowance for doubtful accounts: | ||||||||||||||||
Year ended June 30, 2013 | $ | 4,600 | $ | 33,294 | ($ | 30,394 | ) | $ | 7,500 | |||||||
Year ended June 30, 2012 | $ | 3,700 | $ | 24,742 | ($ | 23,842 | ) | $ | 4,600 | |||||||
Year ended June 30, 2011 | $ | 4,400 | $ | 16,183 | ($ | 16,883 | ) | $ | 3,700 | |||||||
| (1) | Represents amounts written off against the allowance. |
See report of independent registered public accounting firm.
EXHIBIT INDEX
Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
2.1 | Agreement and Plan of Merger dated as of April 27, 2011, by and among the Company, Myriad RBM, Inc., Rules-Based Medicine, Inc. and Mark Chandler, Ph.D.** | 8-K (Exhibit 2.1) | 05/03/11 | 000-26642 | ||||||||
3.1 | Restated Certificate of Incorporation, as amended | 10-K (Exhibit 3.1) | 08/15/11 | 000-26642 | ||||||||
3.2 | Restated By-Laws | 8-K (Exhibit 3.1) | 02/28/11 | 000-26642 | ||||||||
4.1 | Specimen common stock certificate | 10-K (Exhibit 4.1) | 08/15/11 | 000-26642 | ||||||||
| Lease Agreements | ||||||||||||
10.1 | Lease Agreement, dated October 12, 1995, between the Registrant and Boyer Research Park Associates V, by its general partner, the Boyer Company | 10-Q (Exhibit 10.2) | 11/08/96 | 000-26642 | ||||||||
10.2 | Amendment to Lease Agreement, dated March 29, 1996 between the Registrant and Boyer Research Park Associates V, by its general partner, the Boyer Company | 10-Q (Exhibit 10.3) | 11/08/96 | 000-26642 | ||||||||
10.3 | Lease Agreement-Research Park Building Phase II, dated March 6, 1998, between the Registrant and Research Park Associated VI, by its general partner, the Boyer Company, L.C. | 10-K (Exhibit 10.44) | 09/24/98 | 000-26642 | ||||||||
10.4 | Memorandum of Lease, dated August 24, 1998, between the Registrant and Boyer Foothill Associates, Ltd. | 10-Q (Exhibit 10.1) | 11/12/98 | 000-26642 | ||||||||
10.5 | Memorandum of Lease, dated August 24, 1998, between the Registrant and Boyer Research Park Associates VI, L.C. | 10-Q (Exhibit 10.2) | 11/12/98 | 000-26642 | ||||||||
10.6 | Subordination Agreement and Estoppel, Attornment and Non-Disturbance Agreement (Lease to Deed of Trust), dated June 24, 1998, between the Registrant and Wells Fargo Bank, National Association | 10-Q (Exhibit 10.3) | 11/12/98 | 000-26642 | ||||||||
10.7 | Lease Agreement, dated March 31, 2001, between the Registrant and Boyer Research Park Associates VI, by its general partner, The Boyer Company, L.C. | 10-Q (Exhibit 10.1) | 05/15/01 | 000-26642 | ||||||||
Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
10.8 | Agreement, dated March 31, 2001, between the Registrant and Boyer Research Park Associates VI, by its general partner, The Boyer Company, L.C. | 10-Q (Exhibit 10.2) | 05/15/01 | 000-26642 | ||||||||
10.9 | Lease Agreement, dated June 29, 2005, between the Registrant and Boyer Research Park Associates VIII, by its general partner, The Boyer Company, L.C. | 8-K (Exhibit 99.1) | 07/05/05 | 000-26642 | ||||||||
10.10 | Letter of Understanding regarding Lease Agreement, dated June 29, 2005, between the Registrant and Boyer Research Park Associates VIII, by its general partner, The Boyer Company, L.C. | 8-K (Exhibit 99.2) | 07/05/05 | 000-26642 | ||||||||
10.11 | .1 | Lease Agreement, dated March 11, 2008, between the Registrant and Boyer Research Park Associates IX, by its general partner, The Boyer Company, L.C. | 10-K (Exhibit 10.32) | 08/28/08 | 000-26642 | |||||||
| .2 | Amendment to Lease Agreement, dated February 12, 2010 between the Registrant and Boyer Research Park Associates IX, L.C.. | 10-Q (Exhibit 10.4) | 05/05/10 | 000-26642 | ||||||||
10.12 | .1 | Sublease Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | 8-K (Exhibit 10.2) | 07/07/09 | 000-26642 | |||||||
| .2 | Amendment No. 1, dated November 11, 2009, to Sublease Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | 10-K (Exhibit 10.12.2) | 08/12/10 | 000-26642 | ||||||||
| .3 | Amendment No. 2, dated February 19, 2010, to Sublease Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | 10-K (Exhibit 10.12.3) | 08/12/10 | 000-26642 | ||||||||
| Agreements with Respect to Collaborations, Licenses, Research and Development | ||||||||||||
10.13 | Exclusive License Agreement, dated October 8, 1991, between the Registrant and the University of Utah Research Foundation, as amended (Breast Cancer-BRCA1)* | S-1 (Exhibit 10.13) | 10/05/95 | 33-95970 | ||||||||
10.14 | Exclusive License Agreement, dated November 23, 1994, between the Registrant and the University of Utah Research Foundation (Breast Cancer-BRCA2)* | S-1 (Exhibit 10.17) | 10/05/95 | 33-95970 | ||||||||
Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
10.15 | Exclusive License Agreement, dated March 15, 1995, between the Registrant and the Hospital for Sick Children* | 10-Q (Exhibit 10.1) | 11/01/07 | 000-26642 | ||||||||
10.16 | Exclusive License Agreement, dated January 6, 1995, between the Registrant and Endorecherche* | 10-Q (Exhibit 10.2) | 11/01/07 | 000-26642 | ||||||||
10.17 | Exclusive License Agreement, dated March 13, 1996, between the Registrant and The Trustees of the University of Pennsylvania* | 10-Q (Exhibit 10.3) | 11/01/07 | 000-26642 | ||||||||
| Agreements with Executive Officers and Directors | ||||||||||||
10.18 | Employment Agreement, dated May 15, 1993, between the Registrant, Myriad Genetic Laboratories, Inc. and Peter D. Meldrum+ | S-1 (Exhibit 10.3) | 10/05/95 | 33-95970 | ||||||||
10.19 | Employment Agreement between Myriad Genetics, Inc., Myriad Genetic Laboratories, Inc. and James S. Evans dated March 3, 1995+ | 8-K (Exhibit 10.1) | 11/06/07 | 000-26642 | ||||||||
10.20 | Employment Agreement, dated November 5, 2002, between the Registrant, Myriad Genetic Laboratories, Inc. and Richard M. Marsh+ | 10-K (Exhibit 10.27) | 08/25/09 | 000-26642 | ||||||||
10.21 | Employment Agreement, dated October 1, 2002, between the Registrant, Myriad Genetic Laboratories, Inc. and Mark. C. Capone+ | 10-K (Exhibit 10.28) | 08/25/09 | 000-26642 | ||||||||
10.22 | Employment Agreement, dated September 2, 2002, between the Registrant, Myriad Genetic Laboratories, Inc. and Jerry S. Lanchbury, Ph.D.+ | 10-K (Exhibit 10.22) | 08/15/2011 | 000-26642 | ||||||||
10.23 | .1 | Form of Executive Retention Agreement+@ | 10-Q (Exhibit 10.1) | 05/05/10 | 000-26642 | |||||||
| .2 | Form of Amendment to Form of Executive Retention Agreement+@ | 10-Q (Exhibit 10.2) | 05/05/10 | 000-26642 | ||||||||
10.24 | Executive Retention Agreement, dated November 17, 2006, between the Registrant and Mark. C. Capone+ | 10-Q (Exhibit 10.1) | 02/06/07 | 000-26642 | ||||||||
10.25 | Non-Employee Director Compensation Policy+ | 10-Q (Exhibit 10.1) | 11/02/11 | 000-26642 | ||||||||
10.26 | Form of director and executive officer indemnification agreement | 10-K (Exhibit 10.34) | 08/25/09 | 000-26642 | ||||||||
Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
| Equity Compensation Plans | ||||||||||||
10.27 | .1 | 2002 Amended and Restated Employee, Director and Consultant Stock Option Plan (the "2002 Plan")+ | 10-K (Exhibit 10.1) | 09/27/02 | 000-26642 | |||||||
| .2 | Form of Incentive Stock Option Agreement under the 2002 Plan+ | 10-Q (Exhibit 10.9) | 11/01/07 | 000-26642 | ||||||||
| .3 | Form of Non-Qualified Stock Option Agreement under the 2002 Plan+ | 10-Q (Exhibit 10.10) | 11/01/07 | 000-26642 | ||||||||
10.28 | .1 | 2003 Employee, Director and Consultant Stock Option Plan, as amended (the "2003 Plan")+ | 10-Q (Exhibit 10.1) | 02/3/10 | 000-26642 | |||||||
| .2 | Form of Incentive Stock Option Agreement under the 2003 Plan+ | 10-Q (Exhibit 10.7) | 11/01/07 | 000-26642 | ||||||||
| .3 | Form of Non-Qualified Stock Option Agreement under the 2003 Plan+ | 10-Q (Exhibit 10.8) | 11/01/07 | 000-26642 | ||||||||
10.29 | .1 | Myriad Genetics, Inc. 2010 Employee, Director and Consultant Equity Incentive Plan, as amended (the "2010 Plan")+ | 8-K (Exhibit 10.1) | 12/07/12 | 000-26642 | |||||||
| .2 | Form of Stock Option Agreement under the 2010 Equity Incentive Plan+ | 10-Q (Exhibit 10.3) | 02/01/11 | 000-26642 | ||||||||
| .3 | Form of Director Stock Option Agreement under the 2010 Equity Incentive Plan+ | 10-Q (Exhibit 10.4) | 02/01/11 | 000-26642 | ||||||||
10.30 | 2012 Employee Stock Purchase Plan+ | 8-K (Exhibit 10.2) | 12/07/12 | 000-26642 | ||||||||
10.31 | 2013 Executive Incentive Plan+ | 8-K (Exhibit 10.3) | 12/07/12 | 000-26642 | ||||||||
| Other | ||||||||||||
21.1 | List of Subsidiaries of the Registrant | X | ||||||||||
23.1 | Consent of Independent Registered Public Accounting Firm (Ernst & Young LLP) | X | ||||||||||
31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
32 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
101 | The following materials from Myriad Genetics, Inc.'s Annual Report on Form 10-K for the fiscal year ended June 30, 2013, formatted in XBRL (Xtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Comprehensive Income, (iii) Consolidated Statements of Stockholders' Equity, (iv) Consolidated Statements of Cash Flows, and (v) Notes to Consolidated Financial Statements. | X | ||||||||||
| (+) | Management contract or compensatory plan arrangement. |
| (@) | The agreements with all executives are identical except for the executive who is a party to the agreement and the date of execution, which are listed at the end of the exhibit |
| (*) | Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions. |
| (**) | The schedules and exhibits to the Agreement and Plan of Merger have been omitted from this filing pursuant to Item 601(b)(2) of Regulation S-K. The Company will furnish supplemental copies of any such schedules or exhibits to the U.S. Securities and Exchange Commission upon request. |