UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2010
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 0-26642
MYRIAD GENETICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 87-0494517 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
| 320 Wakara Way, Salt Lake City, UT | 84108 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant's telephone number, including area code: (801) 584-3600
Securities registered pursuant to Section 12(b) of the Exchange Act:
Title of each class | Name of each exchange on which registered | |
Common Stock, $.01 Par Value Per Share | The NASDAQ Stock Market LLC | |
Preferred Share Purchase Rights |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a "smaller reporting company". See the definitions of "accelerated filer", "large accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer x | Accelerated filer ¨ | |
| Non-accelerated filer ¨ (do not check if a smaller reporting company) | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold on December 31, 2009, the last business day of the registrant's most recently completed second fiscal quarter, was $2,504,021,854.
As of August 9, 2010 the registrant had 94,074,946 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the Registrant's Proxy Statement for the Annual Meeting of Stockholders to be held on December 3, 2010.
PART I
| Item 1. | BUSINESS |
Overview
Myriad Genetics, Inc. is a leading molecular diagnostic company focused on developing and marketing novel predictive medicine, personalized medicine and prognostic medicine products. We believe that the future of medicine lies in a shift from a treatment paradigm to a prevention paradigm. By understanding the genetic basis of disease, we believe that individuals who have a greater risk of developing disease can be identified and physicians can use this information to improve patient outcomes and better manage patient healthcare. We employ a number of proprietary technologies that help us to understand the genetic basis of human disease and the role that genes and their related proteins may play in the onset and progression of disease. We use this information to guide the development of new molecular diagnostic products that are designed to assess an individual's risk for developing disease later in life (predictive medicine), identify a patient's likelihood of responding to drug therapy and guide a patient's dosing to ensure optimal treatment (personalized medicine), or assess a patient's risk of disease progression and disease recurrence (prognostic medicine).
Our goal is to provide physicians with this critical information that may guide the healthcare management of their patients to prevent disease, delay the onset of disease, or diagnose the disease at an earlier stage when it is more treatable. We are also committed to assisting the physician in managing their patient's healthcare to ensure that they receive the most appropriate therapy based on the patient's individual genetic makeup and the specific cause of their disease.
To date we have launched eight commercial molecular diagnostic products, including four predictive medicine, three personalized medicine and one prognostic medicine product. We market these products through our own 300-person sales force in the United States and we have entered into marketing collaborations with other organizations in selected foreign countries. Revenue was $362.6 million for the year ended June 30, 2010, an increase of 11% over the prior fiscal year. BRACAnalysis, which provides a comprehensive analysis of the BRCA1 and BRCA2 genes for assessing a woman's risk of developing hereditary breast and ovarian cancer accounts for most of our revenue.
During the fiscal year ended June 30, 2010, we devoted our resources to supporting our predictive medicine, personalized medicine and prognostic medicine products, as well as to the research and development of future molecular diagnostic candidates. For the year ended June 30, 2010, we had net income of $152.3 million. As of June 30, 2010, we had an accumulated deficit of $10.5 million. For the years ended June 30, 2010, 2009 and 2008, we had research and development expense from continuing operations of $21.9 million, $17.9 million and $18.5 million, respectively.
Spin-off of Our Research and Drug Development Businesses
On June 30, 2009, we transferred our research and drug development businesses along with $188.0 million of cash and marketable securities into our then wholly-owned subsidiary, Myriad Pharmaceuticals, Inc. ("MPI"). All outstanding shares of MPI were then distributed to our stockholders as a pro-rata, tax-free dividend on June 30, 2009 by issuing one share of MPI common stock for every four shares of our common stock to stockholders of record on June 17, 2009. The separation resulted in MPI operating as an independent entity with its own publicly-traded stock. The results of operations for the former research and drug development activities conducted by us and by MPI until June 30, 2009 are included as part of this report for the periods prior to that date as discontinued operations. We do not have any ownership in MPI subsequent to the separation. MPI has recently changed its name to Myrexis, Inc. and is traded on the NASDAQ Global Market under the ticker symbol "MYRX".
2
Our Business Strategy
Our business strategy is to understand the relationship between genes and human diseases in order to develop the next generation of molecular diagnostic products. Through our proprietary technologies, we believe we are positioned to identify important disease genes, the proteins they produce, and the biological pathways in which they are involved to better understand the underlying molecular basis for the cause of human disease. We believe that identifying these genes, proteins, and pathways will enable us to develop novel molecular diagnostic products. Our business strategy includes the following key elements:
| • | Discover important disease genes, understand their function and determine their role in human disease. We will continue to use our proprietary technologies, including our bioinformatics and robotic technologies, in an effort to efficiently discover important genes and proteins and to understand their role in human disease. We believe our technologies provide us with a significant competitive advantage and numerous product opportunities. |
| • | Acquire promising biomarkers/genes from other organizations. We intend to continue to take advantage of in-licensing or acquisition opportunities to augment our in-house product development programs. We recognize that we cannot meet all of our research discovery goals internally and can benefit from the research performed by other organizations. We hope to leverage our financial strength, product development expertise, and sales and marketing presence to acquire new product opportunities in molecular diagnostic areas of focus. |
| • | Grow our molecular diagnostic business in the United States. We will continue to seek to increase the market penetration of our existing molecular diagnostic products in the U.S. Additionally, we will pursue new product opportunities in the areas of predictive, personalized, and prognostic medicine to capitalize on our leadership position. We believe that molecular diagnostics will play an increasingly important role in the future of healthcare in the U.S. |
| • | Expand our molecular diagnostic business internationally. While we have collaborations in several foreign countries, we intend to establish our own commercial operations in Europe in the future. We believe the market for our molecular diagnostic products in the major market countries in Europe represents an attractive commercial opportunity. We believe that personalized medicine and prognostic medicine products in particular would benefit the national insurers of the major countries in the European Union. We are exploring a variety of strategic alternatives to accomplish this expansion including partnering, acquisitions, and building operations in Europe independently. Once we have established a presence in Europe, we will look to expand to other important international markets. |
Molecular Diagnostic Products
Our molecular diagnostic products are designed to analyze genes and their mutations to assess an individual's risk for developing disease later in life, determine a patient's likelihood of responding to a particular drug, assess a patient's risk of disease progression and disease recurrence, and measure a patient's exposure to drug therapy to ensure optimal dosing and reduced drug toxicity. Armed with this valuable information, physicians can more effectively manage their patients healthcare to prevent or delay the onset of disease and ensure that patients receive the most appropriate treatment of their disease.
To date, we have launched eight commercial molecular diagnostic products and are developing and intend to launch in the first half of the 2011 fiscal year our ninth molecular diagnostic product for the genetic pre-disposition to pancreatic cancer. We market these products through our own 300-person sales force in the United States and we have entered into marketing collaborations with other organizations in selected foreign countries. Our current commercial molecular diagnostic products are:
| • | BRACAnalysis ® : predictive medicine product for hereditary breast and ovarian cancer. BRACAnalysis is a comprehensive analysis of the BRCA1 and BRCA2 genes for assessing a woman's risk of developing hereditary breast and ovarian cancer. A woman who tests positive for a deleterious |
3
mutation with the BRACAnalysis test has an 82% risk of developing breast cancer and a 44% risk of developing ovarian cancer during her lifetime. As published in the Journal of the National Cancer Institute , researchers have shown that pre-symptomatic individuals who have a high risk of developing breast cancer can reduce their risk by approximately 50% with appropriate preventive therapies. Additionally, as published in the New England Journal of Medicine , researchers have shown that pre-symptomatic individuals who carry gene mutations can lower their risk of developing ovarian cancer by approximately 60% with appropriate preventive therapies. |
According to the American Cancer Society, in 2010 there will be approximately 229,000 women in the United States diagnosed with breast cancer or ovarian cancer and an estimated 68,000 women will die from these cancers. The test is currently priced at $3,340 and is covered by all major health maintenance organizations and health insurance providers in the United States. We own or have licensed rights to 23 U.S. patents covering BRACAnalysis.
| • | COLARIS ® : predictive medicine product for hereditary colorectal cancer and uterine cancer. COLARIS is a comprehensive analysis of the MLH1, MSH2, and MSH6 genes for assessing a person's risk of developing colorectal cancer or uterine cancer. Individuals who carry a deleterious mutation in one of the colon cancer genes in the COLARIS test have a greater than 80% lifetime risk of developing colon cancer and women have up to a 71% lifetime chance of developing uterine cancer. Highly effective preventive measures for colon cancer include colonoscopy and the removal of precancerous polyps. Through proper application of screening and polyp removal, colon cancer is a preventable disease. |
According to the American Cancer Society, approximately 186,000 new cases of colorectal or uterine cancer will be diagnosed this year and approximately 59,000 Americans will die of the disease. According to the American Society of Clinical Oncologists, familial forms of colorectal cancer are estimated to account for 10% to 30% of all cases. The test is currently priced at $3,150 and is covered by all major health maintenance organizations and health insurance providers in the United States. We own or have licensed rights to eight U.S. patents covering COLARIS.
| • | COLARIS AP ® : predictive medicine product for hereditary colorectal cancer. COLARIS AP detects mutations in the APC and MYH genes, which cause a colon polyp-forming syndrome known as Familial Adenomatous Polyposis (FAP), a more common variation of the syndrome known as attenuated FAP, and the MYH-associated polyposis signature (MAP). Individuals who carry a deleterious mutation in the APC or MYH gene may have a greater than 90% lifetime risk of developing colon cancer. Effective preventive measures include colonoscopy and the removal of pre-cancerous polyps and prophylactic surgery. |
COLARIS AP is currently priced at $2,050 and is covered by all major health maintenance organizations and health insurance providers in the United States. We own or have licensed rights to nine U.S. patents covering COLARIS AP.
| • | MELARIS ® : predictive medicine product for hereditary melanoma. MELARIS analyzes mutations in the p16 gene to determine genetic susceptibility to malignant melanoma, a deadly form of skin cancer. Individuals who test positive for a deleterious mutation in the p16 gene for MELARIS have a 75-fold increased risk of developing melanoma during their lifetimes as compared to the general population. Melanoma can be prevented through appropriate screening and a specific threshold of action for mutation carriers, in which pre-cancerous lesions are removed before cancer can develop. |
According to the American Cancer Society, approximately 68,000 new cases of melanoma will be diagnosed in the United States in 2010. Melanoma is lethal within five years in 86% of cases where it has spread to another site in the body. However, when melanoma is diagnosed at an early stage, fewer than 10% of patients die within five years. MELARIS is currently priced at $900 and is covered by most major health maintenance organizations and health insurance providers in the United States. We own or have licensed rights to five U.S. patents covering MELARIS.
4
| • | THERAGUIDE ® 5-FU: personalized medicine product for drug toxicity. THERAGUIDE 5-FU analyzes mutations in the DPYD gene and variations in the TYMS gene to assess patient risk of 5-FU (flourouracil) toxicity. Cancer patients who test positive for a deleterious mutation in the DPYD gene and variations in the TYMS gene for THERAGUIDE 5-FU have an increased risk of suffering toxicity from 5-FU chemotherapy and should be considered for either a reduced dose of 5-FU or other chemotherapy regimens. 5-FU is widely prescribed for the treatment of colorectal cancer, metastatic breast cancer, skin cancer, and head and neck cancers and up to 20% of patients will experience medically significant toxicity issues (grade 3 or 4 toxicity). |
According to IMS prescription data, there are approximately 347,000 patients that receive 5-FU therapy each year in the United States. THERAGUIDE 5-FU is currently priced at $1,175 and is covered by many health maintenance organizations and health insurance providers in the United States. We own or have licensed rights to one U.S. patent application covering THERAGUIDE 5-FU.
| • | OnDose™: A personalized medicine product for colon cancer. OnDose is a nanoparticle immuno assay that assists oncologists in optimizing infusional 5-FU therapy on an individual basis. OnDose provides pharmacokinetic data to the oncologist to guide dose adjustments of 5-FU to ensure the potential cancer is being treated appropriately with reduced side effects and toxicity. As published in the Journal of Clinical Oncology, a prospective clinical study in 208 colon cancer patients demonstrated an increase in median overall survival of 6 months and a reduction in grade 3 or 4 toxic events of 40% in those patients who were dosed using the OnDose technology compared to patients dosed using current standard of care. |
According to IMS prescription data, there are 179,000 patients diagnosed with colorectal cancer that receive 5-FU chemotherapy each year. OnDose is currently priced at $300 per test, and multiple tests per patient are required to determine and maintain the optimum 5-FU dose for each patient, and is covered by many health maintenance organizations and health insurance providers in the United States. We own or have licensed patent rights to two U.S. patents covering OnDose.
| • | PREZEON™: A personalized and prognostic medicine product for cancer. PREZEON is an immunohistochemistry test that analyzes the PTEN gene and assesses loss of PTEN function in many cancer types. The PTEN gene is one of the most important tumor suppressor genes and its loss of function is associated with more aggressive disease progression and poorer survival. The PTEN gene plays a role in the disease progression of the four major cancers – breast, prostate, colon, and lung cancer. The PTEN gene also plays a critical role in cell signaling pathways that are the target of a number of cancer drugs such as EGFR inhibitors and mTOR inhibitors and PIK3CA inhibitor. Analysis of PTEN function can help oncologists in identifying patients who may not respond to these classes of cancer drugs. |
According to the American Cancer Society, approximately 790,000 new cases of these cancers will be diagnosed this year. PREZEON is currently priced at $500. We own or have licensed patent rights to four U.S. patent covering PREZEON.
| • | PROLARIS™: A prognostic medicine product for prostate cancer. PROLARIS is a 46-gene molecular diagnostic assay that quantitatively determines the risk of prostate cancer recurrence in patients who have undergone prostatectomy surgery and assists the physician in assessing whether a man's prostate cancer is aggressive or slow growing. PROLARIS is based on the understanding of cell division and tumor growth and provides rigorous, quantitative measures of the expression levels of multiple genes related to progression of the cell cycle. As presented at the 2010 Genitourinary Cancers Symposium, the test identifies patients at low risk of disease recurrence with 95% certainty giving these men confidence that additional aggressive treatment with the accompanying toxicity and adverse events is likely unwarranted. Conversely, men with high PROLARIS scores would be considered for more intensive screening and adjuvant therapy to address their more aggressive disease. |
According to the American Cancer Society, in the United States approximately 218,000 men are expected to be diagnosed with prostate cancer this year and 80,000 men will undergo a radical
5
prostatectomy, a surgical procedure that removes the prostate gland and some surrounding tissue. Approximately 35% of these men will eventually have a biochemical recurrence indicating the return of their prostate cancer. PROLARIS is currently priced at $3,400. We own or have licensed patent rights to two U.S. patent applications covering PROLARIS.
Patents and Proprietary Rights
We own or have exclusive license rights to 175 issued patents as well as numerous patent applications in the United States and foreign countries. These patents and patent applications cover a variety of subject matter including, diagnostic biomarkers such as genes, proteins, gene expression signatures, antibodies, disease-associated genetic mutations and single-nucleotide polymorphisms, diagnostic methods involving novel biomarkers, diagnostic kits and assays, general molecular diagnostic techniques, correlation claims, and methods for disease treatment.
The following is a summary of key U.S. patents covering our current molecular diagnostic products. Many of the issued U.S. patents relating to BRACAnalysis, Colaris, Colaris AP, Melaris and Prezeon also have related foreign issued patents in various countries, including in Europe, Canada, Japan, Australia and New Zealand, claiming similar subject matter and having similar expiration dates. For many of the patents, we hold rights through exclusive or non-exclusive license agreements, which are summarized in the following section under the caption "License Agreements." We also own additional patent applications and hold other non-exclusive license rights to patents which cover various aspects of our products or processes.
BRACAnalysis. We own or have exclusive license rights to 23 issued U.S. patents relating to BRACAnalysis. These U.S. patents have terms that are expected to expire commencing in 2014, with the last patent expected to expire in 2025. These patents contain multiple claims, including claims relating to BRCA2 compositions of matter on isolated BRCA nucleic acids, methods of detecting genetic mutations in the BRCA1 and BRCA2 genes and their use thereof for diagnosing predisposition to breast or ovarian cancer, and general molecular diagnostic technology applicable to BRACAnalysis. We are a defendant in a lawsuit brought by the Association for Molecular Pathology, et al . (the "Plaintiffs") on May 12, 2009 in the United States District Court for the Southern District of New York (the "Court") before Judge Robert W. Sweet. The Plaintiffs sought a declaratory ruling that 15 claims of seven patents relating to the BRCA1 and BRCA2 genes, which patents are exclusively licensed to us, are invalid and unenforceable. On April 19, 2010, Judge Sweet entered a Judgment in this lawsuit, ruling that these 15 claims are invalid. On June 16, 2010, Myriad filed a Notice of Appeal with the United States Court of Appeals for the Federal Circuit appealing the District Court decision. Apart from the 15 claims being challenged in this lawsuit, there are 164 separate claims under these seven patents which also cover the intellectual property utilized in, or related to, our BRACAnalysis predictive medicine product for breast and ovarian cancer which are not subject to this lawsuit. Additionally, there are 16 other issued U.S. patents which also cover the intellectual property utilized in, or related to, our BRACAnalysis predictive medicine product for breast and ovarian cancer which are not subject to this lawsuit. Accordingly, we do not believe that this lawsuit will have a material adverse impact on the Company.
COLARIS . We own or have non-exclusive license rights to eight issued U.S. patents relating to Colaris. These U.S. patents have terms that are expected to expire commencing in 2013, with the last patent expected to expire in 2023. These patents contain multiple claims, including but not limited to claims relating to MLH1 and MSH2 compositions of matter on isolated MLH1 and MSH2 nucleic acids, methods of detecting mutations in the MLH1 and MSH2 genes, MLH1 and MSH2-related predisposition to cancer, such as Lynch Syndrome cancers, and general molecular diagnostic technology applicable to Colaris.
COLARIS AP. We own or have exclusive license rights to nine issued U.S. patents relating to Colaris AP. These U.S. patents have terms that are expected to expire commencing in 2017, with the last patent expected to expire in 2026. These patents contain multiple claims, including claims relating to MYH compositions of matter on isolated MYH nucleic acids, methods of detecting MYH mutations and methods of detecting a predisposition to colorectal cancer using MYH , and general molecular diagnostic technology applicable to Colaris AP.
6
MELARIS. We own or have exclusive license rights to five issued U.S. patents relating to Melaris. These U.S. patents have terms that are expected to expire commencing in 2014, with the last patent expected to expire in 2023. These patents contain multiple claims, including claims relating to methods of detecting mutations in the p16 gene and their use for diagnosing predisposition to melanoma, and general molecular diagnostic technology applicable to Melaris.
PREZEON . We have exclusive license rights to four issued U.S. patents relating to Prezeon. These U.S. patents have terms that are expected to expire commencing in 2017, with the last patent expected to expire in 2018. These patents contain multiple claims, including but not limited to claims relating to PTEN compositions of matter on isolated PTEN nucleic acids and antibodies, methods of detecting PTEN expression and PTEN mutations, and methods of detecting cancer or a predisposition to cancer using PTEN.
OnDose. We have exclusive license rights to two issued U.S. patents relating to OnDose. The U.S. patents have terms that are expected to expire commencing in 2025, with the last patent expected to expire in 2026, and contain multiple claims, including but not limited to claims relating to methods, antibodies, and kits for performing immunoassays to measure 5-fluorouracil levels in a sample.
THERAGUIDE 5-FU. We own one U.S. patent and one U.S. patent application relating to TheraGuide 5-FU. The patent will expire in 2023. Subject to applicable extensions, we anticipate that the expiration date of the U.S.patent application, if issued, will commence in 2027. The patent and application disclose varied subject matter, including but not limited to subject matter relating to compositions of matter on DPYD nucleic acids containing specific mutations, diagnostic methods relating to DPYD mutations, and general molecular diagnostic technology applicable to TheraGuide 5-FU.
PROLARIS. We own or have exclusive license rights to two U.S. provisional patent applications and one international patent application relating to Prolaris. Subject to applicable extensions, we anticipate that the expiration dates of these patent applications, if issued, will commence in 2030. These patent applications disclose varied subject matter, including but not limited to composition of matter claims on gene expression signatures and methods of determining risk of cancer recurrence based on gene expression signatures.
We intend to seek patent protection in the United States and major foreign jurisdictions for genes, proteins, antibodies, diagnostic markers, technologies, methods, processes and other inventions which we believe are patentable and where we believe our interests would be best served by seeking patent protection. However, any patents issued to us or our licensors may not afford meaningful protection for our products or technology or may be subsequently circumvented, invalidated or narrowed or found unenforceable. Any patent applications which we have filed or will file or to which we have licensed or will license rights may not issue, and patents that do issue may not contain commercially valuable claims. In addition, others may obtain patents having claims which cover aspects of our products or processes which are necessary for or useful to the development, use or performance of our diagnostic products. Should any other group obtain patent protection with respect to our discoveries, our commercialization of our molecular diagnostic products could be limited or prohibited.
Our products and processes may also conflict with patents which have been or may be granted to competitors, academic institutions or others. As the molecular diagnostic industries expand and more patents are issued, the risk increases that our products and processes may give rise to interferences filed by others in the U.S. Patent and Trademark Office or foreign patent offices, or to claims of patent infringement by other companies, institutions or individuals. In addition, third parties could bring legal actions against us seeking to invalidate our owned or licensed patents, claiming damages, or seeking to enjoin clinical testing, developing and marketing of our products or processes. If any of these actions are successful, in addition to any potential liability for damages, we could lose patent coverage for our products, be required to cease the infringing activity or obtain a license in order to continue to develop or market the relevant product or process. We may not prevail in any such action, and any license required under any such patent may not be made available on acceptable terms, if at all. Our
7
failure to maintain patent protection for our products and processes or to obtain a license to any technology that we may require to commercialize our products and technologies could have a material adverse effect on our business.
We also rely upon unpatented proprietary technology, and in the future may determine in some cases that our interests would be better served by reliance on trade secrets or confidentiality agreements rather than patents or licenses. These include some of our genomic, proteomic, RNA expression, mutation analysis, IHC, robotic and bioinformatic technologies which may be used in discovering and characterizing new genes and proteins and ultimately used in the development or analysis of molecular diagnostic products. We also maintain a database of gene mutations and their status as either harmful or benign for all of our predictive medicine products. To further protect our trade secrets and other proprietary information, we require that our employees and consultants enter into confidentiality and invention assignment agreements. However, those confidentiality and invention assignment agreements may not provide us with adequate protection. We may not be able to protect our rights to such unpatented proprietary technology and others may independently develop substantially equivalent technologies. If we are unable to obtain strong proprietary rights to our processes or products, competitors may be able to market competing processes and products.
License Agreements
We are a party to multiple license agreements which give us the rights to use certain technologies in the research, development, testing processes, and commercialization of our molecular diagnostic products. We may not be able to continue to license these technologies on commercially reasonable terms, if at all. Additionally, patents underlying our license agreements may not afford meaningful protection for our technology or products or may be subsequently circumvented, invalidated or narrowed, or found unenforceable. Our failure to maintain rights to this technology could have a material adverse effect on our business.
In October 1991, we entered into a license agreement with the University of Utah Research Foundation (the "University"), for the exclusive rights to utilize certain intellectual property rights of the University, including issued patents that relate to the BRCA1 gene, on a world-wide basis. Under this license agreement we pay the University a royalty based on net sales of our BRACAnalysis molecular diagnostic products. This license agreement ends on the last to expire patent covered by the license agreement which presently is not anticipated to expire until April 2018. The Licensors have the right to terminate the license agreement for the uncured breach of any material term of the license agreement.
We entered into separate license agreements with the University, Endorecherche, Inc., The Hospital for Sick Children and The Trustees of the University of Pennsylvania (collectively referred to as the "BRAC Licensors") in November 1994, January 1995, March 1995 and march 1996, respectively, for exclusive rights to utilize certain intellectual property rights of the respective BRAC Licensors, including issued patents that relate to the BRCA2 gene, on a world-wide basis. Under these license agreements we pay each of the BRAC Licensors a royalty based on net sales of our BRACAnalysis molecular diagnostic product. Each of these license agreements ends on the expiration date of the last to expire patent covered by the respective license agreements which presently is not anticipated to expire until December 2015. The BRAC Licensors have the right to terminate the license agreements for the uncured breach of any material term of the license agreements.
In April, 2000, we entered into a license agreement with Dana-Farber Cancer Institute, Inc., Oregon Health Sciences University, University of Vermont and State Agricultural College and Yale University (collectively the "Colaris Licensors") for the non-exclusive rights to utilize certain intellectual property rights of the Colaris Licensors, including issued patents that relate to the MLH1, MLH2 and PMS1 genes, on a world-wide basis. Under this license agreement we pay the Colaris Licensors a royalty based on net sales of our Colaris molecular diagnostic product. This license agreement ends on the expiration date of the last to expire patent covered by the license agreement, which presently is not anticipated to expire until October 2023. The Colaris Licensors have the right to terminate the license agreement for the uncured breach of any material term of the license agreement.
8
In April, 2000, we entered into a license agreement with Genzyme Corporation ("Genzyme") for the non-exclusive rights to utilize certain intellectual property rights of Genzyme, including issued patents that relate to the MSH2 gene, on a world-wide basis. Under this license agreement we pay Genzyme a royalty based on net sales of our Colaris molecular diagnostic product. This license agreement ends, on a country by country basis, on the expiration date of the last to expire patent covered by the license agreement, which presently is not anticipated to expire until October 2023. Either party has the right to terminate the license agreement for the uncured breach of any material term of the license agreement.
In March 2004 and June 2007, we entered into separate license agreements with the University of Wales and Human Genome Sciences , Inc. ("HGSI") respectively (collectively referred to as the "Colaris AP Licensors") for the exclusive rights to certain intellectual property rights of the respective licensors, including issued patents that relate to the MYH gene, on a world-wide basis. Under these license agreements we pay each of the Colaris AP Licensors a royalty based on net sales of our COLARIS AP molecular diagnostic product. Each of these license agreements ends on the expiration date of the last to expire patent covered by the respective license agreements which presently is not anticipated to expire until February of 2018 for the HGSI license and April 2023 for the University of Wales license. The licensors have the right to terminate the license agreements for the uncured breach of any material term of the license agreements.
In May 2002, we entered into a license agreement with the University of Utah Research Foundation for the exclusive right to utilize certain intellectual property rights of the University, including issued patents that relate to the APC gene, on a world-wide basis. Under this license agreement we made a one-time payment to the University for a fully paid up, exclusive, irrevocable license for our COLARIS AP molecular diagnostic product. This license agreement ends on the expiration date of the last to expire patent covered by the license agreement, which presently is not anticipated to expire until July 2014.
Competition
Competition is intense in our existing and potential markets. Our competitors in the United States and abroad are numerous and include, other molecular diagnostic companies, diagnostic reference laboratories, large multi-national healthcare companies, and universities and other research institutions. Many of our potential competitors have considerably greater financial, technical, marketing and other resources than we do. We expect competition to intensify in our current fields as technical advances occur and become more widely known.
The technologies for discovering genes that cause major diseases, are involved in disease progression, or are themselves the targets of pharmaceuticals as well as the approaches for commercializing those discoveries are rapidly evolving. Rapid technological developments could result in our potential products or processes becoming obsolete before we recover a significant portion of our related research and development costs and associated capital expenditures. If we do not discover additional disease-causing genes, characterize their functions, develop molecular diagnostic products and related information services based on such discoveries, obtain regulatory and other approvals, and launch such services or products before our competitors, we could be adversely affected. Moreover, any molecular diagnostic products that we may develop could be made obsolete by less expensive or more effective tests or methods that may be developed in the future.
Governmental Regulation
CLIA and other laboratory licensure
Laboratories that perform testing on human specimens for the purpose of providing information for diagnosis, prevention or treatment of disease or assessment of health are subject to federal state and local regulation. The Clinical Laboratory Improvement Amendments of 1988, or CLIA, imposes quality standards for laboratory testing to ensure the accuracy, reliability and timeliness of reporting patient test results. The FDA is responsible for the categorization of commercially marketed in vitro diagnostic, or IVD, tests under CLIA into one of three categories based upon the potential risk to public health in reporting erroneous results. The
9
categories, which were devised on the basis of the complexity of the test, include waived tests, tests of moderate complexity, and tests of high complexity. Under CLIA, certified laboratories are required to hold a certificate applicable to the type of tests that they perform and to comply with standards covering personnel, facilities administration, quality systems and proficiency testing. CLIA-certified laboratories are typically subject to survey and inspection every two years to assess compliance with program standards. Our laboratory is certified by CLIA, CAP accrediated and all applicable states to perform high complexity tests. Our laboratory in Salt Lake City, Utah is CLIA certified to perform high complexity tests.
In addition to CLIA certification, laboratories offering clinical testing are required to hold other licenses, certifications and permits. A clinical laboratory is required to be licensed by the state in which it is located and many CLIA-certified laboratories also seek accreditation by the College of American Pathologists, or CAP. The CAP Laboratory Accreditation Program is an internationally recognized program that utilizes teams of practicing laboratory professionals as inspectors, and accreditation by CAP can often be used to meet CLIA and state certification requirements. In addition, some states, such as New York, require that a laboratory that intends to test clinical samples from residents of that state be licensed by that state even if the laboratory is not located there and that each test that is offered to residents of that state be approved.
Food and Drug Administration
Although the Food and Drug Administration (FDA) has consistently claimed that is has the regulatory authority to regulate laboratory-developed tests, or LDTs, that are validated by the developing laboratory and performed only by that laboratory, it has generally exercised enforcement discretion in not otherwise regulating most tests developed and performed by high complexity CLIA-certified laboratories. Recently, however, the FDA indicated that it was reviewing the regulatory requirements that will apply to LDTs, and held a two-day public meeting on July 19 and July 20, 2010 to obtain input from stakeholders on how it should apply its authority to implement a reasonable, risk-based, and effective regulatory framework for LDTs, including genetic tests. Although the FDA did not indicate when or how those changes would be implemented, it left little doubt that the changes are forthcoming.
HIPAA and other privacy laws
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, established for the first time comprehensive United States protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations, or "Covered Entities": health plans, healthcare clearing houses, and healthcare providers which conduct certain healthcare transactions electronically. Covered Entities must have in place administrative, physical, and technical standards to guard against the misuse of individually identifiable health information. Specifically, Title II of HIPAA, the Administrative Simplification Act, contains four provisions that address the privacy of health data, the security of health data, the standardization of identifying numbers used in the healthcare system and the standardization of data content, codes and formats used in healthcare transactions. The privacy regulations protect medical records and other personal health information by limiting their use and release, giving patients the right to access their medical records and limiting most disclosures of health information to the minimum amount necessary to accomplish an intended purpose. The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures.
On February 17, 2009, Congress enacted Subtitle D of the Health Information Technology for Economic and Clinical Health Act ("HITECH") provisions of the American Recovery and Reinvestment Act of 2009. HITECH amends HIPAA and, among other things, expands and strengthens HIPAA enforcement, imposes new penalties for noncompliance and establishes new breach notification requirements for Covered Entities and Business Associates.
Under HITECT's new breach notification requirements, Covered Entities must, within 60 days of discovery, notify each individual whose information has been, or is reasonably believed to have been, accessed, acquired, or
10
disclosed as a result of a breach. Covered Entities must also report breaches to the Department of Health and Human Services ("HHS"), and in some cases, publish information about the breach in local or prominent media outlets. Consequently, we must ensure that breaches of PHI are promptly detected and reported within the company, so that we can make all required notifications.
We are currently subject to the HIPAA regulations and maintain an active program designed to address regulatory compliance issues. We are subject to prosecution or administrative enforcement and increased civil and criminal penalties for non-compliance, including a new, four-tiered system of monetary penalties. We are also subject to enforcement by state attorneys general who were given authority to enforce HIPAA under HITECH.
In addition to the federal privacy regulations, there are a number of state laws regarding the confidentiality of health information that are applicable to clinical laboratories. The penalties for violation of state privacy laws may vary widely and new privacy laws in this area are pending. We believe that we have taken the steps required of us to comply with health information privacy and confidentiality statutes and regulations in all jurisdictions, both state and federal. However, we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal laws regarding privacy, could result in civil and/or criminal penalties and could have a material adverse effect on our business.
Other laws
Our business is also subject to regulation under state and federal laws regarding environmental protection and hazardous substances control, such as the Occupational Safety and Health Act, the Environmental Protection Act, and the Toxic Substance Control Act. We believe that we are in material compliance with these and other applicable laws and that the costs of our ongoing compliance will not have a material adverse effect on our business. However, statutes or regulations applicable to our business may be adopted which impose substantial additional costs to assure compliance or otherwise materially adversely affect our operations.
Reimbursement
In the United States, revenue for diagnostic tests comes from several sources, including third-party payors such as insurance companies, health maintenance organizations and government healthcare programs, such as Medicare and Medicaid. To date, most third-party payors have agreed to pay for our marketed tests. It is time consuming and expensive for us to obtain reimbursement from third-party payors and even if a third-party payor decides to offer any test as a covered benefit, the amount that it is willing to pay for that test may be insufficient to allow us to sell our test on a competitive and profitable basis.
Available Information
We are a Delaware corporation with our principal executive offices located at 320 Wakara Way, Salt Lake City, Utah 84108. Our telephone number is (801) 584-3600 and our web site address is www.myriad.com. We make available free of charge through the Investor Relations section of our web site our Corporate Code of Conduct and Ethics, our Audit Committee and other committee charters and our other corporate governance policies, as well as our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission. We include our web site address in this Annual Report on Form 10-K only as an inactive textual reference and do not intend it to be an active link to our web site.
Human Resources
As of August 4, 2010, we had 870 full-time equivalent employees, including 31 persons holding doctoral or medical doctor degrees. Most of our employees are engaged directly in research, development, production, sales and marketing activities. We believe that the success of our business will depend, in part, on our ability to attract and retain qualified personnel. Our employees are not covered by a collective bargaining agreement, and we consider our relations with our employees to be good.
11
| Item 1A. | RISK FACTORS |
Risks Related to Our Business and Our Strategy
We may not be able to generate sufficient revenue from our existing products or develop new products to maintain profitability and may never achieve the goals of our business plan.
Although we have developed and marketed several molecular diagnostic products to date, we believe our future success is dependent upon our ability to successfully market our existing molecular diagnostic products to new patients and to develop and commercialize new molecular diagnostic products. The demand for our existing molecular diagnostic products may decrease or may not continue to increase at historical rates for a number of reasons. For example, we believe that revenue for our fiscal 2010 was impacted by the economic recession, which is driving higher unemployment levels and as a consequence there was a resulting loss of insurance coverage and patients delaying or cancelling doctor visits. In addition, because BRACAnalysis and most of our molecular diagnostic products are only utilized once per patient, we will need to sell our services through physicians to new patients or develop new molecular diagnostic products in order to continue to generate revenue. Our pipeline of new molecular diagnostic candidates is in various stages of development and may take several more years to develop and must undergo extensive clinical validation. We may be unable to discover or develop any additional molecular diagnostic products through the utilization of our technologies or technologies we license from others. Even if we develop products for commercial use, we may not be able to develop products that:
| • | meet applicable regulatory standards, in a timely manner or at all; |
| • | successfully compete with other technologies and products; |
| • | avoid infringing the proprietary rights of others; |
| • | are adequately reimbursed by third-party payors; |
| • | can be performed at commercial levels or at reasonable cost; or |
| • | can be successfully marketed. |
We must generate significant revenue to maintain profitability. Even if we succeed in marketing our existing molecular diagnostic products to new patients and in developing and commercializing any additional molecular diagnostic products, we may not be able to generate sufficient revenue and we may not be able to maintain profitability.
We have a history of operating losses.
Until our fiscal year ended June 30, 2008, we had experienced net losses since our inception in 1992. We had net income of $152.3 million and an accumulated deficit of $10.5 million as of June 30, 2010. In order to develop and commercialize our molecular diagnostic product candidates, we expect to incur significant expenses over the next several years as we increase our research and development activities, expand clinical validation trials for our molecular diagnostic product candidates currently in development and engage in commercialization activities in anticipation of the launch of our product candidates. Because of the numerous risks and uncertainties associated with developing our product candidates and their potential for commercialization, we are unable to predict the extent of any future profits. Additionally, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to sustain or increase profitability, the market value of our common stock will likely decline. Our ability to maintain profitability will depend upon numerous factors, including:
| • | our ability to sell our existing molecular diagnostic products to new patients; |
| • | our ability to identify biomarkers that may lead to future molecular diagnostic products; |
| • | our ability to develop product candidates and receive required regulatory approvals; |
| • | our ability to successfully commercialize our products; |
12
| • | the approval and introduction of competitive products; |
| • | the willingness of third-party payors to provide full or even partial reimbursement for our products; |
| • | our ability to maintain and grow our sales force and marketing team to market our products; |
| • | our ability to increase commercial acceptance of our current molecular diagnostic products; and |
| • | our ability to maintain or grow our current product revenues. |
If our current operating plan changes and we find that our existing capital resources will not meet our needs, we may find it necessary to raise additional funding, which may not be available.
We anticipate that our existing capital resources and expected net cash to be generated from sales of our molecular diagnostic products will enable us to maintain our currently planned operations for at least the next two years. However, we base this expectation on our current operating plan, which may change. We have incurred, and will continue to incur, significant costs in the discovery, development and marketing of current and prospective molecular diagnostic products. Our ongoing efforts to develop products will require substantial cash resources. If, for example, a new disease gene is discovered through our research efforts, we would require funds in addition to our current operating plan to demonstrate clinical utility and develop and launch a new molecular diagnostic product. If, due to changes in our current operating plan, adequate funds are not available, we may be required to raise additional funds. Sources of potential additional capital resources may include, but are not limited to, public or private equity financings, establishing a credit facility, or selling convertible debt securities. This additional funding, if necessary, may not be available to us on reasonable terms, or at all.
Because of our potential long-term capital requirements, we may access the public or private equity or debt markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. Under SEC rules, we currently qualify as a well-known seasoned issuer, or WKSI, and can at any time file a registration statement registering securities to be sold to the public which would become effective upon filing. If additional funds are raised by issuing equity securities, existing shareholders may suffer significant dilution. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or products, or grant licenses on terms that are not favorable to us.
If we do not continue to generate sufficient revenue from sales of our molecular diagnostic products and are unable to secure additional funding, we may have to reduce or discontinue operations.
As of June 30, 2010, we had $488.4 million in cash, cash equivalents and marketable securities. For the fiscal year ended June 30, 2010 our revenues were approximately $362.6 million, and net cash from operating activities was approximately $155.1 million. To develop and bring new molecular diagnostic products to market, we must commit substantial resources to costly and time-consuming research, development testing and clinical testing. While we anticipate that our existing cash, cash equivalents and marketable securities and expected net cash to be generated from sales of our molecular diagnostic products will be sufficient to fund our current operations for at least the next two years, we may need or want to raise additional financing within this period of time. If we are unable to secure additional funding, we may be required to reduce research and development projects, limit sales and marketing activities, reduce headcount or discontinue operations. Our future capital requirements will depend on many factors that are currently unknown to us, including:
| • | our ability to maintain the existing licenses to our molecular diagnostic products and enter into collaborations, licensing or other arrangements favorable to us; |
| • | the scope, progress, results and cost of development, clinical testing and pre-market studies of any new molecular diagnostic products that we may discover or acquire; |
| • | the progress, results, and costs to develop additional molecular diagnostic products; |
13
| • | the costs by us or our licensors of preparing, filing and prosecuting patent applications, maintaining and enforcing our current issued patents, and defending intellectual property-related claims; |
| • | the costs of acquiring other molecular diagnostic companies; |
| • | the cost of international expansion efforts; |
| • | the costs of expanding our sales and marketing functions and commercial operation facilities; and |
| • | the costs to satisfy our current and future obligations. |
If we were successfully sued for product liability, we could face substantial liabilities that exceed our resources.
Our business exposes us to potential liability risks inherent in the testing, marketing and processing of molecular diagnostic products, including possible misdiagnoses. Although we are insured against such risks in amounts that we believe to be commercially reasonable, our present professional and product liability insurance may be inadequate. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on our business. Any successful product liability claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable or reasonable terms. An inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of our products.
Our business involves environmental risks that may result in liability for us.
In connection with our research and development activities, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens, chemicals and wastes. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Although we believe that our safety procedures for handling and disposing of controlled materials comply with the standards prescribed by state and federal regulations, accidental contamination or injury from these materials may occur. In the event of such an occurrence, we could be held liable for any damages that result and any such liability could exceed our resources.
Changes in healthcare policy could increase our costs, decrease our revenues and impact sales of and reimbursement for our tests.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the Healthcare Reform Act became law. This law substantially changes the way health care is financed by both governmental and private insurers, and significantly impacts our industry. The Healthcare Reform Act contains a number of provisions that are expected to impact our business and operations, some of which in ways we cannot currently predict, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse, which will impact existing government healthcare programs and will result in the development of new programs. Additional provisions of the Healthcare Reform Act, some of which become effective in 2011, may negatively affect our revenues.
In addition to the Healthcare Reform Act, there will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payors to reduce costs while expanding individual healthcare benefits. Certain of these changes could impose additional limitations on the prices we will be able to charge for our tests or the amounts of reimbursement available for our tests from governmental agencies or third-party payors. While in general it is too early to predict specifically what effect the Health Reform Act and its implementation or any future healthcare reform legislation or policies will have on our business, current and future healthcare reform legislation and policies could have a material adverse effect on our business and financial condition.
14
If we are unable to comply with applicable governmental regulations, we may not be able to continue our operations.
The establishment and operation of our laboratory and the production and marketing of services and products developed through our technologies, as well as our ongoing research and development activities, are subject to regulation by numerous federal, state and local governmental authorities in the United States. Our laboratory in Salt lake City, Utah is accredited by the Department of Health and Human Services under CLIA. CLIA is a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for diagnostic, preventative or treatment purposes. Laboratories are subject to survey and inspection every two years in order to maintain their CLIA certifications. Moreover, CLIA inspectors may make random inspections of these laboratories. If we were to lose our CLIA certification, whether as a result of a revocation, suspension or limitation, we would no longer be able to continue our molecular diagnostic testing operations which would have a material adverse effect on our business. We have also been accredited under the Clinical Laboratory Evaluation Program by the Department of Health of the State of New York. Where necessary or material to our operations, we hold licenses from other states to operate a clinical laboratory. Failure to maintain state regulatory compliance, or changes in state regulatory schemes, could result in a substantial curtailment or even prohibition of our molecular diagnostic testing operations and could have a material adverse effect on our business.
Furthermore, while the FDA has elected not to substantially regulate the activities or tests performed by laboratories like our clinical laboratory, the FDA has stated that it has the right to do so, and the FDA may seek to regulate or require clearance or approval of our molecular diagnostic or personalized medicine tests in the future. On July 19-20, 2010, the FDA's office of In-Vitro Diagnostics held a public meeting to discuss oversight of laboratory developed tests. The FDA highlighted the lack of standardized clinical validation at the assay level under current CLIA regulatory guidelines and noted that CLIA does not require post-market surveillance on monitoring of laboratory developed tests. While no decision has been made as to the overall frameworks, the FDA is cognizant of the issue associated with disrupting existing tests and creating duplicative surveillance and inspection of laboratories. The comment period for providing the FDA with written comments expires on August 15, 2010, and over the next several months the FDA intends to develop draft guidance on the oversight of laboratory developed tests and plans to solicit comments before finalizing it. We cannot provide any assurance, however, that FDA regulation, including pre-market review, will not be required in the future for our molecular diagnostic products. If pre-market review is required, our business could be negatively impacted if we are required to stop selling molecular diagnostic products pending their clearance or approval or new product commercialization could be delayed by new requirements.
Risks Related to Commercialization of Our Products and Product Candidates
We may not be able to maintain or increase revenue growth and profitability for our molecular diagnostic products.
We have experienced revenue growth in our molecular diagnostic business over past years; however, we may not be able to continue this revenue growth or maintain existing revenue levels. Presently, our molecular diagnostic business operates profitably providing a cash contribution to our current funding and operational needs. We may not, however, be able to continue to operate our molecular diagnostic business on a profitable basis. We launched our first molecular diagnostic product, BRACAnalysis, our product for hereditary breast and ovarian cancer, in November 1996. Sales of BRACAnalysis account for most of our revenues. An interruption or cessation of BRACAnalysis sample flow would have a material impact on our revenues and future profitability. Other potential events or factors that may have a significant impact on our ability to sustain revenue growth and profitability for our molecular diagnostic business include the following:
| • | increased costs of reagents and other consumables required for molecular diagnostic testing; |
| • | increased licensing or royalty costs; |
| • | increased personnel and facility costs; |
15
| • | our inability to hire competent, trained staff, including laboratory directors required to review and approve all reports we issue in our molecular diagnostic business, and sales personnel; |
| • | our inability to obtain necessary equipment or reagents to perform molecular diagnostic testing; |
| • | our inability to increase production capacity as demand increases; |
| • | changes in intellectual propriety laws of our patents or enforcement in the United States and foreign countries; |
| • | potential obsolescence of our products; |
| • | our inability to increase commercial acceptance of our molecular diagnostic products; and |
| • | increased regulatory requirements. |
If the government and third-party payors fail to provide coverage and adequate payment for our products and future products, if any, our revenue and prospects for profitability will be harmed.
In both domestic and foreign markets, sales of our molecular diagnostic products or any future diagnostic products will depend in part, upon the availability of reimbursement from third-party payors. Such third-party payors include government healthcare programs such as Medicare, managed care providers, private health insurers and other organizations. These third-party payors are increasingly attempting to contain healthcare costs by demanding price discounts or rebates and limiting both coverage on which diagnostic tests they will pay for and the amounts that they will pay for new molecular diagnostic products. The fact that a diagnostic product has been approved for reimbursement in the past, for any particular indication or in any particular jurisdiction, does not guarantee that such a diagnostic product will remain approved for reimbursement or that similar or additional diagnostic products will be approved in the future. As a result, third-party payors may not cover or provide adequate payment for our current or future molecular diagnostic products. We might need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of any future products to such payors' satisfaction. Such studies might require us to commit a significant amount of management time and financial and other resources. Our future products might not ultimately be considered cost-effective. Adequate third-party reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
U.S. and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. For example, in some foreign markets, the government controls the pricing of many healthcare products. In the United States, the way that healthcare is provided is under consideration by Congress and has been the subject of vigorous debate. We expect that there will continue to be federal and state proposals to implement governmental controls or imposed healthcare requirements. In addition, the Medicare program and increasing emphasis on managed care in the United States will continue to put pressure on product pricing. Cost control initiatives could decrease the price that we would receive for any products in the future, which would limit our revenue and profitability. The American Medical Association ("AMA") formed a Molecular Pathology Coding Workgroup ("MPCW") to make recommendations for modernizing the reimbursement codes for molecular diagnostic and genetic tests. On February 2010, the MPCW met to draft a proposal for reforming the current procedural terminology ("CPT") codes that determine fee schedules for molecular diagnostics tests, including our BRACAnalysis and other products. It is expected that the MPCW will make their recommendations to the AMA CPT Editorial Panel by the end of calendar 2010 and any change to the CPT codes would likely become effective in 2012. The new codes and fee schedule may not allow us to maintain price levels sufficient to realize an appropriate return on investment in product development and could limit the revenue and profitability of our existing products, which could seriously harm our business.
We rely on a single laboratory facility to process our molecular diagnostic tests.
We rely on a single CLIA-certified laboratory facility in Salt Lake City, Utah to perform our molecular diagnostic tests. This facility and certain pieces of laboratory equipment would be difficult to replace and may require significant replacement lead-time. If the laboratory was to lose its CLIA certification or was affected by
16
natural disasters such as earthquakes, floods and fires. In the event our clinical testing facility or equipment is affected by man-made or natural disasters, we would be unable to continue our molecular diagnostic business and meet customer demands for a significant period of time. Although we maintain insurance on this facility, including business interruption insurance, it may not be adequate to protect us from all potential losses if this facility were damaged or destroyed. In addition, any interruption in our molecular diagnostic business would result in a loss of goodwill, including damage to our reputation. If our molecular diagnostic business were interrupted, it would seriously harm our business.
Our current molecular diagnostic product candidates in development may never achieve significant commercial market acceptance.
We may not succeed in achieving significant commercial market acceptance of our product and service candidates. While we have marketed several of our molecular diagnostic products for several years and have gained some market acceptance we need to convince physicians and consumers of the benefits of our current molecular diagnostic products in order to increase our sales of those products. Our ability to successfully commercialize our current molecular diagnostic products, as well as any future molecular diagnostic products that we may develop, will depend on several factors, including:
| • | our ability to convince the medical community of the safety and clinical efficacy of our products and their potential advantages over existing products; |
| • | our ability to sell our molecular diagnostic products to patients who have not previously used our products; |
| • | the agreement by third-party payors to reimburse our products, the scope and extent of which will affect patients willingness or ability to pay for our products and will likely heavily influence physicians' decisions to recommend our products; and |
| • | the willingness of physicians and patients to utilize our products, which can be difficult to interpret. This difficulty is caused by a combination of factors, including the large number, sometimes thousands, of different mutations in the genes which our tests analyze, the need to characterize each specific mutation, and the ability of our products to predict only as to a statistical probability, not certainty, that a tested individual will develop the disease that the test is intended to predict. |
These factors present obstacles to commercial acceptance of our products, which we will have to spend substantial time and money to overcome, if we can do so at all. Our inability to successfully do so will harm our business.
If we do not compete effectively with scientific and commercial competitors, we may not be able to successfully commercialize our products.
The biotechnology and genetics testing fields are intense and highly competitive. Tests that are developed are characterized by rapid technological change. Our competitors in the United States and abroad are numerous and include, among others, major diagnostic companies, reference laboratories, molecular diagnostic firms, universities and other research institutions. Many of our potential competitors have considerably greater financial, technical, marketing and other resources than we do, which may allow these competitors to discover important genes and determine their function before we do. We could be adversely affected if we do not discover genes, proteins or biomarkers and characterize their function, develop molecular diagnostic products based on these discoveries, obtain required regulatory and other approvals and launch these products and their related services before our competitors. We also expect to encounter significant competition with respect to any molecular diagnostic products that we may develop or commercialize. Those companies that bring to market new molecular diagnostic products before we do may achieve a significant competitive advantage in marketing and commercializing their products. We may not be able to develop additional molecular diagnostic products successfully and we or our licensors may not obtain patents covering these products that provide protection against our competitors. Moreover, our competitors may succeed in developing molecular diagnostic products
17
that circumvent our technologies or products. Furthermore, our competitors may succeed in developing technologies or products that are more effective than those developed by us or that would render our technologies or products less competitive or obsolete. We expect competition to intensify in the fields in which we are involved as technical advances in these fields occur and become more widely known.
If our current research collaborators or scientific advisors terminate their relationships with us or develop relationships with a competitor, our ability to discover genes, proteins, and biomarkers, and to commercialize molecular diagnostic products could be adversely affected.
We have relationships with research collaborators at academic and other institutions who conduct research at our request. These research collaborators are not our employees. As a result, we have limited control over their activities and, except as otherwise required by our collaboration agreements, can expect only limited amounts of their time to be dedicated to our activities. Our ability to discover genes, proteins, and biomarkers involved in human disease and commercialize molecular diagnostic products will depend in part on the continuation of these collaborations. If any of these collaborations are terminated, we may not be able to enter into other acceptable collaborations. In addition, our existing collaborations may not be successful.
Our research collaborators and scientific advisors may have relationships with other commercial entities, some of which could compete with us. Our research collaborators and scientific advisors sign agreements which provide for the confidentiality of our proprietary information and the results of studies conducted at our request. We may not, however, be able to maintain the confidentiality of our technology and other confidential information related to all collaborations. The dissemination of our confidential information could have a material adverse effect on our business.
If we fail to retain our key personnel and hire, train and retain qualified employees and consultants, we may not be able to successfully continue our business.
Because of the specialized scientific nature of our business, we are highly dependent upon our ability to attract and retain qualified management, scientific and technical personnel. We are currently recruiting additional qualified management, scientific and technical personnel. Competition for such personnel is intense. Loss of the services of or failure to recruit additional key management, scientific and technical personnel would adversely affect our research and development programs and molecular diagnostic business and may have a material adverse effect on our business as a whole.
Our agreements with our employees generally provide for employment that can be terminated by either party without cause at any time, subject to specified notice requirements. Further, the non-competition provision to which each employee is subject expires for certain key employees on the applicable date of termination of employment.
As we expand our commercial products we may be required to incur significant costs and devote significant efforts to expand our existing products sales and marketing capabilities.
We have limited sales and marketing experience and capabilities. These capabilities consist primarily of our sales force that markets our cancer-related molecular diagnostic products to oncologists, Ob/Gyns and urologists in the United States. If in the future we elect to expand our sales and marketing functions for our products, or as we expand our business operations internationally, we would face a number of additional costs and risks, including the need to recruit a large number of additional experienced marketing and sales personnel.
We depend on a limited number of third parties for some of our supplies of equipment and reagents. If these supplies become unavailable, then we may not be able to successfully perform our research or operate our business at all or on a timely basis.
We currently rely on a small number of suppliers to provide our gene sequencing machines, robots, and specialty reagents required in connection with our research. We believe that currently there are limited alternative
18
suppliers of gene sequencing machines, robots, and reagents. The gene sequencing machines, robots, or the reagents may not remain available in commercial quantities at acceptable costs. If we are unable to obtain when needed additional gene sequencing machines, robots, or an adequate supply of reagents or other ingredients at commercially reasonable rates, our ability to continue to identify genes and perform molecular diagnostic testing would be adversely affected.
Risks Related to Our Intellectual Property
If we fail to comply with our obligations under license or technology agreements with third parties, we could lose license rights that are critical to our business.
We license intellectual property that is critical to our business, including licenses underlying the technology in our BRACAnalysis and other molecular diagnostic products, and in the future we may enter into additional agreements that provide us with licenses to valuable intellectual property or technology. These licenses impose various royalty payments, milestones, and other obligations on us. If we fail to comply with any of these obligations, the licensor may have the right to terminate the license. Termination by the licensor would cause us to lose valuable rights, and could prevent us from distributing our current products, or inhibit our ability to commercialize future product candidates. Our business would suffer if any current or future licenses terminate, if the licensors fail to abide by the terms of the license, if the licensors fail to prevent infringement by third parties, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms.
If we are not able to protect our proprietary technology, others could compete against us more directly, which would harm our business.
As of June 30, 2010, our patent portfolio included 175 issued patents owned or licensed by us and numerous patent applications in the United States and other countries with claims covering our intellectual property rights. Our commercial success will depend, in part, on our ability to obtain additional patents and licenses and protect our existing patent position, both in the United States and in other countries, for predisposing genes we identify and related technologies, processes, methods and other inventions that we believe are patentable. Our ability to preserve our trade secrets and other intellectual property is also critical to our long-term success. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to maintain profitability. Patents may also issue to third parties which could interfere with our ability to bring our molecular diagnostic products to market. The laws of some foreign countries do not protect our proprietary rights to the same extent as U.S. laws, and we may encounter significant problems in protecting our proprietary rights in these countries.
The patent positions of diagnostic companies, including our patent position, are generally highly uncertain and involve complex legal and factual questions, and, therefore, any patents issued to us may be challenged, deemed unenforceable, invalidated or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets. To date there has not emerged from the U.S. Patent and Trademark Office, or PTO, the U.S. courts, or from patent offices or courts in foreign countries, a consistent policy regarding the breadth of claims allowed in genetic patents. Our patent applications may never issue as patents, and the claims of any issued patents may not afford meaningful protection for our technology or products. In addition, any patents issued to us or our licensors may be challenged, and subsequently narrowed, invalidated or circumvented. Specifically, as disclosed in Part I Item 3, of this Annual Report on Form 10-K, we are a defendant in a lawsuit brought by the Association for Medical Pathology and others, or the Plaintiffs, in the United States District Court for the Southern District of New York before Judge Robert W. Sweet. The Plaintiffs brought a declaratory ruling that 15 claims of seven patents relating to the BRCA1 and BRCA2 genes, which patents are exclusively licensed to us, are invalid and unenforceable. These patents, along with 16 other issued patents which are not subject to the lawsuit, cover the intellectual property utilized in our BRACAnalysis predictive medicine product for breast and ovarian cancer,
19
which accounts for most of our revenues. On April 19, 2010, Judge Sweet entered a judgment in this lawsuit, ruling that these 15 claims are invalid. On June 16, 2010, we filed a Notice of Appeal with the United States Court of Appeals for the Federal Circuit appealing the District Court ruling. If the Federal Circuit upholds Judge Sweets' decision, others may be able to commercialize genetic tests that are competitive with our BRACAnalysis product, and our business could be seriously harmed.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| • | we or our licensors were the first to make the inventions covered by each of our patent applications; |
| • | we or our licensors were the first to file patent applications for these inventions; |
| • | others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | any of our or our licensors' patent applications will result in issued patents; |
| • | any of our or our licensors' patents will be valid or enforceable; |
| • | any patents issued to us or our licensors and collaborators will provide a basis for commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
| • | we will develop additional proprietary technologies or products that are patentable; |
| • | the patents of others will not have an adverse effect on our business; or |
| • | our patents or patents that we license from others will survive legal challenges, such as the lawsuit challenging the patents covering our BRACAnalysis predictive medicine product described above, and remain valid and enforceable. |
If a third party files a patent application with claims to a gene, protein, or biomarker we have discovered, the PTO may declare an interference between competing patent applications. If an interference is declared, we may not prevail in the interference. If the other party prevails in the interference, we may be precluded from commercializing services or products based on the gene, protein, or biomarker or may be required to seek a license. A license may not be available to us on commercially acceptable terms, if at all.
We also rely upon unpatented proprietary technologies. Although we require employees, consultants and collaborators to sign confidentiality agreements, we may not be able to adequately protect our rights in such unpatented proprietary technologies, which could have a material adverse effect on our business. For example, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to our proprietary technologies or disclose our technologies to our competitors.
If we were sued for patent infringement by third parties, we might incur significant costs and delays in product introduction.
Our products may also conflict with patents that have been or may be granted to others. Our industry includes many organizations seeking to rapidly identify genes and proteins through the use of genomic, proteomic and other technologies. To the extent any patents are issued to those organizations on genes or proteins or uses for such genes and proteins, the risk increases that the sale of our molecular diagnostic products currently being marketed or under development, may give rise to claims of patent infringement. Others may have filed and in the future are likely to file patent applications covering genes or proteins that are similar or identical to our products. Any of these patent applications may have priority over our patent applications and these entities or persons could bring legal proceedings against us seeking damages or seeking to enjoin us from testing or marketing our products. Patent litigation is costly, and even if we prevail, the cost of such litigation could have a material adverse effect on us. If the other parties in any such actions are successful, in addition to any liability for damages, we could be required to cease the infringing activity or obtain a license. Any license required may not be available to us on commercially acceptable terms, if at all. Our failure to obtain a license to any technology
20
that we may require to commercialize our products could have a material adverse effect on our business. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights. If we become involved in this litigation, it could consume a substantial portion of our managerial and financial resources. In general, we are responsible for enforcing and defending our patents.
We may be unable to adequately prevent disclosure of trade secrets, proprietary databases, and other proprietary information.
We rely on trade secrets to protect our proprietary technologies and databases, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and others to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy if unauthorized disclosure of confidential information occurs. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive position.
We may be subject to claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is commonplace in our industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Our Common Stock
Our stock price is highly volatile, and our stock may lose all or a significant part of its value.
The market prices for securities of molecular diagnostic and other life science companies have been volatile. This volatility has significantly affected the market prices for these securities for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our common stock. The market price for our common stock has fluctuated significantly since public trading commenced in October 1995, and it is likely that the market price will continue to fluctuate in the future. In the two years ended June 30, 2010, our stock price has ranged from $14.91 per share to $47.08 per share, adjusted for the 2-for-1 stock split effected by a stock dividend paid on March 25, 2009 to stockholders of record on March 9, 2009. In addition, the stock market has experienced extreme price and volume fluctuations. Events or factors that may have a significant impact on our business and on the market price of our common stock include the following:
| • | termination of the licenses underlying our molecular diagnostic products; |
| • | delays or other problems with operating our laboratory facilities; |
| • | failure of any of our research and development programs; |
| • | changes in intellectual property laws of our patents or enforcement in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights involving us directly or otherwise affecting the industry as a whole; |
| • | introduction of technological innovations or new commercial products by us or our competitors; |
21
| • | missing or changing the financial guidance we provide; |
| • | changes in estimates or recommendations by securities analysts relating to our common stock or the securities of our competitors; |
| • | changes in the governmental regulatory approved process for our exiting and new products: |
| • | failure to meet estimates or recommendations by securities analysts that cover our common stock; |
| • | public concern over our approved products and any product candidates; |
| • | litigation; |
| • | future sales or anticipated sales of our common stock by us or our stockholders; |
| • | general market conditions; |
| • | changes in the structure of healthcare payment systems and changes in the governmental or private insurers reimbursement levels for our molecular diagnostic products; |
| • | failure to sustain revenue growth or margins in our molecular diagnostic business; |
| • | failure of any of our product candidates to achieve commercial success; |
| • | seasonal slowness in sales, particularly in the quarters ending September 30 and March 31, the effects of which may be difficult to understand during periods of growth; |
| • | economic, healthcare and diagnostic trends, disasters or crises and other external factors; and |
| • | period-to-period fluctuations in our financial results. |
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit regardless of the outcome. Such a lawsuit could also divert the time and attention of our management.
Anti-takeover provisions of Delaware law, provisions in our charter and bylaws and our stockholders' rights plan, or poison pill, could make a third-party acquisition of us difficult.
Because we are a Delaware corporation, the anti-takeover provisions of Delaware law could make it more difficult for a third party to acquire control of us, even if the change in control would be beneficial to stockholders. We are subject to the provisions of Section 203 of the General Corporation Law of Delaware, which prohibits us from engaging in certain business combinations, unless the business combination is approved in a prescribed manner. In addition, our restated certificate of incorporation and restated bylaws also contain certain provisions that may make a third-party acquisition of us difficult, including:
| • | a classified board of directors, with three classes of directors each serving a staggered three-year term; |
| • | the ability of the board of directors to issue preferred stock; |
| • | a 70% super-majority shareholder vote to amend our bylaws and certain provisions of our certificate of incorporation; and |
| • | the inability of our stockholders to call a special meeting or act by written consent. |
We also have implemented a stockholders' rights plan, also called a poison pill, which could make it uneconomical for a third party to acquire our company on a hostile basis. These provisions, as well as Section 203, may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over then current market price, and may limit the ability of our stockholders to approve transactions that they think may be in their best interests.
22
| Item 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| Item 2. | PROPERTIES |
Our headquarters and facilities are located in Salt Lake City, Utah. We currently lease a total of 220,000 square feet of building space dedicated to research and development, administration and laboratory space that has received federal certification under CLIA. Activity related to our molecular diagnostic business is performed at this location. We have also entered into an agreement to lease an additional 87,000 square feet adjacent to our existing facilities. We have signed an agreement with Myrexis, Inc, to sublease the additional 87,000 square foot building for an initial term of three years renewable at the subleasee election for an additional 12 years in 3-year increments. The leases on our existing facilities have terms of fifteen years, expiring from 2017 through 2025, and provide for renewal options for up to ten additional years.
We believe that our existing facilities and equipment are well maintained and in good working condition. We believe our current facilities and those planned or under construction will provide adequate capacity for at least the next two years. We continue to make investments in capital equipment as needed to meet the anticipated demand for our molecular diagnostic products.
| Item 3. | LEGAL PROCEEDINGS |
We are a defendant in a lawsuit brought by the Association for Molecular Pathology, et al . (the "Plaintiffs") on May 12, 2009 in the United States District Court for the Southern District of New York (the "Court") before Judge Robert W. Sweet. The Plaintiffs sought a declaratory ruling that 15 claims of seven patents relating to the BRCA1 and BRCA2 genes, which patents are exclusively licensed to us, are invalid and unenforceable, and enjoining us (and the other defendants) from taking any actions to enforce these claims of these patents. The 15 claims at issue in the lawsuit are part of the intellectual property relating to our BRACAnalysis predictive medicine product for breast and ovarian cancer. On April 19, 2010, Judge Sweet entered a judgment in this lawsuit ruling that these 15 claims at issue are invalid. On June 16, 2010, we filed a Notice to Appeal with the United States Court of Appeals for the Federal Circuit appealing the District Court decision. Apart from the 15 claims being challenged in this lawsuit, there are 164 separate claims under these seven patents which also cover the intellectual property utilized in, or related to, our BRACAnalysis predictive medicine product for breast and ovarian cancer which are not subject to this lawsuit. Additionally, there are 16 other issued U.S. patents which also cover the intellectual property utilized in, or related to, our BRACAnalysis predictive medicine product for breast and ovarian cancer which are not subject to this lawsuit. Accordingly, we do not believe that this lawsuit will have a material adverse impact on the Company.
We are not a party to any other legal proceedings that we believe will have a material impact on our financial position or results of operations.
| Item 4. | REMOVED AND RESERVED |
23
PART II
| Item 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on The NASDAQ Global Select Market under the symbol "MYGN." The following table sets forth the high and low sales prices for our common stock, as reported by The NASDAQ Global Select Market for the last two fiscal years, adjusted for the 2-for-1 stock split effected by a stock dividend paid on March 25, 2009 to stockholders of record on March 9, 2009:
| High | Low | |||||
Fiscal Year Ended June 30, 2010: | ||||||
Fourth Quarter | $ | 24.52 | $ | 14.91 | ||
Third Quarter | $ | 27.13 | $ | 20.62 | ||
Second Quarter | $ | 27.52 | $ | 22.38 | ||
First Quarter | $ | 31.95 | $ | 24.28 | ||
Fiscal Year Ended June 30, 2009: | ||||||
Fourth Quarter | $ | 46.57 | $ | 30.10 | ||
Third Quarter | $ | 47.08 | $ | 32.41 | ||
Second Quarter | $ | 36.23 | $ | 26.25 | ||
First Quarter | $ | 34.57 | $ | 22.42 | ||
Stockholders
As of August 4, 2010, there were approximately 114 stockholders of record of our common stock and, according to our estimates, approximately 22,999 beneficial owners of our common stock.
Dividends
We have not paid cash dividends to our stockholders since our inception. While we periodically evaluate returning cash to our shareholders, such as the payment of cash dividends, we currently intend to continue to reinvest the majority of our earnings in the business.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
On May 4, 2010, we announced a plan to repurchase up to $100,000,000 of company stock with the intention to complete the plan by December 31, 2010. All purchases of our securities during the quarter ended June 30, 2010 were made pursuant to an announced plan and in open market transactions. The details of the activity during the fourth quarter were as follows:
Period | (a) Total Number of Shares Purchased | (b) Average Price Paid per Share | (c) Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | (d) Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs | ||||||
May 1, 2010 to May 31, 2010 | 2,591,031 | $ | 18.30 | 2,591,031 | $ | 52,582,422 | ||||
June 1, 2010 to June 30, 2010 | 1,348,989 | $ | 17.77 | 1,348,989 | $ | 28,606,418 | ||||
Total | 3,940,020 | $ | 18.12 | 3,940,020 | ||||||
24
Stock Performance Graph
The graph set forth below compares the annual percentage change in our cumulative total stockholder return on our common stock, as adjusted for a two-for-one stock split effected on March 25, 2009, during a period commencing on June 30, 2005 and ending on June 30, 2010 (as measured by dividing (A) the difference between our share price at the end and the beginning of the measurement period; by (B) our share price at the beginning of the measurement period) with the cumulative total return of The NASDAQ Stock Market, Inc. and the NASDAQ Health Services Stock Index during such period. We have not paid any cash dividends on our common stock, and we do not include cash dividends in the representation of our performance.
The price of a share of common stock is based upon the closing price per share as quoted on The NASDAQ Global Select Market on the last trading day of the year shown. The graph lines merely connect year-end values and do not reflect fluctuations between those dates. The comparison assumes $100 was invested on June 30, 2005 in our common stock and in each of the foregoing indices. The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
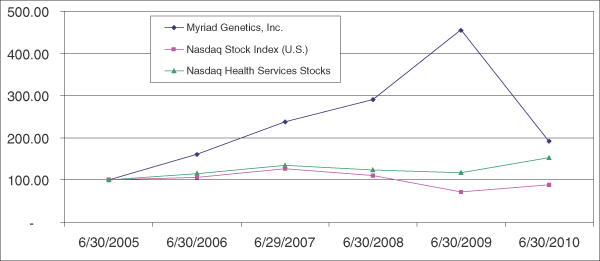
| 6/30/2005 | 6/30/2006 | 6/29/2007 | 6/30/2008 | 6/30/2009 | 6/30/2010 | |||||||
Myriad Genetics, Inc. | 100.00 | 161.34 | 237.64 | 290.86 | 455.59 | 191.05 | ||||||
NASDAQ Stock Index (U.S.) | 100.00 | 106.32 | 126.72 | 110.84 | 71.47 | 88.57 | ||||||
NASDAQ Health Services Stocks | 100.00 | 114.71 | 134.51 | 123.42 | 116.98 | 152.65 |
Note: Information used on the graph was obtained from the CRSP Total Return Indexes, a source believed to be a reliable, but we are not responsible for any errors or omission in such information.
The performance graph shall not be deemed to be incorporated by reference by means of any general statement incorporating by reference this Form 10-K into any filing under the Securities Act of 1933, as amended or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate such information by reference, and shall not otherwise be deemed filed under such acts.
25
| Item 6. | SELECTED CONSOLIDATED FINANCIAL DATA |
The following table sets forth our selected consolidated financial data and has been derived from our audited consolidated financial statements. Consolidated balance sheets as of June 30, 2010 and 2009, as well as consolidated statements of operations for the years ended June 30, 2010, 2009, and 2008 and the reports thereon are included elsewhere in this Annual Report on Form 10-K. The information below should be read in conjunction with our audited consolidated financial statements (and notes thereon) and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included in Item 7.
| Years Ended June 30, | ||||||||||||||||||||
| In thousands, except per share amounts | 2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||||
Consolidated Statement of Operations Data: | ||||||||||||||||||||
Revenue | $ | 362,648 | $ | 326,527 | $ | 222,855 | $ | 145,285 | $ | 100,621 | ||||||||||
Costs and expenses: | ||||||||||||||||||||
Costs of revenue | 44,286 | 43,267 | 32,340 | 30,813 | 27,644 | |||||||||||||||
Research and development expense | 21,873 | 17,914 | 18,482 | 11,639 | 12,619 | |||||||||||||||
Selling, general and administrative expense | 161,414 | 138,884 | 110,428 | 70,520 | 46,483 | |||||||||||||||
Total costs and expenses | 227,573 | 200,065 | 161,250 | 112,972 | 86,746 | |||||||||||||||
Operating income | 135,075 | 126,462 | 61,605 | 32,313 | 13,875 | |||||||||||||||
Other income (expense): | ||||||||||||||||||||
Interest income | 5,660 | 12,478 | 13,709 | 12,112 | 7,412 | |||||||||||||||
Other | 99 | (2,493 | ) | (320 | ) | 663 | (12 | ) | ||||||||||||
Income from continuing operations before income taxes | 140,834 | 136,447 | 74,994 | 45,088 | 21,275 | |||||||||||||||
Income tax provision ( benefit) | (11,469 | ) | 193 | 608 | - | - | ||||||||||||||
Income (loss) from continuing operations | 152,303 | 136,254 | 74,386 | 45,088 | 21,275 | |||||||||||||||
Loss from discontinued operations (1) | - | (51,639 | ) | (26,541 | ) | (80,050 | ) | (59,464 | ) | |||||||||||
Net income (loss) | $ | 152,303 | $ | 84,615 | $ | 47,845 | $ | (34,962 | ) | $ | (38,189 | ) | ||||||||
Earnings (loss) per basic share: | ||||||||||||||||||||
Continuing operations | $ | 1.58 | $ | 1.46 | $ | 0.84 | $ | 0.55 | $ | 0.33 | ||||||||||
Discontinued operations | - | (0.55 | ) | (0.30 | ) | (0.98 | ) | (0.86 | ) | |||||||||||
Earnings (loss) per basic share | $ | 1.58 | $ | 0.91 | $ | 0.54 | $ | (0.43 | ) | $ | (0.53 | ) | ||||||||
Earnings (loss) per diluted share: | ||||||||||||||||||||
Continuing operations | $ | 1.54 | $ | 1.38 | $ | 0.80 | $ | 0.52 | $ | 0.32 | ||||||||||
Discontinued operations | - | (0.52 | ) | (0.29 | ) | (0.92 | ) | (0.83 | ) | |||||||||||
Earnings (loss) per diluted share | $ | 1.54 | $ | 0.86 | $ | 0.51 | $ | (0.40 | ) | $ | (0.51 | ) | ||||||||
Weighted average shares outstanding | ||||||||||||||||||||
Basic | 96,338 | 93,492 | 88,378 | 82,110 | 72,556 | |||||||||||||||
Diluted | 99,152 | 98,573 | 93,408 | 86,399 | 75,425 | |||||||||||||||
| As of June 30, | ||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||||||||||
Cash, cash equivalents and marketable investment securities | $ | 488,382 | $ | 392,225 | $ | 420,056 | $ | 308,312 | $ | 227,744 | ||||||||||
Working capital | 446,510 | 333,951 | 303,616 | 217,357 | 225,465 | |||||||||||||||
Total assets | 593,847 | 466,421 | 499,342 | 375,540 | 276,603 | |||||||||||||||
Stockholders' equity | 557,581 | 434,219 | 425,655 | 340,363 | 249,781 | |||||||||||||||
26
Quarterly Financial Data (Unaudited)
| Quarters Ended | ||||||||||||||
| In thousands, except per share amounts | June 30, 2010 | March 31, 2010 | December 31, 2009 | September 30, 2009 | ||||||||||
Consolidated Statement of Operations Data: | ||||||||||||||
Revenue | $ | 93,929 | $ | 90,830 | $ | 92,768 | $ | 85,122 | ||||||
Costs and expenses: | ||||||||||||||
Costs of revenue | 11,262 | 10,880 | 11,083 | 11,062 | ||||||||||
Research and development expense | 5,254 | 5,885 | 5,059 | 5,676 | ||||||||||
Selling, general and administrative expense | 39,798 | 40,840 | 42,104 | 38,672 | ||||||||||
Total costs and expenses | 56,314 | 57,605 | 58,246 | 55,410 | ||||||||||
Operating income | 37,615 | 33,225 | 34,522 | 29,712 | ||||||||||
Other income (expense): | ||||||||||||||
Interest income | 984 | 1,232 | 1,531 | 1,913 | ||||||||||
Other | 5 | 23 | 286 | (215 | ) | |||||||||
Total other income | 989 | 1,255 | 1,817 | 1,698 | ||||||||||
Income from continuing operations before income taxes | 38,604 | 34,480 | 36,339 | 31,410 | ||||||||||
Income tax provision (benefit) | (14,646 | ) | 1,229 | 980 | 968 | |||||||||
Net income | $ | 53,250 | $ | 33,251 | $ | 35,359 | $ | 30,442 | ||||||
Earnings per basic share | $ | 0.55 | $ | 0.34 | $ | 0.37 | $ | 0.32 | ||||||
Earnings per diluted share | $ | 0.54 | $ | 0.33 | $ | 0.36 | $ | 0.31 | ||||||
Weighted average shares outstanding | ||||||||||||||
Basic | 96,269 | 96,853 | 96,270 | 95,970 | ||||||||||
Diluted | 98,259 | 99,674 | 99,426 | 99,492 | ||||||||||
27
| Quarters Ended | ||||||||||||||||
| In thousands, except per share amounts | June 30, 2009 | March 31, 2009 | December 31, 2008 | September 30, 2008 | ||||||||||||
Consolidated Statement of Operations Data: | ||||||||||||||||
Revenue | $ | 86,079 | $ | 86,531 | $ | 83,952 | $ | 69,965 | ||||||||
Costs and expenses: | ||||||||||||||||
Costs of revenue | 11,185 | 11,232 | 11,060 | 9,790 | ||||||||||||
Research and development expense | 4,381 | 4,543 | 4,615 | 4,375 | ||||||||||||
Selling, general and administrative expense | 36,017 | 35,496 | 34,960 | 32,411 | ||||||||||||
Total costs and expenses | 51,583 | 51,271 | 50,635 | 46,576 | ||||||||||||
Operating income | 34,496 | 35,260 | 33,317 | 23,389 | ||||||||||||
Other income (expense): | ||||||||||||||||
Interest income | 2,661 | 2,946 | 3,437 | 3,434 | ||||||||||||
Other | (455 | ) | (33 | ) | - | (2,005 | ) | |||||||||
Total other income | 2,206 | 2,913 | 3,437 | 1,429 | ||||||||||||
Income from continuing operations before income taxes | 36,702 | 38,173 | 36,754 | 24,818 | ||||||||||||
Income tax provision (benefit) | - | (94 | ) | - | 287 | |||||||||||
Net income from continuing operations | $ | 36,702 | $ | 38,267 | $ | 36,754 | $ | 24,531 | ||||||||
Loss from discontinued operations (1) | (13,062 | ) | (12,949 | ) | (15,551 | ) | (10,077 | ) | ||||||||
Net income | $ | 23,640 | $ | 25,318 | $ | 21,203 | $ | 14,454 | ||||||||
Earnings (loss) per basic share: | ||||||||||||||||
Continuing operations | $ | 0.38 | $ | 0.41 | $ | 0.40 | $ | 0.27 | ||||||||
Discontinued operations . | (0.13 | ) | (0.14 | ) | (0.17 | ) | (0.11 | ) | ||||||||
Earnings (loss) per basic share | $ | 0.25 | $ | 0.27 | $ | 0.23 | $ | 0.16 | ||||||||
Earnings (loss) per diluted share: | ||||||||||||||||
Continuing operations | $ | 0.37 | $ | 0.38 | $ | 0.38 | $ | 0.25 | ||||||||
Discontinued operations | (0.13 | ) | (0.13 | ) | (0.16 | ) | (0.10 | ) | ||||||||
Earnings (loss) per diluted share | $ | 0.24 | $ | 0.25 | $ | 0.22 | $ | 0.15 | ||||||||
Weighted average shares outstanding | ||||||||||||||||
Basic | 95,656 | 94,327 | 93,184 | 90,796 | ||||||||||||
Diluted | 100,192 | 99,594 | 97,716 | 96,618 | ||||||||||||
| (1) | The financial results associated with the research and drug development business operations conducted by us and by our former subsidiary Myriad Pharmaceuticals, Inc. prior to its spin-off effected on June 30, 2009 have been presented as discontinued operations in our Consolidated Statements of Operations. See Notes 1b, 14 and 15 to the Consolidated Financial Statements for further details. |
28
| Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
We are a leading molecular diagnostics company focused on the development and marketing of novel predictive medicine, personalized medicine and prognostic medicine products. We employ a number of proprietary technologies that help us to understand the genetic basis of human disease and the role that genes and their related proteins may play in the onset, progression and treatment of disease. We use this information to guide the development of new molecular diagnostic products that are designed to assess an individual's risk for developing disease later in life (predictive medicine), identify a patient's likelihood of responding to drug therapy and guide a patient's dosing to ensure optimal treatment (personalized medicine), or assess a patient's risk of disease progression and disease recurrence (prognostic medicine).
On June 30, 2009, we separated our main molecular diagnostic business from our research and drug development businesses by transferring our research and drug development businesses along with $188.0 million of cash and marketable securities into our then wholly-owned subsidiary, Myriad Pharmaceuticals, Inc. ("MPI"). All outstanding shares of MPI were then distributed to our stockholders as a pro-rata, tax-free dividend on June 30, 2009 by issuing one share of MPI common stock for every four shares of our common stock to stockholders of record on June 17, 2009. The separation resulted in MPI operating as an independent entity with its own publicly-traded stock. The results of operations for the former research and drug development activities conducted by us and by MPI until June 30, 2009 are included as part of this report for the periods prior to that date as discontinued operations. We do not have any ownership in MPI subsequent to the separation. MPI has recently changed its name to Myrexis, Inc.
During the fiscal year ended June 30, 2010, we devoted our resources to supporting our predictive medicine, personalized medicine and prognostic medicine products, as well as to the research and development of future molecular diagnostic candidates. See Note 9 "Segment and Related Information" in the notes to our consolidated financial statements for information regarding our operating segments. Our revenues consisted primarily of sales of molecular diagnostic products. During the year ended June 30, 2010, we reported income from continuing operations before income tax of $140.8 million and total net income of $152.3 million. As of June 30, 2010, we had an accumulated deficit of $10.5 million.
We incurred research and development expenses from continuing operations of $21.9 million, $17.9 million, and $18.5 million for the years ended June 30, 2010, 2009, and 2008, respectively. Our research and development expenses include costs incurred in the development, maintenance and improvement of our eight current molecular diagnostic product offerings BRACAnalysis, COLARIS, COLARIS AP, MELARIS, THERAGUIDE 5-FU, OnDose, PREZEON and PROLARIS and for costs incurred for the discovery, development and validation of other molecular diagnostic product candidates.
Our sales and marketing expenses and general and administrative expenses include costs associated with building our molecular diagnostic business. We expect that these costs will fluctuate from quarter to quarter and that such fluctuations may be substantial.
Critical Accounting Policies
Critical accounting policies are those policies which are both important to the portrayal of a company's financial condition and results and require management's most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies are as follows:
| • | revenue recognition; |
| • | allowance for doubtful accounts; |
29
| • | share-based payment expense; and |
| • | income taxes |
Revenue Recognition . Revenue includes the sale of molecular diagnostic products for our predictive, personalized and prognostic medicine products, and is recorded at the invoiced amount net of any discounts or allowances. Revenue is recognized upon completion of the test, communication of results, and when collectability is reasonably assured.
Allowance for Doubtful Accounts. The preparation of our financial statements in accordance with U.S. GAAP requires us to make estimates and assumptions that affect the reported amount of assets at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Trade accounts receivable are comprised of amounts due from sales of our molecular diagnostic products, which are recorded net of any discounts or contractual allowances. We analyze trade accounts receivable and consider historic experience, customer creditworthiness, facts and circumstances specific to outstanding balances, and payment terms when evaluating the adequacy of the allowance for doubtful accounts.
We periodically evaluate and adjust the allowance for doubtful accounts through a charge or credit to expense when trends or significant events indicate that a change in estimate is appropriate. Such changes in estimate could materially affect our results of operations or financial position; however, to date these changes have not been material. It is possible that we may need to adjust our estimates in future periods.
After a review of our allowance for doubtful accounts as of June 30, 2010 and 2009, we have determined that a hypothetical ten percent increase in our allowance for doubtful accounts would result in additional bad debt expense and an increase to our allowance for doubtful accounts of $440,000 and $385,000, respectively.
Share-Based Payment Expense. We recognize share-based equity compensation in our consolidated statements of operations at the grant-date fair value of our stock options and other equity-based compensation. The determination of grant-date fair value is estimated using an option-pricing model, which includes variables such as the expected volatility of our share price, the exercise behavior of our employees, interest rates, and dividend yields. These variables are projected based on our historical data, experience, and other factors. Changes in any of these variables could result in material increases to the valuation of options granted in future periods and increases in the expense recognized for share-based payments.
Income taxes . Our income tax provision is based on income before taxes and is computed using the liability method in accordance with Accounting Standards Codification ("ASC") 740 – Income Taxes . Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using tax rates projected to be in effect for the year in which the differences are expected to reverse. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations, or the expected results from any future tax examinations. Various internal and external factors may have favorable or unfavorable effects on our future provision for income taxes. Those factors include, but are not limited to, changes in tax laws, regulations and/or rates, the results of any future tax examinations, changing interpretations of existing tax laws or regulations, changes in estimates of prior years' items, past levels of R&D spending, acquisitions, changes in our corporate structure, and changes in overall levels of income before taxes all of which may result in periodic revisions to our provision for income taxes.
Developing our provision for income taxes, including our effective tax rate and analysis of potential uncertain tax positions, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowance we deem necessary to offset deferred tax assets. During the fiscal year ended June 30, 2010, we determined that a valuation allowance was not required for our deferred tax assets because we have established a sufficient history of taxable income from operations. However, if we do not maintain taxable
30
income from operations in future periods, we may increase the valuation allowance for our deferred tax assets and record material adjustments to our income tax expense. Our judgment and tax strategies are subject to audit by various taxing authorities. While we believe we have provided adequately for our uncertain income tax positions in our consolidate financial statements, adverse determination by these taxing authorities could have a material adverse effect on our consolidated financial condition, results of operations or cash flows. Interest and penalties on income tax items are included as a component of overall income tax expense.
Recent Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2010-06, "Improving Disclosures about Fair Value Measurements". ASU 2010-06 requires additional disclosures about fair value measurements including transfers in and out of Levels 1 and 2 and a higher level of disaggregation for the different types of financial instruments. For the reconciliation of Level 3 fair value measurements, information about purchases, sales, issuances and settlements are presented separately. This standard is effective for interim and annual reporting periods beginning after December 15, 2009 with the exception of revised Level 3 disclosure requirements which are effective for interim and annual reporting periods beginning after December 15, 2010. Comparative disclosures are not required in the year of adoption. We adopted the provisions of the standard on January 1, 2010, which did not have a material impact on our financial statements.
Results of Operations
Years ended June 30, 2010 and 2009
Revenue is comprised primarily of sales of our molecular diagnostic products. Revenue for the fiscal year ended June 30, 2010 was $362.6 million compared to $326.5 million for the prior fiscal year, an increase of 11%. Sales of BRACAnalysis account for most of our revenues. This 11% increase in revenue is primarily attributable to increased testing volume. Increased sales, marketing, and education efforts resulted in wider acceptance of our products by the medical community and patients and increased testing volumes for the fiscal year ended June 30, 2010. We are currently in the process of expanding our sales force, executing a public awareness campaign and increasing our market penetration in the U.S. OB/Gyn market. Through these and other efforts we are attempting to broaden utilization of BRACAnalysis with current physician customers and increase the number of new physician customers prescribing our products. We believe these efforts may allow us to continue to grow molecular diagnostic revenue in future periods; however, the markets in which we operate are still experiencing high unemployment, reduced physician office visits, higher health insurance deductibles, and other economic challenges. We believe that there continues to be a negative impact on our revenue growth due to these difficult economic conditions. In addition, because BRACAnalysis and most of our molecular diagnostic products are only utilized once per patient, we will need to sell our services through physicians to new patients or develop new molecular diagnostic products in order to continue to generate revenue. Therefore, there can be no assurance that molecular diagnostic revenue will continue to increase at historical rates or at all.
Cost of revenue is comprised primarily of salaries and related personnel costs, laboratory supplies, royalty payments, equipment costs and facilities expense. Cost of revenue for the fiscal year ended June 30, 2010 was $44.3 million compared to $43.3 million for the prior fiscal year. This increase of 2% in cost of revenue is primarily due to the 11% increase in revenues for the fiscal year ended June 30, 2010 compared to the prior fiscal year. Our gross profit margin was 88% for the fiscal year ended June 30, 2010 compared to 87% for the prior fiscal year. This modest increase in gross profit margins is primarily attributable to technology improvements and efficiency gains in the operation of our molecular diagnostic laboratory. There can be no assurance that molecular diagnostic gross profit margins will continue to increase and we expect that our gross profit margins will fluctuate from quarter to quarter based on the introduction of new products as well as new technologies and operating systems in our molecular diagnostic laboratory.
Research and development expenses from continuing operations are comprised primarily of salaries and related personnel costs, laboratory supplies, and equipment and facility costs. Research and development
31
expenses for continuing operations incurred during the fiscal year ended June 30, 2010 were $21.9 million compared to $17.9 million for the prior fiscal year. This increase of 22% was primarily due to increased research and development costs associated with clinical studies to support our existing molecular diagnostic products, internal molecular diagnostic product discovery and development and clinical studies undertaken to support our existing products and the launch of new products. We expect our research and development expenses will increase over the next several years as we work to develop our product pipeline and expand our offerings of molecular diagnostic products.
Selling, general and administrative expenses for continuing operations consist primarily of salaries, commissions and related personnel costs for sales, marketing, customer service, billing and collections, executive, legal, finance and accounting, information technology, human resources, and allocated facilities expenses. Selling, general and administrative expenses for the fiscal year ended June 30, 2010 were $161.4 million compared to $138.9 million for the prior fiscal year. This increase of 16% was primarily attributable to:
| • | increased sales and marketing expense of approximately $14.0 million to support the continued expansion of our Ob/Gyn sales, DTC campaign in strategic southern and midwestern states, and other marketing initiatives; |
| • | increased share-based payment expense of approximately $4.9 million; |
| • | an increase of $2.5 million in bad debt expense associated with 11% increase in molecular diagnostic sales; and |
| • | general increase in administrative costs of approximately $1.1 million to support the 11% growth in our revenues. |
We expect our selling, general and administrative expenses will continue to fluctuate depending on the number and scope of any new product launches and efforts in support of our existing molecular diagnostic products.
Interest income for the fiscal year ended June 30, 2010 was $5.7 million, compared to $12.5 million for the prior fiscal year. The decrease was due primarily to lower interest rates during the 2010 period, the repurchase of approximately $71 million of Myriad common stock, and the contribution of approximately $188 million of cash and marketable securities to MPI on June 30, 2009.
Other income for the fiscal year ended June 30, 2010 changed $2.6 million from an expense of $2.5 million for the fiscal year ended June 30, 2009 to $0.1 million income for the fiscal year ended June 30, 2010. The change was due to an other-than-temporary impairment on marketable investment securities from our holding of Lehman Brothers Holdings, Inc. ("Lehman") bonds recorded in fiscal 2009. Due to Lehman's bankruptcy filing in fiscal 2009 we determined that our investment in certain Lehman bonds was impaired.
The income tax benefit of approximately $11.5 million for the fiscal year ended June 30, 2010 represents the reversal in full of our valuation allowance previously offsetting our deferred tax assets that was netted against our alternative miniumum tax and state tax expense (see Note 7 in the Consolidated Financial Statements).
Years ended June 30, 2009 and 2008
Revenue for the fiscal year ended June 30, 2009 was $326.5 million compared to $222.9 million for the prior fiscal year, an increase of 47%. Sales of BRACAnalysis account for most of our revenues. This 47% increase in revenue is primarily attributable to increased testing volume. Increased sales, marketing, and education efforts resulted in wider acceptance of our products by the medical community and patients and increased testing volumes for the fiscal year ended June 30, 2009.
Cost of revenue for the fiscal year ended June 30, 2009 was $43.3 million compared to $32.3 million for the prior fiscal year. This increase of 34% in cost of revenue is primarily due to the 47% increase in revenues for the fiscal year ended June 30, 2009 compared to the prior fiscal year. Our gross profit margin was 87% for the fiscal
32
year ended June 30, 2009 compared to 85% for the prior fiscal year. This increase in gross profit margins is primarily attributable to technology improvements and efficiency gains in the operation of our molecular diagnostic laboratory.
Research and development expenses for continuing operations incurred during the fiscal year ended June 30, 2009 were $17.9 million compared to $18.5 million for the prior fiscal year. This decrease of 3% was primarily due to decreased expenses associated with internal research projects of approximately $1.6 million offset by an increase in share-based payment expense of approximately $1.0 million.
Selling, general and administrative expenses for the fiscal year ended June 30, 2009 were $138.9 million compared to $110.4 million for the prior fiscal year. This increase of 26% was primarily attributable to:
| • | increased sales and marketing expense of approximately $10.5 million to support the 47% growth in our revenues, which included the continued expansion of our oncology and Ob/Gyn sales force and our direct-to-consumer marketing campaign in Florida and Texas; |
| • | general increases in administrative support and facility expenses of approximately $3.3 million to support growth in molecular diagnostic sales and market expansion efforts; |
| • | general increases in sales support costs of approximately $3.9 million to support growth in our molecular diagnostic business; |
| • | an increase of $4.4 million in bad debt expense associated with increased molecular diagnostic sales; |
| • | increased share-based payment expense of approximately $6.4 million. |
Interest income for the fiscal year ended June 30, 2009 was $12.5 million, compared to $13.7 million for the prior fiscal year. The decrease was due primarily to lower market interest rates during the fiscal year.
Other expense for the fiscal year ended June 30, 2009 increased $2.2 million from an expense of $0.3 million for the fiscal year ended June 30, 2008 to $2.5 million expense for the fiscal year ended June 30, 2009. This increase was primarily attributed to due to an other-than-temporary impairment in 2008 on marketable investment securities from our holding of Lehman. Due to Lehman's bankruptcy filing in 2008 we determined that our investment in certain Lehman bonds was impaired.
As noted above under "Overview," on June 30, 2009, we separated the research and drug development businesses conducted by us and by MPI and spun-off MPI to our stockholders. We do not have any ownership or other form of interest in MPI subsequent to the separation. As a result of the separation, we have classified the operations from the research and drug development businesses that were conducted by us and MPI until June 30, 2009 as discontinued operations in our Consolidated Statements of Operations. During the year ended June 30, 2009, losses from discontinued operations increased by $25.1 million, from $26.5 million for the year ended June 30, 2008 to $51.6 million for the year ended June 30, 2009. The increase is primarily due to the lack of the one-time $100 million non-refundable upfront fee recognized in the year ended June 30, 2008 related to the co-marketing agreement with H. Lundbeck A/S for our former Alzheimer's disease program that partially offset our drug development costs. On June 30, 2008, we discontinued our Alzheimers disease program.
Liquidity and Capital Resources
Cash, cash equivalents, and marketable investment securities increased $96.2 million, or 25%, from $392.2 million at June 30, 2009 to $488.4 million at June 30, 2010. This increase is primarily attributable to cash generated from sales of our molecular diagnostic products. This increase was partially offset by expenditures for our internal research and development programs, purchase of capital assets, sales and marketing expense for our molecular diagnostic products, our share repurchase program and other expenditures incurred in the ordinary course of business.
Net cash provided by operating activities was $155.1 million, $84.0 million and $103.7 million during the fiscal years ended June 30, 2010, 2009 and 2008, respectively. Net trade receivables increased $21.7 million
33
(excluding bad debt write-offs/reserves) between June 30, 2009 and June 30, 2010, primarily due to the increase in product sales during the same period. Accounts payable and accrued liabilities decreased by $5.7 million between June 30, 2009 and June 30, 2010, primarily due to payments made from our accounts payable related to our discontinued operations following the spin-off of our former research and drug development businesses to MPI on June 30, 2009.
Our investing activities used cash of $76.7 million, $206.3 million and $31.3 million during the fiscal years ended June 30, 2010, 2009 and 2008, respectively. The decrease in cash used in investing activities from the prior fiscal year ended June 30, 2009 was primarily due to the proceeds from sales and maturities of marketable investment securities during the year. For the fiscal year ended June 30, 2010, purchases of marketable investment securities used cash of $477.6 million, maturities and sales of marketable investment securities provided cash of $408.6 million, and capital expenditures for research equipment used cash of $7.9 million.
Financing activities used cash of $49.1 million, $51.9 million during the fiscal year ended June 30, 2010 and 2009 and provided cash of $21.9 million during the fiscal year ended June 30, 2008. Cash utilized from financing activities in 2010 was primarily due to the purchase of $71.4 million of our common stock through a share repurchase program. The cash used in the share purchase was offset by cash provided by the exercise of stock options and sales of our shares under our Employee Stock Purchase Plan of $22.3 million.
We believe that with our existing capital resources and expected net cash to be generated from sales of our molecular diagnostic products, we will have adequate funds to maintain our current and planned operations for at least the next two years, although no assurance can be given that changes will not occur that would consume available capital resources before such time and we may need or want to raise additional financing within this period of time. Our future capital requirements, cash flows, and results of operations could be affected by and will depend on many factors that are currently unknown to us, including:
| • | failure to sustain revenue growth or margins in our molecular diagnostic business; |
| • | termination of the licenses underlying our molecular diagnostic products or failure to enter into product or technology licensing or other arrangements favorable to us; |
| • | delays or other problems with operating our laboratory facilities; |
| • | the costs and expenses incurred in supporting our existing molecular diagnostic products; |
| • | the progress, results and cost of developing and launching additional molecular diagnostic products for our molecular diagnostic business; |
| • | the costs, timing, outcome, and enforcement of any regulatory review of our existing or future molecular diagnostic products; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our issued patents and defending intellectual property-related claims; |
| • | the costs, timing and outcome of any litigation against us; |
| • | the introduction of technological innovations or new commercial products by our competitors; |
| • | changes in intellectual property laws of our patents or enforcement in the United States and foreign countries; |
| • | changes in the governmental or private insurers reimbursement levels for our products; |
| • | changes in structure of the healthcare system or healthcare payment systems; and |
| • | the impact of current economic conditions and job loss resulting in fewer doctor visits and loss of employer provided insurance coverage. |
Off-Balance Sheet Arrangements
None.
34
Contractual Obligations
The following table represents our consolidated contractual obligations as of June 30, 2010 (in thousands):
| Total | Less than one year | 1-3 Years | 4-5 Years | More than 5 years | |||||||||||
Operating leases | $ | 92,357 | $ | 8,605 | $ | 17,028 | $ | 16,938 | $ | 49,786 | |||||
Purchase obligations | 2,558 | 2,558 | - | - | - | ||||||||||
Total | $ | 94,915 | $ | 11,163 | $ | 17,028 | $ | 16,938 | $ | 49,786 | |||||
The expected timing of payment for the obligations listed above is estimated based on current information. Actual payment timing and amounts may differ depending on the timing of goods or services received or other changes. The table above only includes payment obligations that are fixed or determinable. The table excludes royalties to third parties based on future sales of any of our product candidates that are approved for sale, as the amounts, timing, and likelihood of any such payments are based on the level of future sales of products and are unknown.
Effects of Inflation
We do not believe that inflation has had a material impact on our business, revenues, or operating results during the periods presented.
Certain Factors That May Affect Future Results of Operations
The Securities and Exchange Commission encourages companies to disclose forward-looking information so that investors can better understand a company's future prospects and make informed investment decisions. This Annual Report on Form 10-K contains such "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995.
Words such as "may," "anticipate," "estimate," "expects," "projects," "intends," "plans," "believes" and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements. All forward-looking statements are management's present expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to: the risk that sales and profit margins of our existing molecular diagnostic products may decline or will not continue to increase at historical rates; the risk that we may be unable to develop additional molecular diagnostic products; the risk that licenses to the technology underlying our molecular diagnostic products and any future products are terminated or cannot be maintained on satisfactory terms; risks related to delays or other problems with operating our laboratory testing facilities; risks related to public concern over our products; risks related to regulatory developments or enforcement in the United States and foreign countries and changes in the structure of healthcare payment systems; uncertainties about our ability to obtain new corporate collaborations and acquire new technologies on satisfactory terms, if at all; the development of competing products and services; the risk that we or our licensors may be unable to protect the proprietary technologies underlying our products; the risk of patent-infringement claims; risks of new, changing and competitive technologies and regulations in the United States and internationally; and other factors discussed under the heading "Risk Factors" contained in Item 1A of this Annual Report.
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Annual Report or in any document incorporated by reference might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this Annual Report. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
35
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We maintain an investment portfolio in accordance with our written investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any single issue, issuer or type of investment.
Our investments consist of securities of various types and maturities of three years or less, with a maximum average maturity of 12 months. These securities are classified as available for sale. Available-for-sale securities are recorded on the balance sheet at fair market value with unrealized gains or losses reported as part of accumulated other comprehensive income/loss. Realized gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned. A decline in the market value of any available-for-sale security below cost that is deemed other-than-temporary results in a charge to earnings and establishes a new cost basis for the security.
Although our investment policy guidelines are intended to ensure the preservation of principal, current market conditions have resulted in high levels of uncertainty. Our ability to trade or redeem the marketable investment securities in which we invest, including certain corporate bonds and auction rate securities, has become difficult. Valuation and pricing of these securities has also become variable and subject to uncertainty.
As of June 30, 2010 we have net unrealized gains of $0.1 million in our investment portfolio. For the year ended June 30, 2010 we have experienced fluctuations in our portfolio value primarily from our investments in bonds of financial institutions. If interest rates rise, the market value of our investments may decline, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. A hypothetical increase in interest rates by 25 basis points would have resulted in a decrease in the fair value of our net investment position of approximately $1.2 million and $0.9 million as of June 30, 2010 and 2009, respectively. We do not utilize derivative financial instruments to manage our interest rate risks.
36
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
MYRIAD GENETICS, INC.
Index to Financial Statements | Number | |
Report of Independent Registered Public Accounting Firm | F-1 | |
Consolidated Balance Sheets as of June 30, 2010 and 2009 | F-2 | |
Consolidated Statements of Operations for the Years Ended June 30, 2010, 2009 and 2008 | F-3 | |
Consolidated Statements of Stockholders' Equity and Comprehensive Income for the Years Ended June 30, 2010, 2009 and 2008 | F-4 | |
Consolidated Statements of Cash Flows for the Years Ended June 30, 2010, 2009 and 2008 | F-5 | |
Notes to Consolidated Financial Statements | F-6 | |
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| Item 9A. | CONTROLS AND PROCEDURES |
| 1. | Disclosure Controls and Procedures |
We maintain disclosure controls and procedures (Disclosure Controls) within the meaning of Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Our Disclosure Controls are designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act, such as this Annual Report on Form 10-K, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms. Our Disclosure Controls are also designed to ensure that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our Disclosure Controls, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily applied its judgment in evaluating and implementing possible controls and procedures.
As of the end of the period covered by this Annual Report on Form 10-K, we evaluated the effectiveness of the design and operation of the Company's Disclosure Controls, which was done under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer. Based on the evaluation of our Disclosure Controls, our Chief Executive Officer and Chief Financial Officer have concluded that, as of June 30, 2010, our Disclosure Controls were effective to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms and is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
| 2. | Internal Control Over Financial Reporting |
| a. | Management's Report on Internal Control over Financial Reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, a company's principal executive and
37
principal financial officers and effected by the company's board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that:
| • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| • | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2010. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework . Based on our assessment, management believes that, as of June 30, 2010, our internal control over financial reporting is effective based on those criteria.
| b. | Report of the Independent Registered Public Accounting Firm |
The Board of Directors and Stockholders
Myriad Genetics, Inc.:
We have audited Myriad Genetics, Inc.'s internal control over financial reporting as of June 30, 2010, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Myriad Genetics, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable
38
assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Myriad Genetics, Inc. maintained, in all material respects, effective internal control over financial reporting as of June 30, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Myriad Genetics, Inc. as of June 30, 2010 and 2009, and the related consolidated statements of operations, stockholders' equity and comprehensive income, and cash flows for each of the three years in the period ended June 30, 2010, and our report dated August 12, 2010 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
Salt Lake City, Utah
August 12, 2010
| c. | Change in Internal Control over Financial Reporting |
There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during the fourth quarter of our last fiscal year that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | OTHER INFORMATION |
None.
39
PART III
| Item 10. | DIRECTORS AND EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Management," "Section 16(a) Beneficial Ownership Reporting Compliance" and "Code of Conduct and Ethics" in our Proxy Statement for the 2010 Annual Meeting of Stockholders to be held on December 3, 2010.
| Item 11. | EXECUTIVE COMPENSATION |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Compensation Discussion and Analysis," "Executive Compensation," "Management-Committees of the Board of Directors and Meetings-Compensation Committee Interlocks and Insider Participation," "Director Compensation" and "Compensation Committee Report" and "Compensation Practices and Policies Relating to Risk Management" in our Proxy Statement for the 2010 Annual Meeting of Stockholders to be held on December 3, 2010.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Security Ownership of Certain Beneficial Owners and Management" and "Executive Compensation-Equity Compensation Plan Information" in our Proxy Statement for the 2010 Annual Meeting of Stockholders to be held on December 3, 2010.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the caption "Certain Relationships and Related Transactions" and "Management-The Board of Directors" in our Proxy Statement for the 2010 Annual Meeting of Stockholders to be held on December 3, 2010.
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The response to this item is incorporated by reference from the discussion responsive thereto in the proposal entitled "Independent Public Accountants" in our Proxy Statement for the 2010 Annual Meeting of the Stockholders to be held on December 3, 2010.
40
PART IV
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| (a) | The following documents are included as part of this Annual Report on Form 10-K. |
| 1. | Financial Statements |
See "Index to Consolidated Financial Statements" at Item 8 to this Annual Report on Form 10-K.
| 2. | Financial Statement Schedule |
The following schedule is filed as part of this Form 10-K:
Schedule II-Schedule of Valuation and Qualifying Accounts for the Years Ended June 30, 2010, 2009, and 2008
Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
| 3. | Exhibits |
The exhibits which are filed with or incorporated by reference into this Annual Report on Form 10-K are set forth in the Exhibit Index beginning on page A-1, which is incorporated herein by reference."
41
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on August 12, 2010.
| MYRIAD GENETICS, INC. | ||
| By: | /s/ P ETER D. M ELDRUM | |
| Peter D. Meldrum | ||
| President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
Signatures | Title | Date | ||||
| By: | /s/ P ETER D. M ELDRUM Peter D. Meldrum | President, Chief Executive | August 12, 2010 | |||
| B Y : | /s/ J AMES S. E VANS James S. Evans | Chief Financial Officer | August 12, 2010 | |||
| By: | /s/ J OHN T. H ENDERSON John T. Henderson, M.D. | Chairman of the Board | August 12, 2010 | |||
| By: | /s/ W ALTER G ILBERT Walter Gilbert, Ph.D. | Vice Chairman of the Board | August 12, 2010 | |||
| By: | /s/ D ENNIS L ANGER Dennis Langer, M.D., J.D. | Director | August 12, 2010 | |||
| By: | /s/ L AWRENCE C. B EST Lawrence C. Best | Director | August 12, 2010 | |||
| By: | /s/ L INDA S. W ILSON Linda S. Wilson, Ph.D. | Director | August 12, 2010 | |||
| By: | /s/ S. L OUISE P HANSTIEL S. Louise Phanstiel | Director | August 12, 2010 | |||
| By: | /s/ H EINER D REISMANN Heiner Dreismann, Ph.D. | Director | August 12, 2010 | |||
42
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Myriad Genetics, Inc.
We have audited the accompanying consolidated balance sheets of Myriad Genetics, Inc. and subsidiaries as of June 30, 2010 and 2009, and the related consolidated statements of operations, shareholders' equity and comprehensive income, and cash flows for each of the three years in the period ended June 30, 2010. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Myriad Genetics, Inc. and subsidiaries at June 30, 2010 and 2009, and the consolidated results of their operations and their cash flows for each of the three years in the period ended June 30, 2010, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Myriad Genetics, Inc.'s internal control over financial reporting as of June 30, 2010, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated August 12, 2010 expressed an unqualified opinion thereon.
Ernst & Young LLP
Salt Lake City, Utah
August 12, 2010
F-1
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Balance Sheets
June 30, 2010 and 2009
(In thousands, except per share amounts)
| 2010 | 2009 | |||||||
| Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 92,840 | $ | 63,510 | ||||
Marketable investment securities | 310,388 | 253,345 | ||||||
Prepaid expenses | 4,054 | 3,993 | ||||||
Trade accounts receivable, less allowance for doubtful accounts of $4,400 in 2010 and $3,850 in 2009 | 47,801 | 44,617 | ||||||
Deferred taxes | 18,560 | - | ||||||
Other assets | 333 | 655 | ||||||
Total current assets | 473,976 | 366,120 | ||||||
Equipment and leasehold improvements: | ||||||||
Equipment | 48,941 | 49,116 | ||||||
Leasehold improvements | 16,332 | 11,942 | ||||||
| 65,273 | 61,058 | |||||||
Less accumulated depreciation | 42,012 | 38,435 | ||||||
Net equipment and leasehold improvements | 23,261 | 22,623 | ||||||
Long-term marketable investment securities | 85,154 | 75,370 | ||||||
Long-term deferred taxes | 9,404 | - | ||||||
Other assets | 2,052 | 2,275 | ||||||
| $ | 593,847 | $ | 466,388 | |||||
| Liabilities and Stockholders' Equity | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 8,870 | $ | 14,177 | ||||
Accrued liabilities | 18,596 | 17,992 | ||||||
Total current liabilities | 27,466 | 32,169 | ||||||
Unrecognized tax benefits | 8,800 | - | ||||||
Total liabilities | 36,266 | 32,169 | ||||||
Commitments and contingencies (Note 12) | - | - | ||||||
Stockholders' equity: | ||||||||
Preferred stock, $0.01 par value, authorized 5,000 shares; no shares issued and outstanding | - | - | ||||||
Common stock, $0.01 par value, authorized 150,000 shares; issued and outstanding 94,046 shares in 2010 and 95,896 shares in 2009 | 940 | 959 | ||||||
Additional paid-in capital | 566,967 | 550,432 | ||||||
Accumulated other comprehensive income | 139 | 2,768 | ||||||
Accumulated deficit | (10,465 | ) | (119,940 | ) | ||||
Total stockholders' equity | 557,581 | 434,219 | ||||||
| $ | 593,847 | $ | 466,388 | |||||
See accompanying notes to consolidated financial statements.
F-2
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Operations
Years ended June 30, 2010, 2009, and 2008
(In thousands, except per share amounts)
| 2010 | 2009 | 2008 | ||||||||||
Revenue | $ | 362,648 | $ | 326,527 | $ | 222,855 | ||||||
Costs and expenses: | ||||||||||||
Cost of revenue | 44,286 | 43,267 | 32,340 | |||||||||
Research and development expense | 21,873 | 17,914 | 18,482 | |||||||||
Selling, general, and administrative expense | 161,414 | 138,884 | 110,428 | |||||||||
Total costs and expenses | 227,573 | 200,065 | 161,250 | |||||||||
Operating income | 135,075 | 126,462 | 61,605 | |||||||||
Other income (expense): | ||||||||||||
Interest income | 5,660 | 12,478 | 13,709 | |||||||||
Other | 99 | (2,493 | ) | (320 | ) | |||||||
Total other income | 5,759 | 9,985 | 13,389 | |||||||||
Income from continuing operations before income taxes | 140,834 | 136,447 | 74,994 | |||||||||
Income tax provision (benefit) | (11,469 | ) | 193 | 608 | ||||||||
Income from continuing operations | $ | 152,303 | $ | 136,254 | $ | 74,386 | ||||||
Discontinued operations (Note 15) | ||||||||||||
Loss from discontinued operations | - | (51,639 | ) | (26,541 | ) | |||||||
Net income | $ | 152,303 | $ | 84,615 | $ | 47,845 | ||||||
Earnings (loss) per basic share | ||||||||||||
Continuing operations | $ | 1.58 | $ | 1.46 | $ | 0.84 | ||||||
Discontinued operations | - | (0.55 | ) | (0.30 | ) | |||||||
Earnings (loss) per basic share | $ | 1.58 | $ | 0.91 | $ | 0.54 | ||||||
Earnings (loss) per diluted share | ||||||||||||
Continuing operations | $ | 1.54 | $ | 1.38 | $ | 0.80 | ||||||
Discontinued operations | - | (0.52 | ) | (0.29 | ) | |||||||
Earnings (loss) per diluted share | $ | 1.54 | $ | 0.86 | $ | 0.51 | ||||||
Weighted average shares outstanding | ||||||||||||
Basic | 96,338 | 93,492 | 88,378 | |||||||||
Diluted | 99,152 | 98,573 | 93,408 | |||||||||
See accompanying notes to consolidated financial statements.
F-3
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Stockholders' Equity and Comprehensive Income
Years ended June 30, 2010, 2009, and 2008
(In thousands)
| Common stock | Additional paid-in capital | Accumulated other comprehensive income (loss) | Accumulated deficit | Comprehensive income | Stockholders' equity | ||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||
Balances at June 30, 2007 | 86,880 | $ | 868 | $ | 592,293 | $ | (398 | ) | $ | (252,400 | ) | $ | 340,363 | ||||||||||||||
Issuance of common stock for cash upon exercise of options and employee stock purchase plan | 2,548 | 26 | 20,645 | - | - | 20,671 | |||||||||||||||||||||
Issuance of common stock for cash upon exercise of warrants | 60 | - | 1,200 | - | - | 1,200 | |||||||||||||||||||||
Share-based payment expense | - | - | 15,415 | - | - | 15,415 | |||||||||||||||||||||
Net income | - | - | - | - | 47,845 | 47,845 | 47,845 | ||||||||||||||||||||
Change in unrealized losses on marketable investment securities | - | - | - | - | - | 161 | - | ||||||||||||||||||||
Other comprehensive income | - | - | - | 161 | - | 161 | 161 | ||||||||||||||||||||
Comprehensive income | 48,006 | ||||||||||||||||||||||||||
Balances at June 30, 2008 | 89,488 | 894 | 629,553 | (237 | ) | (204,555 | ) | 425,655 | |||||||||||||||||||
Issuance of common stock for cash upon exercise of options and employee stock purchase plan | 6,408 | 65 | 84,144 | - | - | 84,209 | |||||||||||||||||||||
Share-based payment expense | - | - | 25,682 | - | - | 25,682 | |||||||||||||||||||||
Separation of Myriad Pharmaceuticals, Inc. | - | - | (188,947 | ) | - | - | (188,947 | ) | |||||||||||||||||||
Net income | - | - | - | - | 84,615 | 84,615 | 84,615 | ||||||||||||||||||||
Change in unrealized gains on marketable investment securities | - | - | - | - | - | 3,005 | - | ||||||||||||||||||||
Other comprehensive income | - | - | - | 3,005 | - | 3,005 | 3,005 | ||||||||||||||||||||
Comprehensive income | $ | 87,620 | |||||||||||||||||||||||||
Balances at June 30, 2009 | 95,896 | $ | 959 | $ | 550,432 | $ | 2,768 | $ | (119,940 | ) | $ | 434,219 | |||||||||||||||
Issuance of common stock for cash upon exercise of options and employee stock purchase plan | 2,090 | 20 | 22,285 | - | - | 22,305 | |||||||||||||||||||||
Share-based payment expense | - | - | 22,776 | - | - | 22,776 | |||||||||||||||||||||
Repurchase and retirement of common stock | (3,940 | ) | (39 | ) | (28,526 | ) | - | (42,828 | ) | (71,393 | ) | ||||||||||||||||
Net income | - | - | - | - | 152,303 | 152,303 | 152,303 | ||||||||||||||||||||
Change in unrealized gains on marketable investment securities, net of tax | - | - | - | - | - | (2,629 | ) | - | |||||||||||||||||||
Other comprehensive income, net of tax | - | - | - | (2,629 | ) | - | (2,629 | ) | (2,629 | ) | |||||||||||||||||
Comprehensive income | $ | 149,674 | |||||||||||||||||||||||||
Balances at June 30, 2010 | 94,046 | $ | 940 | $ | 566,967 | $ | 139 | $ | (10,465 | ) | $ | 557,581 | |||||||||||||||
See accompanying notes to consolidated financial statements.
F-4
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Years ended June 30, 2010, 2009, and 2008
(In thousands)
| 2010 | 2009 | 2008 | ||||||||||
Cash flows from operating activities: | ||||||||||||
Net income | $ | 152,303 | $ | 84,615 | $ | 47,845 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation and amortization | 7,084 | 9,449 | 8,781 | |||||||||
Loss on disposition of assets | 496 | 506 | 337 | |||||||||
Share-based compensation expense | 22,776 | 25,682 | 15,415 | |||||||||
Bad debt expense | 18,476 | 15,947 | 11,500 | |||||||||
Deferred income taxes | (28,050 | ) | - | - | ||||||||
Unrecognized tax benefits | 9,797 | - | - | |||||||||
Loss on cost-basis investment | - | - | 3,000 | |||||||||
(Gain) loss on sale of marketable investment securities | (397 | ) | 1,986 | - | ||||||||
Changes in operating assets and liabilities: | ||||||||||||
Prepaid expenses | (61 | ) | (1,090 | ) | 2,829 | |||||||
Trade accounts receivable | (21,660 | ) | (19,901 | ) | (21,060 | ) | ||||||
Other assets | 22 | 4,081 | (3,421 | ) | ||||||||
Accounts payable | (5,307 | ) | (10,707 | ) | 9,121 | |||||||
Accrued liabilities | (393 | ) | (24,526 | ) | 27,739 | |||||||
Deferred revenue | - | (2,000 | ) | 1,650 | ||||||||
Net cash provided by operating activities | 155,086 | 84,042 | 103,736 | |||||||||
Cash flows from investing activities: | ||||||||||||
Capital expenditures for equipment and leasehold improvements | (7,895 | ) | (7,525 | ) | (13,675 | ) | ||||||
Purchase of other assets | (100 | ) | (2,100 | ) | (349 | ) | ||||||
Proceeds from sale of intellectual property | 300 | |||||||||||
Purchases of marketable investment securities | (477,558 | ) | (308,566 | ) | (191,701 | ) | ||||||
Proceeds from maturities marketable investment securities | 356,425 | 111,849 | 174,420 | |||||||||
Proceeds from sales marketable investment securities | 52,160 | - | - | |||||||||
Net cash used in investing activities | (76,668 | ) | (206,342 | ) | (31,305 | ) | ||||||
Cash flows from financing activities: | ||||||||||||
Cash and cash equivilants contributed to Myriad Pharmaceuticals, Inc. | - | (136,133 | ) | - | ||||||||
Net proceeds from common stock issued under share-based compensation plans | 22,305 | 84,209 | 20,671 | |||||||||
Repurchase and retirement of common stock | (71,393 | ) | - | 1,200 | ||||||||
Net cash provided by (used in) financing activities | (49,088 | ) | (51,924 | ) | 21,871 | |||||||
Net increase (decrease) in cash and cash equivalents | 29,330 | (174,224 | ) | 94,302 | ||||||||
Cash and cash equivalents at beginning of year | 63,510 | 237,734 | 143,432 | |||||||||
Cash and cash equivalents at end of year | $ | 92,840 | $ | 63,510 | $ | 237,734 | ||||||
Supplemental cash flow information: | ||||||||||||
Cash paid during the year for income taxes | $ | 3,697 | $ | 801 | $ | - | ||||||
Non-cash investing and financing activities: | ||||||||||||
Fair value adjustment on marketable investment securities recorded to stockholders' equity | $ | (2,629 | ) | $ | 3,005 | $ | 161 | |||||
Transfer of assets, net of liabilities to Myriad Pharmaceuticals, Inc. | $ | - | $ | 52,814 | $ | - | ||||||
See accompanying notes to consolidated financial statements.
F-5
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2010, 2009, and 2008
| (1) | Organization and Summary of Significant Accounting Policies |
| (a) | Organization and Business Description |
Myriad Genetics, Inc. and subsidiaries (collectively, the Company) is a leading healthcare company focused on the development and marketing of novel predictive medicine, personalized medicine and prognostic medicine products. The Company employs a number of proprietary technologies that help it to understand the genetic basis of human disease and the role that genes and their related proteins may play in the onset, progression and treatment of disease. The Company uses this information to guide the development of new molecular diagnostic products that are designed to assess an individual's risk for developing disease later in life, identify a patient's likelihood of responding to drug therapy and guide a patient's dosing to ensure optimal treatment, or assess a patient's risk of disease progression and disease recurrence. The Company's operations are located in Salt Lake City, Utah.
| (b) | Separation of Research and Pharmaceutical Businesses |
On June 30, 2009, the Company separated its molecular diagnostic business from its research and drug development businesses by transferring its research and drug development businesses into its then wholly-owned subsidiary Myriad Pharmaceuticals Inc. ("MPI"). The Company contributed $188 million of cash and marketable securities to MPI and all outstanding shares of MPI were then distributed to the Company's stockholders as a pro-rata, tax-free dividend on June 30, 2009 by issuing one share of MPI common stock for every four shares of the Company's common stock to stockholders of record on June 17, 2009. The separation resulted in MPI operating as an independent entity with its own publicly-traded stock. The results of operations for the former research and drug development businesses conducted until June 30, 2009 are designated as discontinued operations in the accompanying financial statements. The Company does not have any ownership or other form of interest in MPI subsequent to the separation (see notes 14 and 15). MPI has recently changed its name to Myrexis, Inc. and is traded on the NASDAQ Global Market under the ticker symbol "MYRX".
| (c) | Principles of Consolidation |
The consolidated financial statements presented herein include the accounts of Myriad Genetics, Inc. and its wholly owned subsidiaries, Myriad Genetic Laboratories, Inc., Myriad Financial, Inc., and Myriad Therapeutics, Inc. through June 30, 2010. All intercompany amounts have been eliminated in consolidation.
Certain reclassifications have been made to prior period amounts to conform to the current period presentation.
| (d) | Recent Accounting Pronouncements |
In January 2010, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2010-06, "Improving Disclosures about Fair Value Measurements". ASU 2010-06 requires additional disclosures about fair value measurements including transfers in and out of Levels 1 and 2 and a higher level of disaggregation for the different types of financial instruments. For the reconciliation of Level 3 fair value measurements, information about purchases, sales, issuances and settlements are presented separately. This standard is effective for interim and annual reporting periods beginning after December 15, 2009 with the exception of revised Level 3 disclosure requirements which are effective for interim and annual reporting periods beginning after December 15, 2010. Comparative disclosures are not required in the year of adoption. The Company adopted the provisions of the standard on January 1, 2010, which did not have a material impact on its financial statements.
F-6
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
| (e) | Marketable Investment Securities |
The Company has classified its marketable investment securities as available for sale. These securities are carried at estimated fair value with unrealized holding gains and losses, net of the related tax effect, included in accumulated other comprehensive income (loss) in stockholders' equity until realized. Gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned.
A decline in the market value of any available-for-sale security below cost that is deemed other than temporary results in a charge to earnings and establishes a new cost basis for the security. Losses are charged against "Other income" when a decline in fair value is determined to be other than temporary. We review several factors to determine whether a loss is other than temporary. These factors include but are not limited to: (i) the extent to which the fair value is less than cost and the cause for the fair value decline, (ii) the financial condition and near term prospects of the issuer, (iii) the length of time a security is in an unrealized loss position and (iv) our ability to hold the security for a period of time sufficient to allow for any anticipated recovery in fair value. The Company recognized no impairments on available-for-sale securities for the years ended June 30, 2010, and 2008. The Company recorded a $2.0 million other–than-temporary impairment on marketable investment securities for the year ended June 30, 2009. Available-for-sale investment securities with remaining maturities of greater than one year are classified as long-term. Available-for-sale investment securities with remaining maturities of greater than one year are classified as long-term.
| (f) | Trade Accounts Receivable and Allowance for Doubtful Accounts |
Trade accounts receivable are comprised of amounts due from sales of the Company's molecular diagnostic products and are recorded at the invoiced amount, net of discounts and contractual allowances. The allowance for doubtful accounts is based on the Company's best estimate of the amount of probable losses in the Company's existing accounts receivable, which is based on historical write-off experience, customer creditworthiness, facts and circumstances specific to outstanding balances, and payment terms. Account balances are charged against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers.
| (g) | Equipment and Leasehold Improvements |
Equipment and leasehold improvements are stated at cost. Depreciation and amortization are computed using the straight-line method based on the lesser of estimated useful lives of the related assets or lease terms. Equipment items have depreciable lives of five years. Leasehold improvements are depreciated over the shorter of the estimated useful lives or the associated lease terms, which range from three to fifteen years. For the years ended June 30, 2010, 2009, and 2008, the Company recorded depreciation expense of $6.8 million, $9.0 million, and $8.2 million, respectively.
| (h) | Impairment of Long-Lived Assets |
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be
F-7
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. No impairments of long-lived assets were recorded for the years ended June 30, 2010, 2009, and 2008.
| (i) | Other Assets |
Other assets as of June 30, 2010 are comprised of purchased intellectual property. Management reviews the valuation of these intangible assets for possible impairment as changes in facts and circumstances indicate that impairment should be assessed. The purchased intellectual property is being amortized ratably over the expected useful life of approximately 16 years. For the years ended June 30, 2010, 2009, and 2008, the Company recorded amortization expense of $0.3 million, $0.4 million, and $0.6 million, respectively.
| (j) | Revenue Recognition |
Revenue includes the sale of molecular diagnostic products and related marketing agreements, and is recorded at the invoiced amount net of any discounts or contractual allowances. Revenue is recognized upon completion of the test, communication of results to the patient, and when collectability is reasonably assured.
| (k) | Income Taxes |
The Company recognizes income taxes under the asset and liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities.
The provision for income taxes, including the effective tax rate and analysis of potential tax exposure items, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowances deemed necessary to recognize deferred tax assets at an amount that is more likely than not to be realized. The Company's filings, including the positions taken therein, are subject to audit by various taxing authorities. While the Company believes it has provided adequately for its income tax liabilities in the consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on the consolidated financial condition, results of operations or cash flows.
| (l) | Earnings Per Share |
Basic earnings per share is computed based on the weighted-average number of shares of common stock outstanding. Diluted earnings per share is computed based on the weighted-average number of shares of common stock, including common stock equivalents outstanding. Certain common shares consisting of stock options that would have an antidilutive effect were not included in the diluted earnings per share attributable to common stockholders for the years ended June 30, 2010, 2009 and 2008.
F-8
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
The following is a reconciliation of the denominators of the basic and diluted earnings (loss) per share computations ( in thousands ):
| Years Ended June 30, | ||||||
| 2010 | 2009 | 2008 | ||||
Demoninator: | ||||||
Weighted-average shares outstanding used to compute basic EPS | 96,338 | 93,492 | 88,378 | |||
Effect of dilutive stock options | 2,814 | 5,081 | 5,030 | |||
Weighted-average shares outstanding and dilutive securities used to compute diluted EPS | 99,152 | 98,573 | 93,408 | |||
For the years ended June 30, 2010, 2009, and 2008, there were outstanding stock options of 6,434,090, 3,091,555, and 2,603,051, respectively, that were excluded from the computation of diluted earnings per share because the effect would have been anti-dilutive. These potential dilutive common shares may be dilutive to future diluted earnings per share.
| (m) | Use of Estimates |
The preparation of the consolidated financial statements in accordance with U.S. generally accepted accounting principles requires Company management to make estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the period. Significant items subject to such estimates and assumptions include the carrying amount of fixed assets, valuation allowances for receivables and deferred income tax assets, certain accrued liabilities and share-based compensation. Actual results could differ from those estimates.
| (2) | Marketable Investment Securities |
The amortized cost, gross unrealized holding gains, gross unrealized holding losses, and fair value for available-for-sale securities by major security type and class of security at June 30, 2010 and 2009 were as follows (in thousands):
| Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Estimated fair value | ||||||||||
At June 30, 2010: | |||||||||||||
Cash and cash equivalents: | |||||||||||||
Cash | $ | 23,314 | $ | - | $ | - | $ | 23,314 | |||||
Cash equivalents | 69,525 | 1 | - | 69,526 | |||||||||
Total cash and cash equivalents | 92,839 | 1 | - | 92,840 | |||||||||
Available-for-sale: | |||||||||||||
Corporate bonds and notes | 272,371 | 658 | (339 | ) | 272,690 | ||||||||
Federal agency issues | 121,448 | 55 | (1 | ) | 121,502 | ||||||||
Auction rate securities | 1,500 | - | (150 | ) | 1,350 | ||||||||
Total | $ | 488,158 | $ | 714 | $ | (490 | ) | $ | 488,382 | ||||
F-9
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
| Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Estimated fair value | ||||||||||
At June 30, 2009: | |||||||||||||
Cash and cash equivalents: | |||||||||||||
Cash | $ | 30,668 | $ | - | $ | - | $ | 30,668 | |||||
Cash equivalents | 32,842 | - | - | 32,842 | |||||||||
Total cash and cash equivalents | 63,510 | - | - | 63,510 | |||||||||
Available-for-sale: | |||||||||||||
Corporate bonds and notes | 213,187 | 2,331 | (58 | ) | 215,460 | ||||||||
Federal agency issues | 110,660 | 705 | 0 | 111,365 | |||||||||
Auction rate securities | 2,100 | - | (210 | ) | 1,890 | ||||||||
Total | $ | 389,457 | $ | 3,036 | $ | (268 | ) | $ | 392,225 | ||||
The following summarizes the maturities of debt securities classified as cash equivalents and available-for-sale at June 30, 2010 (in thousands):
| Amortized cost | Estimated fair value | |||||
Cash equivalents: | $ | 69,525 | $ | 69,526 | ||
Available-for-sale: | ||||||
Due within one year | 310,113 | 310,388 | ||||
Due after one year through three years | 83,706 | 83,804 | ||||
Due after three years | 1,500 | 1,350 | ||||
Total | $ | 464,844 | $ | 465,068 | ||
Debt securities in an unrealized loss position as of June 30, 2010 were not impaired at acquisition and the declines in fair not attributed to declines in credit quality. Management believes that it is more likely than not that the securities will be held until a recovery of par value. All securities in an unrealized loss position as of June 30, 2010 are debt securities. During the period ended June 30, 2009, the Company recorded a $2.0 million other–than-temporary impairment on marketable investment securities related to its investment in Lehman Brothers. Debt securities available for sale in a gross unrealized loss position as of June 30, 2010 and 2009 are summarized as follows (in thousands):
| Less than 12 months | More than 12 months | Total | |||||||||||||||||||
| Fair value | Unrealized losses | Fair value | Unrealized losses | Fair value | Unrealized losses | ||||||||||||||||
At June 30, 2010: | |||||||||||||||||||||
Debt securities: | |||||||||||||||||||||
Corporate bonds and notes | $ | 88,329 | $ | (228 | ) | $ | 28,713 | $ | (111 | ) | $ | 117,042 | $ | (339 | ) | ||||||
Federal agency issues | 10,093 | (1 | ) | - | - | 10,093 | (1 | ) | |||||||||||||
Auction Rate Securities | - | - | 1,350 | (150 | ) | 1,350 | (150 | ) | |||||||||||||
| $ | 98,422 | $ | (229 | ) | $ | 30,063 | $ | (261 | ) | $ | 128,485 | $ | (490 | ) | |||||||
F-10
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
| Less than 12 months | More than 12 months | Total | |||||||||||||||||||
| Fair value | Unrealized losses | Fair value | Unrealized losses | Fair value | Unrealized losses | ||||||||||||||||
At June 30, 2009: | |||||||||||||||||||||
Debt securities: | |||||||||||||||||||||
Corporate bonds and notes | $ | 43,298 | $ | (35 | ) | $ | 9,918 | $ | (23 | ) | $ | 53,216 | $ | (58 | ) | ||||||
Federal agency issues | - | - | - | - | - | - | |||||||||||||||
Auction Rate Securities | - | - | 1,890 | (210 | ) | 1,890 | (210 | ) | |||||||||||||
| $ | 43,298 | $ | (35 | ) | $ | 11,808 | $ | (233 | ) | $ | 55,106 | $ | (268 | ) | |||||||
| (3) | Fair Value Measurements |
The fair value of the Company's financial instruments reflects the amounts that the Company estimates to receive in connection with the sale of an asset or paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The fair value hierarchy prioritizes the use of inputs used in valuation techniques into the following three levels:
Level 1-quoted prices in active markets for identical assets and liabilities.
Level 2-observable inputs other than quoted prices in active markets for identical assets and liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Some of the Company's marketable securities primarily utilize broker quotes in a non-active market for valuation of these securities.
Level 3-unobservable inputs.
The substantial majority of the Company's financial instruments are valued using quoted prices in active markets or based on other observable inputs. The following table sets forth the fair value of our financial assets that the Company re-measured:
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||
at June 30, 2010 | ||||||||||||
Money market funds (a) | $ | 29,929 | $ | - | $ | - | $ | 29,929 | ||||
Corporate bonds and notes | - | 296,987 | - | 296,987 | ||||||||
Federal agency issues | - | 136,802 | - | 136,802 | ||||||||
Auction rate securities | - | - | 1,350 | 1,350 | ||||||||
Total | $ | 29,929 | $ | 433,789 | $ | 1,350 | $ | 465,068 | ||||
| (a) | Money market funds are primarily comprised of government and agency obligations and accrued interest |
F-11
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||
at June 30, 2009 | ||||||||||||
Money market funds (a) | $ | 18,623 | $ | - | $ | - | $ | 18,623 | ||||
Corporate bonds and notes | - | 229,680 | - | 229,680 | ||||||||
Federal agency issues | - | 111,364 | - | 111,364 | ||||||||
Auction rate securities | - | - | 1,890 | 1,890 | ||||||||
Total | $ | 18,623 | $ | 341,044 | $ | 1,890 | $ | 361,557 | ||||
| (a) | Money market funds are primarily comprised of government and agency obligations and accrued interest |
Our Level 1 assets include cash and money market instruments. Level 2 assets consist of our marketable investment securities that include federal agency issues, commercial paper, corporate bonds, and euro bonds. As of June 30, 2010, the Company held $1.4 million of investments which were measured using unobservable (Level 3) inputs. These investments represent less than 1% of our investments portfolio and were classified as Level 3 assets as of June 30, 2010. Our Level 3 assets consist of auction rate securities and the value is determined based on valuations which approximate fair value. The composition of our Level 3 assets decreased by $0.5 million from $1.9 million at June 30, 2009 to $1.4 million at June 30, 2010. The decrease was due to the settlement of a portion of the Company's holdings in the auction rate securities for which it received par value.
| (4) | Leases |
The Company leases office and laboratory space under five non-cancelable operating leases, with terms that expire between 2017 and 2025. The Company also leases information technology equipment under one non-cancelable operating lease, with terms that expire in 2012. Future minimum lease payments under these leases as of June 30, 2010 are as follows (in thousands):
Fiscal year ending: | |||
2011 | $ | 8,605 | |
2012 | 8,651 | ||
2013 | 8,377 | ||
2014 | 8,442 | ||
2015 | 8,496 | ||
Thereafter | 49,786 | ||
| $ | 92,357 | ||
The Company entered into a sublease agreement on July 1, 2009 with MPI that provides for the sublease of certain office and laboratory space for a period of three years from the commencement date with the option to extend for an additional four three-year periods. The Company received approximately $2.9 million during the period ended June 30, 2010 from MPI that offset rental expense. Future minimum lease payments receivable under the sublease agreements are approximately $2.5 million, $2.5 million and $1.5 million in the fiscal years ended June 30, 2011, 2012 and 2013.
Rental expense was $3.6 million, $5.3 million, and $5.2 million for the fiscal years ended June 30, 2010, 2009, and 2008, respectively.
F-12
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
| (5) | Share-Based Compensation |
In 2003, the Company adopted and the shareholders approved the 2003 Employee, Director and Consultant Stock Option Plan (the 2003 Plan). As most recently amended in November 2009, 18.8 million shares of common stock have been reserved for issuance upon the exercise of options that the Company grants from time to time. Additional shares represented by options previously granted under the Company's 2002 Amended and Restated Employee, Director and Consultant Stock Option Plan (the 2002 Plan) which are canceled or expire after November 12, 2003, which was the date of stockholder approval of the 2003 Plan without delivery of shares of stock by the Company and any shares which have been reserved but not granted under the 2002 Plan as of that date are also available for grant under the 2003 Plan. As of June 30, 2010, approximately 2.0 million shares represented by options that remain outstanding under the 2002 Plan will transfer to the 2003 Plan if they are cancelled or expire without delivery of the shares of stock by the Company.
The exercise price of options granted in 2010, 2009, and 2008 was equivalent to the fair market value of the stock at the date of grant. The number of shares, terms, and vesting periods are determined by the Company's board of directors or a committee thereof on an option-by-option basis. Options generally vest ratably over service periods of four years and expire ten years from the date of grant. As of June 30, 2010, 2,217,083 shares are available for future grant under the 2003 Plan.
The fair value of each option grant is estimated on the date of the grant using the Black-Scholes option-pricing model with the following weighted-average assumptions used for grants for the fiscal year ended June 30:
| 2010 | 2009 | 2008 | ||||
Risk-free interest rate | 1.8% | 2.4% | 3.4% | |||
Expected dividend yield | 0% | 0% | 0% | |||
Expected lives (in years) | 4.1 - 4.3 | 4.7 - 5.7 | 4.9 - 5.7 | |||
Expected volatility | 45% | 42% | 45% |
Expected option lives and volatilities are based on historical data of the Company and other factors.
A summary of activity is as follows:
| 2010 | 2009 | 2008 | ||||||||||||||||
| Number of shares | Weighted average exercise price | Number of shares | Weighted average exercise price | Number of shares | Weighted average exercise price | |||||||||||||
Options outstanding at beginning of year | 14,372,056 | $ | 15.66 | 17,706,066 | $ | 11.34 | 16,923,724 | $ | 9.61 | |||||||||
Options granted | 2,728,080 | 26.58 | 3,480,780 | 25.99 | 3,806,550 | 15.51 | ||||||||||||
Less: | ||||||||||||||||||
Options exercised | (1,968,450 | ) | 10.28 | (6,271,853 | ) | 9.14 | (2,430,914 | ) | 5.41 | |||||||||
Options canceled or expired | (1,014,748 | ) | 22.53 | (542,937 | ) | 17.13 | (593,294 | ) | 13.15 | |||||||||
Options outstanding at end of year | 14,116,938 | 18.03 | 14,372,056 | 15.66 | 17,706,066 | 11.34 | ||||||||||||
Options exercisable at end of year | 7,741,122 | 13.93 | 7,212,040 | 12.10 | 11,256,670 | 10.47 | ||||||||||||
Options vested and expected to vest | 12,830,455 | 17.46 | 13,176,035 | 15.36 | 16,483,150 | 11.25 | ||||||||||||
Weighted average fair value of options granted during the year | 10.00 | 14.87 | 9.91 | |||||||||||||||
F-13
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
The following table summarizes information about stock options outstanding at June 30, 2010:
| Options outstanding | Options exercisable | |||||||||
Range of exercise prices | Number outstanding at June 30, 2010 | Weighted average remaining contractual life (years) | Weighted average exercise price | Number exercisable at June 30, 2010 | Weighted average exercise price | |||||
$ 3.80 - 9.04 | 3,662,520 | 4.58 | $ 7.33 | 3,457,876 | $ 7.22 | |||||
9.15 - 18.06 | 3,870,474 | 6.83 | 14.54 | 2,283,380 | 14.25 | |||||
18.13 - 24.79 | 3,528,880 | 7.72 | 22.76 | 1,081,149 | 22.02 | |||||
25.42 - 33.05 | 3,055,064 | 7.34 | 29.80 | 918,717 | 28.88 | |||||
| 14,116,938 | 6.58 | 7,741,122 | 13.93 | |||||||
Share-based compensation expense recognized and included in the consolidated statements of operations for the fiscal years ended June 30, 2010, 2009 and 2008 was as follows ( in thousands, except per share data ):
| Years Ended June 30, | |||||||||
| 2010 | 2009 | 2008 | |||||||
Cost of revenue | $ | 1,005 | $ | 824 | $ | 560 | |||
Research and development | 3,689 | 3,135 | 2,087 | ||||||
Selling, general, and administrative | 18,082 | 13,192 | 6,791 | ||||||
Stock-based compensation expense for continuing operations | 22,776 | 17,151 | 9,438 | ||||||
Discontinued operations | - | 8,531 | 5,977 | ||||||
Total employee stock-based compensation expense | $ | 22,776 | $ | 25,682 | $ | 15,415 | |||
As of June 30, 2010, there was approximately $41.6 million of total unrecognized share-based compensation cost related to share-based compensation granted under our plans that will be recognized over a weighted-average period of 2.4 years. The total intrinsic value of options exercised during the fiscal years ended June 30, 2010, 2009 and 2008 was approximately $26.8 million, $141.1 million and $37.5 million, respectively. The aggregate intrinsic value of options outstanding was approximately $33.4 million and the aggregate intrinsic value options fully vested was approximately $30.3 million as of June 30, 2010.
F-14
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
The Company also has an Employee Stock Purchase Plan (the Plan) which was adopted and approved by the board of directors and stockholders in December 1994. As most recently amended in November 2006, a maximum of 2,000,000 shares of common stock may be purchased by eligible employees under the Plan. At June 30, 2010, 1,600,218 shares of common stock had been purchased under the Plan. For the years ended June 30, 2010, 2009, and 2008, shares purchased under the Plan were 120,740, 136,252, and 117,034, respectively. Compensation expense associated with the Plan was approximately $823,000, $952,000, and $605,000, for the years ended June 30, 2010, 2009, and 2008, respectively. The fair value of shares issued under the Plan was calculated using the Black-Scholes option-pricing model with the following weighted-average assumptions for the fiscal years ended June 30:
| 2010 | 2009 | 2008 | |||||||
Risk-fee interest rate | 0.2 | % | 0.5 | % | 3.3 | % | |||
Expected dividend yield | 0 | % | 0 | % | 0 | % | |||
Expected lives (in years) | 0.5 | 0.5 | 0.5 | ||||||
Expected volatility | 50 | % | 54 | % | 34 | % |
In connection with the separation of MPI, the Company issued a dividend of one MPI stock option for every four stock options held by Company option holders as of June 30, 2009. Accordingly, the Company adjusted the exercise price of its stock options to adjust for the spin-off of MPI. All other terms of the stock options remain the same. However, the vesting and expiration of the options are based on the option holder's continuing employment or service with the Company or MPI, as applicable. The adjusted exercise price of each revalued option was determined in accordance with Section 409A and Section 422 of the Internal Revenue Code.
As a result of the option modifications that occurred in connection with the separation of MPI from the Company, the Company measured the potential accounting impact of these option modifications. Based upon the analysis that included a comparison of the fair value of the modified options granted to our employees and directors immediately after the modification with the fair value of the original option immediately prior to the modification, the Company determined there was no incremental compensation expense. All remaining unrecognized compensation expense at the separation, from options granted to MPI employees and directors from the Company, will not be recognized by the Company.
| (6) | Stockholders' Equity |
Comprehensive Income
The components of the Company's comprehensive income, net of tax of $0.1 million and $0, respectively, are as follows:
| June 30, | |||||||
| (In thousands) | 2010 | 2009 | |||||
Net income | $ | 152,303 | $ | 84,615 | |||
Unrealized gain (loss) on available-for-sale securities, net of tax | (2,629 | ) | 3,005 | ||||
Comprehensive income | $ | 149,674 | $ | 87,620 | |||
F-15
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
Stock Repurchase Program
On May 4, 2010, the Company board of directors authorized the repurchase of $100 million of the Company's outstanding common stock. As of June 30, 2010, the Company had received and retired approximately 3.9 million shares of its common stock. The Company uses the par value method of accounting for its stock repurchases. As a result of the stock repurchases the Company reduced common stock and additional paid-in capital by an aggregate of $28.6 million and charged $42.8 million to retained earnings.
Warrants
During 2008, 60,000 warrants to purchase common stock previously granted to placement agents were exercised at a price of $20.00 per share for total proceeds of $1,200,000. The Company has no warrants outstanding as of June 30, 2010.
| (7) | Income Taxes |
Income tax expense (benefit) consists of the following:
| (In thousands) | Year ended Jun. 30, | |||||||||||
| 2010 | 2009 | 2008 | ||||||||||
Current: | ||||||||||||
Federal | $ | 8,072 | $ | 193 | $ | 608 | ||||||
State | 8,509 | - | - | |||||||||
Total Current | 16,581 | 193 | 608 | |||||||||
Deferred: | ||||||||||||
Federal | 38,162 | 24,352 | 14,188 | |||||||||
State | 4,701 | 3,957 | 2,335 | |||||||||
Change in valuation allowance | (70,913 | ) | (28,309 | ) | (16,523 | ) | ||||||
Total Deferred | (28,050 | ) | - | - | ||||||||
Total income tax expense (benefit) | $ | (11,469 | ) | $ | 193 | $ | 608 | |||||
F-16
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
The differences between income taxes at the statutory federal income tax rate and income taxes reported in the consolidated statements of operations were as follows:
| Year ended Jun. 30, | |||||||||
| 2010 | 2009 | 2008 | |||||||
Federal income tax expense at the statutory rate | 35.0 | % | 35.0 | % | 35.0 | % | |||
State income taxes, net of federal benefit | 4.3 | 3.0 | 3.1 | ||||||
Research and development credits, net of | |||||||||
the federal tax on state credits | (4.7 | ) | (6.2 | ) | (5.8 | ) | |||
Uncertain tax positions, net of federal benefit | |||||||||
on state positions | 6.1 | - | - | ||||||
Change in valuation allowance | (50.3 | ) | (33.4 | ) | (34.0 | ) | |||
Other, net | 1.5 | 1.8 | 3.0 | ||||||
| (8.1 | )% | 0.2 | % | 1.3 | % | ||||
The significant components of the Company's deferred tax assets and liabilities were comprised of the following at June 30, 2010 and 2009:
| (In thousands) | Year ended Jun. 30, | |||||||
| 2010 | 2009 | |||||||
Net operating loss carryforwards | $ | 37,934 | $ | 63,217 | ||||
Property, plant and equipment | 1,867 | 2,787 | ||||||
Accrued vacation | 1,122 | 985 | ||||||
Allowance for doubtful accounts | 1,672 | 1,436 | ||||||
Stock compensation expense | 8,753 | 6,606 | ||||||
Capital loss carryover | 1,852 | 2,014 | ||||||
Research and development credits | 9,707 | 32,413 | ||||||
Alternative minimum tax credit | 2,772 | 801 | ||||||
Uncertain state tax positions | 1,156 | - | ||||||
Other, net | 915 | 440 | ||||||
Total gross deferred tax assets | 67,750 | 110,699 | ||||||
Less valuation allowance | (39,786 | ) | (110,699 | ) | ||||
Net deferred tax assets | $ | 27,964 | $ | - | ||||
Due to sustained positive operating performance and the availability of expected future taxable income, the Company has concluded that it is more likely than not that the benefits of deferred income tax assets will be realized. Accordingly, we reversed the valuation allowances on the majority of the Company gross deferred income tax assets during the year.
The net change in the total valuation allowance was a decrease of $70.9 million, $28.3 million and $16.5 million for the years ended June 30, 2010, 2009 and 2008, respectively.
At June 30, 2010, the Company had total federal, alternative minimum tax, and state tax net operating loss carryforwards of approximately $251.6 million. If not utilized, these operating loss carryforwards will expire
F-17
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
beginning in 2013 through 2029. None of the net operating loss carryforwards are subject to the limitations imposed by Section 382 of the Internal Revenue Code. The Company had approximately $1.6 million of federal research and development tax credits, which can be carried forward to reduce federal income taxes. Additionally, the Company had approximately $12.5 million of Utah research and development tax credits, which can be carried forward to reduce Utah income taxes. Upon utilization to reduce Utah income tax, there will be a corresponding federal tax due resulting in net Utah credits of $8.1 million. If not utilized, the federal and Utah research and development tax credit carryforwards expire beginning in 2012 through 2029.
Approximately $95.6 million of net operating loss tax benefits are ‘excess tax benefits' as defined by ASC guidance and, if recognizable in future years, will be recognized as additional paid-in capital. Approximately, $37.9 million of the ‘excess tax benefits' are attributable to periods prior to adoption of guidance limiting recognition of the deferred tax asset until realized and are included in deferred tax assets (prior to any offset by valuation allowance.) The remaining $57.7 million of ‘excess tax benefits' are not included in deferred tax assets and will be recognized only upon realization of the tax benefit.
The Company's deferred tax asset for the ‘excess tax benefits' attributable to periods prior to the adoption of the standard are offset by a valuation allowance of approximately $37.9 million. If the ‘excess tax benefits' are recognized as additional paid-in-capital in future years, the corresponding valuation allowance will be reversed. The Company also had a valuation allowance of $1.9 million offsetting its capital loss carryover. The capital loss carryforward expires in the year ended June 30, 2014 and the Company does not expect to have capital gains to offset the loss prior to expiration of the carryforward period.
On June 30, 2009, the Company separated its research and drug development businesses from its molecular diagnostic business (see notes 1a and 16). The historical net operating loss carryforwards generated by MPI have been retained by the Company upon separation. The Company also received a Private Letter Ruling from the Internal Revenue Service indicating that the dividend of common stock of Myriad Pharmaceuticals to Myriad Genetics shareholders qualifies as a tax free distribution for U.S. income tax purposes.
In July 2006, the FASB issued ASC Topic 740 Subtopic 10 Section 05, which clarifies the accounting for uncertainty in tax positions. ASC Topic 740 Subtopic 10 Section 05 requires that the impact of a tax position be recognized in the financial statements if that position is more likely than not of being sustained on audit, based on the technical merits of the position. The Company adopted the provisions of ASC Topic 740 Subtopic 10 Section 05 on January 1, 2007. Accordingly, there was no net impact on retained earnings for the cumulative effect of adopting ASC Topic 740 Subtopic 10 Section 05. As a result of the Company's having recorded a full valuation allowance against its deferred tax assets and the significant net operating losses available to offset any taxes due, there have been no uncertain tax positions for the years ended June 30, 2009 and 2008. As a result, the Company recorded no unrecognized tax benefits. For the year ended June 30, 2010, the Company recorded unrecognized tax benefits of approximately $9.8 million, $1.0 million of which is recorded as current, net of any federal benefits on state income tax positions and interest.
F-18
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Year ended Jun. 30, | |||||||||
| (In thousands) | 2010 | 2009 | 2008 | ||||||
Gross increases - current year tax positions | $ | 9,797 | $ | - | $ | - | |||
Unrecognized tax benefits at end of year | $ | 9,797 | $ | - | $ | - | |||
Interest and penalties related to uncertain tax positions are included as a component of income tax expense. Of the $9.8 million unrecognized tax benefits at June 30, 2010, approximately $0.9 million represents interest and penalties.
The Company files U.S. and state income tax returns in jurisdictions with various statutes of limitations. The 2006 through 2009 tax years remain subject to examination at June 30, 2010. The Company's consolidated federal tax return and any significant state tax returns are not currently under examination. Annual tax provisions include amounts considered necessary to pay assessments that may result from examination of prior year tax returns, however, the amount ultimately paid upon resolution of issues may differ materially from the amount accrued.
| (8) | Employee Deferred Savings Plan |
The Company has a deferred savings plan which qualifies under Section 401(k) of the Internal Revenue Code. Substantially all of the Company's employees are covered by the plan. The Company makes matching contributions of 50% of each employee's contribution with the employer's contribution not to exceed 4% of the employee's compensation. The Company's contributions to the plan were $2,041,000, $2,602,000, and $2,149,000 for the years ended June 30, 2010, 2009, and 2008, respectively.
| (9) | Segment and Related Information |
The Company's business units from continuing operations have been aggregated into two reportable segments: (i) genetics, and (ii) molecular diagnostics. The genetics segment is focused on the discovery of genes related to major common diseases and includes corporate services such as finance, human resources, legal, and information technology. The molecular diagnostics segment provides testing to determine predisposition to common diseases.
On June 30, 2009, the Company spun-off its research and drug development businesses to MPI. The results from the former research and drug development businesses are reflected as discontinued operations in the Consolidated Statements of Operations (see notes 1b, 14 and 15).
The accounting policies of the segments are the same as those described in the summary of significant accounting policies (note 1). The Company evaluates segment performance based on income (loss) from continuing operations before interest income and other income and expense (in thousands).
F-19
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
| Genetics | Molecular diagnostics | Total | ||||||||||
Year ended June 30, 2010: | ||||||||||||
Revenues | $ | - | $ | 362,648 | $ | 362,648 | ||||||
Depreciation and amortization | 2,215 | 4,869 | 7,084 | |||||||||
Segment operating income (loss) | (44,182 | ) | 179,257 | 135,075 | ||||||||
Year ended June 30, 2009: | ||||||||||||
Revenues | $ | - | $ | 326,527 | $ | 326,527 | ||||||
Depreciation and amortization | 2,301 | 4,385 | 6,686 | |||||||||
Segment operating income (loss) | (40,711 | ) | 167,173 | 126,462 | ||||||||
Year ended June 30, 2008: | ||||||||||||
Revenues | $ | - | $ | 222,855 | $ | 222,855 | ||||||
Depreciation and amortization | 2,388 | 3,495 | 5,883 | |||||||||
Segment operating income (loss) | (33,633 | ) | 95,238 | 61,605 | ||||||||
| Years Ended June 30, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
Total operating income for continuing reportable segments | $ | 135,075 | $ | 126,462 | $ | 61,605 | ||||||
Unallocated amounts: | ||||||||||||
Interest income | 5,660 | 12,478 | 13,709 | |||||||||
Other | 99 | (2,493 | ) | (320 | ) | |||||||
Income from continuing operation before income taxes | 140,834 | 136,447 | 74,994 | |||||||||
Income tax provision (benefit) | (11,469 | ) | 193 | 608 | ||||||||
Income from continuing operations | $ | 152,303 | $ | 136,254 | $ | 74,386 | ||||||
The following table sets forth a comparison of balance sheet assets by operating segment:
| June 30, | ||||||
| (In thousands) | 2010 | 2009 | ||||
Net equipment and leasehold improvements: | ||||||
Genetics | $ | 8,968 | $ | 5,720 | ||
Molecular diagnostics | 14,293 | 16,903 | ||||
Total | $ | 23,261 | $ | 22,623 | ||
Total Assets: | ||||||
Genetics | $ | 41,376 | $ | 11,050 | ||
Molecular diagnostics | 64,089 | 63,146 | ||||
Total | $ | 105,465 | $ | 74,196 | ||
F-20
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
The following table reconciles assets by operating segment to total assets:
| June 30, | ||||||
| (In thousands) | 2010 | 2009 | ||||
Total assets by segment | $ | 105,465 | $ | 74,196 | ||
Cash, cash equivalents, and marketable investment securities (1) | 488,382 | 392,225 | ||||
Total | $ | 593,847 | $ | 466,421 | ||
| (1) | The Company manages cash, cash equivalents, and marketable investment securities at the consolidated level for all segments |
The Company's revenues from continuing operations were derived from the sale of molecular diagnostic products. Additionally, all of the Company's long-lived assets are located in the United States.
| (10) | Stockholder Rights Plan |
In July 2001, the Company adopted a stockholder rights plan (the Rights Plan). The Rights Plan provides registered holders of the Company's common stock one preferred share purchase right for each outstanding share of the Company's common stock. Each right entitles the holder to purchase one one-hundredth of a share of a new series of junior participating preferred stock. The rights have certain anti-takeover effects and allow the Company's stockholders (other than the acquiror) to purchase common stock in the Company or in the acquiror at a substantial discount. Prior to the ten days following the acquisition by a person or group of beneficial ownership of 15% or more of the Company's common stock, the Board of Directors may redeem the rights in whole, but not in part, at a price of $0.01 per right. The purchase rights under the Plan expire on July 17, 2011.
| (11) | Investment in Prolexys Pharmaceuticals, Inc. |
In April 2001, the Company contributed technology to Prolexys Pharmaceuticals, Inc. (Prolexys), in exchange for a 49% ownership interest and investors contributed a combined $82 million in cash in exchange for the remaining 51% ownership in Prolexys. At June 30, 2009, the Company's ownership percentage in Prolexys was 12.46%. On June 30, 2009, the Company's investment in Prolexys Pharmaceuticals, Inc. was transferred to MPI in connection with the spin-off (see notes 1b, 14 and 15).
| (12) | Commitments and Contingencies |
The Company is subject to various claims and legal proceedings covering matters that arise in the ordinary course of its business activities. Management believes any liability that may ultimately result from the resolution of these matters will not have a material adverse effect on the Company's consolidated financial position, operating results, or cash flows.
| (13) | Asset Acquisition |
On January 20, 2009, the Company's then wholly-owned subsidiary, MPI, purchased certain in-process research and development assets related to the HIV drug candidate that the Company has labeled MPC-4326 from Panacos Pharmaceuticals, Inc. The assets were determined to be in-process research and development assets and were charged to expense on the acquisition date. MPI assumed control of all clinical and commercial
F-21
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2010, 2009, and 2008
development of MPC-4326. The aggregate purchase price was $7 million, which represented cash consideration. On June 30, 2009, the Company completed the spin-off of MPI. Accordingly, the associated in-process research and development expense is included in discontinued operations in the Consolidated Statements of Operations (see notes 1b, 14 and 15).
| (14) | Spin-off of Myriad Pharmaceuticals, Inc. |
On June 30, 2009, the Company separated its molecular diagnostic business from its research and drug development businesses. The Company contributed substantially all of the assets and certain liabilities from the research and drug development businesses and $188 million of cash and marketable securities to MPI. All outstanding shares of MPI were then distributed to the Company's stockholders of record on June 17, 2009 as a pro-rata, tax-free dividend of one MPI common stock for every four shares of the Company's common stock.
On June 30, 2009, the Company entered into a Separation and Distribution Agreement with MPI that set forth the terms and conditions of the separation of MPI from the Company. The Separation and Distribution Agreement sets forth a framework for the relationship between the Company and MPI following the separation regarding principal transactions necessary to separate MPI from the Company, including: (i) the contribution of substantially all of the assets and certain liabilities of the Company's research and drug development businesses and cash and cash equivalents and marketable securities of approximately $188 million to MPI; and (ii) the distribution by the Company, as of 11:59 p.m. (EDT) on June 30, 2009, of all outstanding shares of MPI common stock to the Company's stockholders in the form of a pro rata dividend of one share of MPI common stock for every four shares of the Company's common stock outstanding to stockholders of record on June 17, 2009. This agreement also sets forth other provisions that govern certain aspects of the Company's relationship with MPI after the completion of the separation from the Company and provides for the allocation of assets, liabilities and obligations between MPI and the Company in connection with the separation.
In addition, on June 30, 2009 the Company entered into other definitive agreements in connection with the spin-off, including (1) a Tax Sharing Agreement that generally governs the parties' respective rights, responsibilities and obligations after the separation with respect to taxes (2) a Sublease Agreement, as amended on November 11, 2009 and February 19, 2010, that provides for the sublease from the Company to MPI of certain office and laboratory space to be utilized by MPI in its operations and (3) an Employee Matters Agreement that allocates liabilities and responsibilities relating to employee compensation, benefit plans, programs and other related matters in connection with the separation, including the treatment of outstanding incentive awards and certain retirement and welfare benefit obligations. These arrangements contain the provisions related to the spin-off of MPI and the distribution of MPI's common stock to the Company's stockholders.
F-22
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2009, 2008, and 2007
The total amount of the MPI stock dividend of $188.9 million was based on the net book value of the net assets that were transferred to MPI in connection with the spin-off, as follows (in thousands):
| June 30, 2009 | ||||
Net book value of assets transferred: | ||||
Cash and cash equivalents | $ | 136,133 | ||
Marketable investment securities | 51,344 | |||
Prepaid and other current assets | 240 | |||
Equipment, net | 5,390 | |||
Other assets, net | 94 | |||
Accrued liabilities | (4,254 | ) | ||
Net assets transferred | $ | 188,947 | ||
MPI's historical results of operations have been presented as discontinued operations in the Consolidated Statement of Operations. See note 15 for further detail of the discontinued operations results.
| (15) | Discontinued Operations |
On June 30, 2009, the Company separated its former research and drug development businesses from its molecular diagnostic business. For further information on the separation see notes 1b and 14. The significant components of the research and drug development operations, which are presented as discontinued operations, were as follows (in thousands):
| Year Ended June 30, | |||||||||||
| 2010 | 2009 | 2008 | |||||||||
Pharmaceutical revenue (1) | $ | - | $ | - | $ | 100,000 | |||||
Research and other revenues (2) | - | 5,456 | 10,774 | ||||||||
Operating expenses (3) | - | (57,095 | ) | (137,315 | ) | ||||||
Total loss from discontinued operations | $ | - | $ | (51,639 | ) | $ | (26,541 | ) | |||
| (1) | Revenue from discontinued operations from non-refundable upfront license fees where the Company has continuing involvement is recognized ratably over the development or agreement period or upon termination of a development or license agreement when the Company has no ongoing obligation. |
In May 2008, the Company entered into a collaboration agreement with Lundbeck granting certain marketing rights for the Company's therapeutic candidate Flurizan. Under the terms of the agreement Lundbeck paid the Company a $100 million, non-refundable fee, and agreed to pay future royalties, sales-based milestones, and share certain development costs.
On June 30, 2008, based on results from the Company's U.S. phase III clinical trial, the Company announced its intention to discontinue all Flurizan development activities. Both the Company and Lundbeck concluded that Flurizan had no future economic value and that the Company had no continuing substantive obligations to Lundbeck. Based on this conclusion, the Company recognized the $100 million as pharmaceutical revenue for the year ended June 30, 2008.
| (2) | Research revenue from discontinued operations includes revenue from research agreements, milestone payments, and technology licensing agreements. In applying the principles of SAB 104 and ASC 605-25 to |
F-23
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements-(Continued)
June 30, 2009, 2008, and 2007
research and technology license agreements we consider the terms and conditions of each agreement separately to arrive at a proportional performance methodology of recognizing revenue. Such methodologies involve recognizing revenue on a straight-line basis over the term of the agreement, as underlying research costs are incurred, or on the basis of contractually defined output measures such as units delivered. We make adjustments, if necessary, to the estimates used in our calculations as work progresses and we gain experience. The principal costs under these agreements are for personnel expenses to conduct research and development but also include costs for materials and other direct and indirect items necessary to complete the research under these agreements. Actual results may vary from our estimates. Payments received on uncompleted long-term contracts may be greater than or less than incurred costs and estimated earnings and have been recorded as other receivables or deferred revenues. Revenue from milestone payments for which we have no continuing performance obligations is recognized upon achievement of the related milestone. When we have continuing performance obligations, the milestone payments are deferred and recognized as revenue over the term of the arrangement as we complete our performance obligations. We recognize revenue from up-front nonrefundable license fees on a straight-line basis over the period of our continued involvement in the research and development project. |
Net research and other revenues include revenues recognized under collaboration agreements. In June 2006, the Company entered into a research collaboration to apply its high-speed genomic sequencing capability and bioinformatics expertise to deliver molecular genetic information to the collaborator. Revenue related to this collaboration is recognized when completed information is delivered to the collaborator. Under this agreement the Company recognized research revenue of $3.1 million, $0 and $7.0 million for the fiscal year ended June 30, 2009, 2008 and 2007, respectively.
In June 2004, the Company entered into a five-year, research agreement to utilize its expertise to characterize pathogen-host protein interactions. Revenue related to this collaboration is being recognized on a cost-to-cost basis. Under this agreement the Company recognized research revenue of $2.2 million, $3.3 million and $2.4 million for the fiscal years ended June 30, 2009, 2008, and 2007, respectively.
| (3) | Included within the loss from discontinued operations for the period ended June 30, 2008 is a one-time sublicense fee of $20 million which represented the maximum amount that may be payable to Encore Pharmaceuticals, Inc. ("Encore") arising from the Company's receipt of a $100 million non-refundable upfront payment from H. Lundbeck A/S. In 2009, the Company negotiated a reduced sublicense fee with Encore for $11 million. Accordingly, the Company recorded a reduction of research and development expense of $9 million for the year ended June 30, 2009. In addition, the Company recorded a $3 million write-off in its preferred stock investment in Encore Pharmaceuticals as a result of our discontinuation of the Flurizan development program for the year ended June 30, 2008. In 2009, the Company purchased certain in-process research and development assets that were expensed from Panacos Pharmaceuticals, Inc. for $7 million (see note 13). |
F-24
Schedule II
MYRIAD GENETICS, INC.
Schedule of Valuation and Qualifying Accounts
Years Ended June 30, 2010, 2009, and 2008
(In thousands)
| Balance at Beginning of Period | Addition Charged to Cost and Expenses | Deductions (1) | Balance at End of Period | ||||||||||
Allowance for doubtful accounts: | |||||||||||||
Year ended June 30, 2010 | $ | 3,850 | $ | 18,475 | ($ | 17,925 | ) | $ | 4,400 | ||||
Year ended June 30, 2009 | $ | 4,100 | $ | 15,947 | ($ | 16,197 | ) | $ | 3,850 | ||||
Year ended June 30, 2008 | $ | 2,600 | $ | 11,500 | ($ | 10,000 | ) | $ | 4,100 | ||||
| (1) | Represents amounts written off against the allowance. |
See report of independent registered public accounting firm.
EXHIBIT INDEX
Exhibit | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
2.1 | Separation and Distribution Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | 8-K (Exhibit 2.1) | 07/07/09 | 000-26642 | ||||||||
3.1 | Restated Certificate of Incorporation, as amended | 10-Q (Exhibit 3.1) | 02/03/09 | 000-26642 | ||||||||
3.2 | Restated By-Laws | 8-K (Exhibit 3.1) | 11/16/07 | 000-26642 | ||||||||
4.1 | Specimen common stock certificate | 10-K (Exhibit 4.2) | 09/27/02 | 000-26642 | ||||||||
4.2 | Rights Agreement, dated as of July 17, 2001, between the Registrant and Mellon Investor Services, LLC | 8-K (Exhibit 4.1) | 07/18/01 | 000-26642 | ||||||||
4.3 | Agreement of Substitution and Amendment of Common Shares Rights Agreement, dated August 16, 2002, between the Registrant and American Stock Transfer and Trust Company | 10-K (Exhibit 4.4) | 09/27/02 | 000-26642 | ||||||||
Lease Agreements | ||||||||||||
10.1 | Lease Agreement, dated October 12, 1995, between the Registrant and Boyer Research Park Associates V, by its general partner, the Boyer Company | 10-Q (Exhibit 10.2) | 11/08/96 | 000-26642 | ||||||||
10.2 | Amendment to Lease Agreement, dated March 29, 1996 between the Registrant and Boyer Research Park Associates V, by its general partner, the Boyer Company | 10-Q (Exhibit 10.3) | 11/08/96 | 000-26642 | ||||||||
10.3 | Lease Agreement-Research Park Building Phase II, dated March 6, 1998, between the Registrant and Research Park Associated VI, by its general partner, the Boyer Company, L.C. | 10-K (Exhibit 10.44) | 09/24/98 | 000-26642 | ||||||||
10.4 | Memorandum of Lease, dated August 24, 1998, between the Registrant and Boyer Foothill Associates, Ltd. | 10-Q (Exhibit 10.1) | 11/12/98 | 000-26642 | ||||||||
10.5 | Memorandum of Lease, dated August 24, 1998, between the Registrant and Boyer Research Park Associates VI, L.C. | 10-Q (Exhibit 10.2) | 11/12/98 | 000-26642 | ||||||||
10.6 | Subordination Agreement and Estoppel, Attornment and Non-Disturbance Agreement (Lease to Deed of Trust), dated June 24, 1998, between the Registrant and Wells Fargo Bank, National Association | 10-Q (Exhibit 10.3) | 11/12/98 | 000-26642 | ||||||||
Exhibit | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
10.7 | Lease Agreement, dated March 31, 2001, between the Registrant and Boyer Research Park Associates VI, by it general partner, The Boyer Company, L.C. | 10-Q (Exhibit 10.1) | 05/15/01 | 000-26642 | ||||||||
10.8 | Agreement, dated March 31, 2001, between the Registrant and Boyer Research Park Associates VI, by its general partner, The Boyer Company, L.C. | 10-Q (Exhibit 10.2) | 05/15/01 | 000-26642 | ||||||||
10.9 | Lease Agreement, dated June 29, 2005, between the Registrant and Boyer Research Park Associates VIII, by it general partner, The Boyer Company, L.C. | 8-K (Exhibit 99.1) | 07/05/05 | 000-26642 | ||||||||
10.10 | Letter of Understanding regarding Lease Agreement, dated June 29, 2005, between the Registrant and Boyer Research Park Associates VIII, by it general partner, The Boyer Company, L.C. | 8-K (Exhibit 99.2) | 07/05/05 | 000-26642 | ||||||||
10.11 | Lease Agreement, dated March 11, 2008, between the Registrant and Boyer Research Park Associates IX, by it general partner, The Boyer Company, L.C. | 10-K (Exhibit 10.32) | 08/28/08 | 000-26642 | ||||||||
10.12 | .1 | Sublease Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | 8-K (Exhibit 10.2) | 07/07/09 | 000-26642 | |||||||
| .2 | Amendment No. 1, dated November 11, 2009, to Sublease Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | X | ||||||||||
| .3 | Amendment No. 2, dated February 19, 2010, to Sublease Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | X | ||||||||||
10.13 | Amendment to Lease Agreement, dated February 12, 2010 between the Registrant and Boyer Research Park Associates IX, L.C.. | 10-Q (Exhibit 10.4) | 05/05/10 | 000-26642 | ||||||||
Agreements with Respect to Collaborations, Licenses, Research and Development | ||||||||||||
10.14 | Exclusive License Agreement, dated October 8, 1991, between the Registrant and the University of Utah Research Foundation, as amended (Breast Cancer-BRCA1)* | S-1 (Exhibit 10.13) | 10/05/95 | 33-95970 | ||||||||
Exhibit | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
10.15 | Exclusive License Agreement, dated November 23, 1994, between the Registrant and the University of Utah Research Foundation (Breast Cancer-BRCA2)* | S-1 (Exhibit 10.17) | 10/05/95 | 33-95970 | ||||||||
10.16 | Exclusive License Agreement, dated March 15, 1995, between the Registrant and the Hospital for Sick Children* | 10-Q (Exhibit 10.1) | 11/01/07 | 000-26642 | ||||||||
10.17 | Exclusive License Agreement, dated January 6, 1995, between the Registrant and Endorecherche* | 10-Q (Exhibit 10.2) | 11/01/07 | 000-26642 | ||||||||
10.18 | Exclusive License Agreement, dated March 13, 1996, between the Registrant and The Trustees of the University of Pennsylvania* | 10-Q (Exhibit 10.3) | 11/01/07 | 000-26642 | ||||||||
Agreements with Myriad Pharmaceuticals, Inc. | ||||||||||||
| Separation and Distribution Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | See Exhibit 2.1 | |||||||||||
| Sublease Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | See Exhibit 10.12 | |||||||||||
10.19 | Tax Sharing Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | 8-K (Exhibit 10.1) | 07/07/09 | 000-26642 | ||||||||
10.20 | Employee Matters Agreement, dated June 30, 2009, between the Registrant and Myriad Pharmaceuticals, Inc. | 8-K (Exhibit 10.3) | 07/07/09 | 000-26642 | ||||||||
Agreements with Executive Officers and Directors | ||||||||||||
10.21 | Employment Agreement, dated May 15, 1993, between the Registrant, Myriad Genetic Laboratories, Inc. and Peter D. Meldrum+ | S-1 (Exhibit 10.3) | 10/05/95 | 33-95970 | ||||||||
10.22 | Employment Agreement, dated January 1, 1994, between the Registrant, Myriad Genetic Laboratories, Inc. and Mark H. Skolnick, Ph.D. + | S-1 (Exhibit 10.4) | 10/05/95 | 33-95970 | ||||||||
10.23 | Employment Agreement, dated September 14, 1998, between the Registrant., Myriad Genetic Laboratories, Inc. and Gregory C. Critchfield, M.D. + | 10-K (Exhibit 10.7) | 09/10/04 | 000-26642 | ||||||||
10.24 | Resignation Agreement between Myriad Genetics, Inc. and Gregory C. Critchfield, dated February 1, 2010+. | 8-K (Exhibit 10.1) | 02/02/10 | 000-26642 | ||||||||
Exhibit | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
10.25 | Employment Agreement between Myriad Genetics, Inc., Myriad Genetic Laboratories, Inc. and James S. Evans dated March 3, 1995+ | 8-K (Exhibit 10.1) | 11/06/07 | 000-26642 | ||||||||
10.26 | Employment Agreement, dated November 5, 2002, between the Registrant, Myriad Genetic Laboratories, Inc. and Richard M. Marsh+ | 10-K (Exhibit 10.27) | 08/25/09 | 000-26642 | ||||||||
10.27 | Employment Agreement, dated October 1, 2002, between the Registrant, Myriad Genetic Laboratories, Inc. and Mark. C. Capone+ | 10-K (Exhibit 10.28) | 08/25/09 | 000-26642 | ||||||||
10.28 | .1 | Form of Executive Retention Agreement+@ | 10-Q (Exhibit 10.1) | 05/05/10 | 000-26642 | |||||||
| .2 | Form of Amendment to Form of Executive Retention Agreement+@ | 10-Q (Exhibit 10.2) | 05/05/10 | 000-26642 | ||||||||
10.29 | Executive Retention Agreement, dated November 17, 2006, between the Registrant and Mark. C. Capone+ | 10-Q (Exhibit 10.1) | 02/06/07 | 000-26642 | ||||||||
10.30 | Management Performance-Incentive Bonus Program Fiscal Year 2010+ | 8-K (Exhibit 10.1) | 06/08/09 | 000-26642 | ||||||||
10.31 | Non-Employee Director Compensation Policy+ | 10-Q (Exhibit 10.6) | 11/01/07 | 000-26642 | ||||||||
10.32 | Form of director and executive officer indemnification agreement | 10-K (Exhibit 10.34) | 08/25/09 | 000-26642 | ||||||||
Equity Compensation Plans | ||||||||||||
10.33 | .1 | 2002 Amended and Restated Employee, Director and Consultant Stock Option Plan (the "2002 Plan")+ | 10-K (Exhibit 10.1) | 09/27/02 | 000-26642 | |||||||
| .2 | Form of Incentive Stock Option Agreement under the 2002 Plan+ | 10-Q (Exhibit 10.9) | 11/01/07 | 000-26642 | ||||||||
| .3 | Form of Non-Qualified Stock Option Agreement under the 2002 Plan+ | 10-Q (Exhibit 10.10) | 11/01/07 | 000-26642 | ||||||||
10.34 | .1 | 2003 Employee, Director and Consultant Stock Option Plan, as amended (the "2003 Plan")+ | 10-Q (Exhibit 10.1) | 2/3/10 | 000-26642 | |||||||
| .2 | Form of Incentive Stock Option Agreement under the 2003 Plan + | 10-Q (Exhibit 10.7) | 11/01/07 | 000-26642 | ||||||||
| .3 | Form of Non-Qualified Stock Option Agreement under the 2003 Plan + | 10-Q (Exhibit 10.8) | 11/01/07 | 000-26642 | ||||||||
10.35 | Employee Stock Purchase Plan, as amended+ | 8-K (Exhibit 99.2) | 11/20/06 | 000-26642 | ||||||||
21.1 | List of Subsidiaries of the Registrant | X | ||||||||||
23.1 | Consent of Independent Registered Public Accounting Firm (Ernst & Young LLP) | X | ||||||||||
Exhibit | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||
31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
32 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X |
| (+) | Management contract or compensatory plan arrangement. |
| (@) | The agreements with all executives are identical except for the executive who is a party to the agreement and the date of execution, which are listed at the end of the exhibit |
| (*) | Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions. |
| (**) | Confidential treatment has been requested from the Securities and Exchange Commission as to certain portions. |
