UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2018
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File Number: 000-50484
MEI Pharma, Inc.
(Exact name of registrant as specified in its charter)
| DELAWARE | 51-0407811 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
3611 Valley Centre Drive, Suite 500, San Diego, CA 92130
(Address of principal executive offices) (Zip Code)
(858) 369-7100
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.00000002 par value | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by a check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by a check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (Section 229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definition of "large accelerated filer," "accelerated filer," "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ☐ | Accelerated filer | ☒ | |||
| Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☐ | |||
| Emerging growth company | ☐ | |||||
If an emerging growth company, indicate by checkmark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting common equity held by non-affiliates of the registrant was approximately $77.7 million as of December 31, 2017, based on the closing price of the registrant's Common Stock as reported on the NASDAQ Capital Market on such date. For purposes of this calculation, shares of the registrant's common stock held by directors and executive officers have been excluded. This number is provided only for purposes of this Annual Report on Form 10-K and does not represent an admission that any particular person or entity is an affiliate of the registrant.
As of August 27, 2018, there were 71,086,404 shares of the registrant's common stock, par value $0.00000002 per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information required by Part III of this Annual Report on Form 10-K is incorporated by reference from the registrant's definitive proxy statement for the annual meeting of stockholders to be held in November 2018, which will be filed with the Securities and Exchange Commission within 120 days after the close of the registrant's fiscal year ended June 30, 2018.
MEI PHARMA, INC.
TABLE OF CONTENTS
| Page | ||||||
PART I | ||||||
Item 1: | Business | 3 | ||||
Item 1A: | Risk Factors | 13 | ||||
Item 1B: | Unresolved Staff Comments | 23 | ||||
Item 2: | Properties | 23 | ||||
Item 3: | Legal Proceedings | 24 | ||||
Item 4: | Mine Safety Disclosures | 24 | ||||
PART II | ||||||
Item 5: | Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 25 | ||||
Item 6: | Selected Financial Data | 26 | ||||
Item 7: | Management's Discussion and Analysis of Financial Condition and Results of Operations | 27 | ||||
Item 7a: | Quantitative and Qualitative Disclosures about Market Risk | 33 | ||||
Item 8: | Financial Statements and Supplementary Data | 33 | ||||
Item 9: | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 50 | ||||
Item 9A: | Controls and Procedures | 50 | ||||
Item 9B: | Other Information | 52 | ||||
PART III | ||||||
Item 10: | Directors, Executive Officers and Corporate Governance | 52 | ||||
Item 11: | Executive Compensation | 52 | ||||
Item 12: | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 52 | ||||
Item 13: | Certain Relationships and Related Transactions, and Director Independence | 52 | ||||
Item 14: | Principal Accountant Fees and Services | 52 | ||||
PART IV | ||||||
Item 15: | Exhibits, Financial Statement Schedules | 53 | ||||
Item 16: | Form 10-K Summary | 54 | ||||
2
Forward-Looking Statements
This Annual Report on Form 10-K, or Annual Report, includes forward-looking statements, which involve a number of risks and uncertainties. These forward-looking statements can generally be identified as such because the context of the statement will include words such as "may," "will," "intend," "plan," "believe," "anticipate," "expect," "estimate," "predict," "potential," "continue," "likely," or "opportunity," the negative of these words or other similar words. Similarly, statements that describe our future plans, strategies, intentions, expectations, objectives, goals or prospects and other statements that are not historical facts are also forward-looking statements. Discussions containing these forward-looking statements may be found, among other places, in "Business" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in this Annual Report. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. Readers of this Annual Report are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the time this Annual Report was filed with the Securities and Exchange Commission, or SEC. These forward-looking statements are based largely on our expectations and projections about future events and future trends affecting our business, and are subject to risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. These risks and uncertainties include, without limitation, those discussed in "Risk Factors" and in "Management's Discussion and Analysis of Financial Condition and Results of Operations" of this Annual Report. In addition, past financial or operating performance is not necessarily a reliable indicator of future performance, and you should not use our historical performance to anticipate results or future period trends. We can give no assurances that any of the events anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on our results of operations and financial condition. Except as required by law, we undertake no obligation to update publicly or revise our forward-looking statements to reflect events or circumstances that arise after the filing of this Annual Report or documents incorporated by reference herein that include forward-looking statements.
Unless the context requires otherwise, references in this Annual Report to "MEI Pharma," "we," "us" and "our" refer to MEI Pharma, Inc.
MEI Pharma, Inc. and our corporate logo are registered service marks of MEI Pharma. Any other brand names or trademarks appearing in this Annual Report are the property of their respective holders.
PART I
| Item 1. | Business |
Overview
We are a pharmaceutical company focused on leveraging our extensive development and oncology expertise to identify and advance new therapies intended to meaningfully improve the treatment of cancer. Our portfolio of drug candidates contains four clinical-stage candidates, including one candidate in an ongoing Phase 3 global registration trial and another candidate that is anticipated to advance, in the fourth calendar quarter of calendar year 2018, into a Phase 2 clinical trial that we intend to submit to the U.S. Food and Drug Administration ("FDA") as support for accelerated approval of a marketing application. Our common stock is listed on the NASDAQ Capital Market under the symbol "MEIP".
Our Strategy
Our focus is on developing and commercializing cancer treatments intended to effectively leverage the potential of a mechanism of action to provide increased benefit to patients. Since each of our drug candidates utilizes a different mechanism of action, we are able to approach cancer treatment through multiple pathways; this creates opportunities across various cancer indications. Our candidates are intended to demonstrate potency and safety as standalone treatments with the potential to deliver synergies in combination with existing medicines.
We emphasize efficient clinical translation of our drug candidates by implementing development paths designed to expeditiously validate therapeutic potential and clinical utility. We also prioritize efficient resource allocation, seeking to advance and commercialize drug candidates either independently or via partnership to strategically and optimally build value.
Clinical Development Programs
Cancer is an intractable and highly adaptable disease capable of evading the body's defenses and resisting treatment to grow and spread. Despite new treatments that leverage actionable insights into cancer biology, effective treatments remain elusive and even the most cutting-edge therapies still struggle to balance potency with safety. As a result, the oncology community strives to improve on existing therapies and search for new and better options to optimize benefits for patients. This approach includes medicines that not only act alone, but also work well in combination with other therapies to deliver the best-possible outcomes.
We currently have four clinical-stage development programs with diverse approaches to inhibiting cancer, including epigenetics, cell signaling and cancer metabolism:
| • | Pracinostat, an oral histone deacetylase ("HDAC") inhibitor; |
| • | ME-401, an oral phosphatidylinositol 3-kinase ("PI3K") delta inhibitor; |
| • | Voruciclib, an oral cyclin-dependent kinase ("CDK") inhibitor; and |
| • | ME-344, a mitochondrial inhibitor targeting the OXPHOS complex. |
3
Each of our four clinical-stage oncology programs is in active development, one of which is in a pivotal registration clinical trial and one that is anticipated to advance, in the fourth calendar quarter of calendar year 2018, into a Phase 2 clinical trial that we intend to submit to the FDA as support for accelerated approval of a marketing application.
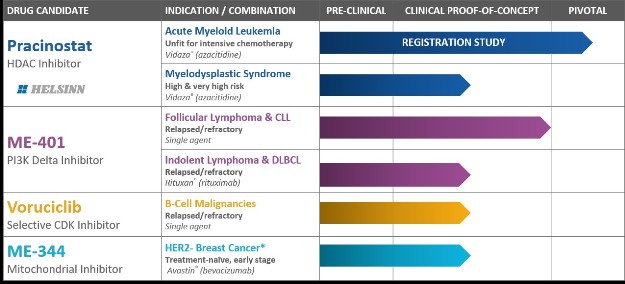
Pracinostat: HDAC Inhibitor Candidate in a Phase 3 Global Registration Clinical Trial
Pracinostat is an oral HDAC inhibitor being evaluated in a pivotal Phase 3 global registration clinical trial for the treatment of adults with newly diagnosed acute myeloid leukemia ("AML") who are unfit to receive intensive chemotherapy. Pracinostat is also being evaluated in a Phase 2 study in patients with high or very high-risk myelodysplastic syndrome ("MDS"). In August 2016, we entered into an exclusive worldwide license, development, manufacturing and commercialization agreement with Helsinn Healthcare SA, a Swiss pharmaceutical corporation ("Helsinn") for pracinostat in AML, MDS and other potential indications (the "Helsinn Agreement). As compensation for such grant of rights, we received upfront payments of $20.0 million in fiscal 2017. We are also eligible to receive up to $444 million in potential regulatory and sales-based milestones, along with royalty payments on the net sales of pracinostat, which, in the U.S., are tiered and begin in the mid-teens. Under the agreement, Helsinn is primarily responsible for funding global development and commercialization costs for pracinostat. We are responsible for conducting the Phase 2 MDS study, the cost of which will be shared equally with Helsinn.
Breakthrough Therapy Designation for pracinostat was granted by the FDA in 2016, and in January 2018 the European Medicines Agency ("EMA") granted Orphan Drug Designation to pracinostat for the treatment of AML. The designations in the US and European Union ("EU") are supported by data from a Phase 2 study of pracinostat plus azacitidine in elderly patients with newly diagnosed AML who are not candidates for induction chemotherapy. The study showed a median overall survival of 19.1 months and a complete remission ("CR") rate of 42% (21 of 50 patients). These data compare favorably to an international Phase 3 study of azacitidine (AZA-001; Dombret et al. Blood. 2015 May 18), which showed a median overall survival of 10.4 months with azacitidine alone and a CR rate of 19.5% in a similar patient population. The combination of pracinostat and azacitidine was generally well tolerated, with no unexpected toxicities. The most common grade 3/4 treatment-emergent adverse events included febrile neutropenia, thrombocytopenia, anemia and fatigue.
Pracinostat Scientific Overview; Epigenetics
HDACs play a key role in epigenetic regulation of gene expression by regulating chromatin structure. Acetylation of positively charged lysine residues present in histone proteins by the histone acetyltransferase ("HATs") reduces the affinity between histones and negatively charged DNA, resulting in the opening of the chromatin structure. This makes it easier for the transcriptional machinery to access the DNA, enhancing RNA transcription. Conversely, deacetylation by the HDACs closes the chromatin structure leading to a repression of gene transcription. In normal cells, HDACs and HATs together control histone acetylation levels to maintain a balance. In diseases such as cancer, this regulation can be disturbed. HDAC inhibitors cause accumulation of acetylated histones, enhance transcription and result in changes to a variety of cellular responses including differentiation, proliferation, migration, survival and response to metabolic and hypoxic stress. In general, tumor cells are more susceptible than normal cells to the anti-proliferative and pro-apoptotic effects of HDAC inhibitors.
There are currently three HDAC inhibitors, one oral and two injectable, approved by the FDA for the treatment of T-cell lymphoma and a fourth orally administered HDAC inhibitor approved for multiple myeloma. Other HDAC inhibitors are being evaluated in clinical trials as single agents and in combination for the treatment of various hematologic diseases and solid tumors.
Pracinostat is an orally available, potent HDAC inhibitor with potentially improved physicochemical, pharmaceutical and pharmacokinetic properties when compared to other compounds of this class, including increased bioavailability and increased half-life.
4
Clinical Program
The ongoing pivotal Phase 3 registration study, which is being run by Helsinn and initiated in June of 2017, is a randomized, double-blind, placebo-controlled study that will enroll worldwide approximately 500 adults with newly diagnosed AML who are unfit to receive intensive chemotherapy. Patients are randomized 1:1 to receive pracinostat or placebo with azacitidine as background therapy. The primary endpoint of the study is overall survival. Secondary endpoints include morphologic CR rate, event-free survival and duration of CR.
Additionally, pracinostat is being investigated in a Phase 2 dose optimization study evaluating patients with high and very high-risk MDS who are previously untreated with hypomethylating agents. This patient group represents the highest unmet need in MDS, with median survival estimates of 1.6 years and 0.8 years, respectively. The ongoing Phase 2 open-label study is evaluating a 45mg dose of pracinostat in combination with the standard dose of azacitidine. The study is designed to improve tolerability and retain patients in the study longer than in an earlier Phase 2 study evaluating a 60 mg dose. A prolonged treatment may result in a systemic exposure to pracinostat sufficient to achieve the desired treatment effect; data from the earlier Phase 2 study suggested that insufficient exposure to treatment may have limited overall efficacy of the combination.
A successful pre-planned interim analysis of the Phase 2 MDS study demonstrated a 10% discontinuation rate among the first 20 evaluable patients treated, beating the predefined threshold in the first 3 treatment cycles. The 10% rate is consistent with the established discontinuation rate for azacitidine given as a monotherapy. Having met this threshold, the study expanded open-label enrollment to 60 patients. Patients will be followed for one year to evaluate safety and efficacy. If the expanded open-label study is successful, a global registration study is anticipated. The primary endpoints of the study are 1) safety and tolerability and 2) overall response rate, defined as CR, partial remission ("PR") and marrow CR. Secondary endpoints include CR rate, overall hematologic improvement ("HI") response rate, clinical benefit rate (defined as rate of CR + PR + HI + Marrow CR), rate of cytogenetic complete response/remission, duration of response, rate of leukemic transformation, event-free survival, progression-free survival and overall survival.
Pracinostat has been previously investigated in more than 300 patients in multiple Phase 1 and Phase 2 clinical trials and found to be generally well tolerated with manageable side effects often associated with drugs of this class, including fatigue, myelosuppression and gastrointestinal toxicity.
ME-401: PI3K Delta Inhibitor Entering Phase 2 Study to Support Accelerated Approval in Relapsed or Refractory Follicular Lymphoma
We own exclusive worldwide rights to ME-401, a selective oral inhibitor of PI3K delta. In the fourth quarter of calendar year 2018, we plan to initiate an ME-401 single-agent Phase 2 clinical trial for the treatment of adults with relapsed or refractory follicular lymphoma ("FL"). We intend to submit the results of this trial to the FDA for accelerated approval of the marketing application under 21CFR314, Subpart H.
We believe ME-401 holds best-in-class potential as a PI3K delta inhibitor based on clinical data observed to date. Clinical data from an ongoing Phase 1b, open-label, dose-escalation study in relapsed/refractory FL, chronic lymphocytic leukemia ("CLL") and small lymphocytic lymphoma ("SLL") demonstrate an objective response rate of 90%. ME-401 was generally well-tolerated in the Phase 1b trial and no dose-limiting toxicities were identified at any dose level.
The clinical data generated to date, along with important differentiating pharmaceutical properties of ME-401, support its potential as a single-agent therapy and the potential to be used in combination with existing or emerging therapies to treat multiple difficult-to-treat oncology indications.
ME-401 Scientific Overview: Cell Cycle Signaling
The PI3K/AKT/mTOR pathway is an important signaling pathway for many cellular functions such as cell survival, cell cycle progression and cellular growth. PI3Ks are a family of enzymes within this pathway that have been shown to play a critical role in the proliferation and survival of certain cancer cells. There are several isoforms of PI3K that are expressed in different types of cells. The PI3K delta isoform is believed to be important for survival of certain B-cell leukemias and lymphomas.
PI3K delta Inhibitors and B-Cell Malignancies
As a class of therapies, PI3K delta inhibitors may have application across a range of B-cell malignancies and compare favorably to other therapeutic approaches.
PI3K delta inhibitors as a group demonstrate promise in the treatment of B-cell malignancies. However, the FDA and EMA approved oral PI3K delta inhibitor idealelisib (marketed as Zydelig ® ), the FDA approved intravenous PI3K alpha/delta inhibitor copanlisib (marketed as Aliqopa ® ), as well as other candidates in development, are challenged by treatment limiting toxicities which may compromise their overall efficacy. We believe this provides an opportunity for the development of a next generation candidate with superior pharmaceutical properties that can provide efficacy and that better maximizes the biological potential of PI3K delta without being limited by toxicities that reduce clinical utility.
Through our extensive pre-clinical and ongoing clinical work, we have demonstrated that ME-401 has important pharmaceutical properties, including prolonged target binding, preferential cellular accumulation, significant distribution throughout the body tissues, and a 28-hour half-life suitable for once daily oral administration. We believe these positive attributes support the promising clinical results observed to date and the continued clinical advancement of ME-401 as an attractive drug candidate with single-agent activity and the potential to be used in combination with existing or emerging therapies to treat multiple difficult-to-treat oncology indications.
5
Clinical Program
ME-401 is being evaluated in an ongoing Phase 1b dose escalation study in patients with relapsed or refractory FL, CLL and SLL. In June 2018, at the American Society of Clinical Oncology ("ASCO") Annual Meeting, we reported data indicating that ME-401 administered as a single-agent achieved a high response rate of 90% among 30 evaluable patients as well as a high response rate of 86% in the group of 21 patients with FL. In addition to the overall high response rate, other notable observations include:
| • | Responses that were generally early in treatment: 85% of responses (23/27) occurred at the first disease assessment after 2 cycles (56 days); |
| • | A 100% (10/10) objective response rate was observed in the group of FL patients with progression of disease within 24 months (POD24) of initial immunochemotherapy. While this group of patients generally received one prior line of therapy, progression of disease within 24 months after initial treatment is associated with very poor outcomes; only about 50% of POD24 patients survive for 5 years compared to about 90% of patients that do not have early disease progression. ("Casulo et al, JCO 2015); |
| • | High objective responses that were independent of the line of treatment: 86% (18/21) of patients treated in ³ 2 nd line therapy and 82% (9/11) of FL patients treated in ³ 3 rd line therapy; |
| • | Durable responses: median follow-up was 8 months (range: 2.4-16.5 months) and only 1 responder had disease progression, and 13 of 18 active patients had a response duration ongoing for more than 6 months. |
ME-401 was generally well-tolerated. No dose-limiting toxicities were identified at any dose level. Among the most common adverse events, Grade 3 adverse events of interest were diarrhea 19% (6/31), rash 13% (4/31), colitis 6% (2/31) and stomatitis 3% (1/31), all of which were reported in Cycle 3 or later cycles and all of which resolved with drug interruption and corticosteroids allowing multiple patients to resume treatment on an intermittent schedule without apparent loss of response. No opportunistic infections or non-infectious pneumonitis were reported. There have been no Grade 4-5 adverse events. Four patients discontinued due to an adverse event. Rates of adverse events across the doses studied were comparable.
Laboratory abnormalities were infrequent. Grade ³ 3 laboratory abnormalities reported were: neutropenia 10% (4/31) and AST/ALT increase 6% (2/31). Myelosuppression was not associated with febrile neutropenia. Based on the data, we determined that no further dose escalation was required. An expansion cohort of up to 30 patients with FL, CLL and SLL was added to further evaluate the safety and efficacy of ME-401 as a single agent at the 60 mg dose. An additional 15 patients are enrolled in the study arm evaluating ME-401 (60 mg) in combination with rituximab (marketed as Rituxan ® ) in patients with various B cell malignancies, including diffuse large B-cell lymphoma ("DLBCL").
Accelerated Approval Registration Study
In July 2018 the Company discussed with FDA a ME-401 monotherapy accelerated approval strategy in patients with relapsed or refractory follicular lymphoma. The FDA communicated support for the Company's proposed randomized Phase 2 trial. Accelerated approval of ME-401 will be subject to FDA review of the improvement provided by ME-401 over other therapies available at the time of the regulatory action.
Informed by our communications with the FDA, we are planning to initiate by the end of calendar year 2018, a global randomized Phase 2 study to evaluate the efficacy, safety, and tolerability of ME-401 in patients with FL after failure of at least two prior systemic therapies including chemotherapy and an anti-CD20 antibody. The study will evaluate two different ME-401 single agent dosing regimens; in one arm, ME-401 will be administered once daily continuously and in the other arm, ME-401 will be administered once daily for two cycles (i.e., eight weeks) followed by an intermittent schedule whereby ME-401 will be administered once daily for the first seven days of a 28 day cycle followed by 21 days placebo. Approximately 150 patients will be randomized in the study and the primary efficacy endpoint will be the rate of objective response to therapy.
Voruciclib: CDK Inhibitor with CDK9 Inhibition in Phase 1 Studies
In September 2017, we entered into a license agreement with Presage Biosciences, Inc. ("Presage"). Under the terms of such license agreement (the "Presage License Agreement"), Presage granted to us exclusive worldwide rights to develop, manufacture and commercialize voruciclib, a clinical-stage, oral and selective CDK inhibitor, and related compounds. Voruciclib is an orally administered CDK inhibitor differentiated by its potent in vitro inhibition of CDK9 in addition to CDK6, 4 and 1. Voruciclib is currently being evaluated in a Phase 1b dose ranging study in patients with B-cell malignancies.
Voruciclib Scientific Overview: Cell Cycle Signaling
The CDK family of proteins are important cell cycle regulators. CDK9 is a transcriptional regulator of the myeloid leukemia cell differentiation protein ("MCL1"), a member of the family of anti-apoptotic proteins which, when elevated, may prevent the cell from undergoing cell death. Inhibition of CDK9 blocks the production of MCL1, which is an established resistance mechanism to the B-cell lymphoma ("BCL2") inhibitor venetoclax (marketed as Venclexta™).
In pre-clinical studies voruciclib shows dose-dependent suppression of MCL1; in December 2017 a study of voruciclib published in the journal Nature Scientific Reports reported that the combination of voruciclib plus the BCL-2 inhibitor venetoclax was capable of inhibiting two master regulators of cell survival, MCL-1 and BCL-2, and achieved synergistic antitumor efficacy in an aggressive subset of Diffuse Large B-cell Lymphoma (DLBCL) pre-clinical models. (Scientific Reports. (2017) 7:18007. DOI:10.1038/s41598-017-18368-w).
6
CDK9 is also a transcriptional regulator of MYC, a transcription factor regulating cell proliferation and growth which contributes to many human cancers and is frequently associated with poor prognosis and unfavorable patient survival. Targeting MYC directly has historically been difficult, but CDK9 is a transcriptional regulator of MYC and is a promising approach to target this oncogene.
Clinical Program
In January 2018, we announced the FDA cleared the voruciclib Investigational New Drug Application ("IND") for hematologic malignancies. In August 2018 we dosed our first patient in a dose ranging Phase 1b clinical trial of voruciclib as a single agent in patients with relapsed and/or refractory B-cell malignancies after failure of prior standard therapies to determine the safety, preliminary efficacy and maximum tolerated dose. We also plan to evaluate voruciclib in combination with venetoclax to assess synergies and the opportunity for combination treatments across multiple indications.
Voruciclib was previously evaluated in more than 70 patients in multiple Phase 1 studies with a tolerability profile consistent with other drugs in its class. In pre-clinical studies, voruciclib shows dose-dependent suppression of MCL1 at concentrations achievable with doses that appear to be generally well tolerated in earlier Phase 1 studies. Pre-clinical studies additionally show inhibition of MYC protein expression.
ME-344: Mitochondrial Inhibitor with Combinatorial Potential
ME-344 is our novel and tumor selective, isoflavone-derived mitochondrial inhibitor drug candidate. It directly targets the OXPHOS complex 1, a pathway involved in ATP production in the mitochondria. ME-344 is currently in an ongoing investigator-initiated, multi-center, randomized study in combination with the VEGF inhibitor bevacizumab (marketed as Avastin ® ) in a total of 40 patients with HER2 negative breast cancer.
ME-344 Scientific Overview: Cancer Metabolism
Tumor cells often display a high metabolic rate to support cell division and growth. This heightened metabolism requires a continual supply of energy in the form of adenosine triphosphate ("ATP"). The two major sources of ATP are the specialized cellular organelles termed mitochondria and through the metabolism of carbohydrates, proteins and lipids.
ME-344 was identified through a screen of more than 400 new chemical structures originally created based on the central design of naturally occurring plant isoflavones. We believe that some of these synthetic compounds, including our drug candidate ME-344, interact with specific mitochondrial enzyme targets, resulting in the inhibition of ATP generation. When these compounds interact with their target, a rapid reduction in ATP occurs, which leads to a cascade of biochemical events within the cell and ultimately to cell death.
Clinical Program
ME-344 demonstrated evidence of single agent activity against refractory solid tumors in a Phase 1 study, and in pre-clinical studies tumor cells treated with ME-344 resulted in a rapid loss of ATP and cancer cell death. In addition to single agent activity, ME-344 may also have significant potential in combination with antiangiogenic therapeutics. While antiangiogenics reduce the rate of glycolysis in tumors as a mechanism to block growth, tumor metabolism often shifts to mitochondrial metabolism to continue energy production to support continued tumor proliferation. In such cases of tumor plasticity in the presence of treatment with antiangiogenics, targeting the alternative metabolic source with ME-344 may open an important therapeutic opportunity.
We are investigating this approach in an ongoing, multicenter, investigator-initiated, randomized, open-label, clinical trial, which is evaluating ME-344 in a total of up to 40 patients with HER2-negative breast cancer in combination with the vascular endothelial growth factor inhibitor bevacizumab (marketed as Avastin ® ). Patients are randomized one-to-one to either ME-344 in combination with bevacizumab or saline in combination with bevacizumab. The interim data review was predefined to take place after 20 patients were randomized. The primary efficacy endpoint is inhibition of cell proliferation as measured by Ki-67 reductions.
Interim data presented from the study at the ASCO Annual Meeting in June 2018 demonstrate evidence of inhibition of tumor proliferation. Mean absolute (relative) Ki-67 decreases were 5.13 (29%) and 1.2 (9%) in the active versus control arms (P=0.06). Patients with standardized uptake values via PET scan ³ 10% experienced an absolute average Ki-67 decrease of 16.6 vs. 2.3 in the active versus control arms (P=0.19). Treatment was generally well tolerated; two Grade 3 adverse events (high blood pressure) were reported, one in each arm, and deemed related to bevacizumab. These interim data are consistent with pre-clinical results indicating ME-344's potential to reverse resistance to anti-angiogenic therapy, thereby warranting the continuation of the ongoing study.
Results from our earlier, first-in-human, single-agent Phase 1 clinical trial of ME-344 in patients with refractory solid tumors were published in the April 1, 2015 issue of Cancer . The results indicated that eight of 21 evaluable patients (38%) treated with ME-344 achieved stable disease or better, including five who experienced progression-free survival that was at least twice the duration of their last prior treatment before entry into the study. In addition, one of these patients, a heavily pre-treated patient with small cell lung cancer, achieved a confirmed partial response and remained on study for two years. ME-344 was generally well tolerated at doses equal to or less than 10 mg/kg delivered on a weekly schedule for extended durations. Treatment-related adverse events included nausea, dizziness and fatigue. Dose-limiting toxicities were observed at both the 15 mg/kg and 20 mg/kg dose levels, consisting primarily of grade three peripheral neuropathy.
7
In June 2016, pre-clinical data from a collaboration with the Spanish National Cancer Research Centre in Madrid showing mitochondria-specific effects of ME-344 in cancer cells, including substantially enhanced anti-tumor activity when combined with agents that inhibit the activity of VEGF, were published in Cell Reports . These data demonstrate that the anti-cancer effects when combining ME-344 with a VEGF inhibitor are due to an inhibition of both mitochondrial and glycolytic metabolism and provided a basis for commencement of the ongoing investigator-initiated study of ME-344 in combination with the VEGF inhibitor bevacizumab (marketed as Avastin ® ) in HER2 negative breast cancer patients.
Competition
The marketplace for our drug candidates is highly competitive. A number of other companies have products or drug candidates in various stages of pre-clinical or clinical development that are intended for the same therapeutic indications for which our drug candidates are being developed. Some of these potential competing drug candidates are further advanced in development than our drug candidates and may be commercialized sooner. Even if we are successful in developing products that receive regulatory approval, such products may not compete successfully with products produced by our competitors or with products that may subsequently receive regulatory approval.
Our competitors include pharmaceutical companies and biotechnology companies, as well as universities and public and private research institutions. In addition, companies active in different but related fields represent substantial competition for us. Many of our competitors developing oncology drugs have significantly greater capital resources, larger research and development staffs and facilities, and greater experience in drug development, regulation, manufacturing, marketing and commercialization than we do. They compete with us in recruiting sites and eligible patients to participate in clinical studies and in attracting development and/or commercialization partners. They also license technologies that are competitive with our technologies. As a result, our competitors may be able to more easily develop technologies and products that would render our technologies or our drug candidates obsolete or non-competitive.
Intellectual Property
We own, by assignment or exclusive license, worldwide rights to each of our current drug candidates. Our intellectual property portfolio includes approximately 29 issued U.S. patents, 186 issued foreign patents, 14 pending U.S. patent applications, and 61 pending foreign applications.
We have acquired, by assignment, patents and patent applications from S*Bio Pte Ltd ("S*Bio") relating to a family of heterocyclic compounds, which include pracinostat, that inhibit histone deacetylases. The U.S. Patent and Trademark Office ("USPTO") has issued six patents covering a number of these heterocyclic-based compounds, including pracinostat, and their composition of matter, pharmaceutical compositions, and methods of use to treat proliferative diseases. The composition of matter claims covering pracinostat are projected to expire in May 2028, not including patent term extension. In our License Agreement, we granted to Helsinn an exclusive (subject to certain retained rights to perform obligations under the agreement), sublicenseable, payment-bearing, license under and to certain patents and know-how controlled by us to develop, manufacture and commercialize pracinostat and any pharmaceutical product containing pracinostat for all human and animal indications.
We have acquired by assignment worldwide rights to ME-401 and other related compounds from Pathway Therapeutics, Inc. The USPTO has issued two patents covering the composition of matter and pharmaceutical compositions of ME-401 which are projected to expire in January 2031 and December 2032, not including any patent term extension. There are currently three U.S. and 15 foreign applications for ME-401 and related compounds pending.
Under the terms of the Presage License Agreement, we acquired, by assignment, exclusive worldwide rights to develop, manufacture and commercialize voruciclib. The USPTO has issued three patents covering the composition of matter and pharmaceutical compositions of voruciclib which are projected to expire in April 29, 2024 and September 2028, not including any patent term extension.
We have acquired, by assignment, patents and patent applications from Novogen, our former majority shareholder, which relate to a large family of isoflavonoid compounds, including ME-344. The USPTO has issued seven patents covering ME-344, including its composition of matter, pharmaceutical compositions and methods of use to treat cancer. The composition of matter and pharmaceutical composition claims covering ME-344 are expected to expire in March 2027 and November 2031, not including patent term extension.
8
Our success depends in large part on our ability to protect our proprietary technologies, compounds and information, and to operate without infringing the proprietary rights of third parties. We rely on a combination of patent, trade secret, copyright, and trademark laws, as well as confidentiality, licensing and other agreements, to establish and protect our proprietary rights. We seek patent protection for our key inventions, including drug candidates we identify, routes for chemical synthesis and pharmaceutical formulations. There is no assurance that any of our pending patent applications will issue, or that any of our patents will be enforceable or will cover a drug or other commercially significant product or method. In addition, we regularly review our patent portfolio to identify patents and patent applications that we deem to have relatively low value to our ongoing business operations for potential abandonment. There is also no assurance that we will correctly identify which of our patents and patent applications should be maintained and which should be abandoned. The term of most of our other current patents commenced, and most of our future patents, if any, will commence, on the date of issuance and terminate 20 years from the earliest effective filing date of the patent application. Because any marketing and regulatory approval for a drug often occurs several years after the related patent application is filed, the resulting market exclusivity afforded by any patent on our drug candidates and technologies will likely be substantially less than 20 years.
As most patent applications in the U.S. are maintained as confidential until published by the USPTO at 18 months from filing for all cases filed after November 29, 2000, or at issue, for cases filed prior to November 29, 2000, we cannot be certain that we or Presage Biosciences, Inc. were the first to make the inventions covered by the patents and applications referred to above. Additionally, publication of discoveries in the scientific or patent literature often lags behind the actual discoveries. Moreover, pursuant to the terms of the Uruguay Round Agreements Act, patents filed on or after June 8, 1995 have a term of twenty years from the date of such filing except for provisional applications, irrespective of the period of time it may take for such patent to ultimately issue. This may shorten the period of patent protection afforded to therapeutic uses of pracinostat, ME-401, voruciclib or ME-344 as patent applications in the biopharmaceutical sector often take considerable time to issue. However, in some countries the patent term may be extended.
In order to protect the confidentiality of our technology, including trade secrets and know-how and other proprietary technical and business information, we require all of our consultants, advisors and collaborators to enter into agreements that prohibit the use or disclosure of information that is deemed confidential. These agreements also oblige our consultants, advisors and collaborators to assign to us, or negotiate a license to developments, discoveries and inventions made by such persons in connection with their work relating to our products. We cannot be sure that confidentiality will be maintained by those from whom we have acquired technology or disclosure prevented by these agreements. We also cannot be sure that our proprietary information or intellectual property will be protected by these agreements or that others will not independently develop substantially equivalent proprietary information or intellectual property.
The pharmaceutical industry is highly competitive, and patents may have been applied for by, and issued to, other parties relating to products competitive with pracinostat, ME-401, voruciclib, or ME-344. Use of these compounds and any other drug candidates may give rise to claims that they infringe the patents or proprietary rights of other parties, existing now and in the future. An adverse claim could subject us to significant liabilities to such other parties and/or require disputed rights to be licensed from such other parties. We cannot be sure that any license required under any such patents or proprietary rights would be made available on terms acceptable to us, if at all. If we do not obtain such licenses, we may encounter delays in product market introductions, or may find that the development, manufacture or sale of products requiring such licenses may be precluded.
Research and Development
The objective of our research and development program is the generation of data sufficient to achieve regulatory approval of our drug candidates in one or more dosage forms in major markets such as the U.S., to meet medical needs and develop a clinical and commercial profile with attractive attributes, and/or to allow us to enter into a development and/or commercial relationship with another party. The data are generated by our pre-clinical studies and clinical trial programs.
The key aspects of our research and development program are to provide more complete characterization of the following:
| • | the relevant molecular targets of action of our drug candidates; |
| • | the relative therapeutic benefits and indications for use of our drug candidates as a monotherapy or as part of combinational therapy with other agents; and |
| • | the most appropriate therapeutic indications and dosage forms for pracinostat, ME-401, voruciclib and ME-344. |
Government Regulation
U.S. Regulatory Requirements
The FDA, and comparable regulatory agencies in other countries, regulate and impose substantial requirements upon the research, development, pre-clinical and clinical testing, labeling, manufacture, quality control, storage, approval, advertising, promotion, marketing, distribution and export of pharmaceutical products including biologics, as well as significant reporting and record-keeping obligations. State governments may also impose obligations in these areas.
In the U.S., pharmaceutical products are regulated by the FDA under the Federal Food, Drug, and Cosmetic Act ("FDCA") and other laws, including in the case of biologics, the Public Health Service Act. We believe, but cannot be certain, that our products will be regulated as drugs by the FDA. The process required by the FDA before drugs may be marketed in the U.S. generally involves the following:
9
| • | pre-clinical laboratory evaluations, including formulation and stability testing, and animal tests performed under the FDA's Good Laboratory Practices regulations to assess pharmacological activity and toxicity potential; |
| • | submission and approval of an IND, including results of pre-clinical tests, manufacturing information, and protocols for clinical tests, which must become effective before clinical trials may begin in the U.S.; |
| • | obtaining approval of Institutional Review Boards ("IRB"), to administer the products to human subjects in clinical trials; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the product for the product's intended use; |
| • | development of manufacturing processes which conform to FDA current Good Manufacturing Practices ("cGMP"), as confirmed by FDA inspection; |
| • | submission of results for pre-clinical and clinical studies, and chemistry, manufacture and control information on the product to the FDA in a New Drug Approval Application ("NDA"); and |
| • | FDA review and approval of a NDA, prior to any commercial sale or shipment of a product. |
The testing and approval process requires substantial time, effort, and financial resources, and we cannot be certain that any approval will be granted on a timely basis, if at all.
The results of the pre-clinical studies, together with initial specified manufacturing information, the proposed clinical trial protocol, and information about the participating investigators are submitted to the FDA as part of an IND, which must become effective before we may begin human clinical trials in the U.S. Additionally, an independent IRB must review and approve each study protocol and oversee conduct of the trial. An IND becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day period, raises concerns or questions about the conduct of the trials as outlined in the IND and imposes a clinical hold. If the FDA imposes a clinical hold, the IND sponsor must resolve the FDA's concerns before clinical trials can begin. Pre-clinical tests and studies can take several years to complete, and there is no guarantee that an IND we submit based on such tests and studies will become effective within any specific time period, if at all.
Human clinical trials are typically conducted in three sequential phases that may overlap.
| • | Phase 1: The drug is initially introduced into healthy human subjects or patients and tested for safety and dosage tolerance. Absorption, metabolism, distribution, and excretion testing is generally performed at this stage. |
| • | Phase 2: The drug is studied in controlled, exploratory therapeutic trials in a limited number of subjects with the disease or medical condition for which the new drug is intended to be used in order to identify possible adverse effects and safety risks, to determine the preliminary or potential efficacy of the product for specific targeted diseases or medical conditions, and to determine dosage tolerance and the optimal effective dose. |
| • | Phase 3: When Phase 2 studies demonstrate that a specific dosage range of the drug is likely to be effective and the drug has an acceptable safety profile, controlled, large-scale therapeutic Phase 3 trials are undertaken at multiple study sites to demonstrate clinical efficacy and to further test for safety in an expanded patient population. |
We cannot be certain that we will successfully complete clinical testing of our products within any specific time period, if at all. Furthermore, the FDA, the IRB or we may suspend or terminate clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
Results of pre-clinical studies and clinical trials, as well as detailed information about the manufacturing process, quality control methods, and product composition, among other things, are submitted to the FDA as part of a NDA seeking approval to market and commercially distribute the product on the basis of a determination that the product is safe and effective for its intended use. Before approving a NDA, the FDA will inspect the facilities at which the product is manufactured and will not approve the product unless cGMP compliance is satisfactory. If applicable regulatory criteria are not satisfied, the FDA may deny the NDA or require additional testing or information. As a condition of approval, the FDA also may require post-marketing testing or surveillance to monitor the product's safety or efficacy. Even after a NDA is approved, the FDA may impose additional obligations or restrictions (such as labeling changes, or clinical post-marketing requirements), or even suspend or withdraw a product approval on the basis of data that arise after the product reaches the market, or if compliance with regulatory standards is not maintained. We cannot be certain that any NDA we submit will be approved by the FDA on a timely basis, if at all. Also, any such approval may limit the indicated uses for which the product may be marketed. Any refusal to approve, delay in approval, suspension or withdrawal of approval, or restrictions on indicated uses could have a material adverse impact on our business prospects.
Each NDA must be accompanied by a user fee, pursuant to the requirements of the Prescription Drug User Fee Act ("PDUFA"), and its amendments. According to the FDA's fee schedule, the user fee for an application requiring clinical data, such as an NDA, is $2,421,495. PDUFA also imposes an annual product fee for prescription drugs and biologics $97,750, and an annual establishment fee $512,200 on facilities used to manufacture prescription drugs and biologics. The FDA adjusts the PDUFA user fees on an annual basis. A written request can be submitted for a waiver for the application fee for the first human drug application that is filed by a small business, but there are no waivers for product or establishment fees. We are not at the stage of development with our products where we are subject to these fees, but they are significant expenditures that may be incurred in the future and must be paid at the time of application submissions to the FDA.
10
Satisfaction of FDA requirements typically takes several years. The actual time required varies substantially, based upon the type, complexity, and novelty of the pharmaceutical product, among other things. Government regulation imposes costly and time-consuming requirements and restrictions throughout the product life cycle and may delay product marketing for a considerable period of time, limit product marketing, or prevent marketing altogether. Success in pre-clinical or early stage clinical trials does not ensure success in later stage clinical trials. Data obtained from pre-clinical and clinical activities are not always conclusive and may be susceptible to varying interpretations that could delay, limit, or prevent marketing approval. Even if a product receives marketing approval, the approval is limited to specific clinical indications. Further, even after marketing approval is obtained, the discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
After product approval, there are continuing significant regulatory requirements imposed by the FDA, including record-keeping requirements, obligations to report adverse side effects in patients using the products, and restrictions on advertising and promotional activities. Quality control and manufacturing procedures must continue to conform to cGMPs, and the FDA periodically inspects facilities to assess cGMP compliance. Additionally, post-approval changes in ingredient composition, manufacturing processes or facilities, product labeling, or other areas may require submission of a NDA Supplement to the FDA for review and approval. New indications will require additional clinical studies and submission of a NDA Supplement. Failure to comply with FDA regulatory requirements may result in an enforcement action by the FDA, including Warning Letters, product recalls, suspension or revocation of product approval, seizure of product to prevent distribution, impositions of injunctions prohibiting product manufacture or distribution, and civil and criminal penalties. Maintaining compliance is costly and time-consuming. We cannot be certain that we, or our present or future suppliers or third-party manufacturers, will be able to comply with all FDA regulatory requirements, and potential consequences of noncompliance could have a material adverse impact on our business prospects.
The FDA's policies may change, and additional governmental regulations may be enacted that could delay, limit, or prevent regulatory approval of our products or affect our ability to manufacture, market, or distribute our products after approval. Moreover, increased attention to the containment of healthcare costs in the U.S. and in foreign markets could result in new government regulations that could have a material adverse effect on our business. Our ability to commercialize future products will depend in part on the extent to which coverage and reimbursement for the products will be available from government and health administration authorities, private health insurers, and other third-party payers. European Union member states and U.S. government and other third-party payers increasingly are attempting to contain healthcare costs by consideration of new laws and regulations limiting both coverage and the level of reimbursement for new drugs. Our failure to obtain coverage, an adequate level of reimbursement, or acceptable prices for our future products could diminish any revenues we may be able to generate. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the U.S. or abroad.
Our activities also may be subject to state laws and regulations that affect our ability to develop and sell our products. We are also subject to numerous federal, state, and local laws relating to such matters as safe working conditions, clinical, laboratory, and manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future, and the failure to comply may have a material adverse impact on our business prospects.
The FDCA includes provisions designed to facilitate the development and expedite the review of drugs and biological products intended for treatment of serious or life-threatening conditions that demonstrate the potential to address unmet medical needs for such conditions. These provisions set forth a procedure for designation of a drug as a "fast track product". The fast track designation applies to the combination of the product and specific indication for which it is being studied. A product designated as fast track is ordinarily eligible for additional programs for expediting development and review, but products that are not in fast track drug development programs may also be able to take advantage of these programs. These programs include priority review of NDAs and accelerated approval. Drug approval under the accelerated approval regulations may be based on evidence of clinical effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. A post-marketing clinical study will be required to verify clinical benefit, and other restrictions to assure safe use may be imposed. We do not currently have fast track designation for any of our clinical programs. If we should seek such designation for any of our programs, however, we cannot be assured that it will be granted by the FDA.
Under the Drug Price Competition and Patent Term Restoration Act of 1984, a sponsor may obtain marketing exclusivity for a period of time following FDA approval of certain drug applications, regardless of patent status, if the drug is a new chemical entity or if new clinical studies were required to support the marketing application for the drug. This marketing exclusivity prevents a third party from obtaining FDA approval for an identical or nearly identical drug under an Abbreviated New Drug Application or a "505(b)(2) New Drug Application". The statute also allows a patent owner to obtain an extension of applicable patent terms for a period equal to one-half the period of time elapsed between the filing of an IND and the filing of the corresponding NDA plus the period of time between the filing of the NDA and FDA approval, with a five year maximum patent extension. We cannot be certain that we will be able to take advantage of either the patent term extension or marketing exclusivity provisions of these laws.
The Best Pharmaceuticals for Children Act ("BPCA"), signed into law on January 4, 2002, was reauthorized and amended by the FDA Amendments Act of 2007 ("FDAAA"). The reauthorization of BPCA provides an additional six months of patent protection to NDA applicants that conduct acceptable pediatric studies of new and currently-marketed drug products for which pediatric information would be beneficial, as identified by the FDA in a Pediatric Written Request. The Pediatric Research Equity Act ("PREA"), signed into law on December 3, 2003, also was reauthorized and amended by the FDAAA. The reauthorization of PREA requires that most applications for drugs and biologics include a pediatric assessment (unless waived or deferred) to ensure the drugs' and biologics' safety and effectiveness in children. Such pediatric assessment must contain data, gathered using appropriate
11
formulations for each age group for which the assessment is required, that are adequate to assess the safety and effectiveness of the drug or the biological product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the drug or the biological product is safe and effective. The pediatric assessments can only be deferred provided there is a timeline for the completion of such studies. The FDA may waive (partially or fully) the pediatric assessment requirement for several reasons, including if the applicant can demonstrate that reasonable attempts to produce a pediatric formulation necessary for that age group have failed. The Food and Drug Administration Safety and Innovation Act signed into law on July 9, 2012, permanently renewed and strengthened BPCA and PREA.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a "rare disease or condition," which generally is a disease or condition that affects fewer than 200,000 individuals in the U.S. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. If a product which has an orphan drug designation subsequently receives the first FDA approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, i.e., the FDA may not approve any other applications to market the same drug for the same indication for a period of seven years, except in limited circumstances. Pracinostat has been granted orphan drug designation by the FDA for the treatment of AML, but it may not receive orphan designation for other indications. Our other products may not be eligible for orphan drug status or be designated as orphan drugs. Even if designated as orphan drugs, our products may not be approved before other applications or granted orphan drug exclusivity if approved.
Foreign Regulatory Requirements
Outside the U.S., our ability to market our products will also be contingent upon receiving marketing authorizations from the appropriate regulatory authorities and compliance with applicable post-approval regulatory requirements. Although the specific requirements and restrictions vary from country to country, as a general matter, foreign regulatory systems include risks similar to those associated with FDA regulation, described above.
Under European Union regulatory systems, marketing authorizations may be submitted either under a centralized or a national procedure. Under the centralized procedure, a single application to the EMA leads to an approval granted by the European Commission which permits the marketing of the product throughout the EU. The centralized procedure is mandatory for certain classes of medicinal products, but optional for others. For example, all medicinal products developed by certain biotechnological means, and those developed for cancer and other specified diseases and disorders, must be authorized via the centralized procedure. We assume that the centralized procedure will apply to our products that are developed by means of a biotechnology process or are intended for treatment of cancer. The national procedure is used for products that are not required to be authorized by the centralized procedure. Under the national procedure, an application for a marketing authorization is submitted to the competent authority of one member state of the EU. The holders of a national marketing authorization may submit further applications to the competent authorities of the remaining member states via either the decentralized or mutual recognition procedure. The decentralized procedure enables applicants to submit an identical application to the competent authorities of all member states where approval is sought at the same time as the first application, while under the mutual recognition procedure, products are authorized initially in one member state, and other member states where approval is sought are then requested to recognize the original authorization based upon an assessment report prepared by the original authorizing competent authority. Both the decentralized and mutual recognition procedures should take no longer than 90 days, but if one member state makes an objection, which under the legislation can only be based on a possible risk to human health, the application will be automatically referred to the Committee for Medicinal Products for Human Use ("CHMP") of the EMA. If a referral for arbitration is made, the procedure is suspended. However, member states that have already approved the application may, at the request of the applicant, authorize the product in question without waiting for the result of the arbitration. Such authorizations will be without prejudice to the outcome of the arbitration. For all other concerned member states, the opinion of the CHMP, which is binding, could support or reject the objection or alternatively could reach a compromise position acceptable to all EU countries concerned. The arbitration procedure may take an additional year before a final decision is reached and may require the delivery of additional data.
As with FDA approval, we may not be able to secure regulatory approvals in Europe in a timely manner, if at all. Additionally, as in the U.S., post-approval regulatory requirements, such as those regarding product manufacture, marketing, or distribution, would apply to any product that is approved in Europe, and failure to comply with such obligations could have a material adverse effect on our ability to successfully commercialize any product.
The conduct of clinical trials in the European Union is governed by the European Clinical Trials Directive, which was implemented in May 2004. This Directive governs how regulatory bodies in member states control clinical trials. No clinical trial may be started without a clinical trial authorization granted by the national competent authority and favorable ethics approval. New legislation to revise and replace the European Clinical Trials Directive is currently proposed by the European Commission and is under consideration by EU institutions.
Accordingly, there is a marked degree of change and uncertainty both in the regulation of clinical trials and in respect of marketing authorizations which we face for our products in Europe.
Manufacturing
We do not have the facilities or capabilities to commercially manufacture any of our drug candidates. We are and expect to continue to be dependent on contract manufacturers for supplying our existing and future candidates for clinical trials and commercial scale manufacturing of our candidates in accordance with regulatory requirements, including cGMP. Contract manufacturers may utilize their own technology, technology developed by us, or technology acquired or licensed from third parties. FDA approval of the manufacturing procedures and the site will be required prior to commercial distribution.
12
Employees
As of June 30, 2018, we had 32 employees, ten of whom hold a Ph.D. or M.D. degree. Other personnel resources are used from time to time as consultants or third party service organizations on an as-needed basis. All members of our senior management team have prior experience with pharmaceutical, biotechnology or medical product companies. We believe that we have been successful in attracting skilled and experienced personnel, but there can be no assurance that we will be able to attract and retain the individuals needed. None of our employees are represented by a collective bargaining agreement, nor have we experienced work stoppages. We believe that our relations with our employees are good.
Available Information
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed with or furnished to the Securities and Exchange Commission pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, are available free of charge through our website at www.meipharma.com as soon as reasonably practicable after they are electronically filed with, or furnished to, the Securities and Exchange Commission.
| Item 1A. | Risk Factors |
Investment in our securities involves a high degree of risk. You should consider carefully the risks described below, together with other information in this Annual Report and other public filings, before making investment decisions regarding our securities. If any of the following events actually occur, our business, operating results, prospects or financial condition could be materially and adversely affected. This could cause the trading price of our common stock to decline and you may lose all or part of your investment. Moreover, the risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business, operating results, prospects or financial condition.
Risks Related to Our Business and Industry
We will need substantial additional funds to progress the clinical trial program for our drug candidates, and to develop new compounds. The actual amount of funds we will need will be determined by a number of factors, some of which are beyond our control.
We will need substantial additional funds to progress the clinical trial program for our drug candidates and to develop any additional compounds. The factors that will determine the actual amount of funds that we will need to progress the clinical trial programs may include, but are not limited to, the following:
| • | the therapeutic indications for use being developed; |
| • | the clinical trial endpoint required to achieve regulatory approval; |
| • | the number of clinical trials required to achieve regulatory approval; |
| • | the number of sites included in the trials; |
| • | the length of time required to enroll suitable patients; |
| • | the number of patients who participate in the trials and the rate that they are recruited; |
| • | the number of treatment cycles patients complete while they are enrolled in the trials; |
| • | costs and potential difficulties encountered in manufacturing sufficient drug product for the trials; and |
| • | the efficacy and safety profile of the product. |
We have been opportunistic in our efforts to obtain cash, and we expect to continue to evaluate various funding alternatives from time to time. If we obtain additional funding, it may adversely affect the market price of our common stock and may be dilutive to existing stockholders. If we are unable to obtain additional funds on favorable terms or at all, we may be required to cease or reduce our operations. We may sell additional shares of common stock, and securities exercisable for or convertible into shares of our common stock, to satisfy our capital and operating needs; however, such transactions will be subject to market conditions and there can be no assurance any such transactions will be completed.
If Helsinn does not satisfy its obligations under our collaboration agreement or if it terminates the collaboration agreement, we may not be able to develop or commercialize pracinostat.
In August 2016, we entered into an exclusive license, development and commercialization agreement with Helsinn to collaborate on the global development, manufacturing and commercialization of pracinostat. In connection with this agreement, we granted to Helsinn certain rights regarding the use of our patents and technology with respect to pracinostat. Helsinn will be primarily responsible for the global development of pracinostat and, subject to certain exceptions, will be solely responsible for all costs related thereto, and will also be solely responsible for the global commercialization of pracinostat and shall be solely responsible for the costs related thereto.
Helsinn might not fulfill all of its obligations under the agreement. Our ability to receive revenue from pracinostat is dependent upon Helsinn's efforts. If Helsinn fails to devote adequate resources or otherwise does not successfully develop and commercialize pracinostat, we may not receive the future milestone payments or royalties provided for in the agreement. In addition, under certain circumstances, including our failure to satisfy our obligations under the agreement, Helsinn has the right to terminate the agreement.
13
We could also become involved in disputes with Helsinn, which could lead to delays in or termination of the agreement and time-consuming and expensive litigation or arbitration.
If Helsinn is unwilling or unable to fulfill its obligations or if the agreement is terminated, we may lack sufficient resources to develop and commercialize pracinostat on our own and may be unable to reach agreement with a suitable alternative collaborator. The failure to develop and commercialize pracinostat would have a material adverse effect on our business, operating results, prospects and financial condition.
We are subject to significant obligations to Presage in connection with our license of voruciclib, which could adversely affect the overall profitability of any products we may seek to commercialize, and our license of voruciclib, the development and commercialization of which we are solely responsible for, may never become profitable.
In September 2017, we entered into the Presage License Agreement. Under the terms of the agreement, Presage granted us exclusive worldwide rights to develop, manufacture and commercialize voruciclib, a clinical-stage, oral and selective CDK inhibitor, and related compounds. In exchange, we paid Presage $2.9 million and are obligated for additional potential payments of up to $181 million upon the achievement of certain development, regulatory and commercial milestones. We will also pay mid-single-digit tiered royalties on the net sales of any product successfully developed pursuant to such agreement. We may be obligated to make milestone or royalty payments when we do not have the cash on hand to make these payments or have available cash for our other development efforts. These milestone and royalty payments could adversely affect the overall profitability for us of any products that we may seek to commercialize. In addition, if we fail to comply with our obligations under the license agreement, Presage may have the right to terminate the agreement. In such a case, we would lose our rights to the intellectual property covered by the license agreement and we would not be able to develop, manufacture or commercialize voruciclib and may face other penalties.
The profitability of our license agreement with Presage depends on the successful development, regulatory approval and commercialization of voruciclib. We are solely responsible for the development and commercialization of voruciclib, including the related costs. Drug development is a long, expensive and uncertain process and delay or failure can occur at any stage of our clinical trials. We cannot be certain that we will ever receive regulatory approval for voruciclib or that it will be successfully commercialized, even if approved.
Negative U.S. and global economic conditions may pose challenges to our business strategy, which relies on funding from the financial markets or collaborators.
Negative conditions in the U.S. or global economy, including financial markets, may adversely affect our business and the business of current and prospective vendors, licensees and collaborators, and others with whom we do or may conduct business. The duration and severity of these conditions is uncertain. If negative economic conditions occur, we may be unable to secure funding on terms satisfactory to us to sustain our operations or to find suitable collaborators to advance our internal programs, even if we achieve positive results from our drug development programs.
We are a clinical research and development stage company and are likely to incur operating losses for the foreseeable future.
You should consider our prospects in light of the risks and difficulties frequently encountered by clinical research stage and developmental companies. We have incurred net losses of $214.4 million from our inception through June 30, 2018, including a net loss of $30.4 million for the year ended June 30, 2018 (excluding a $9.7 million non-cash expense resulting from a change in the fair value of our warrant liability), net income of $2.7 million for the year ended June 30, 2017, and a net loss of $20.9 million for the year ended June 30, 2016. We anticipate that we will incur operating losses and negative operating cash flow for the foreseeable future. We have not yet commercialized any drug candidates and cannot be sure that we will ever be able to do so, or that we may ever become profitable.
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current drug candidates may not have favorable results in later studies or trials.
Pre-clinical studies and Phase 1 and Phase 2 clinical trials are an expensive and uncertain process that may take years to complete. Pre-clinical studies and Phase 1 and Phase 2 clinical trials are not primarily designed to test the efficacy of a drug candidate, but rather to test safety, to study pharmacokinetics and pharmacodynamics, and to understand the drug candidate's side effects at various doses and schedules. Favorable results in early studies or trials may not be repeated in later studies or trials, including ongoing pre-clinical studies, large-scale Phase 3 clinical trials, or other studies intended as registration trials, and our drug candidates in later-stage trials may fail to show desired safety and efficacy despite having progressed through earlier-stage trials. Unfavorable results from ongoing pre-clinical studies or clinical trials could result in delays, modifications or abandonment of ongoing or future clinical trials, or abandonment of a clinical program. Pre-clinical and clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals or commercialization. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be delayed, repeated or terminated, or a clinical program to be abandoned.
14
Final approval by regulatory authorities of our drug candidates for commercial use may be delayed, limited or prevented, any of which would adversely affect our ability to generate operating revenues.
We will not generate any operating revenue until we, a licensee, or a potential collaborator successfully commercialize one of our drug candidates. Currently, we have drug candidates at different stages of development, and each will need to successfully complete certain clinical studies and obtain regulatory approval before potential commercialization.
The pre-clinical and clinical development, manufacturing, labeling, packaging, storage, recordkeeping, export, marketing and distribution, and other possible activities relating to our drug candidates are subject to extensive regulation by the FDA and other regulatory agencies. Failure to comply with applicable regulatory requirements may, either before or after product approval, subject us to administrative or judicially imposed sanctions that may negatively impact the approval of one or more of our drug candidates or otherwise negatively impact our business.
Neither collaborators, licensees nor we are permitted to market a drug candidate in the United States until the particular drug candidate is approved for marketing by the FDA. Specific pre-clinical data, chemistry, manufacturing and controls data, a proposed clinical trial protocol and other information must be submitted to the FDA as part of an IND application, and clinical trials may commence only after the IND application becomes effective. To market a new drug in the United States, we must submit to the FDA and obtain FDA approval of an NDA. An NDA must be supported by extensive clinical and pre-clinical data, as well as extensive information regarding chemistry, manufacturing and controls to demonstrate the safety and effectiveness of the drug candidate.
Obtaining approval of an NDA can be a lengthy, expensive and uncertain process. Regulatory approval of an NDA is not guaranteed. The number and types of pre-clinical studies and clinical trials that will be required for FDA approval varies depending on the drug candidate, the disease or condition that the drug candidate is designed to target and the regulations applicable to any particular drug candidate. Despite the time and expense exerted in pre-clinical and clinical studies, failure can occur at any stage, and we could encounter problems that cause us to abandon clinical trials or to repeat or perform additional pre-clinical studies and clinical trials. The FDA can delay, limit or deny approval of a drug candidate for many reasons, including but not limited to, the following:
| • | a drug candidate may not be deemed adequately safe or effective for an intended use; |
| • | the FDA may not find the data from pre-clinical studies and clinical trials sufficient; |
| • | the FDA's interpretation and our interpretation of data from pre-clinical studies and clinical trials may differ significantly; |
| • | our or our contractors' or collaborators' failure to comply with applicable FDA and other regulatory requirements, including those identified in other risk factors; |
| • | the FDA may not approve the manufacturing processes or facilities; |
| • | the FDA may change its approval policies or adopt new regulations; or |
| • | the FDA may not accept an NDA or other submission due to, among other reasons, the content or formatting of the submission. |
Our pre-clinical and clinical data, other information and procedures relating to a drug candidate may not be sufficient to support approval by the FDA or any other U.S. or foreign regulatory authority, or regulatory interpretation of these data and procedures may be unfavorable. Our efforts to take advantage of expedited regulatory pathways for serious or life-threatening illnesses, such as accelerated approval, to secure marketing authorization more quickly may not be successful. Securing accelerated approval requires demonstrating a meaningful therapeutic benefit over existing treatments, and FDA may require post-marketing studies to verify clinical benefit. Our business and reputation may be harmed by any failure or significant delay in receiving regulatory approval for the sale of any drugs resulting from our drug candidates. As a result, we cannot predict when or whether regulatory approval will be obtained for any drug we develop.
Additionally, other factors may serve to delay, limit or prevent the final approval by regulatory authorities of our drug candidates for commercial use, including, but not limited to:
| • | Pracinostat, ME-401, voruciclib, and ME-344 are in various stages of development, and we or our licensees will need to conduct significant clinical testing to demonstrate safety and efficacy of these drug candidates before applications for marketing can be filed with the FDA, or with the regulatory authorities of other countries; |
| • | development and testing of product formulation, including identification of suitable excipients, or chemical additives intended to facilitate delivery of our drug candidates; |
| • | it may take us many years to complete the testing of our drug candidates, and failure can occur at any stage of this process; and |
| • | negative or inconclusive results or adverse medical events during a clinical trial could cause us to delay or terminate our development efforts. |
The successful development of any of these drug candidates is uncertain and, accordingly, we may never commercialize any of these drug candidates or generate significant revenue.
Failure to obtain regulatory approval in foreign jurisdictions would prevent us from marketing our products internationally.
We intend to have our drug candidates marketed outside the United States. In order to market our products in many non-U.S. jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. To date, we have not filed for marketing approval for any of our drug candidates and may not receive the approvals necessary to commercialize our drug candidates in any market.
15
The approval procedure varies among countries and may include all of the risks associated with obtaining FDA approval. Further, the time required to obtain foreign regulatory approval may differ from that required to obtain FDA approval, and additional pre-clinical studies, clinical trials, other testing and data review may be required. We may not obtain foreign regulatory approvals on a timely basis, if at all. Additionally, approval by the FDA does not ensure approval by regulatory agencies in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory agencies in other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in other jurisdictions, including approval by the FDA. The failure to obtain regulatory approval in foreign jurisdictions could limit commercialization of our products, reduce our ability to generate profits and harm our business.
The Breakthrough Therapy Designation granted by the FDA to pracinostat for the treatment of AML and any additional Breakthrough Therapy Designation granted by the FDA for any of our product candidates or other indications of pracinostat, may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that our product candidates will receive marketing approval.
In August 2016, the FDA granted Breakthrough Therapy Designation to pracinostat for the treatment of AML. We may also seek such designation for pracinostat for the treatment of MDS or for our other drug product candidates if our clinical data supports such a designation. A Breakthrough Therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. For drugs that have been designated as Breakthrough Therapies, interaction and communication between the FDA and the sponsor can help to identify the most efficient path for development.
Designation as a Breakthrough Therapy is within the discretion of the FDA. Accordingly, even if we believe that one of our product candidates meets the criteria for designation as a Breakthrough Therapy, the FDA may disagree and instead determine not to make such designation. However, the reduced timelines may introduce significant chemistry, manufacturing and controls challenges for product development. In any event, the receipt of a Breakthrough Therapy Designation for a product candidate may not result in a faster development process, review or approval compared to drugs considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even after granting a Breakthrough Therapy Designation, the FDA may later decide that such product candidates no longer meet the conditions for qualification and rescind such designations or decide that the time period for FDA review or approval will not be shortened.
Any orphan drug designations we receive may not confer marketing exclusivity or other benefits.
In the United States, under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product intended to treat a rare disease or condition. Such diseases and conditions are those that affect fewer than 200,000 individuals in the United States, or if they affect more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making a drug available in the United States for these types of diseases or conditions will be recovered from sales of the drug. Orphan drug designation must be requested before submitting an NDA. If the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by that agency. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process, but it can lead to financial incentives, such as opportunities for grant funding toward clinical trial costs, tax advantages and user-fee waivers.
If a drug that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the drug is entitled to orphan drug marketing exclusivity for a period of seven years. Orphan drug marketing exclusivity generally prevents the FDA from approving another application, including a full NDA, to market the same drug or biological product for the same indication for seven years, except in limited circumstances, including if the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care. For purposes of small molecule drugs, the FDA defines "same drug" as a drug that contains the same active chemical entity and is intended for the same use as the drug in question. A designated orphan drug may not receive orphan drug marketing exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. Orphan drug marketing exclusivity rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
In January 2018, the EMA granted orphan drug designation to pracinostat for the treatment of AML. The European exclusivity period is ten years but can be reduced to six years if the drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. Orphan drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
16
A Fast Track Designation by the FDA, even if granted for any of our product candidates, may not lead to a faster development or regulatory review or approval process and does not increase the likelihood that our product candidates will receive marketing approval.
We do not currently have Fast Track Designation for any of our product candidates but may seek Fast Track Designation if our clinical data supports such a designation. If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply to the FDA for Fast Track Designation. The FDA has broad discretion whether to grant this designation. Even if we believe a specific product candidate is eligible for this designation, we cannot assure you that the FDA would decide to grant it. Even if we do receive Fast Track Designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program. Many drugs that have received Fast Track Designation have failed to obtain drug approval.
Even if we or our licensees receive regulatory approval to commercialize our drug candidates, our ability to generate revenues from any resulting products will be subject to a variety of risks, many of which are out of our control.
Even if our drug candidates obtain regulatory approval, resulting products may not gain market acceptance among physicians, patients, healthcare payers or the medical community. We believe that the degree of market acceptance and our ability to generate revenues from such products will depend on a number of factors, including, but not limited to, the following:
| • | timing of market introduction of our drugs and competitive drugs; |
| • | actual and perceived efficacy and safety of our drug candidates; |
| • | prevalence and severity of any side effects; |
| • | potential or perceived advantages or disadvantages over alternative treatments; |
| • | potential post-marketing commitments imposed by regulatory authorities, such as patient registries; |
| • | strength of sales, marketing and distribution support; |
| • | price of our future products, both in absolute terms and relative to alternative treatments; |
| • | the effect of current and future healthcare laws on our drug candidates; and |
| • | availability of coverage and reimbursement from government and other third-party payers. |
If any of our drugs are approved and fail to achieve market acceptance, we may not be able to generate significant revenue to achieve or sustain profitability.
If any products we develop become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, our ability to successfully commercialize our products will be impaired.
Our future revenues, profitability and access to capital will be affected by the continuing efforts of governmental and private third-party payers to manage, contain or reduce the costs of health care through various means. We expect a number of federal, state and foreign proposals to control or influence the cost of drugs through government regulation. We are unsure of the impact that the potential repeal of health care reform legislation may have on our business or what actions federal, state, foreign and private payers may take or reforms that may be implemented in the future. Therefore, it is difficult to predict the effect of any potential reform on our business. Our ability to commercialize our drug candidates successfully will depend, in part, on the extent to which reimbursement for the cost of such drug candidates and related treatments will be available from government health administration authorities, such as Medicare and Medicaid in the United States, private health insurers and other organizations. Significant uncertainty exists as to the reimbursement status of newly approved health care products, particularly for indications for which there is no current effective treatment or for which medical care typically is not sought. Adequate third-party coverage may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in research and development. If adequate coverage and reimbursement levels are not provided by government and third-party payers for use of our products, our products may fail to achieve market acceptance without a substantial reduction in price or at all and our results of operations will be harmed.
Even if our drug candidates obtain regulatory approval, we and our collaborators will be subject to ongoing government regulation.
Even if regulatory authorities approve any of our drug candidates, the manufacture, marketing and sale of these drugs will be subject to strict and ongoing regulation. Compliance with such regulations may consume substantial financial and management resources and expose us and our collaborators to the potential for other adverse circumstances. For example, a regulatory authority can place restrictions on the sale or marketing of a drug in order to manage the risks identified during initial clinical trials or after the drug is on the market. A regulatory authority can condition the approval for a drug on costly post-marketing follow-up studies. Based on these studies, if a regulatory authority does not believe that the drug demonstrates a clinical benefit to patients or an acceptable safety profile, it could limit the indications for which a drug may be sold or revoke the drug's marketing approval. In addition, identification of certain side effects either during clinical trials or after a drug is on the market may result in reformulation of a drug, additional pre-clinical and clinical trials, labeling changes, termination of ongoing clinical trials or withdrawal of approval. Any of these events could delay or prevent us from generating revenue from the commercialization of these drugs and cause us to incur significant additional costs.
We may not be able to establish the contractual arrangements necessary to develop, market and distribute our drug candidates.
A key part of our strategy is to establish contractual relationships with third parties to package, market and distribute our drug candidates. There is no assurance that we will be able to negotiate commercially acceptable licensing or other agreements for the future exploitation of our drug candidates, including continued clinical development, manufacture or marketing. If we are unable to successfully contract for these services, or if arrangements for these services are terminated, we may have to delay our commercialization program which will adversely affect our ability to generate operating revenues.
17
Our commercial opportunity will be reduced or eliminated if competitors develop and market products that are more effective, have fewer side effects or are less expensive than our drug candidates.
The development of drug candidates is highly competitive. A number of other companies have products or drug candidates that have either been approved or are in various stages of pre-clinical or clinical development that are intended for the same therapeutic indications for which our drug candidates are being developed. Some of these potential competing drug candidates are further advanced in development than our drug candidates and may be commercialized sooner. Even if we are successful in developing effective drugs, our compounds may not compete successfully with products produced by our competitors.
Our competitors include pharmaceutical companies and biotechnology companies, as well as universities and public and private research institutions. In addition, companies active in different but related fields represent substantial competition for us. Many of our competitors developing oncology drugs have significantly greater capital resources, larger research and development staffs and facilities and greater experience in drug development, regulation, manufacturing and marketing than we do. These organizations also compete with us and our service providers, to recruit qualified personnel, and with us to attract partners for joint ventures and to license technologies that are competitive with us. As a result, our competitors may be able to more easily develop technologies and products that would render our technologies or our drug candidates obsolete or non-competitive.
We rely on third parties to conduct our clinical trials and pre-clinical studies. If those parties do not successfully carry out their contractual duties or meet expected deadlines, our drug candidates may not advance in a timely manner or at all.
In the course of our pre-clinical testing and clinical trials, we rely on third parties, including laboratories, investigators, clinical contract research organizations ("CROs"), and manufacturers, to perform critical services for us. For example, we rely on third parties to conduct our clinical trials and many of our pre-clinical studies, which are required to be conducted consistent with regulations on Good Laboratory Practice ("GLP"). CROs are responsible for many aspects of the trials, including finding and enrolling subjects for testing and administering the trials. Although we rely on these third parties to conduct our clinical trials, we are responsible for ensuring that each of our clinical trials is conducted in accordance with its investigational plan and protocol. Moreover, the FDA and foreign regulatory authorities require us to comply with regulations and standards, commonly referred to as Good Clinical Practices ("GCPs"), for conducting, monitoring, recording, and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate, and that the trial subjects are adequately informed of the potential risks of participating in clinical trials. Our reliance on third parties does not relieve us of these responsibilities and requirements. These third parties may not be available when we need them or, if they are available, may not comply with all regulatory and contractual requirements or may not otherwise perform their services in a timely or acceptable manner, and we may need to enter into new arrangements with alternative third parties and our clinical trials may be extended, delayed or terminated. These independent third parties may also have relationships with other commercial entities, some of which may compete with us. In addition, if such third parties fail to perform their obligations in compliance with our clinical trial protocols or GCPs, our clinical trials may not meet regulatory requirements or may need to be repeated. As a result of our dependence on third parties, we may face delays, failures or cost increases outside of our direct control. These risks also apply to the development activities of collaborators, and we do not control their research and development, clinical trial or regulatory activities.
We will depend on third party suppliers and contract manufacturers for the manufacturing of our drug candidates and have no direct control over the cost of manufacturing our drug candidates. Increases in the cost of manufacturing our drug candidates would increase our costs of conducting clinical trials and could adversely affect our future profitability.
We do not intend to manufacture our drug candidates ourselves, and we will rely on third parties for our drug supplies both for clinical trials and for commercial quantities in the future. We have taken the strategic decision not to manufacture active pharmaceutical ingredients ("API") for our drug candidates, as these can be more economically supplied by third parties with particular expertise in this area. We have identified contract facilities that are registered with the FDA, have a track record of large scale API manufacture, and have already invested in capital and equipment. We have no direct control over the manufacturing of our drug candidates, or the cost thereof. If the contract manufacturers are unable to produce sufficient quantities of our drug candidates, as a result of a lack of available materials or otherwise, our future profitability would be adversely affected. If the cost of manufacturing increases, or if the cost of the materials used increases, these costs will be passed on to us, making the cost of conducting clinical trials more expensive. Increases in manufacturing costs could adversely affect our future profitability if we are unable to pass all of the increased costs along to our customers.
Further, we, along with our contract manufacturers, are required to comply with FDA requirements for cGMPs, related to product testing, quality assurance, manufacturing and documentation. Our contract manufacturers may not be able to comply with the applicable FDA regulatory requirements. They may be required to pass an FDA preapproval inspection for conformity with cGMPs before we can obtain approval to manufacture our drug candidates and will be subject to ongoing, periodic, unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMP, and other applicable government regulations and corresponding foreign standards. If we and our contract manufacturers fail to achieve and maintain high manufacturing standards in compliance with cGMP, we may experience manufacturing errors resulting in defective products that could be harmful to patients, product recalls or withdrawals, delays or interruptions of production or failures in product testing or delivery, delay or prevention of filing or approval of marketing applications for our products, cost overruns or other problems that could seriously harm our business. Not complying with FDA requirements could result in a product recall, or prevent commercialization of our drug candidates and delay our business development activities. In addition, such failure could be the basis for the FDA to issue a warning or untitled letter or take other regulatory or legal enforcement action, including recall or seizure, total or partial suspension of production, suspension of ongoing clinical trials, refusal to approve pending applications or supplemental applications, and potentially civil and/or criminal penalties depending on the matter.
18
Our business strategy may include entry into additional collaborative or license agreements. We may not be able to enter into collaborative or license agreements or may not be able to negotiate commercially acceptable terms for these agreements.
Our current business strategy may include the entry into additional collaborative or license agreements for the development and commercialization of our drug and drug candidates. The negotiation and consummation of these types of agreements typically involve simultaneous discussions with multiple potential collaborators or licensees and require significant time and resources. In addition, in attracting the attention of pharmaceutical and biotechnology company collaborators or licensees, we compete with numerous other third parties with product opportunities as well as the collaborators' or licensees' own internal product opportunities. We may not be able to consummate collaborative or license agreements, or we may not be able to negotiate commercially acceptable terms for these agreements.
If we do enter into such arrangements, we could be dependent upon the subsequent success of these other parties in performing their respective responsibilities and the cooperation of our partners. Our collaborators may not cooperate with us or perform their obligations under our agreements with them. We cannot control the amount and timing of our collaborators' resources that will be devoted to researching our product candidates pursuant to our collaborative agreements with them. Our collaborators may choose to pursue existing or alternative technologies in preference to those being developed in collaboration with us.
Under agreements with any collaborators or licensees we may work with in the future, we may rely significantly on them to, among other activities:
| • | fund research and development activities with us; |
| • | pay us fees upon the achievement of milestones; and |
| • | market for or with us any commercial products that result from our collaborations. |
If we do not consummate collaborative or license agreements, we may use our financial resources more rapidly on our drug development efforts, continue to defer certain development activities or forego the exploitation of certain geographic territories, any of which could have a material adverse effect on our business prospects. Further, we may not be successful in overseeing any such collaborative arrangements. If we fail to establish and maintain necessary collaborative or license relationships, our business prospects could suffer.
We rely on acquisitions or licenses from third parties to expand our pipeline of drug candidates.
We are not presently engaged in drug discovery activities. In order to expand our pipeline of drug candidates for future development, we may need to purchase or in-license any such drug candidates. The success of this strategy depends in large part on the combination of our regulatory and development capabilities and expertise and our ability to identify, select and acquire or in-license clinically-enabled product candidates on terms that are acceptable to us. Identifying, selecting and acquiring or in-licensing promising product candidates requires substantial technical expertise, and we have limited experience in identifying and integrating any acquired product candidates into our current infrastructure. Efforts to do so may not result in the actual acquisition or in-license of a particular drug candidate, potentially resulting in a diversion of our management's time and the expenditure of our resources with no resulting benefit. If we are unable to identify, select and acquire or license suitable product candidates from third parties on terms acceptable to us, our business and prospects may be limited.
We face a risk of product liability claims and claims may exceed our insurance limits.
Our business exposes us to the risk of product liability claims. This risk is inherent in the manufacturing, testing and marketing of human therapeutic products. Our product liability insurance coverage is subject to deductibles and coverage limitations. We may not be able to obtain or maintain adequate protection against potential liabilities, or claims may exceed our insurance limits. If we cannot or do not sufficiently insure against potential product liability claims, we may be exposed to significant liabilities, which may materially and adversely affect our business development and commercialization efforts.
Our efforts will be seriously jeopardized if we are unable to retain and attract key employees.
Our success depends on the continued contributions of our principal management, development and scientific personnel. We face competition for such personnel, and we believe that risks and uncertainties related to our business, including the timing and risk associated with research and development, our available and anticipated cash resources, and the volatility of our stock price, may impact our ability to hire and retain key and other personnel. The loss of services of our Chief Executive Officer or other key employees could adversely impact our operations and ability to generate or raise additional capital.
Laws, rules and regulations relating to public companies may be costly and impact our ability to attract and retain directors and executive officers.
Laws and regulations affecting public companies, including rules adopted by the Securities and Exchange Commission ("SEC") and by the National Association of Securities Dealers Automated Quotations ("NASDAQ"), may result in increased costs to us. These laws, rules and regulations could make it more difficult or costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on our board committees or as executive officers. We cannot estimate accurately the amount or timing of additional costs we may incur to respond to these laws, rules and regulations.
19
If we fail to establish and maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired, which would adversely affect our operating results, our ability to operate our business, and our stock price, and could result in litigation or similar actions.
Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Failure on our part to have effective internal financial and accounting controls would cause our financial reporting to be unreliable, could have a material adverse effect on our business, operating results, and financial condition, and could cause the trading price of our common stock to fall dramatically. Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with accounting standards generally accepted in the United States of America ("U.S. GAAP"). A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the company will be detected.
We cannot be certain that the actions we have taken to ensure we have adequate internal controls over financial reporting will be sufficient. In future periods, if the process required by Section 404 of the Sarbanes-Oxley Act reveals any material weaknesses or significant deficiencies, the correction of any such material weaknesses or significant deficiencies could require remedial measures which could be costly and time-consuming. In addition, in such a case, we may be unable to produce accurate financial statements on a timely basis. Any associated accounting restatement could create a significant strain on our internal resources and cause delays in our release of quarterly or annual financial results and the filing of related reports, increase our costs and cause management distraction. Any of the foregoing could cause investors to lose confidence in the reliability of our financial statements, which could cause the market price of our common stock to decline and make it more difficult for us to finance our operations and growth.
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of our suppliers, as well as personally identifiable information of clinical trial participants and employees. Similarly, our third-party providers possess certain of our sensitive protected health data. The secure maintenance of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen.
The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect our business, including compliance with the Health Insurance Portability and Accountability Act of 1996 and recently enacted laws in a majority of states requiring security breach notification. The collection and use of personal health data of individuals in the European Union is also governed by strict data protection laws. In addition to existing laws, since May 25, 2018, the General Data Protection Regulation has imposed new obligations with respect to European Union data and substantial fines for breaches of the data protection rules. It will increase our responsibility and potential liability in relation to personal data that we process, and we will be required to put in place additional mechanisms ensuring compliance with the new European Union data protection rules. This may be onerous and adversely affect our business, operating results, prospects and financial condition.
Thus, any access, disclosure or other loss of information, including our data being breached at our partners or third-party providers, could result in legal claims or proceedings and liability under laws that protect the privacy of personal information, disrupt our operations and damage our reputation, which could adversely affect our business.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. From time to time and in the future, our operations may involve the use of hazardous and flammable materials, including chemicals and biological materials, and may also produce hazardous waste. Even if we contract with third parties for the disposal of these materials and waste, we cannot completely eliminate the risk of contamination or injury resulting from these materials. In the event of contamination or injury resulting from the use or disposal of our hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
20
We maintain workers' compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, but this insurance may not provide adequate coverage against potential liabilities. However, we do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. Current or future environmental laws and regulations may impair our research, development or production efforts. In addition, failure to comply with these laws and regulations may result in substantial fines, penalties or other sanctions.
The recently passed comprehensive tax reform bill could adversely affect our business and financial condition.
On December 22, 2017, the U.S. government enacted comprehensive tax legislation that significantly revised the Internal Revenue Code of 1986, as amended. The newly enacted federal income tax law, among other things, contains significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, limitation of the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses), limitation of the deduction for net operating losses to 80% of current year taxable income and elimination of net operating loss carrybacks, one time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, elimination of U.S. tax on foreign earnings (subject to certain important exceptions), immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifying or repealing many business deductions and credits. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the new federal tax law is uncertain and our business and financial condition could be adversely affected. In addition, it is uncertain how various states will respond to the newly enacted federal tax law. The impact of this tax reform on holders of our common stock is also uncertain and could be adverse. We urge our stockholders to consult with their legal and tax advisors with respect to this legislation and the potential tax consequences of investing in or holding our common stock.
Risks Relating to Our Intellectual Property
Our commercial success is dependent, in part, on obtaining and maintaining patent protection and preserving trade secrets, which cannot be guaranteed.
Patent protection and trade secret protection are important to our business and our future will depend, in part on our ability to maintain trade secret protection, obtain patents and operate without infringing the proprietary rights of others both in the United States and abroad. Litigation or other legal proceedings may be necessary to defend against claims of infringement, to enforce our patents or to protect our trade secrets. Such litigation could result in substantial costs and diversion of our management's attention.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions. In August 2012, we acquired patents and patent applications related to pracinostat from S*Bio. In September 2013, we acquired patents and patent applications related to ME-401 from Pathway Therapeutics, Inc. In September 2017, we acquired patents and patent applications related to voruciclib from Presage. In 2011 we acquired both issued patents and pending patent applications related to ME-344 from Novogen in relation to our Isoflavone-based compounds, which we previously licensed from Novogen. Additionally, Novogen had previously applied for patents in a number of countries with respect to the use of their isoflavone compounds, including ME-344. The patent applications may not proceed to grant or may be amended to reduce the scope of protection of any patent granted. The applications and patents may also be opposed or challenged by third parties. Our commercial success will depend, in part, on our ability to obtain and maintain effective patent protection for our compounds and their use in treating, preventing, or curing cancer, and to successfully defend patent rights in those technologies against third-party challenges. As patent applications in the United States are maintained in secrecy until published or issued and as publication of discoveries in the scientific or patent literature often lag behind the actual discoveries, we cannot be certain that we or Presage were the first to make the inventions covered by the pending patent applications or issued patents referred to above or that we or they were the first to file patent applications for such inventions. Additionally, the breadth of claims allowed in biotechnology and pharmaceutical patents or their enforceability cannot be predicted. We cannot be sure that, should any patents issue, we will be provided with adequate protection against potentially competitive products. Furthermore, we cannot be sure that should patents issue, they will be of commercial value to us, or that private parties, including competitors, will not successfully challenge our patents or circumvent our patent position in the United States or abroad.
Claims by other companies that we infringe on their proprietary technology may result in liability for damages or stop our development and commercialization efforts.
The pharmaceutical industry is highly competitive, and patents have been applied for by, and issued to, other parties relating to products competitive with the compounds that we have acquired. Therefore, pracinostat, ME-401, voruciclib, and ME-344 and any other drug candidates may give rise to claims that they infringe the patents or proprietary rights of other parties existing now and in the future.
Furthermore, to the extent that we or our consultants or research collaborators use intellectual property owned by others in work performed for us, disputes may also arise as to the rights in such intellectual property or in resulting know-how and inventions. An adverse claim could subject us to significant liabilities to such other parties and/or require disputed rights to be licensed from such other parties.
21
We have contracted formulation development and manufacturing process development work for our product candidates. This process has identified a number of excipients, or additives to improve drug delivery, which may be used in the formulations. Excipients, among other things, perform the function of a carrier of the active drug ingredient. Some of these identified excipients or carriers may be included in third party patents in some countries. We intend to seek a license if we decide to use a patented excipient in the marketed product or we may choose one of those excipients that does not have a license requirement.
We cannot be sure that any license required under any such patents or proprietary rights would be made available on terms acceptable to us, if at all. If we do not obtain such licenses, we may encounter delays in product market introductions, or may find that the development, manufacture or sale of products requiring such licenses may be precluded.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees and the employees of Helsinn and third parties upon which we rely to conduct our clinical trials were previously employed at universities or at other biotechnology or pharmaceutical companies, some of which may be competitors or potential competitors. Some of these employees executed proprietary rights, non-disclosure and non-competition agreements, or similar agreements, in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such third party. Litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms or at all. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while we typically require our employees, consultants, advisors and collaborators who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own, which may result in claims by or against us related to the ownership of such intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our senior management and scientific personnel.
We may be subject to substantial costs stemming from our defense against third-party intellectual property infringement claims.
Third parties may assert that we are using their proprietary information without authorization. Third parties may also have or obtain patents and may claim that technologies licensed to or used by us infringe their patents. If we are required to defend patent infringement actions brought by third parties, or if we sue to protect our own patent rights, we may be required to pay substantial litigation costs and managerial attention may be diverted from business operations even if the outcome is not adverse to us. In addition, any legal action that seeks damages or an injunction to stop us from carrying on our commercial activities relating to the affected technologies could subject us to monetary liability and require us or any third party licensors to obtain a license to continue to use the affected technologies. We cannot predict whether we would prevail in any of these types of actions or that any required license would be made available on commercially acceptable terms or at all.
Risks Related to Securities Markets and Investment in Our Stock
The trading price of the shares of our common stock has been and may continue to be highly volatile and could decline in value and we may incur significant costs from class action litigation.
The trading price of our common stock could be highly volatile in response to various factors, many of which are beyond our control, including, but not limited to, the following:
| • | failure to successfully develop our drug candidates; |
| • | design, results and timing of clinical trials and pre-clinical studies; |
| • | announcements of technological innovations by us or our competitors; |
| • | new products introduced or announced by us or our competitors; |
| • | changes in financial estimates by securities analysts; |
| • | actual or anticipated variations in operating results; |
| • | expiration or termination of licenses, research contracts or other collaboration agreements; |
| • | conditions or trends in the regulatory climate and the biotechnology, pharmaceutical and genomics industries; |
| • | instability in the stock market as a result of current or future domestic and global events; |
| • | changes in the market valuations of similar companies; |
| • | the liquidity of any market for our securities; and |
| • | threatened or actual delisting of our common stock from a national stock exchange. |
22
Equity markets in general, and the market for biotechnology and life sciences companies in particular, have experienced substantial price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies traded in those markets. In addition, changes in economic conditions in the U.S., Europe or globally, particularly in the context of current global events, could impact upon our ability to grow profitably. Adverse economic changes are outside our control and may result in material adverse impacts on our business or our results of operations. These broad market and industry factors may materially affect the market price of shares of our common stock, regardless of our development and operating performance. In the past, following periods of volatility in the market price of a company's securities, securities class-action litigation has often been instituted against that company. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management's attention and resources.
Future sales of our common stock, including common stock issued upon exercise of outstanding warrants or options, may depress the market price of our common stock and cause stockholders to experience dilution.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market, including upon exercise of outstanding warrants or stock options, and any subsequent sales of such shares. As of June 30, 2018, we had outstanding warrants issued in our May 2018 private placement exercisable to purchase 16,501,645 shares of common stock at an exercise price of $2.54 per share, which expire in May 2023. We also had outstanding options to purchase 6,281,615 shares of common stock and restricted stock units ("RSUs") representing the right to receive 332,193 shares of common stock, which vested and were issued in August 2018. We may seek additional capital through one or more additional equity transactions in the future; however, such transactions will be subject to market conditions and there can be no assurance any such transactions will be completed. If we sell shares in the future, the prices at which we sell these future shares will vary, and these variations may be significant. Stockholders will experience significant dilution if we sell these future shares at prices significantly below the price at which previous stockholders invested.
Because we do not intend to pay, and have not paid, any cash dividends on our shares of common stock, our stockholders will not be able to receive a return on their shares unless the value of our common stock appreciates and they sell their shares.
We have never paid or declared any cash dividends on our common stock, and we intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Therefore, our stockholders will not be able to receive a return on their investment unless the value of our common stock appreciates and they sell their shares.
We will have broad discretion over the use of the net proceeds from any exercise of outstanding warrants and options.
We will have broad discretion to use the net proceeds to us upon any exercise of outstanding warrants and options, and investors in our stock will be relying on the judgment of our board of directors and management regarding the application of these proceeds. Although we expect to use a substantial portion of the net proceeds from any exercise of the warrants and options for general corporate purposes and progression of our clinical trial programs, we have not allocated these net proceeds for specific purposes.
We are authorized to issue blank check preferred stock, which could adversely affect the holders of our common stock.
Our restated certificate of incorporation allows us to issue blank check preferred stock with rights potentially senior to those of our common stock without any further vote or action by the holders of our common stock. The issuance of a class of preferred stock could decrease the amount of earnings and assets available for distribution to the holders of our common stock or could adversely affect the rights and powers, including voting rights, of such holders. In certain circumstances, such issuance could have the effect of decreasing the market price of our shares, or making a change in control of the Company more difficult.
Our executive officers and directors may sell shares of their stock, and these sales could adversely affect our stock price.
Sales of our stock by our executive officers and directors, or the perception that such sales may occur, could adversely affect the market price of our stock. Our executive officers and directors may sell stock in the future, either as part, or outside, of trading plans under Rule 10b5-1 under the Securities Exchange Act of 1934, as amended (the "Exchange Act").
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
We have leased approximately 13,700 square feet of office space, located at 3611 Valley Centre Drive, Suite 500, San Diego, California 92130. The location houses our executive and administrative offices. The lease commenced in July 2017 and expires in May 2020. The monthly rental rate is approximately $46,000 per month over the remaining term of the lease, plus a pro rata share of certain building expenses.
23
| Item 3. | Legal Proceedings |
None.
| Item 4. | Mine Safety Disclosures |
Not applicable.
24
PART II
| Item 5. | Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock is listed on the NASDAQ Capital Market under the symbol "MEIP". The following table sets forth, for the periods indicated, the high and low sale prices of our common stock for each quarterly period within the two most recent fiscal years.
| Prices | ||||||||
| High | Low | |||||||
| $ | $ | |||||||
Year Ended June 30, 2018 | ||||||||
First Quarter | 3.26 | 2.34 | ||||||
Second Quarter | 2.90 | 1.79 | ||||||
Third Quarter | 2.49 | 2.02 | ||||||
Fourth Quarter | 5.14 | 1.89 | ||||||
Year Ended June 30, 2017 | ||||||||
First Quarter | 2.28 | 1.32 | ||||||
Second Quarter | 1.89 | 1.34 | ||||||
Third Quarter | 1.88 | 1.40 | ||||||
Fourth Quarter | 2.50 | 1.48 | ||||||
Holders
As of August 27, 2018, there were 71,086,404 shares of our common stock outstanding and 1,099 holders of record of our common stock. This number was derived from our stockholder records and does not include beneficial owners of our common stock whose shares are held in the name of various dealers, clearing agencies, banks, brokers and other fiduciaries.
For a discussion of outstanding warrants and other securities exercisable for or convertible into shares of our common stock, see Notes 6 and 7 under Item 8 in this Annual Report.
Dividends
We have never declared or paid any cash dividends on our common stock and do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain all available funds and future earnings, if any, to support operations and finance the growth and development of our business. Any future determination related to our dividend policy will be made at the discretion of our board of directors.
Securities authorized for issuance under equity compensation plans
The table below shows, as of June 30, 2018, information for equity compensation plans previously approved by stockholders and for compensation plans not previously approved by stockholders.
Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||
Equity compensation plans approved by security holders (1) | 6,613,808 | $ | 3.08 | 2,896,206 | ||||||||
Equity compensation plans not approved by security holders | - | - | - | |||||||||
|
|
|
|
|
| |||||||
Total | 6,613,808 | $ | 3.08 | 2,896,206 | ||||||||
|
|
|
|
|
| |||||||
| (1) | Consists of 6,281,615 shares of common stock issuable upon exercise of options and 332,193 shares of common stock which were issued in August 2018 upon vesting of RSUs, in each case, granted or that may be granted under the MEI Pharma, Inc. Amended and Restated 2008 Stock Omnibus Equity Compensation Plan ("the Plan"), under which 10,186,000 shares of common stock are authorized for issuance. The Plan provides for the grant of options and/or other stock-based or stock-denominated awards to our non-employee directors, officers, employees and advisors. The weighted-average exercise price presented is the weighted-average exercise price of vested and unvested options. The RSUs have no exercise price. For purposes of determining the number of shares available for future grant, there were 332,193 RSUs outstanding as of June 30, 2018 which are calculated as 1.25 shares of common stock under the terms of the Plan. |
25
Performance graph
The graph below compares the cumulative five-year total return on our common stock from July 1, 2013, through June 30, 2018, to the cumulative total return over such period for (i) the NASDAQ Composite Index and (ii) the NASDAQ Biotechnology Index. The graph assumes the investment of $100 on July 1, 2013, with the reinvestment of dividends, although dividends have not been declared on our common stock, and is calculated according to the SEC's methodology. We caution that the stock price performance shown in the graph may not be indicative of future stock price performance. The graph, including each of the graph lines, was provided by Zacks Investment Research, Inc.
This information, including the graph below, is not deemed to be "soliciting material" or to be "filed" with the SEC, or subject to the SEC's proxy rules, other than as provided in such rules, or to the liabilities of Section 18 of the Exchange Act, and shall not be deemed incorporated by reference into any prior or subsequent filing by us under the Securities Act of 1933, as amended (the "Securities Act") or the Exchange Act, except to the extent that we specifically incorporate it by reference into any such filing.
COMPARISON OF FIVE-YEAR CUMULATIVE TOTAL RETURN
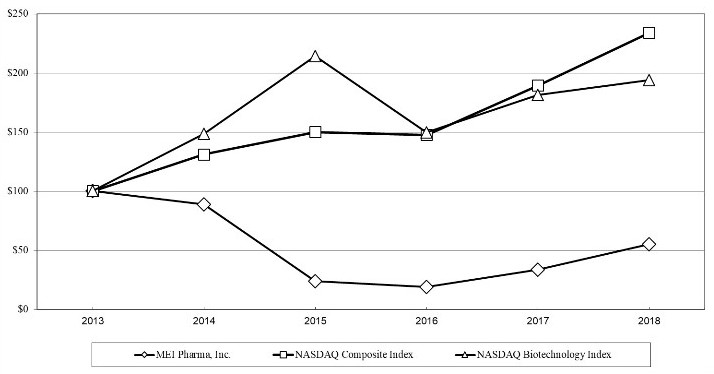
| Item 6. | Selected Financial Data |
The following Selected Financial Data should be read in conjunction with "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Item 8. Financial Statements and Supplementary Data" included below in this Annual Report.
The following table presents our selected historical condensed financial data. The condensed statements of operations data for fiscal years ended June 30, 2018, 2017 and 2016 and the condensed balance sheet data as of June 30, 2018 and 2017 are derived from our audited financial statements included elsewhere in this Annual Report. The condensed statements of operations data for the fiscal years ended June 30, 2015 and 2014 and the condensed balance sheet data as of June 30, 2016, 2015 and 2014 are derived from our audited financial statements that are not included in this Annual Report.
26
| Years Ended June 30, | ||||||||||||||||||||
| 2018 | 2017 | 2016 | 2015 | 2014 | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
Revenues: | ||||||||||||||||||||
License revenue | $ | - | $ | 20,880 | $ | - | $ | - | $ | - | ||||||||||
Research and development revenue | 1,622 | 2,369 | - | - | - | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total revenues | 1,622 | 23,249 | - | - | - | |||||||||||||||
Operating expenses: | ||||||||||||||||||||
Cost of research and development revenue | 3,383 | 5,000 | - | - | - | |||||||||||||||
Research and development | 17,038 | 7,237 | 13,403 | 23,823 | 19,331 | |||||||||||||||
General and administrative | 9,787 | 8,628 | 7,601 | 8,948 | 7,897 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total operating expenses | 30,208 | 20,865 | 21,004 | 32,771 | 27,228 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
(Loss) income from operations | (28,586 | ) | 2,384 | (21,004 | ) | (32,771 | ) | (27,228 | ) | |||||||||||
Change in fair value of warrant liability | (9,705 | ) | - | - | - | - | ||||||||||||||
Financing costs | (2,367 | ) | - | - | - | - | ||||||||||||||
Other income and expense, net | 590 | 286 | 142 | 77 | 80 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net (loss) income | $ | (40,068 | ) | $ | 2,670 | $ | (20,862 | ) | $ | (32,694 | ) | $ | (27,148 | ) | ||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net (loss) income per share, basic | $ | (0.97 | ) | $ | 0.07 | $ | (0.61 | ) | $ | (1.16 | ) | $ | (1.35 | ) | ||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Net (loss) income per share, diluted | $ | (0.97 | ) | $ | 0.07 | $ | (0.61 | ) | $ | (1.16 | ) | $ | (1.35 | ) | ||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Shares used to calculate net (loss) income per share | ||||||||||||||||||||
Basic | 41,431 | 36,813 | 34,400 | 28,204 | 20,061 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Diluted | 41,431 | 36,938 | 34,400 | 28,204 | 20,061 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
| As of June 30, | ||||||||||||||||||||
| 2018 | 2017 | 2016 | 2015 | 2014 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||
Cash, cash equivalents and short-term investments | $ | 102,743 | $ | 53,565 | $ | 45,918 | $ | 63,779 | $ | 48,793 | ||||||||||
Total assets | $ | 104,657 | $ | 55,704 | $ | 47,164 | $ | 64,750 | $ | 49,808 | ||||||||||
Warrant liability | $ | 46,313 | $ | - | $ | - | $ | - | $ | - | ||||||||||
Total liabilities | $ | 54,198 | $ | 4,866 | $ | 5,512 | $ | 4,959 | $ | 4,616 | ||||||||||
Accumulated deficit | $ | (214,399 | ) | $ | (174,331 | ) | $ | (177,001 | ) | $ | (156,139 | ) | $ | (123,445 | ) | |||||
Total stockholders' equity | $ | 50,459 | $ | 50,838 | $ | 41,652 | $ | 59,791 | $ | 45,192 | ||||||||||
| Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations. |
The following discussion and analysis should be read in conjunction with "Item 8. Financial Statements and Supplementary Data" included below in this Annual Report. Operating results are not necessarily indicative of results that may occur in future periods.
This discussion and analysis contains forward-looking statements that involve a number of risks, uncertainties and assumptions. Actual results may differ materially from those anticipated in the forward-looking statements as a result of many factors including, but not limited to, those set forth under "Cautionary Statement About Forward-Looking Statements" and "Risk Factors" in Item 1A. included above in this Annual Report. All forward-looking statements included in this Annual Report are based on the information available to us as of the time we file this Annual Report, and except as required by law, we undertake no obligation to update publicly or revise any forward-looking statements.
Overview
We are a pharmaceutical company focused on leveraging our extensive development and oncology expertise to identify and advance new therapies intended to meaningfully improve the treatment of cancer. Our focus is on developing and commercializing cancer treatments, either as single-agents or as combination therapies that effectively leverage the potential of a mechanism of action to improve existing treatment paradigms and provide increased benefit to patients.
Our portfolio of drug candidates contains four clinical-stage candidates, including one candidate in an ongoing global registration trial (pracinostat), and another candidate (ME-401) that is anticipated to advance, in the fourth quarter of calendar year 2018, into a Phase 2 clinical trial that we intend to submit to the FDA to support accelerated approval of a marketing application. Our drug candidates target different mechanisms critical to overcoming cancer progression and drug resistance:
| • | Pracinostat, an oral HDAC inhibitor; |
| • | ME-401, an oral PI3K delta inhibitor; |
27
| • | Voruciclib, an oral CDK inhibitor: and |
| • | ME-344, a mitochondrial inhibitor targeting the OXPHOS complex. |
Since each of our drug candidates utilizes a different mechanism of action, we are able to approach cancer treatment through multiple pathways; this creates opportunities across various cancer indications. Our candidates are intended to demonstrate potency and safety as standalone treatments, but importantly they are also intended to have a favorable therapeutic index with the potential to deliver synergies in combination with existing medicines.
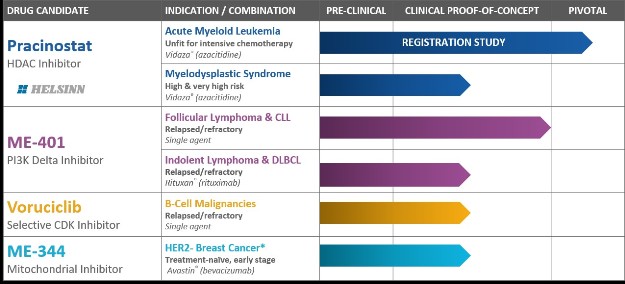
Recent Developments
In July 2018, we announced that David M. Urso, J.D., our senior vice president of corporate development and general counsel, was promoted to chief operating officer. Mr. Urso is also continuing as our general counsel and head of corporate development.
In June 2018, we presented Phase 1b clinical trial data from our highly differentiated PI3K delta inhibitor, ME-401 at the ASCO Annual Meeting and the European Hematology Association Annual Congress. The reported clinical data from the ongoing Phase 1b, open-label, dose-escalation study in relapsed/refractory FL and CLL demonstrated a class-leading objective response rate of 90%. ME-401 was also generally well-tolerated in the Phase 1b and no dose-limiting toxicities were identified at any dose level.
In June 2018, we also presented data from an investigator-initiated clinical trial of ME-344, our novel and tumor selective, isoflavone-derived mitochondrial inhibitor drug candidate. In the ongoing study patients are randomized one-to-one to either ME-344 in combination with bevacizumab or saline in combination with bevacizumab. The reported interim data review was from a predefined interim analysis of 20 patients and demonstrated evidence of inhibition of tumor proliferation.
In May 2018, we jointly announced with Helsinn, our partner in the development of pracinostat, the successful interim analysis from a clinical trial evaluating patients with MDS. The phase 2 dose optimization study is evaluating patients with high and very high-risk MDS who are previously untreated with hypomethylating agents. The pre-planned interim analysis of the study demonstrated a 10% discontinuation rate among the first 20 evaluable patients treated, achieving the predefined threshold in the first three treatment cycles. The 10% rate is consistent with the established discontinuation rate for azacitidine given as a monotherapy. Having met this threshold, the study expanded open-label enrollment to 60 patients. Patients will be followed for one year to evaluate safety and efficacy. If the expanded open-label study is successful, a global registration study is anticipated.
In May 2018, we also announced a private placement of common stock, along with warrants to purchase common stock, resulting in net proceeds of approximately $70.2 million (the "May 2018 Private Placement"). Proceeds from the financing are primarily being used to fund continued clinical development of ME-401 and for general corporate purposes.
For a more complete discussion of our business, see the section of this Annual Report "Item 1- Business" above.
28
Equity Transactions
May 2018 Private Placement
In May 2018, we sold 33,003,296 shares of our common stock, together with warrants to purchase 16,501,645 shares of common stock, in a private offering for approximately $70.2 million, after deducting offering costs.
Shelf Registration Statement
In May 2017, we filed a shelf registration statement on Form S-3 with the SEC ("shelf registration statement"). The shelf registration statement was declared effective by the SEC in May 2017 and permits us to sell, from time to time, up to $150.0 million of common stock, preferred stock and warrants. In November 2017, we entered into an At-The-Market Equity Offering Sales Agreement (the "ATM Sales Agreement"), pursuant to which we may sell an aggregate of up to $30.0 million of our common stock pursuant to the shelf registration statement. As of June 30, 2018, we have not sold any shares under the ATM Sales Agreement. As of June 30, 2018, there is $150.0 million aggregate value of securities available under the shelf registration statement.
Helsinn Equity Investment
In August 2016, we entered into a Common Stock Purchase Agreement with Helsinn Investment Fund SA (the "Helsinn Equity Agreement"). Pursuant to the terms of the Helsinn Equity Agreement, we issued 2,616,431 shares of common stock on August 16, 2016 in exchange for a $5.0 million investment. The transaction was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
Critical Accounting Policies and Management Estimates
Management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and related disclosures. Actual results could differ from those estimates. We believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
Payments received under commercial arrangements, such as licensing technology rights, may include non-refundable fees at the inception of the arrangements, milestone payments for specific achievements designated in the agreements, and royalties on the sale of products. We consider a variety of factors in determining the appropriate method of accounting under our license agreements, including whether the various elements can be separated and accounted for individually as separate units of accounting.
Multiple Element Arrangements
Deliverables under an arrangement will be separate units of accounting, provided (i) a delivered item has value to the customer on a standalone basis; and (ii) the arrangement includes a general right of return relative to the delivered item, and delivery or performance of the undelivered item is considered probable and substantially in our control.
We account for revenue arrangements with multiple elements by separating and allocating consideration according to the relative selling price of each deliverable. If an element can be separated, an amount is allocated based upon the relative selling price of each element. We determine the relative selling price of a separate deliverable using the price we charge other customers when we sell that element separately. If the element is not sold separately and third party pricing evidence is not available, we will use our best estimate of selling price.
License Revenue
Non-refundable, up-front fees that are not contingent on any future performance by us and require no consequential continuing involvement on our part are recognized as revenue when the license term commences and the licensed data, technology or product is delivered. We defer recognition of non-refundable upfront license fees if we have continuing performance obligations, without which the licensed data, technology, or product has no utility to the licensee separate and independent of our performance under the other elements of the applicable arrangement. The specific methodology for the recognition of the revenue is determined on a case-by-case basis according to the facts and circumstances of the applicable agreement.
Research and Development Revenue
Research and development revenue represents ratable recognition of fees allocated to research and development activities. We defer recognition of research and development revenue until the performance of the related research and development activities has occurred. Research and development revenue for the years ended June 30, 2018 and 2017 related to services provided by third-party vendors related to research and development activities performed under the Helsinn License Agreement (See Note 2 to the Financial Statements in Item 8 of this Annual Report).
29
Cost of Research and Development Revenue
Cost of research and development revenue primarily includes external costs paid to third-party contractors to perform research, conduct clinical trials and develop and manufacture drug materials, and internal compensation and related personnel expenses to support our research and development revenue.
Research and Development Costs
Research and development costs are expensed as incurred and include costs paid to third-party contractors to perform research, conduct clinical trials and develop and manufacture drug materials. Clinical trial costs, including costs associated with third-party contractors, are a significant component of research and development expenses. We expense research and development costs based on work performed. In determining the amount to expense, management relies on estimates of total costs based on contract components completed, the enrollment of subjects, the completion of trials, and other events. Costs incurred related to the purchase of in-process research and development for early-stage products or products that are not commercially viable and ready for use, or have no alternative future use, are charged to expense in the period incurred. Costs incurred related to the licensing of products that have not yet received regulatory approval to be marketed, or that are not commercially viable and ready for use, or have no alternative future use, are charged to expense in the period incurred.
Share-Based Compensation
Share-based compensation expense for employees and directors is recognized in the statement of operations based on estimated amounts, including the grant date fair value and the expected service period. For stock options, we estimate the grant date fair value using a Black-Scholes valuation model, which requires the use of multiple subjective inputs including estimated future volatility, expected forfeitures and the expected term of the awards. We estimate the expected future volatility based on the stock's historical price volatility. The stock's future volatility may differ from the estimated volatility at the grant date. For RSU equity awards, we estimate the grant date fair value using our closing stock price on the date of grant. We recognize the effect of forfeitures in compensation expense when the forfeitures occur. The estimated forfeiture rates may differ from actual forfeiture rates which would affect the amount of expense recognized during the period. We recognize the value of the awards over the awards' requisite service or performance periods. The requisite service period is generally the time over which our share-based awards vest.
Warrant Liability
In May 2018, we issued warrants in connection with the May 2018 Private Placement. Pursuant to the terms of the warrants, we could be required to settle the warrants in cash in the event of an acquisition of the Company and, as a result, the warrants are required to be measured at fair value and reported as a liability in the balance sheet. We recorded the fair value of the warrants of $36.6 million upon issuance using the Black-Scholes valuation model, and are required to revalue the warrants at each reporting date with any changes in fair value recorded on our statement of operations. Inputs used to determine estimated fair value of the warrant liabilities include the estimated fair value of the underlying stock at the valuation date, the estimated term of the warrants, risk-free interest rates, expected dividends and the expected volatility of the underlying stock. As of June 30, 2018, the fair value of these warrants of $46.3 million was recorded as a long-term liability on our balance sheet. This resulted in a change in fair value of $9.7 million for the year ended June 30, 2018. Additionally, we allocated $2.4 million of the transaction costs associated with our May 2018 Private Placement to financing expense on our statement of operations. The remaining $2.4 million of transaction costs were offset against the proceeds allocated to our common stock.
Results of Operations
Comparison of Years Ended June 30, 2018 and 2017
License Revenue: We recognized no license revenue for the year ended June 30, 2018 compared to $20.9 million for the year ended June 30, 2017. The license revenue in 2017 resulted from the completion of the performance obligations related to the upfront license fees in accordance with the Helsinn License Agreement.
Research and Development Revenue: We recognized research and development revenue of $1.6 million for the year ended June 30, 2018 compared to $2.4 million for the year ended June 30, 2017. Research and development revenue resulted from the recognition of fees allocated to research and development activities in accordance with the Helsinn License Agreement. Revenue decreased due to lower levels of research and development activities during the year ended June 30, 2018.
Cost of Research and Development Revenue: We recognized cost of research and development revenue of $3.4 million for the year ended June 30, 2018 compared to $5.0 million for the year ended June 30, 2017. The cost of research and development revenue includes external costs paid to third-party contractors to perform research, conduct clinical trials and develop and manufacture drug materials, and internal compensation and related personnel expenses to support our research and development revenue. All costs of research and development revenue relate to expenses for pracinostat incurred in connection with our development activities in accordance with the Helsinn License Agreement, including both Helsinn's share and our share of costs related to the POC study (as defined below), which we are responsible for conducting.
30
Research and Development: The following is a summary of our research and development expenses to supplement the more detailed discussion below (in thousands):
| Years Ended June 30, | ||||||||
Research and development expenses | 2018 | 2017 | ||||||
ME-401 | $ | 5,766 | $ | 2,565 | ||||
Voruciclib | 4,121 | - | ||||||
Pracinostat | 22 | (945 | ) | |||||
ME-344 | 685 | 66 | ||||||
Other | 6,444 | 5,551 | ||||||
|
|
|
| |||||
Total research and development expenses | $ | 17,038 | $ | 7,237 | ||||
|
|
|
| |||||
Research and development expenses consist primarily of clinical trial costs (including payments to CROs), pre-clinical study costs, and costs to manufacture our drug candidates for non-clinical and clinical studies. Other research and development expenses consist primarily of salaries and other personnel costs, share-based compensation, legal costs, and other costs not allocated to specific drug programs. Research and development expenses increased by $9.8 million to $17.0 million for the year ended June 30, 2018 compared to $7.2 million for the year ended June 30, 2017. The increase was primarily due to an increase of $4.1 million in costs associated with voruciclib, including a $2.9 million upfront payment made for the license of voruciclib. Additionally, drug manufacturing costs related to ME-401 increased by $2.6 million to supply planned clinical trials and there was a prior year reduction of clinical trial costs of $1.9 million due to revisions in estimates of amounts that were owed to contract research organizations for clinical trials for pracinostat and ME-344 that were at or near completion. Other research and development costs increased by $0.9 million due to increased personnel costs and share-based compensation.
General and Administrative: General and administrative expenses increased by $1.2 million to $9.8 million for the year ended June 30, 2018 compared to $8.6 million for the year ended June 30, 2017. The increase primarily relates to professional services expenses, share-based compensation, and general corporate expenses incurred during the year ended June 30, 2018.
Other income or expense: We recognized other expense of $11.5 million for the year ended June 30, 2018 and other income of $286,000 for the year ended June 30, 2017. The expense in 2018 primarily consisted of a $9.7 million change in the fair value of outstanding warrants and $2.4 million in financing costs related to the May 2018 Private Placement, offset by interest on cash, cash equivalents, and short-term investments. The other income for the year ended June 30, 2017 consisted of interest on cash, cash equivalents and short-term investments.
Comparison of Years Ended June 30, 2017 and 2016
License Revenue: We recognized license revenue of $20.9 million for the year ended June 30, 2017 compared to no license revenue for the year ended June 30, 2016. The license revenue resulted from the completion of the performance obligations related to the upfront license fees in accordance with the Helsinn License Agreement.
Research and Development Revenue: We recognized research and development revenue of $2.4 million for the year ended June 30, 2017 compared to no research and development revenue for the year ended June 30, 2016. The research and development revenue resulted from the recognition of fees allocated to research and development activities in accordance with the Helsinn License Agreement.
Cost of Research and Development Revenue: We recognized cost of research and development revenue of $5.0 million for the year ended June 30, 2017 compared to no cost of research and development revenue for the year ended June 30, 2016. The cost of research and development revenue includes external costs paid to third-party contractors to perform research, conduct clinical trials and develop and manufacture drug materials, and internal compensation and related personnel expenses to support our research and development revenue. All costs of research and development revenue relate to expenses incurred in connection with our development activities in accordance with the Helsinn License Agreement.
Research and Development: The following is a summary of our research and development expenses to supplement the more detailed discussion below (in thousands):
| Years Ended June 30, | ||||||||
Research and development expenses | 2017 | 2016 | ||||||
ME-401 | $ | 2,565 | $ | 3,041 | ||||
Pracinostat | (945 | ) | 3,405 | |||||
ME-344 | 66 | 1,972 | ||||||
Other | 5,551 | 4,985 | ||||||
|
|
|
| |||||
Total research and development expenses | $ | 7,237 | $ | 13,403 | ||||
|
|
|
| |||||
31
Research and development expenses consist primarily of clinical trial costs (including payments to CROs), pre-clinical study costs, cost to manufacture our drug candidates for non-clinical and clinical studies, and salaries and other personnel costs. Research and development expenses decreased $6.2 million to $7.2 million for the year ended June 30, 2017 compared to $13.4 million for the year ended June 30, 2016. The decrease was primarily due to a reduction of $2.5 million in costs associated with clinical trials for pracinostat pursuant to the Helsinn License Agreement whereby Helsinn is primarily responsible for the costs to continue the development of pracinostat. There was also a reduction in clinical trial costs of $1.9 million due to revisions in estimates of amounts that were owed to CROs for clinical trials for pracinostat and ME-344 that were at or near completion. Additionally, costs related to our other clinical trials decreased by $1.0 million and the cost of drug manufacturing for Pracinostat decreased by $0.7 million.
General and Administrative: General and administrative expenses increased by $1.0 million to $8.6 million for the year ended June 30, 2017 compared to $7.6 million for the year ended June 30, 2016. The increase primarily relates to higher levels of professional services expenses related to the Helsinn License Agreement.
Other income or expense: We received interest on cash, cash equivalents and short-term investments of $287,000 for the year ended June 30, 2017 and $143,000 for the year ended June 30, 2016. The increase was due to higher yields on investments during the year ended June 30, 2017 compared to the year ended June 30, 2016.
New Accounting Pronouncements
See Note 1 to the Financial Statements included in Item 8 of this Annual Report.
Off-Balance Sheet Arrangements
We do not currently have any off-balance sheet arrangements.
Liquidity and Capital Resources
We have accumulated losses of $214.4 million since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future. As of June 30, 2018, we had $102.7 million in cash and cash equivalents and short-term investments, which we believe will be sufficient to fund our operations into calendar year 2020. Our current business operations are focused on continuing the clinical development of our drug candidates. Changes to our research and development plans or other changes affecting our operating expenses may affect actual future use of existing cash resources. Our research and development expenses are expected to increase in the foreseeable future. We cannot determine with certainty costs associated with ongoing and future clinical trials or the regulatory approval process. The duration, costs and timing associated with the development for our product candidates will depend on a variety of factors, including uncertainties associated with the results of our clinical trials.
To date, we have obtained cash and funded our operations primarily through equity financings. In order to continue the development of our drug candidates, at some point in the future we expect to pursue one or more capital transactions, whether through the sale of equity securities, license agreements or entry into strategic partnerships.
Sources and Uses of Our Cash
Net cash used in operations for the year ended June 30, 2018 was $21.3 million compared to net cash provided by operations of $3.5 million for the year ended June 30, 2017 due to an increase in operating expenses incurred for research and development and general and administrative costs as described above. Cash provided by operations for the year ended June 30, 2017 was due to license fee revenues earned from the Helsinn License Agreement. Net cash provided by operations for the year ended June 30, 2017 was $3.5 million compared to net cash used in operations of $17.9 million in the year ended June 30, 2016 due to the license revenue earned from the Helsinn License Agreement, a decrease in operating expenses incurred for research and development and general and administrative costs as described above.
Net cash used in investing activities for the year ended June 30, 2018 was $44.3 million compared to $10.1 million used in investing activities for the year ended June 30, 2017. The change was primarily due to higher purchases of short-term investments in 2018, as a result of the May 2018 Private Placement compared to 2017, net of maturities. Net cash used in investing activities for the year ended June 30, 2017 was $10.1 million compared to $10.0 million provided by investing activities for the year ended June 30, 2016. The decrease was primarily due to higher purchases of short-term investments in 2016 compared to 2017, net of maturities.
There was $70.5 million provided by financing activities during the year ended June 30, 2018 compared with $4.2 million provided by financing activities during the year ended June 30, 2017. Cash raised during the year ended June 30, 2018 reflected the May 2018 Private Placement. There was $4.2 million provided by financing activities during the year ended June 30, 2017 compared with no cash during the year ended June 30, 2016. Cash raised during the year ended June 30, 2017 reflected the equity investment by Helsinn.
Contractual Obligations
We have contracted with various consultants and third parties to assist us in pre-clinical research and development and clinical trials work for our leading drug compounds. The contracts are terminable at any time, but obligate us to reimburse the providers for any time or costs incurred through the date of termination. Additionally, we have employment agreements with certain of our current employees that provide for severance payments and accelerated vesting for share-based awards if their employment is terminated under specified circumstances.
We have leased approximately 13,700 square feet of office space, located at 3611 Valley Centre Drive, San Diego, California 92130. The location houses our executive and administrative offices. The lease commenced in June 2017 and expires in May 2020. The monthly rental rate is approximately $46,000 over the remaining lease term, plus a pro rata share of certain building expenses. The remaining contractual obligation for the lease is $1.1 million.
32
Presage License Agreement
In September 2017, we entered into the Presage License Agreement. Under the terms of the Presage License Agreement, Presage granted to us exclusive worldwide rights to develop, manufacture and commercialize voruciclib, a clinical-stage, oral and selective CDK inhibitor, and related compounds. In exchange, we paid Presage $2.9 million. With respect to the first indication, an incremental $2.0 million payment, due upon dosing the first subject in the first registration trial will be owed to Presage, for total payments of $4.9 million up to receipt of marketing approval of the first indication in the U.S., E.U. or Japan. Additional potential payments of up to $179 million will be due upon the achievement of certain development, regulatory and commercial milestones. We will also pay mid-single-digit tiered royalties on the net sales of any product successfully developed. As an alternative to milestone and royalty payments related to countries in which we sublicense product rights, we will pay to Presage a tiered percent (which decreases as product development progresses) of amounts received from such sublicensees. As of June 30, 2018, we have not accrued any amounts for potential future payments as they are not probable.
S*Bio Purchase Agreement
We are party to a definitive asset purchase agreement with S*Bio, pursuant to which we acquired certain assets comprised of intellectual property and technology including rights to pracinostat. We agreed to make certain milestone payments to S*Bio based on the achievement of certain clinical, regulatory and net sales-based milestones, as well as to make certain contingent earnout payments to S*Bio. Milestone payments will be made to S*Bio up to an aggregate amount of $75.2 million if certain U.S., E.U. and Japanese regulatory approvals are obtained and if certain net sales thresholds are met in North America, the E.U. and Japan. The first milestone payment of $200,000 plus 166,527 shares of our common stock having a value of $500,000 was paid in August 2017 upon the first dosing of a patient in a Phase 3 clinical trial. Subsequent milestone payments will be due upon certain regulatory approvals and sales-based events. As of June 30, 2018, we have not accrued any amounts for potential future payments as they are not probable.
CyDex License Agreement
We are party to a license agreement with CyDex Pharmaceuticals, Inc. ("CyDex"). Under the license agreement, CyDex granted to us an exclusive, nontransferable license to intellectual property rights relating to Captisol ® for use with our two isoflavone-based drug compounds (currently ME-344). We agreed to pay to CyDex a non-refundable license issuance fee, future milestone payments, and royalties at a low, single-digit percentage rate on future sales of our approved drugs utilizing Captisol. Contemporaneously with the license agreement, CyDex entered into a commercial supply agreement with us, pursuant to which we agreed to purchase 100% of our requirements for Captisol from CyDex. We may terminate both the license agreement and the supply agreement for convenience at any time upon 90 days' prior written notice. As of June 30, 2018, we have not accrued any amounts for potential future payments as they are not probable.
| Item 7a. | Quantitative and Qualitative Disclosures about Market Risk |
Interest Rate Risk
Our exposure to market interest rates relates primarily to the investments of cash balances and short-term investments. We have cash reserves held in U.S. dollars and we place funds on deposit with financial institutions, which are readily available. Our short-term investments consist solely of U.S. government securities with a maturity of three to twelve months.
We place our cash deposits with high credit quality financial institutions and by policy limit the amount of credit exposure to any one corporation or bank. These deposits are in excess of the Federal Deposit Insurance Corporation ("FDIC") insurance limits. We are adverse to principal loss and we ensure the safety and preservation of our invested funds by limiting default risk, market risk and reinvestment risk. We seek to mitigate default risk by depositing funds with high credit quality financial institutions, by limiting the amount of credit exposure to any one corporation or bank, by purchasing short-term investments consisting of U.S. government securities, and by positioning our portfolio to respond appropriately to a significant reduction in a credit rating of any such financial institution.
We do not consider the effects of interest rate movements to be a material risk to our financial condition.
| Item 8. | Financial Statements and Supplementary Data |
MEI Pharma, Inc.
Index to Financial Statements
Report of Independent Registered Public Accounting Firm
Balance Sheets
Statements of Operations
Statements of Stockholders' Equity
Statements of Cash Flows
Notes to Financial Statements
33
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
MEI Pharma, Inc.
San Diego, California
Opinion on the Financial Statements
We have audited the accompanying balance sheets of MEI Pharma, Inc. (the "Company") as of June 30, 2018 and 2017, the related statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended June 30, 2018, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at June 30, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2018 , in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) ("PCAOB"), the Company's internal control over financial reporting as of June 30, 2018, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") and our report dated August 29, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, LLP
We have served as the Company's auditor since 2011.
San Diego, California
August 29, 2018
34
MEI PHARMA, INC.
BALANCE SHEETS
(In thousands, except per share amounts)
| June 30, | ||||||||
| 2018 | 2017 | |||||||
| ASSETS | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 13,309 | $ | 8,458 | ||||
Short-term investments | 89,434 | 45,107 | ||||||
|
|
|
| |||||
Total cash, cash equivalents and short-term investments | 102,743 | 53,565 | ||||||
Prepaid expenses and other current assets | 1,586 | 1,758 | ||||||
|
|
|
| |||||
Total current assets | 104,329 | 55,323 | ||||||
Intangible assets, net | 296 | 331 | ||||||
Property and equipment, net | 32 | 50 | ||||||
|
|
|
| |||||
Total assets | $ | 104,657 | $ | 55,704 | ||||
|
|
|
| |||||
| LIABILITIES AND STOCKHOLDERS' EQUITY |
| |||||||
Current liabilities: | ||||||||
Accounts payable | $ | 3,643 | $ | 585 | ||||
Accrued liabilities | 3,454 | 3,285 | ||||||
Deferred revenues | 788 | 996 | ||||||
|
|
|
| |||||
Total current liabilities | 7,885 | 4,866 | ||||||
Warrant liability | 46,313 | - | ||||||
|
|
|
| |||||
Total liabilities | 54,198 | 4,866 | ||||||
|
|
|
| |||||
Commitments and contingencies (Note 9) | ||||||||
Stockholders' equity: | ||||||||
Preferred stock, $0.01 par value; 100 shares authorized; none outstanding | - | - | ||||||
Common stock, $0.00000002 par value; 113,000 shares authorized; 70,406 and 36,772 shares issued and outstanding at June 30, 2018 and 2017, respectively. | - | - | ||||||
Additional paid-in-capital | 264,858 | 225,169 | ||||||
Accumulated deficit | (214,399 | ) | (174,331 | ) | ||||
|
|
|
| |||||
Total stockholders' equity | 50,459 | 50,838 | ||||||
|
|
|
| |||||
Total liabilities and stockholders' equity | $ | 104,657 | $ | 55,704 | ||||
|
|
|
| |||||
See accompanying notes to financial statements.
35
MEI PHARMA, INC.
STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| Years Ended June 30, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Revenues: | ||||||||||||
License revenue | $ | - | $ | 20,880 | $ | - | ||||||
Research and development revenue | 1,622 | 2,369 | - | |||||||||
|
|
|
|
|
| |||||||
| 1,622 | 23,249 | - | ||||||||||
|
|
|
|
|
| |||||||
Operating expenses: | ||||||||||||
Cost of research and development revenue | 3,383 | 5,000 | - | |||||||||
Research and development | 17,038 | 7,237 | 13,403 | |||||||||
General and administrative | 9,787 | 8,628 | 7,601 | |||||||||
|
|
|
|
|
| |||||||
Total operating expenses | 30,208 | 20,865 | 21,004 | |||||||||
|
|
|
|
|
| |||||||
(Loss) income from operations | (28,586 | ) | 2,384 | (21,004 | ) | |||||||
Other income (expense): | ||||||||||||
Change in fair value of warrant liability | (9,705 | ) | - | - | ||||||||
Financing costs associated with warrants | (2,367 | ) | - | - | ||||||||
Interest and dividend income | 591 | 287 | 143 | |||||||||
Income tax expense | (1 | ) | (1 | ) | (1 | ) | ||||||
|
|
|
|
|
| |||||||
Net (loss) income | $ | (40,068 | ) | $ | 2,670 | $ | (20,862 | ) | ||||
|
|
|
|
|
| |||||||
Net (loss) income per share, basic | $ | (0.97 | ) | $ | 0.07 | $ | (0.61 | ) | ||||
|
|
|
|
|
| |||||||
Net (loss) income per share, diluted | $ | (0.97 | ) | $ | 0.07 | $ | (0.61 | ) | ||||
|
|
|
|
|
| |||||||
Shares used in computing net (loss) income per share: |
| |||||||||||
Basic | 41,431 | 36,813 | 34,400 | |||||||||
|
|
|
|
|
| |||||||
Diluted | 41,431 | 36,938 | 34,400 | |||||||||
|
|
|
|
|
| |||||||
See accompanying notes to financial statements.
36
MEI PHARMA, INC.
STATEMENTS OF STOCKHOLDERS' EQUITY
(In thousands)
| Common Shares | Additional paid in capital | Accumulated Deficit | Total Stockholders' Equity | |||||||||||||
Balance at June 30, 2015 | 34,156 | $ | 215,930 | $ | (156,139 | ) | $ | 59,791 | ||||||||
Net loss | - | - | (20,862 | ) | (20,862 | ) | ||||||||||
Share-based compensation expense | - | 2,723 | - | 2,723 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Balance at June 30, 2016 | 34,156 | 218,653 | (177,001 | ) | 41,652 | |||||||||||
Net income | - | - | 2,670 | 2,670 | ||||||||||||
Issuance of common stock | 2,616 | 4,212 | - | 4,212 | ||||||||||||
Share-based compensation expense | - | 2,304 | - | 2,304 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Balance at June 30, 2017 | 36,772 | 225,169 | (174,331 | ) | 50,838 | |||||||||||
Net loss | - | - | (40,068 | ) | (40,068 | ) | ||||||||||
Issuance of common stock in private placement (Note 7) | 33,003 | 35,643 | - | 35,643 | ||||||||||||
Issuance of common stock for milestone payment (Note 9) | 167 | 500 | - | 500 | ||||||||||||
Issuance of common stock for vested restricted stock units | 271 | - | - | - | ||||||||||||
Exercise of stock options | 193 | 329 | - | 329 | ||||||||||||
Share-based compensation expense | - | 3,217 | - | 3,217 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Balance at June 30, 2018 | 70,406 | $ | 264,858 | $ | (214,399 | ) | $ | 50,459 | ||||||||
|
|
|
|
|
|
|
| |||||||||
See accompanying notes to financial statements.
37
MEI PHARMA, INC.
STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended June 30, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Cash flows from operating activities: | ||||||||||||
Net (loss) income | $ | (40,068 | ) | $ | 2,670 | $ | (20,862 | ) | ||||
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: | ||||||||||||
Change in fair value of warrant liability | 9,705 | - | - | |||||||||
Financing costs associated with warrants | 2,367 | - | - | |||||||||
Share-based compensation | 3,217 | 2,304 | 2,723 | |||||||||
Depreciation and amortization | 53 | 85 | 58 | |||||||||
Changes in operating assets and liabilities: | ||||||||||||
Prepaid expenses and other current assets | 172 | (927 | ) | (329 | ) | |||||||
Accounts payable | 3,058 | (494 | ) | 216 | ||||||||
Accrued liabilities | 402 | (1,148 | ) | 337 | ||||||||
Deferred revenue | (208 | ) | 996 | - | ||||||||
|
|
|
|
|
| |||||||
Net cash (used in) provided by operating activities | (21,302 | ) | 3,486 | (17,857 | ) | |||||||
|
|
|
|
|
| |||||||
Cash flows from investing activities: | ||||||||||||
Purchases of property and equipment | - | (51 | ) | (4 | ) | |||||||
Purchases of short-term investments | (114,233 | ) | (60,123 | ) | (55,238 | ) | ||||||
Proceeds from maturity of short-term investments | 69,906 | 50,097 | 65,214 | |||||||||
|
|
|
|
|
| |||||||
Net cash (used in) provided by investing activities | (44,327 | ) | (10,077 | ) | 9,972 | |||||||
|
|
|
|
|
| |||||||
Cash flows from financing activities: | ||||||||||||
Proceeds from exercise of stock options | 329 | - | - | |||||||||
Issuance of common stock and warrants | 70,151 | 4,212 | - | |||||||||
|
|
|
|
|
| |||||||
Net cash provided by financing activities | 70,480 | 4,212 | - | |||||||||
|
|
|
|
|
| |||||||
Net increase (decrease) in cash and cash equivalents | 4,851 | (2,379 | ) | (7,885 | ) | |||||||
Cash and cash equivalents at beginning of the period | 8,458 | 10,837 | 18,722 | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents at end of the period | $ | 13,309 | $ | 8,458 | $ | 10,837 | ||||||
|
|
|
|
|
| |||||||
Supplemental cash flow information: | ||||||||||||
Income taxes paid | $ | (1 | ) | $ | (1 | ) | $ | (1 | ) | |||
|
|
|
|
|
| |||||||
See accompanying notes to financial statements.
38
MEI PHARMA, INC.
NOTES TO FINANCIAL STATEMENTS
June 30, 2018
Note 1. The Company and Summary of Significant Accounting Policies
The Company
We are a pharmaceutical company focused on leveraging our extensive development and oncology expertise to identify and advance new therapies intended to meaningfully improve the treatment of cancer. Our portfolio of drug candidates contains four clinical-stage candidates, including one candidate in an ongoing Phase 3 global registration trial and another candidate that is anticipated to advance, in the fourth calendar quarter of calendar year 2018, into a Phase 2 clinical trial that we intend to submit to the U.S. Food and Drug Administration ("FDA") to support accelerated approval of a marketing application. Our common stock is listed on the NASDAQ Capital Market under the symbol "MEIP".
Clinical Development Programs
Our approach to building our pipeline is to license promising cancer agents and build value in programs through development and commercialization, or strategic partnerships, as appropriate. Our drug candidate pipeline includes:
| • | Pracinostat, an oral histone deacetylase ("HDAC") inhibitor; |
| • | ME-401, an oral phosphatidylinositol 3-kinase ("PI3K") delta inhibitor; |
| • | Voruciclib, an oral cyclin-dependent kinase ("CDK") inhibitor; and |
| • | ME-344, a mitochondrial inhibitor targeting the OXPHOS complex. |
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current drug candidates may not have favorable results in later studies or trials. The commercial opportunity will be reduced or eliminated if competitors develop and market products that are more effective, have fewer side effects or are less expensive than our drug candidates. We will need substantial additional funds to progress the clinical trial programs for the drug candidates pracinostat, ME-401, voruciclib and ME-344, and to develop new compounds. The actual amount of funds that will be needed are determined by a number of factors, some of which are beyond our control. Negative U.S. and global economic conditions may pose challenges to our business strategy, which relies on funding from the financial markets or collaborators.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and disclosures made in the accompanying notes to the financial statements. We use estimates that affect the reported amounts (including assets, liabilities, revenues and expenses) and related disclosures. Actual results could materially differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist of cash and highly liquid investments with original maturities of three months or less when purchased. Cash is maintained at financial institutions and, at times, balances may exceed federally insured limits. We have never experienced any losses related to these balances.
Short-Term Investments
Investments that have maturities of greater than three months but less than one year are classified as short-term investments. As of June 30, 2018 and 2017, our short-term investments consisted of $89.4 million and $45.1 million, respectively, in U.S. government securities. The short-term investments held as of June 30, 2018 and 2017 had maturity dates of less than one year, are considered to be "held to maturity" and are carried at amortized cost. Due to the short-term maturities of these instruments, the amortized cost approximates the related fair values. As of June 30, 2018 and 2017, the gross holding gains and losses were immaterial.
Fair Value Measurements
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value is as follows:
| • Level 1 - | Observable inputs such as quoted prices in active markets for identical assets or liabilities. |
39
| • Level 2 - | Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| • Level 3 - | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
We measure the following financial instruments at fair value on a recurring basis. The fair values of these financial instruments were as follows (in thousands):
| June 30, 2018 | June 30, 2017 | |||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | |||||||||||||||||||
Assets: | ||||||||||||||||||||||||
Cash and cash equivalents | $ | 13,309 | $ | - | $ | - | $ | 8,458 | $ | - | $ | - | ||||||||||||
U.S. government treasury bills | 89,434 | - | - | 45,107 | - | - | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total | $ | 102,743 | $ | - | $ | - | $ | 53,565 | $ | - | $ | - | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Liabilities: | ||||||||||||||||||||||||
Warrant liability | $ | - | $ | - | $ | (46,313 | ) | $ | - | $ | - | $ | - | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total | $ | - | $ | - | $ | (46,313 | ) | $ | - | $ | - | $ | - | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
The carrying amounts of financial instruments such as cash equivalents, short-term investments and accounts payable approximate the related fair values due to the short-term maturities of these instruments. We invest our excess cash in financial instruments which are readily convertible into cash, such as money market funds and U.S. government securities. Cash equivalents, where applicable, and short-term investments are classified as Level 1 as defined by the fair value hierarchy.
In May 2018, we issued warrants in connection with our private placement of common shares. Pursuant to the terms of the warrants, we could be required to settle the warrants in cash in the event of an acquisition of the Company and, as a result, the warrants are required to be measured at fair value and reported as a liability in the balance sheet. We recorded the fair value of the warrants upon issuance using the Black-Scholes valuation model and are required to revalue the warrants at each reporting date with any changes in fair value recorded on our statement of operations. The valuation of the warrants is considered under Level 3 of the fair value hierarchy due to the need to use assumptions in the valuation that are both significant to the fair value measurement and unobservable. Inputs used to determine estimated fair value of the warrant liabilities include the estimated fair value of the underlying stock at the valuation date, the estimated term of the warrants, risk-free interest rates, expected dividends and the expected volatility of the underlying stock. The significant unobservable inputs used in the fair value measurement of the warrant liabilities were the volatility rate and the estimated term of the warrants. Generally, increases (decreases) in the fair value of the underlying stock and estimated term would result in a directionally similar impact to the fair value measurement. The change in the fair value of the Level 3 warrant liability is reflected in the statement of operations for the year ended June 30, 2018.
To calculate the fair value of the warrant liability, the following assumptions were used:
| June 30, 2018 | May 16, 2018 | |||||||
Risk-free interest rate | 2.7 | % | 2.9 | % | ||||
Expected life (years) | 4.8 | 5.0 | ||||||
Expected volatility | 77.3 | % | 75.6 | % | ||||
Dividend yield | 0.0 | % | 0.0 | % | ||||
Black-Scholes Fair Value | $ | 2.81 | $ | 2.22 | ||||
The following table sets forth a summary of changes in the estimated fair value of our Level 3 warrant liability for the year ended June 30, 2018 (in thousands):
| Fair Value of Warrants Using Significant Unobservable Inputs (Level 3) | ||||
Balance at July 1, 2017 | $ | - | ||
Issuance of liability classified warrants | 36,608 | |||
Change in estimated fair value of liability classified warrants | 9,705 | |||
|
| |||
Balance at June 30, 2018 | $ | 46,313 | ||
|
| |||
40
Intangible Assets
Intangible assets consist of patents acquired from S*Bio in August 2012, relating to a family of heterocyclic compounds that inhibit HDACs. Capitalized amounts are amortized on a straight-line basis over the expected life of the intellectual property of 14 years from the date of acquisition. The carrying values of intangible assets are periodically reviewed to determine if the facts and circumstances suggest that a potential impairment may have occurred. Results of operations for the years ended June 30, 2018, 2017 and 2016 do not reflect any write-downs associated with the potential impairment of intangible assets.
Property and Equipment
Property and equipment are stated at cost and depreciated over the estimated useful lives of the assets (generally three to seven years) using the straight-line method. Leasehold improvements are stated at cost and are amortized over the shorter of the estimated useful lives of the assets or the lease term.
Revenue Recognition
Payments received under commercial arrangements, such as licensing technology rights, may include non-refundable fees at the inception of the arrangements, milestone payments for specific achievements designated in the agreements, and royalties on the sale of products. We consider a variety of factors in determining the appropriate method of accounting under our license agreements, including whether the various elements can be separated and accounted for individually as separate units of accounting.
Multiple Element Arrangements
Deliverables under an arrangement will be separate units of accounting, provided (i) a delivered item has value to the customer on a standalone basis; and (ii) the arrangement includes a general right of return relative to the delivered item, and delivery or performance of the undelivered item is considered probable and substantially in our control.
We account for revenue arrangements with multiple elements by separating and allocating consideration according to the relative selling price of each deliverable. If an element can be separated, an amount is allocated based upon the relative selling price of each element. We determine the relative selling price of a separate deliverable using the price we charge other customers when we sell that element separately. If the element is not sold separately and third party pricing evidence is not available, we will use our best estimate of selling price.
License Fee Revenue
Non-refundable, up-front fees that are not contingent on any future performance by us and require no consequential continuing involvement on our part are recognized as revenue when the license term commences and the licensed data, technology or product is delivered. We defer recognition of non-refundable upfront license fees if we have continuing performance obligations, without which the licensed data, technology, or product has no utility to the licensee separate and independent of our performance under the other elements of the applicable arrangement. The specific methodology for the recognition of the revenue is determined on a case-by-case basis according to the facts and circumstances of the applicable agreement.
Research and Development Revenue
Research and development revenue represents ratable recognition of fees allocated to research and development activities. We defer recognition of research and development revenue until the performance of the related research and development activities has occurred. Research and development revenue for the year ended June 30, 2018 and 2017 related to services provided by third-party vendors related to research and development activities performed under the Helsinn License Agreement (Note 2).
Cost of Research and Development Revenue
Cost of research and development revenue primarily includes external costs paid to third-party contractors to perform research, conduct clinical trials and develop and manufacture drug materials, and internal compensation and related personnel expenses to support our research and development revenue. All cost of research and development revenue relates to expenses incurred in connection with our development activities in accordance with the Helsinn License Agreement.
Research and Development Costs
Research and development costs are expensed as incurred and include costs paid to third-party contractors to perform research, conduct clinical trials and develop and manufacture drug materials. Clinical trial costs, including costs associated with third-party contractors, are a significant component of research and development expenses. We expense research and development costs based on work performed. In determining the amount to expense, management relies on estimates of total costs based on contract components completed, the enrollment of subjects, the completion of trials, and other events. Costs incurred related to the purchase of in-process research and development for early-stage products or products that are not commercially viable and ready for use, or have no alternative future use, are charged to expense in the period incurred. Costs incurred related to the licensing of products that have not yet received regulatory approval to be marketed, or that are not commercially viable and ready for use, or have no alternative future use, are charged to expense in the period incurred.
41
Share-based Compensation
Share-based compensation expense for employees and directors is recognized in the statement of operations based on estimated amounts, including the grant date fair value and the expected service period. For stock options, we estimate the grant date fair value using a Black-Scholes valuation model, which requires the use of multiple subjective inputs including estimated future volatility, expected forfeitures and the expected term of the awards. We estimate the expected future volatility based on the stock's historical price volatility. The stock's future volatility may differ from our estimated volatility at the grant date. For restricted stock unit ("RSU") equity awards, we estimate the grant date fair value using our closing stock price on the date of grant. Share-based compensation recorded in the statement of operations is based on the awards expected to ultimately vest and has been reduced for estimated forfeitures. The estimated forfeiture rates may differ from actual forfeiture rates which would affect the amount of expense recognized during the period. We recognize the value of the awards over the awards' requisite service or performance periods. The requisite service period is generally the time over which the share-based awards vest.
Interest and Dividend Income
Interest on cash balances is recognized when earned. Dividend income is recognized when the right to receive the payment is established.
Income Taxes
Our income tax expense consists of current and deferred income tax expense or benefit. Current income tax expense or benefit is the amount of income taxes expected to be payable or refundable for the current year. A deferred income tax asset or liability is recognized for the future tax consequences attributable to tax credits and loss carryforwards and to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. As of June 30, 2018 and 2017, we have established a valuation allowance to fully reserve our net deferred tax assets. Tax rate changes are reflected in income during the period such changes are enacted. Changes in our ownership may limit the amount of net operating loss carry-forwards that can be utilized in the future to offset taxable income.
On December 22, 2017 the U.S government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the "Tax Act"). The Tax Act reduces the corporate tax rate from 34% to 21%, effective for tax years beginning January 1, 2018. We are subject to the provisions of the Financial Accounting Standards Board ("FASB") Accounting Standards Codification 740-10, Income Taxes, which requires that the effect on deferred tax assets and liabilities of a change in tax rates be recognized in the period the tax rate change was enacted. The rate change is administratively effective at the beginning of our fiscal year, using a blended rate for the annual period. As a result, the blended statutory tax rate for the year ended June 30, 2018 is 27.5%. The enacted reduction in the corporate federal income tax rate resulted in a re-measurement of our net deferred tax assets and liabilities. Consequently, we have recorded a decrease related to deferred tax assets $15.9 million with a corresponding change to our valuation allowance.
Changes in our ownership may limit the amount of net operating loss carry-forwards that can be utilized in the future to offset taxable income.
The FASB Topic on Income Taxes prescribes a recognition threshold and measurement attribute criteria for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. There were no unrecognized tax benefits as of June 30, 2018 and 2017.
Net (Loss) Income Per Share
Basic and diluted net (loss) income per share are computed using the weighted-average number of shares of common stock outstanding during the period, less any shares subject to repurchase or forfeiture. There were no shares of common stock subject to repurchase or forfeiture for the years ended June 30, 2018, 2017 and 2016.
Our potentially dilutive shares, which include outstanding stock options, restricted stock units, and warrants, are considered to be common stock equivalents and are only included in the calculation of diluted net loss per share when their effect is dilutive. For the years ended June 30, 2018, 2017 and 2016, we did not have any items that would be classified as other comprehensive income or losses.
42
Shares used in calculating net (loss) income per share was determined as follows (in thousands):
| Years ended June 30, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Weighted average shares outstanding | 41,064 | 36,435 | 34,156 | |||||||||
Effect of vested restricted stock units | 367 | 378 | 244 | |||||||||
|
|
|
|
|
| |||||||
Weighted average shares used in calculating basic (loss) earnings per share | 41,431 | 36,813 | 34,400 | |||||||||
Effect of potentially dilutive common shares from equity awards | - | 125 | - | |||||||||
|
|
|
|
|
| |||||||
Weighted average shares used in calculating diluted (loss) earnings per share | 41,431 | 36,938 | 34,400 | |||||||||
|
|
|
|
|
| |||||||
The following potentially dilutive shares (in thousands) that have been excluded from the calculation of net (loss) income per share because of their anti-dilutive effect:
| Years ended June 30, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Stock options | 5,606 | 3,749 | 2,687 | |||||||||
Restricted stock units | 336 | - | 179 | |||||||||
Warrants | 3,532 | 3,582 | 3,762 | |||||||||
|
|
|
|
|
| |||||||
Total anti-dilutive shares | 9,474 | 7,331 | 6,628 | |||||||||
|
|
|
|
|
| |||||||
Recent Accounting Pronouncements
Adopted Accounting Standards
In January 2017, the FASB issued Accounting Standards Update ("ASU") 2017-01 ("ASU 2017-01"), Business Combinations (Topic 804): Clarifying the Definition of a Business . This ASU clarifies the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions or disposals of assets or businesses. We adopted ASU 2017-01 as of July 1, 2017, and this guidance was used in our assessment of the Presage License Agreement (Note 3).
In March 2016, the FASB issued ASU 2016-09 Improvements to Employee Share-Based Payment Accounting , which simplifies several aspects of accounting for share-based payment transactions including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. We adopted this ASU as of July 1, 2017, and it did not have a material impact on our financial statements.
In May 2017, the FASB issued ASU 2017-09, Compensation-Stock Compensation: Scope of Modification Accounting, which provides clarification on when modification accounting should be used for changes to the terms or conditions of a share-based payment award. This ASU does not change the accounting for modifications but clarifies that modification accounting guidance should only be applied if there is a change to the value, vesting conditions or award classification and would not be required if the changes are considered non-substantive. We adopted this ASU as of July 1, 2017, and it did not have a material impact on our financial statements.
Accounting Standards Not Yet Adopted
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers . The standard provides companies with a single model for accounting for revenue arising from contracts with customers and supersedes current revenue recognition guidance, including industry-specific revenue guidance. The core principle of the model is to recognize revenue when control of the goods or services transfers to the customer, as opposed to recognizing revenue when the risks and rewards transfer to the customer under the existing revenue guidance. The guidance permits companies to either apply the requirements retrospectively to all prior periods presented, or apply the requirements in the year of adoption, through a cumulative adjustment. The following ASUs were subsequently issued by the FASB to clarify the implementation guidance in some areas and add practical expedients: In March 2016, ASU 2016-08, Revenue from Contracts with Customers, Principal versus Agent Considerations ; in April 2016, ASU 2016-10, Revenue from Contracts with Customers, Identifying Performance Obligations and Licensing ; in May 2016, ASU 2016-11, Revenue from Contracts with Customers and Derivatives and Hedging – Rescission of SEC Guidance ; and ASU 2016-12, Revenue from Contracts with Customers – Narrow Scope Improvements and Practical Expedients . ASU 2014-09 will be effective for us in our first quarter of fiscal 2019. We plan to adopt this standard on a modified retrospective basis. We have evaluated the impact of adoption of this standard and determined that it will not have a material impact on our financial statements.
In February 2016, the FASB issued ASU 2016-02 Leases , which introduces the recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous guidance. The new standard establishes a right-of-use ("ROU") model that requires a lessee to record an ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. The new standard is effective for fiscal years beginning after December 15, 2018 and interim periods within those fiscal years with early adoption permitted. We are evaluating the impact that the adoption of this standard will have on our financial statements.
43
Note 2. Helsinn License Agreement
In August 2016, we entered into the Helsinn License Agreement. Under the terms of the agreement, Helsinn was granted a worldwide exclusive license to develop, manufacture and commercialize pracinostat, and is primarily responsible for funding its global development and commercialization. As compensation for such grant of rights, we received payments of $20.0 million in fiscal 2017. In addition, we are eligible to receive up to $444 million in potential regulatory and sales-based milestones, along with royalty payments on the net sales of pracinostat, which, in the U.S., are tiered and begin in the mid-teens.
We determined that the exclusive license, development and commercialization agreement represents a multiple-element arrangement for purposes of revenue recognition. We identified the following elements, based upon deliverables under the agreement: (i) worldwide license and transfer of technology and data; (ii) completion of the conduct of certain identified clinical trials related to pracinostat; (iii) coordination of services provided by third-party vendors related to research and development activities, for which Helsinn has agreed to reimburse such third-party expenses; and (iv) the conduct of the Phase 2 dose-optimization study of pracinostat in combination with azacitidine in patients with high and very high risk MDS who are previously untreated with hypomethylating agents (the "POC study"), for which Helsinn has agreed to share third-party expenses. The license was determined to represent a separate element as it has stand-alone value and is not dependent upon the performance of the research and development activities. The research and development elements, related to the conduct of clinical trials and services provided by third-party vendors, were determined to represent separate elements as they primarily represent pass through of services performed by third parties and therefore are sold separately by other vendors. We allocated the proceeds related to the agreement to the units of accounting using the relative selling price method. We determined the estimated selling price for the license using an income approach. We determined the estimated selling price for the research and development elements based on estimated fulfillment costs plus a normal profit margin. Revenues related to the research and development elements of the arrangement are recognized based on the proportional performance of each research and development activity. Research and development revenues are recognized on a gross basis as we are the primary obligor and have discretion in supplier selection. As of June 30, 2018, we have substantially completed all of the deliverables under the agreement, with the exception of the POC study, which is ongoing.
Note 3. Presage License Agreement
In September 2017, we entered into a license agreement with Presage Biosciences, Inc. ("Presage"). Under the terms of such license agreement (the "Presage License Agreement"), Presage granted to us exclusive worldwide rights to develop, manufacture and commercialize voruciclib, a clinical-stage, oral and selective CDK inhibitor, and related compounds. In exchange, we paid Presage an up-front payment of $1.9 million in September 2017 and an additional payment of $1.0 million in January 2018. With respect to the first indication, an incremental $2.0 million payment, due upon dosing of the first subject in the first registration trial will be owed to Presage, for total payments of $4.9 million prior to receipt of marketing approval of the first indication in the U.S., E.U. or Japan. Additional potential payments of up to $179 million will be due upon the achievement of certain development, regulatory and commercial milestones. We will also pay mid-single-digit tiered royalties on the net sales of any product successfully developed. As an alternative to milestone and royalty payments related to countries in which we sublicense product rights, we will pay to Presage a tiered percent (which decreases as product development progresses) of amounts received from such sublicensees. The payments totaling $2.9 million are included in research and development expenses for the year ended June 30, 2018.
Note 4. Intangible Assets
Intangible assets consisted of the following, in thousands:
| June 30, | ||||||||
| 2018 | 2017 | |||||||
S*Bio Patents – Gross | $ | 500 | $ | 500 | ||||
Less: accumulated amortization | (204 | ) | (169 | ) | ||||
|
|
|
| |||||
Intangible assets, net | $ | 296 | $ | 331 | ||||
|
|
|
| |||||
Amortization expense of intangible assets for the years ended June 30, 2018, 2017 and 2016 was $35,000, $35,000 and $35,000, respectively. We expect to record amortization of $35,000 per year through 2026 for our S*Bio patents.
Note 5. Property and Equipment
Property and equipment consisted of the following, in thousands:
| June 30, | ||||||||
| 2018 | 2017 | |||||||
Furniture and equipment | $ | 81 | $ | 95 | ||||
Less: accumulated depreciation | (49 | ) | (45 | ) | ||||
|
|
|
| |||||
Property and equipment, net | $ | 32 | $ | 50 | ||||
|
|
|
| |||||
Depreciation expense of property and equipment for the years ended June 30, 2018, 2017 and 2016 was $18,000, $50,000 and $23,000, respectively.
44
Note 6. Accrued Liabilities
Accrued liabilities consisted of the following, in thousands:
| June 30, | ||||||||
| 2018 | 2017 | |||||||
Accrued pre-clinical and clinical trial expenses | $ | 1,234 | $ | 1,334 | ||||
Accrued compensation and benefits | 1,766 | 1,546 | ||||||
Accrued legal and professional services expenses | 251 | 267 | ||||||
Other | 203 | 138 | ||||||
|
|
|
| |||||
Total accrued liabilities | $ | 3,454 | $ | 3,285 | ||||
|
|
|
| |||||
Note 7. Stockholders' Equity
Equity Transactions
May 2018 Private Placement
In May 2018, we raised $70.2 million, net of transaction costs, in a private placement of common shares and warrants. We issued and sold 33,003,296 shares of common stock at a purchase price of $2.27 per share, as well as warrants to purchase 16,501,645 shares. The warrants are exercisable at a price of $2.54 per share and expire in May 2023. In the event of a sale of the Company, the terms of the warrants require us to use our best efforts to ensure the holders of such warrants will have a continuing right to purchase shares of the acquirer and, if our efforts are unsuccessful, to make a payment to such warrant holders based on a Black-Scholes valuation (using variables as specified in the warrants). Therefore we are required to account for these warrants as liabilities and record them at fair value. We recorded the fair value of the warrants of $36.6 million upon issuance using the Black-Scholes valuation model. The warrants were revalued as of June 30, 2018 at $46.3 million; the change in fair value of $9.7 million was recorded in our statement of operations for the year ended June 30, 2018. Additionally, we allocated $2.4 million of the transaction costs to financing expense on our statement of operations. The remaining $2.4 million of transaction costs were offset against the proceeds allocated to our common stock.
Shelf Registration Statement
In May 2017, we filed a shelf registration statement on Form S-3 with the SEC ("shelf registration statement"). The shelf registration statement was declared effective by the SEC in May 2017. The shelf registration statement permits us to sell, from time to time, up to $150.0 million of common stock, preferred stock and warrants. In November 2017, we entered into an At-The-Market Equity Offering Sales Agreement (the "ATM Sales Agreement"), pursuant to which we may sell an aggregate of up to $30.0 million of our common stock pursuant to the shelf registration statement. As of June 30, 2018, we have not sold any shares under the ATM Sales Agreement there is $150.0 million aggregate value of securities available under the shelf registration statement.
Helsinn Equity Investment
On August 5, 2016, we entered into the Helsinn Equity Agreement. Pursuant to the terms of the Helsinn Equity Agreement, we issued 2,616,431 shares of common stock on August 16, 2016 in exchange for a $5.0 million investment. The transaction was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
Description of Capital Stock
Our total authorized share capital is 113,100,000 shares consisting of 113,000,000 shares of common stock, $0.00000002 par value per share, and 100,000 shares of preferred stock, $0.01 par value per share.
Common Stock
The holders of common stock are entitled to one vote per share. In the event of a liquidation, dissolution or winding up of our affairs, holders of the common stock will be entitled to share rateably in all our assets that are remaining after payment of our liabilities and the liquidation preference of any outstanding shares of preferred stock. All outstanding shares of common stock are fully paid and non-assessable. The rights, preferences and privileges of holders of common stock are subject to any series of preferred stock that we have issued or that we may issue in the future. The holders of common stock have no pre-emptive rights and are not subject to future calls or assessments by us.
Preferred Stock
Our Board of Directors has the authority to issue up to 100,000 shares of preferred stock with par value of $.01 per share in one or more series and to fix the rights, preferences, privileges and restrictions in respect of that preferred stock, including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption (including sinking fund provisions), redemption prices and liquidation preferences, and the number of shares constituting such series and the designation of any such series, without future vote or action by the stockholders. Therefore, the board without the approval of the stockholders could authorize the issue of preferred stock with voting, conversion and other rights that could affect the voting power, dividend and other rights of the holders of shares or that could have the effect of delaying, deferring or preventing a change of control. There were no shares of preferred stock outstanding as of June 30, 2018 or 2017.
45
Warrants
As of June 30, 2018, there were outstanding warrants to purchase 16,501,645 shares of our common stock at an exercise price of $2.54 per share, which expire in May 2023, issued in conjunction with our May 2018 Private Placement. The warrants were fully vested upon issuance in May 2018.
Note 8. Share-based Compensation
We use equity-based compensation programs to provide long-term performance incentives for our employees. These incentives consist primarily of stock options and RSUs. In December 2008, we adopted the MEI Pharma, Inc. 2008 Stock Omnibus Equity Compensation Plan ("2008 Plan"), as amended and restated in 2011, 2013 and 2016, under which 10,186,000 shares of common stock are authorized for issuance. The 2008 Plan provides for the grant of options and/or other stock-based or stock-denominated awards to our non-employee directors, officers, employees and advisors. As of June 30, 2018, there were 2,896,206 shares available for future grant under the 2008 Plan.
Total share-based compensation expense for all stock awards consists of the following, in thousands:
| Years Ended June 30, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Research and development | $ | 1,176 | $ | 839 | $ | 1,871 | ||||||
General and administrative | 2,041 | 1,465 | 852 | |||||||||
|
|
|
|
|
| |||||||
Total share-based compensation | $ | 3,217 | $ | 2,304 | $ | 2,723 | ||||||
|
|
|
|
|
| |||||||
Stock Options
Stock options granted to employees vest ratably each month for a period of 36 months, or vest 25% one year from the date of grant and ratably each month thereafter for a period of 36 months and expire either five years or ten years from the date of grant. Stock options granted to directors vest ratably each month for periods ranging from seven to 36 months from the date of grant and expire either five years or ten years from the date of grant. As of June 30, 2018, there were a total of 6,281,615 options outstanding.
A summary of our stock option activity and related data follows:
| Number of Options | Weighted-Average Exercise Price | Weighted-Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value | |||||||||||||
Outstanding at June 30, 2017 | 4,259,083 | $ | 3.21 | |||||||||||||
Granted | 2,772,333 | $ | 3.14 | |||||||||||||
Exercised | (192,937 | ) | $ | 1.71 | ||||||||||||
Forfeited / Cancelled | (318,615 | ) | $ | 3.44 | ||||||||||||
Expired | (238,249 | ) | $ | 6.76 | ||||||||||||
|
| |||||||||||||||
Outstanding at June 30, 2018 | 6,281,615 | $ | 3.08 | 7.3 | $ | 8,942,979 | ||||||||||
|
| |||||||||||||||
Vested and exercisable at June 30, 2018 | 3,048,685 | $ | 3.40 | 5.5 | $ | 4,846,546 | ||||||||||
As of June 30, 2018, the aggregate intrinsic value of outstanding options is calculated as the difference between the exercise price of the underlying options and the closing price of our common stock of $3.94 on that date. The total fair value of options that vested during the years ended June 30, 2018, 2017 and 2016 was $2.4 million, $2.4 million and $3.1 million, respectively.
A summary of our nonvested stock option activity:
| Number of Options | Weighted-Average Grant Date Fair Value | |||||||
Nonvested at June 30, 2017 | 2,019,593 | $ | 1.57 | |||||
Granted | 2,772,333 | $ | 2.40 | |||||
Forfeited | (237,627 | ) | $ | 1.89 | ||||
Vested | (1,321,369 | ) | $ | 1.84 | ||||
|
| |||||||
Nonvested at June 30, 2018 | 3,232,930 | $ | 2.15 | |||||
|
| |||||||
Unrecognized compensation expense related to non-vested stock options totalled $4.3 million as of June 30, 2018. Such compensation expense is expected to be recognized over a weighted-average period of 1.9 years. As of June 30, 2018, we expect all outstanding options to vest.
46
We use a Black-Scholes valuation model to estimate the grant date fair value of stock options. To calculate these fair values, the following weighted-average assumptions were used:
| Years ended June 30, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Risk-free interest rate | 2.3 | % | 1.3 | % | 1.7 | % | ||||||
Expected life (years) | 6.0 | 5.9 | 5.8 | |||||||||
Expected volatility | 93.7 | % | 107.4 | % | 116.7 | % | ||||||
Dividend yield | 0.0 | % | 0.0 | % | 0.0 | % | ||||||
Weighted-average grant date fair value | $ | 2.40 | $ | 1.15 | $ | 1.35 | ||||||
Restricted Stock Units
In March 2013, the Compensation Committee of the Board of Directors granted 400,000 RSUs to our Chief Executive Officer. Each RSU represented the contingent right to receive one share of our common stock. The shares underlying the RSUs were delivered on March 29, 2018, and we issued 271,080 shares of common stock, net of shares withheld to cover taxes and fees. The fair value of the RSUs on the date of grant was $3.5 million.
We had 332,193 unvested RSUs outstanding as of June 30, 2018. Each RSU represents the contingent right to receive one share of our common stock. The RSUs were subject to performance criteria that were met in August 2016. The RSUs vested in August 2018. The fair value of the RSUs was measured at $1.61 per unit on the date the performance criteria were met. As of June 30, 2018, unrecognized compensation expense related to the unvested portion of our RSUs was approximately $26,000 and is expected to be recognized over approximately 0.1 years.
Note 9. Commitments and Contingencies
We have contracted with various consultants and third parties to assist us in pre-clinical research and development and clinical trials work for our leading drug compounds. The contracts are terminable at any time, but obligate us to reimburse the providers for any time or costs incurred through the date of termination. We also have employment agreements with certain of our current employees that provide for severance payments and accelerated vesting for share-based awards if their employment is terminated under specified circumstances.
We have leased approximately 13,700 square feet of office space, located at 3611 Valley Centre Drive, San Diego, California 92130. The location houses our executive and administrative offices. The lease commenced in June 2017 and expires in May 2020. The monthly rental rate is approximately $46,000 over the remaining lease term, plus a pro rata share of certain building expenses. The remaining contractual obligation for the lease is $1.1 million.
Presage License Agreement
As discussed in Note 3, we are party to a license agreement with Presage under which we may be required to make future payments upon the achievement of certain development, regulatory and commercial milestones, as well as potential future royalties based upon net sales. As of June 30, 2018, we have not accrued any amounts for potential future payments as they are not probable.
S*Bio Purchase Agreement
We are party to a definitive asset purchase agreement with S*Bio, pursuant to which we acquired certain assets comprised of intellectual property and technology including rights to pracinostat. We agreed to make certain milestone payments to S*Bio based on the achievement of certain clinical, regulatory and net sales-based milestones, as well as to make certain contingent earnout payments to S*Bio. Milestone payments will be made to S*Bio up to an aggregate amount of $75.2 million if certain U.S., E.U. and Japanese regulatory approvals are obtained and if certain net sales thresholds are met in North America, the E.U. and Japan. The first milestone payment of $200,000 plus 166,527 shares of our common stock having a value of $500,000 was paid in August 2017 upon the first dosing of a patient in a Phase 3 clinical trial. Subsequent milestone payments will be due upon certain regulatory approvals and sales-based events. As of June 30, 2018, we have not accrued any amounts for potential future payments as they are not probable.
CyDex License Agreement
We are party to a license agreement with CyDex Pharmaceuticals, Inc. ("CyDex"). Under the license agreement, CyDex granted to us an exclusive, nontransferable license to intellectual property rights relating to Captisol ® for use with our isoflavone-based drug compounds (currently ME-344). We agreed to pay to CyDex a non-refundable license issuance fee, future milestone payments, and royalties at a low, single-digit percentage rate on future sales of our approved drugs utilizing Captisol. Contemporaneously with the license agreement, CyDex entered into a commercial supply agreement with us, pursuant to which we agreed to purchase 100% of our requirements for Captisol from CyDex. We may terminate both the license agreement and the supply agreement at any time upon 90 days' prior written notice. As of June 30, 2018, we have not accrued any amounts for potential future payments as they are not probable.
47
Note 10. Segment Information
We have one operating segment, the development of pharmaceutical compounds. All of our assets and liabilities were located in the United States of America as of June 30, 2018, 2017 and 2016.
Note 11. Income Taxes
Pre-tax income (loss) consists of the following jurisdictions (in thousands):
| Years ended June 30, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Domestic | $ | (40,068 | ) | $ | 2,670 | $ | (20,862 | ) | ||||
Foreign | - | - | - | |||||||||
|
|
|
|
|
| |||||||
Pre-tax loss | $ | (40,068 | ) | $ | 2,670 | $ | (20,862 | ) | ||||
|
|
|
|
|
| |||||||
The reconciliation of income tax computed at the U.S. federal statutory tax rates to income tax expense is as follows (in thousands):
| Years Ended June 30, | ||||||||||||||||||||||||
| 2018 | 2017 | 2016 | ||||||||||||||||||||||
| $ | % | $ | % | $ | % | |||||||||||||||||||
Tax benefit (expense) at U.S. statutory rates | $ | 11,019 | 28% | $ | (908 | ) | 34% | $ | 7,093 | 34% | ||||||||||||||
State tax | (5,370 | ) | -13% | (158 | ) | 6% | 1,215 | 6% | ||||||||||||||||
Other | (537 | ) | -1% | (208 | ) | 8% | (356 | ) | -2% | |||||||||||||||
Capital loss carryover expiration | - | 0% | (26,382 | ) | 988% | - | 0% | |||||||||||||||||
Decrease (increase) in valuation allowance | 14,914 | 37% | 27,655 | -1036% | (7,953 | ) | -38% | |||||||||||||||||
Revaluation of deferred taxes | (15,870 | ) | -40% | - | 0% | - | 0% | |||||||||||||||||
Equity compensation | (837 | ) | -2% | - | 0% | - | 0% | |||||||||||||||||
Equity financing costs | (3,320 | ) | -8% | - | 0% | - | 0% | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| $ | (1 | ) | 0% | $ | (1 | ) | 0% | $ | (1 | ) | 0% | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Deferred tax liabilities and assets are comprised of the following (in thousands):
| June 30, | ||||||||
| 2018 | 2017 | |||||||
Deferred tax assets: | ||||||||
Fixed and intangible assets | $ | 17,790 | $ | 29,660 | ||||
Tax carried forward losses | 8,893 | 7,886 | ||||||
Share-based payments | 2,354 | 5,621 | ||||||
Compensation accruals | 367 | 630 | ||||||
Capital lease obligation | 171 | 413 | ||||||
Consultant and other accruals | 35 | 325 | ||||||
Charitable contributions | 11 | - | ||||||
|
|
|
| |||||
Total deferred tax assets | 29,621 | 44,535 | ||||||
Valuation allowance for deferred tax assets | (29,621 | ) | (44,535 | ) | ||||
|
|
|
| |||||
Net deferred tax assets and liabilities | $ | - | $ | - | ||||
|
|
|
| |||||
We evaluate the recoverability of the deferred tax assets and the amount of the required valuation allowance. Due to the uncertainty surrounding the realization of the tax deductions in future tax returns, we have recorded a valuation allowance against our net deferred tax assets as of June 30, 2018 and 2017. At such time as it is determined that it is more likely than not that the deferred tax assets will be realized, the valuation allowance would be reduced.
We had federal and state net operating loss carryforwards of approximately $36.4 million and $17.8 million as of June 30, 2018. Under the new tax law, the Federal net operating loss generated subsequent to December 31, 2017 will be carried forward indefinitely. The Federal and state net operating losses generated prior to December 31, 2017 will begin to expire in 2022 and 2029, respectively.
48
Our ability to utilize our net operating loss carryforwards may be substantially limited due to ownership changes that have occurred or that could occur in the future under Section 382 of the Internal Revenue Code and similar state laws. During 2017, we completed a study to analyze whether one or more ownership changes had occurred through August 31, 2016 and determined that two such ownership changes did occur. While those ownership changes do limit the amount of net operating loss we are able to use each year, all of our net operating losses were expected to be available for utilization prior to expiring. We are in the process of updating our analysis of owner shifts to determine whether there are any additional limitations on the utilization of our net operating loss carryforwards since August 31, 2016.
None of our prior income tax returns have been selected for examination by a major taxing jurisdiction; however, the statutes of limitations for various filings remain open. The oldest filings subject to potential examination for federal and state purposes are 2015 and 2014, respectively. If we utilize a net operating loss related to a closed year, the amount of the net operating loss may still be adjusted by the taxing authority. We have not reduced any tax benefit on our financial statements due to uncertain tax positions as of June 30, 2018 and we are not aware of any circumstance that would significantly change this result through the end of fiscal year 2019. To the extent we incur income-tax related penalties or interest, we will recognize them as additional income tax expense.
Note 12. Selected Quarterly Financial Information (Unaudited)
The following table presents our unaudited quarterly results of operations for the years ended June 30, 2018 and 2017 (in thousands, except per share amounts).
| Quarters Ended | ||||||||||||||||
| June 30, 2018 | March 31, 2018 | December 31, 2017 | September 30, 2017 | |||||||||||||
Total revenues | $ | 548 | $ | 433 | $ | 358 | $ | 283 | ||||||||
Net loss (1) | $ | (19,253 | ) | $ | (5,948 | ) | $ | (6,079 | ) | $ | (8,788 | ) | ||||
Basic loss per share | $ | (0.36 | ) | $ | (0.16 | ) | $ | (0.16 | ) | $ | (0.24 | ) | ||||
Diluted loss per share | $ | (0.36 | ) | $ | (0.16 | ) | $ | (0.16 | ) | $ | (0.24 | ) | ||||
| Quarters Ended | ||||||||||||||||
| June 30, 2017 | March 31, 2017 | December 31, 2016 | September 30, 2016 | |||||||||||||
Total revenues | $ | 449 | $ | 4,505 | $ | 17,199 | $ | 1,096 | ||||||||
Net income (loss) (1) | $ | (4,343 | ) | $ | (602 | ) | $ | 11,885 | $ | (4,270 | ) | |||||
Basic income (loss) per share | $ | (0.12 | ) | $ | (0.02 | ) | $ | 0.32 | $ | (0.12 | ) | |||||
Diluted income (loss) per share | $ | (0.12 | ) | $ | (0.02 | ) | $ | 0.32 | $ | (0.12 | ) | |||||
| (1) | We have experienced large changes in our net (loss) income which relates to the fair value of the warrant liability for the year ended June 30, 2018 and revenues associated with the Helsinn License Agreement for the year ended June 30, 2017. Refer to Notes 7 and 2 respectively, for further discussion. |
49
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
(a) Disclosure Controls and Procedures
At the end of the period covered by this Annual Report on Form 10-K, our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer's management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective to ensure that the information required to be disclosed by the Company in reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms.
A control system no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within the Company are detected. Accordingly, our disclosure controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our disclosure control system are met and, as set forth above, our Chief Executive Officer and Chief Financial Officer have concluded, based on their evaluation as of the end of the period covered by this Annual Report on Form 10-K, that our disclosure controls and procedures were effective to provide reasonable assurance that the objectives of our disclosure control system were met.
(b) Management's Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Exchange Act. Our internal control was designed to provide reasonable assurance to our management and Board of Directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Management maintains a comprehensive system of controls intended to ensure that transactions are executed in accordance with management's authorization, assets are safeguarded and financial records are reliable. Management also takes steps to ensure that information and communication flows are effective, and to monitor performance, including performance of internal control procedures.
Management assessed the effectiveness of our internal control over financial reporting as of June 30, 2018, based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework). Based on this assessment, management believes that our internal control over financial reporting is effective as of June 30, 2018.
There were no changes in internal control over financial reporting during the quarter ended June 30, 2018, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
The effectiveness of our internal control over financial reporting as of June 30, 2018 has been audited by BDO USA, LLP, an independent registered public accounting firm, as stated in their report which is included herein.
50
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
MEI Pharma, Inc.
San Diego, California
Opinion on Internal Control over Financial Reporting
We have audited MEI Pharma, Inc. (the "Company's") internal control over financial reporting as of June 30, 2018, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2018, based on the COSO criteria .
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) ("PCAOB"), the balance sheets of the Company as of June 30, 2018 and 2017, and the related statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended June 30, 2018, and the related notes and our report dated August 29, 2018 expressed an unqualified opinion thereon.
Basis for Opinion
The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying, Item 9A, Management's Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit of internal control over financial reporting in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ BDO USA, LLP
San Diego, California
August 29, 2018
51
| Item 9B. | Other Information |
Not applicable.
PART III
| Item 10. | Directors and Executive Officers of the Registrant |
Code of Ethics
We have adopted a Code of Business and Ethics policy that applies to our directors and employees (including our principal executive officer and our principal financial officer), and have posted the text of our policy on our website (www.meipharma.com). In addition, we intend to promptly disclose (i) the nature of any amendment to the policy that applies to our principal executive officer and principal financial officer and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
The other information required by this item is incorporated herein by reference to our proxy statement for the fiscal year ended June 30, 2018 (the "Proxy Statement").
| Item 11. | Executive Compensation |
The information required by this item is incorporated herein by reference to the Proxy Statement.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item is incorporated herein by reference to the Proxy Statement.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item is incorporated herein by reference to the Proxy Statement.
| Item 14. | Principal Accountant Fees and Services. |
The information required by this item is incorporated herein by reference to the Proxy Statement.
52
PART IV
| Item 15. | Exhibits, Financial Statement Schedules |
| (a) | 1. Financial Statements |
Reference is made to the Financial Statements under Item 8 in Part II hereof.
2. Financial Statement Schedules
The Financial Statement Schedules have been omitted either because they are not required or because the information has been included in the financial statements or the notes thereto included in this Annual Report on Form 10-K.
3. Exhibits
Exhibit Index
3.1 | Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Registrant's Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129)). | |
3.2 | Certificate of Amendment to the Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1.1 to the Registrant's Current Report on Form 8-K filed on March 31, 2010 (File No. 000-50484)). | |
3.3 | Certificate of Amendment to the Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on December 19, 2012 (File No. 000-50484)). | |
3.4 | Certificate of Ownership and Merger (incorporated by reference to Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on July 2, 2012 (File No. 000-50484)). | |
3.5 | Certificate of Designation of Series A Convertible Preferred Stock of Marshall Edwards, Inc. (incorporated by reference to Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on May 11, 2011 (File No. 000-50484)) | |
3.6 | Certificate of Designation of Series B Preferred Stock of Marshall Edwards, Inc. (incorporated by reference to Exhibit 4 to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on March 18, 2011 (File No. 000-50484)) | |
3.7 | Second Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the Registrant's Quarterly Report on Form 10-Q filed on May 4, 2017 (File No. 000-50484)). | |
4.1 | Specimen Stock Certificate (incorporated by reference to Exhibit 4.1 to Amendment No. 1 to the Registrant's Registration Statement on Form S-1 filed on October 31, 2003 (Reg. No. 333-109129)). | |
4.2 | Form of Warrant (incorporated by reference to Exhibit B to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on May 16, 2018 (File No. 000-50484)). | |
10.1 | Employment letter dated April 23, 2010, between Marshall Edwards, Inc. and Daniel Gold (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on April 26, 2010 (File No. 000-50484)). | |
10.2 | Employment letter dated June 1, 2011, between Marshall Edwards, Inc. and Robert D. Mass (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on June 2, 2011 (File No. 000-50484)). | |
10.3 | Employment letter dated March 6, 2014, between MEI Pharma, Inc. and David M. Urso (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on April 8, 2014 (File No. 000-50484)). | |
10.4 | Amendment No. 1, dated July 12, 2018, to the Employment Letter dated March 6, 2014, between MEI Pharma, Inc. and David M. Urso. (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on July 16, 2018 (File No. 000-50484)). | |
10.5 | Employment letter dated February 1, 2017, between MEI Pharma, Inc. and Brian G. Drazba (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on April 3, 2017 (File No. 000-50484)). | |
10.6 | MEI Pharma, Inc. Amended and Restated 2008 Stock Omnibus Equity Compensation Plan (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on December 2, 2016 (File No. 000-50484)). | |
10.7 | Form of Indemnification Agreement (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on August 29, 2011 (File No. 000-50484)). | |
10.8 | Asset Purchase Agreement, dated as of August 7, 2012, between MEI Pharma, Inc. and S*Bio Pte Ltd. (incorporated by reference to Exhibit 2.1 to the Registrant's Current Report on Form 8-K filed on August 8, 2012 (File No. 000-50484)). | |
53
10.9** | License Agreement, dated September 28, 2012, between Cydex Pharmaceuticals, Inc. and the Company (incorporated by reference to Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-Q filed on November 13, 2012 (File No. 000-50484)). | |
10.10** | Supply Agreement, dated September 28, 2012, between Cydex Pharmaceuticals, Inc. and the Company (incorporated by reference to Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q filed on November 13, 2012 (File No. 000-50484)). | |
10.11** | License, Development and Commercialization Agreement, dated August 5, 2016, by and between the Company and Helsinn Healthcare SA (incorporated by reference to Exhibit 10.1 to Amendment No. 1 to the Registrant's Quarterly Report on Form 10-Q/A filed on February 16, 2017 (File No. 000-50484)). | |
10.12 | Common Stock Purchase Agreement, dated as of August 5, 2016, by and between MEI Pharma, Inc. and Helsinn Investment Fund SA (incorporated by reference to Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q filed on November 9, 2016 (File No. 000-50484)). | |
10.13** | License Agreement, dated as of September 5, 2017, by and between MEI Pharma, Inc. and Presage Biosciences, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-Q filed on November 8, 2017 (File No. 000-50484)). | |
10.14 | At-The-Market Equity Offering Sales Agreement, dated November 8, 2017 between MEI Pharma, Inc. and Stifel, Nicolaus & Company, Inc. (incorporated by reference to Exhibit 1.1 to the Registrant's Current Report on Form 8-K filed on November 8, 2017 (File No. 000-50484)). | |
10.15 | Securities Purchase Agreement, dated May 11, 2018, between MEI Pharma, Inc. and the purchasers identified in Exhibit A therein (incorporated by reference to Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on May 16, 2018 (File No. 000-50484)). | |
10.16 | Registration Rights Agreement dated May 16, 2018, between MEI Pharma, Inc. and the purchasers identified in Exhibit A therein. (incorporated by reference to Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed on May 16, 2018 (File No. 000-50484)). | |
23.1 | Consent of Independent Registered Accounting Firm* | |
31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14(A) promulgated under the Securities Exchange Act of 1934* | |
31.2 | Certification of Chief Financial Officer pursuant to Rule 13a-14(A) promulgated under the Securities Exchange Act of 1934* | |
32.1 | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350 and Rule 13a-14(B) promulgated under the Securities Exchange Act of 1934* | |
101.INS | XBRL Instance Document* | |
101.SCH | XBRL Taxonomy Extension Schema Document* | |
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document* | |
101.DEF | XBRL Taxonomy Extension Definition Linkbase Document* | |
101.LAB | XBRL Taxonomy Extension Label Linkbase Document* | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document* | |
| (*) | Filed herewith. |
| (**) | Portions of this exhibit have been redacted pursuant to a confidential treatment request filed with the Securities and Exchange Commission. |
| Item 16. | Form 10-K Summary |
None.
54
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, on August 29, 2018.
| MEI PHARMA, INC. | ||
| A Delaware Corporation | ||
| By: | /s/ Daniel P. Gold | |
| Daniel P. Gold | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities indicated on August 29, 2018.
Signatures | Title | |||||
| By: | /s/ Daniel P. Gold | President, Chief Executive Officer and Director (Principal Executive Officer) | ||||
| Daniel P. Gold | ||||||
| By: | /s/ Brian G. Drazba | Secretary, Chief Financial Officer (Principal Financial and Accounting Officer) | ||||
| Brian G. Drazba | ||||||
| By: | /s/ Christine A. White | Chairman | ||||
| Christine A. White | ||||||
| By: | /s/ William D. Rueckert | Director | ||||
| William D. Rueckert | ||||||
| By: | /s/ Charles V. Baltic III | Director | ||||
| Charles V. Baltic | ||||||
| By: | /s/ Thomas C. Reynolds | Director | ||||
| Thomas C. Reynolds | ||||||
| By: | /s/ Nicholas R. Glover | Director | ||||
| Nicholas R. Glover | ||||||
| By: | /s/ Kevan E. Clemens | Director | ||||
| Kevan E. Clemens | ||||||
| By: | /s/ Frederick W. Driscoll | Director | ||||
| Frederick W. Driscoll | ||||||
55