WASHINGTON, D. C. 20549
FORM�10-K
| (MARK ONE) | ||
� | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
| For the Fiscal Year Ended December 31, 2009 | ||
or | ||
o | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
| For the transition period from to | ||
Merck�& Co., Inc.
One Merck DriveWhitehouse Station, N. J. 08889-0100
(908)�423-1000
Incorporated in New Jersey | I.R.S. Employer Identification No. 22-1918501 |
| Name of Each Exchange | ||
Title of Each Class | on which Registered | |
Common Stock ($0.50�par value) | New York Stock Exchange | |
Mandatory Convertible Preferred Stock | New York Stock Exchange |
Number of shares of Common Stock ($0.50�par value) outstanding as of January�29, 2010: 3,115,317,260.
Aggregate market value of Common Stock ($0.50�par value) held by non-affiliates on June�30, 2009 based on closing price on June�30, 2009: $41,003,000,000.
Indicate by check mark if disclosure of delinquent filers pursuant to Item�405 of Regulation�S-K (��229.405) is not contained herein, and will not be contained, to the best of registrant�s knowledge, in definitive proxy or information statements incorporated by reference in Part�III of this Form�10-K or any amendment to this Form�10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of �large accelerated filer,� �accelerated filer� and �smaller reporting company� in Rule�12b-2 of the Exchange Act. (Check One):
| Large accelerated filer � | Accelerated filer o | Non-accelerated filer o | Smaller reporting company o |
(Do not check if a smaller reporting company)
Document | Part of Form 10-K | |
Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010, to be filed with the Securities and Exchange Commission within 120�days after the close of the fiscal year covered by this report | Part�III |
Page | ||||||||
Part�I | ||||||||
Item 1. | Business | 2 | ||||||
Item 1A. | Risk Factors | 23 | ||||||
| Cautionary Factors that May Affect Future Results | 35 | |||||||
Item 1B. | Unresolved Staff Comments | 36 | ||||||
Item 2. | Properties | 37 | ||||||
Item 3. | Legal Proceedings | 37 | ||||||
| Executive Officers of the Registrant | 54 | |||||||
Item 4. | Reserved | |||||||
Part�II | ||||||||
Item 5. | Market for Registrant�s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 58 | ||||||
Item 6. | Selected Financial Data | 62 | ||||||
Item 7. | Management�s Discussion and Analysis of Financial Condition and Results of Operations | 63 | ||||||
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 107 | ||||||
Item 8. | Financial Statements and Supplementary Data | 108 | ||||||
| (a)���Financial Statements | 108 | |||||||
| Notes to Consolidated Financial Statements | 112 | |||||||
| Report of Independent Registered Public Accounting Firm | 181 | |||||||
| (b)���Supplementary Data | 182 | |||||||
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 183 | ||||||
Item 9A. | Controls and Procedures | 183 | ||||||
| Management�s Report | 183 | |||||||
Item 9B. | Other Information | 184 | ||||||
Part�III | ||||||||
Item 10. | Directors, Executive Officers and Corporate Governance | 185 | ||||||
Item 11. | Executive Compensation | 185 | ||||||
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 185 | ||||||
Item 13. | Certain Relationships and Related Transactions, and Director Independence | 186 | ||||||
Item 14. | Principal Accountant Fees and Services | 186 | ||||||
Part�IV | ||||||||
Item 15. | Exhibits and Financial Statement Schedules | 186 | ||||||
| Signatures | 204 | |||||||
| Consent of Independent Registered Public Accounting Firm | 206 | |||||||
| Independent Auditors� Consent | 207 | |||||||
| EX-10.10 | ||||||||
| EX-10.11 | ||||||||
| EX-10.12 | ||||||||
| EX-10.13 | ||||||||
| EX-10.41 | ||||||||
| EX-10.42 | ||||||||
| EX-10.43 | ||||||||
| EX-10.44 | ||||||||
| EX-10.46 | ||||||||
| EX-12 | ||||||||
| EX-21 | ||||||||
| EX-31.1 | ||||||||
| EX-31.2 | ||||||||
| EX-32.1 | ||||||||
| EX-32.2 | ||||||||
| EX-101 INSTANCE DOCUMENT | ||||||||
| EX-101 SCHEMA DOCUMENT | ||||||||
| EX-101 CALCULATION LINKBASE DOCUMENT | ||||||||
| EX-101 LABELS LINKBASE DOCUMENT | ||||||||
| EX-101 PRESENTATION LINKBASE DOCUMENT | ||||||||
| EX-101 DEFINITION LINKBASE DOCUMENT | ||||||||
| Item�1. | Business. |
On November�3, 2009, Merck�& Co., Inc. (�Old Merck�) and Schering-Plough Corporation (�Schering-Plough�) completed their previously-announced merger (the �Merger�). In the Merger, Schering-Plough acquired all of the shares of Old Merck, which became a wholly-owned subsidiary of Schering-Plough and was renamed Merck Sharp�& Dohme Corp. Schering-Plough continued as the surviving public company and was renamed Merck�& Co., Inc. (�New Merck� or the �Company�). However, for accounting purposes only, the Merger was treated as an acquisition with Old Merck considered the accounting acquirer. Accordingly, the accompanying financial statements reflect Old Merck�s stand-alone operations as they existed prior to the completion of the Merger. The results of Schering-Plough�s business have been included in New Merck�s financial statements only for periods subsequent to the completion of the Merger. Therefore, New Merck�s financial results for 2009 do not reflect a full year of legacy Schering-Plough operations. References in this report and in the accompanying financial statements to �Merck� for periods prior to the Merger refer to Old Merck and for periods after the completion of the Merger to New Merck.
The Company is a global health care company that delivers innovative health solutions through its medicines, vaccines, biologic therapies, and consumer and animal products, which it markets directly and through its joint ventures. The Company�s operations are principally managed on a products basis and are comprised of one reportable segment, which is the Pharmaceutical segment. The Pharmaceutical segment includes human health pharmaceutical and vaccine products marketed either directly by the Company or through joint ventures. Human health pharmaceutical products consist of therapeutic and preventive agents, sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Vaccine products consist of preventative pediatric, adolescent and adult vaccines, primarily administered at physician offices. The Company sells these human health vaccines primarily to physicians, wholesalers, physician distributors and government entities. The Company�s professional representatives communicate the effectiveness, safety and value of its pharmaceutical and vaccine products to health care professionals in private practice, group practices and managed care organizations. The Company also has animal health operations that discover, develop, manufacture and market animal health products, including vaccines. The Company�s professional representatives communicate the safety and value of the Company�s animal health products to veterinarians, distributors and animal producers. Additionally, the Company has consumer health care operations that develop, manufacture and market Over-the-Counter (�OTC�), foot care and sun care products, which are sold through wholesale and retail drug, food chain and mass merchandiser outlets in the United States and Canada.
For financial information and other information about the Pharmaceutical segment, see Item�7. �Management�s Discussion and Analysis of Financial Condition and Results of Operations� and Item�8. �Financial Statements and Supplementary Data� below.
As discussed above, the Merger was completed on November 3, 2009. In the Merger, Old Merck shareholders received one share of common stock of New Merck for each share of Old Merck stock that they owned, and Schering-Plough shareholders received 0.5767 of a share of common stock of New Merck and $10.50 in cash for each share of Schering-Plough stock that they owned. The consideration in the Merger was valued at $49.6 billion in the aggregate. Schering-Plough was Old Merck�s long-term partner in the Merck/Schering-Plough cholesterol partnership (the �MSP Partnership�). The cash portion of the consideration was funded with a
2
combination of existing cash, including proceeds from the sale of Old Merck�s interest in Merial Limited, the sale or redemption of investments and the issuance of debt.
The combined company has a research and development pipeline with greater depth and breadth and many promising drug candidates, a significantly broader portfolio of medicines and an expanded presence in key international markets, particularly in high-growth emerging markets. The Company anticipates that the efficiencies gained from the Merger will allow it to invest in promising pipeline candidates, as well as strategic external research and development opportunities.
The combination increased the Company�s pipeline of early, mid- and late stage product candidates, including a significant increase in the number of potential medicines the Company has in Phase�III development to 19 candidates. Additionally, a number of candidates are currently under review in the United States and internationally.
3
As a result of the Merger, the Company expects to achieve substantial cost savings across all areas, including from consolidation in both sales and marketing and research and development, the application of the Company�s lean manufacturing and sourcing strategies to the expanded operations, and the full integration of the MSP Partnership.
In February 2010, the Company announced the first phase of a new global restructuring program (the �Merger Restructuring Program�) in conjunction with the integration of the legacy Merck and legacy Schering-Plough businesses. This Merger Restructuring Program is intended to optimize the cost structure of the combined Company. As part of the first phase of the Merger Restructuring Program, by the end of 2012, the Company expects to reduce its total workforce by approximately 15% across all areas of the Company worldwide. The Company also plans to eliminate 2,500 vacant positions as part of the first phase of the program. These workforce reductions will primarily come from the elimination of duplicative positions in sales, administrative and headquarters organizations, as well as from the consolidation of certain manufacturing facilities and research and development operations. The Company will continue to hire new employees in strategic growth areas of the business during this period. Certain actions, such as the ongoing reevaluation of manufacturing and research and development facilities worldwide, have not yet been completed, but will be included later in 2010 in other phases of the Merger Restructuring Program. In connection with the first phase of the Merger Restructuring Program, separation costs under the Company�s existing severance programs worldwide were recorded in the fourth quarter of 2009 to the extent such costs were probable and reasonably estimable. The Company recorded pretax restructuring costs of $1.5�billion, primarily employee separation costs, related to the Merger Restructuring Program in the fourth quarter of 2009. This first phase of the Merger Restructuring Program is expected to be completed by the end of 2012 with the total pretax costs estimated to be $2.6�billion to $3.3�billion. The Company estimates that approximately 85% of the cumulative pretax costs relate to cash outlays, primarily related to employee separation expense. Approximately 15% of the cumulative pretax costs are non-cash, relating primarily to the accelerated depreciation of facilities to be closed or divested.
The Company expects this first phase of the Merger Restructuring Program to yield annual savings in 2012 of approximately $2.6�billion to $3.0�billion. These anticipated savings relate only to the first phase of the Merger Restructuring Program and therefore are only a portion of the estimated $3.5�billion of incremental annual savings originally disclosed when the Merger was announced. The Company expects that additional savings will be generated by subsequent phases of the Merger Restructuring Program that will be announced later this year, as well as by non-restructuring related activities, such as procurement savings initiatives. These cost savings, which are expected to come from all areas of the Company�s pharmaceutical business, are in addition to the previously announced ongoing cost reduction initiatives at both legacy companies.
Earnings per common share (�EPS�) assuming dilution for 2009 were $5.65, which reflect a net impact of $2.40 resulting from gains related to the MSP Partnership and the sale of Merial, partially offset by increased expenses from the amortization of purchase accounting adjustments, restructuring and merger-related costs. EPS in 2009 were also affected by the dilutive impact of shares issued in the Merger.
4
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Pharmaceutical: | ||||||||||||
Bone, Respiratory, Immunology and Dermatology | ||||||||||||
Singulair | $ | 4,659.7 | $ | 4,336.9 | $ | 4,266.3 | ||||||
Fosamax | 1,099.8 | 1,552.7 | 3,049.0 | |||||||||
Propecia | 440.3 | 429.1 | 405.4 | |||||||||
Remicade | 430.7 | � | � | |||||||||
Arcoxia | 357.5 | 377.3 | 329.1 | |||||||||
Nasonex | 164.9 | � | � | |||||||||
Clarinex | 100.6 | � | � | |||||||||
Asmanex | 37.0 | � | � | |||||||||
Cardiovascular | ||||||||||||
Vytorin | 440.8 | 84.2 | 84.3 | |||||||||
Zetia | 402.9 | 6.4 | 6.5 | |||||||||
Integrilin | 45.9 | � | � | |||||||||
Diabetes and Obesity | ||||||||||||
Januvia | 1,922.1 | 1,397.1 | 667.5 | |||||||||
Janumet | 658.4 | 351.1 | 86.4 | |||||||||
Infectious Disease | ||||||||||||
Isentress | 751.8 | 361.1 | 41.3 | |||||||||
Primaxin | 688.9 | 760.4 | 763.5 | |||||||||
Cancidas | 616.7 | 596.4 | 536.9 | |||||||||
Invanz | 292.9 | 265.0 | 190.2 | |||||||||
Crixivan/Stocrin | 206.1 | 275.1 | 310.2 | |||||||||
PegIntron | 148.7 | � | � | |||||||||
Avelox | 66.2 | � | � | |||||||||
Rebetol | 36.1 | � | � | |||||||||
Mature Brands | ||||||||||||
Cozaar/Hyzaar | 3,560.7 | 3,557.7 | 3,350.1 | |||||||||
Zocor | 558.4 | 660.1 | 876.5 | |||||||||
Vasotec/Vaseretic | 310.8 | 356.7 | 494.6 | |||||||||
Proscar | 290.9 | 323.5 | 411.0 | |||||||||
Claritin Rx | 71.1 | � | � | |||||||||
Proventil | 26.2 | � | � | |||||||||
Neurosciences and Ophthalmology | ||||||||||||
Maxalt | 574.5 | 529.2 | 467.3 | |||||||||
Cosopt/Trusopt | 503.5 | 781.2 | 786.8 | |||||||||
Remeron | 38.5 | � | � | |||||||||
Subutex/Suboxone | 36.3 | � | � | |||||||||
Oncology | ||||||||||||
Emend | 313.1 | 259.7 | 201.7 | |||||||||
Temodar | 188.1 | � | � | |||||||||
Caelyx | 46.5 | � | � | |||||||||
Intron A | 38.4 | � | � | |||||||||
Vaccines (2) | ||||||||||||
ProQuad/M-M-R II/Varivax | 1,368.5 | 1,268.5 | 1,347.1 | |||||||||
Gardasil | 1,118.4 | 1,402.8 | 1,480.6 | |||||||||
RotaTeq | 521.9 | 664.5 | 524.7 | |||||||||
Pneumovax | 345.6 | 249.3 | 233.2 | |||||||||
Zostavax | 277.4 | 312.4 | 236.0 | |||||||||
Women�s Health and Endocrine | ||||||||||||
Follistim/Puregon | 96.5 | � | � | |||||||||
NuvaRing | 88.3 | � | � | |||||||||
Other
Pharmaceutical (3) | 1,294.9 | 922.9 | 1,136.6 | |||||||||
| 25,236.5 | 22,081.3 | 22,282.8 | ||||||||||
Other segment
revenues (4) | 2,114.0 | 1,694.1 | 1,848.1 | |||||||||
Total segment revenues | 27,350.5 | 23,775.4 | 24,130.9 | |||||||||
Other (5) | 77.8 | 74.9 | 66.8 | |||||||||
| $ | 27,428.3 | $ | 23,850.3 | $ | 24,197.7 | |||||||
| ||||||||||||
| (1) | Sales of legacy Schering-Plough products only reflect results for the post-Merger period through December�31, 2009. Sales of MSP Partnership products Zetia and Vytorin represent sales for the post-Merger period through December�31, 2009. Prior to the Merger, sales of Zetia and Vytorin were primarily recognized by the MSP Partnership and the results of Old Merck�s interest in the MSP Partnership were recorded in Equity income from affiliates. Sales of Zetia and Vytorin in 2008 and 2007 reflect Old Merck�s sales of these products in Latin America which was not part of the MSP Partnership. |
| (2) | These amounts do not reflect sales of vaccines sold in most major European markets through the Company�s joint venture, Sanofi Pasteur MSD, the results of which are reflected in Equity income from affiliates. These amounts do, however, reflect supply sales to Sanofi Pasteur MSD. |
| (3) | Other pharmaceutical primarily includes sales of other human pharmaceutical products, including products within the franchises not listed separately. |
| (4) | Reflects other non-reportable segments, including animal health and consumer health care, and revenue from the Company�s relationship with AZLP primarily relating to sales of Nexium, as well as Prilosec. Revenue from AZLP was $1.4�billion, $1.6�billion and $1.7�billion in 2009, 2008 and 2007, respectively. |
| (5) | Other revenues are primarily comprised of miscellaneous corporate revenues, third party manufacturing sales, sales related to divested products or businesses and other supply sales not included in segment results. |
5
The Company�s pharmaceutical products include therapeutic and preventive agents, generally sold by prescription, for the treatment of human disorders. Among these are:
The Animal Health segment discovers, develops, manufactures and markets animal health products, including vaccines. Principal marketed products in this segment include:
6
The Consumer Health Care segment develops, manufactures and markets OTC, foot care and sun care products. Principal products in this segment include:
For a further discussion of sales of the Company�s products, see Item�7. �Management�s Discussion and Analysis of Financial Condition and Results of Operations� below.
7
8
In 1998, Old Merck and Astra restructured the joint venture whereby Old Merck acquired Astra�s interest in the joint venture, renamed KBI Inc. (�KBI�), and contributed KBI�s operating assets to a new U.S.�limited partnership named Astra Pharmaceuticals, L.P. (the �Partnership�), in exchange for a 1% limited partner interest. Astra contributed the net assets of its wholly owned subsidiary, Astra USA, Inc., to the Partnership in exchange for a 99% general partner interest. The Partnership, renamed AstraZeneca LP (�AZLP�) upon Astra�s 1999 merger with Zeneca Group Plc (the �AstraZeneca merger�), became the exclusive distributor of the products for which KBI retained rights.
The Company earns certain Partnership returns as well as ongoing revenue based on sales of current and future KBI products. The Partnership returns include a priority return provided for in the Partnership Agreement, variable returns based, in part, upon sales of certain former Astra USA, Inc. products, and a preferential return representing the Company�s share of undistributed Partnership AZLP generally accepted accounting principles (�GAAP�) earnings. The AstraZeneca merger triggered a partial redemption in March 2008 of Old Merck�s interest in certain AZLP product rights. Upon this redemption, Old Merck received $4.3�billion from AZLP. This amount was based primarily on a multiple of Old Merck�s average annual variable returns derived from sales of the former Astra USA, Inc. products for the three years prior to the redemption (the �Limited Partner Share of Agreed Value�). Old Merck recorded a $1.5�billion pretax gain on the partial redemption in 2008. The partial redemption of Old Merck�s interest in the product rights did not result in a change in Old Merck�s 1% limited partnership interest. As described in Item�7. �Management�s Discussion and Analysis of Financial Condition and Results of Operations� below, after certain adjustments, Old Merck recorded an aggregate pretax gain of $2.2�billion in 2008.
In 1994, Old Merck and Pasteur M�rieux Connaught (now Sanofi Pasteur S.A.) formed a joint venture to market human vaccines in Europe and to collaborate in the development of combination vaccines for distribution in the then existing EU and the European Free Trade Association. Old Merck and Sanofi Pasteur contributed, among other things, their European vaccine businesses for equal shares in the joint venture, known as Pasteur M�rieux MSD, S.N.C. (now Sanofi Pasteur MSD, S.N.C.). The joint venture maintains a presence, directly or through affiliates or branches, in Belgium, Italy, Germany, Spain, France, Austria, Ireland, Sweden, Portugal, the Netherlands, Switzerland and the United Kingdom and through distributors in the rest of its territory.
9
In 1997, Old Merck and Rh�ne-Poulenc S.A. (now sanofi-aventis) combined their respective animal health businesses to form�Merial Limited (�Merial�), a fully integrated animal health company, which was a stand-alone joint venture, 50% owned by each party. Merial provides a comprehensive range of pharmaceuticals and vaccines to enhance the health, well-being and performance of a wide range of animal species.
10
The markets in which the Company conducts its business and the pharmaceutical industry are highly competitive and highly regulated. The Company�s operations may be affected by technological advances of competitors, industry consolidation, patents granted to competitors, competitive combination products, new products of competitors, new information from clinical trials of marketed products or post-marketing surveillance and generic competition as the Company�s products mature. In addition, patent positions are increasingly being challenged by competitors, and the outcome can be highly uncertain. An adverse result in a patent dispute can preclude commercialization of products or negatively affect sales of existing products and could result in the recognition of an impairment charge with respect to certain products. Competitive pressures have intensified as pressures in the industry have grown. The effect on operations of competitive factors and patent disputes cannot be predicted.
Pharmaceutical competition involves a rigorous search for technological innovations and the ability to market these innovations effectively. With its long-standing emphasis on research and development, the Company is well positioned to compete in the search for technological innovations. Additional resources to meet market challenges include quality control, flexibility to meet customer specifications, an efficient distribution system and a strong technical information service. The Company is active in acquiring and marketing products through external alliances, such as joint ventures, and licenses and has been refining its sales and marketing efforts to further address changing industry conditions. However, the introduction of new products and processes by competitors may result in price reductions and product displacements, even for products protected by patents. For example, the number of compounds available to treat a particular disease typically increases over time and can result in slowed sales growth for the Company�s products in that therapeutic category.
Global efforts toward healthcare cost containment continue to exert pressure on product pricing and access. In addressing cost containment pressure, the Company makes a continuing effort to demonstrate that its medicines provide value to patients and to those who pay for health care. In addition, pricing flexibility across the Company�s product portfolio has encouraged growing use of its medicines and mitigated the effects of increasing cost pressures on individual medicines.
Outside the United States, in difficult government budgetary environments, the Company has worked with payers to encourage allocation of scarce resources to optimize healthcare outcomes, limiting the potentially detrimental effects of government policies on sales growth and access to innovative medicines and vaccines, and to support the discovery and development of innovative products to benefit patients. The Company also is working with governments in many emerging markets in Eastern Europe, Latin America and Asia to encourage them to increase their investments in health and thereby improve their citizens� access to medicines. In addition, certain countries within the EU, recognizing the economic importance of the research-based pharmaceutical industry and the value of innovative medicines to society, are working with industry representatives to improve the competitive climate through a variety of means including market deregulation.
The Company anticipates that the worldwide trend toward cost containment will continue, resulting in ongoing pressures on healthcare budgets. In the United States, major healthcare reform has been introduced and passed in both houses of Congress. A final revised bill which unifies both versions may be considered and adopted into law. The impact of such actions, as well as budget pressures on governments in the United States and other nations, cannot be predicted at this time. As the Company continues to successfully launch new products, contribute to health care debates and monitor reforms, its new products, policies and strategies should enable it to maintain a strong position in the changing economic environment.
Although no one can predict the outcome of these and other legislative, regulatory and advocacy initiatives, the Company believes that it is well positioned to respond to the evolving health care environment and market forces.
The Company is also committed to improving access to medicines and enhancing the quality of life for people around the world. To cite just one example, The African Comprehensive HIV/AIDS Partnerships in
11
Botswana, a partnership between the government of Botswana, the Bill�& Melinda Gates Foundation and The Merck Company Foundation/Merck�& Co., Inc., is supporting Botswana�s response to HIV/AIDS through a comprehensive and sustainable approach to HIV prevention, care, treatment, and support.
The pharmaceutical industry is subject to regulation by regional, country, state and local agencies around the world. Of particular importance is the FDA in the United States, which administers requirements covering the testing, approval, safety, effectiveness, manufacturing, labeling, and marketing of prescription pharmaceuticals. In many cases, the FDA requirements have increased the amount of time and resources necessary to develop new products and bring them to market in the United States. In 1997, the Food and Drug Administration Modernization Act (the �FDA Modernization Act�) was passed and was the culmination of a comprehensive legislative reform effort designed to streamline regulatory procedures within the FDA and to improve the regulation of drugs, medical devices, and food. The legislation was principally designed to ensure the timely availability of safe and effective drugs and biologics by expediting the premarket review process for new products. A key provision of the legislation is the re-authorization of the Prescription Drug User Fee Act of 1992, which permits the continued collection of user fees from prescription drug manufacturers to augment FDA resources earmarked for the review of human drug applications. This helps provide the resources necessary to ensure the prompt approval of safe and effective new drugs.
In the United States, the government expanded access for senior citizens to prescription drug coverage by enacting the Medicare Prescription Drug Improvement and Modernization Act of 2003, which was signed into law in December 2003. Prescription drug coverage began on January�1, 2006. This legislation supports the Company�s goal of improving access to medicines by expanding insurance coverage, while preserving market-based incentives for pharmaceutical innovation. At the same time, the legislation has helped control the cost of prescription drug costs through competitive pressures and by encouraging the appropriate use of medicines. As mentioned above, in the United States major healthcare reform has been introduced and passed in both houses of Congress. A final revised bill which unifies both versions may be considered and adopted into law. The U.S.�Congress has also considered, and may consider again, proposals to increase the government�s role in pharmaceutical pricing in the Medicare program. These proposals may include removing the current legal prohibition against the Secretary of the Health and Human Services intervening in price negotiations between Medicare drug benefit program plans and pharmaceutical companies. They may also include mandating the payment of rebates for some or all of the pharmaceutical utilization in Medicare drug benefit plans. In addition, Congress may again consider proposals to allow, under certain conditions, the importation of medicines from other countries.
For many years, the pharmaceutical industry has been under federal and state oversight with the approval process for new drugs, drug safety, advertising and promotion, drug purchasing and reimbursement programs, and formularies. The Company believes that it will continue to be able to conduct its operations, including the introduction of new drugs to the market, in this regulatory environment.
The Company continues to work with private and public payors to slow increases in healthcare spending. Also, U.S.�federal and state governments have pursued methods to directly reduce the cost of drugs and vaccines for
12
which they pay. For example, federal laws require the Company to pay specified rebates for medicines reimbursed by Medicaid, to provide discounts for outpatient medicines purchased by certain Public Health Service entities and �disproportionate share� hospitals (hospitals meeting certain criteria), and to provide minimum discounts of 24% off of a defined �non-federal average manufacturer price� for purchases by certain components of the federal government such as the Department of Veterans Affairs and the Department of Defense.
Initiatives in some states seek rebates beyond the minimum required by Medicaid legislation, in some cases for patients beyond those who are eligible for Medicaid. Under the Federal Vaccines for Children entitlement program, the U.S.�Centers for Disease Control and Prevention (�CDC�) funds and purchases recommended pediatric vaccines at a public sector price for the immunization of Medicaid-eligible, uninsured, Native American and certain underinsured children. Old Merck was awarded a CDC contract in 2009 for the supply of pediatric vaccines for the Vaccines for Children program.
Outside the United States, the Company encounters similar regulatory and legislative issues in most of the countries where it does business. There, too, the primary thrust of governmental inquiry and action is toward determining drug safety and effectiveness, often with mechanisms for controlling the prices of or reimbursement for prescription drugs and the profits of prescription drug companies. The EU has adopted directives concerning the classification, labeling, advertising, wholesale distribution and approval for marketing of medicinal products for human use. The Company�s policies and procedures are already consistent with the substance of these directives; consequently, it is believed that they will not have any material effect on the Company�s business.
In January 2008, the EC launched a sector inquiry in the pharmaceutical industry under the rules of EU competition law. As part of this inquiry, Old Merck�s offices in Germany were inspected by the authorities beginning in January 2008. The preliminary report of the EC was issued on November�28, 2008, and following the public consultation period, the final report was issued in July 2009. The final report confirmed that there has been a decline in the number of novel medicines reaching the market and instances of delayed market entry of generic medicines and discussed industry practices that may have contributed to these phenomena. While the EC has issued further inquiries with respect to the subject of the investigation, the EC has not alleged that the Company or any of its subsidiaries have engaged in any unlawful practices.
The Company is subject to the jurisdiction of various regulatory agencies and is, therefore, subject to potential administrative actions. Such actions may include seizures of products and other civil and criminal sanctions. Under certain circumstances, the Company on its own may deem it advisable to initiate product recalls. The Company believes that it should be able to compete effectively within this environment.
The Company is subject to a number of privacy and data protection laws and regulations globally. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing attention to privacy and data protection issues with the potential to affect directly the Company�s business, including recently enacted laws and regulations in the United States and internationally requiring notification to individuals and government authorities of security breaches involving certain categories of personal information.
The Company sells its human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Human health vaccines are sold primarily to physicians, wholesalers, physician distributors and government entities. The Company�s professional representatives communicate the effectiveness, safety and value of the Company�s pharmaceutical and vaccine products to health care professionals in private practice, group practices and managed care organizations. The Company�s professional representatives communicate the safety and value of the Company�s animal health products to veterinarians, distributors and animal producers. The Company�s OTC, foot care and sun care products are sold through wholesale and retail drug, food chain and mass merchandiser outlets.
13
Raw materials and supplies, which are generally available from multiple sources, are purchased worldwide and are normally available in quantities adequate to meet the needs of the Company�s business.
The FDA Modernization Act includes a Pediatric Exclusivity Provision that may provide an additional six months of market exclusivity in the United States for indications of new or currently marketed drugs if certain agreed upon pediatric studies are completed by the applicant. These exclusivity provisions were re-authorized by the Prescription Drug User Fee Act passed in September 2007. Current U.S.�patent law provides additional patent term under Patent Term Restoration for periods when the patented product was under regulatory review before the FDA.
14
Patent portfolios developed for products introduced by the Company normally provide market exclusivity. The Company has the following key U.S.�patent protection (including Patent Term Restoration and Pediatric Exclusivity) for major marketed products:
Product (1) | Year of Expiration (in
U.S.) | |
Cozaar | 2010 | |
Hyzaar | 2010 | |
Crixivan | 2012 (compound)/2018 (formulation) | |
Maxalt | 2012 (compound)/2014 (other) | |
Singulair | 2012 | |
Cancidas | 2013 (compound)/2015 (composition) | |
Propecia (2) | 2013 (formulation/use) | |
Asmanex | 2014 (use)/2018 (formulation) | |
Avelox | 2014 | |
Integrilin | 2014 (compound)/2015 (use/formulation) | |
Nasonex | 2014 (use/formulation)/2018(formulation) | |
Temodar (3) | 2014 | |
Emend | 2015 | |
Follistim/Puregon | 2015 | |
PegIntron | 2015 (conjugates)/2020 (Mature IFN-alpha) | |
Zolinza | 2015 | |
Invanz | 2016 (compound)/2017 (composition) | |
Zostavax | 2016 | |
Zetia/Vytorin | 2017 | |
NuvaRing | 2018 (delivery system) | |
Noxafil | 2019 | |
RotaTeq | 2019 | |
Clarinex (4 ) | 2020 (formulation) | |
Comvax | 2020 (method of making/vectors) | |
Intron A | 2020 | |
Recombivax | 2020 (method of making/vectors) | |
Saphris/Sycrest | 2020 (use/formulation) (subject to pending Patent Term Restoration application) | |
Januvia/Janumet | 2022 (compound)/2026 (salt) | |
Isentress | 2023 | |
Gardasil | 2026 (method of making/use/product by process) |
| (1) | Compound patent unless otherwise noted. |
| (2) | By agreement, Dr.�Reddy�s Laboratories may launch a generic on January�1, 2013. |
| (3) | In January 2010, a court held the patent for Temodar to be unenforceable. That decision is being appealed. See Item�3. �Legal Proceedings�� Patent Litigation� below. |
| (4) | By virtue of litigation settlement, generics have been given the right to enter the market as of 2012. |
While the expiration of a product patent normally results in a loss of market exclusivity for the covered pharmaceutical product, commercial benefits may continue to be derived from: (i)�later-granted patents on processes and intermediates related to the most economical method of manufacture of the active ingredient of such product; (ii)�patents relating to the use of such product; (iii)�patents relating to novel compositions and formulations; and (iv)�in the United States and certain other countries, market exclusivity that may be available under relevant law. The effect of product patent expiration on pharmaceutical products also depends upon many other factors such as the nature of the market and the position of the product in it, the growth of the market, the complexities and economics of the process for manufacture of the active ingredient of the product and the requirements of new drug provisions of the Federal Food, Drug and Cosmetic Act or similar laws and regulations in other countries.
15
Additions to market exclusivity are sought in the United States and other countries through all relevant laws, including laws increasing patent life. Some of the benefits of increases in patent life have been partially offset by a general increase in the number of incentives for and use of generic products. Additionally, improvements in intellectual property laws are sought in the United States and other countries through reform of patent and other relevant laws and implementation of international treaties.
For further information with respect to the Company�s patents, see Item�1A. �Risk Factors� and Item�3. �Legal Proceedings�� Patent Litigation� below.
Worldwide, all of the Company�s important products are sold under trademarks that are considered in the aggregate to be of material importance. Trademark protection continues in some countries as long as used; in other countries, as long as registered. Registration is for fixed terms and can be renewed indefinitely.
Royalties received during 2009 on patent and know-how licenses and other rights amounted to $218.9�million. Merck also paid royalties amounting to $1.27�billion in 2009 under patent and know-how licenses it holds.
The Company�s business is characterized by the introduction of new products or new uses for existing products through a strong research and development program. Approximately 17,200�people are employed in the Company�s research activities. Research and development expenses (which included restructuring costs) were $5.8�billion in 2009, $4.8�billion in 2008 and $4.9�billion in 2007. The Company maintains its ongoing commitment to research over a broad range of therapeutic areas and clinical development in support of new products.
The Company maintains a number of long-term exploratory and fundamental research programs in biology and chemistry as well as research programs directed toward product development. The Company�s research and development model is designed to increase productivity and improve the probability of success by prioritizing the Company�s research and development resources on disease areas of unmet medical needs, scientific opportunity and commercial opportunity. Merck is managing its research and development portfolio across diverse approaches to discovery and development by balancing investments appropriately on novel, innovative targets with the potential to have a major impact on human health, on developing best-in-class approaches, and on delivering maximum value of its new medicines and vaccines through new indications and new formulations. Another important component of the Company�s science-based diversification is based on expanding the Company�s portfolio of modalities to include not only small molecules and vaccines, but also biologics, peptides and RNAi. Further, Merck moved to diversify its portfolio by creating a new division, Merck BioVentures, which has the potential to harness the market opportunity presented by biological medicine patent expiries by delivering high quality follow-on biologic products to enhance access for patients worldwide. The Company will continue to pursue appropriate external licensing opportunities.
The integration plans for research and development are focused on integrating the research operations of the legacy companies, including providing an effective transition for employees, realizing projected merger synergies in the form of cost savings and revenue growth opportunities, and maintaining momentum in the Company�s late-stage pipeline. During 2009, Merck continued implementing a new model for its basic research global operating strategy at legacy Merck Research Laboratories sites. The new model will align franchise and function as well as align resources with disease area priorities and balance capacity across discovery phases and allow the Company to act upon those programs with the highest probability of success. Additionally, across all
16
disease area priorities, the Company�s strategy is designed to expand access to worldwide external science and incorporate external research as a key component of the Company�s early discovery pipeline in order to translate basic research productivity into late-stage clinical success.
The Company�s clinical pipeline includes candidates in multiple disease areas, including anemia, atherosclerosis, cancer, diabetes, heart disease, hypertension, infectious diseases, inflammatory/autoimmune diseases, migraine, neurodegenerative diseases, ophthalmics, osteoporosis, psychiatric diseases, respiratory disease and women�s health. The Company supplements its internal research with an aggressive licensing and external alliance strategy focused on the entire spectrum of collaborations from early research to late-stage compounds, as well as new technologies.
In the development of human health products, industry practice and government regulations in the United�States and most foreign countries provide for the determination of effectiveness and safety of new chemical compounds through preclinical tests and controlled clinical evaluation. Before a new drug or vaccine may be marketed in the United States, recorded data on preclinical and clinical experience are included in the New Drug Application (�NDA�) for a drug or the Biologics License Application (�BLA�) for a vaccine or biologic submitted to the FDA for the required approval.
Once the Company�s scientists discover a new small molecule compound that they believe has promise to treat a medical condition, the Company commences preclinical testing with that compound. Preclinical testing includes laboratory testing and animal safety studies to gather data on chemistry, pharmacology and toxicology. Pending acceptable preclinical data, the Company will initiate clinical testing in accordance with established regulatory requirements. The clinical testing begins with Phase I studies, which are designed to assess safety, tolerability, pharmacokinetics, and preliminary pharmacodynamic activity of the compound in humans. If favorable, additional, larger Phase�II studies are initiated to determine the efficacy of the compound in the affected population, define appropriate dosing for the compound, as well as identify any adverse effects that could limit the compound�s usefulness. If data from the Phase�II trials are satisfactory, the Company commences large-scale Phase�III trials to confirm the compound�s efficacy and safety. Upon completion of those trials, if satisfactory, the Company submits regulatory filings with the appropriate regulatory agencies around the world to have the product candidate approved for marketing. There can be no assurance that a compound that is the result of any particular program will obtain the regulatory approvals necessary for it to be marketed.
Vaccine development follows the same general pathway as for drugs. Preclinical testing focuses on the vaccine�s safety and ability to elicit a protective immune response (immunogenicity). Pre-marketing vaccine clinical trials are typically done in three phases. Initial Phase I clinical studies are conducted in normal subjects to evaluate the safety, tolerability and immunogenicity of the vaccine candidate. Phase�II studies are dose-ranging studies. Finally, Phase�III trials provide the necessary data on effectiveness and safety. If successful, the Company submits regulatory filings with the appropriate regulatory agencies. Also during this stage, the proposed manufacturing facility undergoes a pre-approval inspection during which production of the vaccine as it is in progress is examined in detail.
In the United States, the FDA review process begins once a complete NDA is submitted and received by the FDA. Pursuant to the Prescription Drug User Fee Act, the FDA review period targets for NDAs or supplemental NDAs is either six months, for priority review, or ten months, for a standard review. Within 60�days after receipt of an NDA, the FDA determines if the application is sufficiently complete to permit a substantive review. The FDA also assesses, at that time, whether the application will be granted a priority review or standard review. Once the review timelines are defined, the FDA will generally act upon the application within those timelines, unless a major amendment has been submitted (either at the Company�s own initiative or the FDA�s request) to the pending application. If this occurs, the FDA may extend the review period to allow for review of the new information, but by no more than 180�days. Extensions to the review period are communicated to the Company. The FDA can act on an application by issuing an approval letter or a complete response letter.
In connection with the Merger, the Company is assessing its pipeline to identify the most promising, high-potential compounds for development. The Company has completed the prioritization of its clinical development
17
programs. The Company is continuing to work on the prioritization of its value adding programs related to currently marketed products and to its preclinical/discovery programs. The Company anticipates that the full prioritization process will be completed by the first half of 2010. In connection with this process, the Company may recognize non-cash impairment charges for the cancellation of certain legacy Schering-Plough pipeline programs that were measured at fair value and capitalized in connection with the Merger. These non-cash impairment charges, which are anticipated to be excluded from the Company�s non-GAAP earnings, could be material to the Company�s future GAAP earnings.
The Company currently has a number of candidates under regulatory review in the United States and internationally. Additionally, the Company has 19 drug candidates in Phase�III development.
MK-6621, vernakalant (IV), is an investigational candidate for the treatment of atrial fibrillation currently undergoing regulatory review in the EU. In April 2009, Old Merck and Cardiome Pharma Corp. (�Cardiome�) announced a collaboration and license agreement for the development and commercialization of vernakalant which provides Merck exclusive rights outside of the United States, Canada and Mexico to the intravenous formulation of vernakalant. Vernakalent (oral) is currently in Phase�II development. Merck has exclusive global rights to the oral formulation of vernakalent for the maintenance of normal heart rhythm in patients with atrial fibrillation.
SCH 900121, NOMAC/E2, is an oral contraceptive that combines a selective progestin with estradiol, the estrogen that women produce naturally. The drug is currently under regulatory review in the EU. It is in Phase�III development for the U.S.�market.
SCH 503034, boceprevir, is a hepatitis�C protease inhibitor currently under development. Boceprevir is fully enrolled in its Phase�III program, which the Company expects to conclude in mid-2010. The Company expects to submit an NDA to the FDA for boceprevir by the end of 2010 for both treatment-experienced and treatment-na�ve patients with hepatitis�C.
MK-8669, ridaforolimus, is a novel mTOR (mammalian target of rapamycin) inhibitor being evaluated for the treatment of cancer. The drug candidate is being jointly developed and commercialized with ARIAD Pharmaceuticals, Inc., under an agreement entered into in 2007. A Phase�III study (SUCCEED) in patients with metastatic soft-tissue or bone sarcomas is underway. The Company continues to anticipate filing an NDA for ridaforolimus with the FDA in 2010, subject to a review of the results from the planned interim analysis of SUCCEED.
SCH 697243, an allergy immunotherapy sublingual tablet (�AIT�) for grass pollen allergy, is being developed by the Company. In November 2009, SCH 697243 met the primary endpoint in a Phase�III study of adult subjects in the United States with a history of grass pollen induced rhinoconjunctivitis with or without asthma. The investigational grass AIT treatment is designed to work by inducing a protective immune response against grass pollen allergy and providing sustained prevention of allergy symptoms, treating both the symptoms and the underlying cause of the disease.
SCH 039641, an AIT for ragweed allergy, is also in Phase�III development for the U.S.�market.
18
SCH 530348, vorapaxar, is a thrombin receptor antagonist or antiplatelet protease activated receptor-1 inhibitor being studied for the prevention and treatment of thrombosis. In November 2009, Merck announced completion of patient enrollment of more than 26,000�patients in the TRA 2�P-TIMI 50 clinical trial, a Phase III, randomized, double-blind, placebo-controlled, multinational study. The trial will assess the ability of SCH 530348 to prevent major cardiovascular events when added to current antiplatelet regimens (aspirin or aspirin plus an ADP inhibitor) in patients who have previously experienced a heart attack or stroke or who have peripheral arterial disease. SCH 530348 is also being studied in the treatment of patients with acute coronary syndrome in the ongoing Phase�III Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome trial, led by the Duke Clinical Research Institute. The Company anticipates filing an NDA for vorapaxar with the FDA in 2011.
MK-2452, tafluprost, is a preservative free, synthetic analogue of the prostaglandin F2\u03b1 for the reduction of elevated intraocular pressure in appropriate patients with primary open-angle glaucoma and ocular hypertension. In April 2009, Old Merck and Santen announced a worldwide licensing agreement for tafluprost.
As previously disclosed, Old Merck submitted for filing an NDA with the FDA for MK-0653C, ezetimibe combined with atorvastatin, which is an investigational medication for the treatment of dyslipidemia, and the FDA refused to file the application. The FDA has identified additional manufacturing and stability data that are needed and the Company is assessing the FDA�s response and anticipates filing in 2011.
MK-0822, odanacatib, is an oral, once-weekly investigational treatment for osteoporosis. Osteoporosis is a disease which reduces bone density and strength and results in an increased risk of bone fractures. Odanacatib is a cathepsin K inhibitor that selectively inhibits the cathepsin K enzyme. Cathepsin K is known to play a central role in the function of osteoclasts, which are cells that break down existing bone tissue, particularly the protein components of bone. Inhibition of cathepsin K is a novel approach to the treatment of osteoporosis. In September 2009, data from a Phase IIB clinical study of odanacatib were presented at the 31st�Annual Meeting of the American Society for Bone and Mineral Research which showed that when stopping treatment after two years the increases in lower back (lumbar spine) bone mineral density (�BMD�) were reversed over the next year, while BMD at the hip (femoral neck) remained above levels observed at the start of the study. Additionally, three years of treatment with odanacatib 50�mg demonstrated increases in BMD at key fracture sites and minimal impact on the formation of new bone as measured by biochemical markers of bone turnover. Odanacatib is currently in Phase�III clinical trials and is being evaluated in a large-scale, global outcomes study to determine its effects on vertebral, hip and non-vertebral fractures. The Company continues to anticipate filing an NDA with the FDA in 2012.
V503 is a nine-valent HPV vaccine in development to expand protection against cancer-causing HPV types. The Phase�III clinical program is underway and Merck anticipates filing a BLA with the FDA in 2012.
19
MK-0524B is a drug candidate that combines the novel approach to raising HDL-C and lowering triglycerides from ER niacin combined with laropiprant with the proven benefits of simvastatin in one combination product. Merck will not seek approval for MK-0524B in the United States until it files its complete response relating to MK-0524A.
MK-0859, anacetrapib, is an inhibitor of the cholesteryl ester transfer protein that has shown promise in lipid management by raising HDL-C and reducing LDL-C without raising blood pressure. In November 2009, Merck announced that in a Phase IIb study in 589�patients with primary hypercholesterolemia or mixed hyperlipidemia treated with anacetrapib as monotherapy or co-administered with atorvastatin, there were persistent lipid effects in the higher dose arms in both the monotherapy and co-administration treatment groups eight weeks after stopping active therapy with anacetrapib. The effect of CETP inhibition on cardiovascular risk has yet to be established. A Phase�III trial, titled DEFINE, is ongoing to further evaluate the safety and efficacy of anacetrapib in patients with coronary heart disease. The Company anticipates filing an NDA with the FDA beyond 2015.
As previously disclosed, in 2009, Old Merck announced it was delaying the filing of the U.S.�application for telcagepant (MK-0974), the Company�s investigational calcitonin gene-related peptide (�CGRP�)-receptor antagonist for the intermittent treatment of acute migraine. The decision was based on findings from a Phase IIa exploratory study in which a small number of patients taking telcagepant twice daily for three months for the prevention of migraine were found to have marked elevations in liver transaminases. The daily dosing regimen in the prevention study was different than the dosing regimen used in Phase�III studies in which telcagepant was intermittently administered in one or two doses to treat individual migraine attacks as they occurred. Other studies with telcagepant for the acute, intermittent treatment of migraine continue. Following meetings with regulatory agencies at the end of 2009, Merck is planning to conduct an additional safety study as part of the overall Phase�III program for telcagepant. The results of this study will inform planned filings for approval.
SCH 900395, acadesine, is a potential first-in class adenosine regulating agent for ischemia reperfusion-injury in patients undergoing heart bypass surgery. Patient enrollment in the RED CABG Phase�III clinical trial was initiated in 2009.
SCH 417690, vicriviroc, for the treatment of HIV infection (treatment experienced) was evaluated in two Phase�III studies in this patient population, and it was announced in January 2010 that the primary efficacy endpoint was not met. Merck will not submit an NDA to the FDA for vicriviroc in treatment-experienced HIV-infected patients at this time but will continue to evaluate vicriviroc as first-line therapy for treatment-naive patients.
As previously disclosed, in 2007, Cubist Pharmaceuticals, Inc. (�Cubist�) entered into a license agreement with Old Merck for the development and commercialization of Cubicin (daptomycin for injection, MK-3009) in Japan. Merck will develop and commercialize Cubicin through its wholly-owned subsidiary, Banyu Pharmaceutical Co., Ltd. Cubist commercializes Cubicin in the United States. MK-3009 is currently in Phase�III development.
MK-4305 is an orexin receptor antagonist, a potential new approach to the treatment of chronic insomnia, currently in Phase�III development.
Merck has terminated the internal clinical development program for esmirtazapine (SCH 900265)�for hot flashes and insomnia for strategic reasons.
As previously disclosed, in 2009, Old Merck announced that preliminary results for the pivotal Phase�III study of rolofylline (MK-7418), its investigational medicine for the treatment of acute heart failure, showed that rolofylline did not meet the primary or secondary efficacy endpoints. Old Merck terminated the clinical development program for rolofylline.
The chart below reflects the Company�s current research pipeline as of February�12, 2010. Candidates shown in Phase�III include specific products. Candidates shown in Phase�II include the most advanced compound with a specific mechanism or, if listed compounds have the same mechanism, they are each currently intended for
20
commercialization in a given therapeutic area. Small molecules and biologics are given MK-number or SCH-number designations and vaccine candidates are given V-number designations. Candidates in Phase I, additional indications in the same therapeutic area and additional claims, line extensions or formulations for in-line products are not shown.
SCH 900237 (2)
MK-2578
MK-0476C
MK-6621 (vernakalant [oral])
MK-0646 (dalotuzumab)
SCH 727965 (dinaciclib)
SCH 900776
MK-3415A
SCH 527123
MK-0941
MK-3577
MK-7009 (vaniprevir)
SCH 417690 (vicriviroc)
MK-6913
MK-0736
MK-6096
MK-5442
SCH 420814 (preladenant)
V419
SCH 066336, Sarasar (lonafarnib)
MK-8998
SCH 900435
V710
MK-4448 (betrixaban)
SCH 697243, Grass pollen (2)
SCH 039641, Ragweed (2)
SCH 900616 (sugammadex) (U.S.) (4)
MK-0524A (extended-release
niacin/ laropiprant)
(U.S.) (3)
MK-0524B (extended-release
niacin/ laropiprant/simvastin)
MK-0859 (anacetrapib)
V503
SCH 900121 (NOMAC/E2) (U.S.)
MK-0431C ( Januvia /pioglitazone)
SCH 900962 (corifollitropin alfa
injection)
(U.S.) (3)
MK-2452 (tafluprost) (U.S.) (4)
SCH 503034 (boceprevir)
MK-4305
SCH 900395 (acadesine)
MK-0974 (telcagepant)
MK-0822 (odanacatib)
MK-8669 (ridaforolimus)
MK-3009 (daptomycin for injection) (5)
SCH 530348 (vorapaxar) (TRA)
SCH 418131 (momestasone/ formoterol combination) (U.S./EU)
MK-6621 (vernakalant [IV]) (EU) (1)
SCH 900121 (NOMAC/E2) (EU)
SCH 900274 (asenapine) (EU)
| (1) | Exclusive rights outside of the United�States, Canada and Mexico to vernakalant (IV) |
| (2) | North American rights only |
| (3) | Approved in certain countries in Europe |
| (4) | Approved in certain countries in Europe and Japan |
| (5) | Japanese rights only |
| (6) | MK-0653C fixed dose combination of ezetimibe and atorvastatin is anticipated to be submitted to the U.S. FDA in 2011 and commercialized when regulatory and legal requirements have been satisfied |
As of December�31, 2009, the Company had approximately 100,000�employees worldwide, with approximately 42,000 employed in the United States, including Puerto Rico. Approximately 28% of worldwide employees of the Company are represented by various collective bargaining groups.
In October 2008, Old Merck announced a global restructuring program (the �2008 Restructuring Program�) to reduce its cost structure, increase efficiency, and enhance competitiveness. As part of the 2008 Restructuring Program, the Company expects to eliminate approximately 7,200 positions ��6,800 active employees and 400 vacancies�� across all areas of the Company worldwide by the end of 2011. About 40% of the total
21
reductions will occur in the United States. As part of the 2008 Restructuring Program, Old Merck is streamlining management layers by reducing its total number of senior and mid-level executives globally.
Prior to the Merger, Schering-Plough commenced a Productivity Transformation Program, which was designed to reduce and avoid costs and increase productivity.
In February 2010, the Company announced the first phase of a new global restructuring program (the �Merger Restructuring Program�) in conjunction with the integration of the legacy Merck and legacy Schering-Plough businesses. This Merger Restructuring Program is intended to optimize the cost structure of the combined Company. As part of the first phase of the Merger Restructuring Program, by the end of 2012, the Company expects to reduce its total workforce by approximately 15% across all areas of the Company worldwide. The Company also plans to eliminate 2,500 vacant positions as part of the first phase of the program. These workforce reductions will primarily come from the elimination of duplicative positions in sales, administrative and headquarters organizations, as well as from the consolidation of certain manufacturing facilities and research and development operations.
The Company believes that there are no compliance issues associated with applicable environmental laws and regulations that would have a material adverse effect on the Company. In 2009, Merck incurred capital expenditures of approximately $33.6�million for environmental protection facilities. The Company is also remediating environmental contamination resulting from past industrial activity at certain of its sites. Expenditures for remediation and environmental liabilities were $16.6�million in 2009, $34.5�million in 2008, $19.5�million in 2007, and are estimated at $55�million for the years 2010 through 2013. These amounts do not consider potential recoveries from other parties. The Company has taken an active role in identifying and providing for these costs and, in management�s opinion, the liabilities for all environmental matters, which are probable and reasonably estimable, have been accrued and totaled $161.8�million at December�31, 2009. Although it is not possible to predict with certainty the outcome of these environmental matters, or the ultimate costs of remediation, management does not believe that any reasonably possible expenditures that may be incurred in excess of the liabilities accrued should exceed $170.0�million in the aggregate. Management also does not believe that these expenditures should have a material adverse effect on the Company�s financial position, results of operations, liquidity or capital resources for any year.
The Company�s operations outside the United States are conducted primarily through subsidiaries. Sales worldwide by subsidiaries outside the United States were 47% of sales in 2009, 44% of sales in 2008 and 39% of sales in 2007. The increase in proportion of sales outside the United States in 2009 is primarily due to the inclusion of results of Schering-Plough following the close of the Merger.
The Company�s worldwide business is subject to risks of currency fluctuations, governmental actions and other governmental proceedings abroad. The Company does not regard these risks as a deterrent to further expansion of its operations abroad. However, the Company closely reviews its methods of operations and adopts strategies responsive to changing economic and political conditions.
As a result of the Merger, Merck has expanded its operations in countries located in Latin America, the Middle East, Africa, Eastern Europe and Asia Pacific. Business in these developing areas, while sometimes less stable, offers important opportunities for growth over time.
Financial information about geographic areas of the Company�s business is discussed in Item�8. �Financial Statements and Supplementary Data� below.
22
Section�13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after such reports are electronically filed with, or furnished to, the Securities and Exchange Commission (�SEC�).
| Item�1A. | Risk Factors. |
Investors should carefully consider all of the information set forth in this Form�10-K, including the following risk factors, before deciding to invest in any of the Company�s securities. The risks below are not the only ones the Company faces. Additional risks not currently known to the Company or that the Company presently deems immaterial may also impair its business operations. The Company�s business, financial condition, results of operations or prospects could be materially adversely affected by any of these risks. This Form�10-K also contains forward-looking statements that involve risks and uncertainties. The Company�s results could materially differ from those anticipated in these forward-looking statements as a result of certain factors, including the risks it faces as described below and elsewhere. See �Cautionary Factors that May Affect Future Results� below.
A chart listing the U.S.�patent protection for the Company�s major marketed products is set forth above in Item�1. �Business�� Patents, Trademarks and Licenses.�
23
For a description of the research and development process, see �Research and Development� above. Each phase of testing is highly regulated, and during each phase there is a substantial risk that the Company will encounter serious obstacles or will not achieve its goals, and accordingly the Company may abandon a product in which it has invested substantial amounts of time and resources. Some of the risks encountered in the research and development process include the following: pre-clinical testing of a new compound may yield disappointing results; clinical trials of a new drug may not be successful; a new drug may not be effective or may have harmful side effects; a new drug may not be approved by the FDA for its intended use; it may not be possible to obtain a patent for a new drug; or sales of a new product may be disappointing.
Products that appear promising in development may fail to reach market for numerous reasons, including the following:
| � | findings of ineffectiveness, superior safety or efficacy of competing products, or harmful side effects in clinical or pre-clinical testing; |
| � | failure to receive the necessary regulatory approvals, including delays in the approval of new products and new indications, and increasing uncertainties about the time required to obtain regulatory approvals and the benefit/risk standards applied by regulatory agencies in determining whether to grant approvals; |
| � | lack of economic feasibility due to manufacturing costs or other factors;�and |
| � | preclusion from commercialization by the proprietary rights of others. |
In connection with the Merger, the Company is assessing its pipeline to identify the most promising, high-potential compounds for development. The Company has completed the prioritization of its clinical development programs. The Company is continuing to work on the prioritization of its value adding programs related to currently marketed products and to its preclinical/discovery programs. The Company anticipates that the full prioritization process will be completed by the first half of 2010. In connection with this process, the Company may recognize non-cash impairment charges for the cancellation of certain legacy Schering-Plough pipeline programs that were measured at fair value and capitalized in connection with the Merger. These non-cash impairment charges, which are anticipated to be excluded from the Company�s non-GAAP earnings, could be material to the Company�s future GAAP earnings.
24
The Company�s activities, including research, preclinical testing, clinical trials and manufacturing and marketing its products, are subject to extensive regulation by numerous federal, state and local governmental authorities in the United States, including the FDA, and by foreign regulatory authorities, including the EC. In the United States, the FDA is of particular importance to the Company, as it administers requirements covering the testing, approval, safety, effectiveness, manufacturing, labeling and marketing of prescription pharmaceuticals. In many cases, the FDA requirements have increased the amount of time and money necessary to develop new products and bring them to market in the United States. Regulation outside the United States also is primarily focused on drug safety and effectiveness and, in many cases, cost reduction. The FDA and foreign regulatory authorities have substantial discretion to require additional testing, to delay or withhold registration and marketing approval and to mandate product withdrawals.
Even if the Company is successful in developing new products, it will not be able to market any of those products unless and until it has obtained all required regulatory approvals in each jurisdiction where it proposes to market the new products. Once obtained, the Company must maintain approval as long as it plans to market its new products in each jurisdiction where approval is required. The Company�s failure to obtain approval, significant delays in the approval process, or its failure to maintain approval in any jurisdiction will prevent it from selling the new products in that jurisdiction until approval is obtained, if ever. The Company would not be able to realize revenues for those new products in any jurisdiction where it does not have approval.
Patent protection is considered, in the aggregate, to be of material importance in the Company�s marketing of human health products in the United States and in most major foreign markets. Patents covering products that it has introduced normally provide market exclusivity, which is important for the successful marketing and sale of its products. The Company seeks patents covering each of its products in each of the markets where it intends to sell the products and where meaningful patent protection is available.
Even if the Company succeeds in obtaining patents covering its products, third parties or government authorities may challenge or seek to invalidate or circumvent its patents and patent applications. It is important for the Company�s business to defend successfully the patent rights that provide market exclusivity for its products. The Company is often involved in patent disputes relating to challenges to its patents or infringement and similar claims against the Company. The Company aggressively defends its important patents both within and outside the United�States, including by filing claims of infringement against other parties. See Item�3. �Legal Proceedings ��Patent Litigation� below. In particular, manufacturers of generic pharmaceutical products from time to time file Abbreviated New Drug Applications (�ANDA�) with the FDA seeking to market generic forms of the Company�s products prior to the expiration of relevant patents owned by the Company. The Company normally responds by vigorously defending its patent, including by filing lawsuits alleging patent infringement. Patent litigation and other challenges to the Company�s patents are costly and unpredictable and may deprive the Company of market exclusivity for a patented product or, in some cases, third party patents may prevent the Company from marketing and selling a product in a particular geographic area.
Additionally, certain foreign governments have indicated that compulsory licenses to patents may be granted in the case of national emergencies, which could diminish or eliminate sales and profits from those regions and negatively affect the Company�s results of operations. Further, recent court decisions relating to other companies� U.S.�patents, potential U.S.�legislation relating to patent reform, as well as regulatory initiatives may result in further erosion of intellectual property protection.
If one or more important products lose patent protection in profitable markets, sales of those products are likely to decline significantly as a result of generic versions of those products becoming available and, in the case of certain products, such a loss could result in an impairment charge. The Company�s results of operations may be adversely affected by the lost sales unless and until the Company has successfully launched commercially successful replacement products.
25
In general, the Company faces increasing competition from lower-cost generic products. The patent rights that protect its products are of varying strengths and durations. In addition, in some countries, patent protection is significantly weaker than in the United States or the EU. In the United States, political pressure to reduce spending on prescription drugs has led to legislation which encourages the use of generic products. Although it is the Company�s policy to actively protect its patent rights, generic challenges to the Company�s products can arise at any time, and it may not be able to prevent the emergence of generic competition for its products.
Loss of patent protection for a product typically is followed promptly by generic substitutes, reducing the Company�s sales of that product. Availability of generic substitutes for the Company�s drugs may adversely affect its results of operations and cash flow. In addition, proposals emerge from time to time in the United States and other countries for legislation to further encourage the early and rapid approval of generic drugs. Any such proposal that is enacted into law could worsen this substantial negative effect on the Company�s sales and, potentially, its business, cash flow, results of operations, financial position and prospects.
The Company�s products face intense competition from competitors� products. This competition may increase as new products enter the market. In such an event, the competitors� products may be safer or more effective or more effectively marketed and sold than the Company�s products. Alternatively, in the case of generic competition, they may be equally safe and effective products that are sold at a substantially lower price than the Company�s products. As a result, if the Company fails to maintain its competitive position, this could have a material adverse effect on its business, cash flow, results of operations, financial position and prospects.
The Company faces increasing pricing pressure globally from managed care organizations, institutions and government agencies and programs that could negatively affect the Company�s sales and profit margins. In the United States, these include (i)�practices of managed care groups and institutional and governmental purchasers and (ii)�U.S.�federal laws and regulations related to Medicare and Medicaid, including the Medicare Prescription Drug Improvement and Modernization Act of 2003 (the �2003 Act�). The 2003 Act included a prescription drug benefit for individuals that first went into effect on January�1, 2006. The increased purchasing power of entities that negotiate on behalf of Medicare beneficiaries could result in further pricing pressures.
Outside the United States, numerous major markets have pervasive government involvement in funding healthcare and, in that regard, fix the pricing and reimbursement of pharmaceutical and vaccine products. Consequently, in those markets, the Company is subject to government decision making and budgetary actions with respect to its products.
The Company expects pricing pressures to increase in the future.
The Company believes that the healthcare industry will continue to be subject to increasing regulation as well as political and legal action, as future proposals to reform the healthcare system are considered by Congress and state legislatures. Recently, major health care reform has been introduced and passed in both houses of Congress. A final revised bill which unifies both versions may be considered and adopted into law. Congress may
26
also choose not to take action on comprehensive reform and instead move to consider more incremental health care proposals that may or may not involve pharmaceutical-related issues.
Some of the proposals included in the House and Senate versions of health reform could adversely affect the Company�s sales and profit margins. If enacted, these proposals could call for government intervention in pharmaceutical pricing, changes in government reimbursement, or increased rebates and discounts on sales related to state and federal government programs among other changes. Other proposals that might be enacted that would adversely affect our business include legalization of commercial drug importation into the United States, and involuntary approval of medicines for OTC use. In addition, individual states have enacted or proposed regulations that restrict certain sales and marketing activities and/or require tracking and disclosure of payments and other financial support to healthcare professionals. Similar regulations may be proposed at the federal level. Such regulations could adversely affect the Company�s sales and profit margins.
Any of these new legislative initiatives, if enacted, may further increase government regulation of or other government involvement in healthcare, lower reimbursement rates and otherwise change the operating environment for healthcare companies. Government regulations applicable to the Company�s current or future products, or the interpretation of existing regulations, might change and thereby prevent the Company from marketing some or all of its products and services for a period of time or indefinitely.
The Company cannot predict the likelihood of all future changes in the healthcare industry in general, or the pharmaceutical industry in particular, or what impact they may have on the Company�s results of operations, financial condition or business.
In addition to the difficulties that the Company is experiencing currently, the Company may experience difficulties and delays inherent in manufacturing its products, such as (i)�failure of the Company or any of its vendors or suppliers to comply with Current Good Manufacturing Practices and other applicable regulations and quality assurance guidelines that could lead to manufacturing shutdowns, product shortages and delays in product manufacturing; (ii)�construction delays related to the construction of new facilities or the expansion of existing facilities, including those intended to support future demand for the Company�s products; and (iii)�other manufacturing or distribution problems including changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, or physical limitations that could impact continuous supply. Manufacturing difficulties can result in product shortages, leading to lost sales.
27
28
29
Under Section�8.2(c) of the Distribution Agreement, �If either party is acquired by a third party or otherwise comes under Control (as defined in Section�1.4 [of the Distribution Agreement]) of a third party, it will promptly notify the other party not subject to such change of control. The party not subject to such change of control will have the right, however not later than thirty (30)�days from such notification, to notify in writing the party subject to the change of Control of the termination of the Agreement taking effect immediately. As used herein �Change of Control� shall mean (i)�any merger, reorganization, consolidation or combination in which a party to this Agreement is not the surviving corporation; or (ii)�any �person� (within the meaning of Section�13(d) and Section�14(d)(2) of the Securities Exchange Act of 1934), excluding a party�s Affiliates, is or becomes the beneficial owner, directly or indirectly, of securities of the party representing more than fifty percent (50%) of either (A)�the then-outstanding shares of common stock of the party or (B)�the combined voting power of the party�s then-outstanding voting securities; or (iii)�if individuals who as of the Effective Date [April�3, 1998] constitute the Board of Directors of the party (the �Incumbent Board�) cease for any reason to constitute at least a majority of the Board of Directors of the party; provided, however, that any individual becoming a director subsequent to the Effective Date whose election, or nomination for election by the party�s shareholders, was approved by a vote of at least a majority of the directors then comprising the Incumbent Board shall be considered as though such individual were a member of the Incumbent Board, but excluding, for this purpose, any such individual whose initial assumption of office occurs as a result of an actual or threatened election contest with respect to the election or removal of directors or other actual or threatened solicitation of proxies or consents by or on behalf of a person other than the Board; or (iv)�approval by the shareholders of a party of a complete liquidation or the complete dissolution of such party.�
Section�1.4 of the Distribution Agreement defines �Control� to mean �the ability of any entity (the �Controlling� entity), directly or indirectly, through ownership of securities, by agreement or by any other method, to direct the manner in which more than fifty percent (50%) of the outstanding voting rights of any other entity (the �Controlled� entity), whether or not represented by securities, shall be cast, or the right to receive over fifty percent (50%) of the profits or earnings of, or to otherwise control the management decisions of, such other entity (also a �Controlled� entity).�
The Company is vigorously contesting Centocor�s attempt to terminate the Distribution Agreement as a result of the Merger. However, if the arbitrator were to conclude that Centocor is permitted to terminate the Distribution Agreement as a result of the Merger and Centocor in fact terminates the Distribution Agreement, the
30
Finally, due to the uncertainty surrounding the outcome of the arbitration, the parties may choose to settle the dispute under mutually agreeable terms but any agreement reached with Centocor to resolve the dispute under the Distribution Agreement may result in the terms of the Distribution Agreement being modified in a manner that may reduce the benefits of the Distribution Agreement to the Company.
Unexpected safety or efficacy concerns can arise with respect to marketed products, whether or not scientifically justified, leading to product recalls, withdrawals, or declining sales, as well as product liability, consumer fraud and/or other claims.
All aspects of the Company�s business, including research and development, manufacturing, marketing, pricing, sales, litigation and intellectual property rights, are subject to extensive legislation and regulation. Changes in applicable federal and state laws and agency regulations could have a material adverse effect on the Company�s business.
The Company depends on third parties, including suppliers, alliances with other pharmaceutical and biotechnology companies, and third party service providers, for key aspects of its business including development, manufacture and commercialization of its products and support for its information technology systems. Failure of these third parties to meet their contractual, regulatory and other obligations to the Company or the development of factors that materially disrupt the relationships between the Company and these third parties could have a material adverse effect on the Company�s business.
The Company is increasingly dependent on sophisticated information technology and infrastructure. Any significant breakdown, intrusion, interruption or corruption of these systems or data breaches could have a material adverse effect on our business. In addition, the Company currently is proceeding with a multi-year implementation of an enterprise wide resource planning system, which includes modification to the design, operation and documentation of its internal controls over financial reporting, and intends to implement the resource planning system in the United States in 2010. Any material problems in the implementation could have a material adverse effect on the Company�s business.
Even after a product reaches market, certain developments following regulatory approval, including results in post-marketing Phase�IV trials, may decrease demand for the Company�s products, including the following:
| � | the re-review of products that are already marketed; | |
| � | new scientific information and evolution of scientific theories; | |
| � | the recall or loss of marketing approval of products that are already marketed; | |
| � | changing government standards or public expectations regarding safety, efficacy or labeling changes;�and | |
| � | greater scrutiny in advertising and promotion. |
31
In the past several years, clinical trials and post-marketing surveillance of certain marketed drugs of the Company and of competitors within the industry have raised safety concerns that have led to recalls, withdrawals or adverse labeling of marketed products. Clinical trials and post-marketing surveillance of certain marketed drugs also have raised concerns among some prescribers and patients relating to the safety or efficacy of pharmaceutical products in general that have negatively affected the sales of such products. In addition, increased scrutiny of the outcomes of clinical trials have led to increased volatility in market reaction. Further, these matters often attract litigation and, even where the basis for the litigation is groundless, considerable resources may be needed to respond.
In addition, following the wake of product withdrawals and other significant safety issues, health authorities such as the FDA, the European Medicines Agency (�EMEA�) and the Pharmaceutical and Medical Device Agency have increased their focus on safety when assessing the benefit/risk balance of drugs. Some health authorities appear to have become more cautious when making decisions about approvability of new products or indications and are re-reviewing select products that are already marketed, adding further to the uncertainties in the regulatory processes. There is also greater regulatory scrutiny, especially in the United States, on advertising and promotion and, in particular, direct-to-consumer advertising.
If previously unknown side effects are discovered or if there is an increase in negative publicity regarding known side effects of any of the Company�s products, it could significantly reduce demand for the product or require the Company to take actions that could negatively affect sales, including removing the product from the market, restricting its distribution or applying for labeling changes. Further, in the current environment in which all pharmaceutical companies operate, the Company is at risk for product liability claims for its products.
Future sales of key animal health products could be adversely impacted by a number of risk factors including certain risks that are specific to the animal health business. For example, the outbreak of disease carried by animals, such as Bovine Spongiform Encephalopathy (�BSE�) or mad cow disease, could lead to their widespread death and precautionary destruction as well as the reduced consumption and demand for animals, which could adversely impact the Company�s results of operations. Also, the outbreak of any highly contagious diseases near the Company�s main production sites could require the Company to immediately halt production of vaccines at such sites or force the Company to incur substantial expenses in procuring raw materials or vaccines elsewhere. Other risks specific to animal health include epidemics and pandemics, government procurement and pricing practices, weather and global agribusiness economic events. As the Animal Health segment of the Company�s business becomes more significant, the impact of any such events on future results of operations would also become more significant.
The successful development, testing, manufacturing and commercialization of biologics, particularly human and animal health vaccines, is a long, expensive and uncertain process. There are unique risks and uncertainties with biologics, including:
| � | There may be limited access to and supply of normal and diseased tissue samples, cell lines, pathogens, bacteria, viral strains and other biological materials. In addition, government regulations in multiple jurisdictions, such as the United States and European states within the EU, could result in restricted access to, or transport or use of, such materials. If the Company loses access to sufficient sources of such materials, or if tighter restrictions are imposed on the use of such materials, the Company may not be able to conduct research activities as planned and may incur additional development costs. | |
| � | The development, manufacturing and marketing of biologics are subject to regulation by the FDA, the EMEA and other regulatory bodies. These regulations are often more complex and extensive than the regulations applicable to other pharmaceutical products. For example, in the United States, a BLA, including both preclinical and clinical trial data and extensive data regarding the manufacturing |
32
| procedures, is required for human vaccine candidates and FDA approval is required for the release of each manufactured lot. |
| � | Manufacturing biologics, especially in large quantities, is often complex and may require the use of innovative technologies to handle living micro-organisms. Each lot of an approved biologic must undergo thorough testing for identity, strength, quality, purity and potency. Manufacturing biologics requires facilities specifically designed for and validated for this purpose, and sophisticated quality assurance and quality control procedures are necessary. Slight deviations anywhere in the manufacturing process, including filling, labeling, packaging, storage and shipping and quality control and testing, may result in lot failures, product recalls or spoilage. When changes are made to the manufacturing process, the Company may be required to provide pre-clinical and clinical data showing the comparable identity, strength, quality, purity or potency of the products before and after such changes. | |
| � | Biologics are frequently costly to manufacture because production ingredients are derived from living animal or plant material, and most biologics cannot be made synthetically. In particular, keeping up with the demand for vaccines may be difficult due to the complexity of producing vaccines. | |
| � | The use of biologically derived ingredients can lead to allegations of harm, including infections or allergic reactions, or closure of product facilities due to possible contamination. Any of these events could result in substantial costs. | |
| � | There currently is no process in the United States for the submission or approval of generic biologics based upon abbreviated data packages or a showing of sameness to another approved biologic, but there is public dialogue at the FDA and in Congress regarding the scientific and statutory basis upon which such products, known as biosimilars or follow-on biologics, could be approved and marketed in the United States. The Company cannot be certain when Congress will create a statutory pathway for the approval of biosimilars, and the Company cannot predict what impact, if any, the approval of biosimilars would have on the sales of Company products in the United States. In Europe, however, the EMEA has issued guidelines for approving biological products through an abbreviated pathway, and biosimilars have been approved in Europe. If a biosimilar version of one of the Company�s products were approved in Europe, it could have a negative effect on sales of the product. |
The Company operates in multiple jurisdictions and, as such, virtually all sales are denominated in currencies of the local jurisdiction. Additionally, the Company has entered and will enter into acquisition, licensing, borrowings or other financial transactions that may give rise to currency and interest rate exposure.
Since the Company cannot, with certainty, foresee and mitigate against such adverse fluctuations, fluctuations in currency exchange rates and interest rates could negatively affect the Company�s results of operations, financial position and cash flows.
In order to mitigate against the adverse impact of these market fluctuations, the Company will from time to time enter into hedging agreements. While hedging agreements, such as currency options and interest rate swaps, may limit some of the exposure to exchange rate and interest rate fluctuations, such attempts to mitigate these risks may be costly and not always successful.
The Company is subject to evolving and complex tax laws in the jurisdictions in which it operates. Significant judgment is required for determining the Company�s tax liabilities, and the Company�s tax returns are periodically examined by various tax authorities. The Company believes that its accrual for tax contingencies is adequate for all open years based on past experience, interpretations of tax law, and judgments about potential actions by tax authorities; however, due to the complexity of tax contingencies, the ultimate resolution of any tax matters may result in payments greater or less than amounts accrued.
In February 2010, President Obama�s administration proposed significant changes to the U.S.�international tax laws, including changes that would limit U.S.�tax deductions for expenses related to un-repatriated
33
foreign-source income and modify the U.S.�foreign tax credit rules. We cannot determine whether these proposals will be enacted into law or what, if any, changes may be made to such proposals prior to their being enacted into law. If these or other changes to the U.S.�international tax laws are enacted, they could have a significant impact on the financial results of the Company.
In addition, the Company may be impacted by changes in tax laws, including tax rate changes, changes to the laws related to the remittance of foreign earnings (deferral), or other limitations impacting the U.S.�tax treatment of foreign earnings, new tax laws, and revised tax law interpretations in domestic and foreign jurisdictions.
The success of the Merger will depend, in part, on the Company�s ability to successfully combine the businesses of Old Merck and Schering-Plough and realize the anticipated benefits and cost savings from the combination of the two companies. If the combined company is not able to achieve these objectives within the anticipated time frame, or at all, the value of the Company�s common stock may be adversely affected.
It is possible that the integration process could result in the loss of key employees, result in the disruption of each legacy company�s ongoing businesses or identify inconsistencies in standards, controls, procedures and policies that adversely affect our ability to maintain relationships with customers, suppliers, distributors, creditors, lessors, clinical trial investigators or managers or to achieve the anticipated benefits of the Merger.
Specifically, issues that must be addressed in integrating the operations of the two legacy companies in order to realize the anticipated benefits of the Merger include, among other things:
| � | integrating the research and development, manufacturing, distribution, marketing and promotion activities and information technology systems of Old Merck and Schering-Plough; | |
| � | conforming standards, controls, procedures and accounting and other policies, business cultures and compensation structures between the companies; | |
| � | identifying and eliminating redundant and underperforming operations and assets; and | |
| � | managing tax costs or inefficiencies associated with integrating the operations of the combined company. |
Integration efforts between the two companies will also divert management attention and resources. An inability to realize the full extent of, or any of, the anticipated benefits of the Merger, as well as any delays encountered in the integration process, could have an adverse effect on the Company�s business and results of operations, which may affect the value of the shares of Company common stock.
In addition, the actual integration may result in additional and unforeseen expenses, and the anticipated benefits of the integration plan may not be realized. Actual cost and sales synergies, if achieved at all, may be lower than the Company expects and may take longer to achieve than anticipated. If the Company is not able to adequately address these challenges, it may be unable to successfully integrate the operations of the two legacy companies, or to realize the anticipated benefits of the integration of the two legacy companies.
Delays encountered in the integration process could have a material adverse effect on the revenues, expenses, operating results and financial condition of the Company. Although the Company expects significant benefits, such as increased cost savings, to result from the Merger, there can be no assurance that the Company will realize any of these anticipated benefits.
The Company will incur significant costs in connection with consummating the Merger and integrating the operations of the two companies, with a significant portion of such costs being incurred through the first year after completion of the Merger. The Company continues to assess the magnitude of these costs, and additional unanticipated costs may be incurred in the integration of the businesses of the two legacy companies. Although the Company believes that the elimination of duplicative costs, as well as the realization of other efficiencies related to the integration of the businesses, will offset incremental transaction and Merger-related costs over time, no assurance can be given that this net benefit will be achieved in the near term, or at all.
34
While the Company�s financing strategy for the Merger was focused on preserving financial strength and flexibility to continue to invest in the Company�s business and key growth drivers post-merger, debt obligations incurred to finance the Merger could affect the Company�s flexibility in planning for, or reacting to, changes in its business and the industry in which it operates, thereby placing it at a competitive disadvantage compared to competitors that have less indebtedness. Further, if the Company decides to retire or pay down indebtedness early it may be required to dedicate a substantial portion of its cash flow from operations to do so, thereby reducing the availability of its cash flow for other purposes, including business development efforts and mergers and acquisitions.
As a result of a number of factors, product liability insurance has become less available while the cost has increased significantly. With respect to product liability, the Company self-insures substantially all of its risk, as the availability of commercial insurance has become more restrictive. The Company has evaluated its risks and has determined that the cost of obtaining product liability insurance outweighs the likely benefits of the coverage that is available and, as such, has no insurance for certain product liabilities effective August�1, 2004, including liability for legacy Merck products first sold after that date. The Company will continually assess the most efficient means to address its risk; however, there can be no guarantee that insurance coverage will be obtained or, if obtained, will be sufficient to fully cover product liabilities that may arise.
The extent of the Company�s operations outside the United States will be significant due to the fact that the majority of Schering-Plough�s legacy operations are outside the United States. Risks inherent in conducting a global business include:
| � | changes in medical reimbursement policies and programs and pricing restrictions in key markets; | |
| � | multiple regulatory requirements that could restrict the Company�s ability to manufacture and sell its products in key markets; | |
| � | trade protection measures and import or export licensing requirements; | |
| � | foreign exchange fluctuations; | |
| � | diminished protection of intellectual property in some countries;�and | |
| � | possible nationalization and expropriation. |
As discussed below, the Venezuelan economy was recently determined to be hyperinflationary which requires the Company to remeasure its local currency operations there to U.S. dollars which remeasurement will be recorded in the first quarter of 2010. In addition, the Venezuelan government recently devalued its currency. These actions will have an adverse effect on the Company�s results of operations, financial position and cash flows.
In addition, there may be changes to the Company�s business and political position if there is instability, disruption or destruction in a significant geographic region, regardless of cause, including war, terrorism, riot, civil insurrection or social unrest; and natural or man-made disasters, including famine, flood, fire, earthquake, storm or disease.
(Cautionary Statements Under the Private Securities Litigation Reform Act of 1995)
This report, including the Annual Report, and other written reports and oral statements made from time to time by the Company may contain so-called �forward-looking statements,� all of which are based on management�s current expectations and are subject to risks and uncertainties which may cause results to differ materially from those set forth in the statements. One can identify these forward-looking statements by their use of words such as �expects,� �plans,� �will,� �estimates,� �forecasts,� �projects� and other words of similar meaning. One can also identify them by the fact that they do not relate strictly to historical or current facts. These statements are likely to address the Company�s growth strategy, financial results, product development, product approvals, product
35
potential, and development programs. One must carefully consider any such statement and should understand that many factors could cause actual results to differ materially from the Company�s forward-looking statements. These factors include inaccurate assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward-looking statement can be guaranteed and actual future results may vary materially. The Company does not assume the obligation to update any forward-looking statement. The Company cautions you not to place undue reliance on these forward-looking statements. Although it is not possible to predict or identify all such factors, they may include the following:
| � | Competition from generic products as the Company�s products lose patent protection. |
| � | Increased �brand� competition in therapeutic areas important to the Company�s long-term business performance. |
| � | The difficulties and uncertainties inherent in new product development. The outcome of the lengthy and complex process of new product development is inherently uncertain. A drug candidate can fail at any stage of the process and one or more late-stage product candidates could fail to receive regulatory approval. New product candidates may appear promising in development but fail to reach the market because of efficacy or safety concerns, the inability to obtain necessary regulatory approvals, the difficulty or excessive cost to manufacture and/or the infringement of patents or intellectual property rights of others. Furthermore, the sales of new products may prove to be disappointing and fail to reach anticipated levels. |
| � | Pricing pressures, both in the United States and abroad, including rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and health care reform, pharmaceutical reimbursement and pricing in general. |
| � | Changes in government laws and regulations and the enforcement thereof affecting the Company�s business. |
| � | Efficacy or safety concerns with respect to marketed products, whether or not scientifically justified, leading to product recalls, withdrawals or declining sales. |
| � | Significant litigation related to Vioxx , and Vytorin and Zetia . |
| � | The arbitration proceeding involving the Company�s right to distribute Remicade and Simponi . |
| � | Legal factors, including product liability claims, antitrust litigation and governmental investigations, including tax disputes, environmental concerns and patent disputes with branded and generic competitors, any of which could preclude commercialization of products or negatively affect the profitability of existing products. |
| � | Lost market opportunity resulting from delays and uncertainties in the approval process of the FDA and foreign regulatory authorities. |
| � | Increased focus on privacy issues in countries around the world, including the United States and the EU. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect directly the Company�s business, including recently enacted laws in a majority of states in the United States requiring security breach notification. |
| � | Changes in tax laws including changes related to the taxation of foreign earnings. |
| � | Changes in accounting pronouncements promulgated by standard-setting or regulatory bodies, including the Financial Accounting Standards Board and the SEC, that are adverse to the Company. |
| � | Economic factors over which the Company has no control, including changes in inflation, interest rates and foreign currency exchange rates. |
This list should not be considered an exhaustive statement of all potential risks and uncertainties. See �Risk Factors� above.
| Item�1B. | Unresolved Staff Comments. |
None
36
| Item�2. | Properties. |
The Company�s corporate headquarters is located in Whitehouse Station, New Jersey. The Company�s U.S.�commercial operations are headquartered in Upper Gwynedd, Pennsylvania. The Company�s U.S.�pharmaceutical business is conducted through divisional headquarters located in Upper Gwynedd and Whitehouse Station, New Jersey. The Company�s vaccines business is conducted through divisional headquarters located in West Point, Pennsylvania. Merck�s Animal Health global headquarters is located in Boxmeer, the Netherlands. Principal U.S.�research facilities are located in Rahway, Kenilworth, Summit and Union, New Jersey, West Point, Palo Alto, California, and Nebraska (Animal Health). Principal research facilities outside the U.S.�are located in the Netherlands and Scotland. The Company also has production facilities for human health products at 14 locations in the United States and Puerto Rico. Outside the United States, through subsidiaries, the Company owns or has an interest in manufacturing plants or other properties in Australia, Canada, Japan, Singapore, South Africa, and other countries in Western Europe, Central and South America, and Asia.
Capital expenditures for 2009 were $1.5�billion compared with $1.3�billion for 2008. In the United States, these amounted to $981.6�million for 2009 and $946.6�million for 2008. Abroad, such expenditures amounted to $479.0�million for 2009 and $351.7�million for 2008.
The Company and its subsidiaries own their principal facilities and manufacturing plants under titles that they consider to be satisfactory. The Company considers that its properties are in good operating condition and that its machinery and equipment have been well maintained. Plants for the manufacture of products are suitable for their intended purposes and have capacities and projected capacities adequate for current and projected needs for existing Company products. Some capacity of the plants is being converted, with any needed modification, to the requirements of newly introduced and future products.
| Item�3. | Legal Proceedings. |
The Company is involved in various claims and legal proceedings of a nature considered normal to its business, including product liability, intellectual property, and commercial litigation, as well as additional matters such as antitrust actions.
37
claims of over 35,600 plaintiffs had been dismissed as of December�31, 2009, the vast majority of which were dismissed as a result of the settlement process discussed below.
Interim and final payments have been made to certain qualifying claimants. It is expected that the remainder of the full $4.85�billion will be distributed in the first half of 2010. The Company has completed making payments into the settlement funds.
38
$80�million in the fourth quarter of 2009. Since the settlement, one additional TPP case has been filed which is pending in the MDL proceeding.
Plaintiffs also filed a class action in California state court seeking certification of a class of California third-party payors and end-users. The trial court denied the motion for class certification on April�30, 2009, and the Court of Appeal affirmed that ruling on December�15, 2009. On January�25, 2010, plaintiffs filed a petition for review with the California Supreme Court.
Old Merck�s motion for summary judgment was granted in November 2009 in a case brought by the Attorney General of Texas that was scheduled to go to trial in early 2010. The Texas Attorney General did not appeal. In the Michigan Attorney General case, Old Merck is currently seeking appellate review of the trial court�s order denying Old Merck�s motion to dismiss. The trial court has entered a stay of proceedings (including discovery) pending the result of that appeal. Finally, the Attorney General actions in the MDL described in the previous paragraph are in the discovery phase. The Louisiana Attorney General case is currently scheduled for trial in the MDL court on April�12, 2010.
39
40
41
Ontario certification order. Leave to appeal was granted, the appeal was filed on May�20, 2009 and, in accordance with the court�s decision, Old Merck sought leave to appeal to the Divisional Court, which was denied on December�7, 2009. These procedural decisions in the Canadian litigation do not address the merits of the plaintiffs� claims and litigation in Canada remains in an early stage.
42
43
44
As previously disclosed, Old Merck was joined in ongoing litigation alleging manipulation by pharmaceutical manufacturers of Average Wholesale Prices (�AWP�), which are sometimes used in calculations that determine public and private sector reimbursement levels. The complaints allege violations of federal and state law, including fraud, Medicaid fraud and consumer protection violations, among other claims. The outcome of these litigations and investigations could include substantial damages, the imposition of substantial fines, penalties and injunctive or administrative remedies. In 2002, the JPML ordered the transfer and consolidation of all pending federal AWP cases to federal court in Boston, Massachusetts. Plaintiffs filed one consolidated class action complaint, which aggregated the claims previously filed in various federal district court actions and also expanded the number of manufacturers to include some which, like Old Merck, had not been defendants in any prior pending case. In May 2003, the court granted Old Merck�s motion to dismiss the consolidated class action and dismissed Old Merck from the class action case. Old Merck and many other pharmaceutical manufacturers are defendants in similar complaints pending in federal and state court including cases brought individually by a number of counties in the State of New York. Fifty of the county cases have been consolidated in New York state court. Old Merck was dismissed from the Suffolk County case, which was the first of the New York county cases to be filed. In addition to the New York county cases, as of December�31, 2008, Old Merck was a defendant in state cases brought by the Attorneys General of eleven states, all of which are being defended. In February 2009, the Kansas Attorney General filed suit against Old Merck and several other manufacturers. AWP claims brought by the Attorney General of Arizona against Old Merck were dropped in 2009. The court in the AWP cases pending in Hawaii listed Old Merck and others to be set for trial in mid-2010.
The Company continues to respond to litigation brought by certain states and private payors and to investigations initiated by the Department of Health and Human Services, the Department of Justice and several states regarding AWP. The Company is cooperating with these investigations.
45
$1.9�billion. The arbitration process involves a number of steps, including the selection of independent arbitrators, information exchanges and hearings, before a final decision will be reached. A hearing in the arbitration is scheduled to commence in late September 2010. An unfavorable outcome in the arbitration would have a material adverse effect on the Company�s financial position, liquidity and results of operations. For more information about this matter, see Item�1A. �Risk Factors� above.
As previously disclosed, in February 2008, Old Merck entered into a Corporate Integrity Agreement (�CIA�) with the U.S.�Department of Health and Human Services Office of Inspector General (�HHS-OIG�) for a five-year term. The CIA requires, among other things, that Old Merck maintain its ethics training program and policies and procedures governing promotional practices and Medicaid price reporting. Further, as required by the CIA, Old Merck has retained an Independent Review Organization (�IRO�) to conduct a systems review of its promotional policies and procedures and to conduct, on a sample basis, transactional reviews of Old Merck�s promotional programs and certain Medicaid pricing calculations. Old Merck is also required to provide regular reports and certifications to the HHS-OIG regarding its compliance with the CIA.
Similarly, as previously disclosed by Schering-Plough, in 2004 Schering-Plough entered into a CIA with HHS-OIG for a five-year term, and in August 2006, it entered into an addendum to the CIA also effective for five years. The requirements of Old Merck and Schering-Plough CIAs are similar, although not identical. Failure to comply with the CIAs� requirements can result in financial penalties or exclusion from participation in federal health care programs. The Company believes that its promotional practices and Medicaid price reports meet the requirements of each of the CIAs.
46
action lawsuits. The MSP Partnership recorded these charges in the second quarter of 2009. On February�9, 2010, Judge Cavanaugh granted final approval of the settlements.
47
motion to dismiss was filed on December�16, 2009, and the motion was fully briefed on January�15, 2010. A decision remains pending.
The Company intends to defend the lawsuits referred to in this section. Unfavorable outcomes resulting from the government investigations or the civil litigations could have a material adverse effect on the Company�s financial position, liquidity and results of operations.
On March�31, 2003, Schering-Plough was served with a putative class action complaint filed in the U.S.�District Court in New Jersey alleging that Schering-Plough, its Employee Savings Plan (the �Plan�) administrator, several current and former directors, and certain former corporate officers breached their fiduciary obligations to certain participants in the Plan. The complaint seeks damages in the amount of losses allegedly
48
suffered by the Plan. The complaint was dismissed on June�29, 2004. The plaintiffs appealed. On August�19, 2005 the U.S.�Court of Appeals for the Third Circuit reversed the dismissal by the District Court and the matter has been remanded back to the District Court for further proceedings. On September�30, 2008, the District Court entered an order granting in part, and denying in part, the named putative class representative�s motion for class certification. Schering-Plough thereafter petitioned the U.S.�District Court of Appeals for the Third Circuit for leave to appeal the class certification decision. Schering-Plough�s petition was granted on December�10, 2008. On December�21, 2009, the Third Circuit vacated the District Court�s order and remanded the case for further proceedings consistent with the court�s ruling.
In June 1997 and January 1998, Schering-Plough settled patent litigation with Upsher-Smith, Inc. (�Upsher-Smith�) and ESI Lederle, Inc. (�Lederle�), respectively, relating to generic versions of K-DUR, Schering-Plough�s long-acting potassium chloride product supplement used by cardiac patients, for which Lederle and Upsher-Smith had filed Abbreviated New Drug Applications. Following the commencement of an administrative proceeding by the United States Federal Trade Commission (the �FTC�) alleging anti-competitive effects from those settlements (which has been resolved in Schering-Plough�s favor), alleged class action suits were filed in federal and state courts on behalf of direct and indirect purchasers of K-DUR against Schering-Plough, Upsher-Smith and Lederle. These suits claim violations of federal and state antitrust laws, as well as other state statutory and common law causes of action. These suits seek unspecified damages. In February 2009, a special master recommended that the U.S.�District Court for the District of New Jersey dismiss the class action lawsuits on summary judgment. The U.S.�District Court judge has not yet ruled on the recommendation.
As discussed above, in July 2004, in connection with the settlement of an investigation with the DOJ and the U.S.�Attorney�s Office for the Eastern District of Pennsylvania, Schering-Plough entered into a five-year CIA. The CIA was amended in August 2006 in connection with the $435�million settlement of an investigation by the State of Massachusetts involving certain of Schering-Plough�s sales, marketing and clinical trial practices and programs (�Massachusetts Investigation�). Several purported class action litigations have been filed following the announcement of the settlement of the Massachusetts Investigation. Plaintiffs in these actions seek damages on behalf of third-party payors resulting from the allegations of off-label promotion and improper payments to physicians that were at issue in the Massachusetts Investigation. The actions have been consolidated in a multidistrict litigation in federal District Court for the District of New Jersey. In July 2009, the District Court dismissed the consolidated class action complaint but granted plaintiffs leave to refile. In September 2009, plaintiffs filed amended complaints, and the Company�s motion to dismiss those complaints is pending.
As previously disclosed, Old Merck is a party to individual and class action product liability lawsuits and claims in the United States involving pediatric vaccines (e.g., hepatitis B vaccine) that contained thimerosal, a preservative used in vaccines. As of December�31, 2009, there were approximately 200 thimerosal related lawsuits pending in which Old Merck is a defendant, although the vast majority of those lawsuits are not currently active. Other defendants include other vaccine manufacturers who produced pediatric vaccines containing thimerosal as well as manufacturers of thimerosal. In these actions, the plaintiffs allege, among other things, that they have suffered neurological injuries as a result of exposure to thimerosal from pediatric vaccines. There are no cases currently scheduled for trial. The Company will defend against these lawsuits; however, it is possible that unfavorable outcomes could have a material adverse effect on the Company�s financial position, liquidity and results of operations.
Old Merck has been successful in having cases of this type either dismissed or stayed on the ground that the action is prohibited under the National Childhood Vaccine Injury Act (the �Vaccine Act�). The Vaccine Act prohibits any person from filing or maintaining a civil action (in state or federal court) seeking damages against a vaccine manufacturer for vaccine-related injuries unless a petition is first filed in the United States Court of Federal Claims (hereinafter the �Vaccine Court�). Under the Vaccine Act, before filing a civil action against a vaccine manufacturer, the petitioner must either (a)�pursue his or her petition to conclusion in Vaccine Court and then timely
49
file an election to proceed with a civil action in lieu of accepting the Vaccine Court�s adjudication of the petition or (b)�timely exercise a right to withdraw the petition prior to Vaccine Court adjudication in accordance with certain statutorily prescribed time periods. Old Merck is not a party to Vaccine Court proceedings because the petitions are brought against the United States Department of Health and Human Services.
50
In May 2005, the Federal Court of Canada Trial Division issued a decision refusing to bar the approval of generic alendronate on the grounds that Old Merck�s patent for weekly alendronate was likely invalid. This decision cannot be appealed and generic alendronate was launched in Canada in June 2005. In July 2005, Old Merck was sued in the Federal Court of Canada by Apotex Corp. (�Apotex�) seeking damages for lost sales of generic weekly alendronate due to the patent proceeding. In October 2008, the Federal Court of Canada issued a decision awarding Apotex its lost profits for its generic alendronate product for the period of time that it was held off the market due to Old Merck�s lawsuit. In June 2009, the trial court decision was upheld in part and both companies sought leave to appeal to the Supreme Court of Canada. In January 2010, the Supreme Court of Canada declined to hear the appeal, leaving intact the decision that Apotex is entitled to damages for the discrete period of time that its market entry was postponed due to the litigation launched by Old Merck.
In addition, as previously disclosed, in Japan after a proceeding was filed challenging the validity of Old Merck�s Japanese patent for the once-weekly administration of alendronate, the patent office invalidated the patent. The decision is under appeal.
51
52
In connection with the Merger, separate class action lawsuits were brought against Old Merck and Schering-Plough challenging the Merger and seeking other forms of relief. As previously disclosed, both lawsuits have been settled pending court approval.
These settlements, if approved by the court, will resolve and release all claims that were or could have been brought by any shareholder of Old Merck or Schering-Plough challenging any aspect of the proposed merger, including any merger disclosure claims.
There are various other legal proceedings, principally product liability and intellectual property suits involving the Company, that are pending. While it is not feasible to predict the outcome of such proceedings or the proceedings discussed in this Item, in the opinion of the Company, all such proceedings are either adequately covered by insurance or, if not so covered, should not ultimately result in any liability that would have a material adverse effect on the financial position, liquidity or results of operations of the Company, other than proceedings for which a separate assessment is provided in this Item.
53
any reduction for anticipated recoveries of cleanup costs from former site owners or operators or other recalcitrant potentially responsible parties.
As previously disclosed, approximately 2,200 plaintiffs have filed an amended complaint against Old Merck and 12 other defendants in U.S.�District Court, Eastern District of California asserting claims under the Clean Water Act, the Resource Conservation and Recovery Act, as well as negligence and nuisance. The suit seeks damages for personal injury, diminution of property value, medical monitoring and other alleged real and personal property damage associated with groundwater and soil contamination found at the site of a former Old Merck subsidiary in Merced, California. Old Merck intends to defend itself against these claims.
In management�s opinion, the liabilities for all environmental matters that are probable and reasonably estimable have been accrued and totaled $161.8�million and $89.5�million at December�31, 2009 and 2008, respectively. These liabilities are undiscounted, do not consider potential recoveries from other parties and will be paid out over the periods of remediation for the applicable sites, which are expected to occur primarily over the next 15�years. Although it is not possible to predict with certainty the outcome of these matters, or the ultimate costs of remediation, management does not believe that any reasonably possible expenditures that may be incurred in excess of the liabilities accrued should exceed $170.0�million in the aggregate. Management also does not believe that these expenditures should result in a material adverse effect on the Company�s financial position, results of operations, liquidity or capital resources for any year.
RICHARD T. CLARK�� Age�63
November 2009�� Chairman, President and Chief Executive Officer, Merck�& Co., Inc. (formerly Schering-Plough Corporation)
April 2007�� Chairman, President and Chief Executive Officer, Old Merck
May 2005�� Chief Executive Officer and President, Old Merck
June 2003�� President, Merck Manufacturing Division, Old Merck�� responsible for the Company�s manufacturing, information services and operational excellence organizations worldwide
ADELE D. AMBROSE�� Age�53
November 2009�� Senior Vice President and Chief Communications Officer, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Global Communications organization
December 2007�� Vice President and Chief Communications Officer, Old Merck�� responsible for the Global Communications organization
April, 2005�� On sabbatical
Prior to April 2005, Ms.�Ambrose was Executive Vice President, Public Relations�& Investor Communications at AT&T Wireless (wireless services provider) from September 2001 to April 2005
STANLEY F. BARSHAY�� Age�70
November 2009�� Executive Vice President and President, Consumer Health Care, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Consumer Health Care organization. Mr.�Barshay will retire effective April�1, 2010.
Prior to November 2009, Mr.�Barshay was Chairman, Consumer Health Care, Schering-Plough Corporation since June 2003
54
RICHARD S. BOWLES III�� Age�58
November 2009�� Executive Vice President and Chief Compliance Officer, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Company�s compliance function, including Global Safety�& Environment, Systems Assurance, Ethics and Privacy
Prior to November 2009, Dr.�Bowles was Senior Vice President, Global Quality Operations, Schering-Plough Corporation since March 2001
JOHN CANAN�� Age�53
November 2009�� Senior Vice President and Global Controller, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Company�s global control organization including all accounting, controls, external reporting and financial standards and policies
January 2008�� Senior Vice President and Controller, Old Merck��responsible for the Corporate Controller�s Group
September 2006�� Vice President, Controller, Old Merck�� responsible for the Corporate Controller�s Group
WILLIE A. DEESE�� Age�54
November 2009�� Executive Vice President and President, Merck Manufacturing Division (�MMD�), Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Company�s global manufacturing, procurement, and distribution and logistics functions
January 2008�� Executive Vice President and President, MMD, Old Merck�� responsible for the Company�s global manufacturing, procurement, and distribution and logistics functions
May 2005�� President, MMD, Old Merck�� responsible for the Company�s global manufacturing, procurement, and operational excellence functions
January 2004�� Senior Vice President, Global Procurement
KENNETH C. FRAZIER�� Age�55
November 2009�� Executive Vice President and President, Global Human Health, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Company�s marketing and sales organizations worldwide, including the global pharmaceutical and vaccine franchises
August 2007�� Executive Vice President and President, Global Human Health, Old Merck�� responsible for the Company�s marketing and sales organizations worldwide, including the global pharmaceutical and vaccine franchises
November 2006�� Executive Vice President and General Counsel, Old Merck�� responsible for legal and public affairs functions and The Merck Company Foundation (a not-for-profit charitable organization affiliated with the Company)
December 1999�� Senior Vice President and General Counsel, Old Merck�� responsible for legal and public affairs functions and The Merck Company Foundation (a not-for-profit charitable organization affiliated with the Company)
MIRIAN M. GRADDICK-WEIR�� Age�55
November 2009�� Executive Vice President, Human Resources, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Global Human Resources organization
January 2008�� Executive Vice President, Human Resources, Old Merck�� responsible for the Global Human Resources organization
September 2006�� Senior Vice President, Human Resources, Old Merck
55
Prior to September 2006, Dr.�Graddick-Weir was Executive Vice President of Human Resources and Employee Communications at AT&T (communications services provider), and held several other senior Human Resources leadership positions at AT&T for more than 20�years.
BRIDGETTE HELLER�� Age�48
Effective March�1, 2010�� Executive Vice President and President, Consumer Health Care, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Consumer Health Care organization
Prior to March�1, 2010, Ms.�Heller was President, Johnson�& Johnson�s Baby Global Business Unit (2007�� 2010)�and Global President for Baby, Kids and Wound Care (2005�� 2007).
Prior to joining Johnson�& Johnson, Ms.�Heller was founder and managing partner at Heller Associates from 2004 to 2005.
PETER N. KELLOGG�� Age�53
November 2009�� Executive Vice President and Chief Financial Officer, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Company�s worldwide financial organization, investor relations, corporate development and licensing, and the Company�s joint venture relationships
August 2007�� Executive Vice President and Chief Financial Officer, Old Merck�� responsible for the Company�s worldwide financial organization, investor relations, corporate development and licensing, and the Company�s joint venture relationships
Prior to August 2007, Mr.�Kellogg was Executive Vice President, Finance and Chief Financial Officer of Biogen Idec (biotechnology company) since November 2003, from the merger of Biogen, Inc. and IDEC Pharmaceuticals Corporation.
PETER S. KIM�� Age�51
November 2009�� Executive Vice President and President, Merck Research Laboratories, Merck�& Co., Inc. (formerly Schering-Plough Corporation) (since January 2003)�� responsible for the Company�s research and development efforts worldwide
January 2008�� Executive Vice President and President, Merck Research Laboratories, Old Merck�� responsible for the Company�s research and development efforts worldwide
January 2003�� President, Merck Research Laboratories, Old Merck�� responsible for the Company�s research and development efforts worldwide
RAUL E. KOHAN�� Age�57
November 2009�� Executive Vice President and President, Animal Health, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for the Company�s Animal Health organization
October 2008�� Senior Vice President and President, Intervet/Schering-Plough Animal Health, Schering-Plough Corporation
October 2007�� Deputy Head, Animal Health and Senior Vice President, Corporate Excellence and Administrative Services, Schering-Plough Corporation.
February 2007�� Senior Vice President and President, Animal Health, Schering-Plough Corporation
Prior to February 2007, Mr.�Kohan was Group Head of Global Specialty Operations and President, Animal Health, Schering-Plough Corporation (since 2003).
56
BRUCE N. KUHLIK�� Age�53
November 2009�� Executive Vice President and General Counsel, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for legal, communications, and public policy functions and The Merck Company Foundation (a not-for-profit charitable organization affiliated with the Company)
January 2008�� Executive Vice President and General Counsel, Old Merck�� responsible for legal, communications, and public policy functions and The Merck Company Foundation (a not-for-profit charitable organization affiliated with the Company)
August 2007�� Senior Vice President and General Counsel, Old Merck�� responsible for legal, communications, and public policy functions and The Merck Company Foundation (a not-for-profit charitable organization affiliated with the Company)
Prior to May 2005, Mr.�Kuhlik was Senior Vice President and General Counsel for the Pharmaceutical Research and Manufacturers of America since October, 2002
MICHAEL ROSENBLATT�� Age�62
December 2009�� Executive Vice President and Chief Medical Officer, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� the Company�s primary voice to the global medical community on critical issues such as patient safety and will oversee the Company�s Global Center for Scientific Affairs
Prior to December 2009, Dr.�Rosenblatt was the Dean of Tufts University School of Medicine since 2003
J.�CHRIS SCALET�� Age�51
November 2009�� Executive Vice President, Global Services, and Chief Information Officer (�CIO�), Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for Global Shared Services across the human resources, finance, site services and information services function; and the enterprise business process redesign initiative
January 2008�� Executive Vice President, Global Services, and CIO, Old Merck�� responsible for Global Shared Services across the human resources, finance, site services and information services function; and the enterprise business process redesign initiative
January 2006�� Senior Vice President, Global Services, and CIO, Old Merck�� responsible for Global Shared Services across the human resources, finance, site services and information services function; and the enterprise business process redesign initiative
March 2003�� Senior Vice President, Information Services, and CIO, Old Merck�� responsible for all areas of information technology and services including application development, technical support, voice and data communications, and computer operations worldwide
MERVYN TURNER�� Age�63
November 2009�� Chief Strategy Officer and Senior Vice President, Emerging Markets Research�& Development, Merck Research Laboratories, Merck�& Co., Inc. (formerly Schering-Plough Corporation)�� responsible for leading the formulation and execution of the Company�s long term strategic plan and additional responsibilities in Licensing�& External Research within Merck Research Laboratories
November 2008�� Chief Strategy Officer and Senior Vice President, Worldwide Licensing and External Research, Merck Research Laboratories, Old Merck
October 2002�� Senior Vice President, Worldwide Licensing and External Research, Old Merck
All officers listed above serve at the pleasure of the Board of Directors. None of these officers was elected pursuant to any arrangement or understanding between the officer and the Board.
57
| Item�5. | Market for Registrant�s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
The principal market for trading of the Company�s Common Stock is the New York Stock Exchange (�NYSE�) under the symbol SGP prior to the Merger, and then MRK after the Merger. The Common Stock market price information set forth in the table below is based on historical NYSE market prices.
The following table also sets forth, for the calendar periods indicated, the dividend per share information.
| Year | 4th Q | 3rd Q | 2nd Q | 1st Q | ||||||||||||||||
2009 | $ | 0.26 | $ | 0.065 | $ | 0.065 | $ | 0.065 | $ | 0.065 | ||||||||||
2008 | $ | 0.26 | $ | 0.065 | $ | 0.065 | $ | 0.065 | $ | 0.065 | ||||||||||
| 2009 | 4th Q | 3rd Q | 2nd Q | 1st Q | ||||||||||||
High | $ | 38.42 | $ | 28.68 | $ | 25.12 | $ | 24.42 | ||||||||
Low | $ | 27.97 | $ | 24.34 | $ | 21.67 | $ | 16.32 | ||||||||
2008 | ||||||||||||||||
High | $ | 18.48 | $ | 22.32 | $ | 20.72 | $ | 27.73 | ||||||||
Low | $ | 12.76 | $ | 17.51 | $ | 13.86 | $ | 14.41 | ||||||||
| (1) | In each of 2009 and 2008, Old Merck paid quarterly cash dividends per common share of $0.38 for an annual amount of $1.52. |
As of January�31, 2010, there were approximately 176,000�shareholders of record.
The following table summarizes information about the options, warrants and rights and other equity compensation under the Company�s legacy Merck and legacy Schering-Plough equity plans as of the close of business on December�31, 2009. The table does not include information about tax qualified plans such as the MSD Employee Savings and Security Plan and the Schering-Plough Employees� Savings Plan.
| Number of | ||||||||||||
| securities | ||||||||||||
| Number of | remaining available | |||||||||||
| securities to be | for future issuance | |||||||||||
| issued upon | Weighted-average | under equity | ||||||||||
| exercise of | exercise price of | compensation plans | ||||||||||
| outstanding | outstanding | (excluding | ||||||||||
| options, warrants | options, warrants | securities | ||||||||||
| and rights | and rights | reflected in column (a)) | ||||||||||
Plan Category | (a) | (b) | (c) | |||||||||
Equity compensation plans approved by security
holders (1) | 313,784,854 | (2) | $ | 43.01 | 134,004,583 | |||||||
Equity compensation plans not approved by security
holders (3) | � | � | � | |||||||||
Total | 313,784,854 | $ | 43.01 | 134,004,583 | ||||||||
| (1) | Includes options to purchase shares of Company Common Stock and other rights under the following shareholder-approved plans: the Merck Sharp & Dohme 1996, 2001, 2004 and 2007 Incentive Stock Plans, the Merck & Co., Inc. 1996, 2001 and 2006 Non-Employee Directors Stock Option Plans, and the Schering-Plough Corporation 1997, 2002 and 2006 Stock Incentive Plans. |
58
| (2) | Excludes approximately 7,453,426�shares of restricted stock units and 3,695,024 performance share units (assuming maximum payouts) under the Merck Sharp & Dohme 2004 and 2007 Incentive Stock Plans and 7,665,296�shares of restricted stock units and 475,077 performance share units (excluding accrued dividends) under the Schering-Plough Corporation 2006 Stock Incentive Plan. Also excludes 350,473�shares of phantom stock deferred under the Merck & Co., Inc. Deferral Program. | |
| (3) | The table does not include information for equity compensation plans and options and other warrants and rights assumed by the Company in connection with mergers and acquisitions and pursuant to which there remain outstanding options or other warrants or rights (collectively, �Assumed Plans�), which include the Rosetta Inpharmatics, Inc. 1997 and 2000 Employee Stock Option Plans. A total of 69,934�shares of Merck Common Stock may be purchased under the Assumed Plans, at a weighted average exercise price of $37.90. No further grants may be made under any Assumed Plans. |
59
The following graph assumes a $100 investment on December�31, 2004, and reinvestment of all dividends, in each of the Company�s Common Shares, the S&P 500 Index, and a composite peer group of the major U.S.-based pharmaceutical companies, which are: Abbott Laboratories, Bristol-Myers Squibb Company, Johnson�& Johnson, Eli Lilly and Company, and Pfizer Inc.
Merck�& Co., Inc., Composite Peer Group and S&P 500 Index
| End of | 2009/2004 | |||||||
| Period Value | CAGR** | |||||||
MERCK | $ | 170 | 11 | % | ||||
PEER GRP.*** | 104 | 1 | ||||||
S&P 500 | 102 | 0 | ||||||
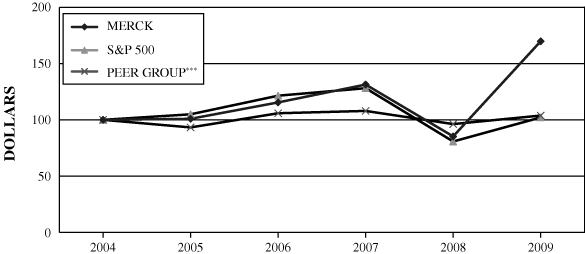
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||||||||||||||
MERCK | 100.00 | 100.91 | 115.48 | 131.36 | 85.26 | 169.87 | ||||||||||||||||||||||||
PEER GRP. | 100.00 | 93.24 | 105.85 | 107.91 | 96.21 | 103.80 | ||||||||||||||||||||||||
S&P 500 | 100.00 | 104.91 | 121.46 | 128.13 | 80.73 | 102.10 | ||||||||||||||||||||||||
| * | The Performance Graph reflects Schering-Plough�s stock performance from December�31, 2004 through the close of the Merger and New Merck�s stock performance from November�3, 2009 through December�31, 2009. Assumes the cash component of the merger consideration was reinvested in New Merck stock at the closing price on November�3, 2009. |
| ** | Compound Annual Growth Rate | |
| *** | On October�15, 2009, Wyeth and Pfizer Inc. completed its previously announced merger (the �Pfizer/Wyeth Merger�) where Wyeth became a wholly-owned subsidiary of Pfizer Inc. As discussed, on November�3, 2009, Old Merck and Schering-Plough completed the Merger (together with the Pfizer/Wyeth Merger, the �Transactions�) where Old Merck (subsequently renamed Merck Sharp & Dohme Corp.) became a wholly-owned subsidiary of Schering-Plough (subsequently renamed Merck & Co., Inc.). As a result of the Transactions, Wyeth and Old Merck no longer exist as publicly traded entities and ceased all trading of their common stock as of the close of business on their respective merger dates. Wyeth and Old Merck have been permanently removed from the peer group index. |
60
Between November�3, 2009 and January�22, 2010, the Company inadvertently issued a total of approximately 834,000 unregistered shares of common stock to certain former directors and former employees of Old Merck upon the exercise of stock options they held. The aggregate of the exercise prices paid in connection with the stock option exercises was approximately $26.6�million.
In addition, on January�8, 2010, the Company inadvertently issued a total of approximately 66,000 unregistered shares of common stock to certain former senior employees of Old Merck upon the vesting of performance share units they held.
61
| Item�6. | Selected Financial Data. |
The following selected financial data should be read in conjunction with Item�7. �Management�s Discussion and Analysis of Financial Condition and Results of Operations� and consolidated financial statements and notes thereto contained in Item�8. �Financial Statements and Supplementary Data� of this report.
Merck�& Co., Inc. and Subsidiaries
| 2009 (1) | 2008 (2) | 2007 (3) | 2006 (4) | 2005 (5) | ||||||||||||||||
Results for Year: | ||||||||||||||||||||
Sales | $27,428.3 | $23,850.3 | $24,197.7 | $22,636.0 | $22,011.9 | |||||||||||||||
Materials and production costs | 9,018.9 | 5,582.5 | 6,140.7 | 6,001.1 | 5,149.6 | |||||||||||||||
Marketing and administrative expenses | 8,543.2 | 7,377.0 | 7,556.7 | 8,165.4 | 7,155.5 | |||||||||||||||
Research and development expenses | 5,845.0 | 4,805.3 | 4,882.8 | 4,782.9 | 3,848.0 | |||||||||||||||
Restructuring costs | 1,633.9 | 1,032.5 | 327.1 | 142.3 | 322.2 | |||||||||||||||
Equity income from affiliates | (2,235.0 | ) | (2,560.6 | ) | (2,976.5 | ) | (2,294.4 | ) | (1,717.1 | ) | ||||||||||
U.S. Vioxx Settlement Agreement charge | � | � | 4,850.0 | � | � | |||||||||||||||
Other (income) expense, net | (10,669.5 | ) | (2,318.1 | ) | (75.2 | ) | (503.2 | ) | (232.0 | ) | ||||||||||
Income before taxes | 15,291.8 | 9,931.7 | 3,492.1 | 6,341.9 | 7,485.7 | |||||||||||||||
Taxes on income | 2,267.6 | 1,999.4 | 95.3 | 1,787.6 | 2,732.6 | |||||||||||||||
Net income | 13,024.2 | 7,932.3 | 3,396.8 | 4,554.3 | 4,753.1 | |||||||||||||||
Net income attributable to noncontrolling interests | 122.9 | 123.9 | 121.4 | 120.5 | 121.8 | |||||||||||||||
Net income attributable to Merck�& Co., Inc. | 12,901.3 | 7,808.4 | 3,275.4 | 4,433.8 | 4,631.3 | |||||||||||||||
Preferred stock dividends | 2.1 | � | � | � | � | |||||||||||||||
Net income available to common shareholders | 12,899.2 | 7,808.4 | 3,275.4 | 4,433.8 | 4,631.3 | |||||||||||||||
Basic earnings per common share available to common shareholders | $5.67 | $3.65 | $1.51 | $2.03 | $2.10 | |||||||||||||||
Earnings per common share assuming dilution available to common shareholders | $5.65 | $3.63 | $1.49 | $2.02 | $2.10 | |||||||||||||||
Cash dividends declared | 3,599.8 | 3,250.4 | 3,310.7 | 3,318.7 | 3,338.7 | |||||||||||||||
Cash dividends paid per common share | $1.52 | (6) | $1.52 | $1.52 | $1.52 | $1.52 | ||||||||||||||
Capital expenditures | 1,460.6 | 1,298.3 | 1,011.0 | 980.2 | 1,402.7 | |||||||||||||||
Depreciation | 1,654.3 | 1,445.1 | 1,752.4 | 2,098.1 | 1,544.2 | |||||||||||||||
Average common shares outstanding (millions) | 2,268.2 | 2,135.8 | 2,170.5 | 2,177.6 | 2,197.0 | |||||||||||||||
Average common shares outstanding assuming dilution (millions) | 2,273.2 | 2,142.5 | 2,189.8 | 2,184.1 | 2,199.2 | |||||||||||||||
Year-End Position: | ||||||||||||||||||||
Working capital | 12,677.9 | $4,793.9 | $2,787.2 | $2,507.5 | $7,806.9 | |||||||||||||||
Property, plant and equipment, net | 18,273.5 | 11,999.6 | 12,346.0 | 13,194.1 | 14,398.2 | |||||||||||||||
Total assets | 112,089.7 | 47,195.7 | 48,350.7 | 44,569.8 | 44,845.8 | |||||||||||||||
Long-term debt | 16,074.9 | 3,943.3 | 3,915.8 | 5,551.0 | 5,125.6 | |||||||||||||||
Total equity | 61,492.6 | 21,167.1 | 20,591.4 | 19,965.8 | 20,384.9 | |||||||||||||||
Year-End Statistics: | ||||||||||||||||||||
Number of stockholders of record | 175,600 | 165,700 | 173,000 | 184,200 | 198,200 | |||||||||||||||
Number of employees | 100,000 | 55,200 | 59,800 | 60,000 | 61,500 | |||||||||||||||
| ||||||||||||||||||||
| (1) | Amounts for 2009 include the impact of the merger with Schering-Plough Corporation on November�3, 2009, including the recognition of a gain representing the fair value step-up of Merck�s previously held interest in the Merck/Schering-Plough partnership as a result of obtaining a controlling interest and increased materials and production costs as a result of the amortization of intangible assets and inventory step-up. Also included in 2009, is a gain on the sale of Merck�s interest in Merial Limited, the favorable impact of certain tax items, the impact of restructuring actions and additional legal defense costs. |
| (2) | Amounts for 2008 include a gain on distribution from AstraZeneca LP, a gain related to the sale of the remaining worldwide rights to Aggrastat , the favorable impact of certain tax items, the impact of restructuring actions, additional legal defense costs and an expense for a contribution to the Merck Company Foundation. |
| (3) | Amounts for 2007 include the impact of the U.S. Vioxx Settlement Agreement charge, restructuring actions, a civil governmental investigations charge, an insurance arbitration settlement gain, in-process research and development expense resulting from an acquisition, additional Vioxx legal defense costs, gains on sales of assets and product divestitures, as well as a net gain on the settlements of certain patent disputes. |
| (4) | Amounts for 2006 include the impact of restructuring actions, in-process research and development expenses resulting from acquisitions, additional Vioxx legal defense costs and the adoption of a new accounting standard requiring the expensing of stock options. |
| (5) | Amounts for 2005 include the impact of the net tax charge primarily associated with the American Jobs Creation Act repatriation, restructuring actions and additional Vioxx legal defense costs. |
| (6) | Amount reflects dividends paid to common shareholders of Old Merck. In addition, approximately $144�million of dividends were paid subsequent to the merger with Schering-Plough, and $431 million were paid prior to the merger, relating to common stock and preferred stock dividends declared by Schering-Plough in�2009. |
62
| Item�7. | Management�s Discussion and Analysis of Financial Condition and Results of Operations. |
On November�3, 2009, Merck�& Co., Inc. (�Old Merck�) and Schering-Plough Corporation (�Schering-Plough�) completed their previously-announced merger (the �Merger�). In the Merger, Schering-Plough acquired all of the shares of Old Merck, which became a wholly-owned subsidiary of Schering-Plough and was renamed Merck Sharp�& Dohme Corp. Schering-Plough continued as the surviving public company and was renamed Merck�& Co., Inc. (�New Merck� or the �Company�). However, for accounting purposes only, the Merger was treated as an acquisition with Old Merck considered the accounting acquirer. Accordingly, the accompanying financial statements reflect Old Merck�s stand-alone operations as they existed prior to the completion of the Merger. The results of Schering-Plough�s business have been included in New Merck�s financial statements only for periods subsequent to the completion of the Merger. Therefore, New Merck�s financial results for 2009 do not reflect a full year of legacy Schering-Plough operations. References in this report and in the accompanying financial statements to �Merck� for periods prior to the Merger refer to Old Merck and for periods after the completion of the Merger to New Merck.
The Company is a global health care company that delivers innovative health solutions through its medicines, vaccines, biologic therapies, and consumer and animal products, which it markets directly and through its joint ventures. The Company�s operations are principally managed on a products basis and are comprised of one reportable segment, which is the Pharmaceutical segment. The Pharmaceutical segment includes human health pharmaceutical and vaccine products marketed either directly by the Company or through joint ventures. Human health pharmaceutical products consist of therapeutic and preventive agents, sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Vaccine products consist of preventative pediatric, adolescent and adult vaccines, primarily administered at physician offices. The Company sells these human health vaccines primarily to physicians, wholesalers, physician distributors and government entities. The Company�s professional representatives communicate the effectiveness, safety and value of its pharmaceutical and vaccine products to health care professionals in private practice, group practices and managed care organizations. The Company also has animal health operations that discover, develop, manufacture and market animal health products, including vaccines. The Company�s professional representatives communicate the safety and value of the Company�s animal health products to veterinarians, distributors and animal producers. Additionally, the Company has consumer health care operations that develop, manufacture and market Over-the-Counter (�OTC�), foot care and sun care products, which are sold through wholesale and retail drug, food chain and mass merchandiser outlets in the United States and Canada.
As discussed above, the Merger was completed on November�3, 2009. In the Merger, Old Merck shareholders received one share of common stock of New Merck for each share of Old Merck stock that they owned, and Schering-Plough shareholders received 0.5767 of a share of common stock of New Merck and $10.50 in cash for each share of Schering-Plough stock that they owned. The consideration in the Merger was valued at $49.6�billion in the aggregate. Schering-Plough was Old Merck�s long-term partner in the Merck/Schering-Plough cholesterol partnership (the �MSP Partnership�). The cash portion of the consideration was funded with a combination of existing cash, including proceeds from the sale of Old Merck�s interest in Merial Limited, the sale or redemption of investments and the issuance of debt.
The combined company has a research and development pipeline with greater depth and breadth and many promising drug candidates, a significantly broader portfolio of medicines and an expanded presence in key international markets, particularly in high-growth emerging markets. The Company anticipates that the efficiencies gained from the Merger will allow it to invest in promising pipeline candidates, as well as strategic external research and development opportunities.
The combination increased the Company�s pipeline of early, mid- and late stage product candidates, including a significant increase in the number of potential medicines the Company has in Phase�III development to
63
19 candidates. Additionally, a number of candidates are currently under review in the United States and internationally.
As a result of the Merger, the Company expects to achieve substantial cost savings across all areas, including from consolidation in both sales and marketing and research and development, the application of the Company�s lean manufacturing and sourcing strategies to the expanded operations, and the full integration of the MSP Partnership.
In February�2010, the Company announced the first phase of a new global restructuring program (the �Merger Restructuring Program�) in conjunction with the integration of the legacy Merck and legacy Schering-Plough businesses. This Merger Restructuring Program is intended to optimize the cost structure of the combined Company. As part of the first phase of the Merger Restructuring Program, by the end of 2012, the Company expects to reduce its total workforce by approximately 15% across all areas of the Company worldwide. The Company also plans to eliminate 2,500 vacant positions as part of the first phase of the program. These workforce reductions will primarily come from the elimination of duplicative positions in sales, administrative and headquarters organizations, as well as from the consolidation of certain manufacturing facilities and research and development operations. The Company will continue to hire new employees in strategic growth areas of the business during this period. Certain actions, such as the ongoing reevaluation of manufacturing and research and development facilities
64
worldwide have not yet been completed, but will be included later in 2010 in other phases of the Merger Restructuring Program. In connection with the first phase of the Merger Restructuring Program, separation costs under the Company�s existing severance programs worldwide were recorded in the fourth quarter of 2009 to the extent such costs were probable and reasonably estimable. The Company recorded pretax restructuring costs of $1.5�billion, primarily employee separation costs, related to the Merger Restructuring Program in the fourth quarter of 2009. This first phase of the Merger Restructuring Program is expected to be completed by the end of 2012 with the total pretax costs estimated to be $2.6�billion to $3.3�billion. The Company estimates that approximately 85% of the cumulative pretax costs relate to cash outlays, primarily related to employee separation expense. Approximately 15% of the cumulative pretax costs are non-cash, relating primarily to the accelerated depreciation of facilities to be closed or divested.
The Company expects this first phase of the Merger Restructuring Program to yield annual savings in 2012 of approximately $2.6�billion to $3.0�billion. These anticipated savings relate only to the first phase of the Merger Restructuring Program and therefore are only a portion of the estimated $3.5�billion of incremental annual savings originally disclosed when the Merger was announced. The Company expects that additional savings will be generated by subsequent phases of the Merger Restructuring Program that will be announced later this year, as well as by non-restructuring related activities, such as procurement savings initiatives. These cost savings, which are expected to come from all areas of the Company�s pharmaceutical business, are in addition to the previously announced ongoing cost reduction initiatives at both legacy companies.
Earnings per common share (�EPS�) assuming dilution for 2009 were $5.65, which reflect a net impact of $2.40 resulting from gains related to the MSP Partnership and the sale of Merial, partially offset by increased expenses from the amortization of purchase accounting adjustments, restructuring and merger-related costs. EPS in 2009 were also affected by the dilutive impact of shares issued in the Merger.
The markets in which the Company conducts its business and the pharmaceutical industry are highly competitive and highly regulated. The Company�s operations may be affected by technological advances of competitors, industry consolidation, patents granted to competitors, competitive combination products, new products of competitors, new information from clinical trials of marketed products or post-marketing surveillance and generic competition as the Company�s products mature. In addition, patent positions are increasingly being challenged by competitors, and the outcome can be highly uncertain. An adverse result in a patent dispute can preclude commercialization of products or negatively affect sales of existing products and could result in the recognition of an impairment charge with respect to certain products. Competitive pressures have intensified as pressures in the industry have grown. The effect on operations of competitive factors and patent disputes cannot be predicted.
Pharmaceutical competition involves a rigorous search for technological innovations and the ability to market these innovations effectively. With its long-standing emphasis on research and development, the Company is well positioned to compete in the search for technological innovations. Additional resources to meet market challenges include quality control, flexibility to meet customer specifications, an efficient distribution system and a strong technical information service. The Company is active in acquiring and marketing products through external alliances such as joint ventures and licenses and has been refining its sales and marketing efforts to further address changing industry conditions. However, the introduction of new products and processes by competitors may result in price reductions and product displacement, even for products protected by patents. For example, the number of
65
compounds available to treat a particular disease typically increases over time and can result in slowed sales growth for the Company�s products in that therapeutic category.
Global efforts toward healthcare cost containment continue to exert pressure on product pricing and access. In addressing cost containment pressure, the Company makes a continuing effort to demonstrate that its medicines provide value to patients and to those who pay for healthcare. In addition, pricing flexibility across the Company�s product portfolio has encouraged growing use of its medicines and mitigated the effects of increasing cost pressures on individual medicines.
Outside the United States, in difficult government budgetary environments, the Company has worked with payers to encourage allocation of scarce resources to optimize healthcare outcomes, limiting the potentially detrimental effects of government policies on sales growth and access to innovative medicines and vaccines, and to support the discovery and development of innovative products to benefit patients. The Company also is working with governments in many emerging markets in Eastern Europe, Latin America and Asia to encourage them to increase their investments in health and thereby improve their citizens� access to medicines. In addition, certain countries within the European Union (�EU�), recognizing the economic importance of the research-based pharmaceutical industry and the value of innovative medicines to society, are working with industry representatives to improve the competitive climate through a variety of means including market deregulation.
The Company anticipates that the worldwide trend toward cost containment will continue, resulting in ongoing pressures on healthcare budgets. In the United States, major healthcare reform has been introduced and passed in both houses of Congress. A final revised bill which unifies both versions may be considered and adopted into law. The impact of such actions, as well as budget pressures on governments in the United States and other nations, cannot be predicted at this time. As the Company continues to successfully launch new products, contribute to healthcare debates and monitor reforms, its new products, policies and strategies should enable it to maintain a strong position in the changing economic environment.
Although no one can predict the outcome of these and other legislative, regulatory and advocacy initiatives, the Company believes that it is well positioned to respond to the evolving healthcare environment and market forces.
The Company is also committed to improving access to medicines and enhancing the quality of life for people around the world. To cite just one example, The African Comprehensive HIV/AIDS Partnerships in Botswana, a partnership between the government of Botswana, the Bill�& Melinda Gates Foundation and The Merck Company Foundation/Merck�& Co., Inc., is supporting Botswana�s response to HIV/AIDS through a comprehensive and sustainable approach to HIV prevention, care, treatment, and support.
The pharmaceutical industry is subject to regulation by regional, country, state and local agencies around the world. Of particular importance is the U.S.�Food and Drug Administration (�FDA�) in the United States, which administers requirements covering the testing, approval, safety, effectiveness, manufacturing, labeling, and marketing of prescription pharmaceuticals. In many cases, the FDA requirements have increased the amount of
66
time and resources necessary to develop new products and bring them to market in the United States. In 1997, the Food and Drug Administration Modernization Act (the �FDA Modernization Act�) was passed and was the culmination of a comprehensive legislative reform effort designed to streamline regulatory procedures within the FDA and to improve the regulation of drugs, medical devices, and food. The legislation was principally designed to ensure the timely availability of safe and effective drugs and biologics by expediting the premarket review process for new products. A key provision of the legislation is the re-authorization of the Prescription Drug User Fee Act of 1992, which permits the continued collection of user fees from prescription drug manufacturers to augment FDA resources earmarked for the review of human drug applications. This helps provide the resources necessary to ensure the prompt approval of safe and effective new drugs.
In the United States, the government expanded access for senior citizens to prescription drug coverage by enacting the Medicare Prescription Drug Improvement and Modernization Act of 2003, which was signed into law in December 2003. Prescription drug coverage began on January�1, 2006. This legislation supports the Company�s goal of improving access to medicines by expanding insurance coverage, while preserving market-based incentives for pharmaceutical innovation. At the same time, the legislation has helped control the cost of prescription drug costs through competitive pressures and by encouraging the appropriate use of medicines. As mentioned above, in the United States major healthcare reform has been introduced and passed in both houses of Congress. A final revised bill which unifies both versions may be considered and adopted into law. The U.S.�Congress also considered, and may consider again, proposals to increase the government�s role in pharmaceutical pricing in the Medicare program. These proposals may include removing the current legal prohibition against the Secretary of the Health and Human Services intervening in price negotiations between Medicare drug benefit program plans and pharmaceutical companies. They may also include mandating the payment of rebates for some or all of the pharmaceutical utilization in Medicare drug benefit plans. In addition, Congress may again consider proposals to allow, under certain conditions, the importation of medicines from other countries.
For many years, the pharmaceutical industry has been under federal and state oversight with the approval process for new drugs, drug safety, advertising and promotion, drug purchasing and reimbursement programs, and formularies. The Company believes that it will continue to be able to conduct its operations, including the introduction of new drugs to the market, in this regulatory environment.
The Company continues to work with private and public payors to slow increases in healthcare spending. Also, U.S.�federal and state governments have pursued methods to directly reduce the cost of drugs and vaccines for which they pay. For example, federal laws require the Company to pay specified rebates for medicines reimbursed by Medicaid, to provide discounts for outpatient medicines purchased by certain Public Health Service entities and �disproportionate share� hospitals (hospitals meeting certain criteria), and to provide minimum discounts of 24% off of a defined �non-federal average manufacturer price� for purchases by certain components of the federal government such as the Department of Veterans Affairs and the Department of Defense.
Initiatives in some states seek rebates beyond the minimum required by Medicaid legislation, in some cases for patients beyond those who are eligible for Medicaid. Under the Federal Vaccines for Children entitlement program, the U.S.�Centers for Disease Control and Prevention (�CDC�) funds and purchases recommended pediatric vaccines at a public sector price for the immunization of Medicaid-eligible, uninsured, Native American and certain underinsured children. Old Merck was awarded a CDC contract in 2009 for the supply of pediatric vaccines for the Vaccines for Children program.
Outside the United States, the Company encounters similar regulatory and legislative issues in most of the countries where it does business. There, too, the primary thrust of governmental inquiry and action is toward determining drug safety and effectiveness, often with mechanisms for controlling the prices of or reimbursement for prescription drugs and the profits of prescription drug companies. The EU has adopted directives concerning the classification, labeling, advertising, wholesale distribution and approval for marketing of medicinal products for human use. The Company�s policies and procedures are already consistent with the substance of these directives; consequently, it is believed that they will not have any material effect on the Company�s business.
In January 2008, the European Commission (�EC�) launched a sector inquiry in the pharmaceutical industry under the rules of EU competition law. As part of this inquiry, Old Merck�s offices in Germany were inspected by the authorities beginning in January 2008. The preliminary report of the EC was issued on November�28, 2008, and following the public consultation period, the final report was issued in July 2009. The final report confirmed that there has been a decline in the number of novel medicines reaching the market and instances of delayed market entry of generic medicines and discussed industry practices that may have contributed
67
to these phenomena. While the EC has issued further inquiries with respect to the subject of the investigation, the EC has not alleged that the Company or any of its subsidiaries have engaged in any unlawful practices.
The Company is subject to the jurisdiction of various regulatory agencies and is, therefore, subject to potential administrative actions. Such actions may include seizures of products and other civil and criminal sanctions. Under certain circumstances, the Company on its own may deem it advisable to initiate product recalls. The Company believes that it should be able to compete effectively within this environment.
The Company is subject to a number of privacy and data protection laws and regulations globally. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing attention to privacy and data protection issues with the potential to affect directly the Company�s business, including recently enacted laws and regulations in the United States and internationally requiring notification to individuals and government authorities of security breaches involving certain categories of personal information.
68
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Pharmaceutical: | ||||||||||||
Bone, Respiratory, Immunology and Dermatology | ||||||||||||
Singulair | $ | 4,659.7 | $ | 4,336.9 | $ | 4,266.3 | ||||||
Fosamax | 1,099.8 | 1,552.7 | 3,049.0 | |||||||||
Propecia | 440.3 | 429.1 | 405.4 | |||||||||
Remicade | 430.7 | � | � | |||||||||
Arcoxia | 357.5 | 377.3 | 329.1 | |||||||||
Nasonex | 164.9 | � | � | |||||||||
Clarinex | 100.6 | � | � | |||||||||
Asmanex | 37.0 | � | � | |||||||||
Cardiovascular | ||||||||||||
Vytorin | 440.8 | 84.2 | 84.3 | |||||||||
Zetia | 402.9 | 6.4 | 6.5 | |||||||||
Integrilin | 45.9 | � | � | |||||||||
Diabetes and Obesity | ||||||||||||
Januvia | 1,922.1 | 1,397.1 | 667.5 | |||||||||
Janumet | 658.4 | 351.1 | 86.4 | |||||||||
Infectious Disease | ||||||||||||
Isentress | 751.8 | 361.1 | 41.3 | |||||||||
Primaxin | 688.9 | 760.4 | 763.5 | |||||||||
Cancidas | 616.7 | 596.4 | 536.9 | |||||||||
Invanz | 292.9 | 265.0 | 190.2 | |||||||||
Crixivan/Stocrin | 206.1 | 275.1 | 310.2 | |||||||||
PegIntron | 148.7 | � | � | |||||||||
Avelox | 66.2 | � | � | |||||||||
Rebetol | 36.1 | � | � | |||||||||
Mature Brands | ||||||||||||
Cozaar/Hyzaar | 3,560.7 | 3,557.7 | 3,350.1 | |||||||||
Zocor | 558.4 | 660.1 | 876.5 | |||||||||
Vasotec/Vaseretic | 310.8 | 356.7 | 494.6 | |||||||||
Proscar | 290.9 | 323.5 | 411.0 | |||||||||
Claritin Rx | 71.1 | � | � | |||||||||
Proventil | 26.2 | � | � | |||||||||
Neurosciences and Ophthalmology | ||||||||||||
Maxalt | 574.5 | 529.2 | 467.3 | |||||||||
Cosopt/Trusopt | 503.5 | 781.2 | 786.8 | |||||||||
Remeron | 38.5 | � | � | |||||||||
Subutex/Suboxone | 36.3 | � | � | |||||||||
Oncology | ||||||||||||
Emend | 313.1 | 259.7 | 201.7 | |||||||||
Temodar | 188.1 | � | � | |||||||||
Caelyx | 46.5 | � | � | |||||||||
Intron A | 38.4 | � | � | |||||||||
Vaccines (2) | ||||||||||||
ProQuad/M-M-R II/Varivax | 1,368.5 | 1,268.5 | 1,347.1 | |||||||||
Gardasil | 1,118.4 | 1,402.8 | 1,480.6 | |||||||||
RotaTeq | 521.9 | 664.5 | 524.7 | |||||||||
Pneumovax | 345.6 | 249.3 | 233.2 | |||||||||
Zostavax | 277.4 | 312.4 | 236.0 | |||||||||
Women�s Health and Endocrine | ||||||||||||
Follistim/Puregon | 96.5 | � | � | |||||||||
NuvaRing | 88.3 | � | � | |||||||||
Other
Pharmaceutical (3) | 1,294.9 | 922.9 | 1,136.6 | |||||||||
| 25,236.5 | 22,081.3 | 22,282.8 | ||||||||||
Other segment revenues (4) | 2,114.0 | 1,694.1 | 1,848.1 | |||||||||
Total segment revenues | 27,350.5 | 23,775.4 | 24,130.9 | |||||||||
Other (5) | 77.8 | 74.9 | 66.8 | |||||||||
| $ | 27,428.3 | $ | 23,850.3 | $ | 24,197.7 | |||||||
| ||||||||||||
| (1) | Sales of legacy Schering-Plough products only reflect results for the post-Merger period through December�31, 2009. Sales of MSP Partnership products Zetia and Vytorin represent sales for the post-Merger period through December�31, 2009. Prior to the Merger, sales of Zetia and Vytorin were primarily recognized by the MSP Partnership and the results of Old Merck�s interest in the MSP Partnership were recorded in Equity income from affiliates. Sales of Zetia and Vytorin in 2008 and 2007 reflect Old Merck�s sales of these products in Latin America which was not part of the MSP Partnership. |
| (2) | These amounts do not reflect sales of vaccines sold in most major European markets through the Company�s joint venture, Sanofi Pasteur MSD, the results of which are reflected in Equity income from affiliates. These amounts do, however, reflect supply sales to Sanofi Pasteur MSD. |
| (3) | Other pharmaceutical primarily includes sales of other human pharmaceutical products, including products within the franchises not listed separately. |
| (4) | Reflects other non-reportable segments, including animal health and consumer health care, and revenue from the Company�s relationship with AZLP primarily relating to sales of Nexium, as well as Prilosec. Revenue from AZLP was $1.4�billion, $1.6�billion and $1.7�billion in 2009, 2008 and 2007, respectively. |
| (5) | Other revenues are primarily comprised of miscellaneous corporate revenues, third-party manufacturing sales, sales related to divested products or businesses and other supply sales not included in segment results. |
69
70
71
Merck�s mature brands are human health pharmaceutical products that are approaching the expiration of their marketing exclusivity or are no longer protected by patents in developed markets, but continue to be a core part of the Company�s offering in other markets around the world.
72
73
74
Global sales of Animal Health products, which include livestock, poultry, companion animal and aquaculture products that prevent and treat animal diseases, totaled $494.2�million for the post-Merger period through December�31, 2009. Animal Health sales are affected by intense competition and the frequent introduction of generic products.
75
| ($ in millions) | 2009 | Change | 2008 | Change | 2007 | |||||||||||||||
Materials and production | $ | 9,018.9 | 62 | % | $ | 5,582.5 | -9 | % | $ | 6,140.7 | ||||||||||
Marketing and administrative | 8,543.2 | 16 | % | 7,377.0 | -2 | % | 7,556.7 | |||||||||||||
Research and development | 5,845.0 | 22 | % | 4,805.3 | -2 | % | 4,882.8 | |||||||||||||
Restructuring costs | 1,633.9 | 58 | % | 1,032.5 | * | 327.1 | ||||||||||||||
Equity income from affiliates | (2,235.0 | ) | \u221213 | % | (2,560.6 | ) | -14 | % | (2,976.5 | ) | ||||||||||
U.S. Vioxx Settlement Agreement charge | � | � | � | * | 4,850.0 | |||||||||||||||
Other (income) expense, net | (10,669.5 | ) | * | (2,318.1 | ) | * | (75.2 | ) | ||||||||||||
| $ | 12,136.5 | \u221213 | % | $ | 13,918.6 | -33 | % | $ | 20,705.6 | |||||||||||
| ||||||||||||||||||||
| * | 100% or greater. |
In 2009, materials and production costs were $9.0�billion compared with $5.6�billion in 2008. Materials and production costs include expenses related to the sale of legacy Schering-Plough products in the post-Merger period. Additionally, these costs were unfavorably affected by $1.5�billion of amortization of purchase accounting adjustments to Schering-Plough�s inventories and $0.8�billion of expense for the amortization of intangible assets recognized in the Merger. Also included in materials and production costs in 2009 were $115.2�million of costs associated with restructuring activities, substantially all of which represents accelerated depreciation associated with the planned sale or closure of manufacturing facilities. (See Note�4 to the consolidated financial statements.)
In 2008, materials and production costs declined 9% compared with a 1% decline in sales primarily reflecting lower restructuring costs. Included in materials and production costs in 2008 were $123.2�million of restructuring costs comprised of $88.7�million of accelerated depreciation and $34.5�million of other costs, primarily asset write-offs. This compares with restructuring costs of $483.1�million in 2007 representing $460.6�million of accelerated depreciation and $22.5�million of asset impairments.
76
Research and development expenses increased 22% in 2009 as compared with 2008, due in part to the incremental expenditures associated with the inclusion of Schering-Plough�s results in the post-Merger period. Additionally, expenses in 2009 reflect $231.6�million of costs associated with restructuring activities, including the closure or sale of research facilities in connection with the 2008 Restructuring Program, substantially all of which represent accelerated depreciation. (See Note�4 to the consolidated financial statements.) In addition, research and development expenses in 2009 as compared with 2008 reflect an increase in development spending in support of the continued advancement of the research pipeline, including investments in late-stage clinical trials.
Research and development expenses declined 2% in 2008 compared with 2007. Expenses in 2008 reflect $128.4�million of costs related to restructuring activities. Expenses in 2007 reflect $325.1�million of in-process research and development expense related to the NovaCardia acquisition. Research and development expenses in 2008 compared with 2007 reflect an increase in development spending in support of the continued advancement of the research pipeline.
Total pretax share-based compensation expense was $415.5�million in 2009, $348.0�million in 2008 and $330.2�million in 2007. At December�31, 2009, there was $521.8�million of total pretax unrecognized compensation expense related to nonvested stock option, restricted stock unit and performance share unit awards which will be recognized over a weighted average period of 1.5�years. For segment reporting, share-based compensation costs are unallocated expenses.
77
78
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Pharmaceutical segment profits | $ | 15,714.6 | $ | 14,110.3 | $ | 14,558.7 | ||||||
Other non-reportable segment profits | 1,735.1 | 1,691.0 | 2,027.6 | |||||||||
Other | (2,157.9 | ) | (5,869.6 | ) | (13,094.2 | ) | ||||||
Income before income taxes | $ | 15,291.8 | $ | 9,931.7 | $ | 3,492.1 | ||||||
| ||||||||||||
Net income available to common shareholders was $12.9�billion in 2009 compared with $7.8�billion in 2008 and $3.3�billion in 2007. Earnings per common share assuming dilution available to common shareholders (�EPS�) were $5.65 in 2009 compared with $3.63 in 2008 and $1.49 in 2007. The increases in net income and earnings per share in 2009 were largely driven by the gain associated with the MSP Partnership recognized in conjunction with the Merger, as well as the gain recorded on the sale of Old Merck�s interest in Merial, partially offset by incremental charges associated with the Merger, including the amortization of intangible assets and
79
Non-GAAP income and non-GAAP�EPS are alternative views of the Company�s performance used by management that Merck is providing because management believes this information enhances investors� understanding of the Company�s results. Non-GAAP income and non-GAAP earnings per share exclude certain items because of the nature of these items and the impact that they have on the analysis of underlying business performance and trends. The excluded items are certain purchase accounting items related to the Merger, restructuring activities, merger-related costs, and certain other items. These excluded items are significant components in understanding and assessing financial performance. Therefore, the information on non-GAAP income and non-GAAP�EPS should be considered in addition to, but not in lieu of, net income and earnings per share prepared in accordance with generally accepted accounting principles in the United States (�GAAP�). Additionally, since non-GAAP income and non-GAAP�EPS are not measures determined in accordance with GAAP, they have no standardized meaning prescribed by GAAP and, therefore, may not be comparable to the calculation of similar measures of other companies.
Non-GAAP income and non-GAAP�EPS are important internal measures for the Company. Senior management receives a monthly analysis of operating results that includes non-GAAP income and non-GAAP�EPS and the performance of the Company is measured on this basis along with other performance metrics. Senior management�s annual compensation is derived in part using non-GAAP income and non-GAAP�EPS.
80
A reconciliation between GAAP financial measures and non-GAAP financial measures is as follows:
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Pretax income as reported under GAAP | $ | 15,292 | $ | 9,932 | $ | 3,492 | ||||||
Increase (decrease) for excluded items: | ||||||||||||
Purchase accounting | 2,286 | � | � | |||||||||
Restructuring activities | 1,981 | 1,284 | 810 | |||||||||
Merger-related costs | 544 | � | � | |||||||||
Other items: | ||||||||||||
Gain related to the MSP Partnership | (7,530 | ) | � | � | ||||||||
Gain on Merial | (3,163 | ) | � | � | ||||||||
Gain on distribution from AZLP | � | (2,223 | ) | |||||||||
U.S. Vioxx settlement agreement charge | � | � | 4,850 | |||||||||
Civil governmental investigations charge | � | � | 671 | |||||||||
Insurance arbitration gain | � | � | (455 | ) | ||||||||
| 9,410 | 8,993 | 9,368 | ||||||||||
Taxes on income as reported under GAAP | 2,268 | 1,999 | 95 | |||||||||
Tax (benefit) expense on excluded items | (390 | ) | (472 | ) | 2,134 | |||||||
Non-GAAP taxes on income | 1,878 | 1,527 | 2,229 | |||||||||
Non-GAAP net income | $ | 7,532 | $ | 7,466 | $ | 7,139 | ||||||
| ||||||||||||
| ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
EPS assuming dilution as reported under GAAP | $ | 5.65 | $ | 3.63 | $ | 1.49 | ||||||
EPS impact of excluded items | (2.40 | ) | (0.21 | ) | 1.71 | |||||||
Non-GAAP�EPS assuming dilution | $ | 3.25 | $ | 3.42 | $ | 3.20 | ||||||
| ||||||||||||
Non-GAAP income and non-GAAP�EPS exclude certain amounts recorded in connection with the Merger (see Note�3 to the consolidated financial statements). These amounts include the amortization of intangible assets and inventory step-up.
Non-GAAP income and non-GAAP�EPS exclude restructuring activities, including restructuring activities related to the Merger (see Note�4 to the consolidated financial statements). These amounts include employee separation costs and accelerated depreciation associated with facilities to be sold or closed. The Company has undertaken restructurings of different types during the covered periods and therefore these charges should not to be considered non-recurring; however, management excludes these amounts from non-GAAP income and non-GAAP�EPS because it believes it is helpful for understanding the performance of the continuing business.
Non-GAAP income and non-GAAP�EPS exclude transaction costs associated directly with the Merger, as well as integration costs. These costs are excluded because management believes that these costs are unique to the Merger transaction and are not representative of ongoing normal business activities. Integration costs associated with the Merger will occur over several years, however, the impacts within each year will vary as the integration progresses.
Non-GAAP income and non-GAAP�EPS exclude certain other items. These items represent substantive, unusual items that are evaluated on an individual basis. Such evaluation considers both the quantitative and the
81
qualitative aspect of their unusual nature and generally represent items that, either as a result of their nature or magnitude, management would not anticipate that they would occur as part of the Company�s normal business on a regular basis. Certain other items include, among other items, the gain on the fair value adjustment of Merck�s existing interest in the MSP Partnership as a result of the Merger, the gain on the divestiture of the interest in Merial, the gain on a distribution from AZLP and certain legal settlements.
In connection with the Merger, the Company is assessing its pipeline to identify the most promising, high-potential compounds for development. The Company has completed the prioritization of its clinical development programs. The Company is continuing to work on the prioritization of its value adding programs related to currently marketed products and to its preclinical/discovery programs. The Company anticipates that the full prioritization process will be completed by the first half of 2010. In connection with this process, the Company may recognize non-cash impairment charges for the cancellation of certain legacy Schering-Plough pipeline programs that were measured at fair value and capitalized in connection with the Merger. These non-cash impairment charges, which are anticipated to be excluded from the Company�s non-GAAP earnings, could be material to the Company�s future GAAP earnings.
The Company currently has a number of candidates under regulatory review in the United States and internationally. Additionally, the Company has 19 drug candidates in Phase�III development.
MK-6621, vernakalant (IV), is an investigational candidate for the treatment of atrial fibrillation currently undergoing regulatory review in the EU. In April 2009, Old Merck and Cardiome Pharma Corp. (�Cardiome�) announced a collaboration and license agreement for the development and commercialization of vernakalant which provides Merck exclusive rights outside of the United States, Canada and Mexico to the intravenous formulation of vernakalant (see below). Vernakalent (oral) is currently in Phase�II development. Merck has exclusive global rights to the oral formulation of vernakalent for the maintenance of normal heart rhythm in patients with atrial fibrillation.
SCH 900121, NOMAC/E2, is an oral contraceptive that combines a selective progestin with estradiol, the estrogen that women produce naturally. The drug is currently under regulatory review in the EU. It is in Phase�III development for the U.S.�market.
SCH 503034, boceprevir, is a hepatitis�C protease inhibitor currently under development. Boceprevir is fully enrolled in its Phase�III program, which the Company expects to conclude in mid-2010. The Company expects to submit an NDA to the FDA for boceprevir by the end of 2010 for both treatment-experienced and treatment-na�ve patients with hepatitis�C.
82
MK-8669, ridaforolimus, is a novel mTOR (mammalian target of rapamycin) inhibitor being evaluated for the treatment of cancer. The drug candidate is being jointly developed and commercialized with ARIAD Pharmaceuticals, Inc., under an agreement entered into in 2007. A Phase�III study (SUCCEED) in patients with metastatic soft-tissue or bone sarcomas is underway. The Company continues to anticipate filing an NDA for ridaforolimus with the FDA in 2010, subject to a review of the results from the planned interim analysis of SUCCEED.
SCH 697243, an allergy immunotherapy sublingual tablet (�AIT�) for grass pollen allergy, is being developed by the Company. In November 2009, SCH 697243 met the primary endpoint in a Phase�III study of adult subjects in the United States with a history of grass pollen induced rhinoconjunctivitis with or without asthma. The investigational grass AIT treatment is designed to work by inducing a protective immune response against grass pollen allergy and providing sustained prevention of allergy symptoms, treating both the symptoms and the underlying cause of the disease.
SCH 039641, an AIT for ragweed allergy, is also in Phase�III development for the U.S.�market.
SCH 530348, vorapaxar, is a thrombin receptor antagonist or antiplatelet protease activated receptor-1 inhibitor being studied for the prevention and treatment of thrombosis. In November 2009, Merck announced completion of patient enrollment of more than 26,000�patients in the TRA 2�P-TIMI 50 clinical trial, a Phase III, randomized, double-blind, placebo-controlled, multinational study. The trial will assess the ability of SCH 530348 to prevent major cardiovascular events when added to current antiplatelet regimens (aspirin or aspirin plus an ADP inhibitor) in patients who have previously experienced a heart attack or stroke or who have peripheral arterial disease. SCH 530348 is also being studied in the treatment of patients with acute coronary syndrome in the ongoing Phase�III Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome trial, led by the Duke Clinical Research Institute. The Company anticipates filing an NDA for vorapaxar with the FDA in 2011.
MK-2452, tafluprost, is a preservative free, synthetic analogue of the prostaglandin F2\u03b1 for the reduction of elevated intraocular pressure in appropriate patients with primary open-angle glaucoma and ocular hypertension. In April 2009, Old Merck and Santen announced a worldwide licensing agreement for tafluprost (see below).
As previously disclosed, Old Merck submitted for filing an NDA with the FDA for MK-0653C, ezetimibe combined with atorvastatin, which is an investigational medication for the treatment of dyslipidemia, and the FDA refused to file the application. The FDA has identified additional manufacturing and stability data that are needed and the Company is assessing the FDA�s response and anticipates filing in 2011.
MK-0822, odanacatib, is an oral, once-weekly investigational treatment for osteoporosis. Osteoporosis is a disease which reduces bone density and strength and results in an increased risk of bone fractures. Odanacatib is a cathepsin K inhibitor that selectively inhibits the cathepsin K enzyme. Cathepsin K is known to play a central role in the function of osteoclasts, which are cells that break down existing bone tissue, particularly the protein components of bone. Inhibition of cathepsin K is a novel approach to the treatment of osteoporosis. In September 2009, data from a Phase IIB clinical study of odanacatib were presented at the 31st�Annual Meeting of the American Society for Bone and Mineral Research which showed that when stopping treatment after two years the increases in lower back (lumbar spine) bone mineral density (�BMD�) were reversed over the next year, while BMD at the hip (femoral neck) remained above levels observed at the start of the study. Additionally, three years of treatment with odanacatib 50�mg demonstrated increases in BMD at key fracture sites and minimal impact on the formation of new bone as measured by biochemical markers of bone turnover. Odanacatib is currently in Phase�III clinical trials and is being evaluated in a large-scale, global outcomes study to determine its effects on vertebral, hip and non-vertebral fractures. The Company continues to anticipate filing an NDA with the FDA in 2012.
V503 is a nine-valent HPV vaccine in development to expand protection against cancer-causing HPV types. The Phase�III clinical program is underway and Merck anticipates filing a Biologics License Application (�BLA�) with the FDA in 2012.
83
MK-0524B is a drug candidate that combines the novel approach to raising HDL-C and lowering triglycerides from ER niacin combined with laropiprant with the proven benefits of simvastatin in one combination product. Merck will not seek approval for MK-0524B in the United States until it files its complete response relating to MK-0524A.
MK-0859, anacetrapib, is an inhibitor of the cholesteryl ester transfer protein that has shown promise in lipid management by raising HDL-C and reducing LDL-C without raising blood pressure. In November 2009, Merck announced that in a Phase IIb study in 589�patients with primary hypercholesterolemia or mixed hyperlipidemia treated with anacetrapib as monotherapy or co-administered with atorvastatin, there were persistent lipid effects in the higher dose arms in both the monotherapy and co-administration treatment groups eight weeks after stopping active therapy with anacetrapib. The effect of CETP inhibition on cardiovascular risk has yet to be established. A Phase�III trial, titled DEFINE, is ongoing to further evaluate the safety and efficacy of anacetrapib in patients with coronary heart disease. The Company anticipates filing an NDA with the FDA beyond 2015.
As previously disclosed, in 2009, Old Merck announced it was delaying the filing of the U.S.�application for telcagepant (MK-0974), the Company�s investigational calcitonin gene-related peptide (�CGRP�)-receptor antagonist for the intermittent treatment of acute migraine. The decision was based on findings from a Phase IIa exploratory study in which a small number of patients taking telcagepant twice daily for three months for the prevention of migraine were found to have marked elevations in liver transaminases. The daily dosing regimen in the prevention study was different than the dosing regimen used in Phase�III studies in which telcagepant was intermittently administered in one or two doses to treat individual migraine attacks as they occurred. Other studies with telcagepant for the acute, intermittent treatment of migraine continue. Following meetings with regulatory agencies at the end of 2009, Merck is planning to conduct an additional safety study as part of the overall Phase�III program for telcagepant. The results of this study will inform planned filings for approval.
SCH 900395, acadesine, is a potential first-in class adenosine regulating agent for ischemia reperfusion-injury in patients undergoing heart bypass surgery. Patient enrollment in the RED CABG Phase�III clinical trial was initiated in 2009.
SCH 417690, vicriviroc, for the treatment of HIV infection (treatment experienced) was evaluated in two Phase�III studies in this patient population, and it was announced in January 2010 that the primary efficacy endpoint was not met. Merck will not submit an NDA to the FDA for vicriviroc in treatment-experienced HIV-infected patients at this time but will continue to evaluate vicriviroc as first-line therapy for treatment-naive patients.
As previously disclosed, in 2007, Cubist Pharmaceuticals, Inc. (�Cubist�) entered into a license agreement with Old Merck for the development and commercialization of Cubicin (daptomycin for injection, MK3009) in Japan. Merck will develop and commercialize Cubicin through its wholly-owned subsidiary, Banyu Pharmaceutical Co., Ltd. Cubist commercializes Cubicin in the United States. MK-3009 is currently in Phase�III development.
84
MK-4305 is an orexin receptor antagonist, a potential new approach to the treatment of chronic insomnia, currently in Phase�III development.
Merck has terminated the internal clinical development program for esmirtazapine (SCH 900265)�for hot flashes and insomnia for strategic reasons.
As previously disclosed, in 2009, Old Merck announced that preliminary results for the pivotal Phase�III study of rolofylline (MK-7418), its investigational medicine for the treatment of acute heart failure, showed that rolofylline did not meet the primary or secondary efficacy endpoints. Old Merck terminated the clinical development program for rolofylline.
In connection with the Merger, the Company recorded the fair value of human and animal health research projects underway at Schering-Plough. Approximately $5.0�billion of the consideration transferred in the Merger was allocated to Pharmaceutical segment IPR&D projects and $1.3�billion was allocated to Animal Health operating segment IPR&D projects.
The fair values of identifiable intangible assets related to IPR&D were determined by using an income approach, through which fair value is estimated based on each asset�s probability adjusted future net cash flows, which reflect the different stages of development of each product and the associated probability of successful completion. The net cash flows are then discounted to present value using discount rates which range from 12% to 15%. Actual cash flows are likely to be different than those assumed.
Additional research and development will be required before any of the programs reach technological feasibility. The costs to complete the research projects will depend on whether the projects are brought to their final stages of development and are ultimately submitted to the FDA or other regulatory agencies for approval. As of December�31, 2009, the estimated costs to complete projects in Phase�III development for human health and the analogous stage of development for animal health were approximately $1.6�billion. All of the IPR&D projects are subject to the inherent risks and uncertainties in drug development and it is possible that the Company will not be able to successfully develop and complete the IPR&D programs and profitably commercialize the underlying product candidates. The time periods to receive approvals from the FDA and other regulatory agencies are subject to uncertainty. Significant delays in the approval process, or the Company�s failure to obtain approval at all, will delay or prevent the Company from realizing revenues from these products. Additionally, if certain of the IPR&D programs fail or are abandoned during development as a result of the Company�s portfolio prioritization process or otherwise, then the Company will not realize the future cash flows it has estimated and recorded as IPR&D on the Merger date, and the Company may also not recover the research and development expenditures made since the Merger to further develop that program. If such circumstances were to occur, the Company�s future operating results could be adversely affected.
Merck continues to remain focused on augmenting its internal efforts by capitalizing on growth opportunities that will drive both near- and long-term growth. During 2009, transactions across a broad range of therapeutic categories, as well as early�� stage technology transactions were completed. Merck is actively
85
monitoring the landscape for growth opportunities that meet the Company�s strategic criteria. Highlights from these activities include:
In December 2009, Merck and Avecia Investments Limited announced a definitive agreement under which Merck would acquire the biologics business of the Avecia group for a total purchase price of $180�million. Avecia Biologics is a contract manufacturing organization with specific expertise in microbial-derived biologics. Under the terms of the agreement, Merck would acquire Avecia Biologics Limited (�Avecia�) and all of its assets, including all Avecia�s process development and scale-up, manufacturing, quality and business support operations located in Billingham, United Kingdom. This transaction closed on February�1, 2010, and accordingly, the results of operations of the acquired business will be included in Merck�s results of operations beginning as of the acquisition date.
In July 2009, Old Merck and Portola Pharmaceuticals, Inc. (�Portola�) signed an exclusive global collaboration and license agreement for the development and commercialization of betrixaban (MK-4448), an investigational oral Factor Xa inhibitor anticoagulant currently in Phase�II clinical development for the prevention of stroke in patients with atrial fibrillation. In return for an exclusive worldwide license to betrixaban, Old Merck paid Portola an initial fee of $50�million at closing, which was recorded as research and development expense. Portola is eligible to receive additional cash payments totaling up to $420�million upon achievement of certain development, regulatory and commercialization milestones, as well as double-digit royalties on worldwide sales of betrixaban, if approved. The Company will assume all development and commercialization costs, including the costs of Phase�III clinical trials. Portola retained an option (a)�to co-fund�Phase�III clinical trials in return for additional royalties and (b)�to co-promote betrixaban with Merck in the United States. The term of the agreement commenced in August 2009 and, unless terminated earlier, will continue until there are no remaining royalty payment obligations in a country, at which time the agreement will expire in its entirety in such country. The agreement may be terminated by either party in the event of a material uncured breach or bankruptcy of a party. The agreement may be terminated by Merck in the event that the parties or Merck decide to cease development of betrixaban for safety or efficacy. In addition, Merck may terminate the agreement at any time upon 180�days prior written notice. Portola may terminate the agreement in the event that Merck challenges any Portola patent covering betrixaban. Upon termination of the agreement, depending upon the circumstances, the parties have varying rights and obligations with respect to the continued development and commercialization of betrixaban and, in the case of termination for cause by Merck, certain royalty obligations.
86
In addition, in April 2009, Old Merck and Cardiome Pharma Corp. (�Cardiome�) announced a collaboration and license agreement for the development and commercialization of vernakalant (MK-6621), an investigational candidate for the treatment of atrial fibrillation. The agreement provides Merck with exclusive global rights to the oral formulation of vernakalant (�vernakalant (oral)�) for the maintenance of normal heart rhythm in patients with atrial fibrillation, and provides a Merck affiliate, Merck Sharp�& Dohme (Switzerland) GmbH, with exclusive rights outside of the United States, Canada and Mexico to the intravenous (�IV�) formulation of vernakalant (�vernakalant (IV)�) for rapid conversion of acute atrial fibrillation to normal heart rhythm. Under the terms of the agreement, Old Merck paid Cardiome an initial fee of $60�million upon closing, which was recorded as research and development expense. In addition, Cardiome is eligible to receive up to $200�million in payments based on achievement of certain milestones associated with the development and approval of vernakalant products (including $15�million for submission for regulatory approval in Europe of vernakalant (IV), which Old Merck paid in 2009 as a result of that submission, and $20�million for initiation of a planned Phase�III program for vernakalant (oral)) and up to $100�million for milestones associated with approvals in other subsequent indications of both the intravenous and oral formulations. Also, Cardiome will receive tiered royalty payments on sales of any approved products and has the potential to receive up to $340�million in milestone payments based on achievement of significant sales thresholds. Cardiome has retained an option to co-promote vernakalant (oral) with Merck through a hospital-based sales force in the United States. Merck will be responsible for all future costs associated with the development, manufacturing and commercialization of these candidates. Merck has granted Cardiome a secured, interest-bearing credit facility of up to $100�million that Cardiome may access in tranches over several years commencing in 2010. Cardiome�s co-development partner in North America, Astellas Pharma U.S., Inc., submitted an NDA with the FDA for Kynapid (vernakalant hydrochloride) Injection in December 2006 that included results from two pivotal Phase�III clinical trials. In December 2007, the Cardiovascular and Renal Drugs Advisory Committee recommended that the FDA approve vernakalant (IV)�for rapid conversion of atrial fibrillation. In August 2008, the FDA issued an Approvable action letter requesting additional information. A Phase IIb double-blind, placebo-controlled, randomized, dose-ranging clinical trial in patients at risk of recurrent atrial fibrillation showed that, at the 500�mg dose, vernakalant (oral) significantly reduced the rate of atrial fibrillation relapse as compared to placebo. This agreement continues in effect until the expiration of Cardiome�s co-promotion rights and all royalty and milestone payment obligations. This agreement may be terminated in the event of insolvency or a material uncured breach by either party. Additionally, the collaboration may be terminated by Merck in the event that Merck determines (in good faith) that it is not advisable to continue the development or commercialization of a vernakalant product as a result of a serious safety issue. In addition, Merck may terminate the agreement at any time upon 12�months prior written notice. Cardiome may terminate the agreement in the event that Merck challenges any Cardiome patent covering vernakalant. Upon termination of the agreement, depending upon the circumstances, the parties have varying rights and obligations with respect to the continued development and commercialization of vernakalant and in some cases continuing royalty obligations.
87
In March 2009, Old Merck acquired Insmed Inc.�s (�Insmed�) portfolio of follow-on biologic therapeutic candidates and its commercial manufacturing facilities located in Boulder, Colorado. Under the terms of the agreement, Old Merck paid Insmed an aggregate of $130�million in cash to acquire all rights to the Boulder facilities and Insmed�s pipeline of follow-on biologic candidates. Insmed�s follow-on biologics portfolio includes two clinical candidates: MK-4214, an investigational recombinant granulocyte-colony stimulating factor (�G-CSF�) that will be evaluated for its ability to prevent infections in patients with cancer receiving chemotherapy, and MK-6302, a pegylated recombinant G-CSF designed to allow for less frequent dosing. The transaction was accounted for as a business combination; accordingly, the assets acquired and liabilities assumed were recorded at their respective fair values as of the acquisition date. The determination of fair value requires management to make significant estimates and assumptions. In connection with the acquisition, substantially all of the purchase price was allocated to Insmed�s follow-on biologics portfolio (MK-4214 and MK-6302) and an indefinite-lived intangible asset was recorded. The fair value was determined based upon the present value of expected future cash flows of new product candidates resulting from Insmed�s follow-on biologics portfolio adjusted for the probability of their estimated technical and marketing success utilizing an income approach reflecting appropriate risk-adjusted discount rates. The ongoing activity related to MK-4214 and MK-6302 is not expected to be material to the Company�s research and development expense. The remaining net assets acquired were not material and there were no other milestone or royalty obligations associated with the acquisition. This transaction closed on March�31, 2009, and accordingly, the results of operations of the acquired business have been included in Merck�s results of operations beginning April�1, 2009.
The Company maintains a number of long-term exploratory and fundamental research programs in biology and chemistry as well as research programs directed toward product development. The Company�s research and development model is designed to increase productivity and improve the probability of success by prioritizing the Company�s research and development resources on disease areas of unmet medical needs, scientific opportunity and commercial opportunity. Merck is managing its research and development portfolio across diverse approaches to discovery and development by balancing investments appropriately on novel, innovative targets with the potential to have a major impact on human health, on developing best-in-class approaches, and on delivering maximum value of its new medicines and vaccines through new indications and new formulations. Another important component of the Company�s science-based diversification is based on expanding the Company�s portfolio of modalities to include not only small molecules and vaccines, but also biologics, peptides and RNAi. Further, Merck moved to diversify its portfolio by creating a new division, Merck BioVentures, which has the potential to harness the market opportunity presented by biological medicine patent expiries by delivering high quality follow-on biologic products to enhance access for patients worldwide. The Company will continue to pursue appropriate external licensing opportunities.
The integration plans for research and development are focused on integrating the research operations of the legacy companies, including providing an effective transition for employees, realizing projected merger synergies in the form of cost savings and revenue growth opportunities, and maintaining momentum in the Company�s late-state pipeline. During 2009, Merck continued implementing a new model for its basic research global operating strategy at legacy Merck Research Laboratories sites. The new model will align franchise and function as well as align resources with disease area priorities and balance capacity across discovery phases and allow the Company to act upon those programs with the highest probability of success. Additionally, across all disease area priorities, the Company�s strategy is designed to expand access to worldwide external science and incorporate external research as a key component of the Company�s early discovery pipeline in order to translate basic research productivity into late-stage clinical success.
The Company�s clinical pipeline includes candidates in multiple disease areas, including anemia, atherosclerosis, cancer, diabetes, heart disease, hypertension, infectious diseases, inflammatory/autoimmune diseases, migraine, neurodegenerative diseases, ophthalmics, osteoporosis, psychiatric diseases, respiratory disease and women�s health. The Company supplements its internal research with an aggressive licensing and external alliance strategy focused on the entire spectrum of collaborations from early research to late-stage compounds, as well as new technologies.
88
To expand its research base and realize synergies from combining capabilities, opportunities and assets, in previous years Old Merck formed a number of joint ventures. (See Note�10 to the consolidated financial statements.)
| 2009 | ||||||||||||||||||||
| ($ in millions) | Pre-Merger | Post-Merger | Total | 2008 | 2007 | |||||||||||||||
Vytorin | $ | 1,689.5 | $ | 370.6 | $ | 2,060.1 | $ | 2,360.0 | $ | 2,779.1 | ||||||||||
Zetia | 1,697.7 | 370.3 | 2,068.0 | 2,201.1 | 2,407.1 | |||||||||||||||
| $ | 3,387.2 | $ | 740.9 | $ | 4,128.1 | $ | 4,561.1 | $ | 5,186.2 | |||||||||||
| ||||||||||||||||||||
| (1) | Amounts exclude sales of these products by the Partners outside of the MSP Partnership. |
89
simvastatin on cardiovascular morbidity and mortality over and above that demonstrated for simvastatin has been established. In January 2009, the FDA announced that it had completed its review of the final clinical study report of ENHANCE. The FDA stated that the results from ENHANCE did not change its position that elevated LDL cholesterol is a risk factor for cardiovascular disease and that lowering LDL cholesterol reduces the risk for cardiovascular disease.
The financial statements of the MSP Partnership for 2008 are included in Item�15. (a) (2)��Financial Statement Schedules� below.
In 1998, Old Merck and Astra completed the restructuring of the ownership and operations of the joint venture whereby Old Merck acquired Astra�s interest in AMI, renamed KBI Inc. (�KBI�), and contributed KBI�s operating assets to a new U.S.�limited partnership, Astra Pharmaceuticals L.P. (the �Partnership�), in exchange for a 1% limited partner interest. Astra contributed the net assets of its wholly owned subsidiary, Astra USA, Inc., to the Partnership in exchange for a 99% general partner interest. The Partnership, renamed AstraZeneca LP (�AZLP�) upon Astra�s 1999 merger with Zeneca Group Plc (the �AstraZeneca merger�), became the exclusive distributor of the products for which KBI retained rights.
90
The AstraZeneca merger constituted a Trigger Event under the KBI restructuring agreements. As a result of the merger, in exchange for Old Merck�s relinquishment of rights to future Astra products with no existing or pending U.S.�patents at the time of the merger, Astra paid $967.4�million (the �Advance Payment�). The Advance Payment was deferred as it remained subject to a true-up calculation (the �True-Up Amount�) that was directly dependent on the fair market value in March 2008 of the Astra product rights retained by Old Merck. The calculated True-Up Amount of $243.7�million was returned to AZLP in March 2008 and a pretax gain of $723.7�million was recognized related to the residual Advance Payment balance.
91
In 1997, Old Merck and Rh�ne-Poulenc S.A. (now sanofi-aventis) combined their animal health businesses to form�Merial Limited (�Merial�), a fully integrated animal health company, which was a stand-alone joint venture, 50% owned by each party. Merial provides a comprehensive range of pharmaceuticals and vaccines to enhance the health, well-being and performance of a wide range of animal species.
Also, in connection with the sale of Merial, Old Merck, sanofi-aventis and Schering-Plough signed a call option agreement. Under the terms of the call option agreement, following the closing of the Merger, sanofi-aventis has an option to require the Company to combine its Intervet/Schering-Plough Animal Health business with Merial to form an animal health joint venture that would be owned equally by the Company and sanofi-aventis. As part of the call option agreement, the value of Merial has been fixed at $8�billion. The minimum total value received by the Company and its affiliates for contributing Intervet/Schering-Plough to the combined entity would be $9.25�billion (subject to customary transaction adjustments), consisting of a floor valuation of Intervet/Schering-Plough which is fixed at a minimum of $8.5�billion (subject to potential upward revision based on a valuation exercise by the two parties) and an additional payment by sanofi-aventis of $750�million. Based on the valuation exercise of Intervet/Schering-Plough and the customary transaction adjustments, if Merial and Intervet/Schering-Plough are combined, a payment may be required to be paid by either party to make the joint venture equally owned by the Company and sanofi-aventis. This payment would true-up the value of the contributions so that they are equal. Any formation of a new animal health joint venture with sanofi-aventis is subject to customary closing conditions including antitrust review in the United States and Europe. Prior to the closing of the Merger, the agreements provided Old Merck with certain rights to terminate the call option for a fee of $400�million. The recognition of the termination fee was deferred until the fourth quarter of 2009 when the conditions that could have triggered its payment lapsed.
Sales of joint venture products were as follows:
| ($ in millions) | 2009 (1) | 2008 | 2007 | |||||||||
Fipronil products | $ | 783.9 | $ | 1,053.0 | $ | 1,033.3 | ||||||
Biological products | 524.5 | 789.7 | 674.9 | |||||||||
Avermectin products | 341.4 | 511.8 | 478.4 | |||||||||
Other products | 199.7 | 288.2 | 262.2 | |||||||||
| $ | 1,849.5 | $ | 2,642.7 | $ | 2,448.8 | |||||||
| ||||||||||||
In 1994, Old Merck and Pasteur Merieux Connaught (now Sanofi Pasteur S.A.) established a 50% owned joint venture to market vaccines in Europe and to collaborate in the development of combination vaccines for distribution in Europe.
Sales of joint venture products were as follows:
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Gardasil | $ | 549.2 | $ | 865.3 | $ | 476.0 | ||||||
Influenza vaccines | 249.4 | 229.9 | 232.5 | |||||||||
Other viral vaccines | 112.1 | 105.1 | 86.8 | |||||||||
Hepatitis vaccines | 44.2 | 72.6 | 72.9 | |||||||||
RotaTeq | 42.2 | 28.4 | 15.7 | |||||||||
Other vaccines | 591.5 | 583.5 | 554.1 | |||||||||
| $ | 1,588.6 | $ | 1,884.8 | $ | 1,438.0 | |||||||
| ||||||||||||
92
Sales of joint venture products were as follows:
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Gastrointestinal products | $ | 202.0 | $ | 210.7 | $ | 218.5 | ||||||
Other products | 1.2 | 1.4 | 1.2 | |||||||||
| $ | 203.2 | $ | 212.1 | $ | 219.7 | |||||||
| ||||||||||||
Capital expenditures were $1.5�billion in 2009, $1.3�billion in 2008 and $1.0�billion in 2007. Expenditures in the United States were $981.6�million in 2009, $946.6�million in 2008 and $788.0�million in 2007. Expenditures during 2009 included $801.5�million for production facilities, $161.2�million for research and development facilities, $33.6�million for environmental projects, and $464.3�million for administrative, safety and general site projects, of which approximately 25% represents capital investments related to a multi-year initiative to standardize the Company�s information systems.
Depreciation expense was $1.7�billion in 2009, $1.4�billion in 2008 and $1.8�billion in 2007 of which $1.0�billion, $1.0�billion and $1.4�billion, respectively, applied to locations in the United States. Total depreciation expense in 2009, 2008 and 2007 included accelerated depreciation of $348.6�million, $216.7�million and $460.6�million, respectively, associated with restructuring activities (see Note�4 to the consolidated financial statements).
Merck�s strong financial profile enables it to fully fund research and development, focus on external alliances, support in-line products and maximize upcoming launches while providing significant cash returns to shareholders.
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Working capital | $ | 12,677.9 | $ | 4,793.9 | $ | 2,787.2 | ||||||
Total debt to total liabilities and equity | 15.6 | % | 13.2 | % | 11.9 | % | ||||||
Cash provided by operations to total debt | 0.2:1 | 1.1:1 | 1.2:1 | |||||||||
| ||||||||||||
The $18�billion cash portion of the consideration for the Merger was funded with a combination of existing cash, including the proceeds from the sale of Old Merck�s interest in Merial discussed above, the sale or redemption of short-term investments and the issuance of debt. In preparation for the Merger, during 2009, Old Merck closed an underwritten public offering of $4.25�billion senior unsecured notes as discussed below. Additionally, a significant portion of the long-term investments as of December�31, 2008 were liquidated in anticipation of the Merger.
93
Cash used by financing activities was $1.6�billion in 2009 compared with $5.5�billion in 2008 reflecting the 2009 issuance of $4.25�billion senior unsecured notes, no purchases of treasury stock and lower payments on debt, partially offset by a net decrease in short-term borrowings. Cash used in financing activities was $5.5�billion in 2008 compared with $4.9�billion in 2007 reflecting higher purchases of treasury stock, lower proceeds from the exercise stock options and higher payments on debt in connection with the settlement of a note due to Astra, partially offset by a net increase in short-term borrowings. Dividends paid to stockholders were $3.2�billion in 2009 and $3.3�billion in 2008 and 2007.
At December�31, 2009, the total of worldwide cash and investments was $10.0�billion, including $9.6�billion of cash, cash equivalents and short-term investments, and $432.3�million of long-term investments. In addition, the Company has $290�million of cash and investments restricted under certain collateral arrangements as discussed below. Working capital levels are more than adequate to meet the operating requirements of the Company.
As previously disclosed, the IRS has completed its examination of Old Merck�s tax returns for the years 1993 to 2001. As a result of the examination, Old Merck made an aggregate payment of $2.79�billion in February 2007. This payment was offset by (i)�a tax refund of $165�million received in 2007 for amounts previously paid for these matters and (ii)�a federal tax benefit of approximately $360�million related to interest included in the payment, resulting in a net cash cost to Old Merck of approximately $2.3�billion in 2007. The impact for years subsequent to 2001 for items reviewed as part of the examination was included in the payment although those years remain open in all other respects. The closing of the IRS examination did not have a material impact on results of operations in 2007 as these amounts had been previously accrued for.
As previously disclosed, in October 2006, the CRA issued Old Merck a notice of reassessment containing adjustments related to certain intercompany pricing matters. In February 2009, Old Merck and the CRA negotiated a settlement agreement in regard to these matters. In accordance with the settlement, Old Merck paid an additional tax of approximately $300�million (U.S.�dollars) and interest of approximately $360�million (U.S.�dollars) with no
94
additional amounts or penalties due on this assessment. The settlement was accounted for in the first quarter of 2009. Old Merck had previously established reserves for these matters. A significant portion of the taxes paid is expected to be creditable for U.S.�tax purposes. The resolution of these matters did not have a material effect on financial position or liquidity, other than with respect to the associated collateral as discussed below.
In addition, in July 2007 and November 2008, the CRA proposed additional adjustments for 1999 and 2000, respectively, relating to other intercompany pricing matters. The adjustments would increase Canadian tax due by approximately $312�million (U.S.�dollars) plus $314�million (U.S.�dollars) of interest through December�31, 2009. It is possible that the CRA will propose similar adjustments for later years. The Company disagrees with the positions taken by the CRA and believes they are without merit. The Company intends to contest the assessments through the CRA appeals process and the courts if necessary. Management believes that resolution of these matters will not have a material effect on the Company�s financial position or liquidity.
The IRS is examining Old Merck�s 2002 to 2005 federal income tax returns. In addition, various state and foreign tax examinations are in progress. For most of its other significant tax jurisdictions (both U.S.�state and foreign), the Company�s income tax returns are open for examination for the period 1999 through 2009.
During the second quarter of 2007, the IRS completed its examination of Schering-Plough�s 1997-2002 federal income tax returns. The Company is seeking resolution of an issue raised during this examination through the IRS administrative appeals process. In July 2007, Schering-Plough made a payment of $98�million to the IRS pertaining to the 1997-2002 examination. The Company�s income tax returns remain open with the IRS for the 1997-2009 tax years. During 2008, the IRS commenced its examination of the 2003-2006 federal income tax returns. This examination is expected to be completed in 2010. For most of its other significant tax jurisdictions (both U.S.�state and foreign), the Company�s income tax returns are open for examination for the period 2002 through 2009.
The Company�s contractual obligations as of December�31, 2009 are as follows:
| ($ in millions) | Total | 2010 | 2011 - 2012 | 2013 -2014 | Thereafter | |||||||||||||||
Purchase obligations | $ | 3,734.9 | $ | 2,380.8 | $ | 730.4 | $ | 523.0 | $ | 100.7 | ||||||||||
Loans payable and current portion of long-term debt | 1,362.3 | 1,362.3 | � | � | � | |||||||||||||||
Long-term debt | 15,329.1 | � | 2,378.5 | 3,966.5 | 8,984.1 | |||||||||||||||
Interest related to debt obligations | 9,665.4 | 778.3 | 1,422.1 | 1,243.3 | 6,221.7 | |||||||||||||||
Unrecognized tax benefits (1) | 324.0 | 324.0 | � | � | � | |||||||||||||||
Operating leases | 944.8 | 281.6 | 393.4 | 195.1 | 74.7 | |||||||||||||||
| $ | 31,360.5 | $ | 5,127.0 | $ | 4,924.4 | $ | 5,927.9 | $ | 15,381.2 | |||||||||||
| ||||||||||||||||||||
| (1) | As of December�31, 2009, the Company�s Consolidated Balance Sheet reflects liabilities for unrecognized tax benefits, interest and penalties of $5.7�billion, including $324.0�million reflected as a current liability. Due to the high degree of uncertainty regarding the timing of future cash outflows of liabilities for unrecognized tax benefits beyond one year, a reasonable estimate of the period of cash settlement for years beyond 2010 can not be made. |
95
Purchase obligations consist primarily of goods and services that are enforceable and legally binding and include obligations for minimum inventory contracts, research and development and advertising. Amounts reflected for research and development obligations do not include contingent milestone payments. Loans payable and current portion of long-term debt also reflects $298.2�million of long-dated notes that are subject to repayment at the option of the holders on an annual basis. Required funding obligations for 2010 relating to the Company�s pension and other postretirement benefit plans are not expected to be material. However, the Company currently anticipates contributing approximately $950�million and $50�million, respectively, to its pension plans and other postretirement benefit plans during 2010.
On June�25, 2009, Old Merck closed an underwritten public offering of $4.25�billion senior unsecured notes consisting of $1.25�billion aggregate principal amount of 1.875%�notes due 2011, $1.0�billion aggregate principal amount of 4.00%�notes due 2015, $1.25�billion aggregate principal amount of 5.00%�notes due 2019 and $750�million aggregate principal amount of 5.85%�notes due 2039. Interest on the notes is payable semi-annually. The notes of each series are redeemable in whole or in part at any time, at the Company�s option at the redemption prices specified in each notes associated prospectus. Proceeds from the notes were used to fund a portion of the cash consideration of the Merger.
In December 2009, the Company filed a securities registration statement with the Securities and Exchange Commission (�SEC�) under the automatic shelf registration process available to �well-known seasoned issuers� which is effective for three years.
Also, in connection with the Merger, on March�8, 2009, Old Merck entered into a financing commitment letter with JPMorgan Chase Bank, N.A. and J.P.�Morgan Securities Inc. (collectively �JPMorgan�), under which JPMorgan committed to provide $7�billion of financing. On May�6, 2009, Old Merck entered into a $3�billion 364-day senior unsecured interim term loan facility (the �bridge loan facility�); a $3�billion 364-day asset sale revolving credit facility (the �asset sale facility�); and a $1�billion 364-day corporate revolving credit facility (the �incremental facility�). In connection with the above $4.25�billion offering, the bridge loan facility was terminated and the commitment of the lenders under the 364-day asset sale facility was reduced. Upon completion of the sale of Merial to sanofi-aventis (see Note�10 to the consolidated financial statements), the asset sale facility was terminated. The incremental facility is available to backstop commercial paper and for general corporate purposes. This facility has not been drawn on and will expire in November 2010. Merck has incurred commitment fees of approximately $150 million associated with these facilities which are being amortized over the commitment period.
In April 2009, Old Merck amended its $1.5�billion, 5-year revolving credit facility maturing in April 2013 to allow the facility to remain in place after the Merger. The Company�s existing $2.0�billion credit facility maturing in August 2012 remains outstanding. These facilities provide backup liquidity for the Company�s commercial paper borrowing facility and are for general corporate purposes. The Company has not drawn funding from either facility.
Also, in connection with the Merger, effective as of November�3, 2009, New Merck executed a full and unconditional guarantee of the then existing debt of Old Merck and Old Merck executed a full and unconditional guarantee of the then existing debt of New Merck (excluding commercial paper), including for payments of principal and interest.
The Company�s long-term credit ratings assigned by Moody�s Investors Service and Standard�& Poor�s are Aa3 with a stable outlook and AA- with a positive outlook, respectively. These ratings continue to allow access to the capital markets and flexibility in obtaining funds on competitive terms. The Company continues to maintain a conservative financial profile. The Company places its cash and investments in instruments that meet high credit quality standards, as specified in its investment policy guidelines. These guidelines also limit the amount of credit exposure to any one issuer. Despite this strong financial profile, certain contingent events, if realized, which are discussed in Note�12 to the consolidated financial statements, could have a material adverse impact on the Company�s liquidity and capital resources. The Company does not participate in any off-balance sheet arrangements involving unconsolidated subsidiaries that provide financing or potentially expose the Company to unrecorded financial obligations.
In November 2009 and February 2010, the Board of Directors declared a quarterly dividend of $0.38 per share on the Company�s common stock for the first and second quarter of 2010, respectively, and declared a
96
quarterly dividend of $3.75 per share on the 6% mandatory convertible preferred stock for the first and second quarter of 2010, respectively.
In November 2009, the Board of Directors approved purchases over time of up to $3.0�billion of Merck�s common stock for its treasury. No purchases of treasury stock were made in 2009. Old Merck purchased $2.7�billion and $1.4�billion of treasury stock in 2008 and 2007, respectively, under a previous program approved by Old Merck�s Board of Directors in July 2002.
The Company manages the impact of foreign exchange rate movements and interest rate movements on its earnings, cash flows and fair values of assets and liabilities through operational means and through the use of various financial instruments, including derivative instruments.
A significant portion of the Company�s revenues and earnings in foreign affiliates is exposed to changes in foreign exchange rates. The objectives and accounting related to the Company�s foreign currency risk management program, as well as its interest rate risk management activities are discussed below.
A significant portion of the Company�s revenues are denominated in foreign currencies. Merck relies on sustained cash flows generated from foreign sources to support its long-term commitment to U.S.�dollar-based research and development. To the extent the dollar value of cash flows is diminished as a result of a strengthening dollar, the Company�s ability to fund research and other dollar-based strategic initiatives at a consistent level may be impaired. The Company has established revenue hedging and balance sheet risk management programs to protect against volatility of future foreign currency cash flows and changes in fair value caused by volatility in foreign exchange rates at its U.S. functional currency entities.
The objective of the revenue hedging program is to reduce the potential for longer-term unfavorable changes in foreign exchange to decrease the U.S.�dollar value of future cash flows derived from foreign currency denominated sales, primarily the euro and Japanese yen. To achieve this objective, the Company will partially hedge forecasted foreign currency denominated third party and intercompany distributor entity sales that are expected to occur over its planning cycle, typically no more than three years into the future. The Company will layer in hedges over time, increasing the portion of third party and intercompany distributor sales hedged as it gets closer to the expected date of the forecasted foreign currency denominated sales, such that it is probable the hedged transaction will occur. The portion of sales hedged is based on assessments of cost-benefit profiles that consider natural offsetting exposures, revenue and exchange rate volatilities and correlations, and the cost of hedging instruments. The hedged anticipated sales are a specified component of a portfolio of similarly denominated foreign currency-based sales transactions, each of which responds to the hedged risk in the same manner. The Company manages its anticipated transaction exposure principally with purchased local currency put options, which provide the Company with a right, but not an obligation, to sell foreign currencies in the future at a predetermined price. If the U.S.�dollar strengthens relative to the currency of the hedged anticipated sales, total changes in the options� cash flows offset the decline in the expected future U.S.�dollar cash flows of the hedged foreign currency sales. Conversely, if the U.S.�dollar weakens, the options� value reduces to zero, but the Company benefits from the increase in the value of the anticipated foreign currency cash flows. The Company also utilizes forward contracts in its revenue hedging program. If the U.S.�dollar strengthens relative to the currency of the hedged anticipated sales, the increase in the fair value of the forward contracts offsets the decrease in the expected future U.S.�dollar cash flows of the hedged foreign currency sales. Conversely, if the U.S.�dollar weakens, the decrease in the fair value of the forward contracts offsets the increase in the value of the anticipated foreign currency cash flows. While a weaker U.S.�dollar would result in a net benefit, the market value of Merck�s hedges would have declined by $245.0�million and $194.7�million, respectively, from a uniform 10% weakening of the U.S.�dollar at December�31, 2009 and 2008. The market value was determined using a foreign exchange option pricing model and holding all factors except exchange rates constant. Because Merck principally uses purchased local currency put options, a uniform weakening of the U.S.�dollar will yield the largest overall potential loss in the market value of these options. The sensitivity measurement assumes that a change in one foreign currency relative to the U.S.�dollar would not affect other foreign currencies relative to the U.S.�dollar. Although not predictive in nature, the Company believes that a
97
10% threshold reflects reasonably possible near-term changes in Merck�s major foreign currency exposures relative to the U.S.�dollar. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
The Venezuelan economy was recently determined to be hyperinflationary which requires the Company to remeasure its local currency operations there to U.S. dollars. Accordingly, in accordance with U.S. GAAP, the Company will remeasure its monetary assets and liabilities for those operations in earnings in the first quarter. Effective January�11, 2010, the Venezuelan government devalued its currency from BsF at 2.15 per U.S.�dollar to a two-tiered official exchange rate at (1)��the essentials rate� at BsF�2.60 per U.S.�dollar and (2)��the non-essentials rate� at BsF 4.30 per U.S.�dollar. The Company�s products are expected to be classified as �essentials� and anticipates that the majority of its transactions will be settled at the essential rate of BsF�2.60 per U.S. dollar. These actions will have an adverse effect on the Company�s results of operations, financial position and cash flows.
In addition to the revenue hedging and balance sheet risk management programs, the Company may use interest rate swap contracts on certain investing and borrowing transactions to manage its net exposure to interest rate changes and to reduce its overall cost of borrowing. The Company does not use leveraged swaps and, in general, does not leverage any of its investment activities that would put principal capital at risk. At December�31, 2009, the Company was a party to seven pay-floating, receive-fixed interest rate swap contracts designated as fair value hedges of fixed-rate notes in which the notional amounts match the amount of the hedged fixed-rate notes. There are two swaps maturing in 2011 with notional amounts of $125�million each that effectively convert the Company�s $250�million, 5.125% fixed-rate notes due 2011 to floating rate instruments and five swaps maturing in 2015 with notional amounts of $150�million each that effectively convert $750�million of the Company�s $1.0�billion, 4.0% fixed-rate notes due 2015 to floating rate instruments. The fair value changes in the notes attributable to changes in the benchmark interest rate are recorded in interest expense and offset by the fair value changes in the swap
98
contracts. In 2008, Old Merck terminated four interest rate swap contracts with notional amounts of $250�million each, and terminated one interest rate swap contract with a notional amount of $500�million. These swaps had effectively converted its $1.0�billion, 4.75% fixed-rate notes due 2015 and its $500�million, 4.375% fixed-rate notes due 2013 to variable rate debt. As a result of the swap terminations, Old Merck received $128.3�million in cash, excluding accrued interest which was not material. The corresponding gains related to the basis adjustment of the debt associated with the terminated swap contracts were deferred and are being amortized as a reduction of interest expense over the remaining term of the notes. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
The Company�s investment portfolio includes cash equivalents and short-term investments, the market values of which are not significantly affected by changes in interest rates. The market value of the Company�s medium- to long-term fixed-rate investments is modestly affected by changes in U.S.�interest rates. Changes in medium- to long-term U.S.�interest rates have a more significant impact on the market value of the Company�s fixed-rate borrowings, which generally have longer maturities. A sensitivity analysis to measure potential changes in the market value of Merck�s investments, debt and related swap contracts from a change in interest rates indicated that a one percentage point increase in interest rates at December�31, 2009 and 2008 would have positively affected the net aggregate market value of these instruments by $990.1�million and $98.9�million, respectively. A one percentage point decrease at December�31, 2009 and 2008 would have negatively affected the net aggregate market value by $1,152.7�million and $156.3�million, respectively. The increased sensitivity to interest rate movements from the prior year is attributable to the sale or redemption of long-term fixed rate investments to fund the merger, as well as an increase in debt, which included existing Schering-Plough debt as well as debt issued in 2009 to fund the Merger. The fair value of Merck�s debt was determined using pricing models reflecting one percentage point shifts in the appropriate yield curves. The fair values of the Merck�s investments were determined using a combination of pricing and duration models.
The Company�s consolidated financial statements include certain amounts that are based on management�s best estimates and judgments. Estimates are used when accounting for amounts recorded in connection with mergers and acquisitions, including fair value determinations of assets and liabilities. Additionally, estimates are used in determining such items as provisions for sales discounts and returns, depreciable and amortizable lives, recoverability of inventories, including those produced in preparation for product launches, amounts recorded for contingencies, environmental liabilities and other reserves, pension and other postretirement benefit plan assumptions, share-based compensation assumptions, restructuring costs, impairments of long-lived assets (including intangible assets and goodwill) and investments, and taxes on income. Because of the uncertainty inherent in such estimates, actual results may differ from these estimates. Application of the following accounting policies result in accounting estimates having the potential for the most significant impact on the financial statements.
On January�1, 2009, new guidance issued by the FASB was adopted which changes the way in which the acquisition method is to be applied in a business combination and also changes the way assets and liabilities are recognized in purchase accounting on a prospective basis. The acquisition method of accounting requires that the assets acquired and liabilities assumed be recorded at the date of acquisition at their respective fair values with limited exceptions. Assets acquired and liabilities assumed in a business combination that arise from contingencies are recognized at fair value if fair value can reasonably be estimated. If the acquisition date fair value of an asset acquired or liability assumed that arises from a contingency cannot be determined, the asset or liability is recognized if probable and reasonably estimable; if these criteria are not met, no asset or liability is recognized. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Accordingly, the Company may be required to value assets at fair value measures that do not reflect the Company�s intended use of those assets. Any excess of the purchase price (consideration transferred) over the estimated fair values of net assets acquired is recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred. If the Company determines the asset acquired does not meet the definition of a business under the acquisition method of accounting, the transaction will
99
be accounted for as an acquisition of assets rather than a business combination, and therefore, no goodwill will be recorded. The fair value of intangible assets, including acquired in-process research and development, is based on significant judgments made by management, and accordingly, for significant items, the Company typically obtains assistance from third party valuation specialists. Amounts are allocated to acquired in-process research and development are capitalized and accounted for similar to indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of each project, Merck will make a separate determination as to the then useful life of the asset and begin amortization. The valuations and useful life assumptions are based on information available near the merger or acquisition date and are based on expectations and assumptions that are deemed reasonable by management. The judgments made in determining estimated fair values assigned to assets acquired and liabilities assumed, as well as asset lives, can materially affect the Company�s results of operations.
The fair values of identifiable intangible assets related to currently marketed products and product rights are primarily determined by using an �income approach,� through which fair value is estimated based on each asset�s discounted projected net cash flows. The Company�s estimates of market participant net cash flows consider historical and projected pricing, margins and expense levels; the performance of competing products where applicable; relevant industry and therapeutic area growth drivers and factors; current and expected trends in technology and product life cycles; the time and investment that will be required to develop products and technologies; the ability to obtain marketing and regulatory approvals; the ability to manufacture and commercialize the products; the extent and timing of potential new product introductions by the Company�s competitors; and the life of each asset�s underlying patent, if any. The net cash flows are then probability-adjusted where appropriate to consider the uncertainties associated with the underlying assumptions, as well as the risk profile of the net cash flows utilized in the valuation. The probability-adjusted future net cash flows of each product are then discounted to present value utilizing an appropriate discount rate.
The fair values of identifiable intangible assets related to IPR&D are determined using an income approach, through which fair value is estimated based on each asset�s probability adjusted future net cash flows, which reflect the different stages of development of each product and the associated probability of successful completion. The net cash flows are then discounted to present value using an appropriate discount rate.
Revenues from sales of products are recognized at the time of delivery and when title and risk of loss passes to the customer. Recognition of revenue also requires reasonable assurance of collection of sales proceeds and completion of all performance obligations. Domestically, sales discounts are issued to customers as direct discounts at the point-of-sale or indirectly through an intermediary wholesaler, known as chargebacks, or indirectly in the form of rebates. Additionally, sales are generally made with a limited right of return under certain conditions. Revenues are recorded net of provisions for sales discounts and returns, which are established at the time of sale.
The provision for aggregate indirect customer discounts covers chargebacks and rebates. Chargebacks are discounts that occur when a contracted customer purchases directly through an intermediary wholesaler. The contracted customer generally purchases product at its contracted price plus a mark-up from the wholesaler. The wholesaler, in turn, charges the Company back for the difference between the price initially paid by the wholesaler and the contract price paid to the wholesaler by the customer. The provision for chargebacks is based on expected sell-through levels by the Company�s wholesale customers to contracted customers, as well as estimated wholesaler inventory levels. Rebates are amounts owed based upon definitive contractual agreements or legal requirements with private sector and public sector (Medicaid and Medicare Part�D)�benefit providers, after the final dispensing of the product by a pharmacy to a benefit plan participant. The provision is based on expected payments, which are driven by patient usage and contract performance by the benefit provider customers.
The Company uses historical customer segment mix, adjusted for other known events, in order to estimate the expected provision. Amounts accrued for aggregate indirect customer discounts are evaluated on a quarterly basis through comparison of information provided by the wholesalers, health maintenance organizations, pharmacy benefit managers and other customers to the amounts accrued. Adjustments are recorded when trends or significant events indicate that a change in the estimated provision is appropriate.
100
The Company continually monitors its provision for aggregate indirect customer discounts. There were no material adjustments to estimates associated with the aggregate indirect customer discount provision in 2009, 2008 or 2007.
Summarized information about changes in the aggregate indirect customer discount accrual is as follows:
| ($ in millions) | 2009 | 2008 | ||||||
Balance, January 1 | $ | 616.3 | $ | 699.4 | ||||
Current provision | 2,541.9 | 2,037.5 | ||||||
Schering-Plough accrual assumed in the Merger | 584.1 | � | ||||||
Adjustments to prior years | (22.3 | ) | (13.7 | ) | ||||
Payments | (2,347.4 | ) | (2,106.9 | ) | ||||
Balance, December 31 | $ | 1,372.6 | $ | 616.3 | ||||
| ||||||||
The Company maintains a returns policy that allows its customers to return product within a specified period prior to and subsequent to the expiration date (generally, three to six months before and twelve months after product expiration). The estimate of the provision for returns is based upon historical experience with actual returns. Additionally, the Company considers factors such as levels of inventory in the distribution channel, product dating and expiration period, whether products have been discontinued, entrance in the market of additional generic competition, changes in formularies or launch of over-the-counter products, among others. The product returns provision, as well as actual returns, were less than 1.0% of net sales in 2009, 2008 and 2007.
Through its distribution programs with U.S.�wholesalers, the Company encourages wholesalers to align purchases with underlying demand and maintain inventories below specified levels. The terms of the programs allow the wholesalers to earn fees upon providing visibility into their inventory levels as well as by achieving certain performance parameters, such as, inventory management, customer service levels, reducing shortage claims and reducing product returns. Information provided through the wholesaler distribution programs includes items such as sales trends, inventory on-hand, on-order quantity and product returns.
Wholesalers generally provide only the above mentioned data to the Company, as there is no regulatory requirement to report lot level information to manufacturers, which is the level of information needed to determine the remaining shelf life and original sale date of inventory. Given current wholesaler inventory levels, which are generally less than a month, the Company believes that collection of order lot information across all wholesale customers would have limited use in estimating sales discounts and returns.
The Company capitalizes inventories produced in preparation for product launches sufficient to support estimated initial market demand. Typically, capitalization of such inventory does not begin until the related product candidates are in Phase�III clinical trials and are considered to have a high probability of regulatory approval. The Company monitors the status of each respective product within the regulatory approval process; however, the Company generally does not disclose specific timing for regulatory approval. If the Company is aware of any specific risks or contingencies other than the normal regulatory approval process or if there are any specific issues identified during the research process relating to safety, efficacy, manufacturing, marketing or labeling, the related inventory would generally not be capitalized. Expiry dates of the inventory are affected by the stage of completion. The Company manages the levels of inventory at each stage to optimize the shelf life of the inventory in relation to anticipated market demand in order to avoid product expiry issues. For inventories that are capitalized, anticipated future sales and shelf lives support the realization of the inventory value as the inventory shelf life is sufficient to meet initial product launch requirements. Inventories produced in preparation for product launches capitalized at December�31, 2009 and 2008 were not significant.
101
The Company is involved in various claims and legal proceedings of a nature considered normal to its business, including product liability, intellectual property and commercial litigation, as well as additional matters such as antitrust actions. (See Note�12 to the consolidated financial statements.) The Company records accruals for contingencies when it is probable that a liability has been incurred and the amount can be reasonably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. For product liability claims, a portion of the overall accrual is actuarially determined and considers such factors as past experience, number of claims reported and estimates of claims incurred but not yet reported. Individually significant contingent losses are accrued when probable and reasonably estimable.
102
The Company and its subsidiaries are parties to a number of proceedings brought under the Comprehensive Environmental Response, Compensation and Liability Act, commonly known as Superfund, and other federal and state equivalents. When a legitimate claim for contribution is asserted, a liability is initially accrued based upon the estimated transaction costs to manage the site. Accruals are adjusted as site investigations, feasibility studies and related cost assessments of remedial techniques are completed, and as the extent to which other potentially responsible parties who may be jointly and severally liable can be expected to contribute is determined.
The Company is also remediating environmental contamination resulting from past industrial activity at certain of its sites and takes an active role in identifying and providing for these costs. In the past, Old Merck performed a worldwide survey to assess all sites for potential contamination resulting from past industrial activities. Where assessment indicated that physical investigation was warranted, such investigation was performed, providing a better evaluation of the need for remedial action. Where such need was identified, remedial action was then initiated. As definitive information became available during the course of investigations and/or remedial efforts at each site, estimates were refined and accruals were established or adjusted accordingly. These estimates and related accruals continue to be refined annually. A similar process is being followed for legacy Schering-Plough sites.
The Company believes that there are no compliance issues associated with applicable environmental laws and regulations that would have a material adverse effect on the Company. Expenditures for remediation and environmental liabilities were $16.6�million in 2009, and are estimated at $55�million for the years 2010 through 2014. In management�s opinion, the liabilities for all environmental matters that are probable and reasonably estimable have been accrued and totaled $161.8�million and $89.5�million at December�31, 2009 and December�31, 2008, respectively. These liabilities are undiscounted, do not consider potential recoveries from other parties and will be paid out over the periods of remediation for the applicable sites, which are expected to occur primarily over the next 15�years. Although it is not possible to predict with certainty the outcome of these matters, or the ultimate costs of remediation, management does not believe that any reasonably possible expenditures that may be incurred in excess of the liabilities accrued should exceed $170.0�million in the aggregate. Management also does not believe that these expenditures should result in a material adverse effect on the Company�s financial position, results of operations, liquidity or capital resources for any year.
The Company expenses all share-based payment awards to employees, including grants of stock options, over the requisite service period based on the grant date fair value of the awards. The Company determines the fair value of certain share-based awards using the Black-Scholes option-pricing model which uses both historical and current market data to estimate the fair value. This method incorporates various assumptions such as the risk-free interest rate, expected volatility, expected dividend yield and expected life of the options.
Net pension and other postretirement benefit cost totaled $511.1�million in 2009, $376.6�million in 2008 and $489.3�million in 2007. The increase in 2009 as compared with 2008 is primarily due to $118.2�million of costs associated with Schering-Plough benefit plans from the date of the Merger through December�31, 2009. The decrease in 2008 as compared with 2007 is primarily due to the lower amortization of actuarial net losses and higher expected return on plan assets which were partially offset by an increase in termination benefits attributable to restructuring actions. Pension and other postretirement benefit plan information for financial reporting purposes is calculated using actuarial assumptions including a discount rate for plan benefit obligations and an expected rate of return on plan assets.
The Company reassesses its benefit plan assumptions on a regular basis. For both the pension and other postretirement benefit plans, the discount rate is evaluated on measurement dates and modified to reflect the
103
prevailing market rate of a portfolio of high-quality fixed-income debt instruments that would provide the future cash flows needed to pay the benefits included in the benefit obligation as they come due. At December�31, 2009, the discount rates for the Company�s U.S.�pension and other postretirement benefit plans ranged from 4.60% to 6.00% compared with a range of 6.00% to 6.40% at December�31, 2008.
The expected rate of return for both the pension and other postretirement benefit plans represents the average rate of return to be earned on plan assets over the period the benefits included in the benefit obligation are to be paid. In developing the expected rate of return, the Company considers long-term compound annualized returns of historical market data as well as actual returns on the Company�s plan assets. Using this reference information, the Company develops forward-looking return expectations for each asset category and a weighted average expected long-term rate of return for a target portfolio allocated across these investment categories. The expected portfolio performance reflects the contribution of active management as appropriate. As a result of this analysis, for 2010, the Company�s expected rate of return will range from 8.00% to 8.75% compared to a range of 7.50% to 8.75% in 2009 for its U.S.�pension and other postretirement benefit plans.
The Company has established investment guidelines for its U.S. pension and other postretirement plans to create an asset allocation that is expected to deliver a rate of return sufficient to meet the long-term obligation of each plan, given an acceptable level of risk. The target investment portfolio of the Company�s U.S.�pension and other postretirement benefit plans is allocated 45% to 60% in U.S.�equities, 20% to 30% in international equities, 15% to 25% in fixed-income investments, and up to 8% in cash and other investments. The portfolio�s equity weighting is consistent with the long-term nature of the plans� benefit obligations. The expected annual standard deviation of returns of the target portfolio, which approximates 13%, reflects both the equity allocation and the diversification benefits among the asset classes in which the portfolio invests. For non-U.S.�pension plans, the targeted investment portfolio varies based on the duration of pension liabilities and local government rules and regulations. Although a significant percentage of plan assets are invested in U.S.�equities, concentration risk is mitigated through the use of strategies that are diversified within management guidelines.
Actuarial assumptions are based upon management�s best estimates and judgment. A reasonably possible change of plus (minus) 25�basis points in the discount rate assumption, with other assumptions held constant, would have an estimated $54.3�million favorable (unfavorable) impact on its net pension and postretirement benefit cost. A reasonably possible change of plus (minus) 25�basis points in the expected rate of return assumption, with other assumptions held constant, would have an estimated $23.9�million favorable (unfavorable) impact on its net pension and postretirement benefit cost. Required funding obligations for 2010 relating to the Company�s pension and other postretirement benefit plans are not expected to be material. The preceding hypothetical changes in the discount rate and expected rate of return assumptions would not impact the Company�s funding requirements.
104
The Company assesses changes in economic, regulatory and legal conditions and makes assumptions regarding estimated future cash flows in evaluating the value of the Company�s property, plant and equipment, goodwill and other intangible assets.
The Company periodically evaluates whether current facts or circumstances indicate that the carrying values of its long-lived assets to be held and used may not be recoverable. If such circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets, or appropriate asset groupings, is compared to the carrying value to determine whether an impairment exists. If the asset is determined to be impaired, the loss is measured based on the difference between the asset�s fair value and its carrying value. If quoted market prices are not available, the Company will estimate fair value using a discounted value of estimated future cash flows approach.
The Company tests its goodwill for impairment at least annually using a fair value based test. Goodwill represents the excess of the consideration transferred over the fair value of net assets of businesses purchased and is assigned to reporting units. Other acquired intangibles (excluding in-process research and development) are recorded at fair value and amortized on a straight-line basis over their estimated useful lives. When events or circumstances warrant a review, the Company will assess recoverability from future operations using pretax undiscounted cash flows derived from the lowest appropriate asset groupings. Impairments are recognized in operating results to the extent that the carrying value of the intangible asset exceeds its fair value, which is determined based on the net present value of estimated cash flows.
The Company tests its indefinite-lived intangibles, including in-process research and development, for impairment at least annually, through a one-step test that compares the fair value of the indefinite lived intangible asset with the asset�s carrying value. For impairment testing purposes, the Company may combine separately recorded indefinite-lived intangible assets into one unit of account based on the relevant facts and circumstances. Generally, the Company will combine indefinite-lived intangible assets for testing purposes if they operate as a single asset and are essentially inseparable. If the fair value is less than the carrying amount, an impairment loss is recognized within the Company�s operating results.
The Company reviews its investments for impairments based on the determination of whether the decline in market value of the investment below the carrying value is other-than-temporary. The Company considers available evidence in evaluating potential impairments of its investments, including the duration and extent to which fair value is less than cost, and for equity securities, the Company�s ability and intent to hold the investments. On April�1, 2009, new authoritative guidance from the FASB was adopted which amended the other-than-temporary recognition guidance for debt securities. Pursuant to this new guidance, an other-than-temporary impairment has occurred if the Company does not expect to recover the entire amortized cost basis of the debt security. If the Company does not intend to sell the impaired debt security, and it is not more likely than not it will be required to sell the debt security before the recovery of its amortized cost basis, the amount of the other-than-temporary impairment recognized in earnings is limited to the portion attributed to credit loss. The remaining portion of the other-than-temporary impairment related to other factors is recognized in other comprehensive income.
The Company�s effective tax rate is based on pretax income, statutory tax rates and tax planning opportunities available in the various jurisdictions in which the Company operates. An estimated effective tax rate for a year is applied to the Company�s quarterly operating results. In the event that there is a significant unusual or one-time item recognized, or expected to be recognized, in the Company�s quarterly operating results, the tax attributable to that item would be separately calculated and recorded at the same time as the unusual or one-time item. The Company considers the resolution of prior year tax matters to be such items. Significant judgment is required in determining the Company�s tax provision and in evaluating its tax positions. The recognition and measurement of a tax position is based on management�s best judgment given the facts, circumstances and information available at the reporting date. The Company evaluates tax positions to determine whether the benefits of tax positions are more likely than not of being sustained upon audit based on the technical merits of the tax
105
position. For tax positions that are more likely than not of being sustained upon audit, the Company recognizes the largest amount of the benefit that is greater than 50% likely of being realized upon ultimate settlement in the financial statements. For tax positions that are not more likely than not of being sustained upon audit, the Company does not recognize any portion of the benefit in the financial statements. If the more likely than not threshold is not met in the period for which a tax position is taken, the Company may subsequently recognize the benefit of that tax position if the tax matter is effectively settled, the statute of limitations expires, or if the more likely than not threshold is met in a subsequent period. (See Note�17 to the consolidated financial statements.)
Tax regulations require items to be included in the tax return at different times than the items are reflected in the financial statements. Timing differences create deferred tax assets and liabilities. Deferred tax assets generally represent items that can be used as a tax deduction or credit in the tax return in future years for which the Company has already recorded the tax benefit in the financial statements. The Company establishes valuation allowances for its deferred tax assets when the amount of expected future taxable income is not likely to support the use of the deduction or credit. Deferred tax liabilities generally represent tax expense recognized in the financial statements for which payment has been deferred or expense for which the Company has already taken a deduction on the tax return, but has not yet recognized as expense in the financial statements. At December�31, 2009, foreign earnings of $31.2�billion have been retained indefinitely by subsidiary companies for reinvestment, therefore no provision has been made for income taxes that would be payable upon the distribution of such earnings.
In June 2009, the FASB issued an amendment to the accounting and disclosure requirements for transfers of financial assets, which is effective January�1, 2010. The amendment eliminates the concept of a qualifying special-purpose entity, changes the requirements for derecognizing financial assets and requires enhanced disclosures to provide financial statement users with greater transparency about transfers of financial assets, including securitization transactions, and an entity�s continuing involvement in and exposure to the risks related to transferred financial assets. The effect of adoption on the Company�s financial position and results of operations is not expected to be material.
Also in June 2009, the FASB amended the existing accounting and disclosure guidance for the consolidation of variable interest entities, which is effective January�1, 2010. The amended guidance requires enhanced disclosures intended to provide users of financial statements with more transparent information about an enterprise�s involvement in a variable interest entity. The effect of adoption on the Company�s financial position and results of operations is not expected to be material.
In October 2009, the FASB issued new guidance for revenue recognition with multiple deliverables, which is effective for revenue arrangements entered into or materially modified in fiscal years beginning on or after June�15, 2010, although early adoption is permitted. This guidance eliminates the residual method under the current guidance and replaces it with the �relative selling price� method when allocating revenue in a multiple deliverable arrangement. The selling price for each deliverable shall be determined using vendor specific objective evidence of selling price, if it exists, otherwise third-party evidence of selling price shall be used. If neither exists for a deliverable, the vendor shall use its best estimate of the selling price for that deliverable. After adoption, this guidance will also require expanded qualitative and quantitative disclosures. The Company is currently assessing the impact of adoption on its financial position and results of operations.
In January 2010, the FASB amended the existing disclosure guidance on fair value measurements, which is effective January�1, 2010, except for disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level�3 fair value measurements, which is effective January�1, 2011. Among other things, the updated guidance requires additional disclosure for the amounts of significant transfers in and out of Level�1 and Level�2 measurements and requires certain Level�3 disclosures on a gross basis. Additionally, the updates amend existing guidance to require a greater level of disaggregated information and more robust disclosures about valuation techniques and inputs to fair value measurements. Since the amended guidance requires only additional disclosures, the adoption will not affect the Company�s financial position or results of operations.
106
This report and other written reports and oral statements made from time to time by the Company may contain so-called �forward-looking statements,� all of which are based on management�s current expectations and are subject to risks and uncertainties which may cause results to differ materially from those set forth in the statements. One can identify these forward-looking statements by their use of words such as �expects,� �plans,� �will,� �estimates,� �forecasts,� �projects� and other words of similar meaning. One can also identify them by the fact that they do not relate strictly to historical or current facts. These statements are likely to address the Company�s growth strategy, financial results, product development, product approvals, product potential and development programs. One must carefully consider any such statement and should understand that many factors could cause actual results to differ materially from the Company�s forward-looking statements. These factors include inaccurate assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward-looking statement can be guaranteed and actual future results may vary materially.
The Company does not assume the obligation to update any forward-looking statement. One should carefully evaluate such statements in light of factors, including risk factors, described in the Company�s filings with the Securities and Exchange Commission, especially on Forms�10-K, 10-Q and 8-K. In Item�1A. �Risk Factors� of this annual report on Form�10-K the Company discusses in more detail various important risk factors that could cause actual results to differ from expected or historic results. The Company notes these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. One should understand that it is not possible to predict or identify all such factors. Consequently, the reader should not consider any such list to be a complete statement of all potential risks or uncertainties.
| Item�7A. | Quantitative and Qualitative Disclosures about Market Risk. |
The information required by this Item is incorporated by reference to the discussion under �Financial Instruments Market Risk Disclosures� in Item�7. �Management�s Discussion and Analysis of Financial Condition and Results of Operations.�
107
| Item�8. | Financial Statements and Supplementary Data. |
| (a) | Financial Statements |
The consolidated balance sheet of Merck�& Co., Inc. and subsidiaries as of December�31, 2009 and 2008, and the related consolidated statements of income, of equity and of cash flows for each of the three years in the period ended December�31, 2009, the notes to consolidated financial statements, and the report dated February�26, 2010 of PricewaterhouseCoopers LLP, independent registered public accounting firm, are as follows:
Merck�& Co., Inc. and Subsidiaries
| 2009 | 2008 | 2007 | ||||||||||
Sales | $ | 27,428.3 | $ | 23,850.3 | $ | 24,197.7 | ||||||
Costs, Expenses and Other | ||||||||||||
Materials and production | 9,018.9 | 5,582.5 | 6,140.7 | |||||||||
Marketing and administrative | 8,543.2 | 7,377.0 | 7,556.7 | |||||||||
Research and development | 5,845.0 | 4,805.3 | 4,882.8 | |||||||||
Restructuring costs | 1,633.9 | 1,032.5 | 327.1 | |||||||||
Equity income from affiliates | (2,235.0 | ) | (2,560.6 | ) | (2,976.5 | ) | ||||||
U.S. Vioxx Settlement Agreement charge | - | - | 4,850.0 | |||||||||
Other (income) expense, net | (10,669.5 | ) | (2,318.1 | ) | (75.2 | ) | ||||||
| 12,136.5 | 13,918.6 | 20,705.6 | ||||||||||
Income Before Taxes | 15,291.8 | 9,931.7 | 3,492.1 | |||||||||
Taxes on Income | 2,267.6 | 1,999.4 | 95.3 | |||||||||
Net Income | 13,024.2 | 7,932.3 | 3,396.8 | |||||||||
Less: Net Income Attributable to Noncontrolling Interests | 122.9 | 123.9 | 121.4 | |||||||||
Net Income Attributable to Merck�& Co., Inc. | 12,901.3 | 7,808.4 | 3,275.4 | |||||||||
Preferred Stock Dividends | 2.1 | - | - | |||||||||
Net Income Available to Common Shareholders | $ | 12,899.2 | $ | 7,808.4 | $ | 3,275.4 | ||||||
| ||||||||||||
Basic Earnings per Common Share Available to Common Shareholders | $5.67 | $3.65 | $1.51 | |||||||||
| ||||||||||||
Earnings per Common Share Assuming Dilution Available to Common Shareholders | $5.65 | $3.63 | $1.49 | |||||||||
| ||||||||||||
108
Merck�& Co., Inc. and Subsidiaries
| 2009 | 2008 | |||||||
Assets | ||||||||
Current Assets | ||||||||
Cash and cash equivalents | $ | 9,311.4 | $ | 4,368.3 | ||||
Short-term investments | 293.1 | 1,118.1 | ||||||
Accounts receivable (net of allowance for doubtful accounts of
$112.6 in 2009 and $58.5 in 2008) | 6,602.9 | 2,907.7 | ||||||
Inventories (excludes inventories of $1,157.2 in 2009 and $587.3 in | ||||||||
2008 classified in Other assets�� see Note�8) | 8,055.3 | 2,091.0 | ||||||
Deferred income taxes and other current assets | 4,165.9 | 8,627.5 | ||||||
Total current assets | 28,428.6 | 19,112.6 | ||||||
Investments | 432.3 | 6,491.3 | ||||||
Property, Plant and Equipment (at cost) | ||||||||
Land | 666.7 | 386.1 | ||||||
Buildings | 12,210.3 | 9,767.4 | ||||||
Machinery, equipment and office furnishings | 16,173.6 | 13,103.7 | ||||||
Construction in progress | 1,817.5 | 871.0 | ||||||
| 30,868.1 | 24,128.2 | |||||||
Less allowance for depreciation | 12,594.6 | 12,128.6 | ||||||
| 18,273.5 | 11,999.6 | |||||||
Goodwill | 11,923.1 | 1,438.7 | ||||||
Other Intangibles, Net | 47,655.8 | 525.4 | ||||||
Other Assets | 5,376.4 | 7,628.1 | ||||||
| $ | 112,089.7 | $ | 47,195.7 | |||||
| ||||||||
Liabilities and Equity | ||||||||
Current Liabilities | ||||||||
Loans payable and current portion of long-term debt | 1,379.2 | $ | 2,297.1 | |||||
Trade accounts payable | 2,236.9 | 617.6 | ||||||
Accrued and other current liabilities | 9,453.8 | 9,174.1 | ||||||
Income taxes payable | 1,285.2 | 1,426.4 | ||||||
Dividends payable | 1,189.0 | 803.5 | ||||||
6% Mandatory convertible preferred stock, $1�par value | ||||||||
Authorized�� 11,500,000�shares; issued and outstanding�� 855,422�shares | 206.6 | - | ||||||
Total current liabilities | 15,750.7 | 14,318.7 | ||||||
Long-Term Debt | 16,074.9 | 3,943.3 | ||||||
Deferred Income Taxes and Noncurrent Liabilities | 18,771.5 | 7,766.6 | ||||||
Merck�& Co., Inc. Stockholders� Equity | ||||||||
Common stock, $0.50�par value�� 2009; $0.01�par value�� 2008 | ||||||||
Authorized�� 6,500,000,000�shares�� 2009; 5,400,000,000�shares�� 2008 | ||||||||
Issued�� 3,562,528,536�shares�� 2009; 2,983,508,675�� 2008 | 1,781.3 | 29.8 | ||||||
Other paid-in capital | 39,682.6 | 8,319.1 | ||||||
Retained earnings | 41,404.9 | 43,698.8 | ||||||
Accumulated other comprehensive loss | (2,766.5 | ) | (2,553.9 | ) | ||||
| 80,102.3 | 49,493.8 | |||||||
Less treasury stock, at cost: | ||||||||
454,305,985�shares�� 2009; | ||||||||
875,818,333�shares�� 2008 | 21,044.3 | 30,735.5 | ||||||
Total Merck�& Co., Inc. stockholders� equity | 59,058.0 | 18,758.3 | ||||||
Noncontrolling Interests | 2,434.6 | 2,408.8 | ||||||
Total equity | 61,492.6 | 21,167.1 | ||||||
| $ | 112,089.7 | $ | 47,195.7 | |||||
| ||||||||
109
Merck�& Co., Inc. and Subsidiaries
| Accumulated | ||||||||||||||||||||||||||||
| Other | Other | Non- | ||||||||||||||||||||||||||
| Common | Paid-In | Retained | Comprehensive | Treasury | controlling | |||||||||||||||||||||||
| Stock | Capital | Earnings | Loss | Stock | Interests | Total | ||||||||||||||||||||||
Balance at January�1, 2007 | $ | 29.8 | $ | 7,166.5 | $ | 39,095.1 | $ | (1,164.3 | ) | $ | (27,567.4 | ) | $ | 2,406.1 | $ | 19,965.8 | ||||||||||||
Net income attributable to Merck�& Co., Inc. | 3,275.4 | 3,275.4 | ||||||||||||||||||||||||||
Total other comprehensive income, net of tax | 338.2 | 338.2 | ||||||||||||||||||||||||||
Comprehensive income, net of tax | 3,613.6 | |||||||||||||||||||||||||||
Cumulative effect of adoption of guidance on accounting for unrecognized tax benefits | 81.0 | 81.0 | ||||||||||||||||||||||||||
Cash dividends declared on common stock ($1.52 per share) | (3,310.7 | ) | (3,310.7 | ) | ||||||||||||||||||||||||
Treasury stock shares purchased | (1,429.7 | ) | (1,429.7 | ) | ||||||||||||||||||||||||
Acquisition of NovaCardia, Inc. | 366.4 | 366.4 | ||||||||||||||||||||||||||
Net income attributable to noncontrolling interests | 121.4 | 121.4 | ||||||||||||||||||||||||||
Distributions attributable to noncontrolling interests | (120.8 | ) | (120.8 | ) | ||||||||||||||||||||||||
Share-based compensation plans and other | 482.0 | 822.4 | 1,304.4 | |||||||||||||||||||||||||
Balance at December�31, 2007 | 29.8 | 8,014.9 | 39,140.8 | (826.1 | ) | (28,174.7 | ) | 2,406.7 | 20,591.4 | |||||||||||||||||||
Net income attributable to Merck�& Co., Inc. | 7,808.4 | 7,808.4 | ||||||||||||||||||||||||||
Total other comprehensive loss, net of tax | (1,727.8 | ) | (1,727.8 | ) | ||||||||||||||||||||||||
Comprehensive income, net of tax | 6,080.6 | |||||||||||||||||||||||||||
Cash dividends declared on common stock ($1.52 per share) | (3,250.4 | ) | (3,250.4 | ) | ||||||||||||||||||||||||
Treasury stock shares purchased | (2,725.0 | ) | (2,725.0 | ) | ||||||||||||||||||||||||
Net income attributable to noncontrolling interests | 123.9 | 123.9 | ||||||||||||||||||||||||||
Distributions attributable to noncontrolling interests | (121.8 | ) | (121.8 | ) | ||||||||||||||||||||||||
Share-based compensation plans and other | 304.2 | 164.2 | 468.4 | |||||||||||||||||||||||||
Balance at December�31, 2008 | 29.8 | 8,319.1 | 43,698.8 | (2,553.9 | ) | (30,735.5 | ) | 2,408.8 | 21,167.1 | |||||||||||||||||||
Net income attributable to Merck�& Co.,
Inc. | 12,901.3 | 12,901.3 | ||||||||||||||||||||||||||
Total other comprehensive loss, net of tax | (212.6 | ) | (212.6 | ) | ||||||||||||||||||||||||
Comprehensive income, net of tax | 12,688.7 | |||||||||||||||||||||||||||
Schering-Plough merger | 1,752.0 | 30,860.7 | (1,964.1 | ) | 22.3 | 30,670.9 | ||||||||||||||||||||||
Cancellations of treasury stock | (4.9 | ) | (11,595.4 | ) | 11,600.3 | - | ||||||||||||||||||||||
Preferred stock conversions | 0.1 | 5.4 | 5.5 | |||||||||||||||||||||||||
Cash dividends declared on common stock ($1.52 per share) | (3,597.7 | ) | (3,597.7 | ) | ||||||||||||||||||||||||
Net income attributable to noncontrolling | ||||||||||||||||||||||||||||
interests | 122.9 | 122.9 | ||||||||||||||||||||||||||
Distributions attributable to noncontrolling interests | (119.4 | ) | (119.4 | ) | ||||||||||||||||||||||||
Share-based compensation plans and other | 4.3 | 497.4 | (2.1 | ) | 55.0 | 554.6 | ||||||||||||||||||||||
Balance at December�31, 2009 | $ | 1,781.3 | $ | 39,682.6 | $ | 41,404.9 | $ | (2,766.5 | ) | $ | (21,044.3 | ) | $ | 2,434.6 | $ | 61,492.6 | ||||||||||||
| ||||||||||||||||||||||||||||
110
Merck�& Co., Inc. and Subsidiaries
| 2009 | 2008 | 2007 | ||||||||||
Cash Flows from Operating Activities | ||||||||||||
Net income | $ | 13,024.2 | $ | 7,932.3 | $ | 3,396.8 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Gain related to Merck/Schering-Plough partnership | (7,529.6 | ) | - | - | ||||||||
Gain on disposition of interest in Merial Limited | (3,162.5 | ) | - | - | ||||||||
Gain on distribution from AstraZeneca LP | - | (2,222.7 | ) | - | ||||||||
Equity income from affiliates | (2,235.0 | ) | (2,560.6 | ) | (2,976.5 | ) | ||||||
Dividends and distributions from equity affiliates | 1,724.3 | 4,289.6 | 2,485.6 | |||||||||
U.S. Vioxx Settlement Agreement charge | - | - | 4,850.0 | |||||||||
Depreciation and amortization | 2,576.0 | 1,631.2 | 1,988.2 | |||||||||
Deferred income taxes | 1,820.2 | 530.1 | (1,781.9 | ) | ||||||||
Share-based compensation | 415.5 | 348.0 | 330.2 | |||||||||
In-process research and development | - | - | 325.1 | |||||||||
Taxes paid for Internal Revenue Service settlement | - | - | (2,788.1 | ) | ||||||||
Other | (534.2 | ) | 607.8 | (186.1 | ) | |||||||
Net changes in assets and liabilities: | ||||||||||||
Accounts receivable | 165.2 | (889.4 | ) | (290.7 | ) | |||||||
Inventories | 1,211.3 | (452.1 | ) | (40.7 | ) | |||||||
Trade accounts payable | (45.2 | ) | - | 117.7 | ||||||||
Accrued and other current liabilities | (4,003.5 | ) | (1,710.9 | ) | 451.1 | |||||||
Income taxes payable | (364.8 | ) | (465.3 | ) | 987.2 | |||||||
Noncurrent liabilities | 231.3 | (108.0 | ) | 26.2 | ||||||||
Other | 98.8 | (358.3 | ) | 105.1 | ||||||||
Net Cash Provided by Operating Activities | 3,392.0 | 6,571.7 | 6,999.2 | |||||||||
Cash Flows from Investing Activities | ||||||||||||
Capital expenditures | (1,460.6 | ) | (1,298.3 | ) | (1,011.0 | ) | ||||||
Purchases of securities and other investments | (3,070.8 | ) | (11,967.3 | ) | (10,132.7 | ) | ||||||
Proceeds from sales of securities and other investments | 10,941.9 | 11,065.8 | 10,860.2 | |||||||||
Proceeds from sale of interest in Merial Limited | 4,000.0 | - | - | |||||||||
Schering-Plough merger, net of cash acquired | (12,842.6 | ) | - | - | ||||||||
Acquisitions of businesses, net of cash acquired | (130.0 | ) | - | (1,135.9 | ) | |||||||
Distribution from AstraZeneca LP | - | 1,899.3 | - | |||||||||
Increase in restricted assets | 5,547.6 | (1,629.7 | ) | (1,401.1 | ) | |||||||
Other | 170.5 | 95.8 | 10.5 | |||||||||
Net Cash Provided by (Used by) Investing Activities | 3,156.0 | (1,834.4 | ) | (2,810.0 | ) | |||||||
Cash Flows from Financing Activities | ||||||||||||
Net change in short-term borrowings | (2,422.0 | ) | 1,859.9 | 11.4 | ||||||||
Proceeds from issuance of debt | 4,228.0 | - | - | |||||||||
Payments on debt | (25.3 | ) | (1,392.0 | ) | (1,195.3 | ) | ||||||
Purchases of treasury stock | - | (2,725.0 | ) | (1,429.7 | ) | |||||||
Dividends paid to stockholders | (3,215.0 | ) | (3,278.5 | ) | (3,307.3 | ) | ||||||
Other dividends paid | (264.1 | ) | (121.8 | ) | (120.8 | ) | ||||||
Proceeds from exercise of stock options | 186.4 | 102.3 | 898.6 | |||||||||
Other | (126.3 | ) | 32.6 | 277.0 | ||||||||
Net Cash Used by Financing Activities | (1,638.3 | ) | (5,522.5 | ) | (4,866.1 | ) | ||||||
Effect of Exchange Rate Changes on Cash and Cash Equivalents | 33.4 | (182.6 | ) | 98.3 | ||||||||
Net Increase (Decrease) in Cash and Cash Equivalents | 4,943.1 | (967.8 | ) | (578.6 | ) | |||||||
Cash and Cash Equivalents at Beginning of Year | 4,368.3 | 5,336.1 | 5,914.7 | |||||||||
Cash and Cash Equivalents at End of Year | $ | 9,311.4 | $ | 4,368.3 | $ | 5,336.1 | ||||||
| ||||||||||||
Supplemental Cash Flow Information (See Note�3) | ||||||||||||
111
Merck�& Co., Inc. and Subsidiaries
| 1. | Nature of Operations |
On November�3, 2009, Merck�& Co., Inc. (�Old Merck�) and Schering-Plough Corporation (�Schering-Plough�) completed their previously-announced merger (the �Merger�). In the Merger, Schering-Plough acquired all of the shares of Old Merck, which became a wholly-owned subsidiary of Schering-Plough and was renamed Merck Sharp�& Dohme Corp. Schering-Plough continued as the surviving public company and was renamed Merck�& Co., Inc. (�New Merck� or the �Company�). However, for accounting purposes only, the Merger was treated as an acquisition with Old Merck considered the accounting acquirer. Accordingly, the accompanying financial statements reflect Old Merck�s stand-alone operations as they existed prior to the completion of the Merger. References in these financial statements to �Merck� for periods prior to the Merger refer to Old Merck and for periods after the completion of the Merger to New Merck.
The results of Schering-Plough�s business have been included in New Merck�s financial statements only for periods subsequent to the completion of the Merger. Therefore, New Merck�s financial results for 2009 do not reflect a full year of legacy Schering-Plough operations. The Merger resulted in the inclusion of Schering-Plough�s assets and liabilities as of the merger date at their respective fair values with limited exceptions. Accordingly, the Merger materially affected Merck�s results of operations and financial position (see Note�3).
The Company is a global health care company that delivers innovative health solutions through its medicines, vaccines, biologic therapies, and consumer and animal products, which it markets directly and through its joint ventures. The Company�s operations are principally managed on a products basis and are comprised of one reportable segment, which is the Pharmaceutical segment. The Pharmaceutical segment includes human health pharmaceutical and vaccine products marketed either directly by the Company or through joint ventures. Human health pharmaceutical products consist of therapeutic and preventive agents, sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Vaccine products consist of preventive pediatric, adolescent and adult vaccines, primarily administered at physician offices. The Company sells these human health vaccines primarily to physicians, wholesalers, physician distributors and government entities. The Company�s professional representatives communicate the effectiveness, safety and value of its pharmaceutical and vaccine products to health care professionals in private practice, group practices and managed care organizations. The Company also has animal health operations that discover, develop, manufacture and market animal health products, including vaccines. The Company�s professional representatives communicate the safety and value of the Company�s animal health products to veterinarians, distributors and animal producers. Additionally, the Company has consumer health care operations that develop, manufacture and market Over-the-Counter, foot care and sun care products, which are sold through wholesale and retail drug, food chain and mass merchandiser outlets in the United States and Canada.
| 2. | Summary of Accounting Policies |
112
business combination. The acquisition method of accounting requires that the assets acquired and liabilities assumed be recorded at the date of the merger or acquisition at their respective fair values with limited exceptions. Assets acquired and liabilities assumed in a business combination that arise from contingencies are recognized at fair value if fair value can reasonably be estimated. If the acquisition date fair value of an asset acquired or liability assumed that arises from a contingency cannot be determined, the asset or liability is recognized if probable and reasonably estimable; if these criteria are not met, no asset or liability is recognized. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Accordingly, the Company may be required to value assets at fair value measures that do not reflect the Company�s intended use of those assets. Any excess of the purchase price (consideration transferred) over the estimated fair values of net assets acquired is recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred. The operating results of the acquired business are reflected in the Company�s consolidated financial statements and results of operations after the date of the merger or acquisition. If the Company determines the assets acquired do not meet the definition of a business under the acquisition method of accounting, the transaction will be accounted for as an acquisition of assets rather than a business combination, and therefore, no goodwill will be recorded.
113
114
Merck will make a determination as to the useful life of the intangible asset and begin amortization. The Company tests its indefinite-lived intangibles, including in-process research and development, for impairment at least annually, through a one-step test that compares the fair value of the indefinite-lived intangible asset with the asset�s carrying value. IPR&D increased as a result of the Merger (see Notes�3 and 9).
On January�1, 2009, new guidance on business combinations was adopted which changes the way in which the acquisition method is to be applied in a business combination. This guidance requires an acquirer to recognize the assets acquired and liabilities assumed at the acquisition date fair values with limited exceptions. Additionally, the guidance requires that contingent consideration be recorded at fair value on the acquisition date, that acquired in-process research and development be capitalized and recorded as intangible assets at the acquisition
115
date, and also requires transaction costs and costs to restructure the acquired company be expensed. On April�1, 2009, additional guidance was issued further amending the accounting for contingencies in a business combination. The Company�s business combination transactions are now being accounted for under this new guidance, including the Merger (see Note�3).
On January�1, 2009, new guidance for the accounting, reporting and disclosure of noncontrolling interests was adopted which requires, among other things, that noncontrolling interests be recorded as equity in the consolidated financial statements. The adoption of this new guidance resulted in the reclassification of $2.4�billion of Noncontrolling interests (formerly referred to as minority interests) to a separate component of equity on the Consolidated Balance Sheet (see Note�13). Additionally, net income attributable to noncontrolling interests is now shown separately from parent net income in the Consolidated Statement of Income. Prior periods have been restated to reflect the presentation and disclosure requirements of the new guidance.
On January�1, 2009, new guidance was adopted requiring enhanced disclosures about derivative instruments and hedging activities to allow for a better understanding of their effects on an entity�s financial position, financial performance, and cash flows. Among other things, the new guidance requires disclosure of the fair values of derivative instruments and associated gains and losses in a tabular format (see Note�7). Since the new guidance requires only additional disclosures about derivatives and hedging activities, the adoption did not affect Merck�s financial position or results of operations.
On January�1, 2009, new guidance was adopted which clarifies that unvested share-based payment awards that contain nonforfeitable rights to dividends or dividend equivalents (whether paid or unpaid) are considered participating securities and shall be included in the computation of earnings per share pursuant to the two class method. The effect of adoption was not material to Merck�s results of operations. The provisions of the guidance are retrospective; therefore prior periods have been restated (see Note�18).
On January�1, 2009, new guidance was adopted which defines collaborative arrangements and establishes reporting requirements for transactions between participants in a collaborative arrangement and between participants in the arrangement and third parties. The effect of adoption was not material to Merck�s financial position or results of operations. See Note�6 for the associated disclosures of the Company�s collaborative arrangements.
On January�1, 2009, new guidance was adopted which clarifies the accounting for certain transactions and impairment considerations involving equity method investments and is effective on a prospective basis.
On January�1, 2009, new guidance was adopted which clarifies that a defensive intangible asset (an intangible asset that the entity does not intend to actively use, but intends to hold to prevent others from obtaining access to the asset) should be accounted for as a separate unit of accounting and should be assigned a useful life that reflects the entity�s consumption of the expected benefits related to the asset. This guidance is effective on a prospective basis.
On April�1, 2009, new guidance was adopted which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. This guidance was subsequently amended on February 24, 2010 to no longer require disclosure of the date through which an entity has evaluated subsequent events. The effect of adoption was not material.
On April�1, 2009, new guidance was adopted which provides additional guidelines for estimating fair value when there has been a significant decrease in the volume and level of activity for an asset or liability in relation to the normal market activity for the asset or liability (or similar assets or liabilities). In addition, the new guidance includes guidelines for identifying circumstances that indicate a transaction for the asset or liability is not orderly, in which case the entity shall place little, if any, weight on that transaction price as an indicator of fair value. The effect of adoption on Merck�s financial position and results of operations was not material.
On April�1, 2009, new guidance was adopted which amended the other-than-temporary recognition guidance for debt securities. The impairment model for equity securities was not affected. An impairment exists when the current fair value of an individual security is less than its amortized cost basis. Pursuant to this new guidance, an other-than-temporary impairment has occurred if the Company does not expect to recover the entire amortized cost basis of the debt security. If the Company does not intend to sell the impaired debt security, and it is
116
As of December�31, 2009, the Company adopted new guidance amending existing authoritative literature which provides guidance on an employer�s disclosures about plan assets of defined pension or other postretirement benefit plans. The amended guidance requires disclosures about plan assets including how investment allocation decisions are made, the major categories of plan assets, the inputs and valuation techniques used to measure the fair value of plan assets, the effect of fair value measurements using significant unobservable inputs (Level�3)�on changes in plan assets for the period, and significant concentrations of risk within plan assets. Since the amended guidance required only additional disclosures about the Company�s pension and other postretirement plan assets (see Note�15), the adoption did not affect the Company�s financial position or results of operations.
In June 2009, the FASB issued an amendment to the accounting and disclosure requirements for transfers of financial assets, which is effective January�1, 2010. The amendment eliminates the concept of a qualifying special-purpose entity, changes the requirements for derecognizing financial assets and requires enhanced disclosures to provide financial statement users with greater transparency about transfers of financial assets, including securitization transactions, and an entity�s continuing involvement in and exposure to the risks related to transferred financial assets. The effect of adoption on the Company�s financial position and results of operations is not expected to be material.
Also in June 2009, the FASB amended the existing accounting and disclosure guidance for the consolidation of variable interest entities, which is effective January�1, 2010. The amended guidance requires enhanced disclosures intended to provide users of financial statements with more transparent information about an enterprise�s involvement in a variable interest entity. The effect of adoption on the Company�s financial position and results of operations is not expected to be material.
In October 2009, the FASB issued new guidance for revenue recognition with multiple deliverables, which is effective for revenue arrangements entered into or materially modified in fiscal years beginning on or after June�15, 2010, although early adoption is permitted. This guidance eliminates the residual method under the current guidance and replaces it with the �relative selling price� method when allocating revenue in a multiple deliverable arrangement. The selling price for each deliverable shall be determined using vendor specific objective evidence of selling price, if it exists, otherwise third-party evidence of selling price shall be used. If neither exists for a deliverable, the vendor shall use its best estimate of the selling price for that deliverable. After adoption, this guidance will also require expanded qualitative and quantitative disclosures. The Company is currently assessing the impact of adoption on its financial position and results of operations.
In January 2010, the FASB amended the existing disclosure guidance on fair value measurements, which is effective January�1, 2010, except for disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level�3 fair value measurements, which is effective January�1, 2011. Among other things, the updated guidance requires additional disclosure for the amounts of significant transfers in and out of Level�1 and Level�2 measurements and requires certain Level�3 disclosures on a gross basis. Additionally, the updates amend existing guidance to require a greater level of disaggregated information and more robust disclosures about valuation techniques and inputs to fair value measurements. Since the amended guidance requires only additional disclosures, the adoption will not impact the Company�s financial position or results of operations.
117
| 3. | Merger with Schering-Plough Corporation |
On November�3, 2009, Old Merck and Schering-Plough completed the Merger. In the Merger, Schering-Plough acquired all of the shares of Old Merck, which became a wholly-owned subsidiary of Schering-Plough and was renamed Merck Sharp�& Dohme Corp. Schering-Plough continued as the surviving public company and was renamed Merck�& Co., Inc. However, for accounting purposes only, the Merger was treated as an acquisition with Old Merck considered the accounting acquirer. Under the terms of the Merger agreement, each issued and outstanding share of Schering-Plough common stock was converted into the right to receive a combination of $10.50 in cash and 0.5767 of a share of the common stock of New Merck. Each issued and outstanding share of Old Merck common stock was automatically converted into a share of the common stock of New Merck. Based on the closing price of Old Merck stock on November�3, 2009, the consideration received by Schering-Plough shareholders was valued at $28.19 per share, or $49.6�billion in the aggregate. The cash portion of the consideration was funded with a combination of existing cash, including from the sale of Old Merck�s interest in Merial Limited, the sale or redemption of investments and the issuance of debt (see Note�11). Upon completion of the Merger, each issued and outstanding share of Schering-Plough 6% Mandatory Convertible Preferred Stock (�Schering-Plough 6% preferred stock�) not converted in accordance with the terms of the preferred stock remained outstanding as one share of Merck 6% Mandatory Convertible Preferred Stock (�6% preferred stock�) having the rights set forth in the New Merck certificate of incorporation (see Note�13)�which rights were substantially similar to the rights of the Schering-Plough 6% preferred stock.
The Merger expanded the Company�s pipeline of product candidates, broadened the Company�s commercial portfolio, expanded its global presence and increased its manufacturing capabilities. Additionally, the Company expects to realize substantial cost savings and synergies, including opportunities for consolidation in both sales and marketing and research and development.
Schering-Plough common stock shares outstanding at November�3, 2009 (net of treasury shares) | 1,641.1 | |||
Units of merger consideration arising from conversion of 6% preferred stock | 74.7 | (1) | ||
Shares and units eligible | 1,715.8 | |||
Cash per share/unit | $ | 10.50 | ||
Cash consideration for outstanding shares/units | $ | 18,015.6 | ||
6% preferred stock make-whole dividend payments | 98.5 | (2) | ||
Value of Schering-Plough deferred stock units settled in cash | 155.9 | (3) | ||
Total cash consideration | $ | 18,270.0 | ||
Shares and units eligible | 1,715.8 | |||
Common stock exchange ratio per share/unit | 0.5767 | |||
Equivalent New Merck shares | 989.5 | |||
Shares issued to settle certain performance-based awards | 0.7 | |||
New Merck shares issued | 990.2 | |||
Old Merck common stock share price on November�3, 2009 | $ | 30.67 | ||
Common stock equity consideration | $ | 30,370.0 | ||
Fair value of 6% preferred stock not converted | 214.7 | |||
Fair value of other share-based compensation awards | 525.2 | (4) | ||
Employee benefit related amounts payable as a result of the Merger | 191.9 | |||
Total consideration transferred | $ | 49,571.8 | ||
| ||||
| (1) | Upon completion of the Merger and for a period of 15�days thereafter, holders of 6% preferred stock were entitled to convert each share of 6% preferred stock into a number of units of merger consideration equal to the �make-whole� conversion rate of 8.2021 determined in |
118
| accordance with the terms of the preferred stock. This amount represents the units of merger consideration relating to the 6% preferred stock converted by those holders in the 15-day period following the Merger. |
| (2) | Represents the present value of all remaining future dividend payments (from the conversion date through the mandatory conversion date on August�13, 2010)�paid to holders of 6% preferred stock that elected to convert in connection with the Merger using the discount rate as stipulated in the terms of the preferred stock. |
| (3) | Represents the cash consideration paid to holders of Schering-Plough deferred stock units issued in 2007 and prior which were converted into the right to receive cash as specified in the Merger agreement attributable to precombination services . |
| (4) | Represents the fair value of Schering-Plough stock option, performance share unit and deferred stock unit replacement awards attributable to precombination service issued to holders of these awards in the Merger. The fair value of outstanding Schering-Plough stock options and performance share units for 2007 awards and prior, which immediately vested at the effective time of the Merger, was attributed to precombination service and included in the consideration transferred. Stock option, performance share unit and deferred stock unit awards for 2008 and 2009, did not immediately vest upon completion of the Merger. For these awards, the fair value of the awards attributed to precombination services was included as part of the consideration transferred and the fair value attributed to postcombination service is being recognized as compensation cost over the requisite service period in the postcombination financial statements of New Merck. |
The following amounts represent the preliminary determination of the fair value of identifiable assets acquired and liabilities assumed in the Merger. The final determination of fair value for certain assets and liabilities will be completed in 2010 which may result in adjustments to the preliminary values presented below:
Cash and cash equivalents | $ | 5,427.4 | ||
Inventories | 7,371.8 | |||
Other current assets | 4,815.5 | |||
Property, plant and equipment | 6,677.6 | |||
Other identifiable intangible assets: (1) | ||||
Products and product rights (10-year weighted average useful life) | 32,955.8 | |||
In-process research and development
(�IPR&D�) (2) | 6,344.5 | |||
Tradenames (26-year weighted average useful life) | 1,538.0 | |||
Other | 74.0 | |||
Other noncurrent assets | 982.1 | |||
Current liabilities | (6,864.0 | ) | ||
Deferred income tax liabilities | (8,907.6 | ) | ||
Long-term debt | (8,089.2 | ) | ||
Other noncurrent liabilities | (3,238.2 | ) | ||
Total identifiable net assets | 39,087.7 | |||
Goodwill (3) | 10,484.1 | |||
Estimated consideration transferred | $ | 49,571.8 | ||
| ||||
| (1) | In connection with the Merger, the Company obtained a controlling interest in the Merck/Schering-Plough partnership. The table above reflects Schering-Plough�s share of the fair value of the Merck/Schering-Plough partnership�s net assets including intangibles and inventories. Not reflected in this table are Merck�s share of the fair value of the Merck/Schering-Plough partnership�s net assets recorded in connection with the fair value adjustment to Merck�s previously held equity interest in the partnership (see �Merck/Schering-Plough Partnership� below). |
| (2) | IPR&D represents the fair value assigned to incomplete research projects, which at the time of the Merger, had not reached technological feasibility. The amounts were capitalized and are being accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of each project, Merck will make a determination as to the useful life of the asset and begin amortization (see �In-Process Research and Development� below). |
| (3) | The goodwill recognized is largely attributable to anticipated synergies expected to arise after the Merger. Approximately $8.7�billion of the goodwill has been allocated to the Pharmaceutical segment. The remainder of the goodwill was allocated to other non-reportable segments. The goodwill is not deductible for tax purposes. |
In order to allocate the Merger consideration, the Company estimated the fair value of the assets and liabilities of Schering-Plough. No contingent assets or liabilities were recognized at fair value as of the Merger date because the fair value of such contingencies could not be determined. Contingent liabilities were recorded to the
119
extent the amounts were probable and reasonably estimable (see Note�12). For accounting and financial reporting purposes, fair value is defined as the price that would be received upon sale of an asset or the amount paid to transfer a liability in an orderly transaction between market participants at the measurement date. Market participants are assumed to be buyers and sellers in the principal (most advantageous) market for the asset or liability. Additionally, fair value measurements for an asset assume the highest and best use of that asset by market participants. Use of different estimates and judgments could yield different results.
The fair values of identifiable intangible assets related to currently marketed products and product rights were primarily determined by using an �income approach,� through which fair value is estimated based on each asset�s discounted projected net cash flows. The Company�s estimates of market participant net cash flows considered historical and projected pricing, margins and expense levels; the performance of competing products where applicable; relevant industry and therapeutic area growth drivers and factors; current and expected trends in technology and product life cycles; the time and investment that will be required to develop products and technologies; the ability to obtain marketing and regulatory approvals; the ability to manufacture and commercialize the products; the extent and timing of potential new product introductions by the Company�s competitors; and the life of each asset�s underlying patent, if any. The net cash flows are then probability-adjusted where appropriate to consider the uncertainties associated with the underlying assumptions, as well as the risk profile of the net cash flows utilized in the valuation. The probability-adjusted future net cash flows of each product are then discounted to present value utilizing an appropriate discount rate.
In connection with the Merger, the Company recorded the fair value of human and animal health research projects. Approximately $5.0�billion of the consideration transferred in the Merger was allocated to human health IPR&D projects and $1.3�billion was allocated to animal health IPR&D projects. The amounts were capitalized and are being accounted for similar to indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of a project, Merck will make a determination as to the then useful life of the asset and begin amortization.
The fair values of identifiable intangible assets related to IPR&D were determined by using an income approach, through which fair value is estimated based on each asset�s probability adjusted future net cash flows, which reflect the different stages of development of each product and the associated probability of successful completion. The net cash flows are then discounted to present value using discount rates which range from 12% to 15%. Actual cash flows are likely to be different than those assumed. All of the IPR&D projects are subject to the inherent risks and uncertainties in drug development and it is possible that the Company will not be able to successfully develop and complete the IPR&D programs and profitably commercialize the underlying product candidates.
120
Schering-Plough�s results of operations have been included in New Merck�s financial statements for periods subsequent to the completion of the Merger. Schering-Plough contributed revenues of $3.4�billion and estimated losses of $2.2�billion to New Merck for the period from the consummation of the Merger through December�31, 2009. The following unaudited supplemental pro forma data presents consolidated information as if the Merger had been completed on January�1, 2008:
| Years Ended December�31 | 2009 | 2008 | ||||||
| (Unaudited) | ||||||||
Sales | $ | 45,970.7 | $ | 46,749.6 | ||||
Earnings attributable to Merck�& Co., Inc. | 6,069.7 | 3,020.4 | ||||||
Earnings available to Merck�& Co., Inc. common shareholders | 5,934.7 | 2,883.2 | ||||||
Basic earnings per common share available to common shareholders | 1.91 | 0.92 | ||||||
Earning per common share assuming dilution available to common shareholders | 1.90 | 0.92 | ||||||
| ||||||||
The unaudited supplemental pro forma data reflect the application of the following adjustments:
| � | The consolidation of the MSP Partnership which is now owned 100% by the Company and the corresponding gain resulting from the Company�s remeasurement of its previously held equity interest in the MSP Partnership; |
| � | Additional depreciation and amortization expense that would have been recognized assuming fair value adjustments to inventory, property, plant and equipment and intangible assets; |
| � | Additional interest expense and financing costs that would have been incurred on borrowing arrangements and loss of interest income on cash and short-term investments used to fund the Merger; |
| � | Transaction costs associated with the Merger;�and |
| � | Conversion of a portion of outstanding 6% preferred stock |
The unaudited supplemental pro forma financial information does not reflect the potential realization of cost savings relating to the integration of the two companies. The pro forma data should not be considered indicative of the results that would have occurred if the Merger and related borrowings had been consummated on January�1, 2008, nor are they indicative of future results.
| 4. | Restructuring |
In February 2010, the Company announced the first phase of a new global restructuring program (the �Merger Restructuring Program�) in conjunction with the integration of the legacy Merck and legacy Schering-Plough businesses. This Merger Restructuring Program is intended to optimize the cost structure of the combined Company. As part of the first phase of the Merger Restructuring Program, by the end of 2012, the Company expects to reduce its total workforce by approximately 15% across all areas of the Company worldwide. The Company also plans to eliminate 2,500 vacant positions as part of the first phase of the program. These workforce reductions will primarily come from the elimination of duplicative positions in sales, administrative and headquarters organizations, as well as from the consolidation of certain manufacturing facilities and research and development operations. The Company will continue to hire new employees in strategic growth areas of the business during this period. Certain actions, such as the ongoing reevaluation of manufacturing and research and development facilities
121
worldwide have not yet been completed, but will be included later in 2010 in other phases of the Merger Restructuring Program. In connection with the Merger Restructuring Program, separation costs under the Company�s existing severance programs worldwide were recorded in the fourth quarter of 2009 to the extent such costs were probable and reasonably estimable. The Company recorded pretax restructuring costs of $1.5�billion, primarily employee separation costs, related to the Merger Restructuring Program in the fourth quarter of 2009. This first phase of the Merger Restructuring Program is expected to be completed by the end of 2012 with the total pretax costs estimated to be $2.6�billion to $3.3�billion. Costs under voluntary programs and enhancement programs will be recorded in 2010 as the relevant criteria are met. The Company estimates that approximately 85% of the cumulative pretax costs relate to cash outlays, primarily related to employee separation expense. Approximately 15% of the cumulative pretax costs are non-cash, relating primarily to the accelerated depreciation of facilities to be closed or divested.
In October 2008, Old Merck announced a global restructuring program (the �2008 Restructuring Program�) to reduce its cost structure, increase efficiency, and enhance competitiveness. As part of the 2008 Restructuring Program, the Company expects to eliminate approximately 7,200 positions ��6,800 active employees and 400 vacancies�� across all areas of the Company worldwide by the end of 2011. About 40% of these total reductions will occur in the United States. As part of the 2008 Restructuring Program, the Company is streamlining management layers by reducing its total number of senior and mid-level executives globally. As of December�31, 2009, approximately 4,910 positions have been eliminated in connection with 2008 Restructuring Program, comprised of employee separations and the elimination of contractors and vacant positions. Merck is rolling out a new, more customer-centric selling model designed to provide Merck with a meaningful competitive advantage and help physicians, patients and payers improve patient outcomes. The Company is now operating its new commercial selling models in the United States and other markets around the world. The Company is also making greater use of outside technology resources, centralizing common sales and marketing activities, and consolidating and streamlining its operations. Merck�s manufacturing division will further focus its capabilities on core products and outsource non-core manufacturing. During 2009, Merck continued implementing a new model for its basic research global operating strategy at legacy Merck Research Laboratories sites. The new model will align franchise and function as well as align resources with disease area priorities and balance capacity across discovery phases to allow the Company to act upon those programs with the highest probability of success. Additionally, across all disease area priorities, the Company�s strategy is designed to expand access to worldwide external science and incorporate external research as a key component of the Company�s early discovery pipeline in order to translate basic research productivity into late-stage clinical success. During 2009, basic research facilities in Pomezia, Italy and Tsukuba, Japan were sold and the operations conducted at the basic research facility in Seattle were closed. Merck has also sold or closed certain other facilities and sold related assets in connection with the 2008 Restructuring Program.
In connection with the 2008 Restructuring Program, separation costs under existing severance programs worldwide were recorded in the third quarter of 2008 to the extent such costs were probable and estimable. Old Merck commenced accruing costs related to one-time termination benefits offered to employees under the 2008 Restructuring Program in the fourth quarter of 2008 as that is when the necessary criteria were met. Pretax restructuring costs of $474.7�million and $921.3�million, respectively, were recorded related to the 2008 Restructuring Program in 2009 and 2008. Since inception of the 2008 Restructuring Program through December�31, 2009, Merck has recorded total pretax accumulated costs of $1.4�billion. The 2008 Restructuring Program is expected to be completed by the end of 2011 with the total pretax costs estimated to be $1.6�billion to $2.0�billion. The Company estimates that two-thirds of the cumulative pretax costs relate to cash outlays, primarily from employee separation expense. Approximately one-third of the cumulative pretax costs are non-cash, relating primarily to the accelerated depreciation of facilities to be closed or divested.
In November 2005, Old Merck announced a global restructuring program (the �2005 Restructuring Program�) designed to reduce the cost structure, increase efficiency and enhance competitiveness which was substantially complete at the end of 2008.
For segment reporting, restructuring charges are unallocated expenses.
122
The following table summarizes the charges related to Merger Restructuring Program and 2008 and 2005 Restructuring Program activities by type of cost:
| Separation | Accelerated | |||||||||||||||
| Year Ended December�31, 2009 | Costs | Depreciation | Other | Total | ||||||||||||
Merger Restructuring Program | ||||||||||||||||
Materials and production | $ | - | $ | 42.6 | $ | - | $ | 42.6 | ||||||||
Research and development | - | - | - | - | ||||||||||||
Restructuring costs | 1,338.3 | - | 78.7 | 1,417.0 | ||||||||||||
| 1,338.3 | 42.6 | 78.7 | 1,459.6 | |||||||||||||
2008 Restructuring Program | ||||||||||||||||
Materials and production | - | 70.5 | (5.6 | ) | 64.9 | |||||||||||
Research and development | - | 227.8 | 3.8 | 231.6 | ||||||||||||
Restructuring costs | 13.6 | - | 164.6 | 178.2 | ||||||||||||
| 13.6 | 298.3 | 162.8 | 474.7 | |||||||||||||
| $ | 1,351.9 | $ | 340.9 | $ | 241.5 | $ | 1,934.3 | |||||||||
| ||||||||||||||||
Year Ended December�31, 2008 | ||||||||||||||||
2008 Restructuring Program | ||||||||||||||||
Materials and production | $ | - | $ | 33.7 | $ | 25.0 | $ | 58.7 | ||||||||
Research and development | - | 127.1 | - | 127.1 | ||||||||||||
Restructuring costs | 684.9 | - | 50.6 | 735.5 | ||||||||||||
| 684.9 | 160.8 | 75.6 | 921.3 | |||||||||||||
2005 Restructuring Program | ||||||||||||||||
Materials and production | - | 55.0 | 9.5 | 64.5 | ||||||||||||
Research and development | - | 0.9 | 0.4 | 1.3 | ||||||||||||
Restructuring costs | 272.4 | - | 24.6 | 297.0 | ||||||||||||
| 272.4 | 55.9 | 34.5 | 362.8 | |||||||||||||
| $ | 957.3 | $ | 216.7 | $ | 110.1 | $ | 1,284.1 | |||||||||
| ||||||||||||||||
Year Ended December�31, 2007 | ||||||||||||||||
2005 Restructuring Program | ||||||||||||||||
Materials and production | $ | - | $ | 460.6 | $ | 22.5 | $ | 483.1 | ||||||||
Research and development | - | - | (0.1 | ) | (0.1 | ) | ||||||||||
Restructuring costs | 251.4 | - | 75.7 | 327.1 | ||||||||||||
| $ | 251.4 | $ | 460.6 | $ | 98.1 | $ | 810.1 | |||||||||
| ||||||||||||||||
Separation costs are associated with actual headcount reductions, as well as those headcount reductions which were probable and could be reasonably estimated. Approximately 3,300 positions were eliminated in 2009 of which approximately 3,160 related to the 2008 Restructuring Program and approximately 140 related to the Merger Restructuring Program. During 2009, certain employees anticipated to be separated as part of planned restructuring actions for the 2008 Restructuring Program were instead transferred to the buyer in conjunction with the sale of a facility. Accordingly, the accrual of separation costs associated with these employees was reversed resulting in a reduction to expenses. Approximately 5,800 positions were eliminated in 2008 of which approximately 1,750 related to the 2008 Restructuring Program and 4,050 related to the 2005 Restructuring Program. Approximately
123
2,400 positions were eliminated in 2007 in connection with the 2005 Restructuring Program. These position eliminations are comprised of actual headcount reductions, and the elimination of contractors and vacant positions.
Accelerated depreciation costs primarily relate to manufacturing and research facilities to be sold or closed as part of the programs. All of the sites have and will continue to operate up through the respective closure dates, and since future cash flows were sufficient to recover the respective book values, Merck was required to accelerate depreciation of the site assets rather than write them off immediately. The site assets include manufacturing and research facilities and equipment.
Other activity in 2009, 2008 and 2007 includes $14.9�million, $29.4�million and $39.4�million, respectively, of asset abandonment, shut-down and other related costs. Additionally, other activity includes $82.3�million, $68.4�million and $18.9�million in 2009, 2008 and 2007, respectively, related to curtailment, settlement and termination charges on pension and other postretirement benefit plans (see Note�15). Other activity also reflects pretax losses resulting from sales of facilities and related assets in 2009 of $57.9�million and pretax gains on such sales in 2008 of $61.5�million.
Adjustments to the recorded amounts were not material in any period.
The following table summarizes the charges and spending relating to Merger Restructuring Program and 2008 and 2005 Restructuring Program activities:
| Separation | Accelerated | |||||||||||||||
| Costs | Depreciation | Other | Total | |||||||||||||
Merger Restructuring Program | ||||||||||||||||
Restructuring reserves as of January�1, 2009 | $ | - | $ | - | $ | - | $ | - | ||||||||
Expense | 1,338.3 | 42.6 | 78.7 | 1,459.6 | ||||||||||||
(Payments) receipts, net | (34.9 | ) | - | (58.4 | ) | (93.3 | ) | |||||||||
Non-cash activity | - | (42.6 | ) | (20.3 | ) | (62.9 | ) | |||||||||
Restructuring reserves as of December�31, 2009 (1) | $ | 1,303.4 | $ | - | $ | - | $ | 1,303.4 | ||||||||
| ||||||||||||||||
2008 Restructuring Program | ||||||||||||||||
Restructuring reserves as of January�1, 2008 | $ | - | $ | - | $ | - | $ | - | ||||||||
Expense | 684.9 | 160.8 | 75.6 | 921.3 | ||||||||||||
(Payments) receipts, net | (77.2 | ) | - | (37.3 | ) | (114.5 | ) | |||||||||
Non-cash activity | - | (160.8 | ) | (38.3 | ) | (199.1 | ) | |||||||||
Restructuring reserves as of December�31, 2008 | $ | 607.7 | $ | - | $ | - | $ | 607.7 | ||||||||
Expense | $ | 13.6 | $ | 298.3 | $ | 162.8 | $ | 474.7 | ||||||||
(Payments) receipts, net | (372.0 | ) | - | (154.5 | ) (2) | (526.5 | ) | |||||||||
Non-cash activity | - | (298.3 | ) | (8.3 | ) | (306.6 | ) | |||||||||
Restructuring reserves as of December�31, 2009 (1) | $ | 249.3 | $ | - | $ | - | $ | 249.3 | ||||||||
| ||||||||||||||||
2005 Restructuring Program | ||||||||||||||||
Restructuring reserves as of January�1, 2008 | $ | 231.5 | $ | - | $ | - | $ | 231.5 | ||||||||
Expense | $ | 272.4 | $ | 55.9 | $ | 34.5 | $ | 362.8 | ||||||||
(Payments) receipts, net | (389.1 | ) | - | (23.2 | ) (2) | (412.3 | ) | |||||||||
Non-cash activity | - | (55.9 | ) | (11.3 | ) | (67.2 | ) | |||||||||
Restructuring reserves as of December�31, 2008 | $ | 114.8 | $ | - | $ | - | $ | 114.8 | ||||||||
(Payments) receipts, net | (77.2 | ) | - | - | (77.2 | ) | ||||||||||
Restructuring reserves as of December�31,
2009 (1) | $ | 37.6 | $ | - | $ | - | $ | 37.6 | ||||||||
| ||||||||||||||||
| (1) | The cash outlays associated with the first phase of the Merger Restructuring Program are expected to be substantially completed by the end of 2012. The cash outlays associated with the remaining restructuring reserve for the 2008 Restructuring Program are expected to be completed by the end of 2011. The cash outlays associated with the remaining restructuring reserve for the 2005 Restructuring Program are expected to be completed by the end of 2010. |
| (2) | Includes proceeds from the sales of facilities in connection with restructuring actions. |
124
| 5. | Acquisitions, Research Collaborations and License Agreements |
In December 2009, Merck and Avecia Investments Limited announced a definitive agreement under which Merck would acquire the biologics business of the Avecia group for a total purchase price of $180�million. Avecia Biologics is a contract manufacturing organization with specific expertise in microbial-derived biologics. Under the terms of the agreement, Merck would acquire Avecia Biologics Limited (�Avecia�) and all of its assets, including all Avecia�s process development and scale-up, manufacturing, quality and business support operations located in Billingham, United Kingdom. This transaction closed on February�1, 2010, and accordingly, the results of operations of the acquired business will be included in Merck�s results of operations after the acquisition date.
125
payments up to $165�million in the aggregate upon achievement of certain milestones associated with the development and approval of a drug candidate covered by this agreement. Upon commercialization, Medarex and MBL will also be eligible to receive double-digit royalties on product sales and milestones if certain sales targets are met. The term of the agreement commenced on the closing date and, unless terminated earlier, will continue until there are no remaining royalty payment obligations in a country, at which time the agreement will expire in its entirety in such country. Either party may terminate this agreement for uncured material breach by the other party, or bankruptcy or insolvency of the other party. Merck may terminate this agreement at any time upon providing 180�days prior written notice to Medarex and MBL.
126
August 2008, the FDA issued an Approvable action letter requesting additional information. A Phase IIb double-blind, placebo-controlled, randomized, dose-ranging clinical trial in patients at risk of recurrent atrial fibrillation showed that, at the 500�mg dose, vernakalant (oral) significantly reduced the rate of atrial fibrillation relapse as compared to placebo. This agreement continues in effect until the expiration of Cardiome�s co-promotion rights and all royalty and milestone payment obligations. This agreement may be terminated in the event of insolvency or a material uncured breach by either party. Additionally, the collaboration may be terminated by Merck in the event that Merck determines (in good faith) that it is not advisable to continue the development or commercialization of a vernakalant product as a result of a serious safety issue. In addition, Merck may terminate the agreement at any time upon 12�months prior written notice. Cardiome may terminate the agreement in the event that Merck challenges any Cardiome patent covering vernakalant. Upon termination of the agreement, depending upon the circumstances, the parties have varying rights and obligations with respect to the continued development and commercialization of vernakalant and in some cases continuing royalty obligations.
In March 2009, Old Merck acquired Insmed Inc.�s (�Insmed�) portfolio of follow-on biologic therapeutic candidates and its commercial manufacturing facilities located in Boulder, Colorado. Under the terms of the agreement, Old Merck paid Insmed an aggregate of $130�million in cash to acquire all rights to the Boulder facilities and Insmed�s pipeline of follow-on biologic candidates. Insmed�s follow-on biologics portfolio includes two clinical candidates: MK-4214, an investigational recombinant granulocyte-colony stimulating factor (�G-CSF�) that will be evaluated for its ability to prevent infections in patients with cancer receiving chemotherapy, and MK-6302, a pegylated recombinant G-CSF designed to allow for less frequent dosing. The transaction was accounted for as a business combination; accordingly, the assets acquired and liabilities assumed were recorded at their respective fair values as of the acquisition date. The determination of fair value requires management to make significant estimates and assumptions. In connection with the acquisition, substantially all of the purchase price was allocated to Insmed�s follow-on biologics portfolio (MK-4214 and MK-6302) and an indefinite-lived intangible asset was recorded. The fair value was determined based upon the present value of expected future cash flows of new product candidates resulting from Insmed�s follow-on biologics portfolio adjusted for the probability of their estimated technical and marketing success utilizing an income approach reflecting appropriate risk-adjusted discount rates. The ongoing activity related to MK-4214 and MK-6302 is not expected to be material to the Company�s research and development expense. The remaining net assets acquired were not material and there were no other milestone or royalty obligations associated with the acquisition. This transaction closed on March�31, 2009, and accordingly, the results of operations of the acquired business have been included in Merck�s results of operations beginning April�1, 2009.
In September 2007, Old Merck completed the acquisition of NovaCardia, Inc. (�NovaCardia�), a privately held clinical-stage pharmaceutical company focused on cardiovascular disease. Old Merck acquired all of the outstanding equity of NovaCardia for a total purchase price of $366.4�million (including $16.4�million of
127
Also in 2007, Old Merck and GTx, Inc. (�GTx�) entered into an agreement providing for a research and development and global strategic collaboration for selective androgen receptor modulators (�SARMs�), a new class of drugs with the potential to treat age-related muscle loss (sarcopenia) as well as other musculoskeletal conditions. Merck has discontinued internal development of MK-2866 (which is a SARM) under this agreement, and is currently discussing next steps with GTx. Also in 2007, Old Merck and ARIAD Pharmaceuticals, Inc. (�ARIAD�) entered into a global collaboration to jointly develop and commercialize ridaforolimus (MK-8669), ARIAD�s novel mTOR inhibitor, for use in cancer. These collaborations generally continue in effect until the expiration of all royalty and milestone payment obligations. These collaborations may generally be terminated in the event of insolvency or a material uncured breach by either party. Additionally, the collaboration agreement between Merck and GTx may be terminated by Merck upon 90 days notice to GTx at any time after December 18, 2009. The collaboration agreement between Merck and ARIAD may be terminated by Merck upon the failure of MK-8669 to meet certain developmental and safety requirements or in the event Merck concludes it is not advisable to continue the development of MK-8669 for use in a cancer indication. In addition, Merck may terminate the ARIAD collaboration agreement on or after the third anniversary of the effective date by providing at least 12�months prior written notice. Upon termination of the ARIAD collaboration agreement, depending upon the circumstances, the parties have varying rights and obligations with respect to the continued development and commercialization of MK-8669 and continuing royalty obligations.
| 6. | Collaborative Arrangements |
The Company continues its strategy of establishing external alliances to complement its substantial internal research capabilities, including research collaborations, licensing preclinical and clinical compounds and technology platforms to drive both near- and long-term growth. The Company supplements its internal research with an aggressive licensing and external alliance strategy focused on the entire spectrum of collaborations from early research to late-stage compounds, as well as new technologies across a broad range of therapeutic areas. These arrangements often include upfront payments and royalty or profit share payments, contingent upon the occurrence of certain future events linked to the success of the asset in development, as well as expense reimbursements or payments to the third party.
As discussed in Note�2, on January�1, 2009, new guidance issued by the FASB was adopted which defines collaborative arrangements and establishes reporting requirements for transactions between participants in a collaborative arrangement and between participants in the arrangement and third parties. The Company reviewed its third party arrangements to determine if any arrangement is within the scope of this new guidance. Each arrangement is unique in nature and the Company�s most significant arrangements are discussed below.
128
| 7. | Financial Instruments |
The Company manages the impact of foreign exchange rate movements and interest rate movements on its earnings, cash flows and fair values of assets and liabilities through operational means and through the use of various financial instruments, including derivative instruments.
A significant portion of the Company�s revenues and earnings in foreign affiliates is exposed to changes in foreign exchange rates. The objectives and accounting related to the Company�s foreign currency risk management program, as well as its interest rate risk management activities are discussed below.
A significant portion of the Company�s revenues are denominated in foreign currencies. Merck relies on sustained cash flows generated from foreign sources to support its long-term commitment to U.S.�dollar-based research and development. To the extent the dollar value of cash flows is diminished as a result of a strengthening dollar, the Company�s ability to fund research and other dollar-based strategic initiatives at a consistent level may be impaired. The Company has established revenue hedging and balance sheet risk management programs to protect against volatility of future foreign currency cash flows and changes in fair value caused by volatility in foreign exchange rates at its U.S. functional currency entities.
The objective of the revenue hedging program is to reduce the potential for longer-term unfavorable changes in foreign exchange to decrease the U.S.�dollar value of future cash flows derived from foreign currency
129
denominated sales, primarily the euro and Japanese yen. To achieve this objective, the Company will partially hedge forecasted foreign currency denominated third party and intercompany distributor entity sales that are expected to occur over its planning cycle, typically no more than three years into the future. The Company will layer in hedges over time, increasing the portion of third party and intercompany distributor entity sales hedged as it gets closer to the expected date of the forecasted foreign currency denominated sales, such that it is probable the hedged transaction will occur. The portion of sales hedged is based on assessments of cost-benefit profiles that consider natural offsetting exposures, revenue and exchange rate volatilities and correlations, and the cost of hedging instruments. The hedged anticipated sales are a specified component of a portfolio of similarly denominated foreign currency-based sales transactions, each of which responds to the hedged risk in the same manner. The Company manages its anticipated transaction exposure principally with purchased local currency put options, which provide the Company with a right, but not an obligation, to sell foreign currencies in the future at a predetermined price. If the U.S.�dollar strengthens relative to the currency of the hedged anticipated sales, total changes in the options� cash flows offset the decline in the expected future U.S.�dollar cash flows of the hedged foreign currency sales. Conversely, if the U.S.�dollar weakens, the options� value reduces to zero, but the Company benefits from the increase in the value of the anticipated foreign currency cash flows. The Company also utilizes forward contracts in its revenue hedging program. If the U.S.�dollar strengthens relative to the currency of the hedged anticipated sales, the increase in the fair value of the forward contracts offsets the decrease in the expected future U.S.�dollar cash flows of the hedged foreign currency sales. Conversely, if the U.S.�dollar weakens, the decrease in the fair value of the forward contracts offsets the increase in the value of the anticipated foreign currency cash flows.
Where the U.S.�dollar is the functional currency of the Company�s foreign subsidiaries, the primary objective of the balance sheet risk management program is to protect the U.S.�dollar value of foreign currency denominated net monetary assets from the effects of volatility in foreign exchange that might occur prior to their conversion to U.S.�dollars. In these instances, Merck principally utilizes forward exchange contracts, which enable the Company to buy and sell foreign currencies in the future at fixed exchange rates and economically offset the consequences of changes in foreign exchange on the amount of U.S.�dollar cash flows derived from the net assets. Where the U.S.�dollar is not the functional currency of the Company�s foreign subsidiaries, Merck executes spot trades to convert foreign currencies into U.S.�dollars based on short-term forecast needs. These U.S.�dollar proceeds are then invested until required by the Company�s foreign subsidiaries. Merck routinely enters into contracts to offset the effects of exchange on exposures denominated in developed country currencies, primarily the euro and Japanese yen. For exposures in developing country currencies, the Company will enter into forward contracts to partially offset the effects of exchange on exposures when it is deemed economical to do so based on a cost-benefit analysis that considers the magnitude of the exposure, the volatility of the exchange rate and the cost of the hedging instrument. The Company will also minimize the effect of exchange on monetary assets and liabilities by managing operating activities and net asset positions at the local level.
130
Foreign exchange risk is also managed through the use of foreign currency debt. The Company�s senior unsecured euro-denominated notes and euro-denominated term loan have been designated as, and are effective as, economic hedges of the net investment in a foreign operation. Accordingly, foreign currency transaction gains or losses on the euro-denominated debt instruments are included in foreign currency translation adjustment within comprehensive income.
At December�31, 2009, the Company was a party to seven pay-floating, receive-fixed interest rate swap contracts designated as fair value hedges of fixed-rate notes in which the notional amounts match the amount of the hedged fixed-rate notes. There are two swaps maturing in 2011 with notional amounts of $125�million each that effectively convert the Company�s $250�million, 5.125% fixed-rate notes due 2011 to floating rate instruments and five swaps maturing in 2015 with notional amounts of $150�million each that effectively convert $750�million of the Company�s $1.0�billion, 4.0% fixed-rate notes due 2015 to floating rate instruments. The interest rate swap contracts are designated hedges of the fair value changes in the notes attributable to changes in the benchmark London Interbank Offered Rate (�LIBOR�) swap rate. The fair value changes in the notes attributable to changes in the benchmark interest rate are recorded in interest expense and offset by the fair value changes in the swap contracts. During 2008, Old Merck terminated four interest rate swap contracts with notional amounts of $250�million each, and terminated one interest rate swap contract with a notional amount of $500�million. These swaps had effectively converted its $1.0�billion, 4.75% fixed-rate notes due 2015 and its $500�million, 4.375% fixed-rate notes due 2013 to variable rate debt. As a result of the swap terminations, Old Merck received $128.3�million in cash, excluding accrued interest which was not material. The corresponding gains related to the basis adjustment of the debt associated with the terminated swap contracts were deferred and are being amortized as a reduction of interest expense over the remaining term of the notes. The cash flows from these contracts are reported as operating activities in the Consolidated Statement of Cash Flows.
Presented in the table below is the fair value of derivatives segregated between those derivatives that are designated as hedging instruments and those that are not designated as hedging instruments as of December�31, 2009.
| Fair Value of Derivative | U.S. Dollar | |||||||||||||
| Balance Sheet Caption | Asset | Liability | Notional | |||||||||||
Derivatives Designated as Hedging Instruments | ||||||||||||||
Foreign Exchange Contracts (current) | Deferred income taxes and other current assets | $ | 139.3 | $ | - | $ | 3,050.5 | |||||||
Foreign Exchange Contracts (non-current) | Other assets | 152.6 | - | 2,118.1 | ||||||||||
Foreign Exchange Contracts (current) | Accrued and other current liabilities | - | 34.0 | 658.6 | ||||||||||
Interest Rate Swaps (non-current) | Other assets | 26.7 | - | 1,000.0 | ||||||||||
| $ | 318.6 | $ | 34.0 | $ | 6,827.2 | |||||||||
| ||||||||||||||
Derivatives Not Designated as Hedging Instruments | ||||||||||||||
Foreign Exchange Contracts (current) | Deferred income taxes and other current assets | $ | 60.3 | $ | - | $ | 2,841.7 | |||||||
Foreign Exchange Contracts (current) | Accrued and other current liabilities | - | 38.6 | 2,104.3 | ||||||||||
| $ | 60.3 | $ | 38.6 | $ | 4,946.0 | |||||||||
| $ | 378.9 | $ | 72.6 | $ | 11,773.2 | |||||||||
| ||||||||||||||
131
The table below provides information on the location and pretax (gain) or loss amounts for derivatives that are: (i)�designated in a fair value hedging relationship, (ii)�designated in a cash flow hedging relationship, and (iii)�not designated in a hedging relationship for the year ended December�31, 2009.
| Amount of | Amount of | |||||||||||||||
| Amount of | Amount of | Pretax | Pretax | |||||||||||||
| Gain (Loss) | Gain (Loss) | (Gain) Loss | (Gain) Loss | |||||||||||||
| Recognized in | Recognized in | Reclassified | Recognized | |||||||||||||
| Earnings on | Earnings on | from AOCI | in OCI on | |||||||||||||
| Derivatives (1) | Hedged Item (1) | into Earnings (2) | Derivatives | |||||||||||||
Derivatives designated in fair value hedging
relationships: | ||||||||||||||||
Interest rate swap contracts | $ | 2.8 | $ | (2.8 | ) | $ | - | $ | - | |||||||
Foreign exchange contracts | 5.2 | (9.1 | ) | - | - | |||||||||||
| $ | 8.0 | $ | (11.9 | ) | $ | - | $ | - | ||||||||
Derivatives designated in cash flow hedging relationships: | ||||||||||||||||
Foreign exchange contracts | $ | - | $ | - | $ | 60.5 | $ | 310.1 | ||||||||
Derivatives not designated in a hedging relationship: | ||||||||||||||||
Foreign exchange
contracts (3) | $ | (40.8 | ) | $ | - | $ | - | $ | - | |||||||
| ||||||||||||||||
| (1) | Recognized in Other (income) expense, net. |
| (2) | Recognized in Sales. |
| (3) | These derivative contracts mitigate changes in the value of remeasured foreign currency denominated monetary assets and liabilities attributable to changes in foreign currency exchange rates. |
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Entities are required to use a fair value hierarchy which maximizes the use of observable inputs and minimizes the use of unobservable inputs when measuring fair value. There are three levels of inputs that may be used to measure fair value:
132
backed securities, as well as certain corporate notes and bonds with limited market activity. At December�31, 2009, $71.5�million, or approximately 7.3%, of the Company�s investment securities were categorized as Level�3 assets (all of which were pledged under certain collateral arrangements (see Note�17)). All of the assets classified as Level�3 at December�31, 2009 were acquired when Old Merck elected to be redeemed-in-kind from a short-term fixed income fund that restricted cash redemptions as described below.
If the inputs used to measure the financial assets and liabilities fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
Financial assets and liabilities measured at fair value on a recurring basis are summarized below:
| Fair Value Measurements Using | Fair Value Measurements Using | |||||||||||||||||||||||||||||||
| Quoted Prices | Significant | Quoted Prices | Significant | |||||||||||||||||||||||||||||
| In Active | Other | Significant | In Active | Other | Significant | |||||||||||||||||||||||||||
| Markets for | Observable | Unobservable | Markets for | Observable | Unobservable | |||||||||||||||||||||||||||
| Identical Assets | Inputs | Inputs | Identical Assets | Inputs | Inputs | |||||||||||||||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | Total | (Level 1) | (Level 2) | (Level 3) | Total | |||||||||||||||||||||||||
| December�31, 2009 | December�31, 2008 | |||||||||||||||||||||||||||||||
Assets | ||||||||||||||||||||||||||||||||
Investments | ||||||||||||||||||||||||||||||||
U.S. government and agency securities | $ | - | $ | 215.6 | $ | - | $ | 215.6 | $ | - | $ | 2,885.7 | $ | - | $ | 2,885.7 | ||||||||||||||||
Corporate notes and bonds | - | 205.2 | - | 205.2 | - | 3,093.2 | - | 3,093.2 | ||||||||||||||||||||||||
Municipal securities | - | 186.7 | - | 186.7 | - | - | - | - | ||||||||||||||||||||||||
Mortgage-backed securities (1) | - | - | - | - | - | 723.9 | - | 723.9 | ||||||||||||||||||||||||
Commercial paper | - | - | - | - | - | 133.0 | - | 133.0 | ||||||||||||||||||||||||
Asset-backed securities (1) | - | 36.0 | - | 36.0 | - | 306.7 | - | 306.7 | ||||||||||||||||||||||||
Foreign government bonds | - | - | - | - | - | 319.4 | - | 319.4 | ||||||||||||||||||||||||
Equity securities | 39.4 | 39.1 | - | 78.5 | 71.1 | 73.6 | - | 144.7 | ||||||||||||||||||||||||
Other debt securities | - | 3.4 | - | 3.4 | - | 2.8 | - | 2.8 | ||||||||||||||||||||||||
| 39.4 | 686.0 | - | 725.4 | 71.1 | 7,538.3 | - | 7,609.4 | |||||||||||||||||||||||||
Other assets | ||||||||||||||||||||||||||||||||
Securities held for employee compensation | 107.7 | 14.2 | - | 121.9 | - | - | - | - | ||||||||||||||||||||||||
Other assets (2) | - | 55.1 | 71.5 | 126.6 | - | 2,877.9 | 96.6 | 2,974.5 | ||||||||||||||||||||||||
| 107.7 | 69.3 | 71.5 | 248.5 | - | 2,877.9 | 96.6 | 2,974.5 | |||||||||||||||||||||||||
Derivative assets (3) | ||||||||||||||||||||||||||||||||
Purchased currency options | - | 291.9 | - | 291.9 | - | 451.3 | - | 451.3 | ||||||||||||||||||||||||
Forward exchange contracts | - | 60.3 | - | 60.3 | - | 73.2 | - | 73.2 | ||||||||||||||||||||||||
Interest rate swaps | - | 26.7 | - | 26.7 | - | 23.9 | - | 23.9 | ||||||||||||||||||||||||
| - | 378.9 | - | 378.9 | - | 548.4 | - | 548.4 | |||||||||||||||||||||||||
Total assets | $ | 147.1 | $ | 1,134.2 | $ | 71.5 | $ | 1,352.8 | $ | 71.1 | $ | 10,964.6 | $ | 96.6 | $ | 11,132.3 | ||||||||||||||||
Liabilities | ||||||||||||||||||||||||||||||||
Derivative liabilities (3) | ||||||||||||||||||||||||||||||||
Written currency options | $ | - | $ | 0.3 | $ | - | $ | 0.3 | $ | - | $ | 1.9 | $ | - | $ | 1.9 | ||||||||||||||||
Forward exchange contracts | - | 72.3 | - | 72.3 | - | 273.1 | - | 273.1 | ||||||||||||||||||||||||
Total liabilities | $ | - | $ | 72.6 | $ | - | $ | 72.6 | $ | - | $ | 275.0 | $ | - | $ | 275.0 | ||||||||||||||||
| ||||||||||||||||||||||||||||||||
| (1) | Substantially all of the asset-backed securities are highly-rated (Standard�& Poor�s rating of AAA and Moody�s Investors Service rating of Aaa), secured primarily by credit card, auto loan, and home equity receivables, with weighted-average lives of primarily 5�years or less. Mortgage-backed securities represent AAA-rated securities issued or unconditionally guaranteed as to payment of principal and interest by U.S. government agencies. |
| (2) | Other assets represent a portion of the pledged collateral discussed below and in Note�17. At December�31, 2009, Level�2 other assets are comprised of $39.5�million of asset-backed securities, $11.6�million of mortgage backed securities and $4.0�million of corporate notes and bonds. At December�31, 2008, Level�2 other assets are comprised of $987.4�million of corporate notes and bonds, $792.5�million of municipal securities, $357.3�million of commercial paper, $276.0�million of mortgage-backed securities, $240.1�million of U.S. government and agency securities and $224.6�million of asset-backed securities. |
| (3) | The fair value determination of derivatives includes an assessment of the credit risk of counterparties to the derivatives and the Company�s own credit risk, the effects of which were not significant. |
133
As of December�31, 2009, the Company had approximately $8.5�billion of cash equivalents.
Financial assets are considered Level�3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable. Level�3 financial assets also include certain investment securities for which there is limited market activity such that the determination of fair value requires significant judgment or estimation. The Company�s Level�3 investment securities at December�31, 2009, primarily include certain mortgage-backed and asset-backed securities, as well as certain corporate notes and bonds for which there was a decrease in the observability of market pricing for these investments. These securities were valued primarily using pricing models for which management understands the methodologies. These models incorporate transaction details such as contractual terms, maturity, timing and amount of future cash inflows, as well as assumptions about liquidity and credit valuation adjustments of marketplace participants at December�31, 2009.
The table below provides a summary of the changes in fair value, including net transfers in and/or out, of all financial assets measured at fair value on a recurring basis using significant unobservable inputs (Level�3):
| 2009 | 2008 | |||||||||||||||||||||||
| Available- | Other | |||||||||||||||||||||||
| for-Sale | Other | Debt | Other | |||||||||||||||||||||
| Investments | Assets | Total | Securities | Assets | Total | |||||||||||||||||||
Beginning balance January 1 | $ | - | $ | 96.6 | $ | 96.6 | $ | 314.5 | $ | 958.6 | $ | 1,273.1 | ||||||||||||
Net transfers in to (out of)
Level�3 (1) | 26.7 | 14.5 | 41.2 | (314.5 | ) | (684.5 | ) | (999.0 | ) | |||||||||||||||
Purchases, sales, settlements, net | (26.9 | ) | (48.8 | ) | (75.7 | ) | - | (132.8 | ) | (132.8 | ) | |||||||||||||
Total realized and unrealized gains (losses) | ||||||||||||||||||||||||
Included in: | ||||||||||||||||||||||||
Earnings (2) | 0.5 | (4.5 | ) | (4.0 | ) | - | (43.6 | ) | (43.6 | ) | ||||||||||||||
Comprehensive income | (0.3 | ) | 13.7 | 13.4 | - | (1.1 | ) | (1.1 | ) | |||||||||||||||
Ending balance at December 31 | $ | - | $ | 71.5 | $ | 71.5 | $ | - | $ | 96.6 | $ | 96.6 | ||||||||||||
Losses recorded in earnings for Level�3 assets still held at December 31 | $ | - | $ | 3.3 | $ | 3.3 | $ | - | $ | (44.3 | ) | $ | (44.3 | ) | ||||||||||
| ||||||||||||||||||||||||
| (1) | Transfers in and out of Level�3 are deemed to occur at the beginning of the quarter in which the transaction takes place. |
| (2) | Amounts are recorded in Other (income) expense, net. |
Some of the Company�s financial instruments are not measured at fair value on a recurring basis but are recorded at amounts that approximate fair value due to their liquid or short-term nature, such as cash and cash equivalents, receivables and payables.
134
The estimated fair value of loans payable and long-term debt (including current portion) at December�31, 2009 was $17.7�billion compared with a carrying value of $17.5�billion and at December�31, 2008 was $6.3�billion compared with a carrying value of $6.2�billion. Fair value was estimated using quoted dealer prices.
| December�31, 2009 | December�31, 2008 | |||||||||||||||||||||||||||||||
| Fair | Amortized | Gross Unrealized | Fair | Amortized | Gross Unrealized | |||||||||||||||||||||||||||
| Value | Cost | Gains (1) | Losses (1) | Value | Cost | Gains (1) | Losses (1) | |||||||||||||||||||||||||
U.S. government and agency securities | $ | 215.6 | $ | 215.7 | $ | 1.1 | $ | (1.2 | ) | $ | 3,125.8 | $ | 3,061.6 | $ | 67.4 | $ | (3.2 | ) | ||||||||||||||
Corporate notes and bonds | 209.2 | 207.1 | 3.3 | (1.2 | ) | 4,124.7 | 4,158.4 | 31.6 | (65.3 | ) | ||||||||||||||||||||||
Municipal securities | 186.7 | 184.8 | 2.9 | (1.0 | ) | 792.5 | 764.4 | 28.4 | (0.3 | ) | ||||||||||||||||||||||
Mortgage-backed securities | 79.4 | 65.9 | 13.8 | (0.3 | ) | 1,031.9 | 1,024.4 | 12.5 | (5.0 | ) | ||||||||||||||||||||||
Asset-backed securities | 79.3 | 69.2 | 10.1 | - | 551.7 | 571.8 | 0.6 | (20.7 | ) | |||||||||||||||||||||||
Foreign government bonds | 0.4 | 0.4 | - | - | 319.4 | 305.9 | 13.5 | - | ||||||||||||||||||||||||
Commercial paper | - | - | - | - | 490.3 | 490.3 | - | - | ||||||||||||||||||||||||
Other debt securities | 21.7 | 19.3 | 9.4 | (7.0 | ) | 46.7 | 48.6 | 1.5 | (3.4 | ) | ||||||||||||||||||||||
Equity securities | 181.6 | 161.4 | 28.4 | (8.2 | ) | 100.9 | 86.3 | 17.7 | (3.1 | ) | ||||||||||||||||||||||
| $ | 973.9 | $ | 923.8 | $ | 69.0 | $ | (18.9 | ) | $ | 10,583.9 | $ | 10,511.7 | $ | 173.2 | $ | (101.0 | ) | |||||||||||||||
| ||||||||||||||||||||||||||||||||
| (1) | At December�31, 2009, gross unrealized gains and gross unrealized losses related to amounts pledged as collateral (see below and Note�17)�were $25.6�million and $(0.3)�million, respectively. At December�31, 2008, gross unrealized gains and gross unrealized losses related to amounts pledged as collateral were $36.1�million and $(30.3)�million, respectively. |
On an ongoing basis, the Company monitors concentrations of credit risk associated with corporate issuers of securities and financial institutions with which it conducts business. Credit exposure limits are established to limit a concentration with any single issuer or institution. Cash and investments are placed in instruments that meet high credit quality standards, as specified in the Company�s investment policy guidelines.
135
The Company�s four largest U.S.�customers, McKesson Corporation, Cardinal Health, Inc., AmerisourceBergen Corporation and Medco Health Solutions, Inc., represented, in aggregate, approximately one-fifth of accounts receivable at December�31, 2009. The Company monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business. Bad debts have been minimal. The Company does not normally require collateral or other security to support credit sales.
| 8. | Inventories |
Inventories at December 31 consisted of:
| 2009 | 2008 | |||||||
Finished goods | $ | 2,475.5 | $ | 432.6 | ||||
Raw materials and work in process | 6,580.9 | 2,147.1 | ||||||
Supplies | 322.8 | 98.6 | ||||||
Total (approximates current cost) | 9,379.2 | 2,678.3 | ||||||
Reduction to LIFO costs | (166.7 | ) | - | |||||
| $ | 9,212.5 | $ | 2,678.3 | |||||
Recognized as: | ||||||||
Inventories | $ | 8,055.3 | $ | 2,091.0 | ||||
Other assets | 1,157.2 | 587.3 | ||||||
| ||||||||
| 9. | Goodwill and Other Intangibles |
As a result of the Merger (see Note�3), the Company recorded $10.5�billion of goodwill and $40.9�billion of acquired identifiable intangible assets, including acquired IPR&D. The Company recorded an additional $7.3�billion of intangible assets in conjunction with the remeasurement of Merck�s previously held equity interest in the MSP Partnership.
The following table summarizes goodwill activity by segment:
| All | ||||||||||||
| Pharmaceutical | Other | Total | ||||||||||
Goodwill balance as of January�1, 2008 | $ | 1,115.2 | $ | 339.6 | $ | 1,454.8 | ||||||
Other | (16.1 | ) | - | (16.1 | ) | |||||||
Goodwill balance as of December�31, 2008 | 1,099.1 | 339.6 | 1,438.7 | |||||||||
Additions | 8,736.0 | 1,748.4 | 10,484.4 | |||||||||
Goodwill balance as of December�31, 2009 | $ | 9,835.1 | $ | 2,088.0 | $ | 11,923.1 | ||||||
| ||||||||||||
136
Other intangibles at December 31 consisted of:
| 2009 | 2008 | |||||||||||||||||||||||
| Gross | Gross | |||||||||||||||||||||||
| Carrying | Accumulated | Carrying | Accumulated | |||||||||||||||||||||
| Amount | Amortization | Net | Amount | Amortization | Net | |||||||||||||||||||
Products and product rights | $ | 41,413.8 | $ | 2,301.5 | $ | 39,112.3 | $ | 1,629.1 | $ | 1,501.2 | $ | 127.9 | ||||||||||||
In-process research and development (1) | 6,650.7 | � | 6,650.7 | � | � | � | ||||||||||||||||||
Tradenames | 1,599.8 | 52.1 | 1,547.7 | 64.0 | 37.5 | 26.5 | ||||||||||||||||||
Other | 816.3 | 471.2 | 345.1 | 742.5 | 371.5 | 371.0 | ||||||||||||||||||
Total identifiable intangible assets | $ | 50,480.6 | $ | 2,824.8 | $ | 47,655.8 | $ | 2,435.6 | $ | 1,910.2 | $ | 525.4 | ||||||||||||
| ||||||||||||||||||||||||
| (1) | Amounts capitalized as in-process research and development are accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of the projects. Upon successful completion of each project, the Company will make a separate determination as to the useful life of the assets and begin amortization. |
Aggregate amortization expense was $921.8�million in 2009, $186.1�million in 2008 and $235.8�million in 2007. The estimated aggregate amortization expense for each of the next five years is as follows: 2010, $4.8�billion; 2011, $4.8�billion; 2012, $4.7�billion; 2013, $4.7�billion; 2014, $4.4�billion.
| 10. | Joint Ventures and Other Equity Method Affiliates |
Equity income from affiliates reflects the performance of the Company�s joint ventures and other equity method affiliates and was comprised of the following:
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Merck/Schering-Plough | $ | 1,195.5 | $ | 1,536.3 | $ | 1,830.8 | ||||||
AstraZeneca LP | 674.3 | 598.4 | 820.1 | |||||||||
Other (1) | 365.2 | 425.9 | 325.6 | |||||||||
| $ | 2,235.0 | $ | 2,560.6 | $ | 2,976.5 | |||||||
| ||||||||||||
| (1) | Primarily reflects results from Merial Limited until disposition on September�17, 2009, Sanofi Pasteur MSD and Johnson�& Johnson�Merck Consumer Pharmaceuticals Company. |
137
However, these costs were reflected in the overall results of each company. Certain research and development expenses were generally shared equally by the Partners, after adjusting for earned milestones.
Summarized financial information for the MSP Partnership is as follows:
| Period from | ||||||||||||
| January�1, | ||||||||||||
| through | Years Ended | |||||||||||
| November�3, | December�31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
Sales | $ | 3,387.2 | $ | 4,561.1 | $ | 5,186.2 | ||||||
Vytorin | 1,689.5 | 2,360.0 | 2,779.1 | |||||||||
Zetia | 1,697.7 | 2,201.1 | 2,407.1 | |||||||||
Materials and production costs | 144.4 | 176.3 | 216.0 | |||||||||
Other expense, net | 848.7 | 1,230.1 | 1,307.2 | |||||||||
Income before taxes | $ | 2,394.1 | $ | 3,154.7 | $ | 3,663.0 | ||||||
Merck�s share of income before taxes (1) | $ | 1,197.7 | $ | 1,489.5 | $ | 1,832.5 | ||||||
| ||||||||||||
| December�31, | ||||||||||||
| 2008 | ||||||||||||
Total assets (2) | $ | 608.0 | ||||||||||
Total liabilities (2) | 488.0 | |||||||||||
| ||||||||||||
| (1) | Old Merck�s share of the MSP Partnership�s income before taxes differs from the equity income recognized from the MSP Partnership primarily due to the timing of recognition of certain transactions between Old Merck and the MSP Partnership during the periods presented, including milestone payments. |
| (2) | Amounts are comprised almost entirely of current balances. |
In 1998, Old Merck and Astra completed the restructuring of the ownership and operations of the joint venture whereby Old Merck acquired Astra�s interest in AMI, renamed KBI Inc. (�KBI�), and contributed KBI�s operating assets to a new U.S.�limited partnership, Astra Pharmaceuticals L.P. (the �Partnership�), in exchange for a 1% limited partner interest. Astra contributed the net assets of its wholly owned subsidiary, Astra USA, Inc., to the Partnership in exchange for a 99% general partner interest. The Partnership, renamed AstraZeneca LP (�AZLP�) upon Astra�s 1999 merger with Zeneca Group Plc (the �AstraZeneca merger�), became the exclusive distributor of the products for which KBI retained rights.
While maintaining a 1% limited partner interest in AZLP, Merck has consent and protective rights intended to preserve its business and economic interests, including restrictions on the power of the general partner to
138
The AstraZeneca merger constituted a Trigger Event under the KBI restructuring agreements. As a result of the merger, in exchange for Old Merck�s relinquishment of rights to future Astra products with no existing or pending U.S.�patents at the time of the merger, Astra paid $967.4�million (the �Advance Payment�). The Advance Payment was deferred as it remained subject to a true-up calculation (the �True-Up Amount�) that was directly dependent on the fair market value in March 2008 of the Astra product rights retained by Old Merck. The calculated True-Up Amount of $243.7�million was returned to AZLP in March 2008 and Old Merck recognized a pretax gain of $723.7�million related to the residual Advance Payment balance.
139
Summarized financial information for AZLP is as follows:
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Sales | $ | 5,743.6 | $ | 5,450.4 | $ | 6,345.4 | ||||||
Materials and production costs | 3,136.6 | 2,682.4 | 3,364.0 | |||||||||
Other expense, net | 1,194.2 | 1,408.1 | 1,090.1 | |||||||||
Income before taxes | 1,412.8 | 1,359.9 | 1,891.3 | |||||||||
| ||||||||||||
| December 31 | 2009 | 2008 | ||||||
Current assets | $ | 2,956.2 | $ | 2,023.9 | ||||
Noncurrent assets | 294.5 | 359.0 | ||||||
Total liabilities (all current) | 3,489.3 | 3,054.4 | ||||||
| ||||||||
In 1997, Old Merck and Rh�ne-Poulenc S.A. (now sanofi-aventis) combined their animal health businesses to form�Merial Limited (�Merial�), a fully integrated animal health company, which was a stand-alone joint venture, 50% owned by each party. Merial provides a comprehensive range of pharmaceuticals and vaccines to enhance the health, well-being and performance of a wide range of animal species.
Merial sales were $1.8�billion for the period from January�1, 2009 until the September�17, 2009 divestiture date, $2.6�billion for 2008 and $2.4�billion for 2007.
In 1994, Old Merck and Pasteur M�rieux Connaught (now Sanofi Pasteur S.A.) established an equally-owned joint venture to market vaccines in Europe and to collaborate in the development of combination vaccines for distribution in Europe. Joint venture vaccine sales were $1.6�billion for 2009, $1.9�billion for 2008 and $1.4�billion for 2007.
140
Summarized information for those affiliates (excluding the MSP Partnership and AZLP disclosed separately above) is as follows:
| Years Ended December 31 | 2009 (1) | 2008 | 2007 | |||||||||
Sales | $ | 3,767.0 | $ | 4,860.4 | $ | 4,218.6 | ||||||
Materials and production costs | 1,225.3 | 1,553.6 | 1,346.9 | |||||||||
Other expense, net | 1,564.1 | 2,297.9 | 1,995.2 | |||||||||
Income before taxes | 977.6 | 1,008.9 | 876.5 | |||||||||
| ||||||||||||
| December 31 | 2009 | 2008 | ||||||
Current assets | $ | 757.2 | $ | 1,935.8 | ||||
Noncurrent assets | 270.7 | 1,174.4 | ||||||
Current liabilities | 601.3 | 1,152.6 | ||||||
Noncurrent liabilities | 84.3 | 266.5 | ||||||
| ||||||||
| (1) | Includes information for Merial until divestiture on September�17, 2009. |
| 11. | Loans Payable, Long-Term Debt and Other Commitments |
Loans payable at December�31, 2009 included $739.1�million of Euro-denominated notes due in 2010 and short-term foreign borrowing of $235.9�million. Also included in loans payable at December�31, 2009 was $106.0�million of long-dated notes that are subject to repayment at the option of the holders beginning in 2010 that were reclassified from long-term debt during 2009. Additionally, loans payable at December�31, 2009 included $298.2�million of long-dated notes that are subject to repayment at the option of the holders on an annual basis. Loans payable at December�31, 2008 included $1.9�billion of commercial paper borrowings, $322.2�million of long-dated notes that are subject to repayment at the option of the holders on an annual basis and $68�million of short-term foreign borrowing.
141
Long-term debt at December 31 consisted of:
| 2009 | 2008 | |||||||
5.375% euro-denominated notes due 2014 | $ | 2,349.6 | $ | � | ||||
5.30%�notes due 2013 | 1,362.7 | � | ||||||
6.50%�notes due 2033 | 1,314.4 | � | ||||||
1.875%�notes due 2011 | 1,249.8 | � | ||||||
5.00%�notes due 2019 | 1,242.5 | � | ||||||
6.55%�notes due 2037 | 1,147.3 | � | ||||||
6.00%�notes due 2017 | 1,118.3 | � | ||||||
4.75%�notes due 2015 | 1,065.5 | 1,078.3 | ||||||
4.00%�notes due 2015 | 1,004.4 | � | ||||||
5.85%�notes due 2039 | 748.5 | � | ||||||
Floating rate euro-denominated term loan due 2012 | 650.0 | � | ||||||
4.375%�notes due 2013 | 522.7 | 530.0 | ||||||
6.4%�debentures due 2028 | 499.4 | 499.3 | ||||||
5.75%�notes due 2036 | 497.8 | 497.8 | ||||||
5.95%�debentures due 2028 | 497.4 | 497.2 | ||||||
5.125%�notes due 2011 | 268.5 | 273.7 | ||||||
6.3%�debentures due 2026 | 248.2 | 248.0 | ||||||
Other | 287.9 | 319.0 | ||||||
| $ | 16,074.9 | $ | 3,943.3 | |||||
| ||||||||
The Company was a party to interest rate swap contracts which effectively convert the 5.125% fixed-rate notes and $750�million of the 4.00% fixed-rate notes to floating-rate instruments (see Note�7).
Other (as presented in the table above) at December�31, 2009 and 2008 consisted primarily of $186.7�million and $292.7�million of borrowings at variable rates averaging 0.0% and 1.1%, respectively. Of these borrowings, $158.7�million is subject to repayment at the option of the holders beginning in 2011. In both years, Other also included foreign borrowings at varying rates up to 11.7%.
On June�25, 2009, Old Merck closed an underwritten public offering of $4.25�billion senior unsecured notes consisting of $1.25�billion aggregate principal amount of 1.875%�notes due 2011, $1.0�billion aggregate principal amount of 4.00%�notes due 2015, $1.25�billion aggregate principal amount of 5.00%�notes due 2019 and $750�million aggregate principal amount of 5.85%�notes due 2039. Interest on the notes is payable semi-annually. The notes of each series are redeemable in whole or in part at any time, at the Company�s option at the redemption prices specified in each notes associated prospectus. Proceeds from the notes were used to fund a portion of the cash consideration of the Merger.
In connection with the Merger, the Company recorded long-term debt with a fair value of $8.0�billion at December�31, 2009, which included $745.8�million representing the remaining excess of the fair value over the recorded value of debt which is being amortized to interest expense over the remaining lives of the underlying debt obligations.
The 5.00% euro-denominated notes due 2010, the 5.375% euro-denominated notes due 2014, the 5.30%�notes due 2013, the 6.50%�notes due 2033, the 6.00%�notes due 2017 and the 6.55%�notes due 2037 are redeemable in whole or in part, at Merck�s option at any time, at the redemption prices specified in each notes associated prospectus. With respect to the euro-denominated notes, the 6.00%�notes and the 6.55%�notes, if a change of control triggering event (as defined therein) occurs, under certain circumstances, as defined in each notes associated prospectus, holders of the notes will have the right to require Merck to repurchase all or any part of the notes for a cash payment equal to 101% of the aggregate principal amount of the notes repurchased plus accrued and unpaid interest, if any, to the date of purchase.
142
The 5.30%�notes due 2013 and the 6.50%�notes due 2033 were subject to interest rate adjustment provisions in the event that the rating assigned to a particular series of notes by either Moody�s Investors Service, Inc. or Standard and Poor�s Rating Services dropped below a predetermined level. Prior to the Merger, this interest rate adjustment was triggered and consequently, at the time of the Merger, the 5.30%�notes due 2013 were paying an interest rate of 5.55% and the 6.50%�notes due 2033 were paying an interest rate of 6.75%. Following the closing of the Merger, the ratings on each of these series of notes were upgraded such that, pursuant to the terms of the indenture, the interest rates payable on these notes reverted back to their stated amounts effective as of December�1, 2009 and the interest rate adjustment provisions on these notes no longer apply.
Also, in connection with the Merger, effective as of November�3, 2009, New Merck executed a full and unconditional guarantee of the existing debt of Old Merck and Old Merck executed a full and unconditional guarantee of the existing debt of New Merck (excluding commercial paper), including for payments of principal and interest.
The aggregate maturities of long-term debt for each of the next five years are as follows: 2010, $748.6�million; 2011, $1.6�billion; 2012, $674.2�million; 2013, $1.9�billion; 2014, $2.4�billion.
Also, in connection with the Merger, on March�8, 2009, Old Merck entered into a financing commitment letter with JPMorgan Chase Bank, N.A. and J.P.�Morgan Securities Inc. (collectively �JPMorgan�), under which JPMorgan committed to provide $7�billion of financing. On May�6, 2009, Old Merck entered into a $3�billion 364-day senior unsecured interim term loan facility (the �bridge loan facility�); a $3�billion 364-day asset sale revolving credit facility (the �asset sale facility�); and a $1�billion 364-day corporate revolving credit facility (the �incremental facility�). In connection with the above $4.25�billion offering, the bridge loan facility was terminated and the commitment of the lenders under the 364-day asset sale facility was reduced. Upon completion of the sale of Merial to sanofi-aventis (see Note�10), the asset sale facility was terminated. The incremental facility is available to backstop commercial paper and for general corporate purposes. This facility has not been drawn on and will expire in November 2010. Merck has incurred commitment fees of approximately $150�million associated with these facilities which are being amortized over the commitment period.
In April 2009, Old Merck amended its $1.5�billion, 5-year revolving credit facility maturing in April 2013 to allow the facility to remain in place after the Merger. The Company�s existing $2.0�billion credit facility maturing in August 2012 remains outstanding. Both facilities provide backup liquidity for the Company�s commercial paper borrowing facility and are to be used for general corporate purposes. The Company has not drawn funding from either facility.
Rental expense under operating leases, net of sublease income, was $236.6�million in 2009, $222.4�million in 2008 and $197.5�million in 2007. The minimum aggregate rental commitments under noncancellable leases are as follows: 2010, $281.6�million; 2011, $231.0�million; 2012, $162.4�million; 2013, $114.3�million and thereafter, $155.4�million. The Company has no significant capital leases.
| 12. | Contingencies and Environmental Liabilities |
The Company is involved in various claims and legal proceedings of a nature considered normal to its business, including product liability, intellectual property and commercial litigation, as well as additional matters such as antitrust actions. The Company records accruals for contingencies when it is probable that a liability has been incurred and the amount can be reasonably estimated. These accruals are adjusted periodically as assessments change or additional information becomes available. For product liability claims, a portion of the overall accrual is actuarially determined and considers such factors as past experience, number of claims reported and estimates of claims incurred but not yet reported. Individually significant contingent losses are accrued when probable and reasonably estimable. Legal defense costs expected to be incurred in connection with a loss contingency are accrued when probable and reasonably estimable.
The Company�s decision to obtain insurance coverage is dependent on market conditions, including cost and availability, existing at the time such decisions are made. As a result of a number of factors, product liability insurance has become less available while the cost has increased significantly. The Company has evaluated its risks and has determined that the cost of obtaining product liability insurance outweighs the likely benefits of the
143
coverage that is available and as such, has no insurance for certain product liabilities effective August�1, 2004, including liability for legacy Merck products first sold after that date. The Company will continue to evaluate its insurance needs and the costs, availability and benefits of product liability insurance in the future.
Interim and final payments have been made to certain qualifying claimants. It is expected that the remainder of the full $4.85�billion will be distributed in the first half of 2010. The Company has completed making payments into the settlement funds.
144
Plaintiffs also filed a class action in California state court seeking certification of a class of California third-party payors and end-users. The trial court denied the motion for class certification on April�30, 2009, and the Court of Appeal affirmed that ruling on December�15, 2009. On January�25, 2010, plaintiffs filed a petition for review with the California Supreme Court.
145
Old Merck�s motion for summary judgment was granted in November 2009 in a case brought by the Attorney General of Texas that was scheduled to go to trial in early 2010. The Texas Attorney General did not appeal. In the Michigan Attorney General case, Old Merck is currently seeking appellate review of the trial court�s order denying Old Merck�s motion to dismiss. The trial court has entered a stay of proceedings (including discovery) pending the result of that appeal. Finally, the Attorney General actions in the MDL described in the previous paragraph are in the discovery phase. The Louisiana Attorney General case is currently scheduled for trial in the MDL court on April�12, 2010.
146
defendants are not required to respond to these complaints until the resolution of any motion to dismiss in the consolidated securities action.
147
148
149
150
Merck in the New Jersey coordinated proceeding. On July�20, 2009, Judge Higbee entered a Case Management Order (and various amendments thereto) setting forth a schedule that contemplates completing fact discovery in an initial group of 10 cases by February�28, 2010, followed by expert discovery in five of those cases, and a projected trial date of July�12, 2010 for the first case to be tried in the New Jersey coordinated proceeding.
As previously disclosed, Old Merck was joined in ongoing litigation alleging manipulation by pharmaceutical manufacturers of Average Wholesale Prices (�AWP�), which are sometimes used in calculations that determine public and private sector reimbursement levels. The complaints allege violations of federal and state law, including fraud, Medicaid fraud and consumer protection violations, among other claims. The outcome of these litigations and investigations could include substantial damages, the imposition of substantial fines, penalties and injunctive or administrative remedies. In 2002, the JPML ordered the transfer and consolidation of all pending federal AWP cases to federal court in Boston, Massachusetts. Plaintiffs filed one consolidated class action complaint, which aggregated the claims previously filed in various federal district court actions and also expanded the number of manufacturers to include some which, like Old Merck, had not been defendants in any prior pending case. In May 2003, the court granted Old Merck�s motion to dismiss the consolidated class action and dismissed Old
151
Merck from the class action case. Old Merck and many other pharmaceutical manufacturers are defendants in similar complaints pending in federal and state court including cases brought individually by a number of counties in the State of New York. Fifty of the county cases have been consolidated in New York state court. Old Merck was dismissed from the Suffolk County case, which was the first of the New York county cases to be filed. In addition to the New York county cases, as of December�31, 2008, Old Merck was a defendant in state cases brought by the Attorneys General of eleven states, all of which are being defended. In February 2009, the Kansas Attorney General filed suit against Old Merck and several other manufacturers. AWP claims brought by the Attorney General of Arizona against Old Merck were dropped in 2009. The court in the AWP cases pending in Hawaii listed Old Merck and others to be set for trial in mid-2010.
The Company continues to respond to litigation brought by certain states and private payors and to investigations initiated by the Department of Health and Human Services, the Department of Justice and several states regarding AWP. The Company is cooperating with these investigations.
As previously disclosed, in February 2008, Old Merck entered into a Corporate Integrity Agreement (�CIA�) with the U.S.�Department of Health and Human Services Office of Inspector General (�HHS-OIG�) for a five-year term. The CIA requires, among other things, that Old Merck maintain its ethics training program and policies and procedures governing promotional practices and Medicaid price reporting. Further, as required by the CIA, Old Merck has retained an Independent Review Organization (�IRO�) to conduct a systems review of its promotional policies and procedures and to conduct, on a sample basis, transactional reviews of Old Merck�s promotional programs and certain Medicaid pricing calculations. Old Merck is also required to provide regular reports and certifications to the HHS-OIG regarding its compliance with the CIA.
Similarly, as previously disclosed by Schering-Plough, in 2004 Schering-Plough entered into a CIA with HHS-OIG for a five-year term, and in August 2006, it entered into an addendum to the CIA also effective for five years. The requirements of Old Merck and Schering-Plough CIAs are similar, although not identical. Failure to comply with the CIAs� requirements can result in financial penalties or exclusion from participation in federal health care programs. The Company believes that its promotional practices and Medicaid price reports meet the requirements of each of the CIAs.
152
153
and the other defendants filed motions to dismiss this lawsuit on the grounds that the plaintiffs failed to state a claim for which relief can be granted. On September�2, 2009, the court issued an opinion and order denying the defendants� motion to dismiss this lawsuit, and on September�17, 2009, the defendants filed a motion for reconsideration of the court�s September�2, 2009 opinion and order denying the motion to dismiss. The motion for reconsideration was fully briefed on October�13, 2009 and a decision remains pending. The defendants filed an answer to the consolidated amended complaint on November�18, 2009.
The Company intends to defend the lawsuits referred to in this section. Unfavorable outcomes resulting from the government investigations or the civil litigations could have a material adverse effect on the Company�s financial position, liquidity and results of operations.
154
On March�31, 2003, Schering-Plough was served with a putative class action complaint filed in the U.S.�District Court in New Jersey alleging that Schering-Plough, its Employee Savings Plan (the �Plan�) administrator, several current and former directors, and certain former corporate officers breached their fiduciary obligations to certain participants in the Plan. The complaint seeks damages in the amount of losses allegedly suffered by the Plan. The complaint was dismissed on June�29, 2004. The plaintiffs appealed. On August�19, 2005 the U.S.�Court of Appeals for the Third Circuit reversed the dismissal by the District Court and the matter has been remanded back to the District Court for further proceedings. On September�30, 2008, the District Court entered an order granting in part, and denying in part, the named putative class representative�s motion for class certification. Schering-Plough thereafter petitioned the U.S.�District Court of Appeals for the Third Circuit for leave to appeal the class certification decision. Schering-Plough�s petition was granted on December�10, 2008. On December�21, 2009, the Third Circuit vacated the District Court�s order and remanded the case for further proceedings consistent with the court�s ruling.
In June 1997 and January 1998, Schering-Plough settled patent litigation with Upsher-Smith, Inc. (�Upsher-Smith�) and ESI Lederle, Inc. (�Lederle�), respectively, relating to generic versions of K-DUR, Schering-Plough�s long-acting potassium chloride product supplement used by cardiac patients, for which Lederle and Upsher-Smith had filed Abbreviated New Drug Applications. Following the commencement of an administrative proceeding by the United States Federal Trade Commission (the �FTC�) alleging anti-competitive effects from those settlements (which has been resolved in Schering-Plough�s favor), alleged class action suits were filed in federal and state courts on behalf of direct and indirect purchasers of K-DUR against Schering-Plough, Upsher-Smith and Lederle. These suits claim violations of federal and state antitrust laws, as well as other state statutory and common law causes of action. These suits seek unspecified damages. In February 2009, a special master recommended that the U.S.�District Court for the District of New Jersey dismiss the class action lawsuits on summary judgment. The U.S.�District Court judge has not yet ruled on the recommendation.
155
As discussed above, in July 2004, in connection with the settlement of an investigation with the DOJ and the U.S.�Attorney�s Office for the Eastern District of Pennsylvania, Schering-Plough entered into a five-year CIA. The CIA was amended in August 2006 in connection with the $435�million settlement of an investigation by the State of Massachusetts involving certain of Schering-Plough�s sales, marketing and clinical trial practices and programs (�Massachusetts Investigation�). Several purported class action litigations have been filed following the announcement of the settlement of the Massachusetts Investigation. Plaintiffs in these actions seek damages on behalf of third-party payors resulting from the allegations of off-label promotion and improper payments to physicians that were at issue in the Massachusetts Investigation. The actions have been consolidated in a multidistrict litigation in federal District Court for the District of New Jersey. In July 2009, the District Court dismissed the consolidated class action complaint but granted plaintiffs leave to refile. In September 2009, plaintiffs filed amended complaints, and the Company�s motion to dismiss those complaints is pending.
As previously disclosed, Old Merck is a party to individual and class action product liability lawsuits and claims in the United States involving pediatric vaccines (e.g., hepatitis B vaccine) that contained thimerosal, a preservative used in vaccines. As of December�31, 2009, there were approximately 200 thimerosal related lawsuits pending in which Old Merck is a defendant, although the vast majority of those lawsuits are not currently active. Other defendants include other vaccine manufacturers who produced pediatric vaccines containing thimerosal as well as manufacturers of thimerosal. In these actions, the plaintiffs allege, among other things, that they have suffered neurological injuries as a result of exposure to thimerosal from pediatric vaccines. There are no cases currently scheduled for trial. The Company will defend against these lawsuits; however, it is possible that unfavorable outcomes could have a material adverse effect on the Company�s financial position, liquidity and results of operations.
Old Merck has been successful in having cases of this type either dismissed or stayed on the ground that the action is prohibited under the National Childhood Vaccine Injury Act (the �Vaccine Act�). The Vaccine Act prohibits any person from filing or maintaining a civil action (in state or federal court) seeking damages against a vaccine manufacturer for vaccine-related injuries unless a petition is first filed in the United States Court of Federal Claims (hereinafter the �Vaccine Court�). Under the Vaccine Act, before filing a civil action against a vaccine manufacturer, the petitioner must either (a)�pursue his or her petition to conclusion in Vaccine Court and then timely file an election to proceed with a civil action in lieu of accepting the Vaccine Court�s adjudication of the petition or (b)�timely exercise a right to withdraw the petition prior to Vaccine Court adjudication in accordance with certain statutorily prescribed time periods. Old Merck is not a party to Vaccine Court proceedings because the petitions are brought against the United States Department of Health and Human Services.
156
In May 2005, the Federal Court of Canada Trial Division issued a decision refusing to bar the approval of generic alendronate on the grounds that Old Merck�s patent for weekly alendronate was likely invalid. This decision cannot be appealed and generic alendronate was launched in Canada in June 2005. In July 2005, Old Merck was sued in the Federal Court of Canada by Apotex Corp. (�Apotex�) seeking damages for lost sales of generic weekly alendronate due to the patent proceeding. In October 2008, the Federal Court of Canada issued a decision awarding Apotex its lost profits for its generic alendronate product for the period of time that it was held off the market due to Old Merck�s lawsuit. In June 2009, the trial court decision was upheld in part and both companies sought leave to appeal to the Supreme Court of Canada. In January 2010, the Supreme Court of Canada declined to hear the appeal, leaving intact the decision that Apotex is entitled to damages for the discrete period of time that its market entry was postponed due to the litigation launched by Old Merck.
157
In addition, as previously disclosed, in Japan after a proceeding was filed challenging the validity of Old Merck�s Japanese patent for the once-weekly administration of alendronate, the patent office invalidated the patent. The decision is under appeal.
158
and Company (�du Pont�). Old Merck and du Pont have filed patent infringement proceedings against various companies in Portugal, Spain, Norway, Finland, Belgium, the Netherlands and Austria.
In connection with the Merger, separate class action lawsuits were brought against Old Merck and Schering-Plough challenging the Merger and seeking other forms of relief. As previously disclosed, both lawsuits have been settled pending court approval.
These settlements, if approved by the court, will resolve and release all claims that were or could have been brought by any shareholder of Old Merck or Schering-Plough challenging any aspect of the proposed merger, including any merger disclosure claims.
159
basis that the Juge des Libert�s et de la D�tention had not examined each document to assess whether it should have been seized and whether it had been lawfully seized. The case is now pending before the Paris Court of Appeal.
There are various other legal proceedings, principally product liability and intellectual property suits involving the Company, that are pending. While it is not feasible to predict the outcome of such proceedings or the proceedings discussed in this Note, in the opinion of the Company, all such proceedings are either adequately covered by insurance or, if not so covered, should not ultimately result in any liability that would have a material adverse effect on the financial position, liquidity or results of operations of the Company, other than proceedings for which a separate assessment is provided in this Note.
As previously disclosed, approximately 2,200 plaintiffs have filed an amended complaint against Old Merck and 12 other defendants in U.S.�District Court, Eastern District of California asserting claims under the Clean Water Act, the Resource Conservation and Recovery Act, as well as negligence and nuisance. The suit seeks damages for personal injury, diminution of property value, medical monitoring and other alleged real and personal property damage associated with groundwater and soil contamination found at the site of a former Old Merck subsidiary in Merced, California. Old Merck intends to defend itself against these claims.
In management�s opinion, the liabilities for all environmental matters that are probable and reasonably estimable have been accrued and totaled $161.8�million and $89.5�million at December�31, 2009 and 2008, respectively. These liabilities are undiscounted, do not consider potential recoveries from other parties and will be paid out over the periods of remediation for the applicable sites, which are expected to occur primarily over the next 15�years. Although it is not possible to predict with certainty the outcome of these matters, or the ultimate costs of remediation, management does not believe that any reasonably possible expenditures that may be incurred in excess of the liabilities accrued should exceed $170.0�million in the aggregate. Management also does not believe that these expenditures should result in a material adverse effect on the Company�s financial position, results of operations, liquidity or capital resources for any year.
160
| 13. | Equity |
In accordance with the New Merck certificate of incorporation there are 6,500,000,000�shares of common stock and 20,000,000�shares of preferred stock authorized. Of the authorized shares of preferred stock, there is a series of 11,500,000�shares which is designated as 6% mandatory convertible preferred stock.
Prior to the Merger, on August�15, 2007, Schering-Plough issued 10,000,000�shares of Schering-Plough 6% preferred stock. In connection with the Merger, holders of the Schering-Plough 6% preferred stock received 6% preferred stock (which rights were substantially similar to the rights of the Schering-Plough 6% preferred stock) in accordance with the New Merck Restated Certificate of Incorporation. As a result of the Merger, the 6% preferred stock became subject to the �make-whole� acquisition provisions of the preferred stock effective as of November�3, 2009. During the make-whole acquisition conversion period that ended on November�19, 2009, the 6% preferred stock was convertible at a make-whole conversion rate of 8.2021. For each share of preferred stock that was converted during this period, the holder received $86.12 in cash and 4.7302 New Merck common shares. Holders also received a dividend make-whole payment of between $10.79 and $10.82 depending on the date of the conversion. A total of 9,110,423�shares of 6% preferred stock were converted into 43,093,881�shares of New Merck common stock and cash payments of approximately $785�million were made to those holders who converted. In addition, make-whole dividend payments of $98.5�million were made to those holders who converted representing the present value of all remaining future dividend payments from the conversion date through the mandatory conversion date on August�13, 2010 using the discount rate as stipulated in the preferred stock designations.
As of December�31, 2009, 855,422�shares of 6% preferred stock remained issued and outstanding. These outstanding shares will automatically convert into common shares of the Company and cash on August�13, 2010, pursuant to the provisions of the New Merck Restated Certificate of Incorporation. The holders may also elect to convert at any time prior to August�13, 2010. The 6% preferred stock of $206.6�million at December�31, 2009 has been classified as a current liability because all conversions will be settled as a combination of cash and common stock. Additionally, under certain conditions, the Company may elect to cause the conversion of all, but not less than all, of the Merck 6% preferred stock then outstanding.
The 6% preferred stock accrues dividends at an annual rate of 6% on shares outstanding. The dividends are cumulative from the date of issuance and, to the extent the Company is legally permitted to pay dividends and the Board of Directors declares a dividend payable, the Company will pay dividends on each dividend payment date. The remaining dividend payment dates are February�15, May 15 and August�13, 2010.
A summary of common stock and treasury stock transactions (shares in millions) is as follows:
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| Common | Treasury | Common | Treasury | Common | Treasury | |||||||||||||||||||
| Stock | Stock | Stock | Stock | Stock | Stock | |||||||||||||||||||
Balance as of January 1 | 2,983.5 | 875.8 | 2,983.5 | 811.0 | 2,976.2 | 808.4 | ||||||||||||||||||
Issuances of shares in connection with the Merger | 1,054.3 | 64.0 | � | � | � | � | ||||||||||||||||||
Issuances of shares in connection with the acquisition of NovaCardia, Inc. | � | � | � | � | 7.3 | � | ||||||||||||||||||
Other issuances (1) | 8.7 | (1.5 | ) | � | (4.7 | ) | � | (23.9 | ) | |||||||||||||||
Purchases of treasury stock | � | � | � | 69.5 | � | 26.5 | ||||||||||||||||||
Cancellations of treasury stock (2) | (484.0 | ) | (484.0 | ) | � | � | � | � | ||||||||||||||||
Balance as of December 31 | 3,562.5 | 454.3 | 2,983.5 | 875.8 | 2,983.5 | 811.0 | ||||||||||||||||||
| ||||||||||||||||||||||||
| (1) | Issuances primarily reflect activity under share-based compensation plans. |
| (2) | Pursuant to the Merger agreement, certain of Old Merck�s treasury shares were cancelled. |
161
| 14. | Share-Based Compensation Plans |
The Company has share-based compensation plans under which employees, non-employee directors and employees of certain of the Company�s equity method investees may be granted options to purchase shares of Company common stock at the fair market value at the time of grant. In addition to stock options, the Company grants performance share units (�PSUs�) and restricted stock units (�RSUs�) to certain management level employees. These plans were approved by the Company�s shareholders.
As a result of the Merger, the Schering-Plough 2006 Stock Incentive Plan (�Schering-Plough 2006 SIP�) was amended and restated. Share-based compensation instruments remain available for future grant under the Schering-Plough 2006 SIP to New Merck employees who were employees of Schering-Plough prior to the Merger. As such, there are outstanding share-based compensation instruments, as well as share-based compensation instruments available for future grant, under Old Merck and New Merck incentive plans.
Also, as a result of the Merger, certain share-based compensation instruments previously granted under the Schering-Plough 2006 SIP and other legacy Schering-Plough incentive plans were exchanged for New Merck replacement awards. Other awards related to precombination services became payable in cash. In addition, certain stock options under Schering-Plough legacy incentive plans contain a �lock-in� feature whereby an award holder can elect to receive a cash payment for those stock options at a fixed amount based on the price of Schering-Plough�s common stock 60�days prior to the Merger. The liability associated with this provision was $246.4�million at December�31, 2009. Upon expiration of the exercise period associated with the �lock-in� feature, the amount was reclassified from liabilities to equity. The fair value of replacement awards attributable to precombination service was $525.2�million and is included in the calculation of consideration transferred (see Note�3). A significant portion of the legacy Schering-Plough awards vested in the opening balance sheet at the time of the Merger. Those Schering-Plough share-based compensation instruments that did not immediately vest upon completion of the Merger were exchanged for New Merck replacement awards that generally vest on the same basis as the original grants made under the Schering-Plough legacy incentive plans and will immediately vest if the employee is terminated by the Company within two years of the Merger under certain circumstances. The fair value of New Merck replacement awards attributed to postcombination services is being recognized as compensation cost subsequent to the Merger over the requisite service period of the awards.
At December�31, 2009, 134.0�million shares collectively were authorized for future grants under the Company�s share-based compensation plans. Prior to the Merger, employee share-based compensation awards were settled primarily with treasury shares. Subsequent to the Merger, these awards are being settled with newly issued shares.
Employee stock options are granted to purchase shares of Company stock at the fair market value at the time of grant. These awards generally vest one-third each year over a three-year period, with a contractual term of 7-10�years. RSUs are stock awards that are granted to employees and entitle the holder to shares of common stock as the awards vest, as well as non-forfeitable dividend equivalents. The fair value of the stock option and RSU awards is determined and fixed on the grant date based on the Company�s stock price. PSUs are stock awards where the ultimate number of shares issued will be contingent on the Company�s performance against a pre-set objective or set of objectives. The fair value of each PSU is determined on the date of grant based on the Company�s stock price. The fair value of stock option, RSU and PSU replacement awards was determined and fixed at the time of the Merger. Over the PSU performance period, the number of shares of stock that are expected to be issued will be adjusted based on the probability of achievement of a performance target and final compensation expense will be recognized
162
based on the ultimate number of shares issued. RSU and PSU distributions will be in shares of Company stock after the end of the vesting or performance period, generally three years, subject to the terms applicable to such awards.
Total pretax share-based compensation cost recorded in 2009, 2008 and 2007 was $415.5�million, $348.0�million and $330.2�million, respectively, with related income tax benefits of $132.4�million, $107.5�million and $104.1�million, respectively.
The Company uses the Black-Scholes option pricing model for determining the fair value of option grants. In applying this model, the Company uses both historical data and current market data to estimate the fair value of its options. The Black-Scholes model requires several assumptions including expected dividend yield, risk-free interest rate, volatility, and term of the options. The expected dividend yield is based on historical patterns of dividend payments. The risk-free rate is based on the rate at grant date of zero-coupon U.S.�Treasury Notes with a term equal to the expected term of the option. Expected volatility is estimated using a blend of historical and implied volatility. The historical component is based on historical monthly price changes. The implied volatility is obtained from market data on the Company�s traded options. The expected life represents the expected amount of time that options granted are expected to be outstanding, based on historical and forecasted exercise behavior.
The weighted average fair value of options granted in 2009, 2008 and 2007 was $4.02, $9.80 and $9.51 per option, respectively, and were determined using the following assumptions:
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Expected dividend yield | 6.3 | % | 3.5 | % | 3.4 | % | ||||||
Risk-free interest rate | 2.2 | % | 2.7 | % | 4.4 | % | ||||||
Expected volatility | 33.8 | % | 31.0 | % | 24.6 | % | ||||||
Expected life (years) | 6.1 | 6.1 | 5.7 | |||||||||
| ||||||||||||
Summarized information relative to stock option plan activity (options in thousands) is as follows:
| Weighted | ||||||||||||||||
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Number | Exercise | Contractual | Intrinsic | |||||||||||||
| of Options | Price | Term | Value | |||||||||||||
Balance as of January�1, 2009 | 247,651.3 | $ | 51.50 | |||||||||||||
Granted | 34,279.2 | 24.31 | ||||||||||||||
Replacement awards | 66,611.8 | 28.23 | ||||||||||||||
Exercised | (8,548.1 | ) | 21.80 | |||||||||||||
Forfeited | (26,139.4 | ) | 68.10 | |||||||||||||
Outstanding as of December�31, 2009 | 313,854.8 | $ | 43.02 | 4.95 | $ | 1,102.7 | ||||||||||
| ||||||||||||||||
Exercisable as of December�31, 2009 | 235,353.6 | $ | 46.48 | 3.90 | $ | 531.4 | ||||||||||
| ||||||||||||||||
Additional information pertaining to stock option plans is provided in the table below:
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Total intrinsic value of stock options exercised | $ | 119.1 | $ | 40.3 | $ | 301.2 | ||||||
Fair value of stock options
vested (1) | $ | 311.2 | $ | 259.0 | $ | 251.1 | ||||||
Cash received from the exercise of stock options | $ | 186.4 | $ | 102.3 | $ | 898.6 | ||||||
| ||||||||||||
| (1) | The fair value of stock options vested excludes the fair value of options that vested as a result of the Merger attributable to precombination service. |
163
A summary of nonvested RSU and PSU activity (shares in thousands) is as follows:
| RSUs | PSUs | |||||||||||||||
| Weighted | Weighted | |||||||||||||||
| Average | Average | |||||||||||||||
| Number | Grant Date | Number | Grant Date | |||||||||||||
| of Shares | Fair Value | of Shares | Fair Value | |||||||||||||
Nonvested as of January�1, 2009 | 6,292.2 | $ | 39.41 | 1,621.4 | $ | 41.86 | ||||||||||
Granted | 2,818.5 | 26.78 | 726.4 | 24.20 | ||||||||||||
Replacement awards | 8,105.6 | 30.67 | 2,049.9 | 30.67 | ||||||||||||
Vested | (1,896.6 | ) | 34.18 | (1,063.7 | ) | 32.10 | ||||||||||
Forfeited | (201.0 | ) | 36.81 | (1,011.4 | ) | 31.46 | ||||||||||
Nonvested at December�31, 2009 | 15,118.7 | $ | 33.06 | 2,322.6 | $ | 35.46 | ||||||||||
| ||||||||||||||||
At December�31, 2009, there was $521.8�million of total pretax unrecognized compensation expense related to nonvested stock options, RSU and PSU awards which will be recognized over a weighted average period of 1.5�years. For segment reporting, share-based compensation costs are unallocated expenses.
| 15. | Pension and Other Postretirement Benefit Plans |
The Company has defined benefit pension plans covering eligible employees in the United States and in certain of its international subsidiaries. Pension benefits in the United States are based on a formula that considers final average pay and years of credited service. In addition, the Company provides medical, dental and life insurance benefits, principally to its eligible U.S.�retirees and similar benefits to their dependents, through its other postretirement benefit plans. The Company uses December 31 as the year-end measurement date for all of its pension plans and other postretirement benefit plans.
The net cost for pension and other postretirement benefit plans consisted of the following components:
| Other Postretirement | ||||||||||||||||||||||||
| Pension Benefits | Benefits | |||||||||||||||||||||||
| Years Ended December 31 | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||||
Service cost | $ | 398.4 | $ | 344.1 | $ | 377.2 | $ | 75.3 | $ | 73.2 | $ | 90.8 | ||||||||||||
Interest cost | 449.7 | 414.2 | 379.9 | 108.3 | 113.8 | 107.7 | ||||||||||||||||||
Expected return on plan assets | (649.2 | ) | (559.4 | ) | (491.4 | ) | (98.0 | ) | (129.0 | ) | (130.5 | ) | ||||||||||||
Net amortization | 122.8 | 70.4 | 149.4 | 18.9 | (22.6 | ) | (16.8 | ) | ||||||||||||||||
Termination benefits | 88.7 | 62.3 | 25.6 | 9.6 | 11.2 | 7.7 | ||||||||||||||||||
Curtailments | (6.2 | ) | 5.7 | 1.1 | (9.9 | ) | (15.9 | ) | (16.8 | ) | ||||||||||||||
Settlements | 2.7 | 8.6 | 5.4 | - | - | - | ||||||||||||||||||
Net pension and other postretirement cost | $ | 406.9 | $ | 345.9 | $ | 447.2 | $ | 104.2 | $ | 30.7 | $ | 42.1 | ||||||||||||
| ||||||||||||||||||||||||
Net pension and other postretirement benefit cost totaled $511.1�million in 2009, $376.6�million in 2008 and $489.3�million in 2007. The increase in 2009 as compared with 2008 is primarily due to $118.2�million of costs associated with Schering-Plough benefit plans from the date of the Merger through December�31, 2009.
The net pension cost attributable to U.S.�plans included in the above table was $288.7�million in 2009, $226.4�million in 2008 and $302.2�million in 2007.
In connection with restructuring actions (see Note�4), termination charges were recorded in 2009, 2008 and 2007 on pension and other postretirement benefit plans related to expanded eligibility for certain employees exiting Merck. Also, in connection with these restructuring activities, net curtailment gains were recorded in 2009 and curtailment losses were recorded in 2008 and 2007 on pension plans and net curtailment gains were recorded in 2009, 2008 and 2007 on other postretirement benefit plans.
164
In addition, settlement losses were recorded in 2009, 2008 and 2007 on certain of its domestic and international pension plans.
Employee benefit plans are an exception to the recognition and fair value measurement principles in business combinations. Employee benefit plan obligations are recognized and measured in accordance with the existing authoritative literature for accounting for benefit plans rather than at fair value. Accordingly, the Company remeasured the benefit plans sponsored by Schering-Plough and recognized an asset or liability for the funded status of these plans as of the Merger date.
Summarized information about the changes in plan assets and benefit obligation, the funded status and the amounts recorded at December�31, 2009 and 2008 is as follows:
| Pension Benefits | Other Postretirement Benefits | |||||||||||||||
| 2009 | 2008 | 2009 | 2008 | |||||||||||||
Fair value of plan assets at January 1 | $ | 5,887.6 | $ | 7,385.4 | $ | 1,088.4 | $ | 1,577.6 | ||||||||
Actual return on plan assets | 1,450.2 | (1,959.4 | ) | 311.4 | (512.0 | ) | ||||||||||
Company contributions | 868.7 | 1,190.8 | 88.8 | 99.5 | ||||||||||||
Schering-Plough merger | 3,041.2 | - | 107.2 | - | ||||||||||||
Effects of exchange rate changes | 72.9 | (90.3 | ) | - | - | |||||||||||
Benefits paid | (511.0 | ) | (643.2 | ) | (73.2 | ) | (76.7 | ) | ||||||||
Other | 25.1 | 4.3 | - | - | ||||||||||||
Fair value of plan assets at December 31 | $ | 10,834.7 | $ | 5,887.6 | $ | 1,522.6 | $ | 1,088.4 | ||||||||
| ||||||||||||||||
Benefit obligation at January 1 | $ | 7,140.1 | $ | 7,049.4 | $ | 1,747.3 | $ | 1,936.8 | ||||||||
Service cost | 398.4 | 344.1 | 75.3 | 73.2 | ||||||||||||
Interest cost | 449.7 | 414.2 | 108.3 | 113.8 | ||||||||||||
Schering-Plough merger | 5,029.7 | - | 586.1 | - | ||||||||||||
Actuarial losses (gains) | 517.9 | 325.8 | 121.1 | (129.8 | ) | |||||||||||
Benefits paid | (511.0 | ) | (643.2 | ) | (73.2 | ) | (76.7 | ) | ||||||||
Effects of exchange rate changes | 88.2 | (158.0 | ) | 5.9 | (6.6 | ) | ||||||||||
Plan amendments | 1.8 | - | - | (180.6 | ) | |||||||||||
Curtailments | (32.6 | ) | (249.6 | ) | 33.7 | 6.0 | ||||||||||
Termination benefits | 88.7 | 62.3 | 9.6 | 11.2 | ||||||||||||
Other | 12.4 | (4.9 | ) | - | - | |||||||||||
Benefit obligation at December 31 | $ | 13,183.3 | $ | 7,140.1 | $ | 2,614.1 | $ | 1,747.3 | ||||||||
| ||||||||||||||||
Funded status at December 31 | $ | (2,348.6 | ) | $ | (1,252.5 | ) | $ | (1,091.5 | ) | $ | (658.9 | ) | ||||
| ||||||||||||||||
Recognized as: | ||||||||||||||||
Other assets | $ | 402.0 | $ | 142.4 | $ | 220.1 | $ | 147.7 | ||||||||
Accrued and other current liabilities | (248.7 | ) | (46.8 | ) | (9.2 | ) | (3.4 | ) | ||||||||
Deferred income taxes and noncurrent liabilities | (2,501.9 | ) | (1,348.1 | ) | (1,302.4 | ) | (803.2 | ) | ||||||||
| ||||||||||||||||
The fair value of U.S.�pension plan assets included in the preceding table was $6.1�billion in 2009 and $3.5�billion in 2008. The pension projected benefit obligation of U.S.�plans included in this table was $7.6�billion in 2009 and $4.6�billion in 2008. The increase in the fair value and the pension projected benefit obligation of the U.S.�plans was primarily related to the Merger. Approximately 42% of the Company�s pension projected benefit obligation relates to international defined benefit plans, of which each individual plan is not significant relative to the total benefit obligation.
165
At December�31, 2009 and 2008, the accumulated benefit obligation was $10.7�billion and $5.7�billion, respectively, for all pension plans. At December�31, 2009 and 2008, the accumulated benefit obligation for U.S.�pension plans was $6.0�billion and $3.4�billion, respectively.
For pension plans with benefit obligations in excess of plan assets at December�31, 2009 and 2008, the fair value of plan assets was $4.9�billion and $4.8�billion, respectively, and the benefit obligation was $7.7�billion and $6.2�billion, respectively. For those plans with accumulated benefit obligations in excess of plan assets at December�31, 2009 and 2008, the fair value of plan assets was $3.5�billion and $414.5�million, respectively, and the accumulated benefit obligation was $5.1�billion and $880.0�million, respectively.
As discussed in Note�2, as of December�31, 2009, the Company adopted new authoritative guidance issued by the FASB which revised the disclosure requirements for plan assets of defined pension and other postretirement plans. This amended guidance requires disclosure of how investment allocation decisions are made, the major categories of plan assets, the inputs and valuation techniques used to measure the fair value of plan assets, the effect of fair value measurements using significant unobservable inputs (Level�3)�on changes in plan assets for the period, and significant concentrations of risk within plan assets.
Entities are required to use a fair value hierarchy which maximizes the use of observable inputs and minimizes the use of unobservable inputs when measuring fair value. There are three levels of inputs that may be used to measure fair value:
If the inputs used to measure the financial assets fall within more than one level described above, the categorization is based on the lowest level input that is significant to the fair value measurement of the instrument.
166
The fair values of the Company�s pension plan assets at December�31, 2009 by asset category are as follows:
| Fair Value Measurements Using | ||||||||||||||||
| Quoted Prices | Significant | |||||||||||||||
| In Active | Other | Significant | ||||||||||||||
| Markets for | Observable | Unobservable | ||||||||||||||
| Identical Assets | Inputs | Inputs | ||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | Total | |||||||||||||
Assets | ||||||||||||||||
Cash and cash equivalents | $ | 95.9 | $ | 544.1 | $ | - | $ | 640.0 | ||||||||
Securities lending collaterial in short-term investments | - | 280.5 | - | 280.5 | ||||||||||||
Equity securities | ||||||||||||||||
U.S. large cap | 469.6 | 1,805.5 | - | 2,275.1 | ||||||||||||
U.S. small/mid cap | 625.3 | 744.5 | - | 1,369.8 | ||||||||||||
Non-U.S. developed markets | 1,274.2 | 1,077.3 | - | 2,351.5 | ||||||||||||
Emerging markets | 93.3 | 449.0 | - | 542.3 | ||||||||||||
Fixed income securities | ||||||||||||||||
Government and agency obligations | 70.3 | 1,599.7 | - | 1,670.0 | ||||||||||||
Corporate obligations | 71.2 | 819.0 | 0.9 | 891.1 | ||||||||||||
Mortgage and asset backed securities | - | 287.8 | 3.0 | 290.8 | ||||||||||||
Other fixed income obligations | - | 22.6 | - | 22.6 | ||||||||||||
Other types of investments | ||||||||||||||||
Insurance contracts | - | 139.2 | 310.0 | 449.2 | ||||||||||||
Real estate | - | 8.7 | 190.4 | 199.1 | ||||||||||||
Derivatives | - | 70.6 | - | 70.6 | ||||||||||||
Other | - | 2.2 | 63.9 | 66.1 | ||||||||||||
| $ | 2,699.8 | $ | 7,850.7 | $ | 568.2 | $ | 11,118.7 | |||||||||
| ||||||||||||||||
Liabilities | ||||||||||||||||
Liability for the return of collateral for securities loaned | $ | - | $ | 280.5 | $ | - | $ | 280.5 | ||||||||
| ||||||||||||||||
The table below provides a summary of the changes in fair value, including net transfers in and/or out, of all financial assets measured at fair value using significant unobservable inputs (Level�3)�during 2009 for the Company�s pension plan assets:
| Actual Return on Plan Assets | ||||||||||||||||||||||||
| Relating to | ||||||||||||||||||||||||
| Beginning | Assets Still | Ending | ||||||||||||||||||||||
| Balance at | Held at | Relating to | Purchases, | Schering- | Balance at | |||||||||||||||||||
| January 1, | December�31, | Assets Sold | Sales, | Plough | December�31, | |||||||||||||||||||
| 2009 | 2009 | During 2009 | Settlements, Net | Merger | 2009 | |||||||||||||||||||
Insurance Contracts | $ | 182.0 | $ | 19.5 | $ | - | $ | (18.0 | ) | $ | 126.5 | $ | 310.0 | |||||||||||
Real Estate | 52.6 | (9.4 | ) | - | (0.3 | ) | 147.5 | 190.4 | ||||||||||||||||
Other | 0.2 | 1.2 | 0.1 | 1.1 | 65.2 | 67.8 | ||||||||||||||||||
Total | $ | 234.8 | $ | 11.3 | $ | 0.1 | $ | (17.2 | ) | $ | 339.2 | $ | 568.2 | |||||||||||
| ||||||||||||||||||||||||
167
The fair values of the Company�s other postretirement benefit plan assets at December�31, 2009 by asset category are as follows:
| Fair Value Measurements Using | ||||||||||||||||
| Quoted Prices | Significant | |||||||||||||||
| In Active | Other | Significant | ||||||||||||||
| Markets for | Observable | Unobservable | ||||||||||||||
| Identical Assets | Inputs | Inputs | ||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | Total | |||||||||||||
Assets | ||||||||||||||||
Cash and cash equivalents | $ | 1.7 | $ | 58.1 | $ | - | $ | 59.8 | ||||||||
Securites lending collateral in short-term investments | - | 65.0 | - | 65.0 | ||||||||||||
Equity securities | ||||||||||||||||
U.S. large cap | 25.2 | 436.4 | - | 461.6 | ||||||||||||
U.S. small/mid cap | 85.5 | 271.6 | - | 357.1 | ||||||||||||
Non-U.S. developed markets | 187.3 | 95.5 | - | 282.8 | ||||||||||||
Emerging markets | 31.2 | 80.7 | - | 111.9 | ||||||||||||
Fixed income securities | ||||||||||||||||
Corporate obligations | 1.6 | 137.9 | - | 139.5 | ||||||||||||
Government and agency obligations | 4.3 | 66.5 | - | 70.8 | ||||||||||||
Mortgage and asset backed securities | 3.4 | 27.8 | - | 31.2 | ||||||||||||
Other fixed income obligations | - | 6.0 | - | 6.0 | ||||||||||||
Total investments | $ | 340.2 | $ | 1,245.5 | $ | - | $ | 1,585.7 | ||||||||
| ||||||||||||||||
Liabilities | ||||||||||||||||
Liability for the return of collateral for securities loaned | $ | - | $ | 65.0 | $ | - | $ | 65.0 | ||||||||
| ||||||||||||||||
Total pension and other postretirement benefit plan assets excluded from the fair value hierarchy include short-term payables and receivables related to the purchase and sale of investments, respectively.
The Company has established investment guidelines for its U.S.�pension and other postretirement plans to create an asset allocation that is expected to deliver a rate of return sufficient to meet the long-term obligation of each plan, given acceptable level of risk. The target investment portfolio of the Company�s U.S.�pension and other postretirement benefit plans is allocated 45% to 60% in U.S.�equities, 20% to 30% in international equities, 15% to 25% in fixed-income investments, and up to 8% in cash and other investments. The portfolio�s equity weighting is consistent with the long-term nature of the plans� benefit obligations. The expected annual standard deviation of returns of the target portfolio, which approximates 13%, reflects both the equity allocation and the diversification benefits among the asset classes in which the portfolio invests. For non-U.S.�pension plans, the targeted investment portfolio varies based on the duration of pension liabilities and local government rules and regulations. Although a significant percentage of plan assets are invested in U.S.�equities, concentration risk is mitigated through the use of strategies that are diversified within management guidelines.
Contributions to the pension plans and other postretirement benefit plans during 2010 are expected to be approximately $950�million and $50�million, respectively.
168
Expected benefit payments are as follows:
| Other | ||||||||
| Pension | Postretirement | |||||||
| Benefits | Benefits | |||||||
2010 | $ | 639.6 | $ | 119.3 | ||||
2011 | 521.3 | 127.4 | ||||||
2012 | 565.7 | 134.2 | ||||||
2013 | 593.1 | 142.7 | ||||||
2014 | 617.0 | 151.2 | ||||||
2015�� 2019 | 4,018.4 | 898.6 | ||||||
| ||||||||
Expected benefit payments are based on the same assumptions used to measure the benefit obligations and include estimated future employee service.
| Other Postretirement | ||||||||||||||||||||||||
| Pension Plans | Benefit Plans | |||||||||||||||||||||||
| Years Ended December 31 | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||||
Net gain (loss) arising during the period | $ | 302.5 | $ | (2,586.0 | ) | $ | 269.1 | $ | 70.9 | $ | (509.3 | ) | $ | (16.5 | ) | |||||||||
Prior service (cost) credit arising during the period | (0.5 | ) | 10.6 | 21.4 | (23.5 | ) | 157.7 | (21.2 | ) | |||||||||||||||
| $ | 302.0 | $ | (2,575.4 | ) | $ | 290.5 | $ | 47.4 | $ | (351.6 | ) | $ | (37.7 | ) | ||||||||||
| ||||||||||||||||||||||||
Net loss amortization included in benefit cost | $ | 127.5 | $ | 50.8 | $ | 139.3 | $ | 67.7 | $ | 26.1 | $ | 26.6 | ||||||||||||
Prior service cost (credit) amortization | ||||||||||||||||||||||||
included in benefit cost | 8.7 | 7.6 | 12.1 | (48.8 | ) | (48.7 | ) | (43.4 | ) | |||||||||||||||
| $ | 136.2 | $ | 58.4 | $ | 151.4 | $ | 18.9 | $ | (22.6 | ) | $ | (16.8 | ) | |||||||||||
| ||||||||||||||||||||||||
The estimated net loss and prior service cost (credit) amounts that will be amortized from AOCI into net pension and postretirement benefit cost during 2010 are $169.6�million and $8.7�million, respectively, for pension plans and are $56.9�million and $(47.2)�million, respectively, for other postretirement benefit plans.
169
The Company reassesses its benefit plan assumptions on a regular basis. The weighted average assumptions used in determining pension plan and U.S.�pension and other postretirement benefit plan information are as follows:
| U.S. Pension and Other | ||||||||||||||||||||||||
| Postretirement | ||||||||||||||||||||||||
| Pension Plans | Benefit Plans | |||||||||||||||||||||||
| December 31 | 2009 | 2008 | 2007 | 2009 | 2008 | 2007 | ||||||||||||||||||
Net cost | ||||||||||||||||||||||||
Discount rate | 5.80% | 5.90% | 5.35% | 6.15% | 6.50% | 6.00% | ||||||||||||||||||
Expected rate of return | ||||||||||||||||||||||||
on plan assets | 7.90% | 7.65% | 7.65% | 8.75% | 8.75% | 8.75% | ||||||||||||||||||
Salary growth rate | 4.30% | 4.30% | 4.20% | 4.50% | 4.50% | 4.50% | ||||||||||||||||||
Benefit obligation | ||||||||||||||||||||||||
Discount rate | 5.50% | 5.75% | 5.90% | 5.90% | 6.20% | 6.50% | ||||||||||||||||||
Salary growth rate | 4.15% | 4.25% | 4.30% | 4.50% | 4.50% | 4.50% | ||||||||||||||||||
| ||||||||||||||||||||||||
The 2009 net cost rates in the preceding table include costs associated with the Schering-Plough benefit plans from the date of the Merger through December�31, 2009.
The expected rate of return for both the pension and other postretirement benefit plans represents the average rate of return to be earned on plan assets over the period the benefits included in the benefit obligation are to be paid and is determined on a country basis. In developing the expected rate of return within each country, long-term historical returns data is considered as well as actual returns on the plan assets and other capital markets experience. Using this reference information, the long-term return expectations for each asset category and a weighted average expected return for each country�s target portfolio is developed, according to the allocation among those investment categories. The expected portfolio performance reflects the contribution of active management as appropriate. For 2010, the Company�s expected rate of return will range from 8.0% to 8.75% compared to a range of 7.50% to 8.75% in 2009 for its U.S.�pension and other postretirement benefit plans.
The health care cost trend rate assumptions for other postretirement benefit plans are as follows:
| December 31 | 2009 | 2008 | ||||||
Health care cost trend rate assumed for next year | 8.6 | % | 9.0 | % | ||||
Rate to which the cost trend rate is assumed to decline | 5.0 | % | 5.0 | % | ||||
Year that the trend rate reaches the ultimate trend rate | 2018 | 2016 | ||||||
| ||||||||
A one percentage point change in the health care cost trend rate would have had the following effects:
| One Percentage | ||||||||
| Point | ||||||||
| Increase | Decrease | |||||||
Effect on total service and interest cost components | $ | 33.5 | $ | (26.5 | ) | |||
Effect on benefit obligation | $ | 387.9 | $ | (315.8 | ) | |||
| ||||||||
The Company also maintains defined contribution savings plans in the United States, including plans assumed in connection with the Merger. The Company matches a percentage of each employee�s contributions consistent with the provisions of the plan for which the employee is eligible. Total employer contributions to these plans in 2009, 2008, and 2007 were $111.5�million, $104.0�million, and $110.0�million, respectively.
170
| 16. | Other (Income) Expense, Net |
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Interest income | $ | (210.2 | ) | $ | (631.4 | ) | $ | (741.1 | ) | |||
Interest expense | 458.0 | 251.3 | 384.3 | |||||||||
Exchange (gains) losses | (12.4 | ) | 147.4 | (54.3 | ) | |||||||
Other, net | (10,904.9 | ) | (2,085.4 | ) | 335.9 | |||||||
| $ | (10,669.5 | ) | $ | (2,318.1 | ) | $ | (75.2 | ) | ||||
| ||||||||||||
Interest paid was $351.4�million in 2009, $247.0�million in 2008 and $406.4�million in 2007, respectively, which excludes commitment fees.
171
| 17. | Taxes on Income |
A reconciliation between the effective tax rate and the U.S.�statutory rate is as follows:
| 2009 | 2008 | 2007 | ||||||||||||||||||||||
| Amount | Tax Rate | Amount | Tax Rate | Amount | Tax Rate | |||||||||||||||||||
U.S. statutory rate applied to income before taxes | $ | 5,352.2 | 35.0 | % | $ | 3,476.1 | 35.0 | % | $ | 1,222.3 | 35.0 | % | ||||||||||||
Differential arising from: | ||||||||||||||||||||||||
Gain on equity investments | (2,539.7 | ) | (16.6 | ) | 29.0 | 0.3 | � | � | ||||||||||||||||
Foreign earnings | (1,189.0 | ) | (7.8 | ) | (1,269.9 | ) | (12.9 | ) | (1,196.0 | ) | (34.3 | ) | ||||||||||||
Tax rate change | (198.0 | ) | (1.3 | ) | � | � | � | � | ||||||||||||||||
State tax settlements | (108.0 | ) | (0.7 | ) | (191.6 | ) | (2.0 | ) | � | � | ||||||||||||||
Foreign tax credit utilization | � | � | (192.0 | ) | (2.0 | ) | � | � | ||||||||||||||||
Amortization of purchase accounting adjustments | 760.0 | 5.0 | � | � | � | � | ||||||||||||||||||
Restructuring | 264.0 | 1.7 | 114.7 | 1.2 | � | � | ||||||||||||||||||
State taxes | 185.1 | 1.2 | 310.9 | 3.2 | 11.6 | 0.3 | ||||||||||||||||||
In-process research and development | � | � | � | � | 113.8 | 3.3 | ||||||||||||||||||
Other (1) | (259.0 | ) | (1.7 | ) | (277.8 | ) | (2.7 | ) | (56.4 | ) | (1.6 | ) | ||||||||||||
| $ | 2,267.6 | 14.8 | % | $ | 1,999.4 | 20.1 | % | $ | 95.3 | 2.7 | % | |||||||||||||
| ||||||||||||||||||||||||
| (1) | Other includes the tax effect of contingency reserves, research credits, export incentives and miscellaneous items. |
Income (loss) before taxes consisted of:
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Domestic | $ | 5,319.5 | $ | 5,210.1 | $ | (2,525.8 | ) | |||||
Foreign | 9,972.3 | 4,721.6 | 6,017.9 | |||||||||
| $ | 15,291.8 | $ | 9,931.7 | $ | 3,492.1 | |||||||
| ||||||||||||
Taxes on income consisted of:
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Current provision | ||||||||||||
Federal | $ | (55.2 | ) | $ | 1,053.6 | $ | 988.1 | |||||
Foreign | 495.4 | 292.4 | 687.0 | |||||||||
State | 7.2 | 123.3 | 202.2 | |||||||||
| 447.4 | 1,469.3 | 1,877.3 | ||||||||||
| ||||||||||||
Deferred provision | ||||||||||||
Federal | 2,094.7 | 419.0 | (1,671.5 | ) | ||||||||
Foreign | (437.3 | ) | 55.8 | 157.2 | ||||||||
State | 162.8 | 55.3 | (267.7 | ) | ||||||||
| 1,820.2 | 530.1 | (1,782.0 | ) | |||||||||
| $ | 2,267.6 | $ | 1,999.4 | $ | 95.3 | |||||||
| ||||||||||||
172
Deferred income taxes at December 31 consisted of:
| 2009 | 2008 | |||||||||||||||
| Assets | Liabilities | Assets | Liabilities | |||||||||||||
Other intangibles | $ | - | $ | 8,503.6 | $ | - | $ | 124.9 | ||||||||
Inventory related | 509.9 | 176.7 | 248.6 | - | ||||||||||||
Accelerated depreciation | 46.5 | 1,458.2 | - | 1,045.1 | ||||||||||||
Unremitted foreign earnings | - | 2,991.1 | - | 16.7 | ||||||||||||
Equity investments | - | 177.9 | - | 75.1 | ||||||||||||
Pensions and other postretirement benefits | 1,345.7 | 103.1 | 796.5 | 129.9 | ||||||||||||
Compensation related | 735.8 | - | 347.5 | - | ||||||||||||
Vioxx Litigation reserve | 42.0 | - | 1,755.1 | - | ||||||||||||
Unrecognized tax benefits | 730.0 | - | 984.1 | - | ||||||||||||
Net operating losses and other tax credit carryforwards | 1,247.3 | - | 224.7 | - | ||||||||||||
Other | 2,201.1 | 58.4 | 1,012.9 | 95.9 | ||||||||||||
Subtotal | 6,858.3 | 13,469.0 | 5,369.4 | 1,487.6 | ||||||||||||
Valuation allowance | (195.6 | ) | (94.2 | ) | ||||||||||||
Total deferred taxes | $ | 6,662.7 | $ | 13,469.0 | $ | 5,275.2 | $ | 1,487.6 | ||||||||
Net deferred income taxes | $ | 6,806.3 | $ | 3,787.6 | ||||||||||||
| ||||||||||||||||
Recognized as: | ||||||||||||||||
Deferred income taxes and other current assets | $ | 1,657.9 | $ | 2,436.9 | ||||||||||||
Other assets | 500.8 | 1,666.7 | ||||||||||||||
Income taxes payable | $ | 167.8 | $ | 3.8 | ||||||||||||
Deferred income taxes and noncurrent liabilities | 8,797.2 | 312.2 | ||||||||||||||
| ||||||||||||||||
Increases in deferred tax assets relating to pensions and other postretirement benefits, compensation related, and net operating losses and other tax credit carryforwards, as well as increases in deferred tax liabilities relating to other intangibles and unremitted foreign earnings primarily reflect the impact of deferred taxes recorded in conjunction with the Merger.
The Company has net operating loss (�NOL�) carryforwards in several jurisdictions. As of the December�31, 2009, the most significant NOL carryforwards are approximately $668�million in the United States that will begin to expire in 2024 and $360�million in the Netherlands that will expire in 2017 if unused. The Company also has other tax credit carryforwards for U.S.�research and development tax credits, U.S.�foreign tax credits and federal alternative minimum tax (�AMT�) credit carryforwards. At December�31, 2009, the Company had $250�million of research and development credit carryforwards that will begin to expire in 2022; $705�million of foreign tax credit carryforwards that will begin to expire in 2011; and $43�million of AMT credit carryforwards that have an indefinite life. The valuation allowance in 2009 primarily relates to various foreign entity NOL carryforwards resulting primarily from losses generated by restructuring actions.
Income taxes paid in 2009, 2008 and 2007 were $957.5�million, $1.8�billion and $3.5�billion, respectively. Stock option exercises reduced income taxes paid by $138.4�million in 2007. Stock option exercises did not have a significant impact on taxes paid in 2009 or 2008.
173
On January�1, 2007, new authoritative guidance issued by the FASB for the accounting and reporting of uncertain tax positions was adopted. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| 2009 | 2008 | 2007 | ||||||||||
Balance as of January 1 | $ | 3,665.0 | $ | 3,689.5 | $ | 5,008.4 | ||||||
Additions related to current year positions | 333.0 | 269.4 | 284.5 | |||||||||
Additions related to prior year positions | 48.9 | 64.2 | 187.8 | |||||||||
Additons related to the merger with Schering-Plough | 1,176.0 | - | - | |||||||||
Reductions for tax positions of prior years | (547.4 | ) | (310.5 | ) | (87.0 | ) | ||||||
Settlements (1) | (331.8 | ) | (38.8 | ) | (1,703.5 | ) | ||||||
Lapse of statute of limitations | (2.6 | ) | (8.8 | ) | (0.7 | ) | ||||||
Balance as of December 31 | $ | 4,341.1 | $ | 3,665.0 | $ | 3,689.5 | ||||||
| ||||||||||||
| (1) | Reflects the settlement with the Internal Revenue Service in 2007 discussed below . |
If the Company were to recognize the unrecognized tax benefits of $4.3�billion at December�31, 2009, the income tax provision would reflect a favorable net impact of $3.7�billion.
The amount of unrecognized tax benefits will change in the next 12�months due primarily to the anticipated closure of various tax examinations. The Company estimates that the change could result in a reduction in unrecognized tax benefits of approximately $760�million.
Interest and penalties associated with uncertain tax positions amounted to a (benefit) expense of $(163)�million in 2009, $101�million in 2008 and $270�million in 2007. Liabilities for accrued interest and penalties were $1.4�billion and $1.7�billion as of December�31, 2009 and 2008, respectively.
As previously disclosed, the Internal Revenue Service (�IRS�) has completed its examination of Old Merck�s tax returns for the years 1993 to 2001. As a result of the examination, Old Merck made an aggregate payment of $2.79�billion in February 2007. This payment was offset by (i)�a tax refund of $165�million received in 2007 for amounts previously paid for these matters and (ii)�a federal tax benefit of approximately $360�million related to interest included in the payment, resulting in a net cash cost to Old Merck of approximately $2.3�billion in 2007. The impact for years subsequent to 2001 for items reviewed as part of the examination was included in the payment although those years remain open in all other respects. The closing of the IRS examination did not have a material impact on Old Merck�s results of operations in 2007 as these amounts had been previously accrued for.
Old Merck reported the results of the IRS adjustments for the years 1993 through 2001 to various state tax authorities. This resulted in additional tax, as well as interest and penalty payments of $20�million and $9�million, respectively, in 2008 and $57�million and $67�million, respectively, in 2007, and an equivalent reduction in the balances of unrecognized tax benefits, accrued interest and penalties.
In October 2001, IRS auditors asserted that two interest rate swaps that Schering-Plough entered into with an unrelated party should be recharacterized as loans from affiliated companies, resulting in additional tax liability for the 1991 and 1992 tax years. In September 2004, Schering-Plough made payments to the IRS in the amount of $194�million for income taxes and $279�million for interest. Schering-Plough filed refund claims for the taxes and interest with the IRS in December 2004. Following the IRS�s denial of Schering-Plough�s claims for a refund, Schering-Plough filed suit in May 2005 in the U.S.�District Court for the District of New Jersey for refund of the full amount of taxes and interest. Schering-Plough�s tax reserves were adequate to cover the above mentioned payments. A decision in favor of the government was announced in August 2009. Schering-Plough has filed a motion for a retrial and if that motion in not granted, Schering-Plough intends to file an appeal to the 3 rd circuit.
As previously disclosed, in October 2006, the Canada Revenue Agency (�CRA�) issued Old Merck a notice of reassessment containing adjustments related to certain intercompany pricing matters. In February 2009, Old Merck and the CRA negotiated a settlement agreement in regard to these matters. In accordance with the settlement, Old Merck paid an additional tax of approximately $300�million (U.S.�dollars) and interest of approximately $360�million (U.S.�dollars) with no additional amounts or penalties due on this assessment. The settlement was accounted for in the first quarter of 2009. Old Merck had previously established reserves for these
174
matters. A significant portion of the taxes paid is expected to be creditable for U.S.�tax purposes. The resolution of these matters did not have a material effect on Old Merck�s financial position or liquidity, other than with respect to the associated collateral as discussed below.
In addition, in July 2007 and November 2008, the CRA proposed additional adjustments for 1999 and 2000, respectively, relating to other intercompany pricing matters. The adjustments would increase Canadian tax due by approximately $312�million (U.S.�dollars) plus $314�million (U.S.�dollars) of interest through December�31, 2009. It is possible that the CRA will propose similar adjustments for later years. The Company disagrees with the positions taken by the CRA and believes they are without merit. The Company intends to contest the assessments through the CRA appeals process and the courts if necessary. Management believes that resolution of these matters will not have a material effect on the Company�s financial position or liquidity.
The IRS is examining Old Merck�s 2002 to 2005 federal income tax returns. In addition, various state and foreign tax examinations are in progress. For most of its other significant tax jurisdictions (both U.S.�state and foreign), the Company�s income tax returns are open for examination for the period 1999 through 2009.
During the second quarter of 2007, the IRS completed its examination of the Schering-Plough�s 1997-2002 federal income tax returns. The Company is seeking resolution of an issue raised during this examination through the IRS administrative appeals process. In July 2007, Schering-Plough made a payment of $98�million to the IRS pertaining to the 1997-2002 examination. The Company�s income tax returns remain open with the IRS for the 1997-2009 tax years. During 2008, the IRS commenced its examination of the 2003-2006 federal income tax returns. This examination is expected to be completed in 2010. For most of its other significant tax jurisdictions (both U.S.�state and foreign), the Company�s income tax returns are open for examination for the period 2002 through 2009.
At December�31, 2009, foreign earnings of $31.2�billion have been retained indefinitely by subsidiary companies for reinvestment, therefore no provision has been made for income taxes that would be payable upon the distribution of such earnings. In addition, the Company has subsidiaries operating in Puerto Rico and Singapore under tax incentive grants that begin to expire in 2013.
| 18. | Earnings per Share |
As discussed in Note�2, effective January�1, 2009, new guidance issued by the FASB was adopted which clarifies that unvested share-based payment awards that contain nonforfeitable rights to dividends or dividend equivalents (whether paid or unpaid) are considered participating securities and shall be included in the computation of earnings per share pursuant to the two-class method. The provisions of this new guidance are retrospective; therefore prior periods have been restated. The two-class method is an earnings allocation formula that determines earnings per share for common stock and participating securities according to dividends declared and participation rights in undistributed earnings. Under this method, all earnings (distributed and undistributed) are allocated to common shares and participating securities based on their respective rights to receive dividends. RSUs and certain PSUs granted to certain management level employees (see Note�14)�participate in dividends on the same basis as common shares and are nonforfeitable by the holder. As a result, these RSUs and PSUs meet the definition of a participating security.
175
The calculations of earnings per share under the two-class method are as follows:
| Years Ended December�31 | 2009 | 2008 | 2007 | |||||||||
Basic Earnings per Common Share | ||||||||||||
Net income available to Merck�& Co., Inc. common shareholders | $ | 12,899.2 | $ | 7,808.4 | $ | 3,275.4 | ||||||
Less: Income allocated to participating securities | 46.3 | 20.8 | 8.6 | |||||||||
Net income allocated to common shareholders | $ | 12,852.9 | $ | 7,787.6 | $ | 3,266.8 | ||||||
Average common shares outstanding | 2,268.2 | 2,135.8 | 2,170.5 | |||||||||
| $ | 5.67 | $ | 3.65 | $ | 1.51 | |||||||
| ||||||||||||
Earnings per Common Share Assuming Dilution | ||||||||||||
Net income available to Merck�& Co., Inc. common shareholders | $ | 12,899.2 | $ | 7,808.4 | $ | 3,275.4 | ||||||
Less: Income allocated to participating securities | 46.2 | 20.8 | 8.6 | |||||||||
Net income allocated to common shareholders | $ | 12,853.0 | $ | 7,787.6 | $ | 3,266.8 | ||||||
Average common shares outstanding | 2,268.2 | 2,135.8 | 2,170.5 | |||||||||
Common shares issuable (1) | 5.0 | 6.7 | 19.3 | |||||||||
Average common shares outstanding assuming dilution | 2,273.2 | 2,142.5 | 2,189.8 | |||||||||
| $ | 5.65 | $ | 3.63 | $ | 1.49 | |||||||
| ||||||||||||
| (1) | Issuable primarily under share-based compensation plans. |
In 2009, 2008 and 2007, 228.0�million, 201.2�million and 123.7�million, respectively, of common shares issuable under share-based compensation plans were excluded from the computation of earnings per common share assuming dilution because the effect would have been antidilutive.
176
| 19. | Comprehensive Income |
| Year Ended December�31, 2009 | Pretax | Tax | After Tax | |||||||||
Net unrealized loss on derivatives | $ | (316.1 | ) | $ | 124.9 | $ | (191.2 | ) | ||||
Net loss realization | 61.2 | (24.1 | ) | 37.1 | ||||||||
Derivatives | (254.9 | ) | 100.8 | (154.1 | ) | |||||||
Net unrealized gain on investments | 208.3 | (31.2 | ) | 177.1 | ||||||||
Net gain realization | (230.5 | ) | 23.6 | (206.9 | ) | |||||||
Investments | (22.2 | ) | (7.6 | ) | (29.8 | ) | ||||||
Benefit plan net (loss) gain and prior service cost (credit),
net of amortization | 504.5 | (219.0 | ) | 285.5 | ||||||||
Cumulative translation adjustment (1) | (314.2 | ) | - | (314.2 | ) | |||||||
| $ | (86.8 | ) | $ | (125.8 | ) | $ | (212.6 | ) | ||||
| ||||||||||||
Year Ended December�31, 2008 | ||||||||||||
Net unrealized gain on derivatives | $ | 291.0 | $ | (116.0 | ) | $ | 175.0 | |||||
Net gain realization | (38.8 | ) | 15.4 | (23.4 | ) | |||||||
Derivatives | 252.2 | (100.6 | ) | 151.6 | ||||||||
Net unrealized loss on investments | (212.9 | ) | 79.2 | (133.7 | ) | |||||||
Net loss realization | 116.9 | (63.7 | ) | 53.2 | ||||||||
Investments | (96.0 | ) | 15.5 | (80.5 | ) | |||||||
Benefit plan net (loss) gain and prior service cost (credit), net of amortization | (2,891.2 | ) | 1,129.5 | (1,761.7 | ) | |||||||
Cumulative translation adjustment (1) | (37.2 | ) | - | (37.2 | ) | |||||||
| $ | (2,772.2 | ) | $ | 1,044.4 | $ | (1,727.8 | ) | |||||
| ||||||||||||
Year Ended December�31, 2007 | ||||||||||||
Net unrealized loss on derivatives | $ | (50.5 | ) | $ | 20.7 | $ | (29.8 | ) | ||||
Net loss realization | 43.0 | (17.6 | ) | 25.4 | ||||||||
Derivatives | (7.5 | ) | 3.1 | (4.4 | ) | |||||||
Net unrealized gain on investments | 106.2 | (24.5 | ) | 81.7 | ||||||||
Net gain realization | (36.1 | ) | 12.4 | (23.7 | ) | |||||||
Investments | 70.1 | (12.1 | ) | 58.0 | ||||||||
Benefit plan net gain (loss) and prior service cost (credit), net of amortization | 387.4 | (147.1 | ) | 240.3 | ||||||||
Cumulative translation adjustment (1) | 34.4 | 9.9 | 44.3 | |||||||||
| $ | 484.4 | $ | (146.2 | ) | $ | 338.2 | ||||||
| ||||||||||||
| (1) | The increase in the cumulative translation adjustment in 2009 is due to the Merger. Amounts in 2008 and 2007 represent cumulative translation adjustments related to equity investees. |
| December 31 | 2009 | 2008 | ||||||
Net unrealized (loss) gain on derivatives | $ | (42.2 | ) | $ | 111.9 | |||
Net unrealized gain on investments | 33.3 | 63.1 | ||||||
Pension plan net loss | (2,191.3 | ) | (2,440.7 | ) | ||||
Other postretirement benefit plan net loss | (521.4 | ) | (596.5 | ) | ||||
Pension plan prior service cost | (20.7 | ) | (26.4 | ) | ||||
Other postretirement benefit plan prior service cost | 264.3 | 309.0 | ||||||
Cumulative translation adjustment | (288.5 | ) | 25.7 | |||||
| $ | (2,766.5 | ) | $ | (2,553.9 | ) | |||
| ||||||||
177
Included in the cumulative translation adjustment are gains of $78.2�million for the post-Merger period from euro-denominated debt which have been designated as, and are effective as, economic hedges of the net investment in a foreign operation.
| 20. | Segment Reporting |
The Company�s operations are principally managed on a products basis and are comprised of one reportable segment, which is the Pharmaceutical segment. The Pharmaceutical segment includes human health pharmaceutical and vaccine products marketed either directly by the Company or through joint ventures. Human health pharmaceutical products consist of therapeutic and preventive agents, sold by prescription, for the treatment of human disorders. The Company sells these human health pharmaceutical products primarily to drug wholesalers and retailers, hospitals, government agencies and managed health care providers such as health maintenance organizations, pharmacy benefit managers and other institutions. Vaccine products consist of preventive pediatric, adolescent and adult vaccines, primarily administered at physician offices. The Company sells these human health vaccines primarily to physicians, wholesalers, physician distributors and government entities. A large component of pediatric and adolescent vaccines is sold to the U.S.�Centers for Disease Control and Prevention Vaccines for Children program, which is funded by the U.S.�government. The Company also has animal health operations that discover, develop, manufacture and market animal health products, including vaccines. Additionally, the Company has consumer health care operations that develop, manufacture and market OTC, foot care and sun care products in the United States and Canada. Segment composition reflects certain managerial changes that have been implemented. Segment disclosures for prior periods have been recast on a comparable basis with 2009.
All other includes other non-reportable segments, including animal health and consumer health care, as well as revenue from the Company�s relationship with AZLP. The accounting policies for the segments described above are the same as those described in Note�2. Revenues and profits for these segments are as follows:
| Pharmaceutical | All Other | Total | ||||||||||
Year Ended December�31, 2009 | ||||||||||||
Segment revenues | $ | 25,236.5 | $ | 2,114.0 | $ | 27,350.5 | ||||||
Segment profits | 15,714.6 | 1,735.1 | 17,449.7 | |||||||||
Included in segment profits: | ||||||||||||
Equity income from affiliates | 1,330.1 | 751.7 | 2,081.8 | |||||||||
Depreciation and amortization | (92.6 | ) | - | (92.6 | ) | |||||||
Year Ended December�31, 2008 | ||||||||||||
Segment revenues | $ | 22,081.3 | $ | 1,694.1 | $ | 23,775.4 | ||||||
Segment profits | 14,110.3 | 1,691.0 | 15,801.3 | |||||||||
Included in segment profits: | ||||||||||||
Equity income from affiliates | 1,655.8 | 668.4 | 2,324.2 | |||||||||
Depreciation and amortization | (101.4 | ) | - | (101.4 | ) | |||||||
Year Ended December�31, 2007 | ||||||||||||
Segment revenues | $ | 22,282.8 | $ | 1,848.1 | $ | 24,130.9 | ||||||
Segment profits | 14,558.7 | 2,027.6 | 16,586.3 | |||||||||
Included in segment profits: | ||||||||||||
Equity income from affiliates | 1,895.9 | 820.0 | 2,715.9 | |||||||||
Depreciation and amortization | (137.1 | ) | - | (137.1 | ) | |||||||
| ||||||||||||
Segment profits are comprised of segment revenues less certain elements of materials and production costs and operating expenses, including components of equity income (loss) from affiliates and depreciation and amortization expenses. For internal management reporting presented to the chief operating decision maker, Merck does not allocate production costs, other than standard costs, research and development expenses and general and administrative expenses, as well as the cost of financing these activities. Separate divisions maintain responsibility for monitoring and managing these costs, including depreciation related to fixed assets utilized by these divisions and, therefore, they are not included in segment profits.
178
| ($ in millions) | 2009 | 2008 | 2007 | |||||||||
Pharmaceutical: | ||||||||||||
Bone, Respiratory, Immunology and Dermatology | ||||||||||||
Singulair | $ | 4,659.7 | $ | 4,336.9 | $ | 4,266.3 | ||||||
Fosamax | 1,099.8 | 1,552.7 | 3,049.0 | |||||||||
Propecia | 440.3 | 429.1 | 405.4 | |||||||||
Remicade | 430.7 | - | - | |||||||||
Arcoxia | 357.5 | 377.3 | 329.1 | |||||||||
Nasonex | 164.9 | - | - | |||||||||
Clarinex | 100.6 | - | - | |||||||||
Asmanex | 37.0 | - | - | |||||||||
Cardiovascular | ||||||||||||
Vytorin | 440.8 | 84.2 | 84.3 | |||||||||
Zetia | 402.9 | 6.4 | 6.5 | |||||||||
Integrilin | 45.9 | - | - | |||||||||
Diabetes and Obesity | ||||||||||||
Januvia | 1,922.1 | 1,397.1 | 667.5 | |||||||||
Janumet | 658.4 | 351.1 | 86.4 | |||||||||
Infectious Disease | ||||||||||||
Isentress | 751.8 | 361.1 | 41.3 | |||||||||
Primaxin | 688.9 | 760.4 | 763.5 | |||||||||
Cancidas | 616.7 | 596.4 | 536.9 | |||||||||
Invanz | 292.9 | 265.0 | 190.2 | |||||||||
Crixivan/Stocrin | 206.1 | 275.1 | 310.2 | |||||||||
PegIntron | 148.7 | - | - | |||||||||
Avelox | 66.2 | - | - | |||||||||
Rebetol | 36.1 | - | - | |||||||||
Mature Brands | ||||||||||||
Cozaar/Hyzaar | 3,560.7 | 3,557.7 | 3,350.1 | |||||||||
Zocor | 558.4 | 660.1 | 876.5 | |||||||||
Vasotec/Vaseretic | 310.8 | 356.7 | 494.6 | |||||||||
Proscar | 290.9 | 323.5 | 411.0 | |||||||||
Claritin Rx | 71.1 | - | - | |||||||||
Proventil | 26.2 | - | - | |||||||||
Neurosciences and Ophthalmology | ||||||||||||
Maxalt | 574.5 | 529.2 | 467.3 | |||||||||
Cosopt/Trusopt | 503.5 | 781.2 | 786.8 | |||||||||
Remeron | 38.5 | - | - | |||||||||
Subutex/Suboxone | 36.3 | - | - | |||||||||
Oncology | ||||||||||||
Emend | 313.1 | 259.7 | 201.7 | |||||||||
Temodar | 188.1 | - | - | |||||||||
Caelyx | 46.5 | - | - | |||||||||
Intron A | 38.4 | - | - | |||||||||
Vaccines (2) | ||||||||||||
ProQuad/M-M-R II/Varivax | 1,368.5 | 1,268.5 | 1,347.1 | |||||||||
Gardasil | 1,118.4 | 1,402.8 | 1,480.6 | |||||||||
RotaTeq | 521.9 | 664.5 | 524.7 | |||||||||
Pneumovax | 345.6 | 249.3 | 233.2 | |||||||||
Zostavax | 277.4 | 312.4 | 236.0 | |||||||||
Women�s Health and Endocrine | ||||||||||||
Follistim/Puregon | 96.5 | - | - | |||||||||
NuvaRing | 88.3 | - | - | |||||||||
Other
Pharmaceutical (3) | 1,294.9 | 922.9 | 1,136.6 | |||||||||
| 25,236.5 | 22,081.3 | 22,282.8 | ||||||||||
Other segment
revenues (4) | 2,114.0 | 1,694.1 | 1,848.1 | |||||||||
Total segment revenues | 27,350.5 | 23,775.4 | 24,130.9 | |||||||||
Other (5) | 77.8 | 74.9 | 66.8 | |||||||||
| $ | 27,428.3 | $ | 23,850.3 | $ | 24,197.7 | |||||||
| ||||||||||||
| (1) | Sales of legacy Schering-Plough products only reflect results for the post-merger period through December�31, 2009. Sales of MSP Partnership products Zetia and Vytorin represent sales for the post-Merger period through December�31, 2009. Prior to the Merger, sales of Zetia and Vytorin were primarily recognized by the MSP Partnership and the results of Old Merck�s interest in the MSP Partnership were recorded in Equity income from affiliates. Sales of Zetia and Vytorin in 2008 and 2007 reflect Old Merck�s sales of these products in Latin America which was not part of the MSP Partnership. | |
| (2) | These amounts do not reflect sales of vaccines sold in most major European markets through the Company�s joint venture, Sanofi Pasteur MSD, the results of which are reflected in Equity income from affiliates. These amounts do, however, reflect supply sales to Sanofi Pasteur MSD. | |
| (3) | Other pharmaceutical primarily includes sales of other human pharmaceutical products, including products within the franchises not listed separately. | |
| (4) | Reflects other non-reportable segments, including animal health and consumer health care, and revenue from the Company�s relationship with AZLP primarily relating to sales of Nexium, as well as Prilosec. Revenue from AZLP was $1.4�billion, $1.6�billion and $1.7�billion in 2009, 2008 and 2007, respectively. | |
| (5) | Other revenues are primarily comprised of miscellaneous corporate revenues, third-party manufacturing sales, sales related to divested products or businesses and other supply sales not included in segment results. |
179
Consolidated revenues by geographic area where derived are as follows:
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
United States | $ | 14,401.2 | $ | 13,370.5 | $ | 14,690.9 | ||||||
Europe, Middle East and Africa | 7,093.1 | 5,773.8 | 5,159.0 | |||||||||
Japan | 2,425.6 | 1,823.5 | 1,533.2 | |||||||||
Other | 3,508.4 | 2,882.5 | 2,814.6 | |||||||||
| $ | 27,428.3 | $ | 23,850.3 | $ | 24,197.7 | |||||||
| ||||||||||||
| Years Ended December 31 | 2009 | 2008 | 2007 | |||||||||
Segment profits | $ | 17,449.7 | $ | 15,801.3 | $ | 16,586.3 | ||||||
Other profits | (136.7 | ) | (92.3 | ) | (56.2 | ) | ||||||
Adjustments | 372.0 | 424.7 | 367.7 | |||||||||
Unallocated: | ||||||||||||
Interest income | 210.2 | 631.4 | 741.1 | |||||||||
Interest expense | (458.0 | ) | (251.3 | ) | (384.3 | ) | ||||||
Equity income from affiliates | 153.2 | 236.5 | 260.6 | |||||||||
Depreciation and amortization | (1,569.6 | ) | (1,529.8 | ) | (1,851.0 | ) | ||||||
Research and development | (5,845.0 | ) | (4,805.3 | ) | (4,882.8 | ) | ||||||
Amortization of purchase accounting adjustments | (2,285.9 | ) | - | - | ||||||||
Gain related to MSP Partnership | 7,529.5 | - | - | |||||||||
Gain on Merial divestiture | 3,162.5 | - | - | |||||||||
Gain on distribution from AstraZeneca LP | - | 2,222.7 | - | |||||||||
U.S. Vioxx Settlement Agreement charge | - | - | (4,850.0 | ) | ||||||||
Other expenses, net | (3,290.1 | ) | (2,706.2 | ) | (2,439.3 | ) | ||||||
| $ | 15,291.8 | $ | 9,931.7 | $ | 3,492.1 | |||||||
| ||||||||||||
Other profits are primarily comprised of miscellaneous corporate profits as well as operating profits related to divested products or businesses and other supply sales. Adjustments represent the elimination of the effect of double counting certain items of income and expense. Equity income from affiliates includes taxes paid at the joint venture level and a portion of equity income that is not reported in segment profits. Other expenses, net, include expenses from corporate and manufacturing cost centers and other miscellaneous income (expense), net.
Property, plant and equipment, net by geographic area where located is as follows:
| December 31 | 2009 | 2008 | 2007 | |||||||||
United States | $ | 11,785.2 | $ | 9,023.2 | $ | 9,249.1 | ||||||
Europe, Middle East and Africa | 2,863.3 | 1,649.0 | 1,625.0 | |||||||||
Japan | 283.9 | 362.0 | 459.0 | |||||||||
Other | 3,341.1 | 965.4 | 1,012.9 | |||||||||
| $ | 18,273.5 | $ | 11,999.6 | $ | 12,346.0 | |||||||
| ||||||||||||
The Company does not disaggregate assets on a products and services basis for internal management reporting and, therefore, such information is not presented.
180
To the Board of Directors and Shareholders of Merck�& Co., Inc.:
As discussed in Note�17 to the consolidated financial statements, Merck changed the manner in which it accounts for unrecognized tax benefits in 2007.
As discussed in Note�3 and Note�5 to the consolidated financial statements, Merck changed the manner in which it accounts for business combinations in 2009.
As discussed in Note�13 to the consolidated financial statements, Merck changed the manner in which it accounts for noncontrolling interests in 2009.
A company�s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company�s internal control over financial reporting includes those policies and procedures that (i)�pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii)�provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii)�provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company�s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
PricewaterhouseCoopers LLP
Florham Park, New Jersey
February�26, 2010
181
| (b) | Supplementary Data |
Selected quarterly financial data for 2009 and 2008 are contained in the Condensed Interim Financial Data table below.
| ($ in millions except per share amounts) | 4th Q (1),(2),(3) | 3rd Q (3),(4) | 2nd Q (3),(5) | 1st Q (3),(6) | ||||||||||||
2009 (7) | ||||||||||||||||
Sales | $10,093.5 | $6,049.7 | $5,899.9 | $5,385.2 | ||||||||||||
Materials and production costs | 4,900.8 | 1,430.3 | 1,353.9 | 1,333.8 | ||||||||||||
Marketing and administrative expenses | 3,455.2 | 1,725.5 | 1,729.5 | 1,632.9 | ||||||||||||
Research and development expenses | 1,971.5 | 1,254.0 | 1,395.3 | 1,224.2 | ||||||||||||
Restructuring costs | 1,489.8 | 42.4 | 37.4 | 64.3 | ||||||||||||
Equity income from affiliates | (373.8 | ) | (688.2 | ) | (587.1 | ) | (585.8 | ) | ||||||||
Other (income) expense, net | (7,814.8 | ) | (2,791.1 | ) | 3.6 | (67.2 | ) | |||||||||
Income before taxes | 6,464.8 | 5,076.8 | 1,967.3 | 1,783.0 | ||||||||||||
Net income available to common shareholders | 6,493.6 | 3,424.3 | 1,556.3 | 1,425.0 | ||||||||||||
Basic earnings per common share available to common
shareholders | $2.36 | $1.62 | $0.74 | $0.67 | ||||||||||||
Earnings per common share assuming dilution available to
common shareholders | $2.35 | $1.61 | $0.74 | $0.67 | ||||||||||||
2008 (7) | ||||||||||||||||
Sales | $6,032.4 | $5,943.9 | $6,051.8 | $5,822.1 | ||||||||||||
Materials and production costs | 1,470.0 | 1,477.9 | 1,396.5 | 1,238.1 | ||||||||||||
Marketing and administrative expenses | 1,862.1 | 1,730.3 | 1,930.2 | 1,854.4 | ||||||||||||
Research and development expenses | 1,386.6 | 1,171.1 | 1,169.3 | 1,078.3 | ||||||||||||
Restructuring costs | 103.1 | 757.5 | 102.2 | 69.7 | ||||||||||||
Equity income from affiliates | (720.0 | ) | (665.6 | ) | (523.0 | ) | (652.1 | ) | ||||||||
Other (income) expense, net | (26.8 | ) | 30.6 | (112.8 | ) | (2,209.2 | ) | |||||||||
Income before taxes | 1,957.4 | 1,442.1 | 2,089.4 | 4,442.9 | ||||||||||||
Net income available to common shareholders | 1,644.8 | 1,092.7 | 1,768.3 | 3,302.6 | ||||||||||||
Basic earnings per common share available to common shareholders | $0.78 | $0.51 | $0.82 | $1.52 | ||||||||||||
Earnings per common share assuming dilution available to common shareholders | $0.78 | $0.51 | $0.82 | $1.52 | ||||||||||||
| ||||||||||||||||
| (1) | Amounts for 2009 include a gain on the fair value adjustment to Merck�s previously held interest in the MSP Partnership (see Note�3). | |
| (2) | The fourth quarter 2008 tax provision reflects the favorable impact of foreign exchange rate changes and a benefit relating to the U.S. research and development tax credit. | |
| (3) | Amounts for third and fourth quarter 2009 and fourth quarter 2008 include the impact of additional Vioxx legal defense reserves (see Note�12). Amounts for third quarter and second quarter 2009 and first quarter 2008 include the impact of additional Fosamax legal defense reserves (see Note�12). | |
| (4) | Amounts for 2009 include a gain on the sale of Old Merck�s interest in Merial Limited (see Note�10). | |
| (5) | Amounts for 2008 reflect the favorable impact of tax settlements. | |
| (6) | Amounts for 2008 include a gain on distribution from AstraZeneca LP (see Note�10), a gain related to the sale of the remaining worldwide rights to Aggrastat , the realization of foreign tax credits and an expense for a contribution to the Merck Company Foundation. | |
| (7) | Amounts for 2009 include the impacts of the Merger, including amortization of intangible assets and merger-related costs (see Note�3). Amounts for 2009 and 2008 include the impact of restructuring actions (see Note�4). |
182
| Item�9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
Prior to the Merger, Old Merck�s historical financial statements were audited by PricewaterhouseCoopers LLP (�PwC�) and Schering-Plough�s historical financial statements were audited by Deloitte�& Touche LLP (�Deloitte�).
On the closing date of the Merger, the Board of Directors of the Company dismissed Deloitte as the Company�s independent registered public accounting firm.
The audit reports of Deloitte on the financial statements of Schering-Plough as of and for each of the two fiscal years ended December�31, 2008 and 2007 did not contain any adverse opinion or disclaimer of opinion, and were not qualified or modified as to uncertainty, audit scope, or accounting principles. During Schering-Plough�s fiscal years ended December�31, 2008 and 2007, and during Schering-Plough�s subsequent interim period from January�1, 2009 through the closing date of the Merger, the date of the dismissal of Deloitte, with regard to the financial statements referred to above, (i)�there were no disagreements with Deloitte on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to Deloitte�s satisfaction, would have caused Deloitte to make reference to the subject matter of the disagreement in connection with its report, and (ii)�there were no reportable events of the type described in Item�304(a)(1)(v) of Regulation�S-K.
| Item�9A. | Controls and Procedures. |
Management of the Company, with the participation of its Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of the Company�s disclosure controls and procedures. Based on their evaluation, as of the end of the period covered by this Form�10-K, the Company�s Chief Executive Officer and Chief Financial Officer have concluded that the Company�s disclosure controls and procedures (as defined in Rules�13a-15(e) or 15d-15(e) under the Securities Exchange Act of 1934, as amended (the �Act�)) are effective.
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule�13a-15(f) of the Act. Management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control�� Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (�COSO�). Based on this evaluation, management concluded that internal control over financial reporting was effective as of December�31, 2009. The effectiveness of the Company�s internal control over financial reporting as of December�31, 2009, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
In November 2009, Merck�& Co, Inc. and Schering-Plough Corporation completed the planned merger of the two entities. During the 2009 period leading up to the Merger, there were no changes to either company�s internal controls over financial reporting that were reasonably likely to have a material effect. For the post-Merger period, management maintained the operational integrity of each company�s legacy controls over financial reporting. In addition, management designed and tested new controls over financial reporting which supported the accuracy of the financial presentation of the merged Company�s operations. To support business integration plans, a process for evaluating and addressing necessary changes to the control environment over financial reporting was adopted. As Old Merck has previously disclosed, it is in the process of a multi-year implementation of an enterprise wide resource planning system. The Company intends to implement this system in the United States in 2010 and further implementation plans are under revision to address the combined Company�s requirements. In 2009, Old Merck entities implemented a worldwide employee data management system. The implementation of this system included modifications to the design and operation of controls validating components of employee master data.
Management�s Responsibility for Financial Statements
Responsibility for the integrity and objectivity of the Company�s financial statements rests with management. The financial statements report on management�s stewardship of Company assets. These statements
183
are prepared in conformity with generally accepted accounting principles and, accordingly, include amounts that are based on management�s best estimates and judgments. Nonfinancial information included in the Annual Report on Form�10-K has also been prepared by management and is consistent with the financial statements.
To assure that financial information is reliable and assets are safeguarded, management maintains an effective system of internal controls and procedures, important elements of which include: careful selection, training and development of operating and financial managers; an organization that provides appropriate division of responsibility; and communications aimed at assuring that Company policies and procedures are understood throughout the organization. A staff of internal auditors regularly monitors the adequacy and application of internal controls on a worldwide basis.
To ensure that personnel continue to understand the system of internal controls and procedures, and policies concerning good and prudent business practices, the Company periodically conducts the Management�s Stewardship Program for key management and financial personnel. This program reinforces the importance and understanding of internal controls by reviewing key corporate policies, procedures and systems. In addition, the Company has compliance programs, including an ethical business practices program to reinforce the Company�s long-standing commitment to high ethical standards in the conduct of its business.
The financial statements and other financial information included in the Annual Report on Form�10-K fairly present, in all material respects, the Company�s financial condition, results of operations and cash flows. Our formal certification to the Securities and Exchange Commission is included in this Form�10-K filing.
Management�s Report on Internal Control Over Financial Reporting
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The effectiveness of the Company�s internal control over financial reporting as of December�31, 2009, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
 Richard T. Clark Chairman, President and Chief Executive Officer |  Peter N. Kellogg Executive Vice President and Chief Financial Officer | |||||
| Item�9B. | Other Information. |
None.
184
| Item�10. | Directors, Executive Officers and Corporate Governance. |
The required information on directors and nominees is incorporated by reference from the discussion under Item�1. Election of Directors of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010. Information on executive officers is set forth in Part�I of this document on pages 54 through 57.
The required information on compliance with Section�16(a) of the Securities Exchange Act of 1934 is incorporated by reference from the discussion under the heading �Section�16(a) Beneficial Ownership Reporting Compliance� of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
The required information on the identification of the audit committee and the audit committee financial expert is incorporated by reference from the discussion under the heading �Board Committees� of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
| Item�11. | Executive Compensation. |
The information required on executive compensation is incorporated by reference from the discussion under the headings �Compensation Discussion and Analysis�, �Summary Compensation Table�, �All Other Compensation� table, �Grants of Plan-Based Awards Table�, �Outstanding Equity Awards at Fiscal Year-End Table�, �Option Exercises and Stock Vested Table�, Retirement Plan Benefits and related �Pension Benefits� table, Nonqualified Deferred Compensation and related tables, Potential Payments Upon Termination or Change-in-Control, including the discussion under the subheadings �Separation�, �Separation Plan Payment and Benefit Estimates� table, �Individual Agreements�, �Change in Control� and �Change in Control Payment and Benefit Estimates� table, as well as all footnote information to the various tables, of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
The required information on director compensation is incorporated by reference from the discussion under the heading �Director Compensation� and related �Director Compensation� table and �Schedule of Director Fees� table of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
The required information under the headings �Compensation Committee Interlocks and Insider Participation� and �Compensation and Benefits Committee Report� is incorporated by reference from the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
| Item�12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
Information with respect to securities authorized for issuance under equity compensation plans is incorporated by reference from the discussion under the heading �Equity Compensation Plan Information� of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010. Information with respect to security ownership of certain beneficial owners and management is incorporated by reference from the discussion under the heading �Security Ownership of Certain Beneficial Owners and Management� of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
185
| Item�13. | Certain Relationships and Related Transactions, and Director Independence. |
The required information on transactions with related persons is incorporated by reference from the discussion under the heading �Related Person Transactions� of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
The required information on director independence is incorporated by reference from the discussion under the heading �Independence of Directors� of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
| Item�14. | Principal Accountant Fees and Services. |
The information required for this item is incorporated by reference from the discussion under �Audit Committee� beginning with the caption �Pre-Approval Policy for Services of Independent Registered Public Accounting Firm� through �All Other Fees� of the Company�s Proxy Statement for the Annual Meeting of Shareholders to be held May�25, 2010.
| Item�15. | Exhibits and Financial Statement Schedules. |
(a)�The following documents are filed as part of this Form�10-K
| 1. | Financial Statements |
Consolidated statement of income for the years ended December�31, 2009, 2008 and 2007
Consolidated statement of retained earnings for the years ended December�31, 2009, 2008 and 2007
Consolidated statement of comprehensive income for the years ended December�31, 2009, 2008 and 2007
Consolidated balance sheet as of December�31, 2009 and 2008
Consolidated statement of cash flows for the years ended December�31, 2009, 2008 and 2007
Notes to consolidated financial statements
Report of PricewaterhouseCoopers LLP, independent registered public accounting firm
186
Partnership Combined Financial Statements
| 2. | Financial Statement Schedules |
| Merck/Schering-Plough Cholesterol Partnership Combined Financial Statements |
Combined Statements of Net Sales and Contractual Expenses
Years Ended December�31,
($ in millions)
| 2008 | 2007 | |||||||
Net sales | $ | 4,561 | $ | 5,186 | ||||
Cost of sales | 176 | 216 | ||||||
Selling, general and administrative | 1,062 | 1,151 | ||||||
Research and development | 168 | 156 | ||||||
| 1,406 | 1,523 | |||||||
Income from operations | $ | 3,155 | $ | 3,663 | ||||
| ||||||||
Combined Balance Sheet
December�31,
($ in millions)
| 2008 | ||||
Assets | ||||
Cash and cash equivalents | $ | 204 | ||
Accounts receivable, net | 311 | |||
Inventories | 79 | |||
Prepaid expenses and other assets | 14 | |||
Total assets | $ | 608 | ||
| ||||
Liabilities and Partners� Capital | ||||
Rebates payable | $ | 263 | ||
Payable to Merck, net | 81 | |||
Payable to Schering-Plough, net | 100 | |||
Accrued expenses and other liabilities | 44 | |||
Total liabilities | 488 | |||
Commitments and contingent liabilities (notes�3 and 5) | ||||
Partners� capital | 120 | |||
Total liabilities and Partners� capital | $ | 608 | ||
| ||||
187
Combined Statements of Cash Flows
Years Ended December�31,
($ in millions)
| 2008 | 2007 | |||||||
Operating Activities: | ||||||||
Income from operations | $ | 3,155 | $ | 3,663 | ||||
Adjustments to reconcile income from operations to net cash provided by operating activities: | ||||||||
Accounts receivable, net | 91 | (109 | ) | |||||
Inventories | 26 | (18 | ) | |||||
Prepaid expenses and other assets | 2 | (2 | ) | |||||
Rebates payable | (114 | ) | 106 | |||||
Payable to Merck and Schering-Plough, net | (53 | ) | 1 | |||||
Accrued expenses and other liabilities | (1 | ) | 38 | |||||
Non-cash charges | 68 | 60 | ||||||
Net cash provided by operating activities | 3,174 | 3,739 | ||||||
Financing Activities: | ||||||||
Contributions from Partners | 407 | 722 | ||||||
Distributions to Partners | (3,868 | ) | (4,006 | ) | ||||
Net cash used for financing activities | (3,461 | ) | (3,284 | ) | ||||
Net increase/(decrease) in cash and cash equivalents | (287 | ) | 455 | |||||
Cash and cash equivalents, beginning of period | 491 | 36 | ||||||
Cash and cash equivalents, end of period | $ | 204 | $ | 491 | ||||
| ||||||||
188
Combined Statements of Partners� Capital (Deficit)
($ in millions)
| Schering- | ||||||||||||
| Plough | Merck | Total | ||||||||||
Balance, January�1, 2007 | 2 | (83 | ) | (81 | ) | |||||||
Contributions from Partners | 276 | 506 | 782 | |||||||||
Income from operations | 1,831 | 1,832 | 3,663 | |||||||||
Distributions to Partners | (1,944 | ) | (2,062 | ) | (4,006 | ) | ||||||
Balance, December�31, 2007 | 165 | 193 | 358 | |||||||||
Contributions from Partners | 143 | 264 | 407 | |||||||||
Income from operations | 1,665 | 1,490 | 3,155 | |||||||||
Distributions to Partners | (1,964 | ) | (1,836 | ) | (3,800 | ) | ||||||
Balance, December�31, 2008 | $ | 9 | $ | 111 | $ | 120 | ||||||
| ||||||||||||
189
Notes to Combined Financial Statements
| 1. | Description of Business and Basis of Presentation |
In May 2000, Merck�& Co., Inc. (�Merck�) and Schering-Plough Corporation (�Schering-Plough�) (collectively the �Partners�) entered into agreements (the �Agreements�) to jointly develop and market in the United States, Schering-Plough�s then investigational cholesterol absorption inhibitor (�CAI�) ezetimibe (marketed today in the United States as ZETIA and as EZETROL in most other countries) (the �Cholesterol Collaboration�) and a fixed-combination tablet containing the active ingredients montelukast sodium and loratadine (the �Respiratory Collaboration�). Montelukast sodium, a leukotriene receptor antagonist, is sold by Merck as SINGULAIR and loratadine, an antihistamine, is sold by Schering-Plough as CLARITIN, both of which are indicated for the relief of symptoms of allergic rhinitis. The Respiratory Collaboration was terminated in 2008 in accordance with the applicable agreements, following the receipt of a not-approvable letter from the U.S.�Food and Drug Administration (�FDA�) for the fixed-combination tablet.
The Cholesterol Collaboration is formally referred to as the Merck/Schering-Plough Cholesterol Partnership (the �Partnership�). In December 2001, the Cholesterol Collaboration Agreements were expanded to include all countries of the world, except Japan. The Cholesterol Collaboration Agreements provide for ezetimibe to be developed and marketed in the following forms:
| � | Ezetimibe, a once daily CAI, non-statin cholesterol reducing medicine used alone or co-administered with any statin drug,�and | |
| � | Ezetimibe and simvastatin (Merck�s existing ZOCOR statin cholesterol modifying medicine) combined into one tablet (marketed today in the United States as VYTORIN and as INEGY in most other countries). |
VYTORIN and ZETIA were approved by the FDA in July 2004 and October 2002, respectively. Together, these products, whether marketed as VYTORIN, ZETIA or under other trademarks locally, are referred to as the �Cholesterol Products.�
Under the Cholesterol Collaboration Agreements, the Partners established jointly-owned, limited purpose legal entities based in Canada and the United States through which to carry out the contractual activities of the Partnership in these countries. An additional jointly-owned, limited purpose legal entity based in Singapore was established to own the rights to the intellectual property and to fund and oversee research and development and manufacturing activities of the Cholesterol Collaboration. In all other markets except Latin America, subsidiaries of Merck or Schering-Plough perform marketing activities for the Cholesterol Products under contract with the Partnership. These legal entity and subsidiary operations are collectively referred to as the �Combined Companies.� In Latin America, the Partnership sells directly to Schering-Plough and Merck�s Latin American subsidiaries and Schering-Plough and Merck compete against one another in the cholesterol market. Consequently, selling, promotion and distribution activities for the Cholesterol Products within Latin America are not included in the Combined Companies.
The Partnership is substantially reliant on the infrastructures of Merck and Schering-Plough. There are a limited number of employees of the legal entities of the Partnership and most activities are performed by employees of either Merck or Schering-Plough under service agreements with the Partnership. Profits, which are shared by the Partners under differing arrangements in countries around the world, are generally defined as net sales minus (1)�agreed upon manufacturing costs and expenses incurred by the Partners and invoiced to the Partnership, (2)�direct promotion expenses incurred by the Partners and invoiced to the Partnership, (3)�expenses for a limited specialty sales force in the United States incurred by the Partners and invoiced to the Partnership, and certain amounts for sales force physician detailing of the Cholesterol Products in the United States, Puerto Rico, Canada and Italy, (4)�administration expenses based on a percentage of Cholesterol Product net sales, which are invoiced by one of the Partners, and (5)�other costs and expenses incurred by the Partners that were not contemplated when the Cholesterol Collaboration Agreements were entered into but that were subsequently agreed to by both Partners.
190
Agreed upon research and development expenses incurred by the Partners and invoiced to the Partnership are shared equally by the Partners, after adjusting for special allocations in the nature of milestones due to one of the Partners.
The Partnership�s future results of operations, financial position, and cash flows may differ materially from the historical results presented herein because of the risks and uncertainties related to the Partnership�s business. The Partnership�s future operating results and cash flows are dependent on the Cholesterol Products. Any events that adversely affect the market for those products could have a significant impact on the Partnership�s results of operations and cash flows. These events could include loss of patent protection, increased costs associated with manufacturing, increased competition from the introduction of new, more effective treatments, exclusion from government reimbursement programs, discontinuation or removal from the market of a product for safety or other reason, and the results of future clinical or outcomes studies (Note�5).
The accompanying combined balance sheet and combined statements of net sales and contractual expenses, cash flows and partners� capital (deficit) include the Cholesterol and Respiratory Collaboration activities of the Combined Companies. The Respiratory Collaboration activities primarily pertained to clinical development work and pre-launch marketing activities. Spending on respiratory-related activities ceased in 2008 following termination of the collaboration, and is not material to the income from operations in any of the years presented.
Net sales include the net sales of the Cholesterol Products sold by the Combined Companies. Expenses include amounts that Merck and Schering-Plough have contractually agreed to directly invoice to the Partnership, or are shared through the contractual profit sharing arrangements between the Partners, as described above.
The accompanying combined financial statements were prepared for the purpose of complying with certain rules and regulations of the Securities and Exchange Commission and reflect the activities of the Partnership based on the contractual agreements between the Partners. Such combined financial statements include only the expenses agreed by the Partners to be shared or included in the calculation of profits under the contractual agreements of the Partnership, and are not intended to be a complete presentation of all of the costs and expenses that would be incurred by a stand-alone pharmaceutical company for the discovery, development, manufacture, distribution and marketing of pharmaceutical products.
Under the Cholesterol Collaboration Agreements, certain activities are charged to the Partnership by the Partners based on contractually agreed upon allocations of Partner-incurred expenses as described below. In the opinion of management, any allocations of expenses described below are made on a basis that reasonably reflects the actual level of support provided. All other expenses are expenses of the Partners and are reflected in their separate consolidated financial statements.
As described above, the profit sharing arrangements under the Cholesterol Collaboration Agreements provide that only certain Partner-incurred costs and expenses be invoiced to the Partnership by the Partners and therefore become part of the profit sharing calculation. The following paragraphs list the typical categories of costs and expenses that are generally incurred in the discovery, development, manufacture, distribution and marketing of the Cholesterol Products and provide a description of how such costs and expenses are treated in the accompanying combined statements of net sales and contractual expenses, and in determining profits under the contractual agreements.
| � | Manufacturing costs and expenses�� All contractually agreed upon manufacturing plant costs and expenses incurred by the Partners related to the manufacture of the Cholesterol Products are included as Cost of sales in the accompanying combined statements of net sales and contractual expenses, including direct production costs, certain production variances, expenses for plant services and administration, warehousing, distribution, materials management, technical services, quality control, and asset utilization. All other manufacturing costs and expenses incurred by the Partners not agreed to be included in the determination of profits under the contractual agreements are not invoiced to the Partnership and, therefore, are excluded from the accompanying combined financial statements. These costs and expenses include, but are not limited to, yield gains and losses in excess of jointly agreed upon yield rates and excess/idle capacity of manufacturing plant assets. |
191
| � | Direct promotion expenses�� Direct promotion represents direct and identifiable out-of-pocket expenses incurred by the Partners on behalf of the Partnership including, but not limited to, contractually agreed upon expenses related to market research, detailing aids, agency fees, direct-to-consumer advertising, meetings and symposia, trade programs, launch meetings, special sales force incentive programs and product samples. All such contractually agreed upon expenses are included in Selling, general and administrative in the accompanying combined statements of net sales and contractual expenses. All other promotion expenses incurred by the Partners not agreed to be included in the determination of profits under the contractual agreements are excluded from the accompanying combined financial statements. | |
| � | Selling expenses�� In the United States, Canada, Puerto Rico and other markets outside the United States (primarily Italy), the general sales forces of the Partners provide a majority of the physician detail activity at an agreed upon cost which is included in Selling, general and administrative in the accompanying combined statements of net sales and contractual expenses. In addition, the agreed upon costs of a limited specialty sales force for the United States market that calls on opinion leaders in the field of cholesterol medicine are also included in Selling, general and administrative. All other selling expenses incurred by the Partners not agreed to be included in the determination of profits under the contractual agreements are excluded from the accompanying combined financial statements. These expenses include the total costs of the general sales forces of the Partners detailing the Cholesterol Products in most countries other than the United States, Canada, Puerto Rico and Italy. | |
| � | Administrative expenses�� Administrative support is primarily provided by one of the Partners. The contractually agreed upon expenses for support are determined based on a percentage of the net sales of the Cholesterol Products. Such amounts are included in Selling, general and administrative in the accompanying combined statements of net sales and contractual expenses. Selected contractually agreed upon direct costs of employees of the Partners for support services and out-of-pocket expenses incurred by the Partners on behalf of the Partnership are also included in Selling, general and administrative. All other expenses incurred by the Partners not agreed to be included in the determination of profits under the contractual agreements are excluded from the accompanying combined financial statements. These expenses include, but are not limited to, certain U.S.�managed care services, Partners� subsidiary management in most international markets, and other indirect expenses such as corporate overhead and interest. | |
| � | Research and development (�R&D�) expenses�� R&D activities are performed by the Partners and agreed upon costs and expenses are invoiced to the Partnership. These agreed upon expenses generally represent an allocation of each Partner�s estimate of full time equivalents devoted to pre-clinical and post-marketing clinical development and regulatory activities and include grants and other third-party expenses. These contractually agreed upon allocated costs are included in Research and development in the accompanying combined statements of net sales and contractual expenses. All other R&D costs that are incurred by the Partners but not jointly agreed upon, are excluded from the accompanying combined financial statements. |
| 2. | Summary of Significant Accounting Policies |
The accompanying combined balance sheet and combined statements of net sales and contractual expenses, cash flows and partners� capital (deficit) include the Cholesterol and Respiratory Collaboration activities of the Combined Companies. Interpartnership balances and profits are eliminated.
The combined financial statements are prepared based on contractual agreements between the Partners, as described above, and include certain amounts that are based on management�s best estimates and judgments. Estimates are used in determining such items as provisions for sales discounts and returns and government and managed care rebates. Because of the uncertainty inherent in such estimates, actual results may differ from these estimates.
192
The net assets of the Partnership�s foreign operations are translated into U.S.�dollars at current exchange rates. The U.S.�dollar effects arising from translating the net assets of these operations are included in Partners� capital, and are not significant.
Cash and cash equivalents primarily consist of highly liquid money market instruments with original maturities of less than three months. In 2007, the Partnership changed certain cash management practices, increasing the amount of cash held by the Partnership. The Partnership�s cash, which is primarily invested in highly liquid money market instruments, is used to fund trade obligations coming due in the month and for distributions to the Partners. Interest income earned on cash and cash equivalents is reported as a reduction to Selling, general and administrative in the accompanying combined statements of net sales and contractual expenses and amounted to $10�million and $8�million in 2008 and 2007, respectively.
Substantially all inventories are valued at the lower of first in, first out cost or market.
Intangible assets consist of licenses, trademarks and trade names owned by the Partnership. These intangible assets were recorded at the Partners� historical cost at the date of contribution, at a nominal value.
Revenues from sales of Cholesterol Products are recognized when title and risk of loss pass to the customer. Recognition of revenue also requires reasonable assurance of collection of sales proceeds and completion of all performance obligations.
Net sales of VYTORIN/INEGY and ZETIA/EZETROL for the years ended December 31 are as follows:
| $ in millions | 2008 | 2007 | ||||||
Vytorin/Inegy | $ | 2,360 | $ | 2,779 | ||||
Zetia/Ezetrol | 2,201 | 2,407 | ||||||
Total | $ | 4,561 | $ | 5,186 | ||||
| ||||||||
In the United States, sales discounts are issued to customers as direct discounts at the point-of-sale or indirectly through an intermediary wholesale purchaser, known as chargebacks, or indirectly in the form of rebates. Additionally, sales are generally made with a limited right of return under certain conditions. Sales are recorded net of provisions for sales discounts and returns for which reliable estimates can be made at the time of sale. Reserves for chargebacks, discounts and returns and allowances are reflected as a direct reduction to accounts receivable and amounted to $34�million at December�31, 2008. Accruals for rebates are reflected as Rebates payable, shown separately in the combined balance sheet.
The Partnership�s concentrations of credit risk consist primarily of accounts receivable. The Partnership does not normally require collateral or other security to support credit sales. Bad debts for the years ended
193
December�31, 2008 and 2007 have been minimal. At December�31, 2008, three customers each represented 25%, 19% and 17% of Accounts receivable, net. The same three customers each accounted for more than 10% of Net sales as shown in the table below.
| Percent of Net Sales | ||||||||
| 2008 | 2007 | |||||||
McKesson Drug Company | 24 | % | 28 | % | ||||
Cardinal Health, Inc. | 21 | % | 26 | % | ||||
Amerisourcebergen Corp. | 16 | % | 17 | % | ||||
The Partnership derived approximately 65% and 75% of its combined Net sales from the United States in 2008 and 2007, respectively.
The Respiratory Collaboration was terminated in 2008 in accordance with the applicable agreements, following the receipt of a not-approvable letter from the FDA for the proposed montelukast/loratadine combination tablet. As a result of termination, Schering-Plough received $105�million in incremental allocations of Partnership profits in 2008. Except for the allocation of certain profits, termination had no other impact on the Cholesterol Collaboration.
| 3. | Inventories |
Inventories at December 31 consisted of:
| $ in millions | 2008 | |||
Finished goods | $ | 31 | ||
Raw materials and work in process | 48 | |||
Total | $ | 79 | ||
| ||||
The Partnership has entered into long-term agreements with the Partners for the supply of active pharmaceutical ingredients (API) and for the formulation and packaging of the Cholesterol Products at an agreed upon cost. In connection with these supply agreements, the Partnership has entered into capacity agreements under which the Partnership has committed to take a specified annual minimum supply of API and formulated tablets or pay a penalty. These capacity agreements are in effect for a period of seven years following the first full year of production by one of the Partners and expire beginning in 2009. The Partnership had no payment obligation under the capacity agreements at December�31, 2008.
| 4. | Related Party Transactions |
The Partnership receives substantially all of its goods and services, including pharmaceutical product, manufacturing services, sales force services, administrative services and R&D services, from its Partners. The Partnership had a net payable to Merck and Schering-Plough for these services of $81�million and $100�million, respectively, at December�31, 2008.
Selling, general and administrative expense includes contractually defined costs for physician detailing provided by Schering-Plough and Merck of $223�million and $201�million, respectively, in 2008 and $242�million and $197�million, respectively, in 2007. These expenses are not necessarily reflective of the actual cost of the Partners� sales efforts in the countries in which the amounts are contractually defined. Included in these amounts are $68�million and $60�million in 2008 and 2007, respectively, relating to contractually defined costs of physician detailing in Italy. These amounts were not invoiced or paid by the Partnership to the Partners, but are a component of the profit sharing calculation.
Cost of sales and selling, general and administrative expense also include contractually defined costs for distribution and administrative services provided by Merck and Schering-Plough of $39�million and $34�million in 2008 and 2007, respectively. These amounts are not necessarily reflective of the actual costs for such distribution and administrative services.
194
The Partnership also sells Cholesterol Products directly to the Partners, principally to Merck and Schering-Plough affiliates in Latin America. In Latin America, where the Partners compete with one another in the cholesterol market, Merck and Schering-Plough purchase Cholesterol Products from the Partnership and sell directly to third parties. Sales to the Partners are included in Net sales at their invoiced price in the accompanying combined statements of net sales and contractual expenses and totaled $74�million and $82�million in 2008 and 2007, respectively.
| 5. | Legal and Other Matters |
The Partnership may become party to claims and legal proceedings of a nature considered normal to its business, including product liability and intellectual property. The Partnership records a liability in connection with such matters when it is probable a liability has been incurred and an amount can be reasonably estimated. Legal costs associated with litigation and investigation activities are expensed as incurred.
The Partnership maintains insurance coverage with deductibles and self-insurance as management believes is cost beneficial. The Partnership self-insures all of its risk as it relates to product liability and accrues an estimate of product liability claims incurred but not reported.
In February 2007, Schering-Plough received a notice from Glenmark Pharmaceuticals Inc. USA (�Glenmark�), a generic pharmaceutical company, indicating that it had filed an Abbreviated New Drug Application (�ANDA�) for a generic form of ZETIA and that it is challenging the U.S.�patents that are listed for ZETIA. In March 2007, Schering-Plough and the Partnership filed a patent infringement suit against Glenmark and its parent company. The lawsuit automatically stays FDA approval of Glenmark�s ANDA until the earlier of October 2010 or an adverse court decision, if any. Schering-Plough and the Partnership intend to vigorously defend its patents, which they believe are valid, against infringement by generic companies attempting to market products prior to the expiration dates of such patents. As with any litigation, there can be no assurances of the outcomes which, if adverse, could result in significantly shortened periods of exclusivity.
In January 2008, the Partners announced the results of the Effect of Combination Ezetimibe and High-Dose Simvastatin vs. Simvastatin Alone on the Atherosclerotic Process in Patients with Heterozygous Familial Hypercholesterolemia (�ENHANCE�) clinical trial, an imaging trial in 720�patients with heterozygous familial hypercholesterolemia, a rare genetic condition that causes very high levels of LDL �bad� cholesterol and greatly increases the risk for premature coronary artery disease. Despite the fact that ezetimibe/simvastatin 10/80 mg (VYTORIN) significantly lowered LDL �bad� cholesterol more than simvastatin 80�mg alone, there was no significant difference between treatment with ezetimibe/simvastatin and simvastatin alone on the pre-specified primary endpoint, a change in the thickness of carotid artery walls over two years as measured by ultrasound. There also were no significant differences between treatment with ezetimibe/simvastatin and simvastatin on the four pre-specified key secondary endpoints: percent of patients manifesting regression in the average carotid artery intima-media thickness (�CA IMT�); proportion of patients developing new carotid artery plaques >1.3 mm; changes in the average maximum CA IMT; and changes in the average CA IMT plus in the average common femoral artery IMT. In ENHANCE, when compared to simvastatin alone, ezetimibe/simvastatin significantly lowered LDL �bad� cholesterol, as well as triglycerides and C-reactive protein (�CRP�). Ezetimibe/simvastatin is not indicated for the reduction of CRP. In the ENHANCE study, the overall safety profile of ezetimibe/simvastatin was generally consistent with the product label. The ENHANCE study was not designed nor powered to evaluate cardiovascular clinical events. The Improved Reduction in High-Risk Subjects Presenting with Acute Coronary Syndrome (�IMPROVE-IT�) trial is underway and is designed to provide cardiovascular outcomes data for ezetimibe/simvastatin in patients with acute coronary syndrome. No incremental benefit of ezetimibe/simvastatin on cardiovascular morbidity and mortality over and above that demonstrated for simvastatin has been established. In March 2008, the results of ENHANCE were reported at the annual Scientific Session of the American College of Cardiology. In January 2009, the FDA announced that it had completed its review of the final clinical study report of ENHANCE. The FDA stated that the results from ENHANCE did not change its position that an elevated LDL cholesterol is a risk factor for cardiovascular disease and that lowering LDL cholesterol reduces the risk for cardiovascular disease. The FDA also stated that, based on current available data, patients should not stop taking VYTORIN or other cholesterol lowering medications and should talk to their doctor if they have any questions about VYTORIN, ZETIA, or the ENHANCE trial.
195
On July�21, 2008, efficacy and safety results from the Simvastatin and Ezetimibe in Aortic Stenosis (�SEAS�) study were announced. SEAS was designed to evaluate whether intensive lipid lowering with VYTORIN 10/40 mg would reduce the need for aortic valve replacement and the risk of cardiovascular morbidity and mortality versus placebo in patients with asymptomatic mild to moderate aortic stenosis who had no indication for statin therapy. VYTORIN failed to meet its primary end point for the reduction of major cardiovascular events. There also was no significant difference in the key secondary end point of aortic valve events; however, there was a reduction in the group of patients taking VYTORIN compared to placebo in the key secondary end point of ischemic cardiovascular events. VYTORIN is not indicated for the treatment of aortic stenosis. No incremental benefit of VYTORIN on cardiovascular morbidity and mortality over and above that demonstrated for simvastatin has been established. In the study, patients in the group who took VYTORIN 10/40 mg had a higher incidence of cancer than the group who took placebo. There was also a nonsignificant increase in deaths from cancer in patients in the group who took VYTORIN versus those who took placebo. Cancer and cancer deaths were distributed across all major organ systems. The Partners and the Partnership believe the cancer finding in SEAS is likely to be an anomaly that, taken in light of all the available data, does not support an association with VYTORIN. In August 2008, the FDA announced that it was investigating the results from the SEAS trial. In this announcement, the FDA also cited interim data from two large ongoing cardiovascular trials of VYTORIN�� the Study of Heart and Renal Protection (�SHARP�) and the IMPROVE-IT clinical trials�� in which there was no increased risk of cancer with the combination of simvastatin plus ezetimibe. The SHARP trial is expected to be completed in 2010. The IMPROVE-IT trial is scheduled for completion around 2012. The FDA determined that, as of that time, these findings in the SEAS trial plus the interim data from ongoing trials should not prompt patients to stop taking VYTORIN or any other cholesterol lowering drug.
The Partners and the Partnership are committed to working with regulatory agencies to further evaluate the available data and interpretations of those data, and do not believe that changes in the clinical use of VYTORIN are warranted.
As previously disclosed, since December 2007, Merck and Schering-Plough have received several letters addressed to both companies from the House Committee on Energy and Commerce, its Subcommittee on Oversight and Investigations (�O&I�), and the Ranking Minority Member of the Senate Finance Committee, collectively seeking a combination of witness interviews, documents and information on a variety of issues related to the ENHANCE clinical trial, the sale and promotion of VYTORIN, as well as sales of stock by corporate officers of Merck and Schering-Plough. In addition, since August 2008, the Partners have received three additional letters from O&I, including one dated February�19, 2009, seeking certain information and documents related to the SEAS clinical trial. Also, as previously disclosed, the Partners and the Partnership have received subpoenas from the New York and New Jersey State Attorneys General Offices and a letter from the Connecticut Attorney General seeking similar information and documents. In addition, the Partners and the Partnership have received five Civil Investigative Demands (�CIDs�) from a multistate group of 35 State Attorneys General who are jointly investigating whether violations of state consumer protection laws occurred when marketing VYTORIN. Finally, in September 2008, Merck and Schering-Plough received a letter from the Civil Division of the U.S.�Department of Justice (�DOJ�) informing them that the DOJ is investigating whether the companies� conduct relating to the promotion of VYTORIN caused false claims to be submitted to federal health care programs. The Partners and the Partnership are cooperating with these investigations and responding to the inquiries. In addition, the Partners and the Partnership have become aware of or been served with approximately 145 civil class action lawsuits alleging common law and state consumer fraud claims in connection with the Partnership�s sale and promotion of VYTORIN and ZETIA. Certain of those lawsuits allege personal injuries and/or seek medical monitoring. These actions, which have been filed in or transferred to federal court, are coordinated in a multidistrict litigation in the U.S.�District Court for the District Court of New Jersey before District Judge Dennis M. Cavanaugh. The parties are presently engaged in motions practice and briefing.
While it is not feasible to predict the outcome of the investigations or lawsuits arising from the ENHANCE and SEAS clinical trials, unfavorable outcomes could have a significant adverse effect on the Partnership�s financial position, results of operations and cash flows.
196
The Partners of the Merck/Schering-Plough Cholesterol Partnership
We have audited the accompanying combined balance sheet of the Merck/Schering-Plough Cholesterol Partnership (the �Partnership�) as of December�31, 2008, as described in Note�1, and the related combined statements of net sales and contractual expenses, partners� capital (deficit) and cash flows, as described in Note�1, for each of the two years in the period ended December�31, 2008. These financial statements are the responsibility of the management of the Partnership, Merck�& Co., Inc., and Schering-Plough Corporation. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with generally accepted auditing standards as established by the Auditing Standards Board (United States) and in accordance with the auditing standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Partnership is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Partnership�s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying statements were prepared for the purpose of complying with certain rules and regulations of the Securities and Exchange Commission and, as described in Note�1, are not intended to be a complete presentation of the financial position, results of operations or cash flows of all the activities of a stand-alone pharmaceutical company involved in the discovery, development, manufacture, distribution and marketing of pharmaceutical products.
In our opinion, the financial statements referred to above present fairly, in all material respects, the combined financial position of the Merck/Schering-Plough Cholesterol Partnership, as described in Note�1, as of December�31, 2008, and the combined results of its net sales and contractual expenses and its combined cash flows, as described in Note�1, for each of the two years in the period ended December�31, 2008, in conformity with accounting principles generally accepted in the United States of America.
Deloitte�& Touche LLP
Parsippany, New Jersey
February 26, 2009
197
Schedules other than those listed above have been omitted because they are either not required or not applicable.
Financial statements of other affiliates carried on the equity basis have been omitted because, considered individually or in the aggregate, such affiliates do not constitute a significant subsidiary.
| 3. | Exhibits |
| Exhibit | ||||||
Number | Description | |||||
| 2 | .1 | � | Master Restructuring Agreement dated as of June�19, 1998 between Astra AB, Merck�& Co., Inc., Astra Merck Inc., Astra USA, Inc., KB USA, L.P., Astra Merck Enterprises, Inc., KBI Sub Inc., Merck Holdings, Inc. and Astra Pharmaceuticals, L.P. (Portions of this Exhibit are subject to a request for confidential treatment filed with the Commission)�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 2 | .2 | � | Agreement and Plan of Merger by and among Merck�& Co., Inc., Spinnaker Acquisition Corp., a wholly owned subsidiary of Merck�& Co., Inc. and Sirna Therapeutics, Inc., dated as of October�30, 2006�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated October�30, 2006 | |||
| 2 | .3 | � | Agreement and Plan of Merger by and among Merck�& Co., Inc., Schering-Plough Corporation, Blue, Inc. and Purple, Inc. dated as of March�8, 2009�� Incorporated by reference to Schering-Plough�s Current Report on Form�8-K filed March�11, 2009 | |||
| 2 | .4 | � | Share Purchase Agreement, dated July�29, 2009, by and among Merck�& Co., Inc., Merck SH Inc., Merck Sharp�& Dohme (Holdings) Limited and sanofi-aventis�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated July�31, 2009 | |||
| 3 | .1 | � | Restated Certificate of Incorporation of Merck�& Co., Inc. (November�3, 2009)�� Incorporated by reference to Current Report on Form�8-K filed November�4, 2009 | |||
| 3 | .2 | � | By-Laws of Merck�& Co., Inc. (effective November�3, 2009)�� Incorporated by reference to Current Report on Form�8-K filed November�4, 2009 | |||
| 4 | .1 | � | Indenture, dated as of April�1, 1991, between Merck�& Co., Inc. and Morgan Guaranty Trust�Company of New York, as Trustee�� Incorporated by reference to Exhibit�4 to Old Merck�s Registration Statement on Form�S-3 (No.�33-39349) | |||
| 4 | .2 | � | First Supplemental Indenture between Merck�& Co., Inc. and First Trust of New York, National Association, as Trustee�� Incorporated by reference to Exhibit�4(b) to Old Merck�s Registration Statement on Form�S-3 (No.�333-36383) | |||
| 4 | .3 | � | Second Supplemental Indenture, dated November�3, 2009, among Merck Sharp�& Dohme Corp., Merck�& Co., Inc. and U.S. Bank Trust�National Association, as Trustee�� Incorporated by reference to Exhibit�4.3 to Current Report on Form�8-K filed November�4, 2009 | |||
| 4 | .4 | � | 1.875%�Notes due 2011 Officers� Certificate of the Company dated June�25, 2009, including form of the 2011 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .5 | � | 4.000%�Notes due 2015 Officers� Certificate of the Company dated June�25, 2009, including form of the 2015 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .6 | � | 5.000%�Notes due 2019 Officers� Certificate of the Company dated June�25, 2009, including form of the 2019 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .7 | � | 5.850%�Notes due 2039 Officers� Certificate of the Company dated June�25, 2009, including form of the 2039 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .8 | � | Indenture, dated November�26, 2003, between Schering-Plough and The Bank of New York as Trustee � Incorporated by reference to Exhibit�4.1 to Schering-Plough�s Current Report on Form�8-K filed November�28, 2003 | |||
198
| Exhibit | ||||||
Number | Description | |||||
| 4 | .9 | � | First Supplemental Indenture (including Form of Note), dated November�26, 2003�� Incorporated by reference to Exhibit�4.2 to Schering-Plough�s Current Report on Form�8-K filed November�28, 2003 | |||
| 4 | .10 | � | Second Supplemental Indenture (including Form of Note), dated November�26, 2003�� Incorporated by reference to Exhibit�4.3 to Schering-Plough�s Current Report on Form�8-K filed November�28, 2003 | |||
| 4 | .11 | � | 5.30% Global Senior Note, due 2013�� Incorporated by reference to Exhibit�4(c)(iv) to Schering-Plough�s Form�10-K Annual Report for the fiscal year ended December�31, 2003 | |||
| 4 | .12 | � | 6.50% Global Senior Note, due 2033�� Incorporated by reference to Exhibit�4(c)(v) to Schering-Plough�s Form�10-K Annual Report for the fiscal year ended December�31, 2003 | |||
| 4 | .13 | � | Third Supplemental Indenture (including Form of Note), dated September�17, 2007�� Incorporated by reference to Exhibit�4.1 to Schering-Plough�s Current Report on Form�8-K filed September�17, 2007 | |||
| 4 | .14 | � | Fourth Supplemental Indenture (including Form of Note), dated October�1, 2007�� Incorporated by reference to Exhibit�4.1 to Schering-Plough�s Current Report on Form�8-K filed October�2, 2007 | |||
| 4 | .15 | � | Fifth Supplemental Indenture, dated November�3, 2009, among Merck Sharp�& Dohme Corp., Merck�& Co., Inc. and The Bank of New York Mellon, as Trustee�� Incorporated by reference to Exhibit�4.4 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .1 | � | Executive Incentive Plan (as amended effective February�27, 1996)�� Incorporated by reference to Old Merck�s Form�10-K Annual Report for the fiscal year ended December�31, 1995 | |||
| *10 | .2 | � | Merck Sharp�& Dohme Corp. Deferral Program, including Base Salary Deferral Plan (effective as amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.15 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .3 | � | Merck Sharp�& Dohme Corp. 1996 Incentive Stock Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.10 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .4 | � | Merck Sharp�& Dohme Corp. 2001 Incentive Stock Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.9 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .5 | � | Merck Sharp�& Dohme Corp. 2004 Incentive Stock Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.8 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .6 | � | Merck Sharp�& Dohme Corp. 2007 Incentive Stock Plan (effective as amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.7 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .7 | � | Amendment One to the Merck Sharp & Dohme Corp. 2007 Incentive Stock Plan (effective February�15, 2010)�� Incorporated by reference to Exhibit 10.2 to Current Report on Form�8-K filed February�18, 2010 | |||
| *10 | .8 | � | Merck�& Co., Inc. Change in Control Separation Benefits Plan�� Incorporated by reference to Current Report on Form�8-K dated November�23, 2009 | |||
| *10 | .9 | � | Amendment One to Merck�& Co., Inc. Change in Control Separation Benefits Plan (effective February�15, 2010)�� Incorporated by reference to Exhibit�10.1 to Current Report on Form�8-K filed February�18, 2010 | |||
| *10 | .10 | � | MSD Separation Benefits Plan for Nonunion Employees (amended and restated effective as of November�3, 2009) | |||
| *10 | .11 | � | MSD Special Separation Program for �Separated� Employees (effective as of November�3, 2009) | |||
| *10 | .12 | � | MSD Special Separation Program for �Bridged� Employees (effective as of November�3, 2009) | |||
| *10 | .13 | � | MSD Special Separation Program for �Separated Retirement Eligible� Employees (effective as of November�3, 2009) | |||
| Exhibit | ||||||
Number | Description | |||||
| *10 | .14 | � | Merck�& Co., Inc. 1996 Non-Employee Directors Stock Option Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.12 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .15 | � | Merck�& Co., Inc. 2001 Non-Employee Directors Stock Option Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.11 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .16 | � | Merck�& Co., Inc. 2006 Non-Employee Directors Stock Option Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.5 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .17 | � | Retirement Plan for the Directors of Merck�& Co., Inc. (amended and restated June�21, 1996)�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1996 | |||
| *10 | .18 | � | Merck�& Co., Inc. Plan for Deferred Payment of Directors� Compensation (effective as amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.6 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .19 | � | Merck�& Co., Inc. Schering-Plough 2006 Stock Incentive Plan (amended and restated as of November�3, 2009�� Incorporated by reference to Exhibit�10.13 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .20 | � | Offer Letter between Merck�& Co., Inc. and Peter S. Kim, dated December�15, 2000�� Incorporated by reference to Old Merck�s Form�10-K Annual Report for the fiscal year ended December�31, 2003 | |||
| *10 | .21 | � | Offer Letter between Merck�& Co., Inc. and Peter N. Kellogg, dated June�18, 2007�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�28, 2007 | |||
| *10 | .22 | � | 1997 Stock Incentive Plan�� Incorporated by reference to Exhibit�10 to Schering-Plough�s 10-Q for the period ended September�30, 1997 | |||
| *10 | .23 | � | Amendment to 1997 Stock Incentive Plan (effective February�22, 1999)�� Incorporated by reference to Exhibit�10(a) to Schering-Plough�s 10-Q for the period ended March�31, 1999 | |||
| *10 | .24 | � | Amendment to the 1997 Stock Incentive Plan (effective February�25, 2003)�� Incorporated by reference to Exhibit�10(c) to Schering-Plough�s 10-K for the year ended December�31, 2002 | |||
| *10 | .25 | � | 2002 Stock Incentive Plan (as amended to February�25, 2003)�� Incorporated by reference to Exhibit�10(d) to Schering-Plough�s 10-K for the year ended December�31, 2002 | |||
| *10 | .26 | � | Merck�& Co., Inc. Schering-Plough 2006 Stock Incentive Plan (as amended and restated, effective November�3, 2009) ��Incorporated by reference to Exhibit�10.13 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .27 | � | Letter agreement dated November�4, 2003 between Robert Bertolini and Schering-Plough�� Incorporated by reference to Exhibit�10(e)(iii) to Schering-Plough�s 10-K for the year ended December�31, 2003 | |||
| *10 | .28 | � | Employment Agreement effective upon a change of control dated as of December�19, 2006 between Robert Bertolini and Schering-Plough Corporation�� Incorporated by reference to Exhibit�99.1 to Schering-Plough�s 8-K filed December�21, 2006 | |||
| *10 | .29 | � | Amendment to Letter Agreement and Employment Agreement between Schering-Plough Corporation and Robert J. Bertolini, dated December�9, 2008�� Incorporated by reference to Exhibit�99.1 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| *10 | .30 | � | Employment Agreement dated as of May�12, 2003 between Carrie Cox and Schering-Plough�� Incorporated by reference to Exhibit�99.6 to Schering-Plough�s 8-K filed May�13, 2003 | |||
| *10 | .31 | � | Amendment to Employment Agreement between Schering-Plough Corporation and Carrie S. Cox, dated December�9, 2008�� Incorporated by reference to Exhibit�99.2 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| *10 | .32 | � | Employment Agreement dated as of April�20, 2003 between Fred Hassan and Schering-Plough�� Incorporated by reference to Exhibit�99.2 to Schering-Plough�s 8-K filed April�21, 2003 | |||
| Exhibit | ||||||
Number | Description | |||||
| *10 | .33 | � | Amendment to Employment Agreement between Schering-Plough Corporation and Fred Hassan, dated December�9, 2008�� Incorporated by reference to Exhibit�99.3 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| *10 | .34 | � | Employment Agreement dated as of December�19, 2006 between Thomas P. Koestler,�Ph.D. and Schering-Plough�� Incorporated by reference to Exhibit�10(e)(v) to Schering-Plough�s 10-K for the year ended December�31, 2006 | |||
| *10 | .35 | � | Amendment to Employment Agreement between Schering-Plough Corporation and Thomas P. Koestler, dated December�9, 2008�� Incorporated by reference to Exhibit�99.4 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| *10 | .36 | � | Form of employment agreement effective upon a change of control between Schering-Plough and certain executives for new agreements beginning in January�1, 2008�� Incorporated by reference to Exhibit�10(e)(xv) to Schering-Plough�s 10-K for the year ended December�31, 2008 | |||
| *10 | .37 | � | Operations Management Team Incentive Plan (as amended and restated effective June�26, 2006)�� Incorporated by reference to Exhibit�10(m)(ii) to Schering-Plough�s 10-Q for the period ended September�30, 2006 | |||
| *10 | .38 | � | Cash Long-Term Incentive Plan (as amended and restated effective January�24, 2005)�� Incorporated by reference to Exhibit�10(n) to Schering-Plough�s 10-K for the year ended December�31, 2004 | |||
| *10 | .39 | � | Long-Term Performance Share Unit Incentive Plan (as amended and restated effective January�24, 2005)�� Incorporated by reference to Exhibit�10(o) to Schering-Plough�s 10-K for the year ended December�31, 2004 | |||
| *10 | .40 | � | Transformational Performance Contingent Shares Program�� Incorporated by reference to Exhibit�10(p) to Schering-Plough�s 10-K for the year ended December�31, 2003 | |||
| *10 | .41 | � | Schering-Plough Corporation Severance Benefit Plan (as amended and restated effective November�3, 2009) | |||
| *10 | .42 | � | Schering-Plough Corporation Savings Advantage Plan (as amended and restated effective November�4, 2009) | |||
| *10 | .43 | � | Schering-Plough Corporation Supplemental Executive Retirement Plan (as amended and restated effective November�4, 2009) | |||
| *10 | .44 | � | Schering-Plough Retirement Benefits Equalization Plan (as amended and restated effective November�4, 2009) | |||
| *10 | .45 | � | Executive Incentive Plan (as amended and restated to October�1, 2000)�� Incorporated by reference to Exhibit�10(a)(i) to Schering-Plough�s 10-K for the year ended December�31, 2000 | |||
| *10 | .46 | � | Schering-Plough Corporation Executive Life Insurance Direct Payment Program (as amended and restated effective November�4, 2009) | |||
| *10 | .47 | � | Amended and Restated Defined Contribution Trust�� Incorporated by reference to Exhibit�10(a)(ii) to Schering-Plough�s 10-K for the year ended December�31, 2000 | |||
| *10 | .48 | � | Amended and Restated SERP Rabbi Trust�Agreement�� Incorporated by reference to Exhibit�10(g) to Schering-Plough�s 10-K for the year ended December�31, 1998 | |||
| 10 | .49 | � | Share Purchase Agreement between Akzo Nobel N.V., Schering-Plough International C.V., and Schering-Plough Corporation�� Incorporated by reference to Exhibit�10.1 to Schering-Plough�s 8-K filed October�2, 2007 | |||
| 10 | .50 | � | Amended and Restated License and Option Agreement dated as of July�1, 1998 between Astra AB and Astra Merck Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .51 | � | KBI Shares�Option Agreement dated as of July�1, 1998 by and among Astra AB, Merck�& Co., Inc. and Merck Holdings, Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .52 | � | KBI-E Asset Option Agreement dated as of July�1, 1998 by and among Astra AB, Merck�& Co., Inc., Astra Merck Inc. and Astra Merck Enterprises Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| Exhibit | ||||||
Number | Description | |||||
| 10 | .53 | � | KBI Supply Agreement dated as of July�1, 1998 between Astra Merck Inc. and Astra Pharmaceuticals, L.P. (Portions of this Exhibit are subject to a request for confidential treatment filed with the Commission).�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .54 | � | Second Amended and Restated Manufacturing Agreement dated as of July�1, 1998 among Merck�& Co., Inc., Astra AB, Astra Merck Inc. and Astra USA, Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .55 | � | Limited Partnership Agreement dated as of July�1, 1998 between KB USA, L.P. and KBI Sub Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .56 | � | Distribution Agreement dated as of July�1, 1998 between Astra Merck Enterprises Inc. and Astra Pharmaceuticals, L.P.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .57 | � | Agreement to Incorporate Defined Terms dated as of June�19, 1998 between Astra AB, Merck�& Co., Inc., Astra Merck Inc., Astra USA, Inc., KB USA, L.P., Astra Merck Enterprises Inc., KBI Sub Inc., Merck Holdings, Inc. and Astra Pharmaceuticals, L.P.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .58 | � | Master Agreement, dated as of December�18, 2001, by and among MSP Technology (U.S.) Company LLC, MSP Singapore Company, LLC, Schering Corporation, Schering-Plough Corporation, and Merck�& Co., Inc. (Portions of this Exhibit are subject to a request for confidential treatment filed with the Commission)�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 2008 | |||
| 10 | .59 | � | Form of Voting Agreement made and entered into as of October�30, 2006 by and between Merck�& Co., Inc. and Sirna Therapeutics, Inc.�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated October�30, 2006 | |||
| 10 | .60 | � | Settlement Agreement, dated November�9, 2007, by and between Merck�& Co., Inc. and The Counsel Listed on the Signature Pages Hereto, including the exhibits thereto�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated November�9, 2007 | |||
| 10 | .61 | � | Commitment Letter by and among Merck�& Co., Inc., J.P.�Morgan Securities Inc. and JPMorgan Chase Bank, N.A. dated as of March�8, 2009�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated March�8, 2009 | |||
| 10 | .62 | � | Stock option terms for a non-qualified stock option under the Merck Sharp�& Dohme Corp. 2007 Incentive Stock Plan and the Schering-Plough 2006 Stock Incentive Plan�� Incorporated by reference to Exhibit�10.3 to Current Report on Form�8-K filed February�15, 2010 | |||
| 10 | .63 | � | Restricted stock unit terms for annual grant under the Merck Sharp�& Dohme Corp. 2007 Incentive Stock Plan and the Schering-Plough 2006 Stock Incentive Plan�� Incorporated by reference to Exhibit�10.4 to Current Report on Form�8-K filed February�15, 2010 | |||
| 10 | .64 | � | Restricted stock unit terms for Leader Shares grant under the Merck�& Co., Inc. 2007 Incentive Stock Plan�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended March�31, 2009 | |||
| 10 | .65 | � | Incremental Credit Agreement dated as of May�6, 2009, among Merck�& Co., Inc., the Guarantors and Lenders party thereto, and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated May�6, 2009 | |||
| 10 | .66 | � | Asset Sale Facility Agreement dated as of May�6, 2009, among Merck�& Co., Inc., the Guarantors and Lenders party thereto, and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated May�6, 2009 | |||
| 10 | .67 | � | Bridge Loan Agreement dated as of May�6, 2009, among Merck�& Co., Inc., the Guarantors and Lenders party thereto, and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated May�6, 2009 | |||
| Exhibit | ||||||
Number | Description | |||||
| 10 | .68 | � | Amendment No.�1 to Amended and Restated Five-Year Credit Agreement dated as of April�20, 2009 among Merck�& Co., Inc., the Lenders party thereto and Citicorp USA, Inc., as Administrative Agent�� Incorporated by reference to Exhibit�10.1 to Current Report on Form�8-K filed November�4, 2009 | |||
| 10 | .69 | � | Guarantee and Joinder Agreement dated as of November�3, 2009 by Merck�& Co., Inc., the Guarantor, for the benefit of the Guaranteed Parties�� Incorporated by reference to Exhibit�10.3 to Current Report on Form�8-K filed November�4, 2009 | |||
| 10 | .70 | � | Guarantor Joinder Agreement dated as of November�3, 2009, by Merck�& Co., Inc., the Guarantor and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Exhibit�10.4 to Current Report on Form�8-K filed November�4, 2009 | |||
| 10 | .71 | � | Call Option Agreement, dated July�29, 2009, by and among Merck�& Co., Inc., Schering-Plough Corporation and sanovi-aventis�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated July�31, 2009 | |||
| 10 | .72 | � | Termination Agreement, dated as of September�17, 2009, by and among Merck�& Co., Inc., Merck SH Inc., Merck Sharp�& Dohme (Holdings) Limited, sanofi-aventis, sanofi 4 and Merial Limited�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated September�21, 2009 | |||
| 10 | .73 | � | Cholesterol Governance Agreement, dated as of May�22, 2000, by and among Schering-Plough, Merck�& Co., Inc. and the other parties signatory thereto�� Incorporated by reference to Exhibit�99.2 to Schering-Plough�s Current Report on Form�8-K dated October�21, 2002� | |||
| 10 | .74 | � | First Amendment to the Cholesterol Governance Agreement, dated as of December�18, 2001, by and among Schering-Plough, Merck�& Co., Inc. and the other parties signatory thereto�� Incorporated by reference to Exhibit�99.3 to Schering-Plough�s Current Report on Form�8-K filed October�21, 2002� | |||
| 10 | .75 | � | Master Agreement, dated as of December�18, 2001, by and among Schering-Plough, Merck�& Co., Inc. and the other parties signatory thereto�� Incorporated by reference to Exhibit�99.4 to Schering-Plough�s Current Report on Form�8-K filed October�21, 2002� | |||
| 10 | .76 | � | Letter Agreement dated April�14, 2003 relating to Consent Decree�� Incorporated by reference to Exhibit�99.3 to Schering-Plough�s 10-Q for the period ended March�31, 2003 | |||
| 10 | .77 | � | Distribution agreement between Schering-Plough and Centocor, Inc., dated April�3, 1998 � Incorporated by reference to Exhibit�10(u) to Schering-Plough�s Amended 10-K for the year ended December�31, 2003, filed May�3, 2004� | |||
| 10 | .78 | � | Amendment Agreement to the Distribution Agreement between Centocor, Inc., CAN Development, LLC, and Schering-Plough (Ireland) Company�� Incorporated by reference to Exhibit�10.1 to Schering-Plough�s Current Report on Form�8-K filed December�21, 2007� | |||
| 12 | � | Computation of Ratios of Earnings to Fixed Charges | ||||
| 21 | � | Subsidiaries of Merck�& Co., Inc. | ||||
| 23 | .1 | � | Consent of Independent Registered Public Accounting Firm�� Contained on page�206 of this Report | |||
| 23 | .2 | � | Independent Auditor�s Consent�� Contained on page�207 of this Report | |||
| 31 | .1 | � | Rule�13a-14(a)/15d-14(a) Certification of Chief Executive Officer | |||
| 31 | .2 | � | Rule�13a-14(a)/15d-14(a) Certification of Chief Financial Officer | |||
| 32 | .1 | � | Section�1350 Certification of Chief Executive Officer | |||
| 32 | .2 | � | Section�1350 Certification of Chief Financial Officer | |||
| 101 | � | The following materials from Merck�& Co., Inc.�s Annual Report on Form�10-K for the fiscal year ended December�31, 2009, formatted in XBRL (Extensible Business Reporting Language):(i)�the Consolidated Statement of Income, (ii)�the Consolidated Balance Sheet, (iii)�the Consolidated Statement of Cash Flow, and (iv)�Notes to Consolidated Financial Statements, tagged as blocks of text. | ||||
| * | Management contract or compensatory plan or arrangement. | |
| � | Certain portions of the exhibit have been omitted pursuant to a request for confidential treatment. The non-public information has been filed separately with the Securities and Exchange Commission pursuant to rule�24b-2 under the Securities Exchange Act of 1934, as amended. |
Pursuant to the requirements of Section�13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: March�1, 2010
MERCK�& CO., INC.
| By: | /s/��Richard T. Clark |
Richard T. Clark
Chairman, President and Chief Executive Officer
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signatures | Title | Date | ||||
/s/��Richard T. Clark Richard T. Clark | Chairman, President, Chief Executive Officer; Principal Executive Officer; Director | March 1, 2010 | ||||
/s/��Peter N. Kellogg Peter N. Kellogg | Executive Vice President and Chief Financial Officer; Principal Financial Officer | March 1, 2010 | ||||
/s/��John Canan John Canan | Senior Vice President and Global Controller; Principal Accounting Officer | March 1, 2010 | ||||
/s/��Leslie A. Brun Leslie A. Brun | Director | March 1, 2010 | ||||
/s/��Thomas R. Cech Thomas R. Cech | Director | March 1, 2010 | ||||
/s/��Thomas H. Glocer Thomas H. Glocer | Director | March 1, 2010 | ||||
/s/��Steven F. Goldstone Steven F. Goldstone | Director | March 1, 2010 | ||||
/s/��William B. Harrison, Jr. William B. Harrison, Jr. | Director | March 1, 2010 | ||||
Harry R. Jacobson | Director | March 1, 2010 | ||||
/s/��William N. Kelley William N. Kelley | Director | March 1, 2010 | ||||
/s/��C. Robert Kidder C. Robert Kidder | Director | March 1, 2010 | ||||
204
Signatures | Title | Date | ||||
/s/��Rochelle B. Lazarus Rochelle B. Lazarus | Director | March 1, 2010 | ||||
Carlos E. Represas | Director | March 1, 2010 | ||||
/s/��Patricia F. Russo Patricia F. Russo | Director | March 1, 2010 | ||||
/s/��Thomas E. Shenk Thomas E. Shenk | Director | March 1, 2010 | ||||
/s/��Anne M. Tatlock Anne M. Tatlock | Director | March 1, 2010 | ||||
/s/��Samuel O. Thier Samuel O. Thier | Director | March 1, 2010 | ||||
/s/��Craig B. Thompson Craig B. Thompson | Director | March 1, 2010 | ||||
/s/��Wendell P. Weeks Wendell P. Weeks | Director | March 1, 2010 | ||||
/s/��Peter C. Wendell Peter C. Wendell | Director | March 1, 2010 | ||||
205
We hereby consent to the incorporation by reference in the Registration Statements on Form S-3 (Nos. 333-164482, 333-163546 and 333-163858) and on Form S-8 (Nos. 333-162882, 333-162883, 333-162884, 333-162885, 333-162886, 033-57111, 333-112421, 333-134281, 333-121089, 333-30331, 333-87077, 333-153542, 333-162007, 333-91440 and 333-105567) of Merck & Co., Inc. of our report dated February�26, 2010 relating to the financial statements and the effectiveness of internal control over financial reporting, which appears in this Form 10-K.
PricewaterhouseCoopers LLP
Florham Park, New Jersey
February�26, 2010
206
We consent to the incorporation by reference in Registration Statement Nos.�333-164482, 333-163546, 333-163858, 333-145055, 333-12909 and 333-30355 on Form�S-3 and Registration Statement Nos. 333-162882, 333-162883, 333-162884, 333-162885, 333-162886, 033-57111, 333-112421, 333-134281, 333-121089, 333-30331, 333-87077, 333-153542, 333-162007, 333-91440, 333-105567, 2-84723, 2-83963, 333-105568, 333-104714 and 33-50606 on Form�S-8 of Merck�& Co., Inc. of our report dated February�26, 2009, relating to the combined financial statements of the Merck/Schering-Plough Cholesterol Partnership appearing in this Annual Report on Form�10-K of Merck�& Co., Inc. for the year ended December�31, 2009.
Deloitte�& Touche LLP
Parsippany, New Jersey
February�26, 2010
207
| Exhibit | ||||||
Number | Description | |||||
| 2 | .1 | � | Master Restructuring Agreement dated as of June�19, 1998 between Astra AB, Merck�& Co., Inc., Astra Merck Inc., Astra USA, Inc., KB USA, L.P., Astra Merck Enterprises, Inc., KBI Sub Inc., Merck Holdings, Inc. and Astra Pharmaceuticals, L.P. (Portions of this Exhibit are subject to a request for confidential treatment filed with the Commission)�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 2 | .2 | � | Agreement and Plan of Merger by and among Merck�& Co., Inc., Spinnaker Acquisition Corp., a wholly owned subsidiary of Merck�& Co., Inc. and Sirna Therapeutics, Inc., dated as of October�30, 2006�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated October�30, 2006 | |||
| 2 | .3 | � | Agreement and Plan of Merger by and among Merck�& Co., Inc., Schering-Plough Corporation, Blue, Inc. and Purple, Inc. dated as of March�8, 2009�� Incorporated by reference to Schering-Plough�s Current Report on Form�8-K filed March�11, 2009 | |||
| 2 | .4 | � | Share Purchase Agreement, dated July�29, 2009, by and among Merck�& Co., Inc., Merck SH Inc., Merck Sharp�& Dohme (Holdings) Limited and sanofi-aventis�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated July�31, 2009 | |||
| 3 | .1 | � | Restated Certificate of Incorporation of Merck�& Co., Inc. (November�3, 2009)�� Incorporated by reference to Current Report on Form�8-K filed November�4, 2009 | |||
| 3 | .2 | � | By-Laws of Merck�& Co., Inc. (effective November�3, 2009)�� Incorporated by reference to Current Report on Form�8-K filed November�4, 2009 | |||
| 4 | .1 | � | Indenture, dated as of April�1, 1991, between Merck�& Co., Inc. and Morgan Guaranty Trust�Company of New York, as Trustee�� Incorporated by reference to Exhibit�4 to Old Merck�s Registration Statement on Form�S-3 (No.�33-39349) | |||
| 4 | .2 | � | First Supplemental Indenture between Merck�& Co., Inc. and First Trust of New York, National Association, as Trustee�� Incorporated by reference to Exhibit�4(b) to Old Merck�s Registration Statement on Form�S-3 (No.�333-36383) | |||
| 4 | .3 | � | Second Supplemental Indenture, dated November�3, 2009, among Merck Sharp�& Dohme Corp., Merck�& Co., Inc. and U.S. Bank Trust�National Association, as Trustee�� Incorporated by reference to Exhibit�4.3 to Current Report on Form�8-K filed November�4, 2009 | |||
| 4 | .4 | � | 1.875%�Notes due 2011 Officers� Certificate of the Company dated June�25, 2009, including form of the 2011 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .5 | � | 4.000%�Notes due 2015 Officers� Certificate of the Company dated June�25, 2009, including form of the 2015 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .6 | � | 5.000%�Notes due 2019 Officers� Certificate of the Company dated June�25, 2009, including form of the 2019 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .7 | � | 5.850%�Notes due 2039 Officers� Certificate of the Company dated June�25, 2009, including form of the 2039 Notes�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�25, 2009 | |||
| 4 | .8 | � | Indenture, dated November�26, 2003, between Schering-Plough and The Bank of New York as Trustee � Incorporated by reference to Exhibit�4.1 to Schering-Plough�s Current Report on Form�8-K filed November�28, 2003 | |||
| 4 | .9 | � | First Supplemental Indenture (including Form of Note), dated November�26, 2003�� Incorporated by reference to Exhibit�4.2 to Schering-Plough�s Current Report on Form�8-K filed November�28, 2003 | |||
| 4 | .10 | � | Second Supplemental Indenture (including Form of Note), dated November�26, 2003�� Incorporated by reference to Exhibit�4.3 to Schering-Plough�s Current Report on Form�8-K filed November�28, 2003 | |||
| 4 | .11 | � | 5.30% Global Senior Note, due 2013�� Incorporated by reference to Exhibit�4(c)(iv) to Schering-Plough�s Form�10-K Annual Report for the fiscal year ended December�31, 2003 | |||
208
| Exhibit | ||||||
Number | Description | |||||
| 4 | .12 | � | 6.50% Global Senior Note, due 2033�� Incorporated by reference to Exhibit�4(c)(v) to Schering-Plough�s Form�10-K Annual Report for the fiscal year ended December�31, 2003 | |||
| 4 | .13 | � | Third Supplemental Indenture (including Form of Note), dated September�17, 2007�� Incorporated by reference to Exhibit�4.1 to Schering-Plough�s Current Report on Form�8-K filed September�17, 2007 | |||
| 4 | .14 | � | Fourth Supplemental Indenture (including Form of Note), dated October�1, 2007�� Incorporated by reference to Exhibit�4.1 to Schering-Plough�s Current Report on Form�8-K filed October�2, 2007 | |||
| 4 | .15 | � | Fifth Supplemental Indenture, dated November�3, 2009, among Merck Sharp�& Dohme Corp., Merck�& Co., Inc. and The Bank of New York Mellon, as Trustee�� Incorporated by reference to Exhibit�4.4 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .1 | � | Executive Incentive Plan (as amended effective February�27, 1996)�� Incorporated by reference to Old Merck�s Form�10-K Annual Report for the fiscal year ended December�31, 1995 | |||
| *10 | .2 | � | Merck Sharp�& Dohme Corp. Deferral Program, including Base Salary Deferral Plan (effective as amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.15 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .3 | � | Merck Sharp�& Dohme Corp. 1996 Incentive Stock Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.10 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .4 | � | Merck Sharp�& Dohme Corp. 2001 Incentive Stock Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.9 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .5 | � | Merck Sharp�& Dohme Corp. 2004 Incentive Stock Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.8 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .6 | � | Merck Sharp�& Dohme Corp. 2007 Incentive Stock Plan (effective as amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.7 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .7 | � | Amendment One to the Merck Sharp & Dohme Corp. 2007 Incentive Stock Plan (effective February�15, 2010)�� Incorporated by reference to Exhibit 10.2 to Current Report on Form�8-K filed February�18, 2010 | |||
| *10 | .8 | � | Merck�& Co., Inc. Change in Control Separation Benefits Plan�� Incorporated by reference to Current Report on Form�8-K dated November�23, 2009 | |||
| *10 | .9 | � | Amendment One to Merck�& Co., Inc. Change in Control Separation Benefits Plan (effective February�15, 2010)�� Incorporated by reference to Exhibit�10.1 to Current Report on Form�8-K filed February�18, 2010 | |||
| *10 | .10 | � | MSD Separation Benefits Plan for Nonunion Employees (amended and restated effective as of November�3, 2009) | |||
| *10 | .11 | � | MSD Special Separation Program for �Separated� Employees (effective as of November�3, 2009) | |||
| *10 | .12 | � | MSD Special Separation Program for �Bridged� Employees (effective as of November�3, 2009) | |||
| *10 | .13 | � | MSD Special Separation Program for �Separated Retirement Eligible� Employees (effective as of November�3, 2009) | |||
| *10 | .14 | � | Merck�& Co., Inc. 1996 Non-Employee Directors Stock Option Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.12 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .15 | � | Merck�& Co., Inc. 2001 Non-Employee Directors Stock Option Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.11 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .16 | � | Merck�& Co., Inc. 2006 Non-Employee Directors Stock Option Plan (amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.5 to Current Report on Form�8-K filed November�4, 2009 | |||
| Exhibit | ||||||
Number | Description | |||||
| *10 | .17 | � | Retirement Plan for the Directors of Merck�& Co., Inc. (amended and restated June�21, 1996)�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1996 | |||
| *10 | .18 | � | Merck�& Co., Inc. Plan for Deferred Payment of Directors� Compensation (effective as amended and restated as of November�3, 2009)�� Incorporated by reference to Exhibit�10.6 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .19 | � | Merck�& Co., Inc. Schering-Plough 2006 Stock Incentive Plan (amended and restated as of November�3, 2009�� Incorporated by reference to Exhibit�10.13 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .20 | � | Offer Letter between Merck�& Co., Inc. and Peter S. Kim, dated December�15, 2000�� Incorporated by reference to Old Merck�s Form�10-K Annual Report for the fiscal year ended December�31, 2003 | |||
| *10 | .21 | � | Offer Letter between Merck�& Co., Inc. and Peter N. Kellogg, dated June�18, 2007�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated June�28, 2007 | |||
| *10 | .22 | � | 1997 Stock Incentive Plan�� Incorporated by reference to Exhibit�10 to Schering-Plough�s 10-Q for the period ended September�30, 1997 | |||
| *10 | .23 | � | Amendment to 1997 Stock Incentive Plan (effective February�22, 1999)�� Incorporated by reference to Exhibit�10(a) to Schering-Plough�s 10-Q for the period ended March�31, 1999 | |||
| *10 | .24 | � | Amendment to the 1997 Stock Incentive Plan (effective February�25, 2003)�� Incorporated by reference to Exhibit�10(c) to Schering-Plough�s 10-K for the year ended December�31, 2002 | |||
| *10 | .25 | � | 2002 Stock Incentive Plan (as amended to February�25, 2003)�� Incorporated by reference to Exhibit�10(d) to Schering-Plough�s 10-K for the year ended December�31, 2002 | |||
| *10 | .26 | � | Merck�& Co., Inc. Schering-Plough 2006 Stock Incentive Plan (as amended and restated, effective November�3, 2009) ��Incorporated by reference to Exhibit�10.13 to Current Report on Form�8-K filed November�4, 2009 | |||
| *10 | .27 | � | Letter agreement dated November�4, 2003 between Robert Bertolini and Schering-Plough�� Incorporated by reference to Exhibit�10(e)(iii) to Schering-Plough�s 10-K for the year ended December�31, 2003 | |||
| *10 | .28 | � | Employment Agreement effective upon a change of control dated as of December�19, 2006 between Robert Bertolini and Schering-Plough Corporation�� Incorporated by reference to Exhibit�99.1 to Schering-Plough�s 8-K filed December�21, 2006 | |||
| *10 | .29 | � | Amendment to Letter Agreement and Employment Agreement between Schering-Plough Corporation and Robert J. Bertolini, dated December�9, 2008�� Incorporated by reference to Exhibit�99.1 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| *10 | .30 | � | Employment Agreement dated as of May�12, 2003 between Carrie Cox and Schering-Plough�� Incorporated by reference to Exhibit�99.6 to Schering-Plough�s 8-K filed May�13, 2003 | |||
| *10 | .31 | � | Amendment to Employment Agreement between Schering-Plough Corporation and Carrie S. Cox, dated December�9, 2008�� Incorporated by reference to Exhibit�99.2 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| *10 | .32 | � | Employment Agreement dated as of April�20, 2003 between Fred Hassan and Schering-Plough�� Incorporated by reference to Exhibit�99.2 to Schering-Plough�s 8-K filed April�21, 2003 | |||
| *10 | .33 | � | Amendment to Employment Agreement between Schering-Plough Corporation and Fred Hassan, dated December�9, 2008�� Incorporated by reference to Exhibit�99.3 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| *10 | .34 | � | Employment Agreement dated as of December�19, 2006 between Thomas P. Koestler,�Ph.D. and Schering-Plough�� Incorporated by reference to Exhibit�10(e)(v) to Schering-Plough�s 10-K for the year ended December�31, 2006 | |||
| *10 | .35 | � | Amendment to Employment Agreement between Schering-Plough Corporation and Thomas P. Koestler, dated December�9, 2008�� Incorporated by reference to Exhibit�99.4 to Schering-Plough�s 8-K filed December�12, 2008 | |||
| Exhibit | ||||||
Number | Description | |||||
| *10 | .36 | � | Form of employment agreement effective upon a change of control between Schering-Plough and certain executives for new agreements beginning in January�1, 2008�� Incorporated by reference to Exhibit�10(e)(xv) to Schering-Plough�s 10-K for the year ended December�31, 2008 | |||
| *10 | .37 | � | Operations Management Team Incentive Plan (as amended and restated effective June�26, 2006)�� Incorporated by reference to Exhibit�10(m)(ii) to Schering-Plough�s 10-Q for the period ended September�30, 2006 | |||
| *10 | .38 | � | Cash Long-Term Incentive Plan (as amended and restated effective January�24, 2005)�� Incorporated by reference to Exhibit�10(n) to Schering-Plough�s 10-K for the year ended December�31, 2004 | |||
| *10 | .39 | � | Long-Term Performance Share Unit Incentive Plan (as amended and restated effective January�24, 2005)�� Incorporated by reference to Exhibit�10(o) to Schering-Plough�s 10-K for the year ended December�31, 2004 | |||
| *10 | .40 | � | Transformational Performance Contingent Shares Program�� Incorporated by reference to Exhibit�10(p) to Schering-Plough�s 10-K for the year ended December�31, 2003 | |||
| *10 | .41 | � | Schering-Plough Corporation Severance Benefit Plan (as amended and restated effective November�3, 2009) | |||
| *10 | .42 | � | Schering-Plough Corporation Savings Advantage Plan (as amended and restated effective November�4, 2009) | |||
| *10 | .43 | � | Schering-Plough Corporation Supplemental Executive Retirement Plan (as amended and restated effective November�4, 2009) | |||
| *10 | .44 | � | Schering-Plough Retirement Benefits Equalization Plan (as amended and restated effective November�4, 2009) | |||
| *10 | .45 | � | Executive Incentive Plan (as amended and restated to October�1, 2000)�� Incorporated by reference to Exhibit�10(a)(i) to Schering-Plough�s 10-K for the year ended December�31, 2000 | |||
| *10 | .46 | � | Schering-Plough Corporation Executive Life Insurance Direct Payment Program (as amended and restated effective November�4, 2009) | |||
| *10 | .47 | � | Amended and Restated Defined Contribution Trust�� Incorporated by reference to Exhibit�10(a)(ii) to Schering-Plough�s 10-K for the year ended December�31, 2000 | |||
| *10 | .48 | � | Amended and Restated SERP Rabbi Trust�Agreement�� Incorporated by reference to Exhibit�10(g) to Schering-Plough�s 10-K for the year ended December�31, 1998 | |||
| 10 | .49 | � | Share Purchase Agreement between Akzo Nobel N.V., Schering-Plough International C.V., and Schering-Plough Corporation�� Incorporated by reference to Exhibit�10.1 to Schering-Plough�s 8-K filed October�2, 2007 | |||
| 10 | .50 | � | Amended and Restated License and Option Agreement dated as of July�1, 1998 between Astra AB and Astra Merck Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .51 | � | KBI Shares�Option Agreement dated as of July�1, 1998 by and among Astra AB, Merck�& Co., Inc. and Merck Holdings, Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .52 | � | KBI-E Asset Option Agreement dated as of July�1, 1998 by and among Astra AB, Merck�& Co., Inc., Astra Merck Inc. and Astra Merck Enterprises Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .53 | � | KBI Supply Agreement dated as of July�1, 1998 between Astra Merck Inc. and Astra Pharmaceuticals, L.P. (Portions of this Exhibit are subject to a request for confidential treatment filed with the Commission).�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .54 | � | Second Amended and Restated Manufacturing Agreement dated as of July�1, 1998 among Merck�& Co., Inc., Astra AB, Astra Merck Inc. and Astra USA, Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| Exhibit | ||||||
Number | Description | |||||
| 10 | .55 | � | Limited Partnership Agreement dated as of July�1, 1998 between KB USA, L.P. and KBI Sub Inc.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .56 | � | Distribution Agreement dated as of July�1, 1998 between Astra Merck Enterprises Inc. and Astra Pharmaceuticals, L.P.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .57 | � | Agreement to Incorporate Defined Terms dated as of June�19, 1998 between Astra AB, Merck�& Co., Inc., Astra Merck Inc., Astra USA, Inc., KB USA, L.P., Astra Merck Enterprises Inc., KBI Sub Inc., Merck Holdings, Inc. and Astra Pharmaceuticals, L.P.�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 1998 | |||
| 10 | .58 | � | Master Agreement, dated as of December�18, 2001, by and among MSP Technology (U.S.) Company LLC, MSP Singapore Company, LLC, Schering Corporation, Schering-Plough Corporation, and Merck�& Co., Inc. (Portions of this Exhibit are subject to a request for confidential treatment filed with the Commission)�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended June�30, 2008 | |||
| 10 | .59 | � | Form of Voting Agreement made and entered into as of October�30, 2006 by and between Merck�& Co., Inc. and Sirna Therapeutics, Inc.�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated October�30, 2006 | |||
| 10 | .60 | � | Settlement Agreement, dated November�9, 2007, by and between Merck�& Co., Inc. and The Counsel Listed on the Signature Pages Hereto, including the exhibits thereto�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated November�9, 2007 | |||
| 10 | .61 | � | Commitment Letter by and among Merck�& Co., Inc., J.P.�Morgan Securities Inc. and JPMorgan Chase Bank, N.A. dated as of March�8, 2009�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated March�8, 2009 | |||
| 10 | .62 | � | Stock option terms for a non-qualified stock option under the Merck Sharp�& Dohme Corp. 2007 Incentive Stock Plan and the Schering-Plough 2006 Stock Incentive Plan�� Incorporated by reference to Exhibit�10.3 to Current Report on Form�8-K filed February�15, 2010 | |||
| 10 | .63 | � | Restricted stock unit terms for annual grant under the Merck Sharp�& Dohme Corp. 2007 Incentive Stock Plan and the Schering-Plough 2006 Stock Incentive Plan�� Incorporated by reference to Exhibit�10.4 to Current Report on Form�8-K filed February�15, 2010 | |||
| 10 | .64 | � | Restricted stock unit terms for Leader Shares grant under the Merck�& Co., Inc. 2007 Incentive Stock Plan�� Incorporated by reference to Old Merck�s Form�10-Q Quarterly Report for the period ended March�31, 2009 | |||
| 10 | .65 | � | Incremental Credit Agreement dated as of May�6, 2009, among Merck�& Co., Inc., the Guarantors and Lenders party thereto, and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated May�6, 2009 | |||
| 10 | .66 | � | Asset Sale Facility Agreement dated as of May�6, 2009, among Merck�& Co., Inc., the Guarantors and Lenders party thereto, and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated May�6, 2009 | |||
| 10 | .67 | � | Bridge Loan Agreement dated as of May�6, 2009, among Merck�& Co., Inc., the Guarantors and Lenders party thereto, and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated May�6, 2009 | |||
| 10 | .68 | � | Amendment No.�1 to Amended and Restated Five-Year Credit Agreement dated as of April�20, 2009 among Merck�& Co., Inc., the Lenders party thereto and Citicorp USA, Inc., as Administrative Agent�� Incorporated by reference to Exhibit�10.1 to Current Report on Form�8-K filed November�4, 2009 | |||
| 10 | .69 | � | Guarantee and Joinder Agreement dated as of November�3, 2009 by Merck�& Co., Inc., the Guarantor, for the benefit of the Guaranteed Parties�� Incorporated by reference to Exhibit�10.3 to Current Report on Form�8-K filed November�4, 2009 | |||
| 10 | .70 | � | Guarantor Joinder Agreement dated as of November�3, 2009, by Merck�& Co., Inc., the Guarantor and JPMorgan Chase Bank, N.A., as Administrative Agent�� Incorporated by reference to Exhibit�10.4 to Current Report on Form�8-K filed November�4, 2009 | |||
| Exhibit | ||||||
Number | Description | |||||
| 10 | .71 | � | Call Option Agreement, dated July�29, 2009, by and among Merck�& Co., Inc., Schering-Plough Corporation and sanovi-aventis�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated July�31, 2009 | |||
| 10 | .72 | � | Termination Agreement, dated as of September�17, 2009, by and among Merck�& Co., Inc., Merck SH Inc., Merck Sharp�& Dohme (Holdings) Limited, sanofi-aventis, sanofi 4 and Merial Limited�� Incorporated by reference to Old Merck�s Current Report on Form�8-K dated September�21, 2009 | |||
| 10 | .73 | � | Cholesterol Governance Agreement, dated as of May�22, 2000, by and among Schering-Plough, Merck�& Co., Inc. and the other parties signatory thereto�� Incorporated by reference to Exhibit�99.2 to Schering-Plough�s Current Report on Form�8-K dated October�21, 2002� | |||
| 10 | .74 | � | First Amendment to the Cholesterol Governance Agreement, dated as of December�18, 2001, by and among Schering-Plough, Merck�& Co., Inc. and the other parties signatory thereto�� Incorporated by reference to Exhibit�99.3 to Schering-Plough�s Current Report on Form�8-K filed October�21, 2002� | |||
| 10 | .75 | � | Master Agreement, dated as of December�18, 2001, by and among Schering-Plough, Merck�& Co., Inc. and the other parties signatory thereto�� Incorporated by reference to Exhibit�99.4 to Schering-Plough�s Current Report on Form�8-K filed October�21, 2002� | |||
| 10 | .76 | � | Letter Agreement dated April�14, 2003 relating to Consent Decree�� Incorporated by reference to Exhibit�99.3 to Schering-Plough�s 10-Q for the period ended March�31, 2003 | |||
| 10 | .77 | � | Distribution agreement between Schering-Plough and Centocor, Inc., dated April�3, 1998 � Incorporated by reference to Exhibit�10(u) to Schering-Plough�s Amended 10-K for the year ended December�31, 2003, filed May�3, 2004� | |||
| 10 | .78 | � | Amendment Agreement to the Distribution Agreement between Centocor, Inc., CAN Development, LLC, and Schering-Plough (Ireland) Company�� Incorporated by reference to Exhibit�10.1 to Schering-Plough�s Current Report on Form�8-K filed December�21, 2007� | |||
| 12 | � | Computation of Ratios of Earnings to Fixed Charges | ||||
| 21 | � | Subsidiaries of Merck�& Co., Inc. | ||||
| 23 | .1 | � | Consent of Independent Registered Public Accounting Firm�� Contained on page�206 of this Report | |||
| 23 | .2 | � | Independent Auditor�s Consent�� Contained on page�207 of this Report | |||
| 31 | .1 | � | Rule�13a-14(a)/15d-14(a) Certification of Chief Executive Officer | |||
| 31 | .2 | � | Rule�13a-14(a)/15d-14(a) Certification of Chief Financial Officer | |||
| 32 | .1 | � | Section�1350 Certification of Chief Executive Officer | |||
| 32 | .2 | � | Section�1350 Certification of Chief Financial Officer | |||
| 101 | � | The following materials from Merck�& Co., Inc.�s Annual Report on Form�10-K for the fiscal year ended December�31, 2009, formatted in XBRL (Extensible Business Reporting Language):(i)�the Consolidated Statement of Income, (ii)�the Consolidated Balance Sheet, (iii)�the Consolidated Statement of Cash Flow, and (iv)�Notes to Consolidated Financial Statements, tagged as blocks of text. | ||||
| * | Management contract or compensatory plan or arrangement. | |
| � | Certain portions of the exhibit have been omitted pursuant to a request for confidential treatment. The non-public information has been filed separately with the Securities and Exchange Commission pursuant to rule�24b-2 under the Securities Exchange Act of 1934, as amended. |
