UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☑ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2013
¨ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
Commission file number 000-52534

PARALLAX HEALTH SCIENCES, INC.
(Exact name of registrant as specified in its charter)
Nevada | 46-4733512 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
|
|
One Boston Place, Suite 2600, Boston, MA | 02108 |
(Address of principal executive offices) | (Zip Code) |
|
|
Registrant's telephone number, including area code: | (617) 209-7999 |
Copy of all Communications to:
Lawrence I. Washor
Washor & Associates
21800 Oxnard Street, Suite 790
Woodland Hills, CA 91367
(310) 479-2660
|
| |||||
|
| |||||
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 the Securities Act. | Yes ¨ No ☑ | |||||
|
| |||||
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. | Yes ¨ No ☑ | |||||
|
| |||||
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the last 90 days. | Yes ☑ No ¨ | |||||
|
| |||||
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registration statement was required to submit and post such files). | Yes ☑ No ¨ | |||||
|
| |||||
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. | Yes ¨ No ☑ | |||||
| ||||||
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. | ||||||
| ||||||
| Large accelerated filer | ¨ |
| Accelerated filer | ¨ |
|
| Non-accelerated filer | ¨ |
| Smaller reporting company | ☑ |
|
| ||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). | Yes ¨ No ☑ | |||||
|
| |||||
|
| |||||
The aggregate market value of Common Stock held by non-affiliates of the Registrant as of June 30, 2013 was $798,197, based on a closing price of $0.01 for the Common Stock on June 30, 2013, the last business day of the Registrant's most recently completed second fiscal quarter. For purposes of this computation, all executive officers and directors have been deemed to be affiliates. Such determination should not be deemed to be an admission that such executive officers and directors are, in fact, affiliates of the Registrant.
Indicate the number of shares outstanding of each of the registrant's
classes of common stock as of the latest practicable date.
126,303,018 Common Shares issued and outstanding as of March 15, 2014
TABLE OF CONTENTS | ||
| ||
ITEM 1. | BUSINESS | 3 |
|
|
|
ITEM 1A. | RISK FACTORS | 25 |
|
|
|
ITEM 2. | PROPERTIES | 25 |
|
|
|
ITEM 3. | LEGAL PROCEEDINGS | 25 |
|
|
|
ITEM 4. | MINE SAFETY STANDARDS | 25 |
|
|
|
ITEM 5 . | MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS | 26 |
|
|
|
ITEM 6. | SELECTED FINANCIAL DATA | 28 |
|
|
|
ITEM 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 28 |
|
|
|
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS | 33 |
|
|
|
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 34 |
|
|
|
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 35 |
|
|
|
ITEM 9A. | CONTROLS AND PROCEDURES | 35 |
|
|
|
ITEM 9B. | OTHER INFORMATION | 36 |
|
|
|
ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 36 |
|
|
|
ITEM 11. | EXECUTIVE COMPENSATION | 42 |
|
|
|
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 43 |
|
|
|
ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 45 |
|
|
|
ITEM 14. | PRINCIPAL ACCOUNTANTS FEES AND SERVICES | 46 |
|
|
|
ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES | 47 |
2
PART I
ITEM 1. | BUSINESS |
This annual report contains forward-looking statements. These statements relate to future events or the Company's future financial performance, and include statements made by the Company regarding product development and obtaining FDA clearances. In some cases, forward-looking statements can be identified by terminology such as "may", "should", "expects", "plans", "anticipates", "believes", "estimates", "predicts", "potential" or "continue" or negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors" that may cause the Company's or its industry's actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results.
The Company's financial statements are stated in United States Dollars (US$) and are prepared in accordance with United States Generally Accepted Accounting Principles.
In this annual report, unless otherwise specified, all dollar amounts are expressed in United States Dollars and all references to "common shares" refer to the common shares in the Company's capital stock.
As used in this annual report, the terms "we", "us", "our" and "Parallax" mean Parallax Health Sciences, Inc. (formerly Endeavor Power Corp.), and its wholly-owned subsidiary, Endeavor Sciences, Inc. (formerly Parallax Diagnostics, Inc.), unless otherwise indicated.
Corporate Overview
The Company's principal executive office is located at One Boston Place, Suite 2600, Boston, MA 02108, with operations at 1327 Ocean Avenue, Suite M, Santa Monica, California, 90401. The Company's telephone numbers are (617) 209-7999 (Boston) and (310) 899-4442 (Santa Monica).
The Company's website under construction will be at www.parallaxhealthsciences.com . The Company's subsidiary website is at www.endeavorsciences.com .
The Company is currently a fully reporting Company with its stock traded on the OTC Bulletin Board and the OTCQB under the symbol "PRLX".
On March 8, 2013, the Company was notified that the Securities and Exchange Commission ("SEC") had suspended the trading of the Company's securities for 10 days, until March 21, 2013. This temporary suspension of trading arose out of concerns that the Company had incorrectly stated within its public filings and press releases that certain components related to the Company's Target Antigen Detection System ("Target System") are patented, when these patents have expired.
The Company, after discussions with the SEC and with the Company's counsel, has addressed any questions raised regarding the accuracy of assertions in the Company's public filings. The Company has clarified the status of the patents in question, and the way in which the Company refers to its Target System components. The Company has also clarified that it currently has filed four patent applications with the USPTO, which are deemed "patent pending", and the Company has disclosed that there can be no assurance that the patents will be granted. For additional information on the Company's patent applications, please refer to the section entitled "Intellectual Property" contained within this Annual Report.
On May 10, 2013, the Company filed its new form 15c211 with a FINRA Member Market Maker, in order that the Company's shares can resume trading on the OTCBB and OTCQB markets, pursuant to Rule 15c2-11 under the Exchange Act, which states that at the termination of the trading suspension, no quotation may be entered unless and until the Company has strictly complied with all of the provisions of the rule, including the filing of a new Form 15c2-11 and obtaining FINRA approval to commence trading. On August 19, 2013, FINRA approved the resumption of trading.
Table of Contents
3
Corporate History
Formation and Development
Parallax Health Sciences, Inc. (formerly Endeavor Power Corp.) (the "Company") was incorporated in the State of Nevada on July 6, 2005 under the name VB Biotech Laboratories, Inc. On September 21, 2007, the Company filed a Certificate of Amendment with the State of Nevada to change its operating name to VB Trade, Inc., with principal business operations to develop an online website that allowed web designers to sell their website designs in exchange for a commission on all products that were sold through the website. On September 21, 2007, the Company entered into a Plan of Merger (the "Merger") with Endeavor Uranium, Inc., a mineral exploration company with mineral properties in the northwestern United States. Effectively, the Company changed its name to Endeavor Uranium, Inc. as part of the Merger transaction. On December 23, 2008, the Company entered into a Joint Venture Agreement (the "Agreement") with Federated Energy Corporation, a Tennessee corporation, for working interests in prospective oil and gas wells located in Nowata County, Oklahoma. Effectively on December 23, 2008, the Company changed its operating name to Endeavor Power Corporation.
In November 2010, Management assessed a potential business opportunity and determined that in an effort to create value for its Shareholders, the Company should change its business direction. On November 8, 2010, the Company discontinued its operations in its working interests in oil and gas exploration and changed its operating focus to the development of E-Waste processing services aimed at industrial and government clients. The Company's new direction sought to limit the impact of discarded "E-Waste" on the environment, as discarded computers and electronic equipment pose environmental hazards.
On May 26, 2011, Mr. Alfonso Knoll resigned from all positions with the Company, including but not limited to, that of President, Chief Executive Officer, Chief Financial Officer, Treasurer and Secretary. The resignation did not involve any disagreement with the Company. On June 8, 2011, the Company entered into a Settlement Agreement and General Mutual Release ("Settlement Agreement") to terminate Mr. Knoll's Employment Agreement dated November 8, 2010, and to accept his resignation. Pursuant to the Settlement Agreement, Mr. Knoll immediately ceased all services to the Company and, on June 11, 2011, returned to the Company any and all shares of its common stock currently held by him.
On June 2, 2011, Mr. Matthew Carley was appointed as the Company's President, Chief Executive Officer, Chief Financial Officer, Treasurer, Secretary and Director. Mr. Carley accepted the appointment, but effectively resigned his positions on September 27, 2011. The Company's Board of Directors accepted the resignation of Mr. Carley, as well as the resignation of Mr. Keith Kress as a member of the Board of Directors. Simultaneously, the Board of Directors appointed Tom Mackay as the President/Chief Executive Officer, Secretary, Treasurer/Chief Financial Officer and the sole member of the Board of Directors.
In accordance with a change in management effective September 27, 2011, the Company's business operations changed. The Company intended to change its business focus to provide managerial services, and pursue potential funding opportunities for the Company. It also retained consultants to perform due diligence on certain mining properties located in Venezuela, Brazil, Bolivia, Guyana and several other South American countries. Management, however, determined that the outcome of such due diligence did not provide the Company a viable opportunity, nor did it provide sufficient economic benefit for the Company. Management therefore pursued other viable opportunities to increase shareholder market value.
During 2012, the Company's management entered discussions and assessed a potential business opportunity with Parallax Diagnostics, Inc., a Nevada corporation ("Parallax'), whose principal line of business is in the bio-medical sector.
On November 1, 2012, the Company, and its wholly owned subsidiary Endeavor Holdings, Inc. ("Endeavor Holdings") entered into an Agreement and Plan of Merger (the "Parallax Merger") with Parallax and the shareholders of Parallax (the "Parallax Shareholders"), whereby Endeavor Holdings acquired 100% (one hundred percent), or 24,870,000 shares, of Parallax common stock (the "Parallax Stock") from the Parallax Shareholders. In exchange for the Parallax Stock, the Company issued 90,375,750 shares of its common stock to the Parallax Shareholders. The 90,375,750 shares, issued at par value $.001, represent approximately 60% of the Company's total issued and outstanding shares. The Common Stock Purchase Agreement, and subsequent transaction closing, was completed on October 22, 2012. On October 27, 2012, the Common Stock Purchase Agreement was finalized, and a change in control of the Registrant took place.
As a further condition of the Merger Agreement, the then-current officer and director of the Company, Mr. Gardner Williams, resigned from all positions, and Mr. J. Michael Redmond was appointed to serve as Chief Executive Officer and President of the Company, and also as a Director on the Board of Directors. Additionally, Ms. Calli Bucci was appointed to serve as the Company's Treasurer and Chief Financial Officer, Mr. Kyle W. Withrow was appointed to serve as the Company's Corporate Secretary, Dr. Roger Morris was appointed to serve as the Company's Chief Science Officer, and Mr. Mike Contarino was appointed to serve as the Company's Vice President. Mr. Edward W. Withrow III was appointed to serve as Executive Chairman of the Board of Directors, and Mr. Redmond, Dr. Jorn Gorlach, Mr. Anand Kumar, Mr. David Engert and Mr. E. William Withrow Jr. were appointed to serve as Directors.
As a result of the transactions effected by the Merger Agreement, (i) Parallax merged with and into Endeavor Holdings whereupon Endeavor Holdings continued as the surviving entity and the corporate existence of Parallax ceased; (ii) the former business of Parallax is now the Company's primary business, (iii) the Company's existing business activities will continue as ancillary operations, and (iv) there is a change of control whereby the former shareholders of Parallax now own a controlling 60% ownership interest in the Company on a fully diluted basis.
4
On November 26, 2012, Parallax Diagnostics, Inc. changed its name to Endeavor Sciences, Inc. ("ESI"), a wholly owned subsidiary of Parallax Health Sciences, Inc.
On July 22, 2013, the Company entered into a binding Letter of Intent ("LOI") for the acquisition of a privately held California corporation, whose primary operations are in the pharmaceutical industry (the "NewCo"). Since completion of its due diligence, the Company has been working with financial institutions to secure a combined debt-equity financing that will enable the Company to complete the acquisition. There can, however, be no guarantee that satisfactory financing arrangements will be secured to complete the acquisition.
The Company's management and the NewCo management have completed negotiations, the terms of which are confidential and will be disclosed upon completion of the acquisition. On March 28, 2014, the Company's board of directors issued a board resolution authorizing the acquisition.
On January 9, 2014, the Company changed its name from Endeavor Power Corp. (OTCQB.EDVP) to Parallax Health Sciences, Inc. (OTCQB.PRLX).
NOTE : The following sections of this annual report and any further reference made to "the Company", "we", "us", "our" and "Parallax " shall mean Parallax Health Sciences, Inc. (formerly Endeavor Power Corp.) and its wholly-owned subsidiary, Endeavor Sciences, Inc. (formerly Parallax Diagnostics, Inc.), unless otherwise indicated.
Description of Business
The Company is a development stage company whose principal line of business is in the bio-medical sector. More specifically, the Company, through its wholly owned subsidiary, Endeavor Sciences, Inc. ("ESI"), is focused on the exploitation of a diagnostic and monitoring platform and processes.
Product Strategy and Overview
In recent years, there has been a continuing shift from the use of laboratory-based analyzers to point-of-care ("POC") tests that can be performed in a matter of minutes. Unlike the centralized clinical laboratory segment of the diagnostic market, which is mature and highly competitive, the POC market is still in its relatively early stages, According to the recent worldwide research reports, however, such as the 2010 Worldwide IVD Market, by the research firm Kalorama Information, the growth rate of the POC market continues to rise. Although certain simple, single analyte diagnostic tests have been developed, such tests have remained incapable of precise and highly sensitive quantitative measurements. As a result, medical tests that require precise quantization of the target analyte have remained the domain of immunoassay analyzers in the centralized laboratory.
Point-of-care diagnostic kits typically consist of test strips that the health care provider applies a patient's sample to and then reads the strip either visually or with an instrument in order to determine a result. They are simple to use, fast, disposable and reliable within an acceptable range. More sensitive analytes or tests requiring quantitative analysis and definitive antibody screening needed in most situations, must be sent out to a diagnostic lab, and hours or days later results arrive. These tests are comparatively complex, expensive, and time consuming; only centralized diagnostic facilities can manage sample handling and the cost of instruments and reagents. A point-of-care instrument that has the advantage of a test strip device in terms of ease of use and rapid results along with ELISA-like capabilities for major diseases would circumscribe diagnosis routinely within the course of a patient visit. This could disrupt the current model. The Company is developing just such a device that it intends to sell to doctors and health care providers.
The commercial success of the current generation of small, simple to use diagnostic devices which provide rapid results in POC applications has been limited by their inability to provide precise, highly sensitive, quantitative measurement.
Despite these limitations, the rapid increase in discovery of individual markers of disease processes, coupled with the advancements in rapid detection technologies, has made these tools available to medical professionals on a wide scale and POC diagnostics are quickly becoming a high growth industry.
The Company believes that there is market potential for advanced POC diagnostic products that provide quick and accurate diagnosis during a patient visit, shortening the decision time to medical intervention and minimizing the need for additional patient follow-up, thereby reducing overall health care delivery costs.
The Company's Target System (the systems includes the VT-1000 Desktop Analyzer, the Target Antigen Detection Cartridge and associated reagents) technology addresses these limitations by applying sophisticated immunochemical and optical methods to detect and quantify analytes present in various human specimens, including blood, urine, and feces. Data indicates that sensitivity will be comparable to expensive and complicated laboratory-based analyzers.
The Company also believes that there is growth opportunity for the exploitation of its Target System platform in developing nations and regions such as Africa, India, South America, Eastern Europe, Russia and Asia as well as developed markets of North America and Western Europe. One of the first initiatives to be developed for this market will combine the Company's SPARKS Mobile (a portable hand-held diagnostic analyzer based on the VT-1000 Desktop Analyzer technology, but smaller and more portable), currently in development, with a test for the monitoring of AIDS/TB patients through the use of a proprietary rapid point-of-care immunoassay CD4-CD8 test called PROMISE CD4, also in development.
Table of Contents
5
The Products
Overview
The Company's product line will include a previously FDA-cleared VT-1000 Desktop Analyzer and more than a dozen FDA 510(k) cleared diagnostic tests.
The Company's previously FDA-cleared VT-1000 Desktop Analyzer and immunoassay system incorporates a flow-through rapid antigen test platform configuration that has the ability to produce high-performance quantitative blood test results with the ease of rapid qualitative diagnostic strips. The Company has patent applications related to its current and future products, as well as methods for future test development. The Target VT-1000 Desktop Analyzer is ideally suited for rapid development and commercialization of all new tests that may be introduced.
VT-1000 Desktop Analyzer: Quantitative and Qualitative Immunoassay
The Company's VT-1000 Desktop Analyzer was FDA 510(k) cleared and is capable of rapidly detecting qualitative and quantitative data for the Company's FDA-cleared Target Platform tests. The VT-1000 Desktop Analyzer is used for all Target Platform Tests, allowing for clinical personnel to be trained once and also gives consistent results for either qualitative or quantitative testing.
Target Antigen Detection System ("TADS")
The Target Antigen Detection System consists of a unique disposable cartridge with preloaded reagents capable of testing multiple test markers, combined with the VT-1000 Desktop Analyzer. The TADS requires a small amount of sample and provides results in minutes. The simplicity of the fully loaded disposable test cartridge and subsequent ease-of-use of the instrument helps to alleviate the technical burden on medical staff and makes patient diagnosis more efficient.
The Company's Target Antigen Detection System is a departure from the standard devices typical to the rapid testing markets. The device is part of the manufacturer's qualitative and quantitative "Target System Diagnostics Platform," which offers an array of improved modifications and features to the traditional qualitative and semi-quantitative flow-through immunoassay test. With its platform uniformity, vacuum pump, absorption layer for sample overflow, and complete compatibility with single and multi-light source reflectometer technology, the TADS cartridge is a unique collection of tests for qualitative and quantitative detection diseases and of conditions.
The Company is currently developing the SPARKS Mobile, a hand-held analyzer unit, similar in size to a mobile phone/PDA, which will be based on the VT-1000 Desktop Analyzer (see Products in Development).
TADS Vacuum Control Flow Device
The TADS cartridge utilizes a vacuum technology to deposit specimen samples uniformly on test membranes. The Vacuum Control Flow Device provides a vacuum pump action, which reduces test time and ensures maximum contact with the membrane antibodies. This collection device allows for numerous tests to be incorporated. The vacuum specimen filtration and excess specimen absorption is built right in.
Target System Patent Status: The Target System and certain of its related components were previously issued patents by the United States Patent and Trademark Office ("USPTO"). The following previously-issued patents have expired:
USPTO Patent # | Description | Date Filed in US | Date Expired |
US4,748,042 | Target Ringing & Spotting Machine (method and Apparatus for Imprinting membrane with pattern of antibody) | May 31, 1988 | May 31, 2008 |
US4,797,260 | Target Cassette (Antibody testing system) | January 10, 1989 | January 1, 2009 |
US5,137,691 | Target Cassette with Removable Air Gap (Antibody testing system with removable air gap) | August 11, 1992 | August 11, 2012 |
The Company's Current Technology
The Company currently holds the rights to certain technology related to the VT-1000 Desktop Analyzer and the Target System, for which four patent applications have been filed and are pending with the USPTO.
The Company also retained the services of Marathon Patent Group to perform an analysis of the Company's intellectual property ("Patent Report"). The Patent Report, included in this filing as Exhibit 99.2, was issued on April 1, 2013, and was intended to inform the Company and its shareholders of the accurate and current state of the commercial patent pending coverage and, where possible, to identify the existence of novel and patentable inventions present in the current innovation initiative. The Patent Report concluded that the Company has a strong patent portfolio protecting its business, and recommended that the Company aggressively proceed with additional patent applications to protect the Company's inventions and innovations. As a result, the Company has initiated the drafting of a minimum of one (potentially two) additional USPTO and International patent applications.
6
There can be no assurance that the Company will be granted patents for any of the patent applications it has filed with the USPTO.
For more information on the Company's patent applications, please see the section entitled "Intellectual Property" contained within this Annual Report.
The Company's previously FDA-Cleared Tests in the area of Infectious Diseases
Rubella
Rubella, German measles, is a highly contagious disease, which is generally transmitted by direct contact with infected persons. Rubella is generally a mild disease. However, when a pregnant woman becomes infected with rubella, the virus may infect the placenta, multiply and induce serious damage to the fetus. Rubella and congenital rubella syndrome became nationally notifiable diseases in 1966. The largest annual total of cases of rubella in the United States was in 1969, when 57,686 cases were reported (58 cases per 100,000 populations). Following vaccine licensure in 1969, rubella incidence fell rapidly. By 1983, fewer than 1,000 cases per year were reported (<0.5 cases per 100,000 population). A moderate resurgence of rubella occurred in 1990-1991, primarily due to outbreaks in California (1990) and among the Amish in Pennsylvania (1991). In 2002 a record low annual total of 18 cases were reported.
Rotavirus
Human rotavirus is recognized as a major cause of gastroenteritis in infants, young children, and the elderly. During the winter months a portion of gastroenteritis in children is due to rotavirus infection. The disease manifests with the symptoms of vomiting, diarrhea, and fever. Rapid and accurate diagnosis is important to avoid inappropriate antibiotic therapy, provide proper treatment early, and to prevent spread of nosocomial infection.
Globally, rotavirus accounts for an estimated 125 million cases of diarrhea each year and represents 30% - 40% of hospitalizations for diarrhea in children less than five years. In developing countries, between 600,000 and 800,000 children die from rotavirus each year (or approximately 2,000 children each day.) This accounts for about one quarter of the deaths from diarrhea and about 5% of all deaths among children less than five years of age.
CMV- Herpes
Cytomegalovirus (CMV) is a human viral pathogen belonging to the Herpes family. Infection in humans is widespread and usually results in asymptomatic disease. However, severe symptomatic infections are a very significant risk in infants and Immuno-compromised individuals. An important primary source of such infection is via blood transfusion and allograft transfer. The serological status of donor and recipient is, therefore, important in patient management.
The United States is not unique in its high rates of CMV seroprevalence. Virtually every country in the world presents similar numbers. Since recurrences are often mild and few patients are aware that they are infected, the infection is likely to continue to rise at double-digit rates without an intervention.
Group A Streptococci: Strep A, Strep Throat, Necrotizing Fasciitis, impetigo
Strep throat is an infection of the pharynx (the part of the throat between the tonsils and the larynx) caused by streptococcus bacteria. The infection is spread by person-to-person contact with nasal secretions or saliva, often among family or household members. Even though the sore throat usually gets better on its own, people who have strep throat should take antibiotics to prevent some of the more serious complications of this infection, particularly acute rheumatic fever.
Approximately 15% of children who have a sore throat and fever are infected by Group A streptococci. CDC estimates that approximately 9,100 cases of invasive GAS disease (rate: 3.2/100,000) and 1,350 deaths occurred nationally during 2002. Disease incidence was highest among children aged <1 year (6.9/100,000) and adults aged >65 years.
Infectious Mononucleosis: EB, Epstein-Barr Viral Syndrome, Mono
Infectious mononucleosis (IM) is a viral infection causing high temperature, sore throat, and swollen lymph glands, especially in the neck. The Epstein-Barr virus typically causes it. Infectious mononucleosis may begin slowly with fatigue, malaise, headache, and sore throat. The sore throat becomes progressively worse, often with enlarged tonsils covered with a whitish-yellow fibrinous exudate. The lymph nodes in the neck are frequently enlarged and painful. Symptoms of mononucleosis gradually subside over a period of weeks to a month. The disease is generally self-limited.
Tests Currently in Development for the Target System Diagnostic Platform
The Company is in the process of developing and obtaining FDA clearance for the following products. There can be no assurance that the Company will be successful in developing such tests or in obtaining the required FDA clearance.
Table of Contents
7
HIV 1 & 2 TADS Rapid Test
Today, 42 million people are estimated to be living with HIV/AIDS. Of these, 38.6 million are adults. 19.2 million are women, and 3.2 million are children under 15. During 2002, AIDS caused the deaths of an estimated 3.1 million people, including 1.2 million women and 610,000 children under 15. With the recent advent of Rapid HIV testing, HIV detection and prevention programs around the world have become increasingly effective by reducing their time and costs of detecting the virus, thus allowing for a far greater number of individuals to be screened. The FDA has approved several rapid Immunoassay tests for the detection of HIV, but none of these tests are designed for HIV 1 and 2. The current "rate" of these "rapid" tests is from 15 minutes to hours and only a few can produce results less than 15 minutes.
The Company has an HIV 1 & 2 qualitative rapid test in development. The Company realizes that there are numerous competing HIV 1 & 2 tests but has decided to develop the test so that health care providers utilizing the Target VT-1000 Desktop Analyzer and Platform would have the opportunity to incorporate the Company's HIV 1 & 2 test into their use of the Company's platform. The Company has prepared a Clinical Trial Protocol to test the accuracy of the test and the efficacy and specificity of the results. The Company will need to secure additional capital to conduct the FDA-required trials and to prepare the test for commercialization if the FDA approves it for commercialization.
Additional Products in Development
The Company also believes that there is growth opportunity for the exploitation of the Target System platform in developing nations and regions such as Africa, India, South America, Eastern Europe, Russia and Asia as well as developed markets of North America and Western Europe.
One of the first initiatives for the development of this specific market will be to combine the SPARKS Mobile, the Company's hand-held analyzer (the portable version of its VT-1000 Desktop Analyzer), currently in development, with a test for the monitoring of HIV/AIDS patients and Tuberculosis patients, through the use of the Company's proprietary rapid POC immunoassay PROMISE CD4 quantitative test, also in development.
The Target System Hand-Held Analyzer: SPARKS Mobile
The Company's next generation Target System Analyzer, the SPARKS Mobile, a hand-held analyzer currently under development, will include a small, rapid testing format, in conjunction with a hand-held data acquisition and test reading device. The SPARKS Mobile will be a re-engineered version of the Company's previously FDA-approved VT-1000 Desktop Analyzer.
Whether searching for markers in the blood stream, or diagnosing a pathogen in urine, the Company's SPARKS Mobile will be a portable tool for rapid diagnostics. The SPARKS Mobile will also provide an improvement in POC diagnostics and applications in countries with limited health care infrastructures and geographic limitations, both of which are of paramount importance in the combat against infectious diseases and in the fight against proliferation of endemic and pandemic diseases.
This innovative SPARKS Mobile will allow for a fast (minutes instead of hours or days) performance of tests at the point-of-care, and will only require a test cartridge and a small number of ready-to-use solutions in preformatted quantities. Moreover, the SPARKS Mobile will include the ability to store patient information, test data, and QC data, and transmit data through wireless connections.
The SPARKS Mobile is being developed to:
a)
achieve a portable monitoring system, which is compatible with proven and reliable ELISA-based target system technology.
b)
expand readout capabilities to provide a mobile testing and monitoring platform.
c)
increase the economy of scale and scope of the diagnostics and monitoring platform by the development of additional utility of the device without redundant infrastructure investments (additional data acquisition of patients, additional tests for other, predominant diseases).
The basic design of the Company's SPARKS Mobile is based on the same 510(k) cleared technology employed in its VT-1000 Desktop Analyzer and is compatible with existing Test Cartridges. However, a number of innovative features will be integrated into the design to meet customer and patient needs:
1.
High Infrared Light Spectrum : Multiple light source system providing variable light wave analysis into the infra-red spectrum. This diversity in light source and detection will allow for the simultaneous identification and diagnosis of a broader spectrum of different targets within the same sample and assay. It will also allow for very specific test development, without having to develop a new analyzer to read the results.
2.
Easy Field Upgrades : Field software upgrades made through memory chip (SIMM) or Flash memory stick.
8
3.
No Change of Equipment : The same Analyzer will be used for all Target System Tests (example: Cardiac Panel., infectious disease), and will be able to be used on all future tests, allowing for training personnel only once, and displaying consistent test results on an easy to read LCD screen.
4.
Printer Hook-up Capability : When hooked to a printer, the SPARKS Mobile will be able to provide printed results for any Target System Test, both Qualitative (when written results must be stored with original test for HIPPA and other compliance issues), or Quantitative (measured amount analysis must be printed and maintained in the patient chart folder).
5.
Low Entry Cost for New Test Development and Analysis : Due to the technologies' broad capability, a new analyzer will not have to be developed for different samples types (blood, serum, plasma, urine, soil or human skin).
6.
Safety, Security and Accuracy by design: For all tests, the SPARKS Mobile bar code activation system will identify the test to be analyzed.
7.
Desk to Docking Station : The SPARKS Mobile will be able to be configured with or without a desk-to-docking station. The docking station will provide a stationary platform when in use in an office or non-mobile application. It will also provide the user to set up multiple tests samples while the analyzer is processing tests.
8.
Smart Phone Capability: The SPARKS Mobile will have many Smart Phone Capabilities including, but not limited to, Bluetooth, WiFi, MMS messaging, SMS, Memory Cards, internal memory expandable to 8GB, data transfer, and more.
The SPARKS Mobile is being specifically designed to work with the Company's Target System Diagnostics Platform to provide reliable quantitative results within minutes, right at the point-of-care or site of testing. The continuity of the Company's Platform upgrades and the continuous development of new tests based on an increasing point-of-care market paradigm, points to the VT-1000 Desktop Analyzer and the SPARKS Mobile as low cost alternatives to large laboratory analyzers and specialized training of personnel on multiple machinery. The ultimate value to the clinician or the attending physician is the ease of use, reproducibility and the history of accuracy of this type of rapid immunoassay principle in the area of quantitative analysis.
PROMISE CD4: CD4-CD8 Rapid Monitoring Test
The Company has initiated the development of the CD4-CD8 monitoring rapid test using an immunoassay test the Company has named PROMISE CD4, which the Company believes has the potential to enhance the testing, monitoring and treatment of AIDS patients in developing economies such as South Africa, Sub-Saharan countries, India and other nations struggling to deal with the treatment of AIDS. The PROMISE CD4 monitoring test is being developed in conjunction with some of the leaders of research in the HIV testing community.
AIDS Diagnostics and Immune Status
The Company's aim is to use markers for a disease progression instead of using the cell count method that is associated with and based on those markers. The PROMISE CD4 will include quantification of CD4-CD8 protein in either total blood or CD3 + pre-selected cell populations. This quantification can directly be used to assess an individual's immune status.
AIDS Immune Status: Value Proposition
The treatment of AIDS patients represents a challenge in the developed world and much more so in developing countries. The current methodology to determine the status of an HIV-positive individual involves elaborate technologies to determine the immune status of an individual as well as the presence of the HIV virus in the individual's blood (called the "viral load").
Determination of the immune status is usually performed through so-called cell counts of T-cells, in particular the determination of CD4 + cell count or the relationship of CD4 and CD3 positive cells. This diagnostic procedure requires high-tech machinery (e.g. cell counters), and well-educated laboratory personnel in a stationary laboratory setting. In addition, the cell counting method presently employed and defined by the Western medical community as the "Gold Standard" has shortcomings, which limit its reproducibility and reliability. These factors might cause changes in diagnostic procedures even within those communities in the future. The determination of the amount of virus populating the blood of a person infected with HIV is currently performed through quantitative PCR, again a method requiring stationary settings, as well as highly educated personnel and sophisticated machinery. These setting are usually not available in developing economies. While, in the Western economic environment, the medical care of HIV positive individuals and AIDS patients involves a combination of the above mentioned medical diagnostics in combination with additional, patient dependent procedures, the situation in developing countries looks to the contrary.
Table of Contents
9
In South Africa, the country with one of the highest infection rates with HIV in the world, treatment is only available to a small number of infected people. Even under those medication-limited circumstances, treatment is usually administered without any diagnostic procedures concerning the immune status or the viral load of an individual in question, leading to unnecessary treatment of otherwise non-immuno-compromised individuals and the lack of treatment for others with AIDS at later progression. Countries like China have only recently begun to diagnose for HIV positive individuals, and have not moved into the AIDS diagnostic either. The same can be said for many other countries in Africa and Asia.
Requirements for "appropriate" AIDS diagnostics have been defined by many national and international, organizations, amongst them the World Health Organization ("WHO"), under strong influence of scientists mainly from the US and the EU. These requirements have led to the above described situation in developing counties: No appropriate diagnosis of AIDS patients caused by requirements that cannot be achieved under the given circumstances and a strong increase of HIV infection in most of these countries over the last years.
Furthermore, the lack of financial resources are limiting to the expansion of suitable points of diagnostics. Cell counts require elaborate machinery (like FACS or alike) and there are no low-cost or highly portable testing systems available to date. There is an overwhelming demand and urgent need to reduce the costs for cell counting or other methods to determine the immune status, and to increase their usefulness in non-laboratory settings.
In addition, the geographic and social structures of many countries require a more POC oriented approach, as opposed to the dominating centralized care found in highly populated countries in North America and Europe. Therefore, it would by highly desirable to reduce the measurements used as a guide for disease progression or treatment to more simple technologies, like an ELISA performed on a handheld device or similar.
For the Company and its efforts to design a handheld diagnostics device for optimal market use, this means:
·
development of a testing system which is compatible with proven and reliable ELISA based target system technology.
·
expansion of the capabilities of handheld device to provide a mobile testing platform.
·
increase the economy of the diagnostics platform by the development of additional utility of the device without redundant infrastructure investments (additional data acquisition of patients, additional tests for other, predominant diseases).
·
acceptance of the Company's testing system as well as the platform within the medical community of African, Asian, and other countries with mounting problems in the field of HIV and other infectious diseases.
Tests for Other Diseases
The Company's testing system is not limited to HIV or AIDS diagnostics. The test format has been applied in the past to viral and bacterial infections (e.g., Rubella, Rotavirus, Strep. A), and can easily be adopted toward other epidemics. Diseases like malaria, cholera, hepatitis, yellow fever, or West Nile virus and other viral diseases present increasing health threats to large populations in the world, with major existing problems at the stage of proper diagnosis. The Company believes that it can adapt its VT-1000 Desktop Analyzer and SPARKS Mobile to the rapid, simple, point-of-care diagnosis of almost all of these diseases without the requirement of additional equipment. Further, the Company believes that the combination of a mobile, hand-held testing device with a large number of different tests provided by a family of cartridges will improve the ability of current health care and disease diagnostics in a fast majority of today's underserved regions. In addition, the Target System Platform also allows for the monitoring of environmental components influencing the health of populations, including the presence of toxins in soil and drinking water as well as contamination of food supply.
The Company's innovative process for the development of new antibodies, which is patent pending, will also be used in the identification of test markers for its Target System Platform, adding a dimension that further distinguishes the Company from its competition. The Company believes that the innovative antibody development process will allow it to create new barriers to entry on certain antigens that it identifies by subsequent patent application filings for use in conjunction the Company's VT-1000 Desktop Analyzer and SPARKS Mobile.
.
Ease of Use
The Company's platform provides tremendous flexibility in sample requirements, clinician training and result interpretation. The Company's "train once" system means the clinician can now perform a number of single use tests on a wide variety of conditions with the interpretation of results consistent through the platform paradigm. The "while you watch" speed of the test development, results in a significant cost saving in time and training.
Application and Economy of Scale
The Company's unique vacuum pump action reduces test time and ensures maximum contact with the membrane antibodies. This collection device is versatile in the number of different tests that can be incorporated. The economy of scale is provided to health care provider or any other customer group by being able to utilize a single test system for multiple tests with varies little variance in training needed. A clinician can move from one test to the next in a matter of minutes.
Furthermore, the capability of acquiring and transmitting patient related data in addition to the tests performed at the point-of-care will enable the Company's SPARKS Mobile Analyzer to become the central diagnostic device in a decentralized, patient oriented, and cost-conscious environment to provide or maintain a high level of health care in the face of threatening epidemics.
10
Safety and Accuracy by Design
For all tests, the Company's bar code activation system will identify the test to be analyzed, allowing only those medical personal that possess that test will be aware that it is available. Without the Company's specific Target System Test Cartridge, read by the bar code reader, the analyzer will not calibrate to that test. This precludes mistakes by the user or erroneous results by the device.
Each Test Cartridge bar code must be read to initialize the analyzer, and load the appropriate algorithm from the software table. This provides a level of security for patient related tests and eliminates errors based on operator's mistakes.
The Company will provide a combination of innovative, fast, and inexpensive diagnostic testing products with a highly mobile data collection and transfer test reader. In this regard, the Company's Target System is suitable for rapid, point-of-care testing in almost every environment, which includes emergency situations, remote locations within the US as well as other parts of the world, immediate response teams, personal testing in a home setting, and many more.
Advantageously, many different tests can be performed using the same reader, e.g., either the Company's VT-1000 Desktop Analyzer or its SPARKS Mobile, currently in development, at any location.
Mobility
The Company's mobile testing, data acquisition, and data transmission system being developed is intended to meet the needs for diagnostics in particular in areas where either no structured health care systems exist or where, due to geographic nature and population density and distribution, a more decentralized approach is necessary. The highly mobile test reader can be used in basically all environments, and will be suited to use power sources independent of an electric network (rechargeable batteries, solar panel, running on motor vehicle voltage and power supply). Furthermore, the tests can be performed within short time periods, and do not require the performing medical personnel, the physician, or the patient to return for test results or potential initiation of treatment.
New Product Screening Criteria
The Target System Platform is broken down into three categories and their associated sub-groups. The categories are:
1. | Qualitative |
2. | Quantitative |
3. | Specialized |
The following list represents a general representation of targeted test to be developed in the area of infectious or highly contagious diseases:
· | Trichomoniasis |
· | Chlamydia |
· | Gonorrhea |
· | Genital herpes (herpes simplex virus of JSV) |
· | Genital warts (human papilloma virus or HPV) |
· | Hepatitis B |
· | H. pylori |
· | Human immunodeficiency virus (HIV) |
· | Lyme Disease |
· | Rocky Mountain Spotted Fever |
· | West Nile |
· | Asian Bird Flu |
Raw substances as well as fully developed and commercially available disease markers and antibodies for the diseases listed above as well as many others can be purchased by the Company from a variety of sources and incorporated into the Target System Platform.
This in no way represents the complete segment of Qualitative or Semi-Quantitative tests available for rapid development on the Target System Platform.
New Product Identification
·
Track all CDC, FDA, WHO, relevant reports of medical diagnostic requirements. Provide analysis of whether the test should be Specialized, Quantitative or Qualitative.
·
Determine human capital requirements: project management, outsourcing needed political needs (if any) and social needs (affiliations with association or non-profit groups).
·
Determine the market size and utilization of device needed to address identified diagnostic needs.
·
Determine from source venders what antibodies and antigens are available to use in the Company's device with minimal regulatory and manufacturing hurdles.
Table of Contents
11
·
Perform cost analysis of device manufacture, to include: regulatory application time estimates, clinical requirements, third party and vendor involvement for regulatory support.
·
Identify and prepare pre-market distributor (government or commercial) analysis for market penetration timetable and/or government contract fulfillment.
·
Identify new partnership resources if necessary for specialty devices.
·
On all Quantitative Devices the Company will determine the Biohazard level at which it is to perform its algorithm development. For highly contagious diseases, the Company will outsource its complete process to a certified lab.
·
In the development of standard quantitative test the Company will determine through the protocol process: how many tests must be performed for an I.R.B. for both the algorithm development (quantitative controls for each test process) and the accuracy of the variable light analysis.
·
All new quantitative tests will be videotaped during algorithm development (light source verification and reflectivity of known sample), and equivalency testing (where the Company compares its device to another like kind device).
·
All software developed for the Company's tests, that are not modifications of existing source code, will be previewed via written outline to the FDA.
Market Opportunities
In recent years, there has been a continuing shift from the use of laboratory-based analyzers to more technologically advanced point-of-care tests that can be performed in a matter of minutes. Unlike the centralized clinical laboratory segment, which is mature and highly competitive, the point-of-care market is still a relatively early stage market. Although certain simple single analyte diagnostic tests have been developed, such tests have remained incapable of precise and highly sensitive quantitative measurements. As a result, medical tests that require precise quantization of the target analyte have remained the domain of immunoassay analyzers.
Global Statistics
The global In Vitro Diagnostics ("IVD") market was valued at $44 billion in the year 2011, growing at a CAGR of 7.8% from 2011 to 2016. The U.S. represented the biggest market for the IVD equipment's accounting for a share of 47% of the total IVD market in the year 2011. The European region accounted for 31% of the global IVD market, with Germany accounting for the largest share of 23.24%, followed by France (16.89%) and Italy (16.41%) of the total IVD market. Asia is the fastest growing region of the global market and accounts to be 22.88% of the global market and is estimated to reach the market of $17.20 billion with a CAGR 11.3% from 2011 to 2016. China is the fastest growing market within Asia and is growing at a CAGR of 18.8% to reach the market of $1.24 billion by 2016. The Asian region is expected to be ruled by the emerging economies such as China and India, showing the highest CAGR by the year 2016. The Chinese IVD market is taking frog leap amongst the emerging nations, followed by India, Russia, and Brazil.
Budget constraints causing and unfavorable reimbursement scenario for tests especially for severe conditions like cancer are prime reasons for slow growth in the U.S. and Canadian markets. However, the condition is reverse in the Latin American countries like Brazil and Mexico. There has been huge funding from the Brazilian government and the public sector, with increased efforts being taken to prevent infectious diseases in the country by conducting all the preventive tests.
Molecular diagnostics is the largest growing segment of the global IVD market with a highest CAGR for year 2011 to 2016. The major players in the IVD market are Roche Diagnostics (Germany), Abbott Diagnostics (U.S.), Beckman Coulter (U.S.), BD Diagnostics (U.S.), and Siemens Diagnostics (Germany).
The major driving factor for the IVD industry to boom in the emerging countries is the government funding and improved healthcare facilities. However, the condition is completely reverse in the developed countries such as North America and Eu-5 as these countries are facing major financial, thus hampering the growth of the IVD industry. The major factors driving the growth of the IVD market are:
· | increased patient awareness, patient self testing, and increasing baby booming population across the globe. |
· | advancement in the technology bringing more of automated tests is also one of the major drivers for the growth of IVD market. |
Other major drivers for the growth of the IVD industry is rise in the number of diseases, like respiratory infections, hospital acquired infections, and sexually transmitted diseases. Similarly, rises in the chronic diseases such as diabetes, hypertension, cardiovascular diseases, and cancer are driving the overall IVD market.
12
Point-of-Care Diagnostics
The trend is moving toward POC diagnostics using systems and procedures, which do not require extensive laboratory equipment. Here, direct read-out technology will provide a suitable tool, which can be used in basically every environment. The global point-of-care (POC) diagnostics market reached $13.4 billion in 2010 and is expected to reach $13.8 billion in 2011. It will further grow to $16.5 billion in 2016 for a compound annual growth rate (CAGR) of 3.7% between 2011 and 2016. The growth in the POC market is expected to continue through the end of the decade.
The point-of-care market includes hospitals, clinicians, laboratories, assisted living facilities, retirement communities and geriatric facilities and the international market. The Company's system provides the platform for the development of a series of quantitative tests for important diagnostic applications that can provide results at a patient's bedside, in a doctor's office, in the emergency room, in a clinic or in an ambulance.
The two factors that are significant to the rapid growth of POC testing are technology advancements and health care economics. The development of new and improved technologies has resulted in the ability to make evidence-based medical decisions that improve patient outcomes and reduce patient acuity, criticality, morbidity and mortality. Quicker diagnosis of infectious agents can also permit the earlier prescription of appropriate medications, thereby potentially shortening the duration of illness.
Additionally, the economic climate is driving significant changes in the manner in which patients will be tested and how results are delivered. Recent revisions to government regulations, together with growing patient and insurer pressures on hospitals and physicians have increased incentives to reduce overall patient healthcare costs while providing a higher level of care to a greater number of patients. One cost-cutting measure is to reduce the high cost of diagnostic testing carried out in central laboratory sites.
The Target System provides the platform for the development of a series of quantitative tests for important diagnostic applications that can provide results at a patient's bedside, in a doctor's office, in the emergency room, in a clinic, in an ambulance, on the battlefield, on site agri-business locations, rural and economically disadvantaged areas.
The Target System expects to meet the POC diagnostic market criteria as follows:
·
Rapid turnaround time
·
Direct application of a non-critical volume or placement of sample directly into instrument
·
Disposable device or minimal maintenance required
·
Minimal technical expertise required
·
Positive identification and specimen tracking strategy that eliminates specimen identification errors
·
Simple strategy for calibration and QC
·
Transferability of data to the LIS or HIS
·
Agreement of result with accepted "Gold Standard" tests
·
Affordable cost
Immunoassays: Defined
Immunoassays are chemical tests used to detect or quantify a specific substance, the analyte, in a blood or body fluid sample, using an immunological reaction. Immunoassays are highly sensitive and specific. Their high specificity results from the use of antibodies and purified antigens as reagents. An antibody is a protein (immunoglobulin) produced by B-lymphocytes (immune cells) in response to stimulation by an antigen. Immunoassays measure the formation of antibody-antigen complexes and detect them via an indicator reaction. High sensitivity is achieved by using an indicator system (e.g., enzyme label) that results in amplification of the measured product. Immunoassays may be qualitative (positive or negative) or quantitative (amount measured). An example of a qualitative assay is an immunoassay test for pregnancy. Pregnancy tests detect the presence of human chorionic gonadotropin (hCG) in urine or serum. Highly purified antibodies can detect pregnancy within two days of fertilization. Measuring the signal produced by the indicator reaction performs quantitative immunoassays. This same test for pregnancy can be made into a quantitative assay of hCG by measuring the concentration of product formed.
The purpose of an immunoassay is to measure (or, in a qualitative assay, to detect) an analyte. Immunoassay is the method of choice for measuring analytes normally present at very low concentrations that cannot be determined accurately by other less expensive tests. Common uses include measurement of drugs, hormones, specific proteins, tumor markers, and markers of cardiac injury. Qualitative immunoassays are often used to detect antigens on infectious agents and antibodies that the body produces to fight them. For example, immunoassays are used to detect antigens on Hemophilus, Cryptococcus, and Streptococcus organisms in the cerebrospinal fluid (CSF) of meningitis patients. They are also used to detect antigens associated with organisms that are difficult to culture, such as hepatitis B virus and Chlamydia trichomatis. Immunoassays for antibodies produced in viral hepatitis, HIV, and Lyme disease are commonly used to identify patients with these diseases.
Quantitative Immunoassay Analysis
Immunoassays are powerful techniques for understanding the role of specific components in complex systems. They work on the basis of the recognition of a specific component (target X) by an antibody or equivalent (affibody, RNA aptamer, recombinant antibody, etc.), which results in the production of a detectable signal. In most cases immunoassays are qualitative, providing information in terms of signal intensity. What is really wanted, however, is quantitative assay providing information in absolute chemical terms, namely the concentration of target X.
Table of Contents
13
Quantitative Immunoassays would allow:
·
Detection of the absolute concentration of components
·
Reduce inter-assay variation in data
·
Permit successful statistical analysis of smaller sample sets
·
Permits direct comparison of data generated at independent sites or occasions.
Quantitative Immunoassays are simple to construct. They require the simultaneous analysis of experimental (or test) samples and calibration standards. The signal intensity generated by calibration standards of known concentration permits conversion of the signals generated by the test samples into absolute units of concentration.
Calibration curve
A calibration curve (or standard curve) establishes the relationship between the amount of material present and the signal intensity measured. In the case of immunoassays, this would represent the relationship between the epitope concentration and the signal intensity obtained. This relationship is often non-linear, and in many applications displays a dynamic range (or response range) of approximately two orders of magnitude in the concentration of target X.
To perform a Quantitative Immunoassay, a set of "calibration standards" containing the epitope in various concentrations, are deployed in the immunoassay alongside experimental "test samples". Densitometry is performed on all data from the assay, and curve fitting used to define the relationship between epitope concentration and signal intensity. This mathematical relationship is then used to convert the signals generated by experimental samples into concentration of target X, which in the Company's experience is highly accurate.
Molecular Identity of Calibration Standards
For Western Blot applications, a calibration standard is a molecule which contains the epitope feature of an immunoassay covalently bonded to a protein of known molecular weight. Two configurations of this structure are possible (Figure 1), where the epitope structure is either linked to the amino acid backbone (Fig 1a) in the form of a fusion protein or linked to a side chain of a specific amino acid (Fig 1b).
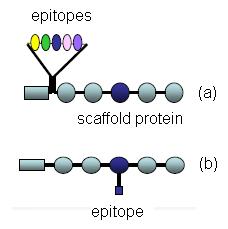
Figure
Figure 1 : Schematic representation of calibration standard molecules.
A set of calibration standards to common epitope tags (His6, c-myc, HA, FLAG, AU1, AU5, glu-glu,) was analyzed by SDS-PAGE/Western blotting (detected via the His6 tag). A single band of 55kDa was detected, and the intensity of signal decreased with decreasing calibration standard loading as expected (Figure 2).
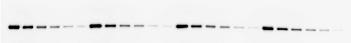
Figure
Figure 2 : Immunodetection of a serial dilution of His6-calibration standard.
Densitometry of the data was performed and the data plotted to define the relationship between epitope amount and signal intensity (Figure 3). Mathematical fitting of the data was performed, with the best fit achieved by "one site-specific binding" analysis (GraphPad Prism) as shown in Figure 3. An excellent fit of the data was achieved using 6 calibration standard concentrations each analyzed in quadruplicate. Similar excellent fits could also be achieved by analysis of fewer standards, with indistinguishable results obtained from 3 calibration standard samples analyzed in triplicate.
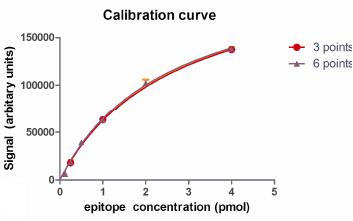
Figure
Figure 3 : Mathematical description of a calibration curve.
14
To determine the epitope concentration of an experimental sample, the mathematical description of the calibration curve is rearranged to calculate epitope concentration from raw signal intensity. Figure 4 displays the quantitative measurement of three "test" samples. Test samples of 2pmol and 0.5 pmol were analyzed and the results obtained were 2.153± 0.127 pmol (mean ± standard error, n=4), 0.552± 0.045 pmol (mean ± standard error, n=4), confirming the accuracy of the measure (Figure 4). Samples should only be analyzed which fall within the calibration range, as errors are higher for observations beyond the confines of the calibration curve e.g. 0.125 pmol in this example.
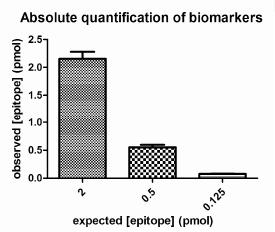
Figure
Figure 4 : Accuracy of Quantitative Immunoassays.
In summary, Quantitative Immunoassays are easy to construct and offer several valuable benefits to the researcher. They permit calculation of the absolute concentration of the component of study with high accuracy (error <10%) and high reproducibility. This enhances the quality of research results and also the productivity of research programs by facilitating the direct comparison of data obtained on separate occasions.
The Immunoassay Market
Overview
Immunoassays have been used since the mid-1960's by hospitals, laboratories and research facilities. With the widespread usage of blood and other biological specimen tests for disease and medical condition diagnoses, there is a growing need for new and better technologies to achieve fast and accurate test results. Though the traditional laboratory testing of blood samples has been an acceptable form of screening for certain conditions for quite some time, it has only been in the last twenty years that rapid POC qualitative (yes/no) and semi quantitative (based on predetermined cutoff levels) testing has become an acknowledged source of accurate information.
Rapid Immunoassay Test
With continuing breakthroughs in detectable markers in the body that can identify the presence of a growing number of diseases and conditions, coupled with the advancements in rapid detection technologies, the tools available to medical professionals is quickly becoming a booming industry.
Rapid immunoassays generally come in two configurations: Lateral Flow and Flow-Through. Examples of the rapid immunoassay test are the home pregnancy test and the on-site drug screening test. While both of these examples are based on urine specimens, many of the new rapid screening devices have been developed to use blood as the specimen. The different types of rapid immunoassay test include:
·
Lateral Flow Test
·
Solid Phase Test
·
Agglutination Assay
·
Flow Through Immunoassay
Lateral Flow Tests
A popular testing method used by both professional and over-the-counter tests, the lateral flow test is quick and efficient. Depending on the specific test kit, a sample of urine, whole blood, blood plasma, and in some cases feces, may be mixed with diluting substances, reactive agents or other solutions that are provided for the conduct of the test. Most of the tests are classified as solid phase enzyme immunoassays.
Most home pregnancy tests utilize lateral-flow technology, whereby urine is absorbed through the exposed sample application pad and is allowed (by natural wicking) to migrate to the analytical membrane and react with an embedded agent designed to change color if hormones associated with pregnancy are present in the urine. This is a direct specimen application and does not require dilution or other agents to be added for results to appear. The typical finished product in general use encases all but the application pad in plastic with view openings for the test line or dot and the control line.
Lateral flow devices have been used for home pregnancy tests, drugs of abuse testing in clinical laboratories and, more recently, for home use. Manufactured in continuous membrane strips cut to the desired length and batch tested for accuracy, the manufacture of test kits is highly automated and inexpensive, making lateral flow tests well suited for mass market applications.
Table of Contents
15
Lateral flow devices, however, can suffer in performance when the sample being tested is not handled within strict conditions. Test samples may be affected by environmental conditions (barometric pressure, temperature and humidity), thereby requiring special care in sample preparation, exact dilution controls and controlled time for the test to develop properly. Test development time, for example, can vary from a few minutes (3 to 5) for urine-based tests and up to 20 minutes for whole blood or plasma.
Solid Phase Tests
Solid phase assays include the so-called "dipstick" or "dipstick comb" tests. As its title suggests, the detection materials are in a solid state affixed to a solid, non-porous base. The dipstick is then incubated with the patient's specimen.
The solid phase tests use urine, saliva, serum, plasma, or whole blood as its specimen and the same patient can be tested for multiple parameters with a single assay. Results are usually provided in an hour or less. The sensitivity and accuracy is generally lower than the flow through and lateral flow tests.
Agglutination Test
The basic principle of an agglutination test is the formation of clumps (agglutination) of small particles coated with antigens when exposed to antibodies specific for the antigen. The test particles and the patient antibodies combine to form a visible precipitate. This usually is observed under a microscope.
Some of the advantages of the agglutination test are that its individual test cost is low, the results are semi-quantitative, and it takes a relatively short time to obtain results. However, results can vary as the test reaction depends upon careful control of the test re-agents and environmental conditions. The sensitivity/accuracy is lower than the flow through or lateral flow tests.
Flow-Through Immunoassay
The flow through test procedure requires a number of steps, and includes a vacuum device that deposits fluid containing the test sample through a porous membrane and into an absorbent pad. A second layer, or sub-membrane, inhibits the immediate back-flow of fluids, which can obscure results.
Positive, uniform deposition of test samples on a membrane containing selected diagnostic reagents provides for a flexible, inexpensive and reproducible platform technology to test for a large number of diseases.
The flow-through platform technology can be used to detect both antibodies and antigens. To perform the test, a sample is applied to the membrane followed by a wash step, the addition of the signal reagent, and a second wash to clear the membrane. The solutions can be added as rapidly as the previous liquids are absorbed into the cassette.
The time it takes for a test to display results is subject to the viscosity of the sample, which can be affected by environmental conditions, such as humidity and barometric pressure, further interfering with the time the test takes is the amount of sample used.
Limitations
Each of the screening devices described above have limitations in their utility and range of application. Many screening devices have been adopted from their use in clinical laboratories and, when applied to POC application, required special handling of the specimen samples (blood, urine, and feces) and decreased sensitivity and/or specificity.
Competition
There are approximately 40 to 50 companies in the point-of care ("POC") diagnostic industry in the U.S. and approximately another 100 outside the U.S. The POC space can be broken down into various sub-sets such as molecular biologist developing reagents, and markers to diagnostic equipment and test development companies, as well as companies who do neither and focus on marketing tests, equipment and assays. Most notably in the POC space are the large pharmaceutical companies such as Bayer, Roche, Abbot Labs and others. The Company's specific competitive landscape is tied to its patent pending process involving its SPARKS Mobile Analyzer and Target System Platform In-Vitro Tests. There are a number of companies developing mobile devices to perform a host of health industry-related services and the Company believes that more companies will enter the mobile diagnostic space in the next few years. The industry has yet to develop a standardized point of care immunoassay platform for any device to be integrated into. The goal of the Company's SPARKS Mobile Analyzer is to deliver a device that adds immediate value to health providers, patients and health insurance companies. The Company's primary goal is to create a mobile platform that could integrate and utilize the flow-through process of the Company's Target System Platform and offer the healthcare provider a system that is fully interoperable and ubiquitous with a potentially large number of in vitro tests. There are other test platforms in the space but the Company has filed a patent on the process of its SPARKS Mobile Analyzer and its TADS Cartridge. There can be no assurance that the Company's POC device will prove to be competitive with the other POC devices under development.
16
Until the Company secures a minimum of two million dollars ($2,000,000) of additional capital to operate for the next eighteen months the Company will remain highly vulnerable from the Company's competition. The Company anticipates the need for a minimum of an additional four million ($4,000,000) dollars of investment capital will be required for it to achieve its goal of developing a commercially viable rapid point of care CD4-8 immune status test and its proposed mobile SPARKS Mobile version of its FDA Approved VT 1000 Desktop Analyzer. There can be no assurance that such amount will prove adequate to develop the Company' products. Furthermore, the Company's competition has significantly greater resources that it can deploy and anytime to head off competition.
In Vitro Diagnostic Sales Leaders
·
Roche Diagnostics, Switzerland www.roche.com
·
Abbott Diagnostics, Abbott Park, IL 60064 www.abbott.com
·
Siemens Medical Solutions Diagnostics, Deerfield, IL www.diagnostics.siemens.com
·
Johnson & Johnson, Ortho Clinical Diagnostics (OCD) division, Raritan, NJ www.jnj.com
·
Beckman Coulter Inc., Fullerton, CA www.beckmancoulter.com
·
Becton, Dickinson & Co., Franklin Lakes, NJ www.bd.com
·
bioMerieux SA, Marcy l'Etoile, France www.biomerieux.com
·
Bio-Rad Laboraties Inc., Hercules, CA www.bio-rad.com
·
Arkray Inc., Kyoto, Japan www.arkray.co.jp
·
Mitsubishi,Japan www.mitsubishi.com
Top Medical Device Manufacturers
Rank | Company | Earnings |
| Rank | Company | Earnings |
1. | Johnson and Johnson | $17.7B |
| 16. | St. Jude Medical | $2.9B |
2. | GE Healthcare | $12.1B |
| 17. | Kodak Health Group | $2.7B |
3. | Medtronic | $10.1B |
| 18. | Hospira | $2.6B |
4. | Baxter International | $9.8B |
| 19. | Fresenius | $2.5B |
4. | Cardinal Health | $9.8B |
| 20. | Smith & Nephew | $2.4B |
6. | Tyco Healthcare | $9.5B |
| 21. | Synthes | $2.1B |
7. | Siemens Medical Solutions | $9.2B |
| 22. | Alcon | $2.0B |
8. | Philips Medical Systems | $7.5B |
| 23. | Biomet | $1.9B |
9. | Boston Scientific | $6.3B |
| 24. | C. R. Bard | $1.8B |
10. | Stryker | $4.9B |
| 24. | Terumo | $1.8B |
11. | B. Braun | $3.9B |
| 26. | Dentsply International | $1.7B |
12. | Guidant Corp. | $3.6B |
| 27. | Invacare | $1.5B |
13. | 3M Healthcare | $3.5B |
| 28. | Gambro | $1.4B |
14. | Zimmer Holdings | $3.3B |
| 29. | Dräger Medical | $1.3B |
15. | Becton, Dickinson & Co. | $3.0B |
| 30. | Varian Medical | $1.2B |
Barriers to Use
The main barriers and constraints to the use of rapid diagnostic tests can be put into three main categories:
·
Acceptability : Rapid tests need to be acceptable to policymakers, clinicians, and patients. Tests need to have sufficient sensitivity and specificity and need to have an adequate predictive value. Ease-of-use is critical for point-of-care use by clinicians. Culturally appropriate specimens and credible results are important if rapid tests are to be accepted by patients.
·
Affordability: Many rapid diagnostic tests are more expensive than the tests or syndromic algorithms they are intended to replace. Decreasing per-test costs, carefully designing diagnostic algorithms, and educating end users about the cost-savings of more efficient use of therapeutic drugs are important means of maximizing rapid test affordability.
·
Availability: Rapid diagnostic tests are not always available in developing countries. Most tests have limited shelf lives, and many countries have weak public and private sector procurement and distribution systems. The consistency and quality of imported tests can also be issues. To address these constraints, local government regulations, quality assurance, shelf life testing, and distribution systems all need to be assessed and improved. The Company will initially control all of the manufacturing of its Target System test cartridges and Desk Top Analyzer and SPARKS Mobile Analyzer in conjunction with Montecito.
Intellectual Property
The Company's products include a previously FDA-cleared VT-1000 Desktop Analyzer and more than a dozen previously FDA 510(k)-cleared tests. The Company holds the rights in perpetuity to a number of pending USPTO Patent Applications on the Company's products, as well as methods for future test development.
Table of Contents
17
USPTO and International Patent Pending Applications:
The Company acquired exclusive rights in perpetuity to certain USPTO Patent Applications in the area of Infectious Diseases through a License Agreement with Montecito BioSciences, Ltd. The Company is currently prosecuting its pending USPTO Patent Applications under the following application numbers:
Application # | Description | Date Filed in US | Date Published |
US2006051348 - 11/221,252 | Method of Producing a Plurality of Isolated Antibodies | September 7, 2005 | March 9, 2006 |
US2006052948 - 11/221,038 | Method of Producing Drugs, Targeting Moieties or Diagnostics | September 7, 2005 | March 9, 2006 |
US11/856,925 | Method for Determining the Immune State of a Subject | September 18, 2007 | April 10, 2008 |
US11/924,033 [1] | Portable Apparatus for Improved Sample Analysis | September 27, 2006 | May 15, 2008 |
[1] Patent Application US11/924,033 is being prosecuted worldwide. EPO Application No. 07854420.2 has been filed in the following designee countries; Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Monaco, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Switzerland, Liechtenstein, Turkey and the United Kingdom. The Company filed in Hong Kong its Hong Kong Patent Application No. 10103654.9. The Company filed in India under a New Indian Patent Application based on the PCT Application No. PCT/US2007/082499. | |||
The Company also filed to prosecute its Patent Pending Application in China through NPA International, Inc., and with the U.S. Office of China SINDA Intellectual Property Ltd. under the Chinese Patent Application No. 200780039901.X, and is published under the publication No. CN 1015558302A in No. 41 of Vol. 25 of Patent Gazette.
On August 23, 2013 the Company was notified that its Chinese Patent Application No. 200780039901.X "Portable Apparatus for Improved Sample Analysis" had been granted by the States Intellectual Property Office of the Peoples Republic of China. This Patent Application covers the Company's SPARKS Mobile hand held analyzer in conjunction with the TARGET System Test Cartridge.
For further information on the exclusive license of these Patent Applications, and the complete text of the License Agreement and subsequent Modification, please refer to Exhibits 10.20 and 10.22, respectively, to the Company's Current Report filed November 15, 2012 on Form 8-K.
The Company considers its US Patent Application US 11/924,033 "Portable Apparatus for Improved Sample Analysis" an important Patent Application for the potential protection of its Test Platform and its mobile version of its VT-1000 Desktop Analyzer. The US Patent Application US 11/924,033 "Portable Apparatus for Improved Sample Analysis" was filed to protect the process of testing with the Company's Target Antigen Detection System Cartridge and a mobile version of the Company's VT-1000 Desktop Analyzer. Currently, Patent Application US 11/924,033 is the only Patent Application that the Company has sought to protect outside of the U.S. However, the Company intends to seek Intellectual Property protection for all of its products, both domestically and internationally. Moreover, the Company intends to seek Intellectual Property protection for all supporting products such as novel biomarker candidates, antibodies, proteins, and diagnostic tests surrounding the Company's core indication areas, in order to create a barrier to entry for its competitors.
The Company retained the services of Marathon Patent Group to perform an analysis of the Company's intellectual property ("Patent Report"). The Patent Report, included as Exhibit 99.2 to the Company's Annual Report filed April 16, 2013 on Form 10-K, was issued on April 1, 2013, and was intended to inform the Company and its shareholders of the accurate and current state of the commercial patent pending coverage and where possible to identify the existence of novel and patentable inventions present in the current innovation initiative. The Patent Report concluded that the Company has a strong patent portfolio protecting its business, and recommended that the Company aggressively proceed with additional patent applications to protect the Company's inventions and innovations. As a result, the Company has initiated the drafting of a minimum of one (potentially two) additional USPTO and International patent applications.
There can be no assurance that the Company will be granted patents for any of the patent applications it has filed with the USPTO.
18
USPTO Patent Applications Abstracts
Following are the Company's Patent Application Abstracts, which provide information on the methods and practice underlying the Company's pending Patent Application(s):
Abstract to U.S. Patent Application No. 11/221,252 "Method of Producing a Plurality of Isolated Antibodies to a Plurality of Cognate Antigens "
The present invention relates to a method for producing high affinity antibodies that are antigen-specific. The method involves binding a plurality of antibody-producing B-cells from a mammal to a plurality of cognate antigens; sorting the bound antibody-producing B-cell and cognate antigen; amplifying nucleic acid sequences encoding each antibody, or fragment thereof, from the B-cells; and expressing the each antibody in a protein expression system. Antibodies produced in this manner are useful in diagnostic and therapeutic applications.
Abstract to U.S. Patent Application No. 11/221,038 "Method of Identifying Drugs, Targeting Moieties or Diagnostics "
The present invention relates to a method for identifying a binding agent or epitope for use in drug design, drug targeting or diagnostics. The method employs contacting and sorting binding agents and cognate epitopes from collections thereof, characterizing the binding agent and cognate epitope, detecting the level or location of the epitope in a sample using the binding agent, and correlating the level or location of the epitope in the sample with the presence or stage of a disease or condition to identify novel drugs, targeting moieties, or diagnostic agents.
Abstract to U.S. Patent Application No. 11/856,925 "Method for Determining the Immune Status of a Subject"
The present invention is a method for using levels of soluble Clusters of Differentiation (CD) proteins, or cell surface-localized CD proteins extracted from T lymphocytes for determining the immune status of a subject. The present invention also a kit containing a CD protein extraction means and at least one antibody which specifically binds a CD protein for use in carrying out the method of the invention.
Abstract to U.S. Patent Application No. 11/924,033 "Portable Apparatus for Improved Sample Analysis"
The present invention is an improved apparatus for sample analysis. The apparatus employs an assay component containing a membrane having one or a plurality of analyte-specific binding agents attached thereto, a means for absorbing liquid, and a piston means for drawing analytes through said membrane into said means for absorbing liquid. The apparatus is configured to be portable and provide a detector for detecting binding of an analyte to an analyte-specific binding agent, a plurality of data acquisition components, and a computer for integrating, analyzing and storing the detected analyte specific binding and acquired data.
Expired Patents :
The Target System and certain of its related components were previously issued patents by the USPTO. The following previously-issued patents have expired:
USPTO Patent # | Description | Date Filed in US | Date Expired |
US4,748,042 | Target Ringing & Spotting Machine (method and Apparatus for Imprinting membrane with pattern of antibody) | May 31, 1988 | May 31, 2008 |
US4,797,260 | Target Cassette (Antibody testing system) | January 10, 1989 | January 1, 2009 |
US5,137,691 | Target Cassette with Removable Air Gap (Antibody testing system with removable air gap) | August 11, 1992 | August 11, 2012 |
The Company's Previously FDA-Cleared Tests
Table of Contents
19
The Company acquired the exclusive rights in perpetuity to the following FDA-approved 510(k) tests (the "Tests") through an Assignment Agreement with Montecito BioSciences, Ltd.:
No. | Test/Device Name | 510(k) Number |
| No. | Test/Device Name | 510(k) Number |
1 | Rotacube (Rotavirus) | K884017 |
| 14 | First Sign (Pregnancy, Hcg) | K973208 |
2 | Rubella-Cube TM | K892051 |
| 15 | Target Hcg | K914303 |
3 | Cmv-Cube TM | K884842 |
| 16 | Target Quantitative Hog One Step | K903937 |
4 | Target Quantitative Hcg | K890131 |
| 17 | V-Trend Target Rf Test | K904105 |
5 | Target Strep A (Streptococcus Spp.) | K880460 |
| 18 | Blue Dot Test for Pregnancy | K884017 |
6 | V-Trend Target Im Test (infect mononucleosis) | K890041 |
| 19 | Target Cocaine Metabolites-R Test | K910122 |
7 | Target Reader | K885254 |
| 20 | Target Cocaine Metabolites-V Test | K910123 |
8 | Target Cardiac Ck-Mb | K890295 |
| 21 | Target Cannabinoids-R Test | K910893 |
9 | Target Cardiac Troponin 1 | K972094 |
| 22 | Target Cannabinoids-V Test | K910892 |
10 | Target C-Reactive Protein Test | K892231 |
| 23 | Target Amphetamines/Methamphetamines-R Test | K910739 |
11 | Target C-Reactive Protein Test | K890423 |
| 24 | Target Amphetamines/Methamphetamines-V Test | K910740 |
12 | Target Myoglobin | K963680 |
| 25 | Target Opiate-R Test | K890978 |
13 | Target Aso Test | K910073 |
| 26 | Target Opiate-V Test | K890979 |
For further information on the exclusive rights to these Tests, and the complete text of the Assignment Agreement and subsequent Modification, please refer to Exhibits 10.19 and 10.21, respectively, to the Company's Current Report filed November 15, 2012 on Form 8-K.
It is expected that after successful re-introduction of the Target System and the introduction of its novel PROMISE CD4 immune status test, additional tests will be developed and protected by the Company. Generally, the Company and Montecito BioSciences, Ltd., will own improvements to the basic technology platform in exclusivity.
Trademarks
The Company will also utilize trademark applications to protect its Intellectual Property that may not be suitable for patent protection. Unlike patent applications, which in many cases must be filed in advance of a particular date, there is no specific date by which a trademark application must be filed. Instead, the time constraint is in a different direction. In the United States, an ordinary so-called "use" trademark application can only be filed after the goods or services have been in interstate commerce.
Government Regulations
The long legal journey toward medical device regulation began with the Pure Food and Drugs Act of 1906. Medical devices were not included, as no one envisioned how technology would grow increasingly complex, and would ultimately require regulation.
The Medical Device Amendments of 1976 gave FDA authority to ensure the safety and effectiveness of a range of life-saving medical devices, while also protecting the public from fraudulent devices. The Amendments:
·
defined a medical device,
·
established three device classes (I, II, and III),
·
identified pathways to market,
·
established Advisory Panels, and
·
set clinical investigation requirements.
20
Subsequent legislation strengthened the FDA's regulatory authority. The following table identifies the legislation and significance for the Major Medical Device:
Legislation | Significance |
Safe Medical Devices Act of 1990 | · established Quality System requirements |
· supported post market surveillance | |
· allowed FDA discretion for PMAs brought to panel | |
FDA Modernization Act of 1997 | · supported for early collaboration, expanded Class I and Class II exemptions |
· set the "least burdensome provision"* | |
· supported dispute resolution | |
· established evaluation of automatic Class III designation (giving the sponsor the opportunity to request lower classification due to a minimal risk device, known as "de novo" review) | |
· mandated free and open participation by all interested persons | |
Medical Device User Fee and Modernization Act (MDUFMA) of 2002 | · established a fee schedule for most types of device submissions to achieve shorter review times |
· requires FDA to include pediatric experts on the panel for a product intended for pediatric use | |
FDA Modernization Act of 2007 | · reauthorized and expanded MDUFMA |
The least burdensome provision allows industry and FDA to consider the least burdensome appropriate means of evaluating a device's effectiveness when there is a reasonable likelihood of its approval. The intent is to help expedite the availability of new device technologies without compromising scientific integrity in the decision-making process or FDA's ability to protect the public health. This provision does not lower the standard for premarket clearance.
Medical Devices: Defined
The definition has several components. A medical device:
·
diagnoses, cures, lessens, treats, or prevents disease
·
affects the function or structure of the body
·
does not achieve primary intended purposes through chemical action
FDA's Center for Devices and Radiological Health regulates companies that design, manufacture, repackage, relabeling, and/or import medical devices into the United States. The agency does not regulate the practice of medicine – how and which physicians can use a device. The only exception is FDA's regulation of mammography facilities under the Mammography Quality Standards Act.
Combination Products
Combination products are therapeutic and diagnostic products that combine drugs, devices, and/or biological products. The term acknowledges the role technological advancements have made in merging medical product types. Examples of combination products include a drug-eluting stent, a nicotine patch, and surgical mesh with antibiotic coating, prefilled syringes, and a steroid-eluting pacing lead.
Combination products raise regulatory challenges because they involve components that were normally regulated under different types of authorities and often by different FDA Centers. Differences in regulatory pathways for each component can affect the regulatory processes for all aspects of product development and management, including preclinical testing, clinical investigation, marketing applications, manufacturing and quality control, adverse event reporting, promotion and advertising, and post-approval modifications.
Three classes of regulatory control
The three device classes are based on the degree of regulatory control necessary to ensure their safety and effectiveness:
Class I:
devices present a low risk of harm to the user and are subject to general controls that are sufficient to protect the user. Most are exempt from the regulatory process.
Examples: non-powered breast pumps, elastic bandages, tongue depressors, examination gloves, most hearing aids, arm slings, microbial analyzers, keratoscopes
Class II:
devices are more complicated and require special controls for labeling, guidance, tracking, design, performance standards, and post market monitoring. Most require Premarket Notification 510(k).
Examples: powered wheelchairs, CT scanners, and contact lens care products, endolymphatic shunts
Table of Contents
21
Class III:
devices usually sustain or support life, are implanted, or present potential unreasonable risk of illness or injury. They have the toughest regulatory controls. Most of these devices require Premarket Approval because general and special controls alone cannot reasonably assure their safety and effectiveness.
Examples: pacemakers, implanted weight loss devices, non-invasive glucose testing devices, medical imaging analyzers, cochlear implants, breast implants
FDA Review of Medical Devices
Investigational Device Exemptions (IDE )
An IDE allows an investigational device to be used in a clinical study to collect the safety and effectiveness data required for a Premarket Approval (PMA) application or a Premarket Notification (510(k)) submission to FDA. Both FDA and an Institutional Review Board (IRB) must approve clinical studies with devices of significant risk before the study can begin. Studies with devices posing non-significant risk must be approved by an IRB before the study can begin.
FDA observes a 30-day review period for IDE applications. The agency focuses its review on the data provided to demonstrate the safety and anticipated benefits of the device for use in humans, as well as the scientific validity of the proposed clinical trial protocol.
Following clinical studies, a device's journey to market can take one of four major pathways:
1. | Investigational Device Exemptions (IDE) |
2. | Premarket Notification (510(k)) |
3. | Premarket Approval Application (PMA) |
4. | Humanitarian Device Exemption (HDE) |
Premarket Notification (510(k))
510(k) is required when demonstrating substantial equivalence to a legally marketed device, when making significant modifications to a marketed device, and when a person required to register with FDA introduces a device for the first time. If a device requires the submission of a 510(k), it cannot be commercially distributed until the FDA authorizes it.
Substantial Equivalence
A device is substantially equivalent (SE) if it has the same intended use and same technological characteristics as a legally marketed device, known as the predicate. A legally marketed device:
1. | was legally marketed prior to May 28, 1976 ("pre-amendments device"), for which a PMA is not required |
| or |
2. | was reclassified from Class III to Class II or Class I, |
| or |
3. | was found SE through the 510(k) process |
Applicants must compare their device to one or more similar legally marketed devices and make and support their SE claims. If the device is SE to a predicate, it is placed in the same class. If it is not SE, it becomes non-SE and is placed into Class III.
Examples of 510(k)s include x-ray machines, dialysis machines, fetal monitors, lithotripsy machines, and muscle stimulators.
Premarket Approval (PMA)
PMA refers to the scientific and regulatory review necessary to evaluate the safety and effectiveness of Class III devices or devices that were found not substantially equivalent to a Class I or II predicate through the 510(k) process.
PMA is the most involved process. To reasonably assure that a device is safe and effective, PMA requires valid scientific evidence that the probable benefits to health from the intended use of a device outweigh the probable risks, and that the device will significantly help a large portion of the target population. Sources of valid scientific evidence may include well controlled investigations, partially controlled studies, historical controls, well documented case histories by qualified experts, and robust human experience.
Independence is an important concept for PMAs, meaning that each PMA should establish the safety and effectiveness of the device under review, and that data about one device cannot be used to support another. Examples of PMAs include digital mammography, minimally invasive and non-invasive glucose testing devices, implanted defibrillators, and implantable middle ear devices.
22
Summary Comparison of 510(k) and PMA
510(k) Submissions | PMA Submissions |
· primarily for Class II devices · a Class I or II pre-amendment or legally marketed device (predicate) exists · third party review option is available for devices not requiring clinical data · documented proof of Substantial Equivalence to a predicate is required | · primarily for Class III devices · a Class I or II pre-amendment or legally marketed device (predicate) does not exist · device is life supporting and/or has potential risk to patient · documented safety and effectiveness data for the device is required |
Humanitarian Device Exemption (HDE)
An HDE is a device that is intended to benefit patients by treating or diagnosing a disease or condition that affects fewer than 4,000 individuals in the United States per year. HDEs are exempt from requirements to demonstrate effectiveness. Still, they must pose no unreasonable risks, or, at least, the probable benefits should outweigh the risks. And the device must be used at a facility with an Institutional Review Board.
HDEs provide a powerful incentive for device manufacturers to develop devices that help diagnose or treat patients with rare conditions. Otherwise, a company's research and development costs would likely exceed the market returns for serving such small patient populations.
Examples of HDEs include a fetal bladder stent, iris replacement, radioactive microspheres for cancer treatment, and semi-constructed finger joints.
Post-Approval Studies
The FDA can impose requirements at the time of approval of a PMA or HDE, or by regulation afterwards. One requirement may be the need for post-approval studies. The CDRH Post-Approval Studies Program helps ensure that well designed post-approval studies are conducted effectively and efficiently and in the least burdensome manner. Post-approval studies should not be used to evaluate unresolved premarket issues that are important to the initial establishment of device safety and effectiveness.
With post-approval studies, FDA can evaluate device performance and potential problems when the device is used more widely than in clinical trials and over a longer period of time. This allows FDA to build in accountability and gather essential post market information, including:
·
longer-term performance of the device (for example, effects of re-treatments and product changes)
·
community performance (clinicians and patients)
·
effectiveness of training programs
·
sub-group performance
·
outcomes of concern – real and potential
Manufacturing
The Company does not intend to manufacture in house for at least three years, with the exception of prototype and small batch production of tests for clinical trials and in-house testing. The Company is required to use manufacturers who operate under Good Manufacturing Practices ("GMP").
A GMP is a production and testing practice that helps to ensure a quality product. Many countries have legislated that pharmaceutical and medical device companies must follow GMP procedures, and have created their own GMP guidelines that correspond with their legislation. Basic concepts of all of these guidelines remain more or less similar to the ultimate goals of safeguarding the health of the patient as well as producing good quality medicine, medical devices or active pharmaceutical products. In the U.S. a drug may be deemed adulterated if it has passed all of the specifications tests but is found to be manufactured in a condition which violates current good manufacturing practice guidelines. Therefore, complying with GMP is a mandatory aspect in pharmaceutical manufacturing.
Although there are a number of them, all guidelines follow a few basic principles:
a.
Manufacturing processes are clearly defined and controlled. All critical processes are validated to ensure consistency and compliance with specifications.
b.
Manufacturing processes are controlled, and any changes to the process are evaluated. Changes that have an impact on the quality of the drug are validated as necessary.
c.
Instructions and procedures are written in clear and unambiguous language. (Good Documentation Practices)
d.
Operators are trained to carry out and document procedures.
e.
Records are made, manually or by instruments, during manufacture that demonstrate that all the steps required by the defined procedures and instructions were in fact taken and that the quantity and quality of the drug was as expected. Deviations are investigated and documented.
f.
Records of manufacture (including distribution) that enable the complete history of a batch to be traced are retained in a comprehensible and accessible form.
Table of Contents
23
g.
The distribution of the drugs minimizes any risk to their quality.
h.
A system is available for recalling any batch of drug from sale or supply.
i.
Complaints about marketed drugs are examined, the causes of quality defects are investigated, and appropriate measures are taken with respect to the defective drugs and to prevent recurrence.
j.
GMP guidelines are not prescriptive instructions on how to manufacture products. They are a series of general principles that must be observed during manufacturing. When a company is setting up its quality program and manufacturing process, there may be many ways it can fulfill GMP requirements. It is the company's responsibility to determine the most effective and efficient quality process.
Food & Drug Administration ("FDA") and International Regulatory recognition of GMP [1]
GMPs are enforced in the United States by the US FDA, under Section 501(B) of the 1938 Food, Drug, and Cosmetic Act (21 USCS § 351). The regulations use the phrase "current good manufacturing practices" ("cGMP") to describe these guidelines. Courts may theoretically hold that a drug product is adulterated even if there is no specific regulatory requirement that was violated as long as the process was not performed according to industry standards. As of June 2010, a different set of cGMP requirements apply to all manufacturers of dietary supplements.
The World Health Organization ("WHO") version of GMP is used by pharmaceutical regulators and the pharmaceutical industry in over one hundred countries worldwide, primarily in the developing world. The European Union's GMP enforces similar requirements to the WHO GMP, as does the Food and Drug Administration's version in the US. Similar GMPs are used in other countries, with Australia, Canada, Japan, Singapore, Philippines and others having highly developed/sophisticated GMP requirements. In the United Kingdom, the Medicines Act (1968) covers most aspects of GMP in what is commonly referred to as "The Orange Guide", which is named so because of the color of its cover; it is officially known as Rules and Guidance for Pharmaceutical Manufacturers and Distributors .
Since the 1999 publication of GMPs for Active Pharmaceutical Ingredients , by the International Conference on Harmonization (ICH), GMPs now apply in those countries and trade groupings that are signatories to ICH (the EU, Japan and the U.S.), and applies in other countries (e.g., Australia, Canada, Singapore) which adopt ICH guidelines for the manufacture and testing of active raw materials.
[1] Source: " Guideline Versions ", Good Manufacturing Practices, Wikipedia, 2012.
Distribution
As the Company is still in the development stages and has not yet commenced commercial operations, it has yet to develop methods of distribution for its products beyond the business plan stage.
In order to commercially sell the Company's VT-1000 Desktop Analyzer, the Company must have it manufactured under GMP. The Company can and will provide demonstrations of its VT-1000 Desktop Analyzer capabilities to potential customers.
The Company will need to secure additional capitalization before it can acquire additional antibody markers, produce additional Target System cartridges or produce its VT-1000 Desktop Analyzer under GMP.
Principal Suppliers
Corder Engineering, LLC : Located in Chesterton, Indiana, Corder Engineering provides the Company with engineering services related to the Company's medical testing equipment, and manufactured the Company's evaluation units, including software, hardware and instrumentation.
Meyers Stevens Group, Inc. : Located in Montebello, California, Meyers Stevens Group manufactures and supplies the Company's diagnostic assays related to the Company's medical testing equipment.
The Company relies upon these suppliers to provide all of its test cartridges and materials used in association with the development of its products. If these suppliers were to cease providing test cartridges and materials to the Company, the Company's ability to develop its products may be adversely affected.
Facilities
In addition to its corporate headquarters, the Company maintains operations at 1327 Ocean Avenue Suite M, Santa Monica, California 90401.
Employees
As of December 31, 2013, the Company had no employees, exclusive of its directors and executive officers.
The Company currently has no employees, exclusive of its directors and executive officers.
24
Research and Development
The Company has incurred no expenditures relating to the research and development of its proprietary medical diagnostic equipment during 2013 and 2012, including costs for consultants, costs to develop and manufacture prototype units, and assays, costs to conduct clinical trials, and costs incurred to develop new products.
Reports to Security Holders
The Company is not required to deliver an annual report to its stockholders, but will voluntarily send an annual report, together with the Company's annual audited financial statements upon request. The Company is required to file annual, quarterly and current reports, proxy statements, and other information with the Securities and Exchange Commission. The Company's Securities and Exchange Commission filings are available to the public over the Internet at the SEC's website at www.sec.gov .
The public may read and copy any materials filed by the Company with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The Company is an electronic filer. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The Internet address of the site is www.sec.gov .
ITEM 1A. | RISK FACTORS |
The Company is a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and is not required to provide the information under this item.
ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
ITEM 2. | PROPERTIES |
The Company's principal executive offices are located at One Boston Place, Suite 2600, Boston, MA 02108, with operations at 1327 Ocean Avenue, Suite M, Santa Monica, CA 90401. The Company currently rents the Boston space for approximately $300 a month, and sub-leases the Ocean Avenue space for $1,000 per month. Currently, this space is sufficient to meet the Company's needs. However, once the Company expands its business to a significant degree, it will require additional space. The Company does not currently own any real estate.
ITEM 3. | LEGAL PROCEEDINGS |
The Company knows of no material, existing or pending legal proceedings against it, nor is the Company involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which its director, officer or any affiliates, or any registered or beneficial shareholder, is an adverse party or has a material interest adverse to its interest.
ITEM 4. | MINE SAFETY STANDARDS |
Not applicable.
Table of Contents
25
PART II
ITEM 5. | MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS |
The Company's Common Stock is quoted on the OTC Quotation Board "OTCQB – U.S. Registered" under the trading symbol PRLX.QB. The following table sets forth the high and low bid prices for its Common Stock per quarter as reported by the OTCQB for the last two years. These prices represent quotations between dealers without adjustment for retail mark-up, markdown or commission and may not represent actual transactions.
The high and low prices of the Company's common shares for the periods indicated below are as follows:
Quarter Ended | High | Low |
December 31, 2013 | $0.10 | $0.10 |
September 30, 2013 | $0.08 | $0.08 |
June 30, 2013 | $0.01 | $0.01 |
March 31, 2013 | $0.10 | $0.10 |
December 31, 2012 | $0.05 | $0.05 |
September 30, 2012 | $0.05 | $0.05 |
June 30, 2012 | $0.05 | $0.05 |
March 31, 2012 | $0.06 | $0.06 |
The Company's common stock is subject to rules adopted by the Commission regulating broker dealer practices in connection with transactions in "penny stocks." Those disclosure rules applicable to "penny stocks" require a broker dealer, prior to a transaction in a "penny stock" not otherwise exempt from the rules, to deliver a standardized list disclosure document prepared by the Securities and Exchange Commission. That disclosure document advises an investor that investment in "penny stocks" can be very risky and that the investor's salesperson or broker is not an impartial advisor but rather paid to sell the shares. The disclosure contains further warnings for the investor to exercise caution in connection with an investment in "penny stocks," to independently investigate the security, as well as the salesperson with whom the investor is working and to understand the risky nature of an investment in this security. The broker dealer must also provide the customer with certain other information and must make a special written determination that the "penny stock" is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction. Further, the rules require that, following the proposed transaction, the broker provide the customer with monthly account statements containing market information about the prices of the securities.
On March 8, 2013, the Company was notified that the Securities and Exchange Commission ("SEC") had suspended the trading of the Company's securities for 10 days, until March 21, 2013. This temporary suspension of trading arose out of concerns that the Company had incorrectly stated within its public filings and press releases that certain components related to the Company's Target Antigen Detection System ("Target System") are patented, when these patents have expired.
The Company, after discussions with the SEC and with the Company's counsel, has addressed any questions raised regarding the accuracy of assertions in the Company's public filings. The Company has clarified the status of the patents in question, and the way in which the Company refers to its Target System components. The Company has also clarified that it currently has filed four Patent Applications with the USPTO, which are deemed "patent pending", and the Company has disclosed that there are no guarantees that the patents will be granted. For additional information on the Company's patent applications, please refer to the section entitled "Intellectual Property" contained within this Annual Report.
On May 10, 2013, the Company filed its new form 15c211 with a FINRA Member Market Maker, in order that the Company's shares can resume trading on the OTCBB and OTCQB markets, pursuant to Rule 15c2-11 under the Exchange Act, which states that at the termination of the trading suspension, no quotation may be entered unless and until the Company has strictly complied with all of the provisions of the rule, including the filing of a new Form 15c2-11 and obtaining FINRA approval to commence trading. On August 19, 2013, FINRA approved the resumption of trading.
These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for its common stock. Many brokers may be unwilling to engage in transactions in its common stock because of the added disclosure requirements, thereby making it more difficult for stockholders to dispose of their shares.
Record Holders
The Company's common shares are issued in registered form. Action Stock Transfer Corp., 2469 E. Fort Union Blvd., Suite 214, Salt Lake City, UT 84121 (Telephone 801-274-1088, Facsimile 801-274-1099) is the registrar and transfer agent for the Company's common shares.
As of December 31, 2013, pursuant to Action Stock Transfer Corp., the shareholders' list of the Company's common shares showed 53 registered shareholders and 151,306,558 shares outstanding. The total number of shares outstanding stated in the Action Stock Transfer Corp. list of 151,306,558, does not include the 11,459,279 common shares recorded by the Company on December 31, 2013.
26
As of December 31, 2013, an aggregate of 823,691 shares of the Company's preferred stock were issued and outstanding and are held by 4 shareholders. All preferred shares are convertible into the Company's common stock at a conversion ratio of 20 shares of common stock and 20 warrants for each preferred share held (see Warrants).
Dividends
The Company has not declared any dividends on its common stock since the Company's inception. There is no restriction in the Company's Articles of Incorporation and Bylaws that will limit its ability to pay dividends on its common stock. However, the Company does not anticipate declaring and paying dividends to its shareholders in the near future.
Dividends are payable semi-annually on the Company's preferred stock at a rate of 7% per annum. Dividends may be paid in kind, at the option of the Company, to the extent that if the Company is not legally permitted to distribute cash dividends, it shall pay dividends in the form of preferred shares equal to the amount of the dividend. No dividends have been declared on the Company's preferred stock.
Equity Compensation Plan Information
On June 30, 2010, the board of directors of the Company adopted the 2010 Employee Stock Option Plan (the "2010 Plan"). Under the 2010 Plan, 2,800,000 restricted shares of common stock have been reserved for issuance upon exercise of options granted from time to time under the stock option plan. The 2010 plan is intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. Under the 2010 Plan, the Company may grant incentive stock options only to key employees and employee directors, or the Company may grant non-qualified options to employees, officers, directors and consultants. Subject to the provisions of the 2010 Plan, the board of directors will determine who shall receive options, the number of shares of common stock that may be purchased under the options.
On October 1, 2010, the Company, under the 2010 Plan, granted the CEO of the Company qualified stock options to purchase 1,375,000 shares of the Company's common stock at $0.10 per share for a period of 10 years. The options, which vested quarterly over a period of 18 months, were valued at $137,500, and have been fully expensed. On November 30, 2010, the Company, under the 2010 Plan, granted two officers of the Company qualified stock options to purchase 300,000 shares of the Company's common stock at $0.25 per share for a period of 5 years. The options, which vested quarterly over a period of 18 months, were valued at $75,000, and have been fully expensed. On January 11, 2011, the Company, under the 2010 Plan, granted two consultants qualified stock options to purchase 225,000 shares of the Company's common stock at $0.25 per share for a period of 5 years. The options, which vest quarterly over a period of 18 months, were valued at $56,250, and have been fully expensed. On February 28, 2011, the Company, under the 2010 Plan, granted the former Chief Financial Officer of the Company qualified stock options to purchase 50,000 shares of the Company's common stock at $0.25 per share for a period of 5 years. The options, which vest quarterly over a period of 18 months, were valued at $12,500, and have been fully expensed, but were subsequently cancelled due to the holder's death in October 2012. In connection with the total options granted, $281,250 was recorded as deferred compensation and amortized over a 12 to18 month period.
On March 26, 2011, the Company adopted and approved the 2011 Equity Incentive Plan ("the 2011 Plan"), wherein twenty million (20,000,000) restricted shares of common stock were reserved for issuance. The 2011 Plan was intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. The 2011 Plan is currently administered by the Company's board of directors. Subject to the provisions of the plan, the board will determine who shall receive options, the number of shares of common stock that may be purchased under the options. Under the 2011 Plan, no options have been granted.
As of December 31, 2013, the Company has granted options to purchase a total of 1,900,000 shares. In connection with the options granted, a total of $281,250 was recorded as deferred compensation, which was fully expensed in prior years.
Warrants
Prior to the Parallax Merger, and in connection with the issuance of 230,000 shares of preferred stock, warrants were issued to purchase 4,600,000 shares of common stock. Of these warrants, 4,000,000 were issued at an exercise price of $1.00 per share, and 600,000 were issued at an exercise price of $1.50 per share. The number of shares of common stock underlying the warrants and the exercise price were subject to adjustment upon certain events.
As part of the Merger Agreement, the warrants were adjusted at an exchange ratio of 3.633926 warrants for each warrant. As a result, the 4,600,000 warrants were converted to 16,716,061 warrants. In addition, the $1.00 exercise price was adjusted to $0.27518 per share, and the $1.50 exercise price was adjusted to $0.41278 per share.
On April 1, 2013, in connection with a Notice to Convert, 12,112 shares of preferred stock were converted into common stock. As a result, the 242,660 underlying warrants were cancelled.
On June 17, 2013, 14,535,706 warrants underlying 726,786 shares of preferred stock, which were to expire on June 17, 2013, were extended for a period of 2 years. The warrants now expire on June 17, 2015.
On September 30, 2013, 726,785 warrants underlying 36,339 shares of preferred stock, which were to expire on September 30, 2013, were extended for a period of 2 years. The warrants now expire on September 30, 2015.
Table of Contents
27
On December 6, 2013, 726,785 warrants underlying 36,339 shares of preferred stock, which were to expire on December 6, 2013, were extended for a period of 2 years. The warrants now expire on December 6, 2015.
As of December 31, 2013, the Company had 16,473,401 warrants issued and outstanding.
Purchase of Equity Securities by the Issuer and Affiliated Purchasers
The Company did not purchase any shares of its common stock or other securities during the year ended December 31, 2013.
On January 7, 2014, 36,462,819 shares of the Company's common stock were purchased from certain shareholders by its Chairman, Edward W. Withrow III. On the same day, Mr. Withrow III cancelled the shares. As a result of this transaction, the total issued and outstanding shares of common stock was reduced from 162,765,837 shares to 126,303,018 shares.
Recent Sales of Unregistered Securities
The following represents all unregistered securities issued by the registrant during the current period, including sales of reacquired securities, as well as new issues, securities issued in exchange for property, services, or other securities, and new securities resulting from the modification of outstanding securities:
On December 31, 2013, pursuant to a Stock Purchase Agreement, the Company issued 11,459,279 shares of its common stock for cash in the amount of $1,146. As a result, additional paid in capital was reduced by $10,313.
Exemption From Registration . The shares of Common Stock referenced herein were issued in reliance upon an exemption from registration afforded either under Section 4(2) of the Securities Act for transactions by an issuer not involving a public offering, or Regulation D promulgated thereunder, or Regulation S for offers and sales of securities outside the U.S.
ITEM 6. | SELECTED FINANCIAL DATA |
As a "smaller reporting company", the Company is not required to provide the information required by this Item.
ITEM 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Forward-Looking Statements
This annual report contains forward-looking statements. These statements relate to future events or the Company's future financial performance. In some cases, forward-looking statements can be identified by terminology such as "may", "should", "expects", "plans", "anticipates", "believes", "estimates", "predicts", "potential" or "continue" or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors that may cause the Company's or the Company's industry's actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results..
The Company's consolidated audited financial statements are stated in United States Dollars and are prepared in accordance with United States Generally Accepted Accounting Principles.
Corporate History
The Company was incorporated in the State of Nevada on July 6, 2005, and began its development activities in the field of online website design and commerce. In September 2007, it merged with Endeavor Uranium, Inc. and began activities in the mineral exploration field, with mineral properties in the northwestern United States. Furthering the Company's development, on December 23, 2008, the Company entered into a Joint Venture Agreement with Federated Energy Corporation, a Tennessee corporation, for working interests in prospective oil and gas wells, and changed its operating name to Endeavor Power Corporation. The Company's development activities in oil and gas exploration continued until November, 2010.
In November 2010, management assessed a potential business opportunity and determined that in an effort to create value for its Shareholders, the Company should change its business direction. On November 8, 2010, the Company discontinued its operations in its working interests in oil and gas exploration and changed its operating focus to the development of E-Waste processing services aimed at industrial and government clients. The Company's new direction sought to limit the impact of discarded "E-Waste" on the environment, as discarded computers and electronic equipment pose environmental hazards. However, in accordance with a change in management effective September 27, 2011, the Company's business operations changed, and its activities in the e-waste were discontinued.
28
The Company began developing activities in managerial services, and pursued potential funding opportunities for the Company. It also retained consultants to perform due diligence on certain mining properties located in Venezuela, Brazil, Bolivia, Guyana and several other South American countries. Management, however, determined that the outcome of such due diligence did not provide the Company a viable opportunity and that it did not provide sufficient economic benefit for the Company. Management therefore ceased its due diligence, and continued its operations in managerial services whilst pursuing other viable business opportunities to increase shareholder value.
During 2012, the Company's management entered discussions and assessed a potential business opportunity with Parallax Diagnostics, Inc., a Nevada corporation ("Parallax'), whose principal business is in the bio-medical sector. More specifically, Parallax is focused on the exploitation of a proprietary diagnostic and monitoring platform and processes in the area of infectious disease. Parallax, in its development stage, holds the right, title, and interest to certain FDA 510(k) approved tests in perpetuity. In addition, Parallax acquired the exclusive license to a suite of medical devices, tests and utility processes in perpetuity.
On November 1, 2012, the Company, and its wholly owned subsidiary Endeavor Holdings, Inc. entered into an Agreement and Plan of Merger (the "Merger Agreement") with Parallax and the shareholders of Parallax. As a result of the transactions effected by the Merger Agreement, (i) Parallax merged with and into Endeavor Holdings whereupon Endeavor Holdings continued as the surviving entity and the corporate existence of Parallax ceased; (ii) the former business of Parallax is now the Company's primary business, (iii) the Company's existing business in managerial services will continue as ancillary operations, and (iv) there is a change of control whereby the former shareholders of Parallax now own a controlling 60% ownership interest in the Company on a fully diluted basis. On November 26, 2012, Parallax Diagnostic, Inc. changed its name to Endeavor Sciences, Inc. ("ESI"), a wholly owned subsidiary of Parallax Health Sciences, Inc., (formerly Endeavor Power Corp.)
On July 22, 2013, the Company entered into a binding Letter of Intent ("LOI") for the acquisition of a privately held California corporation, whose primary operations are in the pharmaceutical industry (the "NewCo"). Since completion of its due diligence, the Company has been working with financial institutions to secure a combined debt-equity financing that will enable the Company to complete the acquisition. There can, however, be no guarantee that satisfactory financing arrangements will be secured to complete the acquisition.
The Company's management and the NewCo management have completed negotiations, the terms of which are confidential and will be disclosed upon completion of the acquisition. On March 28, 2014, the Company's board of directors issued a board resolution authorizing the acquisition.
On January 9, 2014, the Company changed its name from Endeavor Power Corp. (OTCQB.EDVP) to Parallax Health Sciences, Inc. (OTCQB.PRLX)
The Company is a development stage company as defined by ASC 915-10, "Accounting and Reporting by Development Stage Enterprises". A development stage enterprise is one in which planned principal operations have not commenced or if its operations have commenced, there has been no significant revenues there from. At December 31, 2013, the Company has not yet commenced its principal operations, which is in the bio-medical and diagnostics sector.
Results of Operations
The following summary of the Company's results of operations should be read in conjunction with the Company's audited consolidated financial statements for the years ended December 31, 2013 and 2012, which are included herein. The financial information provided includes the accounts of the Company and its wholly owned subsidiary, Endeavor Sciences, Inc., formerly Parallax Diagnostics, Inc. ("ESI"), on a consolidated basis. All significant inter-company accounts and transactions have been eliminated.
As a result of the Share Exchange, a change in control occurred whereby the Company is the legal parent/accounting subsidiary of ESI, and ESI is the legal subsidiary/accounting parent of the Company. The Share Exchange has therefore been identified and recorded as a reverse acquisition and, pursuant to ASC 805-40-45-1, the historical and cumulative financial information are a continuation of the financial information of the legal subsidiary, ESI. For cumulative purposes, the date of inception is that of ESI, December 30, 2008.
|
|
| Cumulative from |
| |||||
|
|
| December 30, 2008 |
| |||||
| For the year ended |
| (inception) to |
| |||||
| December 31, 2013 |
| December 31, 2012 |
| December 31, 2013 |
| |||
Revenue | $ | – |
| $ | – |
| $ | – |
|
Cost of Sales | $ | – |
| $ | – |
| $ | – |
|
Gross profit (loss) | $ | – |
| $ | – |
| $ | – |
|
General and administrative expenses | $ | 473,403 |
| $ | 482,568 |
| $ | 1,539,112 |
|
Operating (loss) | $ | (473,403 | ) | $ | (482,568 | ) | $ | (1,539,112 | ) |
Insurance claim refund | $ | 1,695 |
| $ | – |
| $ | 1,695 |
|
Depreciation and amortization | $ | (12,232 | ) | $ | (18,550 | ) | $ | (43,145 | ) |
Amortization of deferred compensation | $ | – |
| $ | (42,370 | ) | $ | (281,250 | ) |
Interest expense | $ | (20,554 | ) | $ | (11,180 | ) | $ | (31,734 | ) |
Net (loss) | $ | (504,494 | ) | $ | (554,668 | ) | $ | (1,893,546 | ) |
Table of Contents
29
Revenue
As a development stage company, the Company has not yet launched its major business activity, which is medical diagnostics and testing.
Cost of sales
As a development stage company, the Company has not yet launched its major business activity, which is medical diagnostics and testing.
General and Administrative Expenses
| For the year ended |
|
|
|
| ||||
| December 31, 2013 |
| December 31, 2012 |
| Variances |
| |||
Legal, accounting, and management services | $ | 217,813 |
| $ | 191,505 |
| $ | 26,308 |
|
Salaries, taxes and benefits |
| 246,573 |
|
| 236,724 |
|
| 9,849 |
|
Travel, meals and entertainment |
| – |
|
| 22,360 |
|
| (22,360 | ) |
Office supplies and miscellaneous expenses |
| 9,017 |
|
| 31,979 |
|
| (22,962 | ) |
Total general and administrative expenses | $ | 473,403 |
| $ | 482,568 |
| $ | (9,165 | ) |
General and administrative expenses in the amount of $473,403 for the year ended December 31, 2013, were comprised of $217,813 of legal and accounting fees, $246,573 of salaries and related taxes and benefits, and $9,017 of office, overhead and other general and administrative expenses.
General and administrative expenses in the amount of $482,568 for the year ended December 31, 2012, were comprised of $191,505 of legal and accounting fees, $236,724 of salaries and related taxes and benefits, $22,360 of travel, meals and entertainment $31,979 of office overhead and other general and administrative expenses.
General and administrative expenses for the year ended December 31, 2013 were $ 473,403 as compared to $ 482,568 for the year ended December 31, 2012, which resulted in a decrease in general and administrative expenses for the current period of $ 9,165 .
Significant changes in general and administrative expenses of $9,165 for continuing operations during the years 2013 compared to 2012 were attributable to the following items
·
an increase in legal, accounting and consulting services of $26,308, due to a increase in legal costs of $20,012 resulting from a change in legal counsel; and an increase in accounting fees of $6,296 resulting from a change in accounting department staffing;
·
an increase in management salaries and fees, and related taxes and benefits of $9,849, due to an increase of $8,654 in salaries resulting from a contractual increase in the base salary for the Company's CEO; and an increase in related taxes and benefits of $4,425;
·
a decrease in travel, meals and entertainment of $22,360 resulting from no expenses incurred in the current year versus $17,334 in travel and $5,026 in meals and entertainment incurred in 2012; and
·
a decrease in office supplies and miscellaneous expenses of $22,962, due to a decrease in filing fees of $14,993 resulting from a change in firms; a decrease in escrow account fees due to funding received in escrow in 2012 resulting in an escrow fee of $3,500 compared to none in the current year; an increase in publicity and promotion of $2,560 resulting from press releases costs incurred in 2013, compared to none in the prior year; and a decrease in general office expenses of $7,029.
General and administrative expenses for both 2013 and 2012 were incurred primarily for the purpose of advancing the Company closer to its goal of financing and operating an environment-friendly car rental business.
Net Loss
During the year ended December 31, 2013, the Company incurred a net loss of $504,494 compared with a net loss of $554,668 for the year ended December 31, 2012. The decrease in net loss of $50,174 is attributable to an increase in miscellaneous income of $1,695; a decrease in stock option amortization of $42,370 resulting from stock options fully amortized in 2012; a decrease in general and administrative expenses of $9,165; a decrease in depreciation expense of $6,318; and an increase in interest expense of $9,374.
30
Liquidity and Capital Resources
Working Capital | At December 31, |
| Increase |
| |||||
| 2013 |
| 2012 |
| (Decrease) |
| |||
Current assets | $ | 569 |
| $ | 9,397 |
| $ | (8,828 | ) |
Current liabilities |
| 1,179,821 |
|
| 713,987 |
|
| 465,834 |
|
Working capital (deficit) | $ | (1,179,252 | ) | $ | (704,590 | ) | $ | (474,662 | ) |
|
|
|
|
|
|
|
|
|
|
As at December 31, 2013, the Company had cash in the amount of $569 compared to $9,397 as of December 31, 2012.
The Company had a working capital deficit of $1,179,252 as of December 31, 2013, compared to a working capital deficit of $704,590 at December 31, 2012. The increase in working capital deficit of $474,662 is primarily attributable to a decrease in cash of $8,828; an increase in accounts payable and accrued expenses of $57,293; and an increase in related party payable of $408,541.
Cash Flows | For the year ended |
| Increase |
| |||||
| December 31, 2013 |
| December 31, 2012 |
| (Decrease) |
| |||
Net cash used in operating activities | $ | (26,428 | ) | $ | (238,983 | ) | $ | 212,555 |
|
Net cash used in investing activities |
| – |
|
| (2,186 | ) |
| 2,186 |
|
Net cash provided by financing activities |
| 17,600 |
|
| 114,500 |
|
| (96,900 | ) |
Increase (decrease) in cash | $ | (8,828 | ) | $ | (126,669 | ) | $ | 117,841 |
|
Cash Flows from Operating Activities
During the year ended December 31, 2013, the Company used $26,428 of cash flow for operating activities, compared with $238,983 for the year ended December 31, 2012. The decrease in cash used for operating activities of $212,555 is primarily attributable to a reduction in the net loss from operations of $50,174, a decrease in depreciation and amortization of $6,318, a decrease in stock compensation of $5,000, a decrease in stock option amortization of $42,370, an increase in accounts payable and accrued expenses of $58,970 and an increase in related party payables of $157,099.
Cash Flows from Investing Activities
During the year ended December 31, 2013 the Company used $0 of cash flow for investing activities, compared with $2,186 for the year ended December 31, 2012. The decrease in cash used for investing activities of $2,186 is attributable to the decrease in the purchase of equipment.
Cash Flows from Financing Activities
During the year ended December 31, 2013, the Company was provided with $17,600 of cash flow from financing activities compared with $114,500 during the year ended December 31, 2012. The decrease in cash flows provided from financing activities of $96,900 is attributable to an increase in related party loans of $3,100, and a decrease in the proceeds from the sale of preferred stock of $100,000.
As at December 31, 2013 and 2012, respectively, related parties are due a total of $921,693 and $513,152, consisting of $795,127 and $389,961 in accrued compensation, and $126,566 and $123,191 in cash advances to the Company for operating expenses. The amounts owing are unsecured, are non-interest bearing, and due upon demand. As at December 31, 2013 and 2012, the principal balance owed for related party notes payable is $190,100 and $172,500, respectively, and a total of $22,994 and $10,080, respectively, of interest has been accrued.
The Company's principal sources of funds have been from sales of the Company's common stock and loans from related parties.
Future Financings
The Company has suffered recurring losses from operations. The continuation of the Company's operations is dependent upon the Company's attaining and maintaining profitable operations and raising additional capital as needed. The Company anticipates that it will have to raise additional funds through private placements of the Company's equity securities and/or debt financing to complete its business plan.
The Company will require additional financing in order to proceed with its plan of operations, including approximately $2,000,000 over the next 12 months to pay for its ongoing expenses. These cash requirements include working capital, general and administrative expenses, the development of the Company's product line, and the pursuit of acquisitions. These cash requirements are in excess of the Company's current cash and working capital resources. Accordingly, the Company will require additional financing in order to continue operations and to repay its liabilities. There is no assurance that the financing will be completed as planned or at all. If the Company is unable to secure adequate capital to continue the Company's planned operations, the Company's shareholders may lose some or all of their investment and the Company's business may fail.
Table of Contents
31
The Company anticipates continuing to rely on equity sales of its common stock in order to continue to fund its business operations. Issuances of additional shares will result in dilution to the Company's existing stockholders. There is no assurance that the Company will achieve any additional sales of its equity securities or arrange for debt or other financing to fund its planned business activities.
Personnel
As of December 31, 2013, the Company had no employees, excluding its directors and executive officers. Currently, the Company has no employees, excluding its directors and executive officers.
Contractual Obligations
As a "smaller reporting company", the Company is not required to provide tabular disclosure obligations.
Going Concern
The Company has incurred losses since inception resulting in an accumulated deficit of $1,893,546, and further losses are anticipated in the development of its business. The Company's ability to continue as a going concern is dependent upon its ability to generate profitable operations in the future and/or to obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due, which may not be available at commercially reasonable terms There can be no assurance that the Company will be able to continue to raise funds, in which case the Company may be unable to meet its obligations and the Company may cease operations. These factors, among others, raise substantial doubt about the Company's ability to continue as a going concern.
The consolidated audited financial statements included with this annual report have been prepared on the going concern basis which assumes that adequate sources of financing will be obtained as required and that the Company's assets will be realized and liabilities settled in the ordinary course of business. Accordingly, the consolidated audited financial statements do not include any adjustments related to the recoverability of assets and classification of assets and liabilities that might be necessary should the Company be unable to continue as a going concern.
Off-Balance Sheet Arrangements
The Company has no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on the Company's financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Critical Accounting Policies
The discussion and analysis of the Company's financial condition and results of operations are based upon the Company's consolidated audited financial statements, which have been prepared in accordance with the accounting principles generally accepted in the United States of America. Preparing financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. These estimates and assumptions are affected by management's application of accounting policies. The Company believes that understanding the basis and nature of the estimates and assumptions involved with the following aspects of the Company's financial statements is critical to an understanding of its consolidated financial statements.
Net Income (Loss) Per Share
The Company computes earnings per share under Accounting Standards Codification subtopic 260-10, Earnings Per Share ("ASC 260-10"). Net earnings (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and dilutive common stock equivalents outstanding during the period. Dilutive common stock equivalents consist of shares issuable upon conversion of convertible preferred shares and the exercise of the Company's stock options and warrants.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States and requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company's estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will be affected.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary, Endeavor Sciences, Inc., formerly Parallax Diagnostics, Inc. ("ESI"). All significant inter-company accounts and transactions have been eliminated.
32
In November 2012, the Company acquired 100% of ESI's common stock in exchange for, among other things, approximately 60% of the Company's common stock (the "Share Exchange"). As a result of the Share Exchange, a change in control occurred whereby the Company is the legal parent/accounting subsidiary of ESI, and ESI is the legal subsidiary/accounting parent of the Company. The Share Exchange has therefore been identified and recorded as a reverse acquisition and, pursuant to ASC 805-40-45-1, the consolidated financial statements' historical and cumulative data are a continuation of the financial statements of the legal subsidiary, ESI. For cumulative purposes, the date of inception is that of ESI, December 30, 2008.
Revenue Recognition
The Company recognizes revenue in accordance with ASC 605, Revenue Recognition . Revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service has been provided, and collectability is reasonably assured.
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, Share-Based Payments , using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued.
Recently Adopted Accounting Standards :
The Company evaluates the pronouncements of various authoritative accounting organizations, primarily the Financial Accounting Standards Board ("FASB"), the US Securities and Exchange Commission ("SEC"), and the Emerging Issues Task Force ("EITF"), to determine the impact of new pronouncements on US GAAP and the impact on the Company. The Company has adopted the following new accounting standards during 2012:
Trouble Debt Restructuring: Issued in April, 2011, ASU 2011-02 clarifies which loan modifications constitute troubled debt restructurings. It is intended to assist creditors in determining whether a modification of the terms of a receivable meets the criteria to be considered a troubled debt restructuring, both for purposes of recording an impairment loss and for disclosure of troubled debt restructurings. The new guidance is effective for interim and annual periods beginning on or after June 15, 2011, and applies retrospectively to restructurings occurring on or after the beginning of the fiscal year of adoption. Early adoption is permitted.
Comprehensive Income : Issued in June, 2011, ASU 2011-05 eliminates the current option to present other comprehensive income and its components in the statement of changes in equity. It will require companies to report the total of comprehensive income including the components of net income and the components of other comprehensive income in either a single continuous statement of comprehensive income or in two separate but consecutive statements. The amendments in the ASU are effective for interim and annual periods beginning on or after December 15, 2011, and should be applied retrospectively. Early adoption is permitted.
Intangibles: Issued in September, 2011, ASU 2011-08 permits entities to first perform a qualitative assessment to determine whether it is more likely than not (a likelihood of more than 50 percent) that the fair value of a reporting unit is less than its carrying amount. If the entity determines that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, it would then perform the first step of the goodwill impairment test; otherwise, no further impairment test would be required. The amendments will be effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. Early adoption is permitted.
Disclosures about Offsetting Assets and Liabilities : Issued in December, 2011, ASU 2011-11 requires disclosures to provide information to help reconcile differences in the offsetting requirements under U.S. GAAP and IFRS. The differences in the offsetting requirements account for a significant difference in the amounts presented in statements of financial position prepared in accordance with U.S. GAAP and IFRS for certain entities. An entity is required to apply the amendments for annual reporting periods beginning on or after January 1, 2013, and interim periods within those annual periods.
Impairment Testing-Intangibles: Issued in July 2012, ASU 2012-02 reduces the cost and complexity of performing an impairment test for indefinite-lived intangible assets by simplifying how an entity tests those assets for impairment, and improves consistency in impairment testing guidance among long-lived asset categories. The amendments will be effective for annual and interim impairment tests performed for fiscal years beginning after September 15, 2012. Early adoption is permitted.
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
As a "smaller reporting company", the Company is not required to provide the information required by this Item.
Table of Contents
33
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The Company's consolidated audited financial statements are stated in United States dollars (US$) and are prepared in accordance with United States Generally Accepted Accounting Principles.
The following consolidated audited financial statements are filed as part of this annual report:
Report of Independent Registered Public Accounting Firm | F-1 |
|
|
Consolidated Balance Sheets as at December 31, 2013 and 2012 | F-2 |
|
|
Consolidated Statements of Operations for the years ended December 31, 2013 and 2012, and for the period from inception (December 30, 2008) to December 31, 2013 | F-3 |
|
|
Consolidated Statements of Changes in Stockholders' Deficit for the period from inception (December 30, 2008 ) to December 31, 2013 | F -4 |
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2013 and 2012, and from inception (December 30, 2008) to December 31, 2013 | F-5 |
|
|
Notes to the Consolidated Financial Statements for the years ended December 31, 2013 and 2012. | F-6 |
34
SEALE AND BEERS, CPAs
PCAOB & CPAB REGISTERED AUDITORS
www.sealebeers.com
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Parallax Health Sciences, Inc.
(A Development Stage Company)
We have audited the accompanying balance sheets of Parallax Health Sciences, Inc. (A Development Stage Company) as of December 31, 2013 and 2012, and the related statements of income, stockholders' equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2013 and since inception on December 30, 2008 through December 31, 2013. Parallax Health Sciences, Inc.'s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Parallax Health Sciences, Inc. (A Development Stage Company) as of December 31, 2013, and 2012, and the related statements of income, stockholders' equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2013, and since inception on December 30, 2008 through December 31, 2013, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has no revenues, has negative working capital at December 31, 2013, has incurred recurring losses and recurring negative cash flow from operating activities, and has an accumulated deficit which raises substantial doubt about its ability to continue as a going concern. Management's plans concerning these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Seale and Beers, CPAs
Seale and Beers, CPAs
Las Vegas, Nevada
April 11, 2014
50 S. Jones Blvd, Suite 201 - Las Vegas, NV 89107 Phone: (888)727-8251 Fax: (888)782-2351
Table of Contents
F-1
PARALLAX HEALTH SCIENCES, INC. | ||||||
(formerly to Endeavor Power Corp.) | ||||||
(A DEVELOPMENT STAGE COMPANY) | ||||||
CONSOLIDATED BALANCE SHEETS | ||||||
|
|
|
|
|
|
|
| December 31, 2013 |
| December 31, 2012 |
| ||
ASSETS |
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
Cash and cash equivalents | $ | 569 |
| $ | 9,397 |
|
Total current assets |
| 569 |
|
| 9,397 |
|
|
|
|
|
|
|
|
Property and equipment, net |
| 15,851 |
|
| 26,417 |
|
|
|
|
|
|
|
|
Intangible assets, net |
| 19,584 |
|
| 21,250 |
|
|
|
|
|
|
|
|
TOTAL ASSETS | $ | 36,004 |
| $ | 57,064 |
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT |
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
Accounts payable and accrued expenses | $ | 174,053 |
| $ | 116,760 |
|
Notes and loans payable |
| 84,075 |
|
| 84,075 |
|
Related party payables |
| 921,693 |
|
| 513,152 |
|
Total current liabilities |
| 1,179,821 |
|
| 713,987 |
|
|
|
|
|
|
|
|
Related party loans |
| 190,100 |
|
| 172,500 |
|
Total liabilities |
| 1,369,921 |
|
| 886,487 |
|
|
|
|
|
|
|
|
Stockholders' deficit |
|
|
|
|
|
|
Preferred stock, $.001 par, 10,000,000 shares authorized, 823,691 and 835,803 issued and outstanding at December 31, 2013 and 2012, respectively |
| 824 |
|
| 836 |
|
Common stock: 250,000,000 shares authorized, $.001 par, 162,765,837 and 151,063,898 issued and outstanding as of December 31, 2013 and 2012, respectively |
| 162,766 |
|
| 151,064 |
|
Additional paid in capital-preferred |
| 465,843 |
|
| 499,164 |
|
Additional paid in capital-common |
| (68,658 | ) |
| (91,435 | ) |
Subscriptions receivable |
| (1,146 | ) |
| – |
|
Accumulated deficit |
| (1,893,546 | ) |
| (1,389,052 | ) |
Total stockholders' deficit |
| (1,333,917 | ) |
| (829,423 | ) |
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS' (DEFICIT) | $ | 36,004 |
| $ | 57,064 |
|
The accompanying notes are an integral part of these financial statements
Table of Contents
F-2
PARALLAX HEALTH SCIENCES, INC. | |||||||||
(formerly to Endeavor Power Corp.) | |||||||||
(A DEVELOPMENT STAGE COMPANY) | |||||||||
CONSOLIDATED STATEMENTS OF OPERATIONS | |||||||||
| |||||||||
|
|
|
|
|
|
| Cumulative from |
| |
|
|
|
|
|
|
| December 30, 2008 |
| |
| For the year ended |
| (inception) to |
| |||||
| December 31, 2013 |
| December 31, 2012 |
| December 31, 2013 |
| |||
|
|
|
|
|
|
|
|
|
|
Revenue | $ | – |
| $ | – |
| $ | – |
|
Cost of sales |
| – |
|
| – |
|
| – |
|
Gross Profit |
| – |
|
| – |
|
| – |
|
|
|
|
|
|
|
|
|
|
|
General and administrative expenses |
| 473,403 |
|
| 482,568 |
|
| 1,539,112 |
|
|
|
|
|
|
|
|
|
|
|
Operating (loss) |
| (473,403 | ) |
| (482,568 | ) |
| (1,539,112 | ) |
|
|
|
|
|
|
|
|
|
|
Other income (expenses) |
|
|
|
|
|
|
|
|
|
Insurance claim refund |
| 1,695 |
|
| – |
|
| 1,695 |
|
Depreciation and amortization |
| (12,232 | ) |
| (18,550 | ) |
| (43,145 | ) |
Amortization of deferred compensation |
| – |
|
| (42,370 | ) |
| (281,250 | ) |
Interest expense |
| (20,554 | ) |
| (11,180 | ) |
| (31,734 | ) |
Total other income (expenses) |
| (31,091 | ) |
| (72,100 | ) |
| (354,434 | ) |
|
|
|
|
|
|
|
|
|
|
Net (loss) | $ | (504,494 | ) | $ | (554,668 | ) | $ | (1,893,546 | ) |
|
|
|
|
|
|
|
|
|
|
Net (loss) per common share - basic and diluted | $ | (0.003 | ) | $ | (0.005 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding - basic and diluted |
| 150,699,093 |
|
| 114,600,585 |
|
|
|
|
The accompanying notes are an integral part of these financial statements
Table of Contents
F-3
PARALLAX HEALTH SCIENCES, INC. | ||||||||||||||||||||||||||||
(formerly Endeavor Power Corp.) | ||||||||||||||||||||||||||||
(A DEVELOPMENT STAGE COMPANY) | ||||||||||||||||||||||||||||
STATEMENT OF STOCKHOLDERS' (DEFICIT) | ||||||||||||||||||||||||||||
PERIOD FROM DECEMBER 30, 2008 (INCEPTION) TO DECEMBER 31, 2013 | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (DEFICIT) |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ACCUMULATED |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DURING THE |
|
|
|
| |
| PREFERRED STOCK |
| COMMON STOCK |
| PAID IN CAPITAL |
| SUBSCRIPTIONS |
| DEFERRED |
| EXPLORATION |
|
|
|
| |||||||||||||
| SHARES |
| AMOUNT |
| SHARES |
| AMOUNT |
| PREFERRED |
| COMMON |
| RECEIVABLE |
| COMP |
| STAGE |
| TOTAL |
| ||||||||
Balance, December 30, 2008 (date of inception) | – |
| $ | – |
| – |
| $ | – |
| $ | – |
| $ | – |
| $ | – |
| $ | – |
| $ | – |
| $ | – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock to founders @ .0001, December 2008 |
|
|
|
|
| 1,500 |
|
|
|
|
|
|
|
| 50 |
|
| (50 | ) |
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (411 | ) |
| (411 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2008 | – |
|
| – |
| 1,500 |
|
| – |
|
| – |
|
| 50 |
|
| (50 | ) |
| – |
|
| (411 | ) |
| (411 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subscriptions received |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 50 |
|
|
|
|
|
|
|
| 50 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (132,050 | ) |
| (132,050 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2009 | – |
|
| – |
| 1,500 |
|
| – |
|
| – |
|
| 50 |
|
| – |
|
| – |
|
| (132,461 | ) |
| (132,411 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Surrender of stock to treasury |
|
|
|
|
| (1,500 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock pursuant to Assignment Agreement @ $.001, September, 2010 |
|
|
|
|
| 750 |
|
|
|
|
|
|
|
| 12,500 |
|
|
|
|
|
|
|
|
|
|
| 12,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock pursuant to License Agreement @ $.001, September, 2010 |
|
|
|
|
| 750 |
|
|
|
|
|
|
|
| 12,500 |
|
|
|
|
|
|
|
|
|
|
| 12,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock Split – 1,500 shares @ 19,999 |
|
|
|
|
| 29,998,500 |
|
| 3,000 |
|
|
|
|
| (3,000 | ) |
|
|
|
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recapitalization due to merger December 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 29,222 |
|
|
|
|
|
|
|
|
|
|
| 29,222 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock to officer for cash |
|
|
|
|
| 125,000 |
|
| 13 |
|
|
|
|
| 112 |
|
| (125 | ) |
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
| (41,640 | ) |
| (41,640 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2010 | – |
|
| – |
| 30,125,000 |
|
| 3,013 |
|
| – |
|
| 51,384 |
|
| (125 | ) |
| – |
|
| (174,101 | ) |
| (119,829 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recapitalization due to merger, January 2011 |
|
|
|
|
| (2,000,000 | ) |
| (200 | ) |
|
|
|
| (53,274 | ) |
|
|
|
|
|
|
|
|
|
| (53,474 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 281,250 |
|
|
|
|
| (182,985 | ) |
|
|
|
| 98,265 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 140,615 |
|
|
|
|
| 140,615 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of preferred stock | 220,000 |
|
| 22 |
|
|
|
|
|
|
| 399,978 |
|
|
|
|
| (50,000 | ) |
|
|
|
|
|
|
| 350,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Subscriptions received |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 50,125 |
|
|
|
|
|
|
|
| 50,125 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of stock as settlement of debt |
|
|
|
|
| 20,000 |
|
| 2 |
|
|
|
|
| 19,998 |
|
|
|
|
|
|
|
|
|
|
| 20,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (660,283 | ) |
| (660,283 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2011 | 220,000 |
|
| 22 |
| 28,145,000 |
|
| 2,815 |
|
| 399,978 |
|
| 299,358 |
|
| – |
|
| (42,370 | ) |
| (834,384 | ) |
| (174,581 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 42,370 |
|
|
|
|
| 42,370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of preferred stock | 10,000 |
|
| 1 |
|
|
|
|
|
|
| 99,999 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 100,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for services |
|
|
|
|
| 75,000 |
|
| 8 |
|
|
|
|
| 4,992 |
|
|
|
|
|
|
|
|
|
|
| 5,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancellation of shares |
|
|
|
|
| (3,350,000 | ) |
| (335 | ) |
|
|
|
| 335 |
|
|
|
|
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cancellation of related party debt |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 42,500 |
|
|
|
|
|
|
|
|
|
|
| 42,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recapitalization upon merger | 605,803 |
|
| 813 |
| 126,193,898 |
|
| 148,576 |
|
| (813 | ) |
| (438,620 | ) |
|
|
|
|
|
|
|
|
|
| (290,044 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (554,668 | ) |
| (554,668 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2012 | 835,803 |
|
| 836 |
| 151,063,898 |
|
| 151,064 |
|
| 499,164 |
|
| (91,435 | ) |
| – |
|
| – |
|
| (1,389,052 | ) |
| (829,423 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Conversion of preferred stock to common stock | (12,112 | ) |
| (12 | ) | 242,660 |
|
| 243 |
|
| (33,321 | ) |
| 33,090 |
|
|
|
|
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of common stock for cash |
|
|
|
|
| 11,459,279 |
|
| 11,459 |
|
|
|
|
| (10,313 | ) |
| (1,146 | ) |
|
|
|
|
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (504,494 | ) |
| (504,494 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2013 | 823,691 |
| $ | 824 |
| 162,765,837 |
| $ | 162,766 |
| $ | 465,843 |
| $ | (68,658 | ) | $ | (1,146 | ) | $ | – |
| $ | (1,893,546 | ) | $ | (1,333,917 | ) |
The accompanying notes are an integral part of these financial statements
Table of Contents
F-4
PARALLAX HEALTH SCIENCES, INC. | |||||||||
(formerly to Endeavor Power Corp.) | |||||||||
(A DEVELOPMENT STAGE COMPANY) | |||||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS | |||||||||
|
| ||||||||
|
| Cumulative from |
| ||||||
|
|
|
|
|
|
| December 30, 2008 |
| |
| For the twelve months ended |
| (Inception) to |
| |||||
| December 31, 2013 |
| December 31, 2012 |
| December 31, 2013 |
| |||
Cash flows from operations: |
|
|
|
|
|
|
|
|
|
Net (loss) | $ | (504,494 | ) | $ | (554,668 | ) | $ | (1,893,546 | ) |
Adjustments to reconcile net (loss) to net cash (used in) operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation expense |
| 10,566 |
|
| 16,883 |
|
| 37,728 |
|
Amortization expense |
| 1,666 |
|
| 1,667 |
|
| 5,417 |
|
Stock compensation |
| – |
|
| 5,000 |
|
| 5,000 |
|
Stock options / amortization of stock options |
| – |
|
| 42,370 |
|
| 281,250 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
| – |
|
Increase in accounts payable and accrued expenses |
| 57,293 |
|
| (1,677 | ) |
| 257,170 |
|
Increase in related party payables |
| 408,541 |
|
| 251,442 |
|
| 812,754 |
|
Net cash (used in) operating activities |
| (26,428 | ) |
| (238,983 | ) |
| (494,227 | ) |
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Cash (used in) purchase of property and equipment |
| – |
|
| (2,186 | ) |
| (53,579 | ) |
Net cash (used in) investing activities |
| – |
|
| (2,186 | ) |
| (53,579 | ) |
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
Proceeds from related party loans |
| 17,600 |
|
| 14,500 |
|
| 46,100 |
|
Repayment of related party loans |
| – |
|
| – |
|
| (1,450 | ) |
Proceeds from issuance of preferred shares |
| – |
|
| 100,000 |
|
| 500,000 |
|
Cash received upon merger |
| – |
|
| – |
|
| 3,600 |
|
Subscription payment |
| – |
|
| – |
|
| 125 |
|
Net cash provided by financing activities |
| 17,600 |
|
| 114,500 |
|
| 548,375 |
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash |
| (8,828 | ) |
| (126,669 | ) |
| 569 |
|
|
|
|
|
|
|
|
|
|
|
Cash – beginning of period |
| 9,397 |
|
| 136,066 |
|
| – |
|
|
|
|
|
|
|
|
|
|
|
Cash – end of period | $ | 569 |
| $ | 9,397 |
| $ | 569 |
|
|
|
|
|
|
|
|
|
|
|
NON–CASH ACTIVITIES |
|
|
|
|
|
|
|
|
|
Recapitalization of equity upon merger | $ | – |
| $ | 290,044 |
| $ | 265,793 |
|
Conversion of accounts payable to notes payable | $ | – |
| $ | (144,000 | ) | $ | – |
|
Conversion of debt into common stock | $ | – |
| $ | – |
| $ | 20,000 |
|
Conversion of preferred stock into common stock | $ | 33,333 |
| $ | – |
| $ | 33,333 |
|
Assignment of note payable to related party note | $ | – |
| $ | 144,000 |
| $ | 144,000 |
|
Cancellation of related party debt | $ | – |
| $ | 43,500 |
| $ | 43,500 |
|
Subscriptions receivable | $ | (1,146 | ) | $ | – |
| $ | (1,271 | ) |
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL INFORMATION |
|
|
|
|
|
|
|
|
|
Interest paid | $ | – |
| $ | – |
| $ | – |
|
Income taxes paid | $ | – |
| $ | – |
| $ | – |
|
The accompanying notes are an integral part of these financial statements
Table of Contents
F-6
PARALLAX HEALTH SCIENCES, INC.
(formerly Endeavor Power Corp.)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2013
NOTE 1. OVERVIEW AND NATURE OF BUSINESS
The accompanying audited consolidated financial statements of Parallax Health Sciences, Inc., (formerly Endeavor Power Corp.) (the "Company") have been prepared in accordance with generally accepted accounting principles. The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and that effect the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates .
The Company is a development stage company as defined by ASC 915-10, "Accounting and Reporting by Development Stage Enterprises". A development stage enterprise is one in which planned principal operations have not commenced or if its operations have commenced, there has been no significant revenues there from. At December 31, 2013, the Company has not yet commenced operations. All activity from December 30, 2008 (date of inception) through December 31, 2013, relates to the Company's formation and ongoing development.
Parallax Health Sciences, Inc. was incorporated in the State of Nevada on July 6, 2005 under the name VB Biotech Laboratories, Inc. On September 21, 2007, the Company filed a Certificate of Amendment with the State of Nevada to change its operating name to VB Trade, Inc., with principal business operations to develop an online website that allowed web designers to sell their website designs in exchange for a commission on all products that were sold through the website. On September 21, 2007, the Company entered into a Plan of Merger (the "Merger") with Endeavor Uranium, Inc., a mineral exploration company with mineral properties in the northwestern United States. Effectively, the Company changed its name to Endeavor Uranium, Inc. as part of the Merger transaction. On December 23, 2008, the Company entered into a Joint Venture Agreement (the "Agreement") with Federated Energy Corporation, a Tennessee corporation, for working interests in prospective oil and gas wells located in Nowata County, Oklahoma. Effectively, on December 23, 2008, the Company changed its operating name to Endeavor Power Corporation.
In November 2010, Management assessed a potential business opportunity and determined that in an effort to create value for its Shareholders, the Company should change its business direction. On November 8, 2010, the Company discontinued its operations in its working interests in oil and gas exploration and changed its operating focus to the development of E-Waste processing services aimed at industrial and government clients. The Company's new direction sought to limit the impact of discarded "E-Waste" on the environment, as discarded computers and electronic equipment pose environmental hazards.
On May 26, 2011, Mr. Alfonso Knoll resigned from all positions with the Company, including but not limited to, that of President, Chief Executive Officer, Chief Financial Officer, Treasurer and Secretary. The resignation did not involve any disagreement with the Company. On June 8, 2011, the Company entered into a Settlement Agreement and General Mutual Release ("Settlement Agreement") to terminate Mr. Knoll's Employment Agreement dated November 8, 2010, and to accept his resignation. Pursuant to the Settlement Agreement, Mr. Knoll immediately ceased all services to the Company and, on June 11, 2011, returned to the Company any and all shares of its common stock currently held by him.
On June 2, 2011, Mr. Matthew Carley was appointed as the Company's President, Chief Executive Officer, Chief Financial Officer, Treasurer, Secretary and Director. Mr. Carley accepted the appointment, but effectively resigned his positions on September 27, 2011. The Company's Board of Directors accepted the resignation of Mr. Carley, as well as the resignation of Mr. Keith Kress as a member of the Board of Directors. Simultaneously, the Board of Directors appointed Tom Mackay as the President/Chief Executive Officer, Secretary, Treasurer/Chief Financial Officer and the sole member of the Board of Directors.
In accordance with a change in management effective September 27, 2011, the Company's business operations changed. The Company intended to change its business focus to provide managerial services, and pursue potential funding opportunities for the Company. It also retained consultants to perform due diligence on certain mining properties located in Venezuela, Brazil, Bolivia, Guyana and several other South American countries. Management, however, determined that the outcome of such due diligence did not provide the Company a viable opportunity, nor did it provide sufficient economic benefit for the Company. Management therefore pursued other viable opportunities to increase shareholder market value.
During 2012, the Company's management entered discussions and assessed a potential business opportunity with Parallax Diagnostics, Inc., a Nevada corporation ("Parallax'), whose principal line of business is in the bio-medical sector. More specifically, Parallax is focused on the exploitation of a proprietary diagnostic and monitoring platform and processes in the area of infectious disease.
On August 15, 2012, the Company entered into a non-binding Letter of Intent ("LOI") with Parallax, that outlined the terms and conditions for a proposed merger of the companies as understood by their respective boards. The terms of the LOI included, but were not limited to, an exchange of common stock, and a replacement of management.
Table of Contents
F-7
On November 1, 2012, the Company, and its wholly owned subsidiary Endeavor Holdings, Inc. ("Endeavor Holdings") entered into an Agreement and Plan of Merger (the "Merger Agreement") with Parallax and the shareholders of Parallax (the "Parallax Shareholders"), whereby Endeavor Holdings acquired 100% (one hundred percent), or 24,870,000 shares, of Parallax common stock (the "Parallax Stock") from the Parallax Shareholders (the "Parallax Merger"). In exchange for the Parallax Stock, the Company issued 90,375,750 shares of its common stock to the Parallax Shareholders. The 90,375,750 shares, issued at par value $.0001, represent approximately 60% of the Company's total issued and outstanding shares. The Common Stock Purchase Agreement, and subsequent transaction closing, was completed on October 22, 2012. On October 27, 2012, the Common Stock Purchase Agreement was finalized, and a change in control took place.
As a result of the transactions effected by the Merger Agreement, (i) Parallax merged with and into Endeavor Holdings whereupon Endeavor Holdings continued as the surviving entity and the corporate existence of Parallax ceased; (ii) the former business of Parallax is now the Company's primary business, (iii) the Company's existing business activities will continue as ancillary operations, and (iv) there is a change of control whereby the former shareholders of Parallax now own a controlling 60% ownership interest in the Company on a fully diluted basis.
As a further condition of the Merger Agreement, the then-current officer and director of the Company, Mr. Gardner Williams, resigned from all positions, and Mr. J. Michael Redmond was appointed to serve as Chief Executive Officer and President of the Company, and also as a Director on the Board of Directors. Additionally, Ms. Calli Bucci was appointed to serve as the Company's Treasurer, Chief Financial Officer, Dr. Roger Morris was appointed to serve as the Company's Chief Science Officer, and Mr. Mike Contarino was appointed to serve as the Company's Vice President. Mr. Edward W. Withrow III was appointed to serve as Executive Chairman of the Board of Directors, and Mr. Redmond, Dr. Jorn Gorlach, Mr. Anand Kumar, Mr. David Engert and Mr. E. William Withrow Jr. were appointed to serve as Directors.
About Parallax
Parallax was incorporated in the state of Delaware on December 30, 2008 under the name Roth Kline, Inc. ("Roth Kline"). In September 2010, Roth Kline acquired the right, title, and interest to certain FDA 510(k) cleared tests from Montecito BioSciences, Ltd., a Nevada corporation ("Montecito"), in perpetuity (the "Assignment") in exchange for cash compensation in the form of royalties, and common stock. In addition, Roth Kline acquired the exclusive license to a suite of medical devices, tests and utility processes from Montecito in perpetuity (the ‘License") in exchange for cash compensation in the form of royalties, and common stock. The Assignment and License agreements were modified in September 2011 to mitigate the payment terms, and increase the royalty percentages due to Montecito. On December 29, 2010, Roth Kline changed its name to Parallax Diagnostics, Inc. On January 11, 2011 (the "Closing Date"), Parallax entered into and closed a share exchange agreement (the "Share Exchange Agreement") with ABC Acquisition Corp. ("ABC") a Nevada corporation. On the Closing Date, pursuant to the terms and conditions of the Share Exchange Agreement, (i) ABC acquired 100% of the issued and outstanding shares of common stock of Parallax in exchange for the issuance of 21,000,000 shares of its common stock. Parallax merged with and into ABC whereupon ABC continued as the surviving entity and the corporate existence of Parallax ceased (the "ABC Merger"). Subsequent to the Closing Date, ABC changed its name to Parallax Diagnostics, Inc.
On November 26, 2012, Parallax changed its name to Endeavor Sciences, Inc. ("ESI"), and is a wholly owned subsidiary of Parallax Health Sciences, Inc.
On January 9, 2014, the Company changed its name from Endeavor Power Corp. (OTCQB.EDVP) to Parallax Health Sciences, Inc. (OTCQB.PRLX).
Going Concern
The Company has incurred losses since inception resulting in an accumulated deficit of $1,893,546, and a working capital deficit of $1,179,252, and further losses are anticipated in the development of its business. The Company's ability to continue as a going concern is dependent upon its ability to generate profitable operations in the future and/or to obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due, which may not be available at commercially reasonable terms There can be no assurance that the Company will be able to continue to raise funds, in which case the Company may be unable to meet its obligations and the Company may cease operations. These factors, among others, raise substantial doubt about the Company's ability to continue as a going concern.
The consolidated financial statements reflect all adjustments consisting of normal recurring adjustments, which, in the opinion of management, are necessary for a fair presentation of the results for the periods shown. The financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts of and classification of liabilities that might be necessary in the event the Company cannot continue as a going concern.
NOTE : The following notes and any further reference made to "the Company", "we", "us", "our" and "Parallax" shall mean Parallax Health Sciences, Inc.(formerly Endeavor Power Corp.) and its wholly-owned subsidiary, Endeavor Sciences, Inc. (formerly Parallax Diagnostics, Inc.), unless otherwise indicated.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
This summary of significant accounting policies is presented to assist in understanding the Company's financial statements. These accounting policies conform to accounting principles, generally accepted in the United States of America, and have been consistently applied in the preparation of the financial statements.
The Company's fiscal year-end is December 31.
Table of Contents
F-8
Development Stage Company
The Company is a development stage company as defined by ASC 915-10-05, "Development Stage Entity." The Company is still devoting substantially all of its efforts on establishing the business, and its planned principal operations, namely its car rental operations, have not commenced. All losses accumulated since inception have been considered as part of the Company's development stage activities.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary, Endeavor Sciences, Inc., formerly Parallax Diagnostics, Inc. ("ESI"). All significant inter-company accounts and transactions have been eliminated. In November 2012, the Company acquired 100% of ESI's common stock in exchange for, among other things, approximately 60% of the Company's common stock (the "Share Exchange").
As a result of the Share Exchange, a change in control occurred whereby the Company is the legal parent/accounting subsidiary of ESI, and ESI is the legal subsidiary/accounting parent of the Company. The Share Exchange has therefore been identified and recorded as a reverse acquisition and, pursuant to ASC 805-40-45-1, the consolidated financial statements' historical and cumulative data are a continuation of the financial statements of the legal subsidiary, ESI. For cumulative purposes, the date of inception is that of ESI, December 30, 2008.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with maturity of three months or less at the time of issuance to be cash equivalents. As at December 31, 2013, the Company had no cash equivalents.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States and requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to the valuation of its mineral properties, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company's estimates. To the extent there are material differences between the estimates and the actual results, future results of operations will be affected.
Net Income (Loss) Per Share
The Company computes earnings per share under Accounting Standards Codification subtopic 260-10, Earnings Per Share ("ASC 260-10"). Net earnings (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and dilutive common stock equivalents outstanding during the period. Dilutive common stock equivalents consist of shares issuable upon conversion of convertible preferred shares and the exercise of the Company's stock options and warrants.
Comprehensive Loss
ASC 220, Comprehensive Income, establishes standards for the reporting and display of comprehensive loss and its components in the financial statements. As at December 31, 2013, the Company has no items that represent comprehensive loss and, therefore, has not included a schedule of comprehensive loss in the financial statements.
Revenue Recognition
The Company recognizes revenue in accordance with ASC 605, Revenue Recognition . Revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, the service has been provided, and collectability is reasonably assured. As at December 31, 2013, the Company has not commenced its principal operations and, therefore, has not recognized any revenue.
Property and Equipment
Property and equipment is comprised of office equipment and medical testing and prototype equipment, recorded at cost and depreciated using the double declining balance method over the estimated useful lives of 5 to 7 years. Maintenance and repairs are charged to expense as incurred. Significant renewals and betterments are capitalized.
Impairment of Long-Lived Assets
The Company's long-lived assets, including intangibles, are reviewed for impairment whenever events or changes in circumstances indicate that the historical-cost carrying value of an asset may no longer be appropriate. The Company assesses recoverability of the asset by comparing the undiscounted future net cash flows expected to result from the asset to its carrying value. If the carrying value exceeds the undiscounted future net cash flows of the asset, an impairment loss is measured and recognized. An impairment loss is measured as the difference between the net book value and the fair value of the long-lived asset.
Due to the Company's recurring losses, its patents were evaluated for impairment and it was determined that future cash flows were sufficient for recoverability of the asset.
Income Taxes
Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. These assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which the temporary differences are expected to reverse.
Table of Contents
F-9
The Company has net operating loss carryforwards available to reduce future taxable income. Future tax benefits for these net operating loss carryforwards are recognized to the extent that realization of these benefits is considered more likely than not. To the extent that the Company will not realize a future tax benefit, a valuation allowance is established.
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, Share-Based Payments , using the fair value method. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued.
Financial Instruments
Pursuant to ASC 820, Fair Value Measurements and Disclosures and ASC 825, Financial Instruments , an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 and 825 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument's categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 and 825 prioritizes the inputs into three levels that may be used to measure fair value:
Ÿ | Level 1 | Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities. |
Ÿ | Level 2 | Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.. |
Ÿ | Level 3 | Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities. |
The Company ' s financial instruments consist principally of cash, accounts payable, and accrued liabilities. Pursuant to ASC 820 and 825, the fair value of cash is determined based on "Level 1" inputs, which consist of quoted prices in active markets for identical assets. The recorded values of all other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
Recently Adopted Accounting Standards :
The Company evaluates the pronouncements of various authoritative accounting organizations, primarily the Financial Accounting Standards Board ("FASB"), the US Securities and Exchange Commission ("SEC"), and the Emerging Issues Task Force ("EITF"), to determine the impact of new pronouncements on US GAAP and the impact on the Company. The Company has recently adopted the following new accounting standards:
Adopted :
Effective January 2012, the Company adopted ASU No. 2011-04, Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs (ASU 2011-04). ASU 2011-04 represents the converged guidance of the Financial Accounting Standards Board (FASB) and the International Accounting Standards Board (IASB) on fair value measurement. A variety of measures are included in the update intended to either clarify existing fair value measurement requirements, change particular principles requirements for measuring fair value or for disclosing information about fair value measurements. For many of the requirements, the FASB does not intend to change the application of existing requirements under Accounting Standards Codification (ASC) Topic 820, Fair Value Measurements. ASU 2011-04 was effective for interim and annual periods beginning after December 15, 2011. The adoption of this update did not have a material impact on the consolidated financial statements.
Effective January 2012, the Company adopted ASU No. 2011-05, Presentation of Comprehensive Income (ASU 2011-05). ASU 2011-05 is intended to increase the prominence of items reported in other comprehensive income and to facilitate convergence of accounting guidance in this area with that of the IASB. The amendments require that all non-owner changes in shareholders' equity be presented in a single continuous statement of comprehensive income or in two separate but consecutive statements. In December 2011, the FASB issued ASU No. 2011-12, Comprehensive Income (Topic 220): Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05 (ASU 2011-12). ASU 2011-12 defers the provisions of ASU 2011-05 that require the presentation of reclassification adjustments on the face of both the statement of income and statement of other comprehensive income. Amendments under ASU 2011-05 that were not deferred under ASU 2011-12 will be applied retrospectively for fiscal years, and interim periods within those years, beginning after December 15, 2011. The adoption of this update did not have a material impact on the consolidated financial statements.
Table of Contents
F-10
Not Yet Adopted:
In February 2013, the FASB issued ASU No. 2013-02, Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive (ASU 2013-02). This guidance is the culmination of the FASB's deliberation on reporting reclassification adjustments from accumulated other comprehensive income (AOCI). The amendments in ASU 2013-02 do not change the current requirements for reporting net income or other comprehensive income. However, the amendments require disclosure of amounts reclassified out of AOCI in its entirety, by component, on the face of the statement of operations or in the notes thereto. Amounts that are not required to be reclassified in their entirety to net income must be cross-referenced to other disclosures that provide additional detail. This standard is effective prospectively for annual and interim reporting periods beginning after December 15, 2012. The Company is evaluating the effect, if any, the adoption of ASU 2013-02 may have on its consolidated financial statements.
In April 2013, the FASB issued ASU No. 2013-07, Presentation of Financial Statements (Top 205): Liquidation Basis of Accounting. The objective of ASU No. 2013-07 is to clarify when an entity should apply the liquidation basis of accounting and to provide principles for the measurement of assets and liabilities under the liquidation basis of accounting, as well as any required disclosures. The amendments in this standard is effective prospectively for entities that determine liquidation is imminent during annual reporting periods beginning after December 15, 2013, and interim reporting periods therein. The Company is evaluating the effect, if any, the adoption of ASU No. 2013-07 may have on its consolidated financial statements.
In July 2013, the FASB issued ASU No 2013-11, Presentation of an Unrecognized Tax Benefit When Net Operating Loss Carryforward Exists. The objective of ASU 2013-11 is to reduce diversity in practice by providing guidance on the presentation of unrecognized tax benefits, and will better reflect the manner in which an entity would settle at the reporting date any additional income taxes that would result from the disallowance of a tax position when net operating loss carryforwards, similar tax losses, or tax credit carryforwards exist. The amendments in this Update are effective for fiscal years, and interim periods within those years, beginning after December 15, 2013, and interim reporting periods therein. Early adoption is permitted. The Company is evaluating the effect, if any, adoption of ASU No. 2013-11 will have on its consolidated financial statements.
Recently Issued Accounting Standards Updates:
There were various updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries. None of the updates are expected to a have a material impact on the Company's consolidated financial position, results of operations or cash flows.
NOTE 3. PROPERTY AND EQUIPMENT
Property and equipment consists of the following:
| December 31, 2013 |
| December 31, 2012 |
| ||
Office equipment | $ | 8,385 |
| $ | 8,385 |
|
Medical devices and instruments |
| 45,194 |
|
| 45,194 |
|
Sub-total |
| 53,579 |
|
| 53,579 |
|
Accumulated depreciation |
| (37,728 | ) |
| (27,162 | ) |
Property and equipment, net | $ | 15,851 |
| $ | 26,417 |
|
Depreciation expense for the year ended December 31, 2013 and 2012 was $10,566 and $16,883, respectively.
NOTE 4. INTANGIBLE ASSETS
Intangible assets consists of the following:
| December 31, 2013 |
| December 31, 2012 |
| ||
Products and processes | $ | 12,500 |
| $ | 12,500 |
|
Trademarks and patents |
| 12,500 |
|
| 12,500 |
|
Sub-total |
| 25,000 |
|
| 25,000 |
|
Accumulated amortization |
| (5,416 | ) |
| (3,750 | ) |
Intangible assets, net | $ | 19,584 |
| $ | 21,250 |
|
Amortization expense for the year ended December 31, 2013 and 2012 was $1,666 and $1,667, respectively.
Table of Contents
F-11
NOTE 5. NOTES AND LOANS PAYABLE
Notes and loans payable consists of the following:
| December 31, 2013 |
| December 31, 2012 |
| ||
Rast Trade | $ | 65,000 |
| $ | 65,000 |
|
THI Inc. |
| 10,000 |
|
| 10,000 |
|
Phillip Knight |
| 9,075 |
|
| 9,075 |
|
Total notes and loans payable-principal |
| 84,075 |
|
| 84,075 |
|
Accrued interest |
| 22,212 |
|
| 14,572 |
|
Total notes and loans payable | $ | 106,287 |
| $ | 98,647 |
|
In June 2010, the Company issued a note payable in the principal amount of $9,075 to a non-related party. Under the terms of the note, the amount is unsecured, non-interest bearing, and due upon demand.
In June 2010, the Company issued a note payable in the principal amount of $10,000 to a non-related party. Under the terms of the note, the amount is unsecured, accrues interest at a rate of 8% per annum, and is due upon demand. The Company has recorded $3,015 and $2,213 in accrued interest as of December 31, 2013 and 2012, respectively.
On April 21, 2011, the Company issued a note payable in the principal amount of $65,000 to a non-related party. The note is unsecured, bears interest at a rate of 10% per annum, and is due upon demand. The Company has recorded $19,197 and $12,359 in accrued interest as of December 31, 2013 and 2012, respectively.
As at December 31, 2013 and 2012, a total of $22,212 and $14,572, respectively, of interest on notes payable has been accrued and is included as part of accrued expenses on the balance sheet.
NOTE 6. RELATED PARTY TRANSACTIONS
On November 15, 2010, the Company entered into an employment agreement with its CEO, Mr. J. Michael Redmond (the "Employment Agreement"). Under the Employment Agreement, Mr. Redmond agrees to serve as the President, CEO, and Director of the Company for a term of three years. As compensation for his services, Mr. Redmond's base salary will be $200,000 per annum for the first year, increasing to $225,000 in year 2, and $250,000 in year 3, contingent upon the Company meeting certain goals. In addition, Mr. Redmond was granted one million three hundred seventy five thousand (1,375,000) options at an exercise price of $0.10 per share. As of December 31, 2013 and 2012, respectively, $411,827 and $185,961 has been recorded as accrued compensation.
On January 2, 2012, the Company entered into a consulting agreement with Huntington Chase Financial Group LLC ("HCFG"), a Nevada corporation, whose principal is a related party. The consulting agreement provides for HCFG to provide advisory services to the Company for a period of three years for a fee of $12,500 per month, which has been deferred until such time as the Company reaches certain funding goals. As of December 31, 2013 and 2012, respectively, $300,000 and $150,000 has been recorded as accrued compensation.
As at December 31, 2013 and 2012, respectively, related parties are due a total of $921,693 and $513,152, consisting of $795,127 and $389,961 in accrued compensation, and $126,566 and $123,191 in cash advances to the Company for operating expenses. The amounts owing are unsecured, non-interest bearing, and due upon demand.
Related party payable consists of the following:
| December 31, 2013 |
| December 31, 2012 |
| ||
Accrued compensation |
|
|
|
|
|
|
J. Michael Redmond | $ | 411,827 |
| $ | 185,961 |
|
Huntington Chase Financial Group, Ltd. |
| 300,000 |
|
| 150,000 |
|
MJ Management, LLC |
| 45,800 |
|
| 16,500 |
|
Tom Mackay |
| 26,250 |
|
| 26,250 |
|
Gardner Williams |
| 11,250 |
|
| 11,250 |
|
Total accrued compensation |
| 795,127 |
|
| 389,961 |
|
|
|
|
|
|
|
|
Cash advances |
|
|
|
|
|
|
Edward W. Withrow, III |
| 11,217 |
|
| 7,842 |
|
Tom Mackay |
| 11,900 |
|
| 11,900 |
|
Regal Capital Development |
| 103,449 |
|
| 103,449 |
|
Total cash advances |
| 126,566 |
|
| 123,191 |
|
Total related party payable | $ | 921,693 |
| $ | 513,152 |
|
On September 30, 2010, the Company issued a Convertible Note Payable in the amount of $144,000 to a non-related party pursuant to a Confidential Settlement Agreement. On January 1, 2012, the note was assigned and purchased by The Kasper Group. Ltd., a related party, and a new Convertible Promissory Note was issued (the "Kasper Note"). The Kasper Note bears interest at a rate of 7% per annum, is due by January 1, 2015, and contains a repayment provision to convert the debt into common stock of the Company at a rate of $0.25 per share. As of December 31, 2013 and 2012, respectively, interest in the amount of $20,160 and $10,080 has been accrued, and is included as an accrued expense on the accompanying consolidated balance sheets.
Table of Contents
F-12
On December 31, 2012, the Company issued a Convertible Note Payable in the amount of $28,500 to a related party for cash loans made to the Company of $14,000 during 2011 and $14,500 during 2012. The note bears interest at a rate of 7% per annum, is due by December 31, 2015, and contains a repayment provision to convert the debt into common stock of the Company at a rate of $0.25 per share. The note has been modified to increase the principal balance by $17,600 for additional cash loans made to the Company through December 31, 2013, for a total principal balance owing of $46,100. As of December 31, 2013 and 2012, respectively, interest in the amount of $2,834 and $0 has been accrued, and is included as an accrued expense on the accompanying consolidated balance sheets.
As at December 31, 2013 and 2012, the principal balance owed for related party notes payable is $190,100 and $172,500, respectively, and a total of $22,994 and $10,080, respectively, of interest has been accrued.
| December 31, 2013 |
| December 31, 2012 |
| ||
Long-term related party notes payable |
|
|
|
|
|
|
The Kasper Group, Ltd. | $ | 144,000 |
| $ | 144,000 |
|
Huntington Chase Financial Group, Ltd. |
| 46,100 |
|
| 28,500 |
|
Total related party notes payable | $ | 190,100 |
| $ | 172,500 |
|
NOTE 7. COMMITMENTS AND CONTINGENCIES
In September 2010, the Company, through its wholly owned subsidiary, Endeavor Sciences, Inc. ("ESI"), acquired the exclusive rights, title, and interest in perpetuity (the "Rights") to certain FDA 510(k) cleared tests held by Montecito BioSciences, Ltd., a Nevada corporation ("Montecito"), a related party, in the area of infectious diseases (the "Assignment Agreement"). Pursuant to the Assignment Agreement, in exchange for these Rights, Montecito received 50% of issued and outstanding shares of ESI's common stock, a $750,000 assignment fee (the "Assignment Fee"), and a royalty of 4% of all gross revenues earned from the Rights. The Assignment Fee is payable once the Company reaches certain funding goals. In addition, in September 2010, the Company acquired the exclusive license in perpetuity (the "License") to certain Patent Applications initiated by Montecito for a suite of medical devices, tests and utility processes (the ‘License Agreement"). Pursuant to the License Agreement, and in exchange for this License, Montecito received 50% of issued and outstanding shares of ESI's common stock, a $750,000 license fee (the "License Fee"), and a royalty of 3½% of all gross revenues earned from the License. The License Fee is payable once the Company reaches certain funding goals. In September, 2011, the Assignment Agreement and License Agreement were modified (the "Modifications") to i) mitigate the payment terms of the Assignment Fee and the License Fee (the "Fees"), and ii) increase the royalty rates by 1% respectively, until such time as the royalties paid exceed an aggregate sum of $1,500,000, at which point the royalty rates will revert back to their original rates. The 1% royalty earned under this increase shall be applied towards the respective Fees until such time as the Fees are paid in full. As a result of the Assignment Agreement, License Agreement, and Modifications, the Company has a contingent liability of $1,500,000 payable to Montecito.
On July 1, 2011, the Company entered into a Development and Supply Agreement with Corder Engineering, LLC for certain engineering services as well as the development and manufacturing of ten (10) evaluation units, including software, hardware and instrumentation, related to the Company's medical testing equipment (the "Corder Agreement"). The Corder Agreement is for an estimated term of twelve weeks, or until delivery of the evaluation units, and includes compensation in the amount of $35,000, which is payable upon certain stages of production over the twelve week term. As of December 31, 2013, payments totaling $22,500 have been made, and $12,500 has been accrued.
On July 1, 2011, the Company entered into a Supply Agreement with Meyers Stevens Group, Inc. for the manufacturing of diagnostic assays related to the Company's medical testing equipment (the "Meyer Agreement"). The Meyer Agreement is for an estimated term of eight weeks, or until delivery of the assays, and includes compensation in the amount of $10,194, which is payable in two installments over the eight week term. As of December 31, 2013, payments totaling $8,980 have been made, and $1,214 has been accrued.
On January 10, 2013, the Company entered into a consulting agreement with Capital Group Communications, Inc. (the "CGC Agreement") for certain advisory services. The term of the CGC Agreement was for a period of twelve months, and contained consideration for such advisory services in the form of 1,500,000 shares of the Company's restricted common stock (the "CGC shares"). The CGC Agreement was cancelled due to non-performance by CGC. At September 30, 2013, the CGC shares, valued at $90,000, were unissued, and $42,580 previously expensed as stock compensation through June 30, 2013, was reversed. No further consideration, in the form of stock or other compensation, is payable under this Agreement.
NOTE 8. CONVERTIBLE PREFERRED STOCK
The total number of authorized shares of preferred stock that may be issued by the Company is 10,000,000 with a par value of $0.001 per share.
On June 17, 2011, pursuant to a Convertible Preferred Purchase Agreement, the Company issued 100,000 shares of its preferred stock at $1 per share to Hamburg Investment Company, LLC, whose principal is Jorn Gorlach, a director of the Company, for cash in the amount of $100,000. As a result, $99,990 was recorded as paid in capital.
On June 17, 2011, pursuant to a Convertible Preferred Purchase Agreement, the Company issued 100,000 shares of its preferred stock at $1 per share to Huntington Chase Financial Group, LLC, whose principal is Edward W. Withrow III, the executive chairman of the Company, for cash in the amount of $100,000. As a result, $99,990 was recorded as paid in capital.
Table of Contents
F-13
On September 30, 2011, pursuant to a Convertible Preferred Purchase Agreement, the Company issued 10,000 shares of its preferred stock at $10 per share to Hamburg Investment Company, LLC, whose principal is Jorn Gorlach, a director of the Company, for cash in the amount of $100,000. As a result, $99,999 was recorded as paid in capital.
On December 6, 2011, pursuant to a Convertible Preferred Purchase Agreement, the Company issued 10,000 shares of its preferred stock at $10 per share to David Engert, a director of the Company, for cash in the amount of $100,000. As a result, $99,999 was recorded as paid in capital.
On May 3, 2012, pursuant to a Convertible Preferred Purchase Agreement, the Company issued 10,000 shares of its preferred stock at $10 per share to a non-related party, for cash in the amount of $100,000. As a result, $99,999 was recorded as paid in capital. On April 4, 2013, the Company received a Notice to Convert from one of its preferred shareholders, requesting that 1/3 (one-third) of his 36,339 shares of preferred stock holdings be converted into common shares. As a result, 12,112 shares of preferred stock were converted into 242,660 shares of the Company's restricted common stock were issued at a conversion ratio of 20 common shares for each preferred share held, and preferred paid in capital was reduced by $33,321.
All preferred shares are convertible into the Company's common stock at a rate of 20 shares of common stock for each preferred share held, and were issued with 100% warrant coverage (Note 10). The number of shares of common stock underlying the warrants and the exercise price were subject to adjustment upon certain events.
On November 1, 2012, in connection with the Merger Agreement, the Company converted all its formerly outstanding preferred shares at an exchange ratio of 3.633926 shares for each share held. As a result, the issued and outstanding preferred stock increased by 605,803 shares, from 230,000 shares to 835,803 shares.
As of December 31, 2013 and 2012, the Company had 823,691 and 835,803 shares of preferred stock issued and outstanding, respectively.
NOTE 9. COMMON STOCK
The total number of authorized shares of common stock that may be issued by the Company is 250,000,000 with a par value of $0.001 per share.
On July 11, 2012, the Company issued 75,000 shares of its common stock in exchange for services valued at $5,000. As a result, $4,992 was recorded as paid in capital.
In November 2012, as part of the Merger Agreement, 3,350,000 shares of common stock were cancelled and returned to treasury. As a result, $335 was recorded as paid in capital.
On April 1, 2013, in connection with a Notice to Convert, 12,112 shares of preferred stock were converted into common stock at a rate of 20 common shares to 1 preferred share. As a result, 242,660 restricted shares of common stock were issued, and $33,090 was recorded as paid in capital.
On November 1, 2012, in connection with the Merger Agreement, the Company converted all its formerly outstanding shares at an exchange ratio of 3.633926 shares for each share held. As a result, the issued and outstanding common stock increased by 126,193,898 shares, from 24,870,000 shares to 151,063,898 shares, and paid in capital was reduced by $438,621.
On December 31, 2013, pursuant to a Stock Purchase Agreement, the Company issued 11,459,279 shares of its common stock for cash in the amount of $1,145.96. As a result, additional paid in capital was reduced by $10,313.
As of December 31, 2013 and 2012, the Company has 162,765,837 and 151,063,898 common shares issued and outstanding, respectively.
NOTE 10. WARRANTS AND OPTIONS
As of December 31, 2013, the Company had 16,473,401 warrants and 1,900,000 options issued and outstanding.
On April 1, 2013, in connection with a Notice to Convert, 12,112 shares of preferred stock were converted into common stock. As a result, the 242,660 underlying warrants were cancelled.
On June 17, 2013, 14,535,706 warrants underlying 726,786 shares of preferred stock, which were to expire on June 17, 2013, were extended for a period of 2 years. The warrants now expire on June 17, 2015.
On September 30, 2013, 726,785 warrants underlying 36,339 shares of preferred stock, which were to expire on September 30, 2013, were extended for a period of 2 years. The warrants now expire on September 30, 2015.
Table of Contents
F-14
Warrants Outstanding |
| |||||||||
|
| Number of |
| Remaining |
| Exercise Price |
| Weighted |
| |
|
| Common |
| Contractual Life |
| times Number |
| Average |
| |
Exercise Price |
| Shares |
| (in years) |
| of Shares |
| Exercise Price |
| |
|
|
|
|
|
|
|
|
|
|
|
$0.27518 |
| 14,535,706 |
| 1.46 |
| $ | 4,000,000 |
| $0.27518 |
|
$0.41278 |
| 726,785 |
| 1.75 |
|
| 300,000 |
| $0.41278 |
|
$0.41278 |
| 726,785 |
| 0.16 |
|
| 300,000 |
| $0.41278 |
|
$0.41278 |
| 484,125 |
| 0.57 |
|
| 199,837 |
| $0.41278 |
|
|
| 16,473,401 |
|
|
| $ | 4,799,837 |
| $0.41278 |
|
Warrant Activity | ||||||
| Number of Shares |
| Weighted Average Exercise Price |
| ||
Outstanding at December 31, 2012 |
| 16,716,061 |
|
| $0.41278 |
|
Issued |
| - |
|
| - |
|
Exercised |
| - |
|
| - |
|
Expired / Cancelled |
| (242,660 | ) |
| $0.27518 |
|
Outstanding at December 31, 2013 |
| 16,473,401 |
|
| $0.41278 |
|
Options Outstanding | ||||||||||
|
|
|
| Remaining |
| Exercise Price |
| Weighted |
| |
|
| Number of |
| Contractual Life |
| times Number |
| Average |
| |
Exercise Price |
| Shares |
| (in years) |
| of Shares |
| Exercise Price |
| |
|
|
|
|
|
|
|
|
|
|
|
$0.10 |
| 1,375,000 |
| 7.00 |
| $ | 137,500 |
| $0.10 |
|
$0.25 |
| 225,000 |
| 2.25 |
|
| 56,250 |
| $0.25 |
|
$0.25 |
| 225,000 |
| 2.08 |
|
| 56,250 |
| $0.25 |
|
|
| 1,900,000 |
|
|
| $ | 250,000 |
| $0.20 |
|
Options Activity | |||||||
| Number of Shares |
| Weighted Average Exercise Price |
| |||
Outstanding at December 31, 2012 |
| 1,900,000 |
|
| $0.20 |
| |
Issued |
| - |
|
| - |
| |
Exercised |
| - |
|
| - |
| |
Expired / Cancelled |
| - |
|
| - |
| |
Outstanding at December 31, 2013 |
| 1,900,000 |
|
| $0.20 |
| |
NOTE 11. INCOME TAXES
The components of the cumulative net deferred tax asset at December 31, 2013 and 2012, the statutory tax rate, the effective tax rate and the amount of the valuation allowance are indicated below:
| December 31, 2013 |
| December 31, 2012 |
| ||
|
|
|
|
|
|
|
Income (loss) before taxes | $ | (504,494 | ) | $ | (554,668 | ) |
Statutory rate |
| 34% |
|
| 34% |
|
|
|
|
|
|
|
|
Computed expected tax payable (recovery) | $ | 171,500 |
| $ | 188,600 |
|
Non-deductible expenses |
| - |
|
| (900 | ) |
Change in valuation allowance |
| (171,500 | ) |
| (187,700 | ) |
|
|
|
|
|
|
|
Reported income taxes | $ | – |
| $ | – |
|
The significant components of deferred income tax assets and liabilities at December 31, 2013 and 2012 are as follows:
| December 31, 2013 |
| December 31, 2012 |
| ||
|
|
|
|
|
|
|
Net operating loss carried forward | $ | 642,500 |
| $ | 471,000 |
|
|
|
|
|
|
|
|
Valuation allowance |
| (642,500 | ) |
| (471,000 | ) |
|
|
|
|
|
|
|
Net deferred income tax asset | $ | – |
| $ | – |
|
As at December 31, 2013, the Company had approximately $1,890,000 of federal net operating losses which expire commencing in the year 2026.
Table of Contents
F-15
NOTE 12. SUBSEQUENT EVENTS
On July 22, 2013, the Company entered into a binding Letter of Intent ("LOI") for the acquisition of a privately held California corporation, whose primary operations are in the pharmaceutical industry (the "NewCo"). Since completion of its due diligence, the Company has been working with financial institutions to secure a combined debt-equity financing that will enable the Company to complete the acquisition. There can, however, be no guarantee that satisfactory financing arrangements will be secured to complete the acquisition. The Company's management and the NewCo management have completed negotiations, the terms of which are confidential and will be disclosed upon completion of the acquisition. On March 28, 2014, the Company's board of directors issued a board resolution authorizing the acquisition.
On January 7, 2014, 36,462,819 shares of the Company's common stock were purchased from certain shareholders by its Chairman, Edward W. Withrow III. On the same day, said shares were cancelled, for the benefit of the Company. As a result of this transaction, the total issued and outstanding shares of common stock were reduced to 126,303,018 shares, and $36,462 was recorded as additional paid in capital.
On January 9, 2014, pursuant to a majority vote of the shareholders and resolution of the board of directors, the Company changed its name to Parallax Health Sciences, Inc. (OTCQB.PRLX). A Certificate of Amendment to the Company's Articles of Incorporation has been filed with the Secretary of State of Nevada.
On March 31, 2014, the Company issued a Convertible Promissory Note Modification to that certain Convertible Promissory Note payable to Huntington Chase Financial Group dated December 31, 2012. The Modification was made to increase the principal amount to $383,600, to include $337,500 in compensation owed through March 31, 2014, pursuant to that certain Consulting Agreement dated January 2, 2012.
* * * * *
Table of Contents
F-16
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
The Company has not changed its auditors since its last year end and has not had any disagreements with its auditors.
ITEM 9A. | CONTROLS AND PROCEDURES |
Management's Report on Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures that are designed to ensure that information required to be disclosed in the Company's reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to the Company's management, including the Company's president, chief executive officer and chief financial officer to allow for timely decisions regarding required disclosure. In designing and evaluating the Company's disclosure controls and procedures, the Company's management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and the Company's management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As of December 31, 2013, the end of the Company's fiscal year covered by this report, the Company carried out an evaluation, under the supervision and with the participation of the Company's president, chief executive officer and chief financial officer of the effectiveness of the design and operation of the Company's disclosure controls and procedures. Based on the foregoing, the Company's president, chief executive officer and chief financial officer concluded that the Company's disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Management's Report on Internal Control over Financial Reporting
The Company's management is responsible for establishing and maintaining adequate internal control over financial reporting. Responsibility, estimates and judgments by management are required to assess the expected benefits and related costs of the Company's control procedures. The objectives of internal control include providing management with reasonable, but not absolute, assurance that assets are safeguarded against loss from unauthorized use or disposition, and that transactions are executed in accordance with management's authorization and recorded properly to permit the preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States. The Company's management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2013. In making this assessment, the Company's management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") in Internal Control-Integrated Framework . The Company's management has concluded that, as of December 31, 2013, the Company's internal control over financial reporting is effective in providing reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with the Company generally accepted accounting principles. The Company's management reviewed the results of their assessment with the Company's Board of Directors.
This annual report does not include an attestation report of the Company's registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by the Company's registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit the Company to provide only management's report in this annual report.
Inherent limitations on effectiveness of controls
Internal control over financial reporting has inherent limitations which include but is not limited to the use of independent professionals for advice and guidance, interpretation of existing and/or changing rules and principles, segregation of management duties, scale of organization, and personnel factors. Internal control over financial reporting is a process which involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements on a timely basis, however these inherent limitations are known features of the financial reporting process and it is possible to design into the process safeguards to reduce, though not eliminate, this risk. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
There have been no changes in the Company's internal controls over financial reporting that occurred during the year ended December 31, 2013 that have materially or are reasonably likely to materially affect, the Company's internal controls over financial reporting.
Table of Contents
35
ITEM 9B. | OTHER INFORMATION |
None
PART III
ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Identification of Directors and Executive Officers
The following table represents the directors and executive officers of the Company as of December 31, 2013:
Name | Position(s) Held | Age | Date first Elected or Appointed |
J. Michael Redmond | President, Chief Executive Officer, Director | 53 | November 1, 2012 |
Calli Bucci | Chief Financial Officer Corporate Secretary | 49 | November 1, 2012 March 31, 2014 |
Edward W. Withrow III | Executive Chairman | 49 | November 1, 2012 |
Dr. Jorn Gorlach | Director | 52 | November 1, 2012 |
Anand Kumar | Director | 71 | November 1, 2012 |
David Engert | Director | 61 | November 1, 2012 |
E. William Withrow Jr. | Director | 76 | November 1, 2012 |
Term of Office
The Board of Directors elects officers and their terms of office are at the discretion of the Board of Directors. Each officer serves until the earlier occurrence of the election of his or her successor at the next meeting of stockholders, death, resignation or removal by the Board of Directors. At the present time, members of the board of directors are not compensated for their services to the board. Each Director shall hold office until the next annual meeting of stockholders and until his/her successor shall have been duly elected and qualified.
On November 1, 2012, Mr. J. Michael Redmond was appointed the Company's President, Chief Executive Officer and member of the Board of Directors.
On November 1, 2012, Ms. Calli Bucci was appointed the Company's Chief Financial Officer, and Mr. Kyle Withrow was appointed Corporate Secretary.
On November 1, 2012, Mr. Edward W. Withrow III was appointed Executive Chairman, and Dr. Jorn Gorlach, Mr. Anand Kumar, Mr. David Engert and Mr. E. William Withrow Jr. were appointed to serve as Directors.
On March 31, 2014, Mr. Kyle Withrow resigned as the Company's Corporate Secretary. Ms. Calli Bucci was appointed as his successor.
Background and Business Experience
J. Michael Redmond – President, Chief Executive Officer, Director
Mr. J. Michael Redmond, age 53, has over twenty-five years of experience in the medical device and biotech markets. In May of 2009, Mr. Redmond founded JMR, Inc. and has served as its President since that time. JMR, Inc. provides business development and marketing services to diagnostic and biotech companies. As President, Mr. Redmond is responsible for developing and implementing the business plan of the company.
From May 2007 to June 2009, Mr. Redmond served as the Vice President of Marketing and Business Development for DxTech, Inc., a startup company focused on a disruptive model for point of care diagnostic testing. As the Vice President of Marketing and Business Development, Mr. Redmond was responsible for creating and implementing the company's business plan, raising capital and forming strategic alliances with industry partners.
36
From 1996 to 2007, Mr. Redmond worked in various titles and capacities for Bioject, Inc. ("Bioject"), an early stage drug delivery company. From 1996 to 1997, Mr. Redmond served as Bioject's Vice President of Sales and Marketing. From 1998 to 2002, Mr. Redmond served as Bioject's Vice President of Business Development, and from 2003 to 2007, Mr. Redmond served as Bioject's Senior Vice President of Business Development, Sales and Marketing. In these positions, Mr. Redmond's responsibilities included negotiating corporate partnerships with major pharmaceutical and biotech companies, launching new products, securing distribution channels, P&L responsibility and raising capital.
From 1989 to 1996, Mr. Redmond was employed with KMC Systems, a private label developer and manufacturer of medical devices and instruments. At KMC Systems, Mr. Redmond served as the Director of Sales and Marketing and the Director of Business Development, Sales and Marketing. Mr. Redmond was responsible for developing new business in the U.S. and Europe as well as negotiating long-term product development and production contracts. Additionally, from 1983 to 1989, Mr. Redmond was employed with Abbott Laboratories in the diagnostic division. While at Abbott Laboratories, Mr. Redmond served as Product Manager, Account Executive, and Diagnostics Systems Sales Specialist.
Mr. Redmond earned a Bachelor of Arts degree from Denison University in 1983. He lives with his family in Windham, New Hampshire.
Mr. Redmond is qualified to be the Company's President, CEO, and Director because of his extensive experience in a multitude of different capacities in the medical device and biotech markets.
Calli Bucci, Chief Financial Officer, Corporate Secretary
Ms. Bucci has over 25 years experience in the field of finance and business management. Prior to holding the position of Chief Financial Officer, Ms. Bucci was Controller of the Company since January 2010, and was responsible for general ledger, quarterly certified reviews, annual audits, preparation for SEC filings, customer billing and invoicing, multi-state payroll, licenses and consolidated corporate income taxes.
Before joining the Company, Ms. Bucci held the position of Chief Financial Officer at InstaSave, Inc., a promotional incentive company, from December 2007, where she was responsible for financial reporting, capital structure strategy and modeling, financial transactions with consumers, consumer product goods companies and retailers, investor relations, audits, payroll and corporate income taxes.
In addition to her public accounting background, Ms. Bucci held the position of Manager/Senior Accountant at Gelfand Rennert & Feldman, a division of PriceWaterhouseCoopers, where she was responsible for all financial transactions for high net worth clientele, was liaison for annual audits, general ledger reviews and annual tax preparation.
Ms. Bucci held the position of Director of Accounting and Contract Administration at Intercontinental Releasing Corporation (IRC), a Los Angeles based Motion Picture Distribution Company. Ms. Bucci was responsible for all functions within the company's accounting department, from financial statements and forecasting, to annual audits and corporate taxes. During her tenure with IRC, Ms. Bucci also designed and implemented a custom computerized availabilities system for the film rights of over 35 film properties distributed to foreign territories throughout the world. She was also responsible for the administration and facilitation of all client contracts, dealing heavily in foreign currencies and international import regulations.
Ms. Bucci attended the University of California at Berkley, majoring in Accounting. She lives with her family in Santa Monica, California.
Ms. Bucci is qualified to be the Company's Chief Financial Officer because of her extensive experience in a multitude of different capacities in accounting and finance.
Ms. Bucci is qualified to be the Company's Secretary because of her knowledge of and prior experience working in corporate business and in public markets.
Edward W. Withrow III, Executive Chairman
Mr. Edward W. Withrow III, age 49, currently serves as a director for ABC Acquisition Corp 1502. In addition to serving as a director for ABC Acquisition Corp 1502, Mr. Withrow III currently serves as the Chairman of the Board for Ecologic Transportation, Inc., a company founded by Mr. Withrow III and dedicated to providing environmentally friendly transportation services. Mr. Withrow III also currently serves as the President and CEO of Montecito Bio Sciences, Ltd., a bio-medical diagnostics company. As President and CEO, Mr. Withrow III is responsible for creating and implementing the company's business plan, raising capital and forming strategic alliances with industry partners.
Table of Contents
37
From 2002 to 2005, Mr. Withrow III served as the CEO for Addison-Davis Diagnostics, Inc. Addison Davis Diagnostics, Inc. offers point-of-care screening tests to the global health care market. As CEO, Mr. Withrow III was responsible for corporate governance, strategic planning, capitalization, and business development. From 2002 to 2004, Mr. Withrow III served as the CEO for Reward Enterprises, Inc., a diversified financial services company specializing in subprime consumer lending. As CEO, Mr. Withrow III was responsible for developing and implementing the overall business plan of the company.
Mr. Withrow III graduated from Alameda High School in Alameda California in 1982 and attended Santa Barbara City College from 1982 to 1985 where he majored in Business. He lives with his family in Malibu, California.
Mr. Withrow III is qualified to be the Company's Executive Chairman because of his knowledge of and prior experience working in the diagnostics products market.
Dr. Jorn Gorlach, Director
Dr. Jorn Gorlach, age 52, has over twenty years of experience in the bio-medical field. In 2001, Dr. Gorlach co-founded AAvantgarde, a management consulting firm focused on the development and support of start-up companies. Since the inception of AAvantgarde in 2001, Dr. Gorlach has also served as one of its directors. As a co-founder and director of AAvantgarde, Dr. Gorlach is responsible for management consulting, licensing, and general operations. Since 2006, Dr. Gorlach has also served as a co-founder and director of Montecito Bio Sciences, Ltd., a diagnostics and testing company with proprietary technology for point of care diagnostics, testing, and data communication. Dr. Gorlach, in his role as co-founder and director, is responsible for developing and implementing the business plan of the company.
In 2002, Dr. Gorlach co-founded AAvantgarde Laboratories AG and has served as its CEO since that time. AAvantgarde Laboratories AG is a research, development, and licensing company of biotechnology products, particularly in the field of diagnostics, biological prognostics, and diseases. As CEO, Dr. Gorlach is responsible for developing the company's business plan, developing outlines for product concept, research, and development, and leading financing activities and investor relations. In 2001, Dr. Gorlach co-founded Arcanum Discovery, Inc., a proteomics and drug discovery company focusing on novel drug target identifiers and validation. Additionally, from 2001 to 2002, Dr. Gorlach served as head of business development and finances for Arcanum Discovery, Inc. where he developed the company's product concept, research and development, and business plan as well as managed financing activities and investor relations. In 2001, Dr. Gorlach co-founded Ercole Biotech, Inc., a research stage biopharmaceutical company involved in the creation of oligonucleotide drugs. Since its inception until 2003, Dr. Gorlach served as a director of the company where he was responsible for developing business strategy, financial planning, and contract negotiation strategy.
In 1997, Dr. Gorlach co-founded Paradigm Genetics, Inc., a bio-technology research company. From 1997 to 1999, Dr. Gorlach served as the company's Director of Research where he was responsible for developing concepts regarding novel functional genomics platform, focusing on high throughput, industrialization, systematization, and biology/IT integration. From 1999 to 2000, Dr. Gorlach served as the Director of Project Management for Paradigm Genetics, Inc. As Director of Project Management, Dr. Gorlach managed customer projects and research progress. From 2000 to 2001, Dr. Gorlach served as the company's vice president of business development. As a member of the company's executive team, Dr. Gorlach was responsible for new projects and the development of plans in future key business fields. Beginning in 2001 and continuing through 2002, Dr. Gorlach served as a consultant for Paradigm Genetics, Inc., where he supported the company's agricultural project initiatives and customer negotiations.
From 1996 to 1997, Dr. Gorlach served as the Group Leader of Combinatorial Biochemistry for Novartis, Inc., a healthcare and scientific research company. As Group Leader of Combinatorial Biochemistry, Dr. Gorlach led team efforts in developing pharmaceutically active macrolide and cloning multiple polyketides genes.
From 1994 to 1996, Dr. Gorlach was a research scientist for Ciba-Geigy, Inc., a chemical company. As a research scientist, Dr. Gorlach focused on acquired immunity and chemical regulation in wheat.
From 1991 to 1994, Dr. Gorlach was a research fellow for the Swiss Federal Institute in Zurich, Switzerland. As a research fellow, Dr. Gorlach focused his attention on gene regulation of amino acid biosynthetic pathways.
Dr. Gorlach has a Bachelor of Science Degree in Chemistry and Biology as well as a Bachelor of Science Degree in Biochemistry from the University of Hannover. In 1991, Dr. Gorlach obtained a Master in Science from the University of Hannover in Biochemistry. In 1994, Dr. Gorlach received a Ph.D. in Molecular Biology from ETH Zurich, and in 2000, received a MBA from the Kenan-Flagler Business School at the University of North Carolina-Chapel Hill.
Dr. Gorlach is qualified to be a director of the Company because of his extensive experience in business development, project management, strategic planning, and business management in a multitude of different capacities in the bio-technology field.
38
Anand Kumar, Director
Mr. Kumar, age 71, has over twenty-five years of experience in international business development. In 1999, Mr. Kumar founded Global Telesolutions, a company responsible for creating partnerships and in-country relationships for various companies in Asia and the Indian subcontinent. From 1999 to 2010, Mr. Kumar served as the CEO for Global Telesolutions where, among other things, he developed presence and business in the Middle East and Indian, built global network partnerships for telecommunications and traffic, and oversaw international staff for operations.
From 1995 to 1999, Mr. Kumar served as the Executive Vice President for Facilicom International, a leading international telecommunications carrier. As Executive Vice President, Mr. Kumar developed multi-country business and network presence for operations, negotiated with vendors, regulators, and partners, and oversaw Europe and Asia managers and assisted in multi-national sales closings.
From 1986 to 1993, Mr. Kumar served as the President for Washington International Teleport. As President, Mr. Kumar built the first direct international earth station after U.S. de-regulation, obtained new national and international video and data clients, and created the satellite, fiber hybrid network video concept. From 1981 to 1986, Mr. Kumar served as the President of Communications Strategies Group, a company that delivers comprehensive public relations and strategic communications services to organizations. As President, Mr. Kumar investigated technology business opportunities for international clients and ran special training sessions in various areas of telecommunications practice.
Mr. Kumar earned a B.S.E.E. from Jadavpur University and a M.S.E.E. and PhD candidacy degree from the University of Connecticut.
Mr. Kumar is qualified to be a director of the Company because of his extensive experience in international operations and strategic business development.
David Engert
Mr. Engert, age 61, has served as the President and Chief Executive Officer of NightHawk Radiology Holdings, Inc. since November 2008 and as a member of its board of directors since April 30, 2008. Mr. Engert also sits on the Board of Directors of Healthation, Inc., a healthcare information technology company. Mr. Engert was the founder and owner of ES3, a strategic consulting and investment company since 2007. From 2002 to 2006, Mr. Engert served as the president, chief executive officer and director of Quality Care Solutions, Inc., one of the nation's leading providers of advanced healthcare payer enterprise application solutions, which was acquired by Trizetto, Inc. in January 2007. Prior to 2002, Mr. Engert held a number of senior level management positions in the healthcare industry over the previous 10 years, including senior vice president & general manager at McKesson Corporation's Managed Care Division.
Mr. Engert is qualified to be a director of the Company because of his extensive experience in healthcare technology and strategic business development.
E. William Withrow Jr., Director
Mr. Withrow Jr., age 76, has nearly twenty years of experience in the financial investment industry, twenty-four years of experience in the logistics field, and twenty years of experience in civic leadership. From 1997 to 2002, Mr. Withrow Jr. served as a financial consultant for Wells Fargo, a provider of personal banking and investing services. From 1993 to 1997, Mr. Withrow Jr. served as a financial consultant for Merrill Lynch, a financial management and advisory company. From 1987 to 1989, Mr. Withrow Jr. served as a sales manager for Paine Webber, a stock brokerage and asset management firm, and from 1983 to 1987, Mr. Withrow Jr. served as a financial consultant for Drexel Burnham Lambert, an investment banking firm. As a financial consultant and sales manager for the aforementioned financial institutions, Mr. Withrow Jr. examined financial statements, evaluated investment opportunities, provided advice to clients about possible investment opportunities and provided advice to stockbrokers and other individuals attempting to sell securities.
Additionally, Mr. Withrow Jr. served twenty-four years on active duty in the U.S. Navy as a professional logistician, retiring with the rank of Captain.
Mr. Withrow Jr. has been very active in civic leadership for the past 20 years serving in a number of elected and appointed positions, including Mayor of Alameda, California. Mr. Withrow Jr. is currently serving as the regionally elected President of the Governing Board of The Peralta Colleges, an institution consisting of 2,000 faculty and staff and approximately 30,000 students.
Mr. Withrow Jr. received a Bachelor of Business in Finance and Accounting from the University of Colorado in 1959, and in 1972, received a Master in Business Administration from Harvard University.
Mr. Withrow Jr. is qualified to be a director of the Company because of his extensive experience in financial consulting and strategic business development.
Table of Contents
39
Kyle W. Withrow – Former Secretary
Mr. Withrow was appointed Secretary of the Company on November 1, 2012. He resigned his position as Secretary on March 31, 2014. He is succeeded by Ms. Calli Bucci.
Identification of Significant Employees
Dr. Roger Morris, Chief Science Officer
Dr. Roger Morris, age 61, has experience providing over all guidance for technology development, intellectual property and scientific communications strategies. Dr. Morris has twenty years management and technical experience in developing clinical diagnostic systems with Baxter Healthcare Corporation, bioMerieux and most recently XL TechGroup. During this time he has developed and launched a number of successful automated diagnostic system products.
Dr. Morris received his B.Sc. and PhD in biochemistry from the University of Salford, UK and did his Post Doctorate work at Cornell and has published over 20 scientific articles and received 14 patents.
Mike Contarino, Vice President
Mr. Mike Contarino, age 58, has extensive experience developing, integrating and driving complex programs to meet corporate goals. Additionally, Mr. Contarino has directed Regulatory Affairs, Quality Assurance and Manufacturing. As a technical and operations professional, Mr. Contarino brings over 30 years successful leadership in product development, commercialization of complex medical/diagnostic instrumentation, and operations management.
Mr. Contarino was employed by Tecan-Boston, a subsidiary of Tecan AG, where he held the positions of Vice President of R&D, and led all aspects of its novel, automated micro fluidic system platform for Drug Discovery, including overall site management, R&D and Operations.
Previously, Mr. Contarino held the position of Vice President of Systems Development for Instrumentation Laboratory (IL), a global leader in medical diagnostic systems. Mr. Contarino accelerated product development cycles by introducing design control processes and procedures in R&D. At IL, Mr. Contarino was a member of the Executive Committee and Scientific Advisory Boards for homeostasis and critical care, was technical lead of the Merger & Acquisition (M&A) team, and had responsibility for R&D sites in the US, Italy and Spain.
Prior to IL, Mr. Contarino was the Director of Engineering at KMC Systems Inc. a recognized leader in systems development of diagnostic platforms. Mr. Contarino successfully commercialized immunoassay, clinical chemistry and robotic pipetting systems.
Mr. Contarino earned his Mechanical Engineering degree from the University of Lowell, completed the Management Development Program at Boston University and is a Member of the Society of Automotive Engineers (SAE).
Dr. David Stark, Consultant
Dr. Stark has 18 years experience from the toxicology labs to the investigator site and has been essential to all aspects clinical and device research. Dr. Stark is the President and CEO of Stark-SMO, a Site Management Organization whose services go far beyond that of an ordinary SMO. Due to his extensive and broad experiences in the inner workings of the research and regulatory aspects of clinical trials, Dr. Stark brings a unique vision to the industry and the Company as a motivated designer of superior approaches to research challenges. Most importantly, Dr. Stark is highly qualified to manage the development opportunities of the Company.
Formerly the Director of the National Institute of Clinical Research (NICR), he has been responsible for the design, organization and implementation of clinical trials for pharmaceutical and device companies. He has a broad background in designing, conducting, and monitoring clinical trials of new pharmaceuticals and devices. He is one of the few that has worked in the manufacturing validation of pharmaceuticals, the clinical field, and the regulatory (IRB) arenas, and therefore possesses a big-picture understanding of pharmaceutical development.
Through Dr. Stark's diverse and devoted networking within the industry, Stark-SMO has assembled a wide network of more than 5000 physicians throughout the United States, which extends to the international community. Currently, he is negotiating a unique DMF partnership with drug manufacturers in China.
In addition to his significant accomplishments on the industry side of clinical drug and device development, Dr. Stark has experience with the FDA (major focus on IND's NDA's and 510K applications). Prior to his employment at NICR, Dr. Stark was the President and Chief Executive Officer of Powder Ice, Inc a medical products company. Additionally, Dr. Stark is a California state licensed Qualified Medical Examiner and Certified Clinical Research Associate.
The Company does not expect any other individuals to make a significant contribution to the Company's business.
40
Family Relationships
There are no family relationships among its directors or executive officers, with the exception of the following:
Mr. E. William Withrow Jr. is the father of Mr. Edward W. Withrow III and Mr. Kyle Withrow. Mr. Edward W. Withrow III and Mr. Kyle Withrow are brothers.
Involvement in Certain Legal Proceedings
The Company's directors, executive officers and control persons have not been involved in any of the following events during the past five years:
1.
any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
2.
any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences);
3.
being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or
4.
being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated.
Audit Committee and Audit Committee Financial Expert
The Company intends to establish an audit committee of the board of directors. The audit committee's duties will be to recommend to the Company's board of directors the engagement of an independent registered public accounting firm to audit the Company's financial statements and to review the Company's accounting and auditing principles. The audit committee will review the scope, timing and fees for the annual audit and the results of audit examinations performed by the internal auditors and independent registered public accounting firm, including their recommendations to improve the system of accounting and internal controls. The audit committee will at all times be composed exclusively of directors who are, in the opinion of the Company's board of directors, free from any relationship which would interfere with the exercise of independent judgment as a committee member and who possess an understanding of financial statements and generally accepted accounting principles.
Code of Ethics
The Company has adopted a Code of Ethics within the meaning of Item 406(b) of Regulation S-K of the Securities Exchange Act of 1934. The Code of Ethics applies to directors and senior officers, such as the principal executive officer, principal financial officer, controller, and persons performing similar functions.
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Securities Exchange Act of 1934 requires the Company's directors and executive officers and persons who beneficially own more than ten percent of a registered class of the Company's equity securities to file with the SEC initial reports of ownership and reports of change in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent stockholders are required by SEC regulations to furnish the Company with copies of all Section 16(a) forms they file. Based solely upon a review of Forms 3 and 4 and amendments thereto furnished to the Company under Rule 16a-3(e) during the year ended December 31, 2013, Forms 5 and any amendments thereto furnished to the Company with respect to the year ended December 31, 2013, and the representations made by the reporting persons to the Company, the Company believes that during the year ended December 31, 2013, its executive officers and directors and all persons who own more than ten percent of a registered class of the Company's equity securities complied with all Section 16(a) filing requirements.
ITEM 11. | EXECUTIVE COMPENSATION |
Summary Compensation Table
The table below summarizes the compensation paid by the Company to the following persons:
(a)
its principal executive officer;
(b)
each of the Company's two most highly compensated executive officers who were serving as executive officers at the end of the years ended December 31, 2013 and 2012; and
(c)
up to two additional individuals for whom disclosure would have been provided under (b) but for the fact that the individual was not serving as the Company's executive officer at the end of the years ended December 31, 2013 and 2012.
No disclosure is provided for any named executive officer, other than the Company's principal executive officers, whose total compensation did not exceed $100,000 for the respective fiscal year:
Table of Contents
41
SUMMARY COMPENSATION TABLE | ||||||||||||||
|
|
| Salary | Bonus | Stock Award | Option Awards | Non-Equity Incentive Plan Compensation | Change in Pension Value and Nonqualified Deferred Compensation Earnings | All Other Compensation | Total | ||||
Name and Principal Position | Year | ($) | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||
J. Michael Redmond President, CEO, Director | 2013 | None |
| None | None |
| None |
| None | None | 225,865 | [1] | 225,865 | |
2012 | 130,770 |
| None | 330 |
| None |
| None | None | 86,442 | [1] | 217,542 | ||
Calli Bucci CFO, Secretary | 2013 | None |
| None | None |
| None |
| None | None | 30,000 | [1] | 30,000 | |
2012 | None |
| None | 382 |
| None |
| None | None | 16,500 | [1] | 16,882 | ||
Edward W. Withrow III Executive Chairman | 2013 | None |
| None | None |
| None |
| None | None | 150,000 | [1] | 150,000 | |
2012 | None |
| None | 18,343 |
| None |
| None | None | 150,000 | [1] | 168,343 | ||
Gardner Williams Former President, CEO, CFO, | 2013 | None |
| None | None |
| None |
| None | None | None |
| None | |
2012 | None |
| None | None |
| None |
| None | None | 11,250 | [1] | 11,250 | ||
Tom Mackay Former President, CEO, CFO, | 2013 | None |
| None | None |
| None |
| None | None | None |
| None | |
2012 | None |
| None | None |
| None |
| None | None | 26,250 | [1] | 26,250 | ||
[1] | Compensation earned, but accrued and deferred until the Company reaches certain funding goals. | |||||||||||||
Employment Contracts and Termination of Employment and Change in Control Arrangements
On November 15, 2010, the Company entered into an employment agreement with its CEO, Mr. J. Michael Redmond (the "Employment Agreement"). Under the Employment Agreement, Mr. Redmond agrees to serve as the President, CEO, and Director of the Company for a term of three years. Mr. Redmond shall have such authority, and the Company's board of directors may reasonably assign responsibility to him. As compensation for his services, Mr. Redmond's base salary will be $200,000 per annum for the first year, increasing to $225,000 in year 2, and $250,000 in year 3, contingent upon the Company meeting certain goals . In addition, Mr. Redmond was granted one million three hundred seventy five thousand (1,375,000) options of the Company's common stock under the Company's 2010 Employee Stock Option Plan. The options vest on a quarterly basis over a three year period at an exercise price of ten cents ($0.10) per share.
There are no other employment contracts, compensatory plans or arrangements, including payments to be received from the Company with respect to any executive officer, that would result in payments to such person because of his or her resignation, retirement or other termination of employment with the Company, or its subsidiaries, any change in control, or a change in the person's responsibilities following a change in control of the Company.
There are no agreements or understandings for any executive officer to resign at the request of another person. None of the Company's executive officers acts or will act on behalf of or at the direction of any other person.
Equity Compensation Plan
On October 1, 2010, the board of directors of the Company adopted the 2010 Employee Stock Option Plan (the "2010 Plan"). Under the 2010 Plan, 2,800,000 restricted shares of common stock have been reserved for issuance upon exercise of options granted from time to time under the stock option plan. The 2010 plan is intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. Under the 2010 Plan, the Company may grant incentive stock options only to key employees and employee directors, or the Company may grant non-qualified options to employees, officers, directors and consultants. Subject to the provisions of the 2010 Plan, the board of directors will determine who shall receive options, the number of shares of common stock that may be purchased under the options. As of December 31, 2013, the Company has granted options to purchase a total of 1,950,000 shares, of which 50,000 were cancelled in October 2012. In connection with the options granted, a total of $281,250 was recorded as deferred compensation, and was amortized over a 12-18 month vesting period.
On March 26, 2011, the Company adopted and approved the 2011 Equity Incentive Plan ("the 2011 Plan"), wherein 20,000,000 restricted shares of common stock were reserved for issuance. The 2011 Plan was intended to assist the Company in securing and retaining key employees, directors and consultants by allowing them to participate in the Company's ownership and growth through the grant of incentive and non-qualified options. The 2011 Plan is currently administered by the Company's board of directors. Subject to the provisions of the plan, the board will determine who shall receive options, the number of shares of common stock that may be purchased under the options. Under the 2011 Plan, no options have been granted.
42
Stock Options/SAR Grants
There were no stock options granted to directors and officers through the 2010 ESOP during the years ended December 31, 2013 or 2012.
Aggregated Option Exercised in Last Fiscal Year
There were no options exercised during the years ended December 31, 2013 or 2012, by any officer or director of the Company.
Outstanding Equity Awards at Fiscal Year End
| Number of | Number of |
|
|
| Options | Options | Exercise | Expiration |
Name | Exercisable | Unexercisable | Price | Date |
J Michael Redmond | 1,375,000 | - | $0.10 | 10/31/2020 |
Dr Roger Morris | 150,000 | - | $0.25 | 11/15/2015 |
Michael Contarino | 150,000 | - | $0.25 | 11/15/2015 |
Dr. David Stark | 75,000 | - | $0.25 | 01/10/2016 |
Ricky Richardson | 150,000 | - | $0.25 | 01/28/2016 |
| 1,900,000 |
|
|
|
On October 31, 2010, the Company, under its 2010 Plan, granted qualified stock options to its Chief Executive Officer to purchase 1,375,000 shares of the Company's common stock for five years at $0.10 per share, which vest quarterly over a period of twelve months. As of the date of this filing, the options are fully vested, and were expensed by the Company over the vesting period of 12 months, at an aggregate value of $137,500.
On November 15, 2010, the Company, under its 2010 Plan, granted qualified stock options to two of its consultants to purchase 300,000 shares of the Company's common stock for five years at $0.25 per share, which vest quarterly over a period of eighteen months. As of the date of this filing, the options are fully vested, and were expensed by the Company over the vesting period of 18 months, at an aggregate value of $75,000.
On January 10, 2011, the Company, under its 2010 Plan, granted qualified stock options to one of its consultants to purchase 75,000 shares of the Company's common stock at a price of $0.25 per share, vesting quarterly over a period of eighteen months. As of the date of this filing, the options are fully vested, and were expensed by the Company over the vesting period of 18 months, at an aggregate value of $18,750.
On January 28, 2011, the Company, under its 2010 Plan, granted qualified stock options to one of its consultants to purchase 150,000 shares of the Company's common stock at a price of $0.25 per share, vesting quarterly over a period of eighteen months. As of the date of this filing, the options are fully vested, and were expensed by the Company over the vesting period of 18 months, at an aggregate value of $37,500.
Compensation of Directors
The Company reimburses its directors for expenses incurred in connection with attending board meetings. The Company has no formal plan for compensating its directors for their service in their capacity as directors. However, certain directors and officers of the Company have received stock options to purchase common shares under the Company's 2010 Plan, and may receive additional stock options at the discretion of the Company's board of directors. The Company has not paid any other cash compensation or director's fees for services rendered as a director since the Company's inception to the date of this filing.
Pension, Retirement or Similar Benefit Plans
As of December 31, 2013, the Company had no pension plans or compensatory plans or other arrangements which provide compensation in the event of termination of employment or change in control of us. There are no arrangements or plans in which the Company provides pension, retirement or similar benefits for directors or executive officers. The Company has no material bonus or profit sharing plans pursuant to which cash or non-cash compensation is or may be paid to its directors or executive officers, except that stock options may be granted at the discretion of the Board of Directors or a committee thereof.
Compensation Committee
The Company currently does not have a compensation committee of the Board of Directors. The Board of Directors as a whole determines executive compensation.
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth, as of March 31, 2014, certain information with respect to the beneficial ownership of its common stock by each stockholder known by the Company to be the beneficial owner of more than 5% of its common stock and by each of its current directors and executive officers. Each person has sole voting and investment power with respect to the shares of common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated.
Table of Contents
43
Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership [1] | Percentage of Shares of Common Stock | ||
Montecito BioSciences, Ltd. 1327 Ocean Avenue, Suite M Santa Monica, CA 90401 | 38,156,227 | [2] | 30.21% | |
Shahla Melamed 465 Roxbury Drive Beverly Hills, CA 90210 | 11,459,279 |
| 9.07% | |
Jorn & Jennifer Gorlach 3194 Quarry Road Manchester, NJ 08759 | 10,683,743 | [3] | 8.46% | |
Edward W. Withrow III 1327 Ocean Avenue, Suite B Santa Monica, CA 90401 | 7,631,245 | [2] [4] [5] | 6.04% | |
Johann Bastian Deuterstrasse 11B4 Neusass, Germany D-86359 | 7,500,000 |
| 5.94% | |
Alexander Hoffman Deuterstrasse 11B4 Neusass, Germany D-86359 | 7,500,000 |
| 5.94% | |
Withrow Sinclair & Co. 1327 Ocean Avenue, Suite M Santa Monica, CA 90401 | 5,721,900 | [4] | 4.53% | |
M. Katsuka Sandoval 1327 Ocean Avenue, Suite B Santa Monica, CA 90401 | 5,000,000 | [5] | 3.96% | |
AvanteGarde LLC 3194 Quarry Road Manchester, NJ 08759 | 4,960,310 | [3] | 3.93% | |
J. Michael Redmond 10 Canterbury Road Windham, NH 03087 | 454,241 1,375,000 | [7] | 0.36% | |
Calli Bucci 1327 Ocean Avenue, Suite M Santa Monica, CA 90401 | 381,562 |
| 0.30% | |
Edward W. Withrow Jr. 133 Cumberland Way Alameda, CA 94502 | 152,625 |
| 0.12% | |
Total | 99,601,132 |
| 78.86% | |
1,375,000 |
| ESOP | ||
| ||||
[1] | Based upon 126,303,018 shares issued and outstanding at March 31, 2014 [6] . The number and percentage of shares beneficially owned is determined under rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. | |||
[2] | 5% shareholder Montecito BioSciences Ltd. controlled by Edward W. Withrow III, the Company's Executive Chairman (45%) and Dr. Jorn Gorlach, a director of the Company (45%) | |||
[3] | 5% shareholder Avantgarde LLC controlled by to Dr. Jorn Gorlach, a director of the Company. | |||
[4] | 5% shareholder Withrow Sinclair & Co. controlled by to Edward W. Withrow III, the Company's Executive Chairman | |||
[5] | 5% shareholder M. Katsuka Sandoval by marriage to Edward W. Withrow III | |||
[6] | Represents shares held after cancellation of 36,462,819 shares on January 7, 2014. | |||
[7] | Stock options granted under 2010 ESOP, fully vested | |||
|
|
|
| |
|
|
|
| |
Directors and officers as a group (5 shareholders) | 19,303,416 |
| 15.28% | |
More than 5% ownership (7 shareholders) | 80,297,716 |
| 63.58% | |
|
|
|
| |
Total | 90,601,132 |
| 78.86% | |
|
|
|
| |
44
Changes in Control
On November 1, 2012, the Company, and its wholly owned subsidiary Endeavor Holdings, Inc. ("Endeavor Holdings") entered into an Agreement and Plan of Merger (the "Merger Agreement") with Parallax Diagnostics, Inc, a Nevada corporation ("Parallax") and the shareholders of Parallax (the "Parallax Shareholders"), whereby Endeavor Holdings acquired 24,870,000 shares of common stock (100%) of Parallax (the "Parallax Stock") from the Parallax Shareholders. In exchange for the Parallax Stock, the Company issued 90,375,750 shares of its common stock to the Parallax Shareholders. The 90,375,750 shares, issued at par value $.001, represent approximately 60% of the Company's total issued and outstanding shares. The Common Stock Purchase Agreement, and subsequent transaction closing, was completed on October 22, 2012. On October 27, 2012, the Common Stock Purchase Agreement was finalized, and a change in control of the Registrant took place.
As a result of the transactions effected by the Merger Agreement, (i) Parallax merged with and into Endeavor Holdings whereupon Endeavor Holdings continued as the surviving entity and the corporate existence of Parallax ceased; (ii) the former business of Parallax is now the Company's primary business, (iii) the Company's existing business activities will continue as ancillary operations, and (iv) there is a change of control whereby the former shareholders of Parallax now own a controlling 60% ownership interest in the Company on a fully diluted basis.
As a further condition of the Merger Agreement, the current officer and director of the Company, Mr. Gardner Williams, resigned from all positions, and Mr. J. Michael Redmond was appointed to serve as Chief Executive Officer and President of the Company, and also as a Director on the Board of Directors. Additionally, Ms. Calli Bucci was appointed to serve as the Company's Treasurer and Chief Financial Officer, Mr. Kyle W. Withrow was appointed to serve as the Company's Corporate Secretary, Dr. Roger Morris was appointed to serve as the Company's Chief Science Officer, and Mr. Mike Contarino was appointed to serve as the Company's Vice President. Mr. Edward W. Withrow III was appointed to serve as Executive Chairman of the Board of Directors, and Mr. Redmond, Dr. Jorn Gorlach, Mr. Anand Kumar, Mr. David Engert and Mr. E. William Withrow Jr. were appointed to serve as Directors.
On November 26, 2012, Parallax Diagnostics, Inc. changed its name to Endeavor Sciences, Inc. ("ESI"), and is a wholly owned subsidiary of Parallax Health Sciences, Inc.
The Company is unaware of any contract or other arrangement or provisions of its Articles or Bylaws the operation of which may at a subsequent date result in a change of control of the Company. There are not any provisions in its Articles or Bylaws, the operation of which would delay, defer, or prevent a change in control of its company.
ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Related Party Transactions
None of the directors or executive officers of the Company, nor any person who owned of record or was known to own beneficially more than 5% of the Company's outstanding shares of its Common Stock, nor any associate or affiliate of such persons or companies, has any material interest, direct or indirect, in any transaction that has occurred during the year ended December 31, 2013, or in any proposed transaction, which has materially affected or will affect the Company, with the exception of the following:
Montecito BioSciences, Ltd. ("Montecito") is a beneficial ownership shareholder of the Company. The President of Montecito is Edward W. Withrow III, the Company's Executive Chairman. Calli Bucci, the Company's Chief Financial Officer and Secretary, is the Corporate Secretary of Montecito.
Withrow Sinclair & Company ("Withrow Sinclair") is a beneficial ownership shareholder of the Company. The President of Withrow Sinclair is Edward W. Withrow III, the Company's Executive Chairman. Calli Bucci, the Company's Chief Financial Officer and Secretary, is the Chief Financial Officer of Withrow Sinclair.
On November 15, 2010, the Company entered into an employment agreement with its CEO, Mr. J. Michael Redmond (the "Employment Agreement"). Under the Employment Agreement, Mr. Redmond agrees to serve as the President, CEO, and Director of the Company for a term of three years. As compensation for his services, Mr. Redmond's base salary will be $200,000 per annum for the first year, increasing to $225,000 in year 2, and $250,000 in year 3, contingent upon the Company meeting certain goals. In addition, Mr. Redmond was granted one million three hundred seventy five thousand (1,375,000) options at an exercise price of $0.10 per share. As of December 31, 2013, $411,827 has been recorded as accrued compensation.
On September 30, 2010, the Company issued a Convertible Note Payable in the amount of $144,000 to a non-related party pursuant to a Confidential Settlement Agreement. On January 1, 2012, the note was assigned and purchased by The Kasper Group. Ltd., a related party, and a new Convertible Promissory Note was issued (the "Kasper Note"). The Kasper Note bears interest at a rate of 7% per annum, is due by January 1, 2015, and contains a repayment provision to convert the debt into common stock of the Company at a rate of $0.25 per share. As of December 31, 2013, interest in the amount of $20,160 has been accrued.
Table of Contents
45
On January 2, 2012, the Company entered into a consulting agreement with Huntington Chase Financial Group LLC ("HCFG"), a Nevada corporation, whose principal is a related party. The consulting agreement provides for HCFG to provide advisory services to the Company for a period of three years for a fee of $12,500 per month, which has been deferred until such time as the Company reaches certain funding goals. As of December 31, 2013, $300,000 has been recorded as accrued compensation. On December 31, 2012, the Company issued a Convertible Note Payable in the amount of $28,500 to HCFG for cash loans made to the Company of $14,000 during 2011 and $14,500 during 2012. The note bears interest at a rate of 7% per annum, is due by December 31, 2015, and contains a repayment provision to convert the debt into common stock of the Company at a rate of $0.25 per share. On September 30, 2013, the Company modified the note to increase the principal balance by $17,600 for additional cash loans made to the Company. On March 31, 2014, the Company modified the note to 1) increase the principal amount to $383,600, to include $337,500 in compensation owed to HCFG under the consulting agreement through March 31, 2014; and 2) modify the conversion strike price to $0.10. As of December 31, 2013, interest in the amount of $2,834 has been accrued.
On April 4, 2013, the Company received a Notice to Convert from one of its preferred shareholders, requesting that 1/3 (one-third) of his 36,339 shares of preferred stock holdings be converted into common shares. As a result, 12,112 shares of preferred stock were converted into 242,660 shares of the Company's restricted common stock were issued at a conversion ratio of 20 common shares for each preferred share held, and preferred paid in capital was reduced by $33,321.
On January 7, 2014, 36,462,819 shares of the Company's common stock were purchased from certain shareholders by its Chairman, Edward W. Withrow III. On the same day, said shares were cancelled, for the benefit of the Company.
Director Independence
For purposes of determining director independence, the Company have applied the definitions set out in NASDAQ Rule 5605(a)(2). The OTCQB on which shares of Common Stock are quoted does not have any director independence requirements. The NASDAQ definition of "Independent Officer" means a person other than an Executive Officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company's Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. According to the NASDAQ definition, J. Michael Redmond is not an independent director of the Company.
ITEM 14. | PRINCIPAL ACCOUNTANTS FEES AND SERVICES |
The aggregate fees billed or to be billed for the most recently completed fiscal year ended December 31, 2013 and 2012 for professional services rendered by the principal accountant for the audit of its annual financial statements and review of the financial statements included in its quarterly reports on Form 10-Q and services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for these fiscal periods were as follows:
| Year Ended | |
| December 31, 2013 $ | December 31, 2012 $ |
Audit Fees | 11,250 | 9,000 |
Audit Related Fees | 46 | 0 |
Tax Fees | 0 | 0 |
All Other Fees | 0 | 0 |
Total | 11,296 | 9,000 |
The Company's board of directors pre-approves all services provided by its independent auditors. All of the above services and fees were reviewed and approved by the board of directors either before or after the respective services were rendered.
The Company's board of directors has considered the nature and amount of fees billed by its independent auditors and believes that the provision of services for activities unrelated to the audit is compatible with maintaining its independent auditors' independence.
46
PART IV
ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
Exhibits required by Item 601 of Regulation S-B
Exhibit Number | Description of Exhibit | Filing Reference |
(2) | Plan of Purchase, Sale, Reorganization, Arrangement, Liquidation or Succession | |
2.1 | Share Exchange Agreement between Endeavor Power Corporation, Endeavor Holdings, Inc. and Parallax Diagnostics, Inc. and the Parallax Shareholders dated October 1, 2012 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
2.2 | Letter of Intent between Parallax Diagnostics, Inc. and Endeavor Power Corporation dated August 15, 2012 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
(3) | Articles of Incorporation and Bylaws | |
3.1 | Articles of Incorporation | Filed with the SEC on March 5, 2007 as part of the Company's Registration Statement on Form SB-2. |
3.1(a) | Amended and Restated Articles of Incorporation | Filed with the SEC on May 17, 2010 as part of the Company's Annual Report on Form 10-K. |
3.2 | Bylaws | Filed with the SEC on March 5, 2007 as part of the Company's Registration Statement on Form SB-2. |
3.2(a) | Amended Bylaws | Filed with the SEC on May 17, 2010 as part of the Company's Annual Report on Form 10-K. |
3.3 | Articles of Merger between Endeavor Power Corporation and Parallax Diagnostics, Inc. filed with Secretary of State of Nevada on November 6, 2012 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
3.4* | Certificate of Amendment filed with the Secretary of State of Nevada on January 9, 2014 | Filed herewith. |
(4) | Instruments Defining the Rights of Security Holders | |
4.1 | 2011 Equity Incentive Plan dated March 26, 2011 | Filed with the SEC on March 31, 2011 as part of the Company's Current Report on Form 8-K. |
4.2 | Sample Stock Option Agreement | Filed with the SEC on March 31, 2011 as part of the Company's Current Report on Form 8-K. |
4.3 | Sample Stock Award Agreement for Stock Units | Filed with the SEC on March 31, 2011 as part of the Company's Current Report on Form 8-K. |
4.4 | Sample Stock Award Agreement for Restricted Stock | Filed with the SEC on March 31, 2011 as part of the Company's Current Report on Form 8-K. |
4.5 | 2010 Employee Stock Option Plan of Parallax Diagnostics, Inc, dated October 1, 2010 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
4.6 | Sample Stock Option Agreement | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
(10) | Material Contracts | |
10.1 | Second Amendment to Joint Venture Agreement between the Company and Federated Energy Corporation dated September 15, 2009 | Filed with the SEC on September 19, 2009 as part of the Company's Current Report on Form 8-K. |
10.2 | Farmount Agreement between the Company and Togs Energy, Inc. and M-C Production & Drilling Co, Inc. dated July 21, 2009 | Filed with the SEC on July 23, 2009 as part of the Company's Current Report on Form 8-K. |
10.3 | Convertible Promissory Note to Regal Capital Development, Inc. dated August 25, 2009 | Filed with the SEC on September 4, 2009 as part of the Company's Current Report on Form 8-K. |
10.4 | Common Stock Purchase Warrant to Regal Capital Development, Inc. dated August 25, 2009 | Filed with the SEC on September 4, 2009 as part of the Company's Current Report on Form 8-K. |
10.5 | Settlement Agreement between the Company and Regal Capital Development, Inc. dated September 11, 2010 | Filed with the SEC on July 12, 2010 as part of the Company's Current Report on Form 8-K. |
10.6 | Promissory Note to Regal Capital Development, Inc. dated September 11, 2010 | Filed with the SEC on July 12, 2010 as part of the Company's Current Report on Form 8-K. |
10.7 | Amended Promissory Note to Regal Capital Development, Inc. dated September 11, 2010 | Filed with the SEC on April 14, 2011 as part of the Company's Annual Report on Form 10-K. |
10.8 | Settlement Agreement between the Company and Andrew I. Telsey, P.C., dated August 3, 2010 | Filed with the SEC on August 22, 2011 as part of the Company's Quarterly Report on Form 10-Q. |
10.9 | Settlement Agreement between the Company and Regal Capital Development, Inc. dated September 17, 2010 | Filed with the SEC on October 21, 2010 as part of the Company's Current Report on Form 8-K. |
10.10 | Promissory Note to Regal Capital Development, Inc. dated September 17, 2010 | Filed with the SEC on October 21, 2010 as part of the Company's Current Report on Form 8-K. |
10.11 | Employment Agreement between the Company and Alfonso Knoll dated November 8, 2010 | Filed with the SEC on November 12, 2010 as part of the Company's Current Report on Form 8-K. |
10.12 | Promissory Note to Regal Capital Development, Inc. dated November 23, 2010 | Filed with the SEC on November 30, 2010 as part of the Company's Current Report on Form 8-K. |
10.13 | Amendment to Employment Agreement between the Company and Alfonso Knoll dated November 17, 2010 | Filed with the SEC on November 30, 2010 as part of the Company's Current Report on Form 8-K. |
10.14 | Consulting Agreement between the Company and The Musser Group, LLC dated February 21, 2011 | Filed with the SEC on February 25, 2011 as part of the Company's Current Report on Form 8-K. |
10.15 | Promissory Note to Marans Invest & Finance S.A. dated April 8, 2011 | Filed with the SEC on August 22, 2011 as part of the Company's Quarterly Report on Form 10-Q. |
10.16 | Promissory Note to Rast Trade Corp. dated April 21, 2011 | Filed with the SEC on August 22, 2011 as part of the Company's Quarterly Report on Form 10-Q. |
10.17 | Settlement Agreement between the Company and Mr. Alfonso Knoll dated June 8, 2011 | Filed with the SEC on June 16, 2011 as part of the Company's Current Report on Form 8-K. |
10.18 | Settlement Agreement between the Company and The Musser Group, LLC dated July 19, 2011 | Filed with the SEC on August 22, 2011 as part of the Company's Quarterly Report on Form 10-Q. |
10.19 | Assignment of Intellectual Property between Roth Kline, Inc. and Montecito BioSciences, Ltd. dated September 10, 2010 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.20 | License of Intellectual Property between Roth Kline Inc. and Montecito BioSciences, Ltd. dated September 10, 2010 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.21 | Modification to the Assignment of Intellectual Property between Roth Kline, Inc. and Montecito BioSciences, Ltd. | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.22 | Modification to the License of Intellectual Property between Roth Kline Inc. and Montecito BioSciences, Ltd. dated September 10, 2010 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.23 | Employment Agreement between Roth Kline, Inc. and Michael Redmond dated November 15, 2010 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.24 | Development and Supply Agreement between Parallax Diagnostics, Inc. and Corder Engineering, LLC dated July 1, 2011 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.25 | Supply Agreement between Parallax Diagnostics, Inc. and Meyer Stevens Group, Inc. dated July 1, 2011 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.26 | Consulting Agreement between Parallax Diagnostics, Inc. and Huntington Chase Financial Group, LLC dated January 2, 2012 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.27 | Consulting Agreement between Parallax Diagnostics, Inc. and Greg Suess dated July 11, 2012 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.28 | Convertible Preferred Purchase Agreement between Parallax Diagnostics, Inc. and Hamburg Investment Company, LLC, dated June 17, 2011 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.29 | Convertible Preferred Purchase Agreement between Parallax Diagnostics, Inc. and Huntington Chase Financial Group, LLC, dated June 17, 2011 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.30 | Convertible Preferred Purchase Agreement between Parallax Diagnostics, Inc. and Huntington Chase Financial Group, LLC, dated September 30, 2011 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
10.31 | Consulting Agreement between Endeavor Power Corporation and Capital Group Communications, Inc. dated January 10, 2013 | Filed with the SEC on May 15, 2013 as part of the Company's Quarterly Report on Form 10-Q. |
(14) | Code of Ethics | |
14.1 | Code of Ethics | Filed with the SEC on April 14, 2011 as part of the Company's Annual Report on Form 10-K. |
(16) | Letter Re Change in Certifying Accountant | |
16.1 | Letter from Moore and Associates, Chartered dated August 13, 2009 | Filed with the SEC on August 13, 2009 as part of the Company's Current Report on Form 8-K. |
16.2 | Letter from Seale & Beers, CPAs dated August 26, 2009 | Filed with the SEC on August 27, 2009 as part of the Company's Current Report on Form 8-K. |
16.3 | Letter from M&K CPAs, PLLC dated March 12, 2010 | Filed with the SEC on March 12, 2010 as part of the Company's Current Report on Form 8-K. |
16.4 | Letter from Ron Chadwick, P.C. dated August 3, 2010 | Filed with the SEC on August 4, 2010 as part of the Company's Current Report on Form 8-K. |
16.5 | Letter from Davis Accounting Group, P.C. dated November 29, 2010 | Filed with the SEC on November 30, 2010 as part of the Company's Current Report on Form 8-K. |
16.6 | Letter from M&K CPAs, PLLC dated October 23, 2012 | Filed with the SEC on October 25, 2012 as part of the Company's Current Report on Form 8-K. |
(23) | Consent Letters | |
23.1* | Letter from Seale & Beers, CPAs dated April 14, 2014 | Filed herewith. |
(31) | Section 302 Certifications | |
31.1* | Section 302 Certification of J. Michael Redmond | Filed herewith. |
31.2* | Section 302 Certification of Calli Bucci | Filed herewith. |
(32) | Section 906 Certifications | |
32.1* | Section 906 Certification of J. Michael Redmond | Filed herewith. |
32.2* | Section 906 Certification of Calli Bucci | Filed herewith. |
(99) | Other Documents | |
99.1 | Confidential Private Placement Memorandum for Parallax Diagnostics dated July 1, 2012 | Filed with the SEC on November 15, 2012 as part of the Company's Current Report on Form 8-K. |
99.2 | Patent Report issued by Marathon Patent Group on April 1, 2013 | Filed with the SEC on April 16, 2013 as part of the Company's Annual Report on Form 10-K. |
(100) | XBRL Related Documents | |
101.INS** | XBRL Instance Document | Filed herewith. |
101.SCH** | XBRL Taxonomy Extension Schema Document | Filed herewith. |
101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document | Filed herewith. |
101.LAB** | XBRL Taxonomy Extension Labels Linkbase Document | Filed herewith. |
101.PRE** | XBRL Taxonomy Extension Presentation Linkbase Document | Filed herewith. |
101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document | Filed herewith. |
*
Filed herewith .
**
Pursuant to Regulation S-T, this interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections.
Table of Contents
47
SIGNATURES
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| PARALLAX HEALTH SCIENCES, INC. |
|
|
|
|
Dated: April 14, 2014 | /s/ J. Michael Redmond |
| J. Michael Redmond |
| President, Chief Executive Officer, and Director |
| (Principal Executive Officer) |
|
|
|
|
Dated: April 14, 2014 | /s/ Calli Bucci |
| Calli Bucci |
| Chief Financial Officer |
| (Principal Financial Officer and Principal Financial Officer) |
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Dated: April 14, 2014 | /s/ J. Michael Redmond |
| J. Michael Redmond |
| President, Chief Executive Officer, and Director |
| (Principal Executive Officer) |
|
|
Dated: April 14, 2014 | /s/ Calli Bucci |
| Calli Bucci |
| Chief Financial Officer |
| (Principal Financial Officer and Principal Accounting Officer) |
|
|
|
|
Dated: April 14, 2014 | /s/ Edward W. Withrow III |
| Edward W. Withrow III |
| Executive Chairman |
|
|
|
|
Dated: April 14, 2014 | /s/ Dr. Jorn Gorlach |
| Dr. Jorn Gorlach |
| Director |
|
|
|
|
Dated: April 14, 2014 | /s/ Anand Kumar |
| Anand Kumar |
| Director |
|
|
|
|
Dated: April 14, 2014 | /s/ David Engert |
| David Engert |
| Director |
|
|
|
|
Dated: April 14, 2014 | /s/ E. William Withrow Jr. |
| E. William Withrow Jr. |
| Director |
|
|
48
