Table of Contents
|
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________
FORM 10-K
______________________________
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2016
or
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-36587
______________________________
CATALENT, INC.
(Exact name of registrant as specified in its charter)
______________________________
Delaware | 20-8737688 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
14 Schoolhouse Road Somerset, New Jersey | 08873 |
(Address of principal executive offices) | (Zip Code) |
______________________________
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered |
Common Stock, $0.01 par value per share | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
______________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Large accelerated filer | x |
| Accelerated filer | o |
Non-accelerated filer | o | (Do not check if a smaller reporting company) | Smaller reporting company | o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
As of December 31, 2015, the aggregate market value of the registrant's voting and non-voting common equity held by non-affiliates was $1.4 billion. On August 22, 2016 there were 124,744,437 shares of the Registrant's Common Stock, par value $0.01 per share, issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant's Proxy Statement relating to the 2016 Annual Meeting of Shareholders are incorporated by reference into Part III of this report.
|
1
Table of Contents
CATALENT, INC.
INDEX TO ANNUAL REPORT ON FORM 10-K
For the Year Ended June 30, 2016
Item |
| Page |
| PART I |
|
|
|
|
| Special Note Regarding Forward-Looking Statements | 3 |
|
|
|
Item 1. | Business | 6 |
|
|
|
Item 1A. | Risk Factors | 19 |
|
|
|
Item 1B. | Unresolved Staff Comments | 32 |
|
|
|
Item 2. | Properties | 33 |
|
|
|
Item 3. | Legal Proceedings | 34 |
|
|
|
Item 4. | Mine Safety Disclosures | 34 |
|
|
|
| PART II |
|
|
|
|
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 35 |
|
|
|
Item 6. | Selected Financial Data | 37 |
|
|
|
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | 39 |
|
|
|
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 63 |
|
|
|
Item 8. | Financial Statements and Supplementary Data | 64 |
|
|
|
Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 110 |
|
|
|
Item 9A. | Controls and Procedures | 110 |
|
|
|
Item 9B. | Other Information | 111 |
|
|
|
| PART III |
|
|
|
|
Item 10. | Directors, Executive Officers and Corporate Governance | 112 |
|
|
|
Item 11. | Executive Compensation | 112 |
|
|
|
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 112 |
|
|
|
Item 13. | Certain Relationships and Related Transactions, and Director Independence | 112 |
|
|
|
Item 14. | Principal Accountant Fees and Services | 112 |
|
|
|
| PART IV |
|
|
|
|
Item 15. | Exhibits and Financial Statement Schedules | 113 |
|
|
|
Signatures | 117 | |
2
Table of Contents
PART I
Special Note Regarding Forward-Looking Statements
In addition to historical information, this Annual Report on Form 10-K of Catalent, Inc. ("Catalent" or the "Company") contains "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), which are subject to the "safe harbor" created by those sections. All statements, other than statements of historical facts, included in this Annual Report on Form 10-K are forward-looking statements. In some cases, you can identify these forward-looking statements by the use of words such as "outlook," "believes," "expects," "potential," "continues," "may," "will," "should," "could," "seeks," "predicts," "intends," "plans," "estimates," "anticipates" or the negative version of these words or other comparable words.
These statements are based on assumptions and assessments made by our management in light of their experience and, their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate. Any forward-looking statement is subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements.
Some of the factors that may cause actual results, developments and business decisions to differ materially from those contemplated by such forward-looking statements include, but are not limited to, those described under the section entitled "Risk Factors" in this Annual Report on Form 10-K for the fiscal year ended June 30, 2016 and the following:
• | We participate in a highly competitive market, and increased competition may adversely affect our business. |
• | The demand for our offerings depends in part on our customers' research and development and the clinical and market success of their products. Our business, financial condition and results of operations may be harmed if our customers spend less on, or are less successful, in these activities. |
• | We are subject to product and other liability risks that could adversely affect our results of operations, financial condition, liquidity, and cash flows. |
• | Failure to comply with existing and future regulatory requirements could adversely affect our results of operations and financial condition. |
• | Failure to provide quality offerings to our customers could have an adverse effect on our business and subject us to regulatory actions and costly litigation. |
• | The services and offerings we provide are highly exacting and complex, and if we encounter problems providing the services or support required, our business could suffer. |
• | Our global operations are subject to economic, political and regulatory risks. |
• | If we do not enhance our existing or introduce new technology or service offerings in a timely manner, our offerings may become obsolete over time, customers may not buy our offerings and our revenue and profitability may decline. |
• | We and our customers depend on patents, copyrights, trademarks, trade secrets and other forms of intellectual property protections, but these protections may not be adequate. |
• | Our future results of operations are subject to fluctuations in the costs, availability, and suitability of the components of the products we manufacture, including active pharmaceutical ingredients, excipients, purchased components, and raw materials. |
• | Changes in market access or healthcare reimbursement for our customers' products in the United States or internationally could adversely affect our results of operations and financial condition by affecting demand for our offerings. |
• | As a global enterprise, fluctuations in the exchange rate of the U.S. dollar against foreign currencies could have a material adverse effect on our financial performance and results of operations. |
3
Table of Contents
• | Tax legislation initiatives or challenges to our tax positions could adversely affect our results of operations and financial condition. |
• | Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. |
• | We are dependent on key personnel. |
• | Risks generally associated with information and communications systems could adversely affect our results of operations. |
• | We have in the past engaged and may in the future engage in acquisitions and other transactions that may complement or expand our business or divest of non-strategic businesses or assets. We may not be able to complete such transactions, and such transactions, if executed, pose significant risks and could have a negative effect on our operations. |
• | Our offerings and our customers' products may infringe on the intellectual property rights of third parties. |
• | We are subject to environmental, health and safety laws and regulations, which could increase our costs and restrict our operations in the future. |
• | We are subject to labor and employment laws and regulations, which could increase our costs and restrict our operations in the future. |
• | Certain of our pension plans are underfunded, and additional cash contributions we may make will reduce the cash available for our business, such as the payment of our interest expense. |
• | Our substantial leverage could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or in our industry, expose us to interest-rate risk to the extent of our variable rate debt and prevent us from meeting our obligations under our indebtedness. |
• | Affiliates of The Blackstone Group L.P. ( " Blackstone " ) have substantial influence over us and their interests may conflict with ours or yours in the future. |
We caution you that the risks, uncertainties and other factors referenced above may not contain all of the risks, uncertainties and other factors that are important to you. In addition, we cannot assure you that we will realize the results, benefits or developments that we expect or anticipate or, even if substantially realized, that they will result in the consequences or affect us or our business in the way expected. There can be no assurance that (i) we have correctly measured or identified all of the factors affecting our business or the extent of these factors' likely impact, (ii) the available information with respect to these factors on which such analysis is based is complete or accurate, (iii) such analysis is correct or (iv) our strategy, which is based in part on this analysis, will be successful. All forward-looking statements in this report apply only as of the date of this report or as of the date they were made and we undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as required by law.
Social Media
We use our website (www.catalent.com), corporate Facebook page (https://www.facebook.com/CatalentPharmaSolutions) and corporate Twitter account (@catalentpharma) as channels of distribution of Company information. The information we post through these channels may be deemed material. Accordingly, investors should monitor these channels, in addition to following our press releases, Securities and Exchange Commission ("SEC") filings and public conference calls and webcasts. The contents of our website and social media channels are not, however, a part of this report.
4
Table of Contents
Trademarks and Service Marks
We have U.S. or foreign registration in the following marks, among others: ADVASEPT ® , OptiForm ® , GPEx ® , Liqui-Gels ® , Vegicaps ® , and Zydis ® . This Annual Report on Form 10-K also includes trademarks and trade names owned by other parties, and these trademarks and trade names are the property of their respective owners. We use certain other trademarks and service marks, including PEEL-ID™, Fastchain™, OptiShell™, OptiPact™, SMARTag™, OptiGel™, OptiGel™ Bio, Easyburst™, Savorgel™, Galacorin™ and Softdrop™ on an unregistered basis in the United States and abroad.
Solely for convenience, the trademarks, service marks and trade names identified in this Annual Report on Form 10-K may appear without the ® and ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks, and trade names.
5
Table of Contents
ITEM 1. | BUSINESS |
Overview
We are the leading global provider of advanced delivery technologies and development solutions for drugs, biologics and consumer and animal health products. Our oral, injectable, and respiratory delivery technologies address the full diversity of the pharmaceutical industry, including small molecules, large molecule biologics and consumer and animal health products. Through our extensive capabilities and deep expertise in product development, we help our customers take products to market faster, including nearly half of new drug products approved by the Food and Drug Administration (the "FDA") in the last decade. Our advanced delivery technology platforms, including those in our Softgel Technologies and Drug Delivery Solutions segments, our proven formulation, manufacturing and regulatory expertise, and our broad and deep intellectual property enable our customers to develop more products and better treatments for patients and consumers. Across both development and delivery, our commitment to reliably supply our customers' and their patients' needs is the foundation for the value we provide; annually, we produce more than 70 billion doses for nearly 7,000 customer products, or approximately 1 in every 20 doses of such products taken each year by patients and consumers around the world. We believe that through our investments in growth-enabling capacity and capabilities, our ongoing focus on operational and quality excellence, the sales of existing customer products, the introduction of new customer products, our innovation activities and patents, and our entry into new markets, we will continue to benefit from attractive and differentiated margins, and realize the growth potential from these areas.
We continue to make investments to expand our sales and marketing activities, leading to growth in the number of active development programs for our customers in both of our two main strategic areas. This has further enhanced our extensive, long-duration relationships and long-term contracts with a broad and diverse range of industry-leading customers. In the fiscal year ended June 30, 2016 , we did business with 87 of the top 100 branded drug marketers, 22 of the top 25 generics marketers, 24 of the top 25 biologics marketers, and 21 of the top 25 consumer health marketers globally . Selected key customers include Pfizer, Johnson & Johnson, GlaxoSmithKline, Novartis, Roche and Teva. We have many long-standing relationships with our customers, particularly in advanced delivery technologies, where we tend to follow a prescription molecule through all phases of its lifecycle, from the development and launch of the original brand prescription, to generics or over-the-counter switch. A prescription pharmaceutical product relationship with an innovator will often last many years, in several cases nearly two decades or more, extending from pre-clinical development through the end of the product's life cycle. We serve customers who require innovative product development, superior quality, advanced manufacturing and skilled technical services to support their development and marketed product needs. Our broad and diverse range of technologies closely integrates with our customers' molecules to yield final dose forms, and this generally results in the inclusion of Catalent in our customers' prescription product regulatory filings. Both of these factors translate to long-duration supply relationships at an individual product level.
We believe our customers value us because our depth of development solutions and advanced delivery technologies, intellectual property, consistent and reliable supply, geographic reach, and substantial expertise enable us to create a broad range of business and product solutions that can be customized to fit their individual needs. Today we employ approximately 1,400 scientists and technicians and hold approximately 1,100 patents and patent applications in advanced delivery, drug and biologics formulation and manufacturing. The aim of our offerings is to allow our customers to bring more products to market faster, and develop and market differentiated new products that improve patient outcomes. We believe our leading market position, significant global scale, and diversity of customers, offerings, regulatory categories, products, and geographies reduce our exposure to potential strategic and product shifts within the industry.
We provide a number of proprietary, differentiated technologies, products and service offerings to our customers across our advanced delivery technologies and development solutions platforms. The core technologies within our advanced delivery technologies platform include softgel capsules, our Zydis oral dissolving tablets, blow-fill-seal unit dose liquids and a range of other oral, injectable and respiratory technologies. The technologies and service offerings within our development solutions platform span the drug development process, ranging from our OptiForm Solutions Suite for bioavailability enhancement of early-stage molecules, and GPEx and SMARTag platforms for development of biologics and antibody-drug conjugates (ADCs), to formulation, analytical services, early stage clinical development, and clinical trials supply, including our unique FastChain demand-led clinical supply solution. Our offerings serve a critical need in the development and manufacturing of difficult-to-formulate products across a number of product types.
We have advanced our technologies and grown our service offerings over more than 80 years through internal development, strategic alliances, in-licensing and acquisitions. We initially introduced our softgel capsule technology in the 1930s and have continued to expand our range of new, technologically enhanced offerings. Since fiscal 2013, we have launched OptiShell, OptiMelt, Zydis Nano and Zydis Bio and OptiPact. In fiscal 2016, we launched OptiForm Solutions Suite and our FastChain demand-led clinical supply solution. Also in 2016, our customers received regulatory approval for first-to-market
6
Table of Contents
products using the OptiShell and ADVASEPT technologies. To extend the reach of our technologies and services, we have also formed several active partnerships, including partnerships with BASF (Germany), CEVEC (Germany), and CTC Bio (South Korea), and have active relationships with research universities around the world. We have also augmented our portfolio through nine acquisitions since fiscal 2012, including significantly expanding our scale through the acquisition of the Aptuit CTS business in February 2012, adding an ADC business through the completion of our acquisition of the Redwood Bioscience business in October 2014, and extending our particle engineering capabilities via our November 2014 acquisition of Micron Technologies, a leader in the category. We believe our own internal innovation, supplemented by current and future external partnerships and acquisitions, will continue to strengthen and extend our leadership positions in the delivery and development of drugs, biologics and consumer and animal health products.
History
Catalent was formed in April 2007, when affiliates of Blackstone acquired the core of the Pharmaceutical Technologies and Services ("PTS") segment of Cardinal Health, Inc. ("Cardinal"). Cardinal had created PTS through a series of acquisitions beginning with R.P. Scherer Corporation in 1998, with the intent of creating the world's leading outsourcing provider of specialized, market-leading solutions to the global pharmaceutical and biotechnology industry. We are a holding company that indirectly owns Catalent Pharma Solutions, Inc. (the "Operating Company"), which owns, directly or indirectly, all of our operating subsidiaries. Since our 2007 acquisition, we have regularly reviewed our portfolio of offerings and operations in the context of our strategic growth plan, and, as a result, we have sold five businesses and consolidated operations at five facilities, integrating them into the remaining facility network. We have also actively acquired new businesses and facilities, completing nine transactions since fiscal 2012. In July 2014, we completed the initial public offering of our common stock (the "IPO"), which is now listed on the New York Stock Exchange (the "NYSE") under the symbol "CTLT."
Our Competitive Strengths
Leading Provider of Advanced Delivery Technologies and Development Solutions
We are the leading global provider of advanced delivery technologies and development solutions for drugs, biologics and consumer and animal health products. In the last decade, we have earned revenue with respect to nearly half of the drugs based on new molecular entities ("NMEs") approved by the FDA, and over the past three years with respect to nearly 80% of the top 200 largest-selling compounds globally. With approximately 1,400 scientists and technicians worldwide and approximately 1,100 patents and patent applications, our expertise is in providing differentiated technologies and solutions that help our customers bring more products and better treatments to market faster. For example, in the high-value area of NCEs, approximately 90% of NCE softgel approvals by the FDA over the last 25 years have been developed and supplied by us .
Diversified Operating Platform
We are diversified by virtue of our geographic scope, our large customer base, the extensive range of products we produce, our broad service offerings, and our ability to provide solutions at nearly every stage of a product ' s lifecycle. We produce nearly 7,000 distinct items across multiple categories, including brand and generic prescription drugs and biologics, over-the-counter, consumer health and veterinary products, medical devices and diagnostics. In fiscal 2016 , our top 20 products represented approximately 25% of total revenue, with no single customer accounting for greater than 10% of revenue and with no individual product greater than 3%. We serve more than 1,000 customers in approximately 80 countries, with a majority of our fiscal 2016 revenues coming from outside the United States. This diversity, combined with long product lifecycles and close customer relationships, has contributed to the stability of our business. It has also allowed us to reduce our exposure to potential strategic, customer and product shifts as well as to payer-driven pricing pressures experienced by our branded drug and biologic customers.
Longstanding, Extensive Relationships with Blue Chip Customers
We have longstanding, extensive relationships with leading pharmaceutical and biotechnology customers. In fiscal 2016 , we did business with 87 of the top 100 branded drug marketers, 22 of the top 25 generics marketers, 24 of the top 25 biologics marketers, and 21 of the top 25 consumer health marketers globally , as well as with more than 1,000 other customers, including emerging and specialty companies, which are often more reliant on outside partners as a result of their more virtual business models. Regardless of size, our customers seek innovative product development, superior quality, advanced manufacturing and skilled technical services to support their development and marketed product needs.
7
Table of Contents
We believe our customers value us because our depth of development solutions and advanced delivery technologies, consistent and reliable supply, geographic reach and substantial expertise enable us to create a broad range of tailored solutions, many of which are unavailable from other individual providers.
Deep, Broad and Growing Technology Foundation
Our breadth of proprietary and patented technologies and long track record of innovation substantially differentiate us from other industry participants. Our leading softgel platforms, including Liqui-Gels, OptiShell and Vegicaps capsules, and our modified release technologies, including the Zydis family, OptiPact and OptiMelt technologies, provide formulation expertise to solve complex delivery challenges for our customers. We offer advanced technologies for delivery of small molecules and biologics via respiratory, ophthalmic and injectable routes, including the blow-fill-seal unit dose technology, ADVASEPT glass-free vials, and prefilled syringes. We also provide advanced biologics formulation options, including Gene Product Expression ("GPEx") cell-line and SMARTag antibody-drug conjugate technologies. We have a market leadership position within respiratory delivery, including metered dose and dry powder inhalers, and intra-nasal forms. We have reinforced our leadership position in advanced delivery technologies over the last four years, as we have launched more than a dozen new technology platforms and applications, including in fiscal 2016 the launch of our Optiform Solutions Suite, a dose form-agnostic bioavailability enhancement for early-stage molecules. Our culture of creativity and innovation is grounded in our advanced delivery technologies, our scientists and engineers, and our patents and proprietary manufacturing processes throughout our global network. Our global product development team drives a focused application of resources to our highest priority opportunities for both new customer product introductions and platform technology development. As of June 30, 2016 , we had approximately 700 product development programs in active development across our businesses.
Long-Duration Relationships Provide Sustainability
Our broad and diverse range of technologies closely integrates with our customers' molecules to yield final dose forms, and this generally results in the inclusion of Catalent in our customers' prescription product regulatory filings. Both of these factors translate to long-duration supply relationships at an individual product level, to which we apply our expertise in contracting to produce long-duration commercial supply agreements. These agreements typically have initial terms of three to ten years with regular renewals of one to three years (see "Contractual Arrangements" for more detail). Nearly two-thirds of our fiscal 2016 advanced delivery technology platform revenues (comprised of our Softgel Technologies and Drug Delivery Solutions reporting segments) were covered by such long-term contractual arrangements. We believe this base provides us with a sustainable competitive advantage.
Significant Recent Growth Investments
We have made significant investments over time to establish a global manufacturing network, and today employ 5.1 million square feet of manufacturing and laboratory space across five continents. We have invested approximately $630 million in the last five fiscal years in gross capital expenditures. Growth-related investments in facilities, capacity and capabilities across our businesses have positioned us for future growth in areas aligned with anticipated future demand. Through our focus on operational, quality and regulatory excellence, we drive ongoing and continuous improvements in safety, productivity and reliable supply to customer expectations, which we believe further differentiate us. Our manufacturing network and capabilities allow us the flexibility to reliably supply the changing needs of our customers while consistently meeting their quality, delivery and regulatory compliance expectations.
High Standards of Regulatory Compliance and Operational and Quality Excellence
We operate our plants in accordance with current good manufacturing practices ("cGMP"), following our own high standards that are consistent with those of many of our large global pharmaceutical and biotechnology customers. We have more than 1,100 employees around the globe focused on quality and regulatory compliance. More than half of our facilities are registered with the FDA , with the remaining facilities registered with other applicable regulatory agencies, such as the European Medicines Agency (the "EMA"). In some cases, facilities are registered with multiple regulatory agencies. In fiscal 2016 , we were subject to 49 regulatory audits and, over the last five fiscal years, we successfully completed more than 250 regulatory audits . We also undergo more than 400 customer and internal audits annually. We believe our quality and regulatory track record to be a competitive differentiator for Catalent.
8
Table of Contents
Strong and Experienced Management Team
Our executive leadership team collectively has more than 200 years of combined and diverse experience within the pharmaceutical and healthcare industries. With an average of more than 20 years of functional experience, this team possesses deep knowledge and a wide network of industry relationships.
Our Strategy
We are pursuing the following key growth initiatives:
"Follow the Molecule" by Providing Solutions to our Customers across all Phases of the Product Lifecycle
We intend to use our advanced delivery technologies and development solutions across the entire lifecycle of our customers' products to drive future growth. Our development solutions span the drug development process, starting with our platforms for development of small molecules, biologics and antibody-drug conjugates, to formulation and analytical services, through early stage clinical development and manufacturing of clinical trials supply, to regulatory consulting. Once a molecule is ready for late-stage trials and subsequent commercialization, we provide our customers with a range of advanced delivery technologies and manufacturing expertise that allow them to deliver their molecules to the end-users in appropriate dosage forms. The relationship between a molecule and our advanced delivery technologies typically starts with developing and manufacturing the innovator product, then extends throughout the molecule's commercial life, including through potential generic launches or over-the-counter conversion. For prescription products, we are typically the sole and/or exclusive provider, and are reflected in customers' new drug applications.
Our breadth of solutions gives us multiple entry points into the lifecycle of our customers' molecules. Our initial commercial opportunity arises during the discovery and development of a molecule, when our development and particle engineering solutions can be applied. Once a product reaches late-stage development, we can provide our customers with drug delivery solutions for the commercialization of their products. We have two additional entry points during the commercial phase: upon loss-of-exclusivity and upon conversion to over-the-counter status. At these points, we partner with the makers and marketers of both generic and over-the-counter products to provide them with advanced delivery technologies that can be applied to their products through these stages of the product lifecycle. Our revenues from our advanced delivery technologies are primarily driven by volumes and, as a result, the loss of exclusivity may not have a significant negative impact if we continue to work with both branded and generic partners.
An example of this can be found in a leading over-the-counter respiratory brand, which today uses both our Zydis fast dissolve and our Liqui-Gels softgel technologies. We originally began development of the prescription format of this product for our multinational pharmaceutical company partner in 1992 to address specific patient sub-segment needs. After four years of development, we then commercially supplied the prescription Zydis product for six years, and we have continued to provide the Zydis form since the switch to over-the-counter status in the United States and other markets in the early 2000s. More recently, we proactively brought a softgel product concept for the brand to the customer, which the customer elected to develop and launch as well. By following this molecule, we have built a strong, 24-year long relationship across multiple formats and markets.
Continue to Grow Through New Product Launches and Projects
We intend to grow by supplementing our existing diverse base of commercialized advanced delivery technology products with new development programs. As of June 30, 2016 , our product development teams were working on approximately 700 new customer programs. Our base of active development programs has expanded in recent years from growing market demand, as well as from our investments since 2010 to expand our global sales and marketing function; once developed and approved in the future, we expect these programs to add to long-duration commercial revenues under long-term contracts and grow our existing product base. In the year ended June 30, 2016 , we introduced 184 new products, which is up 12% versus new product introductions in the year ended June 30, 2015 . We also expect that our expanded offerings and capacity, such as our OptiForm Solutions Suite bioavailability enhancement offering, expanded bioanalytical testing and commercial-scale metered dose inhaler production, ongoing service offering and geographic network expansion in our clinical supply services business, our expanded presence in Brazil, and our continued growth in China, will further expand our active advanced delivery technologies development programs, and position us for future growth. Our development solutions business is driven by thousands of projects annually, ranging from individual short-duration analytical projects to multi-year clinical supply programs.
Catalent continues to be the global leader in providing chemistry, manufacturing and controls-based product development services to the global pharmaceutical, biotechnology and consumer health industry. In the year ended June 30, 2016, we recognized approximately $330 million of revenue related to the development of products on behalf of customers, included in our Softgel Technologies and Drug Delivery Solutions reporting segments, up 19% from the prior year. In addition,
9
Table of Contents
substantially all of the revenues associated with the Clinical Supply Services segment relate to our support of customer products in development.
Accelerate Growth with Existing Customers through Increased Penetration and Broadening of Services
While we have a broad presence across the pharmaceutical and biotechnology industries, we believe there are significant opportunities for additional revenue growth in our existing customer base, by providing advanced delivery solutions for new pipeline or commercial molecules, and by expanding the range and depth of our development solutions used by those customers. Within our top 50 customers, nearly 75% use less than half of our individual offerings. In order to ensure we provide the most value to our customers, we have increased our field sales and marketing force by approximately 20% since fiscal 2009. We have continued to follow a targeted account strategy, designating certain accounts as global accounts, based on current materiality, partnering approach and growth potential. We also designate other accounts as growth accounts, based primarily on partnering approach and potential to become global accounts in the future. In both cases, we assign incremental business development product development resources to identify and pursue new opportunities to partner. Global accounts represented nearly 29% of our revenues in fiscal 2016 , while growth accounts represented approximately 8% of revenues in that same period.
Enter Into and Expand Into Attractive Technologies and Geographies
We have made a number of internal investments in new geographies and markets, including the construction and ongoing expansion of a state-of-the-art biomanufacturing facility in Wisconsin to serve the growing global biologics development market, a recently completed significant expansion of oral solid controlled release production capacity in Kentucky, the scaling-up of commercial manufacturing capacity for metered-dose inhalers and continuing development and scale-up of the SMARTag™ antibody-drug conjugate technology to address the growing need for improved targeted delivery of therapeutic compounds directly to tumor sites.
In addition, we intend to increase our presence in emerging/high-growth geographies and other markets where we are currently only narrowly represented, including China, Brazil, Japan, and the animal health market.
Capitalize on Our Substantial Technology Platform
We have a broad and diverse technology platform that is supported by approximately 1,100 patents and patent applications in approximately 100 families across advanced delivery technologies, drug and biologics formulation and manufacturing. This platform is supported by substantial know-how and trade secrets that provide us with additional competitive advantages. For example, we have significant softgel fill and formulation databases and substantial softgel regulatory approval expertise, and as a result, approximately 90% of NCE softgel approvals by the FDA over the last 25 years have been developed and supplied by us .
In addition to resolving product challenges for our customers' molecules, for more than two decades we have applied our technology platforms and development expertise to proactively develop proof-of-concept products, whether improved versions of existing drugs, new generic formulations or innovative consumer health products. In the consumer health area, we file product dossiers with regulators in relevant jurisdictions for self-created products, which help contribute sustainable growth to our consumer health business. We expect to continue to seek proactive development and other non-traditional relationships to increase demand for and value realized from our technology platforms. These activities have provided us with opportunities to capture an increased share of end-market value through out-licensing, profit-sharing and other arrangements.
Leverage Existing Infrastructure and Operational Discipline to Drive Profitable Growth
Through our existing infrastructure, including our global network of operating locations and programs, we promote operational discipline and drive margin expansion. With our Lean Manufacturing and Lean Six Sigma programs, a global procurement function and conversion cost productivity metrics in place, we have created a culture of functional excellence and cost accountability. We intend to continue to apply this discipline to further leverage our operational network for profitable growth. Since fiscal 2009, we have expanded gross margin by over 400 basis points and Adjusted EBITDA margin by over 200 basis points .
10
Table of Contents
Pursue Strategic Acquisitions and Licensing to Build upon our Existing Platform
We operate in highly fragmented markets in both our advanced delivery technologies and development solutions businesses. Within those markets, the five top players represent nearly 35% and 10% of the total market share, respectively, by revenue. Our broad platform, global infrastructure and diversified customer base provide us with a strong foundation from which to consolidate within these markets and to generate operating leverage through such acquisitions. Since fiscal 2012, we have executed nine transactions, investing more than $700 million, and have demonstrated an ability to efficiently and effectively integrate these acquisitions.
While we are rigorously focused on driving Catalent's organic growth, we intend to continue to opportunistically source and execute bolt-on strategic acquisitions within our existing business areas, as well as to undertake transactions that provide us with expansion opportunities within new geographic markets or adjacent market segments. We have a dedicated corporate development team in place to identify these opportunities and have a rigorous and financially disciplined process for evaluating, executing and integrating such acquisitions.
Our Reportable Segments
In fiscal 2016, the Company engaged in a business reorganization which was finalized in the fourth quarter to better align its internal business unit structure with its "Follow the Molecule" strategy. Under the revised structure, we have created a Drug Delivery Solutions ("DDS") operating segment which encompasses all of our modified release technologies; prefilled syringes and other injectable formats; blow-fill seal unit dose development and manufacturing; biologic cell line development; analytical services; micronization technologies; and other conventional oral dose forms under a single DDS management team. Additionally, as part of the re-alignment, we have created a stand-alone Clinical Supply Services ("CSS") operating segment and management team with a sole focus on providing global clinical supply chain management services that aim to speed our customers' drugs to market. Further, as a result of the business unit re-alignment, our Softgel Technologies business now reports as a distinct operating segment. Our operating segments are the same as our reporting segments. All prior period comparative segment information has been restated to reflect the current reportable segments in accordance with ASC 280 Segment Reporting as discussed in Note 1 to the Consolidated Financial Statements included in this Annual Report on Form 10-K (the "Consolidated Financial Statements"). Our offerings and services are summarized below by reporting segment.
(Dollars in millions)
Segment | Offerings and Services | Fiscal 2016 Revenue* | ||
Softgel Technologies | Formulation, development and manufacturing of prescription and consumer health soft capsules, or "softgels" including traditional softgel capsules (in which the shell is made from animal-derived materials) and Vegicaps and OptiShell capsules (in which the shell is made from vegetable-derived materials). | $ | 775.0 | |
Drug Delivery Solutions | Formulation, development and manufacturing of prescription and consumer and animal health products using our proprietary OptiMelt, OptiPact, OptiForm and Zydis technologies, other proprietary and conventional drug delivery technologies such as prefilled syringes; blow-fill seal unit dose manufacturing including our ADVASEPT technology; biologic cell line development including our GPEx and SMARTag technologies; and analytical and bioanalytical development; and testing services. | $ | 806.4 | |
Clinical Supply Services | Manufacturing, packaging, labeling, storage, distribution and inventory management for global clinical trials of drugs and biologics for customer required patient kits; FastChain demand-led clinical supply service; clinical e-solutions and informatics; and global comparator sourcing services. | $ | 307.5 | |
*Segment Revenue includes inter-segment revenue of $40.8 million .
This table should be read in conjunction with Note 17 to the Consolidated Financial Statements.
11
Table of Contents
Softgel Technologies
Through our Softgel Technologies segment, we provide formulation, development and manufacturing services for soft capsules, or "softgels," which we first commercialized in the 1930s and have continually enhanced. We are the market leader in overall softgel manufacturing, and hold the leading market position in the prescription arena. Our principal softgel technologies include traditional softgel capsules, in which the shell is made of animal-derived gelatin, and Vegicaps and OptiShell capsules, in which the shell is made from vegetable-derived materials. Softgel capsules are used in a broad range of customer products, including prescription drugs, over-the-counter medications, dietary supplements and unit-dose cosmetics. Softgel capsules encapsulate liquid, paste or oil-based active compounds in solution or suspension within an outer shell, filling and sealing the capsule simultaneously. We typically perform all encapsulation for a product within one of our softgel facilities, with active ingredients provided by customers or sourced directly by us. Softgels have historically been used to solve formulation challenges or technical issues for a specific drug, to help improve the clinical performance of compounds, to provide important market differentiation, particularly for over-the-counter compounds, and to provide safe handling of hormonal, potent and cytotoxic drugs. We also participate in the softgel vitamin, mineral and supplement business in selected regions around the world. With the 2001 introduction of our vegetable-derived softgel shell, Vegicaps capsules, consumer health manufacturers have been able to extend the softgel dose form to a broader range of active ingredients and serve patient/consumer populations that were previously inaccessible due to religious, dietary or cultural preferences. In recent years, we have extended this platform to pharmaceutical products via our OptiShell offering. Our Vegicaps and OptiShell capsules are protected by patents in most major global markets. Physician and patient studies we have conducted have demonstrated a preference for softgels versus traditional tablet and hard capsule dose forms in terms of ease of swallowing, real or perceived speed of delivery, ability to remove or eliminate unpleasant odor or taste and, for physicians, perceived improved patient adherence with dosing regimens. Representative customers of Softgel Technologies include Pfizer, Novartis, Bayer, GlaxoSmithKline, Teva, Johnson & Johnson and Allergan.
Our Softgel Technologies segment represents 41% , 42%, and 46% of the segments' aggregate revenue before inter-segment eliminations for fiscal 2016 , 2015 and 2014, respectively.
Drug Delivery Solutions
Our Drug Delivery Solutions segment provides various complex advanced formulation delivery technologies, and related integrated solutions including: development and manufacturing of a broad range of oral dose forms including fast-dissolve tablets and both proprietary and conventional controlled release products, and delivery of pharmaceuticals, biologics and biosimilars administered via injection, inhalation and ophthalmic routes, using both traditional and advanced technologies. Representative customers of DDS include Pfizer, GlaxoSmithKline, Roche, Teva, Eli Lilly, Johnson & Johnson and Allergan.
We provide comprehensive pre-formulation, development, and both clinical and commercial scale for most traditional and advanced oral solid dose formats, including uncoated and coated tablets, powder/pellet/bead-filled two piece hard capsules, lozenges, powders and other forms for immediate and modified release prescription, consumer and animal health products. We have substantial experience developing and scaling up products requiring accelerated development timelines, requiring specialized handling, complex technology transfers, or specialized manufacturing processes.
We launched our orally dissolving tablet business in 1986 with the introduction of Zydis tablets, a unique oral dosage form that is freeze-dried in its package, can be swallowed without water, and typically dissolves in the mouth in less than three seconds. Most often used for indications, drugs and patient groups that can benefit from rapid oral disintegration, the Zydis technology is utilized in a wide range of products and indications, including treatments for a variety of central nervous system-related conditions such as migraines, Parkinson's Disease, schizophrenia, and pain relief and consumer healthcare products targeting allergy relief. Zydis tablets continue to be used in new ways by our customers as we extend the application of the technology to new categories, such as for immunotherapies, vaccines and biologics delivery.
Our range of injectable manufacturing offerings includes filling drugs or biologics into pre-filled syringes and glass-free ADVASEPT vials, with flexibility to accommodate other formats within our existing network, increasingly focused on complex pharmaceuticals and biologics. With our range of technologies we are able to meet a wide range of specifications, timelines and budgets. The complexity of the manufacturing process, the importance of experience and know-how, regulatory compliance, and high start-up capital requirements create significant barriers to entry and, as a result, limit the number of competitors in the market. For example, blow-fill-seal is an advanced aseptic processing technology, which uses a continuous process to form, fill with drug, and seal a plastic container in a sterile environment. Blow-fill-seal units are currently used for a variety of pharmaceuticals in liquid form, such as respiratory, ophthalmic and otic products. We are a leader in the outsourced blow-fill-seal market, and operate one of the largest capacity commercial manufacturing blow-fill-seal facilities in the world. Our sterile blow-fill-seal manufacturing has significant capacity and flexibility of manufacturing configurations. This business provides
12
Table of Contents
flexible and scalable solutions for unit-dose delivery of complex formulations such as suspensions and emulsions. Further, the business provides engineering and manufacturing solutions related to complex containers. Our regulatory expertise can lead to decreased time to commercialization, and our dedicated development production lines support feasibility, stability and clinical runs. We plan to continue to expand our product line in existing and new markets, and in higher margin specialty products with additional respiratory, ophthalmic, injectable and nasal applications.
Our fast-growing biologics offerings include our formulation development and cell-line manufacturing based on our advanced and patented GPEx technology, which is used to develop stable, high-yielding mammalian cell lines for both innovator and biosimilar biologic compounds. Our GPEx technology can provide rapid cell-line development, high biologics production yields, flexibility and versatility. We believe our development-stage SMARTag next-generation antibody-drug conjugate technology will provide more precision targeting for delivery of drugs to tumors or other locations, with improved safety versus existing technologies. Our biologics facility in Madison, Wisconsin has the capability and capacity to produce clinical-scale biologic supplies; combined with offerings from our other businesses and external partners, we provide the broadest range of technologies and services supporting the development and launch of new biologic entities, biosimilars or biobetters to bring a product from gene to market commercialization, faster.
We also offer analytical chemical and cell-based testing and scientific services, stability testing, respiratory products formulation and manufacturing, micronization and particle engineering services, regulatory consulting, and bioanalytical testing for biologic products. Our respiratory product capabilities include development and manufacturing services for inhaled products for delivery via metered dose inhalers, dry powder inhalers and intra-nasal sprays. We also provide formulation development and clinical and commercial manufacturing for conventional and specialty oral dose forms. We provide global regulatory and clinical support services for our customers' regulatory and clinical strategies during all stages of development. Demand for our offerings is driven by the need for scientific expertise and depth and breadth of services offered, as well as by the reliable supply thereof, including quality, execution and performance.
Our Drug Delivery Solutions segment represents 43% , 43% and 38% of the segments' aggregate revenue before inter-segment eliminations for fiscal 2016 , 2015 and 2014, respectively.
Clinical Supply Services
Our Clinical Supply Services segment provides manufacturing, packaging, storage and inventory management for drugs and biologics in clinical trials. We offer customers flexible solutions for clinical supplies production, and provide distribution and inventory management support for both simple and complex clinical trials. This includes dose form manufacturing or over-encapsulation where needed; supplying placebos, comparator drug procurement and clinical packages and kits for physicians and patients; inventory management; investigator kit ordering and fulfillment; and return supply reconciliation and reporting. We support trials in all regions of the world through our facilities and distribution network. In fiscal 2016, we commenced an expansion of our Singapore facility by building new flexible cGMP space and we introduced clinical supply services at our 200,000 square foot facility in Japan, expanding our Asia Pacific capabilities. Additionally, in fiscal 2013, we established our first clinical supply services facility in China as a joint venture and assumed full ownership in fiscal 2015. We are the leading provider of integrated development solutions and one of the leading providers of clinical trial supplies and respiratory products. Representative customers of Clinical Supply Services include Astellas, GlaxoSmithKline, Eli Lilly, Merck, Pfizer and Shire.
Our Clinical Supply Services segment represents 16% , 15% and 16% of the segments' aggregate revenue before inter-segment eliminations for fiscal 2016 , 2015 and 2014, respectively.
Development and Product Supply Chain Solutions
In addition to our proprietary offerings, we are also differentiated in the market by our ability to bring together our development solutions and advanced delivery technologies to offer innovative development and product supply solutions which can be combined or tailored in many ways to enable our customers to take their drugs, biologics and consumer and animal health products from laboratory to market. Once a product is on the market, we can provide comprehensive integrated product supply, from the sourcing of the bulk active ingredient to comprehensive manufacturing and packaging to the testing required for release to distribution. Customer solutions we develop are flexible, scalable and creative, so that they meet the unique needs of both large and emerging companies, and for products of all sizes. We believe that our development and product supply solutions will continue to contribute to our future growth.
13
Table of Contents
Sales and Marketing
Our target customers include large pharmaceutical and biotechnology companies, mid-size, emerging and specialty pharmaceutical and biotechnology companies, and consumer health companies, along with companies in other selected healthcare market segments such as animal health and medical devices and companies in adjacent industries, such as cosmetics. We have longstanding, extensive relationships with leading pharmaceutical and biotechnology customers. In fiscal 2016 , we did business with 87 of the top 100 branded drug marketers, 22 of the top 25 generics marketers, 24 of the top 25 biologics marketers, and 21 of the top 25 consumer health marketers globally , as well as with more than 1,000 other customers. Faced with access, pricing and reimbursement pressures as well as other market challenges, large pharmaceutical and biotechnology companies have increasingly sought partners to enhance the clinical competitiveness of their drugs and biologics and improve the productivity of their research and development activities, while reducing their fixed cost base. Many mid-size, emerging and specialty pharmaceutical and biotechnology companies, while facing the same pricing and market pressures, have chosen not to build a full infrastructure, but rather to partner with other companies through licensing agreements or outsourcing to access the critical skills, technologies and services required to bring their products to market. Consumer health companies require rapidly developed, innovative dose forms and formulations to keep up in the fast-paced over-the-counter medication and vitamins markets. These market segments are all critically important to our growth, but require distinct solutions, marketing and sales approaches, and market strategy.
We follow a hybrid demand generation organization model, with global and growth account teams offering the full breadth of Catalent's solutions to selected accounts, and technical specialist teams providing the in-depth technical knowledge and practical experience essential for each individual offering. All business development and field sales representatives ultimately report to a single sales head, and significant ongoing investments are made to enhance their skills and capabilities. Our sales organization currently consists of more than 150 full-time, experienced sales professionals, supported by inside sales and sales operations. We also have built a dedicated strategic marketing team, providing strategic market and product planning and management for our offerings. As part of our marketing efforts, we participate in major trade shows relevant to the offerings globally and ensure adequate visibility to our offerings and solutions through a comprehensive print and on-line advertising and publicity program. We believe that Catalent is a strong brand with high overall awareness in our established markets and target customers, and that our brand identity has become a competitive advantage for us.
Global Accounts
We manage selected accounts globally due to their substantial current business and growth potential by establishing strategic plans, goals and targets. We recorded approximately 29% of our total revenue in fiscal 2016 from these global accounts. Each global account is assigned a dedicated business development professional with substantial industry experience. These account leaders, along with the leadership of the sales and marketing function and other members of the executive leadership team, are responsible for managing and extending the overall account relationship. Growing sales, profitability, and increasing account penetration are key goals and are directly linked to compensation. Account leaders also work closely with the rest of the sales organization to ensure alignment around critical priorities for the accounts.
Emerging, Specialty and Virtual Accounts
Emerging, specialty and virtual pharmaceutical and biotechnology companies are expected to be critical drivers of industry growth globally. Historically, many of these companies have chosen not to build a full infrastructure, but rather partner with other companies to produce their products. We expect them to continue to do so in the future, providing a critical source for future integrated solutions demand. We expect to continue to increase our penetration of geographic clusters of emerging companies in North America, Europe, South America and Asia. We regularly use active pipeline and product screening and customer targeting to identify the optimal candidates for partnering based on product profiles, funding status, and relationships, to ensure that our technical sales specialists and field sales representatives develop custom solutions designed to address the specific needs of customers in the market.
Contractual Arrangements
We generally enter into a broad range of contractual arrangements with our customers, including agreements with respect to feasibility, development, supply, licenses, and quality. The terms of these contracts vary significantly depending on the offering and customer requirements. Some of our agreements may include a variety of revenue arrangements such as fee-for-service, royalties, profit-sharing and fixed fees. We employ a range of capacity access approaches, from standard to completely dedicated capacity models, based on consumer and product needs. We generally secure pricing and contract mechanisms in our supply agreements that allow for periodic resetting of pricing terms and, in some cases, these agreements provide for our ability to renegotiate pricing in the event of certain price increases for the raw materials utilized in the products we make. Our typical supply agreements include indemnification from our customers for product liability and intellectual property matters and caps on our contractual liabilities, subject in each case to negotiated exclusions. In addition, our manufacturing supply agreement
14
Table of Contents
terms range from three to ten years with regular renewals of one to three years, although some of our agreements are terminable upon much shorter notice periods, such as 30 or 90 days. For our development solutions offerings, we may enter into master service agreements, which provide for standardized terms and conditions and make it easier and faster for customers with multiple development needs to access our offerings.
Backlog
While we generally have long-term supply agreements that provide for a revenue stream over a period of years, our backlog represents, as of a point in time, future service revenues from work not yet completed. For our Softgel and DDS segments, backlog represents firm orders for manufacturing services and includes minimum volumes, where applicable. For our Clinical Supply Services segment, backlog represents estimated future service revenues from work not yet completed under signed contracts. Using these methods of reporting backlog, as of June 30, 2016 , our backlog was approximately $827.5 million , as compared to approximately $827.6 million as of June 30, 2015 , including approximately $292.1 million and $246.0 million , respectively, related to our Clinical Supply Services segment. We expect to recognize approximately 85% of revenue from the backlog in existence as of June 30, 2016 by the completion of the fiscal year ending June 30, 2017 .
To the extent projects are delayed, the timing of our revenue could be affected. If a customer cancels an order, we may be reimbursed for the costs we have incurred. For orders that are placed inside a contractual firm period, we generally have a contractual right to payment in the event of cancellation. Fluctuations in our reported backlog levels also result from the timing and order pattern of our customers who often seek to manage their level of inventory on hand. Because of customer ordering patterns, our backlog reported for certain periods may fluctuate and may not be indicative of future results.
Manufacturing Capabilities
We operate manufacturing facilities, development centers and sales offices throughout the world. We have thirty-three facilities (three locations each operate as two facilities for different reporting segments) on five continents with 5.1 million square feet of manufacturing, lab and related space. Our manufacturing capabilities include the full suite of competencies relevant to support each site's activities, including regulatory, quality assurance and in-house validation.
We operate our plants in accordance with cGMP. More than half of our facilities are registered with the FDA , with the remaining facilities being registered with other applicable regulatory agencies, such as the EMA. In some cases, our facilities are registered with multiple regulatory agencies.
We have invested approximately $403.0 million of cash outflows in our manufacturing facilities since fiscal 2014 through improvements and expansions in our facilities, including approximately $139.6 million on capital expenditures in fiscal 2016 . We believe that our facilities and equipment are in good condition, are well maintained and are able to operate at or above present levels for the foreseeable future, in all material respects.
Our manufacturing operations are focused on employee health and safety, regulatory compliance, operational excellence, continuous improvement, and process standardization across the organization. In fiscal 2016 , we achieved approximately 97% on-time shipment delivery versus customer request date across our network as a result of this focus. Our manufacturing operations are structured around an enterprise management philosophy and methodology that utilizes principles and tools common to a number of quality management programs, including Lean Six Sigma and Lean Manufacturing.
Raw Materials
We use a broad and diverse range of raw materials in the design, development and manufacture of our products. This includes, but is not limited to, key materials such as gelatin, starch, and iota carrageenan for our Softgel Technologies segment; packaging films for our Clinical Supply Services segment, and resin for our blow-fill-seal business in our Drug Delivery Solutions segment. The raw materials that we use are sourced externally on a global basis. Globally, our supplier relationships could be interrupted due to natural disasters and international supply disruptions, including those caused by pandemics, geopolitical and other issues. For example, the supply of gelatin is obtained from a limited number of sources. In addition, much of the gelatin we use is bovine-derived. Past concerns of contamination from Bovine Spongiform Encephalopathy ("BSE") have narrowed the number of possible sources of particular types of gelatin. If there were a future disruption in the supply of gelatin from any one or more key suppliers, there can be no assurance that we could obtain an alternative supply from our other suppliers. If future restrictions were to emerge on the use of bovine-derived gelatin from certain geographic sources due to concerns of contamination from BSE, any such restriction could hinder our ability to timely supply our customers with products and the use of alternative non-bovine-derived gelatin for specific customer products could be subject to lengthy formulation, testing and regulatory approval.
15
Table of Contents
We work very closely with our suppliers to assure continuity of supply while maintaining excellence in material quality and reliability, and we have an active and effective supplier audit program. We continually evaluate alternate sources of supply, although we do not frequently pursue regulatory qualification of alternative sources for key raw materials due to the strength of our existing supplier relationships, the reliability of our current supplier base and the time and expense associated with the regulatory process. Although a change in suppliers could require significant effort or investment by us in circumstances where the items supplied are integral to the performance of our products or incorporate specialized material such as gelatin, we do not believe that the loss of any existing supply arrangement would have a material adverse effect on our business. See "Risk Factors-Risks Relating to Our Business and Industry-Our future results of operations are subject to fluctuations in the costs, availability, and suitability of the components of the products we manufacture, including active pharmaceutical ingredients, excipients, purchased components, and raw materials."
Competition
We compete on several fronts both domestically and internationally, including with other companies that offer advanced delivery technologies, outsourced dose form manufacturing, or development services to pharmaceutical, biotechnology and consumer health companies based in North America, South America, Europe and the Asia-Pacific region. We also may compete with the internal operations of those pharmaceutical, biotechnology and consumer health manufacturers that choose to source these services internally, where possible.
Competition is driven by proprietary technologies and know-how (where relevant), consistency of operational performance, quality, price, value and speed. While we do have competitors that compete with us in our individual offerings, we do not believe we have competition from any directly comparable companies.
Research and Development Costs
Our research activities are primarily directed toward the development of new offerings and manufacturing process improvements. Costs incurred in connection with the development of new offerings and manufacturing process improvements are recorded within selling, general, and administrative expenses. Such research and development costs included in selling, general, and administrative expenses amounted to $7.6 million , $12.2 million and $17.5 million for the fiscal years ended June 30, 2016 , June 30, 2015 and June 30, 2014 , respectively. Costs incurred in connection with research and development services we provide to customers and services performed in support of the commercial manufacturing process for customers are recorded within cost of sales. Such research and development costs included in cost of sales amounted to $47.4 million , $41.3 million and $34.0 million for the fiscal years ended June 30, 2016 , June 30, 2015 and June 30, 2014 , respectively.
Employees
As of June 30, 2016 , we had approximately 9,200 employees in thirty-three facilities on five continents: eleven facilities are in the United States, with certain employees at one facility being represented by a labor organization with their terms and conditions of employment being subject to a collective bargaining agreement. National works councils and/or labor organizations are active at all twelve of our European facilities consistent with labor environments/laws in European countries. Similar relationships with labor organizations or national works councils exist in our plants in Argentina, Brazil, and Australia. Our management believes that our employee relations are satisfactory.
| North America | Europe | South America | Asia Pacific | Total |
Approximate Number of Employees | 3,900 | 3,600 | 900 | 800 | 9,200 |
Intellectual Property
We rely on a combination of know-how, trade secrets, patents, copyrights and trademarks and other intellectual property laws, nondisclosure and other contractual provisions and technical measures to protect a number of our offerings, services and intangible assets. These proprietary rights are important to our ongoing operations. Certain of our operations and products are under intellectual property licenses from third parties, and in certain instances we license our technology to third parties. We also have a long track record of innovation across our lines of business, and, to further encourage active innovation, we have developed incentive compensation systems linked to patent filings and other recognition and reward programs for scientists and non-scientists alike.
We have applied in the United States and certain foreign countries for registration of a number of trademarks, service marks and patents, some of which have been registered and issued, and also hold common law rights in various trademarks and
16
Table of Contents
service marks. We hold approximately 1,100 patents and patent applications worldwide in advanced drug delivery and biologics formulations and technologies, and manufacturing and other areas.
We hold patents and license rights relating to certain aspects of our formulations, nutritional and pharmaceutical dosage forms, mammalian cell engineering, and sterile manufacturing services. We also hold patents relating to certain processes and products. We have a number of pending patent applications in the United States and certain foreign countries, and intend to pursue additional patents as appropriate. We have enforced and will continue to enforce our intellectual property rights in the United States and worldwide.
We do not consider any particular patent, trademark, license, franchise or concession to be material to our overall business.
Regulatory Matters
The manufacture, distribution and marketing of healthcare products are subject to extensive ongoing regulation by the FDA, other United States ("U.S.") governmental authorities and foreign regulatory authorities. Certain of our subsidiaries are required to register for permits and/or licenses with, and must comply with the operating, cGMP, quality and security standards of, applicable domestic and foreign healthcare regulators, including the FDA, the Drug Enforcement Agency (the "DEA"), the Department of Health and Human Services (the "DHHS"), the equivalent agencies of European Union (the "EU") member states and various state boards of pharmacy, state health departments and comparable foreign agencies, as well as various accrediting bodies, each depending upon the type of operations and the locations of distribution and sale of the products manufactured or services provided by those subsidiaries.
In addition, certain of our subsidiaries are subject to other healthcare laws, including the United States Federal Food, Drug, and Cosmetic Act, the Public Health Service Act, the Controlled Substances Act and comparable state and foreign laws and regulations in certain of their activities.
We are also subject to various federal, state, local, foreign and transnational laws, regulations and recommendations, both in the United States and abroad, relating to safe working conditions, laboratory and distribution practices and the use, transportation and disposal of hazardous or potentially hazardous substances. In addition, U.S. and international import and export laws and regulations require us to abide by certain standards relating to the cross-border transit of finished goods, raw materials and supplies and the handling of information. We are also subject to various other laws and regulations concerning the conduct of our foreign operations, including the U.S. Foreign Corrupt Practices Act, the U.K. Anti-Bribery Act and other anti-bribery laws and laws pertaining to the accuracy of our internal books and records.
The costs associated with complying with the various applicable federal, state, local, foreign and transnational regulations could be significant and the failure to comply with such legal requirements could have an adverse effect on our results of operations and financial condition. See "Risk Factors-Risks Relating to Our Business and Industry- Failure to comply with existing and future regulatory requirements could adversely affect our results of operations and financial condition ," for additional discussion of the costs associated with complying with the various regulations.
In fiscal 2016 , we were subject to 49 regulatory audits and, over the last five fiscal years, we successfully completed more than 250 regulatory audits , with more than 50% resulting in no reported observations.
Quality Assurance
We are committed to ensuring and maintaining the highest standard of regulatory compliance while providing high quality products to our customers. To meet these commitments, we have developed and implemented a Catalent-wide quality management system throughout the organization. We have more than 1,100 employees around the globe focusing on quality and regulatory compliance. Our senior management team is actively involved in setting quality policies, standards and internal position papers as well as managing internal and external quality performance. Our quality assurance department provides quality leadership and supervises our quality systems programs. An internal audit program monitors compliance with applicable regulations, standards and internal policies. In addition, our facilities are subject to periodic inspection by the FDA, the DEA and other equivalent local, state and foreign regulatory authorities and customers. All FDA, DEA and other regulatory inspectional observations have been resolved or are on track to be completed at the prescribed timeframe provided in commitments to the applicable agency. We believe that our operations are in compliance in all material respects with the regulations under which our facilities are governed.
17
Table of Contents
Environmental Matters
Our operations are subject to a variety of environmental, health and safety laws and regulations, including those of the U.S. Environmental Protection Agency (the "EPA") and equivalent state, local and foreign regulatory agencies in each of the jurisdictions in which we operate. These laws and regulations govern, among other things, air emissions, wastewater discharges, the use, handling and disposal of hazardous substances and wastes, soil and groundwater contamination and employee health and safety. Our manufacturing facilities use, in varying degrees, hazardous substances in their processes. These substances include, among others, chlorinated solvents, and in the past chlorinated solvents were used at one or more of our facilities, including a number we no longer own or operate. As at our current facilities, contamination at such formerly owned or operated properties can result and has resulted in liability to us, for which we have recorded appropriate reserves as needed. We believe that our operations are in compliance in all material respects with the environment, health and safety regulations applicable to our facilities.
Available Information
We file annual, quarterly and special reports and other information with the SEC. Our filings with the SEC are available to the public on the SEC's website at www.sec.gov. Those filings are also available to the public on, or accessible through, our website for free via the "Investors" section at www.catalent.com.
The information we file with the SEC or contained on or accessible through our corporate website or any other website that we may maintain is not incorporated by reference and is not part of this Annual Report on Form 10-K. You may also read and copy, at SEC prescribed rates, any document we file with the SEC at the SEC's Public Reference Room located at 100 F Street, N.E., Washington D.C. 20549. You can call the SEC at 1-800-SEC-0330 to obtain information on the operation of the Public Reference Room.
18
Table of Contents
ITEM 1A. | RISK FACTORS |
If any of the following risks actually occur, our business, financial condition, operating results or cash flow could be materially and adversely affected. Additional risks or uncertainties not presently known to us, or that we currently believe are immaterial, may also impair our business operations.
Risks Relating to Our Business and Industry
We participate in a highly competitive market, and increased competition may adversely affect our business.
We operate in a market that is highly competitive. We compete on several fronts, both domestically and internationally, including competing with other companies that provide similar offerings to pharmaceutical, biotechnology and consumer and animal health companies based in North America, Latin America, Europe and the Asia-Pacific region. We also may compete with the internal operations of those pharmaceutical, biotechnology and consumer and animal health manufacturers that choose to source these offerings internally, where possible.
We face material competition in each of our markets. Competition is driven by proprietary technologies and know-how, capabilities, consistency of operational performance, quality, price, value and speed. Some competitors may have greater financial, research and development, operational and marketing resources than we do. Competition may also increase as additional companies enter our markets or use their existing resources to compete directly with ours. Expanded competition from companies in low-cost jurisdictions, such as India and China, may in the future adversely affect our results of operations or limit our growth. Greater financial, research and development, operational and marketing resources may allow our competitors to respond more quickly with new, alternative or emerging technologies. Changes in the nature or extent of our customer requirements may render our offerings obsolete or non-competitive and could adversely affect our results of operations and financial condition.
The demand for our offerings depends in part on our customers' research and development and the clinical and market success of their products. Our business, financial condition and results of operations may be harmed if our customers spend less on, or are less successful in, these activities.
Our customers are engaged in research, development, production and marketing of pharmaceutical, biotechnology and consumer and animal health products. The amount of customer spending on research, development, production and marketing, as well as the outcomes of such research, development, and marketing activities, have a large impact on our sales and profitability, particularly the amount our customers choose to spend on our offerings. Our customers determine the amounts that they will spend based upon, among other things, available resources and their need to develop new products, which, in turn, is dependent upon a number of factors, including their competitors' research, development and production initiatives, and the anticipated market uptake, clinical and reimbursement scenarios for specific products and therapeutic areas. In addition, consolidation in the industries in which our customers operate may have an impact on such spending as customers integrate acquired operations, including research and development departments and their budgets. Our customers finance their research and development spending from private and public sources. A reduction in spending by our customers could have a material adverse effect on our business, financial condition and results of operations. If our customers are not successful in attaining or retaining product sales due to market conditions, reimbursement issues or other factors, our results of operations may be materially adversely affected.
We are subject to product and other liability risks that could adversely affect our results of operations, financial condition, liquidity and cash flows.
We are subject to potentially significant product liability and other liability risks that are inherent in the design, development, manufacture and marketing of our offerings. We may be named as a defendant in product liability lawsuits, which may allege that our offerings have resulted or could result in an unsafe condition or injury to consumers. Such lawsuits could be costly to defend and could result in reduced sales, significant liabilities and diversion of management's time, attention and resources. Even claims without merit could subject us to adverse publicity and require us to incur significant legal fees.
Furthermore, product liability claims and lawsuits, regardless of their ultimate outcome, could have a material adverse effect on our business operations, financial condition and reputation and on our ability to attract and retain customers. We have historically sought to manage this risk through the combination of product liability insurance and contractual indemnities and liability limitations in our agreements with customers and vendors. The availability of product liability insurance for companies in the pharmaceutical industry is generally more limited than insurance available to companies in other industries. Insurance carriers providing product liability insurance to those in the pharmaceutical and biotechnology industries generally limit the
19
Table of Contents
amount of available policy limits, require larger self-insured retentions and exclude coverage for certain products and claims. We maintain product liability insurance with annual aggregate limits in excess of $25 million. There can be no assurance that a successful product liability claim or other liability claim would be adequately covered by our applicable insurance policies or by any applicable contractual indemnity or liability limitations.
Failure to comply with existing and future regulatory requirements could adversely affect our results of operations and financial condition .
The healthcare industry is highly regulated. We are subject to various local, state, federal, foreign and transnational laws and regulations, which include the operating, quality and security standards of the FDA, the DEA, various state boards of pharmacy, state health departments, the DHHS, similar bodies of the EU and its member states and other comparable agencies around the world, and, in the future, any changes to such laws and regulations could adversely affect us. Among other rules affecting us, we are subject to laws and regulations concerning cGMP and drug safety. Our subsidiaries may be required to register for permits and/or licenses with, and may be required to comply with, the laws and regulations of the FDA, the DEA, the DHHS, foreign agencies including the EMA, and other various state boards of pharmacy, state health departments and/or comparable state and foreign agencies as well as certain accrediting bodies depending upon the type of operations and locations of distribution and sale of the products manufactured or services provided by those subsidiaries.
The manufacture, distribution and marketing of our offerings are subject to extensive ongoing regulation by the FDA, the DEA, the EMA, and other equivalent local, state, federal, foreign and transnational regulatory authorities. Failure by us or by our customers to comply with the requirements of these regulatory authorities could result in warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture or distribution, restrictions on our operations, civil or criminal sanctions, or withdrawal of existing or denial of pending approvals, permits or registrations, including those relating to products or facilities. In addition, any such failure relating to the products or services we provide could expose us to contractual or product liability claims as well as contractual claims from our customers, including claims for reimbursement for lost or damaged active pharmaceutical ingredients, which cost could be significant. Customers may also claim loss of profits due to lost or delayed sales, although our contracts generally place substantial limits on such claims. There can be no assurance that any such contractual limitation will be applicable or sufficient or fully enforced in any given situation.
In addition, any new offering or product classified as a pharmaceutical product must undergo lengthy and rigorous clinical testing and other extensive, costly and time-consuming procedures mandated by the FDA, the EMA and other equivalent local, state, federal and foreign regulatory authorities. We or our customers may elect to delay or cancel anticipated regulatory submissions for current or proposed new products for any number of reasons.
Although we believe that we comply in all material respects with applicable laws and regulations, there can be no assurance that a regulatory agency or tribunal would not reach a different conclusion concerning the compliance of our operations with applicable laws and regulations. In addition, there can be no assurance that we will be able to maintain or renew existing permits, licenses or other regulatory approvals or obtain, without significant delay, future permits, licenses or other approvals needed for the operation of our businesses. Any noncompliance by us with applicable laws and regulations or the failure to maintain, renew or obtain necessary permits and licenses could have an adverse effect on our results of operations and financial condition. Furthermore, loss of a permit, license or other approval in any one portion of our business may have indirect consequences in another portion of our business if regulators or customers adjust their reviews of such other portion as a result or customers cease business with such other portion due to fears that such loss is a sign of broader concerns about our ability to deliver products or services of sufficient quality.
Failure to provide quality offerings to our customers could have an adverse effect on our business and subject us to regulatory actions and costly litigation.
Our results depend on our ability to execute and improve when necessary our quality management strategy and systems, and effectively train and maintain our employee base with respect to quality management. Quality management plays an essential role in determining and meeting customer requirements, preventing defects and improving our offerings. While we have a network of quality systems throughout our business units and facilities that relate to the design, formulation, development, manufacturing, packaging, sterilization, handling, distribution and labeling of the products we supply, quality and safety issues may occur with respect to any of our offerings. A quality or safety issue could have an adverse effect on our business, financial condition and results of operations and may subject us to regulatory actions, including product recalls, product seizures, injunctions to halt manufacture or distribution, restrictions on our operations, or civil sanctions, including monetary sanctions and criminal actions. In addition, such an issue could subject us to costly litigation, including claims from our customers for reimbursement for the cost of lost or damaged active pharmaceutical ingredients or other related losses, the cost of which could be significant.
20
Table of Contents
The services and offerings we provide are highly exacting and complex, and, if we encounter problems providing the services or support required, our business could suffer.
The offerings we provide are highly exacting and complex, particularly in our Softgel Technologies and Drug Delivery Solutions segments, due in part to strict regulatory requirements. From time to time, problems may arise in connection with facility operations or during preparation or provision of an offering, in both cases for a variety of reasons including, but not limited to, equipment malfunction, sterility variances or failures, failure to follow specific protocols and procedures, problems with raw materials, environmental factors and damage to, or loss of, manufacturing operations due to fire, flood or similar causes. Such problems could affect production of a particular batch or series of batches, require the destruction of or otherwise result in the loss of product or materials used in the production of product, or could halt facility production altogether. This could, among other things, lead to increased costs, lost revenue, damage to customer relations, reimbursement to customers for lost active pharmaceutical ingredients or other related losses, time and expense spent investigating the cause, lost production time, and, depending on the cause, similar losses with respect to other batches or products. Production problems in our drug and biologic manufacturing operations could be particularly significant because the cost of raw materials is often higher than in our other businesses. If problems are not discovered before the product is released to the market, recall and product liability costs may also be incurred. In addition, such risks may be greater at facilities that are new or going through significant expansion or renovation.
Our global operations are subject to a number of economic, political and regulatory risks.
We conduct our operations in various regions of the world, including North America, South America, Europe and the Asia-Pacific region. Global and regional economic and regulatory developments affect businesses such as ours in many ways. Our operations are subject to the effects of global and regional competition, including potential competition from manufacturers in low-cost jurisdictions such as India and China. Local jurisdiction risks include regulatory risks arising from local laws. Our global operations are also affected by local economic environments, including inflation and recession. Political changes, some of which may be disruptive, and related hostilities can interfere with our supply chain and customers and some or all of our activities in a particular location. While some of these risks can be hedged using derivatives or other financial instruments and some are insurable, such attempts to mitigate these risks are costly and not always successful. Also, fluctuations in foreign currency exchange rates can adversely affect our consolidated financial results.
The recent referendum in the U.K. and resulting decision of the U.K. government to consider exiting from the European Union could have future adverse effects on our revenues and costs, and therefore our profitability.
In June 2016, the United Kingdom (the "U.K.") held a referendum in which a majority of voters approved the U.K.'s exit from the EU, and the U.K. government has publicly announced that it intends to honor that vote and seek an exit. There is no immediate change in either the U.K. or the EU as a result of this referendum, and the U.K. government must now decide, through legislative action and through negotiations with the EU and other affected parties, what changes will result from the decision to exit. Four of our thirty-three facilities, employing hundreds of workers, are located in the U.K., and these facilities, as well as others in our network, source goods, manufacture goods and provide services from or intended for the U.K. Due to future changes in the U.K. resulting from the eventual exit, or in anticipation of such changes, our suppliers, customers or employees may change their interactions with us, including changes in imports to or exports from the U.K., changes in the requested utilization of our facilities, both within and without the U.K., and changes in our relationships with our workforce in the U.K. We cannot anticipate the nature of these changes, as they largely depend on factors outside our control, but the changes may result in adverse changes in our future revenues and costs, and therefore our future profitability.
If we do not enhance our existing or introduce new technology or service offerings in a timely manner, our offerings may become obsolete or uncompetitive over time, customers may not buy our offerings and our revenue and profitability may decline.
The healthcare industry is characterized by rapid technological change. Demand for our offerings may change in ways we may not anticipate because of evolving industry standards as well as a result of evolving customer needs that are increasingly sophisticated and varied and the introduction by others of new offerings and technologies that provide alternatives to our offerings. Several of our higher margin offerings are based on proprietary technologies. To the extent that our proprietary rights are based on patents, patents are inherently of limited longevity and therefore will ultimately expire, and such offerings may then become subject to competition. Without the timely introduction of enhanced or new offerings, our offerings may become obsolete or uncompetitive over time, in which case our revenue and operating results would suffer. For example, if we are unable to respond to changes in the nature or extent of the technological or other needs of our pharmaceutical customers through enhancing our offerings, our competition may develop offerings that are more competitive than ours and we could find it more difficult to renew or expand existing agreements or obtain new agreements. Potential innovations intended to facilitate
21
Table of Contents
enhanced or new offerings generally will require a substantial investment before we can determine their commercial viability, and we may not have financial resources sufficient to fund all desired innovations.
The success of enhanced or new offerings will depend on several factors, including our ability to:
• | properly anticipate and satisfy customer needs, including increasing demand for lower cost products; |
• | enhance, innovate, develop and manufacture new offerings in an economical and timely manner; |
• | differentiate our offerings from competitors' offerings; |
• | achieve positive clinical outcomes for our customers' new products; |
• | meet safety requirements and other regulatory requirements of governmental agencies; |
• | obtain valid and enforceable intellectual property rights; and |
• | avoid infringing the proprietary rights of third parties. |
Even if we succeed in creating enhanced or new offerings from these innovations, they may still fail to result in commercially successful offerings or may not produce revenue in excess of the costs of development, and they may be rendered obsolete by changing customer preferences or the introduction by our competitors of offerings embodying new technologies or features. Finally, innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice, the need for regulatory clearance and uncertainty over market access or government or third-party reimbursement.
We and our customers depend on patents, copyrights, trademarks, trade secrets and other forms of intellectual property protections, but these protections may not be adequate.
We rely on a combination of know-how, trade secrets, patents, copyrights and trademarks and other intellectual property laws, nondisclosure and other contractual provisions and technical measures to protect a number of our offerings and intangible assets. These proprietary rights are important to our ongoing operations. There can be no assurance that these protections will prove meaningful against competitive offerings or otherwise be commercially valuable or that we will be successful in obtaining additional intellectual property or enforcing our intellectual property rights against unauthorized users. Our exclusive rights under certain of our offerings are protected by patents, some of which will expire in the near term. When patents covering an offering expire, loss of exclusivity may occur and this may force us to compete with third parties, thereby affecting our revenue and profitability. We do not currently expect any material loss of revenue to occur as a result of the expiration of any patent currently protecting our business.
Our proprietary rights may be invalidated, circumvented or challenged. We may in the future be subject to proceedings seeking to oppose or limit the scope of our patent applications or issued patents. In addition, in the future, we may need to take legal actions to enforce our intellectual property rights, to protect our trade secrets or to determine the validity or scope of the proprietary rights of others. Legal proceedings are inherently uncertain, and the outcome of any such legal action may be unfavorable to us.
Any legal action regardless of outcome might result in substantial costs and diversion of resources and management attention. Although we use reasonable efforts to protect our proprietary and confidential information, there can be no assurance that our confidentiality and non-disclosure agreements will not be breached, our trade secrets will not otherwise become known by competitors or that we will have adequate remedies in the event of unauthorized use or disclosure of proprietary information. Even if the validity and enforceability of our intellectual property is upheld, an adjudicator might construe our intellectual property not to cover the alleged infringement. In addition, intellectual property enforcement may be unavailable or practically ineffective in some foreign countries. There can be no assurance that our competitors will not independently develop technologies that are substantially equivalent or superior to our technology or that third parties will not design around our intellectual property claims to produce competitive offerings. The use of our technology or similar technology by others could reduce or eliminate any competitive advantage we have developed, cause us to lose sales or otherwise harm our business.
We have applied in the United States and certain foreign countries for registration of a number of trademarks, service marks and patents, some of which have been registered or issued, and also claim common law rights in various trademarks and service marks. In the past, third parties have occasionally opposed our applications to register intellectual property and there can be no assurance that they will not do so in the future. It is possible that in some cases we may be unable to obtain the registrations for trademarks, service marks and patents for which we have applied and a failure to obtain trademark and patent
22
Table of Contents
registrations in the United States or other countries could limit our ability to protect our trademarks and proprietary technologies and impede our marketing efforts in those jurisdictions.
Our use of certain intellectual property rights is also subject to license agreements with third parties for certain patents, software and information technology systems and proprietary technologies. If these license agreements were terminated for any reason, it could result in the loss of our rights to this intellectual property, our operations may be materially adversely affected and we may be unable to commercialize certain offerings.
In addition, many of our branded pharmaceutical customers rely on patents to protect their products from generic competition. Because incentives exist in some countries, including the United States, for generic pharmaceutical companies to challenge these patents, pharmaceutical and biotechnology companies are under the ongoing threat of challenges to their patents. If our customers' patents were successfully challenged and as a result subjected to generic competition, the market for our customers' products could be significantly adversely affected, which could have an adverse effect on our results of operations and financial condition. We attempt to mitigate these risks by making our offerings available to generic as well as branded manufacturers and distributors, but there can be no assurance that we will be successful in marketing these offerings.
Our future results of operations are subject to fluctuations in the costs, availability, and suitability of the components of the products we manufacture, including active pharmaceutical ingredients, excipients, purchased components, and raw materials.
We depend on various active pharmaceutical ingredients, components, compounds, raw materials, and energy supplied primarily by others for our offerings. This includes, but is not limited to, gelatin, starch, iota carrageenan, petroleum-based products and resin. Also, our customers frequently provide to us their active pharmaceutical or biologic ingredient for formulation or incorporation in the finished product. It is possible that any of our or our customers' supplier relationships could be interrupted due to changing regulatory requirements, import or export restrictions, natural disasters, international supply disruptions caused by pandemics, geopolitical issues and other events, or could be terminated in the future.
For example, gelatin is a critical component in most of the products produced in our Softgel Technologies segment. Gelatin is available from only a limited number of sources. In addition, much of the gelatin we use is bovine-derived. Past concerns of contamination from BSE have narrowed the number of possible sources of particular types of gelatin. If there were a future disruption in the supply of gelatin from any one or more key suppliers, we may not be able to obtain an adequate alternative supply from our other suppliers. If future restrictions were to emerge on the use of bovine-derived gelatin due to concerns of contamination from BSE or otherwise, any such restriction could hinder our ability to timely supply our customers with products and the use of alternative non-bovine-derived gelatin could be subject to lengthy formulation, testing and regulatory approval.
Any sustained interruption in our receipt of adequate supplies could have an adverse effect on us. In addition, while we have processes intended to reduce volatility in component and material pricing, we may not be able to successfully manage price fluctuations and future price fluctuations or shortages may have an adverse effect on our results of operations.
Changes in market access or healthcare reimbursement for our customers' products in the United States or internationally could adversely affect our results of operations and financial condition.
The healthcare industry has changed significantly over time, and we expect the industry to continue to evolve. Some of these changes, such as ongoing healthcare reform, adverse changes in governmental or private funding of healthcare products and services, legislation or regulations governing patient access to care and privacy, or the delivery, pricing or reimbursement approval of pharmaceuticals and healthcare services or mandated benefits, may cause healthcare industry participants to change the amount of our offerings they purchase or the price they are willing to pay for our offerings. Changes in the healthcare industry's pricing, selling, inventory, distribution or supply policies or practices could also significantly reduce our revenue and results of operations. In particular, volatility in individual product demand may result from changes in public or private payer reimbursement or coverage.
Fluctuations in the exchange rate of the U.S. dollar and other foreign currencies could have a material adverse effect on our financial performance and results of operations.
As a company with many international operations, certain revenues, costs, assets and liabilities, including a portion of our senior secured credit facilities, are denominated in currencies other than the U.S. dollar. As a result, changes in the exchange rates of these currencies or any other applicable currency to the U.S. dollar will affect our revenues, earnings and cash flows. There has been, and may continue to be, volatility in currency exchange rates as a result of the U.K.'s referendum in which
23
Table of Contents
voters approved the U.K.'s exit from the EU. Such volatility and other changes in the exchange rates could result in unrealized and realized exchange losses despite any effort we may undertake to manage or mitigate our exposure to foreign currency fluctuations.
Tax legislation initiatives or challenges to our tax positions could adversely affect our results of operations and financial condition.
We are a large multinational corporation with operations in the United States and international jurisdictions, including North America, South America, Europe and the Asia-Pacific region. As such, we are subject to the tax laws and regulations of the United States federal, state and local governments and of many international jurisdictions. From time to time, various legislative initiatives may be proposed that could adversely affect our tax positions. There can be no assurance that our effective tax rate or tax payments will not be adversely affected by these initiatives. In addition, United States federal, state and local, as well as international tax laws and regulations are extremely complex and subject to varying interpretations. There can be no assurance that our tax positions will not be challenged by relevant tax authorities or that we would be successful in any such challenge.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have net operating loss carryforwards available to reduce future taxable income. Utilization of our net operating loss carryforwards may be subject to a substantial limitation under Section 382 of the Internal Revenue Code of 1986, as amended (the "Code"), and comparable provisions of state, local and foreign tax laws due to changes in ownership of our company that may occur in the future. Under Section 382 of the Code and comparable provisions of state, local and foreign tax laws, if a corporation undergoes an "ownership change," generally defined as a greater than 50% change by value in its equity ownership over a three-year period, the corporation's ability to carry forward its pre-change net operating losses to reduce its post-change income may be limited. We may experience ownership changes in the future as a result of future changes in our stock ownership. As a result, our ability to use our pre-change net operating loss carryforwards to reduce U.S. federal and state taxable income we produce in the future years may be subject to limitations, which could result in increased future tax liability to us.
We may be required to establish an additional valuation allowance against our U.S. deferred tax assets in the future.
We have deferred tax assets for net operating loss carryforwards and other temporary differences. We currently do not maintain a valuation allowance for a portion of our U.S. net deferred tax assets. We may experience, in the future, a decline in U.S. federal taxable income, resulting from a decline in profitability of our U.S. operations, an increased level of debt in the U.S. or other factors. In assessing our ability to realize our U.S. deferred tax assets, we may conclude that it is more likely than not that some portion or all of our U.S. deferred tax assets will not be realized. As a result, we may be required to record an additional valuation allowance against our U.S. deferred tax assets, which could adversely affect our effective income tax rate and therefore our financial results.
We are dependent on key personnel.
We depend on our executive officers and other key personnel, including our technical personnel, to operate and grow our business and to develop new enhancements, offerings and technologies. The loss of any of these officers or other key personnel combined with a failure to attract and retain suitably skilled technical personnel could adversely affect our operations.
In addition to our executive officers, we rely on approximately 150 senior employees to lead and direct the Company. Our senior leadership team ("SLT") is comprised of our executive officers and other vice presidents and directors who hold critical positions and possess specialized talents and capabilities that give us a competitive advantage in the market. The members of the SLT hold positions such as facility general manager, vice president/general manager of business unit commercial development, vice president of quality and regulatory activities and vice president-finance.
With respect to our technical talent, we have approximately 1,400 scientists and technicians whose areas of expertise and specialization cover subjects such as advanced delivery, drug and biologics formulation and manufacturing. Many of our sites and laboratories are located in competitive labor markets like those in which our Morrisville, North Carolina; Brussels, Belgium; Woodstock, Illinois; Madison, Wisconsin; and Schorndorf, Germany facilities are located. Global and regional competitors and, in some cases, customers and suppliers, compete for the same skills and talent as we do.
24
Table of Contents
Risks generally associated with information and communications systems could adversely affect our results of operations.
We rely on information systems in our business to obtain, rapidly process, analyze and manage data to:
• | facilitate the manufacture and distribution of thousands of inventory items in, to and from our facilities; |
• | receive, process and ship orders on a timely basis; |
• | manage the accurate billing and collections for roughly one thousand customers; |
• | manage the accurate accounting and payment for thousands of vendors; |
• | schedule and operate our global network of development, manufacturing and packaging facilities; and |
• | communicate among our 9,200 employees spread across thirty-three facilities over five continents. |
Our results of operations could be adversely affected if these systems are interrupted, damaged by unforeseen events or fail for any extended period of time, including due to the actions of third parties. We deploy defenses against cyber-attack and work to secure the integrity of our data systems using techniques, hardware and software typical of companies of our size and scope, but there can be no assurance that such defenses and efforts will be sufficient to combat increasingly sophisticated intruders and others who regularly try to cause harm to or interfere with our normal use of our systems.
We may engage from time to time in acquisitions and other transactions that may complement or expand our business or divest of non-strategic businesses or assets. We may not be able to complete such transactions, and such transactions, if executed, pose significant risks and could have a negative effect on our operations.
Our future success may depend in part on opportunities to buy or otherwise acquire rights to other businesses or technologies or enter into joint ventures or otherwise enter into strategic arrangements with business partners that could complement, enhance or expand our current business or offerings and services or that might otherwise offer us growth opportunities. We may face competition from other companies in pursuing acquisitions and similar transactions in the pharmaceutical and biotechnology industry. Our ability to complete such transactions may also be limited by applicable antitrust and trade regulation laws and regulations in the United States and foreign jurisdictions in which we or the operations or assets we seek to acquire carry on business. To the extent that we are successful in making acquisitions, we may have to expend substantial amounts of cash, incur debt or assume loss-making divisions as consideration. We may not be able to complete such transactions for reasons including, but not limited to, a failure to secure financing. Any transaction that we are able to identify and complete may involve a number of risks, including, but not limited to, the diversion of management's attention to integrate the acquired businesses or joint ventures, the possible adverse effects on our operating results during the integration process, the potential loss of customers or employees in connection with the acquisition, delays or reduction in realizing expected synergies, unexpected liabilities and our potential inability to achieve our intended objectives for the transaction. In addition, we may be unable to maintain uniform standards, controls, procedures and policies, and this may lead to operational inefficiencies.
To the extent that we are not successful in completing divestitures, as such may be determined by future strategic plans and business performance, we may have to expend substantial amounts of cash, incur debt and continue to absorb the costs of loss-making or under-performing divisions. Any divestiture, whether we are able to complete it or not may involve a number of risks, including diversion of management's attention, a negative impact on our customer relationships, costs associated with maintaining the business of the targeted divestiture during the disposition process, and the costs of closing and disposing of the affected business or transferring the operations of the business to other facilities.
Our offerings or our customers' products may infringe on the intellectual property rights of third parties.
From time to time, third parties have asserted intellectual property infringement claims against us and our customers, and there can be no assurance that third parties will not assert infringement claims against either us or our customers in the future. While we believe that our offerings do not infringe in any material respect upon proprietary rights of other parties and/or that meritorious defenses would exist with respect to any assertion to the contrary, there can be no assurance that we could successfully avoid being found to infringe on the proprietary rights of others. Patent applications in the United States and some foreign countries are generally not publicly disclosed until the patent is issued or published, and we and our customers may not be aware of currently filed patent applications that relate to our or their products, offerings or processes. If patents later issue on these applications, we or they may be found liable for subsequent infringement. There has been substantial litigation in the pharmaceutical and biotechnology industries with respect to the manufacture, use and sale of products that are the subject of conflicting patent rights.
25
Table of Contents
Any claim that our offerings or processes infringe third-party intellectual property rights (including claims arising through our contractual indemnification of our customers), regardless of the claim's merit or resolution, could be costly and may divert the efforts and attention of our management and technical personnel. We may not prevail against any such claim given the complex technical issues and inherent uncertainties in intellectual property matters. If any such claim results in an adverse outcome, we could, among other things, be required to:
• | pay substantial damages (potentially including treble damages in the United States); |
• | cease the manufacture, use or sale of the infringing offerings or processes; |
• | discontinue the use of the infringing technology; |
• | expend significant resources to develop non-infringing technology; |
• | license technology from the third party claiming infringement, which license may not be available on commercially reasonable terms, or may not be available at all; and |
• | lose the opportunity to license our technology to others or to collect royalty payments based upon successful protection and assertion of our intellectual property against others. |
In addition, our customers' products may be subject to claims of intellectual property infringement and such claims could materially affect our business if their products cease to be manufactured or they have to discontinue the use of the infringing technology.
Any of the foregoing could affect our ability to compete or have a material adverse effect on our business, financial condition and results of operations.
We are subject to environmental, health and safety laws and regulations, which could increase our costs and restrict our operations in the future.
Our operations are subject to a variety of environmental, health and safety laws and regulations, including those of the EPA and the U.S. Occupational Safety & Health Administration and equivalent local, state, and foreign regulatory agencies in each of the jurisdictions in which we operate. These laws and regulations govern, among other things, air emissions, wastewater discharges, the use, handling and disposal of hazardous substances and wastes, soil and groundwater contamination and employee health and safety. Any failure by us to comply with environmental, health and safety requirements could result in the limitation or suspension of production or subject us to monetary fines or civil or criminal sanctions, or other future liabilities in excess of our reserves. We are also subject to laws and regulations governing the destruction and disposal of raw materials and non-compliant products, the handling of regulated material that are included in our offerings, and the disposal of our products or their components at the end of their useful lives. In addition, compliance with environmental, health and safety requirements could restrict our ability to expand our facilities or require us to acquire costly environmental or safety control equipment, incur other significant expenses or modify our manufacturing processes. Our manufacturing facilities may use, in varying degrees, hazardous substances in their processes. These substances include, among others, chlorinated solvents, and in the past chlorinated solvents were used at one or more of our facilities, including a number we no longer own or operate. As at our current facilities, contamination at such formerly owned or operated properties can result and has resulted in liability to us. In the event of the discovery of new or previously unknown contamination either at our facilities or at third-party locations, including facilities we formerly owned or operated, the issuance of additional requirements with respect to existing contamination, or the imposition of other cleanup obligations for which we are responsible, we may be required to take additional, unplanned remedial measures for which no reserves have been recorded. We are conducting monitoring and cleanup of contamination at certain facilities currently or formerly owned or operated by us. We have established accounting reserves for certain contamination liabilities but cannot assure that such liabilities will not exceed our reserves.
We are subject to labor and employment laws and regulations, which could increase our costs and restrict our operations in the future.
We employ approximately 9,200 employees worldwide, including approximately 3,900 employees in North America, 3,600 in Europe, 900 in South America and 800 in the Asia/Pacific region. Certain employees at one of our North American facilities are represented by a labor organization, and national works councils and/or labor organizations are active at all of our European facilities and certain of our other facilities consistent with local labor environments/laws. Our management believes that our employee relations are satisfactory. However, further organizing activities, collective bargaining or changes in the regulatory framework for employment may increase our employment-related costs or may result in work stoppages or other labor disruptions. Moreover, as employers are subject to various employment-related claims, such as individual and class actions relating to alleged employment discrimination and wage-hour and labor standards issues, such actions, if brought
26
Table of Contents
against us and successful in whole or in part, may affect our ability to compete or have a material adverse effect on our business, financial condition and results of operations.
Certain of our pension plans are underfunded, and additional cash contributions we may make will reduce the cash available for our business or to discharge our financial obligations.
Certain of our current and former employees in the U.S., the U.K., Germany, France, Japan and Australia are participants in defined benefit pension plans that we sponsor. As of June 30, 2016 , the underfunded amount of our pension plans on a worldwide basis was approximately $109.0 million , primarily related to our pension plans in the U.K. and Germany. In addition, we have an estimated obligation of approximately $39.3 million , as of June 30, 2016 , related to our withdrawal from a multiemployer pension plan in which we formerly participated, resulting in total obligations related to our pension plans of $148.3 million as of June 30, 2016 . In general, the amount of future contributions to the underfunded plans will depend upon asset returns, applicable actuarial assumptions, prevailing and expected interest rates and other factors, and, as a result, the amount we may be required to contribute in the future to fund the obligations associated with such plans may vary. Such cash contributions to the plans will reduce the cash available for our business, including the funds available to pursue strategic growth initiatives or the payment of interest expense on our indebtedness.
Risks Relating to Our Indebtedness
Our substantial leverage could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or in our industry or to deploy capital to grow our business, expose us to interest-rate risk to the extent of our variable rate debt and prevent us from meeting our obligations under our indebtedness.
We are highly leveraged. As of June 30, 2016 , we had $1,799.4 million (dollar equivalent) of senior indebtedness; an additional $186.1 million of un-utilized capacity and $13.9 million of outstanding letters of credit under our revolving credit facility.
Our high degree of leverage could have important consequences for us, including:
• | increasing our vulnerability to adverse economic, industry or competitive developments; |
• | exposing us to the risk of increased interest rates because certain of our borrowings, including borrowings under our senior secured credit facilities, are at variable rates of interest; |
• | exposing us to the risk of fluctuations in exchange rates because certain of our borrowings, including certain of our senior secured term loan facilities, are denominated in euros; |
• | making it more difficult for us to satisfy our obligations with respect to our indebtedness, and any failure to comply with the obligations of any of our debt instruments, including restrictive covenants and borrowing conditions, could result in an event of default under the agreements governing such indebtedness; |
• | restricting us from making strategic acquisitions or capital investments or causing us to make non-strategic divestitures; |
• | limiting our ability to obtain additional financing for working capital, capital expenditures, product development, debt service requirements, acquisitions and general corporate or other purposes; and |
• | limiting our flexibility in planning for, or reacting to, changes in our business or market conditions and placing us at a competitive disadvantage compared to our competitors who are less highly leveraged and who, therefore, may be able to take advantage of opportunities that our leverage prevents us from exploiting. |
Our total interest expense, net was $88.5 million , $105.0 million and $163.1 million for fiscal years 2016 , 2015 and 2014 , respectively. After taking into consideration our ratio of fixed-to-floating rate debt, an increase of 100 basis points in such rates would increase our annual interest expense by approximately $6.9 million .
Despite our high indebtedness level, we and our subsidiaries will still be able to incur significant additional debt, which could further exacerbate the risks associated with our substantial indebtedness.
We and our subsidiaries may be able to incur substantial additional indebtedness in the future. Although the agreements governing our indebtedness contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of significant qualifications and exceptions, and, under certain circumstances, the amount of indebtedness that we may incur while remaining in compliance with these restrictions could be substantial.
27
Table of Contents
Our debt agreements contain restrictions that limit our flexibility in operating our business.
The agreements governing our outstanding indebtedness contain various covenants that limit our ability to engage in specified types of transactions. These covenants limit the ability of the Operating Company and those of its subsidiaries to which these covenants apply (which our credit agreements call "restricted subsidiaries") to, among other things:
• | incur additional indebtedness and issue certain preferred stock; |
• | pay certain dividends on, repurchase or make distributions in respect of capital stock or make other restricted payments; |
• | pay distributions from restricted subsidiaries; |
• | issue or sell capital stock of restricted subsidiaries; |
• | guarantee certain indebtedness; |
• | make certain investments; |
• | sell or exchange assets; |
• | enter into transactions with affiliates; |
• | create certain liens; and |
• | consolidate, merge or transfer all or substantially all of their assets and the assets of their subsidiaries, when considered on a consolidated basis. |
A breach of any of these covenants could result in a default under one or more of these agreements, including as a result of cross-default provisions, and, in the case of our revolving credit facility, permit the lenders to cease making loans to us.
We may use derivative financial instruments to reduce our exposure to market risks from changes in interest rates on our variable-rate indebtedness and any such instruments may expose us to risks related to counterparty credit worthiness or non-performance of these instruments.
We may enter into interest-rate swap agreements or other hedging transactions in an attempt to limit our exposure to changes in variable interest rates. Such instruments may result in economic losses if, for example, prevailing interest rates decline to a point lower than any applicable fixed-rate commitment. Any such swap will expose us to credit-related risks that, if realized could adversely affect our results of operations or financial condition.
Risks Related to Ownership of Our Common Stock
Our stock price may change significantly, and you may not be able to resell shares of our common stock at or above the price you paid or at all, and you could lose all or part of your investment as a result.
The trading price of our common stock has been and continues to be volatile. Since shares of our common stock were offered for sale in our initial public offering on July 31, 2014 through June 30, 2016, our common stock price ranged from $18.92 to $34.42 . The trading price of our common stock may be adversely affected due to a number of factors such as those listed in "Risks Related to Our Business and Our Industry" and the following:
• | results of operations that vary from the expectations of securities analysts or investors; |
• | results of operations that vary from those of our competitors; |
• | changes in expectations as to our future financial performance, including financial estimates and investment recommendations by securities analysts or investors; |
• | declines in the market prices of stocks generally, or those of pharmaceutical or other healthcare companies; |
• | strategic actions by us or our competitors; |
• | announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships or capital commitments; |
• | changes in general economic or market conditions or trends in our industry or markets; |
• | changes in business or regulatory conditions or regulatory actions taken with respect to our business or the business of any of our competitors or customers; |
28
Table of Contents
• | future sales of our common stock or other securities; |
• | investor perceptions of the investment opportunity associated with our common stock relative to other investment alternatives; |
• | the public response to press releases or other public announcements by us or third parties, including our filings with or documents furnished to the SEC; |
• | announcements relating to litigation; |
• | guidance, if any, that we provide to the public, any change in this guidance or any failure to meet this guidance; |
• | the development and sustainability of an active trading market for our stock; |
• | changes in accounting principles or our application of these principles to our business; and |
• | other events or factors, including those resulting from natural disasters, hostilities, acts of terrorism, geopolitical activity or responses to these events. |
Broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our actual operating performance. In addition, price volatility may be greater if the public float or trading volume of our common stock is low, and the amount of public float on any given day can vary depending on whether our stockholders choose to hold for the long term.
Following periods of market volatility, stockholders have been known to institute securities class action litigation in order to recover their resulting losses. If we become involved in securities litigation, it could have a substantial cost and divert resources and the attention of senior management from our business regardless of the outcome of such litigation.
Because we have no plan to pay cash dividends on our common stock for the foreseeable future, you may not receive any return on your investment in your stock unless you sell it for a net price greater than that which you paid for it.
We currently intend to retain future earnings, if any, for future operations, expansion and debt repayment and have no current plan to pay any cash dividend for the foreseeable future. Our board of directors has also authorized a stock buyback program that we may use from time to time to purchase our common stock. Any future decision to pay a dividend, and the amount and timing of any future dividend on shares of our common stock will be at the sole discretion of our board of directors. Our board of directors may take into account, when deciding whether or how to pay a dividend, numerous factors, including general and economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, possible future alternative deployments of our cash, our future capital requirements, contractual, legal, tax and regulatory restrictions and implications on the payment of dividends by us to our stockholders or by our subsidiaries to us and such other factors as our board of directors may deem relevant. In addition, our ability to pay dividends is limited by covenants in the agreements governing our outstanding indebtedness and may be limited by covenants of any future indebtedness we or our subsidiaries incur. As a result, you may not receive any return on an investment in our common stock unless you sell our common stock for a price greater than that which you paid for it, taking into account any applicable commission or other costs of acquisition or sale.
If securities analysts do not publish research or reports about our business or if they downgrade our stock or our sector, our stock price and trading volume could decline.
The trading market for our common stock has been affected in part by the research and reports that industry and financial analysts publish about us or our business. We do not control these analysts. Furthermore, if one or more of the analysts who cover us downgrade our stock or our industry, change their views regarding the stock of any of our competitors or other healthcare sector companies, or publish inaccurate or unfavorable research about our business, the market price of our stock could decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, we could lose visibility in the market, which in turn could cause our stock price or trading volume to decline.
Future sales, or the perception of future sales of common stock, by us or our existing stockholders could cause the market price for our common stock to decline.
The sale of shares of our common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our common stock. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
As of August 22, 2016 , 131,704 shares of our common stock, representing less than 1% of our total outstanding shares of common stock, are "restricted securities" within the meaning of the SEC's Rule 144 promulgated under the Securities Act
29
Table of Contents
("Rule 144") and subject to certain restrictions on resale. Restricted securities may be sold in the public market only if they are registered under the Securities Act or are sold pursuant to an exemption from registration such as Rule 144.
In addition, 1,423,389 shares of common stock may become eligible for sale upon exercise of vested options and restricted share units. A total of 6,700,000 shares of common stock were reserved for issuance under the 2014 Omnibus Incentive Plan, of which 3,177,262 shares of common stock remain available for future issuance at August 22, 2016 . These shares can be sold in the public market upon issuance, subject to restrictions under the securities laws applicable to resales by affiliates.
Pursuant to a registration rights agreement, certain holders of restricted shares, subject to certain conditions, may require us to register or otherwise facilitate the sale under the Securities Act of their shares of common stock. Any exercise of their registration rights, or any sale by one or more of them of a substantial number of shares, could cause the then-prevailing market price of our common stock to decline. The shares subject to the registration rights agreement represent approximately 15.6% of our outstanding shares of common stock.
The market price of shares of our common stock could drop significantly if the holders of these shares sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of shares of our common stock or other equity securities that we wish to issue. In the future, we may also issue our securities in connection with investments or acquisitions. The number of shares of our common stock issued in connection with an investment or acquisition could constitute a material portion of then-outstanding shares of our common stock, subject to limitations on issuance of new shares without stockholder approval imposed by the NYSE. Any issuance of additional securities in connection with investments or acquisitions may result in dilution to you.
Anti-takeover provisions in our organizational documents could delay or prevent a change of control.
Certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws may have an anti-takeover effect and may delay, defer or prevent a merger, acquisition, tender offer, takeover attempt or other change of control transaction that may otherwise be in the best interests of our stockholders, including transactions that might otherwise result in the payment of a premium over the market price for the shares held by our stockholders.
These provisions provide for, among other things:
• a classified board of directors with staggered three-year terms;
• the ability of our board of directors to issue one or more series of preferred stock;
• | advance notice for nominations of directors by stockholders and for stockholders to include matters to be considered at our annual meetings; |
• | certain limitations on convening special stockholder meetings; |
• | the removal of directors only for cause and only upon the affirmative vote of holders of at least 66-2/3% of the shares of common stock entitled to vote generally in the election of directors; and |
• | any amendment of certain provisions only by the affirmative vote of at least 66-2/3% of the shares of common stock entitled to vote generally in the election of directors. |
These anti-takeover provisions could make it more difficult for a third party to acquire us, even if the third-party's offer may be considered beneficial by many of our stockholders. As a result, our stockholders may be limited in their ability to obtain a premium for their shares.
Affiliates of Blackstone have substantial influence over us, and their interests may conflict with ours or yours in the future.
Affiliates of Blackstone beneficially own approximately 13.7% of our common stock. As a result, investment funds associated with or designated by affiliates of Blackstone have the ability to influence the election of the members of our board of directors and thereby affect our policies and operations, including the appointment of management, future issuances of our common stock or other securities, the payment of dividends, if any, on our common stock, the incurrence or modification of debt by us, amendments to our amended and restated certificate of incorporation and amended and restated bylaws and the entering into of extraordinary transactions, and their interests may not in all cases be aligned with your interests. In addition, Blackstone may have an interest in pursuing acquisitions, divestitures and other transactions that, in its judgment, could enhance its investment, even though such transactions might involve risks to you.
Blackstone is in the business of making investments in companies and may from time to time acquire and hold interests in businesses that compete directly or indirectly with us. For example, Blackstone has made investments in Biomet, Inc., Emcure Pharmaceuticals Ltd., Apria Healthcare Group Inc., Nycomed Holding A/S, DJO Global LLC, Independent Clinical
30
Table of Contents
Services Ltd, Southern Cross Healthcare Group PLC, Stiefel Laboratories, Inc., Team Health Holdings, Inc. and Vanguard Health Systems, Inc.
Our amended and restated certificate of incorporation provides that none of Blackstone, any of its affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his director and officer capacities) or his or her affiliates has any duty to refrain from engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. Blackstone also may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. So long as Blackstone continues to own a significant amount of our combined voting power, Blackstone will continue to be able to exercise substantial influence over our decisions, and, so long as Blackstone and its affiliates collectively own at least 5% of all outstanding shares of our stock entitled to vote generally in the election of directors, it will be able to appoint individuals to our board of directors under a stockholders agreement. The concentration of ownership could deprive you of an opportunity to receive a premium for your shares of common stock as part of a sale of the Company and ultimately might affect the market price of our common stock.
31
Table of Contents
ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
32
Table of Contents
ITEM 2. | PROPERTIES |
Our principal executive offices are located at 14 Schoolhouse Road, Somerset, New Jersey. We also operate manufacturing operations, development centers, and sales offices throughout the world. We have thirty-three facilities (three locations each operate as two facilities for different reporting segments) with manufacturing capabilities located on five continents with approximately 5.1 million square feet of manufacturing, lab and related space. Our manufacturing capabilities encompass a full suite of competencies including regulatory, quality assurance and in-house validation at all of the production sites. The following table sets forth our manufacturing and laboratory facilities by area and region as of June 30, 2016 :
| Facility Sites |
| Country |
| Region |
| Segment |
| Total Square Footage |
| Leased/Owned | |
1 | Eberbach |
| Germany |
| Europe |
| Softgel |
| 370,580 | |
| Leased |
2 | St. Petersburg, FL |
| USA |
| North America |
| Softgel |
| 328,073 | |
| Owned |
3 | Buenos Aires |
| Argentina |
| South America |
| Softgel |
| 265,000 | |
| Owned |
4 | Haining |
| China |
| Asia Pacific |
| Softgel |
| 219,930 | |
| Owned |
5 | Braeside |
| Australia |
| Asia Pacific |
| Softgel |
| 163,100 | |
| Owned |
6 | Sorocaba |
| Brazil |
| South America |
| Softgel |
| 124,685 | |
| Owned |
7 | Kakegawa (2) |
| Japan |
| Asia Pacific |
| Softgel |
| 104,500 | |
| Owned |
8 | Aprilia |
| Italy |
| Europe |
| Softgel |
| 92,010 | |
| Owned |
9 | Beinheim |
| France |
| Europe |
| Softgel |
| 78,100 | |
| Owned |
10 | Dee Why |
| Australia |
| Asia Pacific |
| Softgel |
| 59,836 | |
| Leased |
11 | Indaiatuba |
| Brazil |
| South America |
| Softgel |
| 53,800 | |
| Owned |
12 | Woodstock, IL |
| USA |
| North America |
| Drug Delivery Solutions |
| 421,665 | |
| Owned |
13 | Kansas City, MO (2) |
| USA |
| North America |
| Drug Delivery Solutions |
| 329,394 | |
| Owned |
14 | Brussels |
| Belgium |
| Europe |
| Drug Delivery Solutions |
| 265,287 | |
| Owned |
15 | Somerset, NJ |
| USA |
| North America |
| Drug Delivery Solutions / Corporate HQ |
| 265,000 | |
| Owned |
16 | Swindon |
| United Kingdom |
| Europe |
| Drug Delivery Solutions |
| 253,314 | |
| Owned |
17 | Morrisville, NC |
| USA |
| North America |
| Drug Delivery Solutions |
| 186,406 | |
| Leased |
18 | Winchester, KY |
| USA |
| North America |
| Drug Delivery Solutions |
| 180,000 | |
| Owned |
19 | Limoges |
| France |
| Europe |
| Drug Delivery Solutions |
| 179,000 | |
| Owned |
20 | Schorndorf (2) |
| Germany |
| Europe |
| Drug Delivery Solutions |
| 166,027 | |
| Owned |
21 | Madison, WI |
| USA |
| North America |
| Drug Delivery Solutions |
| 102,723 | |
| Leased |
22 | Malvern, PA |
| USA |
| North America |
| Drug Delivery Solutions |
| 84,000 | |
| Leased |
23 | Dartford |
| United Kingdom |
| Europe |
| Drug Delivery Solutions |
| 20,250 | |
| Leased |
24 | Emeryville, CA |
| USA |
| North America |
| Drug Delivery Solutions |
| 6,418 | |
| Leased |
25 | Philadelphia, PA |
| USA |
| North America |
| Clinical Supply Services |
| 206,878 | |
| Leased/Owned |
26 | Bathgate |
| United Kingdom |
| Europe |
| Clinical Supply Services |
| 191,000 | |
| Owned |
27 | Deeside (1) |
| United Kingdom |
| Europe |
| Clinical Supply Services |
| 127,533 | |
| Leased |
28 | Kansas City, MO (2) |
| USA |
| North America |
| Clinical Supply Services |
| 80,606 | |
| Owned |
29 | Bolton |
| United Kingdom |
| Europe |
| Clinical Supply Services |
| 60,830 | |
| Owned |
30 | Schorndorf (2) |
| Germany |
| Europe |
| Clinical Supply Services |
| 54,693 | |
| Owned |
31 | Shanghai |
| China |
| Asia Pacific |
| Clinical Supply Services |
| 31,000 | |
| Leased |
32 | Singapore |
| Singapore |
| Asia Pacific |
| Clinical Supply Services |
| 13,379 | |
| Leased |
33 | Kakegawa (2) | | Japan | | Asia Pacific | | Clinical Supply Services |
| 2,800 | | | Owned |
| Total |
|
|
|
|
|
|
| 5,087,817 | |
|
|
(1) As of June 30, 2016, the Company has ceased commercial activities at its Deeside location.
(2) Represents sites where multiple segments operate.
33
Table of Contents
ITEM 3. | LEGAL PROCEEDINGS |
From time to time, we may be involved in legal proceedings arising in the ordinary course of business, including, without limitation, inquiries and claims concerning environmental contamination as well as litigation and allegations in connection with acquisitions, product liability, manufacturing or packaging defects, claims for reimbursement for the cost of lost or damaged active pharmaceutical ingredients or delayed production of customer product and employment-related claims, the cost of any of which could be significant. We intend to vigorously defend ourselves against any such litigation and do not currently believe that the outcome of any such litigation will have a material adverse effect on our financial condition or results of operations. In addition, the healthcare industry is highly regulated and government agencies continue to scrutinize certain practices affecting government programs and otherwise.
From time to time, we receive subpoenas or requests for information from private parties and various governmental agencies, including from state attorneys general and the U.S. Department of Justice relating to the business practices of customers or suppliers. We generally respond to such subpoenas and requests in a timely and thorough manner, which responses sometimes require considerable time and effort and can result in considerable costs being incurred by us. We expect to incur costs in the future in connection with future requests.
During the period November 2015 through April 2016, the primary French drug regulatory agency (the "ANSM") temporarily suspended operations at the Company's softgel manufacturing facility in Beinheim, France, subject to exemptions for certain types of operations. Due to the temporary suspension, the Company was unable to use certain raw materials, work in process and finished goods, and took a charge of $1.0 million , net of insurance recoveries, during the year ended June 30, 2016, in connection with such loss of use and recorded remediation associated costs of $6.0 million . Further, certain of the customers of the facility have presented claims against the Company for losses they have allegedly suffered due to the temporary suspension or have reserved their right to do so subsequently. The Company is unable to estimate at this time either the total value of these claims or the likely cost to resolve them. Changes to the operations at the facility to address the issues leading to the suspension have increased and may in the future additionally increase the cost and therefore decrease the profitability of its operation and may also require the Company to incur additional costs.
ITEM 4. | MINE SAFETY DISCLOSURES |
Not Applicable.
34
Table of Contents
PART II
ITEM 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Common Stock Market Prices | 4th Quarter |
| 3rd Quarter |
| 2nd Quarter |
| 1st Quarter |
Fiscal year ended June 30, 2016 |
|
|
|
|
|
|
|
High | $32.24 |
| $27.60 |
| $28.75 |
| $34.42 |
Low | $20.94 |
| $18.92 |
| $23.63 |
| $24.05 |
As of August 22, 2016 we had approximately 29 holders of record of our common stock. This number does not include beneficial owners whose shares were held in street name.
We have no current plans to pay dividends on our common stock. Any decision to declare and pay dividends in the future will be made at the sole discretion of our board of directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions and other factors that our board of directors may deem relevant. Because we are a holding company and have no direct operations, we will only be able to pay dividends from funds we receive from our subsidiaries. In addition, our ability to pay dividends will be limited by covenants in our existing indebtedness and may be limited by the agreements governing other indebtedness we or our subsidiaries incur in the future. See "Management's Discussion and Analysis of Financial Condition and Results of Operations-Debt Covenants."
We did not declare or pay any dividends on our common stock in fiscal 2016 or fiscal 2015 .
Recent Sales of Unregistered Securities
We did not sell any unregistered securities during the period covered by this Annual Report on Form 10-K.
Purchases of Equity Securities
On October 29, 2015, our Board of Directors authorized a share repurchase program to use up to $100.0 million to repurchase outstanding shares of our common stock. We may repurchase shares under the program through open market purchases, privately negotiated transactions or otherwise as permitted by applicable federal securities laws. There was no purchase by us, on our behalf, or on behalf of any affiliate of our registered equity securities during the period covered by this Annual Report on Form 10-K.
35
Table of Contents
Performance Graph
Set forth below is a line graph comparing the cumulative total shareholder return on the Company's common stock since July 31, 2014 (the date our common stock commenced trading on the NYSE) through June 30, 2016, based on the market price of the Company's common stock and assuming reinvestment of dividends, with the cumulative total shareholder return of companies on the S&P Composite 1500 Index and S&P Composite 1500 Healthcare Index. The graph assumes that $100 was invested in the Company's common stock and in each index at the market close on July 31, 2014. The stock price performance of the following graph is not necessarily indicative of future stock performance.
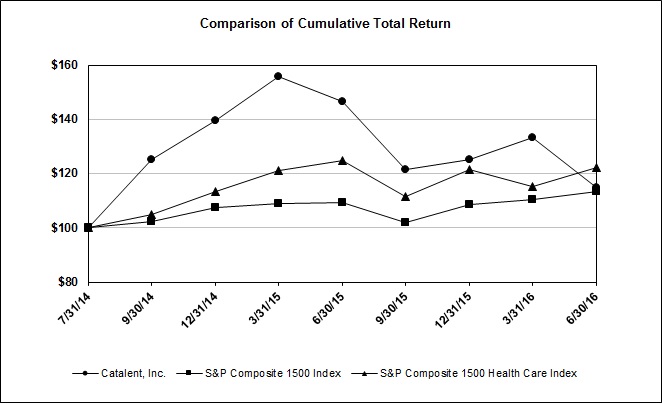
36
Table of Contents
ITEM 6. | SELECTED FINANCIAL DATA |
The following table sets forth our selected historical financial and operating data for, or as of the end of, each of the five years ended June 30, 2016 . The selected financial data as of June 30, 2016 and 2015 , and for the fiscal years ended June 30, 2016 , 2015 and 2014 has been derived from our audited consolidated financial statements included in "Financial Statements and Supplementary Data." The financial data as of June 30, 2014 , 2013 and 2012 and for the fiscal years ended June 30, 2013 and 2012 have been derived from our audited consolidated financial statements not included in this Annual Report on Form 10-K. This table should be read in conjunction with the Consolidated Financial Statements and the Notes thereto.
| Year Ended June 30, | ||||||||||||||||||
(Dollars in millions, except as noted) | 2016 |
| 2015 |
| 2014 |
| 2013 |
| 2012 | ||||||||||
Statement of Operations Data: |
|
|
|
|
|
|
|
|
| ||||||||||
Net revenue | $ | 1,848.1 | |
| $ | 1,830.8 | |
| $ | 1,827.7 | |
| $ | 1,800.3 | |
| $ | 1,694.8 | |
Cost of sales | 1,260.5 | |
| 1,215.5 | |
| 1,229.1 | |
| 1,231.7 | |
| 1,136.2 | | |||||
Gross margin | 587.6 | |
| 615.3 | |
| 598.6 | |
| 568.6 | |
| 558.6 | | |||||
Selling, general and administrative expenses | 358.1 | |
| 337.3 | |
| 334.8 | |
| 340.6 | |
| 348.1 | | |||||
Impairment charges and (gain)/loss on sale of assets | 2.7 | |
| 4.7 | |
| 3.2 | |
| 5.2 | |
| 1.8 | | |||||
Restructuring and other | 9.0 | |
| 13.4 | |
| 19.7 | |
| 18.4 | |
| 19.5 | | |||||
Property and casualty (gain)/loss, net (1) | - | |
| - | |
| - | |
| - | |
| (8.8 | ) | |||||
Operating earnings/(loss) | 217.8 | |
| 259.9 | |
| 240.9 | |
| 204.4 | |
| 198.0 | | |||||
Interest expense, net | 88.5 | |
| 105.0 | |
| 163.1 | |
| 203.2 | |
| 183.2 | | |||||
Other (income)/expense, net | (15.6 | ) |
| 42.4 | |
| 10.4 | |
| 25.1 | |
| (3.8 | ) | |||||
Earnings/(loss) from continuing operations before income taxes | 144.9 | |
| 112.5 | |
| 67.4 | |
| (23.9 | ) |
| 18.6 | | |||||
Income tax expense/(benefit) | 33.7 | |
| (97.7 | ) |
| 49.5 | |
| 27.0 | |
| 0.5 | | |||||
Earnings/(loss) from continuing operations | 111.2 | |
| 210.2 | |
| 17.9 | |
| (50.9 | ) |
| 18.1 | | |||||
Earnings/(loss) from discontinued operations, net of tax | - | |
| 0.1 | |
| (2.7 | ) |
| 1.2 | |
| (41.3 | ) | |||||
Net earnings/(loss) | 111.2 | |
| 210.3 | |
| 15.2 | |
| (49.7 | ) |
| (23.2 | ) | |||||
Less: Net earnings/(loss) attributable to noncontrolling interest, net of tax | (0.3 | ) |
| (1.9 | ) |
| (1.0 | ) |
| (0.1 | ) |
| 1.2 | | |||||
Net earnings/(loss) attributable to Catalent | $ | 111.5 | |
| $ | 212.2 | |
| $ | 16.2 | |
| $ | (49.6 | ) |
| $ | (24.4 | ) |
|
|
|
|
|
|
|
|
|
| ||||||||||
Basic earnings per share attributable to Catalent common shareholders: |
|
|
|
|
|
|
|
|
| ||||||||||
Earnings/(loss) from continuing operations | $ | 0.89 | |
| $ | 1.77 | |
| $ | 0.25 | |
| $ | (0.68 | ) |
| $ | 0.23 | |
Net earnings/(loss) | 0.89 | |
| 1.77 | |
| 0.22 | |
| (0.66 | ) |
| (0.33 | ) | |||||
Diluted earnings per share attributable to Catalent common shareholders: |
|
|
|
|
|
|
|
|
| ||||||||||
Earnings/(loss) from continuing operations | $ | 0.89 | |
| $ | 1.75 | |
| $ | 0.25 | |
| $ | (0.68 | ) |
| $ | 0.22 | |
Net earnings/(loss) | 0.89 | |
| 1.75 | |
| 0.21 | |
| (0.66 | ) |
| (0.32 | ) | |||||
(1) | In March 2011, a U.K. based packaging facility was damaged by fire. The 2012 amounts reported are net of insurance recovery. |
37
Table of Contents
| Year Ended June 30, | ||||||||||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2014 |
| 2013 |
| 2012 | ||||||||||
Balance Sheet Data (at period end): |
|
|
|
|
|
|
|
|
| ||||||||||
Cash and cash equivalents | $ | 131.6 | |
| $ | 151.3 | |
| $ | 74.4 | |
| $ | 106.4 | |
| $ | 139.0 | |
Goodwill | 996.5 | |
| 1,061.5 | |
| 1,097.1 | |
| 1,023.4 | |
| 1,029.9 | | |||||
Total assets (2) | 3,091.1 | |
| 3,138.3 | |
| 3,073.4 | |
| 2,931.3 | |
| 3,009.4 | | |||||
Long term debt, including current portion and other short term borrowing (2) | 1,860.5 | |
| 1,880.8 | |
| 2,693.8 | |
| 2,673.4 | |
| 2,660.8 | | |||||
Total liabilities (2) | 2,455.2 | |
| 2,498.5 | |
| 3,440.7 | |
| 3,341.6 | |
| 3,360.1 | | |||||
Total shareholders' equity/(deficit) | $ | 635.9 | |
| $ | 634.0 | |
| $ | (371.8 | ) |
| $ | (410.3 | ) |
| $ | (350.7 | ) |
| Year Ended June 30, | ||||||||||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2014 |
| 2013 |
| 2012 | ||||||||||
Other Financial Data: |
|
|
|
|
|
|
|
|
| ||||||||||
Capital expenditures | $ | 139.6 | |
| $ | 141.0 | |
| $ | 122.4 | |
| $ | 122.5 | |
| $ | 104.2 | |
Ratio of Earnings to Fixed Charges (3) | 2.5x | |
| 2.0x | |
| 1.4x | |
| - | |
| 1.1x | | |||||
Net cash provided by/(used in) continuing operations: |
|
|
|
|
|
|
|
|
| ||||||||||
Operating activities | 155.3 | |
| 171.7 | |
| 180.2 | |
| 139.1 | |
| 87.7 | | |||||
Investing activities | (137.7 | ) |
| (271.8 | ) |
| (175.2 | ) |
| (122.1 | ) |
| (538.2 | ) | |||||
Financing activities | (30.8 | ) |
| 196.5 | |
| (42.1 | ) |
| (49.3 | ) |
| 352.9 | | |||||
Net cash provided by/(used in) discontinued operations: | - | |
| 0.1 | |
| 2.1 | |
| (1.4 | ) |
| 43.9 | | |||||
Effect of foreign currency on cash | $ | (6.5 | ) |
| $ | (19.6 | ) |
| $ | 3.0 | |
| $ | 1.1 | |
| $ | (12.4 | ) |
(2) | In connection with the Company's adoption of ASU 2015-03, Simplifying the Presentation of Debt Issuance Costs , as of January 1, 2016, prior year debt balances have been retrospectively adjusted to include a direct deduction of unamortized debt issuance costs, resulting in a reclassification of $7.1 million, $16.8 million, $18.2 million and $22.7 million of debt issuance costs as of June 30, 2015, 2014, 2013, and 2012, respectively, to long-term debt, including current portion and other short term borrowing for the respective periods. Prior to the adoption of ASU 2015-03, the unamortized debt issuance costs were included in other assets on the Company's consolidated balance sheets. The unamortized debt issuance costs associated with the Company's revolving credit facility continues to be included within other assets. |
(3) | The ratio of earnings to fixed charges is calculated by dividing the sum of earnings from continuing operations before income taxes, equity in earnings (loss) from non-consolidated investments and fixed charges, by fixed charges. Fixed charges consist of interest expenses, capitalized interest and imputed interest on our leased obligations. For fiscal year 2013, earnings were insufficient to cover fixed charges by $25.9 million. |
38
Table of Contents
ITEM 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with "Item 6. Selected Financial Data" and our consolidated financial statements and related notes that appear elsewhere in this Annual Report on Form 10-K. In addition to historical consolidated financial information, the following discussion contains forward-looking statements that reflect our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this Annual Report on Form 10-K, particularly in "Item 1A. Risk Factors."
Overview
We are the leading global provider of advanced delivery technologies and development solutions for drugs, biologics and consumer and animal health products. Our oral, injectable, and respiratory delivery technologies provide delivery solutions across the full diversity of the pharmaceutical industry, including small molecules, large molecule biologics and consumer and animal health products. Through our extensive capabilities and deep expertise in product development, we help our customers take products to market faster, including nearly half of new drug products approved by the FDA in the last decade. Our advanced delivery technology platforms, which include those in our Softgel Technologies and our Drug Delivery Solutions segments, our proven formulation, manufacturing and regulatory expertise and our broad and deep intellectual property enable our customers and their patients' needs to develop more products and better treatments for patients and consumers. Across both development and delivery, our commitment to reliably supply our customers' needs is the foundation for the value we provide; annually, we produce more than 70 billion doses for nearly 7,000 customer products or approximately one in every twenty doses of such products taken each year by patients and consumers around the world. We believe that through our investments in growth-enabling capacity and capabilities, our ongoing focus on operational and quality excellence, the sales of existing customer products, the introduction of new customer products, our innovation activities and patents, and our entry into new markets, we will continue to benefit from attractive and differentiated margins, and realize the growth potential from these areas.
In the fourth quarter of fiscal 2016, we engaged in a business reorganization to better align our internal business unit structure with our "Follow the Molecule" strategy. Under the revised structure, we have created a Drug Delivery Solutions ("DDS") operating segment which encompasses all of our modified release technologies; prefilled syringes and other injectable formats; blow-fill seal unit dose development and manufacturing; biologic cell line development; analytical services; micronization technologies; and other conventional oral dose forms under a single DDS management team. Additionally, as part of the re-alignment, we have created a stand-alone Clinical Supply Services ("CSS") operating segment and management team with a sole focus on providing global clinical supply chain management services that aim to speed our customers' drugs to market. Further, as a result of the business unit re-alignment, our Softgel Technologies business now reports as a distinct operating segment. Our operating segments are the same as our reporting segments. All prior period comparative segment information has been restated to reflect the current reportable segments in accordance with ASC 280 Segment Reporting as discussed in Note 1 to the Consolidated Financial Statements. Our offerings and services are summarized below by reporting segment.
Advanced Delivery Technology Platforms
Softgel Technologies
Through our Softgel Technologies segment, we provide formulation, development and manufacturing services for soft capsules, or "softgels," which we first commercialized in the 1930s and have continually enhanced. We are the market leader in overall softgel manufacturing, and hold the leading market position in the prescription arena. Our principal softgel technologies include traditional softgel capsules, in which the shell is made of animal-derived gelatin, and Vegicaps and OptiShell capsules, in which the shell is made from vegetable-derived materials. Softgel capsules are used in a broad range of customer products, including prescription drugs, over-the-counter medications, dietary supplements and unit-dose cosmetics. Softgel capsules encapsulate liquid, paste or oil-based active compounds in solution or suspension within an outer shell, filling and sealing the capsule simultaneously. We typically perform all encapsulation for a product within one of our softgel facilities, with active ingredients provided by customers or sourced directly by us. Softgels have historically been used to solve formulation challenges or technical issues for a specific drug, to help improve the clinical performance of compounds, to provide important market differentiation, particularly for over-the-counter compounds, and to provide safe handling of hormonal, potent and cytotoxic drugs. We also participate in the softgel vitamin, mineral and supplement business in selected regions around the world. With the 2001 introduction of our vegetable-derived softgel shell, Vegicaps capsules, consumer health manufacturers have been able to extend the softgel dose form to a broader range of active ingredients and serve patient/consumer populations
39
Table of Contents
that were previously inaccessible due to religious, dietary or cultural preferences. In recent years, we have extended this platform to pharmaceutical products via our OptiShell offering. Our Vegicaps and OptiShell capsules are protected by patents in most major global markets. Physician and patient studies we have conducted have demonstrated a preference for softgels versus traditional tablet and hard capsule dose forms in terms of ease of swallowing, real or perceived speed of delivery, ability to remove or eliminate unpleasant odor or taste and, for physicians, perceived improved patient adherence with dosing regimens. Representative customers of Softgel Technologies include Pfizer, Novartis, Bayer, GlaxoSmithKline, Teva, Johnson & Johnson and Allergan.
We have eleven Softgel Technologies facilities in nine countries, including one in North America, three in Europe, three in South America and four in the Asia-Pacific region. Our Softgel Technologies segment represents 41% of the segments' aggregate revenue before inter-segment eliminations for fiscal 2016 .
Drug Delivery Solutions
Our Drug Delivery Solutions segment provides various complex advanced formulation delivery technologies, and related integrated solutions including: development and manufacturing of a broad range of oral dose forms including fast-dissolve tablets and both proprietary and conventional controlled release products, and delivery of pharmaceuticals, biologics and biosimilars administered via injection, inhalation and ophthalmic routes, using both traditional and advanced technologies. Representative customers of DDS include Pfizer, GlaxoSmithKline, Roche, Teva, Eli Lilly, Johnson & Johnson and Allergan.
We provide comprehensive pre-formulation, development, and both clinical and commercial scale for most traditional and advanced oral solid dose formats, including uncoated and coated tablets, powder/pellet/bead-filled two piece hard capsules, lozenges, powders and other forms for immediate and modified release prescription, consumer and animal health products. We have substantial experience developing and scaling up products requiring accelerated development timelines, requiring specialized handling, complex technology transfers, or specialized manufacturing processes.
We launched our orally dissolving tablet business in 1986 with the introduction of Zydis tablets, a unique oral dosage form that is freeze-dried in its package, can be swallowed without water, and typically dissolves in the mouth in less than three seconds. Most often used for indications, drugs and patient groups that can benefit from rapid oral disintegration, the Zydis technology is utilized in a wide range of products and indications, including treatments for a variety of central nervous system-related conditions such as migraines, Parkinson's Disease, schizophrenia, and pain relief and consumer healthcare products targeting allergy relief. Zydis tablets continue to be used in new ways by our customers as we extend the application of the technology to new categories, such as for immunotherapies, vaccines and biologics delivery.
Our range of injectable manufacturing offerings includes filling drugs or biologics into pre-filled syringes and glass-free ADVASEPT vials, with flexibility to accommodate other formats within our existing network, increasingly focused on complex pharmaceuticals and biologics. With our range of technologies we are able to meet a wide range of specifications, timelines and budgets. The complexity of the manufacturing process, the importance of experience and know-how, regulatory compliance, and high start-up capital requirements create significant barriers to entry and, as a result, limit the number of competitors in the market. For example, blow-fill-seal is an advanced aseptic processing technology, which uses a continuous process to form, fill with drug, and seal a plastic container in a sterile environment. Blow-fill-seal units are currently used for a variety of pharmaceuticals in liquid form, such as respiratory, ophthalmic and otic products. We are a leader in the outsourced blow-fill-seal market, and operate one of the largest capacity commercial manufacturing blow-fill-seal facilities in the world. Our sterile blow-fill-seal manufacturing has significant capacity and flexibility of manufacturing configurations. This business provides flexible and scalable solutions for unit-dose delivery of complex formulations such as suspensions and emulsions. Further, the business provides engineering and manufacturing solutions related to complex containers. Our regulatory expertise can lead to decreased time to commercialization, and our dedicated development production lines support feasibility, stability and clinical runs. We plan to continue to expand our product line in existing and new markets, and in higher margin specialty products with additional respiratory, ophthalmic, injectable and nasal applications.
Our fast-growing biologics offerings include our formulation development and cell-line manufacturing based on our advanced and patented GPEx technology, which is used to develop stable, high-yielding mammalian cell lines for both innovator and biosimilar biologic compounds. Our GPEx technology can provide rapid cell line development, high biologics production yields, flexibility and versatility. We believe our development stage SMARTag next-generation antibody-drug conjugate technology will provide more precision targeting for delivery of drugs to tumors or other locations, with improved safety versus existing technologies. Our biologics facility in Madison, Wisconsin has the capability and capacity to produce clinical-scale biologic supplies; combined with offerings from our other businesses and external partners, we provide the broadest range of technologies and services supporting the development and launch of new biologic entities, biosimilars or biobetters to bring a product from gene to market commercialization, faster.
40
Table of Contents
We also offer analytical chemical and cell-based testing and scientific services, stability testing, respiratory products formulation and manufacturing, micronization and particle engineering services, regulatory consulting, and bioanalytical testing for biologic products. Our respiratory product capabilities include development and manufacturing services for inhaled products for delivery via metered dose inhalers, dry powder inhalers and intra-nasal sprays. We also provide formulation development and clinical and commercial manufacturing for conventional and specialty oral dose forms. We provide global regulatory and clinical support services for our customers' regulatory and clinical strategies during all stages of development. Demand for our offerings is driven by the need for scientific expertise and depth and breadth of services offered, as well as by the reliable supply thereof, including quality, execution and performance.
We have thirteen Drug Delivery Solutions manufacturing facilities, including eight in North America and five in Europe. Our Drug Delivery Solutions segment represents 43% of the segments' aggregate revenue before inter-segment eliminations for fiscal 2016 .
Clinical Supply Services
Our Clinical Supply Services segment provides manufacturing, packaging, storage and inventory management for drugs and biologics in clinical trials. We offer customers flexible solutions for clinical supplies production, and provide distribution and inventory management support for both simple and complex clinical trials. This includes dose form manufacturing or over-encapsulation where needed; supplying placebos, comparator drug procurement and clinical packages and kits for physicians and patients; inventory management; investigator kit ordering and fulfillment; and return supply reconciliation and reporting. We support trials in all regions of the world through our facilities and distribution network. In fiscal 2016, we commenced an expansion of our Singapore facility by building new flexible cGMP space and we introduced clinical supply services at our 200,000 square foot facility in Japan, expanding our Asia Pacific capabilities. We completed a site consolidation in pursuit of synergies in our Clinical Supply Services segment within our U.K. operations in fiscal 2016. Additionally, in fiscal 2013, we established our first clinical supply services facility in China as a joint venture and assumed full ownership in fiscal 2015. We are the leading provider of integrated development solutions and one of the leading providers of clinical trial supplies and respiratory products. Representative customers of Clinical Supply Services include Astellas, GlaxoSmithKline, Eli Lilly, Merck, Pfizer and Shire.
We have nine Clinical Supply Service facilities, including two in North America, four in Europe and three in the Asia Pacific region. Our Clinical Supply Services segment represents 16% of the segments' aggregate revenue before inter-segment eliminations for fiscal 2016 .
Critical Accounting Policies and Estimates
The following disclosure supplements the descriptions of our accounting policies contained in Note 1 to our Consolidated Financial Statements (the "Consolidated Financial Statements") included elsewhere in this Annual Report on Form 10-K in regard to significant areas of judgment. Management made certain estimates and assumptions during the preparation of the Consolidated Financial Statements in accordance with generally accepted accounting principles. These estimates and assumptions affect the reported amount of assets and liabilities and disclosures of contingent assets and liabilities in the Consolidated Financial Statements. These estimates also affect the reported amount of net earnings during the reporting periods. Actual results could differ from those estimates. Because of the size of the financial statement elements to which they relate, some of our accounting policies and estimates have a more significant impact on the Consolidated Financial Statements than others.
Management has discussed the development and selection of these critical accounting policies and estimates with the audit committee of the board of directors. A discussion of some of our more significant accounting policies and estimates follows.
Revenues and Expenses
Net Revenue
We sell products and services directly to our pharmaceutical, biotechnology and consumer and animal health customers. The majority of our business is conducted through supply or development agreements. The majority of our revenue is charged on a price-per-unit basis and is recognized either upon shipment or delivery of the product or service. Revenue generated from development arrangements are generally priced by project and are recognized either upon completion of the required service or achievement of a specified project phase or milestone.
41
Table of Contents
Our overall net revenue is generally affected by the following factors:
• | Changes in the level or timing of research and development activities and sales activities by our customers; |
• | Fluctuations in overall economic activity within the geographic markets in which we operate; |
• | Change in the level of competition we face from our competitors; |
• | New intellectual property we develop and expiration of our patents; |
• | Changes in prices of our products and services, which are generally relatively stable due to our long-term contracts; and |
• | Fluctuations in exchange rates between foreign currencies, in which a substantial portion of our revenues and expenses are denominated, and the U.S. dollar. |
Operating Expenses
Cost of sales consists of direct costs incurred to manufacture and package products and costs associated with supplying other revenue-generating services. Cost of sales includes labor costs for employees involved in the production process and the cost of raw materials and components used in the process or product. Cost of sales also includes labor costs of employees supporting the production process, such as production management, quality, engineering, and other support services. Other costs in this category include the external research and development costs on behalf of our customers, depreciation of fixed assets, utility costs, freight, operating lease expenses and other general manufacturing expenses.
Selling, general and administrative expenses consist of all expenditures incurred in connection with the sales and marketing of our products, as well as administrative expenses to support our businesses. The category includes salaries and related benefit costs of employees supporting our sales and marketing, finance, human resources, information technology and legal functions, research and development costs in pursuit of our own proactive development and costs related to executive management. Other costs in this category include depreciation of fixed assets, amortization of our intangible assets, professional fees, and marketing and other expenses to support selling and administrative areas.
Direct expenses incurred by a segment are included in that segment's results. Shared sales and marketing, information technology services and general administrative costs are allocated to each segment based upon the specific activity being performed for each segment or are charged on the basis of the segment's respective revenues or other applicable measurement. Certain corporate expenses are not allocated to the segments. We do not allocate the following costs to the segments:
• | Impairment charges and (gain)/loss on sale of assets; |
• | Equity compensation; |
• | Restructuring expenses and other special items; |
• | Sponsor advisory fee and the related termination fee incurred in connection with our initial public offering; |
• | Noncontrolling interest; and |
• | Other income/(expense), net. |
Our operating expenses are generally affected by the following factors:
• | The utilization rate of our facilities: as our utilization rate increases, we achieve greater economies of scale as fixed manufacturing costs are spread over a larger number of units produced; |
• | Production volumes: as volumes change, the level of resources employed also fluctuate, including raw materials, component costs, employment costs and other related expenses, and our utilization rate may also be affected; |
• | The mix of different products or services that we sell; |
• | The cost of raw materials, components and general expense; |
• | Implementation of cost control measures and our ability to effect cost savings through our Operational Excellence, Lean Manufacturing and Lean Six Sigma programs; and |
• | Fluctuations in exchange rates between foreign currencies, in which a substantial portion of our revenues and expenses are denominated, and the U.S. dollar. |
42
Table of Contents
Allowance for Inventory Obsolescence
We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of the inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected, additional inventory write-downs may be required resulting in a charge to income in the period such determination was made.
Long-lived and Other Definite-Lived Intangible Assets
We allocate the cost of an acquired company to the tangible and identifiable intangible assets and liabilities acquired, with the remaining amount being recorded as goodwill. Certain intangible assets are amortized over their estimated useful life.
We assess the impairment of identifiable intangibles if events or changes in circumstances indicate that the carrying value of the asset may not be recoverable. Factors that we consider important that could trigger an impairment review include the following:
• | Significant under-performance relative to historical or projected future operating results; |
• | Significant changes in the manner of use of the acquired assets or the strategy of the overall business; |
• | Significant negative industry or economic trends; and |
• | Recognition of goodwill impairment charges. |
If we determine that the carrying value of intangibles and/or long-lived assets may not be recoverable based on the existence of one or more of the above indicators of impairment, we measure any impairment based on fair value, which we derive either by the estimated cash flows expected to result from the use of the asset and its eventual disposition or on assumptions we believe marketplace participants would utilize and comparable marketplace information in similar arm's length transactions. We then compare weighted values to the asset's carrying amount. Any impairment loss recognized would represent the excess of the asset's carrying value over its estimated fair value. Significant estimates and judgments are required when estimating such fair values. If it is determined that assets are impaired, an impairment charge would be recorded and the amount could be material. See Note 4 to the Consolidated Financial Statements.
Goodwill
We account for purchased goodwill and intangible assets with indefinite lives in accordance with Accounting Standard Codification ("ASC") 350 Goodwill, Intangible and Other Assets . Under ASC 350, goodwill and intangible assets with indefinite lives are tested for impairment at least annually utilizing both qualitative and quantitative assessments. Our annual goodwill impairment test was conducted as of April 1, 2016 . We assess goodwill for possible impairment by comparing the carrying value of our reporting units to their fair values. We determine the fair value of our reporting units utilizing estimated future discounted cash flows and incorporate assumptions that we believe marketplace participants would utilize. In addition, we use comparative market information and other factors to corroborate the discounted cash flow results. No reporting units were at risk of failing step one in the goodwill impairment test under the provisions of ASC 350 as of April 1, 2016 . See Note 3 to the Consolidated Financial Statements.
Income Taxes
In accordance with ASC 740 Income Taxes , we account for income taxes using the asset and liability method. The asset and liability method requires recognition of deferred tax assets and liabilities for expected future tax consequences of temporary differences that currently exist between tax bases and financial reporting bases of our assets and liabilities. Deferred tax assets and liabilities are measured using enacted tax rates in the respective jurisdictions in which we operate. Deferred taxes are not provided on the undistributed earnings of subsidiaries outside of the United States when it is expected that these earnings will be permanently reinvested. We have not made any provision for U.S. income taxes on the undistributed earnings of foreign subsidiaries as those earnings are considered permanently reinvested in the operations of those foreign subsidiaries.
We had valuation allowances of $69.9 million and $82.4 million as of June 30, 2016 and 2015 , respectively, against our deferred tax assets. We considered all available evidence, both positive and negative, in assessing the need for a valuation allowance for deferred tax assets. We evaluated three possible sources of taxable income when assessing the realization of deferred tax assets:
• | Future reversals of existing taxable temporary differences; |
• | Tax planning strategies; and |
43
Table of Contents
• | Future taxable income exclusive of reversing temporary differences and carryforwards. |
We considered the need to maintain a valuation allowance on deferred tax assets based on management's assessment of whether it is more likely than not that deferred tax assets would be realized based on future reversals of existing taxable temporary differences and the ability to generate sufficient taxable income within the carryforward period available under the applicable tax law. The deferred tax liabilities are expected to reverse in the same period and jurisdiction and are of the same character as the temporary differences giving rise to a portion of the deferred tax assets.
During the year ended June 30, 2015, we released the majority of our U.S. federal valuation allowance of $136.7 million based on projected U.S. future earnings in excess of the $294.1 million required to realize its net U.S. federal deferred tax assets. Of the $294.1 million, $329.5 million relates to the federal net operating loss carryforward (NOL), which expires in the years 2028 to 2032. The remaining $35.4 million related to other net deferred tax liabilities.
The reversal of the valuation allowance was the result of a continuing trend of U.S. taxable income and the expectation that this trend will continue, rather than relying on tax planning strategies to support the realization of deferred tax assets. We had experienced three consecutive years of positive U.S. taxable earnings as of June 30, 2015 and expect to sustain this position in the future, due to the positive impact on U.S. earnings from reduced interest expense resulting from a reduction in our external debt, among other factors.
While the U.S. federal valuation allowance was reversed, the U.S. state valuation allowance on $375.7 million of apportioned state net operating losses was maintained. Due to uncertainty around earnings, apportionment, certain restrictions at the state level, and the history of tax losses, anticipated utilization rates were not sufficient to overcome the negative evidence and allow a release.
ASC 740 provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolution of any related appeal or litigation process, based on the technical merits. We recognized no material adjustment in the liability for unrecognized income tax benefits.
The calculation of our income tax liabilities involves dealing with uncertainties in the application of complex domestic and foreign income tax regulations. Unrecognized tax benefits are generated when there are differences between tax positions taken in a tax return and amounts recognized in the Consolidated Financial Statements. Tax benefits are recognized in the Consolidated Financial Statements when it is more likely than not that a tax position will be sustained upon examination. To the extent we prevail in matters for which liabilities have been established, or are required to pay amounts in excess of our liabilities, our effective income tax rate in a given period could be materially affected. An unfavorable income tax settlement may require the use of cash and result in an increase in our effective income tax rate in the year it is resolved. A favorable income tax settlement would be recognized as a reduction in the effective income tax rate in the year of resolution. At June 30, 2016 and 2015 , we recorded unrecognized tax benefits and related interest and penalties of $67.1 million and $73.2 million , respectively.
The anticipated future trends included in our assessment of the realizability of our deferred tax assets are the same assumptions and anticipated future trends that were incorporated into the estimated fair value of our reporting units for purposes of testing goodwill for impairment. Such assumptions and anticipated future trends were also incorporated into other assessments of our tangible and intangible assets for impairment, as applicable. We are not currently relying on any tax-planning strategy to support the realization of deferred tax assets.
In the fourth quarter of fiscal 2016, we adopted Accounting Standard Update ("ASU") 2015-17 Balance Sheet Classification of Deferred Taxes , which requires that all deferred tax assets and liabilities, along with any related valuation allowance, be classified as noncurent on the balance sheet and applied its provisions prospectively without retrospective adjustment.
Also, in the fourth quarter of fiscal 2016, we adopted ASU 2016-09 Improvements to Employee Share-Based Payment Accounting , which requires all excess tax benefits and deficiencies to be recognized in income tax expense or benefit in earnings and applied its provision on a modified retrospective basis. Accordingly, we recognized the previously unrecognized excess tax benefits, which resulted in a cumulative-effect tax benefit adjustment of $19.9 million recorded as part of accumulated deficit, with the tax effects recorded as deferred tax assets at the beginning of the 2016 fiscal year.
New Accounting Pronouncements
Refer to Note 1 to the Consolidated Financial Statements for a description of recent accounting pronouncements.
44
Table of Contents
Factors Affecting our Performance
Fluctuations in Operating Results
Our financial reporting periods operate on a June 30 fiscal year end. Our revenue and net earnings are generally higher in our third and fourth quarters of each fiscal year. These fluctuations are primarily the result of the timing of our, and our customers', annual operational maintenance periods at locations in continental Europe and the United Kingdom, the seasonality associated with pharmaceutical and biotechnology budgetary spending decisions, clinical trial and research and development schedules and, to a lesser extent, the time of the year some of our customers' products are in higher demand.
Acquisition and Related Integration Efforts
Our growth and profitability are affected by the acquisitions we are able to complete and the speed at which we integrate those acquisitions into our existing operating platforms. Since January 1, 2012, we have completed nine acquisitions, the largest of which was the February 2012 purchase of the Aptuit CTS business. Since that acquisition, we consolidated three operations including one in December 2012, December 2013 and June 2016, respectively. In February 2012, we acquired the remaining 49% ownership interest in our German softgel joint venture. We commenced two joint ventures in China in fiscal 2013 and 2014, and completed the acquisition of the partner's interest in one venture in fiscal 2015 and the other venture in fiscal 2016. We purchased a softgel operation in Brazil in fiscal 2014 and have integrated it into our softgel business. Further, in October 2014, the Company acquired the remaining shares of Redwood Bioscience Inc. ("Redwood") and its SMARTag ADC technology platform. The acquired business is based in the U.S. and is included in the Drug Delivery Solutions segment. Additionally, in November 2014, the Company acquired 100% of the shares of MTI Pharma Solutions, Inc. ("Micron Technologies"), a company specializing in particle size reduction (micronization), milling and analytical contract services. The acquired business is based in the U.S. and the U.K. and is included in the Drug Delivery Solutions segment.
Foreign Exchange Rates
Significant portions of our revenues and costs are affected by changes in foreign exchange rates. Our operating network is global and, as a result, our revenues and operating expenses are influenced by changes in foreign exchange rates. In fiscal 2016 , approximately 54% of our revenue was generated from our operations outside the United States. Much of the revenue generated outside the United States and many of the expenses associated with our operations outside the United States are denominated in currencies other than the U.S. dollar, particularly the British pound, the euro, the Brazilian real, the Argentine peso, the Japanese yen and the Australian dollar. Changes in those currencies relative to the U.S. dollar will affect our revenues and expenses.
Trends Affecting Our Business
Industry
We participate in nearly every sector of the $900 billion annual revenue global pharmaceutical industry, including but not limited to the prescription drug and biologic sectors as well as consumer health, which includes the over-the-counter and vitamins and nutritional supplement sectors, and animal health. Innovative pharmaceuticals continue to play a critical role in the global market, while generic drug share is increasing in both developed and developing markets. Sustained developed market demand and rapid growth in emerging economies is driving the consumer health product growth rate to more than double that for pharmaceuticals. Payors, both public and private, have sought to limit the economic impact of such demand through greater use of generic drugs, access and spending controls and health technology assessment techniques, favoring products that deliver truly differentiated outcomes.
New Molecule Development and R&D Sourcing
Continued strengthening in early-stage development pipelines for drugs and biologics, compounded by increasing clinical trial breadth and complexity, sustain our belief in the attractive growth prospects for development solutions. Large companies are in many cases reconfiguring their R&D resources, increasingly involving the appointment of strategic partners for important outsourced functions. Additionally, an increasing portion of compounds in development are from companies that do not have full R&D infrastructure, and thus are more likely to need strategic development solutions partners.
Demographics
Aging population demographics in developed countries, combined with health care reforms in many global markets that are expanding access to treatments to a greater proportion of their populations, will continue to drive increases in demand for
45
both pharmaceutical and consumer health products. Increasing economic affluence in developing regions will further increase demand for healthcare treatments, and we are taking active steps to allow us to participate effectively in these growth regions and product categories.
Finally, we believe the market access and payor pressures our customers face, global supply chain complexity, and the increasing demand for improved treatments will continue to escalate the need for product differentiation, improved outcomes and treatment cost reduction, all of which can often be addressed using our advanced delivery technologies.
Non-GAAP Performance Metrics
Use of EBITDA from continuing operations
Management measures operating performance based on consolidated earnings from continuing operations before interest expense, expense/(benefit) for income taxes and depreciation and amortization, which is further adjusted for the income or loss attributable to noncontrolling interests ("EBITDA from continuing operations"). EBITDA from continuing operations is not defined under U.S. GAAP and is not a measure of operating income, operating performance or liquidity presented in accordance with U.S. GAAP and is subject to important limitations.
We believe that the presentation of EBITDA from continuing operations enhances an investor's understanding of our financial performance. We believe this measure is a useful financial metric to assess our operating performance from period to period and use this measure for business planning purposes. In addition, given the significant investments that we have made in the past in property, plant and equipment, depreciation and amortization expenses represent a meaningful portion of our cost structure. We believe that disclosing EBITDA from continuing operations will provide investors with a useful tool for assessing the comparability between periods of our ability to generate cash from operations sufficient to pay taxes, to service debt and to undertake capital expenditures because it eliminates depreciation and amortization expense. We present EBITDA from continuing operations in order to provide supplemental information that we consider relevant for the readers of the Consolidated Financial Statements, and such information is not meant to replace or supersede U.S. GAAP measures. Our definition of EBITDA from continuing operations may not be the same as similarly titled measures used by other companies.
In addition, we evaluate the performance of our segments based on segment earnings before noncontrolling interest, other (income)/expense, impairments, restructuring costs, interest expense, income tax expense/(benefit), and depreciation and amortization ("Segment EBITDA").
Use of Constant Currency
As exchange rates are an important factor in understanding period-to-period comparisons, we believe the presentation of results on a constant currency basis in addition to reported results helps improve investors' ability to understand our operating results and evaluate our performance in comparison to prior periods. Constant currency information compares results between periods as if exchange rates had remained constant period-over-period. We use results on a constant currency basis as one measure to evaluate our performance. In this Annual Report on Form 10-K, we calculate constant currency by calculating current-year results using prior-year foreign currency exchange rates. We generally refer to such amounts calculated on a constant currency basis as excluding the impact of foreign exchange. These results should be considered in addition to, not as a substitute for, results reported in accordance with U.S. GAAP. Results on a constant currency basis, as we present them, may not be comparable to similarly titled measures used by other companies and are not measures of performance presented in accordance with U.S. GAAP.
46
Table of Contents
Fiscal Year Ended June 30, 2016 compared to the Fiscal Year Ended June 30, 2015
Results for the fiscal year ended June 30, 2016 compared to the fiscal year ended June 30, 2015 were as follows:
| Fiscal Year Ended |
| FX impact (unfavorable) / favorable |
| Constant Currency Increase/(Decrease) | |||||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| |
| Change $ |
| Change % | |||||||||
Net revenue | $ | 1,848.1 | |
| $ | 1,830.8 | |
| $ | (95.4 | ) |
| $ | 112.7 | |
| 6 | % |
Cost of sales | 1,260.5 | |
| 1,215.5 | |
| (69.1 | ) |
| 114.1 | |
| 9 | % | ||||
Gross margin | 587.6 | |
| 615.3 | |
| (26.3 | ) |
| (1.4 | ) |
| * | | ||||
Selling, general and administrative expenses | 358.1 | |
| 337.3 | |
| (9.5 | ) |
| 30.3 | |
| 9 | % | ||||
Impairment charges and (gain)/loss on sale of assets | 2.7 | |
| 4.7 | |
| 0.2 | |
| (2.2 | ) |
| (47 | )% | ||||
Restructuring and other | 9.0 | |
| 13.4 | |
| (0.6 | ) |
| (3.8 | ) |
| (28 | )% | ||||
Operating earnings | 217.8 | |
| 259.9 | |
| (16.4 | ) |
| (25.7 | ) |
| (10 | )% | ||||
Interest expense, net | 88.5 | |
| 105.0 | |
| (1.5 | ) |
| (15.0 | ) |
| (14 | )% | ||||
Other (income)/expense, net | (15.6 | ) |
| 42.4 | |
| (2.6 | ) |
| (55.4 | ) |
| * | | ||||
Earnings from continuing operations before income taxes | 144.9 | |
| 112.5 | |
| (12.3 | ) |
| 44.7 | |
| 40 | % | ||||
Income tax expense/(benefit) | 33.7 | |
| (97.7 | ) |
| (4.0 | ) |
| 135.4 | |
| * | | ||||
Earnings from continuing operations | 111.2 | |
| 210.2 | |
| (8.3 | ) |
| (90.7 | ) |
| (43 | )% | ||||
Net earnings from discontinued operations, net of tax | - | |
| 0.1 | |
| - | |
| (0.1 | ) |
| * | | ||||
Net earnings | 111.2 | |
| 210.3 | |
| (8.3 | ) |
| (90.8 | ) |
| (43 | )% | ||||
Less: Net earnings/(loss) attributable to noncontrolling interest, net of tax | (0.3 | ) |
| (1.9 | ) |
| - | |
| 1.6 | |
| (84 | )% | ||||
Net earnings attributable to Catalent | $ | 111.5 | |
| $ | 212.2 | |
| $ | (8.3 | ) |
| $ | (92.4 | ) |
| (44 | )% |
* Percentage not meaningful
Net Revenue
Net revenue increased by $112.7 million , or 6%, as compared to the twelve months ended June 30, 2015 , excluding the impact of foreign exchange. The increase in net revenue was driven by increased sales across all three reportable segments, led primarily by our Softgel Technologies segment. The increase in net revenue was primarily driven by higher end market volume demand for consumer health products using our softgel offering, increased sales volume across our Drug Delivery Solutions segment platforms and increased comparator sourcing volume and increased sales volume related to storage and distribution revenue within our Clinical Supply Services segment. Revenue increases were partially offset by a decrease in volume as a result of the temporary suspension of operations at our softgel manufacturing facility in Beinheim, France, which occurred between November 2015 and April 2016.
Gross Margin
Gross margin decreased by $1.4 million , as compared to the twelve months ended June 30, 2015 , excluding the impact of foreign exchange. The decrease in gross margin was primarily driven by lower volumes resulting in reduced end customer demand of certain higher margin offerings within our Drug Delivery Solutions segment and decreased revenue resulting from the temporary suspension of operations at our softgel manufacturing facility in Beinheim, France during the period within our Softgel Technologies segment, partially offset by higher sales volumes across all three segments and more effective absorption of fixed costs through higher capacity utilization within our Softgel Technologies segment. On a constant currency basis, gross margin, as a percentage of revenue, decreased 200 basis points to 31.6% in the twelve months ended June 30, 2016 as compared to the prior year primarily driven by an unfavorable shift in revenue mix in our Drug Delivery Solutions segment and in our Clinical Supply Services segment.
Selling, General and Administrative Expense
Selling, general and administrative expense increased by $30.3 million , or 9% , as compared to the twelve months ended June 30, 2015 , excluding the impact of foreign exchange, primarily due to incremental employee compensation costs of approximately $13 million, inclusive of certain severance payments, inflationary increases and an increase in our non-cash equity compensation plans as a result of a change from a cash-based long-term incentive plan to an equity-based long-term
47
Table of Contents
incentive plan. Selling, general and administrative expense also increased due to acquisition-related transaction costs of approximately $5 million, and increased costs of approximately $6 million related to the temporary suspension of operations at our softgel manufacturing facility in Beinheim, France from November 2015 to April 2016. Selling, general and administrative expense increased approximately $5 million because of entities we acquired during the prior year.
Restructuring and Other
Restructuring and other charges of $9.0 million for the twelve months ended June 30, 2016 decreased by $4.4 million , or 33% , compared to the twelve months ended June 30, 2015 . The twelve months ended June 30, 2016 included restructuring activities enacted to improve cost efficiency, including employee severance expenses and costs related to a site consolidation in pursuit of synergies in our Clinical Supply Services segment within our U.K. operations. The prior period charges included restructuring initiatives enacted to improve cost efficiency at sites across our global network. Restructuring expense will vary period to period based on the level of acquisitions during the year and site consolidation efforts to further streamline the business.
Interest Expense, net
Interest expense, net, of $88.5 million for the twelve months ended June 30, 2016 decreased by $16.5 million , or 16%, compared to the twelve months ended June 30, 2015 , primarily driven by lower levels of outstanding debt as compared to the prior year. We redeemed $350 million of Senior Notes due 2018 (the "Senior Notes") and $275 million of Senior Subordinated Notes due 2017 (the "Senior Subordinated Notes") on August 28, 2014 and September 4, 2014, respectively. In addition, we reduced an aggregate of $234.5 million of outstanding borrowings under an unsecured term loan during the first quarter of fiscal 2015, partially offset by incremental borrowings of $191 million during the second quarter of fiscal 2015 in support of completed acquisitions. The funds utilized to reduce our debt levels were generated by proceeds from our IPO, which was completed during the first quarter of fiscal 2015.
Other (Income)/Expense, net
Other income , net of $15.6 million for the twelve months ended June 30, 2016 was primarily driven by non-cash net gains from foreign exchange translation recorded during the period plus earnings from our available for sale investments related to our deferred compensation plans. Other expense, net of $42.4 million in the twelve months ended June 30, 2015 was primarily driven by a sponsor advisory fee agreement termination fee of $29.8 million, which we agreed to pay in connection with our IPO. In addition, we incurred $21.8 million of expense in fiscal 2015 associated with the early redemption of our Senior Notes and pre-payment of an unsecured term loan, of which $9.8 million was a cash expense. Offsetting these other expense items were non-recurring non-cash purchase accounting gains, net, of $8.9 million related to acquisitions completed during the period and $2.4 million of non-cash net gains associated with foreign exchange.
Provision/(Benefit) for Income Taxes
Our provision for income taxes for the twelve months ended June 30, 2016 was $33.7 million relative to earnings before income taxes of $144.9 million . Our benefit for income taxes for the twelve months ended June 30, 2015 was $97.7 million relative to earnings before income taxes of $112.5 million . The income tax provision for the current period is not comparable to the same period of the prior year due to changes in pretax income over many jurisdictions and the impact of discrete items. Generally, fluctuations in the effective tax rate are primarily due to changes in our geographic pretax income resulting from our business mix and changes in the tax impact of permanent differences, restructuring, other special items and other discrete tax items, which may have unique tax implications depending on the nature of the item. Our effective tax rate at June 30, 2016 reflects the release of the U.S. federal valuation allowance and an increase in a tax reserve related to an adjustment to inter-company interest income in Germany, partially offset by a corresponding deduction in the United Kingdom.
48
Table of Contents
Segment Review
All prior period comparative segment information has been restated to reflect the current reportable segments in accordance with ASC 280 Segment Reporting as discussed in Note 1 to the Consolidated Financial Statements. The Company's results on a segment basis for the fiscal year ended June 30, 2016 compared to the twelve months ended June 30, 2015 were as follows:
| Fiscal Year Ended |
| FX impact (unfavorable) / favorable |
| Constant Currency Increase/(Decrease) | |||||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| |
| Change $ |
| Change % | |||||||||
Softgel Technologies |
|
|
|
|
|
|
|
|
| |||||||||
Net revenue | $ | 775.0 | |
| $ | 787.5 | |
| $ | (68.2 | ) |
| $ | 55.7 | |
| 7 | % |
Segment EBITDA | 163.8 | |
| 173.6 | |
| (15.9 | ) |
| 6.1 | |
| 4 | % | ||||
Drug Delivery Solutions |
|
|
|
|
|
|
|
|
| |||||||||
Net revenue | 806.4 | |
| 798.3 | |
| (20.4 | ) |
| 28.5 | |
| 4 | % | ||||
Segment EBITDA | 215.2 | |
| 230.7 | |
| (5.2 | ) |
| (10.3 | ) |
| (4 | )% | ||||
Clinical Supply Services |
|
|
|
|
|
|
|
|
| |||||||||
Net revenue | 307.5 | |
| 288.4 | |
| (9.4 | ) |
| 28.5 | |
| 10 | % | ||||
Segment EBITDA | 53.2 | |
| 56.7 | |
| (2.4 | ) |
| (1.1 | ) |
| (2 | )% | ||||
Inter-segment revenue elimination | (40.8 | ) |
| (43.4 | ) |
| 2.6 | |
| - | |
| * | | ||||
Unallocated Costs (1) | (57.9 | ) |
| (100.8 | ) |
| 3.3 | |
| 39.6 | |
| (39 | )% | ||||
Combined Total |
|
|
|
|
|
|
|
|
| |||||||||
Net revenue | $ | 1,848.1 | |
| $ | 1,830.8 | |
| $ | (95.4 | ) |
| $ | 112.7 | |
| 6 | % |
|
|
|
|
|
|
|
|
|
| |||||||||
EBITDA from continuing operations | $ | 374.3 | |
| $ | 360.2 | |
| $ | (20.2 | ) |
| $ | 34.3 | |
| 10 | % |
(1) | Unallocated costs includes equity-based compensation, certain acquisition-related costs, impairment charges, certain other corporate directed costs, and other costs that are not allocated to the segments as follows: |
| Fiscal Year Ended | ||||||
(Dollars in millions) | 2016 |
| 2015 | ||||
Impairment charges and gain/(loss) on sale of assets | $ | (2.7 | ) |
| $ | (4.7 | ) |
Equity compensation | (10.8 | ) |
| (9.0 | ) | ||
Restructuring and other special items (2) | (27.2 | ) |
| (27.2 | ) | ||
Noncontrolling interest | 0.3 | |
| 1.9 | | ||
Other income/(expense), net (3) | 15.6 | |
| (42.4 | ) | ||
Non-allocated corporate costs, net | (33.1 | ) |
| (19.4 | ) | ||
Total unallocated costs | $ | (57.9 | ) |
| $ | (100.8 | ) |
(2) | Segment results do not include restructuring and certain acquisition-related costs. |
(3) | Amounts for fiscal 2015 primarily relate to the expense associated with the termination of the sponsor advisory fee agreement of $29.8 million resulting from the IPO, expenses related to financing transactions of $21.8 million and non-recurring non-cash purchase accounting gains of approximately $8.9 million related to acquisitions completed. |
49
Table of Contents
Provided below is a reconciliation of earnings/(loss) from continuing operations to EBITDA from continuing operations:
| Fiscal Year Ended | ||||||
(Dollars in millions) | 2016 |
| 2015 | ||||
Earnings from continuing operations | $ | 111.2 | |
| $ | 210.2 | |
Depreciation and amortization | 140.6 | |
| 140.8 | | ||
Interest expense, net | 88.5 | |
| 105.0 | | ||
Income tax (benefit)/expense | 33.7 | |
| (97.7 | ) | ||
Noncontrolling interest | 0.3 | |
| 1.9 | | ||
EBITDA from continuing operations | $ | 374.3 | |
| $ | 360.2 | |
Softgel Technologies segment
| 2016 vs. 2015 | ||||
Factors Contributing to Year-Over-Year Change | Fiscal Year Ended | ||||
| Net Revenue |
| Segment EBITDA | ||
Organic revenue / Segment EBITDA | 6 | % |
| 4 | % |
Impact of acquisitions | 1 | % |
| - | % |
Impact of divestitures / business restructuring | - | % |
| - | % |
Constant currency change | 7 | % |
| 4 | % |
Foreign exchange fluctuation | (8 | )% |
| (9 | )% |
Total % Change | (1 | )% |
| (5 | )% |
Softgel Technologies' net revenue increased $55.7 million , or 7% , excluding the impact of foreign exchange. The primary driver was higher end market volume demand for lower margin consumer health products using our softgel offering primarily in Asia Pacific. Partially offsetting the segment's increased revenue was a decrease in volume of prescription products of approximately $35 million primarily in Europe due to the temporary suspension of operations at our facility in Beinheim, France, which occurred between November 2015 and April 2016. See below for further discussion.
Softgel Technologies' Segment EBITDA increased by $6.1 million , or 4%, as compared to the twelve months ended June 30, 2015 , excluding the impact of foreign exchange. The increase was primarily driven by increased sales volumes of our lower margin consumer health products and more effective absorption of fixed costs through higher capacity utilization, partially offset by the temporary suspension of operations at our facility in Beinheim, France resulting in a decrease of approximately $32 million. See below for further discussion.
On November 13, 2015, the primary French drug regulatory agency (the "ANSM") issued an order temporarily suspending operations at our softgel manufacturing facility in Beinheim, France, which was lifted on April 28, 2016. The suspension order permitted the facility to apply for exemptions for certain types of operations. Due to the temporary suspension, we were unable to use certain raw materials, work in process and finished goods and took a charge of $1.0 million in fiscal 2016 in connection with such loss of use. We recorded remediation associated costs of $6.0 million in the same period. Further, certain customers of the facility have presented claims against us for losses they have allegedly suffered due to the temporary suspension or have reserved their right to do so subsequently. We are unable to estimate at this time either the total value of these claims or the likely cost to resolve them. Changes to the operations at the facility to address the issues leading to the suspension have increased and may in the future additionally increase the cost and therefore decrease the profitability of its operation.
50
Table of Contents
Drug Delivery Solutions segment
| 2016 vs. 2015 | ||||
Factors Contributing to Year-Over-Year Change | Fiscal Year Ended | ||||
| Net Revenue |
| Segment EBITDA | ||
Organic revenue / Segment EBITDA | 3 | % |
| (5 | )% |
Impact of acquisitions | 1 | % |
| 1 | % |
Impact of divestitures / business restructuring | - | % |
| - | % |
Constant currency change | 4 | % |
| (4 | )% |
Foreign exchange fluctuation | (3 | )% |
| (3 | )% |
Total % Change | 1 | % |
| (7 | )% |
Net revenue in our Drug Delivery Solutions segment increased by $28.5 million , or 4%, as compared to the twelve months ended June 30, 2015 , excluding the impact of foreign exchange. Net revenue increased approximately 3% from our analytical services platform driven by increased sales volumes related to fee for service development work and analytical testing in the U.S. Net revenue also increased approximately 2% as a result of increased volume from our biologics offerings and increased volume of products utilizing our blow-fill-seal technology platform of approximately 1%. Offsetting revenue was decreased volumes from our oral delivery solutions platform of 3% due to reduced end customer volume demand for certain higher margin offerings primarily in our U.S. operations and lower revenue from product participation related activities. Finally, net revenue increased approximately 1% as a result of the Micron Technologies acquisition completed during the second quarter of fiscal 2015.
Drug Delivery Solutions' Segment EBITDA decreased by $10.3 million , or 4% , as compared to the twelve months ended June 30, 2015 , excluding the impact of foreign exchange, primarily due to lower volumes driven by reduced end customer demand of certain higher margin offerings and lower absorption of fixed manufacturing costs within our oral delivery solutions platform, partially offset by increased profit generated by our biologics offering and from products utilizing our blow-fill-seal technology platform.
| 2016 vs. 2015 | ||||
Factors Contributing to Year-Over-Year Change | Fiscal Year Ended | ||||
| Net Revenue |
| Segment EBITDA | ||
Organic revenue / Segment EBITDA | 10 | % |
| (2 | )% |
Impact of acquisitions | - | % |
| - | % |
Impact of divestitures / business restructuring | - | % |
| - | % |
Constant currency change | 10 | % |
| (2 | )% |
Foreign exchange fluctuation | (3 | )% |
| (4 | )% |
Total % Change | 7 | % |
| (6 | )% |
Clinical Supply Services' net revenue increased by $28.5 million , or 10% , as compared to the twelve months ended June 30, 2015 , excluding the impact of foreign exchange, primarily due to increased lower-margin comparator sourcing volume of $13 million, or 4%, and increased volume related to storage and distribution revenue.
Clinical Supply Services' Segment EBITDA decreased by $1.1 million , or 2%, excluding the impact of foreign exchange, as compared to the twelve months ended June 30, 2015 , primarily due to a shift to increased lower-margin comparator sourcing volume within our revenue mix in addition to increased cost related to a business update to enhance operational efficiency.
51
Table of Contents
Fiscal Year Ended June 30, 2015 compared to Fiscal Year Ended June 30, 2014
| Fiscal Year Ended |
| FX impact (unfavorable) / favorable |
| Constant Currency Increase/(Decrease) | |||||||||||||
(Dollars in millions) | 2015 |
| 2014 |
|
|
| Change $ |
| Change % | |||||||||
Net revenue | $ | 1,830.8 | |
| $ | 1,827.7 | |
| $ | (117.9 | ) |
| $ | 121.0 | |
| 7 | % |
Cost of products sold | 1,215.5 | |
| 1,229.1 | |
| (82.2 | ) |
| 68.6 | |
| 6 | % | ||||
Gross margin | 615.3 | |
| 598.6 | |
| (35.7 | ) |
| 52.4 | |
| 9 | % | ||||
Selling, general and administrative expenses | 337.3 | |
| 334.8 | |
| (11.0 | ) |
| 13.5 | |
| 4 | % | ||||
Impairment charges and (gain)/loss on sale of assets | 4.7 | |
| 3.2 | |
| (0.1 | ) |
| 1.6 | |
| 50 | % | ||||
Restructuring and other | 13.4 | |
| 19.7 | |
| (2.0 | ) |
| (4.3 | ) |
| (22 | )% | ||||
Operating earnings | 259.9 | |
| 240.9 | |
| (22.6 | ) |
| 41.6 | |
| 17 | % | ||||
Interest expense, net | 105.0 | |
| 163.1 | |
| (1.2 | ) |
| (56.9 | ) |
| (35 | )% | ||||
Other (income)/expense, net | 42.4 | |
| 10.4 | |
| (5.2 | ) |
| 37.2 | |
| * | | ||||
Earnings from continuing operations before income taxes | 112.5 | |
| 67.4 | |
| (16.2 | ) |
| 61.3 | |
| 91 | % | ||||
Income tax expense/(benefit) | (97.7 | ) |
| 49.5 | |
| (4.3 | ) |
| (142.9 | ) |
| * | | ||||
Earnings from continuing operations | 210.2 | |
| 17.9 | |
| (11.9 | ) |
| 204.2 | |
| * | | ||||
Net earnings/(loss) from discontinued operations, net of tax | 0.1 | |
| (2.7 | ) |
| - | |
| 2.8 | |
| * | | ||||
Net earnings | 210.3 | |
| 15.2 | |
| (11.9 | ) |
| 207.0 | |
| * | | ||||
Less: Net earnings/(loss) attributable to noncontrolling interest, net of tax | (1.9 | ) |
| (1.0 | ) |
| 0.1 | |
| (1.0 | ) |
| * | | ||||
Net earnings attributable to Catalent | $ | 212.2 | |
| $ | 16.2 | |
| $ | (12.0 | ) |
| $ | 208.0 | |
| * | |
* Percentage not meaningful
Net Revenue
Net revenue increased by $121.0 million, or 7%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange. The increase in net revenue was driven primarily by increased volume within our integrated oral solids development and manufacturing capabilities, higher revenue from product participation related activities and increased volume from our biologics offerings within our Drug Delivery Solutions segment.
Gross Margin
Gross margin increased by $52.4 million, or 9%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange. On a constant currency basis, gross margin, as a percentage of revenue, increased 60 basis points to 33.4% in the twelve months ended June 30, 2015 as compared to 32.8% in the prior year. The increase in gross margin was primarily due to increased sales across three reportable segments and a favorable shift in revenue mix within our Drug Delivery Solutions segment. The increase in gross margin was partially offset by an unfavorable product mix from our softgel offering within our Softgel Technologies segment.
Selling, General and Administrative Expense
Selling, general and administrative expense increased by $13.5 million, or 4%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange, primarily due to $12 million of incremental expense related to entities we acquired during the year. The $12 million was primarily comprised of non-cash depreciation and amortization expense of $7 million, integration costs of $4 million, and employee compensation costs of $1 million. In addition, selling, general and administrative expense increased $4.5 million related to our non-cash equity compensation plans as a result of a change from a cash-based long-term incentive plan to an equity-based long-term incentive plan. These costs were partially offset by a $12.9 million reduction in expense due to the elimination of the recurring sponsor advisory fee agreement as a result of our IPO during the first quarter of fiscal 2015.
52
Table of Contents
Restructuring and Other
Restructuring and other charges of $13.4 million for the twelve months ended June 30, 2015 decreased by $6.3 million, or 32%, compared to the twelve months ended June 30, 2014. The twelve months ended June 30, 2015 included restructuring initiatives enacted to improve cost efficiency primarily related to employee severance expenses. The prior period charges included restructuring initiatives across several of our operations enacted to improve cost efficiency, including site consolidation in pursuit of synergies related to the Aptuit CTS acquisition and employee-related severance expenses during the twelve months ended June 30, 2014.
Interest Expense, net
Interest expense, net, of $105.0 million for the twelve months ended June 30, 2015 decreased by $58.1 million, or 36%, compared to the twelve months ended June 30, 2014, primarily driven by lower levels of outstanding debt as compared to the prior year. The Company redeemed $350 million of Senior Notes and $275 million of Senior Subordinated Notes on August 28, 2014 and September 4, 2014, respectively. In addition, we reduced an aggregate of $234.5 million of outstanding borrowings under an unsecured term loan during the first quarter of fiscal 2015. The funds utilized to reduce our debt levels were generated by proceeds from our IPO, which was completed during the first quarter of fiscal 2015. The decrease in interest expense, net was partially offset by incremental borrowings of $191 million during the second quarter of fiscal 2015 in support of acquisitions.
Other (Income)/Expense, net
Other expense, net of $42.4 million for the twelve months ended June 30, 2015 increased from $10.4 million in the twelve months ended June 30, 2014. The increase was primarily driven by a sponsor advisory fee agreement termination fee of $29.8 million, which we agreed to pay in connection with our IPO. In addition, we incurred $21.8 million of expense associated with the early redemption of our Senior Notes and pre-payment of an unsecured term loan in fiscal 2015, of which $9.8 million was a cash expense. Offsetting these other expense items were non-recurring non-cash purchase accounting gains, net, of approximately $8.9 million related to acquisitions completed during the period and $2.4 million of non-cash net gains associated with foreign exchange. Other expense, net for the twelve months ended June 30, 2014, was primarily driven by expenses of approximately $11 million related to the May 2014 refinancing of our Senior Secured Credit Facility and the write off of unamortized deferred financing fees. Also included were non-cash unrealized gains related to foreign currency translation, partially offset by realized losses related to foreign currency translation.
Provision/(Benefit) for Income Taxes
Our benefit for income taxes for the twelve months ended June 30, 2015 was $97.7 million relative to earnings before income taxes of $112.5 million. Our provision for income taxes for the twelve months ended June 30, 2014 was $49.5 million relative to earnings before income taxes of $67.4 million. The income tax benefit for the current period is not comparable to the same period of the prior year due to changes in pretax income over many jurisdictions and the impact of discrete items. Generally, fluctuations in the effective tax rate are primarily due to changes in our geographic pretax income resulting from our business mix and changes in the tax impact of permanent differences, restructuring, other special items and other discrete tax items, which may have unique tax implications depending on the nature of the item. Our effective tax rate at June 30, 2015 reflects the release of the U.S. federal valuation allowance and an increase in a tax reserve related to an adjustment to inter-company interest income in Germany, partially offset by a corresponding deduction in the United Kingdom.
53
Table of Contents
Segment Review
| Fiscal Year Ended |
| FX impact (unfavorable) / favorable |
| Constant Currency Increase/(Decrease) | |||||||||||||
(Dollars in millions) | 2015 |
| 2014 |
|
|
| Change $ |
| Change % | |||||||||
Softgel Technologies |
|
|
|
|
|
|
|
|
| |||||||||
Net revenue | $ | 787.5 | |
| $ | 857.5 | |
| $ | (83.6 | ) |
| $ | 13.6 | |
| 2 | % |
Segment EBITDA | 173.6 | |
| 214.8 | |
| (22.6 | ) |
| (18.6 | ) |
| (9 | )% | ||||
Drug Delivery Solutions |
|
|
|
|
|
| |
|
| |||||||||
Net revenue | 798.3 | |
| 719.2 | |
| (33.1 | ) |
| 112.2 | |
| 16 | % | ||||
Segment EBITDA | 230.7 | |
| 182.2 | |
| (7.9 | ) |
| 56.4 | |
| 31 | % | ||||
Clinical Supply Services |
|
|
|
|
|
|
|
|
| |||||||||
Net revenue | 288.4 | |
| 291.7 | |
| (6.4 | ) |
| 3.1 | |
| 1 | % | ||||
Segment EBITDA | 56.7 | |
| 59.5 | |
| (2.0 | ) |
| (0.8 | ) |
| (1 | )% | ||||
Inter-segment revenue elimination | (43.4 | ) |
| (40.7 | ) |
| 5.2 | |
| (7.9 | ) |
| 19 | % | ||||
Unallocated Costs (1) | (100.8 | ) |
| (82.1 | ) |
| 7.6 | |
| (26.3 | ) |
| 32 | % | ||||
Combined Total |
|
|
|
|
|
|
|
|
| |||||||||
Net revenue | $ | 1,830.8 | |
| $ | 1,827.7 | |
| $ | (117.9 | ) |
| $ | 121.0 | |
| 7 | % |
|
|
|
|
|
|
|
|
|
| |||||||||
EBITDA from continuing operations | $ | 360.2 | |
| $ | 374.4 | |
| $ | (24.9 | ) |
| $ | 10.7 | |
| 3 | % |
*Percentage not meaningful
(1) | Unallocated costs includes equity-based compensation, certain acquisition-related costs, impairment charges, certain other corporate directed costs, and other costs that are not allocated to the segments as follows: |
| Fiscal Year Ended | ||||||
(Dollars in millions) | 2015 |
| 2014 | ||||
Impairment charges and gain/(loss) on sale of assets | $ | (4.7 | ) |
| $ | (3.2 | ) |
Equity compensation | (9.0 | ) |
| (4.5 | ) | ||
Restructuring and other special items (2) | (27.2 | ) |
| (29.4 | ) | ||
Sponsor advisory fee | - | |
| (12.9 | ) | ||
Noncontrolling interest | 1.9 | |
| 1.0 | | ||
Other income/(expense), net (3) | (42.4 | ) |
| (10.4 | ) | ||
Non-allocated corporate costs, net | (19.4 | ) |
| (22.7 | ) | ||
Total unallocated costs | $ | (100.8 | ) |
| $ | (82.1 | ) |
(2) | Segment results do not include restructuring and certain acquisition-related costs |
(3) | Amounts for fiscal 2015 primarily relate to the expense associated with the termination of the sponsor advisory fee agreement of $29.8 million resulting from the IPO, expenses related to financing transactions of $21.8 million, non-recurring non-cash purchase accounting gains of approximately $8.9 million related to acquisitions completed. |
54
Table of Contents
Provided below is a reconciliation of earnings/(loss) from continuing operations to EBITDA from continuing operations:
| Fiscal Year Ended | ||||||
(Dollars in millions) | 2015 |
| 2014 | ||||
Earnings from continuing operations | $ | 210.2 | |
| $ | 17.9 | |
Depreciation and amortization | 140.8 | |
| 142.9 | | ||
Interest expense, net | 105.0 | |
| 163.1 | | ||
Income tax (benefit)/expense | (97.7 | ) |
| 49.5 | | ||
Noncontrolling interest | 1.9 | |
| 1.0 | | ||
EBITDA from continuing operations | $ | 360.2 | |
| $ | 374.4 | |
Softgel Technologies segment
| 2015 vs. 2014 | ||||
Factors Contributing to Year-Over-Year Change | Fiscal Year Ended | ||||
| Net Revenue |
| Segment EBITDA | ||
Organic Growth / Segment EBITDA | 1 | % |
| (9 | )% |
Impact of acquisitions | 1 | % |
| - | % |
Impact of divestitures / business restructuring | - | % |
| - | % |
Constant currency change | 2 | % |
| (9 | )% |
Foreign exchange fluctuation | (10 | )% |
| (10 | )% |
Total % Change | (8 | )% |
| (19 | )% |
Net Revenue in our Softgel Technologies segment increased by $13.6 million or 2%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange, primarily driven by higher end market volume demand for lower margin consumer health products primarily in Latin America and Asia Pacific, partially offset by decreased consumer health product volume in Europe. Profit participation related activities decreased by approximately $6.0 million.
Softgel Technologies' Segment EBITDA decreased by $18.6 million, or 9%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange, primarily due to a shift to lower-margin consumer health product within our offering mix.
| 2015 vs. 2014 | ||||
Factors Contributing to Year-Over-Year Change | Fiscal Year Ended | ||||
| Net Revenue |
| Segment EBITDA | ||
Organic Growth / Segment EBITDA | 14 | % |
| 30 | % |
Impact of acquisitions | 2 | % |
| 1 | % |
Impact of divestitures / business restructuring | - | % |
| - | % |
Constant currency change | 16 | % |
| 31 | % |
Foreign exchange fluctuation | (5 | )% |
| (4 | )% |
Total % Change | 11 | % |
| 27 | % |
55
Table of Contents
Drug Delivery Solutions' net revenue increased $112.2 million or 16%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange, primarily driven by increased revenue from our oral delivery solutions platform of approximately 11% due to higher revenue from product participation related activities and increased volume within our integrated oral solids development and manufacturing capabilities. Net revenue also increased approximately 1% as a result of increased volume from our biologics offerings and increased volume of products utilizing our blow-fill-seal technology platform of approximately 2%. Finally, net revenue increased approximately 2% as a result of the Micron Technologies acquisition completed during the second quarter of fiscal 2015.
Drug Delivery Solutions' Segment EBITDA increased by $56.4 million, or 31%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange, primarily driven by increased profit from our product related activities coupled with increased volume related to our integrated oral solids development and manufacturing capabilities within our oral delivery technologies business.
| 2015 vs. 2014 | ||||
Factors Contributing to Year-Over-Year Change | Fiscal Year Ended | ||||
| Net Revenue |
| Segment EBITDA | ||
Organic Growth / Segment EBITDA | 1 | % |
| (1 | )% |
Impact of acquisitions | - | % |
| - | % |
Impact of divestitures / business restructuring | - | % |
| - | % |
Constant currency change | 1 | % |
| (1 | )% |
Foreign exchange fluctuation | (2 | )% |
| (4 | )% |
Total % Change | (1 | )% |
| (5 | )% |
Clinical Supply Services' net revenue increased by $3.1 million, or 1%, as compared to the twelve months ended June 30, 2014, excluding the impact of foreign exchange, primarily due to increased lower-margin comparator sourcing volume of $7.0 million, or 2%, partially offset by decreased manufacturing and packaging sales volume.
Clinical Supply Services' Segment EBITDA decreased by $0.8 million, or 1%, excluding the impact of foreign exchange, as compared to the twelve months ended June 30, 2014 primarily due to a shift to increased lower-margin comparator sourcing volume.
Liquidity and Capital Resources
Overview
Our principal source of liquidity has been cash flow generated from operations. The principal uses of cash are to fund planned operating and capital expenditures, business or asset acquisitions and any mandatory or discretionary principal payments on our debt. As of June 30, 2016 , our financing needs were supported by a five-year $200 million revolving credit facility that matures in May 2019 and is reduced by $13.9 million in letters of credit. The revolving credit facility includes borrowing capacity available for letters of credit and for short-term borrowings, referred to as swing-line borrowings. As of June 30, 2016 , we had no outstanding borrowings under our revolving credit facility.
We continue to believe that our cash from operations and available borrowings under the revolving credit facility will be adequate to meet our future liquidity needs for at least the next twelve months. We have no significant debt maturity until the senior secured term loans mature in May 2021.
Cash Flows
Fiscal Year Ended June 30, 2016 Compared to the Fiscal Year Ended June 30, 2015
The following table summarizes our Consolidated Statement of Cash Flows from continuing operations for the fiscal year ended June 30, 2016 compared with the fiscal year ended June 30, 2015 :
56
Table of Contents
| Fiscal Year Ended |
|
| ||||||||
(in millions) | 2016 |
| 2015 |
| $ Change | ||||||
Net cash provided by/(used in): |
|
|
|
|
| ||||||
Operating activities from continuing operations | $ | 155.3 | |
| $ | 171.7 | |
| $ | (16.4 | ) |
Investing activities from continuing operations | $ | (137.7 | ) |
| $ | (271.8 | ) |
| $ | 134.1 | |
Financing activities from continuing operations | $ | (30.8 | ) |
| $ | 196.5 | |
| $ | (227.3 | ) |
Operating Activities
For the fiscal year ended June 30, 2016 , cash provided by operating activities from continuing operations was $ 155.3 million compared to $ 171.7 million for the comparable prior-year period. The decrease of $16.4 million was primarily driven by net cash outflows associated with working capital changes in the current period compared to the previous period.
Investing Activities
For the fiscal year ended June 30, 2016 , cash used in investing activities from continuing operations was $137.7 million , which is primarily related to acquisitions of property, plant and equipment of $139.6 million . Cash used in investing activities from continuing operations for the comparable prior-year period was $271.8 million , which consisted of acquisition of property, plant and equipment and intangible asset additions of $141.0 million and $130.8 million for business acquisition activities. In the prior-year period, we acquired the remaining interest in Redwood and purchased the stock of MTI Pharma Solutions, Inc. (Micron Technologies).
Financing Activities
For the fiscal year ended June 30, 2016 , cash used in financing activities was $30.8 million compared to cash provided by financing activities of $196.5 million in the same period a year ago. The current year activity includes $18.6 million of long-term debt payments as well as $8.7 million paid for minimum tax withholding obligations associated with equity award settlements. Additionally, we closed on the purchase of the redeemable non-controlling interest in the softgel manufacturing facility in Haining, China from the non-controlling interest shareholders, at a purchase price of $5.8 million in the second quarter. In the prior year, the net proceeds raised in connection with our IPO of $948.8 million were primarily used to fund debt payments of $863.8 million. In addition, the prior year period activities included $150.4 million of net proceeds from borrowing on our secured term loan facilities pursuant to Amendment No. 1 to our Amended and Restated Credit Agreement.
Fiscal Year Ended June 30, 2015 Compared to the Fiscal Year Ended June 30, 2014
The following table summarizes our Consolidated Statement of Cash Flows from continuing operations for the fiscal year ended June 30, 2015 compared with the fiscal year ended June 30, 2014:
| Fiscal Year Ended |
|
| ||||||||
(in millions) | 2015 |
| 2014 |
| $ Change | ||||||
Net cash provided by/(used in): |
|
|
|
|
| ||||||
Operating activities from continuing operations | $ | 171.7 | |
| $ | 180.2 | |
| $ | (8.5 | ) |
Investing activities from continuing operations | $ | (271.8 | ) |
| $ | (175.2 | ) |
| $ | (96.6 | ) |
Financing activities from continuing operations | $ | 196.5 | |
| $ | (42.1 | ) |
| $ | 238.6 | |
Operating Activities
For the fiscal year ended June 30, 2015, cash provided by operating activities from continuing operations was $171.7 million compared to $180.2 million for the fiscal year 2014 period. Cash provided by operating activities decreased compared to the same period in fiscal year 2014 by $8.5 million driven by net cash outflows associated with working capital changes compared to the previous period. These cash outflows were offset by higher earnings from continuing operations in the fiscal year ended June 30, 2015 as compared to the year ended June 30, 2014, which benefited from lower interest expense in the fiscal year 2015 as a result of paying down high-interest debt with proceeds from the IPO.
57
Table of Contents
Investing Activities
For the fiscal year ended June 30, 2015, cash used in investing activities from continuing operations was $271.8 million, which primarily related to acquisitions of property, plant and equipment of $138.2 million, intangible asset additions of $2.8 million, and business acquisitions of $130.8 million. We acquired the remaining interest in Redwood and purchased the stock of MTI Pharma Solutions, Inc. (Micron Technologies). Cash used in investing activities from continuing operations for the fiscal year 2014 period was $175.2 million, which was primarily related to the acquisition of property, plant and equipment of $122.4 million and $53.7 million for business acquisition activities, including the purchases of a softgel manufacturing business in Brazil and a 67% controlling interest in a softgel manufacturing facility located in Haining, China.
Financing Activities
For the fiscal year ended June 30, 2015, cash provided by financing activities was $196.5 million compared to cash used in financing activities of $42.1 million in the same period a year ago. The net proceeds raised in connection with our IPO of $948.8 million were primarily used to fund debt payments of $863.8 million in fiscal year 2015. The activities as of June 30, 2015, also included $12.6 million of call premiums paid in connection with the early termination of certain debt instruments in the period. Additionally, on December 1, 2014, we entered into Amendment No. 1 to our Amended and Restated Credit Agreement to provide additional senior secured financing of incremental dollar and euro-denominated term loan facilities of $100 million and €72.8 million ($91 million), respectively. The proceeds of the borrowing were primarily used to pay the remaining $40.5 million outstanding on the unsecured term loans, fund acquisitions completed in the second quarter of $111.6 million and for general corporate purposes. Although we completed two secondary offerings of our common stock during fiscal 2015, we did not sell shares of our common stock in these offerings and did not receive any of the proceeds.
Debt and Financing Arrangements
Senior Secured Credit Facilities
On May 20, 2014, we entered into the Amended and Restated Credit Agreement (as amended, the "Credit Agreement") to provide senior secured financing consisting of a seven-year $1,400.0 million term loan (the "Dollar Term Loan"), a seven-year €250.0 million term loan (together with the Dollar Term Loan, the "Term Loan Facilities") and a five-year $200 million revolving credit facility (the "Revolving Credit Facility"), the proceeds of which were used to prepay in full all outstanding all term loans under our previous senior secured facility. The Revolving Credit Facility replaced a prior revolving credit facility and includes borrowing capacity available for letters of credit and for short-term borrowings, referred to as the swing line borrowings. Borrowings under the Term Loan Facilities and the Revolving Credit Facility bear interest, at our option, at a rate equal to a margin over either (a) a base rate determined by reference to the higher of (1) the rate of interest published by The Wall Street Journal as its "prime lending rate" and (2) the federal funds rate plus 1 / 2 of 1% or (b) a LIBOR rate determined by reference to the London Interbank Offered Rate set by ICE Benchmark Administration (or any successor thereto). The applicable margin for the Term Loan Facilities and borrowings under the Revolving Credit Facility may be reduced if we attain a certain total net leverage ratio. The applicable margin for borrowings is 3.50% for loans based on a LIBOR rate and 2.50% for loans based on the base rate. The LIBOR rate for the Term Loan Facilities is subject to a floor of 1.00% and the base rate for the Term Loan Facilities is subject to a floor of 2.00%.
On December 1, 2014, we entered into Amendment No. 1 to the Credit Agreement to provide additional senior secured financing of incremental Dollar and Euro denominated term loan facilities of $100 million and €72.8 million ($91 million), respectively. The incremental term loans have substantially similar terms as the Term Loan Facilities under the original version of the Credit Agreement. The proceeds of the borrowing were used during fiscal 2015 primarily to pay the remaining $40.5 million outstanding on unsecured term loans under the senior unsecured term loan facility entered into on April 29, 2013, fund acquisitions completed in the second quarter of $111.6 million and general corporate purposes.
As of June 30, 2016 , there were $13.9 million in outstanding letters of credit, which reduced the borrowing capacity under the Revolving Credit Facility.
Redemption of Notes and Unsecured Term Loan Prepayment
On July 29, 2014, we provided notice of our election to redeem the entire $350.0 million aggregate principal amount outstanding the Senior Notes and redeemed them on August 28, 2014 at a redemption price of 101.5% of their principal amount plus accrued and unpaid interest. The redemption was funded with proceeds from the IPO.
58
Table of Contents
On August 5, 2014, we provided notice of our election to redeem the entire €225.0 million aggregate principal amount outstanding of 9.75% senior subordinated notes due 2017 and redeemed them on September 4, 2014 at a redemption price of 101.625% of their principal amount plus accrued and unpaid interest. The redemption was funded with proceeds from the IPO.
On August 6, 2014, we repaid $114.5 million of the then outstanding borrowings under unsecured term loans with proceeds from the IPO. On September 12, 2014, we repaid $120.0 million of the outstanding borrowings under the unsecured term loans with proceeds from the additional shares purchased by the representatives of the underwriters in connection with the IPO. On December 1, 2014, we repaid the remaining $40.5 million then outstanding on the unsecured term loans with proceeds from the incremental Term Loan Facilities.
Guarantees and Security
All obligations under the Credit Agreement, and the guarantees of those obligations, are secured by substantially all of the following assets of the Company and each guarantor, subject to certain exceptions:
• | a pledge of 100% of the capital stock of the borrower and 100% of the equity interests directly held by the borrower and each guarantor in any wholly owned material subsidiary of the borrower or any guarantor (which pledge, in the case of any non-U.S. subsidiary of a U.S. subsidiary, will not include more than 65% of the voting stock of such non-U.S. subsidiary); and |
• | a security interest in, and mortgages on, substantially all tangible and intangible assets of the borrower and of each guarantor, subject to certain limited exceptions. |
Debt Covenants
The Credit Agreement contains a number of covenants that, among other things, restrict, subject to certain exceptions, our (and our restricted subsidiaries') ability to incur additional indebtedness or issue certain preferred shares; create liens on assets; engage in mergers and consolidations; sell assets; pay dividends and distributions or repurchase capital stock; engage in certain transactions with affiliates; make investments, loans or advances; make certain acquisitions; enter into sale and leaseback transactions and change our lines of business.
The Credit Agreement also contains change of control provisions and certain customary affirmative covenants and events of default. The revolving credit facility requires compliance with a net leverage covenant when there is a 30% or more draw outstanding at a period end. As of June 30, 2016 , we were in compliance with all material covenants related to our long-term debt obligations.
Subject to certain exceptions, our Credit Agreement permits us and our restricted subsidiaries to incur certain additional indebtedness, including secured indebtedness. None of our non-U.S. subsidiaries or Puerto Rico subsidiaries is a guarantor of the loans.
Liquidity in Foreign Subsidiaries
As of June 30, 2016 and June 30, 2015 , the amounts of cash and cash equivalents held by foreign subsidiaries were $129.1 million and $116.3 million, respectively, out of the total consolidated cash and cash equivalents of $131.6 million and $151.3 million, respectively. We believe that the amount of funds held by foreign subsidiaries as of such dates not readily convertible into other foreign currencies, including U.S. dollars, was $5.9 million and $1.7 million, respectively. As of June 30, 2016 , there is an additional $7.0 million of highly liquid investments purchased with original maturities greater than three months but less than one year, held by a foreign subsidiary, which are classified as other current assets. Based on our domestic cash flows from operations and our other sources of liquidity, we believe we have sufficient access to funds for our expected future domestic liquidity needs. Our intent is to continue to reinvest undistributed earnings of our foreign local entities and we do not currently plan to repatriate them to fund our operations in the United States. In the event we need to repatriate funds from outside of the United States, such repatriation would likely be subject to restrictions by local laws and/or tax consequences including foreign withholding taxes or U.S. income taxes. It is not feasible to estimate the amount of U.S. tax that might be payable on the remittance of such earnings.
59
Table of Contents
Historical and Adjusted EBITDA
Under the Credit Agreement, the ability of the Operating Company to engage in certain activities such as incurring certain additional indebtedness, making certain investments and paying certain dividends is tied to ratios based on Adjusted EBITDA (which is defined as "Consolidated EBITDA" in the credit agreement). Adjusted EBITDA is a covenant compliance measure in our Credit Agreement, particularly those covenants governing debt incurrence and restricted payments. Adjusted EBITDA is not defined under U.S. GAAP and is subject to important limitations. Because not all companies use identical calculations, our presentation of Adjusted EBITDA may not be comparable to other similarly titled measures of other companies.
The measure under U.S. GAAP most directly comparable to EBITDA from continuing operations and Adjusted EBITDA is earnings/(loss) from continuing operations. In calculating Adjusted EBITDA, we add back certain non-cash, non-recurring and other items that are included in the definitions of EBITDA from continuing operations and consolidated net income, as required in the Credit Agreement. Adjusted EBITDA, among other things:
• | does not include non-cash stock-based employee compensation expense and certain other non-cash charges; |
• | does not include cash and non-cash restructuring, severance and relocation costs incurred to realize future cost savings and enhance our operations; |
• | adds back noncontrolling interest expense, which represents minority investors' ownership of certain of our consolidated subsidiaries and is, therefore, not available to us; and |
• | includes estimated cost savings that have not yet been fully reflected in our results. |
A reconciliation between earnings / (loss) from continuing operations and Adjusted EBITDA, which also shows the adjustments from EBITDA from continuing operations, follows:
| Twelve Months Ended | ||||||
| June 30, |
| June 30, | ||||
Earnings from continuing operations | $ | 111.2 | |
| $ | 210.2 | |
Interest expense, net | 88.5 | |
| 105.0 | | ||
Income tax expense/(benefit) (1) | 33.7 | |
| (97.7 | ) | ||
Depreciation and amortization | 140.6 | |
| 140.8 | | ||
Noncontrolling interest | 0.3 | |
| 1.9 | | ||
EBITDA from continuing operations | 374.3 | |
| 360.2 | | ||
Equity compensation | 10.8 | |
| 9.0 | | ||
Impairment charges and (gain)/loss on sale of assets | 2.7 | |
| 4.7 | | ||
Financing related expenses and other (2) | - | |
| 21.8 | | ||
U.S. GAAP Restructuring | 9.0 | |
| 13.4 | | ||
Acquisition, integration and other special items | 18.2 | |
| 13.8 | | ||
Foreign exchange loss/(gain) (included in other, net) (3) | (10.5 | ) |
| (2.7 | ) | ||
Other adjustments (4) | (3.3 | ) |
| 22.9 | | ||
Subtotal | 401.2 | |
| 443.1 | | ||
Estimated cost savings | - | |
| - | | ||
Adjusted EBITDA | $ | 401.2 | |
| $ | 443.1 | |
FX impact (unfavorable) | $ | (20.8 | ) |
|
| ||
Adjusted EBITDA - Constant Currency | $ | 422.0 | |
| | ||
(1) | Represents the amount of income tax-related expense/(benefit) recorded within our net earnings/(loss) that may not result in cash payment or receipt. |
60
Table of Contents
(2) | Financing-related expenses for the three months ended September 30, 2014 include $20.6 million of early debt termination expenses incurred in connection with the repayment of debt with the net proceeds of the IPO. See footnote 4 below for an additional $29.8 million of IPO-related costs, totaling $50.4 million. |
(3) | Foreign exchange gain of $10.5 million for the twelve months ended June 30, 2016 included $16.3 million of unrealized foreign currency exchange rate gains primarily driven by gains of $9.0 million related to inter-company loans denominated in a currency different from the functional currency of either the borrower or the lender, partially offset by foreign currency exchange gains of $3.8 million driven by the ineffective portion of the net investment hedge related to the Euro-denominated debt. The foreign exchange adjustment was also affected by the exclusion of realized foreign currency exchange rate losses from the non-cash and cash settlement of inter-company loans of $5.8 million . Inter-company loans are between our entities and do not reflect the ongoing results of the Company's trade operations. |
Foreign exchange gain of $2.7 million for the twelve months ended June 30, 2015 included $16.4 million of unrealized foreign currency exchange rate gains primarily driven by losses of $31.4 million related to inter-company loans denominated in a currency different from the functional currency of either the borrower or the lender, partially offset by foreign currency exchange gains of $47.8 million driven by the ineffective portion of the net investment hedge related to the Euro-denominated debt. The foreign exchange adjustment was also affected by the exclusion of realized foreign currency exchange rate losses from the non-cash and cash settlement of inter-company loans of $13.7 million. Inter-company loans are between our entities and do not reflect the ongoing results of the company's trade operations.
(4) | Other Adjustments for the twelve months ended June 30, 2015 includes $29.8 million for a sponsor advisory agreement termination fee paid in connection with the IPO. See footnote 2 above for an additional $20.6 million of IPO-related costs, totaling $50.4 million. |
Interest Rate Risk Management
A portion of the debt used to finance our operations is exposed to interest-rate fluctuations. We may use various hedging strategies and derivative financial instruments to create an appropriate mix of fixed-and floating-rate assets and liabilities. Historically, we have used interest-rate swaps to manage the economic effect of variable rate interest obligations associated with our floating rate term loans so that the interest payable on the term loans effectively becomes fixed at a certain rate, thereby reducing the impact of future interest-rate changes on our future interest expense. As of June 30, 2016 , we did not have any interest-rate swap agreements in place that would have the economic effect of modifying the variable interest obligations associated with our floating-rate term loans.
Currency Risk Management
We are exposed to fluctuations in the EUR-USD exchange rate on our investments in foreign operations in Europe. While we do not actively hedge against changes in foreign currency, we have mitigated the exposure of our investments in our European operations by denominating a portion of our debt in euros. At June 30, 2016 , we had $345.2 million of euro-denominated debt outstanding that qualifies as a hedge of a net investment in foreign operations. Refer to Note 8 to our Consolidated Financial Statements for further discussion of net investment hedge activity in the period.
Periodically, we may utilize forward currency exchange contracts to manage our exposure to the variability of cash flows primarily related to the foreign exchange rate changes of future foreign currency transaction costs. In addition, we may utilize foreign currency forward contracts to protect the value of existing foreign currency assets and liabilities. Currently, we do not utilize foreign currency exchange contracts. We expect to continue to evaluate hedging opportunities for foreign currency in the future.
61
Table of Contents
Contractual Obligations
The following table summarizes our significant contractual obligations as of June 30, 2016 :
(Dollars in millions) | Total |
| Fiscal 2017 |
| Fiscal 2018 - Fiscal 2019 |
| Fiscal 2020 - Fiscal 2021 |
| Thereafter | |||||||||||
Long-term debt obligations (1) | $ | 1,828.7 | |
| $ | 25.9 | |
| $ | 39.5 | |
| $ | 1,763.3 | |
| $ | - | | |
Interest on long-term obligations (2) | 431.8 | |
| 83.8 | |
| 163.9 | |
| 151.8 | |
| 32.3 | | ||||||
Capital lease obligations (3) | 51.4 | |
| 2.0 | |
| 4.6 | |
| 5.5 | |
| 39.3 | | ||||||
Operating lease obligations (4) | 34.1 | |
| 9.2 | |
| 12.5 | |
| 8.0 | |
| 4.4 | | ||||||
Purchase obligations (5) | 51.4 | |
| 44.8 | |
| 4.1 | |
| 2.5 | |
| - | | ||||||
Other long-term liabilities (6) | 61.3 | |
| 4.6 | |
| 7.1 | |
| 7.2 | |
| 42.4 | | ||||||
Total | $ | 2,458.7 | |
| $ | 170.3 | |
| $ | 231.7 | |
| $ | 1,938.3 | |
| $ | 118.4 | | |
(1) | Represents gross maturities of our long-term debt obligations excluding capital lease obligations as of June 30, 2016 . |
(2) | Represents estimated interest payments relating to our long-term obligations including capital lease obligations. Estimated future interest payments on our variable-rate debt obligations were calculated using the interest and exchange rates as of June 30, 2016 . |
(3) | Represents maturities of our capital lease obligations included within long-term debt as of June 30, 2016 . |
(4) | Represents minimum rental payments for operating leases having initial or remaining non-cancelable lease terms. |
(5) | Purchase obligations includes agreements to purchase goods or services that are enforceable, specify all significant terms, including the following: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and approximate timing of the transaction. Purchase obligations disclosed above may include estimates of the time period in which cash outflows will occur. Purchase orders entered into in the normal course of business and authorizations to purchase that involve no firm commitment from either party are excluded from the above table. In addition, contracts that can be unilaterally canceled with no termination fee or with proper notice are excluded from our total purchase obligations except for the amount of the termination fee or the minimum amount of goods that must be purchased during the requisite notice period. |
(6) | Primarily relates to certain long-term employee-related liabilities for operations under programs that we have discontinued. |
The table excludes our retirement and other post-retirement benefits ("OPEB") obligations. The timing and amount of payments for these obligations may be affected by a number of factors, including the funded status of the plans. In fiscal 2017 , we are not required to make contributions to our plans to satisfy regulatory funding standards. Beyond fiscal 2017 , the actual amounts required to be contributed are dependent upon, among other things, interest rates, underlying asset returns and the impact of legislative or regulatory actions related to pension funding obligations. Payments due under our OPEB plans are not required to be funded in advance, but are paid as medical costs are incurred by covered retiree populations, and are principally dependent upon the future cost of retiree medical benefits under our plans. Refer to Note 10 to the Consolidated Financial Statements for further discussion.
The table also excludes approximately $11.0 million of funded deferred compensation payments owed as of June 30, 2016 to certain employees participating in our deferred compensation plan. The timing and amount of payments for these obligations are dependent on employee directed distributions, withdrawals and employment status. As part of the deferred compensation plan, we have a corresponding $11.1 million of deferred compensation investments as of June 30, 2016 , which will be used to fund future obligations to the participating employees.
Off-Balance Sheet Arrangements
Other than operating leases and letters of credit under the senior secured credit facility, we do not have any material off-balance sheet arrangements as of June 30, 2016 . See Note 6 to the Consolidated Financial Statements for further detail.
62
Table of Contents
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to cash flow and earnings fluctuations as a result of certain market risks. These market risks primarily relate to changes in interest rates associated with our long-term debt obligations and foreign exchange rate changes.
Interest Rate Risk
The Company has historically used interest-rate swaps to manage the economic effect of variable rate interest obligations associated with our floating-rate term loans so that the interest payable on the term loans effectively becomes fixed at a certain rate, thereby reducing the impact of future interest-rate changes on our future interest expense. As of June 30, 2016 , we did not have any interest-rate swap agreements in place that would either have the economic effect of modifying the variable interest obligations associated with our floating-rate term loans or would be considered effective cash flow hedges for financial reporting purposes.
Foreign Currency Exchange Risk
By the nature of our global operations, we are exposed to cash flow and earnings fluctuations resulting from foreign exchange rate variation. These exposures are transactional and translational in nature. Since we manufacture and sell our products throughout the world, our foreign currency risk is diversified. Principal drivers of this diversified foreign exchange exposure include the European euro, British pound, Argentinean peso, Brazilian real and Australian dollar. Our transactional exposure arises from the purchase and sale of goods and services in currencies other than the functional currency of our operational units. We also have exposure related to the translation of financial statements of our foreign divisions into U.S. dollars, the functional currency of the parent. The financial statements of our operations outside the U.S. are measured using the local currency as the functional currency. Adjustments to translate the assets and liabilities of these foreign operations in U.S. dollars are accumulated as a component of other comprehensive income/(loss) utilizing period-end exchange rates. Foreign currency transaction gains and losses calculated by utilizing weighted average exchange rates for the period are included in the statements of operations in "other expense, net." Such foreign currency transaction gains and losses include inter-company loans denominated in non-U.S. dollar currencies.
63
Table of Contents
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
INDEX TO FINANCIAL STATEMENTS
Consolidated Financial Statements as of June 30, 2016 and 2015 and for the years ended June 30, 2016 , 2015 and 2014
| Reports of Independent Registered Public Accounting Firm | 65 |
|
|
|
| Consolidated Statements of Operations | 67 |
|
|
|
| Consolidated Statements of Comprehensive Income/(Loss) | 68 |
|
|
|
| Consolidated Balance Sheets | 69 |
|
|
|
| Consolidated Statement of Changes in Shareholders' Equity/(Deficit) | 70 |
|
|
|
| Consolidated Statements of Cash Flows | 71 |
|
|
|
| Notes to Consolidated Financial Statements | 72 |
64
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of
Catalent, Inc.
We have audited the accompanying consolidated balance sheets of Catalent, Inc. and subsidiaries as of June 30, 2016 and 2015 , and the related consolidated statements of operations, comprehensive income/(loss), changes in shareholders' equity/(deficit), and cash flows for each of the three years in the period ended June 30, 2016 . Our audits also included the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Catalent, Inc. and subsidiaries at June 30, 2016 and 2015 , and the consolidated results of their operations and their cash flows for each of the three years in the period ended June 30, 2016 , in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
As discussed in Note 1 to the consolidated financial statements, Catalent, Inc. changed the classification of all deferred tax assets and liabilities to noncurrent on the consolidated balance sheet as a result of the adoption of the amendments to the FASB Accounting Standards Codification resulting from Accounting Standards Update No. 2015-17, "Balance Sheet Classification of Deferred Taxes", effective June 30, 2016 and the Company changed its recognition of excess tax benefits and forfeiture of share-based awards as a result of the adoption of the amendments to the FASB Accounting Standards Codification resulting from Accounting Standards Update No. 2016-09, "Improvements to Employee Share-Based Payment Accounting", effective June 30, 2016 .
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Catalent, Inc.'s internal control over financial reporting as of June 30, 2016, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated August 29, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
MetroPark, New Jersey
August 29, 2016
65
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of
Catalent, Inc.
We have audited Catalent, Inc. and subsidiaries' internal control over financial reporting as of June 30, 2016 based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Catalent, Inc. and subsidiaries' management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect and correct misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Catalent, Inc. and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of June 30, 2016 , based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Catalent, Inc. and subsidiaries as of June 30, 2016 and 2015 , and the related consolidated statements of operations, comprehensive income/(loss), changes in shareholders' equity/(deficit), and cash flows for each of the three years in the period ended June 30, 2016 of Catalent, Inc. and subsidiaries and our report dated August 29, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
MetroPark, New Jersey
August 29, 2016
66
Table of Contents
Catalent, Inc. and Subsidiaries
Consolidated Statements of Operations
(Dollars in millions, except per share data)
| Year ended June 30, | ||||||||||
| 2016 |
| 2015 |
| 2014 | ||||||
Net revenue | $ | 1,848.1 | |
| $ | 1,830.8 | |
| $ | 1,827.7 | |
Cost of sales | 1,260.5 | |
| 1,215.5 | |
| 1,229.1 | | |||
Gross margin | 587.6 | |
| 615.3 | |
| 598.6 | | |||
Selling, general and administrative expenses | 358.1 | |
| 337.3 | |
| 334.8 | | |||
Impairment charges and (gain)/loss on sale of assets | 2.7 | |
| 4.7 | |
| 3.2 | | |||
Restructuring and other | 9.0 | |
| 13.4 | |
| 19.7 | | |||
Operating earnings/(loss) | 217.8 | |
| 259.9 | |
| 240.9 | | |||
Interest expense, net | 88.5 | |
| 105.0 | |
| 163.1 | | |||
Other (income)/expense, net | (15.6 | ) |
| 42.4 | |
| 10.4 | | |||
Earnings from continuing operations before income taxes | 144.9 | |
| 112.5 | |
| 67.4 | | |||
Income tax expense/(benefit) | 33.7 | |
| (97.7 | ) |
| 49.5 | | |||
Earnings from continuing operations | 111.2 | |
| 210.2 | |
| 17.9 | | |||
Net earnings/(loss) from discontinued operations, net of tax | - | |
| 0.1 | |
| (2.7 | ) | |||
Net earnings | 111.2 | |
| 210.3 | |
| 15.2 | | |||
Less: Net (loss) attributable to noncontrolling interest, net of tax | (0.3 | ) |
| (1.9 | ) |
| (1.0 | ) | |||
Net earnings attributable to Catalent | $ | 111.5 | |
| $ | 212.2 | |
| $ | 16.2 | |
|
|
|
|
|
| ||||||
Amounts attributable to Catalent: |
| |
|
| |
|
| ||||
Earnings from continuing operations less net income (loss) attributable to noncontrolling interest | 111.5 | |
| 212.1 | |
| 18.9 | | |||
Net earnings attributable to Catalent | 111.5 | |
| 212.2 | |
| 16.2 | | |||
|
|
|
|
| |||||||
Earnings per share attributable to Catalent: |
| |
|
|
|
| |||||
Basic |
| |
|
|
|
| |||||
Earnings continuing operations | 0.89 | |
| 1.77 | |
| 0.25 | | |||
Net earnings | 0.89 | |
| 1.77 | |
| 0.22 | | |||
Diluted |
|
|
|
|
| ||||||
Earnings continuing operations | 0.89 | |
| 1.75 | |
| 0.25 | | |||
Net earnings | 0.89 | |
| 1.75 | |
| 0.21 | | |||
The accompanying notes are an integral part of these consolidated financial statements.
67
Table of Contents
Catalent, Inc. and Subsidiaries
Consolidated Statements of Comprehensive Income/(Loss)
(Dollars in millions)
| Year Ended June 30, | ||||||||||
| 2016 | | 2015 | | 2014 | ||||||
Net earnings | $ | 111.2 | |
| $ | 210.3 | |
| $ | 15.2 | |
Other comprehensive income/(loss), net of tax |
|
|
|
|
| ||||||
Foreign currency translation adjustments | (118.8 | ) |
| (144.0 | ) |
| 32.4 | | |||
Defined benefit pension plan | (9.1 | ) |
| (6.4 | ) |
| (15.5 | ) | |||
Deferred compensation | (3.8 | ) |
| 0.6 | |
| 1.7 | | |||
Other comprehensive income/(loss), net of tax | (131.7 | ) |
| (149.8 | ) |
| 18.6 | | |||
Comprehensive income/(loss) | (20.5 | ) |
| 60.5 | |
| 33.8 | | |||
Comprehensive income/(loss) attributable to noncontrolling interest | (0.3 | ) |
| (1.9 | ) |
| (0.6 | ) | |||
Comprehensive income/(loss) attributable to Catalent | $ | (20.2 | ) |
| $ | 62.4 | |
| $ | 34.4 | |
The accompanying notes are an integral part of these consolidated financial statements.
68
Table of Contents
Catalent, Inc. and Subsidiaries
Consolidated Balance Sheets
(Dollars in millions except per share data)
| June 30, |
| June 30, | ||||
ASSETS |
|
|
| ||||
Current assets: |
|
|
| ||||
Cash and cash equivalents | $ | 131.6 | |
| $ | 151.3 | |
Trade receivables, net | 414.8 | |
| 372.4 | | ||
Inventories | 154.8 | |
| 132.9 | | ||
Prepaid expenses and other | 89.0 | |
| 80.9 | | ||
Total current assets | 790.2 | |
| 737.5 | | ||
Property, plant, and equipment, net | 905.8 | |
| 885.2 | | ||
Other assets: |
|
|
| ||||
Goodwill | 996.5 | |
| 1,061.5 | | ||
Other intangibles, net | 294.0 | |
| 368.7 | | ||
Deferred income taxes | 37.5 | |
| 64.1 | | ||
Other | 67.1 | |
| 21.3 | | ||
Total assets | $ | 3,091.1 | |
| $ | 3,138.3 | |
LIABILITIES, REDEEMABLE NONCONTROLLING INTEREST, AND SHAREHOLDERS' EQUITY | |||||||
Current liabilities: |
|
|
| ||||
Current portion of long-term obligations and other short-term borrowings | $ | 27.7 | |
| $ | 23.8 | |
Accounts payable | 143.7 | |
| 128.2 | | ||
Other accrued liabilities | 219.8 | |
| 247.0 | | ||
Total current liabilities | 391.2 | |
| 399.0 | | ||
Long-term obligations, less current portion | 1,832.8 | |
| 1,857.0 | | ||
Pension liability | 151.0 | |
| 143.7 | | ||
Deferred income taxes | 41.4 | |
| 56.3 | | ||
Other liabilities | 38.8 | |
| 42.5 | | ||
Commitment and contingencies (see Note 16) | - | |
| - | | ||
|
|
|
| ||||
Redeemable noncontrolling interest | - | |
| 5.8 | | ||
|
|
|
| ||||
Shareholders' equity/(deficit): |
|
|
| ||||
Common stock $0.01 par value; 1.0 billion and 1.0 billion shares authorized in 2016 and 2015, respectively; 124,712,240 and 124,319,279 shares issued and outstanding in 2016 and 2015, respectively. | 1.2 | |
| 1.2 | | ||
Preferred stock $0.01 par value; 100 million and 100 million authorized in 2016 and 2015, respectively, 0 issued and outstanding in 2016 and 2015. | - | |
| - | | ||
Additional paid in capital | 1,976.5 | |
| 1,973.7 | | ||
Accumulated deficit | (1,036.1 | ) |
| (1,166.9 | ) | ||
Accumulated other comprehensive income/(loss) | (305.7 | ) |
| (174.0 | ) | ||
Total Catalent shareholders' equity | 635.9 | |
| 634.0 | | ||
Noncontrolling interest | - | |
| - | | ||
Total shareholders' equity | 635.9 | |
| 634.0 | | ||
Total liabilities, redeemable noncontrolling interest and shareholders' equity | $ | 3,091.1 | |
| $ | 3,138.3 | |
The accompanying notes are an integral part of these consolidated financial statements
69
Table of Contents
Catalent, Inc. and Subsidiaries
Consolidated Statement of Changes in Shareholders' Equity/(Deficit)
(Dollars in millions, except share data in thousands)
| Shares of Common Stock |
| Common Stock |
| Additional Paid in Capital |
| Accumulated Deficit |
| Accumulated Other Comprehensive (Loss)/Income |
| Noncontrolling Interest |
| Total Shareholders' Equity/(Deficit) | |||||||||||||
Balance at June 30, 2013 | 74,796.1 | |
| $ | 0.7 | |
| $ | 1,026.7 | |
| $ | (1,395.3 | ) |
| $ | (42.8 | ) |
| $ | 0.4 | |
| $ | (410.3 | ) |
Equity contribution | 25.2 | |
|
|
| 0.2 | |
| |
| |
| (0.4 | ) |
| (0.2 | ) | |||||||||
Equity compensation |
|
|
|
| 4.5 | |
| |
| |
| |
| 4.5 | | |||||||||||
Net earnings |
|
|
|
| |
| 16.2 | |
| |
| (0.6 | ) |
| 15.6 | | ||||||||||
Other comprehensive income /(loss), net of tax |
|
|
|
|
|
|
|
| 18.6 | |
| |
| 18.6 | | |||||||||||
Balance at June 30, 2014 | 74,821.3 | |
| 0.7 | | | 1,031.4 | | | (1,379.1 | ) | | (24.2 | ) | | (0.6 | ) | | (371.8 | ) | ||||||
Equity contribution | 48,875.0 | |
| 0.5 | |
| 946.1 | |
| |
| |
| |
| 946.6 | | |||||||||
Stock option exercises | 623.0 | |
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||
Equity compensation |
|
|
|
| 9.0 | |
| |
| |
| |
| 9.0 | | |||||||||||
Cash paid, in lieu of equity, for tax withholding |
|
|
|
| (10.3 | ) |
|
|
|
|
|
|
| (10.3 | ) | |||||||||||
Noncontrolling interest ownership changes |
|
|
|
| (2.5 | ) |
|
|
|
|
| 1.0 | |
| (1.5 | ) | ||||||||||
Net earnings |
|
|
|
|
|
| 212.2 | |
| |
| (0.4 | ) |
| 211.8 | | ||||||||||
Other comprehensive income /(loss), net of tax |
|
|
|
|
|
|
|
| (149.8 | ) |
|
|
| (149.8 | ) | |||||||||||
Balance at June 30, 2015 | 124,319.3 | |
| 1.2 | |
| 1,973.7 | |
| (1,166.9 | ) |
| (174.0 | ) |
| - | |
| 634.0 | | ||||||
Cumulative effect of stock compensation standard adoption |
|
|
|
| 1.0 | |
| 19.3 | |
|
|
|
|
| 20.3 | | ||||||||||
Stock option exercises | 392.9 | |
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||
Equity compensation |
|
|
|
| 10.8 | |
|
|
|
|
|
|
| 10.8 | | |||||||||||
Cash paid, in lieu of equity, for tax withholding |
|
|
|
| (8.7 | ) |
|
|
|
|
|
|
| (8.7 | ) | |||||||||||
Noncontrolling interest ownership changes |
|
|
|
| (0.3 | ) |
|
|
|
|
| - | |
| (0.3 | ) | ||||||||||
Net earnings |
|
|
|
|
|
| 111.5 | |
|
|
| - | |
| 111.5 | | ||||||||||
Other comprehensive income /(loss), net of tax |
|
|
|
|
|
|
|
| (131.7 | ) |
|
|
| (131.7 | ) | |||||||||||
Balance at June 30, 2016 | 124,712.2 | |
| $ | 1.2 | |
| $ | 1,976.5 | |
| $ | (1,036.1 | ) |
| $ | (305.7 | ) |
| $ | - | |
| $ | 635.9 | |
The accompanying notes are an integral part of these consolidated financial statements
70
Table of Contents
Catalent, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(Dollars in millions)
| Year ended June 30, | ||||||||||
| 2016 |
| 2015 |
| 2014 | ||||||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
| ||||||
Net earnings/(loss) | $ | 111.2 | |
| $ | 210.3 | |
| $ | 15.2 | |
Net earnings/(loss) from discontinued operations | - | |
| 0.1 | |
| (2.7 | ) | |||
Earnings from continuing operations | 111.2 | |
| 210.2 | |
| 17.9 | | |||
Adjustments to reconcile (loss)/earnings from continued operations to net cash from operations: |
|
|
|
|
| ||||||
Depreciation and amortization | 140.6 | |
| 140.8 | |
| 142.9 | | |||
Non-cash foreign currency transaction (gains)/losses, net | (10.9 | ) |
| (16.4 | ) |
| (17.1 | ) | |||
Amortization and write off of debt financing costs | 4.7 | |
| 16.0 | |
| 14.0 | | |||
Asset impairments and (gain)/loss on sale of assets | 2.7 | |
| 4.7 | |
| 3.2 | | |||
Non-cash gain on acquisition | - | |
| (8.9 | ) |
| - | | |||
Call premium and financing fees paid | - | |
| 12.6 | |
| 7.2 | | |||
Equity compensation | 10.8 | |
| 9.0 | |
| 4.5 | | |||
Provision/(benefit) for deferred income taxes | (15.3 | ) |
| (120.7 | ) |
| (15.1 | ) | |||
Provision for bad debts and inventory | 13.2 | |
| 12.7 | |
| 9.8 | | |||
Change in operating assets and liabilities: |
|
|
|
|
| ||||||
(Increase)/decrease in trade receivables | (54.1 | ) |
| (7.5 | ) |
| (38.0 | ) | |||
(Increase)/decrease in inventories | (35.4 | ) |
| (19.2 | ) |
| (8.5 | ) | |||
Increase/(decrease) in accounts payable | 21.4 | |
| (11.7 | ) |
| (7.6 | ) | |||
Other assets/accrued liabilities, net - current and non-current | (33.6 | ) |
| (49.9 | ) |
| 67.0 | | |||
Net cash provided by/(used in) operating activities from continuing operations | 155.3 | |
| 171.7 | |
| 180.2 | | |||
Net cash provided by/(used in) operating activities from discontinued operations | - | |
| 0.1 | |
| (1.9 | ) | |||
Net cash provided by/(used in) operating activities | 155.3 | |
| 171.8 | |
| 178.3 | | |||
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
| ||||||
Acquisition of property and equipment and other productive assets | (139.6 | ) |
| (141.0 | ) |
| (122.4 | ) | |||
Proceeds from sale of property and equipment | 1.9 | |
| - | |
| 0.9 | | |||
Payment for acquisitions, net | - | |
| (130.8 | ) |
| (53.7 | ) | |||
Net cash provided by/(used in) investing activities from continuing operations | (137.7 | ) |
| (271.8 | ) |
| (175.2 | ) | |||
Net cash provided by/(used in) investing activities from discontinued operations | - | |
| - | |
| 4.0 | | |||
Net cash provided by/(used in) investing activities | (137.7 | ) |
| (271.8 | ) |
| (171.2 | ) | |||
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
| ||||||
Net change in other borrowings | 2.3 | |
| - | |
| (17.5 | ) | |||
Proceeds from borrowing, net | - | |
| 150.4 | |
| 1,723.7 | | |||
Payments related to long-term obligations | (18.6 | ) |
| (879.8 | ) |
| (1,741.3 | ) | |||
Call premium and financing fees paid | - | |
| (12.6 | ) |
| (7.2 | ) | |||
Purchase of redeemable noncontrolling interest shares | (5.8 | ) |
| - | |
| - | | |||
Equity contribution | - | |
| 948.8 | |
| 0.2 | | |||
Cash paid, in lieu of equity, for tax withholding obligation | (8.7 | ) |
| (10.3 | ) |
| - | | |||
Net cash (used in)/provided by financing activities from continuing operations | (30.8 | ) |
| 196.5 | |
| (42.1 | ) | |||
Net cash (used in)/provided by financing activities from discontinued operations | - | |
| - | |
| - | | |||
Net cash (used in)/provided by financing activities | (30.8 | ) |
| 196.5 | |
| (42.1 | ) | |||
Effect of foreign currency on cash | (6.5 | ) |
| (19.6 | ) |
| 3.0 | | |||
NET INCREASE/(DECREASE) IN CASH AND EQUIVALENTS | (19.7 | ) |
| 76.9 | |
| (32.0 | ) | |||
CASH AND EQUIVALENTS AT BEGINNING OF PERIOD | 151.3 | |
| 74.4 | |
| 106.4 | | |||
CASH AND EQUIVALENTS AT END OF PERIOD | $ | 131.6 | |
| $ | 151.3 | |
| $ | 74.4 | |
SUPPLEMENTARY CASH FLOW INFORMATION: |
|
|
|
|
| ||||||
Interest paid | $ | 82.4 | |
| $ | 107.1 | |
| $ | 153.8 | |
Income taxes paid, net | $ | 40.6 | |
| $ | 34.0 | |
| $ | 21.0 | |
The accompanying notes are an integral part of these consolidated financial statements
71
Table of Contents
Catalent, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1 . | BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Business
Catalent, Inc. ("Catalent" or the "Company") directly and wholly owns PTS Intermediate Holdings LLC ("Intermediate Holdings"). Intermediate Holdings directly and wholly owns Catalent Pharma Solutions, Inc. (the "Operating Company"). The financial results of Catalent are primarily comprised of the financial results of the Operating Company and its subsidiaries on a consolidated basis.
In July 2014, the Company's board of directors and holders of the requisite number of outstanding shares of its capital stock approved an amendment to the Company's amended and restated certificate of incorporation to effect a 70 -for-1 stock split of its outstanding common stock (the "stock split"). The stock split became effective on July 17, 2014 upon the filing of the Company's Certificate of Amendment of the Amended and Restated Certificate of Incorporation with the Delaware Secretary of State. On the effective date of the stock split, (i) each outstanding share of common stock was increased to seventy shares of common stock, (ii) the number of shares of common stock issuable under each outstanding option to purchase common stock was proportionately increased on a one-to-seventy basis, (iii) the exercise price of each outstanding option to purchase common stock was proportionately decreased on a one-to-seventy basis, and (iv) the number of shares underlying each restricted stock unit was proportionately increased on a one-to-seventy basis. All of the share and per share information referenced throughout the financial statements and notes to the consolidated financial statements have been retroactively adjusted to reflect this stock split.
On July 31, 2014, the Company commenced an initial public offering of its common stock (the "IPO"). As part of its IPO, the Company sold a total of 48.9 million shares at a price of $20.50 per share, before underwriting discounts and commissions. Net of these discounts and commissions and other offering expenses, the Company obtained total proceeds from the IPO, including the underwriters' over-allotment option, of $952.2 million , which it used to fully redeem the outstanding 9.75% senior subordinated notes due 2017 (the "Senior Subordinated Notes"), redeem the outstanding 7.85% senior notes due 2018 (the "Senior Notes"), repay portions of the Company's unsecured term loan, and pay to Blackstone and certain other shareholders an advisory agreement termination fee of $29.8 million (recorded within other income/(expense), net on the Consolidated Statement of Operations), and for other corporate purposes. The Company's common stock began trading on the New York Stock Exchange (the "NYSE") under the symbol "CTLT" as of the IPO. Refer to Note 6 for further discussion regarding debt repayments.
On March 9, 2015, an affiliate of The Blackstone Group, L.P. that owned shares in the Company ("Blackstone"), Genstar Capital and Aisling Capital (collectively the "selling stockholders") completed a secondary offering of 27.3 million shares of the Company's common stock, including 3.6 million shares sold pursuant to the over-allotment option granted to the underwriters at a price of $29.50 per share before underwriting discounts and commissions. On June 2, 2015, the selling stockholders completed an additional secondary offering of 16.1 million shares, including 2.1 million shares sold pursuant to the over-allotment option, at a price of $29.00 per share, before underwriting discounts and commissions. On June 6, 2016, the selling stockholders completed a secondary offering of 10.0 million shares of the Company's common stock at a price of $24.85 per share before underwriting discounts and commissions. The Company did not sell any stock in any of the secondary offerings and did not receive any proceeds of the sales. Blackstone's ownership in the Company was reduced to 32.7% , 20.8% and 13.7% following the March 2015, June 2015 and June 2016 offerings, respectively, and as a result the Company has not qualified as a "controlled company" under applicable NYSE listing standards since March 9, 2015.
The Company is the leading global provider of advanced delivery technologies and development solutions for drugs, biologics and consumer and animal health products. Its oral, injectable, and respiratory delivery technologies address the full diversity of the pharmaceutical industry including small molecules, large molecule biologics and consumer and animal health products. Through its extensive capabilities and deep expertise in product development, it helps its customers take products to market faster, including nearly half of new drug products approved by the Food and Drug Administration (the "FDA") in the last decade. Its advanced delivery technology platforms, its proven formulation, manufacturing and regulatory expertise, and its broad and deep intellectual property enable its customers to develop more products and better treatments for patients and consumers. Across both development and delivery, its commitment to reliably supply its customers' and their patient's needs is the foundation for the value it provides; annually, it produces more than 70 billion doses for nearly 7,000 customer products, or approximately 1 in every 20 doses of such products taken each year by patients and consumers around the world. The Company believes that through its investments in growth-enabling capacity and capabilities, its ongoing focus on operational and quality excellence, the sales of existing customer products, the introduction of new customer products, its innovation activities and patents, and its entry into new markets, it will continue to benefit from attractive and differentiated margins, and realize the growth potential from these areas.
72
Reportable Segments
In fiscal 2016, the Company engaged in a business reorganization which was finalized in the fourth quarter to better align its internal business unit structure with its "Follow the Molecule" strategy. As part of the revised structure, it created a Drug Delivery Solutions ("DDS") reporting segment, which encompasses all of its modified release technologies; prefilled syringes and other injectable formats; blow-fill seal unit dose development and manufacturing; biologic cell line development; analytical services; micronization technologies; and other conventional oral dose forms under a single DDS management team. Additionally, as part of the re-alignment, it created a stand-alone Clinical Supply Services ("CSS") reporting segment and management team with sole focus on providing global clinical supply chain management services that aim to speed its customers' drugs to market. Further, as a result of the business unit re-alignment, the Softgel Technologies reporting segment is now reported separately. For financial reporting purposes, the Company presents three financial reporting segments based on criteria established by those accounting principles generally accepted in the United States ("U.S. GAAP"): Softgel Technologies, Drug Delivery Solutions and Clinical Supply Services. All prior period comparative segment information has been restated to reflect the reportable segments in accordance with ASC 280 Segment Reporting.
Softgel Technologies
Through the Softgel Technologies segment, the Company provides formulation, development and manufacturing services for soft capsules, or "softgels," which it first commercialized in the 1930s and have continually enhanced. The Company is the market leader in overall softgel manufacturing, and hold the leading market position in the prescription arena. Its principal softgel technologies include traditional softgel capsules, in which the shell is made of animal-derived gelatin, and Vegicaps and OptiShell capsules, in which the shell is made from vegetable-derived materials. Softgel capsules are used in a broad range of customer products, including prescription drugs, over-the-counter medications, dietary supplements and unit-dose cosmetics. Softgel capsules encapsulate liquid, paste or oil-based active compounds in solution or suspension within an outer shell, filling and sealing the capsule simultaneously. The Company typically perform all encapsulation for a product within one of its softgel facilities, with active ingredients provided by customers or sourced directly by the Company. Softgels have historically been used to solve formulation challenges or technical issues for a specific drug, to help improve the clinical performance of compounds, to provide important market differentiation, particularly for over-the-counter compounds, and to provide safe handling of hormonal, potent and cytotoxic drugs. The Company also participate in the softgel vitamin, mineral and supplement business in selected regions around the world. With the 2001 introduction of its vegetable-derived softgel shell, Vegicaps capsules, consumer health manufacturers have been able to extend the softgel dose form to a broader range of active ingredients and serve patient/consumer populations that were previously inaccessible due to religious, dietary or cultural preferences. In recent years, the Company has extended this platform to pharmaceutical products via its OptiShell offering. The Company's Vegicaps and OptiShell capsules are protected by patents in most major global markets. Physician and patient studies the Company has conducted have demonstrated a preference for softgels versus traditional tablet and hard capsule dose forms in terms of ease of swallowing, real or perceived speed of delivery, ability to remove or eliminate unpleasant odor or taste and, for physicians, perceived improved patient adherence with dosing regimens.
Drug Delivery Solutions
The Company's Drug Delivery Solutions segment provides various complex advanced formulation delivery technologies, and related integrated solutions including: development and manufacturing of a broad range of oral dose forms including fast-dissolve tablets and both proprietary and conventional controlled release products, and delivery of pharmaceuticals, biologics and biosimilars administered via injection, inhalation and ophthalmic routes, using both traditional and advanced technologies.
The Company provides comprehensive pre-formulation, development, and both clinical and commercial scale for most traditional and advanced oral solid dose formats, including uncoated and coated tablets, powder/pellet/bead-filled two piece hard capsules, lozenges, powders and other forms for immediate and modified release prescription, consumer and animal health products. The Company has substantial experience developing and scaling up products requiring accelerated development timelines, requiring specialized handling, complex technology transfers, or specialized manufacturing processes.
The Company launched its orally dissolving tablet business in 1986 with the introduction of Zydis tablets, a unique oral dosage form that is freeze-dried in its package, can be swallowed without water, and typically dissolves in the mouth in less than three seconds. Most often used for indications, drugs and patient groups that can benefit from rapid oral disintegration, the Zydis technology is utilized in a wide range of products and indications, including treatments for a variety of central nervous system-related conditions such as migraines, Parkinson's Disease, schizophrenia, and pain relief and consumer healthcare products targeting allergy relief. Zydis tablets continue to be used in new ways by the Company's customers as it extends the application of the technology to new categories, such as for immunotherapies, vaccines and biologics delivery.
The Company's range of injectable manufacturing offerings includes filling drugs or biologics into pre-filled syringes and glass-free ADVASEPT vials, with flexibility to accommodate other formats within our existing network, increasingly focused on complex pharmaceuticals and biologics. With its range of technologies, the Company is able to meet a wide range of specifications, timelines and budgets. The complexity of the manufacturing process, the importance of experience and know-how, regulatory compliance, and high start-up capital requirements create significant barriers to entry and, as a result, limit the number of competitors in the market. For example, blow-fill-seal is an advanced aseptic processing technology, which uses a continuous process to form, fill with drug, and seal a plastic container in a sterile environment. Blow-fill-seal units are currently used for a variety of pharmaceuticals in liquid form, such as respiratory, ophthalmic and otic products. The Company is a leader in the outsourced blow-fill-seal market, and operate one of the largest capacity commercial manufacturing blow-fill-seal facilities in the world. Its sterile blow-fill-seal manufacturing has significant capacity and flexibility of manufacturing configurations. This business provides flexible and scalable solutions for unit-dose delivery of complex formulations such as suspensions and emulsions. Further, the business provides engineering and manufacturing solutions related to complex containers. The Company's regulatory expertise can lead to decreased time to commercialization, and its dedicated development production lines support feasibility, stability and clinical runs. The Company plan to continue to expand its product line in existing and new markets, and in higher margin specialty products with additional respiratory, ophthalmic, injectable and nasal applications.
The Company's fast-growing biologics offerings include its formulation development and cell-line manufacturing based on its advanced and patented GPEx technology, which is used to develop stable, high-yielding mammalian cell lines for both innovator and biosimilar biologic compounds. Its GPEx technology can provide rapid cell-line development, high biologics production yields, flexibility and versatility. It believes its development-stage SMARTag next-generation antibody-drug conjugate technology will provide more precision targeting for delivery of drugs to tumors or other locations, with improved safety versus existing technologies. The Company's biologics facility in Madison, Wisconsin has the capability and capacity to produce clinical-scale biologic supplies; combined with offerings from its other businesses and external partners, the Company provides the broadest range of technologies and services supporting the development and launch of new biologic entities, biosimilars or biobetters to bring a product from gene to market commercialization, faster.
The Company also offers analytical chemical and cell-based testing and scientific services, stability testing, respiratory products formulation and manufacturing, micronization and particle engineering services, regulatory consulting, and bioanalytical testing for biologic products. Its respiratory product capabilities include development and manufacturing services for inhaled products for delivery via metered dose inhalers, dry powder inhalers and intra-nasal sprays. The Company also provides formulation development and clinical and commercial manufacturing for conventional and specialty oral dose forms. It provides global regulatory and clinical support services for its customers' regulatory and clinical strategies during all stages of development. Demand for its offerings is driven by the need for scientific expertise and depth and breadth of services offered, as well as by the reliable supply thereof, including quality, execution and performance.
Clinical Supply Services
The Company's Clinical Supply Services segment provides manufacturing, packaging, storage and inventory management for drugs and biologics in clinical trials. It offers customers flexible solutions for clinical supplies production, and provides distribution and inventory management support for both simple and complex clinical trials. This includes dose form manufacturing or over-encapsulation where needed; supplying placebos, comparator drug procurement and clinical packages and kits for physicians and patients; inventory management; investigator kit ordering and fulfillment; and return supply reconciliation and reporting. This business supports trials in all regions of the world through its facilities and distribution network. In fiscal 2016, the Company commenced an expansion of its Singapore facility by building new flexible cGMP space and it introduced clinical supply services at its 200,000 square foot facility in Japan, expanding its Asia Pacific capabilities. Additionally, in fiscal 2013, the Company established its first clinical supply services facility in China as a joint venture and assumed full ownership in fiscal 2015. The Company is the leading provider of integrated development solutions and one of the leading providers of clinical trial supplies and respiratory products.
Basis of Presentation
These financial statements include all of the Company's subsidiaries, including those operating outside the United States ("U.S.") and are prepared in accordance with U.S. GAAP. All significant transactions among the Company's businesses have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect amounts reported in the financial statements and accompanying notes. Such estimates include, but are not limited to, allowance for doubtful accounts, inventory and long-lived asset valuation, goodwill and other intangible asset valuation and impairment, equity-based compensation, income taxes, and pension plan asset and liability valuation. Actual amounts may differ from these estimated amounts.
Foreign Currency Translation
The financial statements of the Company's operations outside the U.S. are generally measured using the local currency as the functional currency. Adjustments to translate the assets and liabilities of these foreign operations into U.S. dollars are accumulated as a component of other comprehensive income/(loss) utilizing period-end exchange rates. The currency fluctuation related to certain long-term inter-company loans deemed to not be repayable in the foreseeable future have been recorded within the cumulative translation adjustment, a component of other comprehensive income/(loss). In addition, the currency fluctuation associated with the portion of the Company's euro-denominated debt designated as a net investment hedge is included as a component of other comprehensive income/(loss). Foreign currency transaction gains and losses calculated by utilizing weighted average exchange rates for the period are included in the statements of operations in "other expense, net." Such foreign currency transaction gains and losses include inter-company loans that are repayable in the foreseeable future.
Revenue Recognition
In accordance with Accounting Standards Codification (" ASC") 605 Revenue Recognition, the Company recognizes revenue when persuasive evidence of an arrangement exists, product delivery has occurred or the services have been rendered, the price is fixed or determinable and collectability is reasonably assured. In cases where the Company has multiple contracts with the same customer, the Company evaluates those contracts to assess if the contracts are linked or are separate arrangements. Factors the Company considers include the timing of negotiation, interdependency with other contracts or elements and payment terms. The Company and its customers generally view each contract as a separate arrangement.
Manufacturing and packaging service revenue is recognized upon delivery of the product in accordance with the terms of the contract, which specify when transfer of title and risk of loss occurs. Some of the Company's manufacturing contracts with its customers have annual minimum purchase requirements. At the end of the contract year, revenue is recognized for the unfilled purchase obligation in accordance with the contract terms. Development service contracts generally take the form of a fee-for-service arrangement. After the Company has evidence of an arrangement, the price is determinable and there is a reasonable expectation regarding payment, the Company recognizes revenue at the point in time the service obligation is completed and accepted by the customer. Examples of output measures include a formulation report, analytical and stability testing, clinical batch production or packaging and the storage and distribution of a customer's clinical trial material. Development service revenue is primarily driven by the Company's Drug Delivery Solutions segment.
Arrangements containing multiple elements, including service arrangements, are accounted for in accordance with the provisions of ASC 605-25 Revenue Recognition-Multiple-Element Arrangements . The Company determines the separate units of account in accordance with ASC 605-25. If the deliverable meets the criteria of a separate unit of accounting, the arrangement consideration is allocated to each element based upon its relative selling price. In determining the best evidence of selling price of a unit of account the Company utilizes vendor-specific objective evidence ("VSOE"), which is the price the Company charges when the deliverable is sold separately. When VSOE is not available, management uses relevant third-party evidence ("TPE") of selling price, if available. When neither VSOE nor TPE of selling price exists, management uses its best estimate of selling price.
Cash and Cash Equivalents
All liquid investments purchased with original maturities of three months or less are considered to be cash and equivalents. The carrying value of these cash equivalents approximates fair value. Liquid investments purchased with original maturities greater than three months but less than one year when purchased are classified as other current assets, and aggregate to $7.0 million as of June 30, 2016 .
Receivables and Allowance for Doubtful Accounts
Trade receivables are primarily comprised of amounts owed to the Company through its operating activities and are presented net of an allowance for doubtful accounts. The Company monitors past due accounts on an ongoing basis and establishes appropriate reserves to cover probable losses. An account is considered past due on the first day after its due date. The Company makes judgments as to its ability to collect outstanding receivables and provides allowances when it concludes that all or a portion of the receivable will not be collected. The Company determines its allowance by considering a number of factors, including the
length of time accounts receivable are past due, the Company's previous loss history, the specific customer's ability to pay its obligation to the Company, and the condition of the general economy and the customer's industry.
Concentrations of Credit Risk and Major Customers
Concentration of credit risk, with respect to accounts receivable, is limited due to the large number of customers and their dispersion across different geographic areas. The customers are primarily concentrated in the pharmaceutical and healthcare industry. The Company normally does not require collateral or any other security to support credit sales. The Company performs ongoing credit evaluations of its customers' financial conditions and maintains reserves for credit losses. Such losses historically have been within the Company's expectations. No single customer exceeded 10% of revenue during the fiscal years ended 2016 , 2015 and 2014 or 10% of accounts receivable as of the years ended 2016 and 2015 .
Inventories
Inventory is stated at the lower of cost or market, using the first-in, first-out ("FIFO") method. The Company provides reserves for excess, obsolete or slow-moving inventory based on changes in customer demand, technology developments or other economic factors. Inventory consists of costs associated with raw material, labor and overhead.
Goodwill
The Company accounts for purchased goodwill and intangible assets with indefinite lives in accordance with ASC 350 Goodwill, Intangible and Other Assets . Under ASC 350, goodwill and intangible assets with indefinite lives are not amortized, but instead are tested for impairment at least annually. The Company's annual goodwill impairment test was conducted as of April 1, 2016 . The Company assesses goodwill for possible impairment by comparing the carrying value of its reporting units to their fair values. The Company determines the fair value of its reporting units utilizing estimated future discounted cash flows and incorporates assumptions that it believes marketplace participants would utilize. In addition, the Company uses comparative market information and other factors to corroborate the discounted cash flow results.
Property and Equipment and Other Definite Lived Intangible Assets
Property and equipment are stated at cost. Depreciation expense is computed using the straight-line method over the estimated useful lives of the assets, including capital lease assets that are amortized over the shorter of their useful lives or the terms of the respective leases. The Company generally uses the following range of useful lives for its property and equipment categories: buildings and improvements- 5 to 50 years; machinery and equipment- 3 to 10 years; and furniture and fixtures- 3 to 7 years. Depreciation expense was $94.2 million for the fiscal year ended June 30, 2016 , $94.3 million for the fiscal year ended June 30, 2015 , and $100.5 million for the fiscal year ended June 30, 2014 . Depreciation expense includes amortization of assets related to capital leases. The Company charges repairs and maintenance costs to expense as incurred. The amount of capitalized interest was immaterial for all periods presented.
Intangible assets with finite lives, primarily including customer relationships, patents and trademarks are amortized over their useful lives. The Company evaluates the recoverability of its other long-lived assets, including amortizing intangible assets, if circumstances indicate impairment may have occurred pursuant to ASC 360 Property, Plant and Equipment . This analysis is performed by comparing the respective carrying values of the assets to the current and expected future cash flows, on an un-discounted basis, to be generated from such assets. If such analysis indicates that the carrying value of these assets is not recoverable, the carrying value of such assets is reduced to fair value through a charge to the Consolidated Statements of Operations. Fair value is determined based on assumptions the Company believes marketplace participants would utilize and comparable marketplace information in similar arm's length transactions. The Company recorded impairment charges related to definite lived intangible assets and property, plant and equipment, net of gains on sale, of approximately $2.7 million , $4.7 million and $3.2 million , for the fiscal years ended June 30, 2016 , June 30, 2015 and June 30, 2014 , respectively.
Post-Retirement and Pension Plans
The Company sponsors various retirement and pension plans, including defined benefit retirement plans and defined contribution retirement plans. The measurement of the related benefit obligations and the net periodic benefit costs recorded each year are based upon actuarial computations, which require management's judgment as to certain assumptions. These assumptions include the discount rates used in computing the present value of the benefit obligations and the net periodic benefit costs, the expected future rate of salary increases (for pay-related plans) and the expected long-term rate of return on plan assets (for funded plans).
Effective June 30, 2016 , the approach used to estimate the service and interest components of net periodic benefit cost for benefit plans was changed to provide a more precise measurement of service and interest costs. Historically, the Company estimated these service and interest components utilizing a single weighted-average discount rate derived from the yield curve used to measure the benefit obligation at the beginning of the period. Going forward, the Company has elected to utilize an approach that discounts the individual expected cash flows using the applicable spot rates derived from the yield curve over the projected cash flow period. The Company has accounted for this change as a change in accounting estimate that is inseparable from a change in accounting principle and accordingly has accounted for it prospectively.
The expected long-term rate of return on plan assets is based on the target asset allocation and the average expected rate of growth for the asset classes invested. The average expected rate of growth is derived from a combination of historic returns, current market indicators, the expected risk premium for each asset class and the opinion of professional advisors. The Company uses a measurement date of June 30 for all its retirement and postretirement benefit plans.
Derivative Instruments, Hedging Activities, and Fair Value
Derivatives Instruments and Hedging Activities
The Company is exposed to certain risks arising from both its business operations and economic conditions. The Company principally manages its exposures to a wide variety of business and operational risks through management of its core business activities. The Company manages economic risks, including interest-rate, liquidity, and credit risk primarily by managing the amount, sources and duration of its debt funding and the use of derivative financial instruments. Specifically, the Company enters into derivative financial instruments to manage exposures that arise from business activities that result in the receipt or payment of future known and uncertain cash amounts, the value of which are determined by interest rates. The Company's derivative financial instruments are used to manage differences in the amount, timing, and duration of the Company's known or expected cash receipts and its known or expected cash payments principally related to the Company's borrowings. The Company does not net any of its derivative positions under master netting arrangements.
Specifically, the Company is exposed to fluctuations in the EUR-USD exchange rate on its investments in foreign operations in Europe. While the Company does not actively hedge against changes in foreign currency, it has mitigated the exposure of investments in its European operations through a net-investment hedge by denominating a portion of its debt in euros.
Fair Value
The Company is required to measure certain assets and liabilities at fair value, either upon initial measurement or for subsequent accounting or reporting. The Company uses fair value extensively in the initial measurement of net assets acquired in a business combination and when accounting for and reporting on certain financial instruments. The Company estimates fair value using an exit price approach, which requires, among other things, that it determine the price that would be received to sell an asset or paid to transfer a liability in an orderly market. The determination of an exit price is considered from the perspective of market participants, considering the highest and best use of assets and, for liabilities, assuming the risk of non-performance will be the same before and after the transfer. A single estimate of fair value results from a complex series of judgments about future events and uncertainties and relies heavily on estimates and assumptions. When estimating fair value, depending on the nature and complexity of the assets or liability, the Company may use one or all of the following approaches:
• | Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities. |
• | Cost approach, which is based on the cost to acquire or construct comparable assets less an allowance for functional and/or economic obsolescence. |
• | Income approach, which is based on the present value of the future stream of net cash flows. |
These fair value methodologies depend on the following types of inputs:
• | Quoted prices for identical assets or liabilities in active markets (called Level 1 inputs). |
• | Quoted prices for similar assets or liabilities in active markets or quoted prices for identical or similar assets or liabilities in markets that are directly or indirectly observable (called Level 2 inputs). |
• | Unobservable inputs that reflect estimates and assumptions (called Level 3 inputs). |
• |
|
Self-Insurance
The Company is partially self-insured for certain employee health benefits and partially self-insured for property losses and casualty claims. The Company accrues for losses based upon experience and actuarial assumptions, including provisions for incurred but not reported losses.
Shipping and Handling
The Company includes shipping and handling costs in cost of sales in the Consolidated Statements of Operations. Shipping and handling revenue received was immaterial for all periods presented and is presented within net revenues.
Accumulated Other Comprehensive Income/(Loss)
Accumulated other comprehensive income/(loss), which is reported in the accompanying Consolidated Statements of Changes in Shareholders' Equity, consists of net earnings/(loss), foreign currency translation, deferred compensation, and minimum pension liability changes.
Research and Development Costs
The Company expenses research and development costs as incurred. It records costs incurred in connection with the development of new offerings and manufacturing process improvements within selling, general, and administrative expenses. Such research and development costs amounted to $7.6 million , $12.2 million and $17.5 million for the fiscal years ended June 30, 2016 , June 30, 2015 and June 30, 2014 , respectively. The Company records within cost of sales the costs it incurred in connection with the research and development services that it provided to customers and services it performed for customers in support of the commercial manufacturing process. This second type of research and development costs amounted to $47.4 million , $41.3 million and $34.0 million for the fiscal years ended June 30, 2016 , June 30, 2015 and June 30, 2014 , respectively.
Earnings / (Loss) Per Share
The Company reports net earnings (loss) per share in accordance with ASC 260 Earnings per Share . Under ASC 260, basic earnings per share, which excludes dilution, is computed by dividing net earnings or loss available to common stockholders by the weighted average number of common shares outstanding for the period. Diluted earnings per share reflects the potential dilution due to securities that could be exercised or converted into common shares, and is computed by dividing net earnings or loss available to common stockholders by the weighted average of common shares outstanding plus the dilutive potential common shares. Diluted earnings per share include as appropriate in-the-money stock options and outstanding restricted stock units using the treasury stock method. During a loss period, the assumed exercise of in-the-money stock options has an anti-dilutive effect and therefore, these instruments are excluded from the computation of diluted earnings per share in a loss period.
Income Taxes
In accordance with ASC 740 Income Taxes, the Company accounts for income taxes using the asset and liability method. The asset and liability method requires recognition of deferred tax assets and liabilities for expected future tax consequences of temporary differences that currently exist between tax bases and financial reporting bases of the Company's assets and liabilities. The Company measures deferred tax assets and liabilities using enacted tax rates in the respective jurisdictions in which it operates. In assessing the ability to realize deferred tax assets, the Company considers whether it is more likely than not that the Company will be able to realize some or all of the deferred tax assets. The calculation of the Company's tax liabilities involves dealing with uncertainties in the application of complex tax regulations in each of its tax jurisdictions. The number of years with open tax audits varies by tax jurisdiction. A number of years may lapse before a particular matter is audited and finally resolved. The Company applies ASC 740 to determine the accounting for uncertain tax positions. This standard clarifies the accounting for income taxes, by prescribing a minimum recognition threshold a tax position is required to meet before the Company may recognize the position in its financial statements. The standard also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
Equity-Based Compensation
The Company accounts for its equity-based compensation in accordance with ASC 718 Compensation-Stock Compensation. Under ASC 718, companies recognize compensation expense using a fair value based method for costs related to share-based payments, including stock options and restricted stock units. The expense is measured based on the grant date fair value of the awards that are expected to vest, and the expense is recorded over the applicable requisite service period. In the absence of an observable market price for a share-based award, the fair value is based upon a valuation methodology that takes into consideration various factors, including the exercise price of the award, the expected term of the award, the current price of the underlying shares, the expected volatility of the underlying share price based on peer companies, the expected dividends on the underlying shares and the risk-free interest rate.
The terms of the Company's equity-based compensation plans permit an employee holding vested stock options to elect to have the Company withhold a portion of the shares otherwise issuable upon the employee's exercise of the option, a so-called "net settlement transaction," as a means of paying the exercise price, meeting tax withholding requirements, or both.
Recent Financial Accounting Standards
In March 2016, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2016-09 Improvements to Employee Share-Based Payment Accounting , which simplifies the accounting for share-based payment transactions, requiring all excess tax benefits and deficiencies to be recognized in income tax expense or benefit in earnings. An entity can make an accounting policy election to either estimate the expected future forfeiture of awards or account for the cost or benefit as forfeitures occur. The guidance will be effective for publicly reporting entities in fiscal periods beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted in any interim or annual period. The Company early-adopted ASU 2016-09 during the fourth quarter of fiscal 2016 on a modified retrospective basis. Accordingly, the Company recognized the previously unrecognized excess tax benefits, which resulted in a cumulative-effect adjustment benefit of $19.9 million recorded as part of accumulated deficit, with the tax effects recorded as deferred tax assets at the beginning of the 2016 fiscal year. In addition, excess tax benefits of $4.3 million generated during fiscal 2016 are recorded as part of income tax expense/(benefit) in the consolidated statement of income. Furthermore, the Company recognized a cumulative-effect adjustment charge of approximately $0.7 million, net of income taxes, to the beginning accumulated deficit for the impact of electing to account for forfeiture as it occurs.
In February 2016, the FASB issued ASU 2016-02 Leases (Topic 842) , which will supersede A SC 840 Leases . The new guidance requires lessees to recognize most leases on their balance sheets for the rights and obligations created by those leases. The guidance requires enhanced disclosures regarding the amount, timing and uncertainty of cash flows arising from leases and will be effective for publicly reporting entities in annual reporting periods beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted. The Company is currently evaluating the impact of adopting this guidance on its consolidated financial statements.
In November 2015, the FASB issued ASU 2015-17 Balance Sheet Classification of Deferred Taxes , which requires that all deferred tax assets and liabilities, along with any related valuation allowance, be classified as noncurrent on the balance sheet. As a result, each jurisdiction will now only have one net noncurrent deferred tax asset or liability. The new guidance will be effective for publicly reporting entities in annual reporting periods beginning after December 15, 2016, including interim periods within those years. Early adoption is permitted for all entities as of the beginning of an interim or annual reporting period. The guidance may be applied either prospectively, for all deferred tax assets and liabilities, or retrospectively. The Company has elected to early adopt this update as of the end of the 2016 fiscal year and applied its provisions prospectively. As a result, the prior period was not retrospectively adjusted.
In April 2015, the FASB issued ASU 2015-03 Simplifying the Presentation of Debt Issuance Costs , which requires that debt issuance costs be presented in the balance sheet as a direct reduction from the carrying value of the associated debt liability, consistent with the presentation of a debt discount. The new guidance is effective for publicly reporting entities for annual and interim periods beginning after December 15, 2015. Early adoption is permitted. The Company early-adopted this guidance as of January 1, 2016, on a retrospective basis, which had an effect on the consolidated balance sheet as of June 30, 2015 and no effect on the consolidated statements of income, comprehensive income (loss), cash flows or changes in stockholders' equity/(deficit) for the year then ended. The unamortized debt issuance costs associated with the Company's revolving credit facility continues to be included within other assets. The following table summarizes the Company's As Reported and As Adjusted changes to the consolidated balance sheet as of June 30, 2015:
| June 30, 2015 | ||||||
(Dollars in millions) | As Reported |
| As Adjusted | ||||
Other assets: |
|
|
| ||||
Other | $ | 28.4 | |
| $ | 21.3 | |
Total assets | $ | 3,145.4 | |
| $ | 3,138.3 | |
|
|
|
| ||||
Long-term obligations, less current portion | $ | 1,864.1 | |
| $ | 1,857.0 | |
Total liabilities, redeemable noncontrolling interest and shareholders' equity | $ | 3,145.4 | |
| $ | 3,138.3 | |
In May 2014, the FASB issued ASU 2014-09 Revenue from Contracts with Customers , which will supersede nearly all existing revenue recognition guidance. The new guidance's core principle is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In doing so, the new guidance creates a five-step model that requires a company to exercise judgment when considering the terms of the contracts and all relevant facts and circumstances. The five steps require a company to identify customer contracts, identify the separate performance obligations, determine the transaction price, allocate the transaction price to the separate performance obligations and recognize revenue when each performance obligation is satisfied. On July 9, 2015, the FASB approved a one-year deferral of the effective date, so that the new guidance will be effective for publicly reporting entities for annual and interim periods beginning after December 15, 2017. The new guidance allows for either full retrospective adoption, where the standard is applied to all periods presented, or modified retrospective adoption where the standard is applied only to the most current period presented in the financial statements. Early adoption is permitted. The Company is currently evaluating the impact of this new guidance on its consolidated results of operations and financial positio n.
2 . | BUSINESS COMBINATIONS |
During the year ended June 30, 2015, the Company completed acquisitions which were immaterial, individually and in the aggregate, to the overall consolidated financial position and results of operations of the Company. Notably, in October 2014, the Company acquired the remaining shares of Redwood Bioscience Inc. and its SMARTag Antibody-Drug Conjugate (ADC) technology platform. The acquired business is based in the U.S. and is included in the Drug Delivery Solutions segment. Additionally, in November 2014, the Company acquired 100% of the shares of MTI Pharma Solutions, Inc. (Micron Technologies), a company specializing in particle size reduction (micronization), milling and analytical contract services. The acquired business is based in the U.S. and the U.K. and is included in the Drug Delivery Solutions segment.
The Company's consolidated balance sheet as of June 30, 2015 includes the fair value allocations for these acquisitions, which were completed in the fiscal year. Aggregate purchase consideration for both acquisitions totaled $110.8 million . As a result of the fair value allocations, the Company recognized intangible assets of $56 million , comprised of $34 million of customer relationships and $22 million of core technology. The remainder of fair value was allocated to tangible assets acquired and goodwill.
Table of Contents
3 . | GOODWILL |
The following table summarizes the changes between June 30, 2014 , June 30, 2015 and June 30, 2016 in the carrying amount of goodwill in total and by reporting segment:
(Dollars in millions) | Softgel Technologies |
| Drug Delivery Solutions |
| Clinical Supply Services |
| Total | ||||||||
Balance at June 30, 2014 | $ | 472.9 | |
| $ | 430.6 | |
| $ | 193.6 | |
| $ | 1,097.1 | |
Additions/(impairments) | 2.3 | |
| 58.7 | |
| - | |
| 61.0 | | ||||
Foreign currency translation adjustments | (64.0 | ) |
| (17.8 | ) |
| (14.8 | ) |
| (96.6 | ) | ||||
Balance at June 30, 2015 | 411.2 | |
| 471.5 | |
| 178.8 | |
| 1,061.5 | | ||||
Additions/(impairments) | - | |
| - | |
| - | |
| - | | ||||
Foreign currency translation adjustments | (5.3 | ) |
| (36.4 | ) |
| (23.3 | ) |
| (65.0 | ) | ||||
Balance at June 30, 2016 | $ | 405.9 | |
| $ | 435.1 | |
| $ | 155.5 | |
| $ | 996.5 | |
No goodwill impairment charges were required during the current or comparable prior year period. When required, impairment charges are recorded within the consolidated statements of operations as impairment charges and (gain)/loss on sale of assets.
4 . | DEFINITE-LIVED LONG-LIVED ASSETS |
The Company's definite-lived long-lived assets include property, plant and equipment as well as other intangible assets with definite lives. Refer to Note 18 Supplemental Balance Sheet Information for details related to property, plant and equipment.
The details of other intangible assets subject to amortization as of June 30, 2016 and June 30, 2015 , are as follows:
(Dollars in millions) | Weighted Average Life |
| Gross Carrying Value |
| Accumulated Amortization |
| Net Carrying Value | ||||||
June 30, 2016 |
|
|
|
|
|
|
| ||||||
Amortized intangibles: |
|
|
|
|
|
|
| ||||||
Core technology | 18 years |
| $ | 170.6 | |
| $ | (64.9 | ) |
| $ | 105.7 | |
Customer relationships | 14 years |
| 230.3 | |
| (90.9 | ) |
| 139.4 | | |||
Product relationships | 12 years |
| 208.6 | |
| (159.7 | ) |
| 48.9 | | |||
Total intangible assets |
|
| $ | 609.5 | |
| $ | (315.5 | ) |
| $ | 294.0 | |
(Dollars in millions) | Weighted Average Life |
| Gross Carrying Value |
| Accumulated Amortization |
| Net Carrying Value | ||||||
June 30, 2015 |
|
|
|
|
|
|
| ||||||
Amortized intangibles: |
|
|
|
|
|
|
| ||||||
Core technology | 18 years |
| $ | 177.6 | |
| $ | (57.6 | ) |
| $ | 120.0 | |
Customer relationships | 14 years |
| 259.2 | |
| (81.8 | ) |
| 177.4 | | |||
Product relationships | 12 years |
| 222.9 | |
| (151.6 | ) |
| 71.3 | | |||
Total intangible assets |
|
| $ | 659.7 | |
| $ | (291.0 | ) |
| $ | 368.7 | |
Amortization expense was $46.4 million , $46.5 million , and $42.4 million for the fiscal year ended June 30, 2016 , June 30, 2015 , and June 30, 2014 , respectively. Future amortization expense for the next five years is estimated to be:
81
Table of Contents
(Dollars in millions) | 2017 |
| 2018 |
| 2019 |
| 2020 |
| 2021 | ||||||||||
Amortization expense | $ | 45.0 | |
| $ | 44.9 | |
| $ | 39.1 | |
| $ | 24.8 | |
| $ | 24.8 | |
The Company impaired definite lived intangible assets of $0.7 million, $3.4 million and zero in the fiscal years ended June 30, 2016, 2015 and 2014, respectively.
5 . | RESTRUCTURING AND OTHER COSTS |
The Company has implemented plans to restructure certain operations, both domestically and internationally. The restructuring plans focused on various aspects of operations, including closing and consolidating certain manufacturing operations, rationalizing headcount and aligning operations in a strategic and more cost-efficient structure. In addition, the Company may incur restructuring charges in the future in cases where a material change in the scope of operation with its business occurs.
The following table summarizes the significant costs recorded within restructuring costs:
| Year ended June 30, | ||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2014 | ||||||
Restructuring costs: |
|
|
|
|
| ||||||
Employee-related reorganization (1) | $ | 3.7 | |
| $ | 11.5 | |
| $ | 16.5 | |
Asset impairments | 0.4 | |
| - | |
| - | | |||
Facility exit and other costs (2) | 4.9 | |
| 1.9 | |
| 3.2 | | |||
Total restructuring costs | $ | 9.0 | |
| $ | 13.4 | |
| $ | 19.7 | |
(1) | Employee-related costs consist primarily of severance costs and also include outplacement services provided to employees who have been involuntarily terminated and duplicate payroll costs during transition periods. |
(2) | Facility exit and other costs consist of accelerated depreciation, equipment relocation costs and costs associated with planned facility expansions and closures to streamline Company operations. |
6 . | LONG-TERM OBLIGATIONS AND OTHER SHORT-TERM BORROWINGS |
Long-term obligations and other short-term borrowings consist of the following at June 30, 2016 and June 30, 2015 :
(Dollars in millions) | Maturity |
| June 30, |
| June 30, | ||||
Senior Secured Credit Facilities |
|
|
|
|
| ||||
Term loan facility dollar-denominated | May 2021 |
| $ | 1,454.2 | |
| $ | 1,465.9 | |
Term loan facility euro-denominated | May 2021 |
| 345.2 | |
| 353.8 | | ||
Capital lease obligations | 2020 to 2032 |
| 51.4 | |
| 55.5 | | ||
Other obligations | 2016 to 2018 |
| 9.7 | |
| 5.6 | | ||
Total |
|
| 1,860.5 | |
| 1,880.8 | | ||
Less: Current portion of long-term obligations and other short-term borrowings |
|
| 27.7 | |
| 23.8 | | ||
Long-term obligations, less current portion |
|
| $ | 1,832.8 | |
| $ | 1,857.0 | |
(1) | In connection with the Company's adoption of ASU 2015-03, prior year debt balances have been retrospectively adjusted to include a direct deduction of unamortized debt issuance costs, resulting in a reclassification of $7.1 million of debt issuance costs to long-term debt obligation, less current portion. Prior to the adoption of ASU 2015-03, the unamortized debt issuance costs were included in other assets on the Company's consolidated balance sheets. The unamortized debt issuance costs associated with the Company's revolving credit facility continues to be included within other assets. |
Senior Secured Credit Facilities
On May 20, 2014, the Operating Company entered into the Amended and Restated Credit Agreement (as amended to date, the "Credit Agreement") to provide senior secured financing consisting of a seven -year $1,400.0 million dollar term loan (the "Dollar Term Loan"), a seven -year €250.0 million euro term loan (the "Euro Term Loan" and, together with the Dollar
82
Table of Contents
Term Loan, the "Term Loan Facilities") and a five-year $200.0 million revolving credit facility (the "Revolving Credit Facility"), the proceeds of which were used to prepay in full all outstanding Refinancing Dollar Term-1 Loans, Refinancing Dollar Term-2 Loans and Extended Euro Term Loans under the prior version of the Credit Agreement. The Revolving Credit Facility includes borrowing capacity available for letters of credit and for short-term borrowings, referred to as the swing line borrowings. Borrowings under the Term Loan Facilities and the Revolving Credit Facility bear interest, at the Company's option, at a rate equal to a margin over either (a) a base rate determined by reference to the higher of (1) the rate of interest published by The Wall Street Journal as its "prime lending rate" and (2) the federal funds rate plus one half of 1% or (b) a LIBOR rate determined by reference to the London Interbank Offered Rate set by ICE Benchmark Administration (or any successor thereto). The applicable margin for the Term Loan Facilities and borrowings under the Revolving Credit Facility may be reduced subject to the Company attaining a certain total net leverage ratio. The applicable margin for borrowings is 3.50% for loans based on a LIBOR rate and 2.50% for loans based on base rate. The LIBOR rate for the Term Loan Facilities is subject to a floor of 1.00% and the base rate for the Term Loan Facilities is subject to a floor of 2.00% . Cash paid associated with this financing activity approximated $23.9 million. The Company expensed $7.2 million of unamortized deferred finance costs and debt discounts.
On December 1, 2014, the Operating Company entered into Amendment No. 1 to the Credit Agreement to provide additional senior secured financing of incremental dollar- and euro- denominated term loan facilities of $100 million and €72.8 million ( $91 million ), respectively. The incremental term loans have substantially similar terms as Catalent's existing Term Loan Facilities. The proceeds of the borrowing were primarily used to pay the remaining $40.5 million outstanding of unsecured term loans, fund acquisitions completed in the second quarter of $111.6 million and general corporate purposes. The Company incurred approximately $2.8 million in financing costs, of which $1.2 million was recorded in other (income) / expense, net in the consolidated statement of operations.
As of June 30, 2016 , there were $13.9 million in outstanding letters of credit that reduced the borrowing capacity under the Revolving Credit Facility.
Redemption of Notes and Unsecured Term Loan Prepayment
In July 2014, the Company provided notice of its election to redeem the entire $350.0 million aggregate principal amount outstanding of Senior Notes and redeemed them in August 2014 at a redemption price of 101.5% of their principal amount plus accrued and unpaid interest. The redemption was funded with proceeds from the IPO. In connection with the redemption the Company recorded $5.3 million in expense related to the call premium and expensed $5.9 million of unamortized debt discount and deferred financing costs, both in other (income) / expense, net in the consolidated statements of operations.
In August 2014, the Company provided notice of its election to redeem the entire €225.0 million aggregate principal amount outstanding of Senior Subordinated Notes and redeemed them in September 2014 at a redemption price of 101.625% of their principal amount plus accrued and unpaid interest. The redemption was funded with proceeds from the IPO. In connection with the redemption the Company recorded $4.5 million in expense related to the call premium and expensed $4.0 million of unamortized debt discount and deferred financing costs, both in other (income) / expense, net in the consolidated statements of operations.
In August 2014, the Company repaid $114.5 million of the outstanding borrowings under unsecured term loans with proceeds from the IPO. In September 2014, the Company repaid $120.0 million of the outstanding borrowings under the unsecured term loans with proceeds from the additional shares purchased by the representatives of the underwriters in connection with the IPO. In connection with the debt payments, the Company expensed $0.9 million of unamortized debt discount and deferred financing costs in other (income) / expense, net in the consolidated statements of operations. In December 2014, the Company paid the remaining $40.5 million outstanding on the unsecured term loans with proceeds from the incremental Term Loan Facility.
Long-Term and Other Obligations
Other obligations consist primarily of capital leases for buildings and other loans for business and working capital needs.
Maturities of long-term obligations, including capital leases of $51.4 million , and other short-term borrowings for future fiscal years are:
(Dollars in millions) | 2017 | 2018 | 2019 | 2020 | 2021 | Thereafter | Total | |||||||||
Maturities of long-term and other obligations | $ | 27.9 | | 23.2 | | 21.0 | | 21.2 | | 1,747.5 | | 39.3 | | $ | 1,880.1 | |
83
Table of Contents
Debt Issuance Costs
Debt issuance costs associated with the Company's Term Loan Facilities are presented as a reduction to the carrying value of the debt while the debt issuance costs associated with the Revolving Credit Facility are capitalized within prepaid expenses and other assets on the balance sheet. All debt issuance costs are amortized over the life of the related obligation through charges to interest expense in the Consolidated Statements of Operations. The unamortized total of debt issuance costs were approximately $7.7 million and $9.5 million as of June 30, 2016 and June 30, 2015, respectively. Amortization of debt issuance costs totaled $1.8 million and $2.2 million for the fiscal years ended June 30, 2016 and June 30, 2015, respectively.
Guarantees and Security
All obligations under the Credit Agreement, and the guarantees of those obligations, are secured by substantially all of the following assets of the Operating Company and each guarantor, subject to certain exceptions:
• | a pledge of 100% of the capital stock of the borrower and 100% of the equity interests directly held by the borrower and each guarantor in any wholly owned material subsidiary of the borrower or any guarantor (which pledge, in the case of any non-U.S. subsidiary of a U.S. subsidiary, will not include more than 65% of the voting stock of such non-U.S. subsidiary); and |
• | a security interest in, and mortgages on, substantially all tangible and intangible assets of the borrower and of each guarantor, subject to certain limited exceptions. |
Debt Covenants
The Credit Agreement contains a number of covenants that, among other things, restrict, subject to certain exceptions, the Company's (and the Company's restricted subsidiaries') ability to incur additional indebtedness or issue certain preferred shares; create liens on assets; engage in mergers and consolidations; sell assets; pay dividends and distributions or repurchase capital stock; engage in certain transactions with affiliates; make investments, loans or advances; make certain acquisitions; enter into sale and leaseback transactions and change its lines of business.
The Credit Agreement also contains change of control provisions and certain customary affirmative covenants and events of default. The revolving credit facility requires compliance with a net leverage covenant when there is a 30% or more draw outstanding at a period end. As of June 30, 2016 , the Company was in compliance with all material covenants related to its long-term obligations.
Subject to certain exceptions, the Credit Agreement permits the Company and its restricted subsidiaries to incur certain additional indebtedness, including secured indebtedness. None of the Company's non-U.S. subsidiaries or Puerto Rico subsidiaries is a guarantor of the loans.
Under the Credit Agreement, the Company's ability to engage in certain activities such as incurring certain additional indebtedness, making certain investments and paying certain dividends is tied to ratios based on Adjusted EBITDA (which is defined as "Consolidated EBITDA" in the Credit Agreement). Adjusted EBITDA is based on the definitions in the Credit Agreement and is not defined under U.S. GAAP, and is subject to important limitations.
Fair Value of Debt Measurements
| June 30, 2016 |
| June 30, 2015 | ||||||||||||
(Dollars in millions) | Carrying Value |
| Estimated Fair Value |
| Carrying Value (1) |
| Estimated Fair Value | ||||||||
Long-term debt and other | $ | 1,860.5 | |
| $ | 1,868.8 | |
| $ | 1,880.8 | |
| $ | 1,854.7 | |
(1) In connection with the Company's adoption of ASU 2015-03, prior year debt balances have been retrospectively adjusted to include a direct deduction of unamortized debt issuance costs, resulting in a reclassification of $7.1 million of debt issuance costs to long-term debt obligation, less current portion. Prior to the adoption of ASU 2015-03, the unamortized debt issuance costs were included in other assets on the Company's consolidated balance sheets. The unamortized debt issuance costs associated with the Company's revolving credit facility continues to be included within other assets.
84
Table of Contents
7 . | EARNINGS PER SHARE |
The reconciliations between basic and diluted earnings per share attributable to Catalent common shareholders for the fiscal years ended June 30, 2016 , 2015 and 2014 are as follows (in millions, except share and per share data):
| Year ended June 30, | ||||||||||
| 2016 |
| 2015 |
| 2014 | ||||||
Earnings from continuing operations less net income / (loss) attributable to noncontrolling interest | $ | 111.5 | |
| $ | 212.1 | |
| $ | 18.9 | |
Earnings / (loss) from discontinued operations | - | |
| 0.1 | |
| (2.7 | ) | |||
Net earnings attributable to Catalent | $ | 111.5 | |
| $ | 212.2 | |
| $ | 16.2 | |
|
|
|
|
|
| ||||||
Weighted average shares outstanding | 124,787,819 | |
| 119,575,568 | |
| 75,045,147 | | |||
Dilutive securities issuable-stock plans | 1,082,275 | |
| 1,773,068 | |
| 1,078,710 | | |||
Total weighted average diluted shares outstanding | 125,870,094 | |
| 121,348,636 | |
| 76,123,857 | | |||
|
|
|
|
|
| ||||||
Basic earnings per share of common stock: |
|
|
| |
|
| | ||||
Earnings from continuing operations | $ | 0.89 | |
| $ | 1.77 | |
| $ | 0.25 | |
Earnings / (loss) from discontinued operations | - | |
| - | |
| (0.03 | ) | |||
Net earnings attributable to Catalent | $ | 0.89 | |
| $ | 1.77 | |
| $ | 0.22 | |
|
|
|
|
|
| ||||||
Diluted earnings per share of common stock-assuming dilution: |
|
|
| |
|
| | ||||
Earnings from continuing operations | $ | 0.89 | |
| $ | 1.75 | |
| $ | 0.25 | |
Earnings / (loss) from discontinued operations | - | |
| - | |
| (0.04 | ) | |||
Net earnings attributable to Catalent | $ | 0.89 | |
| $ | 1.75 | |
| $ | 0.21 | |
The computation of diluted earnings per share for the years ended June 30, 2016 , 2015 and 2014 excludes the effect of potential shares issuable under the Company's pre-IPO employee stock option plan of 2.2 million , 2.1 million and 2.3 million options, respectively, because the vesting provisions of those awards specify performance or market-based conditions that had not been met as of the period end. Further, the computation of diluted earnings per share for the year ended June 30, 2016 excludes the effect of potential common shares issuable under the employee stock option plan and restricted stock units of approximately 0.5 million shares each because they are anti-dilutive.
8 . | DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES |
Risk Management Objective of Using Derivatives
The Company is exposed to fluctuations in the applicable exchange rate on its investments in foreign operations. While the Company does not actively hedge against changes in foreign currency, the Company has mitigated the exposure of its investments in its European operations by denominating a portion of its debt in euros. At June 30, 2016 , the Company had euro-denominated debt outstanding of $345.2 million that qualifies as a hedge of a net investment in foreign operations. For non-derivatives designated and qualifying as net investment hedges, the effective portion of the translation gains or losses are reported in accumulated other comprehensive income/(loss) as part of the cumulative translation adjustment. The ineffective portions of the translation gains or losses are reported in the statement of operations. The following table includes net investment hedge activity during fiscal year ended June 30, 2016 and June 30, 2015 :
(Dollars in millions) | June 30, |
| June 30, | ||||
Unrealized foreign exchange gain/(loss) within other comprehensive income | $ | 1.8 | |
| $ | 30.0 | |
Unrealized foreign exchange gain/(loss) within statement of operations | $ | 3.9 | |
| $ | 47.7 | |
85
Table of Contents
The net accumulated gain of this net investment as of June 30, 2016 within other comprehensive income/(loss) was approximately $81.3 million . Amounts are reclassified out of accumulated other comprehensive income/(loss) into earnings when the entity to which the gains and losses reside is either sold or substantially liquidated.
9 . | INCOME TAXES |
Earnings/(loss) from continuing operations before income taxes and discontinued operations are as follows for the fiscal years ended 2016 , 2015 , and 2014 :
| Fiscal Year Ended | ||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2014 | ||||||
U.S. Operations | $ | 60.0 | |
| $ | 25.8 | |
| $ | (75.6 | ) |
Non-U.S. Operation | $ | 84.9 | |
| $ | 86.7 | |
| $ | 143.0 | |
| $ | 144.9 | |
| $ | 112.5 | |
| $ | 67.4 | |
The provision /(benefit) for income taxes consists of the following for the fiscal years ended 2016 , 2015 , and 2014 :
| Fiscal Year Ended | ||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2014 | ||||||
Current: |
|
|
|
|
| ||||||
Federal | $ | (0.6 | ) |
| $ | - | |
| $ | - | |
State and local | (0.2 | ) |
| (0.8 | ) |
| (1.2 | ) | |||
Non-U.S. | 26.3 | |
| 31.9 | |
| 55.7 | | |||
Total | $ | 25.5 | |
| $ | 31.1 | |
| $ | 54.5 | |
Deferred: |
|
|
|
|
| ||||||
Federal | $ | 19.6 | |
| $ | (125.3 | ) |
| $ | 5.3 | |
State and local | (4.8 | ) |
| (1.1 | ) |
| 0.4 | | |||
Non-U.S. | (6.6 | ) |
| (2.4 | ) |
| (10.7 | ) | |||
Total | 8.2 | |
| (128.8 | ) |
| (5.0 | ) | |||
|
|
|
|
|
| ||||||
Total provision/(benefit) | $ | 33.7 | |
| $ | (97.7 | ) |
| $ | 49.5 | |
86
Table of Contents
A reconciliation of the provision/(benefit) based on the federal statutory income tax rate to the Company's effective income tax rate is as follows for the fiscal years ended 2016 , 2015 , and 2014 :
| Fiscal Year Ended | ||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2014 | ||||||
Provision at U.S. federal statutory tax rate | $ | 50.7 | |
| $ | 39.4 | |
| $ | 23.6 | |
State and local income taxes | (3.0 | ) |
| (2.4 | ) |
| 0.6 | | |||
Foreign tax rate differential | (21.7 | ) |
| (23.9 | ) |
| (25.5 | ) | |||
Permanent items | (2.3 | ) |
| 1.7 | |
| 24.6 | | |||
Unrecognized tax positions | 5.6 | |
| 14.7 | |
| 34.2 | | |||
Tax valuation allowance | 7.2 | |
| (133.2 | ) |
| (9.5 | ) | |||
Withholding tax and other foreign taxes | 0.6 | |
| 1.4 | |
| 6.2 | | |||
Change in tax rate | (3.2 | ) |
| 1.3 | |
| (5.0 | ) | |||
Foreign currency impact on permanently reinvested loans | - | |
| 2.7 | |
| - | | |||
R&D Tax Credit | (1.4 | ) |
| (1.3 | ) |
| (0.8 | ) | |||
Other | 1.2 | |
| 1.9 | |
| 1.1 | | |||
| $ | 33.7 | |
| $ | (97.7 | ) |
| $ | 49.5 | |
The income tax benefit for the current period is not comparable to the same period of the prior year due to changes in pretax income over many jurisdictions and the impact of discrete items. Generally, fluctuations in the effective tax rate are primarily due to changes in the geographic mix of pretax income and changes in the tax impact of permanent differences and other discrete tax items, which may have unique tax implications depending on the nature of the item. The effective tax rate at June 30, 2015 reflects the release of the U.S. federal valuation allowance and an increase in a tax reserve related to an adjustment to inter-company interest income in Germany, partially offset by a corresponding deduction in the United Kingdom due to enacted tax rate changes. The effective tax rate for the fiscal year ended June 30, 2016 reflects the impact of benefits of a valuation allowance release for utilized capital losses prior to expiration this year, a current year deduction related to stock compensation, as well as a deduction related to a further U.K. rate reduction enacted during the current year, countered by valuation allowance builds on current year losses.
As of June 30, 2016 , the Company had $471.9 million of undistributed earnings from non-U.S. subsidiaries that are intended to be permanently reinvested in non-U.S. operations. As these earnings are considered permanently reinvested, no U.S. tax provision has been accrued related to the repatriation of these earnings. It is not feasible to estimate the amount of U.S. tax that might be payable on the eventual remittance of such earnings.
87
Table of Contents
Deferred income taxes arise from temporary differences between financial reporting and tax reporting bases of assets and liabilities, and operating loss and tax credit carryforwards for tax purposes. The components of the deferred income tax assets and liabilities are as follows at June 30, 2016 and 2015 :
| Fiscal Year Ended | ||||||
(Dollars in millions) | 2016 |
| 2015 | ||||
Deferred income tax assets: |
|
|
| | |||
Accrued liabilities | $ | 21.6 | |
| $ | 24.0 | |
Equity compensation | 10.7 | |
| 8.4 | | ||
Loss and tax credit carryforwards | 155.0 | |
| 204.0 | | ||
Foreign currency | 18.8 | |
| 16.2 | | ||
Pension | 45.9 | |
| 42.9 | | ||
Property-related | 9.3 | |
| 9.7 | | ||
Intangibles | 8.0 | |
| 10.5 | | ||
Other | 22.9 | |
| 17.1 | | ||
Total deferred income tax assets | 292.2 | |
| 332.8 | | ||
Valuation allowance | (69.9 | ) |
| (82.4 | ) | ||
Net deferred income tax assets | $ | 222.3 | |
| $ | 250.4 | |
|
|
|
| ||||
|
|
|
| ||||
| Fiscal Year Ended | ||||||
(Dollars in millions) | 2016 |
| 2015 | ||||
Deferred income tax liabilities: |
|
|
| ||||
Accrued liabilities | (0.6 | ) |
| (0.6 | ) | ||
Equity compensation | - | |
| - | | ||
Foreign currency | (0.9 | ) |
| (0.4 | ) | ||
Property-related | (53.9 | ) |
| (44.5 | ) | ||
Goodwill and other intangibles | (142.2 | ) |
| (156.1 | ) | ||
Other | (1.0 | ) |
| (1.8 | ) | ||
Euro Denominated Debt | (27.6 | ) |
| (21.0 | ) | ||
Total deferred income tax liabilities | $ | (226.2 | ) |
| (224.4 | ) | |
|
|
|
| ||||
Net deferred tax asset/(liability) | $ | (3.9 | ) |
| $ | 26.0 | |
Deferred tax assets and liabilities in the preceding table are in the following captions in the balance sheet at June 30, 2016 and 2015 :
| Fiscal Year Ended | ||||||
(Dollars in millions) | 2016 |
| 2015 | ||||
Current deferred tax asset | $ | - | |
| $ | 19.7 | |
Non-current deferred tax asset | 37.5 | |
| 64.1 | | ||
Current deferred tax liability | - | |
| 1.5 | | ||
Non-current deferred tax liability | 41.4 | |
| 56.3 | | ||
Net deferred tax asset/(liability) | $ | (3.9 | ) |
| $ | 26.0 | |
The Company adopted ASU 2015-17 in the fourth quarter of fiscal 2016 on a prospective basis; accordingly, all deferred tax assets and liabilities as of June 30, 2016 , are classified as noncurrent in the balance sheet, and the prior period balances were not retrospectively adjusted.
88
Table of Contents
At June 30, 2016 , the Company has federal net operating loss carryforwards of $230.2 million , all of which are subject to limitations under Section 382 of the Internal Revenue Code of 1986, as amended (the "Internal Revenue Code"); $13.2 million of which, because they were generated in years prior to April 10, 2007, when the Company was owned by Cardinal, and the remainder due to a change in ownership event when Blackstone, Genstar Capital, and Aisling Capital completed a secondary offering of the Company's stock in March 2015. The federal loss carryforwards will expire in fiscal years 2022 through 2033.
The Company adopted ASU 2016-09, during the fourth quarter of fiscal 2016 on a modified retrospective basis; accordingly, the Company recognized the previously unrecognized excess tax benefits, which resulted in a cumulative-effect adjustment tax benefit of $19.9 million recorded as part of accumulated deficit, with the tax effects recorded as deferred tax assets at the beginning of the 2016 fiscal year.
At June 30, 2016 , the Company has state tax loss carryforwards of $376.2 million . Approximately $181.5 million of these losses are state tax losses generated in periods prior to the period ending June 30, 2007. Substantially all state carryforwards have a twenty -year carryforward period. At June 30, 2016 , the Company has international tax loss carryforwards of $129.8 million . Substantially all of these carryforwards are available for at least three years or have an indefinite carryforward period.
The Company had valuation allowances of $69.9 million and $82.4 million as of June 30, 2016 and 2015, respectively, against our deferred tax assets.
The Company considered all available evidence, both positive and negative, in assessing the need for a valuation allowance for deferred tax assets. Three possible sources of taxable income were evaluated when assessing the realization of deferred tax assets:
• | Future reversals of existing taxable temporary differences; |
• | Tax planning strategies; and |
• | Future taxable income exclusive of reversing temporary differences and carryforwards. |
The Company considered the need to maintain a valuation allowance on deferred tax assets based on management's assessment of whether it is more likely than not that deferred tax assets would be realized based on future reversals of existing taxable temporary differences and the ability to generate sufficient taxable income within the carryforward period available under the applicable tax law. The deferred tax liabilities are expected to reverse in the same period and jurisdiction and are of the same character as the temporary differences giving rise to a portion of the deferred tax assets.
During the fiscal year ended June 30, 2015, the Company released the majority of its U.S. federal valuation allowance of $136.7 million based on projected U.S. future earnings in excess of the $294.1 million required to realize its net U.S. federal deferred tax assets. Of the $294.1 million , $329.5 million relates to the federal net operating loss carryforward (NOL) which expires in the years 2028 to 2032. The remaining $35.4 million relates to other net deferred tax liabilities. A valuation allowance of $10.4 million was retained on U.S. federal deferred tax assets for capital losses, which have expired in the current period.
The reversal of the valuation allowance was the result of a continuing trend of U.S. taxable income and the expectation that this trend will continue, rather than relying on tax planning strategies to support the realization of deferred tax assets. We had experienced three consecutive years of positive U.S. taxable earnings as of the current quarter and expect to sustain this position in the future, due to the positive impact on U.S. earnings from reduced interest expense resulting from a reduction in our external debt, among other factors.
While the U.S. federal valuation allowance was reversed, the U.S. state valuation allowance on $375.7 million of pre-apportioned state net operating losses were maintained. Due to uncertainty around earnings, apportionment, certain restrictions at the state level, and the history of tax losses, anticipated utilization rates were not sufficient to overcome the negative evidence and allow a release.
As part of the 2007 acquisition from Cardinal, the Company has been indemnified by Cardinal for tax liabilities that may arise in the future that relate to tax periods prior to April 10, 2007 (the "Formation Date"). The indemnification agreement includes, among other taxes, any and all Federal, state and international income based taxes as well as any interest and penalties that may be related thereto.
Similarly, as part of the 2012 purchase of the CTS business from Aptuit, Inc., the Company has been indemnified by Aptuit, Inc. for tax liabilities relating to the CTS business that may arise in the future that relate to tax periods prior to February 17, 2012. The indemnification agreement includes, among other taxes, any and all Federal, state and international income based taxes as well as any interest and penalties that may be related thereto.
89
Table of Contents
The amount of income taxes the Company may pay is subject to ongoing audits by federal, state and foreign tax authorities, which may result in proposed assessments. The Company's estimate for the potential outcome for any uncertain tax issue is highly judgmental. The Company assesses its income tax positions and record benefits for all years subject to examination based upon management's evaluation of the facts, circumstances and information available at the reporting date. For those tax positions for which it is more likely than not that a tax benefit will be sustained, the Company records the amount that has a greater than 50% likelihood of being realized upon settlement with a taxing authority that has full knowledge of all relevant information. Interest and penalties are accrued, where applicable.
ASC 740 includes guidance on the accounting for uncertainty in income taxes recognized in the financial statements. This standard also provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. As of June 30, 2016 , the Company had a total of $61.5 million of unrecognized tax benefits. A reconciliation of our unrecognized tax benefits, excluding accrued interest, for June 30, 2016 , June 30, 2015 and June 30, 2014 are as follows:
(Dollars in millions) | |||
Balance at June 30, 2014 | $ | 60.6 | |
Additions based on tax positions related to the current year | 7.3 | | |
Additions for tax positions of prior years | 5.5 | | |
Reductions for tax positions of prior years | (5.4 | ) | |
Settlements | (0.5 | ) | |
Lapse of the applicable statute of limitations | (0.6 | ) | |
Balance at June 30, 2015 | $ | 66.9 | |
Additions based on tax positions related to the current year | 6.2 | | |
Additions for tax positions of prior years | - | | |
Reductions for tax positions of prior years | (11.0 | ) | |
Settlements | - | | |
Lapse of the applicable statute of limitations | (0.6 | ) | |
Balance at June 30, 2016 | $ | 61.5 | |
Of this amount, $45.7 million and $46.7 million represent the amount of unrecognized tax benefits that, if recognized, would favorably impact the effective income tax rate as of June 30, 2016 and June 30, 2015 , respectively. An additional $15.8 million represents the amount of unrecognized tax benefits that, if recognized, would not impact the effective income tax rate due to a full valuation allowance.
In the normal course of business, the Company is subject to examination by taxing authorities throughout the world, including major jurisdictions such as Germany, United Kingdom, France, the United States, and various states. The Company is no longer subject to examinations by the relevant tax authorities for years prior to fiscal 2005.
The Company recognizes interest and penalties related to uncertain tax positions in income tax expense. As of June 30, 2016 , the Company has approximately $5.6 million of accrued interest related to uncertain tax positions, a decrease of $0.7 million from the prior year. The Company had approximately $6.3 million and $5.1 million of accrued interest related to uncertain tax positions as of June 30, 2015 and 2014, respectively. The portion of such interest and penalties subject to indemnification by Cardinal is $2.1 million , a decrease of $0.2 million from the prior year.
10 . | EMPLOYEE RETIREMENT BENEFIT PLANS |
The Company sponsors various retirement plans, including defined benefit pension plans and defined contribution plans. Substantially all of the Company's domestic non-union employees are eligible to participate in employer-sponsored retirement savings plans, which include plans covered under Section 401(k) of the Internal Revenue Code, and provide for company matching contributions. The Company's contributions to the plans are discretionary but are subject to certain minimum requirements as specified in the plans under law. The Company uses a measurement date of June 30 for all of its retirement and postretirement benefit plans.
In addition, the Company has recorded obligations related to its withdrawal from a multi-employer pension plan related to a former commercial packaging site, a clinical services site and a former printed components operation. The Company's withdrawal from these multi-employer pension plans has been classified as a mass withdrawal under the Multiemployer Pension Plan Amendments Act of 1980, and, as amended, under the Pension Protection Act of 2006. The withdrawal from the
90
Table of Contents
plan resulted in the recognition of liabilities associated with the Company's long-term obligations in prior year periods not presented, which were primarily recorded as an expense within discontinued operations. The estimated discounted value of the projected contributions related to these plans is $39.3 million and $39.5 million as of June 30, 2016 and June 30, 2015 , respectively. The annual cash impact associated with the Company's long-term obligation approximates $1.7 million per year.
The following table provides a reconciliation of the change in projected benefit obligation and fair value of plan assets for the defined benefit retirement and other retirement plans, excluding the multi-employer pension plan liability:
At June 30, | Retirement Benefits |
| Other Post-Retirement Benefits | ||||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2016 |
| 2015 | ||||||||
Accumulated Benefit Obligation | $ | 328.1 | |
| $ | 316.0 | |
| $ | 3.6 | |
| $ | 3.7 | |
|
|
|
|
|
|
|
| ||||||||
Change in Benefit Obligation |
|
|
|
|
|
|
| ||||||||
Benefit obligation at beginning of year | 323.7 | |
| 333.8 | |
| 3.7 | |
| 4.4 | | ||||
Company service cost | 2.8 | |
| 2.7 | |
| - | |
| - | | ||||
Interest cost | 10.4 | |
| 11.4 | |
| 0.1 | |
| 0.2 | | ||||
Employee contributions | - | |
| - | |
| - | |
| - | | ||||
Plan amendments | (0.7 | ) |
| - | |
| - | |
| - | | ||||
Curtailments | - | |
| (1.6 | ) |
| - | |
| - | | ||||
Settlements | - | |
| - | |
| - | |
| - | | ||||
Special termination benefits | - | |
| - | |
| - | |
| - | | ||||
Divestitures | - | |
| - | |
| - | |
| - | | ||||
Business combinations | - | |
| - | |
| - | |
| - | | ||||
Benefits paid | (11.6 | ) |
| (9.6 | ) |
| (0.2 | ) |
| (0.2 | ) | ||||
Actual expenses | - | |
| - | |
| - | |
| - | | ||||
Actuarial (gain)/loss | 40.5 | |
| 20.8 | |
| - | |
| (0.7 | ) | ||||
Exchange rate gain/(loss) | (28.5 | ) |
| (33.8 | ) |
| - | |
| - | | ||||
Benefit obligation at end of year | 336.6 | |
| 323.7 | |
| 3.6 | |
| 3.7 | | ||||
|
|
|
|
|
|
|
| ||||||||
Change in Plan Assets |
|
|
|
|
|
|
| ||||||||
Fair value of plan assets at beginning of year | 222.0 | |
| 222.2 | |
| - | |
| - | | ||||
Actual return on plan assets | 33.8 | |
| 18.4 | |
| - | |
| - | | ||||
Company contributions | 9.2 | |
| 9.0 | |
| 0.2 | |
| 0.2 | | ||||
Employee contributions | - | |
| - | |
| - | |
| - | | ||||
Settlements | - | |
| - | |
| - | |
| - | | ||||
Special company contributions to fund termination benefits | - | |
| - | |
| - | |
| - | | ||||
Divestitures | - | |
| - | |
| - | |
| - | | ||||
Business combinations | - | |
| - | |
| - | |
| - | | ||||
Benefits paid | (11.6 | ) |
| (9.6 | ) |
| (0.2 | ) |
| (0.2 | ) | ||||
Actual expenses | - | |
| - | |
| - | |
| - | | ||||
Exchange rate gain/(loss) | (25.8 | ) |
| (18.0 | ) |
| - | |
| - | | ||||
Fair value of plan assets at end of year | 227.6 | |
| 222.0 | |
| - | |
| - | | ||||
|
|
|
|
|
|
|
| ||||||||
Funded Status |
|
|
|
|
|
|
| ||||||||
Funded status at end of year | (109.0 | ) |
| (101.7 | ) |
| (3.6 | ) |
| (3.7 | ) | ||||
Employer contributions between measurement date and reporting date | - | |
| - | |
| - | |
| - | | ||||
Net pension asset (liability) | (109.0 | ) |
| (101.7 | ) |
| (3.6 | ) |
| (3.7 | ) | ||||
91
Table of Contents
The following table provides a reconciliation of the net amount recognized in the Consolidated Balance Sheets:
At June 30, | Retirement Benefits |
| Other Post-Retirement Benefits | ||||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2016 |
| 2015 | ||||||||
Amounts Recognized in Statement of Financial Position |
|
|
|
|
|
|
| ||||||||
Noncurrent assets | $ | - | |
| $ | 0.6 | |
| $ | - | |
| $ | - | |
Current liabilities | (0.8 | ) |
| (0.9 | ) |
| - | |
| (0.8 | ) | ||||
Noncurrent liabilities | (108.2 | ) |
| (101.4 | ) |
| (3.6 | ) |
| (2.9 | ) | ||||
Total asset/(liability) | (109.0 | ) |
| (101.7 | ) |
| (3.6 | ) |
| (3.7 | ) | ||||
|
|
|
|
|
|
|
| ||||||||
Amounts Recognized in Accumulated Other Comprehensive Income |
|
|
|
|
|
|
| ||||||||
Transition (asset)/obligation | - | |
| - | |
| - | |
| - | | ||||
Prior service cost | (0.5 | ) |
| 0.1 | |
| - | |
| - | | ||||
Net (gain)/loss | 76.9 | |
| 63.2 | |
| (1.5 | ) |
| (1.6 | ) | ||||
Total accumulated other comprehensive income at the end of the year | 76.4 | |
| 63.3 | |
| (1.5 | ) |
| (1.6 | ) | ||||
|
|
|
|
|
|
|
| ||||||||
Additional Information for Plan with ABO in Excess of Plan Assets |
|
|
|
|
|
|
| ||||||||
Projected benefit obligation | 321.0 | |
| 309.6 | |
| 3.6 | |
| 3.7 | | ||||
Accumulated benefit obligation | 315.7 | |
| 304.1 | |
| 3.6 | |
| 3.7 | | ||||
Fair value of plan assets | 213.3 | |
| 207.3 | |
| - | |
| - | | ||||
|
|
|
|
|
|
|
| ||||||||
Additional Information for Plan with PBO in Excess of Plan Assets |
|
|
|
|
|
|
| ||||||||
Projected benefit obligation | 336.6 | |
| 309.6 | |
| 3.6 | |
| 3.7 | | ||||
Accumulated benefit obligation | 328.1 | |
| 304.1 | |
| 3.6 | |
| 3.7 | | ||||
Fair value of plan assets | 227.6 | |
| 207.3 | |
| - | |
| - | | ||||
|
|
|
|
|
|
|
| ||||||||
Components of Net Periodic Benefit Cost |
|
|
|
|
|
|
| ||||||||
Service Cost | 2.8 | |
| 2.7 | |
| - | |
| - | | ||||
Interest Cost | 10.4 | |
| 11.4 | |
| 0.1 | |
| 0.2 | | ||||
Expected return on plan assets | (9.8 | ) |
| (10.5 | ) |
| - | |
| - | | ||||
Amortization of unrecognized: | |
|
|
|
|
|
| ||||||||
Transition (asset)/obligation | - | |
| - | |
| - | |
| - | | ||||
Prior service cost | - | |
| - | |
| - | |
| - | | ||||
Net (gain)/loss | 2.9 | |
| 1.8 | |
| (0.1 | ) |
| (0.1 | ) | ||||
Ongoing periodic cost | 6.3 | |
| 5.4 | |
| - | |
| 0.1 | | ||||
Settlement/curtailment expense/(income) | - | |
| (0.2 | ) |
| - | |
| - | | ||||
Net periodic benefit cost | 6.3 | |
| 5.2 | |
| - | |
| 0.1 | | ||||
92
Table of Contents
At June 30, | Retirement Benefits |
| Other Post-Retirement Benefits | ||||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2016 |
| 2015 | ||||||||
Other Changes in Plan Assets and Benefit Obligations Recognized in Other Comprehensive Income |
|
|
|
|
|
|
| ||||||||
Net (gain)/loss arising during the year | $ | 16.4 | |
| $ | 13.0 | |
| - | |
| (0.7 | ) | ||
Prior service cost (credit) during the year | (0.7 | ) |
| - | |
| - | |
| - | | ||||
Transition asset/(obligation) recognized during the year | - | |
| - | |
| - | |
| - | | ||||
Prior service cost recognized during the year | - | |
| - | |
| - | |
| - | | ||||
Net gain/(loss) recognized during the year | (2.8 | ) |
| (3.2 | ) |
| 0.1 | |
| 0.1 | | ||||
Exchange rate gain/(loss) recognized during the year | 0.2 | |
| (0.6 | ) |
| - | |
| - | | ||||
Total recognized in other comprehensive income | $ | 13.1 | |
| $ | 9.2 | |
| $ | 0.1 | |
| $ | (0.6 | ) |
Total Recognized in Net Periodic Benefit Cost and Other Comprehensive Income |
|
|
|
|
|
|
| ||||||||
Total recognized in net periodic benefit cost and other comprehensive income | $ | 19.3 | |
| $ | 14.4 | |
| $ | 0.1 | |
| $ | (0.5 | ) |
Estimated Amounts to be Amortized from Accumulated Other Comprehensive Income into Net Periodic Benefit Cost |
|
|
|
|
|
|
| ||||||||
Amortization of: |
|
|
|
|
|
|
| ||||||||
Transition (asset)/obligation | $ | - | |
| $ | - | |
| $ | - | |
| $ | - | |
Prior service cost/(credit) | - | |
| - | |
| - | |
| - | | ||||
Net (gain)/loss | 4.5 | |
| 2.9 | |
| (0.1 | ) |
| (0.1 | ) | ||||
Financial Assumptions Used to Determine Benefit Obligations at the Balance Sheet Date |
|
|
|
|
|
|
| ||||||||
Discount rate (%) | 2.33 | % |
| 3.38 | % |
| 2.89 | % |
| 3.69 | % | ||||
Rate of compensation increases (%) | 2.10 | % |
| 2.06 | % |
| n/a | |
| n/a | | ||||
Financial Assumptions Used to Determine Net Periodic Benefit Cost for Financial Year |
|
|
|
|
|
|
| ||||||||
Discount rate (%) | 3.38 | % |
| 3.73 | % |
| 3.69 | % |
| 3.67 | % | ||||
Rate of compensation increases (%) | 2.10 | % |
| 2.10 | % |
| n/a | |
| n/a | | ||||
Expected long-term rate of return (%) | 4.93 | % |
| 5.11 | % |
| n/a | |
| n/a | | ||||
Expected Future Contributions |
|
|
|
|
|
|
| ||||||||
Financial Year |
|
|
|
|
|
|
| ||||||||
2017 | $ | 8.4 | |
| |
| $ | 0.3 | |
| | | |||
93
Table of Contents
At June 30, | Retirement Benefits |
| Other Post-Retirement Benefits | ||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2016 |
| 2015 | ||||
Expected Future Benefit Payments |
|
|
|
|
|
|
| ||||
Financial Year |
|
|
|
|
|
|
| ||||
2017 | 9.0 | |
| 10.5 | |
| 0.8 | |
| 0.8 | |
2018 | 9.4 | |
| 9.5 | |
| 0.3 | |
| 0.3 | |
2019 | 10.8 | |
| 11.1 | |
| 0.3 | |
| 0.3 | |
2020 | 11.1 | |
| 11.2 | |
| 0.2 | |
| 0.3 | |
2021 | 12.0 | |
| 13.9 | |
| 0.2 | |
| 0.3 | |
2022-2026 | 67.4 | |
| 72.0 | |
| 1.0 | |
| 1.1 | |
|
|
|
|
|
|
|
| ||||
Actual Asset Allocation (%) |
|
|
|
|
|
|
| ||||
Equities | 23.6 | % |
| 34.2 | % |
| - | % |
| - | % |
Government Bonds | 29.9 | % |
| 28.2 | % |
| - | % |
| - | % |
Corporate Bonds | 12.3 | % |
| 17.3 | % |
| - | % |
| - | % |
Property | 2.5 | % |
| 3.1 | % |
| - | % |
| - | % |
Insurance Contracts | 9.0 | % |
| 8.5 | % |
| - | % |
| - | % |
Other | 22.7 | % |
| 8.7 | % |
| - | % |
| - | % |
Total | 100.0 | % |
| 100.0 | % |
| - | % |
| - | % |
|
|
|
|
|
|
|
| ||||
Actual Asset Allocation (Amount) |
|
|
|
|
|
|
| ||||
Equities | 53.7 | |
| 75.7 | |
| - | |
| - | |
Government Bonds | 68.1 | |
| 62.7 | |
| - | |
| - | |
Corporate Bonds | 28.0 | |
| 38.5 | |
| - | |
| - | |
Property | 5.8 | |
| 6.9 | |
| - | |
| - | |
Insurance Contracts | 20.4 | |
| 18.9 | |
| - | |
| - | |
Other | 51.6 | |
| 19.3 | |
| - | |
| - | |
Total | 227.6 | |
| 222.0 | |
| - | |
| - | |
|
|
|
|
|
|
|
| ||||
Target Asset Allocation (%) |
|
|
|
|
|
|
| ||||
Equities | 24.1 | % |
| 34.5 | % |
| - | % |
| - | % |
Government Bonds | 29.8 | % |
| 24.8 | % |
| - | % |
| - | % |
Corporate Bonds | 12.3 | % |
| 22.1 | % |
| - | % |
| - | % |
Property | 2.7 | % |
| 3.5 | % |
| - | % |
| - | % |
Insurance Contracts | 8.9 | % |
| 6.3 | % |
| - | % |
| - | % |
Other | 22.2 | % |
| 8.8 | % |
| - | % |
| - | % |
Total | 100.0 | % |
| 100.0 | % |
| - | % |
| - | % |
The Company employs a building block approach in determining the long-term rate of return for plan assets, with proper consideration of diversification and rebalancing. Historical markets are studied and long-term historical relationships between equities and fixed income are preserved consistent with the widely accepted capital market principle that assets with higher volatility generate a greater return over the long run. Current market factors such as inflation and interest rates are evaluated before long-term capital market assumptions are determined. Peer data are reviewed to check for reasonability and appropriateness.
Plan assets are recognized and measured at fair value in accordance with the accounting standards regarding fair value measurements. The following are valuation techniques used to determine the fair value of each major category of assets:
• | Short-term investments, equity securities, fixed-income securities, and real estate are valued using quoted market prices or other valuation methods, and thus are classified within Level 1 or Level 2. |
94
Table of Contents
• | Insurance contracts and other types of investments include investments with some observable and unobservable prices that are adjusted by cash contributions and distributions, and thus are classified within Level 2 or Level 3. |
• | Other assets as of June 30, 2016 include $28.0 million of investments in hedge funds related to our U.K. pension plan and are classified as Level 2. |
The following table provides a summary of plan assets that are measured in fair value as of June 30, 2016 , aggregated by the level in the fair value hierarchy within which those measurements fall:
(Dollars in millions) | Total Assets |
| Level 1 |
| Level 2 |
| Level 3 | |||||||||
Equity Securities | $ | 53.7 | |
| $ | - | |
| $ | 53.7 | |
| - | | ||
Debt Securities | 96.1 | |
| - | |
| 96.1 | |
| - | | |||||
Real Estate | 5.8 | |
| - | |
| 5.8 | |
| - | | |||||
Other | 72.0 | |
| - | |
| 52.4 | |
| 19.6 | | |||||
Total | $ | 227.6 | |
| $ | - | |
| $ | 208.0 | |
| $ | 19.6 | | |
Level 3 other assets consist of an insurance contract in the UK to fulfill the benefit obligations for a portion of the participant benefits. The value of this commitment is determined using the same assumptions and methods used to value the UK Retirement & Death Benefit Plan pension liability. Level 3 other assets also include the partial funding of a pension liability relating to current and former employees of the Company's Eberbach facility through a Company promissory note or loan with an annual rate of interest of 5% . The value of this commitment fluctuates due to contributions and benefit payments in addition to loan interest.
The following table provides a summary of plan assets that are measured in fair value as of June 30, 2015 , aggregated by the level in the fair value hierarchy within which those measurements fall:
(Dollars in millions) | Total Assets |
| Level 1 |
| Level 2 |
| Level 3 | |||||||||
Equity Securities | $ | 75.7 | |
| $ | - | |
| $ | 75.7 | |
| - | | ||
Debt Securities | 101.2 | |
| - | |
| 101.2 | |
| - | | |||||
Real Estate | 6.9 | |
| - | |
| 0.3 | |
| 6.6 | | |||||
Other | 38.2 | |
| - | |
| 17.3 | |
| 20.9 | | |||||
Total | $ | 222.0 | |
| $ | - | |
| $ | 194.5 | |
| $ | 27.5 | | |
Level 3 real estate assets consist of a U.K. Property fund ("UBS Life Triton Property Fund") that directly invests in properties that are held in the U.K. The funds are priced using the Net Asset Value ("NAV") of the fund and investors also get Bid and Offer prices on a monthly basis. Investment properties are measured at fair value as determined by third-party independent appraisers. Their value is ascertained by reference to the market value, having regard to whether they are let or un-let at the date of valuation, in accordance with the Appraisal and Valuation Manual issued by the Royal Institution of Chartered Surveyors.
95
Table of Contents
The following table provides a reconciliation of the beginning and ending balances of level 3 assets as well as the changes during the period attributable to assets held and those purchases, sales, settlements, contributions and benefits that were paid:
Asset Category Allocations - June 30, 2016
Total (Level 3) | Fair Value Measurement |
| Fair Value Measurement |
| Fair Value Measurement | ||||||
(Dollars in millions) | Using Significant |
| Using Significant |
| Using Significant | ||||||
| Unobservable Inputs |
| Unobservable Inputs |
| Unobservable Inputs | ||||||
| Total (Level 3) |
| Insurance Contracts |
| Other | ||||||
Beginning Balance at June 30, 2015 | $ | 27.5 | |
| $ | 4.7 | |
| $ | 22.8 | |
Actual return on plan assets: |
|
|
|
|
| ||||||
Relating to assets still held at the reporting date | (0.9 | ) |
| (1.3 | ) |
| 0.4 | | |||
Relating to assets sold during the period | - | |
| - | |
| - | | |||
Purchases, sales, settlements, contributions and benefits paid | (7.0 | ) |
| (0.2 | ) |
| (6.8 | ) | |||
Transfers in and/or out of Level 3 | - | |
| - | |
| - | | |||
Ending Balance at June 30, 2016 | $ | 19.6 | |
| $ | 3.2 | |
| $ | 16.4 | |
The investment policy reflects the long-term nature of the plans' funding obligations. The assets are invested to provide the opportunity for both income and growth of principal. This objective is pursued as a long-term goal designed to provide required benefits for participants without undue risk. It is expected that this objective can be achieved through a well-diversified asset portfolio. All equity investments are made within the guidelines of quality, marketability and diversification mandated by the Employee Retirement Income Security Act of 1974, as amended ("ERISA") (for plans subject to ERISA) and other relevant legal requirements. Investment managers are directed to maintain equity portfolios at a risk level approximately equivalent to that of the specific benchmark established for that portfolio. Assets invested in fixed income securities and pooled fixed-income portfolios are managed actively to pursue opportunities presented by changes in interest rates, credit ratings or maturity premiums.
At June 30, | Other Post-Retirement Benefits | ||||||
(Actual dollar amounts) | 2016 |
| 2015 | ||||
|
|
|
| ||||
Assumed Healthcare Cost Trend Rates at the Balance Sheet Date |
|
|
| ||||
Healthcare cost trend rate – initial (%) |
|
|
| ||||
Pre-65 | n/a | |
| n/a | | ||
Post-65 | 10.35 | % |
| 11.35 | % | ||
Healthcare cost trend rate – ultimate (%) |
|
|
| ||||
Pre-65 | n/a | |
| n/a | | ||
Post-65 | 4.84 | % |
| 4.64 | % | ||
Year in which ultimate rates are reached |
|
|
| ||||
Pre-65 | n/a | |
| n/a | | ||
Post-65 | 2022 | |
| 2022 | | ||
Effect of 1% Change in Healthcare Cost Trend Rate |
|
|
| ||||
Healthcare cost trend rate up 1% |
|
|
| ||||
on APBO at balance sheet date | $ | 169,433 | |
| $ | 171,309 | |
on total service and interest cost | 5,721 | |
| 8,181 | | ||
Effect of 1% Change in Healthcare Cost Trend Rate |
|
|
| ||||
Healthcare cost trend rate down 1% |
|
|
| ||||
on APBO at balance sheet date | $ | (151,184 | ) |
| $ | (152,189 | ) |
on total service and interest cost | (5,106 | ) |
| (7,282 | ) | ||
Expected Future Contributions |
|
|
| ||||
Financial Year |
|
|
| ||||
2017 | $ | 259,942 | |
|
| ||
96
Table of Contents
11 . | RELATED PARTY TRANSACTIONS |
Advisor Transaction and Management Fees
Prior to the IPO, the Company was party to a transaction and advisory fee agreement with affiliates of Blackstone and certain other investors in BHP PTS Holdings L.L.C. (the "Investors"), pursuant to which the Company historically paid an annual sponsor advisory fee to Blackstone and the other Investors for certain monitoring, advisory and consulting services to the Company. In connection with the IPO, the Company paid the Investors an advisory agreement termination fee of $29.8 million in August 2014, which was recorded within other (income)/expense, net in the Consolidated Statements of Operations, and terminated the agreement. As a result, the Company did not have management fees for the fiscal years ended June 30, 2016 and 2015. For the fiscal year ended June 30, 2014, this management fee was approximately $12.9 million . This fee was recorded within selling, general and administrative expenses in the Consolidated Statements of Operations.
In connection with each of the secondary offerings of our common stock demanded by Blackstone during fiscal 2015 and 2016 following the IPO, we entered into underwriting agreements with Blackstone, the other shareholders selling in the offerings, and the underwriters managing the offerings setting forth the terms of the offerings and making various representations to the underwriters regarding various facts and circumstances relating to the offerings. The underwriting agreements required us to pay certain expenses relating to the offerings and to indemnify Blackstone, the other sellers, and the underwriters for the offerings against liabilities arising from breaches of our representations and certain other matters relating to the offerings.
Other Related Party Transactions
The Company participates in an employer health program agreement with Equity Healthcare LLC ("Equity Healthcare"). Equity Healthcare negotiates with providers of standard administrative services for health benefit plans and other related services for cost discounts and quality of service monitoring capability by Equity Healthcare. Because of the combined purchasing power of its client participants, Equity Healthcare is able to negotiate pricing terms for providers that are believed to be more favorable than the companies could obtain for themselves on an individual basis. In consideration for these services, the Company paid Equity Healthcare a fee of $3.00 and $2.80 per participating employee per month in calendar years 2016 and 2015 , respectively. As of June 30, 2016 , the Company had approximately 2,700 employees enrolled in its health benefit plans in the United States. Equity Healthcare is an affiliate of Blackstone.
12 . | EQUITY AND ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS) |
Description of Capital Stock and Initial Public Offering
The Company is authorized to issue 1,000,000,000 shares of common stock, par value $0.01 per share, and 100,000,000 shares of preferred stock, par value $0.01 per share. In accordance with the Company's amended and restated certificate of incorporation, each share of common stock has one vote, and the common stock votes together as a single class. In July 2014, the Company's board of directors and holders of the requisite number of outstanding shares of its capital stock approved an amendment to the Company's amended and restated certificate of incorporation to effect a 70 -for-1 stock split. The stock split became effective on July 17, 2014 upon the filing with the Delaware Secretary of State of the amendment. Refer to Note 1 for further discussion of the Company's July 2014 recapitalization and discussion of the Company's public offerings of common stock.
97
Table of Contents
Accumulated other comprehensive income/(loss)
(Dollars in millions) | Foreign Currency Translation Adjustments |
| Deferred Compensation |
| Pension Liability Adjustments |
| Other Comprehensive Income/(Loss) | ||||||||
Balance at June 30, 2013 | $ | (18.4 | ) |
| $ | 1.5 | |
| $ | (25.9 | ) |
| $ | (42.8 | ) |
Activity, net of tax | 32.4 | |
| 1.7 | |
| (15.5 | ) |
| 18.6 | | ||||
Balance at June 30, 2014 | 14.0 | |
| 3.2 | |
| (41.4 | ) |
| (24.2 | ) | ||||
Activity, net of tax | (144.0 | ) |
| 0.6 | |
| (6.4 | ) |
| (149.8 | ) | ||||
Balance at June 30, 2015 | (130.0 | ) |
| 3.8 | |
| (47.8 | ) |
| (174.0 | ) | ||||
Activity, net of tax | (118.8 | ) |
| (3.8 | ) |
| (9.1 | ) |
| (131.7 | ) | ||||
Balance at June 30, 2016 | $ | (248.8 | ) |
| $ | - | |
| $ | (56.9 | ) |
| $ | (305.7 | ) |
Current year activity within deferred compensation includes realized gains associated with the sale of available for sale investments. The components of the changes in the cumulative translation adjustment and minimum pension liability for the fiscal years ended June 30, 2016 , June 30, 2015 and June 30, 2014 consists of:
| Year Ended June 30, | ||||||||||
| 2016 |
| 2015 |
| 2014 | ||||||
Foreign currency translation adjustments: |
|
|
|
|
| ||||||
Net investment hedge | $ | 1.8 | |
| $ | 30.0 | |
| $ | (13.6 | ) |
Long term inter-company loans | (65.0 | ) |
| (9.0 | ) |
| 28.3 | | |||
Translation adjustments | (54.9 | ) |
| (152.7 | ) |
| 17.7 | | |||
Total foreign currency translation adjustments, pretax | (118.1 | ) |
| (131.7 | ) |
| 32.4 | | |||
Tax (1) | (0.7 | ) |
| (12.3 | ) |
| - | | |||
Total foreign currency translation adjustments, net of tax | $ | (118.8 | ) |
| $ | (144.0 | ) |
| $ | 32.4 | |
|
|
|
|
|
| ||||||
Net change in minimum pension liability |
|
|
|
|
| ||||||
Net gain/(loss) arising during the year | $ | (16.4 | ) |
| $ | (12.3 | ) |
| $ | (20.4 | ) |
Net (gain)/loss recognized during the year | 3.4 | |
| 3.1 | |
| 1.5 | | |||
Foreign Exchange Translation and Other | (0.2 | ) |
| 0.6 | |
| (0.5 | ) | |||
Total Pension, pretax | (13.2 | ) |
| (8.6 | ) |
| (19.4 | ) | |||
Tax | 4.1 | |
| 2.2 | |
| 3.9 | | |||
Net change in minimum pension liability, net of tax | $ | (9.1 | ) |
| $ | (6.4 | ) |
| $ | (15.5 | ) |
(1) | Tax related to foreign currency translation adjustments primarily relates to the Net investment hedge activity. |
13 . | EQUITY-BASED COMPENSATION |
The Company's stock-based compensation is comprised of stock options and restricted stock units.
2007 Stock Incentive Plan
Awards issued under the Company's pre-IPO incentive compensation plan, known as the 2007 PTS Holdings Corp. Stock Incentive Plan, as amended (the "2007 Plan"), were generally issued for the purpose of retaining key employees and directors.
2014 Omnibus Incentive Plan
In connection with the IPO, the Company's Board of Directors adopted, and the holder of a majority of the shares approved, the 2014 Omnibus Incentive Plan effective July 31, 2014 (the "2014 Plan"). The 2014 Plan provides certain members of management, employees and directors of the Company and its subsidiaries with the opportunity to obtain various incentives, including grants of stock options and restricted stock units. A maximum of 6,700,000 shares of common stock may be issued under the 2014 Plan.
98
Table of Contents
Stock Compensation Expense
Stock compensation expense recognized in the consolidated statements of income was $10.8 million , $9.0 million and $4.5 million in fiscal 2016 , 2015 and 2014 , respectively. All stock compensation expense is classified in selling, general and administrative expenses along with the wages and benefits of the option participants. Stock compensation expense was based on awards expected to vest, and therefore was reduced by estimated forfeitures in fiscal years 2015 and 2014 prior to the adoption of ASU 2016-09 , whereby the Company elected to account for forfeitures as they occur. The Company recognized a cumulative-effect adjustment charge relating to prior periods of approximately $0.7 million , net of income taxes to accumulated deficit for the impact of electing to account for a forfeiture as it occurs. The Company recorded $0.3 million related to the first nine months of fiscal 2016 to adjust for previously estimated forfeitures for the interim periods. See Note 1 .
Stock Options
The Company adopted two forms of non-qualified stock option agreements (each, a "Form Option Agreement") for awards granted under the 2007 Plan. Under the Company's Form Option Agreement adopted in 2009, a portion of the stock option awards vest in equal annual installments over a five -year period contingent solely upon the participant's continued employment with the Company, or one of its subsidiaries, another portion of the stock option awards vest over a specified performance period upon achievement of pre-determined operating performance targets over time and the remaining portion of the stock option awards vest upon realization of certain internal rates of return or multiple of investment goals. Under the Company's other Form Option Agreement, adopted in 2013, a portion of the stock option awards vest over a specified performance period upon achievement of pre-determined operating performance targets over time while the other portion of the stock option awards vest upon realization of a specified multiple of investment goal. The Form Option Agreements include certain forfeiture provisions upon a participant's separation from service with the Company. Following the IPO, the Company decided not to grant any further awards under the 2007 Plan; however, all outstanding awards granted prior to the IPO remained outstanding in accordance with the terms of the 2007 Plan.
Stock options were also granted under the 2014 Plan during fiscal 2016 and fiscal 2015 for selected executives of the Company, with an aggregate intrinsic value of $0 and $2.3 million , which represents approximately 369,000 and 509,000 shares of common stock for the fiscal 2016 and 2015 grants, respectively. Each stock option vests in equal annual installments over a four-year period from the date of grant, contingent upon the participant's continued employment with the Company.
Methodology and Assumptions
Stock options are granted with an exercise price equal to the fair market value on the date of grant. Stock options granted generally vest in equal annual installments over four or five years from the grant date. All outstanding stock options have a contractual term of 10 years, subject to forfeiture under certain conditions upon separation of employment. The grant-date fair value, adjusted for estimated forfeitures, is recognized as expense on a graded-vesting basis over the vesting period. The fair value of stock options is determined using the Black-Scholes-Merton option pricing model for service and performance based awards, and an adaptation of the Black-Scholes-Merton option valuation model, which takes into consideration the internal rate of return thresholds, for market-based awards. This model adaptation is essentially equivalent to the use of path dependent-lattice model.
The weighted average of assumptions used in estimating the fair value of stock options granted during each year were as follows:
| Year Ended June 30, | ||||
| 2016 |
| 2015 |
| 2014 |
Expected volatility | 28% - 31% |
| 32% |
| 31% |
Expected life (in years) | 6.25 |
| 6.25 |
| 5.66 - 6.50 |
Risk-free interest rates | 1.5% - 1.7% |
| 2% |
| 0.3% - 2.2% |
Dividend yield | None |
| None |
| None |
The Company ended fiscal 2016 in its second year of being public, and as a result has limited relevant historical volatility experience; therefore, the expected volatility assumption is based on the historical volatility of the closing share prices of a comparable peer group. The Company selected peer companies from the pharmaceutical industry with similar characteristics, including market capitalization, number of employees and product focus. In addition, since the Company does not have a pattern of exercise behavior of option holders, the Company used the simplified method to determine the expected life of each option, which is the mid-point between the vesting date and the end of the contractual term. The risk-free interest-rate for the expected life of the option is based on the comparable U.S. Treasury yield curve in effect at the time of grant. The weighted-average grant-date fair value of stock options in fiscal 2016 , 2015 , and 2014 was $10.68 per share, $7.23 per share and $5.41 per share, respectively.
99
Table of Contents
The following table summarizes stock option activity and shares subject to outstanding options for the year ended June 30, 2016 :
|
| Time | Performance | Market | ||||||||||||||||||||
| Weighted |
| Weighted |
|
| Weighted |
|
| Weighted |
| ||||||||||||||
| Average | Number | Average | Aggregate | Number | Average | Aggregate | Number | Average | Aggregate | ||||||||||||||
| Exercise | of | Contractual | Intrinsic | of | Contractual | Intrinsic | of | Contractual | Intrinsic | ||||||||||||||
| Price | shares | Term | Value | shares | Term | Value | shares | Term | Value | ||||||||||||||
Outstanding as of June 30, 2015 | $ | 15.62 | | 2,007,699 | | 6.83 | $ | 25,979,873 | | 1,097,250 | | 6.87 | $ | 14,296,090 | | 2,073,610 | | 5.81 | $ | 30,739,825 | | |||
Granted | $ | 31.80 | | 368,995 | | 9.17 | - | | - | | - | | - | | - | | - | | - | | ||||
Exercised | $ | 12.64 | | (416,183 | ) | - | | 7,700,424 | | (261,042 | ) | - | | 4,450,531 | | - | | - | | - | | |||
Forfeited | $ | 17.26 | | (135,656 | ) | - | | - | | (39,690 | ) | - | | - | | (287,910 | ) | - | | - | | |||
Expired / Canceled | - | | - | | - | | - | | - | | - | | - | | - | | - | | - | | ||||
Outstanding as of June 30, 2016 | $ | 17.26 | | 1,824,855 | | 6.75 | 8,841,470 | | 796,518 | | 6.46 | 4,323,349 | | 1,785,700 | | 5.07 | 15,130,345 | | ||||||
Vest and expected to vest as of June 30, 2016 | $ | 17.57 | | 1,824,855 | | 6.75 | 8,841,470 | | 382,706 | | 5.95 | 2,528,446 | | 693,440 | | 4.08 | 7,387,124 | | ||||||
Vested and exercisable as of June 30, 2016 | $ | 15.23 | | 851,749 | | 5.04 | $ | 7,056,933 | | 382,706 | | 5.95 | $ | 2,528,446 | | - | | - | | - | | |||
In fiscal 2016 , participants exercised options to purchase 212 thousand net settled shares, resulting in $6.4 million of cash paid on behalf of participants for withholding taxes. The intrinsic value of the options exercised in fiscal 2016 was $12.2 million . The total fair value of options vested during the period was $3.1 million .
In fiscal 2015 , participants exercised options to purchase 623 thousand net settled shares, resulting in $10.3 million of cash paid on behalf of participants for withholding taxes. The intrinsic value of the options exercised in fiscal 2015 was $26.8 million . The total fair value of options vested during the period was $3.6 million .
As of June 30, 2016 , $3.3 million of unrecognized compensation cost related to stock options is expected to be recognized as expense over a weighted-average period of approximately 2.7 years.
Restricted Stock Units
Restricted stock units under the 2014 Plan may be granted to members of management and directors. The Company has granted to members of management restricted stock units that vest over specified periods of time as well as restricted stock units that have certain performance-related vesting requirements ("performance share units"). The performance share units granted for fiscal 2016 and 2015 had a grant date fair value of $19.8 million and $14.7 million, respectively, which represents approximately 607,000 and 692,000 shares of common stock, respectively. Under the 2014 Plan, the performance share units vest based on achieving Company financial performance metrics established at the outset of each three -year performance period. The metrics for the fiscal 2015 grant are a mix of cumulative revenue growth and cumulative EBITDA growth targets. The metrics for the fiscal 2016 grant are a mix of earnings-per-share ("EPS") targets and relative total shareholder return ("RTSR") targets. The performance share units vest following the end of the three-year performance period on the third anniversary of the date of grant based on achievement of the targets which are reviewed quarterly to determine the probability of vesting. The time-based restricted stock units awards vest on the third anniversary of the date of grant subject to the participant's continued employment with the Company.
Methodology and Assumptions
The grant-date fair value of restricted stock units is recognized as expense on a graded vesting schedule over the vesting period of three to five years. This fair value is determined based on the number of shares subject to the grant and the fair value of the Company's common stock on the date of grant, as determined by the closing market price.
100
Table of Contents
Time-Based Restricted Stock Units
The following table summarizes non-vested activity in time-based restricted stock units for the year ended June 30, 2016 :
| Time-Based Units |
| Weighted Average Grant-Date Fair Value | |||
Non-vested as of June 30, 2015 | 354,153 | |
| $ | 20.75 | |
Granted | 283,976 | |
| 31.12 | | |
Vested | 57,543 | |
| 21.20 | | |
Forfeited | 76,490 | |
| 24.57 | | |
Non-vested as of June 30, 2016 | 504,096 | |
| $ | 25.96 | |
EPS Performance Share Units
The following table summarizes non-vested EPS performance share unit activity for the year ended June 30, 2016 :
| EPS Units |
| Weighted Average Grant-Date Fair Value | |||
Non-vested as of June 30, 2015 | 419,147 | |
| $ | 22.16 | |
Granted | 174,031 | |
| 31.8 | | |
Vested | - | |
| - | | |
Forfeited | 87,753 | |
| 23.99 | | |
Non-vested as of June 30, 2016 | 505,425 | |
| $ | 25.16 | |
RTSR Performance Share Units
The fair value of the RTSR performance share units is determined using the Monte Carlo pricing model because the number of shares to be awarded is subject to a market condition. The Monte Carlo simulation is a generally accepted statistical technique used to simulate a range of possible future unit prices. Compensation cost is recognized regardless if the market condition is actually satisfied.
The assumptions used in estimating the fair value of the RTSR performance share units granted for the year ended June 30, 2016 are as follows:
Expected volatility | 25% |
Expected life (in years) | 2.84 |
Risk-free interest rates | 0.94% |
Dividend yield | None |
The following table summarizes non-vested RTSR unit activity for the year ended June 30, 2016 :
| RTSR Units |
| Weighted Average Grant-Date Fair Value | |||
Non-vested as of June 30, 2015 | - | |
| $ | - | |
Granted | 148,982 | |
| 37.21 | | |
Vested | - | |
| - | | |
Forfeited | 16,326 | |
| 37.61 | | |
Non-vested as of June 30, 2016 | 132,656 | |
| $ | 37.17 | |
In fiscal 2016 , participants vested and settled 181 thousand net settled shares, resulting in $2.3 million of cash paid on behalf of participants for withholding taxes.
101
Table of Contents
As of June 30, 2016 , $17.2 million of unrecognized compensation cost related to restricted stock units is expected to be recognized as expense over a weighted-average period of approximately 1.9 years. The weighted-average grant-date fair value of restricted stock units in fiscal years 2016 , 2015 and 2014 was $32.82 , $21.49 and $21.64 , respectively. The fair value of restricted stock units vested in fiscal 2016 , 2015 and 2014 was $1.2 million , $0.6 million and $0.6 million , respectively.
14 . | OTHER (INCOME) / EXPENSE, NET |
The components of Other (Income) / Expense, net for the twelve months ended June 30, 2016 , 2015 and 2014 are as follows:
| Twelve Months Ended | ||||||||||
(Dollars in millions) | 2016 |
| 2015 |
| 2014 | ||||||
Other (Income) / Expense, net |
|
|
|
|
| ||||||
Debt extinguishment costs | $ | - | |
| $ | 21.8 | |
| $ | 11.1 | |
Gain on acquisition, net (1) | - | |
| (8.9 | ) |
| - | | |||
Sponsor advisory agreement termination fee (2) | - | |
| 29.8 | |
| - | | |||
Foreign currency (gains) and losses | (12.6 | ) |
| (2.4 | ) |
| (2.5 | ) | |||
Other (3) | (3.0 | ) |
| 2.1 | |
| 1.8 | | |||
Total Other (Income) / Expense | $ | (15.6 | ) |
| $ | 42.4 | |
| $ | 10.4 | |
(1) | Included within Other (income) / expense, net are gains associated with acquisitions completed during the respective periods. Such income events are non-standard in nature and not reflective of the Company's core operating results. During the twelve months ended June 30, 2015 , the Company recorded a gain of $3.2 million on the re-measurement of a cost investment in an entity that became a wholly owned subsidiary as of October 2014, a $7.0 million bargain purchase gain for an acquisition completed in July 2014 , and a $1.3 million loss on the redeemable noncontrolling interest in June 2015 . |
(2) | The Company paid a sponsor advisory agreement termination fee of $29.8 million in connection with its IPO. |
(3) | Included within Other (income) / expense, net are realized gains associated with the sale of available for sale investments of approximately $3.8 million during the fiscal year ended June 30, 2016 . |
15 . | REDEEMABLE NONCONTROLLING INTEREST |
In July 2013, the Company acquired a 67% controlling interest in a softgel manufacturing facility located in Haining, China. The noncontrolling interest shareholders had the right to jointly sell the remaining 33% interest to Catalent during the 30-day period following the third anniversary of closing for a price based on the greater of (1) an amount that would provide the noncontrolling interest shareholders a return on their investment of a predetermined amount per annum on their pro rata share of the initial valuation or (2) a multiple of the sum of the target 's earnings before interest, taxes, depreciation and amortization less net debt for the four quarters immediately preceding such sale. Noncontrolling interest with redemption features, such as the arrangement described above, that are not solely within the Company's control are considered redeemable noncontrolling interests, which is considered temporary equity and is therefore reported outside of permanent equity on the Company ' s consolidated balance sheet at the greater of the initial carrying amount adjusted for the noncontrolling interest ' s share of net income/(loss) or its redemption value.
In June 2015, the Company reached an agreement to acquire the remaining 33% from the noncontrolling interest shareholders for purchase consideration of $5.8 million . As a result of the purchase agreement, the Company recorded a $1.3 million loss in Other Income/Expense, net to reflect the current redemption value as of June 30, 2015. The transaction closed in December 2015.
102
Table of Contents
16 . | COMMITMENTS AND CONTINGENCIES |
Rental Payments and Expense
(Dollars in millions) | 2017 | 2018 | 2019 | 2020 | 2021 | Thereafter | Total | ||||||||||||||
Minimum rental payments | $ | 9.2 | | $ | 6.6 | | $ | 6.0 | | $ | 4.2 | | $ | 3.7 | | $ | 4.4 | | $ | 34.1 | |
Rental expense relating to operating leases was approximately $9.5 million , $10.0 million , and $9.5 million for the fiscal years ended June 30, 2016 , 2015 and 2014, respectively. Sublease rental income was not material for any period presented.
Other Matters
During the period November 2015 through April 2016, the primary French drug regulatory agency (the "ANSM") temporarily suspended operations at the Company's softgel manufacturing facility in Beinheim, France, subject to exemptions for certain types of operations. Due to the temporary suspension, the Company was unable to use certain raw materials, work in process and finished goods, and took a charge of $1.0 million , net of insurance recoveries, during the year ended June 30, 2016, in connection with such loss of use and recorded remediation associated costs of $6.0 million . Further, certain of the customers of the facility have presented claims against the Company for losses they have allegedly suffered due to the temporary suspension or have reserved their right to do so subsequently. The Company is unable to estimate at this time either the total value of these claims or the likely cost to resolve them. Changes to the operations at the facility to address the issues leading to the suspension have increased and may in the future additionally increase the cost and therefore decrease the profitability of its operation and may also require the Company to incur additional costs.
From time to time, we may be involved in legal proceedings arising in the ordinary course of business, including, without limitation, inquiries and claims concerning environmental contamination as well as litigation and allegations in connection with acquisitions, product liability, manufacturing or packaging defects, claims for reimbursement for the cost of lost or damaged active pharmaceutical ingredients and employment-related claims, the cost of any of which could be significant. We intend to vigorously defend ourselves against any such litigation and do not currently believe that the outcome of any such litigation will have a material adverse effect on our financial condition or result of operation. In addition, the healthcare industry is highly regulated and government agencies continue to scrutinize certain practices affecting government programs and otherwise.
From time to time, we receive subpoenas or requests for information from private parties and various governmental agencies, including from state attorneys general and the U.S. Department of Justice, relating to the business practices of customers or suppliers. We generally respond to such subpoenas and requests in a timely and thorough manner, which responses sometimes require considerable time and effort and can result in considerable costs being incurred by us. We expect to incur costs in the future in connection with future requests.
17 . | SEGMENT INFORMATION |
As discussed in Note 1 , the Company conducts its business within the following operating segments: Softgel Technologies, Drug Delivery Solutions, and Clinical Supply Services. The Company evaluates the performance of its segments based on segment earnings before noncontrolling interest, other (income) expense, impairments, restructuring costs, interest expense, income tax (benefit)/expense, and depreciation and amortization ("Segment EBITDA"). EBITDA from continuing operations is consolidated earnings from continuing operations before interest expense, income tax (benefit)/expense, depreciation and amortization and is adjusted for the income or loss attributable to noncontrolling interest. The Company's presentation of Segment EBITDA and EBITDA from continuing operations may not be comparable to similarly titled measures used by other companies.
103
Table of Contents
All prior period comparative segment information has been restated to reflect the current reportable segments in accordance with ASC 280 Segment Reporting as discussed in Note 1 . The following tables include net revenue and Segment EBITDA during the fiscal years ended June 30, 2016 , June 30, 2015 , and June 30, 2014 :
(Dollars in millions) | Fiscal Year Ended | ||||||||||
2016 |
| 2015 |
| 2014 | |||||||
Softgel Technologies |
|
|
|
|
| ||||||
Net revenue | $ | 775.0 | |
| $ | 787.5 | |
| $ | 857.5 | |
Segment EBITDA | $ | 163.8 | |
| $ | 173.6 | |
| $ | 214.8 | |
Drug Delivery Solutions |
|
|
|
|
| ||||||
Net revenue | 806.4 | |
| 798.3 | |
| 719.2 | | |||
Segment EBITDA | 215.2 | |
| 230.7 | |
| 182.2 | | |||
Clinical Supply Services |
|
|
|
|
| ||||||
Net revenue | 307.5 | |
| 288.4 | |
| 291.7 | | |||
Segment EBITDA | 53.2 | |
| 56.7 | |
| 59.5 | | |||
Inter-segment revenue elimination | (40.8 | ) |
| (43.4 | ) |
| (40.7 | ) | |||
Unallocated costs (1) | (57.9 | ) |
| (100.8 | ) |
| (82.1 | ) | |||
Combined Total |
|
|
|
|
| ||||||
Net revenue | $ | 1,848.1 | |
| $ | 1,830.8 | |
| $ | 1,827.7 | |
|
|
|
|
|
| ||||||
EBITDA from continuing operations | $ | 374.3 | |
| $ | 360.2 | |
| $ | 374.4 | |
(1) | Unallocated costs include restructuring and special items, equity-based compensation, impairment charges, certain other corporate directed costs, and other costs that are not allocated to the segments as follows: |
(Dollars in millions) | Fiscal Year Ended | ||||||||||
2016 |
| 2015 |
| 2014 | |||||||
Impairment charges and gain/(loss) on sale of assets | $ | (2.7 | ) |
| $ | (4.7 | ) |
| $ | (3.2 | ) |
Equity compensation | (10.8 | ) |
| (9.0 | ) |
| (4.5 | ) | |||
Restructuring and other items (2) | (27.2 | ) |
| (27.2 | ) |
| (29.4 | ) | |||
Sponsor advisory fee | - | |
| - | |
| (12.9 | ) | |||
Noncontrolling interest | 0.3 | |
| 1.9 | |
| 1.0 | | |||
Other income/(expense), net (3) | 15.6 | |
| (42.4 | ) |
| (10.4 | ) | |||
Non-allocated corporate costs, net | (33.1 | ) |
| (19.4 | ) |
| (22.7 | ) | |||
Total unallocated costs | $ | (57.9 | ) |
| $ | (100.8 | ) |
| $ | (82.1 | ) |
(2) | Segment results do not include restructuring and certain acquisition-related costs. |
(3) | Refer to Note 14 for details. |
Provided below is a reconciliation of earnings/(loss) from continuing operations to EBITDA from continuing operations:
(Dollars in millions) | Fiscal Year Ended | ||||||||||
2016 |
| 2015 |
| 2014 | |||||||
Earnings/(loss) from continuing operations | $ | 111.2 | |
| $ | 210.2 | |
| $ | 17.9 | |
Depreciation and amortization | 140.6 | |
| 140.8 | |
| 142.9 | | |||
Interest expense, net | 88.5 | |
| 105.0 | |
| 163.1 | | |||
Income tax (benefit)/expense | 33.7 | |
| (97.7 | ) |
| 49.5 | | |||
Noncontrolling interest | 0.3 | |
| 1.9 | |
| 1.0 | | |||
EBITDA from continuing operations | $ | 374.3 | |
| $ | 360.2 | |
| $ | 374.4 | |
104
Table of Contents
The following table includes total assets for each segment, as well as reconciling items necessary to total the amounts reported in the Consolidated Financial Statements:
(Dollars in millions) | June 30, |
| June 30, | ||||
Assets |
|
|
| ||||
Softgel Technologies | $ | 1,446.4 | |
| $ | 1,438.8 | |
Drug Delivery Solutions | 1,475.7 | |
| 1,254.0 | | ||
Clinical Supply Services | 578.9 | |
| 575.7 | | ||
Corporate and eliminations | (409.9 | ) |
| (130.2 | ) | ||
Total assets | $ | 3,091.1 | |
| $ | 3,138.3 | |
The following tables include depreciation and amortization expense and capital expenditures for the fiscal years ended June 30, 2016 , June 30, 2015 and June 30, 2014 for each segment, as well as reconciling items necessary to total the amounts reported in the Consolidated Financial statements:
Depreciation and Amortization Expense
(Dollars in millions) | Fiscal Year Ended | ||||||||||
2016 |
| 2015 |
| 2014 | |||||||
Softgel Technologies | $ | 36.7 | |
| $ | 42.8 | |
| $ | 48.3 | |
Drug Delivery Solutions | 72.9 | |
| 66.9 | |
| 63.7 | | |||
Clinical Supply Services | 21.1 | |
| 24.1 | |
| 22.3 | | |||
Corporate | 9.9 | |
| 7.0 | |
| 8.6 | | |||
Total depreciation and amortization expense | $ | 140.6 | |
| $ | 140.8 | |
| $ | 142.9 | |
Capital Expenditures
(Dollars in millions) | Fiscal Year Ended | ||||||||||
2016 |
| 2015 |
| 2014 | |||||||
Softgel Technologies | $ | 20.6 | |
| $ | 29.6 | |
| $ | 24.9 | |
Drug Delivery Solutions | 92.4 | |
| 86.2 | |
| 68.7 | | |||
Clinical Supply Services | 5.1 | |
| 6.4 | |
| 15.7 | | |||
Corporate | 21.5 | |
| 18.8 | |
| 13.1 | | |||
Total capital expenditure | $ | 139.6 | |
| $ | 141.0 | |
| $ | 122.4 | |
The following table presents revenue and long-lived assets by geographic area:
| Net Revenue |
| Long-Lived Assets (1) | ||||||||||||||||
(Dollars in millions) | Fiscal Year Ended |
|
|
|
| ||||||||||||||
2016 |
| 2015 |
| 2014 |
| June 30, |
| June 30, | |||||||||||
United States | $ | 858.6 | |
| $ | 799.3 | |
| $ | 682.3 | |
| $ | 538.9 | |
| $ | 479.0 | |
Europe | 733.2 | |
| 795.4 | |
| 888.8 | |
| 280.2 | |
| 314.6 | | |||||
International Other | 313.5 | |
| 268.6 | |
| 278.8 | |
| 86.7 | |
| 91.6 | | |||||
Eliminations | (57.2 | ) |
| (32.5 | ) |
| (22.2 | ) |
| - | |
| - | | |||||
Total | $ | 1,848.1 | |
| $ | 1,830.8 | |
| $ | 1,827.7 | |
| $ | 905.8 | |
| $ | 885.2 | |
(1) | Long-lived assets include property and equipment, net of accumulated depreciation. |
105
Table of Contents
18 . | SUPPLEMENTAL BALANCE SHEET INFORMATION |
Supplementary balance sheet information at June 30, 2016 and June 30, 2015 is detailed in the following tables.
Inventories
(Dollars in millions) | June 30, |
| June 30, | ||||
Raw materials and supplies | $ | 88.7 | |
| $ | 76.9 | |
Work-in-process | 30.7 | |
| 26.3 | | ||
Finished goods | 55.2 | |
| 43.8 | | ||
Total inventory, gross | 174.6 | |
| 147.0 | | ||
Inventory reserve | (19.8 | ) |
| (14.1 | ) | ||
Inventories | $ | 154.8 | |
| $ | 132.9 | |
Prepaid expenses and other
Prepaid expenses and other current assets consist of the following:
(Dollars in millions) | June 30, |
| June 30, | ||||
Prepaid expenses | $ | 29.3 | |
| $ | 22.0 | |
Spare parts supplies | 10.8 | |
| 11.5 | | ||
Deferred income taxes (1) | - | |
| 19.7 | | ||
Long term tax asset (current portion) (2) | 6.8 | |
| - | | ||
Other current assets | 42.1 | |
| 27.7 | | ||
Prepaid expenses and other | $ | 89.0 | |
| $ | 80.9 | |
(1) The Company early adopted ASU 2015-17 in the year ended June 30, 2016 and accordingly deferred income taxes are now presented as non-current. The prior period was not retrospectively adjusted based on the adoption method.
(2) The Company transferred certain intellectual property assets between jurisdictions in the year ended June 30, 2016 resulting in a deferred tax charge which will be amortized over the remaining 10-year useful life of the asset.
Property, plant, and equipment, net
(Dollars in millions) | June 30, |
| June 30, | ||||
Land, buildings and improvements | $ | 649.6 | |
| $ | 637.6 | |
Machinery, equipment and capitalized software | 757.1 | |
| 727.9 | | ||
Furniture and fixtures | 9.9 | |
| 10.1 | | ||
Construction in progress | 134.1 | |
| 97.6 | | ||
Property and equipment, at cost | 1,550.7 | |
| 1,473.2 | | ||
Accumulated depreciation | (644.9 | ) |
| (588.0 | ) | ||
Property, plant, and equipment, net | $ | 905.8 | |
| $ | 885.2 | |
106
Table of Contents
Other assets
Other assets consist of the following:
(Dollars in millions) | June 30, |
| June 30, | ||||
Long term tax asset (1) | $ | 45.4 | |
| $ | - | |
Deferred compensation investments | 11.1 | |
| 10.1 | | ||
Deferred long-term debt financing costs | 1.8 | |
| 2.4 | | ||
Other | 8.8 | |
| 8.8 | | ||
Total other assets | $ | 67.1 | |
| $ | 21.3 | |
(1) The Company transferred certain intellectual property assets between jurisdictions in the year ended June 30, 2016 resulting in a deferred tax charge which will be amortized over the remaining 10-year useful life of the asset.
Other accrued liabilities
(Dollars in millions) | June 30, |
| June 30, | ||||
Accrued employee-related expenses | $ | 82.8 | |
| $ | 87.8 | |
Restructuring accrual | 6.1 | |
| 7.3 | | ||
Deferred income taxes (1) | - | |
| 1.5 | | ||
Accrued interest | 0.1 | |
| 0.2 | | ||
Deferred revenue and fees | 46.2 | |
| 39.0 | | ||
Accrued income tax | 38.8 | |
| 55.8 | | ||
Other accrued liabilities and expenses | 45.8 | |
| 55.4 | | ||
Other accrued liabilities | $ | 219.8 | |
| $ | 247.0 | |
(1) The Company early adopted ASU 2015-17 in the year ended June 30, 2016 and accordingly deferred income taxes are now presented as non-current. The prior period was not retrospectively adjusted based on the adoption method.
Allowance for doubtful accounts
(Dollars in millions) | June 30, |
| June 30, |
| June 30, | ||||||
Trade receivables allowance for doubtful accounts |
|
|
|
|
| ||||||
Beginning balance | $ | 6.6 | |
| $ | 5.4 | |
| $ | 5.7 | |
Charged to cost and expenses (recoveries) | (0.5 | ) |
| 2.7 | |
| 0.5 | | |||
Deductions | (1.8 | ) |
| (1.1 | ) |
| (1.0 | ) | |||
Impact of foreign exchange | (0.4 | ) |
| (0.4 | ) |
| 0.2 | | |||
Closing balance | $ | 3.9 | |
| $ | 6.6 | |
| $ | 5.4 | |
107
Table of Contents
Inventory reserve
(Dollars in millions) | June 30, |
| June 30, |
| June 30, | ||||||
Inventory reserve |
|
|
|
|
| ||||||
Beginning balance | $ | 14.1 | |
| $ | 12.9 | |
| $ | 11.8 | |
Charged to cost and expenses | 13.6 | |
| 9.5 | |
| 10.2 | | |||
Write offs | (7.2 | ) |
| (6.5 | ) |
| (9.5 | ) | |||
Impact of foreign exchange | (0.7 | ) |
| (1.8 | ) |
| 0.4 | | |||
Closing balance | $ | 19.8 | |
| $ | 14.1 | |
| $ | 12.9 | |
19 . | QUARTERLY FINANCIAL DATA (UNAUDITED) |
The following table summarizes the Company's unaudited quarterly results of operation.
(Dollars in millions, except per share data) | Fiscal Year 2016, By Quarters (as adjusted) (1) | ||||||||||||||
First |
| Second |
| Third |
| Fourth | |||||||||
Net revenue | $ | 423.0 | |
| $ | 454.9 | |
| $ | 438.0 | |
| $ | 532.2 | |
Gross margin | 121.5 | |
| 152.1 | |
| 126.2 | |
| 187.8 | | ||||
Earnings from continuing operations less net income (loss) attributable to noncontrolling interest | 11.9 | |
| 30.8 | |
| 10.7 | |
| 58.1 | | ||||
Net earnings from discontinued operations, net of tax | - | |
| - | |
| - | |
| - | | ||||
Net earnings attributable to Catalent | $ | 11.9 | |
| $ | 30.8 | |
| $ | 10.7 | |
| $ | 58.1 | |
|
|
|
|
|
|
|
| ||||||||
Earnings per share attributable to Catalent: |
|
|
|
|
|
|
| ||||||||
Basic |
|
|
|
|
|
|
| ||||||||
Earnings from continuing operations | $ | 0.10 | |
| $ | 0.25 | |
| $ | 0.09 | |
| $ | 0.47 | |
Net earnings | $ | 0.10 | |
| $ | 0.25 | |
| $ | 0.09 | |
| $ | 0.47 | |
Diluted |
|
|
|
|
|
|
| ||||||||
Earnings from continuing operations | $ | 0.09 | |
| $ | 0.24 | |
| $ | 0.09 | |
| $ | 0.46 | |
Net earnings | $ | 0.09 | |
| $ | 0.24 | |
| $ | 0.09 | |
| $ | 0.46 | |
(1) With the adoption of ASU 2016-09 , the previously filed quarterly data has been updated. The changes to Net Earnings/(loss) attributable to Catalent during the first, second and third quarter reflected above include additional income of $2.8 million, $0.1 million and $0.9 million, respectively. The changes to Basic and Diluted Earnings per share attributable to Catalent during the first, second and third quarter reflected above reflects a change of $0.02, $0, and $0.01, respectively.
108
Table of Contents
(Dollars in millions, except per share data) | Fiscal Year 2015, By Quarters | ||||||||||||||
First |
| Second |
| Third |
| Fourth | |||||||||
Net revenue | $ | 418.3 | |
| $ | 455.8 | |
| $ | 446.6 | |
| $ | 510.1 | |
Gross margin | 125.3 | |
| 156.1 | |
| 152.2 | |
| 181.7 | | ||||
Earnings/(loss) from continuing operations less net income (loss) attributable to noncontrolling interest | (19.9 | ) |
| 46.7 | |
| 31.5 | |
| 153.8 | | ||||
Net earnings/(loss) from discontinued operations, net of tax | 0.4 | |
| (0.2 | ) |
| - | |
| (0.1 | ) | ||||
Net earnings/(loss) attributable to Catalent | $ | (19.5 | ) |
| $ | 46.5 | |
| $ | 31.5 | |
| $ | 153.7 | |
|
|
|
|
|
|
|
| ||||||||
Earnings per share attributable to Catalent: |
|
|
|
|
|
|
| ||||||||
Basic |
|
|
|
|
|
|
| ||||||||
Earnings/(loss) from continuing operations | $ | (0.19 | ) |
| $ | 0.38 | |
| $ | 0.25 | |
| $ | 1.23 | |
Net earnings/(loss) | $ | (0.18 | ) |
| $ | 0.37 | |
| $ | 0.25 | |
| $ | 1.23 | |
Diluted |
|
|
|
|
|
|
| ||||||||
Earnings/(loss) from continuing operations | $ | (0.19 | ) |
| $ | 0.37 | |
| $ | 0.25 | |
| $ | 1.22 | |
Net earnings/(loss) | $ | (0.18 | ) |
| $ | 0.37 | |
| $ | 0.25 | |
| $ | 1.22 | |
109
Table of Contents
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in the Company's reports under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and communicated to the Company's management, including the Company's President and Chief Executive Officer, and the Company's Executive Vice President and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures. Any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. The Company's management, with the participation of the Company's President and Chief Executive Officer, and the Company's Executive Vice President and Chief Financial Officer, has evaluated the effectiveness of the design and operation of the Company's disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based upon that evaluation, the Company's President and Chief Executive Officer and the Company's Executive Vice President and Chief Financial Officer concluded that, as of June 30, 2016 , the Company's disclosure controls and procedures were effective to accomplish their objectives at the reasonable assurance level.
Management's Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. Our internal control over financial reporting is designed to provide reasonable assurances regarding the reliability of financial reporting and the preparation of our consolidated financial statements in accordance with U.S. GAAP.
Our internal control over financial reporting includes those policies and procedures that:
• | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; |
• | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
• | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because either conditions change or the degree of compliance with our policies and procedures may deteriorate.
Our management has assessed the effectiveness of our internal control over financial reporting as of June 30, 2016 . In making this assessment, management used the framework set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013) . Based on this assessment, our management concluded that our internal control over financial reporting was effective as of June 30, 2016 .
The effectiveness of the Company's internal control over financial reporting as of June 30, 2016 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report, which is included in Item 8. Financial Statements and Supplementary Data in this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There was no change in the Company's internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the Company's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting.
110
Table of Contents
ITEM 9B. | OTHER INFORMATION |
Iran Threat Reduction and Syria Human Rights Act of 2012
Pursuant to Section 219 of the Iran Threat Reduction and Syria Human Rights Act of 2012 ("ITRSHRA"), which added Section 13(r) of the Exchange Act, the Company hereby incorporates by reference herein Exhibit 99.1 of this report, which includes disclosures publicly filed and/or provided to Blackstone by Hilton Worldwide Holdings Inc., Travelport Worldwide Limited, and NCR Corporation each of which may be considered our affiliate.
111
Table of Contents
PART III
ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information concerning our Directors and Executive Officers, "Section 16(a) Beneficial Ownership Reporting Compliance," definitive shareholder communications with our Board of Directors, and corporate governance may be found in our Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed within 120 days after June 30, 2016 , the close of our fiscal year covered by this Annual Report on Form 10-K (the "Proxy Statement"). Such information is incorporated by reference.
ITEM 11. | EXECUTIVE COMPENSATION |
Information concerning executive compensation may be found in our Proxy Statement, which will be filed within 120 days after June 30, 2016 , the close of our fiscal year. Such information is incorporated herein by reference.
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information regarding security ownership of certain beneficial owners and management may be found in the Proxy Statement, which will be filed within 120 days after June 30, 2016 , the close of our fiscal year. Such information is incorporated herein by reference.
ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Information regarding certain relationships and related party transactions, and director independence may be found in our Proxy Statement, which will be filed within 120 days after June 30, 2016 , the close of our fiscal year. Such information is incorporated herein by reference.
ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Information regarding the fees paid to and services performed by our independent accountants may be found in our Proxy Statement, which will be filed within 120 days after June 30, 2016 , the close of our fiscal year. Such information is incorporated herein by reference.
112
Table of Contents
PART IV
ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a)(1) Financial Statements. The Financial Statements listed in the Index to Financial Statements, are filed under Item 8 of this Annual Report on Form 10-K.
(a)(2) Financial Statements Schedule.
Deferred Tax Assets - Valuation Allowance
(Dollars in millions) | Beginning Balance |
| Current Period (Charge) / Benefit |
| Deductions and Other |
| Ending Balance | ||||||||
Year ended June 30, 2014 |
|
|
|
|
|
|
| ||||||||
Tax Valuation Allowance | $ | (208.4 | ) |
| $ | (16.1 | ) |
| $ | 6.3 | |
| $ | (218.2 | ) |
|
|
|
|
|
|
|
| ||||||||
Year ended June 30, 2015 |
|
|
|
|
|
|
| ||||||||
Tax Valuation Allowance | $ | (218.2 | ) |
| $ | 107.7 | |
| $ | 28.1 | |
| $ | (82.4 | ) |
|
|
|
|
|
|
|
| ||||||||
Year ended June 30, 2016 |
|
|
|
|
|
|
| ||||||||
Tax Valuation Allowance | $ | (82.4 | ) |
| $ | (2.1 | ) |
| $ | 14.6 | |
| $ | (69.9 | ) |
(b) Exhibits.
The agreements and other documents filed as exhibits to this report are not intended to provide factual information or other disclosure other than with respect to the terms of the agreements or other documents themselves and you should not rely on them for that purpose. In particular, any representation or warranty made by us in these agreements or other documents were made solely within the specific context of the relevant agreement or document and may not describe the actual state of affairs as of the date they were made or at any other time.
Exhibit No. | Description | |
3.1 |
| Amended and Restated Certificate of Incorporation of Catalent, Inc. (incorporated by reference to Exhibit 3.1 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) |
|
| |
3.2 |
| Amended and Restated Bylaws of Catalent, Inc. (incorporated by reference to Exhibit 3.2 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) |
|
|
|
10.1 |
| Stockholders Agreement, dated as of August 5, 2014, between Catalent, Inc. and Blackstone Healthcare Partners L.L.C. (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) |
|
|
|
10.2 |
| Registration Rights Agreement, dated as of August 5, 2014, by and among Catalent, Inc. and certain of its stockholders (incorporated by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) |
|
| |
10.3 |
| Form of Severance Agreement between named executive officers and Catalent Pharma Solutions, Inc. (incorporated by reference to Exhibit 10.3 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 17, 2010, File No. 333-147871) † |
|
| |
10.4 |
| Offer Letter, dated August 25, 2009, between William Downie and Catalent Pharma Solutions, Inc. (incorporated by reference to Exhibit 10.4 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 4, 2012, File No. 333-147871) † |
|
| |
10.5 |
| Letter Agreement, dated November 18, 2010, between Catalent Pharma Solutions, Inc. and William Downie (incorporated by reference to Exhibit 10.6 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 4, 2012, File No. 333-147871) † |
|
|
|
113
Table of Contents
10.6 |
| Employment Agreement, dated as of October 11, 2011, and effective as of September 26, 2011, by and between Catalent Pharma Solutions, Inc. and Matthew Walsh (including Form of Restricted Stock Unit Agreement and Form of Nonqualified Stock Option Agreement) (incorporated by reference to Exhibit 10.42 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 4, 2012, File No. 333-147871) † |
|
| |
10.7 |
| Management Equity Subscription Agreement dated September 8, 2010 by and between Catalent, Inc. (formerly known as PTS Holdings Corp.) and Melvin D. Booth (incorporated by reference to Exhibit 10.7 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 17, 2010, File No. 333-147871) † |
|
|
|
10.8 |
| Amended and Restated Management Equity Subscription Agreement dated as of October 11, 2011 by and between Catalent, Inc. (formerly known as PTS Holdings Corp.) and Matthew Walsh (incorporated by reference to Exhibit 10.43 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 4, 2012, File No. 333-147871) † |
|
| |
10.9 |
| Form of Unit Subscription Agreement (incorporated by reference to Exhibit 10.12 to Catalent Pharma Solutions, Inc.'s Amendment No. 1 to the Registration Statement on Form S-4/A filed on March 3, 2008, File No. 333-147871) † |
|
| |
10.10 |
| Form of Management Equity Subscription Agreement (incorporated by reference to Exhibit 10.13 to Catalent Pharma Solutions, Inc.'s Amendment No. 1 to the Registration Statement on Form S-4/A filed on March 3, 2008, File No. 333-147871) † |
|
| |
10.11 |
| Form of Nonqualified Stock Option Agreement (executives) (incorporated by reference to Exhibit 10.14 to Catalent Pharma Solutions, Inc.'s Amendment No. 1 to the Registration Statement on Form S-4/A filed on March 3, 2008, File No. 333-147871) † |
|
| |
10.12 |
| Form of Nonqualified Stock Option Agreement (non-employee directors) (incorporated by reference to Exhibit 10.15 to Catalent Pharma Solutions, Inc.'s Amendment No. 1 to the Registration Statement on Form S-4/A filed on March 3, 2008, File No. 333-147871) † |
|
| |
10.13 |
| 2007 PTS Holdings Corp. Stock Incentive Plan (incorporated by reference to Exhibit 10.16 to Catalent Pharma Solutions, Inc.'s Registration Statement on Form S-4 filed on December 6, 2007, File No. 333-147871) † |
|
|
|
10.14 |
| Amendment No. 1 to the 2007 PTS Holdings Corp. Stock Incentive Plan, dated September 8, 2010 (incorporated by reference to Exhibit 10.16 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 17, 2010, File No. 333-147871) † |
|
|
|
10.15 |
| Amendment No. 2 to the 2007 PTS Holdings Corp. Stock Incentive Plan, dated June 25, 2013 (incorporated by reference to Exhibit 10.45 to Catalent, Inc.'s Amendment No. 1 to the Registration Statement on Form S-1/A as filed on September 28, 2014, File No. 333-193542) † |
|
|
|
10.16 |
| Form of Nonqualified Stock Option Agreement (executives) approved October 23, 2009 (incorporated by reference to Exhibit 10.1 to Catalent Pharma Solutions, Inc.'s Quarterly Report on Form 10-Q filed on February 12, 2010, File No. 333-147871) † |
|
| |
10.17 |
| Form of Nonqualified Stock Option Agreement Amendment (executives) approved October 23, 2009 (incorporated by reference to Exhibit 10.3 to Catalent Pharma Solutions, Inc.'s Quarterly Report on Form 10-Q filed on February 12, 2010), File No. 333-147871) † |
|
|
|
10.18 |
| Form of Nonqualified Stock Option Agreement (executives) approved June 25, 2013 (incorporated by reference to Exhibit 10.45 of Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 10, 2013, File No. 333-147871) † |
|
|
|
10.19 |
| Form of Nonqualified Stock Option Agreement (Chief Executive Officer) approved June 25, 2013 (incorporated by reference to Exhibit 10.46 of Catalent Pharma Solutions Inc.'s Annual Report on Form 10-K filed on September 10, 2013, File No. 333-147871) † |
|
|
|
10.20 |
| Form of Nonqualified Stock Option Agreement (John R. Chiminski) approved October 23, 2009 (incorporated by reference to Exhibit 10.4 to Catalent Pharma Solutions, Inc.'s Quarterly Report on Form 10-Q filed on February 12, 2010, File No. 333-147871) † |
|
|
|
10.21 |
| Form of Restricted Stock Unit Agreement (John R. Chiminski) approved October 23, 2009 (incorporated by reference to Exhibit 10.5 to Catalent Pharma Solutions, Inc.'s Quarterly Report on Form 10-Q filed on February 12, 2010, File No. 333-147871) † |
|
| |
114
Table of Contents
10.22 |
| Catalent Pharma Solutions, LLC Deferred Compensation Plan (incorporated by reference to Exhibit 10.19 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 28, 2009, File No. 333-147871) † |
|
| |
10.23 |
| First Amendment to the Catalent Pharma Solutions, LLC Deferred Compensation Plan (incorporated by reference to Exhibit 10.1 to Catalent Pharma Solutions, Inc.'s Quarterly Report on Form 10-Q filed on February 17, 2009, File No. 333-147871) † |
|
| |
10.24 |
| Second Amendment to the Catalent Pharma Solutions, LLC Deferred Compensation Plan (incorporated by reference to Exhibit 10.21 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 28, 2009, File No. 333-147871) † |
|
|
|
10.25 |
| Catalent, Inc. 2014 Omnibus Incentive Plan (incorporated by reference to Exhibit 10.3 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) † |
|
|
|
10.26 |
| Form of Stock Option Agreement for U.S. Employees (incorporated by reference to Exhibit 10.4 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) † |
|
|
|
10.27 |
| Form of Restricted Stock Unit Agreement for U.S. Employees (incorporated by reference to Exhibit 10.5 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) † |
|
|
|
10.28 |
| Form of Restricted Stock Unit Agreement for Non-Employee Directors (incorporated by reference to Exhibit 10.6 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) † |
|
|
|
10.29 |
| Form of Stock Option Agreement for Non-U.S. Employees (incorporated by reference to Exhibit 10.7 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) † |
|
|
|
10.30 |
| Form of Restricted Stock Unit Agreement for Non-U.S. Employees (incorporated by reference to Exhibit 10.8 to the Company's Current Report on Form 8-K filed on August 5, 2014, File No. 001-36587) † |
|
| |
10.31 |
| Amended and Restated Credit Agreement, dated as of May 20, 2014, relating to the Credit Agreement, dated as of April 10, 2007, as amended, among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc., as the administrative agent, collateral agent and swing line lender and other lenders as parties thereto (incorporated by reference to Exhibit 10.1 to Catalent Pharma Solutions, Inc.'s Current Report on Form 8-K filed on May 27, 2014, File No. 333-147871) |
|
| |
10.32 |
| Form of Performance Share Unit for U.S. Employees (incorporated by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on November 14, 2014, File No. 001-36587) † |
|
| |
10.33 |
| Form of Performance Share Unit for Non-U.S. Employees (incorporated by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q filed on November 14, 2014, File No. 001-36587)† |
|
| |
10.34 |
| Intellectual Property Security Agreement, dated as of April 10, 2007, among PTS Acquisition Corp., Cardinal Health 409, Inc., PTS Intermediate Holdings LLC, Certain Subsidiaries of Holdings Identified Therein and Morgan Stanley Senior Funding, Inc. (incorporated by reference to Exhibit 10.21 to Catalent Pharma Solutions, Inc.'s Registration Statement on Form S-4 filed on December 6, 2007, File No. 333-147871) |
|
| |
10.35 |
| Intellectual Property Security Agreement Supplement, dated as of July 1, 2008, to the Intellectual Property Security Agreement, dated as of April 10, 2007, among PTS Acquisition Corp., Cardinal Health 409, Inc., PTS Intermediate Holdings LLC, Certain Subsidiaries of Holdings Identified Therein and Morgan Stanley Senior Funding, Inc. (incorporated by reference to Exhibit 10.28 to Catalent Pharma Solutions, Inc.'s Annual Report on Form 10-K filed on September 29, 2008, File No. 333-147871) |
|
| |
10.36 |
| Amendment No. 1, dated December 1, 2014 to Amended and Restated Credit Agreement, dated as of May 20, 2014 among Catalent Pharma Solutions, Inc., PTS Intermediate Holdings LLC, Morgan Stanley Senior Funding, Inc., as the administrative agent, collateral agent and swing line lender and other lenders as parties thereto (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on December 2, 2014, File No. 001-36587) |
|
|
|
10.37 |
| Employment Agreement, dated October 22, 2014 by and among Catalent, Inc. and John R. Chiminski (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on October 24, 2014, File No. 001-36587) † |
|
|
|
115
Table of Contents
10.38 |
| Relocation agreement, dated November 18, 2010, between William Downie and Catalent Pharma Solutions, Inc. (incorporated by reference to Exhibit 10.38 to the Company's Annual Report on Form 10-K filed on September 2, 2015, File No. 001-36587) † |
|
|
|
10.39 |
| Offer letter, dated May 4, 2009, between Stephen Leonard and Catalent Pharma Solutions, Inc. (incorporated by reference to Exhibit 10.38 to the Company's Annual Report on Form 10-K filed on September 2, 2015, File No. 001-36587) † |
|
|
|
10.40 |
| Offer letter, dated October 6, 2014, between Steven Fasman and Catalent Pharma Solutions, Inc. † * |
|
|
|
10.41 |
| Form of Performance Share Unit Agreement for U.S. Employees for the performance period July 1, 2015 through June 30, 2018 † * |
|
|
|
10.42 |
| Form of Performance Share Unit Agreement for Non-U.S. Employees for the performance period July 1, 2015 through June 30, 2018 † * |
|
|
|
10.43 |
| Form of Management Incentive Plan for the fiscal year ended June 30, 2016 † * |
|
| |
10.44 |
| Catalent Pharma Solutions, Inc. Deferred Compensation Plan (formerly the Catalent Pharma Solutions, LLC Deferred Compensation Plan, as amended and restated effective January 1, 2016) † * |
|
|
|
12.1 |
| Statement Regarding Computation of Ratio of Earnings to Fixed Charges* |
|
|
|
21.1 |
| Subsidiaries of the Registrant * |
|
|
|
23.1 |
| Consent of Ernst & Young LLP * |
|
|
|
31.1 |
| Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended* |
|
|
|
31.2 |
| Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as amended* |
|
|
|
32.1 |
| Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002** |
|
|
|
32.2 |
| Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002** |
|
|
|
99.1 |
| Section 13(r) Disclosure* |
|
|
|
101.1 |
| The following materials are formatted in XBRL (eXtensible Business Reporting Language): (i) the Consolidated Statements of Operations, (ii) the Consolidated Statements of Comprehensive Income (Loss), (iii) the Consolidated Balance Sheets, (iv) the Consolidated Statement of Changes in Shareholders' Equity (Deficit), (v) the Consolidated Statements of Cash Flows and (vi) Notes to Consolidated Financial Statements |
|
|
|
* Filed herewith
** Furnished herewith
† Represents a management contract, compensatory plan or arrangement in which directors and/or executive officers are eligible to participate.
116
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| CATALENT, INC. | ||
|
|
|
| |
Date: | August 29, 2016 | By: |
| /s/ STEVEN L. FASMAN |
|
|
|
| Steven L. Fasman |
|
|
|
| Senior Vice President & General Counsel and Secretary |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
| Title | Date |
|
|
|
|
/s/ JOHN R. CHIMINSKI |
| President & Chief Executive Officer (Principal Executive Officer) and Director | August 29, 2016 |
John R. Chiminski |
|
| |
|
|
|
|
/s/ CHINH E. CHU |
| Chairman of the Board and Director | August 29, 2016 |
Chinh E. Chu |
|
|
|
|
|
|
|
/s/ MELVIN D. BOOTH |
| Director | August 29, 2016 |
Melvin D. Booth |
|
|
|
|
|
|
|
/s/ J. MARTIN CARROLL |
| Director | August 29, 2016 |
J. Martin Carroll |
|
|
|
|
|
|
|
/s/ ROLF CLASSON |
| Director | August 29, 2016 |
Rolf Classon |
|
|
|
|
|
|
|
/s/ GREGORY T. LUCIER |
| Director | August 29, 2016 |
Gregory T. Lucier |
|
|
|
|
|
|
|
/s/ BRUCE MCEVOY |
| Director | August 29, 2016 |
Bruce McEvoy |
|
|
|
|
|
|
|
/s/ DONALD E. MOREL |
| Director | August 29, 2016 |
Donald E. Morel |
|
|
|
|
|
|
|
/s/ JAMES QUELLA |
| Director | August 29, 2016 |
James Quella |
|
|
|
|
|
|
|
/s/ JACK STAHL |
| Director | August 29, 2016 |
Jack Stahl |
|
|
|
|
|
|
|
/s/ MATTHEW M. WALSH |
| Executive Vice President & Chief Financial Officer | August 29, 2016 |
Matthew M. Walsh |
| (Principal Financial Officer and Principal Accounting Officer) |
|
117
