UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
or
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 000-50679
CORCEPT THERAPEUTICS INCORPORATED
(Exact Name of Corporation as Specified in Its Charter)
Delaware |
| 77-0487658 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification No.) |
149 Commonwealth Drive
Menlo Park, CA 94025
(Address of principal executive offices) (zip code)
(650) 327-3270
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12 (b) of the Act:
Title of Each Class: |
| Name of Each Exchange on which Registered: |
Common Stock, $0.001 par value |
| The NASDAQ Capital Market |
Securities registered pursuant to Section 12 (g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15 (d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference to Part III of this Form 10-K or any amendment to this Form 10‑K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ |
|
| Accelerated filer | ☒ |
Non-accelerated filer | ☐ |
| (Do not check if a smaller reporting company) | Smaller reporting company | ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of voting and non-voting common equity held by non-affiliates of the Registrant was $427,665,971 as of June 30, 2016 based upon the closing price on the NASDAQ Capital Market reported for such date. This calculation does not reflect a determination that certain persons are affiliates of the Registrant for any other purpose.
On February 28, 2017 there were 112,942,391 shares of common stock outstanding at a par value of $0.001 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant's definitive proxy statement for its 2016 Annual Meeting of Stockholders are incorporated by reference in Items 10, 11, 12, 13 and 14 of Part III.
TABLE OF CONTENTS
Form 10-K
For the year ended December 31, 2016
|
|
|
| Page |
|
|
|
|
|
|
| PART I |
|
|
|
|
|
|
|
ITEM 1. |
| Business |
| 1 |
|
|
|
|
|
ITEM 1A. |
| Risk Factors |
| 11 |
|
|
|
|
|
ITEM 1B. |
| Unresolved Staff Comments |
| 29 |
|
|
|
|
|
ITEM 2. |
| Properties |
| 30 |
|
|
|
|
|
ITEM 3. |
| Legal Proceedings |
| 30 |
|
|
|
|
|
ITEM 4. |
| Mine Safety Disclosures |
| 30 |
|
|
|
|
|
|
| PART II |
|
|
|
|
|
|
|
ITEM 5. |
| Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
| 31 |
|
|
|
|
|
ITEM 6. |
| Selected Financial Data |
| 33 |
|
|
|
|
|
ITEM 7. |
| Management's Discussion and Analysis of Financial Condition and Results of Operations |
| 35 |
|
|
|
|
|
ITEM 7A. |
| Quantitative and Qualitative Disclosures About Market Risk |
| 43 |
|
|
|
|
|
ITEM 8. |
| Financial Statements and Supplementary Data |
| 44 |
|
|
|
|
|
ITEM 9. |
| Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
| 44 |
|
|
|
|
|
ITEM 9A. |
| Controls and Procedures |
| 44 |
|
|
|
|
|
ITEM 9B. |
| Other Information |
| 46 |
|
|
|
|
|
|
| PART III |
|
|
|
|
|
|
|
ITEM 10. |
| Directors, Executive Officers and Corporate Governance |
| 47 |
|
|
|
|
|
ITEM 11. |
| Executive Compensation |
| 47 |
|
|
|
|
|
ITEM 12. |
| Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
| 47 |
|
|
|
|
|
ITEM 13. |
| Certain Relationships and Related Transactions, and Director Independence |
| 47 |
|
|
|
|
|
ITEM 14. |
| Principal Accounting Fees and Services |
| 47 |
|
|
|
|
|
|
| PART IV |
|
|
|
|
|
|
|
ITEM 15. |
| Exhibits, Financial Statement Schedules |
| 48 |
|
|
|
|
|
|
| Signatures and Power of Attorney |
| 52 |
PAR T I
This Annual Report on Form 10-K (Form 10-K) contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (Exchange Act), and Section 27A of the Securities Act of 1933, as amended (Securities Act). All statements contained in this Form 10-K, other than statements of historical fact, are forward-looking statements. When used in this report or elsewhere by management, the words "believe," "anticipate," "intend," "plan," "estimate," "expect," "may," "will," "should, "would," "could," "seek" and similar expressions are forward-looking statements. Such forward-looking statements are based on current expectations. The absence of these words does not mean that a statement is not forward-looking. Forward-looking statements made in this Form 10-K include, but are not limited to, statements about:
| • | our ability to manufacture, market and sell Korlym ® (mifepristone) 300 mg Tablets; |
| • | our estimates regarding enrollment in and the completion dates of our clinical trials and the anticipated results of these trials; |
| • | the progress and timing of our research and development programs and the regulatory activities associated with them; |
| • | our ability to realize the benefits of Orphan Drug designation for Korlym in the United States; |
| • | our estimates for future performance, including revenue and profits; |
| • | the timing of the market introduction of future product candidates, including new uses for Korlym and any compound in our families of selective cortisol modulators; |
| • | our ability to manufacture, market, commercialize and achieve market acceptance for our future product candidates; |
| • | uncertainties associated with obtaining and enforcing patents; and |
| • | estimates regarding our capital requirements. |
Forward-looking statements are not guarantees of future performance. They involve risks and uncertainties. Actual events or results may differ materially from those discussed in the forward-looking statements for many reasons. For a more detailed discussion of the risks and uncertainties that may affect the accuracy of our forward-looking statements, see the "Risk Factors," "Overview" and "Liquidity and Capital Resources" sections of the "Management's Discussion and Analysis of Financial Condition and Results of Operations" section of this Form 10-K. Forward-looking statements reflect our view only as of the date of this report. Except as required by law, we undertake no obligation to update any forward-looking statement. You should carefully consider the other reports and documents that we file with the Securities and Exchange Commission (SEC).
Unless stated otherwise, all references in this document to "we," "us," "our," "Corcept," the "Company," "our company" and similar designations refer to Corcept Therapeutics Incorporated.
ITEM 1. BUSINESS
Overview
We are a pharmaceutical company engaged in the discovery, development and commercialization of drugs that treat severe metabolic, oncologic and psychiatric disorders by modulating the effects of cortisol. Elevated levels and abnormal release patterns of cortisol are implicated in a broad range of human disorders. Since our inception in 1998, we have been developing mifepristone, a compound that modulates the effects of cortisol by acting as a competitive antagonist at the glucocorticoid receptor (GR). We have also discovered three structurally distinct series of proprietary, selective cortisol modulators, all of which share mifepristone's affinity for GR but, unlike mifepristone, do not bind to the progesterone receptor and so do not cause effects associated with progesterone receptor affinity. Development of the lead compounds from these series is in progress .
1
In 2012, the United States Food and Drug Administration (FDA) approved Korlym ® (mifepristone) 300 mg T ablets as a once-daily oral medication for the treatment of hyperglycemia secondary to hypercortisolism in adult p atients with endogenous Cushing syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or are not candidates for surgery .
We are conducting two clinical trials of our proprietary selective cortisol modulator, CORT125134. One trial is investigating CORT125134 as a potential treatment for patients with Cushing syndrome. The second trial is investigating the combination of CORT125134 and nab-paclitaxel (Celgene Corporation's Abraxane ® ) to treat patients with solid-tumor cancers. These trials are enrolling patients.
We plan to begin clinical trials of two other selective cortisol modulators in 2017.
The Role of Cortisol in Disease
Cortisol is a steroid hormone that plays a significant role in the way the body reacts to stressful conditions. It influences metabolism and the immune system and contributes to emotional stability. It is essential for survival. Insufficient cortisol activity may lead to dehydration, hypotension, shock, fatigue, low resistance to infection, trauma, stress and hypoglycemia. Excessive cortisol activity may lead to a suppressed immune response, impaired glucose tolerance, diabetes, obesity, fatty liver disease, depressed mood, psychosis, wasting of the arms and legs, edema, fatigue, hypertension and other problems. Pre-clinical and clinical data suggest that cortisol may reduce a patient's immune response to oncogenesis and shield certain cancer cells from the apoptotic effects of chemotherapy.
The challenge in regulating excessive levels of cortisol is that destroying the ability of the body to make cortisol can cause serious harm. An effective medication must modulate cortisol's effects without suppressing them below normal levels or disrupting the body's normal cortisol rhythm, in which cortisol levels rise at awakening and decrease during the day. The action of cortisol can effectively be modulated by the use of compounds that compete with cortisol as it attempts to bind to GR. Mifepristone, the active ingredient in Korlym, is a competitive GR antagonist, as are Corcept's proprietary compounds.
Because mifepristone works by reducing the binding of excess cortisol to GR, it can modulate the effects of abnormal levels and release patterns of cortisol without compromising cortisol's necessary, normal functions and rhythms. However, mifepristone also binds to the progesterone receptor and thereby terminates pregnancy and sometimes causes other side effects, including irregular vaginal bleeding. Our selective cortisol modulators block GR as potently as mifepristone does, but have no affinity for the progesterone receptor and so do not cause progesterone receptor-related side effects.
Cushing Syndrome
Background. Cushing syndrome is caused by prolonged exposure of the body's tissues to high levels of cortisol. It is relatively uncommon and most often affects adults aged 20 to 50. An estimated 10 to 15 of every one million people are newly diagnosed with this syndrome each year, resulting in approximately 3,000 new patients and an estimated total of 20,000 patients with Cushing syndrome in the United States .
Symptoms vary, but most people with Cushing syndrome have one or more of the following manifestations: high blood sugar, diabetes, high blood pressure, upper body obesity, rounded face, increased fat around the neck, thinning arms and legs, severe fatigue and weak muscles. Irritability, anxiety, cognitive disturbances and depression are also common. Cushing syndrome can affect every organ system in the body and can be lethal if not treated. The preferred treatment for Cushing syndrome patients is surgery, which, if successful, can cure the disease. Depending on the type of tumor, surgery can result in a range of complications and has varying rates of success. In approximately half of the patients, surgery is not successful because the tumor cannot be located or removed completely.
Korlym to Treat Patients with Cushing Syndrome . We have received Orphan Drug designation from the FDA for Korlym in the treatment of patients with endogenous Cushing syndrome. Drugs that receive Orphan Drug
2
designation receive seven years of marketing exclusivity for the approved indication, as well as tax credits for clinical trial costs, marketing application filing fee waivers and assistance from the FDA in the drug development process.
We first made Korlym available to patients on a commercial basis in April 2012. We sell Korlym using experienced sales representatives, who target U.S. endocrinologists who care for a large portion of the patients with Cushing syndrome. In addition, we have a field-based force of medical science liaisons . We also reach patients directly through web-based initiatives and interactions with patient groups. Because a large percentage of the people who suffer from Cushing syndrome remain undiagnosed or are inadequately treated, we have developed and continue to refine and expand programs to educate the medical community and patients about diagnosis of this syndrome and to increase awareness regarding the role of cortisol modulators to treat the disease.
We use a specialty pharmacy and a specialty distributor to distribute Korlym and provide logistical support. We have retained a vendor to help patients with the reimbursement process and to administer our financial assistance programs for uninsured or under-insured patients. We also donate money to independent charitable foundations. These organizations, along with our own programs, help us ensure that no Cushing syndrome patient is denied access to Korlym for financial reasons.
CORT125134 to Treat Patients with Cushing Syndrome. We are enrolling patients in a Phase 2 trial of our proprietary, selective cortisol modulator, CORT125134, to treat patients with Cushing syndrome. CORT125134 shares Korlym's affinity for GR. Data from the compound's Phase 1 trial showed that it potently modulates the effects of the steroid prednisone, a commonly-used GR agonist. Modulating the effect of prednisone is important because it is a strong surrogate for Korlym's modulation of cortisol – the essential quality of an effective treatment for patients with Cushing syndrome. Pharmacokinetic data indicate that CORT125134 is suitable for once-daily oral dosing. We expect to have data from this trial by the end of 2017.
FKBP5 Gene Expression . We are developing a CLIA-validated assay to measure expression of the gene FKBP5 , which is stimulated by cortisol activity at GR. Our hypothesis is that FKBP5 expression increases in patients with Cushing syndrome and falls as their disease is treated. If our hypothesis is correct, our assay would allow physicians to measure the degree to which their patients suffer from excess cortisol activity – the cause of Cushing syndrome – which would help them more easily identify patients with the disease and better treat those already in their care.
Oncology
There is substantial in vitro, in vivo and clinical evidence that cortisol's activity allows certain solid-tumor cancers to resist treatment. In some cancers, cortisol activity promotes tumor growth. Cortisol also stimulates genes that retard cellular apoptosis.
Our oncology development program also seeks to exploit a second mechanism. Cortisol suppresses the body's immune response. Suppression of the immune response is often beneficial, as it lessens the frequency of autoimmune diseases. However, activating, not suppressing, the body's immune system is beneficial in fighting certain cancers . Our hypothesis is that adding a cortisol modulator to a treatment regimen will help the patient's immune system combat the disease .
A range of tumor-types express GR and are potential targets for cortisol modulation therapy, among them triple-negative breast, ovarian, castration-resistant prostate, cervical and pancreatic cancer, as well as sarcoma and melanoma .
Korlym to Treat Patients with Solid-Tumor Cancers. In December 2016, we announced the results of our Phase 1/2 trial of Korlym in combination with eribulin (Eisai's Inc.'s drug, Halaven ® ) to treat patients with metastatic triple-negative breast cancer. The trial studied 21 patients with GR positive tumors, one with GR negative tumors and one with tumors whose GR status was not known. As determined using the Response Evaluation Criteria in Solid Tumors (RECIST), efficacy results were as follows: four patients exhibited a partial response, defined as a 30 percent or greater reduction in tumor size; eight had stable disease; and 11 had progressive
3
disease. Six patients achieved progression-f ree survival (PFS) longer than the upper bound of the 95% confidence interval for PFS (15 weeks) in patients receiving Halav e n ® monotherapy in a comparable population (Aogi et al., Annals of Oncology 23: 1441-1448, 2012). Median PFS in the trial was 11.1 w eeks – compared to 7.2 weeks in the Halaven monotherapy study reported by Aogi. We believe that the addition of Korlym to chemotherapy warrants further study, such as the double-blind, placebo-controlled, multicenter, University of Chicago-led trial descri bed above that Celgene is funding .
Korlym to Treat Patients with Triple-Negative Breast Cancer and Castration-Resistant Prostate Cancer . Investigators at the University of Chicago have initiated a double-blind, placebo-controlled, multicenter Phase 2 study of Korlym in combination with Celgene's drug Abraxane to treat 64 patients with advanced, GR-positive triple-negative breast cancer. Celgene is funding the trial. We are providing Korlym. University of Chicago investigators are also leading a controlled, multicenter Phase 2 study of Korlym combined with the androgen deprivation agent enzalutamide (Astellas Pharma Inc.'s drug, Xtandi ® ) versus Xtandi monotherapy to treat 84 patients with metastatic, castration-resistant prostate cancer. The investigators' hypothesis is that adding cortisol modulation to androgen deprivation therapy will better suppress tumor growth. The Department of Defense and the Prostate Cancer Foundation are funding the trial. Astellas is providing Xtandi. We are providing Korlym .
We have exclusively licensed patents from the University of Chicago covering the use of cortisol modulators in combination with anti-cancer agents to treat triple-negative breast cancer and with androgen deprivation agents to treat castration-resistant prostate cancer.
CORT125134 to Treat Patients with Solid-Tumor Cancers . We are conducting a Phase 1/2 trial of Abraxane (nab-paclitaxel) in combination with CORT125134 to treat any solid-tumor cancer suitable for treatment with Abraxane. Once we identify a recommended dose of this combination, we will open 20-patient cohorts to test the combination's efficacy in one or more solid-tumor cancers. Our likely initial targets will be triple-negative breast cancer and ovarian cancer. Other possible indications include pancreatic cancer, cervical cancer and sarcoma. We may choose to open additional dose-finding cohorts to study CORT125134 in combination with different companion therapeutic agents, including immunotherapy, to treat other solid-tumor cancers .
Development of Our Other Selective Cortisol Modulators
CORT125134 is the lead compound in our portfolio of proprietary selective cortisol modulators, which consists of three structurally distinct series. All of these compounds, like Korlym, potently block GR but do not block the progesterone, estrogen or androgen receptors. In addition to our findings with CORT125134, several of our new compounds have demonstrated positive results in animal or in vitro models that test cortisol modulation. We are advancing the most promising of these compounds towards the clinic and expect to begin clinical trials of CORT118335 and CORT125281 in 2017 . CORT118335 is a potential medication for fatty-liver disease, anti-psychotic-induced weight gain and other metabolic disorders. CORT125281 is a candidate for the treatment of castration-resistant prostate cancer (in combination with an androgen-deprivation agent such as Xtandi) and other indications.
The United States Patent & Trademark Office (USPTO) and the European Patent Office (EPO) have issued to us composition of matter patents related to our selective cortisol modulators. In addition, we own or have exclusively licensed patents for the use of all cortisol modulators (including Korlym) in a broad range of disorders. See "Business – Intellectual Property."
We intend to continue our discovery research program with the goal of identifying new selective cortisol modulators, to manufacture and conduct pre-clinical development of one or more of these compounds and to study the most promising of them in humans.
Studies by Independent Investigators
We have, for many years, sought to advance our understanding of cortisol modulation's therapeutic potential by supporting the work of independent academic investigators. These researchers have studied the utility of our proprietary selective cortisol modulators in pre-clinical studies in a wide range of disorders, including post-traumatic
4
stress disorder , alcoholism , Alzheimer's disease , ALS , muscular dystrophy , Cushing syndrome, metabolic syndrome, fatty liver disease , ovari an cancer, castration-resistant prostate cancer and triple-negative breast cancer .
Clinical Trial Agreements
Some of our clinical trials are conducted through the use of clinical research organizations (CROs). Our Phase 2 trial of CORT125134 for the treatment of patients with Cushing syndrome is being conducted under an agreement with Chiltern International Limited (Chiltern). This agreement may be terminated by us upon 60-days written notice to Chiltern or sooner if the parties mutually agree.
Research and Development Spending
We incurred $23.8 million, $15.4 million and $18.4 million of research and development expenses in the years ended December 31, 2016, 2015 and 2014, which accounted for 33%, 29% and 34%, respectively of our total operating expenses in those years.
Manufacturing Korlym
We do not have manufacturing capabilities and intend to continue to rely on experienced contract manufacturers to produce Korlym and our product candidates. We have a long-term agreement with one contract manufacturer, Produits Chimiques Auxiliaires et de Synthese SA (PCAS), to produce mifepristone, the active pharmaceutical ingredient (API) for Korlym, pursuant to which we agree to purchase a minimum percentage of our mifepristone requirements. The initial term of the agreement is five years, with an automatic extension of one year, unless either party gives 12-months prior written termination notice. We have the right to terminate the agreement if PCAS is unable to manufacture mifepristone for nine consecutive months.
We have one tablet manufacturer for Korlym – Alcami Corporation (formerly known as AAI Pharma Services Corp., or AAI). In April 2014, we entered into an agreement with Alcami for the manufacture and packaging of Korlym tablets. The initial term of this agreement is three years, with consecutive automatic extensions of two years, unless either party gives written termination notice (in the case of Alcami, 18 months prior to the end of the applicable term; in our case, 12 months prior to the end of the applicable term). We have the right to terminate the agreement if (i) Alcami is unable to manufacture our product for four consecutive months or (ii) our product is withdrawn from the market. We have no minimum purchase obligations under this agreement.
Competition for Korlym
Korlym competes with established treatments, including surgery, radiation and other medications, including "off-label" uses of drugs such as ketoconazole, an anti-fungal medication. Korlym also competes with Novartis' drug, Signifor ® (pasireotide) Injection, which the FDA approved in December 2012 for the treatment of adult patients with Cushing disease who are not candidates for pituitary surgery or for whom surgery did not work. (Cushing disease is a subset of Cushing syndrome that afflicts approximately 70 percent of patients with Cushing syndrome.)
Korlym may also experience competition from compounds under development for Cushing syndrome. For example, Strongbridge Biopharma plc has received Orphan Drug designation in the United States and the EU for the use of levoketoconazole, a chiral form of ketoconazole, to treat Cushing syndrome and has begun a Phase 3 clinical trial in Europe and the United States for this indication.
Intellectual Property
Patents and other proprietary rights are important to our business. It is our policy to seek patent protection for our inventions and to rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position.
5
Mifepristone. The composition of matter patent covering mifepristone has expired. The only other FDA-approved use of mifepristone is to terminate pregnancy. The FDA has imposed significa nt restrictions on the use of mifepristone to terminate pregnancy. To protect our market for Korlym we plan to rely on (1) the exclusive marketing rights conferred as a benefit of Orphan Drug designation in the United States, (2) the restrictions imposed b y the FDA on the use of mifepristone to terminate pregnancy, (3) the different patient populations, administering physicians and treatment settings between the use of mifepristone to terminate pregnancy and to treat Cushing syndrome and (4) our method of u se patents described below.
Oncology. We have an exclusive license agreement with the University of Chicago to patents covering the use of all cortisol modulators, including mifepristone, in the treatment of triple-negative breast cancer (in combination with anti-cancer agents) and castration-resistant prostate cancer (in combination with androgen deprivation agents). See "Business – License Agreements."
Other Method of Use Patents. We own issued U.S. patents for the use of cortisol modulators in the treatment of mild cognitive impairment, the prevention and treatment of stress disorders, improving the therapeutic response to electroconvulsive therapy, the treatment of delirium, the treatment of catatonia, the treatment of psychosis with Interferon-Alpha therapy, inhibiting cognitive deterioration in adults with Down's Syndrome, the treatment of weight gain following treatment with antipsychotic medication, the treatment of gastroesophageal reflux disease, the treatment of migraine headaches, the treatment of neurological damage in premature infants, and the treatment of diseases using combination steroid and GR antagonist therapy. We also own a method of use patent for optimizing mifepristone levels in plasma serum in patients suffering from mental disorders, including the mental disorders seen in Cushing syndrome. The expiration dates of these patents and their foreign counterparts range from 2020 to 2034.
In addition, we have six U.S. method-of-use applications covering certain cortisol modulators, including the treatment of patients suffering from mental disorders by optimizing mifepristone absorption, and the treatment of patients suffering from muscular dystrophy and from ALS.
We estimate that the expiration dates of the patents that could issue from these applications and their foreign counterparts range from 2029 to 2036.
Composition of Matter Patents Covering Our Proprietary, Selective Cortisol Modulators. We have eight U.S. composition of matter patents containing claims relating to three structurally distinct series of next-generation cortisol modulators. Four of these patents have issued in Europe, with an additional U.S. application pending. The expiration dates of these patents and their foreign counterparts range from 2026 to 2033.
We have also filed, where we deemed appropriate, foreign patent applications corresponding to our U.S. patents and applications. We cannot assure you that any of our patent applications will result in the issuance of patents, that any issued patent will include claims of the breadth sought in these applications, or that competitors or other third-parties will not successfully challenge or circumvent our patents if they are issued.
We believe that our patents are valid and that we do not currently infringe any third-party's patents or other proprietary rights, and we are not obligated to pay royalties relating to the use of intellectual property to any third-party other than Stanford University and the University of Chicago.
License Agreements
We have exclusively licensed three issued U.S. patents from Stanford University for the use of cortisol modulators, including mifepristone, in the treatment of psychotic depression, cocaine-induced psychosis and early dementia, including early Alzheimer's disease. We are required to make milestone payments and pay royalties to Stanford University on sales of products commercialized under these patents. Milestone payments are creditable against future royalties. Our license will end upon expiration of the related patents in 2018 and 2019 or upon notification by us to Stanford.
We have also exclusively licensed from the University of Chicago two issued U.S. patents for the use of cortisol modulators in the treatment of triple-negative breast cancer, and a second patent family consisting of an
6
issued U.S. patent and applications in Europe having claims directed to the use of cortisol modulators t o treat castration-resistant prostate cancer. We are required to pay the University of Chicago customary milestone fees and royalties on revenue from products commercialized under the issued patents or patents that may issue pursuant to the pending applica tions. Our license will end upon expiration of the related patents in 2031 and 2033 or upon notification by us to the University of Chicago.
Government Regulation
Prescription pharmaceutical products are subject to extensive pre- and post-approval regulation, including regulations that govern the testing, manufacturing, safety, efficacy, labeling, storage, record keeping, advertising, and promotion of the products under the Federal Food, Drug and Cosmetic Act. All of our product candidates require regulatory approval by government agencies prior to commercialization. The process required by the FDA before a new drug may be marketed in the United States generally involves the following: completion of preclinical laboratory and animal testing; submission of an Investigational New Drug ("IND"), which must become effective before clinical trials may begin; performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug's intended use; and approval by the FDA. The process of complying with these and other federal and state statutes and regulations involves significant time and expense.
Preclinical studies are generally conducted in laboratory animals to evaluate the potential safety and the efficacy of a product. Drug developers submit the results of preclinical studies to the FDA as a part of an IND, which must be approved before beginning clinical trials in humans. If it is anticipated that the clinical trial will be conducted in Europe, a Clinical Trial Authorization (CTA) must be submitted and approved by the appropriate European regulatory agency prior to the commencement of the study. Typically, human clinical trials are conducted in three sequential phases that may overlap.
| • | Phase 1 . Clinical trials are conducted with a small number of subjects to determine the early safety profile and pharmacokinetics of the product candidate in human volunteers, and to provide early information about drug effectiveness and/or activity. |
| • | Phase 2 . Clinical trials are conducted with groups of patients afflicted with the targeted disease to determine preliminary efficacy, optimal dosages and expanded evidence of safety. |
| • | Phase 3 . Large-scale, multi-center, trials are conducted with patients afflicted with a target disease to establish the overall risk/benefit ratio of the drug and to demonstrate with substantial evidence the efficacy and safety of the product. |
The FDA and the institutional review boards associated with clinical trial sites closely monitor the progress of clinical trials conducted in the United States and may reevaluate, alter, suspend or terminate the trial at any time for various reasons, including a belief that the subjects are being exposed to unacceptable risks. The FDA may also require that additional studies be conducted.
After Phase 3 trials are completed, drug developers submit the results of preclinical studies, clinical trials, formulation studies and data supporting manufacturing to the FDA in the form of a New Drug Applications ("NDA") for approval to begin commercial sales. The FDA reviews an NDA upon submission, and may request additional information rather than accept an NDA for filing. If the FDA accepts an NDA for filing, it may grant marketing approval, request additional information or deny the application if it determines that the application does not meet the criteria for approval. Once an NDA has been accepted for filing, by law the FDA has 180 days to examine the application and respond to the applicant. However, the review process is often significantly extended by FDA requests for additional information or clarification. Under the Prescription Drug User Fee Act, the FDA has a goal of responding to NDAs within ten months of the filing date for standard review, and six months for priority review if a sponsor shows that its drug candidate provides a significant benefit compared to marketed drugs. FDA approvals may not be granted on a timely basis, or at all.
If the FDA approves an NDA, the subject drug becomes available for physicians to prescribe in the United States. The FDA may withdraw the product approval if compliance with regulatory standards is not maintained. The drug developer must submit periodic reports to the FDA. Adverse patient experiences with the product must be reported to the FDA and could result in the imposition of marketing restrictions through labeling changes or removal
7
of the product from the market . Product approvals may be withdrawn if problems with safety or efficacy occur. In addition, the FD A may require post-marketing studies, referred to as Phase 4 studies, to monitor the effect of approved products, and may limit further marketing of the product based on the results of these post-approval studies.
Facilities involved in the manufacture of drugs are subject to periodic inspection by the FDA and other authorities where applicable, and must comply with FDA-mandated current Good Manufacturing Practices regulations (cGMP). Failure to comply with statutory and regulatory requirements subjects the manufacturer to possible legal or regulatory action, such as suspension of manufacturing, or the seizure or recall of a product.
The FDA imposes complex regulations on entities that advertise and promote pharmaceuticals. These include standards and regulations for direct-to-consumer advertising, off-label promotion, and industry-sponsored scientific and educational activities. The FDA has broad enforcement authority under the Federal Food, Drug and Cosmetic Act. Failure to abide by its regulations can result in penalties including the issuance of a warning letter directing a company to correct deviations from FDA standards, a requirement that future advertising and promotional materials be pre-cleared by the FDA, and state and federal civil and criminal penalties.
In addition to studies requested by the FDA after approval, a drug developer may conduct other preclinical and clinical trials investigating use of the approved compound to treat additional indications. Data supporting the use of a drug for new indications must be approved by the FDA before the drug can be marketed for these indications.
Orphan Drug Designation
We have received Orphan Drug designation for Korlym for the treatment of endogenous Cushing syndrome in the United States. Orphan designation qualifies the sponsor of the product for the tax credit and marketing incentives of the Orphan Drug Act, including seven years of exclusive marketing rights for the specific drug for the orphan indication, if it receives the first regulatory approval for that indication, with limited exceptions. A marketing application for a prescription drug product that has been designated as a drug for a rare disease or condition is not subject to a prescription drug user fee unless the application includes an indication for other than a rare disease or condition. Orphan Drug designation does not prevent competitors from developing or marketing different drugs for an indication. It also does not convey an advantage in, or shorten the duration of, the review and approval process for a drug.
Marketing Approvals Outside the United States
We are not seeking regulatory approval to market Korlym outside the United States. If we do so, we (or our potential future partners) will have to complete an approval process similar to the U.S. approval process in foreign target markets before we can distribute our product candidates in those countries. The approval procedure and the time required for approval vary from country to country and can involve additional preclinical and clinical trials. Foreign approvals may not be granted on a timely basis, or at all. Regulatory approval of pricing is required in most countries other than the United States. The prices approved may be too low to generate an acceptable return.
Coverage and Reimbursement
Sales of our products will depend, in part, on the extent to which they will be covered by government health care programs and commercial insurance and managed healthcare organizations. Although this trend has not had a material impact on the amount or timing of our revenues, these third-party payors are increasingly limiting coverage and reducing reimbursements for medical products and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could limit our net revenue and results. Decreases in third-party reimbursement for our products or a decision by a third-party payor to not cover our products could reduce physician utilization of our products and have a material adverse effect on our sales, results of operations and financial condition.
8
Other Healthcare Laws
We are subject to healthcare regulation and enforcement by the federal government and the states and foreign governments in which we conduct our business. These laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security and physicians' sunshine laws and regulations.
The federal Anti-Kickback Statute prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs. The Anti-Kickback Statute is subject to evolving interpretations. In the past, the government has enforced the Anti-Kickback Statute to reach large settlements with healthcare companies based on sham consulting and other financial arrangements with physicians. Further, the recently enacted Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the PPACA, among other things, amends the intent requirement of the federal Anti-Kickback Statute and the criminal statute governing healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of these statutes or specific intent to violate them. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act or federal civil money penalties statute. The majority of states also have anti-kickback laws which establish similar prohibitions and in some cases may apply to items or services reimbursed by any third-party payor, including commercial insurers.
Additionally, the civil False Claims Act prohibits knowingly presenting or causing the presentation of a false, fictitious or fraudulent claim for payment to the U.S. government. Actions under the False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical and biotechnology companies in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-billion dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. We expect that the government will continue to devote substantial resources to investigating healthcare providers' and manufacturers' compliance with applicable fraud and abuse laws.
In addition, there has been increased federal and state regulation of payments made to physicians and other healthcare providers. The PPACA, among other things, imposes new reporting requirements on drug manufacturers for payments made by them to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year (or up to an aggregate of $1 million per year for "knowing failures"), for all payments, transfers of value or ownership or investment interests that are not timely, accurately and completely reported in an annual submission. Drug manufacturers must report such payments to the government by the 90th day of each calendar year. Certain states also mandate implementation of commercial compliance programs, impose restrictions on drug manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to physicians.
The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance and/or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may violate one or more of the requirements. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Employees
We are managed by a core group of experienced pharmaceutical executives. We also enlist the expertise of associates and advisors with extensive pharmaceutical development experience.
9
As of December 31, 2016, we h ad 103 employees. Six of our employees have M.D.s. We consider our employee relations to be good. None of our employees are covered by a collective bargaining agreement.
About Corcept
We were incorporated in the State of Delaware on May 13, 1998. Our registered trademarks include Corcept ® , Korlym ® and CORLUX ® . Corluxin ® is a registered trademark in the EU; the application for this trademark is pending in the United States. Other service marks, trademarks and trade names referred to in this document are the property of their respective owners.
Available Information
We are subject to the information requirements of the Securities Exchange Act of 1934, as amended, and we therefore file periodic reports, proxy statements and other information with the SEC relating to our business, financial statements and other matters. The reports, proxy statements and other information we file may be inspected and copied at prescribed rates at the SEC's Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549, on official business days during the hours of 10:00 A.M. to 3:00 P.M (EST). You may obtain information on the operation of the SEC's Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet site that contains reports, proxy statements and other information regarding issuers like us that file electronically with the SEC. The address of the SEC's Internet site is www.sec.gov. For more information about us, please visit our website at www.corcept.com . You may also obtain a free copy of our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports on the day the reports or amendments are filed with or furnished to the SEC by visiting our website at www.corcept.com. The information found on, or otherwise accessible through, our website, is not incorporated information, and does not form a part of, this Form 10-K.
10
ITEM 1A. RI SK FACTORS
An investment in our common stock involves significant risks. You should carefully consider the risks described below and the other information in this Annual Report on Form 10-K, including our financial statements and related notes, before investing in our common stock. If any of the following risks or uncertainties actually occurs, our business, results of operations or financial condition could be materially harmed, the price of our common stock could decline and you could lose all or part of your investment. The risks and uncertainties described below are those that we currently believe may materially affect us; however, they may not be the only ones that we face. Additional risks and uncertainties of which we are unaware or currently deem immaterial may become important and may result in harm to our business.
Risks Related to the Commercialization of Korlym
We depend heavily on the success of Korlym ® . If we are unable to increase revenue from the sale of Korlym to the levels investors expect, or experience significant delays in doing so, our stock price will likely decline.
We anticipate that for the foreseeable future our ability to generate meaningful revenue and fund our commercial operations and development programs will be solely dependent on the successful commercialization of Korlym. Many factors could hamper our efforts to commercialize Korlym, including:
| • | an inability to generate sufficient revenue due to low product usage or inadequate insurance coverage and reimbursement; |
| • | competition from Novartis's Signifor and from other companies with greater financial and marketing resources than ours; |
| • | an inability to manufacture Korlym or the active ingredient in Korlym in commercial quantities and at an acceptable cost; |
| • | political concerns relating to other uses of mifepristone that could limit the market acceptance of Korlym; |
| • | previously unknown, serious side effects that may be identified; and |
| • | rapid technological change that makes Korlym obsolete. |
Failure to meet investors' revenue expectations could cause our stock price to decline.
Physicians may accept Korlym slowly or may never accept it, which would adversely affect our financial results.
Physicians will prescribe Korlym only if they determine that it is preferable to other treatments, even if those products are not approved for Cushing syndrome. Because Cushing syndrome is rare, most physicians are inexperienced in the care of patients with the illness and it may be difficult to persuade them to prescribe Korlym, even with clinical trial results that show it is a compelling treatment.
Other factors that may affect the commercial success of Korlym include:
| • | the preference of some physicians for more familiar, long-standing off-label treatments for Cushing syndrome or for Novartis' drug, Signifor, for the treatment of Cushing disease; |
| • | competition from alternative treatment methods, such as surgery and radiation therapy; |
| • | the cost-effectiveness of Korlym and the availability of third-party insurance coverage and reimbursement; |
| • | the product labeling required by the FDA for Korlym; and |
| • | negative publicity concerning Korlym, RU-486, Mifeprex ® or mifepristone. |
11
The failure of Korlym to achieve commercial success would prevent us from generating sufficient revenue to fully fund our commercial and development acti vities.
The Orphan Drug designation for Korlym may not prevent competition from companies that develop other compounds for the treatment of Cushing syndrome. These companies may have significantly more resources than we do. Competition from them could limit our revenue from the commercialization of Korlym for the treatment of Cushing syndrome or other indications.
Although we have received Orphan Drug designation in the United States, we cannot be assured that we will realize the potential benefits of the designation. Even after an orphan drug is approved for its orphan indication, the FDA can subsequently approve a different drug for the same condition if it concludes that the later drug is safer, more effective or makes a major contribution to patient care. Upon expiration of the orphan drug exclusivity period, we may be subject to competition from manufacturers offering a generic form of Korlym at a lower price, in which case our business could be harmed.
In 2012 Novartis received approval in both the United States and the European Union (EU) to market its somatostatin analogue Signifor for adult patients with Cushing disease (a subset of Cushing syndrome that accounts for approximately 70 percent of all patients with Cushing syndrome) for whom pituitary surgery is not an option or has not been curative. In addition, Novartis has received Orphan Drug designation in the United States for the use of the experimental compound osilodrostat to treat Cushing disease and in the EU to treat Cushing syndrome. Novartis has begun a Phase 2 clinical trial in Japan investigating the use of this compound to treat Cushing syndrome due to causes other than Cushing disease and a Phase 3 clinical trial in the EU investigating its use to treat Cushing disease. Novartis has substantially more resources and experience than we do and may provide significant competition.
Laboratoire HRA Pharma (HRA) received Orphan Drug designation in the United States and the EU for the use of mifepristone to treat a subtype of Cushing syndrome. HRA began and terminated a Phase 2 clinical trial in Europe and the United States for this indication. Strongbridge Biopharma plc (Strongbridge) has received Orphan Drug designation in the United States and the EU for the use of levoketoconazole to treat Cushing syndrome. Strongbridge has begun a Phase 3 clinical trial in Europe and the United States for this indication. Exelgyn Laboratories, which operates as a subsidiary of Medi Challenge (Pty) Ltd., received Orphan Drug designation for mifepristone to treat Cushing syndrome in the EU, but has stated that it has not yet conducted any clinical trials.
If we cannot continue to obtain acceptable prices or adequate insurance coverage and reimbursement for Korlym, we will be unable to generate significant revenues.
The commercial success of Korlym depends on whether insurance coverage and reimbursement is available. Government payors, including Medicare, Medicaid and the Veterans Administration, as well as commercial health maintenance organizations and other third-party payors, are increasingly attempting to contain healthcare costs by limiting reimbursement of new medicines. As a result, they may not cover or provide adequate payment for Korlym. Our dependence on the commercial success of Korlym makes us particularly susceptible to cost containment efforts. Unless government and other third-party payors continue to provide adequate and timely coverage and reimbursement, physicians may not prescribe it and patients may not purchase it. In addition, meaningful delays in coverage for individual patients may increase our costs and reduce our revenues.
In some foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the United States, we expect that there will continue to be federal and state proposals for similar controls. Also, the trends toward managed health care in the United States and recent laws and legislation intended to reduce the cost of government insurance programs could significantly influence the purchase of health care services and products and may result in lower prices for Korlym or the exclusion from reimbursement programs.
12
The Patient Protection and Affordable Care Act (PPACA), which was passe d in 2010, substantially changed the way health care is financed by both governmental and private insurers and significantly impacts the U.S. pharmaceutical industry. The PPACA, among other things, expanded Medicaid program eligibility and access to commer cial health insurance coverage, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees and taxes on manufacturers of certain branded prescription drugs , and promo ted a new Medicare Part D coverage gap discount program. The PPACA also appropriated additional funding to comparative clinical effectiveness research , although it rema ins unclear how the research will impact current Medicare coverage and reimbursement or how new information will influence other third-party payor policies.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the PPACA, and we expect there will be additional challenges and amendments to the PPACA in the future, particularly in light of the new presidential administration and U.S. Congress. In addition, Congress could consider subsequent legislation to replace repealed elements of the PPACA. On January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the PPACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the PPACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. At this time, the full effect that the PPACA, the Executive Order and any subsequent legislation would have on our business remains unclear. Any new limitations on, changes to, or uncertainty with respect to the ability of individuals to enroll in governmental reimbursement programs or other third-party payor insurance plans could impact demand for Korlym, which in turn could affect our ability to successfully develop and commercialize our products.
Other legislative and regulatory changes have been proposed and adopted in the United States since the PPACA was enacted. These changes included an aggregate reduction in Medicare payments to providers of up to 2% per fiscal year, which went into effect on April 1, 2013 and will remain in effect through 2025 unless additional Congressional action is taken. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. On February 1, 2016, the Centers for Medicare & Medicaid Services, or CMS, published a final rule that revised certain requirements involved in our calculation of prices we report in connection with our participation in government reimbursement programs so that Korlym will be eligible for purchase by, or qualify for partial or full reimbursement from, Medicaid and other government programs. The extent to which this rule may alter our reported prices and estimated rebates and chargebacks under government programs remains unclear.
These new laws and the regulations and policies implementing them, as well as other healthcare reform measures that may be adopted in the future, may have a material adverse effect on our industry generally and on our ability to successfully develop and commercialize our products.
Public perception of mifepristone may limit our ability to sell Korlym.
The active ingredient in Korlym, mifepristone, is approved by the FDA in another drug for the termination of early pregnancy. As a result, mifepristone has been and continues to be the subject of considerable ethical and political debate in the United States and elsewhere. Public perception of mifepristone may limit our ability to engage alternative manufacturers and may limit the commercial acceptance of Korlym by patients and physicians. Even though we have taken measures to minimize the likelihood of the prescribing of Korlym to a pregnant woman, physicians may choose not to prescribe Korlym to a woman simply to avoid any risk of unintentionally terminating a pregnancy.
13
We have no manufacturing or pharmacy capabilities and currently depend on third-party vendors to manufacture Korlym and dispense it to patients. We also depend on other suppliers to manufacture the API and capsules for CORT125134 and the other selective cortisol modulators we are developing. If these thir d parties are unable or unwilling to continue to manufacture or dispense Korlym for us and we are unable to contract quickly with alternative sources, or if these third-parties fail to comply with FDA or other applicable regulations or otherwise fail to me et our requirements, our business will be harmed.
PCAS, a third-party manufacturer, supplies all of the API in Korlym. Alcami, another third-party manufacturer, produces all of our Korlym tablets. Dohmen Life Science Services, a specialty pharmacy, dispenses Korlym. We have entered into agreements with these vendors that automatically renew. We rely on other third-parties to manufacture the API and capsules of the selective cortisol modulators that we are developing, including CORT125134. If any of these vendors is unable or unwilling to meet our future requirements, we may not be able to manufacture our product in a timely manner. Our current arrangements with these manufacturers are terminable by them, subject to notice provisions.
Our specialty pharmacy is subject to regulation by the FDA and other governmental authorities. We do not control the pharmacy's processes or operations and cannot assure that they will meet all regulatory requirements. In the event it fails to do so, we may be required to identify an alternative pharmacy, which we may not be able to do in a timely manner, which would harm our business.
The facilities used by our vendors to manufacture our product and product candidates must be approved by the FDA. We do not control the manufacturing processes of, and are completely dependent on, our contract manufacturing partners for compliance with the regulatory requirements known as current good manufacturing practices (cGMPs). If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict requirements of the FDA or others, they will not be able to maintain regulatory approval for their manufacturing facilities. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our products or if it withdraws any such approval, we may need to find alternative manufacturing facilities, which would significantly hamper our ability to develop, obtain regulatory approval for or market our products. In addition, sanctions could be imposed on us, including fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of our product candidates, delays, suspension or withdrawal of approvals, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could harm our business. If our suppliers fail to manufacture Korlym or our product candidates on a timely basis in the quantities that we require, or fail to maintain manufacturing capabilities that meet FDA standards, we may exhaust our Korlym inventory and not be able to generate revenue, or our clinical development programs may be delayed.
If we or others identify previously unknown, serious side effects of Korlym, we may be required to perform lengthy clinical trials, change the labeling of Korlym or withdraw it from the market.
The FDA's approval of Korlym requires us to study drug utilization to better characterize the reporting rates of adverse events associated with the long-term use of Korlym. If we or others identify previously unknown, serious side effects of Korlym:
| • | regulatory authorities may withdraw their approvals; |
| • | we may be required to conduct clinical trials, make changes in labeling, implement changes to or obtain re-approvals of our manufacturing facilities; |
| • | we may experience a significant drop in the sales of Korlym; |
| • | our reputation in the marketplace may suffer; and |
| • | we may become the target of lawsuits, including class action lawsuits. |
Any of these events could harm or prevent sales of Korlym or could increase our marketing costs.
14
We may not have adequate insurance to cover our exposure to product liability claims.
We may be subject to product liability or other claims based on allegations that Korlym or one of our product candidates has caused adverse effects. A product liability claim may damage our reputation by raising questions about Korlym or any of our product candidates' safety and could limit our ability to sell a product by preventing or interfering with product commercialization. In some cases, less common adverse effects of a pharmaceutical product are not known until long after the FDA approves the product for marketing. The active ingredient in Korlym is used to terminate pregnancy. Therefore, clinicians using the medicine in our clinical trials and physicians prescribing the medicine to women with childbearing potential must take strict precautions to ensure that the medicine is not administered to pregnant women. The failure to observe these precautions could result in significant product liability claims.
We have product liability insurance with coverage limits we believe to be appropriate for a company marketing a single pharmaceutical product and developing others. However, this insurance may become prohibitively expensive or may not fully cover our potential liabilities. Our inability to obtain adequate insurance coverage at an acceptable cost could prevent or inhibit the commercialization of Korlym or our product candidates, or result in meaningful underinsured or uninsured liability. Defending a lawsuit could be costly and significantly divert management's attention from conducting our business. If we were sued successfully, our liability could exceed our assets.
We are subject to ongoing and continued regulatory review. If we are unable to maintain regulatory approval of Korlym for the treatment of patients with Cushing syndrome, or if we fail to comply with regulatory requirements, we will be unable to generate revenue or may be subject to penalties and our business will be harmed.
The FDA's approval of Korlym was subject to limitations on the indicated uses for which the product may be marketed and requirements for post-marketing information reporting. If we violate any of the FDA's restrictions or other marketing requirements, the FDA could withdraw its approval.
We are subject to ongoing obligations and continued regulatory review by the FDA and other regulatory authorities in the United States and other countries with respect to the research, testing, manufacturing, labeling, distribution, adverse event reporting, storage, selling, advertising, promotion, recordkeeping and marketing of products. These requirements include submissions of safety and other post-marketing information and reports, annual updates on manufacturing activities and continued compliance with cGMPs, and current good clinical practices (cGCPs), for any clinical trials that we conduct post-approval. cGMPs and cGCPs are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities through periodic inspections of manufacturing sites, trial sponsors, clinical investigators and clinical sites. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with FDA regulations and other applicable foreign and U.S. regulatory requirements may result in, among other things, untitled letters, warning letters, civil and criminal penalties, injunctions, holds on clinical trials, product seizure or detention, refusal to permit the import or export of products, restrictions on product marketing, withdrawal of the product from the market, voluntary or mandatory product recalls, total or partial suspension of production, refusal to approve pending NDAs or supplements to approved NDAs, and suspension or revocation of product approvals.
The FDA's policies may change and additional governmental regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. Indeed, we cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. For example, certain policies of the Trump administration may impact our business and industry. Namely, the Trump administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, FDA's ability to engage in routine oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. For example, on January 23, 2017, President Trump ordered a hiring freeze for all executive departments and agencies, including the FDA, which prohibits the FDA from filling employee vacancies or creating new positions. Under the terms of the order, the freeze will remain in effect until implementation of a plan to be recommended by the Director for the Office of Management and Budget, or OMB, in
15
consultation with the Director of the Office of Personnel Management, to reduce the size of the federal workforce through attrition. An under-staffed FDA could result in delays in FDA's responsiveness or in its ability to review submissions or applications, issue regulations or guidance, or implement or enforce regulatory requirements in a timely fashion or at all.
Moreover, on January 30, 2017, President Trump issued an Executive Order directing all executive agencies, including the FDA, that, for each notice of proposed rulemaking or final regulation to be issued in fiscal year 2017, the agency shall identify at least two existing regulations to be repealed, unless prohibited by law. These requirements are referred to as the "two-for-one" provisions. This Executive Order includes a budget neutrality provision that requires the total incremental cost of all new regulations in the 2017 fiscal year, including repealed regulations, to be no greater than zero, except in limited circumstances. For fiscal years 2018 and beyond, the Executive Order requires agencies to identify regulations to offset any incremental cost of a new regulation. In interim guidance issued by the Office of Information and Regulatory Affairs within OMB on February 2, 2017, the administration indicates that the "two-for-one" provisions may apply not only to agency regulations, but also to significant agency guidance documents. It is difficult to predict how these requirements will be implemented, and the extent to which they will impact the FDA's ability to exercise its regulatory authority. If these executive actions impose restrictions on FDA's ability to engage in oversight and implementation activities in the normal course, our business may be negatively impacted. Similarly, if we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may place at risk the FDA marketing approval for Korlym and any other marketing approval that we may obtain, which would adversely affect our business, prospects and ability to sustain profitability.
We may be subject to civil or criminal penalties if we market Korlym in a manner that violates FDA regulations or health care fraud and abuse laws.
In the United States, we are subject to FDA regulations governing the promotion of health care products. Although physicians are permitted, based on their medical judgment, to prescribe drugs for indications other than those approved by the FDA, manufacturers are prohibited from promoting their products for such "off-label" uses. In the United States, we market Korlym for treatment of hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or are not candidates for surgery and provide promotional materials and training programs to physicians regarding the use of Korlym for this indication. Although we believe our marketing materials and training programs for physicians do not constitute "off-label" promotion of Korlym, the FDA may disagree. If the FDA determines that our promotional materials, training or other activities by our employees or agents constitute "off-label" promotion of Korlym, it could request that we modify our training or promotional materials or other activities or subject us to regulatory enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal or state enforcement authorities might take action if they believe that the alleged improper promotion led to the submission and payment of claims for an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. Even if it is later determined that we are not in violation of these laws, we may be faced with negative publicity, incur significant expenses defending our position and have to divert significant management resources from other matters.
16
In add ition, there are health care fraud and abuse regulations and enforcement by both the federal government and the states in whic h we conduct our business. L aws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal health care programs such as the Medicare and Medicaid programs; |
| • | federal false claims laws, including, without limitation, the False Claims Act, which prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Pharmaceutical companies have been prosecuted under these laws for a variety of promotional and marketing activities, such as allegedly providing free product to or entering into "sham" consulting arrangements with customers to induce such customers to purchase, order or recommend the company's products in violation of the Anti-Kickback Statute and federal false claims laws and regulations; reporting to pricing services inflated average wholesale prices that were then used by certain governmental programs to set reimbursement rates; engaging in the promotion of "off-label" uses that caused customers to submit claims to and obtain reimbursement from governmental payors for non-covered "off-label" uses; and submitting inflated best price information to the Medicaid Drug Rebate Program; |
| • | the federal Civil Monetary Penalties law, which prohibits, among other things, offering or transferring remuneration to a federal healthcare beneficiary that a person knows or should know is likely to influence the beneficiary's decision to order or receive items or services reimbursable by the government from a particular provider or supplier; |
| • | the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which created federal criminal laws that prohibit executing a scheme to defraud any health care benefit program or making false statements relating to health care matters; |
| • | federal "sunshine" laws, including the federal Physician Payment Sunshine Act, that require transparency regarding financial arrangements with health care providers, such as the reporting and disclosure requirements imposed by the PPACA on drug manufacturers regarding any "transfer of value" made or distributed to prescribers and other health care providers, and ownership or investment interests held by physicians and their immediate family members. Manufacturers are required to submit reports detailing these financial arrangements by the 90 th day of each calendar year; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
The risk of our being found in violation of these laws and regulations is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts and their provisions are open to a variety of interpretations. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities, including our relationships
17
with physicians and other healthcare providers, s ome of whom recommend, purchase and/or prescribe our products, and the manner in which we promote our products, could be subject to challenge under one or more of such laws. We are also exposed to the risk that our employees, independent contractors, princ ipal investigators, consultants, vendors, distributors, and CROs may engage in fraudulent or other illegal activity. While we have policies and procedures in place prohibiting such activity, it is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits s temming from a failure to be in compliance with such laws or regulations.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from governmental health care programs, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
A break-down or breach of our information technology systems could subject us to liability or interrupt the operation of our business.
We store sensitive data on our computer networks and on the networks of third-party vendors, including our intellectual property and confidential information relating to our business and our employees. Despite the implementation of security measures, our internal computer systems and those of our vendors are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. System failures, unauthorized access to electronic and other confidential information, and other security breaches or accidents could cause interruptions in our operations, and could result in a material disruption of our clinical and commercialization activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. The loss of clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that a disruption or security breach resulted in the theft or loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our product research, development and commercialization efforts could be delayed or otherwise harmed.
The occurrence of a catastrophic disaster or other similar events could cause damage to our own or our manufacturers' facilities and equipment, which could require us to cease or curtail operations.
Our business is vulnerable to damage from various types of disasters or other similarly disruptive events, including earthquake, fire, flood, power loss and communications failures. For example, our headquarters are located in the San Francisco Bay Area, which is earthquake-prone, and our specialty pharmacy and warehouses are located in areas that are subject to severe weather conditions. In addition, political considerations relating to mifepristone may put us and our manufacturers at increased risk for terrorist attacks, protests or other disruptive events. If any disaster or other similar event were to occur, we may not be able to operate our business and our manufacturers may not be able to produce Korlym or our product candidates. Our insurance may not be adequate to cover, and our insurance policies may exclude coverage for, our losses resulting from disasters or other business interruptions.
Risks Related to the Development of our Product Candidates
Clinical drug development is lengthy and expensive and has an uncertain outcome. Results of earlier studies and trials may not be predictive of future trial results.
Clinical development is a long, expensive and uncertain process, and data obtained from clinical trials and supportive studies are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The results from early clinical trials may not be predictive of results eventually obtained in later clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical studies and initial clinical trials. A number of companies have suffered significant setbacks in advanced clinical trials due to the lack of efficacy or adverse safety profile of their medication candidate, despite promising results in earlier trials. Clinical trials may not demonstrate sufficient safety and efficacy to obtain regulatory approval.
18
Our ongoing clinical trials are too small to support marketing approvals for the compounds being studied. Even if these trials generate positive results, those results would have to be con firmed in one or more substantially larger, more expensive and lengthier trials before we could seek regulatory approvals.
The commencement and completion of clinical trials may be delayed by many factors that are beyond our control, including:
| • | delays obtaining regulatory approval to commence a trial; |
| • | reaching agreement on acceptable terms with Clinical Research Organizations ("CROs") and clinical trial sites; |
| • | obtaining institutional review board (IRB) approval at each site; |
| • | slower than anticipated patient enrollment; |
| • | lack of funding; |
| • | negative or inconclusive results; |
| • | patient noncompliance with the protocol; |
| • | adverse medical events or side effects among patients during the clinical trials; |
| • | negative or problematic FDA or other regulatory authority inspections of our clinical operations or manufacturing operations; and |
| • | real or perceived lack of effectiveness or safety. |
We could encounter delays if a clinical trial is suspended or terminated by us, the IRBs of the clinical trial sites where such trials are being conducted, the data safety monitoring board for such trial, or the FDA or other regulatory authorities. Such authorities may suspend or terminate a trial for many reasons, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations, or lack of adequate funding to continue the clinical trial.
Over the course of clinical development of any product candidate, we may decide, or the FDA or other regulatory authorities may require us, to pursue clinical or preclinical studies in addition to those we had initially planned. These may require additional funding, the availability of which is not assured. Also, additional trials or studies that we decide are necessary or desirable may delay or prevent the completion of our development programs or increase their cost. Even if we are able to conduct all of the clinical trials and supportive studies that we consider appropriate, we may never receive regulatory approval to market our product candidates.
We depend on third-parties to conduct and manage many of our clinical trials and to perform related data collection and analysis. Failure of these third-parties to successfully carry out their contractual duties or meet expected timelines may prevent or delay regulatory approval for the commercialization of our product candidates, which could substantially harm our business.
We rely on clinical investigators and clinical sites to enroll patients and other third-parties such as CROs to manage many of our trials and to perform related data collection and analysis. We control only certain aspects of these third-parties' activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the prescribed protocol and the applicable legal, regulatory and scientific standards. Our reliance on third-parties does not relieve us of our regulatory responsibilities. We and these third-parties are required to comply with cGCPs. If we or any of the third-parties working on or conducting our trials fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approval of our marketing applications. We cannot assure you that upon inspection by a regulatory authority, such regulatory authority will determine that any of our clinical trials complies with cGCP requirements. In addition, our clinical trials must be conducted with drug product produced under cGMP regulations. Our failure to comply with these regulations may
19
require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, we may not be able to contro l the timing of identification and selection of appropriate sites for our planned trials and the effectiveness of those sites. If our clinical investigators and clinical sites fail to enroll a sufficient number of patients or fail to enroll them on schedul e, we may be unable to complete our trials as planned, which could delay or prevent us from completing the clinical development of our product candidates.
We have agreements with the CROs and consultants helping to conduct our clinical trials and to perform investigator supervision, data collection and analysis for these trials. We may not be able to maintain relationships with these or other CROs and consultants, or with the clinical investigators and clinical sites conducting our trials. If any of our agreements with these third-parties terminate, we may not be able to enter into alternative arrangements on commercially reasonable terms, or at all. If the third-parties on which we rely do not carry out their contractual duties or fail to meet expected deadlines, or if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised, our clinical trials may be extended, delayed or terminated and we may be unable to obtain regulatory approval for, or successfully commercialize, any of our product candidates.
We may be unable to obtain and maintain regulatory approvals for our product candidates.
We are not permitted to market or promote any products before we receive regulatory approval from the FDA or comparable foreign regulatory authorities. Although we have received FDA approval to market Korlym, we may be unable to maintain such approval. We may not receive regulatory approval for any of our product candidates. Obtaining regulatory approval of a new drug is an uncertain, lengthy and expensive process. Success is never guaranteed. Failure can occur at any stage. In order to receive approval from the FDA for a product candidate, we must demonstrate that the new drug product is safe and effective for its intended use and that our manufacturing processes for the product candidate comply with FDA regulations known as "cGMPs." cGMPs include requirements related to production processes, quality control and assurance, and recordkeeping. Our inability or the inability of our suppliers to comply with applicable FDA and other regulatory requirements can result in, among other things, delays in or denials of new product approvals, warning letters, fines, consent decrees restricting or suspending manufacturing operations, injunctions, civil penalties, recall or seizure of products, total or partial suspension of product sales, and criminal prosecution. Any of these or other regulatory actions could materially harm our business and our financial condition.
Future governmental action or changes in FDA policy or personnel may also result in delays or rejection of an NDA in the United States. As of January 23, 2017, FDA is prohibited from filling employee vacancies or creating new positions pursuant to an Executive Order issued by President Trump. Under the terms of the order, the freeze will remain in effect until implementation of a plan to be recommended by the Director for the OMB, in consultation with the Director of the Office of Personnel Management, to reduce the size of the federal workforce through attrition. An under-staffed FDA could result in delays in FDA's responsiveness or in its ability to review submissions or applications, including NDAs, and may also hinder FDA's ability to issue and implement regulations or guidance in a timely fashion or at all. In addition, because the only other currently FDA-approved use of mifepristone is the termination of pregnancy, we expect that the label for mifepristone for any indication will include, as Korlym's does, some limitations, including a so-called "black-box" warning that it should not be used by pregnant women or women seeking to become pregnant.
If we receive regulatory approval for our future product candidates, we will be subject to ongoing FDA obligations and continued regulatory oversight and review, such as continued safety reporting requirements; and we may also be subject to additional FDA post-marketing restrictions and obligations. If we are not able to maintain regulatory compliance, we may not be permitted to market our product candidates and/or may be subject to product recalls or seizures. Any regulatory approvals that we receive for our future product candidates may also be subject to limitations on the indicated uses for which the medicine may be marketed or contain requirements for potentially costly post-marketing follow-up studies.
Failure to obtain regulatory approval in foreign jurisdictions would prevent us from commercializing our product candidates abroad.
We may seek to commercialize our products and product candidates in international markets with the help of one or more partners or on our own. Outside the United States, we may commercialize a product only if we receive a
20
marketin g authorization and, in many cases, pricing approval, from the appropriate regulatory authorities, whose approval processes include all of the risks associated with the FDA approval process, and, in some cases, additional risks. The approval procedure vari es among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in ot hers. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our product candidates in any foreign market. Although we have received Orphan Drug designation in the EU of Korlym to treat patients with Cu shing syndrome, we are not currently seeking to obtain any foreign approvals.
We face competition from companies with substantial financial, technical and marketing resources.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Our present and potential competitors include major pharmaceutical companies, as well as specialized pharmaceutical firms, universities and public and private research institutions. Moreover, we expect competition to intensify as technical advances are made. These competitors, may develop and commercialize medications that are superior to and more cost-effective than ours.
Many of our competitors and related private and public research and academic institutions have greater experience, more financial and marketing resources and larger research and development staffs than we do. In addition, many of these competitors, either alone or together with their collaborative partners, have significantly greater experience than we do in drug development, obtaining regulatory approvals, manufacturing and commercializing products. They may succeed in developing medicinal products that are superior to our product candidates, which could render our product candidates obsolete or non-competitive.
Our efforts to discover, develop and commercialize product candidates beyond Korlym for the treatment of patients with Cushing syndrome are at an early stage and we may fail to successfully commercialize any of them.
To develop additional sources of revenue, we must identify and develop new product candidates or new therapeutic uses for Korlym. Cortisol modulators may not be effective to treat any additional indications. Moreover, we could discover that the use of cortisol modulators has unacceptable side effects or is otherwise not safe. Due to the potential for lack of efficacy and side effects inherent in novel compounds and in new uses for existing medications, we are entering multiple compounds into development, which will increase our rate of spending with no assurance that we will be successful in developing drugs that are safe and effective.
We may elect to enter into collaboration arrangements with respect to one or more of our product candidates. If we do enter into such an arrangement, we would be dependent on a collaborative partner for the success of the product candidates developed under the arrangement. Any future collaborative partner may fail to successfully develop or commercialize a product candidate under a collaborative arrangement.
We only have significant clinical experience with mifepristone, the active ingredient in Korlym, and we may determine that mifepristone is not desirable for uses other than for the treatment of patients with Cushing syndrome. We may pursue other cortisol modulators for this use. The compounds developed pursuant to our early discovery, preclinical and clinical research programs may fail to become viable product candidates regardless of the resources we dedicate to their development. Even if product candidates are identified, we may abandon further development efforts after expending significant expense and time due to financial constraints, concerns over safety or efficacy, marketing considerations, manufacturing difficulties or other reasons. Moreover, governmental authorities may enact new legislation or regulations that could limit or restrict our development efforts. If we are unable to successfully discover and commercialize new uses for cortisol modulators, we may be unable to generate sufficient revenue to support our operations.
21
We will need to increase the size of our organization and we may experience difficulties in managing growth.
We expect that the further development of our research and development efforts will be constrained by our existing administrative, operational and management resources. Growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. To date, we have relied on a small management team. Our future financial performance and our ability to compete effectively will depend, in part, on our ability to manage growth effectively.
To that end, we must be able to:
| • | manage our sales and marketing efforts, clinical trials, research and development activities and supply chain effectively; |
| • | hire additional management, clinical development, administrative and sales and marketing personnel; and |
| • | develop our administrative, accounting and management information systems and controls. |
We may not be able to accomplish these tasks, and our failure to accomplish any of them could harm our business.
If we lose our key personnel or are unable to attract and retain additional skilled personnel, we may be unable to pursue our product development and commercialization efforts.
Our ability to operate successfully and manage our potential future growth depends significantly upon retaining key managerial, scientific, sales, marketing, and financial personnel, and attracting and retaining additional highly qualified personnel in these areas. We depend substantially on the principal members of our management and scientific staff. We do not have agreements with any of our executive officers that provide for their continued employment with us or employment insurance covering any of our key personnel. Any officer or employee can terminate his or her relationship with us at any time and work for one of our competitors. The loss of these key individuals could result in competitive harm because we could experience delays in our product research, development and commercialization efforts without their expertise.
We face intense competition for qualified personnel from numerous companies, as well as universities and nonprofit research organizations in the highly competitive San Francisco Bay Area. Although we believe that we have been successful in attracting and retaining qualified personnel to date, we may not be able to attract and retain sufficient qualified personnel in the future. The inability to attract and retain these personnel could harm our commercial business or delay the discovery, development and commercialization of our product candidates.
Rapid technological change could make our product and product candidates obsolete.
Pharmaceutical technologies undergo rapid and significant change. Our future will depend in large part on our ability to maintain a competitive position with respect to these technologies. Korlym and any products and processes that we develop may become obsolete or uneconomical before we recover any of the cost of their development. Rapid technological change could make Korlym and our product candidates obsolete or uneconomical, which could materially adversely affect our business, financial condition and results of operations.
Risks Related to Our Capital Needs and Financial Results
We may need additional capital in order to complete the development of Korlym for additional indications or for the development and commercialization of our proprietary, selective cortisol modulators. Additional capital may not be available to us at all or on favorable terms, which could adversely affect our business.
We may need to raise funds to continue the development of our proprietary selective cortisol modulators for any indication or for additional indications for Korlym. We may also raise funds for other research and development activities, including clinical trials, for working capital or for other general corporate purposes, or to acquire or invest in businesses, products and technologies that are complementary to our own.
22
Factors affecting our liquidity include the following:
| • | the pace at which physicians adopt Korlym as a treatment; |
| • | the willingness of insurance companies and the government payors to provide coverage for Korlym; |
| • | the outcome of clinical trials of Korlym and our other product candidates and the further clinical development of those compounds; |
| • | changes in our research and development plans for Korlym and our other product candidates; and |
| • | disputes concerning patents or proprietary rights, including announcements of claims of infringement, interference or litigation against us or our licensors. |
We may also choose to raise additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current and future operating plans.
We cannot be certain that additional funding will be available on acceptable terms or at all. Our sales of common stock and warrants and the exercises of warrants have been dilutive to stockholders and any additional equity financing could cause further dilution. Debt financing, if available, may involve restrictive covenants. If we obtain funds through collaborations with others, these arrangements may be on unfavorable terms or may require us to relinquish certain rights to Korlym or our product candidates. If adequate funds are not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or we may be required to discontinue operations.
We have incurred substantial losses and we may incur losses in the future.
We have financed our operations and internal growth in recent years primarily through revenue from the sale of Korlym, the sale of our common stock and our financing agreement with Biopharma. Prior to that we relied on the public sale of common stock and private placements of preferred and common stock. We may incur additional losses as we continue our discovery and clinical development programs, apply for regulatory approvals, acquire and/or develop treatments in other therapeutic areas, and expand our sales and marketing capabilities.
We may not be able to pursue all of our product research and development opportunities if we are unable to generate sufficient revenue or secure adequate funding for these programs.
The costs required to start or continue many of the programs that our intellectual property allows us to consider for further development are collectively greater than the funds currently available to us. For example, we have successfully discovered three series of compounds that are selective cortisol modulators but do not appear to block the progesterone receptor. Further development of these proprietary compounds or any further development stemming from our method of use patents may be delayed or cancelled if we determine that our expected revenue will be insufficient to support such programs and we are unable to obtain funding from other sources.
Global economic conditions could adversely affect our liquidity and financial condition.
Renewed or increased turbulence in the global markets and economies may cause lenders and institutional investors to reduce, or cease, to provide credit to businesses such as ours, which could adversely affect our liquidity and financial condition.
If we do not have sufficient cash flow to continue operating our business and are unable to borrow funds or raise equity or debt capital, we may need to find alternative ways to increase our liquidity. Such alternatives may include, without limitation, curtailing clinical or drug development activity or limiting our commercial efforts, which would have an adverse effect on our business, results of operations, cash flows and financial condition.
23
If we acquire other selective cortisol modulators or other technologies or potential products, we will incur a variety of costs and may never realize the anticipated benefits of the acquisition.
If appropriate opportunities arise, we may attempt to acquire products or product candidates that are complementary to our operating plan. We currently have no commitments, agreements or plans for any acquisitions. Acquiring rights to another potential product or technology may result in unforeseen difficulties and expenditures and may absorb significant management attention that would otherwise be available for ongoing development of our business. In addition, we may fail to realize the anticipated benefits of any acquired potential product or technology. Future acquisitions could dilute our stockholders' ownership interest in us and could cause us to incur debt, expose us to future liabilities and result in amortization or other expenses related to goodwill and other intangible assets.
Failure to meet our obligations under our Financing Agreement with Biopharma could adversely affect our financial results and liquidity.
Pursuant to our Financing Agreement with Biopharma entered into in August 2012, we are obligated to make payments to Biopharma equal to 20 percent of our net product sales of Korlym, any future mifepristone-based products and our next-generation selective cortisol modulators, subject to certain quarterly caps, as well as an un-capped 20 percent of any upfront, milestone or other contingent payments we receive with respect to Covered Products, until such payments to Biopharma total $45.0 million, at which point the obligation will be extinguished.
Pursuant to this agreement, we may not: (i) incur indebtedness greater than the sum of earnings before interest, taxes, depreciation and amortization, including such items as non-cash stock-based compensation, for the four calendar quarters preceding such incurrence, which we refer to as the Indebtedness Covenant; (ii) pay a dividend or other cash distribution, unless we have cash and cash equivalents in excess of $50.0 million after such payment; (iii) amend or restate our certificate of incorporation or bylaws unless such amendments or restatements do not affect Biopharma's interests under the transaction; and (iv) encumber any of the collateral securing our performance under the agreement.
The percentage used to calculate our payments to Biopharma would increase to 50 percent and any applicable payment caps would lapse if we (i) fail to provide Biopharma with certain information regarding our promotion and sales of Covered Products, (ii) do not devote a commercially reasonable amount of resources to the promotion and marketing of the Covered Products or (iii) violate the Indebtedness Covenant and, in each case, fail to cure within the applicable cure period.
Upon a Corcept change of control transaction, as defined in the agreement, Biopharma will be automatically entitled to receive any amounts not previously paid, up to our maximum repayment obligation of $45.0 million. As defined in the agreement, "Change of Control" includes, among other things, (i) a greater than 50 percent change in the ownership of Corcept, (ii) certain changes in Board composition of Corcept and (iii) the licensing of Korlym to a third-party for sale in the United States.
To secure our obligations under the agreement, we granted Biopharma a security interest in our rights in patents, trademarks, trade names, domain names, copyrights, know-how and regulatory approvals related to the Covered Products, all books and records relating to the foregoing and all proceeds of the foregoing, which we refer to as the Collateral. If we (i) fail to deliver a royalty payment when due and do not remedy that failure within 30 days, (ii) fail to maintain a first-priority perfected security interest in the Collateral in the United States and do not remedy that failure within five business days of receiving notice of such failure or (iii) become subject to an event of bankruptcy, then Biopharma may attempt to recover up to $45.0 million (after deducting any payments we have already made).
We cannot assure that we will not breach the covenants or other terms of, or that an event of default will not occur under this agreement and, if a breach or event of default occurs, we cannot assure that we will be able to cure the event within the time permitted. Any failure to pay our obligations when due, any breach or default of our covenants or other obligations, or any other event that causes an acceleration of payment at a time when we do not have sufficient resources to meet these obligations, could have a material adverse effect on our business, results of operations, financial condition and future viability.
24
The acceleration of the payment obligation in the event of a change of control transaction may make us less attractive to potential acquirers, and the payment of such funds out of our available cash or acquisition proceeds would reduce acquisition proceeds for our stockholders.
Risks Relating to Our Intellectual Property
If Korlym or future product candidates conflict with the patents of others or if we become involved in other intellectual property disputes, we may have to engage in costly litigation or obtain a license and we may be unable to commercialize our product candidates.
The patent positions of companies in the pharmaceutical industry are highly uncertain, involve complex legal and factual questions and have been and continue to be the subject of much litigation. Our product candidates may give rise to claims that our patents are invalid or that we infringe on the products or proprietary rights of others. If it is determined that our product candidates infringe on others' patent rights, we may be required to obtain licenses to those rights. If we fail to obtain licenses when necessary, we may experience delays in commercializing our product candidates while attempting to design around other patents, or determine that we are unable to commercialize our product candidates at all. If we do become involved in intellectual property litigation, we are likely to incur considerable costs in defending or prosecuting the litigation. We believe that we do not currently infringe any third-party's patents or other proprietary rights, and we are not obligated to pay royalties relating to the use of intellectual property except to Stanford University and the University of Chicago.
Our success depends in part on our ability to obtain and maintain adequate patent protection for the use of Korlym for the treatment of triple-negative breast cancer, castration-resistant prostate cancer and other potential uses of cortisol modulators. If we do not adequately protect our intellectual property, competitors may be able to use our intellectual property and erode our competitive advantage.
We own 20 issued U.S. method of use patents and have exclusively licensed six issued U.S. method of use patents. We have six U.S. method of use patent applications pending for our next-generation selective cortisol modulators. We also own eight U.S. composition of matter patents, with one additional U.S. application pending. In addition, we have been issued foreign method of use patents and composition of matter patents around the world. We have applied, and will continue to apply, for patents covering our product candidates as we deem appropriate. We have filed, and will continue to file, where we deem appropriate, foreign patent applications corresponding to our U.S. patents and applications.
We have exclusively licensed three issued U.S. patents from Stanford University for the use of cortisol modulators, including mifepristone, in the treatment of psychotic depression, cocaine-induced psychosis and early dementia, including early Alzheimer's disease. We have also exclusively licensed from the University of Chicago two issued U.S. patents for the use of cortisol modulators in the treatment of triple-negative breast cancer and a third issued U.S. patent covering the use of cortisol modulators to treat castration-resistant prostate cancer.
We bear the costs of prosecuting, protecting and defending these patents. In order to maintain the exclusive license to these patents until their expiration, we are obligated to make milestone and royalty payments to both universities. If we do not comply with our obligations under our agreement with Stanford, we may lose the right to commercialize mifepristone for the treatment of psychotic depression, cocaine-induced psychosis and early dementia. If the University of Chicago were to terminate our licenses, we may not be able to commercialize any cortisol modulators, including mifepristone, for the treatment of triple-negative breast cancer or castration-resistant prostate cancer.
Our patent applications and patents licensed or issued to us may be challenged by third-parties and our patent applications may not result in issued patents. Our presently pending and future patent applications may not issue as patents, and any patent issued to us may be challenged, invalidated, held unenforceable or circumvented. Our patent claims may not be sufficiently broad to prevent third-parties from producing competing products. The laws of foreign countries in which we may someday compete may not protect our intellectual property to the same extent as do the laws of the United States. If we fail to obtain adequate patent protection for our proprietary technology, our competitors may produce competing products based on our technology in these countries, which would impair our ability to succeed.
25
If a third-party successfully asserted an infringement claim against us, we could be forced to pay damages and be prevented from developing, manufacturing or marketing our potential products. We do not have liability in surance for patent infringement . A third-party could require us to obtain a license to use their i ntellectual property, which we may not be able to do on commercially acceptable terms, or at all. If we become involved in litigation, it could consume a substantial portion of our resources and of management's time . Regardless of the merit of any particu lar claim, defending a lawsuit is expensive and diverts management's attention from productive business.
Our ability to compete in the market could be diminished if we are unable to protect our trade secrets and proprietary information.
In addition to patents, we rely on a combination of confidentiality, nondisclosure and other contractual provisions, laws protecting trade secrets and security measures to protect our trade secrets and proprietary information. Nevertheless, these measures may not provide adequate protection, in which case third-parties could use our proprietary information to diminish our ability to compete in the market. In addition, employees, consultants and others who participate in the development of our product candidates may breach their agreements with us regarding our trade secrets and other proprietary information and we may not have adequate remedies for the breach. We also realize that our trade secrets may become known despite our best efforts.
The mifepristone patents that we own cover the use of mifepristone, not its composition, which may make it more difficult for us to prove patent infringement if physicians prescribe another manufacturer's mifepristone or if patients acquire mifepristone from other sources, such as the internet or underground market.
We own or have exclusively licensed issued U.S. patents covering the methods of using cortisol modulators to treat a variety of disorders, including triple-negative breast cancer and castration-resistant prostate cancer. A method of use patent covers only a specified use of a particular compound, not its composition. Because our patents do not cover the composition of mifepristone, we cannot prevent others from commercializing mifepristone to treat disorders not covered by our method of use patents. The availability of mifepristone for these disorders may enable patients to obtain mifepristone for indications covered by our patents. Although any such "off-label" use would violate our patents, effectively monitoring compliance and enforcing our rights may be difficult and costly. In addition, we cannot be assured that patients will not obtain mifepristone from other sources. As with other pharmaceutical products, patients may be able to purchase mifepristone through the internet or underground market. Mifepristone is also sold in the United States by Danco Laboratories for the termination of early pregnancy. While distribution is limited to a single dose provided in the physician's office and covered by other restrictions, we cannot be certain that Cushing syndrome patients will not be able to obtain mifepristone from this source or others, should another company receive approval to market mifepristone for another indication.
Risks Related to Our Stock
The market price of our common stock has been and is likely to continue to be highly volatile due to the limited number of shares of our common stock held by non-affiliates or factors influencing the stock market and opportunities for sale at any given time may be limited.
We cannot assure that an active trading market for our common stock will exist at any time. Holders of our common stock may not be able to sell shares quickly or at the market price if trading in our common stock is not active. During the 52-week period ended February 28, 2017, our average daily trading volume was approximately 453,215 shares and the intra-day sales prices per share of our common stock on The NASDAQ Capital Market ranged from $3.80 to $10.00. As of February 28, 2017, our officers, directors and principal stockholders controlled 22 percent of our common stock. The trading price of our common stock has been and is likely to continue to be highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
| • | actual or anticipated variations in quarterly operating results; |
| • | changes in financial estimates or recommendations by securities analysts or failure of our financial performance to meet the guidance we have provided to the public; |
26
| • | actual or anticipated timing and results of our clinical trials; |
| • | distributions in-kind of our common stock by our venture capital or private equity stockholders, which will increase the supply of our common stock and could decrease its price; |
| • | purchases or sales of our common stock by us, our officers, directors or our stockholders; |
| • | trading volume of our common stock; |
| • | actual or anticipated regulatory approvals of our product candidates or of competing products; |
| • | new products or services introduced or announced by us or our competitors; |
| • | our cash and short-term investment position; |
| • | changes in laws or regulations applicable to our product candidates or our competitors' products; |
| • | changes in the expected or actual timing of our development programs or our competitors' potential development programs; |
| • | announcements of technological innovations by us, our collaborators or our competitors; |
| • | general market and economic conditions; |
| • | conditions or trends in the biotechnology and pharmaceutical industries; |
| • | changes in the market valuations of similar companies; |
| • | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | additions or departures of key personnel; |
| • | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| • | limited number of shares of our common stock held by our non-affiliates; |
| • | developments concerning collaborations; |
| • | maintaining compliance with the listing requirements of the stock exchange on which we are listed; and |
| • | additional financing activities. |
All stock markets, including the NASDAQ Capital Market on which our stock is listed, and the market for biotechnology and life sciences companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. This volatility may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management's attention and resources.
Our stock price may decline if our financial performance does not meet the guidance that we provided to the public, estimates published by research analysts or other investor expectations.
We have provided guidance as to our expected 2017 revenue. Our guidance is only an estimate of what management believes is realizable as of the date of the release of such guidance. Our actual results may vary materially from our guidance.
27
R easons why we might fail t o meet our financial guidance or other investor expectations include, without limitation , the risks and uncertainties described in this report and in our other public filings and public statements. T here are inherent difficulties in predicting the amount o f Korlym that will be sold. For example, the rate of physician adoption of Korlym is uncertain. Research analysts have published a range of revenue estimates, based on their own analyses. We believe research analysts will consider the guidance we have prov ided as one factor in determining their own estimates . Readers of this annual report should rely on our guidance and the estimates of research analysts at their own discretion.
Research analysts may not continue to provide or initiate coverage of our common stock or may issue negative reports.
The trading market for our common stock may be affected in part by the research and reports that industry or financial analysts publish about us or our business. If one or more of the analysts who elects to cover us downgrades our stock, our stock price could decline rapidly and significantly. Securities analysts currently covering our common stock may discontinue research coverage. Additional securities analysts may elect not to provide research coverage of our common stock. A lack of research coverage may adversely affect our common stock's market price.
Sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
Sales of a substantial number of shares of our common stock in the public market could harm the market price of our common stock. As additional shares of our common stock become available for resale in the public market, whether as a result of distributions in-kind of our common stock by our venture capital or private equity stockholders, the exercise of stock options by employees, or equity financing by us, the supply of our common stock will increase, which could decrease the share price. Substantially all of the shares of our common stock are eligible for sale, subject to applicable volume and other resale restrictions.
Our officers, directors and principal stockholders, acting as a group, could significantly influence corporate actions.
As of February 28, 2017, our officers and directors control 22 percent of our common stock. As a result, these stockholders, acting together, will be able to significantly influence all matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of this group of stockholders may not always coincide with our interests or the interests of other stockholders and may prevent or delay a change in control. This significant concentration of share ownership may adversely affect the trading price of our common stock because investors often perceive disadvantages to owning stock in companies with controlling stockholders.
Changes in laws and regulations may significantly increase our costs, which could harm our financial results.
New laws and regulations, as well as changes to existing laws and regulations, affecting our company, including statutes and regulations concerning the development, approval, and marketing of medications, the provisions of the PPACA requiring the reporting of aggregate spending related to health care professionals, the provisions of the Sarbanes-Oxley Act of 2002 and rules adopted by the SEC and by The NASDAQ Capital Market have and will likely continue to result in increased costs to us as we respond to their requirements. We are investing resources to comply with evolving laws and regulations, and this investment may result in increased selling, general and administrative expenses and a diversion of management's time and attention from revenue-generating activities to compliance activities. At present, we cannot predict or estimate the amount of the additional costs related to new rules and regulations or the timing of such costs.
In addition, new rules and regulations could make it more difficult or costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, or our board committees, or as executive officers.
28
We may fail t o comply with public company obligations, including the securities laws and regulations. Such compliance is costly and requires significant management resources.
We are a small company with limited resources. The federal securities laws and regulations, including the corporate governance and other requirements of the Sarbanes-Oxley Act of 2002, impose complex and continually changing regulatory requirements on our operations and reporting. These requirements have increased and will continue to increase our legal compliance costs.
Section 404 of the Sarbanes-Oxley Act of 2002 requires that we evaluate the effectiveness of, and provide a management report with respect to, our internal controls over financial reporting. It also requires that the independent registered public accounting firm auditing our financial statements must attest to and report on the effectiveness of our internal controls over financial reporting. If we are unable to complete management's required assessment and report as to the adequacy of our internal control over financial reporting in or if our independent registered public accounting firm is unable to provide us with an unqualified report as to the effectiveness of our internal control over financial reporting, investors could lose confidence in the reliability of our financial reporting.
Changes in or interpretations of accounting rules and regulations could result in unfavorable accounting charges or require us to change our accounting policies or operating practices.
Accounting methods and policies for business and marketing practices of pharmaceutical companies are subject to continual review, interpretation and guidance from relevant accounting authorities, including the SEC. Although we believe that our accounting practices are consistent with current accounting pronouncements, changes to or interpretations of accounting methods or policies in the future may require us to reclassify, restate or otherwise change or revise our financial statements. Any such changes could result in corresponding changes to the amounts of assets, liabilities, revenues, expenses and income, which could have a material adverse effect on our business, financial position and results of operations and could cause the price of our common stock to decline .
Anti-takeover provisions in our charter and bylaws and under Delaware law and payment acceleration provisions under the Biopharma Financing Agreement may make an acquisition of us or a change in our management more expensive or difficult, even if an acquisition or a management change would be beneficial to our stockholders.
Provisions in our charter and bylaws may delay or prevent an acquisition of us or a change in our management. Some of these provisions allow us to issue preferred stock without any vote or further action by the stockholders, require advance notification of stockholder proposals and nominations of candidates for election as directors and prohibit stockholders from acting by written consent. In addition, a supermajority vote of stockholders is required to amend our bylaws. Our bylaws provide that special meetings of the stockholders may be called only by our Chairman, President or the Board of Directors and that the authorized number of directors may be changed only by resolution of the Board of Directors. These provisions may prevent or delay a change in our Board of Directors or our management, which our Board of Directors appoints. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law. Section 203 may prohibit large stockholders, in particular those owning 15 percent or more of our outstanding voting stock, from merging or combining with us. In addition, our payment obligations to Biopharma accelerate in the event of a change of control transaction. See "Risk Factors – Failure to meet our obligations under our Financing Agreement with Biopharma could adversely affect our financial results and liquidity." These provisions in our charter and bylaws and under Delaware law and the Financing Agreement could reduce the price that investors would be willing to pay for shares of our common stock and result in the market price being lower than it would be without these provisions.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
29
ITEM 2. P ROPERTIES
We lease 20,831 square feet of office space in Menlo Park, California for our corporate facilities. Our current lease extended our occupancy through March 2019.
ITEM 3. LEGAL PROCEEDINGS
We are not currently involved in any material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
30
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on The NASDAQ Capital Market under the symbol "CORT." The following table sets forth the high and low intra-day sale prices per share of our common stock on The NASDAQ Capital Market for the periods indicated. These prices represent quotations among dealers without adjustments for retail mark-ups, markdowns or commissions and may not represent prices of actual transactions.
2016 |
| High |
|
| Low |
| ||
First Quarter |
| $ | 4.92 |
|
| $ | 3.22 |
|
Second Quarter |
| $ | 6.33 |
|
| $ | 4.55 |
|
Third Quarter |
| $ | 6.72 |
|
| $ | 5.24 |
|
Fourth Quarter |
| $ | 10.00 |
|
| $ | 6.11 |
|
|
|
|
|
|
|
|
|
|
2015 |
| High |
|
| Low |
| ||
First Quarter |
| $ | 6.34 |
|
| $ | 2.69 |
|
Second Quarter |
| $ | 7.67 |
|
| $ | 5.40 |
|
Third Quarter |
| $ | 6.15 |
|
| $ | 3.36 |
|
Fourth Quarter |
| $ | 5.71 |
|
| $ | 3.45 |
|
Stockholders of Record and Dividends
As of February 28, 2017, we had 112,942,391 shares of common stock outstanding held by 41 stockholders of record. We have never declared or paid cash dividends. We currently intend to retain any future earnings to finance the growth and development of our business and therefore do not anticipate paying any cash dividends in the foreseeable future. In addition, the Biopharma Financing Agreement prohibits payment of dividends unless we have cash and cash equivalents in excess of $50 million after making such a payment.
Sale of Unregistered Securities
None.
Repurchases of Securities
None.
Market Performance Graph
The graph and the accompanying text below is not "soliciting material," is not deemed filed with the SEC and is not to be incorporated by reference in any filings by us under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in such filing.
The rules of the SEC require that we include a graph comparing cumulative stockholder returns on our common stock with the NASDAQ US Benchmark Total Return Index and either a published industry or line-of-business standard index or an index of peer companies selected by us. We have elected to use the NASDAQ Biotechnology Index (consisting of a group of 120 companies in the biotechnology sector, including us) for purposes of the performance comparison that appears below.
31
The graph shows the cumulative total stockholder return assuming the investment of $100.00 and th e reinvestment of dividends and is based on the returns of the component companies weighted according to their market capitalizations as of the end of the period for which returns are indicated. No dividends have been declared on our common stock.
The stockholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN* AMONG
CORCEPT THERAPEUTICS,
THE NASDAQ US BENCHMARK TOTAL RETURN (TR) INDEX
AND THE NASDAQ BIOTECHNOLOGY INDEX
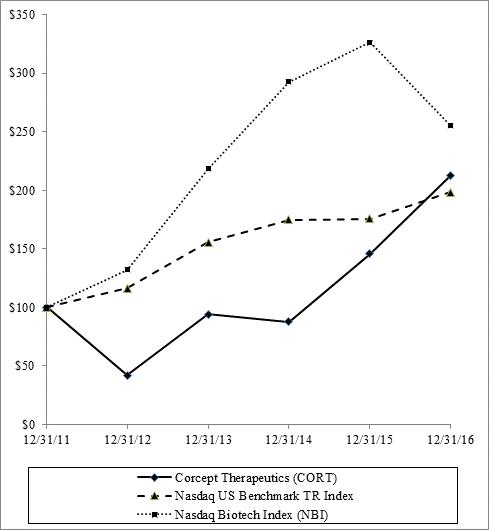
* $100 invested on December 31, 2011 including reinvestment of dividends. Fiscal year ended December 31.
32
ITEM 6. SELECTED FINANCIAL DATA
SELECTED FINANCIAL DATA
(in thousands, except per share data)
The selected financial data set forth below are derived from our financial statements. The statement of operations data for the years ended December 31, 2016, 2015, and 2014 and the balance sheet data as of December 31, 2016 and 2015 are derived from our audited financial statements included in this Annual Report. The statements of operations data for the years ended December 31, 2013 and 2012, and the balance sheet data as of December 31, 2014, 2013 and 2012 have been derived from our audited financial statements, which are not included in this Annual Report. Our historical results are not necessarily indicative of our results expected for 2017 or for any other future period. The selected financial data set forth below should be read in conjunction with our financial statements, the related notes and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this Annual Report.
|
| Year Ended December 31, |
| |||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| 2013 |
|
| 2012 |
| |||||
| (In thousands, except per share data) |
| ||||||||||||||||||
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product sales, net |
| $ | 81,321 |
|
| $ | 50,286 |
|
| $ | 26,551 |
|
| $ | 10,357 |
|
| $ | 3,307 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
| 2,058 |
|
|
| 1,361 |
|
|
| 882 |
|
|
| 143 |
|
|
| 91 |
|
Research and development* |
|
| 23,844 |
|
|
| 15,419 |
|
|
| 18,372 |
|
|
| 20,470 |
|
|
| 14,074 |
|
Selling, general and administrative* |
|
| 45,240 |
|
|
| 36,949 |
|
|
| 34,916 |
|
|
| 31,240 |
|
|
| 25,414 |
|
Total operating expenses |
|
| 71,142 |
|
|
| 53,729 |
|
|
| 54,170 |
|
|
| 51,853 |
|
|
| 39,579 |
|
Income (loss) from operations |
|
| 10,179 |
|
|
| (3,443 | ) |
|
| (27,619 | ) |
|
| (41,496 | ) |
|
| (36,272 | ) |
Non-operating income (expense), net* |
|
| (2,039 | ) |
|
| (2,965 | ) |
|
| (3,764 | ) |
|
| (4,515 | ) |
|
| (1,776 | ) |
Net income (loss) |
| $ | 8,140 |
|
| $ | (6,408 | ) |
| $ | (31,383 | ) |
| $ | (46,011 | ) |
| $ | (38,048 | ) |
Net income (loss) per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
| $ | 0.07 |
|
| $ | (0.06 | ) |
| $ | (0.31 | ) |
| $ | (0.46 | ) |
| $ | (0.41 | ) |
Weighted average shares – basic |
|
| 110,566 |
|
|
| 106,883 |
|
|
| 100,978 |
|
|
| 99,819 |
|
|
| 93,015 |
|
Weighted average shares – diluted |
|
| 116,139 |
|
|
| 106,883 |
|
|
| 100,978 |
|
|
| 99,819 |
|
|
| 93,015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Includes certain non-cash expenses, of the following: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
| $ | 1,312 |
|
| $ | 839 |
|
| $ | 723 |
|
| $ | 618 |
|
| $ | 546 |
|
Selling, general and administrative |
|
| 5,746 |
|
|
| 5,174 |
|
|
| 4,478 |
|
|
| 4,578 |
|
|
| 4,764 |
|
Total stock-based compensation |
|
| 7,058 |
|
|
| 6,013 |
|
|
| 5,201 |
|
|
| 5,196 |
|
|
| 5,310 |
|
Non-operating expense related to accretion of interest on long-term obligation |
|
| 1,929 |
|
|
| 2,848 |
|
|
| 3,678 |
|
|
| 4,410 |
|
|
| 1,680 |
|
Total non-cash expenses |
| $ | 8,987 |
|
| $ | 8,861 |
|
| $ | 8,879 |
|
| $ | 9,606 |
|
| $ | 6,990 |
|
33
|
| As of December 31, |
| |||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| 2013 |
|
| 2012 |
| |||||
|
| (In thousands) |
| |||||||||||||||||
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and investments |
| $ | 51,536 |
|
| $ | 40,435 |
|
| $ | 24,248 |
|
| $ | 54,877 |
|
| $ | 93,032 |
|
Working capital |
|
| 38,315 |
|
|
| 28,104 |
|
|
| 16,675 |
|
|
| 45,573 |
|
|
| 86,703 |
|
Total assets |
|
| 68,753 |
|
|
| 51,937 |
|
|
| 34,630 |
|
|
| 63,077 |
|
|
| 99,166 |
|
Long-term obligation - current portion |
|
| 14,664 |
|
|
| 14,965 |
|
|
| 9,424 |
|
|
| 5,743 |
|
|
| 2,650 |
|
Long-term obligation, net of current portion |
|
| - |
|
|
| 12,528 |
|
|
| 24,405 |
|
|
| 29,234 |
|
|
| 28,907 |
|
Total stockholders' equity (deficit) |
|
| 41,379 |
|
|
| 18,498 |
|
|
| (3,388 | ) |
|
| 21,017 |
|
|
| 61,777 |
|
34
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS O F FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management's Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is intended to help the reader understand our results of operations and financial condition. MD&A is provided as a supplement to, and should be read in conjunction with, our audited Financial Statements and the accompanying Notes to Financial Statements and other disclosures included in this Annual Report on Form 10-K (including the disclosures under Item 1A, Risk Factors). Our Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles and are presented in U.S. dollars.
We make statements in this section that are forward-looking statements within the meaning of the federal securities laws. For a complete discussion of such forward-looking statements and the potential risks and uncertainties that may impact upon their accuracy, see "Forward-Looking Statements" included in "Risk Factors" in Part I, Item 1A of this Form 10-K and the "Overview" and "Liquidity and Capital Resources" sections of this Management's Discussion and Analysis of Financial Condition and Results of Operations.
Overview
We are engaged in the discovery, development and commercialization of drugs that treat severe metabolic, oncologic and psychiatric disorders by modulating the effects of the hormone cortisol. Elevated levels and abnormal release patterns of cortisol are implicated in a broad range of human disorders. Since our inception in 1998, we have been developing mifepristone, a compound that modulates the effects of cortisol by acting as a competitive antagonist at the glucocorticoid receptor (GR). We have also discovered three structurally distinct series of proprietary, selective cortisol modulators, all of which share mifepristone's affinity for GR but, unlike mifepristone, do not bind to the progesterone receptor and so do not cause effects associated with progesterone receptor antagonism. Both pre-clinical and clinical development of the lead compounds from these series are in progress.
In 2012, the United States Food and Drug Administration (FDA) approved Korlym ® (mifepristone) 300 mg tablets as a once-daily oral medication for the treatment of hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or are not candidates for surgery.
We are conducting two clinical trials of our proprietary selective cortisol modulator, CORT125134. One trial is investigating CORT125134 as a potential treatment for patients with Cushing syndrome. The second trial is investigating the combination of CORT125134 and Abraxane as a treatment for patients with a variety of solid-tumor cancers. Both trials are currently enrolling patients.
We are advancing other compounds from our portfolio of selective cortisol modulators towards the clinic and expect to begin clinical trials of two of them in 2017.
Cushing Syndrome
Background . Cushing syndrome is caused by prolonged exposure of the body's tissues to high levels of the stress hormone cortisol. It is relatively uncommon and most often affects adults aged 20 to 50. An estimated 10 to 15 of every one million people are newly diagnosed with this syndrome each year, resulting in approximately 3,000 new patients and an estimated prevalence of 20,000 patients with Cushing syndrome in the United States, approximately half of whom are cured by surgery.
Korlym to Treat Patients with Cushing Syndrome . We have received Orphan Drug designation from the FDA for Korlym for the treatment of patients with endogenous Cushing syndrome. Drugs that receive Orphan Drug designation receive seven years of marketing exclusivity for the approved indication from the date of drug approval, as well as tax credits for clinical trial costs, marketing application filing fee waivers and assistance from the FDA in the drug development process.
35
We first made Korlym available to patients on a commercial basis in April 2012. We sell Korlym using experienced sales representatives, who target U.S. endocrinologists who care for a large portion of t he patients with Cus hing syndrome. We also reach patients directly through web-based initiatives and interactions with patient groups. Because a large percentage of the people who suffer from Cushing syndrome remain undiagnosed or are inadequately treated, we have developed and continue to refine and expand programs to educate the medical community and patients about diagnosis of this syndrome and to increase awareness regarding the role of cortisol modulators to treat the disease. In addition, we have a fi eld-based force of medical science liaisons.
We use a specialty pharmacy and a specialty distributor to distribute Korlym and provide logistical support. We have retained a vendor to help patients with the reimbursement process and to administer our financial assistance programs for uninsured or under-insured patients. We donate money to independent charitable foundations. These organizations, along with our own programs, help us ensure that no Cushing syndrome patient is denied access to Korlym for financial reasons.
CORT125134 to Treat Patients with Cushing Syndrome . In the second quarter of 2016, we began a Phase 2 trial of our proprietary, selective cortisol modulator, CORT125134, to treat patients with Cushing syndrome. CORT125134 shares Korlym's affinity for GR. Data from the compound's Phase 1 trial showed that it can potently modulate the effects of the steroid prednisone, a commonly-used GR agonist, on serum osteocalcin, white blood cell counts, glucose metabolism and expression of the protein FKBP5 – a genetic marker of GR activation. Modulating the effect of prednisone is important because it is a strong surrogate for Korlym's modulation of cortisol – the essential quality of an effective treatment for patients with Cushing syndrome.
We are developing a CLIA-validated assay to measure expression of FKBP5. We believe this assay will allow physicians to measure the degree to which their patients suffer from excess cortisol activity, which would help them more easily identify patients with Cushing syndrome and better treat those already in their care.
Oncology
Background . A range of tumor-types express GR and are potential targets for cortisol modulation therapy, among them triple-negative breast, ovarian, prostate, cervical, and pancreatic cancers, as well as sarcoma and melanoma.
Korlym to Treat Patients with Solid-Tumor Cancers . In December 2016, we announced the results of our Phase 1/2 trial of Korlym in combination with eribulin (Eisai's Inc.'s drug, Halaven ® ) to treat patients with metastatic triple-negative breast cancer. The trial studied 21 patients with GR positive tumors, one with GR negative tumors and one with tumors whose GR status was not known. As determined using the Response Evaluation Criteria in Solid Tumors (RECIST), efficacy results were as follows: four patients exhibited a partial response, defined as a 30 percent or greater reduction in tumor size; eight had stable disease; and 11 had progressive disease. Six patients achieved progression-free survival (PFS) longer than the upper bound of the 95% confidence interval for PFS (15 weeks) in patients receiving Halaven ® monotherapy in a comparable population (Aogi et al., Annals of Oncology 23: 1441-1448, 2012). Median PFS in the trial was 11.1 weeks – compared to 7.2 weeks in the Halaven monotherapy study reported by Aogi. We believe that the addition of Korlym to chemotherapy warrants further study, such as the double-blind, placebo-controlled, multicenter, University of Chicago-led trial described above that Celgene is funding.
CORT125134 to Treat Patients with Solid-Tumor Cancers . We are conducting a Phase 1/2 trial of Abraxane (nab-paclitaxel) in combination with CORT125134 to treat any solid-tumor cancer suitable for treatment with Abraxane. Once we identify a recommended dose of this combination, we will open 20-patient cohorts to test the combination's efficacy in one or more solid-tumor cancers. Our likely initial targets will be triple-negative breast cancer and ovarian cancer. Other possible indications include pancreatic cancer, cervical cancer and sarcoma.
Results of Operations
Net Product Sales – Net product sales are gross product revenue from sales to customers less deductions for estimated government rebates and chargebacks.
36
For the year ended December 31, 2016, we recorded $ 81.3 million in net product sales, as compared to $50.3 million for the year ended December 31, 2015 and $26.6 million for the year ended December 31, 2014. The increases in net product reve nue were primarily driven by increase s in our sales volume and price increases .
We donate cash to the National Organization for Rare Disorders (NORD), a third-party charitable organization that helps patients with financial need pay for the treatment of Cushing syndrome, which treatment may include Korlym. We do not include as net product revenues funds we receive from this organization.
Cost of sales – Cost of sales includes the cost of manufacturing Korlym, including its active pharmaceutical ingredient (API), tableting and packaging costs, indirect personnel and overhead costs, and the cost of stability testing and distribution.
Cost of sales was $2.1 million for the year ended December 31, 2016, as compared to $1.4 million in the corresponding period in 2015. The increase was due to greater sales volumes. For the year ended December 31, 2016, cost of sales was 2.5 percent of our net product revenue, as compared to 2.7 percent in the corresponding period in 2015.
Cost of sales was $1.4 million for the year ended December 31, 2015, as compared to $0.9 million in the corresponding period in 2014. For the year ended December 31, 2015, cost of sales was 2.7 percent of our net product revenue, as compared to 3.3 percent in the corresponding period in 2014.
Cost of sales declined as a percentage of net product revenue for the years ended December 31, 2016 and 2015 due to a decline in the cost of manufacturing Korlym tablets as well as sales price increases.
Research and development expenses – Research and development expenses include the cost of (1) personnel engaged in development activities, including stock-based compensation, (2) clinical trials, including trial preparation, enrollment, site monitoring and data management and analysis expenses, (3) discovery research and pre-clinical studies, (4) acquisition of clinical trial materials and material used in registration and validation batches included in regulatory submissions prior to product approval, (5) manufacturing development, and (6) regulatory activities, including the preparation and prosecution of the regulatory submissions related to Korlym and our other product candidates.
Research and development expenses increased to $23.8 million for the year ended December 31, 2016 from $15.4 million in 2015, an increase of 54.6 percent, primarily due to increased spending on the advancement of CORT125134, which entered clinical trials in patients in the second quarter of 2016, as well as increased compensation expense due to the hiring of additional clinical development employees.
Research and development expenses decreased to $15.4 million for the year ended December 31, 2015 from $18.4 million in 2014, a decline of 16.1 percent, due to the discontinuation of our Phase 3 clinical trial of Korlym to treat psychotic depression in May 2014, which reduced our research and development expenses in the year ended December 31, 2015 by $3.9 million, partially offset by $0.9 million in spending on our Phase 1/2 study in triple-negative breast cancer, an FDA-required drug-drug interaction study and the development of new selective cortisol modulators.
37
Below is a summary of our research and development expenses by major project:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Project |
| (in thousands) |
| |||||||||
Development programs: |
|
|
|
|
|
|
|
|
|
|
|
|
Oncology |
| $ | 4,592 |
|
| $ | 3,494 |
|
| $ | 2,455 |
|
Cushing syndrome |
|
| 3,739 |
|
|
| 811 |
|
|
| 2,157 |
|
Psychotic depression |
|
| - |
|
|
| 190 |
|
|
| 5,971 |
|
Pre-clinical selective cortisol modulators |
|
| 10,393 |
|
|
| 7,431 |
|
|
| 5,607 |
|
Unallocated activities, including pre-clinical, manufacturing and regulatory activities |
|
| 3,808 |
|
|
| 2,654 |
|
|
| 1,459 |
|
Stock-based compensation |
|
| 1,312 |
|
|
| 839 |
|
|
| 723 |
|
Total research and development expense |
| $ | 23,844 |
|
| $ | 15,419 |
|
| $ | 18,372 |
|
We expect research and development expenditures in 2017 to be higher than they were in 2016, as we hire more clinical staff, our research and development programs advance and their costs increase. Research and development expenses in 2017 and beyond will depend on the outcomes of our current trials and future development plans.
Many factors affect the cost and timing of our trials, including inconclusive results requiring more clinical trials, slow patient enrollment, adverse side effects in study patients, insufficient supplies of medicine for our clinical trials and real or perceived lack of effectiveness or safety of the product candidate. The cost and timing of development of our selective cortisol modulators will depend on the success of our efforts and any difficulties we encounter. In addition, the development of our product candidates is subject to extensive governmental regulation. These factors make it difficult for us to predict the timing and costs of developing and securing approval of our product candidates.
Selling, general and administrative expenses – Selling, general and administrative expenses include (1) the cost of personnel, consultancy and contractors engaged in administrative and commercial activities, including stock-based compensation, (2) expenses of third-party vendors used in our commercial activities related to Korlym, including sales, marketing and promotion, pharmacy costs, market research, reimbursement support services, pharmacovigilance, distribution of marketing materials, and logistical requirements and (3) legal, accounting and other professional fees.
Selling, general and administrative expenses for the year ended December 31, 2016 increased 22.4 percent to $45.2 million, from $36.9 million for the comparable period in 2015 . The increases were driven primarily by increased compensation expense due to additional hiring, bonus expense, and commissions related to increased sales.
Selling, general and administrative expenses for the year ended December 31, 2015 increased 5.8 percent to $36.9 million, from $34.9 million for the comparable period in 2014 . The increases were primarily due to the growth of our sales organization.
We expect that selling, general and administrative expenses will be higher in 2017 than in 2016 due to increased sales of Korlym. The level of selling, general and administrative activities and related expenses in 2017 and future years will be dependent on our assessment of the staff and other services necessary to support our commercial efforts and our continued clinical development activities.
See also, "Liquidity and Capital Resources."
Interest and other expense – Interest and other expense for the year ended December 31, 2016 was $2.0 million, as compared to $3.0 million for the year ended December 31, 2015 and $3.8 million for the year ended December 31, 2014. These amounts consisted primarily of interest expense related to our Financing Agreement with
38
Biopharma, which w e entered into in August 2012. Interest expense for 2017 will decrease as our quarterly payments redu ce the outstanding obligation. We expect to make our final payment under t he Financing Agreement in 2017.
Non-GAAP Financial Measures
Our financial statements and footnotes thereto are prepared in accordance with U.S. Generally Accepted Accounting Principles (GAAP) and are included in Part IV, Item 15 of this Annual Report on Form 10-K. To supplement our financial results presented on a GAAP basis, we use non-GAAP measures of net income (loss) and net income (loss) per share that exclude non-cash expenses related to stock-based compensation expense and the accretion of interest expense under our capped royalty financing transaction. We use these non-GAAP measures to manage our business and believe that they may help investors better evaluate our past financial performance and potential future results. Non-GAAP measures should not be considered in isolation or as a substitute for comparable GAAP accounting and investors should read them in conjunction with our financial statements and notes thereto prepared in accordance with GAAP. The non-GAAP measures of net income (loss) and net income (loss) per share we use may be different from, and not directly comparable to, similarly titled measures used by other companies .
The following table reflects the reconciliation of GAAP net income (loss) and net income (loss) per share to non-GAAP net income (loss) and net income (loss) per share for the periods presented.
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
|
| (in thousands, except for per share data) |
| |||||||||
GAAP net income (loss) |
| $ | 8,140 |
|
| $ | (6,408 | ) |
| $ | (31,383 | ) |
Non-cash expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
| 7,058 |
|
|
| 6,013 |
|
|
| 5,201 |
|
Accretion of interest expense related to long -term obligation |
|
| 1,929 |
|
|
| 2,848 |
|
|
| 3,678 |
|
Non-GAAP net income (loss), as adjusted for non-cash expenses |
| $ | 17,127 |
|
| $ | 2,453 |
|
| $ | (22,504 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net income (loss) per share |
| $ | 0.07 |
|
| $ | (0.06 | ) |
| $ | (0.31 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-GAAP basic and diluted net income (loss) per share, as adjusted for non-cash expenses |
| $ | 0.15 |
|
| $ | 0.02 |
|
| $ | (0.22 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding shares used in computing net income (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
| 110,566 |
|
|
| 106,883 |
|
|
| 100,978 |
|
Diluted |
|
| 116,139 |
|
|
| 106,883 |
|
|
| 100,978 |
|
Liquidity and Capital Resources
Until the year ended December, 31, 2016, we had incurred operating losses since inception. At December 31, 2016, we had an accumulated deficit of $322.3 million. Since 2012, we have relied primarily on revenues from the sale of Korlym, and proceeds from the sale of our common stock and our Financing Agreement with Biopharma to fund our operations.
Based on our current plans, which include funding our Cushing syndrome commercial operations, conducting Phase 2 trials of CORT125134 in both Cushing syndrome and solid tumor cancers and advancing to the clinic CORT125281 and CORT118335, we expect to fund our operations without needing to raise additional funds. We may choose to raise additional funds to finance our strategic priorities, however, if we are able to do so on acceptable terms. Any additional equity financing may be dilutive to stockholders. Any debt financing, if available,
39
may involve restrictive covenants. If we obtain funds throug h collaborations with others, these arrangements may be on unfavorable terms or may require us to relinquish certain rights to our technologies or product candidates that we would otherwise seek to develop on our own.
At December 31, 2016, we had cash and cash equivalents of $51.5 million, compared to $40.4 million at December 31, 2015. Net cash provided by operating activities for the year ended December 31, 2016 and December 31, 2015 was $18.4 million and $3.1 million, respectively, primarily due to greater sales volumes. Net cash used in operating activities for the year ended December 31, 2014 was $27.4 million, primarily to fund the commercialization of Korlym and for research and development. Net cash provided by stock option exercises was $7.7 million, $5.2 million, and $1.8 million during the years ended December 31, 2016, 2015, and 2014, respectively. In addition, we made payments under the Biopharma Financing Agreement of $14.8 million, $9.2 million, and $4.9 million during the years ended December 31, 2016, 2015 and 2014, respectively.
We are required to make aggregate payments under the Biopharma Financing Agreement of $45.0 million, with $29.9 million paid through December 31, 2016 and an additional payment of $4.8 million made in February 2017. We will make additional quarterly repayments in 2017 based on the level of our Korlym sales, and expect to fully repay the obligation in 2017.
While we monitor the cash balance in our checking account and transfer the funds into it only as needed, these cash balances and our money market fund could be affected if the underlying financial institution were to fail or were subject to other adverse conditions in the financial markets. We have never experienced a loss or lack of access to cash in our checking account or money market fund.
Contractual Obligations and Commercial Commitments
The following table presents our estimates of obligations under contractual agreements as of December 31, 2016.
Contractual Obligations |
| Total |
|
| Less than 1 year |
|
| 2-3 Years |
|
| 4-5 Years |
|
| More than 5 Years |
| ||||||
|
| (in thousands) |
| ||||||||||||||||||
Long-term obligation (1) |
| $ | 14,664 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other contractual obligations: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development studies (2 to 3) |
| $ | 8,537 |
|
| $ | 5,355 |
|
| $ | 3,182 |
|
| $ | - |
|
| $ |
| - |
|
Operating lease (4) |
|
| 2,331 |
|
|
| 937 |
|
|
| 1,394 |
|
|
| - |
|
|
|
| - |
|
Minimum royalty and license fee payments (5) |
|
|
|
|
|
| 80 |
|
|
| 160 |
|
|
| 60 |
|
|
| 30 per year |
| |
Total other contractual obligations |
|
|
|
|
| $ | 6,372 |
|
| $ | 4,736 |
|
| $ | 60 |
|
| $ | 30 per year |
| |
(1) | As discussed above, in August 2012, we entered into a Financing Agreement with Biopharma under which we received $30.0 million from Biopharma. In consideration of the $30.0 million payment, we are obligated to make payments to Biopharma totaling $45.0 million, of which $29.9 million has been paid through December 31, 2016. The remaining payment obligations will be calculated as follows: |
| • | 20 percent of our net product sales of Covered Products. Payments are due within 30 days of quarter-end for the first, second and third calendar quarters and within 45 days of year-end. |
| • | 20 percent of payments received for upfront, milestone or other contingent fees under co-promotion and out-license agreements for Covered Products. |
| • | The percentage used to calculate our payments to Biopharma would increase to 50 percent if we (i) fail to provide Biopharma with certain information regarding our promotion and sales of Covered Products, (ii) do not devote a commercially reasonable amount of resources to the promotion and marketing of the Covered Products or (iii) violate the indebtedness covenant by incurring indebtedness greater than the sum of earnings before interest, taxes, depreciation and amortization, including such items as non-cash stock-based compensation, for the four calendar quarters preceding such incurrence and, in each case, fail to cure within the applicable cure period. |
| • | Upon the occurrence of a Corcept change of control transaction or the licensing of Korlym to a third-party for promotion and sale in the United States, the entire $45 million, less any amounts already paid by us, would become due. |
40
Under the terms of the Finan cing Agreement, our payments are entirely variable, with no fixed minimums. The timing of our payments is determined by future sales and other receipts as defined. If there are no net sales, upfront, milestone or other contingent payments in a period with respect to Covered Products, then no payment will be due for that period. As noted above, through December 31, 2016, we have made payments of $29.9 million , with an additional payment in the amount of $ 4.8 million in February 2017. Biopharma's right to re ceive payments will expire once it has received cumulative payments of $45 million.
(2) | Amounts reflected for research and development studies exclude amounts included in accounts payable and accrued clinical costs reflected on the balance sheet as of December 31, 2016. |
(3) | In January 2016, we entered into an agreement with Chiltern to assist in the management of a clinical trial evaluating CORT125134 for treatment of Cushing syndrome. The total commitment under this agreement is $2.1 million, but the actual amount to be paid is dependent on actual services provided under this agreement. Approximately $741,000 of the costs under this agreement were incurred through December 31, 2016, with the remainder to be incurred over the course of the trial. |
(4) | In March 2016, we early terminated the lease and replaced it with a new lease effective May 1, 2016 through March 31, 2019. At December 31, 2016, the remaining minimum rental payments under this operating lease were $2.3 million. |
( 5) | Under our cancellable license agreements with the University of Chicago, we are obligated to pay nonrefundable annual license fees of $30,000. Under our cancellable license agreement with Stanford University, we are obligated to make nonrefundable minimum royalty payments of $50,000 annually for as long as we maintain these licenses; however, a portion of these payments are creditable against future royalties. The license agreement with Stanford University will expire in 2019 with the expiration of the patents. |
We also have other contractual payment obligations and purchase commitments, the timing of which are contingent on future events, including our manufacturing. In March 2014, we entered into an agreement with PCAS for the manufacture of mifepristone, the API in Korlym, for an initial term of five years, with an automatic extension of one year unless either party gives 12 months' prior written notice that it does not want an extension. In April 2014, we entered into a manufacturing agreement with Alcami Corporation (formerly known as AAI Pharma Services Corp.) for the manufacture and packaging of Korlym tablets for an initial term of three years, with consecutive automatic extensions of two years unless either party gives written notice of termination – in the case of Alcami, 18 months prior to the end of the applicable term, and in our case 12 months prior to the end of the applicable term. Purchase commitments under these agreements will depend on our future needs; neither agreement requires us to make minimum purchases.
Net Operating Loss Carryforwards
See Note 10 , Income Taxes in our audited financial statements.
Off-Balance Sheet Arrangements
None.
Critical Accounting Policies and Estimates
Our financial statements have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Net Product Sales
We primarily sell Korlym directly to patients through Dohmen Life Science Services (Dohmen), a specialty pharmacy. Prior authorization and confirmation of coverage by the patients' private or government insurance plan or by a third-party charity is a prerequisite for Dohmen to ship Korlym to a patient. We recognize revenue upon the delivery of Korlym to these patients . (See discussion in forth in Part IV – Item 15(1) – Financial Statements, Notes to Financial Statements, Note 2, Significant Agreements – Commercial Agreements. )
We recognize revenue from sales of Korlym upon delivery to patients as long as (i) there is persuasive evidence that an arrangement exists between ourselves and the customer, (ii) collectability is reasonably assured and (iii) the price is fixed or determinable. Prior authorization or confirmation of coverage level by the patient's private
41
insurance plan or government payor is a prerequisite to the shipment of Korlym to a patient. In order to conclude that the price is fixed or determinable, we must be able to (i) calculate gross product revenues from the sales to our customers and (ii) reasonably estimate net product revenues .
Starting January 1, 2016, we began recognizing sales to our specialty distributor (SD) at the time of delivery to the SD. Before that date, we did not recognize these sales until the SD had in turn delivered to its customers. Sales to the SD were less than two percent of our revenue in the year ended December 31, 2016.
We donate cash to NORD, an independent non-profit organization that helps patients with financial need pay for the treatment of Cushing syndrome. We do not include in revenue payments we receive from NORD .
We calculate gross product revenues based on the price we charge our customers. We estimate our net product revenues by deducting from our gross product revenues (a) estimated government rebates and chargebacks, (b) estimated costs of our patient co-pay assistance program, (c) trade allowances, such as discounts for prompt payment and (d) reserves for expected product returns. We initially record estimates for these deductions at the time we recognize the gross revenue. We update our estimates as new information becomes available .
Rebates and Chargebacks
We contract with Medicaid and other government agencies so that Korlym will be eligible for purchase by, or qualify for partial or full reimbursement from, Medicaid and other government programs. We estimate our rebate and chargeback amounts by applying the discount rates applicable to each government-funded program against our sales to patients covered by such program s.
Allowances for Patient Assistance Program
We provide financial assistance to eligible patients whose insurance policies require them to pay high deductibles and co-payments. We calculate the cost of assistance by applying our program guidelines to the eligible sales in the period .
Sales Returns
We estimate the amount of Korlym that we believe will be returned and deduct that estimated amount from gross revenue at the time we recognize such revenue. When estimating future returns, we analyze quantitative and qualitative information including, but not limited to, actual return rates, the amount of product in the distribution channel, the expected shelf life of such product, current and projected product demand, the introduction of competing products that may erode demand, and broad economic and industry-wide indicators. If we cannot reasonably estimate product returns with respect to a particular sale, we defer recognition of revenue from that sale until we can make a reasonable estimate.
Inventory and Cost of Sales
Regulatory approval of product candidates is uncertain. Because product manufactured prior to regulatory approval may not be sold unless regulatory approval is obtained, we expense manufacturing costs for product candidates as research and development expenses at the time such costs are incurred. We capitalize into inventory manufacturing costs related to an approved product that are incurred after regulatory approval.
We value our inventories at the lower of cost or net realizable value. We determine the cost of inventory using the specific identification method, which approximates a first-in, first-out basis. We write down inventory that has become obsolete or has a cost basis in excess of its expected net realizable value. Any expired inventory is disposed of and the related costs are recognized as cost of sales in the statement of comprehensive income (loss) for that period .
42
Cost of sales includes the cost of product (i.e., the cost of manufacturing Korlym, including material, third-party manufacturing costs and indirect personnel and other overhead costs) based on units for which revenue is recognized in the current period, as well as costs of stability testing, logistics and distribution.
Inventory amounts that are not expected to be consumed within 12 months following the balance sheet date are classified as strategic inventory, a noncurrent asset .
Accruals of Research and Development Costs
We recorded accruals for estimated costs of research, pre-clinical and clinical studies, and manufacturing development, which activities represent a major component of our research and development expenses. We make significant judgments and estimates in determining the accrual balance in each reporting period. Accrued clinical trial costs are based on estimates of the work completed under the service agreements, milestones achieved, patient enrollment and past experience with similar contracts and service providers. Our estimate of the work completed, and associated costs to be accrued, includes our assessment of the information received from our third-party contract research organizations and the overall status of our clinical trial activities. In the past, we have not experienced any material deviations between accrued and actual clinical trial expenses. However, actual services performed, number of patients enrolled and the rate of patient enrollment may vary from our estimates, resulting in adjustments to clinical trial expense in future periods.
Stock-based compensation
We have granted stock options to our employees, directors and consultants. We account for stock-based compensation related to option grants under the fair value method based on the value of the award at the grant date using the Black-Scholes option valuation model and we recognize expense over the requisite service period, net of estimated forfeitures . Determining an estimate of the fair value of equity awards using the Black-Scholes valuation model requires the use of subjective assumptions related to expected stock price volatility, term, risk-free interest rate and dividend yield.
We recognize the expense of options granted to non-employees based on their fair-value at the time of vesting .
Long-term obligation
The accounting for the Financing Agreement with Biopharma requires us to make certain estimates and assumptions, including the timing of royalty payments due to Biopharma, the expected rate of return to Biopharma, the split between current and long-term portions of the obligation, and the accretion of interest expense. Actual payment amounts will be based on Korlym receipts during the applicable quarter. In no event will the total amount paid to Biopharma exceed $45.0 million.
Recently Issued Accounting Pronouncements
See Note 1, Basis of Presentation and Summary of Significant Accounting Policies in our audited financial statements.
ITEM 7A. QUANTITATIVE AND QUA LITATIVE DISCLOSURES ABOUT MARKET RISK
Quantitative and Qualitative Disclosures About Market Risk
The primary objective of our investment activities is to preserve principal. As of December 31, 2016, the fair value of our cash and cash equivalents was $51.5 million and consisted primarily of a money market fund maintained at a major U.S. financial institution that invests primarily in short-term U.S. Treasury notes and bills. To minimize our exposure to interest rate and other market risks, we have limited the maturities of our investments to less than two years with an average maturity not to exceed one year. Due to the short-term nature of these instruments, a 10 percent increase or decrease in market interest rates would not have a material impact on the total value of our portfolio as of December 31, 2016.
43
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this item are set forth beginning at page F-1 of this report and are incorporated herein by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC's rules and forms, and that such information is accumulated and discussed with our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable and not absolute assurance of achieving the desired control objectives. In reaching a reasonable level of assurance, management necessarily has applied its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and we cannot assure you that any design will succeed in achieving its stated goals under all potential future conditions; over time, control may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
As of December 31, 2016, our Chief Executive Officer and Chief Financial Officer have evaluated our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) which were designed to ensure that the information required to be disclosed by us in this Annual Report on Form 10-K was recorded, processed, summarized and reported within the time periods specified in the SEC's rules and on Form 10-K. Our disclosure controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our disclosure control system are met. Based on the evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective.
There were no changes in our internal controls over financial reporting during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
(b) Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control system is designed to provide reasonable assurance regarding the preparation and fair presentation of financial statements for external purposes in accordance with generally accepted accounting principles. All internal control systems, no matter how well designed, have inherent limitations and can provide only reasonable assurance that the objectives of the internal control system are met.
Our management, including our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of our internal control over financial reporting, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO)
44
in 2013. Based on this evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2016.
Our independent registered public accounting firm has issued an attestation report on our internal control over financial reporting as included below.
(c) Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Corcept Therapeutics Incorporated
We have audited Corcept Therapeutics Incorporated's internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Corcept Therapeutics Incorporated's management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Corcept Therapeutics Incorporated maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the 2016 financial statements of Corcept Therapeutics Incorporated and our report dated March 6, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Redwood City, California
March 6, 2017
45
ITEM 9B. OTHE R INFORMATION
None.
46
PART III
Certain information required by Part III is omitted from this Annual Report on Form 10-K because we expect to file with the U.S. Securities and Exchange Commission, not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K, a definitive proxy statement (the Proxy Statement), pursuant to Regulation 14A in connection with the solicitation of proxies for our 2017 Annual Meeting of Stockholders, and certain information included therein is incorporated herein by reference.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item will be included in the Proxy Statement and is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
The information required by this Item will be included in the Proxy Statement and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item will be included in the Proxy Statement and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item will be included in the Proxy Statement and is incorporated herein by reference.
ITEM 14. P RINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item will be included in the Proxy Statement and is incorporated herein by reference.
47
PAR T IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
The following documents are filed as part of this Form 10-K
(1) Financial Statements:
|
| Page |
Report of Independent Registered Public Accounting Firm |
| F-2 |
|
|
|
Audited Financial Statements |
|
|
|
|
|
Balance Sheets |
| F-3 |
|
|
|
Statements of Comprehensive Income (Loss) |
| F-4 |
|
|
|
Statement of Stockholders' Equity (Deficit) |
| F-5 |
|
|
|
Statements of Cash Flows |
| F-6 |
|
|
|
Notes to Financial Statements |
| F-7 |
(2) Financial Statement Schedules:
All schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
(3) Exhibits:
Item 601 of Regulation S-K requires the exhibits listed below. Each management contract or compensatory plan or arrangement required to be filed as an exhibit to this Form 10-K has been identified.
(A) EXHIBITS
Exhibit Number |
| Description of Document |
|
|
|
3.1 |
| Amended and Restated Certificate of Incorporation, as amended (incorporated by reference to Exhibit 3.1 to the registrant's Quarterly Report on Form 10-Q filed on August 9 2012). |
|
|
|
3.2 |
| Amended and Restated Bylaws (incorporated by reference to Exhibit 3.1 to the registrant's Current Report on Form 8-K filed on February 13, 2017). |
|
|
|
4.1 |
| Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the registrant's Registration Statement on Form S-1 (Registration No. 333-112676) filed on February 10, 2004). |
|
|
|
4.2 |
| Registration Rights Agreement by and among Corcept Therapeutics Incorporated and the investors signatory thereto, dated March 14, 2008 (incorporated by reference to Exhibit 10.25 to the registrant's Annual Report on Form 10-K filed on March 31, 2008). |
|
|
|
4.3 |
| Amendment to Registration Rights Agreement by and among Corcept Therapeutics Incorporated and the investors signatory thereto, dated November 11, 2008 (incorporated by reference to Exhibit 10.30 to the registrant's Annual Report on Form 10-K filed on March 31, 2009). |
|
|
|
4.4 |
| Registration Rights Agreement dated as of April 21, 2010 by and among Corcept Therapeutics Incorporated and the investors signatory thereto (incorporated by reference to Exhibit 4.2 to the registrant's Current Report on Form 8-K filed on April 23, 2010). |
|
|
|
48
Exhibit Number |
| Description of Document |
|
|
|
4.5 |
| Registration Rights Agreement, dated as of March 29, 2012, by and among Corcept Therapeutics Incorporated and the investors signatory thereto (incorporated by reference to Exhibit 4.2 to the registrant's Current Report on Form 8-K filed on March 29, 2012). |
|
|
|
10.1 |
| License Agreement by and between The Board of Trustees of the Leland Stanford Junior University and Corcept Therapeutics Incorporated, dated as of July 1, 1999 (incorporated by reference to Exhibit 10.6 to the registrant's Registration Statement on Form S-1 (Registration No. 333-112676) filed on February 10, 2004). |
|
|
|
10.2# |
| Manufacturing Agreement with Produits Chimiques Auxiliaires et de Synthese SA, dated November 8, 2006 (incorporated by reference to Exhibit 10.15 to the registrant's Annual Report on Form 10-K filed on April 2, 2007). |
|
|
|
10.3† |
| Form of Indemnification Agreement for directors and officers approved by the Board of Directors on September 24, 2007 (incorporated by reference to Exhibit 10.7 to the registrant's Quarterly Report on Form 10-Q filed on November 14, 2007). |
|
|
|
10.4 |
| Securities Purchase Agreement by and among Corcept Therapeutics Incorporated and the purchasers named therein, dated March 14, 2008 (incorporated by reference to Exhibit 10.24 to the registrant's Annual Report on Form 10-K filed on March 31, 2008). |
|
|
|
10.5† |
| Amended and Restated Severance and Change in Control Agreement by and between Corcept Therapeutics Incorporated and Joseph K. Belanoff, M. D., dated September 19, 2008 (incorporated by reference to Exhibit 10.25 to the registrant's Annual Report on Form 10-K filed on March 31, 2009). |
|
|
|
10.6† |
| Amended and Restated Severance and Change in Control Agreement by and between Corcept Therapeutics Incorporated and James N. Wilson, dated September 19, 2008 (incorporated by reference to Exhibit 10.28 to the registrant's Annual Report on Form 10-K filed on March 31, 2009). |
|
|
|
10.7 |
| Securities Purchase Agreement by and among Corcept Therapeutics Incorporated and the purchasers named therein, dated October 12, 2009 (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed on November 12, 2009). |
|
|
|
10.8† |
| Amended and Restated 2004 Equity Incentive Plan (incorporated by reference to the registrant's Proxy Statement on Schedule 14A filed on May 7, 2009). |
|
|
|
10.9† |
| Form of Option Agreement for options granted pursuant to the Amended and Restated 2004 Equity Incentive Plan (incorporated by reference to Exhibit 10.25 to the registrant's Annual Report on Form 10-K filed on March 15, 2011). |
|
|
|
10.10† |
| Severance and Change in Control Agreement by and between Corcept Therapeutics Incorporated and G. Charles Robb, dated September 1, 2011 (incorporated by reference to Exhibit 10.2 to the registrant's Quarterly Report on Form 10-Q filed on November 8, 2011). |
|
|
|
10.11† |
| Employment offer letter to G. Charles Robb dated August 12, 2011 (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed on November 8, 2011). |
|
|
|
10.12# |
| Commercial Outsourcing Services Agreement with Integrated Commercialization Solutions, Inc., dated as of April 14, 2011 (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed on August 9, 2012). |
|
|
|
10.13† |
| Corcept Therapeutics Incorporated 2012 Incentive Award Plan (incorporated by reference to Appendix A to the registrant's Definitive Proxy Statement on Schedule 14A filed with the SEC on May 21, 2012). |
|
|
|
10.14† |
| Form of 2012 Incentive Award Plan Stock Option Grant Notice and Agreement (incorporated by reference to Exhibit 4.5 to the registrant's Registration Statement on Form S-8 filed with the SEC on August 13, 2012). |
|
|
|
49
Exhibit Number |
| Description of Document |
|
|
|
10.1 5 # |
| Purchase and Sale Agreement with Biopharma Secured Debt Fund II Sub, S.à r.l,, dated as of August 2, 2012 (incorporated by reference to Exhibit 10.4 to the registrant's Quarterly Report on Form 10-Q filed on November 8, 2012). |
|
|
|
10.16 |
| Amendment to Manufacturing Agreement with Produits Chimiques Auxiliaires et de Synthese SA, dated February 21, 2013 (incorporated by reference to Exhibit 10.31 to the registrant's Annual Report on Form 10-K filed on March 15, 2013). |
|
|
|
10.17# |
| Pharmaceutical Manufacturer Services Agreement with Centric Health Resources, Inc., dated May 21, 2013 (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed on August 9, 2013). |
|
|
|
10.18# |
| Amendment to Pharmaceutical Manufacturer Services Agreement with Centric Health Resources, Inc., dated July 22, 2013 (incorporated by reference to Exhibit 10.3 to the registrant's Quarterly Report on Form 10-Q filed on August 9, 2013). |
|
|
|
10.19 |
| Amendment to Manufacturing Agreement with Produits Chimiques Auxiliaires et de Synthese SA, dated August 1, 2013 (incorporated by reference to Exhibit 10.4 to the registrant's Quarterly Report on Form 10-Q filed on August 9, 2013). |
|
|
|
10.20 |
| Amendment to Manufacturing Agreement with Produits Chimiques Auxiliaires et de Synthese SA, dated November 7, 2013 (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed on November 12, 2013). |
|
|
|
10.21 |
| Amendment to Manufacturing Agreement with Produits Chimiques Auxiliaires et de Synthese SA, dated January 27, 2014 (incorporated by reference to Exhibit 10.34 to the registrant's Annual Report on Form 10-K filed on March 14, 2014). |
|
|
|
10.22# |
| Manufacturing and Supply Agreement with Produits Chimiques Auxiliaires et de Synthese SA, dated March 20, 2014 (incorporated by reference to Exhibit 10.2 to the registrant's Quarterly Report on Form 10-Q filed on May 12, 2014). |
|
|
|
10.23 |
| First Amendment to the Commercial Outsourcing Services Agreement with Integrated Commercialization Solutions, Inc., effective as of April 14, 2014 (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed on August 8, 2014). |
|
|
|
10.24# |
| Manufacturing Agreement with AAI Pharma Services Corp., dated April 7, 2014 (incorporated by reference to Exhibit 10.2 to the registrant's Quarterly Report on Form 10-Q filed on August 8, 2014). |
|
|
|
10.25 |
| Second Amendment to the Commercial Outsourcing Services Agreement with Integrated Commercialization Solutions, Inc., effective as of June 11, 2014 (incorporated by reference to Exhibit 10.3 to the registrant's Quarterly Report on Form 10-Q filed on August 8, 2014). |
|
|
|
10.26 |
| Third Amendment to the Commercial Outsourcing Services Agreement with Integrated Commercialization Solutions, Inc., effective as of August 11, 2014 (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed on November 7, 2014). |
|
|
|
10.27# |
| Second Amendment to Pharmaceutical Manufacturer Services Agreement with Dohmen Life Science Services, LLC (as successor in interest to Centric Health Resources, Inc.) dated October 6, 2014 (incorporated by reference to Exhibit 10.41to the registrant's Annual Report on Form 10K filed on March 13, 2015). |
|
|
|
10.28† |
| Employment offer letter to Robert S. Fishman dated September 16, 2015 (incorporated by reference to Exhibit 10.2 to the registrant's Quarterly Report on Form 10-Q filed on November 6, 2015). |
|
|
|
10.29 † |
| Severance and Change in Control Agreement by and between Corcept Therapeutics Incorporated and Robert S. Fishman, dated September 28, 2015 (incorporated by reference to Exhibit 10.3 to the registrant's Quarterly Report on Form 10-Q filed on November 6, 2015). |
|
|
|
23.1 |
| Consent of Independent Registered Public Accounting Firm |
50
Exhibit Number |
| Description of Document |
|
|
|
|
|
|
24.1 |
| Power of Attorney (See signature page) |
|
|
|
31.1 |
| Certification pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934 of Joseph K. Belanoff, M.D. |
|
|
|
31.2 |
| Certification pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934 of G. Charles Robb |
|
|
|
32.1 |
| Certification pursuant to 18 U.S.C. Section 1350 of Joseph K. Belanoff, M.D. |
|
|
|
32.2 |
| Certification pursuant to 18 U.S.C. Section 1350 of G. Charles Robb |
|
|
|
101 |
| The following materials from the registrant's Annual Report on Form 10-K for the year ended December 31, 2016, formatted in Extensible Business Reporting Language (XBRL): (i) Balance Sheets at December 31, 2016 and 2015, (ii) Statements of Comprehensive Income (loss) for the Years Ended December 31, 2016, 2015 and 2014, (iii) Statements of Stockholders' Equity (Deficit) for the Years Ended December 31, 2016, 2015 and 2014, (iv) Statements of Cash Flows for the Years Ended December 31, 2016, 2015 and 2014, and (v) Notes to Financial Statements. |
# | Confidential treatment granted |
† | Management contract or compensatory plan or arrangement |
51
SIGNA TURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| CORCEPT THERAPEUTICS INCORPORATED | |
| By: | /s/ JOSEPH K. BELANOFF |
|
| Joseph K. Belanoff, M.D., |
|
| Chief Executive Officer and President |
| Date: | March 6, 2017 |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Joseph K. Belanoff and G. Charles Robb, and each of them acting individually, as his or her true and lawful attorneys-in-fact and agents, each with full power of substitution, for him or her in any and all capacities, to sign any and all amendments to this report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, with full power of each to act alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Exchange Act, this Annual Report on Form 10-K has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
Signature |
| Title |
| Date |
/s/ JOSEPH K. BELANOFF |
| Chief Executive Officer, President and Director |
| March 6, 2017 |
Joseph K. Belanoff, M.D. |
| (Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ G. CHARLES ROBB |
| Chief Financial Officer and Secretary |
| March 6, 2017 |
G. Charles Robb |
| (Principal Financial Officer) |
|
|
|
|
|
|
|
/s/ JAMES N. WILSON |
| Director and Chairman of the Board of Directors |
| March 6, 2017 |
James N. Wilson |
|
|
|
|
|
|
|
|
|
/s/ G. LEONARD BAKER, JR. |
| Director |
| March 6, 2017 |
G. Leonard Baker, Jr. |
|
|
|
|
|
|
|
|
|
/s/ DANIEL M. BRADBURY |
| Director |
| March 6, 2017 |
Daniel M. Bradbury |
|
|
|
|
|
|
|
|
|
/s/ RENEE D. GALA |
| Director |
| March 6, 2017 |
Renee D. Gala |
|
|
|
|
|
|
|
|
|
/s/ PATRICK G. ENRIGHT |
| Director |
| March 6, 2017 |
Patrick G. Enright |
|
|
|
|
|
|
|
|
|
/s/ DAVID L. MAHONEY |
| Director |
| March 6, 2017 |
David L. Mahoney |
|
|
|
|
|
|
|
|
|
/s/ DANIEL N. SWISHER, JR |
| Director |
| March 6, 2017 |
Daniel N. Swisher, Jr. |
|
|
|
|
52
CORCEPT THERAPEUTICS INCORPORATED
INDEX TO FINANCIAL STATEMENTS
|
| Page |
Report of Independent Registered Public Accounting Firm |
| F-2 |
|
|
|
Audited Financial Statements |
|
|
|
|
|
Balance Sheets |
| F-3 |
|
|
|
Statements of Comprehensive Income (Loss) |
| F-4 |
|
|
|
Statement of Stockholders' Equity (Deficit) |
| F-5 |
|
|
|
Statements of Cash Flows |
| F-6 |
|
|
|
Notes to Financial Statements |
| F-7 |
F-1
REPORT OF INDEPENDENT REGIS TERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Corcept Therapeutics Incorporated
We have audited the accompanying balance sheets of Corcept Therapeutics Incorporated as of December 31, 2016 and 2015 and the related statements of comprehensive income (loss), stockholders' equity (deficit) and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States.) Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Corcept Therapeutics Incorporated at December 31, 2016 and 2015, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Corcept Therapeutics Incorporated's internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 6, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Redwood City, California
March 6, 2017
F-2
CORCEPT THERAPEUTICS INCORPORATED
BALANCE SHEETS
(in thousands, except per share amounts)
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
ASSETS |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
| $ | 51,536 |
|
| $ | 40,435 |
|
Trade receivables, net of allowances |
|
| 9,860 |
|
|
| 6,221 |
|
Inventory |
|
| 2,329 |
|
|
| 1,682 |
|
Prepaid expenses and other current assets |
|
| 1,964 |
|
|
| 642 |
|
Total current assets |
|
| 65,689 |
|
|
| 48,980 |
|
Strategic inventory |
|
| 2,835 |
|
|
| 2,800 |
|
Property and equipment, net of accumulated depreciation |
|
| 205 |
|
|
| 98 |
|
Other assets |
|
| 24 |
|
|
| 24 |
|
Total assets |
| $ | 68,753 |
|
| $ | 51,902 |
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 2,290 |
|
| $ | 1,325 |
|
Accrued clinical expenses |
|
| 1,467 |
|
|
| 1,171 |
|
Other accrued liabilities |
|
| 8,953 |
|
|
| 3,257 |
|
Long-term obligation - current portion |
|
| 14,664 |
|
|
| 14,965 |
|
Deferred revenue |
|
| - |
|
|
| 158 |
|
Total current liabilities |
|
| 27,374 |
|
|
| 20,876 |
|
Long-term obligation, net of current portion |
|
| - |
|
|
| 12,528 |
|
Commitments (Note 11) |
|
|
|
|
|
|
|
|
Stockholders' equity: |
|
|
|
|
|
|
|
|
Preferred stock, par value $0.001 per share, 10,000 shares authorized and no shares outstanding at December 31, 2016 and December 31, 2015 |
|
| - |
|
|
| - |
|
Common stock, par value $0.001 per share, 280,000 shares authorized and 112,710 and 109,642 shares issued and outstanding at December 31, 2016 and December 31, 2015, respectively |
|
| 113 |
|
|
| 110 |
|
Additional paid-in capital |
|
| 363,534 |
|
|
| 348,796 |
|
Accumulated deficit |
|
| (322,268 | ) |
|
| (330,408 | ) |
Total stockholders' equity |
|
| 41,379 |
|
|
| 18,498 |
|
Total liabilities and stockholders' equity |
| $ | 68,753 |
|
| $ | 51,902 |
|
The accompanying notes are an integral part of these financial statements.
F-3
CORCEPT THERAPEUTICS INCORPORATED
STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(in thousands, except per share amounts)
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Product revenue, net |
| $ | 81,321 |
|
| $ | 50,286 |
|
| $ | 26,551 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales |
|
| 2,058 |
|
|
| 1,361 |
|
|
| 882 |
|
Research and development |
|
| 23,844 |
|
|
| 15,419 |
|
|
| 18,372 |
|
Selling, general and administrative |
|
| 45,240 |
|
|
| 36,949 |
|
|
| 34,916 |
|
Total operating expenses |
|
| 71,142 |
|
|
| 53,729 |
|
|
| 54,170 |
|
Income (Loss) from operations |
|
| 10,179 |
|
|
| (3,443 | ) |
|
| (27,619 | ) |
Interest and other expense |
|
| (2,039 | ) |
|
| (2,965 | ) |
|
| (3,764 | ) |
Net income (loss) and comprehensive income (loss) |
| $ | 8,140 |
|
| $ | (6,408 | ) |
| $ | (31,383 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net income (loss) per common share |
| $ | 0.07 |
|
| $ | (0.06 | ) |
| $ | (0.31 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding used in computing net income (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
| 110,566 |
|
|
| 106,883 |
|
|
| 100,978 |
|
Diluted |
|
| 116,139 |
|
|
| 106,883 |
|
|
| 100,978 |
|
The accompanying notes are an integral part of these financial statements.
F-4
CORCEPT THERAPEUTICS INCORPORATED
STATEMENTS OF STOCKHOLDERS EQUITY (DEFICIT)
(in thousands)
| Common Stock |
|
| Additional Paid-in Capital |
|
| Accumulated Deficit |
|
| Total Stockholders' Equity (Deficit) |
| ||||||||
| Shares |
|
| Amount |
|
|
|
|
|
|
|
|
|
|
|
|
| ||
Balance at December 31, 2013 |
| 99,849 |
|
| $ | 100 |
|
| $ | 313,534 |
|
| $ | (292,617 | ) |
| $ | 21,017 |
|
Issuance of common stock upon exercise of options |
| 1,381 |
|
|
| 1 |
|
|
| 1,776 |
|
|
| - |
|
|
| 1,777 |
|
Issuance of common stock upon exercise of warrants |
| 165 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
Stock-based compensation related to employee and director options |
| - |
|
|
| - |
|
|
| 4,731 |
|
|
| - |
|
|
| 4,731 |
|
Stock-based compensation related to non-employee options |
| - |
|
|
| - |
|
|
| 470 |
|
|
| - |
|
|
| 470 |
|
Net loss and comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
| (31,383 | ) |
|
| (31,383 | ) |
Balance at December 31, 2014 |
| 101,395 |
|
|
| 101 |
|
|
| 320,511 |
|
|
| (324,000 | ) |
|
| (3,388 | ) |
Issuance of common stock upon exercise of options |
| 2,041 |
|
|
| 3 |
|
|
| 5,190 |
|
|
| - |
|
|
| 5,193 |
|
Issuance of common stock upon exercise of warrants, net |
| 6,206 |
|
|
| 6 |
|
|
| 17,082 |
|
|
| - |
|
|
| 17,088 |
|
Stock-based compensation related to employee and director options |
| - |
|
|
| - |
|
|
| 5,926 |
|
|
| - |
|
|
| 5,926 |
|
Stock-based compensation related to non-employee options |
| - |
|
|
| - |
|
|
| 87 |
|
|
| - |
|
|
| 87 |
|
Net loss and comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|
| (6,408 | ) |
|
| (6,408 | ) |
Balance at December 31, 2015 |
| 109,642 |
|
|
| 110 |
|
|
| 348,796 |
|
|
| (330,408 | ) |
|
| 18,498 |
|
Issuance of common stock upon exercise of options |
| 3,068 |
|
|
| 3 |
|
|
| 7,680 |
|
|
| - |
|
|
| 7,683 |
|
Stock-based compensation related to employee and director options |
| - |
|
|
| - |
|
|
| 7,002 |
|
|
| - |
|
|
| 7,002 |
|
Stock-based compensation related to non-employee options |
| - |
|
|
| - |
|
|
| 56 |
|
|
| - |
|
|
| 56 |
|
Net income and comprehensive income |
|
|
|
|
|
|
|
|
|
|
|
|
| 8,140 |
|
|
| 8,140 |
|
Balance at December 31, 2016 |
| 112,710 |
|
| $ | 113 |
|
| $ | 363,534 |
|
| $ | (322,268 | ) |
| $ | 41,379 |
|
The accompanying notes are an integral part of these financial statements
F-5
CORCEPT THERAPEUTICS INCORPORATED
STATEMENTS OF CASH FLOWS
(in thousands)
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
| $ | 8,140 |
|
| $ | (6,408 | ) |
| $ | (31,383 | ) |
Adjustments to reconcile net income (loss) to net cash generated from (used in) operations: |
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
| 7,058 |
|
|
| 6,013 |
|
|
| 5,201 |
|
Accretion of interest expense |
|
| 1,929 |
|
|
| 2,848 |
|
|
| 3,678 |
|
Amortization of debt financing costs |
|
| 21 |
|
|
| 22 |
|
|
| 29 |
|
Depreciation and amortization of property and equipment |
|
| 87 |
|
|
| 155 |
|
|
| 141 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Trade receivables |
|
| (3,639 | ) |
|
| (2,887 | ) |
|
| (1,906 | ) |
Inventory |
|
| (682 | ) |
|
| 815 |
|
|
| 249 |
|
Prepaid expenses and other current assets |
|
| (1,322 | ) |
|
| 799 |
|
|
| (531 | ) |
Other assets |
|
| - |
|
|
| (7 | ) |
|
| 10 |
|
Accounts payable |
|
| 965 |
|
|
| (561 | ) |
|
| (495 | ) |
Accrued clinical expenses |
|
| 296 |
|
|
| 835 |
|
|
| (2,952 | ) |
Other accrued liabilities |
|
| 5,696 |
|
|
| 1,381 |
|
|
| 575 |
|
Deferred revenue |
|
| (158 | ) |
|
| 125 |
|
|
| 8 |
|
Net cash provided by (used in) operating activities |
|
| 18,391 |
|
|
| 3,130 |
|
|
| (27,376 | ) |
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
| (194 | ) |
|
| (17 | ) |
|
| (174 | ) |
Cash used in investing activities |
|
| (194 | ) |
|
| (17 | ) |
|
| (174 | ) |
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from exercise of warrants, net of issuance costs |
|
| - |
|
|
| 17,088 |
|
|
| - |
|
Proceeds from exercise of stock options, net of issuance costs |
|
| 7,683 |
|
|
| 5,193 |
|
|
| 1,777 |
|
Payments related to long-term obligation |
|
| (14,779 | ) |
|
| (9,207 | ) |
|
| (4,856 | ) |
Net cash provided by (used in) financing activities |
|
| (7,096 | ) |
|
| 13,074 |
|
|
| (3,079 | ) |
Net increase (decrease) in cash and cash equivalents |
|
| 11,101 |
|
|
| 16,187 |
|
|
| (30,629 | ) |
Cash and cash equivalents, at beginning of period |
|
| 40,435 |
|
|
| 24,248 |
|
|
| 54,877 |
|
Cash and cash equivalents, at end of period |
| $ | 51,536 |
|
| $ | 40,435 |
|
| $ | 24,248 |
|
The accompanying notes are an integral part of these financial statements
F-6
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS
1. Basis of Presentation and Summary of Significant Accounting Policies
Description of Business and Basis of Presentation
Corcept Therapeutics Incorporated was incorporated in the State of Delaware in May 1998, and our headquarters are located in Menlo Park, California. We are a pharmaceutical company engaged in the discovery, development and commercialization of medications that treat severe metabolic, oncologic, and psychiatric disorders by modulating the effect of the stress hormone cortisol. In 2012, the United States Food and Drug Administration (FDA) approved Korlym ® (mifepristone) 300 mg tablets as a once-daily oral medication for treatment of hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing syndrome who have type 2 diabetes mellitus or glucose intolerance and have failed surgery or are not candidates for surgery. We have discovered and patented three structurally distinct series of selective cortisol modulators, consisting of more than 300 compounds. We are developing the lead compounds from these series to treat a broad range of disorders .
Basis of Presentation
The financial statements have been prepared in accordance with U.S. generally accepted accounting principles ("U.S. GAAP").
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ materially from those estimates.
We evaluate our estimates and assumptions on an ongoing basis, including those related to revenue recognition, sales returns, inventory, allowances for doubtful accounts, accrued liabilities including our bonus accrual, clinical trial accruals, stock-based compensation and the timing of payments with respect to our long-term capped royalty obligation, which determines our interest expense. We base our estimates on relevant experience and on other specific assumptions that we believe are reasonable.
Fair Value Measurements
We categorize financial instruments in a fair value hierarchy that prioritizes the information used to develop assumptions for measuring fair value. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities (Level 1 input), then to quoted prices in non-active markets or in active markets for similar assets or liabilities, inputs other than quoted prices that are observable for the asset or liability, and inputs that are not directly observable, but that are corroborated by observable market data for the asset or liability (Level 2 input), then the lowest priority to unobservable inputs, such as our own data about the assumptions that market participants would use in pricing an asset or liability (Level 3 input). Fair value is a market-based measurement and should therefore be based on the assumptions that third-party market participants would use in pricing the asset or liability.
Cash and Cash Equivalents
We consider all highly liquid investments purchased with maturities of three months or less from the date of purchase to be cash equivalents. Cash equivalents are carried at fair value as measured using Level 1 inputs, which approximates cost. As of December 31, 2016 and December 31, 2015, all of our funds were held in checking and money market fund accounts maintained at major U.S. financial institutions.
F-7
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
Credit and Concentration Risks
Our cash and cash equivalents are held in one financial institution. We are exposed to credit and concentration risks in the event of default by the financial institution holding our funds and investments or by the entity or entities that issued the securities held by the funds to the extent of the amount recorded on our balance sheet. We mitigate these risks by investing in money market funds that invest primarily in short-term U.S. Treasury notes and bills. We have never experienced a loss or lack of access to our operating or investment accounts.
Among other services, Dohmen Life Science Services ("Dohmen"), a specialty pharmacy, dispenses Korlym to patients for us, with title to the medicine passing from us to the patient upon the patient's receipt of the drug. Accordingly, our receivables risk is spread among various third-party payors – pharmacy benefit managers, insurance companies, private charities, government programs – and individual patients. We extend credit to third-party payors based on their creditworthiness. We monitor our exposure and record an allowance against uncollectible trade receivables as necessary. To date, we have not incurred any credit losses.
We have a concentration of risk in regard to the manufacture and distribution of our product. As of December 31, 2016, we had one tablet manufacturer for Korlym – Alcami Corporation (formerly known as AAI Pharma Services Corp.). In addition, we have a single-source manufacturer of mifepristone, the active pharmaceutical ingredient (API), in Korlym – Produits Chimiques Auxiliaires et de Synthèse SA (PCAS). If either of these companies is unable to manufacture API or Korlym tablets in the quantities and time frame required, we may not be able to manufacture our product in a timely manner. In order to mitigate these risks related to the manufacture of our product, we purchased and hold in inventory additional quantities of mifepristone API and Korlym tablets. Dohmen is our sole specialty pharmacy. Its unwillingness or inability to dispense Korlym to patients in a timely manner would harm our business.
Inventory
We value our inventories at the lower of cost or net realizable value. We determine the cost of inventory using the specific identification method, which approximates a first-in, first-out basis. We write down inventory that has become obsolete or has a cost basis in excess of its expected net realizable value. Any expired inventory is disposed of and the related costs are recognized as cost of sales in the statement of comprehensive income (loss) in that period.
Inventory amounts that are not expected to be consumed within 12 months following the balance sheet date are classified as strategic inventory, a noncurrent asset.
We expense the manufacturing costs for product candidates incurred prior to regulatory approval as research and development expense as we incur them. We begin capitalizing costs related to the manufacture of a product candidate when we obtain regulatory approval to begin marketing that product.
Long-term Obligation
In August 2012, we entered into a Purchase and Sale Agreement (Financing Agreement) with Biopharma Secured Debt Fund II Sub, S.à r.l (Biopharma), a private limited liability company organized under the laws of Luxembourg. Under the terms of the Financing Agreement, we received $30.0 million from Biopharma, which upon receipt we recorded as a long-term obligation. In return, we are obligated to make payments to Biopharma totaling $45.0 million. These payments equal a percentage of (i) our net product sales, which include sales from any product containing mifepristone or any of our proprietary selective cortisol modulators (Covered Products), and (ii) cash or cash equivalents received from any licensing transaction or co-promotion arrangement involving Covered Products, including any upfront or milestone payments, if any (together, Korlym Receipts). Once we have paid Biopharma a total of $45.0 million, no more payments will be due and the obligation will be extinguished.
F-8
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
We recognize a portion of ea ch quarterly payment under the Financing Agreement as interest expense, which we determine by calculating the interest rate to Biopharma implied by the stream of quarter ly payments we expect to make. The amount shown on our balance sheet as the current por tion is an estimate of the amount we expect to repay Biopharma with in the 12 months follo wing December 31, 2016. We record the balance of the outstanding portion of the obligation, if any, as a long-term liability.
Our estimate of the amount and timing of our quarterly payments to Biopharma is subject to uncertainty and may change. Any changes in our assumed payment stream will change the accretion of interest expense and our split between the current and long-term portions of the obligation, although the total we will pay Biopharma is fixed at $45.0 million.
See Note 5, Long-Term Obligation , for additional information regarding this agreement.
Net Product Sales
We primarily sell Korlym directly to patients through Dohmen, a specialty pharmacy. Prior authorization and confirmation of coverage by the patients' private or government insurance plan or by a third-party charity is a prerequisite for Dohmen to ship Korlym to a patient. We recognize revenue upon the delivery of Korlym to these patients.
We recognize revenue from sales of Korlym upon delivery to patients as long as (i) there is persuasive evidence that an arrangement exists between ourselves and the customer, (ii) collectability is reasonably assured and (iii) the price is fixed or determinable. Prior authorization or confirmation of coverage level by the patient's private insurance plan or government payor is a prerequisite to the shipment of Korlym to a patient. In order to conclude that the price is fixed or determinable, we must be able to (i) calculate gross product revenues from the sales to our customers and (ii) reasonably estimate net product revenues.
Effective January 1, 2016, we recognize sales to our specialty distributor (SD) at the time of sale to the SD. Before that date, we did not recognize these sales until the SD had in turn sold to its customers. Sales to the SD were less than two percent of our revenue in the year ended December 31, 2016.
We donate cash to the National Organization for Rare Disorders ("NORD"), an independent non-profit organization that helps patients with financial need pay for the treatment of Cushing syndrome. We do not include in revenue payments we receive from NORD.
We calculate gross product revenues based on the price we charge our customers. We estimate our net product revenues by deducting from our gross product revenues (a) estimated government rebates and chargebacks, (b) estimated costs of our patient co-pay assistance program, (c) trade allowances, such as discounts for prompt payment and (d) reserves for expected product returns. We initially record estimates for these deductions at the time we recognize the gross revenue. We update our estimates as new information becomes available.
Rebates and Chargebacks: We contract with Medicaid and other government agencies so that Korlym will be eligible for purchase by, or qualify for partial or full reimbursement from, Medicaid and other government programs. We estimate our rebate and chargeback amounts by applying the discount rates applicable to each government-funded program against our sales to patients covered by such programs.
F-9
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
Our reserve activity for d oubtful acco unts, prompt pay cash discounts and chargebacks is summarized as follows:
| Balance at Beginning of Period |
|
| Charges |
|
| Deductions |
|
| Balance at End of Period |
| ||||
| (in thousands) |
| |||||||||||||
Year ended December 31, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable allowances | $ | 12,543 |
|
| $ | 45,401 |
|
| $ | (39,609 | ) |
| $ | 18,335 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2016: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable allowances | $ | 18,335 |
|
| $ | 2,081,419 |
|
| $ | (1,749,074 | ) |
| $ | 350,680 |
|
There were no material changes in reserve estimates relating to prior periods.
Allowances for Patient Assistance Program: We provide financial assistance to eligible patients whose insurance policies require them to pay high deductibles and co-payments. We calculate the cost of assistance by applying our program guidelines to the eligible sales in the period.
Sales Returns : We estimate the amount of Korlym that we believe will be returned and deduct that estimated amount from gross revenue at the time we recognize such revenue. When estimating future returns, we analyze quantitative and qualitative information including, but not limited to, actual return rates, the amount of product in the distribution channel, the expected shelf life of such product, current and projected product demand, the introduction of competing products that may erode demand, and broad economic and industry-wide indicators. If we cannot reasonably estimate product returns with respect to a particular sale, we defer recognition of revenue from that sale until we can make a reasonable estimate.
Research and Development
Research and development expenses consist of direct expenses, such as the cost of discovery research, pre-clinical studies, and clinical trials relating to our portfolio of proprietary, selective cortisol modulators, manufacturing development, preparations for submissions to the FDA or other regulatory agencies and related overhead expenses. We expense nonrefundable payments to third-parties as well as the cost of technologies and materials used in research and development as they are incurred.
We base our cost accruals for research, preclinical activities, and clinical trials on estimates of work completed under service agreements, milestones achieved, patient enrollment and past experience with similar contracts. Our estimates of work completed and associated cost accruals include our assessments of information from third-party contract research organizations and the overall status of clinical trial and other development and administrative activities.
Segment Reporting
We determine our operating segments based on the way we organize our business to make operating decisions and assess performance. We have only one operating segment, which is the discovery, development and commercialization of pharmaceutical products.
Stock-Based Compensation
We account for stock-based compensation related to option grants under the fair value method, based on the value of the award at the grant date using the Black-Scholes option valuation model and we recognize expense over the requisite service period, net of estimated forfeitures. Employee stock-based compensation expense is calculated based on awards ultimately expected to vest and is reduced for estimated forfeitures. Forfeitures are revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates and an adjustment to stock-based compensation expense will be recognized at that time.
F-10
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
We recognize the expense of options granted to non-employees based on the fair-value based measurement of the option grants at the time of vesting.
Recently Issued Accounting Pronouncement
In May 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") No. 2014-09, "Revenue from Contracts with Customers." The standard states that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In August 2015, the FASB issued ASU No. 2015-14, "Revenue from Contracts with Customers: Deferral of the Effective Date," which deferred the effective date of ASU No. 2014-09. ASU No. 2014-09 will now be effective for us beginning January 1, 2018 and can be adopted on a full retrospective basis or on a modified retrospective basis. Early application is permitted in 2017. In March 2016, the FASB issued ASU No. 2016-08, "Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations," which clarifies the implementation guidance on principal versus agent considerations. In April 2016, the FASB issued ASU No. 2016-10, "Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing," which clarifies certain aspects of identifying performance obligations and licensing implementation guidance. In May 2016, the FASB issued ASU No. 2016-12, "Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients," related to disclosures of remaining performance obligations, as well as other amendments to guidance on collectability, non-cash consideration and the presentation of sales and other similar taxes collected from customers. We plan to adopt the accounting standard update using the modified retrospective approach, with the cumulative effect of adopting the update being recorded to our retained earnings on January 1, 2018. At present, we have only one source of revenue: the sale of Korlym to our customers. Our evaluation of the customer contracts governing these sales is still underway. Because each of our arrangements contain variable consideration, we have focused our analysis on how the update will affect our estimate of the transaction price. We are also reviewing our financial policies, procedures and controls and at the time we adopt the update will make appropriate changes to them. We have not completed our assessment of the adoption on our financial statements.
In August 2014, the FASB issued ASU No. 2014-15 (Subtopic 205-40), "Presentation of Financial Statements-Going Concern: Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern" ("ASU 2014-15"), which provides guidance about management's responsibility to evaluate whether or not there is substantial doubt about the Company's ability to continue as a going concern and to provide related footnote disclosure. ASU 2014-15 is effective for fiscal years, and interim periods within those fiscal years, ending after December 15, 2016. The adoption of this standard had no impact on our Financial Statements as we have generated positive cash flow in the years ended December 31, 2015 and 2016 and expect to generate positive cash flow in the year ended December 31, 2017.
In April 2015, the FASB issued ASU No. 2015-03, Simplifying the Presentation of Debt Issuance Costs (ASU 2015-03), which requires an entity to present such costs in the balance sheet as a direct deduction from the related debt liability rather than as an asset. Amortization of the costs will continue to be reported as interest expense. ASU 2015-03 is effective for fiscal years beginning after December 15, 2015 and interim periods within those fiscal years, with early adoption permitted. The new guidance will be applied retrospectively to each prior period presented. We retrospectively adopted ASU 2015-03 as of January 1, 2016, resulting in a $35,000 decrease to long-term assets and long-term debt as of December 31, 2015 on its balance sheets. The adoption of this standard had no impact on our Statement of Comprehensive Income (Loss).
In July 2015, the FASB issued ASU No. 2015-11, Simplifying the Measurement of Inventory (ASU 2015-11), which simplifies the measurement of inventory by requiring certain inventory to be measured at the lower of cost or net realizable value. The amendments in this ASU are effective for fiscal years beginning after December 15, 2016 and for interim periods therein, with early adoption permitted. We plan to adopt this new standard on January 1, 2017, and do not expect this to have a material impact on our Financial Statements.
In November 2015, the FASB issued ASU No. 2015-17 (ASU 2015-17) "Balance Sheet Classification of Deferred Taxes." ASU 2015-17 requires that deferred tax liabilities and assets be classified as noncurrent on the balance sheet. Previous guidance required deferred tax liabilities and assets to be separated into current and
F-11
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
noncurrent amounts on the balance sheet. The guidance will be come effective for us beginning in the first quarter of 2017 and may be applied either prospectively or retrospectively. We plan to adopt this new standard on January 1, 2017 . At the time of adoption, we will reclassify curre nt deferred tax amounts on our Balance Sheets as noncurrent. As we have a full valuation allowance against its deferred tax assets for all periods presented, the adoption is not expected to have a material impact on our Financial Statements.
In February 2016, the FASB issued ASU No. 2016-02, "Leases" (ASU 2016-02), which increases transparency and comparability among organizations by recognizing all lease transactions (with terms in excess of 12 months) on the balance sheet as a lease liability and a right-of-use asset (as defined). ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years, with earlier application permitted. Upon adoption, the lessee will apply the new standard retrospectively to all periods presented or retrospectively using a cumulative effect adjustment in the year of adoption. We plan to adopt this new standard prospectively on January 1, 2019. We are evaluating the impact of the adoption of this standard on our Financial Statements. We expect that it will increase our lease assets and correspondingly increase our lease liabilities.
In March 2016, the FASB issued ASU No. 2016-09, Compensation - Stock Compensation (Topic 718) "Improvements to Employee Share-Based Payment Accounting" (ASU 2016-09), which is intended to simplify several aspects of the accounting for share-based payment award transactions, including the income tax consequences upon settlement , an option to recognize gross stock compensation expense with actual forfeitures recognized as they occur, as well as certain classifications on the statement of cash flows . The guidance will be effective for the fiscal year beginning after December 15, 2016, including interim periods within that year. We plan to adopt this new standard on January 1, 2017 and do not expect a material impact on our Financial Statements given the full valuation allowance position on our deferred tax assets.
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): "Classification of Certain Cash Receipts and Cash Payments," which addresses eight specific cash flow issues with the objective of reducing the existing diversity in practice. The guidance will be effective for the fiscal year beginning after December 15, 2017, including interim periods within that year. We plan to adopt this new standard on January 1, 2018, and do not expect it to have a material impact on our Financial Statements.
2. Significant Agreements
Commercial Agreements
In May 2013, we entered into a services agreement with Dohmen to provide exclusive specialty pharmacy and patient services programs for Korlym beginning July 1, 2013. Under the terms of this agreement, Dohmen acts as the exclusive specialty pharmacy distributor of Korlym in the United States, subject to certain exceptions. Among other services, Dohmen provides services related to pharmacy operations; patient intake, access and reimbursement; patient support; claims management and accounts receivable; and data and reporting. We provide Korlym to Dohmen, which it dispenses to patients. Dohmen does not take title to the product, which passes directly from us to the patient at the time the patient receives the medicine.
The initial term of the agreement is a period of three years, with successive automatic renewal terms of three years unless either party gives at least 180 days' prior notice of non-renewal. The agreement contains customary termination provisions, representations, warranties and covenants. Subject to certain limitations, we have agreed to indemnify Dohmen for certain third-party claims related to the product, and we have each agreed to indemnify the other for certain breaches of representations, warranties, covenants and other specified matters.
Manufacturing Agreements Related to Korlym
Active Pharmaceutical Ingredient
In March 2014, we entered into a new long-term manufacturing and supply agreement with PCAS for the manufacture of mifepristone, the active pharmaceutical ingredient (API) in Korlym. We have agreed to purchase a minimum percentage of our mifepristone requirements from PCAS; the amount of the commitment will depend on
F-12
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
our future needs. The initial term of the agreement is five years, with an automatic extension of one year unless either par ty gives 12 months' prior written notice that it does not want an extension. We have the right to terminate the agreement if PCAS is unable to manufacture the product for a consecutive nine-month period.
Tablet Manufacture
In April 2014, we entered into a new manufacturing agreement with Alcami Corporation for the manufacture and package of Korlym tablets. The initial term of this agreement is a period of three years, with consecutive automatic extensions of two years unless either party gives written notice – in the case of Alcami Corporation, 18 months prior to the end of the applicable term, and in our case 12 months prior to the end of the applicable term – that it does not want such an extension. We have the right to terminate the agreement if Alcami Corporation is unable to manufacture the product for a consecutive four-month period or if the product is withdrawn from the market. There are no minimum purchase obligations under this agreement.
Research and Development Agreements
In 1999, we entered into an agreement with The Board of Trustees of Leland Stanford Junior University (Stanford) in which Stanford granted us an exclusive license to patents covering the use of glucocorticoid receptor antagonists for the treatment of psychotic depression, early dementia, and cocaine-induced psychosis, as specified in the license agreement. This license agreement expires upon the expiration of the related patents or upon notification by us to Stanford. In exchange for the license, we paid Stanford an initial non-refundable fee, immediately issued 30,000 shares of our common stock to Stanford and are obligated to pay Stanford $50,000 per year as a nonrefundable royalty payment. In addition, we are obligated to pay additional milestone payments in the future, which are not material and a portion of which are creditable against future royalties and will pay a royalty based on net revenue generated by any product arising from the patent until its expiration.
We have also exclusively licensed from the University of Chicago two issued U.S. patents for the use of cortisol modulators in the treatment of triple-negative breast cancer and a second patent family with applications in the United States and Europe having claims directed to the use of cortisol modulators to treat castration-resistant prostate cancer. In exchange for these licenses, we paid initial non-refundable fees to the University of Chicago and are committed to additional annual and milestone payments in the future, which are not material and which are creditable against future royalties. We will also pay royalties based on net revenue generated by any product arising from these patents until their expiration.
In January 2016, we entered into an agreement with Chiltern to assist in the management of a clinical trial evaluating CORT125134 for treatment of Cushing syndrome. The total commitment under this agreement is $2.1 million, but the actual amount to be paid is dependent on actual services provided under this agreement. Approximately $741,000 of the costs under this agreement were incurred through December 31, 2016, with the remainder to be incurred over the course of the trial.
In March 2014, we entered into an agreement with Quotient Clinical Limited ("Quotient"), a clinical research organization, to assist in the management and conduct of our Phase 1 study of CORT125134, our lead selective cortisol modulator. The total commitment under the agreement was approximately $3.0 million. All of the costs under this agreement were incurred through December 31, 2016.
In September 2016, we entered into an agreement with Quotient to assist in the management and conduct of our Phase 1 study of CORT122928, a selective cortisol modulator. The total commitment under the agreement was approximately $2.2 million, which is expected to be expended over an approximately two-year period. Approximately $0.1 million of the costs under this agreement were incurred through December 31, 2016.
In December 2016, we entered into an agreement with Quotient to assist in the management and conduct of our Phase 1 study of CORT118335, a selective cortisol modulator. The total commitment under the agreement was approximately $2.2 million, which is expected to be expended over an approximately one-year period. No costs under this agreement were incurred through December 31, 2016.
F-13
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
3. Fair Value of Financial Instruments
As of December 31, 2016 and 2015, we had invested our financial assets in a money market fund that can be converted to cash at par on demand. We measured these funds, which totaled $31.6 million as of both December 31, 2016 and 2015, at fair value, which approximates cost, as of the respective dates and classified them as Level 1 assets in the fair value hierarchy for financial assets.
All cash equivalents and short-term investments held as of December 31, 2016 and 2015 were in active markets and valued based upon their quoted prices. We did not recognize any realized gains or losses on sales of investments for any period presented.
4. Composition of Certain Balance Sheet Items
Inventory
The composition of inventory was as follows:
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
|
| (in thousands) |
| |||||
Raw materials |
| $ | 1,848 |
|
| $ | 2,141 |
|
Work in progress |
|
| 1,414 |
|
|
| 3 |
|
Finished goods |
|
| 1,902 |
|
|
| 2,338 |
|
Total inventory |
|
| 5,164 |
|
|
| 4,482 |
|
Less strategic inventory classified as non-current |
|
| (2,835 | ) |
|
| (2,800 | ) |
Total inventory classified as current |
| $ | 2,329 |
|
| $ | 1,682 |
|
In order to be prepared for potential demand for Korlym and because we have single-source manufacturers of both the API for Korlym and Korlym tablets, we have invested in inventory of both of these materials. Inventory amounts that are not expected to be consumed within 12 months following the balance sheet date are referred to as "Strategic Inventory" and classified as a noncurrent asset.
Property and Equipment
Property and equipment consisted of the following:
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
| (in thousands) |
| ||||||
Furniture and equipment |
| $ | 300 |
|
| $ | 270 |
|
Software |
|
| 351 |
|
|
| 193 |
|
Leasehold improvements |
|
| 6 |
|
|
| - |
|
|
|
| 657 |
|
|
| 463 |
|
Less: accumulated depreciation |
|
| (452 | ) |
|
| (365 | ) |
|
| $ | 205 |
|
| $ | 98 |
|
F-14
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
Other Accrued Liabilities
Other accrued liabilities consisted of the following:
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
|
| (in thousands) |
| |||||
Government rebates |
| $ | 3,426 |
|
| $ | 1,663 |
|
Accrued compensation |
|
| 4,702 |
|
|
| 1,103 |
|
Commercialization costs |
|
| 308 |
|
|
| 111 |
|
Legal fees |
|
| 164 |
|
|
| 69 |
|
Professional fees |
|
| 34 |
|
|
| 220 |
|
Other |
|
| 319 |
|
|
| 91 |
|
Total other accrued liabilities |
| $ | 8,953 |
|
| $ | 3,257 |
|
5. Long-Term Obligation
As discussed in Note 1, Basis of Presentation and Summary of Significant Accounting Policies – Long-term Obligation , under the Financing Agreement with Biopharma, we make payments to Biopharma calculated as a percentage of our Korlym Receipts. Biopharma's right to receive payments will expire once it has received cumulative payments of $45.0 million. Through December 31, 2016, we have paid Biopharma $29.9 million, with an additional payment of $4.8 million made in February 2017.
Under the terms of the Financing Agreement, our payments are variable, with no fixed minimums. If there are no net sales, upfront, milestone or other contingent payments in a period with respect to Covered Products, then no payment will be due for that period.
We are obligated to make future payments as follows:
| • | 20 percent of our net product sales of Covered Products. |
| • | 20 percent of payments received for upfront, milestone or other contingent fees under co-promotion and out-license agreements for Covered Products. |
| • | The percentage used to calculate our payments to Biopharma would increase to 50 percent and any applicable payment caps would lapse if we (i) fail to provide Biopharma with certain information regarding our promotion and sales of Covered Products, (ii) do not devote a commercially reasonable amount of resources to the promotion and marketing of the Covered Products or (iii) violate the indebtedness covenant by incurring indebtedness greater than the sum of earnings before interest, taxes, depreciation and amortization, including such items as non-cash stock-based compensation, for the four calendar quarters preceding such incurrence and, in each case, fail to cure within the applicable cure period. |
| • | Upon the occurrence of a Corcept change of control transaction or the licensing of Korlym to a third-party for promotion and sale in the United States, the entire $45.0 million, less any amounts already paid by us, would become due. |
To secure our obligations in connection with the Financing Agreement, we granted Biopharma a security interest in our rights in patents, trademarks, trade names, domain names, copyrights, know-how and regulatory approvals related to the Covered Products, all books and records relating to the foregoing and all proceeds of the foregoing (together, the Collateral). If we (i) fail to deliver a royalty payment when due and do not remedy that failure within 30 days, (ii) fail to maintain a first-priority perfected security interest in the Collateral in the United States and do not remedy that failure within five business days of receiving notice of such failure or (iii) become subject to an event of bankruptcy, then Biopharma may attempt to recover up to $45.0 million (after deducting any payments we have already made). In addition, pursuant to this agreement, we are not allowed to pay a dividend or other cash distribution, unless we will have cash and cash equivalents in excess of $50.0 million after such payment.
F-15
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
As discussed in Note 1, Basis of Presentation and Summary of Significant Accounting Policies, Long-term Obligation , we recognize a portion of each quarterly payme nt to Biopharma as interest expense, which we determine by calculating the interest rate to Biopharma implied by the stream of payments we expect to make under the Financing Agreement. We recognize the non-interest portion of each payment as a reduction in our obligation to Biopharma. The current portion of the obligation is the amount we expect to pay, exclusive of interest expense, during the next 12 months. The actual amount of each quarterly payment will be based on Korlym Receipts in that quarter and m ay differ from our estimate. Management's estimate of the future product revenue is subject to uncertainty because Korlym Receipts are difficult to predict. While changes in the timing of Korlym Receipts may affect the recognition of interest expense and t he split between the current and long-term portions of the obligation at any balance sheet date, the total we will pay Biopharma is fixed at $45.0 million .
We recorded interest expense of $1.9 million and $2.8 million for the years ended December 31, 2016 and 2015, respectively, and total interest of $14.5 million for the period from August 2012 through December 31, 2016 .
The following table provides a summary of the payment obligations under the Financing Agreement as of December 31, 2016 and 2015, utilizing the payment assumptions discussed above.
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
|
| (in thousands) |
| |||||
Total repayment obligation |
| $ | 45,000 |
|
| $ | 45,000 |
|
Less interest in future periods |
|
| (456 | ) |
|
| (2,385 | ) |
Less unamortized financing costs |
|
| (14 | ) |
|
| (35 | ) |
Less payments made |
|
| (29,866 | ) |
|
| (15,087 | ) |
Less current portion |
|
| (14,664 | ) |
|
| (14,965 | ) |
Long-term obligation, net of current portion |
| $ | - |
|
| $ | 12,528 |
|
The estimated fair value of the long-term obligation, as measured using Level 3 inputs, approximates the carrying amounts as presented on the balance sheet as of December 31, 2016 and 2015. The estimated fair value was calculated using the income method of valuation. The key assumptions required for the calculation were an estimate of the amount and timing of future product sales and an estimated cost of capital.
We capitalized $140,000 of issuance costs related to the Financing Agreement, which are being amortized over the estimated term of the obligation, based on the assumptions discussed above. At December 31, 2016 and 2015, the unamortized issuance costs were $14,000, and $35,000, and are included in long-term obligation, netted against debt on our balance sheets, pursuant to ASU 2015-03 .
6. Lease Obligations
In July 2015, we exercised our option to extend the lease for our office space through December 2016. We subsequently amended the lease agreement in February 2016 to extend the lease through 2019 and to add additional space. In March 2016, we early terminated the lease and replaced it with a new lease effective May 1, 2016 through March 31, 2019. Rent expense for the years ended December 31, 2016, 2015 and 2014 was $885,000, $678,000, and $609,000, respectively.
F-16
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
As of Decemb er 31, 2016, future minimum lease payments under non-cancelable operating leases were as follows:
|
| Lease |
| |
|
| Payments |
| |
2017 |
|
| 937 |
|
2018 |
|
| 1,115 |
|
2019 |
|
| 279 |
|
Thereafter |
| - |
| |
Total |
| $ | 2,331 |
|
7. Related Party Transactions
See discussion below in Note 8, Preferred Stock and Stockholders' Equity , under the caption Common Stock , regarding the sale of securities to various investors, including members of our board of directors and related entities.
8. Preferred Stock and Stockholders' Equity
Preferred Stock
Our Board of Directors is authorized, subject to any limitations prescribed by law, without stockholder approval, to issue up to an aggregate of 10,000,000 shares of preferred stock at $0.001 par value in one or more series and to fix the rights, preferences, privileges and restrictions granted to or imposed upon the preferred stock, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences. The rights of the holders of common stock will be subject to the rights of holders of any preferred stock that may be issued in the future. As of December 31, 2016 and 2015, we had no outstanding shares of preferred stock.
Common Stock
Significant stock transactions
We issued approximately 6.2 million shares of our common stock in March 2015, upon the exercise of warrants that had been issued in two private placement transactions, one in 2008 and the other in 2012, to qualified investors, including members of our board of directors and their affiliates. The transactions generated aggregate net proceeds of approximately $17.1 million, after the deduction of issuance costs. Approximately 3.1 million shares of the securities, which generated aggregate gross proceeds of $5.9 million, were issued in these transactions to venture capital funds, trusts and other entities affiliated with members of our Board of Directors.
We also issued 164,666 shares of common stock related to the exercise of a warrant in May 2014 that had been issued in the 2008 private placement. This warrant was exercised on a cashless net-exercise basis, wherein an unaffiliated investor surrendered a warrant for 529,567 shares in exchange for the issuance of 164,666 shares of common stock.
We have never declared or paid any dividends.
Shares of common stock reserved for future issuance as of December 31, 2016 are as follows:
Common stock: |
| (in thousands) |
| |
Exercise of outstanding options |
|
| 17,663 |
|
Shares available for grant under stock option plans |
|
| 7,920 |
|
|
|
| 25,583 |
|
F-17
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
On February 10 , 2017, our Board of Directors authorized an additional increase of 4. 5 million shares in the number of shares available under the 2012 Equity Incentive Plan (the 2012 Plan), which was equivalent to 4% of the shares of our common stock outstanding at December 31, 2016.
Stock Option Plans
We have two active stock option plans at December 31, 2016 – the 2004 Equity Incentive Plan (the 2004 Plan) and the 2012 Plan.
In 2004, our board of directors and stockholders approved the 2004 Plan, which became effective upon the completion of our initial public offering (IPO). Under the 2004 Plan, options, stock purchase and stock appreciation rights and restricted stock awards can be issued to our employees, officers, directors and consultants. The 2004 Plan provided that the exercise price for incentive stock options will be no less than 100% of the fair value of the Company's common stock, as of the date of grant. Options granted under the 2004 Plan vest over periods ranging from one to five years. The vesting period of the options is generally equivalent to the requisite service period.
In 2012, our board of directors and stockholders approved the 2012 Plan. As of the effective date of the 2012 Plan, 5.3 million shares that remained available for issuance of new grants under the 2004 Plan were transferred to the 2012 Plan. After that date, no additional options were or will be issued under the 2004 Plan. Vested options under the 2004 Plan that are not exercised within the remaining contractual life and any options under the 2004 Plan that do not vest because of terminations after the effective date of the 2012 Plan will be added to the pool of shares available for future grants under the 2012 Plan.
Under the 2012 Plan, we can issue options, stock purchase and stock appreciation rights and restricted stock awards to our employees, officers, directors and consultants. The 2012 Plan provides that the exercise price for incentive stock options will be no less than 100 percent of the fair value of our common stock as of the date of grant. Options granted under the 2012 Plan are expected to vest over periods ranging from one to four years. We expect the vesting period of the options that we grant under the 2012 Plan to be generally equivalent to the requisite service period.
Upon exercise of options, new shares are issued.
On February 26, 2016, our Board of Directors authorized an increase of 4.4 million shares in the number of shares available under the 2012 Plan, which was equivalent to 4% of the shares of our common stock outstanding as of December 31, 2015, pursuant to the terms of the 2012 Plan.
F-18
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
Option activity during 2014, 2015 and 2016
The following table summarizes all stock plan activity:
|
|
|
|
|
| Outstanding Options |
| |||||||||||||
|
| Shares Available For Future Grant |
|
| Options Shares Subject to Options Outstanding |
|
| Weighted- Average Exercise Price |
|
| Weighted- Average Remaining Contractual Life |
|
| Aggregate Intrinsic Value |
| |||||
|
| (in thousands) |
|
| (in thousands) |
|
|
|
|
|
| (in years) |
| (in thousands) |
| |||||
Balance at December 31, 2013 |
|
| 4,926 |
|
|
| 14,712 |
|
| $ | 2.63 |
|
|
|
|
|
|
|
|
|
Increase in shares authorized for grant |
|
| 3,993 |
|
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
Shares granted |
|
| (2,140 | ) |
|
| 2,140 |
|
| $ | 2.62 |
|
|
|
|
|
|
|
|
|
Shares exercised |
|
| - |
|
|
| (1,381 | ) |
| $ | 1.34 |
|
|
|
|
|
|
|
|
|
Shares cancelled and forfeited |
|
| 767 |
|
|
| (767 | ) |
| $ | 5.03 |
|
|
|
|
|
|
|
|
|
Balance at December 31, 2014 |
|
| 7,546 |
|
|
| 14,704 |
|
| $ | 2.62 |
|
|
|
|
|
|
|
|
|
Increase in shares authorized for grant |
|
| 4,056 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares granted |
|
| (4,902 | ) |
|
| 4,902 |
|
| $ | 3.88 |
|
|
|
|
|
|
|
|
|
Shares exercised |
|
| - |
|
|
| (2,041 | ) |
| $ | 2.55 |
|
|
|
|
|
|
|
|
|
Shares cancelled and forfeited |
|
| 1,370 |
|
|
| (1,370 | ) |
| $ | 3.07 |
|
|
|
|
|
|
|
|
|
Balance at December 31, 2015 |
|
| 8,070 |
|
|
| 16,195 |
|
| $ | 2.98 |
|
|
|
|
|
|
|
|
|
Increase in shares authorized for grant |
|
| 4,386 |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares granted |
|
| (5,906 | ) |
|
| 5,906 |
|
| $ | 4.92 |
|
|
|
|
|
|
|
|
|
Shares exercised |
|
| - |
|
|
| (3,068 | ) |
| $ | 2.50 |
|
|
|
|
|
|
|
|
|
Shares cancelled and forfeited |
|
| 1,370 |
|
|
| (1,370 | ) |
| $ | 3.98 |
|
|
|
|
|
|
|
|
|
Balance at December 31, 2016 |
|
| 7,920 |
|
|
| 17,663 |
|
| $ | 3.63 |
|
|
| 6.83 |
|
| $ | 64,122 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options exercisable at December 31, 2016 |
|
|
|
|
|
| 10,471 |
|
| $ | 3.00 |
|
|
| 5.37 |
|
| $ | 44,598 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options fully vested and expected to vest at December 31, 2016 |
|
|
|
|
|
| 16,720 |
|
| $ | 3.56 |
|
|
| 6.70 |
|
| $ | 61,909 |
|
The total intrinsic value of options exercised during the years ended December 31, 2016, 2015 and 2014 was $14.8 million, $5.5 million and $3.0 million, respectively, based on the difference between the closing price of our common stock on the date of exercise of the options and the exercise price.
The total grant date fair value of options to employees and directors that vested during the years ended December 31, 2016, 2015 and 2014 was $7.0 million, $5.4 million and $4.6 million, respectively.
The following is a summary of options outstanding and options exercisable at December 31, 2016.
|
|
|
|
|
| Options Outstanding |
|
| Options Exercisable |
| ||||||||||||||||||||||||||
| Exercise Prices of Options |
|
| Number of Shares |
|
| Weighted- Average Remaining Contractual Life |
|
| Weighted- Average Exercise Price |
|
| Aggregate Intrinsic Value |
|
| Number of Shares |
|
| Weighted- Average Exercise Price |
|
| Aggregate Intrinsic Value |
| |||||||||||||
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
| (in years) |
|
|
|
|
| (in thousands) |
|
| (in thousands) |
|
|
|
|
|
| (in thousands) |
| ||||||
| $ | 0.96 |
| - |
| $ | 2.00 |
|
|
| 2,174 |
|
|
| 3.7 |
|
| $ | 1.40 |
|
| $ | 12,751 |
|
|
| 2,058 |
|
| $ | 1.37 |
|
| $ | 12,116 |
|
| $ | 2.01 |
| - |
| $ | 3.00 |
|
|
| 3,486 |
|
|
| 5.4 |
|
| $ | 2.33 |
|
|
| 17,184 |
|
|
| 3,261 |
|
| $ | 2.31 |
|
|
| 16,141 |
|
| $ | 3.01 |
| - |
| $ | 4.00 |
|
|
| 6,201 |
|
|
| 8.1 |
|
| $ | 3.55 |
|
|
| 22,991 |
|
|
| 2,575 |
|
| $ | 3.48 |
|
|
| 9,745 |
|
| $ | 4.01 |
| - |
| $ | 6.92 |
|
|
| 5,802 |
|
|
| 7.5 |
|
| $ | 5.33 |
|
|
| 11,196 |
|
|
| 2,577 |
|
| $ | 4.70 |
|
|
| 6,596 |
|
|
|
|
|
|
|
|
|
|
|
| 17,663 |
|
|
| 6.8 |
|
| $ | 3.63 |
|
| $ | 64,122 |
|
|
| 10,471 |
|
| $ | 3.00 |
|
| $ | 44,598 |
|
F-19
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value that option holders would have received had all option holders exercised their options on December 31, 2016. The aggregate intrinsic value is the difference between our closing stock price on December 31, 2016 and the exercise price, multiplied by the number of in-the-money options.
Stock-Based Compensation related to Employee and Director Options
Assumptions used in determining fair value-based measurements for options to employees and directors
The following table summarizes the weighted-average assumptions and resultant fair value-based measurements for options granted to employees and directors.
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Weighted-average assumptions for stock options granted: |
|
|
|
|
|
|
|
|
|
|
|
|
Risk-free interest rate |
|
| 1.31% |
|
|
| 1.77% |
|
|
| 1.80% |
|
Expected term |
| 5.8 years |
|
| 7.2 years |
|
| 6.0 years |
| |||
Expected volatility of stock price |
|
| 69.0% |
|
|
| 77.0% |
|
|
| 79.0% |
|
Dividend rate |
|
| 0% |
|
|
| 0% |
|
|
| 0% |
|
Weighted-average grant date fair value-based measurement |
| $2.98 |
|
| $2.72 |
|
| $1.77 |
| |||
The expected term of options reflected in the table above has been based on a formula that considers the expected service period and expected post-vesting termination behavior differentiated by whether the grantee is an employee, an officer or a director.
The expected volatility of our stock used in determining the fair value-based measurement of option grants to employees, officers and directors is based on a weighted-average combination of the volatility of our own stock price and that of a group of peer companies for those grants with expected terms longer than the period of time that we have been a public company. For stock options granted to employees with expected terms of less than the period of time that we have been a public company, the volatility is based on historical data of the price for our common stock for periods of time equivalent to the expected term of these grants.
We calculated employee stock-based compensation expense based on awards ultimately expected to vest and reduced it for estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates .
Summary of compensation expense related to options to employees and directors
We recognized compensation expense of $7.1 million, $6.0 million and $4.7 million related to options to employees and directors during the years ended December 31, 2016, 2015 and 2014, respectively.
As of December 31, 2016, we had $17.0 million of unrecognized compensation expense for employee and director options outstanding as of that date, which had a remaining weighted-average vesting period of 2.97 years.
F-20
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
Stock Options to Non-Employees
We expense stock-based compensation related to service-based option grants to non-employees on a straight-line basis over the vesting period of the options, which approximates the period over which the related services are rendered, based on the fair value-based measurement of the options using the Black-Scholes option pricing model. The assumptions used in these calculations are similar to those used for the determination of fair value-based measurement for options granted to employees and directors, with the exception that, for non-employee options, the remaining contractual term is utilized as the expected term of the option and the fair value-based measurement related to unvested non-employee options is re-measured quarterly, based on the then current stock price as reflected on the NASDAQ Capital Market.
We recorded charges to expense for non-employee stock options of $57,000, $87,000 and $470,000 for the years ended December 31, 2016, 2015 and 2014, respectively.
As of December 31, 2016, there is one award outstanding to a non-employee with an aggregate total of 4,000 shares unvested as of that date.
Summary of Stock-based Compensation Expense
The following table presents a summary of non-cash stock-based compensation by financial statement classification.
|
| Year ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
|
| (in thousands) |
| |||||||||
Research and development |
| $ | 1,312 |
|
| $ | 839 |
|
| $ | 723 |
|
Selling, general and administrative |
|
| 5,746 |
|
|
| 5,174 |
|
|
| 4,478 |
|
Total stock-based compensation |
| $ | 7,058 |
|
| $ | 6,013 |
|
| $ | 5,201 |
|
9. Net Income (Loss) Per Share
Basic and diluted net income (loss) per share is computed by dividing the net income (loss) by the weighted-average number of common shares outstanding during the period. The potential dilutive shares of our common stock resulting from the assumed exercise of outstanding stock options were determined under the treasury stock method. The computation of net income (loss) per share for each period, including the number of weighted-average shares outstanding, is shown on the face of the statements of comprehensive income (loss).
The following table shows the computation of net income (loss) per share for each period, including the number of weighted-average shares outstanding.
|
|
|
|
|
| ||||||||
|
| Year ended December 31, | |||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| |||
|
| (in thousands) | |||||||||||
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
| $ | 8,140 |
|
| $ | (6,408 | ) |
| $ | (31,383 | ) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average shares used to compute basic net income (loss) per share |
|
| 110,566 |
|
|
| 106,883 |
|
|
| 100,978 |
|
|
Dilutive effect of employee stock options |
|
| 5,573 |
|
|
| - |
|
|
| - |
|
|
Weighted-average shares used to compute diluted net income (loss) per share |
|
| 116,139 |
|
|
| 106,883 |
|
|
| 100,978 |
|
|
Net income (loss) per share attributable to common stockholders |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
| $ | 0.07 |
|
| $ | (0.06 | ) |
| $ | (0.31 | ) |
|
F-21
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
We have e xcluded approximately 4,400 weighted average stock options to purchase common stock that were outstanding during the year ended December 31, 2016 from the computation of diluted net income per share because including them would have had an anti-dilutive effect.
We have excluded the impact of all common stock equivalents relating to shares underlying outstanding options and warrants from the calculation of diluted net loss per common share for the years ended December 31, 2015 and 2014 because all such securities are antidilutive .
The following table presents information on securities outstanding as of the end of each period that could potentially dilute the per share data in the future.
|
| December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
|
| (in thousands) |
| |||||||||
Stock options outstanding |
|
| 17,663 |
|
|
| 16,195 |
|
|
| 14,704 |
|
Warrants outstanding |
|
| - |
|
|
| - |
|
|
| 8,044 |
|
Total |
|
| 17,663 |
|
|
| 16,195 |
|
|
| 22,748 |
|
10. Income Taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets are as follows:
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
Deferred tax assets: |
| (in thousands) |
| |||||
Federal and state net operating losses |
| $ | 68,605 |
|
| $ | 68,552 |
|
Capitalized research and patent costs |
|
| 23,575 |
|
|
| 24,876 |
|
Research credits |
|
| 19,058 |
|
|
| 19,208 |
|
Biopharma Financing Agreement |
|
| 5,556 |
|
|
| 10,423 |
|
Stock-based compensation costs |
|
| 6,508 |
|
|
| 6,246 |
|
Other |
|
| 6,067 |
|
|
| 4,345 |
|
Total deferred tax assets |
|
| 129,369 |
|
|
| 133,650 |
|
Valuation allowance |
|
| (129,369 | ) |
|
| (133,650 | ) |
Net deferred tax assets |
| $ | - |
|
| $ | - |
|
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance.
The valuation allowance increased by $15.9 million and decreased by $2.4 million and $4.3 million for the years ended December 31, 2014, 2015 and 2016, respectively.
At December 31, 2016, we had net operating loss carryforwards available to offset any future taxable income that we may generate for federal income tax purposes of $179.3 million, which expire in the years 2019 through 2036, California net operating loss carryforwards of $106.4 million, which expire in the years 2017 through 2036, and net operating loss carryforwards from other states of $32.5 million, which expire in the years 2026 through 2036. Our federal and state net operating loss carryforwards as of December 31, 2016 include amounts resulting from exercises and sales of stock option awards to employees and non-employees. When we realize the tax benefit associated with these stock option exercises as a reduction to taxable income in our returns, we will account for the tax benefit as a credit to stockholders' equity rather than as a reduction of our income tax provision in our financial statements. Based upon our stock option exercise history, we believe such amounts are not a material component of our total net operating loss carryforwards as of December 31, 2016.
F-22
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
At D ecember 31, 2016, we also had federal and California research and development tax credits of $17.2 million and $2.8 million, respectively. The federal research credits will expire in the years 2023 through 2036 and the California research credits have no e xpiration date. Our deferred tax assets have been offset by a full valuation allowance as the realization of such assets is uncertain.
Utilization of our net operating losses and tax credit carryforwards may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such limitations could result in the expiration of the net operating losses and tax credit carryforwards before utilization.
The following table presents a reconciliation from the statutory federal income tax rate to the effective rate.
|
| Year ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
|
| (in thousands) |
| |||||||||
U.S. federal taxes (benefit) at statutory rate |
| $ | 2,840 |
|
| $ | (2,178 | ) |
| $ | (10,670 | ) |
Changes in valuation allowance |
|
| (3,679 | ) |
|
| 2,495 |
|
|
| 11,002 |
|
Unutilized research credits |
|
| (69 | ) |
|
| (445 | ) |
|
| (1,308 | ) |
Non-deductible offset of Orphan Drug Credit |
|
| - |
|
|
| - |
|
|
| 249 |
|
Non-deductible Compensation |
|
| 2,435 |
|
|
| - |
|
|
| - |
|
Stock-based compensation |
|
| (1,660 | ) |
|
| 6 |
|
|
| 673 |
|
Other |
|
| 133 |
|
|
| 122 |
|
|
| 54 |
|
Total |
| $ | - |
|
| $ | - |
|
| $ | - |
|
The Company maintains liabilities for uncertain tax positions. These liabilities involve considerable judgment and estimation and are continuously monitored by management based on the best information available, including changes in tax regulations, the outcome of relevant court cases, and other pertinent information.
No amounts have been recognized as interest or penalties on income tax related matters.
The aggregate annual changes in the balance of gross unrecognized tax benefits are as follows (in thousands):
|
| Year ended December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
Beginning Balance |
| $ | 4,342 |
|
| $ | - |
|
Increase in tax positions for prior years |
|
| 222 |
|
|
| 4,173 |
|
Decreases in tax positions for prior years |
|
| (1,189 | ) |
|
| - |
|
Increase in tax positions for current year |
|
| 152 |
|
|
| 169 |
|
Ending Balance |
| $ | 3,527 |
|
| $ | 4,342 |
|
As of December 31, 2016 and 2015, the Company's total amount of unrecognized tax benefit was approximately $3.5 million and $4.3 million, respectively. There would be no impact to the effective tax rate if these tax benefits were recognized while the Company maintains a full valuation allowance. The Company does not expect its unrecognized tax benefits to change materially over the next 12 months.
While management believes that the Company has adequately provided for all tax positions, amounts asserted by tax authorities could be greater or less than the recorded position. Accordingly, the Company's provisions on federal and state tax-related matters to be recorded in the future may change as revised estimates are made or the underlying matters are settled or otherwise resolved.
All tax years from inception remain open to examination by the Internal Revenue Service, the California Franchise Tax Board and other state taxing authorities until such time as the net operating losses and research credits are either fully utilized or expire.
F-23
CORCEPT THERAPEUTICS INCORPORATED
NOTES TO FINANCIAL STATEMENTS, Continued
11. Commitments
We have entered into a number of agreements to conduct clinical trials and pre-clinical studies for further development of Korlym and our proprietary selective cortisol modulators. See the discussion in Note 2, Significant Agreements , for further discussion regarding the commitments under these agreements.
In the ordinary course of our business, we make certain indemnities, commitments and guarantees under which we may be required to make payments in relation to certain transactions. These include indemnities of clinical investigators and contract research organizations involved in the development of our clinical stage product candidates, indemnities of contract manufacturers and indemnities to our directors and officers to the maximum extent permitted under the laws of the State of Delaware. The duration of these indemnities, commitments and guarantees varies, and in certain cases, is indefinite. The majority of these indemnities, commitments and guarantees do not provide for any limitation of the maximum potential future payments that we could be obligated to make. We have not recorded any liability for these indemnities, commitments and guarantees in the accompanying balance sheets. However, we would accrue for losses for any known contingent liability, including those that may arise from indemnification provisions, when future payment is probable. No such losses have been recorded to date.
12. Quarterly Financial Data (Unaudited)
The following table is in thousands, except per share amounts:
Quarter Ended |
| March 31 |
|
| June 30 |
|
| September 30 |
|
| December 31 |
| ||||
2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product sales, net |
| $ | 16,061 |
|
| $ | 19,724 |
|
| $ | 21,725 |
|
| $ | 23,811 |
|
Gross profit on product sales |
|
| 15,658 |
|
|
| 19,298 |
|
|
| 21,057 |
|
|
| 23,250 |
|
Net income (loss) |
|
| (19 | ) |
|
| 977 |
|
|
| 2,585 |
|
|
| 4,597 |
|
Basic and diluted net income (loss) per share |
|
| (0.00 | ) |
|
| 0.01 |
|
|
| 0.02 |
|
|
| 0.04 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product sales, net |
| $ | 10,102 |
|
| $ | 11,956 |
|
| $ | 13,261 |
|
| $ | 14,967 |
|
Gross profit on product sales |
|
| 9,800 |
|
|
| 11,517 |
|
|
| 13,005 |
|
|
| 14,603 |
|
Net income (loss) |
|
| (4,830 | ) |
|
| (1,936 | ) |
|
| (601 | ) |
|
| 959 |
|
Basic and diluted net income (loss) per share |
|
| (0.05 | ) |
|
| (0.02 | ) |
|
| (0.01 | ) |
|
| 0.01 |
|
F-24
