UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
(Mark One)
☒ | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended September 30, 2018.
☐ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-38042
ARROWHEAD PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
Delaware |
| 46-0408024 |
(State of incorporation) |
| (I.R.S. Employer Identification No.) |
225 S. Lake Avenue, Suite 1050
Pasadena, California 91101
(626) 304-3400
(Address and telephone number of principal executive offices)
Securities registered under Section 12(b) of the Exchange Act:
Title of each class |
| Name of each exchange on which registered |
Common Stock, $0.001 par value |
| The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by a check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by a check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See definitions of "large accelerated filer," "accelerated filer," "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ |
| Accelerated filer ☒ |
| Non-accelerated filer ☐ |
| Smaller Reporting Company ☐ |
Emerging growth company ☐ |
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of issuer's voting and non-voting outstanding Common Stock held by non-affiliates was approximately $619 million based upon the closing stock price of issuer's Common Stock on March 31, 2018. Shares of common stock held by each officer and director and by each person who is known to own 10% or more of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates of the Company. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of December 7, 2018, 92,224,665 shares of the issuer's Common Stock were issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Definitive Proxy Statement to be filed for Arrowhead Pharmaceuticals Inc.'s 2019 Annual Meeting of Stockholders are incorporated by reference into Part III hereof.
PART I |
|
|
|
|
|
|
| ||
I TEM 1. |
| B USINESS |
| 1 |
I TEM 1A. |
| R ISK F ACTORS |
| 22 |
I TEM 1B. |
| U NRESOLVED S TAFF C OMMENTS |
| 34 |
I TEM 2. |
| P ROPERTIES |
| 34 |
I TEM 3. |
| L EGAL P ROCEEDINGS |
| 34 |
I TEM 4. |
| M INE S AFETY D ISCLOSURES |
| 34 |
|
|
| ||
PART II |
|
|
|
|
|
|
| ||
I TEM 5. |
| M ARKET FOR THE R EGISTRANT ' S C OMMON E QUITY , R ELATED S TOCKHOLDER M ATTERS AND I SSUER P URCHASES OF E QUITY S ECURITIES |
| 35 |
I TEM 6. |
| S ELECTED F INANCIAL D ATA |
| 37 |
I TEM 7. |
| M ANAGEMENT ' S D ISCUSSION AND A NALYSIS OF F INANCIAL C ONDITION AND R ESULTS OF O PERATIONS |
| 38 |
I TEM 7A. |
| Q UANTITATIVE AND Q UALITATIVE D ISCLOSURES A BOUT M ARKET R ISK |
| 50 |
I TEM 8. |
| F INANCIAL S TATEMENTS AND S UPPLEMENTARY D ATA |
| 50 |
I TEM 9. |
| C HANGES IN AND D ISAGREEMENTS WITH A CCOUNTANTS ON A CCOUNTING AND F INANCIAL D ISCLOSURE |
| 50 |
I TEM 9A. |
| C ONTROLS AND P ROCEDURES |
| 50 |
I TEM 9B. |
| O THER I NFORMATION |
| 51 |
|
|
| ||
PART III |
|
|
|
|
|
|
| ||
I TEM 10. |
| D IRECTORS , E XECUTIVE O FFICERS , AND C ORPORATE G OVERNANCE |
| 51 |
I TEM 11. |
| E XECUTIVE C OMPENSATION |
| 51 |
I TEM 12. |
| S ECURITY O WNERSHIP OF C ERTAIN B ENEFICIAL O WNERS AND M ANAGEMENT AND R ELATED S TOCKHOLDERS |
| 51 |
I TEM 13. |
| C ERTAIN R ELATIONSHIPS , R ELATED T RANSACTIONS AND D IRECTORS I NDEPENDENCE |
| 51 |
I TEM 14. |
| P RINCIPAL A CCOUNTANT F EES AND S ERVICES |
| 51 |
|
|
| ||
PART IV |
|
|
|
|
|
|
| ||
I TEM 15. |
| E XHIBITS AND F INANCIAL S TATEMENT S CHEDULES |
| 51 |
|
| |||
SIGNATURE |
| 54 | ||
|
| |||
INDEX TO FINANCIAL STATEMENTS AND SCHEDULES |
| F-1 | ||
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and we intend that such forward-looking statements be subject to the safe harbors created thereby. For this purpose, any statements contained in this Annual Report on Form 10-K except for historical information may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as "may," "will," "expect," "believe," "anticipate," "intend," "could," "estimate," or "continue" or the negative or other variations thereof or comparable terminology are intended to identify forward-looking statements. In addition, any statements that refer to projections of our future financial performance, trends in our businesses, or other characterizations of future events or circumstances are forward-looking statements.
The forward-looking statements included herein are based on current expectations of our management based on available information and involve a number of risks and uncertainties, all of which are difficult or impossible to predict accurately and many of which are beyond our control. As such, our actual results may differ significantly from those expressed in any forward-looking statements. Factors that may cause or contribute to such differences include, but are not limited to, those discussed in more detail in Item 1 (Business) and Item 1A (Risk Factors) of Part I and Item 7 (Management's Discussion and Analysis of Financial Condition and Results of Operations) of Part II of this Annual Report on Form 10-K. Readers should carefully review these risks, as well as the additional risks described in other documents we file from time to time with the Securities and Exchange Commission. In light of the significant risks and uncertainties inherent in the forward-looking information included herein, the inclusion of such information should not be regarded as a representation by us or any other person that such results will be achieved, and readers are cautioned not to place undue reliance on such forward-looking information. Except as may be required by law, we disclaim any intent to revise the forward-looking statements contained herein to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
PART I
Unless otherwise noted, (1) the term "Arrowhead" refers to Arrowhead Pharmaceuticals, Inc., a Delaware corporation and its Subsidiaries, (2) the terms "Company," "we," "us," and "our," refer to the ongoing business operations of Arrowhead and its Subsidiaries, whether conducted through Arrowhead or a subsidiary of Arrowhead, (3) the term "Subsidiaries" refers collectively to Arrowhead Madison Inc. ("Arrowhead Madison"), Arrowhead Australia Pty Ltd ("Arrowhead Australia") and Ablaris Therapeutics, Inc. ("Ablaris"), (4) the term "Common Stock" refers to Arrowhead's Common Stock, (5) the term "Preferred Stock" refers to Arrowhead's Preferred Stock and (6) the term "Stockholder(s)" refers to the holders of Arrowhead Common Stock.
ITEM 1. | BUSINESS |
Description of Business
Arrowhead develops medicines that treat intractable diseases by silencing the genes that cause them. Using a broad portfolio of RNA chemistries and efficient modes of delivery, Arrowhead therapies trigger the RNA interference mechanism to induce rapid, deep and durable knockdown of target genes. RNA interference, or RNAi, is a mechanism present in living cells that inhibits the expression of a specific gene, thereby affecting the production of a specific protein. Deemed to be one of the most important recent discoveries in life science with the potential to transform medicine, the discoverers of RNAi were awarded a Nobel Prize in 2006 for their work. Arrowhead's RNAi-based therapeutics leverage this natural pathway of gene silencing.
Pipeline Overview
Arrowhead is focused on developing innovative drugs for diseases with a genetic basis, typically characterized by the overproduction of one or more proteins. The depth and versatility of our RNAi technologies enable us to potentially address conditions in virtually any therapeutic area and pursue disease targets that are not otherwise addressable by small molecules and biologics.
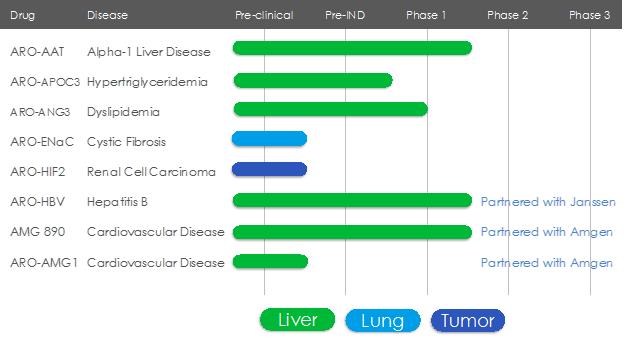
ARO-AAT
ARO-AAT is an RNAi therapeutic candidate for the treatment of liver disease associated with alpha-1 antitrypsin deficiency. ARO-AAT is a second-generation subcutaneously administered compound designed to knock down the Alpha-1 antitrypsin (AAT) gene transcript and reduce the hepatic production of the mutant AAT protein. Arrowhead completed AROAAT1001, a Phase 1 randomized, double-blind, placebo controlled single-ascending dose (SAD) and multiple-ascending dose (MAD) study to evaluate the safety, tolerability, pharmacokinetics, and effect of subcutaneous doses of ARO-AAT on serum alpha-1 antitrypsin levels in healthy adult volunteers in 2018 and plans to initiate a Phase 2 study in 2019.
Initial results from AROAAT1001 were presented in November 2018 at The Liver Meeting®, the Annual Meeting of the American Association for the Study of Liver Disease (AASLD). In the AROAAT1001 study, 45 normal healthy volunteers (NHV)
1
received a single dose of ARO-AAT (n=16), three monthly doses of ARO-AAT (n=12), or placebo (n=17). Key data presented include the following:
| • | ARO-AAT at single- and multiple-doses produced robust and consistent reductions in serum AAT levels |
o Single-doses of 200 and 300 mg resulted in greater than 91% serum AAT reduction with 3 of 4 subjects having concentrations below the level of quantitation (BLQ)
o In 200 and 300 mg single-dose cohorts, an average s erum AAT reduction of greater than 90% was sustained for 6-weeks
o In the multiple-dose cohorts of 200 and 300 mg, for subjects receiving all 3 doses, an average of greater than 90% reduction in serum AAT was sustained for longer than 14 weeks
o The maximum NADIR reduction is 94%
• Monthly serum AAT follow up is ongoing with 9 of 10 subjects at BLQ in the multiple-dose cohorts, including 100% of subjects from the 300 mg cohort
• Duration of response indicates that quarterly or less frequent dosing a ppears feasible
• ARO-AAT has been well tolerated at all doses tested (up to 300 mg) given three times every 28 days
o The most common adverse events (AE) were upper respiratory tract infection (39%) and headache (32%)
Goal of ARO-AAT Treatment
The goal of ARO-AAT treatment is prevention and potential reversal of Z-AAT accumulation-related liver injury and fibrosis. Reduction of inflammatory Z-AAT protein, which has been clearly defined as the cause of progressive liver disease in AATD patients, is important as it is expected to halt the progression of liver disease and allow fibrotic tissue repair.
Alpha-1 Antitrypsin Deficiency (AATD)
AATD is a genetic disorder associated with liver disease in children and adults, and pulmonary disease in adults. AAT is a circulating glycoprotein protease inhibitor that is primarily synthesized and secreted by liver hepatocytes. Its physiologic function is the inhibition of neutrophil proteases to protect healthy tissues during inflammation and prevent tissue damage. The most common disease variant, the Z mutant, has a single amino acid substitution that results in improper folding of the protein. The mutant protein cannot be effectively secreted and accumulates in globules in the hepatocytes. This triggers continuous hepatocyte injury, leading to fibrosis, cirrhosis, and increased risk of hepatocellular carcinoma.
Current Treatments
Individuals with the homozygous PiZZ genotype have severe deficiency of functional AAT leading to pulmonary disease and hepatocyte injury and liver disease. Lung disease in this patient population is frequently treated with AAT augmentation therapy. However, augmentation therapy does nothing to treat liver disease, and there is no specific therapy for hepatic manifestations. There is a significant unmet need as liver transplant, with its attendant morbidity and mortality, is currently the only available cure.
The Alpha-1 Project
Arrowhead has an agreement with The Alpha-1 Project (TAP), the venture philanthropy subsidiary of the Alpha-1 Foundation. TAP's mission is to support organizations in pursuit of cures and therapies for lung and liver disease caused by AATD. Under the terms of the agreement, TAP has partially funded development of ARO-AAT. In addition to the funding, TAP will make its scientific advisors available to Arrowhead, assist with patient recruitment for clinical trials with its Alpha-1 Foundation Patient Research Registry, and engage in other collaborative efforts that support development of ARO-AAT.
ARO-APOC3
ARO-APOC3 is designed to reduce production of Apolipoprotein C-III (apoC-III), a component of triglyceride rich lipoproteins (TRLs) including VLDL and chylomicrons and a key regulator of triglyceride metabolism. The company believes that knocking down the hepatic production of apoC-III may result in reduced VLDL synthesis and assembly, enhanced breakdown of TRLs, and better clearance of VLDL and chylomicron remnants. A CTA is planned for the fourth calendar quarter of 2018.
Hypertriglyceridemia
Elevated triglyceride levels are an independent risk factor for cardiovascular disease. Severely elevated triglycerides (often over 2,000 mg/dL) in patients with familial chylomicronemia syndrome (FCS), a rare genetic disorder, can result in potentially fatal acute pancreatitis.
2
