UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
(Mark One)
☒ | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended September 30, 2016.
☐ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-21898
ARROWHEAD PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
Delaware |
| 46-0408024 |
(State of incorporation) |
| (I.R.S. Employer Identification No.) |
225 S. Lake Avenue, Suite 1050
Pasadena, California 91101
(626) 304-3400
(Address and telephone number of principal executive offices)
Securities registered under Section 12(b) of the Exchange Act:
Title of each class |
| Name of each exchange on which registered |
Common Stock, $0.001 par value |
| The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Exchange Act:
None
Indicate by a check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by a check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of "accelerated filer and large accelerated filer" in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☐ |
| Accelerated filer ☒ |
| Non-accelerated filer ☐ |
| Smaller Reporting Company ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of issuer's voting and non-voting outstanding Common Stock held by non-affiliates was approximately $290 million based upon the closing stock price of issuer's Common Stock on March 31, 2016. Shares of common stock held by each officer and director and by each person who is known to own 10% or more of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates of the Company. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of December 12, 2016, 74,173,484 shares of the issuer's Common Stock were issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Definitive Proxy Statement to be filed for Arrowhead Pharmaceuticals Inc.'s 2016 Annual Meeting of Stockholders are incorporated by reference into Part III hereof.
PART I |
|
|
|
|
|
|
| ||
I TEM 1. |
| B USINESS |
| 1 |
I TEM 1A. |
| R ISK F ACTORS |
| 24 |
I TEM 1B. |
| U NRESOLVED S TAFF C OMMENTS |
| 34 |
I TEM 2. |
| P ROPERTIES |
| 35 |
I TEM 3. |
| L EGAL P ROCEEDINGS |
| 35 |
I TEM 4. |
| M INE S AFETY D ISCLOSURES |
| 35 |
|
|
| ||
PART II |
|
|
|
|
|
|
| ||
I TEM 5. |
| M ARKET FOR THE R EGISTRANT ' S C OMMON E QUITY , R ELATED S TOCKHOLDER M ATTERS AND I SSUER P URCHASES OF E QUITY S ECURITIES |
| 35 |
I TEM 6. |
| S ELECTED F INANCIAL D ATA |
| 37 |
I TEM 7. |
| M ANAGEMENT ' S D ISCUSSION AND A NALYSIS OF F INANCIAL C ONDITION AND R ESULTS OF O PERATIONS |
| 38 |
I TEM 7A. |
| Q UANTITATIVE AND Q UALITATIVE D ISCLOSURES A BOUT M ARKET R ISK |
| 53 |
I TEM 8. |
| F INANCIAL S TATEMENTS AND S UPPLEMENTARY D ATA |
| 53 |
I TEM 9. |
| C HANGES IN AND D ISAGREEMENTS WITH A CCOUNTANTS ON A CCOUNTING AND F INANCIAL D ISCLOSURE |
| 53 |
I TEM 9A. |
| C ONTROLS AND P ROCEDURES |
| 53 |
I TEM 9B. |
| O THER I NFORMATION |
| 54 |
|
|
| ||
PART III |
|
|
|
|
|
|
| ||
I TEM 10. |
| D IRECTORS , E XECUTIVE O FFICERS , AND C ORPORATE G OVERNANCE |
| 54 |
I TEM 11. |
| E XECUTIVE C OMPENSATION |
| 54 |
I TEM 12. |
| S ECURITY O WNERSHIP OF C ERTAIN B ENEFICIAL O WNERS AND M ANAGEMENT AND R ELATED S TOCKHOLDERS |
| 54 |
I TEM 13. |
| C ERTAIN R ELATIONSHIPS , R ELATED T RANSACTIONS AND D IRECTORS I NDEPENDENCE |
| 54 |
I TEM 14. |
| P RINCIPAL A CCOUNTANT F EES AND S ERVICES |
| 54 |
|
|
| ||
PART IV |
|
|
|
|
|
|
| ||
I TEM 15. |
| E XHIBITS AND F INANCIAL S TATEMENT S CHEDULES |
| 54 |
|
| |||
SIGNATURE |
| 58 | ||
|
| |||
INDEX TO FINANCIAL STATEMENTS AND SCHEDULES |
| F-1 | ||
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and we intend that such forward-looking statements be subject to the safe harbors created thereby. For this purpose, any statements contained in this Annual Report on Form 10-K except for historical information may be deemed to be forward-looking statements. Without limiting the generality of the foregoing, words such as "may," "will," "expect," "believe," "anticipate," "intend," "could," "estimate," or "continue" or the negative or other variations thereof or comparable terminology are intended to identify forward-looking statements. In addition, any statements that refer to projections of our future financial performance, trends in our businesses, or other characterizations of future events or circumstances are forward-looking statements.
The forward-looking statements included herein are based on current expectations of our management based on available information and involve a number of risks and uncertainties, all of which are difficult or impossible to predict accurately and many of which are beyond our control. As such, our actual results may differ significantly from those expressed in any forward-looking statements. Factors that may cause or contribute to such differences include, but are not limited to, those discussed in more detail in Item 1 (Business) and Item 1A (Risk Factors) of Part I and Item 7 (Management's Discussion and Analysis of Financial Condition and Results of Operations) of Part II of this Annual Report on Form 10-K. Readers should carefully review these risks, as well as the additional risks described in other documents we file from time to time with the Securities and Exchange Commission. In light of the significant risks and uncertainties inherent in the forward-looking information included herein, the inclusion of such information should not be regarded as a representation by us or any other person that such results will be achieved, and readers are cautioned not to place undue reliance on such forward-looking information. Except as may be required by law, we disclaim any intent to revise the forward-looking statements contained herein to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
PART I
Unless otherwise noted, (1) the term "Arrowhead" refers to Arrowhead Pharmaceuticals, Inc., a Delaware corporation formerly known as Arrowhead Research Corporation and its Subsidiaries, (2) the terms "Company," "we," "us," and "our," refer to the ongoing business operations of Arrowhead and its Subsidiaries, whether conducted through Arrowhead or a subsidiary of Arrowhead, (3) the term "Subsidiaries" refers collectively to Arrowhead Madison Inc. ("Arrowhead Madison"), Arrowhead Australia Pty Ltd ("Arrowhead Australia") and Ablaris Therapeutics, Inc. ("Ablaris"), (4) the term "Common Stock" refers to Arrowhead's Common Stock, (5) the term "Preferred Stock" refers to Arrowhead's Preferred Stock and (6) the term "Stockholder(s)" refers to the holders of Arrowhead Common Stock.
ITEM 1 . | BUSINESS |
Description of Business
Arrowhead develops medicines that treat intractable diseases by silencing the genes that cause them. Using a broad portfolio of RNA chemistries and efficient modes of delivery, Arrowhead therapies trigger the RNA interference mechanism to induce rapid, deep and durable knockdown of target genes. RNA interference, or RNAi, is a mechanism present in living cells that inhibits the expression of a specific gene, thereby affecting the production of a specific protein. Deemed to be one of the most important recent discoveries in life science with the potential to transform medicine, the discoverers of RNAi were awarded a Nobel Prize in 2006 for their work. Arrowhead's RNAi-based therapeutics leverage this natural pathway of gene silencing.
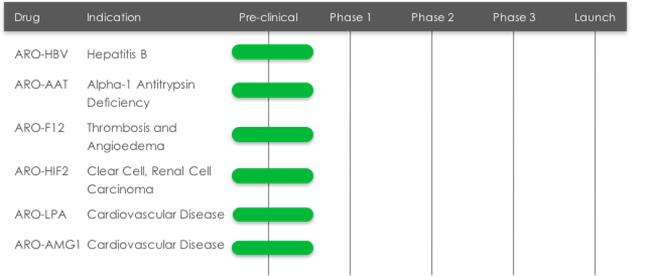
Pre-clinical Stage Drug Candidates
| • | Subcutaneous: |
| • | ARO-HBV is being developed to treat chronic hepatitis B virus infection by reducing the expression and release of new viral particles and key viral proteins with the goal of achieving a functional cure. |
| • | ARO-AAT is being developed to treat liver disease associated with alpha-1 antitrypsin deficiency (AATD), a rare genetic disease that can severely damage the liver and lungs of affected individuals. The goal of treatment with ARO- AAT is to reduce the production of the mutant Z-AAT protein to prevent and potentially reverse accumulation-related liver injury and fibrosis. |
| • | ARO-LPA is designed to reduce production of apolipoprotein A, a key component of lipoprotein(a), which has been genetically linked with increased risk of cardiovascular diseases, independent of cholesterol and LDL levels. Amgen, Inc. ("Amgen") acquired a worldwide, exclusive license in September 2016 to develop and commercialize ARO-LPA. |
| • | ARO-AMG1 is being developed against an undisclosed genetically validated cardiovascular target under a license and collaboration agreement with Amgen. |
| • | ARO-F12 is in preclinical development as a potential treatment for factor 12 (F12) mediated diseases such as hereditary angioedema (HAE) and thromboembolic disorders. Factor 12 initiates the intrinsic coagulation pathway, and reducing its production using Arrowhead's RNAi technology may present opportunities in both disease areas. |
| • | Extra-Hepatic: |
| • | ARO-HIF2 is being developed as a new drug candidate for the treatment of clear cell renal cell carcinoma (ccRCC). ARO-HIF2 is designed to inhibit the production of HIF-2α, which has been linked to tumor progression and metastasis in ccRCC. Arrowhead believes it is an attractive target for intervention because over 90% of ccRCC tumors express a mutant form of the Von Hippel-Landau protein that is unable to degrade HIF-2α, leading to its accumulation during tumor hypoxia and promoting tumor growth. ARO-HIF2 is Arrowhead's first drug candidate using a new delivery vehicle designed to target tissues outside of the liver. |
1
Recent Events
Arrowhead made announcements throughout fiscal 2016 discussing progress towards the company's goals as well as key developments. The following are highlights of those developments:
| • | Discontinued development of ARC-520, ARC-521 and ARC-AAT in November 2016 |
| • | The Company announced that it would be discontinuing these clinical programs, which utilized the intravenously administered DPC iv , or EX1, delivery vehicle, and redeploying its resources and focus toward utilizing the Company's new proprietary subcutaneous and extra-hepatic delivery systems. |
| • | The decision to discontinue development of EX1-containing programs was based primarily on two factors. |
| ▪ | During ongoing discussions with regulatory agencies and outside experts, it became apparent that there would be substantial delays in all clinical programs that utilize EX1, while the Company further explored the cause of deaths in a non-clinical toxicology study in non-human primates exploring doses of EX1 higher than those planned to be used in humans. |
| ▪ | The Company has made substantial advances in RNA chemistry and targeting resulting in large potency gains for subcutaneous administered and extra-hepatic RNAi-based development programs. |
| • | Because of the discontinuation of its existing clinical programs, the Company also reduced its workforce by approximately 30%, while maintaining resources necessary to support current and potential partner-based programs and the Company's pipeline. |
| • | Entered into two collaboration and license agreements with Amgen, Inc. ("Amgen") |
| • | Total deal value of up to $673.5 million |
| • | Arrowhead received $56.5 million upfront: |
| ▪ | $35 million in upfront cash payments, $21.5 million equity investment |
| • | Up to low double-digit royalties for ARO-LPA and single-digit royalties for the undisclosed target, ARO- AMG1 |
| • | Amgen receives: |
| ▪ | Exclusive license to ARO-LPA program |
| ▪ | Option for an additional candidate against an undisclosed target, ARO-AMG1 |
| • | Amgen will be wholly responsible for funding and conducting all clinical development and commercialization |
| • | Continued progress on preclinical candidates including ARO-HBV, ARO-AAT, ARO-F12, ARO-LPA and ARO-HIF2 |
| • | Regarding ARO-F12 and ARO-LPA: |
| ▪ | Presented preclinical data at the American Heart Association's Scientific Sessions 2016 for two development programs using Arrowhead's proprietary subcutaneous delivery platform: |
| ▪ | RNAi triggers against Factor 12 (F12) showed dose dependent reductions in serum F12 |
| ▪ | A statistically significant reduction (p=0.002) in thrombus weight was observed at greater than 95% F12 knockdown in a rat arterio-venous shunt model |
2
| ▪ | There was no increased bleeding risk in AR O - F12 -treated mice, even with greater than 99% knockdown of F12 levels |
| ▪ | RNAi triggers against Lipoprotein (a) [Lp(a)] led to greater than 98% maximum knockdown after a single 3 mg/kg SQ dose in Transgenic mice |
| ▪ | In an atherosclerosis model, data suggest that RNAi triggers can be effectively delivered to a fatty liver using the subcutaneous delivery platform |
| • | Regarding ARO-HIF2 |
| ▪ | Presented preclinical data showing that ARO-HIF2 inhibited renal cell carcinoma growth and promoted tumor cell death in its preclinical studies. |
| • | Strengthened the Company's balance sheet with August 2016 private offering and Amgen agreement upfronts |
| • | In August 2016, the Company sold 7.6 million shares of Common Stock to certain institutional investors and received net proceeds of approximately $43.2 million. |
| • | As part of the collaboration and license agreements as well as a Common Stock Purchase Agreement with Amgen, $14 million of the total $56.5 million upfront cash payments and equity investments were received in September 2016, and the remaining $42.5 million was received in November 2016. |
| • | Continued progress of on former drug candidates prior to the discontinuations |
| • | Presented preclinical and clinical data on former drug candidate ARC-AAT at the Liver Meeting |
| • | In a first-in-human clinical study, ARC-AAT was well tolerated and induced deep and durable reduction of the target AAT protein |
| • | The preclinical data suggest a possible improvement of liver health and arrest of further damage from treatment with ARC-AAT |
| • | Advanced former drug candidate ARC-521 into a Phase 1/2 study |
| • | Conducted multiple dose and combination studies of former drug candidate ARC-520 |
Acquisition of Roche and Novartis RNAi assets
The last five years have brought substantial change to Arrowhead's research and development (R&D) capabilities and strategy. We are now an integrated RNAi therapeutics company, developing novel drugs that silence disease-causing genes based on our broad RNAi technology platform.
The most significant step in this transition was our 2011 acquisition of the RNAi therapeutics business of Hoffmann-La Roche, Inc. and F. Hoffmann-La Roche Ltd. (collectively, "Roche"). Roche built this business unit in a manner that only a large pharmaceutical company is capable of: backed by expansive capital resources, Roche systematically acquired technologies, licensed expansive intellectual property rights, attracted leading scientists, and developed new technologies internally. At a time when the markets were questioning whether RNAi could become a viable therapeutic modality, we saw great promise in the technology broadly and the quality of what Roche built specifically. The acquisition provided us with two primary sources of value:
| • | Broad freedom to operate with respect to key patents directed to the primary RNAi-trigger formats: canonical, UNA, meroduplex, and dicer substrate structures; and |
| • | A large team of scientists experienced in RNAi and oligonucleotide delivery. |
3
In addition, in March 2015 we acquired the entire RNAi research and development portfolio and associated assets of Novartis. Novartis had been working in the R NAi field for over a decade and made some very important advancements in their developments of proprietary oligonucleotide formatting and modifications . Key aspects of the acquisition include the following:
| • | Multiple patent families covering RNAi-trigger design rules and modifications that fall outside of key patents controlled by competitors, which we believe provides freedom to operate for any target and indication; |
| • | Novel intracellular targeting ligands that enhance the activity of RNAi-triggers by targeting the RNA-induced silencing complex (RISC) more effectively and improving stability once RISC is loaded; |
| • | An assignment of Novartis' license from Alnylam Pharmaceuticals, Inc. ("Alnylam") granting Arrowhead access to certain Alnylam intellectual property, excluding delivery, for 30 gene targets; and |
| • | A pipeline of three candidates initiated by Novartis for which Novartis has developed varying amounts of preclinical data. |
We see the Roche and Novartis acquisitions as a powerful combination of intellectual property, R&D infrastructure, and RNAi delivery experts. We believe we are the only company with access to all primary RNAi-trigger structures now including additional novel structures discovered by Novartis. This enables us to optimize our drug candidates on a target-by-target basis and use the structure and modifications that yield the most potent RNAi trigger. Our R&D team and facility enable rapid innovation and drive to the clinic.
RNA Interference & the Benefits of RNAi Therapeutics
RNA interference (RNAi) is a mechanism present in living cells that inhibits the expression of a specific gene, thereby affecting the production of a specific protein. Deemed to be one of the most important recent discoveries in life science with the potential to transform medicine, the discoverers of RNAi were awarded a Nobel Prize in 2006 for their work. RNAi-based therapeutics may leverage this natural pathway of gene silencing to target and shut down specific disease-causing genes.
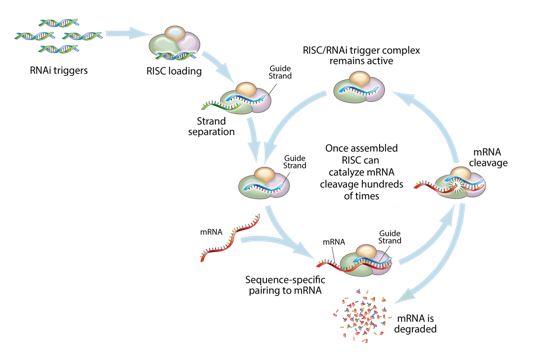
Figure 1: Mechanism of RNA interference
Small molecule and antibody drugs have proven effective at inhibiting certain cell surface, intracellular, and extracellular targets. However, other drug targets such as intranuclear genes and some proteins have proven difficult to inhibit with traditional drug-based and biologic therapeutics. Developing effective drugs for these targets would have the potential to address large underserved
4
marke ts for the treatment of many diseases. Using the ability to specifically silence any gene, RNAi therapeutics may be able to address previously "undruggable" targets, unlocking the market potential of such targets.
Advantages of RNAi as a Therapeutic Modality
| • | Silences the expression of disease causing genes; |
| • | Potential to address any target in the transcriptome including previously "undruggable" targets; |
| • | Rapid lead identification; |
| • | High specificity; |
| • | Opportunity to use multiple RNA sequences in one drug product for synergistic silencing of related targets; and |
RNAi therapeutics are uniquely suited for personalized medicine through target and cell specific delivery and gene knockdown.
Pipeline Overview
Arrowhead is focused on developing innovative drugs for diseases with a genetic basis, characterized by the overproduction of one or more proteins. The depth and versatility of our RNAi technologies enable us to address conditions in virtually any therapeutic area and pursue disease targets that are not otherwise druggable by small molecules and biologics. Our preclinical pipeline of RNAi therapeutics includes both subcutaneously administered liver-targeted candidates and extra-hepatic candidates.
Internal Programs
Hepatitis B Virus Infection
We see the need for a next generation HBV treatment with a finite treatment period and an attractive dosing regimen, and that can be used at earlier stages of disease. We believe a novel therapeutic approach that can effectively treat or provide a functional cure (seroclearance of HBsAg and with or without development of excess patient antibodies against HBsAg) has the potential to take significant market share and may expand the available market to include patients that are currently untreated.
Chronic Hepatitis B Virus
According to the World Health Organization, 240 million people worldwide are chronically infected with hepatitis B virus, of which 500,000 to 1,000,000 people die each year from HBV-related liver disease. Chronic HBV infection is defined by the presence of hepatitis B surface antigen (HBsAg) for more than six months. In the immune tolerant phase of chronic infection, which can last for many years, the infected person typically produces very high levels of viral DNA and viral antigens. However, the infection is not cytotoxic and the carrier may have no symptoms of illness. Over time, the ongoing production of viral antigens causes inflammation
5
and necrosis, leading to elevation of liver enzymes such as alanine and aspartate transaminases, hepa titis, fibrosis, and liver cancer ( hepatocellular carcinoma, or HCC). If untreated, as many as 25% to 40% of chronic HBV carriers ultimately develop cirrhosis or HCC. Antiviral therapy is generally prescribed when liver enzymes become elevated.
Current Treatments
The current standard of care for treatment of chronic HBV infection is a daily oral dose of nucleotide/nucleoside analogs (NUCs) or a regimen of interferon injections for approximately one year. NUCs are generally well tolerated, but patients may need lifetime treatment because viral replication often rebounds upon cessation of treatment. Interferon therapeutics can result in a functional cure in 10-20% of some patient types, but treatment is often associated with significant side effects, including severe flu-like symptoms, marrow suppression, and autoimmune disorders.
ARO-HBV
ARO-HBV is an RNAi therapeutic candidate for the treatment of chronic hepatitis B infection with the goal of achieving a functional cure. This is a next-generation subcutaneously administered compound that follows previous generation HBV compounds ARC-520 and ARC-521, which were in Phase 2b and Phase 1/2 studies respectively when development was discontinued in November 2016.
Goal of ARO-HBV Treatment
ARO-HBV is designed to silence the production of all HBV gene products with the goal of achieving a functional cure. The siRNAs target multiple components of HBV production including the pregenomic RNA that would be reverse transcribed to generate the viral DNA. The siRNAs intervene at the mRNA level, upstream of where NUCs act, and target the mRNAs that produce HBsAg proteins, the viral polymerase, the core protein that forms the capsid, the pre-genomic RNA, the HBeAg, and the hepatitis B X antigen (HBxAg). NUCs are effective at reducing production of viral particles, but are ineffective at controlling production of HBsAg and other HBV gene products. Arrowhead believes that a reduction in the production of HBsAg and other proteins that NUCs are ineffective at controlling is necessary to effective HBV therapy, because those proteins are thought to be major contributors to repression of the immune system and the persistence of liver disease secondary to HBV infection.
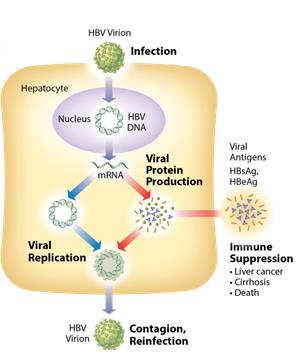
Figure 1: Chronic HBV mechanism untreated
6
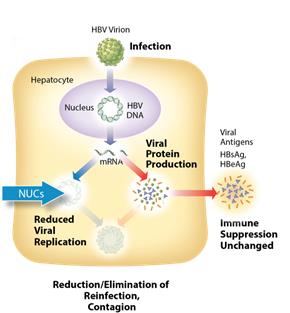
Figure 2: Mechanism of action NUCs
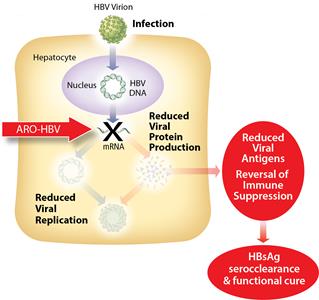
Figure 3: Mechanism of action ARO-HBV
Alpha-1 Antitrypsin Deficiency (AATD)
AATD is a genetic disorder associated with liver disease in children and adults, and pulmonary disease in adults. AAT is a circulating glycoprotein protease inhibitor that is primarily synthesized and secreted by liver hepatocytes. Its physiologic function is the inhibition of neutrophil proteases to protect healthy tissues during inflammation and prevent tissue damage. The most common disease variant, the Z mutant, has a single amino acid substitution that results in improper folding of the protein. The mutant protein cannot be effectively secreted and accumulates in globules in the hepatocytes. This triggers continuous hepatocyte injury, leading to fibrosis, cirrhosis, and increased risk of hepatocellular carcinoma.
Current Treatments
Individuals with the homozygous PiZZ genotype have severe deficiency of functional AAT leading to pulmonary disease and hepatocyte injury and liver disease. Lung disease is frequently treated with AAT augmentation therapy. However, augmentation therapy does nothing to treat liver disease, and there is no specific therapy for hepatic manifestations. There is a significant unmet need as liver transplant, with its attendant morbidity and mortality, is currently the only available cure.
7
ARO -AAT
Arrowhead is developing a therapeutic candidate (ARO-AAT) for the treatment of liver disease associated with AATD. ARO-AAT is designed to knock down the Alpha-1 antitrypsin (AAT) gene transcript and reduce the hepatic production of the mutant AAT protein. This is a next-generation subcutaneously administered compound that follows previous generation AAT compound ARC-AAT, which was in a Phase 2 study when development was discontinued in November 2016.
Goal of ARO-AAT Treatment
The goal of treatment with ARO-AAT is prevention and potential reversal of Z-AAT accumulation-related liver injury and fibrosis. Reduction of inflammatory Z-AAT protein, which has been clearly defined as the cause of progressive liver disease in AATD patients, is important as it is expected to halt the progression of liver disease and allow fibrotic tissue repair.
The Alpha-1 Project
Arrowhead has an agreement with The Alpha-1 Project (TAP), the venture philanthropy subsidiary of the Alpha-1 Foundation. TAP's mission is to support organizations in pursuit of cures and therapies for lung and liver disease caused by AATD. Under the terms of the agreement, TAP has partially funded development of ARO-AAT. In addition to the funding, TAP will make its scientific advisors available to Arrowhead, assist with patient recruitment for clinical trials with its Alpha-1 Foundation Patient Research Registry, and engage in other collaborative efforts that support development of ARO-AAT.
ARO-F12
ARO-F12 is an RNAi-based therapeutic designed to reduce the production of factor 12 with the goal of providing a prophylactic treatment for hereditary angioedema (HAE) and thromboembolic diseases. Arrowhead is conducting relevant disease models and is considering other potential studies to support advancement of ARO-F12 into clinical trials.
ARO-HIF2
ARO-HIF2 is an RNAi-based therapeutic designed to reduce the production of hypoxia-inducible factor 2α (HIF-2α) to treat clear cell renal cell carcinoma. It is the first drug candidate using a new delivery vehicle designed to target tissues outside of the liver.
Partner-based Programs
ARO-LPA and ARO-AMG1
ARO-LPA is designed to reduce production of apolipoprotein A, a key component of lipoprotein(a), which has been genetically linked with increased risk of cardiovascular diseases, independent of cholesterol and LDL levels. Amgen acquired a worldwide, exclusive license in September 2016 to develop and commercialize ARO-LPA.
ARO-AMG1 is being developed against an undisclosed genetically-validated cardiovascular target under a license and collaboration agreement with Amgen.
Under the terms of the agreements taken together for ARO-LPA and ARO-AMG1, the Company will receive $35 million in upfront payments, $21.5 million in the form of an equity investment by Amgen in the Company's Common Stock, and the Company is eligible to receive up to $617 million in option payments and development, regulatory and sales milestone payments. The Company is further eligible to receive single-digit royalties for sales of products under the ARO-AMG1 agreement and up to low double-digit royalties for sales of products under the ARO-LPA agreement.
Intellectual Property and Key Agreements
The Company controls approximately 359 issued patents (including 70 for DPCs; 23 for hydrodynamic gene delivery; 23 for pH labile molecules; 6 for protease cleavable molecules; 7 for polyampholyte; (expired) 18 for delivery polymers; 163 for RNAi trigger molecules; 4 for targeting molecules; 1 for liver expression vector; and 29 for Homing Peptides), including European validations, and 256 patent applications (117 applications in 22 families for DPC-related technologies (includes conjugates, polymers, linkages, etc); 135 applications in 28 families for RNAi trigger targets; and 8 for Homing Peptides). The pending applications have been filed throughout the world, including, in the United States, Argentina, ARIPO (Africa Regional Intellectual Property Organization), Australia, Brazil, Canada, Chile, China, Eurasian Patent Organization, Europe, Hong Kong, Israel, India, Indonesia, Iraq, Jordan, Japan, Republic of Korea, Mexico, New Zealand, OAPI (African Intellectual Property Organization), Peru, Philippines, Russian Federation, Saudi Arabia, Singapore, Thailand, Taiwan, Venezuela, Vietnam, and South Africa.
8
RNAi Triggers
The Company owns patents directed to RNAi triggers targeted to reduce expression of hepatitis B viral proteins as well the RRM2 gene.
Patent Group | Estimated Year of Expiration |
RNAi Triggers | |
Patents directed to HBV RNAi triggers | 2032, 2036 |
Patent directed to RRM2 RNAi triggers | 2031 |
HIF2α | 2034, 2036 |
AAT | 2035 |
Factor 12 | 2036, 2037 |
LPA | 2036 |
Patents directed to α-ENaC | 2028 |
Patents directed to β-ENaC | 2031 |
β-Catenin | 2033 |
KRAS | 2033 |
Patents directed to HSF1 | 2030, 2032 |
APOC3 | 2035 |
Patents directed to Cx43 | 2029 |
Patents directed to HIF1A | 2026 |
Patents directed to FRP-1 | 2026 |
Patents directed to HCV | 2023 |
Patents directed to PDtype4 | 2026 |
Patents directed to PI4Kinase | 2028 |
Patents directed to HRH1 | 2027 |
Patents directed to SYK | 2027 |
Patents directed to TNF-α | 2027, 2028 |
RNAi agent design (26mer) | 2036 |
RNAi agent design (26mer) | 2037 |
RNAi agent design (5′-cyclo-phosphonate) | 2037 |
9
Dynamic Polyconjugates
The DPC-related patents have issued in the United States, Australia, Canada, Europe, France, Germany, Italy, Spain, Switzerland, United Kingdom, India, Japan, Mexico, New Zealand, Philippines, Russia, South Korea, Singapore, and South Africa. The Company also controls a number of patents directed to hydrodynamic nucleic acid delivery, which issued in the United States, Australia and Europe (validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, the United Kingdom, Hungary, Ireland, Italy, Netherlands and Sweden). The approximate year of expiration for each of these various groups of patents are set forth below:
Patent Group | Estimated Year of Expiration |
Dynamic Polyconjugates (DPC) | |
Dynamic Polyconjugates | 2027 |
Dynamic Polyconjugates – second iteration | 2031 |
Membrane Active Polymers | 2027 |
Membrane Active Polymers – Additional Iterations | 2024 |
Membrane Active Polymers – Additional Iterations | 2032 |
Membrane Active Polymers – Additional Iterations | 2033 |
Copolymer Systems | 2024 |
Polynucleotide-Polymer Composition | 2024 |
ARC-520 Polymer | 2031 |
ACR-520 manufacture | 2037 |
ARC-521 Composition | 2036 |
Masking Chemistry | 2031 |
Masking Chemistry | 2032 |
Masking Chemistry | 2036 |
Polyampholyte Delivery | 2017 |
pH Labile Molecules | 2020 |
Endosomolytic Polymers | 2020 |
Targeting groups (Gal trimer-PK) | 2031 |
Targeting groups (αvβ3 integrin) | 2035 |
Targeting groups (αvβ6 integrin) | 2037 |
Targeting groups (GalNAc trimer) | 2037 |
Hydrodynamic delivery | |
Second iteration | 2020 |
Third iteration | 2024 |
The RNAi and drug delivery patent landscapes are complex and rapidly evolving. As such, we may need to obtain additional patent licenses prior to commercialization of our candidates. You should review the factors identified in "Risk Factors" in Part I, Item 1A of this Annual Report on Form 10-K.
Homing Peptides
We also control patents related to our Homing Peptide platforms, related to Adipotide, our drug candidate for the treatment for obesity and related metabolic disorders. Approximately seven of these patents are United States patents and the remaining patents are validated in Belgium, Switzerland, Germany, Spain, France, Japan, the United Kingdom, Ireland, Greece, Italy, Netherlands, Portugal, Sweden and Turkey.
Patent Group | Estimated Year of Expiration |
Adipotide ® | |
Targeting moieties and conjugates | 2021 |
Targeted Pharmaceutical Compositions | 2021 |
Homing Peptides | |
EphA5 Targeting Peptides | 2027 |
IL-11R Targeting Peptides | 2022 |
10
Non-Exclusively Licensed Patent Rights obtained from Roche
Roche and the Company entered into a Stock and Asset Purchase Agreement on October 21, 2011, pursuant to which Roche assigned to Arrowhead its entire rights under certain licenses including: the License and Collaboration Agreement between Roche and Alnylam dated July 8, 2007 (the "Alnylam License"); the Non-Exclusive Patent License Agreement between Roche and MDRNA, Inc. dated February 12, 2009 ("MDRNA License"); and the Non-Exclusive License Agreement between Roche and City of Hope dated September 19, 2011 (the "COH License") (Collectively the "RNAi Licenses"). The RNAi Licenses provide the Company with non-exclusive, worldwide, perpetual, irrevocable, royalty-bearing rights and the right to sublicense a broad portfolio of intellectual property relating to the discovery, development, manufacture, characterization, and use of therapeutic products that function through the mechanism of RNA interference for specified targets.
Terms of the 2007 Alnylam License
The Alnylam License provides us with a non-exclusive, worldwide, perpetual, irrevocable, royalty-bearing right and sublicensable license under Alnylam's rights in certain intellectual property existing as of its effective date, to engage in discovery, development, commercialization and manufacturing activities, including to make, have made, use, offer for sale, sell and import certain licensed products in certain fields. The fields include the treatment or prophylaxis of indications comprising an RNAi compound complementary to, and function in mediating the RNAi of, a target known or believed to be primarily implicated in one or more primary therapeutic areas. The primary therapeutic areas are cancer, hepatic, metabolic disease and pulmonary disease. The hepatic therapeutic area specifically excludes targets of infectious pathogen.
The Alnylam License excludes access to intellectual property specifically related to "Blocked Targets." "Blocked Targets" are those targets that are subject to a contractual obligation of a pre-existing agreement between Alnylam and its alliance partners.
Under the Alnylam License, we may be obligated to pay development and sales milestone payments of up to the mid to upper double-digit millions of dollars for each licensed product that progresses through clinical trials in a particular indication, receives marketing approval for that indication and is the subject of a first commercial sale. Additionally, we may be obligated to pay mid to high single-digit percentage royalties on sales of such products.
Core Patents relating to RNAi
The RNAi Licenses include patents relating to the general structure, architecture, and design of double-stranded oligonucleotide molecules, which engage RNA interference mechanisms in a cell. These rights include the "Tuschl II" patents, including issued U.S. Patent Nos. 7,056,704; 7,078,196; 7,078,196; 8,329,463; 8,362,231; 8,372,968; and 8,445,327; "Tuschl I" patents, including U.S. Patent Nos. 8,394,628 and 8,420,391; and allowed "Tuschl I" patent application, U.S. Publication No. 2011024446; "City of Hope" patents, including U.S. Patent No. 8,084,599; and "Kreutzer-Limmer" patents assigned to Alnylam, including U.S. Patent Nos. 7,829,693; 8,101,594; 8,119,608; 8,202,980; and 8,168,776.
Thomas Tuschl is the first named inventor on "Tuschl I" and "Tuschl II." "Tuschl I" patents refers to the patents arising from the patent application entitled "The Uses of 21-23 Sequence-Specific Mediators of Double-Stranded RNA Interference as a Tool to Study Gene Function and as a Gene-Specific Therapeutic." "Tuschl II" patents refer to the patents and patent applications arising from the patent application entitled "RNA Interference Mediating Small RNA Molecules." "City of Hope" is the first named assignee of certain core RNAi trigger patents. The second named assignee of these patents is Integrated DNA Technologies, Inc. Kreutzer-Limmer patents refer to the Alnylam patents and patent applications, relating to core siRNA IP, which includes inventors Roland Kreutzer and Stefan Limmer.
Chemical modifications of double-stranded oligonucleotides
The RNAi Licenses also include patents related to modifications of double-stranded oligonucleotides, including modifications to the base, sugar, or internucleoside linkage, nucleotide mimetics, and end modifications, which do not abolish the RNAi activity of the double-stranded oligonucleotides. Also included are patents relating to modified double-stranded oligonucleotides, such as meroduplexes described in in U.S. Publication No. 20100209487 assigned to Marina Biotech (f/k/a MDRNA, Inc.), and microRNAs described in U.S. Patent Nos. 7,582,744; 7,674,778, and 7,772,387 assigned to Alnylam as well as U.S. Patent No. 8,314,227 related to unlocked nucleic acids (UNA). The ‘227 patent was assigned by Marina Biotech to Arcturus Therapeutics, Inc. but remains part of the MDRNA License. The RNAi Licenses also include rights from INEX/Tekmira relating to lipid-nucleic acid particles, and oligonucleotide modifications to improve pharmacokinetic activity including resistance to degradation, increased stability, and more specific targeting of cells from Alnylam and ISIS Pharmaceuticals, Inc.
11
Manufacturing techniques for the double-stranded oligonucleotide molecules or chemical modifications
The RNAi Licenses also include patents relating to the synthesis and manufacture of double-stranded oligonucleotide molecules for use in RNA interference, as well as chemical modifications of such molecules, as described above. These include methods of synthesizing the double-stranded oligonucleotide molecules such as in the core "Tuschl I" allowed U.S. Application No. 12/897,749, the core "Tuschl II" U.S. Patent Nos. 7,056,704; 7,078,196; and 8,445,327; and Alnylam's U.S. Patent Nos. 8,168,776, as well as methods of making chemical modifications of the double-stranded oligonucleotides such as described in Alnylam's U.S. Patent No. 7,723,509 and INEX's U.S. Patent Nos. 5,976,567; 6,858,224; and 8,484,282. Patent applications are currently pending that further cover manufacturing techniques for double-stranded oligonucleotide molecules or chemical modifications.
Uses and Applications of Double-Stranded Oligonucleotide Molecules or Chemical modifications
The RNAi Licenses also include patents related to uses of the double-stranded oligonucleotides that function through the mechanism of RNA interference. These include for example, the core "Tuschl I" U.S. Patent No. 8,394,628 and "Tuschl II" U.S. Patent No. 8,329,463; Alnylam's U.S. Patent Nos. 7,763,590; 8,101,594, and 8,119,608, and City of Hope‘s U.S. Patent No. 8,084,599. Other more specific uses have been acquired and patent applications are currently pending that cover additional end uses and applications of double-stranded oligonucleotides functioning through RNA interference.
2012 License from Alnylam
In January 2012, we obtained a license from Alnylam under its rights in certain RNAi intellectual property to develop and commercialize RNAi-based products targeting RNAs encoded by the genome of HBV.
Alnylam granted us a worldwide non-exclusive sublicensable royalty bearing license under Alnylam's general RNAi intellectual property estate to research, develop and commercialize RNAi-based products targeting HBV RNAs in combination with DPC technology. Alnylam further granted us a worldwide sublicensable exclusive royalty bearing license under its target-specific RNAi patent rights to research, develop and commercialize RNAi-based products targeting HBV RNAs in combination with DPC technology. Alnylam further agreed to forego the development of any RNAi-based products targeting HBV RNAs in combination with DPC technology.
Under the license from Alnylam, we may be obligated to pay development and sales milestone payments of up to the low double-digit millions of dollars for each licensed product that progresses through clinical trials, receives marketing approval and is the subject of a first commercial sale. Additionally, we may be obligated to pay low single-digit percentage royalties on sales of such products.
2012 License to Alnylam
In consideration for the licenses from Alnylam, in January 2012 we granted Alnylam a worldwide non-exclusive, sublicensable royalty bearing license under our broad and target-specific DPC intellectual property rights to research, develop and commercialize RNAi-based products against a single undisclosed target in combination with DPC technology. Under the license to Alnylam, Alnylam may be obligated to pay us development and sales milestone payments of up to the low double digit millions of dollars for each licensed product that progresses through clinical trials, receives marketing approval and is the subject of a first commercial sale. Additionally, Alnylam may be obligated to pay us low single digit percentage royalties on sales of such products.
Acquisition of Assets from Novartis
On March 3, 2015, the Company entered into an Asset Purchase and Exclusive License Agreement (the "RNAi Purchase Agreement") with Novartis pursuant to which the Company acquired Novartis' RNAi assets and rights thereunder. Pursuant to the RNAi Purchase Agreement, the Company acquired or licensed certain patents and patent applications owned or controlled by Novartis related to RNAi therapeutics, assignment of Novartis's rights under a license from Alnylam (the "Alnylam-Novartis License"), rights to three pre-clinical RNAi candidates, and a license to certain Novartis assets (the "Licensed Novartis Assets"). The patents acquired from Novartis include multiple patent families covering delivery technologies and RNAi-trigger design rules and modifications. The Licensed Novartis Assets include an exclusive, worldwide right and license, solely in the RNAi field, with the right to grant sublicenses through multiple tiers under or with respect to certain patent rights and know how relating to delivery technologies and RNAi-trigger design rules and modifications. Under the assigned Alnylam-Novartis License, the Company has acquired a worldwide, royalty-bearing, exclusive license with limited sublicensing rights to existing and future Alnylam intellectual property (coming under Alnylam's control on or before March 31, 2016), excluding intellectual property concerning delivery technology, to research, develop and commercialize 30 undisclosed gene targets.
12
Cardiovascular Collaboration and License Agreements with Amgen
On September 28, 2016, the Company entered into two Collaboration and License agreements, and a Common Stock Purchase Agreement with Amgen. Under the First Collaboration and License Agreement, Amgen will receive an option to a worldwide, exclusive license for an RNAi therapy for ARO-AMG1 , an undisclosed genetically validated cardiovascular target. Under the Second Collaboration and License, Amgen will receive a worldwide, exclusive license to Arrowhead's novel, RNAi ARO-LPA program. These RNAi molecules are designed to reduce elevated lipoprotein(a), which is a genetically validated, independent risk factor for atherosclerotic cardiovascular disease. In both agreements, Amgen will be wholly responsible for clinical development and commercialization. Under the terms of the agreements taken together, the Company will receive $35 million in upfront payments, $21.5 million in the form of an equity investment by Amgen in the Company's Common Stock, and up to $617 million in option payments and development, regulatory and sales milestone payments. The Company is further eligible to receive single-digit royalties for sales of products under the ARO-AMG1 agreement and up to low double-digit royalties for sales of products under the ARO-LPA agreement.
Research and Development Facility
Arrowhead's research and development operations are located in Madison, Wisconsin. Substantially all of the Company's assets are located either in this facility or in our corporate headquarters in Pasadena. A summary of our research and development resources is provided below:
| • | Approximately 60 scientists currently; |
| • | State-of-the-art laboratories consisting of 61,000 total sq. ft.; |
| • | Complete small animal facility; |
| • | Primate colony housed at the Wisconsin National Primate Research Center, an affiliate of the University of Wisconsin; |
| • | In-house histopathology capabilities; |
| • | Animal models for metabolic, viral, and oncologic diseases; |
| • | Animal efficacy and safety assessment; |
| • | Polymer, peptide, oligonucleotide and small molecule synthesis and analytics capabilities (HPLC, NMR, MS, etc.); |
| • | Polymer, peptide and oligonucleotide PK, biodistribution, clearance methodologies; and |
| • | Conventional and confocal microscopy, flow cytometry, Luminex platform, qRT-PCR, clinical chemistry analytics. |
Research and Development Expenses
Research and development (R&D) expenses consist of costs incurred in discovering, developing and testing our clinical and preclinical candidates and platform technologies. R&D expenses also include costs related to clinical trials, including costs of contract research organizations to recruit patients and manage clinical trials. Other costs associated with clinical trials include manufacturing of clinical supplies, as well as good laboratory practice ("GLP") toxicology studies necessary to support clinical trials, both of which are outsourced to cGMP-compliant manufacturers and GLP-compliant laboratories. Total research and development expense for fiscal 2016 was $41.5 million, a decrease from $47.3 million in 2015 and an increase from $23.1 million in 2014.
At September 30, 2016, we employed approximately 94 employees in an R&D function, primarily working from our facility in Madison, Wisconsin. Due to the discontinuation of our clinical candidates in November 2016, we reduced our R&D workforce and currently employ approximately 77 employees in an R&D function. These employees are engaged in various areas of research on Arrowhead candidate and platform development including synthesis and analytics, PK/biodistribution, formulation, CMC and analytics, tumor and extra-hepatic targeting, bioassays, live animal research, toxicology/histopathology, clinical and regulatory operations, and other areas. Salaries and payroll-related expenses for our R&D activities were $13.9 million in fiscal 2016, $11.6 million in fiscal 2015, and $7.8 million in fiscal 2014. Laboratory supplies including animal-related costs for in-vivo studies were $4.3 million, $3.1 million, and $2.3 million in fiscal 2016, 2015, and 2014, respectively.
Costs related to manufacture of clinical supplies, GLP toxicology studies and other outsourced lab studies, as well as clinical trial costs were $32.6 million, $41.8 million, and $18.8 million in fiscal 2016, 2015, and 2014, respectively.
Facility-related costs, primarily rental costs for our leased laboratory in Madison, Wisconsin were $1.3 million, $1.0 million, and $0.9 million in fiscal 2016, 2015, and 2014, respectively. Other research and development expenses were $3.2 million, $1.4
13
million, and $1. 2 million in fiscal 201 6 , 201 5 and 201 4 , respectively. These expenses are primaril y related to milestone payments, which can vary from period to period depending on the nature of our various license agreements, and the timing of reaching various development milestones requiring payment.
Government Regulation
Government authorities in the United States, at the federal, state, and local levels, and in other countries and jurisdictions, including the European Union, extensively regulate, among other things, the research, development, testing, product approval, manufacture, quality control, manufacturing changes, packaging, storage, recordkeeping, labeling, promotion, advertising, sales, distribution, marketing, and import and export of drugs and biologic products. All of our foreseeable product candidates are expected to be regulated as drugs. The processes for obtaining regulatory approval in the U.S. and in foreign countries and jurisdictions, along with compliance with applicable statutes and regulations and other regulatory authorities both pre- and post-commercialization, are a significant factor in the production and marketing of our products and our R&D activities and require the expenditure of substantial time and financial resources.
Review and Approval of Drugs in the United States
In the U.S., the FDA and other government entities regulate drugs under the Federal Food, Drug, and Cosmetic Act (the "FDCA"), the Public Health Service Act, and the regulations promulgated under those statutes, as well as other federal and state statutes and regulations. Failure to comply with applicable legal and regulatory requirements in the U.S. at any time during the product development process, approval process, or after approval, may subject us to a variety of administrative or judicial sanctions, such as a delay in approving or refusal by the FDA to approve pending applications, withdrawal of approvals, delay or suspension of clinical trials, issuance of warning letters and other types of regulatory letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil monetary penalties, refusals of or debarment from government contracts, exclusion from the federal healthcare programs, restitution, disgorgement of profits, civil or criminal investigations by the FDA, U.S. Department of Justice, State Attorneys General, and/or other agencies, False Claims Act suits and/or other litigation, and/or criminal prosecutions.
An applicant seeking approval to market and distribute a new drug in the U.S. must typically undertake the following:
(1) completion of pre-clinical laboratory tests, animal studies, and formulation studies in compliance with the FDA's GLP regulations;
(2) submission to the FDA of an Investigational New Drug Application ("IND") for human clinical testing, which must become effective without FDA objection before human clinical trials may begin;
(3) approval by an independent institutional review board ("IRB"), representing each clinical site before each clinical trial may be initiated;
(4) performance of adequate and well-controlled human clinical trials in accordance with the FDA's current good clinical practice ("cGCP") regulations, to establish the safety and effectiveness of the proposed drug product for each indication for which approval is sought;
(5) preparation and submission to the FDA of a New Drug Application ("NDA");
(6) satisfactory review of the NDA by an FDA advisory committee, where appropriate or if applicable,
(7) satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the drug product, and the active pharmaceutical ingredient or ingredients thereof, are produced to assess compliance with current good manufacturing practice ("cGMP") regulations and to assure that the facilities, methods, and controls are adequate to ensure the product's identity, strength, quality, and purity;
(8) payment of user fees, as applicable, and securing FDA approval of the NDA; and
(9) compliance with any post-approval requirements, such as any Risk Evaluation and Mitigation Strategies ("REMS") or post-approval studies required by the FDA.
14
Preclinical Studies and an IND
Preclinical studies can include in vitro and animal studies to assess the potential for adverse events and, in some cases, to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations. Other studies include laboratory evaluation of the purity, stability and physical form of the manufactured drug substance or active pharmaceutical ingredient and the physical properties, stability and reproducibility of the formulated drug or drug product. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical studies, among other things, to the FDA as part of an IND. Some preclinical testing, such as longer-term toxicity testing, animal tests of reproductive adverse events and carcinogenicity, may continue after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to a proposed clinical trial and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Following commencement of a clinical trial under an IND, the FDA may place a clinical hold on that trial. A clinical hold is an order issued by the FDA to the sponsor to delay a proposed clinical investigation or to suspend an ongoing investigation. A partial clinical hold is a delay or suspension of only part of the clinical work requested under the IND. For example, a specific protocol or part of a protocol is not allowed to proceed, while other protocols may do so. No more than 30 days after imposition of a clinical hold or partial clinical hold, the FDA will provide the sponsor a written explanation of the basis for the hold. Following issuance of a clinical hold or partial clinical hold, an investigation may only resume after the FDA has notified the sponsor that the investigation may proceed. The FDA will base that determination on information provided by the sponsor correcting the deficiencies previously cited or otherwise satisfying the FDA that the investigation can proceed.
Human Clinical Studies in Support of an NDA
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with cGCP requirements, which include, among other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written study protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review and reapprove the study at least annually. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical trials must be submitted within specific timeframes to the NIH for public dissemination on its ClinicalTrials.gov website.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
Phase 1: The product candidate is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness.
Phase 2: The product candidate is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
Phase 3: The product candidate is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, Phase 2, and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB's requirements or if the drug has been associated with unexpected serious harm to patients. The FDA will typically inspect one or more clinical sites in late-stage clinical trials to assure compliance with cGCP and the integrity of the clinical data submitted.
15
Submission of an NDA to the FDA
Assuming successful completion of required clinical testing and other requirements, the results of the preclinical and clinical studies, together with detailed information relating to the product's chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the drug product for one or more indications. Under federal law, the submission of most NDAs is additionally subject to an application user fee, currently exceeding $2.4 million, and the sponsor of an approved NDA is also subject to annual product and establishment user fees, currently exceeding $114,450 per product and $585,200 per establishment. These fees are typically increased annually.
Under certain circumstances, the FDA will waive the application fee for the first human drug application that a small business, defined as a company with less than 500 employees, or its affiliate, submits for review. An affiliate is defined as a business entity that has a relationship with a second business entity if one business entity controls, or has the power to control, the other business entity, or a third party controls, or has the power to control, both entities. In addition, an application to market a prescription drug product that has received orphan designation is not subject to a prescription drug user fee unless the application includes an indication for other than the rare disease or condition for which the drug was designated.
The FDA conducts a preliminary review of an NDA within 60 days of its receipt and informs the sponsor by the 74th day after the FDA's receipt of the submission to determine whether the application is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA has agreed to specified performance goals in the review process of NDAs. Most such applications are meant to be reviewed within ten months from the date of filing, and most applications for "priority review" products are meant to be reviewed within six months of filing. The review process may be extended by the FDA for three additional months to consider new information or clarification provided by the applicant to address an outstanding deficiency identified by the FDA following the original submission.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with cGCP.
The FDA also may require submission of an REMS plan to mitigate any identified or suspected serious risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA is required to refer an application for a novel drug to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
The FDA's Decision on an NDA
On the basis of the FDA's evaluation of the NDA and accompanying information, including the results of the inspection of the manufacturing facilities, the FDA may issue an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA's satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
16
If the FDA approves a product, it may limit the approved indications for use for the product, require that contraindicati ons, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess the drug's safety after approval, require testing and surveillance programs to monitor t he product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claim s, are subject to further testing requirements and FDA review and approval.
The product may also be subject to official lot release, meaning that the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release, the manufacturer must submit samples of each lot, together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer's tests performed on the lot, to the FDA. The FDA may in addition perform certain confirmatory tests on lots of some products before releasing the lots for distribution. Finally, the FDA will conduct laboratory research related to the safety and effectiveness of drug products.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for this type of disease or condition will be recovered from sales in the U.S. for that drug. Orphan drug designation must be requested before submitting an NDA, and both the drug and the disease or condition must meet certain criteria specified in the Orphan Drug Act and FDA's implementing regulations at 21 C.F.R. Part 316. The granting of an orphan drug designation does not alter the standard regulatory requirements and process for obtaining marketing approval. Safety and effectiveness of a drug must be established through adequate and well-controlled studies.
After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other application to market the same drug for the same indication, except in very limited circumstances, for seven years.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events or problems with manufacturing processes of unanticipated severity or frequency, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or holds on post-approval clinical trials; |
| • | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
17
| • | product seizure or detention, or refusal to permit the import or export of products; or |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act (PDMA), which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Abbreviated New Drug Applications for Generic Drugs
In 1984, with passage of the Drug Price Competition and Patent Term Restoration Act of 1984 (commonly referred to as the "Hatch-Waxman Amendments") amending the FDCA, Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application (ANDA) to the agency. In support of such applications, a generic manufacturer may rely on the preclinical and clinical testing previously conducted for a drug product previously approved under an NDA, known as the reference listed drug, or RLD. To reference that information, however, the ANDA applicant must demonstrate, and the FDA must conclude, that the generic drug does, in fact, perform in the same way as the RLD it purports to copy. Specifically, in order for an ANDA to be approved, the FDA must find that the generic version is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form, and the strength of the drug.
At the same time, the FDA must also determine that the generic drug is "bioequivalent" to the innovator drug. Under the statute, a generic drug is bioequivalent to a RLD if "the rate and extent of absorption of the generic drug do not show a significant difference from the rate and extent of absorption of the RLD." Upon approval of an ANDA, the FDA indicates that the generic product is "therapeutically equivalent" to the RLD and it assigns a therapeutic equivalence rating to the approved generic drug in its publication "Approved Drug Products with Therapeutic Equivalence Evaluations," also referred to as the "Orange Book." Physicians and pharmacists consider the therapeutic equivalence rating to mean that a generic drug is fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, the FDA's designation of a therapeutic equivalence rating often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or patient.
Under the Hatch-Waxman Amendments, the FDA may not approve an ANDA until any applicable period of nonpatent exclusivity for the RLD has expired. The FDCA provides a period of five years of data exclusivity for new drug containing a new chemical entity. In cases where such exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval. The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or indication.
Hatch-Waxman Patent Certification and the 30 Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant's product or a method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval.
Specifically, the applicant must certify with respect to each patent that:
| • | the required patent information has not been filed; |
| • | the listed patent has expired; |
| • | the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or |
| • | the listed patent is invalid, unenforceable or will not be infringed by the new product. |
18
A certification that the new product will not infringe the already approved product's listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicat e that it is not seeking approval of a patented method of use, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant.
To the extent that a Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. As a result, approval of a 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003, an NDA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the drug product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. With enactment of the Food and Drug Administration Safety and Innovation Act, or FDASIA, in 2012, sponsors must also submit pediatric study plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric study or studies the applicant plans to conduct, including study objectives and design, any deferral or waiver requests, and other information required by regulation. The applicant, the FDA, and the FDA's internal review committee must then review the information submitted, consult with each other, and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the non-patent and orphan exclusivity. This six-month exclusivity may be granted if an NDA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA's request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity or patent protection cover the product are extended by six months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot accept or approve another application.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Amendments. Those Amendments permit a patent restoration of up to five years for patent term lost during product development and the FDA regulatory review. The restoration period granted is typically one-half the time between the effective date of an IND and the submission date of a NDA, plus the time between the submission date of a NDA and ultimate approval. Patent term restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the product's approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. The U.S. Patent and Trademark Office reviews and approves the application for any patent term extension or restoration in consultation with the FDA.
19
Review and Approval of Drug Products in the European Union
In order to market any product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products. Whether or not it obtains FDA approval for a product, the company would need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Pursuant to the European Clinical Trials Directive, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of a European Union member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent ethics committee has issued a favorable opinion. Clinical trial applications must be accompanied by an investigational medicinal product dossier with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member states and further detailed in applicable guidance documents.
To obtain marketing approval of a drug under European Union regulatory systems, an applicant must submit a marketing authorization application (MAA) either under a centralized or decentralized procedure.
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union member states. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the Committee for Medicinal Products for Human Use (CHMP) established at the European Medicines Agency (EMA) is responsible for conducting the initial assessment of a drug. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops, when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. In this circumstance, the EMA ensures that the opinion of the CHMP is given within 150 days.
The decentralized procedure is available to applicants who wish to market a product in various European Union member states where such product has not received marketing approval in any European Union member states before. The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one member state designated by the applicant, known as the reference member state. Under this procedure, an applicant submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference member state and concerned member states. The reference member state prepares a draft assessment report and drafts of the related materials within 120 days after receipt of a valid application. Within 90 days of receiving the reference member state's assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials.
If a member state cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the European Commission, whose decision is binding on all member states.
20
Data and Market Exclusivity in the European Union
In the European Union, new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator's data to assess a generic (abbreviated) application for eight years, after which generic marketing authorization can be submitted, and the innovator's data may be referenced, but not approved for two years. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the sponsor is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the drug if such company can complete a full MAA with a complete database of pharmaceutical test, preclinical tests and clinical trials and obtain marketing approval of its product.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of products approved by the FDA and other government authorities. Sales of products will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs such as, in the United States, Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the product once coverage is approved. Third-party payors may limit coverage to specific products on an approved list, or formulary, which might not include all of the approved products for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, a company may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. A payor's decision to provide coverage for a drug product does not necessarily imply that an adequate reimbursement rate will be approved. Third-party reimbursement may not be sufficient to maintain price levels high enough to realize an appropriate return on our investment in product development.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. Third-party payors are increasingly challenging the prices charged for medical products and services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third-party payors do not consider a product to be cost effective compared to other available therapies, they may not cover the product after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow a company to sell its products at a profit. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, risk sharing, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals. As a result, the marketability of any product which receives regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement.
In addition, an increasing emphasis on managed care in the United States has increased and will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. In particular, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies' share of sales to federal health care programs. Even if favorable coverage and reimbursement status is attained for one or more products that receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
21
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countri es may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system o f direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on healt h care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competi tive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements for any of our products.
Healthcare Laws and Regulation
Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of drug products that are granted marketing approval. Arrangements with third-party payors and customers are subject to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| • | the federal healthcare Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
| • | the federal Foreign Corrupt Practices Act (FCPA) prohibits, among other things, U.S. corporations and their persons acting on their behalf from offering, promising, authorizing or making payments to any foreign government official, including certain healthcare professionals in many countries, in an attempt to obtain or retain business or otherwise seek preferential treatment abroad; |
| • | the federal False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent, making a false statement material to a false or fraudulent claim, or improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA) imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| • | the federal Physician Payment Sunshine Act requires manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment interests; and |
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Certain Financial Information
22
The financial information required in this Item 1 is included in Part II, Item 6 and Part IV, Item 15 of this Annual Report Form 10-K.
Corporate Information
Arrowhead was originally incorporated in South Dakota in 1989, and was reincorporated in Delaware in 2000. In April 2016, Arrowhead changed its name form Arrowhead Research Corporation to Arrowhead Pharmaceuticals, Inc. The Company's principal executive offices are located at 225 South Lake Avenue, Suite 1050, Pasadena, California 91101, and its telephone number is (626) 304-3400. We also operate a research and development facility in Madison, Wisconsin. As of September 30, 2016, Arrowhead had 113 full-time employees.
Investor Information
Our website address is http://www.arrowheadpharmaceuticals.com Our reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, including our annual reports on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K, and amendments to those reports, are accessible through our website, free of charge, as soon as reasonably practicable after these reports are filed electronically with, or otherwise furnished to, the SEC. These SEC reports can be accessed through the "Investors" section of our website.
You may read and copy any materials we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy and information statements, and other information regarding Arrowhead and other issuers that file electronically with the SEC. The SEC's Internet website address is http://www.sec.gov .
23
ITEM 1A. | RISK FACTORS |
You should carefully consider the risks discussed below and all of the other information contained in this report in evaluating us and an investment in our securities. If any of the following risks and uncertainties should occur, they could have a material adverse effect on our business, financial condition or results of operations. In that case, the trading price of our Common Stock could decline. Additionally, we note that we have accrued net losses annually since inception given the stage of our drug development. We urge you to consider our likelihood of success and prospects in light of the risks, expenses and difficulties frequently encountered by entities at similar stages of development.
Risks Related to Our Company
Drug development is time consuming, expensive and risky.
We are focused on technology related to new and improved pharmaceutical candidates. Product candidates that appear promising in the early phases of development, such as in animal and early human clinical trials, often fail to reach the market for a number of reasons, such as:
● | Clinical trial results may be unacceptable, even though preclinical trial results were promising; |
● | Inefficacy and/or harmful side effects in humans or animals; |
● | The necessary regulatory bodies, such as the U.S. Food and Drug Administration, may not approve our potential product for the intended use, or at all; and |
● | Manufacturing and distribution may be uneconomical. |
For example, the positive pre-clinical results in animals for our pre-clinical programs may not be replicated in human clinical studies. These programs may be also found to be unsafe in humans, particularly at higher doses needed to achieve the desired levels of efficacy. Also, the positive safety results from single dose human clinical studies may not be replicated in other human studies, including multiple dose studies. Clinical and pre-clinical study results are frequently susceptible to varying interpretations by scientists, medical personnel, regulatory personnel, statisticians and others, which often delays, limits, or prevents further clinical development or regulatory approvals of potential products. Clinical trials can take many years to complete, including the process of study design, clinical site selection and the recruitment of patients. As a result, we can experience significant delays in completing clinical studies, which can increase the cost of developing a drug candidate and shorten the time that an approved product may be protected by patents. If our drug candidates are not successful in human clinical trials, we may be forced to curtail or abandon certain development programs. If we experience significant delays in commencing or completing our clinical studies, we could suffer from significant cost overruns, which could negatively affect our capital resources and our ability to complete these studies.
There are substantial risks inherent in attempting to commercialize new drugs, and, as a result, we may not be able to successfully develop products for commercial use.
Our research and development efforts involve therapeutics based on RNA interference and our delivery systems, which are largely unproven technologies. Our scientists and engineers are working on developing technology in the early stages. However, such technology's commercial feasibility and acceptance are unknown. Scientific research and development requires significant amounts of capital and takes a long time to reach commercial viability, if it can be achieved at all. To date, our research and development projects have not produced commercially viable drugs, and may never do so. During the research and development process, we may experience technological barriers that we may be unable to overcome. Further, certain underlying premises in our development programs are not proven. For instance, the theory that knockdown of S-antigen in chronic hepatitis B patients will result in a functional cure is unproven. Similarly, the reduction of the production of mutant alpha-1 antitrypsin in the liver may not lead to a reduction of globules in the liver, and even if it leads to a reduction in such globules, this may not lead to other beneficial hepatic changes. It is also unknown at this time what changes in the liver may be required to gain regulatory approval and/or favorable reimbursement. Because of these and similar uncertainties, it is possible that no commercial products will be successfully developed. If we are unable to successfully develop commercial products, we will be unable to generate revenue or build a sustainable or profitable business.
Our drug candidates are in the early stages of development and because we have a short development history with both RNA interference and our delivery technologies, there is a limited amount of information about us upon which you can evaluate our business and prospects.
We have no approved drugs and thus have not begun to market or generate revenues from the commercialization of any products. We have only a limited history upon which one can evaluate our RNAi therapeutic business as our drug candidates are still
24
at an early stage of development. Thus, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and u ncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical area. For example, to execute our business plan, we will need to successfully:
● | Execute product development activities using unproven technologies; |
● | Build, maintain, and protect a strong intellectual property portfolio; |
● | Demonstrate safety and efficacy of our drug candidates in multiple human clinical studies; |
● | Receive FDA approval and approval from similar foreign regulatory bodies; |
● | Gain market acceptance for the development and commercialization of any drugs we develop; |
● | Ensure our products are reimbursed by commercial and/or government payors at a rate that permits commercial viability; |
● | Develop and maintain successful strategic relationships with suppliers, distributors, and commercial licensing partners; and |
● | Manage our spending and cash requirements as our expenses will increase in the near term if we add programs and additional preclinical and clinical trials. |
If we are unsuccessful in accomplishing these objectives, we may not be able to develop products, raise capital, expand our business or continue our operations.
We may be unable to attract additional revenue-generating collaborations with other pharmaceutical and biotech companies to advance our drug candidates.
During fiscal year 2016, we entered into two collaboration and license agreements with Amgen. Our business strategy includes obtaining additional collaborations with other pharmaceutical and biotech companies to support the development of our therapeutic siRNA and other drug candidates. We may not be able to attract such partners, and even if we are able to enter into such partnerships, the terms may be less favorable than anticipated. Further, entering into partnership agreements may limit our commercialization options and/or require us to share revenues and profits with our partners. If we are unable to enter into any of these agreements on commercially attractive terms, we may be unable to develop certain programs due to a limited availability of resources, and we may need to raise additional capital, which could be dilutive to our existing investors.
We will need to achieve commercial acceptance of our drug candidates to generate revenues and achieve profitability.
Even if our research and development efforts yield technologically feasible applications, we may not successfully develop commercial products. Drug development takes years of study in human clinical trials prior to regulatory approval, and, even if we are successful, it may not be on a timely basis. During our drug development period, superior competitive technologies may be introduced which could diminish or extinguish the potential commercial uses for our drug candidates. Additionally, the degree to which the medical community and consumers will adopt any product we develop is uncertain. The rate and degree of market acceptance of our products will depend on a number of factors, including the establishment and demonstration in the medical community of the clinical efficacy and safety of our products, their potential advantage over alternative treatments, and the costs to patients and third-party payors, including insurance companies and Medicare. Recent efforts in the United States and abroad to reduce overall healthcare spending has put significant pressure on the price of prescription drugs and certain companies have been publicly criticized for the relatively high cost of their therapies. These pressures may force us to sell any approved drugs at a lower price than we or analysts may anticipate, or may result in lower levels of reimbursement and coverage from third parties.
We cannot predict whether significant commercial market acceptance for our products, if approved, will ever develop, and we cannot reliably estimate the projected size of any such potential market. Our revenue growth and achievement of profitability will depend substantially on our ability to introduce products that will be accepted by the medical community. If we are unable to cost-effectively achieve acceptance of our technology among the medical establishment and patients, or if the associated products do not achieve wide market acceptance, our business will be materially and adversely affected.
Risks Related to Our Financial Condition
We have a history of net losses, and we expect to continue to incur net losses and may not achieve or maintain profitability.
We have incurred net losses since our inception, including net losses of $81.7 million for the year ended September 30, 2016. We expect that our operating losses will continue for the foreseeable future as we continue our drug development and discovery efforts. To achieve profitability, we must, either directly or through licensing and/or partnering relationships, meet certain milestones, successfully develop and obtain regulatory approval for one or more drug candidates and effectively manufacture, market and sell any
25
drugs we successfully develop. Even if we successfully commercialize drug candidates that receive regulatory approval, we may not be able to realize revenues at a level that would allow us to achieve or sustain profitability.
Accordingly, we may never generate significant revenue and, even if we do generate significant revenue, we may never achieve profitability.
We will require substantial additional funds to complete our research and development activities.
Our business currently does not generate the cash that is necessary to finance our operations. Subject to the success of the research and development programs of our company and our partners, and potential licensing or partnering transactions, we will likely need to raise additional capital to:
● | Fund research and development infrastructure and activities relating to the development of our drug candidates, including pre-clinical and clinical trials and manufacturing to support these efforts; |
● | Fund our general and administrative infrastructure and activities; |
● | Pursue business development opportunities for our technologies; |
● | Add to and protect our intellectual property; and |
● | Retain our management and technical staff. |
Our future capital needs depend on many factors, including:
• | The scope, duration, and expenditures associated with our research and development; |
• | Regulatory requirements for our clinical trials; |
• | The extent to which our R&D and clinical efforts are successful; |
• | The outcome of potential partnering or licensing transactions, if any, and the extent to which our business development efforts result in the acquisition of new programs or technologies; |
• | Competing technological developments; |
• | Our intellectual property positions, if any, in our products; and |
• | The regulatory approval process and regulatory standards for our drug candidates. |
We will need to raise additional funds through public or private equity offerings, debt financings or additional strategic alliances and licensing arrangements in the future to continue our operations. We may not be able to obtain additional financing on terms favorable to us, if at all. General market conditions may make it very difficult for us to seek financing from the capital markets, and the terms of any financing may adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities, further dilution to our stockholders will result, which may substantially dilute the value of your investment. In addition, as a condition to providing additional funds to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. Debt financing, if available, may involve restrictive covenants that could limit our flexibility in conducting future business activities and, in the event of insolvency, would be paid before holders of equity securities received any distribution of corporate assets. In order to raise additional funds through alliance, joint venture or licensing arrangements, we may be required to relinquish rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us. If adequate funds are not available, we may have to further delay, reduce or eliminate one or more of our planned activities. These actions would likely reduce the market price of our common stock.
If the estimates we make, or the assumptions on which we rely, in preparing our consolidated financial statements prove inaccurate, our actual results may vary from those reflected in our accruals.
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP). The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of our assets, liabilities, revenues and expenses, the amounts of charges accrued by us and related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. We cannot assure you, however, that our estimates, or the assumptions underlying them, will be correct.
We may not be able to effectively secure first-tier technologies when competing against other companies or investors.
26
Our future success m ay require that we acquire patent rights and know-how to new or complimentary technologies. However, we compete with a substantial number of other companies that may also compete for technologies we desire. In addition, many venture capital firms and other institutional investors, as well as other pharmaceutical and biotech companies, invest in companies seeking to commercialize various types of emerging technologies. Many of these companies have greater financial, scientific and commercial resources than u s. Therefore, we may not be able to secure the technologies we desire. Furthermore, should any commercial undertaking by us prove to be successful, there can be no assurance competitors with greater financial resources will not offer competitive products a nd/or technologies.
Risks Associated with Reliance on Third Parties
We will need to establish additional relationships with strategic and development partners to fully develop our drug candidates and market any approved products.
We do not possess all of the financial and development resources necessary to develop and commercialize products that may result from our technologies. Unless we expand our product development capacity and enhance our internal marketing capability, we may need to make appropriate arrangements with strategic partners to develop and commercialize any drug candidates that may be approved. If we do not find appropriate partners, or if our existing arrangements or future agreements are not successful, our ability to develop and commercialize products could be adversely affected. Even if we are able to find collaborative partners, the overall success of the development and commercialization of product candidates in those programs will depend largely on the efforts of other parties and will be beyond our control. In addition, in the event we pursue our commercialization strategy through collaboration or licenses to third parties, there are a variety of technical, business and legal risks, including:
● | We may not be able to control the amount and timing of resources that our collaborators may be willing or able to devote to the development or commercialization of our drug candidates or to their marketing and distribution; and |
● | Disputes may arise between us and our collaborators that result in the delay or termination of the research, development or commercialization of our drug candidates or that result in costly litigation or arbitration that diverts our management's resources. |
The occurrence of any of the above events or other related events could impair our ability to generate revenues and harm our business and financial condition.
We may lose a considerable amount of control over our intellectual property and may not receive anticipated revenues in strategic transactions, particularly where the consideration is contingent on the achievement of development or sales milestones.
Our business model has been to develop new technologies and to utilize the intellectual property created through the research and development process to develop commercially successful products. If the acquirers of our technologies fail to achieve performance milestones, we may not receive a significant portion of the total value of any sale, license or other strategic transaction.
We rely on outside sources for various components and processes for our products.
We rely on third parties for various components and processes for our product candidates. We may not be able to achieve multiple sourcing because there may be no acceptable second source, other companies may choose not to work with us, or the component or process sought may be so new that a second source does not exist, or does not exist on acceptable terms. There may be a disruption or delay in the performance of our third-party contractors, suppliers or collaborators which is beyond our control. If such third parties are unable to satisfy their commitments to us, the development of our products would be adversely affected. Therefore, it is possible that our development plans will have to be slowed down or stopped completely at times due to our inability to obtain required raw materials, components, and outsourced processes at an acceptable cost, if at all, or to get a timely response from vendors.
We have limited manufacturing capability and must rely on third-party manufacturers to manufacture our clinical supplies and commercial products, if and when approved, and if they fail to meet their obligations, the development and commercialization of our products could be adversely affected.
We have limited manufacturing capabilities and experience. Our drug candidates are composed of multiple components and require specialized formulations for which scale-up and manufacturing could be difficult. We have limited experience in such scale-up and manufacturing requiring us to depend on a limited number of third parties, who may not be able to deliver in a timely manner, or at all. In order to develop products, apply for regulatory approvals, and commercialize our products, we will need to develop, contract for, or otherwise arrange for the necessary manufacturing capabilities. Our internal manufacturing capabilities are limited to small-scale production of material for use in in vitro and in vivo experiments that is not required to be produced under cGMP standards. There are a limited number of manufacturers that supply synthetic siRNAs. There are risks inherent in pharmaceutical manufacturing that could affect the ability of our contract manufacturers to meet our delivery time requirements or provide adequate amounts of material to meet our needs. Included in these risks are synthesis and purification failures and contamination during the
27
manufacturing process, which could result in unusable product and cause delays in our development process, as we ll as additional expense to us.
Additionally, our product candidates have not yet been manufactured for commercial use. If any of our product candidates become approved for commercial sale, we will need to establish third-party manufacturing capacity. A third-party manufacturing partner may require us to fund capital improvements to support the scale-up of manufacturing and related activities. The third-party manufacturer may not be able to establish scaled manufacturing capacity for an approved product in a timely or economic manner, if at all. If a manufacturer is unable to provide commercial quantities of such an approved product, we will have to successfully transfer manufacturing technology to a different manufacturer. Engaging a new manufacturer for such an approved product could require us to conduct comparative studies or utilize other means to determine bioequivalence of the new and prior manufacturers' products, which could delay or prevent our ability to commercialize such an approved product. If any of these manufacturers is unable or unwilling to increase its manufacturing capacity or if we are unable to establish alternative arrangements on a timely basis or on acceptable terms, the development and commercialization of such an approved product may be delayed or there may be a shortage in supply. Any inability to manufacture our product candidates or future approved drugs in sufficient quantities when needed would seriously harm our business.
Manufacturers of our approved products, if any, must comply with cGMP requirements enforced by the FDA and foreign health authorities through facilities inspection programs. These requirements include quality control, quality assurance, and the maintenance of records and documentation. Manufacturers of our approved products, if any, may be unable to comply with these cGMP requirements and with other FDA, state, and foreign regulatory requirements. We have little control over our manufacturers' compliance with these regulations and standards. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of any quantities supplied is compromised due to our manufacturer's failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our products, which would seriously harm our business.
We rely on third parties to conduct our clinical trials, and if they fail to fulfill their obligations, our the development of our products may be adversely affected.
We rely on independent clinical investigators, contract research organizations and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our clinical trials. We contract with certain third-parties to provide certain services, including site selection, enrollment, monitoring and data management services. Although we depend heavily on these parties, we do not control them and therefore, we cannot be assured that these third-parties will adequately perform all of their contractual obligations to us. If our third party service providers cannot adequately and timely fulfill their obligations to us, or if the quality and accuracy of our clinical trial data is compromised due to failure by such third parties to adhere to our protocols or regulatory requirements or if such third-parties otherwise fail to meet deadlines, our development plans may be delayed or terminated. Further, if clinical study results are compromised, then we may need to repeat the affected studies, which could result in significant additional costs and delays to us.
Risks related to managing our operations
Our success depends on the attraction and retention of senior management and scientists with relevant expertise.
Our future success depends to a significant extent on the continued services of our key employees, including our senior scientific, technical and managerial personnel. We do not maintain key man life insurance for any of our executives and we do not maintain employment agreements with most senior officers or employees. Competition for qualified employees in the pharmaceutical industry is high, and our ability to execute our strategy also will depend on our ability to continue to attract and retain qualified scientists and management. If we are unable to find, hire and retain qualified individuals, we will have difficulty implementing our business plan in a timely manner, or at all.
We may have difficulty expanding our operations successfully as we evolve from a company primarily involved in discovery and pre-clinical testing into one that develops and commercializes drugs.
We expect that as we increase the number of product candidates we are developing we will also need to expand our operations. This expected growth may place a strain on our administrative and operational infrastructure. As product candidates we develop enter and advance through clinical trials, we will need to expand our development, regulatory, manufacturing, marketing, and sales capabilities or contract with other organizations to provide these capabilities for us. As our operations expand due to our development progress, we expect that we will need to manage additional relationships with various collaborators, suppliers, and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and
28
management controls, reporting systems and procedures. We may not be able to implement improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existin g systems and controls.
Our business and operations could suffer in the event of information technology system failures.
Our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war, and telecommunication and electrical failures. Such events could cause interruption of our operations and loss of intellectual property. For example, the loss of pre-clinical trial data or data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of confidential, proprietary or private information, we could incur liability, we could lose valuable trade secret rights, and the development of our product candidates could be delayed.
Risks related to clinical development and regulatory approval of our product candidates
The manufacture and sale of human therapeutic products are governed by a variety of statutes and regulations. There can be no assurance that our product candidates will obtain regulatory approval.
The sale of human therapeutic products in the U.S. and foreign jurisdictions is subject to extensive and time consuming regulatory approval which requires:
• | controlled research and human clinical testing; |
• | establishment of the safety and efficacy of the product; |
• | government review and approval of a submission containing manufacturing, pre-clinical and clinical data; and |
• | adherence to cGMP regulations during production and storage. |
The product candidates we currently have under development will require significant development, pre-clinical and clinical testing and investment of significant funds to gain regulatory approval before they can be commercialized. Some of our product candidates, if approved, will require the completion of post-market studies. There can be no assurance that any of our products will be further developed and approved. The process of completing clinical testing and obtaining required approvals will take a number of years and require the use of substantial resources. Further, there can be no assurance that product candidates employing a new technology will be shown to be safe and effective in clinical trials or receive applicable regulatory approvals. If we fail to obtain regulatory approvals for any or all of our products, we will not be able to market such product and our operations may be adversely affected.
If testing of a particular product candidate does not yield successful results, then we will be unable to commercialize that product candidate.
We must demonstrate our product candidates' safety and efficacy in humans through extensive clinical testing. Our research and development programs are at an early stage of development. We may experience numerous unforeseen events during, or as a result of, the testing process that could delay or prevent commercialization of any products, including the following:
• | the results of pre-clinical studies may be inconclusive, or they may not be indicative of results that will be obtained in human clinical trials; |
• | safety and efficacy results attained in early human clinical trials may not be indicative of results that are obtained in later clinical trials; |
• | after reviewing test results, we may abandon projects that we might previously have believed to be promising; |
• | we or our regulators, may suspend or terminate clinical trials because the participating subjects or patients are being exposed to unacceptable health risks; and |
• | our product candidates may not have the desired effects or may include undesirable side effects or other characteristics that preclude regulatory approval or limit their commercial use if approved. |
Clinical testing is very expensive, takes many years, and the outcomes are uncertain. The data collected from our clinical trials may not be sufficient to support approval of our product candidates by the regulatory authorities. The clinical trials of our product candidates may not be completed on schedule, and the regulatory authorities may not ultimately approve any of our product candidates for commercial sale. If we fail to adequately demonstrate the safety and efficacy of a product candidate, it would prevent regulatory approval of the product candidate, which could prevent us from achieving profitability.
29
It may take us longe r than we project to complete clinical trials, and we may not be able to complete them at all.
Although for planning purposes, we project the commencement, continuation and completion of our clinical trials, a number of factors, including scheduling conflicts with participating clinicians and clinical institutions, and difficulties in identifying or enrolling patients who meet trial eligibility criteria, may cause significant delays. We may not commence or complete clinical trials involving any of our product candidates as projected or may not conduct them successfully.
Even if our clinical studies are successful and we achieve regulatory approval, the approved product label may be more limited than we or analysts anticipate, which could limit the commercial opportunity for our product candidates.
At the time drugs are approved for commercialization, they are given a "product label" from the FDA or other regulatory body. In most countries this label sets forth the approved indication for marketing, and identifies potential safety concerns for prescribing physicians and patients. While we intend to seek as broad a product label as possible for our product candidates, we may receive a narrower label than is expected by either us or third parties, such as stockholders and securities analysts. For example, any approved products may only be indicated to treat refractory patients (i.e., those who have failed some other first-line therapy). Similarly, it is possible that only a specific sub-set of patients safely responds to one or more of our drug candidates. As a result, our product candidates, even if successful in clinical trials, could be approved only for a subset of patients. Additionally, safety considerations may result in contraindications that could further limit the scope of an approved product label. Any of these or other safety and efficacy considerations could limit the commercial opportunity for our product candidates.
Even if our product candidates are approved for commercialization, future regulatory reviews or inspections may result in the suspension or withdrawal of one or more of our products, closure of a facility or enforcement of substantial fines.
If regulatory approval to sell any of our product candidates is received, regulatory agencies will subject any marketed product(s), as well as the manufacturing facilities, to continual review and periodic inspection. If previously unknown problems with a product or manufacturing and laboratory facility are discovered, or we fail to comply with applicable regulatory approval requirements, a regulatory agency may impose restrictions on that product or on us. The agency may require the withdrawal of the product from the market, closure of the facility or enforcement of substantial fines.
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
The use of our product candidates in clinical trials and the sale of any products for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by clinical trial participants, consumers, health-care providers, pharmaceutical companies, or others selling our products. If we cannot successfully defend ourselves against these claims, we may incur substantial liabilities. Regardless of merit or eventual outcomes of such claims, product liability claims may result in:
• | decreased demand for our product candidates; |
• | impairment of our business reputation; |
• | withdrawal of clinical trial participants; |
• | costs of litigation; |
• | substantial monetary awards to patients or other claimants; |
• | loss of revenues; and |
• | the inability to commercialize our product candidates. |
Although we currently have liability insurance for our clinical trials, our insurance coverage may not be sufficient to reimburse us for all expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses.
If a natural or man-made disaster strikes our research and development facility or otherwise affects our business, it could delay our progress developing our product candidates.
We conduct research and development in a facility in Madison, Wisconsin. The facilities and the equipment we use are costly to replace and require substantial lead time to repair or replace. Our facilities may be harmed by natural or man-made disasters, including, without limitation, earthquakes, floods, fires and acts of terrorism; and if our facilities are affected by a disaster, our development efforts would be delayed. Significant delays in our development efforts could materially impact our ability to obtain
30
regulatory approval and to commercialize our products. Any insurance we maintain against damage to our prop erty and the disruption of our business due to disaster may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In addition, our development activities could be harmed or delaye d by a shutdown of the U.S. government, including the FDA.
The successful commercialization of our product candidates, if approved, will depend in part on the extent to which government authorities and health insurers establish adequate reimbursement levels and pricing policies.
Sales of any approved drug candidate will depend in part on the availability of coverage and reimbursement from third-party payers such as government insurance programs, including Medicare and Medicaid, private health insurers, health maintenance organizations and other health care related organizations, who are increasingly challenging the price of medical products and services. Accordingly, coverage and reimbursement may be uncertain. Adoption of any drug by the medical community may be limited if third-party payers will not offer coverage. Additionally, significant uncertainty exists as to the reimbursement status of newly approved drugs. Cost control initiatives may decrease coverage and payment levels for any drug and, in turn, the price that we will be able to charge and/or the volume of our sales. We are unable to predict all changes to the coverage or reimbursement methodologies that will be applied by private or government payers. Any denial of private or government payer coverage or inadequate reimbursement could harm our business and reduce our revenue. If we partner with third parties with respect to any of our product candidates, we may be reliant on that partner to obtain reimbursement from government and private payors for the drug, if approved, and any failure of that partner to establish adequate reimbursement could have a negative impact on our revenues and profitability.
In addition, both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation, regulations, and policies affecting coverage and reimbursement rates, which are designed to contain or reduce the cost of health care. Further federal and state proposals and healthcare reforms are likely, which could limit the prices that can be charged for the product candidates that we develop and may further limit our commercial opportunity. There may be future changes that result in reductions in potential coverage and reimbursement levels for our product candidates, if approved and commercialized, and we cannot predict the scope of any future changes or the impact that those changes would have on our operations.
If future reimbursement for approved product candidates, if any, is substantially less than we project, or rebate obligations associated with them are substantially greater than we expect, our net revenue and profitability could be materially and adversely impacted.
We may not enjoy the market exclusivity benefits of our orphan drug designation.
Although we have obtained an orphan designation in the treatment of alpha-1 antitrypsin deficiency, the designation may not be applicable to any alpha-1 antitrypsin deficiency product we might get approved and that product may not be the first product to receive approval for that indication. Under the Orphan Drug Act, the first product with an orphan designation receives market exclusivity, which prohibits FDA from approving the "same" drug for the same indication. The FDA has stated that drugs can be the "same" even when they are not identical, but has not provided guidance with respect to how it will determine "sameness" for RNAi drugs. It is possible that another RNAi drug could be approved for the treatment of alpha-1 antitrypsin deficiency before ARO-AAT, which means that we may not obtain orphan drug exclusivity and could also potentially be blocked from approval until the first product's orphan drug exclusivity period expires or we demonstrate, if we can, that ARO-AAT is superior. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved and granted orphan drug exclusivity, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care.
Risks Related to Our Intellectual Property Rights
Our ability to protect our patents and other proprietary rights is uncertain, exposing us to the possible loss of competitive advantage.
We have licensed rights to pending patents and have filed and expect to continue to file patent applications. Researchers sponsored by us may also file patent applications that we may need to license. Such patent applications may not be available for licensing or may not be economically feasible to license. Certain of our patents may not be granted or may not contain claims of the necessary breadth because, for example, prior patents exist. If a particular patent is not granted, the value of the invention described in the patent would be diminished. Further, even if these patents are granted, they may be difficult to enforce. Even if ultimately successful, efforts to enforce our patent rights could be expensive, distracting for management, cause our patents to be invalidated or held unenforceable, and thus frustrate commercialization of products. Even if patents are issued and are enforceable, others may develop similar, superior or parallel technologies to any technology developed by us and not infringe on our patents. Our technology may prove to infringe upon patents or rights owned by others. Patent prosecution and maintenance is expensive, and we may be forced to curtail prosecution or maintenance if our cash resources are limited. Thus, the patents held by or licensed to us may not afford us
31
any meaningful competitive advantage. If we are unable to derive value from our licensed or owned intellectual property, the value of your investment may decline.
We are party to technology license agreements with third parties that require us to satisfy obligations to keep them effective and, if these agreements are terminated, our technology and our business would be seriously and adversely affected.
We are party to license agreements with Novartis, Alnylam, City of Hope, and other entities to incorporate their proprietary technologies into our drug products under development. These license agreements require us to pay royalties and satisfy other conditions, including conditions in some cases related to the commercialization of the licensed technology. We may not be able to successfully incorporate these technologies into marketable products or, if we do, sales may not be sufficient to recover the amounts that we are obligated to pay to the licensors. If we fail to satisfy our obligations under these agreements, the terms of the licenses may be materially modified, such as by rendering currently exclusive licenses non-exclusive, or it may give our licensors the right to terminate their respective agreement with us, which would limit our ability to implement our current business plan and harm our business and financial condition.
We may be subject to patent infringement claims, which could result in substantial costs and liability and prevent us from commercializing our potential products.
Because the intellectual property landscape in the fields in which we participate is rapidly evolving and interdisciplinary, it is difficult to conclusively assess our freedom to operate without infringing on third party rights. However, we are currently aware of certain patent rights held by third parties that, if found to be valid and enforceable, could be alleged to render one or more of our drug candidates infringing. If a claim should be brought and is successful, we may be required to pay substantial damages, be forced to abandon any affected drug candidates and/or seek a license from the patent holder. In addition, any patent infringement claims brought against us, whether or not successful, may cause us to incur significant expenses and divert the attention of our management and key personnel from other business concerns. These could negatively affect our results of operations and prospects. We cannot be certain that patents owned or licensed by us will not be challenged, potentially successfully, by others.
In addition, if our product candidates infringe the intellectual property rights of third parties, these third parties may assert infringement claims against our customers, licensees, and other parties with whom we have business relationships and we may be required to indemnify those parties for any damages they suffer as a result of these claims. The claims may require us to initiate or defend protracted and costly litigation on behalf of customers, licensees, and other parties regardless of the merits of these claims. If any of these claims succeed, we may be forced to pay damages on behalf of those parties or may be required to obtain licenses for the products they use. If we cannot obtain all necessary licenses on commercially reasonable terms, we may be unable to continue selling such products.
We license patent rights from third-party owners and we rely on such owners to obtain, maintain and enforce the patents underlying such licenses.
We are a party to a number of licenses that give us rights to third-party intellectual property that is necessary or useful for our business. In particular, we have obtained licenses from, among others, Novartis and Alnylam. We also expect to enter into additional licenses to third-party intellectual property in the future.
Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for our licensed intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications to which we are licensed. Even if patents are issued in respect of these patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than we would. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business prospects.
Our technology licensed from various third parties may be subject to retained rights.
Our licensors often retain certain rights under their agreements with us, including the right to use the underlying technology for noncommercial academic and research use, to publish general scientific findings from research related to the technology, and to make customary scientific and scholarly disclosures of information relating to the technology. It is difficult to monitor whether our licensors limit their use of the technology to these uses, and we could incur substantial expenses to enforce our rights to our licensed technology in the event of misuse.
32
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we rely in part on confidentiality agreements with our collaborators, employees, consultants, outside scientific collaborators and sponsored researchers, and other advisors. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such party. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Risks Related to our Stock
Stockholder equity interest may be substantially diluted in any additional financing.
Our certificate of incorporation authorizes the issuance of 145,000,000 shares of Common Stock and 5,000,000 shares of Preferred Stock, on such terms and at such prices as our Board of Directors may determine. The following serves as a summary of share issuance activity during the fiscal year ended September 30, 2016:
| • | 7,627,119 shares of Common Stock issued in an equity financing in August 2016 that generated $43.2 million of net proceeds to the Company; |
| • | 1,256,983 shares of Common Stock to Amgen, Inc. pursuant to the Common Stock Purchase Agreement executed with Amgen in September 2016; |
| • | 1,317,906 shares of Common Stock pursuant to the exercise of stock options, warrants and exchange rights and the vesting of restricted stock units; |
As of September 30, 2016, we had 69,746,685 shares of Common Stock issued and outstanding. The issuance of additional securities in financing transactions by us or through the exercise of options or warrants will dilute the equity interests of our existing stockholders, perhaps substantially, and could result in dilution in the tangible net book value of a share of our Common Stock, depending upon the price and other terms on which the additional shares are issued.
Our Common Stock price has fluctuated significantly over the last several years and may continue to do so in the future, without regard to our results of operations and prospects.
Because we are early in the stage of our drug development, there are few objective metrics by which our progress may be measured. Consequently, we expect that the market price of our Common Stock will continue to fluctuate significantly. We may not generate substantial revenue from the license or sale of our technology for several years, if at all. In the absence of product revenue as a measure of our operating performance, we anticipate that investors and market analysts will assess our performance by considering factors such as:
● | Announcements of developments related to our business; |
● | Our ability to enter into or extend investigation phase, development phase, commercialization phase and other agreements with new and/or existing partners; |
● | Announcements regarding the status of any or all of our collaborations or products, including clinical trial results; |
● | Market perception and/or investor sentiment regarding our technology; |
● | Announcements of actions taken by regulatory authorities; such as the U.S. Food and Drug Administration; |
● | Announcements regarding developments in the RNA interference or biotechnology fields in general; |
● | Announcements regarding clinical trial results with our products or competitors' products; |
● | Market perception and/or announcements regarding other companies developing products in the field of biotechnology generally or specifically RNA interference; |
● | The issuance of competitive patents or disallowance or loss of our patent rights; |
● | The addition or departure of key executives; and |
● | Variations in our operating results. |
33
We will not have control over many of these factors but expect that they may influence our stock price. As a result, our stock price may be volatile and such volatility could result in the loss of all or part of your investment.
Litigation claims may result in financial losses or harm our reputation and may divert management resources.
When the market price of a stock is volatile, holders of that stock have often initiated securities class action litigation against the company that issued the stock. Certain of our stockholders have recently brought such lawsuits against us, pursuant to which we could incur substantial costs. We cannot predict with certainty the eventual outcome of this or any other litigation, arbitration or third-party inquiry. We may not be successful in defending ourselves or asserting our rights in current or future lawsuits, investigations, or claims that have been or may be brought against us and, as a result, our business could be materially harmed. These lawsuits, arbitrations, investigations or claims may result in large judgments or settlements against us, any of which could have a negative effect on our financial performance and business. Additionally, lawsuits, arbitrations and investigations can be expensive to defend, whether or not the lawsuit, arbitration or investigation has merit, and the defense of these actions may divert the attention of our management and other resources that would otherwise be engaged in running our business.
The market for purchases and sales of our Common Stock may be limited, and the sale of a limited number of shares could cause the price to fall sharply.
Although our Common Stock is listed for trading on the NASDAQ Global Select Market, at various times our securities are relatively thinly traded. Investor trading patterns could serve to exacerbate the volatility of the price of our stock. For example, mandatory sales of our Common Stock by institutional holders could be triggered if an investment in our Common Stock no longer satisfies their investment standards and guidelines. It may be difficult to sell shares of our Common Stock quickly without significantly depressing the value of the stock. Unless we are successful in developing continued investor interest in our stock, sales of our stock could result in major fluctuations in the price of the stock.
If securities or industry analysts do not publish research reports about our business or if they make adverse recommendations regarding an investment in our stock, our stock price and trading volume may decline.
The trading market for our Common Stock can be influenced by the research and reports that industry or securities analysts publish about our business. Currently, coverage of our Company by industry and securities analysts is limited. Investors have many investment opportunities and may limit their investments to companies that receive greater coverage from analysts. If additional industry or securities analysts do not commence coverage of the Company, the trading price of our stock could be negatively impacted. If one or more of the analysts downgrade our stock or comment negatively on our prospects, our stock price may decline. If one or more of these analysts cease to cover our industry or us or fail to publish reports about the Company regularly, our Common Stock could lose visibility in the financial markets, which could also cause our stock price or trading volume to decline. Further, incorrect judgments, estimates or assumptions made by research analysts may adversely affect our stock price, particularly if subsequent performance falls below the levels that were projected by the research analyst(s), even if we did not set or endorse such expectations. Any of these events could cause further volatility in our stock price and could result in substantial declines in the value of our stock.
We do not intend to declare cash dividends on our Common Stock.
We will not distribute cash to our stockholders unless and until we can develop sufficient funds from operations to meet our ongoing needs and implement our business plan. The time frame for that is unpredictable and investors should not expect dividends in the near future, if at all.
Our Board of Directors has the authority to issue shares of "blank check" preferred stock, which may make an acquisition of the Company by another company more difficult.
We have adopted and may in the future adopt certain measures that may have the effect of delaying, deferring or preventing a takeover or other change in control of the Company that a holder of our Common Stock might consider in its best interest. Specifically, our Board of Directors, without further action by our stockholders, currently has the authority to issue up to 5,000,000 shares of preferred stock and to fix the rights (including voting rights), preferences and privileges of these shares ("blank check" preferred). Such preferred stock may have rights, including economic rights, senior to our Common Stock.
ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
34
ITEM 2. | PROPERTIES |
The Company does not own any real property. The following table summarizes the Company's leased facilities as of September 30, 2016.
| Office Space |
|
| Monthly Expenses |
|
| Primary Use |
| Lease Expiration |
|
| Lease Term |
| ||||
Pasadena, California |
| 8,500 sq. ft. |
|
| $ | 26,000 |
|
| Corp. Headqtrs. |
|
| July 31, 2019 |
|
|
| 7 years |
|
Madison, Wisconsin |
| 27,000 sq. ft. |
|
| $ | 83,000 |
|
| Former Research Facility |
|
| October 31, 2016 |
|
|
| 8 years |
|
Madison, Wisconsin |
| 60,000 sq. ft. |
|
| $ | 132,000 |
|
| New Research Facility |
|
| September 30, 2026 |
|
|
| 10 years |
|
Middleton, Wisconsin |
| 2,900 sq. ft |
|
| $ | 18,000 |
|
| Auxiliary Research Facility |
|
| December 31, 2016 |
|
|
| 1.5 years |
|
ITEM 3. | LEGAL PROCEEDINGS |
Legal Proceedings are set forth in our financial statement schedules in Part IV, Item 15 of this Annual Report and are incorporated herein by reference. See Note 7 - Commitments and Contingencies of Notes to Consolidated Financial Statements of Part IV, Item 15. Exhibits and Financial Statement Schedules .
ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
ITEM 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Price Range of Common Stock
Our Common Stock is traded on the NASDAQ Global Select Market under the symbol "ARWR". The following table sets forth the high and low sales prices for a share of the Company's Common Stock during each period indicated.
| Fiscal Year Ended September 30, |
| |||||||||||||
| 2016 |
|
| 2015 |
| ||||||||||
| High |
|
| Low |
|
| High |
|
| Low |
| ||||
1st Quarter | $ | 6.45 |
|
| $ | 4.83 |
|
| $ | 14.80 |
|
| $ | 4.95 |
|
2nd Quarter |
| 6.05 |
|
|
| 3.07 |
|
|
| 9.33 |
|
|
| 6.01 |
|
3rd Quarter |
| 6.55 |
|
|
| 4.68 |
|
|
| 8.90 |
|
|
| 5.99 |
|
4th Quarter |
| 8.22 |
|
|
| 5.29 |
|
|
| 9.36 |
|
|
| 4.35 |
|
Shares Outstanding
At December 12, 2016, 74,173,484 shares of the Company's Common Stock were issued and outstanding, and were owned by 140 stockholders of record, based on information provided by the Company's transfer agent.
Dividends
The Company has never paid dividends on its Common Stock and does not anticipate that it will do so in the foreseeable future.
35
Securities Authorized for Issuance Under the Equity Compensation Plans
The disclosure required under this item related to equity compensation plans is incorporated by reference from Item 12 of Part III of this Annual Report on Form 10-K.
Sales of Unregistered Securities
All information under this Item has been previously reported on our Current Reports on Form 8-K.
Repurchases of Equity Securities
We did not repurchase any shares of our Common Stock during the years ended September 30, 2016, 2015 and 2014.
Performance Graph
The following performance graph shall not be deemed "soliciting material" or to be "filed" with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933 or Securities Exchange Act of 1934, each as amended, except to the extent that we specifically incorporate it by reference into such filing. The graph compares the cumulative 5-year total return to shareholders on our Common Stock relative to the cumulative total returns of the NASDAQ Composite Index and the NASDAQ Biotechnology Index. We selected the NASDAQ Biotechnology Index because we believe the index reflects the market conditions within the industry in which we primarily operate. The comparison of total return on investment, defined as the change in year-end stock price plus reinvested dividends, for each of the periods assumes that $100 was invested on September 30, 2011, in each of our Common Stock, the NASDAQ Composite Index and the NASDAQ Biotechnology Index, with investment weighted on the basis of market capitalization.
The comparisons in the following graph are based on historical data and are not intended to forecast the possible future performance of our Common Stock.
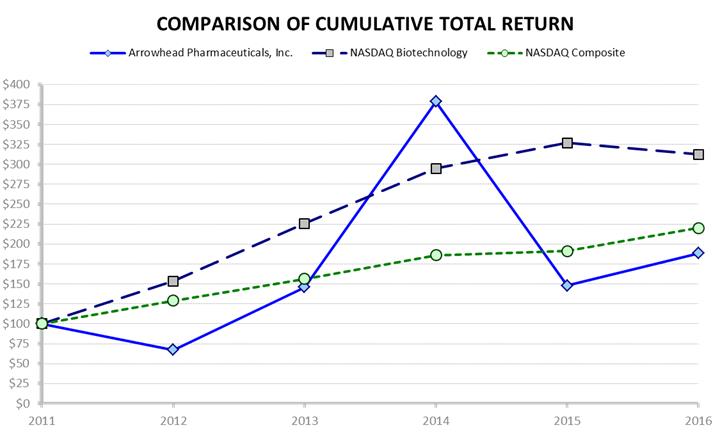
36
ITEM 6. | SELECTED FINA NCIAL DATA |
The following selected financial data has been derived from our audited consolidated financial statements and should be read
in conjunction with the consolidated financial statements, the related notes thereto and the independent auditors' report thereon, and
"Management's Discussion and Analysis of Financial Condition and Results of Operations," which are included elsewhere in this
Form 10-K and in previously filed annual reports on Form 10-K of Arrowhead Pharmaceuticals, Inc.
|
| Year Ended September |
| |||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| 2013 |
|
| 2012 |
| |||||
OPERATING SUMMARY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
REVENUE |
| $ | 158,333 |
|
| $ | 382,000 |
|
| $ | 175,000 |
|
| $ | 290,266 |
|
| $ | 146,875 |
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 41,454,452 |
|
|
| 47,267,361 |
|
|
| 23,138,050 |
|
|
| 8,705,627 |
|
|
| 5,391,463 |
|
Acquired in-process research and development |
|
| - |
|
|
| 10,142,786 |
|
|
| - |
|
|
| - |
|
|
| - |
|
Salaries and payroll-related costs |
|
| 19,461,656 |
|
|
| 16,554,008 |
|
|
| 12,829,355 |
|
|
| 6,667,669 |
|
|
| 6,414,921 |
|
General and administrative expenses |
|
| 9,940,737 |
|
|
| 7,931,184 |
|
|
| 5,894,008 |
|
|
| 3,488,864 |
|
|
| 6,439,323 |
|
Stock-based compensation |
|
| 11,595,816 |
|
|
| 10,232,897 |
|
|
| 5,696,173 |
|
|
| 1,536,271 |
|
|
| 1,241,404 |
|
Depreciation and amortization |
|
| 3,260,045 |
|
|
| 2,336,207 |
|
|
| 1,345,655 |
|
|
| 1,751,412 |
|
|
| 1,748,975 |
|
Impairment expense |
|
| 2,050,817 |
|
|
| - |
|
|
| 2,172,387 |
|
|
| 1,308,047 |
|
|
| - |
|
Contingent consideration - fair value adjustments |
|
| (5,862,464 | ) |
|
| 1,891,533 |
|
|
| 2,375,658 |
|
|
| 1,421,652 |
|
|
| - |
|
TOTAL OPERATING EXPENSES (a) |
|
| 81,901,059 |
|
|
| 96,355,976 |
|
|
| 53,451,286 |
|
|
| 24,879,542 |
|
|
| 21,236,086 |
|
OPERATING LOSS |
|
| (81,742,726 | ) |
|
| (95,973,976 | ) |
|
| (53,276,286 | ) |
|
| (24,589,276 | ) |
|
| (21,089,211 | ) |
LOSS FROM CONTINUING OPERATIONS |
|
| (81,723,002 | ) |
|
| (91,940,882 | ) |
|
| (58,725,412 | ) |
|
| (31,703,079 | ) |
|
| (22,110,643 | ) |
Income (loss) from discontinued operations |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (354 | ) |
|
| (80 | ) |
NET LOSS |
|
| (81,723,002 | ) |
|
| (91,940,882 | ) |
|
| (58,725,412 | ) |
|
| (31,703,433 | ) |
|
| (22,110,723 | ) |
Net (gain) loss attributable to non-controlling interests |
|
| - |
|
|
| - |
|
|
| 95,222 |
|
|
| 560,144 |
|
|
| 984,795 |
|
NET LOSS ATTRIBUTABLE TO ARROWHEAD |
| $ | (81,723,002 | ) |
| $ | (91,940,882 | ) |
| $ | (58,630,190 | ) |
| $ | (31,143,289 | ) |
| $ | (21,125,928 | ) |
EARNINGS PER SHARE (BASIC AND DILUTED): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Income (Loss) attributable to Arrowhead common shareholders |
| $ | (1.34 | ) |
| $ | (1.60 | ) |
| $ | (1.25 | ) |
| $ | (1.30 | ) |
| $ | (1.90 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding – basic and diluted |
|
| 61,050,880 |
|
|
| 57,358,442 |
|
|
| 46,933,030 |
|
|
| 24,002,224 |
|
|
| 11,129,766 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH DIVDEND PAID PER COMMON SHARE |
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
|
| September 30, |
| |||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| 2013 |
|
| 2012 |
| |||||
FINANCIAL POSITION SUMMARY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH AND CASH EQUIVALENTS (b) |
| $ | 85,366,448 |
|
| $ | 81,214,354 |
|
| $ | 132,510,610 |
|
| $ | 19,114,444 |
|
| $ | 3,377,288 |
|
SHORT- AND LONG-TERM INVESTMENTS (c) |
|
| - |
|
|
| 17,539,902 |
|
|
| 44,741,378 |
|
|
| 10,732,414 |
|
|
| 106,500 |
|
TOTAL ASSETS (b) |
|
| 128,176,505 |
|
|
| 132,267,914 |
|
|
| 182,816,756 |
|
|
| 37,329,631 |
|
|
| 16,527,818 |
|
CAPITAL LEASE OBLIGATIONS |
|
| - |
|
|
| 758,340 |
|
|
| 972,331 |
|
|
| 1,282,458 |
|
|
| 1,497,259 |
|
OTHER LONG-TERM OBLIGATIONS |
|
| 7,508,452 |
|
|
| 6,204,917 |
|
|
| 4,226,137 |
|
|
| 1,808,709 |
|
|
| 1,108,563 |
|
| (a) | The decrease in our Total Operating Expenses during the year ended September 30, 2016 is primarily due to a one-time $10.1 million expense related to the acquisition of in-process research and development from the Novartis asset acquisition in March 2015. We also had a $5.8 million reduction in our contingent consideration obligation associated with our acquisition of the Roche RNAi business. This was driven by the Company's decision to discontinue its clinical trial candidates: ARC-520, ARC-AAT and ARC-521. Lastly, the decrease in research and development expenses is primarily due to reduced expenses associated with the drug manufacturing campaign to support our Phase 2 studies for ARC-520. We expect research and development expenses to decrease in the near term with the discontinuation of our clinical candidates; however, as our preclinical candidates progress, these expenses could increase. |
| (b) | The Company's Cash and Cash Equivalents and Total Assets remained relatively consistent as of September 30, 2016 as compared to September 30, 2015. This was due to an equity financing in August 2016 which yielded net proceeds of $43.2 million to the Company, and $14 million of upfront payments and equity investments from the Company's Collaboration and License Agreements and Common Stock Purchase Agreement with Amgen in September 2016. These inflows offset cash outflows for operating expenses. |
| (c) | The Company's Short and Long-Term Investments all matured during the year ended September 30, 2016, and the Company had not reinvested any of its cash balances as of that date. |
37
ITEM 7. | MANAGE MENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Description of Business
Unless otherwise noted, (1) the term "Arrowhead" refers to Arrowhead Pharmaceuticals, Inc., a Delaware corporation formerly known as Arrowhead Research Corporation and its Subsidiaries, (2) the terms "Company," "we," "us," and "our," refer to the ongoing business operations of Arrowhead and its Subsidiaries, whether conducted through Arrowhead or a subsidiary of Arrowhead, (3) the term "Subsidiaries" refers collectively to Arrowhead Madison Inc. ("Arrowhead Madison"), Arrowhead Australia Pty Ltd ("Arrowhead Australia") and Ablaris Therapeutics, Inc. ("Ablaris"), (4) the term "Common Stock" refers to Arrowhead's Common Stock, (5) the term "Preferred Stock" refers to Arrowhead's Preferred Stock and (6) the term "Stockholder(s)" refers to the holders of Arrowhead Common Stock.
Overview
Arrowhead Pharmaceuticals, Inc. develops novel drugs to treat intractable diseases by silencing the genes that cause them. Using a broad portfolio of RNA chemistries and efficient modes of delivery, Arrowhead therapies trigger the RNA interference mechanism to induce rapid, deep and durable knockdown of target genes. RNA interference (RNAi) is a mechanism present in living cells that inhibits the expression of a specific gene, thereby affecting the production of a specific protein. Arrowhead's RNAi-based therapeutics leverage this natural pathway of gene silencing. The company's pipeline includes ARO-HBV for chronic hepatitis B virus, ARO-AAT for liver disease associated with alpha-1 antitrypsin deficiency (AATD), ARO-F12 for hereditary angioedema and thromboembolic disorders, ARO-HIF2 for renal cell carcinoma, and ARO-AMG1 for an undisclosed genetically validate cardiovascular target under a license and collaboration agreement with Amgen, Inc., a Delaware corporation ("Amgen"). ARO-LPA for cardiovascular disease was recently out-licensed to Amgen.
In April 2016, the Company changed its name from Arrowhead Research Corporation to Arrowhead Pharmaceuticals, Inc., to better reflect the Company's focus on advancing products through clinical development to bring innovative new medicines to patients.
Arrowhead operates a lab facility in Madison, Wisconsin, where the Company's research and development activities, including the development of RNAi therapeutics, are based. The Company's principal executive offices are located in Pasadena, California.
During fiscal year 2016, the Company continued to develop its clinical candidates, ARC-520 and ARC-521, for the treatment of chronic hepatitis B infection as well as its second clinical candidate, ARC-AAT, an RNAi therapeutic designed to treat liver disease associated with AATD. However, in November 2016, the Company announced that it would be discontinuing these clinical programs, and redeploying its resources and focus toward utilizing the Company's new proprietary subcutaneous and extra-hepatic delivery systems. Each of these discontinued clinical candidates utilized the intravenously administered DPC iv , or EX1, delivery vehicle. The decision to discontinue development of EX1-containing programs was based primarily on two factors. First, during ongoing discussions with regulatory agencies and outside experts, it became apparent that there would be substantial delays in all clinical programs that utilize EX1, while the Company further explored the cause of deaths in a non-clinical toxicology study in non-human primates. Second, the Company has made substantial advances in RNA chemistry and targeting resulting in large potency gains for subcutaneous administered and extra-hepatic RNAi-based development programs. In preclinical studies with the subcutaneous platform, the Company has obtained depth and duration of target gene knockdown approaching that of intravenously administered EX1-containing candidates, at lower doses and with good safety margins. ARO-HBV and ARO-AAT are the Company's subcutaneous administered preclinical candidates for chronic hepatitis B virus and liver disease associated with AATD, respectively. Because of the discontinuation of its existing clinical programs, the Company has also reduced its workforce by approximately 30%, while maintaining resources necessary to support current and potential future partner-based programs and the Company's pipeline.
38
The Company also continued progressing with its preclinical candidates including AR O -F12 , ARO -HIF2 and AR O -LPA. The Company's most significant development for its preclinical candidates was for AR O -LPA . On September 28, 2016, the Company entered into two Collaboration and License agreements, and a Common Stock Purchase Agreement with Amgen . Under one of the license agreements (the "First Collaboration and License Agreement"), Amgen will receive an option to a worldwide, exclusive license for an RNAi therapy for AR O -AMG1 , an undisclosed genetically validated cardiovascular target. Under the other license agreement (the "Second Collaboration and License Agreement"), Amgen will receive a worldwide, exclusive license to Arrowhead's novel, RNAi AR O -LPA program. These RNAi molecules are designed to reduce elevated lipoprotein(a), which is a geneti cally validated, independent risk factor for atherosclerotic cardiovascular disease. In both agreements, Amgen will be wholly responsible for clinical deve lopment and commercialization. Under the terms of the agreements taken together, the Company will re ceive $35 million in upfront payments, $21.5 million in the form of an equity investment by Amgen in the Company's Common Stock, and up to $617 million in option payments and development, regulatory and sales milestone payments. The Company is further elig ible to receive single-digit royalties for sales of products under the AR O -AMG1 agreement and up to low double-digit royalties for sales of products under the AR O -LPA agreement . Regarding AR O -F12 , in November 2016, the Company presented data showing that its developed triggers gave greater than 95% reduction of serum F12 levels after a single subcutaneous administration with no increased bleeding risk in its preclinical studies. This data illustrates the Company's advancements in its subcutaneous delivery systems. The Company is conducting relevant disease models and is considering other potential studies to support advancement of AR O -F12 into clinical trials. Regarding AR O -HIF2, in April 2016, the Company presented data showing that AR O -HIF2 inhibited renal cell carcinoma growth and promoted tumor cell death in its preclinical studies. AR O -HIF2 is the Company's first RNAi therapeutic to target tissues outside the liver.
In January 2016, the Company entered into a new lease for a Madison, Wisconsin research facility. The 10-year lease is for approximately 60,000 square feet of office and laboratory space located at 502 South Rosa Road, Madison, Wisconsin. This lease will replace the Company's current research facility office lease as of September 30, 2016, also with University Research Park, Inc., for the facility located at 465 Science Drive, Madison Wisconsin. The larger facility is designed to accommodate increased research and development space needed for the Company's pipeline of current and future drug candidates. The initial term of the lease commenced on January 1, 2016, with occupancy occurring in October 2016. The lease payments and payments against a note payable for a tenant improvement allowance, which began on October 1, 2016, will total approximately $15.2 million over the initial 10-year term. We also estimate payments for our pro rata share of certain real estate taxes, operating expenses and common area maintenance expenses to be approximately $0.6 million for the first year of the lease, and these payments will continue throughout the initial 10-year term. We have paid or accrued approximately $7.0 million for leasehold improvements as of September 30, 2016, net of tenant improvement allowances, and the work on the facility was substantially completed in October 2016. The primary tenant improvement allowance of $2.1 million is accounted for as deferred rent and the secondary tenant improvement allowance of $2.7 million is accounted for as a note payable on the Company's Consolidated Balance Sheet. Pursuant to the lease, within six months of the expiration of the initial 10-year term, we have the option to extend the lease for up to two additional five-year terms, with certain annual increases in base rent. Additionally, on January 8, 2016, we entered into an amendment to our research facility office lease for property located at 465 Science Drive, Madison, Wisconsin with University Research Park, Inc. to terminate the lease on this property, effective on October 31, 2016.
Net losses were $81.7 million, $91.9 million and $58.7 million during the years ended September 30, 2016, 2015 and 2014, respectively. Diluted losses per share were $1.34, $1.60 and $1.25 during the years ended September 30, 2016, 2015 and 2014, respectively.
The Company strengthened its liquidity and financial position through an equity offering completed in August 2016, which generated approximately $43.2 million of net cash proceeds for the Company. Additionally, the Company received $14 million of the $56.5 million in upfront payments and equity investments from Amgen in September 2016 and received the remaining proceeds in November 2016. These cash proceeds secured the funding needed to continue to advance our preclinical candidates. The Company had $85.4 million of cash and cash equivalents and $128.2 million of total assets as of September 30, 2016, as compared to $81.2 million and $132.3 million as of September 30, 2015, respectively. Based upon the Company's current cash resources and operating plan, the Company expects to have sufficient liquidity to fund operations for at least the next twelve months.
39
Critical Accounting Policies and Estimates
Management makes certain judgments and uses certain estimates and assumptions when applying GAAP in the preparation of our Consolidated Financial Statements. We evaluate our estimates and judgments on an ongoing basis and base our estimates on historical experience and on assumptions that we believe to be reasonable under the circumstances. Our experience and assumptions form the basis for our judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may vary from what we anticipate and different assumptions or estimates about the future could change our reported results. We believe the following accounting policies are the most critical to us, in that they require our most difficult, subjective or complex judgments in the preparation of our consolidated financial statements. For further information, see Note 1, Organization and Significant Accounting Policies , to our Consolidated Financial Statements, which outlines our application of significant accounting policies.
Revenue Recognition
Revenue from product sales is recorded when persuasive evidence of an arrangement exists, title has passed and delivery has occurred, a price is fixed and determinable, and collection is reasonably assured.
The Company may generate revenue from technology licenses, collaborative research and development arrangements, research grants and product sales. Revenue under technology licenses and collaborative agreements typically consists of nonrefundable and/or guaranteed technology license fees, collaborative research funding, manufacturing and development services and various milestone and future product royalty or profit-sharing payments. These agreements are generally referred to as "multiple element arrangements".
The Company applies the accounting standard on revenue recognition for multiple element arrangements. The fair value of deliverables under the arrangement may be derived using a best estimate of selling price if vendor specific objective evidence and third-party evidence is not available. Deliverables under the arrangement will be separate units of accounting if a delivered item has value to the customer on a standalone basis and if the arrangement includes a general right of return for the delivered item, delivery or performance of the undelivered item is considered probable and substantially in the control of the vendor.
The Company recognizes upfront license payments as revenue upon delivery of the license only if the license has standalone value from any undelivered performance obligations and that value can be determined. The undelivered performance obligations typically include manufacturing or development services or research and/or steering committee services. If the fair value of the undelivered performance obligations can be determined, then these obligations would be accounted for separately. If the license is not considered to have standalone value, then the license and other undelivered performance obligations would be accounted for as a single unit of accounting. In this case, the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed or deferred indefinitely until the undelivered performance obligation is determined.
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, the Company determines the period over which the performance obligations will be performed and revenue will be recognized. Revenue is recognized using a proportional performance or straight-line method. The proportional performance method is used when the level of effort required to complete performance obligations under an arrangement can be reasonably estimated. The amount of revenue recognized under the proportional performance method is determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of milestones, by the ratio of the level of effort performed to date to the estimated total level of effort required to complete performance obligations under the arrangement. If the Company cannot reasonably estimate the level of effort to complete performance obligations under an arrangement, the Company recognizes revenue under the arrangement on a straight-line basis over the period the Company is expected to complete its performance obligations. Under either method, revenue is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned, as calculated under either method, as of the period ending date. Significant management judgment is required in determining the level of effort required under an arrangement and the period over which the Company is expected to complete its performance obligations under an arrangement.
Many of the Company's collaboration agreements entitle the Company to additional payments upon the achievement of development, regulatory and sales performance-based milestones. If the achievement of a milestone is considered probable at the inception of the collaboration, the related milestone payment is included with other collaboration consideration, such as upfront fees and research funding, in the Company's revenue calculation. Typically, these milestones are not considered probable at the inception of the collaboration. As such, milestones will typically be recognized in one of two ways depending on the timing of when the milestone is achieved. If the milestone is achieved during the performance period, then the Company will only recognize revenue to the extent of the proportional performance achieved at that date, or the proportion of the straight-line basis achieved at that date, and the remainder will be recorded as deferred revenue to be amortized over the remaining performance period. If the milestone is
40
achieved after the performance period has completed and all performance obligations have been delivered, then the Company will recognize the milestone payment as revenue in its entirety in the period the milestone was ach ieved.
Deferred revenue will be classified as part of Current or Long-Term Liabilities in the accompanying Consolidated Balance Sheets based on the Company's estimate of the portion of the performance obligations regarding that revenue will be completed within the next 12 months divided by the total performance period estimate. This estimate is based on the Company's current operating plan and, if the Company's operating plan should change in the future, the Company may recognize a different amount of deferred revenue over the next 12-month period.
Collaboration and License Agreements – Amgen, Inc.
On September 28, 2016, the Company entered into two Collaboration and License agreements, and a Common Stock Purchase Agreement with Amgen. Under the Second Collaboration and License Agreement, Amgen will receive a worldwide, exclusive license to Arrowhead's novel, RNAi ARO-LPA program. These RNAi molecules are designed to reduce elevated lipoprotein(a), which is a genetically validated, independent risk factor for atherosclerotic cardiovascular disease. Under the First Collaboration and License Agreement, Amgen will receive an option to a worldwide, exclusive license for ARO-AMG1, an RNAi therapy for an undisclosed genetically validated cardiovascular target. In both agreements, Amgen will be wholly responsible for clinical development and commercialization.
Under the Common Stock Purchase Agreement, the Company sold 3,002,793 shares of Common Stock to Amgen at a price of $7.16 per share, which represents the 30-day volume-weighted average price of the Common Stock on the NASDAQ stock market over the 30 trading days preceding execution. The Common Stock was delivered in two closings per the terms of the agreement. The first tranche of 1,256,983 shares of Common Stock was issued on September 29, 2016, and the Company received proceeds of approximately $9 million. The second tranche of 1,745,810 shares was subject to Hart-Scott-Rodino clearance which was reached on November 18, 2016. The Company received proceeds of approximately $12.5 million from this tranche of shares. Subject to Amgen's exercise of the Option, as defined in the First Collaboration and License Agreement, Amgen has agreed to purchase, and the Company has agreed to sell, an additional $5 million worth of shares of Common Stock based on a 30 trading day formula surrounding the date of the Option exercise.
Under the terms of the agreements taken together, the Company will receive $35 million in upfront payments, $21.5 million in the form of an equity investment by Amgen in the Company's Common Stock, and up to $617 million in option payments and development, regulatory and sales milestone payments. The Company is further eligible to receive single-digit royalties for sales of products under the First Collaboration and License Agreement and up to low double-digit royalties for sales of products under the Second Collaboration and License Agreement.
Under the terms of the First Collaboration and License Agreement, the Company is granting a worldwide, exclusive license to ARO-AMG1, an undisclosed genetically validated cardiovascular target. The collaboration between the Company and Amgen is governed by a joint steering committee comprised of an equal number of representatives from each party. The Company is also responsible for developing, optimizing and manufacturing the candidate through certain preclinical efficacy and toxicology studies to determine whether the candidate the Company has developed meets the required criteria as defined in the agreement (the "Arrowhead Deliverable"). If this is achieved, Amgen will then have the option to an exclusive license for the intellectual property generated through the Company's development efforts, and will likely assume all development, regulatory and commercialization efforts for the candidate upon the option exercise. The Company has determined that the significant deliverables under the First Collaboration and License Agreement include the license, the joint research committee and the development and manufacturing activities toward achieving the Arrowhead Deliverable. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and collective undelivered activities and services do not have standalone value due to the specialized nature of the activities and services to be provided by the Company. Therefore, the deliverables are not separable and, accordingly, the license and undelivered services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, the Company bases its revenue recognition pattern on the final deliverable. The Company will recognize revenue on a straight-line basis from October 1, 2016, through September 30, 2018. The due date for achieving the Arrowhead Deliverable, as defined in the agreement, is September 28, 2018. The Company received the upfront payment of $5 million due under this agreement in September 2016. It has been initially recorded as deferred revenue in the Company's Consolidated Balance Sheets, and will be amortized over the period discussed above.
Under the terms of the Second Collaboration and License Agreement, the Company is granting a worldwide, exclusive license to ARO-LPA. The collaboration between the Company and Amgen is governed by a joint research committee comprised of an equal number of representatives from each party; however, Amgen has the final decision making authority regarding ARO-LPA in this committee. The Company is also responsible for assisting Amgen in the oversight of certain development and manufacturing activities, most of which are to be covered at Amgen's cost. The Company has determined that the significant deliverables under the Second Collaboration and License Agreement include the license and the oversight of certain of the development and manufacturing activities.
41
The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and collective undelivered activities and services do not have standalone value due to the specialized nature of the activities and services to be provided by the Company. Therefore, the deliverables are not separable and, accordingly, the license and undelivered services are being treated as a single unit of accounting. When multiple deliv erables are accounted for as a single unit of accounting, the Company bases its revenue recognition pattern on the final deliverable. The Company will recognize revenue on a straight-line basis from N ovember 18 , 2016 (the Ha rt-Scott-Rodino clearance date) through October 31, 2017, which is the date where the significant development and manufacturing related deliverables are anticipated to be completed. The Company received the upfront payment of $30 million due under this agreement in November 2016. It w ill be initial ly recorded as deferred revenue and amortized over the period discussed above.
Impairment of Long-lived Assets
We review long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of assets may not be fully recoverable or that our assumptions about the useful lives of these assets are no longer appropriate. If impairment is indicated, recoverability is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset.
Impairment of Intangible assets
Intangible assets consist of in-process research and development, license agreements and patents acquired in conjunction with a business or asset acquisition. Intangible assets are monitored for potential impairment whenever events or circumstances indicate that the carrying amount may not be recoverable, and are also reviewed annually to determine whether any impairment is necessary. Based on ASU 2012-02, the annual review of intangible assets is performed via a two-step process. First, a qualitative assessment is performed to determine if it is more likely than not that the intangible asset is impaired. If required, a quantitative assessment is performed and, if necessary, impairment is recorded.
Stock-Based Compensation
We recognize stock-based compensation expense for stock options based on the grant date fair value using the Black-Scholes options pricing model, which requires us to make assumptions regarding certain variables including the risk-free interest rate, expected stock price volatility, assumed forfeitures, and the expected life of the award. The grant date fair value of restricted stock units granted is based upon the quoted closing market price per share on the date of grant, adjusted for assumed forfeitures. For performance-based stock awards, the value of the award is measured at the grant date. Expense for stock options and restricted stock units is recognized over the requisite service period. The assumptions used in calculating stock-based compensation expense represent management's best estimates, but these estimates involve inherent uncertainties, and if factors change or the Company used different assumptions, its stock-based compensation expense could be materially different in the future.
Derivative Assets and Liabilities
We account for warrants and other derivative financial instruments as either equity or assets/liabilities based upon the characteristics and provisions of each instrument. Warrants classified as equity are recorded as additional paid-in capital on our Consolidated Balance Sheet and no further adjustments to their valuation are made. Some of our warrants were determined to be ineligible for equity classification because of provisions that may result in an adjustment to their exercise price. Warrants classified as derivative liabilities and other derivative financial instruments that require separate accounting as assets or liabilities are recorded on our Consolidated Balance Sheet at their fair value on the date of issuance and are revalued on each subsequent balance sheet date until such instruments are exercised or expire, with any changes in the fair value between reporting periods recorded as other income or expense. We estimate the fair value of these assets/liabilities using option pricing models that are based on the individual characteristics of the warrants or instruments on the valuation date, as well as assumptions for expected volatility, expected life and risk-free interest rate. Changes in the assumptions used could have a material impact on the resulting fair value. The primary input affecting the value of our derivatives liabilities is the Company's stock price.
42
Contingent Consideration
The consideration for our acquisitions often includes future payments that are contingent upon the occurrence of a particular event. For example, milestone payments might be based on progress of clinical development, the achievement of various regulatory approvals or future sales milestones, and royalty payments might be based on drug product sales levels. The Company records a contingent consideration obligation for such contingent payments at fair value on the acquisition date. The Company estimates the fair value of contingent consideration obligations through valuation models designed to estimate the probability of the occurrence of such contingent payments based on various assumptions and incorporating estimated success rates. Estimated payments are discounted using present value techniques to arrive at estimated fair value at the balance sheet date. Changes in the fair value of our contingent consideration obligations are recognized within our Consolidated Statements of Operations. Changes in the fair value of the contingent consideration obligations can result from changes to one or multiple inputs, including adjustments to the discount rates, changes in the amount or timing of expected expenditures associated with product development, changes in the amount or timing of cash flows from products upon commercialization, changes in the assumed achievement or timing of any development milestones, changes in the probability of certain clinical events and changes in the assumed probability associated with regulatory approval. These fair value measurements are based on significant inputs not observable in the market. Substantial judgment is employed in determining the appropriateness of these assumptions as of the acquisition date and for each subsequent period. Accordingly, changes in assumptions could have a material impact on the amount of contingent consideration expense the Company records in any given period.
Results of Operations
The following data summarizes our results of operations for the following periods indicated:
|
| Year ended September 30, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Revenue |
| $ | 158,333 |
|
| $ | 382,000 |
|
| $ | 175,000 |
|
Operating Loss |
|
| (81,742,726 | ) |
|
| (95,973,976 | ) |
|
| (53,276,286 | ) |
Loss from Continuing Operations |
|
| (81,723,002 | ) |
|
| (91,940,882 | ) |
|
| (58,725,412 | ) |
Net Loss |
|
| (81,723,002 | ) |
|
| (91,940,882 | ) |
|
| (58,725,412 | ) |
Earnings per Share (Basic and Diluted) |
| $ | (1.34 | ) |
| $ | (1.60 | ) |
| $ | (1.25 | ) |
The decrease in our operating loss during the year ended September 30, 2016 is primarily due to a one-time $10.1 million expense related to the acquisition of in-process research and development from the Novartis asset acquisition in March 2015. We also had a $5.8 million reduction in our contingent consideration obligation associated with our acquisition of the Roche RNAi business. This was driven by the Company's decision to discontinue its clinical trial candidates: ARC-520, ARC-AAT and ARC-521. Lastly, the decrease in research and development expenses is primarily due to reduced expenses associated with the drug manufacturing campaign to support our Phase 2 studies for ARC-520. We expect research and development expenses to decrease in the near term with the discontinuation of our current clinical candidates, however, as our preclinical candidates progress, these expenses could increase.
Results of Operations Comparison for 2016 and 2015
Revenues
Total revenue was $158,333 for the year ended September 30, 2016 and $382,000 for the year ended September 30, 2015. Revenue is primarily related to licensed technology. In addition, the Company had collaboration revenue of $160,000 during the year ended September 30, 2015.
Operating Expenses
The analysis below details the operating expenses and discusses the expenditures of the Company within the major expense categories. Certain reclassifications have been made to prior-period operating expense categories to conform to the current period presentation. For purposes of comparison, the amounts for the years ended September 30, 2016 and 2015 are shown in the tables below.
Research and Development Expenses
R&D expenses are related to the Company's on-going research and development efforts, primarily related to program costs, composed primarily of outsourced costs related to the manufacturing of clinical supplies, toxicity/efficacy studies and clinical trial expenses. Internal costs primarily relate to operations at our research and development facility in Madison, Wisconsin, including facility costs and laboratory-related expenses. The following table provides details of research and development expense for the periods indicated:
43
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
| Twelve Months Ended |
|
| % of Expense |
|
| Twelve Months Ended |
|
| % of Expense |
|
| Increase (Decrease) |
| |||||||||
|
| September 30, 2016 |
|
| Category |
|
| September 30, 2015 |
|
| Category |
|
| $ |
|
|
| % |
| |||||
Laboratory supplies & services |
| $ | 2,706 |
|
|
| 7 | % |
| $ | 2,531 |
|
|
| 5 | % |
| $ | 175 |
|
|
| 7 | % |
In vivo studies |
|
| 1,611 |
|
|
| 4 | % |
|
| 556 |
|
|
| 1 | % |
|
| 1,055 |
|
|
| 190 | % |
Outside labs & contract services |
|
| 155 |
|
|
| 0 | % |
|
| 489 |
|
|
| 1 | % |
|
| (334 | ) |
|
| -68 | % |
Toxicity/efficacy studies |
|
| 7,766 |
|
|
| 19 | % |
|
| 6,572 |
|
|
| 14 | % |
|
| 1,194 |
|
|
| 18 | % |
Drug manufacturing |
|
| 9,855 |
|
|
| 25 | % |
|
| 21,431 |
|
|
| 46 | % |
|
| (11,576 | ) |
|
| -54 | % |
Clinical trials |
|
| 14,800 |
|
|
| 36 | % |
|
| 13,329 |
|
|
| 28 | % |
|
| 1,471 |
|
|
| 11 | % |
License, royalty & milestones |
|
| 3,054 |
|
|
| 6 | % |
|
| 1,065 |
|
|
| 2 | % |
|
| 1,989 |
|
|
| 187 | % |
Facilities and related |
|
| 1,315 |
|
|
| 3 | % |
|
| 977 |
|
|
| 2 | % |
|
| 338 |
|
|
| 35 | % |
Other research expenses |
|
| 192 |
|
|
| 1 | % |
|
| 317 |
|
|
| 1 | % |
|
| (125 | ) |
|
| -39 | % |
Total |
| $ | 41,454 |
|
|
| 100 | % |
| $ | 47,267 |
|
|
| 100 | % |
| $ | (5,813 | ) |
|
| -12 | % |
Laboratory supplies and services expense increased $175,000 from $2,531,000 during the year ended September 30, 2015 to $2,706,000 during the current period. The increase in laboratory supplies and services is a result of additional supply purchases necessary to support the expansion of the Company's preclinical pipeline as well as the development of the subcutaneous versions of its drug candidates.
In vivo studies expense increased $1,055,000 from $556,000 during the year ended September 30, 2015 to $1,611,000 during the current period. In vivo expense can vary depending on the stage of preclinical candidates, the nature and amount of testing required and the varying costs of different in vivo testing models. The expense in both periods relates to studies in connection with the development of new clinical candidates, and the increase in fiscal year 2016 was primarily driven by studies for ARO-LPA before it was licensed to Amgen.
Outside labs and contract services expense decreased $334,000 from $489,000 during the year ended September 30, 2015 to $155,000 during the current period. The decrease in the current period primarily relates to reduced contracted labor services and more functions being performed in house.
Toxicity/efficacy studies expense increased $1,194,000 from $6,572,000 during the year ended September 30, 2015 to $7,766,000 during the current period. This category includes IND-enabling toxicology studies, post-IND toxicology studies, such as long-term toxicology studies, and other efficacy studies. The increase primarily relates to toxicology studies related to ARC-521. We anticipate this expense to decrease in the near term with the discontinuation of our clinical candidates.
Drug manufacturing expense decreased $11,576,000 from $21,431,000 during the year ended September 30, 2015 to $9,855,000 during the current period. The decrease primarily relates to the substantial completion of our ARC-520 Phase 2b manufacturing campaign during fiscal 2015. The current period expense primarily relates to manufacturing for ARC-521 clinical trials. We anticipate this expense to decrease in the near term with the discontinuation of our clinical candidates.
Clinical trials expense increased $1,471,000 from $13,329,000 during the year ended September 30, 2015 to $14,800,000 during the current period. In both periods, the primary driver of the expenses was related to ARC-520 Phase 2b trials. We also incurred costs in fiscal 2016 related to our clinical trials for ARC-AAT and ARC-521. We anticipate this expense to decrease in the near term with the discontinuation of our clinical candidates.
License, royalty and milestones expense increased $1,989,000 from $1,065,000 during the year ended September 30, 2015 to $3,054,000 during the current period. This category can include milestone payments which can vary from period to period depending on the nature of our various license agreements and the timing of reaching various development milestones requiring payment. We reached milestones related to our clinical candidates that required a $3 million payment in fiscal 2016 and a $1 million payment in fiscal 2015.
Facilities expense increased $338,000 from $977,000 during the year ended September 30, 2015 to $1,315,000 during the current period. The increase relates to rent for our additional research and development facility in Middleton, Wisconsin and increased repairs and maintenance costs for our lab equipment. We anticipate this expense to increase in the near term due to the lease we entered into for the new research and development facility in Madison, Wisconsin.
44
Other research expense decreased $12 5 ,000 from $ 317 ,000 during the year ended September 30, 2015 to $19 2 ,000 during the current period. The decrease in the current period p rimarily relates to costs associated with a collaboration agreement to identify muscle targeting peptide molecules, for which the Company has been reimbursed from its collaboration partner.
Salary and Payroll-Related Expenses
The Company employs scientific, technical and administrative staff at its corporate offices and its research facilities. Salaries and payroll-related expense consists of salaries, bonuses, payroll taxes and related benefits. Salary and payroll-related expenses include two major categories, based on the primary activities of each employee: general and administrative (G&A) compensation expense, and research and development (R&D) compensation expense, based on the primary activities of each employee. The following table provides detail of salary and payroll-related expenses for the periods indicated:
(in thousands)
|
| Twelve Months |
|
|
|
|
| Twelve Months |
|
|
|
|
|
|
|
|
|
|
|
| ||||
|
| Ended |
|
| % of |
|
| Ended |
|
| % of |
|
| Increase (Decrease) |
| |||||||||
|
| September 30, 2016 |
|
| Expense Category |
|
| September 30, 2015 |
|
| Expense Category |
|
| $ |
|
| % |
| ||||||
R&D - compensation-related |
| $ | 13,883 |
|
|
| 71 | % |
| $ | 11,605 |
|
|
| 70 | % |
| $ | 2,278 |
|
|
| 20 | % |
G&A - compensation-related |
|
| 5,579 |
|
|
| 29 | % |
|
| 4,949 |
|
|
| 30 | % |
|
| 630 |
|
|
| 13 | % |
Total |
| $ | 19,462 |
|
|
| 100 | % |
| $ | 16,554 |
|
|
| 100 | % |
| $ | 2,908 |
|
|
| 18 | % |
R&D compensation expense increased $2,278,000 from $11,605,000 during the year ended September 30, 2015 to $13,883,000 during the current period. An increase in headcount accounted for the majority of the change in compensation-related expense. We anticipate this expense to decrease in the near term due to the discontinuation of our clinical candidates and reduction in R&D headcount.
G&A compensation expense increased $630,000 from $4,949,000 during the year ended September 30, 2015 to $5,579,000 during the current period. Annual merit increases accounted for the majority of the change in compensation-related expense.
General & Administrative Expenses
The following table provides details of our general and administrative expenses for the periods indicated:
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
| Twelve Months Ended |
|
| % of Expense |
|
| Twelve Months Ended |
|
| % of Expense |
|
| Increase (Decrease) |
| |||||||||
|
| September 30, 2016 |
|
| Category |
|
| September 30, 2015 |
|
| Category |
|
| $ |
|
|
|
|
| |||||
Professional/outside services |
| $ | 4,201 |
|
|
| 43 | % |
| $ | 3,989 |
|
|
| 50 | % |
| $ | 212 |
|
|
| 5 | % |
Patent expense |
|
| 1,529 |
|
|
| 15 | % |
|
| 950 |
|
|
| 12 | % |
|
| 579 |
|
|
| 61 | % |
Facilities and related |
|
| 320 |
|
|
| 3 | % |
|
| 308 |
|
|
| 4 | % |
|
| 12 |
|
|
| 4 | % |
Travel |
|
| 864 |
|
|
| 9 | % |
|
| 841 |
|
|
| 11 | % |
|
| 23 |
|
|
| 3 | % |
Business insurance |
|
| 632 |
|
|
| 6 | % |
|
| 523 |
|
|
| 7 | % |
|
| 109 |
|
|
| 21 | % |
Communication and Technology |
|
| 686 |
|
|
| 7 | % |
|
| 691 |
|
|
| 9 | % |
|
| (5 | ) |
|
| -1 | % |
Office expenses |
|
| 303 |
|
|
| 3 | % |
|
| 270 |
|
|
| 3 | % |
|
| 33 |
|
|
| 12 | % |
Other |
|
| 1,406 |
|
|
| 14 | % |
|
| 359 |
|
|
| 4 | % |
|
| 1,047 |
|
|
| 292 | % |
Total |
| $ | 9,940 |
|
|
| 100 | % |
| $ | 7,931 |
|
|
| 100 | % |
| $ | 2,009 |
|
|
| 25 | % |
Professional/outside services include legal, accounting, consulting and other outside services retained by the Company. All periods include normally recurring legal and audit expenses related to SEC compliance and other corporate matters. Professional/outside services expense increased $212,000 from $3,989,000 during the year ended September 30, 2015 to $4,201,000 during the current period. The increase in professional fees primarily related to higher legal fees related to recent litigation events. See Note 7 – Commitments and Contingencies for further discussion.
45
Patent expense increased $ 579 ,000 from $ 950 ,000 during the year ended September 30, 201 5 to $ 1,529 , 000 during the current period. Patent expense costs increase d due to additional prosecution requirements associated with ne w patents acquired through the Novartis asset acquisition. The Company continues to invest in patent protection for its product candidates and other RNAi technology through patent filings in numerous countries. The Company expects to extend and maintain protection for its current portfolios, as appropriate, and file new patent applications as technologies are developed and improved. Expenses can vary from period to period as patents proceed through their prosecution life cycle .
Facilities-related expense was consistent at $308,000 during the year ended September 30, 2015 and $320,000 in the current period. Facilities expense represents the expense associated with our corporate headquarters in Pasadena.
Travel expense was consistent at $841,000 during the year ended September 30, 2015 and $864,000 during the current period. Travel expense is incurred to support our R&D function, primarily our GMP manufacturing campaign and our clinical trials, as well as other corporate and business development related travel. We anticipate this expense to decrease in the near term due to the discontinuation of our clinical candidates and reduction in R&D headcount.
Business insurance expense increased $109,000 from $523,000 during the year ended September 30, 2015 to $632,000 during the current period. Business insurance costs increased primarily due to added coverage related to the Company's clinical trials, as well as increases in other corporate liability insurance. We anticipate this expense to decrease in the near term due to the discontinuation of our clinical candidates.
Communication and technology expense was consistent at $691,000 during the year ended September 30, 2015 and $686,000 during the current period. This category covers IT equipment and services for our personnel.
Office expense increased $33,000 from $270,000 during the year ended September 30, 2015 to $303,000 during the current period. These expenses relate to conferences/training, office supplies, miscellaneous administrative expenses, and expenses related to office expansions at our R&D facility in Madison and our corporate headquarters in Pasadena. Conference trainings and seminar expenses were increased in the current period.
Other expense increased $1,047,000 from $359,000 during the year ended September 30, 2015 to $1,406,000 during the current period. The increase in the current period pertains to litigation as discussed in Note 7 – Commitments and Contingencies . This category also consists primarily of franchise and property tax expenses and marketing.
Acquired in-process research and development – Novartis pre-clinical candidates
Acquired in-process research and development expense related to the Novartis pre-clinical candidates was $10,142,786 for fiscal year 2015 and zero in the current period. This expense pertains to the acquisition of the Novartis RNAi assets discussed above. The value of the purchase price allocated to certain preclinical candidates was expensed during the period, while certain patents and a third-party license were capitalized as intangible assets.
Stock-based compensation expense
Stock-based compensation expense, a noncash expense, increased $1,362,919 from $10,232,897 during the year ended September 30, 2015 to $11,595,816 during the current period. Stock-based compensation expense is based upon the valuation of stock options and restricted stock units granted to employees, directors, and certain consultants. Many variables affect the amount expensed, including the Company's stock price on the date of the grant, as well as other assumptions. The increase in the current period was primarily due to certain performance based awards that were deemed probable of achievement during the period.
Depreciation and amortization expense
Depreciation and amortization expense, a noncash expense, increased $923,838 from $2,336,207 during the year ended September 30, 2015 to $3,260,045 during the current period. The majority of depreciation and amortization expense relates to depreciation on lab equipment. In addition, the Company records depreciation on leasehold improvements at its Madison research facility and its Pasadena corporate headquarters. The increase in depreciation and amortization expense is primarily due to the amortization of the intangible assets acquired in the Novartis RNAi asset acquisition.
Impairment expense
Impairment expense, a noncash expense, was $2,050,817 in the current period and $0 during the year ended September 30, 2015. During the current period, the Company recognized an impairment expense of $1.1 million related to leasehold improvements
46
at its previous research facility in Madison, Wisconsin. This amount represented the entire net book value remaining for the leasehold improvements associated with the previous facility, an d was recognized during the year ended September 30, 2016 as the Company moved into a larger research facility . During the current period, the Company also recogniz e d a $0.9 million impairment expense related to acquired in-process research and developmen t assets that were acquired in the acquisition of the Roche RNAi business. In November 2016, the Company announced the discontinuation of its clinical trial efforts for ARC-520, ARC-AAT and ARC-521. Given this development, the Company has assessed the fa ir value of this indefinite-lived intangible asset to be $0 at September 30, 2016.
Contingent Consideration – Fair Value Adjustments
Contingent Consideration – Fair Value Adjustments decreased $7,753,997 from $1,891,533 during the year ended September 30, 2015 to ($5,862,464) during the current period. Contingent consideration resulting from the acquisition of Roche's RNAi business is calculated by modeling research and development activities for clinical candidates, forecasting timelines to market, and using "peak sales" estimate modeling, cash flows and potential milestone and royalty payments. The modeling assumes certain success rates, and discount factors related to riskiness of projects and the time value of money to calculate a net present value of future consideration payments to Roche. Each reporting period, the Company re-evaluates its contingent consideration, and if material, makes adjustments to the recorded liability. In November 2016, the Company announced the discontinuation of its clinical trial efforts for ARC-520, ARC-AAT and ARC-521. Given this development, the Company has assessed the fair value of its contingent consideration obligation to be $0 at September 30, 2016.
Other Income / Expense
Other income / expense was income of $4,035,494 during the year ended September 30, 2015 as compared to income of $22,124 during the current period. The largest component of other income / expense is related to the change in the value of derivative liabilities related to certain warrants with a price adjustment feature, which requires derivative accounting. The change in value of derivative liabilities was a reduction of approximately $2.9 million in 2015 and an increase of approximately $0.3 million in the current period. The fluctuations in each period were primarily driven by changes in the Company's stock price, which had a corresponding impact to the valuation of the underlying warrant liability.
Results of Operations Comparison for 2015 and 2014
Revenues
Total revenue was $382,000 for the year ended September 30, 2015 and $175,000 for the year ended September 30, 2014. Revenue is primarily related to licensed technology. In addition, the Company had collaboration revenue of $160,000 during the year ended September 30, 2015.
Operating Expenses
The analysis below details the operating expenses and discusses the expenditures of the Company within the major expense categories. Certain reclassifications have been made to prior-period operating expense categories to conform to the current period presentation. For purposes of comparison, the amounts for the years ended September 30, 2015 and 2014 are shown in the tables below.
Research and Development Expenses
R&D expenses are related to the Company's on-going research and development efforts, primarily related to program costs, composed primarily of outsourced costs related to the manufacturing of clinical supplies, toxicity/efficacy studies and clinical trial expenses. Internal costs primarily relate to operations at our research and development facility in Madison, Wisconsin, including facility costs and laboratory-related expenses. The following table provides details of research and development expense for the periods indicated:
(in thousands)
47
|
| Twelve |
|
|
|
|
|
| Twelve |
|
|
|
|
|
|
|
| |||||||
|
| Months Ended |
|
|
|
|
|
| Months Ended |
|
|
|
|
|
|
|
| |||||||
|
| September 30, 2015 |
|
| % of Total |
|
| September 30, 2014 |
|
| % of Total |
|
| Increase (Decrease) |
| |||||||||
Laboratory supplies & services |
| $ | 2,531 |
|
|
| 5 | % |
| $ | 2,071 |
|
|
| 9 | % |
| $ | 460 |
|
|
| 22 | % |
In vivo studies |
|
| 556 |
|
|
| 1 | % |
|
| 224 |
|
|
| 1 | % |
|
| 332 |
|
|
| 148 | % |
Outside labs & contract services |
|
| 489 |
|
|
| 1 | % |
|
| 691 |
|
|
| 3 | % |
|
| (202 | ) |
|
| -29 | % |
Toxicity/efficacy studies |
|
| 6,572 |
|
|
| 14 | % |
|
| 6,306 |
|
|
| 27 | % |
|
| 266 |
|
|
| 4 | % |
Drug manufacturing |
|
| 21,431 |
|
|
| 46 | % |
|
| 9,142 |
|
|
| 40 | % |
|
| 12,289 |
|
|
| 134 | % |
Clinical trials |
|
| 13,329 |
|
|
| 28 | % |
|
| 2,627 |
|
|
| 11 | % |
|
| 10,702 |
|
|
| 407 | % |
License, royalty & milestones |
|
| 1,065 |
|
|
| 2 | % |
|
| 1,096 |
|
|
| 5 | % |
|
| (31 | ) |
|
| -3 | % |
Facilities and related |
|
| 977 |
|
|
| 2 | % |
|
| 881 |
|
|
| 4 | % |
|
| 96 |
|
|
| 11 | % |
Other research expenses |
|
| 317 |
|
|
| 1 | % |
|
| 100 |
|
|
| 0 | % |
|
| 217 |
|
|
| 217 | % |
Total |
| $ | 47,267 |
|
|
| 100 | % |
| $ | 23,138 |
|
|
| 100 | % |
| $ | 24,129 |
|
|
| 104 | % |
Laboratory supplies and services expense increased $460,000 from $2,071,000 during the year ended September 30, 2014 to $2,531,000 during the year ended September 30, 2015. The increase in laboratory supplies and services is a result of additional supplies necessary to support increased efforts in pre-clinical research.
In vivo studies expense increased $332,000 from $224,000 during the year ended September 30, 2014 to $556,000 during the year ended September 30, 2015. In vivo expense can vary depending on the stage of preclinical candidates, the nature and amount of testing required and the varying costs of different in vivo testing models. The expense in both periods relates to studies in connection with the development of new clinical candidates. During fiscal year 2015, the Company expanded its candidate pipeline which resulted in additional studies conducted.
Outside labs and contract services expense decreased $202,000 from $691,000 during the year ended September 30, 2014 to $489,000 during the year ended September 30, 2015. The decrease during the year ended September 30, 2015 primarily relates to a one-time fee paid during the year ended September 30, 2014 related to access to a certain animal study.
Toxicity/efficacy studies expense increased $266,000 from $6,306,000 during the year ended September 30, 2014 to $6,572,000 during the year ended September 30, 2015. This category includes IND-enabling toxicology studies, post-IND toxicology studies, such as long-term toxicology studies, and other efficacy studies. The full-year expense primarily relates to IND-enabling toxicology studies related to ARC-AAT, long-term toxicology studies to support later clinical trials, and studies related to ARC-520 to support our phase 2b clinical trial. These amounts can vary quarter to quarter based on stage of development.
Drug manufacturing expense increased $12,289,000 from $9,142,000 during the year ended September 30, 2014 to $21,431,000 during the year ended September 30, 2015. The expense during the year ended September 30, 2015 primarily relates to drug manufacturing to supply toxicology studies for our ARC-520 Phase 2b clinical trials and clinical supplies for the ARC-520 Phase 2b clinical trial. The manufacturing campaign for the Phase 2b clinical trial for ARC-520 is largely complete.
Clinical trials expense increased $10,702,000 from $2,627,000 during the year ended September 30, 2014 to $13,329,000 during the year ended September 30, 2015. The increase is primarily driven by costs incurred in preparation for our phase 2b clinical trial for ARC-520. We also incurred costs in fiscal 2015 related to our clinical trial for our second clinical candidate ARC-AAT.
License, royalty and milestones expense was consistent at $1,096,000 during the year ended September 30, 2014 and $1,065,000 during the year ended September 30, 2015. This category can include milestone payments which can vary from period to period depending on the nature of our various license agreements, and the timing of reaching various development milestones requiring payment. We reached milestones related to our clinical candidates that required a $1 million payment in each period.
Facilities expense increased $96,000 from $881,000 during the year ended September 30, 2014 to $977,000 during the year ended September 30, 2015. The increase relates to higher rent in our Madison facility somewhat offset by lower repairs and maintenance costs on our lab equipment.
Other research expense increased $217,000 from $100,000 during the year ended September 30, 2014 to $317,000 during the year ended September 30, 2015. The increase during the year ended September 30, 2015 primarily relates to costs associated with a collaboration agreement to identify muscle targeting peptide molecules in fiscal 2015, for which the Company has been reimbursed from its collaboration partner.
48
Salary and Payroll-Related Expenses
The Company employs scientific, technical and administrative staff at its corporate offices and its research facilities. Salaries and payroll-related expense consists of salaries, bonuses, payroll taxes and related benefits. Salary and payroll-related expenses include two major categories, based on the primary activities of each employee: general and administrative (G&A) compensation expense, and research and development (R&D) compensation expense, based on the primary activities of each employee. The following table provides detail of salary and payroll-related expenses for the periods indicated:
(in thousands)
|
| Twelve months |
|
|
|
|
|
| Twelve months |
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
| Ended |
|
|
|
|
|
| Ended |
|
|
|
|
|
|
|
| |||||||
|
| September 30, 2015 |
|
| % of Total |
|
| September 30, 2014 |
|
| % of Total |
|
| Increase (Decrease) |
| |||||||||
R&D - compensation-related |
| $ | 11,605 |
|
|
| 70 | % |
| $ | 7,828 |
|
|
| 61 | % |
| $ | 3,777 |
|
|
| 48 | % |
G&A - compensation-related |
|
| 4,949 |
|
|
| 30 | % |
|
| 5,001 |
|
|
| 39 | % |
|
| (52 | ) |
|
| -1 | % |
Total |
| $ | 16,554 |
|
|
| 100 | % |
| $ | 12,829 |
|
|
| 100 | % |
| $ | 3,725 |
|
|
| 29 | % |
R&D compensation expense increased $3,777,000 from $7,828,000 during the year ended September 30, 2014 to $11,605,000 during the year ended September 30, 2015. An increase in headcount accounted for the majority of the change in compensation-related expense.
G&A compensation expense was consistent at $5,001,000 during the year ended September 30, 2014 and $4,949,000 during the year ended September 30, 2015. G&A headcount has remained consistent during the periods.
General & Administrative Expenses
The following table provides details of our general and administrative expenses for the periods indicated:
(in thousands)
|
| Twelve |
|
|
|
|
|
| Twelve |
|
|
|
|
|
|
|
| |||||||
|
| Months Ended |
|
|
|
|
|
| Months Ended |
|
|
|
|
|
|
|
| |||||||
|
| September 30, 2015 |
|
| % of Total |
|
| September 30, 2014 |
|
| % of Total |
|
| Increase (Decrease) |
| |||||||||
Professional/outside services |
| $ | 3,989 |
|
|
| 50 | % |
| $ | 2,465 |
|
|
| 42 | % |
| $ | 1,524 |
|
|
| 62 | % |
Patent expense |
|
| 950 |
|
|
| 12 | % |
|
| 632 |
|
|
| 11 | % |
|
| 318 |
|
|
| 50 | % |
Facilities and related |
|
| 308 |
|
|
| 4 | % |
|
| 193 |
|
|
| 3 | % |
|
| 115 |
|
|
| 60 | % |
Travel |
|
| 841 |
|
|
| 11 | % |
|
| 654 |
|
|
| 11 | % |
|
| 187 |
|
|
| 29 | % |
Business insurance |
|
| 523 |
|
|
| 7 | % |
|
| 302 |
|
|
| 5 | % |
|
| 221 |
|
|
| 73 | % |
Communication and Technology |
|
| 691 |
|
|
| 9 | % |
|
| 388 |
|
|
| 7 | % |
|
| 303 |
|
|
| 78 | % |
Office expenses |
|
| 270 |
|
|
| 3 | % |
|
| 380 |
|
|
| 6 | % |
|
| (110 | ) |
|
| -29 | % |
Other |
|
| 359 |
|
|
| 4 | % |
|
| 880 |
|
|
| 15 | % |
|
| (521 | ) |
|
| -59 | % |
Total |
| $ | 7,931 |
|
|
| 100 | % |
| $ | 5,894 |
|
|
| 100 | % |
| $ | 2,037 |
|
|
| 35 | % |
Professional/outside services include legal, accounting, consulting and other outside services retained by the Company. All periods include normally recurring legal and audit expenses related to SEC compliance and other corporate matters. Professional/outside services expense increased $1,524,000 from $2,465,000 during the year ended September 30, 2014 to $3,989,000 during the year ended September 30, 2015. The increase in professional fees primarily related to higher legal fees related to litigation events. See Note 7 – Commitments and Contingencies for further discussion. Additionally, the Company incurred higher recruiting fees to fill new positions.
Patent expense increased $318,000 from $632,000 during the year ended September 30, 2014 to $950,000 during the year ended September 30, 2015. Patent expense costs increase due to additional prosecution requirements associated with patents acquired through the Novartis asset acquisition. The Company continues to invest in patent protection for its product candidates and other RNAi technology through patent filings in multiple countries. The Company expects to extend and maintain protection for its current portfolios, as appropriate, and file new patent applications as technologies are developed and improved. Expenses can vary from period to period as patents proceed through their prosecution life cycle.
49
Facilities-related expense increased $115,000 from $193,000 d uring the year ended September 30, 201 4 to $308,000 during the year ended September 30, 2015 . Facilities expense increased due to the expansion of our corporate headquarters in Pasadena .
Travel expense increased $187,000 from $654,000 during the year ended September 30, 2014 to $841,000 during the year ended September 30, 2015. Travel expense increased due to travel in support of our R&D function, primarily our GMP manufacturing campaign and our clinical trials, as well as other corporate and business development related travel.
Business insurance expense increased $221,000 from $302,000 during the year ended September 30, 2014 to $523,000 during the year ended September 30, 2015. Business insurance costs increased primarily due to added coverage related to the Company's clinical trials, as well as increases in other corporate liability insurance.
Communication and technology expense increased $303,000 from $388,000 during the year ended September 30, 2014 to $691,000 during the year ended September 30, 2015. The increase was related to equipment purchases to replace outdated equipment and new equipment purchases related to new employees.
Office expense decreased $110,000 from $380,000 during the year ended September 30, 2014 to $270,000 during the year ended September 30, 2015. These expenses relate to conferences/training, office supplies, miscellaneous administrative expenses, and expenses related to office expansions at our R&D facility in Madison and our corporate headquarters in Pasadena. During fiscal year 2014, the Company incurred certain charges related to its Madison office expansion which were not repeated during the year ended September 30, 2015.
Other expense decreased $521,000 from $880,000 during the year ended September 30, 2014 to $359,000 during the year ended September 30, 2015. This category consists primarily of conference attendance fees, franchise and property tax expenses and marketing expenses. The decrease was related to a reduction in the provision for franchise taxes as well as an allowance recorded for certain other receivables during fiscal year 2014 that was not repeated in 2015.
Acquired in-process research and development – Novartis pre-clinical candidates
Acquired in-process research and development expense related to the Novartis pre-clinical candidates was $10,142,786 for fiscal year 2015 and zero for the previous period. This expense pertains to the acquisition of the Novartis RNAi assets. The value of the purchase price allocated to certain pre-clinical candidates was expensed during the period, while certain patents and a third-party license were capitalized as intangible assets.
Stock-based compensation expense
Stock-based compensation expense, a noncash expense, increased $4,536,724 from $5,696,173 during the year ended September 30, 2014 to $10,232,897 during the year ended September 30, 2015. Stock-based compensation expense is based upon the valuation of stock options and restricted stock units granted to employees, directors, and certain consultants. Many variables affect the amount expensed, including the Company's stock price on the date of the grant, as well as other assumptions. Based on the additional options and restricted stock units granted to new and existing employees in fiscal 2015, compensation expense has increased from the prior year.
Depreciation and amortization expense
Depreciation and amortization expense, a noncash expense, increased $990,552 from $1,345,655 during the year ended September 30, 2014 to $2,336,207 during the year ended September 30, 2015. The majority of depreciation and amortization expense relates to depreciation on lab equipment obtained as part of the acquisition of Roche's RNAi business in 2011. In addition, the Company records depreciation on leasehold improvements at its Madison research facility and its Pasadena corporate headquarters. The increase in depreciation and amortization expense is primarily due to the amortization of the intangible assets acquired in the Novartis asset acquisition.
Impairment expense
Impairment expense, a noncash expense, decreased $2,172,387 from $2,172,387 during the year ended September 30, 2014 to $0 during the year ended September 30, 2015. During the year ended September 30, 2014, the Company determined that the carrying value of the Alvos in-process research and development (IPR&D) may not be recoverable and should be fully impaired. During the year ended September 30, 2015, the Company did not find any of its intangible or long-lived assets to be impaired.
Contingent Consideration – Fair Value Adjustments
50
Contingent Consideration – Fair Value Adjustments increased $484,125 from $2,375,658 during the year ended September 30, 2014 to $1,891,533 d uring the year ended September 30, 2015 . Contingent consideration resulting from the acquisition of Roche's RNAi business is calculated by modeling research and development activities for clinical candidates, forecasting timelines to market, and using "pe ak sales" estimate modeling, cash flows and potential milestone and royalty payments. The modelin g assumes certain success rates and discount factors related to riskiness of projects and the time value of money to calculate a net present value of future c onsideration payments to Roche. Each reporting period, the Company re-evaluates its contingent consideration, and if material, makes adjustments to the recorded liability. The Company increased this liability by $1.9 million and $2.4 million during the y ears ended September 30, 2015 and 2014, respectively, for a total liability of $5.9 million as of September 30, 2015 , which is recorded on the Company's Consolidated Balance Sheets.
Other Income / Expense
Other income / expense was expense of $5,443,826 during the year ended September 30, 2014 as compared to income of $4,035,494 during the year ended September 30, 2015. The largest component of Other income / expense is related to the change in the value of derivative liabilities related to certain warrants with a price adjustment feature, which requires derivative accounting. The change in value of derivative liabilities was a reduction of approximately $2.9 million in 2015 and an increase of approximately $6.0 million in 2014. The fluctuations in each period were primarily driven by changes in the Company's stock price, which had a corresponding impact to the valuation of the underlying warrant liability.
Liquidity and Cash Resources
Arrowhead has historically financed its operations through the sale of its equity securities. Research and development activities have required significant capital investment since the Company's inception, and are expected to continue to require significant cash expenditure in fiscal year 2017 and beyond .
At September 30, 201 6 , the Company had cash on hand of approximately $85.4 million as compared to $81.2 million at September 30, 2015. Excess cash invested in fixed income securities was $0 million at September 30, 2016, compared to $17.5 million at September 30, 2015. In November 2016, the Company received the upfront payment of $30 million due under the Second Collaboration and License Agreement with Amgen, as well as a $12.5 million equity investment payment from Amgen. The Company believes its current financial resources are sufficient to fund its operations through at least the next twelve months.
A summary of cash flows for the years ended September 30, 2016, 2015, and 2014 is as follows:
|
| Year ended September 30, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Cash Flow from: |
|
|
|
|
|
|
|
|
|
|
|
|
Operating Activities |
| $ | (64,427,486 | ) |
| $ | (65,707,615 | ) |
| $ | (35,416,373 | ) |
Investing Activities |
|
| 13,447,763 |
|
|
| 14,120,838 |
|
|
| (36,481,770 | ) |
Financing Activities |
|
| 55,131,817 |
|
|
| 290,521 |
|
|
| 185,294,309 |
|
Net increase (decrease) in cash |
|
| 4,152,094 |
|
|
| (51,296,256 | ) |
|
| 113,396,166 |
|
Cash at beginning of period |
|
| 81,214,354 |
|
|
| 132,510,610 |
|
|
| 19,114,444 |
|
Cash at end of period |
| $ | 85,366,448 |
|
| $ | 81,214,354 |
|
| $ | 132,510,610 |
|
During the year ended September 30, 2016, the Company used $64.4 million in cash from operating activities, which represents the on-going expenses of its research and development programs and corporate overhead. Cash outlays were primarily composed of the following: research and development costs were $42.6 million, salary and payroll-related expenses were $16.4 million and general and administrative costs were $9.9 million. These expenditures were partially offset by $5 million received under the First Collaboration and License Agreement with Amgen. Cash provided by investing activities was $13.4 million, primarily related to the maturity of certain marketable securities of $17.3 million partially offset by capital expenditures of $3.9 million. Cash provided by financing activities of $55.1 million primarily includes an equity financing in August 2016 which yielded net proceeds of $43.2 million to the Company, and $9 million of equity investments from the Company's Common Stock Purchase Agreement Agreement with Amgen.
During the year ended September 30, 2015, the Company used $65.7 million in cash from operating activities, which represents the on-going expenses of its research and development programs and corporate overhead. Cash outlays were primarily composed of the following: research and development costs were $41.2 million, salary and payroll-related expenses were $16.6 million, and general and administrative costs were $7.9 million. Cash provided by investing activities was $14.1 million, primarily related to the maturity of certain marketable securities partially offset by $10.0 million of cash used to acquire the Novartis assets discussed above. Capital
51
expenditures were $ 2.0 million. Cash provided by financing activities of $0.3 million primarily includes the exercise of warrants and stock opti ons during the year ended September 30, 2015.
During the year ended September 30, 2014, the Company used $35.4 million in cash from operating activities, which represents the on-going expenses of its research and development programs and corporate overhead. Cash outlays were primarily composed of the following: research and development costs were $21.7 million, salary and payroll-related expenses were $9.1 million, and general and administrative costs were $4.6 million. Cash used by investing activities was $36.5 million, primarily related to net cash investments in fixed income securities of $34.8 million. Capital expenditures were $ 1.7 million. Cash provided by financing activities of $185.3 million includes $172.6 million of cash received from equity financings by the Company in October 2013 and February 2014. The exercise of warrants and stock options during fiscal 2014 resulted in additional cash inflow of $12.9 million.
Contractual Obligations
In the table below, we set forth our enforceable and legally binding obligations and future commitments at September 30, 2016 for the categories shown, as well as obligations related to contracts in such categories that we are likely to continue. Some of the figures that we include in this table are based on management's estimates and assumptions about these obligations, including their duration, the possibility of renewal, anticipated actions by third parties and other factors. Because these estimates and assumptions are necessarily subjective, the obligations we will actually pay in future periods may vary from those reflected in the table. The following table does not include any future obligations that may be owed under existing license agreements, as the certainty of achieving the relevant milestones that would trigger these payments is currently unknown.
|
| Payments due by Period |
| |||||||||||||||||
|
| Total |
|
| Less than 1 Year |
|
| 1-3 Years |
|
| 3-5 Years |
|
| More than 5 Years |
| |||||
Debt (a) |
|
| 2,533,455 |
|
|
| - |
|
|
| 432,326 |
|
|
| 498,161 |
|
|
| 1,602,968 |
|
Capital Leases |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
Operating Leases |
|
| 12,407,855 |
|
|
| 1,559,259 |
|
|
| 2,967,174 |
|
|
| 2,114,927 |
|
|
| 5,766,495 |
|
Purchase Obligations (b) |
|
| 10,400,000 |
|
|
| 10,400,000 |
|
|
| - |
|
|
| - |
|
|
| - |
|
Other Long-Term Liabilities (c) |
|
| 2,474,997 |
|
|
| - |
|
|
| 747,915 |
|
|
| 624,319 |
|
|
| 1,102,763 |
|
Total |
| $ | 27,816,307 |
|
| $ | 11,959,259 |
|
| $ | 4,147,415 |
|
| $ | 3,237,407 |
|
| $ | 8,472,226 |
|
| (a) | Includes current portion of $2.7 million Note Payable associated with our lease for our new Madison research facility. |
| (b) | Purchase obligations have decreased due to the discontinuation of the Company's clinical trials. |
| (c) | Excludes $2.5 million Deferred Revenue associated with the Company's collaboration and license agreements with Amgen. |
Off-Balance Sheet Arrangements
As of September 30, 2016, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
Recent Accounting Pronouncements
See Note 1 to our Consolidated Financial Statements of this annual report on Form 10-K for a description of recent accounting pronouncements applicable to our business.
52
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are exposed to market risk related to changes in interest rates, which could adversely affect the value of our interest rate-sensitive assets and liabilities. We do not hold any instruments for trading purposes and investment criteria are governed by the Company's Investment Policy. As of September 30, 2016 and 2015, we had cash and cash equivalents of $85.4 million and $81.2 million, respectively. At times, we have invested our cash reserves in corporate bonds typically with maturities of less than 2 years, and we have historically classified these investments as held-to-maturity. Due to the relatively short-term nature of the investments that we hold, we do not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to our investment portfolio. Our liability instrument sensitive to changes in interest rates is our derivative liability with its fair value determined using an option pricing model, which uses interest rate as an input. However, any change associated with this valuation would result in a noncash expense and would not significantly impact our operations.
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The information required by this item is included in Item 15 of this Annual Report Form 10-K.
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
ITEM 9A. | CONTROLS AND PROCEDURES. |
Our Chief Executive Officer and our Chief Financial Officer, after evaluating our "disclosure controls and procedures" (as defined in Securities Exchange Act of 1934 (the "Exchange Act") Rules 13a-15(e) and 15d-15(e)) as of the end of the period covered by this Annual Report on Form 10-K (the "Evaluation Date") have concluded that as of the Evaluation Date, our disclosure controls and procedures are effective to ensure that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission rules and forms, and to ensure that information required to be disclosed by us in such reports is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, where appropriate, to allow timely decisions regarding required disclosure.
No change in the Company's internal controls over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) of the Exchange Act) occurred during the Company's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management's Annual Report on Internal Control over Financial Reporting
Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States. This process includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of the internal control over financial reporting to future periods are subject to risk that the internal control may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate.
Management's Assessment of the Effectiveness of our Internal Control over Financial Reporting
The Company's management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Management conducted an assessment of the effectiveness of the Company's
53
inte rnal control over financial reporting based on the criteria set forth in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission ( 2013 framework). Based on the Company's assessment, management has concluded that its internal control over financial reporting was effective as of September 30 , 201 6 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP. Th e Company's independent registered public accounting firm, Rose, Snyder and Jacobs LLP, has issued an audit report on the Company's internal control over financial reporting, which appears in Item 15 of this Form 10-K.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the fourth quarter of the year ended September 30, 2016 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. | OTHER INFORMATION |
None.
PART III
ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
The information called for by this Item will be incorporated by reference from our Definitive Proxy Statement to be filed for our 2017 Annual Meeting of Stockholders, which proxy statement will be filed no later than January 28, 2017.
ITEM 11. | EXECUTIVE COMPENSATION |
The information called for by this Item will be incorporated by reference from our Definitive Proxy Statement to be filed for our 2017 Annual Meeting of Stockholders, which proxy statement will be filed no later than January 28, 2017.
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The information called for by this Item will be incorporated by reference from our Definitive Proxy Statement to be filed for our 2017 Annual Meeting of Stockholders, which proxy statement will be filed no later than January 28, 2017.
ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
The information called for by this Item will be incorporated by reference from our Definitive Proxy Statement to be filed for our 2017 Annual Meeting of Stockholders, which proxy statement will be filed no later than January 28, 2017.
ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information called for by this Item will be incorporated by reference from our Definitive Proxy Statement to be filed for our 2017 Annual Meeting of Stockholders, which proxy statement will be filed no later than January 28, 2017.
PART IV
ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
The following documents are filed as part of this Annual Report on Form 10-K:
| (1) | Financial Statements. |
See Index to Financial Statements and Schedule on page F-1.
54
| (2) | Financial Statement Schedules. |
See Index to Financial Statements and Schedule on page F-1. All other schedules are omitted as the required information is not present or is not present in amounts sufficient to require submission of the schedule, or because the information required is included in the consolidated financial statements or notes thereto.
| (3) | Exhibits. |
The following exhibits are filed (or incorporated by reference herein) as part of this Annual Report on Form 10-K:
Exhibit Number |
| Description |
| Incorporated by Reference Herein | ||
|
| Form |
| Date | ||
2.1 |
|
Stock and Asset Purchase Agreement between Arrowhead Research Corporation and Roche entities, dated October 21, 2011† |
|
Annual Report on Form 10-K for the fiscal year ended September 30, 2011, as Exhibit 2.1 |
|
December 20, 2011 |
|
|
|
|
|
|
|
2.2 |
| Asset Purchase and Exclusive License Agreement between Arrowhead Research Corporation and Novartis Institutes for BioMedical Research, Inc., dated March 3, 2015† |
| Quarterly Report on Form 10-Q, as Exhibit 2.1 |
| May 11, 2015 |
|
|
|
|
|
|
|
3.1 |
| Series A Certificate of Designations, filed with the Secretary of State of the State of Delaware on October 25, 2011 |
| Current Report on Form 8-K, as Exhibit 3.1 |
| October 26, 2011 |
|
|
|
|
|
|
|
3.2 |
| Series B Certificate of Designations, filed with the Secretary of State of the State of Delaware on May 1, 2013 |
| Current Report on Form 8-K, as Exhibit 3.1 |
| May 1, 2013 |
|
|
|
|
|
|
|
3.3 |
| Series C Certificate of Designations, filed with the Secretary of State of the State of Delaware on October 10, 2013 |
| Current Report on Form 8-K, as Exhibit 3.1 |
| October 10, 2013 |
|
|
|
|
|
|
|
3.4 |
| Certificate of Elimination of Series A Convertible Preferred Stock of Arrowhead Research Corporation, filed with the Secretary of State of the State of Delaware on April 5, 2016 |
| Current Report on Form 8-K as Exhibit 3.1 |
| April 6, 2016 |
|
|
|
|
|
|
|
3.5 |
| Certificate of Elimination of Series B Convertible Preferred Stock of Arrowhead Research Corporation, filed with the Secretary of State of the State of Delaware on April 5, 2016 |
| Current Report on Form 8-K as Exhibit 3.2 |
| April 6, 2016 |
|
|
|
|
|
|
|
3.6 |
| Amended and Restated Certificate of Incorporation of Arrowhead Research Corporation, a Delaware corporation, filed with the Secretary of State of the State of Delaware on April 5, 2016 |
| Current Report on Form 8-K as Exhibit 3.3 |
| April 6, 2016 |
|
|
|
|
|
|
|
3.7 |
| Amended and Restated Bylaws of Arrowhead Pharmaceuticals, Inc. |
| Current Report on form 8-K as Exhibit 3.4 |
| April 6, 2016 |
|
|
|
|
|
|
|
4.1 |
| Form of Warrant to Purchase Common Stock expiring May 2017 |
| Current Report on Form 8-K, as Exhibit 4.1 |
| May 30, 2007 |
|
|
|
|
|
|
|
4.2 |
| Form of Warrant to Purchase Common Stock expiring December 2015 |
| Current Report on Form 8-K, as Exhibit 4.1 |
| June 18, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.3 |
| Form of Warrant to Purchase Shares of Common Stock Expiring August 13, 2016 |
| Current Report on Form 8-K, as Exhibit 4.1 |
| August 13, 2012 |
|
|
|
|
|
|
|
4.4 |
| Form of Common Stock Certificate of Arrowhead Research Corporation |
| Amendment No. 2 to Registration Statement on Form S-1, as Exhibit 4.7 |
| September 11, 2009 |
|
|
|
|
|
|
|
55
Exhibit Number |
| Description |
| Incorporated by Reference Herein | ||
|
| Form |
| Date | ||
4.5 |
| Form of Series B Preferred Stock Certificate |
| Annual Report on Form 10-K for the fiscal year ended September 30, 2013, as Exhibit 4.15 |
| December 18, 2013 |
|
|
|
|
|
|
|
4.6 |
| Form of Warrant to Purchase Shares of Common Stock expiring August 17, 2016 |
| Current Report on Form 8-K, as exhibit 4.2 |
| August 13, 2012 |
|
|
|
|
|
|
|
4.7 |
| Form of Warrant to Purchase Shares of Common Stock expiring December 12, 2017 |
| Current Report on Form 8-K, as exhibit 4.2 |
| December 12, 2012 |
|
|
|
|
|
|
|
4.8 |
| Form of Warrant to Purchase Shares of Common Stock expiring January 30, 2018 |
| Current Report on Form 8-K, as exhibit 4.2 |
| January 30, 2013 |
|
|
|
|
|
|
|
4.9 |
| Form of Series C Preferred Stock Certificate |
| Annual Report on Form 10-K for the fiscal year ended September 30, 2013, as Exhibit 4.15 |
| December 18, 2013 |
|
|
|
|
|
|
|
4.10 |
| Form of Common Stock Certificate of Arrowhead Pharmaceuticals, Inc. |
| Current Report on Form 8-K, as Exhibit 4.1 |
| April 6, 2016 |
|
|
|
|
|
|
|
4.11 |
| Form of Indenture |
| Registration Statement on Form S-3 (File No. 333-214315) |
| October 28, 2016 |
|
|
|
|
|
|
|
10.1** |
| Arrowhead Research Corporation (fka InterActive, Inc.) 2000 Stock Option Plan |
| Schedule 14C, as Exhibit D |
| December 22, 2000 |
|
|
|
|
|
|
|
10.2** |
| Arrowhead Research Corporation 2004 Equity Incentive Plan, as amended |
| Schedule 14C, as Annex A |
| January 12, 2012 |
|
|
|
|
|
|
|
10.3** |
| Arrowhead Research Corporation 2013 Incentive Plan |
| Schedule 14C, as Annex A |
| December 20, 2013 |
|
|
|
|
|
|
|
10.4** |
| Form of Stock Option Agreement for use with the 2013 Incentive Plan |
| Current Report on Form 8-K, as Exhibit 10.1 |
| February 12, 2014 |
|
|
|
|
|
|
|
10.5** |
| Form of Restricted Stock Unit Agreement for use with the 2013 Incentive Plan |
| Current Report on Form 8-K, as Exhibit 10.2 |
| February 12, 2014 |
|
|
|
|
|
|
|
10.6** |
| Executive Incentive Plan, adopted December 12, 2006 |
| Annual Report on Form 10-K for the fiscal year ended September 30, 2006, as Exhibit 10.11 |
| December 14, 2006 |
|
|
|
|
|
|
|
10.7** |
| Employment Agreement between Arrowhead and Dr. Christopher Anzalone, dated June 11, 2008 |
| Current Report on Form 8-K, as Exhibit 10.1 |
| June 13, 2008 |
|
|
|
|
|
|
|
10.8** |
| Amendment to Employment Agreement between Arrowhead and Dr. Christopher Anzalone, effective May 12, 2009 |
| Annual Report on Form 10-K for the fiscal year ended September 30, 2009, as Exhibit 10.8 |
| December 22, 2009 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9 |
| Non-Exclusive License Agreement between Arrowhead Research Corporation and Roche entities, dated October 21, 2011† |
| Annual Report on Form 10-K for the fiscal year ended September 30, 2011, as Exhibit 10.33 |
| December 20, 2011 |
|
|
|
|
|
|
|
10.10 |
| License and Collaboration Agreement between F. Hoffmann-La Roche Ltd and Hoffman-La Roche Inc. and Alnylam Pharmaceuticals, Inc., dated July 8, 2007 † |
| Annual Report on Form 10-K for the fiscal year ended September 30, 2011, as Exhibit 10.35 |
| December 20, 2011 |
56
Exhibit Number |
| Description |
| Incorporated by Reference Herein | ||
|
| Form |
| Date | ||
|
|
|
|
|
|
|
10.11 |
| Collaboration Agreement by and among Alnylam Pharmaceuticals, Inc. and F. Hoffmann-La Roche Ltd and Hoffman-La Roche Inc., dated October 29, 2009 † |
| Annual Report on Form 10-K for the fiscal year ended September 30, 2011, as Exhibit 10.36 |
| December 20, 2011 |
|
|
|
|
|
|
|
10.12 |
| Office Lease Agreement between South Lake Avenue Investors LLC and the Company, dated April 12, 2012 |
| Quarterly Report on Form 10-Q, as Exhibit 10.1 |
| May 8, 2012 |
|
|
|
|
|
|
|
10.13 |
| Form of Securities Purchase Agreement between Arrowhead Research Corporation and certain Investors |
| Current Report on Form 8-K, as Exhibit 10.1 |
| April 30, 2013 |
|
|
|
|
|
|
|
10.14 |
| Securities Purchase Agreement between the Company and the purchasers listed thereon. |
| Current Report on Form 8-K, as Exhibit 10.1 |
| October 10, 2013 |
|
|
|
|
|
|
|
10.15 |
| License Agreement by and between Alnylam Pharmaceuticals, Inc., Arrowhead Research Corporation and Arrowhead Madison, Inc.† |
| Quarterly Report on Form 10-Q, as Exhibit 10.1 |
| August 12, 2014 |
|
|
|
|
|
|
|
10.16 |
| Lease Agreement between University Research Park, Incorporated and Arrowhead Madison, Inc., dated January 8, 2016 |
| Quarterly Report on Form 10-Q, as Exhibit 10.1 |
| February 2, 2016 |
|
|
|
|
|
|
|
10.17 |
| Securities Purchase Agreement between the Company and the purchasers listed thereon, dated August 8, 2016 |
| Current Report on Form 8-K, as Exhibit 10.1 |
| August 10, 2016 |
|
|
|
|
|
|
|
10.18 |
| First Collaboration and Licensing Agreement between Arrowhead Pharmaceuticals, Inc. and Amgen, Inc., dated September 28, 2016*† |
|
|
|
|
|
|
|
|
|
|
|
10.19 |
| Second Collaboration and Licensing Agreement between Arrowhead Pharmaceuticals, Inc. and Amgen, Inc., dated September 28, 2016*† |
|
|
|
|
|
|
|
|
|
|
|
10.20 |
| Common Stock Purchase Agreement between the Company and Amgen Inc., dated September 28, 2016 |
| Amendment No. 1 to the Registration Statement on Form S-3 (File No. 333-214311) |
| November 25, 2016 |
21.1 |
| List of Subsidiaries* |
|
|
|
|
|
|
|
|
|
|
|
23.1 |
| Consent of Independent Public Registered Accounting Firm* |
|
|
|
|
|
|
|
|
|
|
|
24.1 |
| Power of Attorney (contained on signature page) |
|
|
|
|
|
|
|
|
|
|
|
31.1 |
| Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* |
|
|
|
|
|
|
|
|
|
|
|
31.2 |
| Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* |
|
|
|
|
|
|
|
|
|
|
|
32.1 |
| Certification by Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*** |
|
|
|
|
|
|
|
|
|
|
|
32.2 |
| Certification by Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*** |
|
|
|
|
|
|
|
|
|
|
|
101.INS |
| XBRL Instance Document* |
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
| XBRL Schema Document* |
|
|
|
|
|
|
|
|
|
|
|
101.CAL |
| XBRL Calculation Linkbase Document* |
|
|
|
|
|
|
|
|
|
|
|
101.LAB |
| XBRL Label Linkbase Document* |
|
|
|
|
|
|
|
|
|
|
|
101.PRE |
| XBRL Presentation Linkbase Document* |
|
|
|
|
57
Exhibit Number |
| Description |
| Incorporated by Reference Herein | ||
|
| Form |
| Date | ||
|
|
|
|
|
|
|
101.DEF |
| XBRL Definition Linkbase Document* |
|
|
|
|
* | Filed herewith |
** | Indicates compensation plan, contract or arrangement. |
*** | Furnished herewith |
† | Confidential treatment has been requested with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission. |
SIGNATURE
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, on this 14th day of December 2016.
Dated: December 14, 2016
ARROWHEAD PHARMACEUTICALS, INC. | |||
|
|
| |
By: |
| /s/ Christopher Anzalone | |
|
| Christopher Anzalone | |
|
| Chief Executive Officer | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report on Form 10-K has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
Signature |
| Title |
| Date |
|
|
| ||
/s/ Christopher Anzalone |
| Chief Executive Officer, President and |
| December 14, 2016 |
Christopher Anzalone |
| Director (Principal Executive Officer) |
|
|
|
|
| ||
/s/ Kenneth A. Myszkowski |
| Chief Financial Officer (Principal |
| December 14, 2016 |
Kenneth A. Myszkowski |
| Financial and Accounting Officer) |
|
|
|
|
| ||
/s/ Douglass Given |
| Director, Chairman of the Board of Directors |
| December 14, 2016 |
Douglass Given |
|
|
|
|
|
|
| ||
/s/ Mauro Ferrari |
| Director |
| December 14, 2016 |
Mauro Ferrari |
|
|
|
|
|
|
| ||
/s/ Edward W. Frykman |
| Director |
| December 14, 2016 |
Edward W. Frykman |
|
|
|
|
|
|
| ||
/s/ Michael S. Perry |
| Director |
| December 14, 2016 |
Michael S. Perry |
|
|
|
|
58
INDEX TO FINAN CIAL STATEMENTS AND SCHEDULE
Reports of Independent Registered Public Accounting Firm |
| F-2 |
|
Consolidated Balance Sheets of Arrowhead Pharmaceuticals, Inc., September 30, 2016 and 2015 |
| F-4 |
|
Consolidated Statements of Operations of Arrowhead Pharmaceuticals, Inc. for the years ended September 30, 2016, 2015 and 2014 |
| F-5 |
|
Consolidated Statement of Stockholders' Equity of Arrowhead Pharmaceuticals, Inc. for the years ended September 30, 2016, 2015, and 2014 |
| F-6 |
|
Consolidated Statements of Cash Flows of Arrowhead Pharmaceuticals, Inc. for the years ended September 30, 2016, 2015 and 2014 |
| F-7 |
|
Notes to Consolidated Financial Statements of Arrowhead Pharmaceuticals, Inc . |
| F-8 |
|
F-1
REPORT OF INDEPE NDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Arrowhead Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Arrowhead Pharmaceuticals, Inc. and Subsidiaries (the "Company") as of September 30, 2016 and 2015, and the related consolidated statements of operations and comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended September 30, 2016. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Arrowhead Pharmaceuticals, Inc. and Subsidiaries at September 30, 2016 and 2015, and the consolidated results of their operations and their cash flows for each of the three years in the period ended September 30, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Arrowhead Pharmaceuticals, Inc. and Subsidiaries' internal control over financial reporting as of September 30, 2016, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated December 14, 2016 expressed an unqualified opinion thereon.
Rose, Snyder & Jacobs LLP
Encino, California
December 14, 2016
F-2
REPORT OF INDEPENDENT REG ISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Arrowhead Pharmaceuticals, Inc.
We have audited Arrowhead Pharmaceuticals, Inc. and Subsidiaries' (the "Company") internal control over financial reporting as of September 30, 2016, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) COSO. Arrowhead Pharmaceuticals, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Arrowhead Pharmaceuticals, Inc. and Subsidiaries maintained, in all material respects, effective internal control over financial reporting as of September 30, 2016, based on the criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the 2016 consolidated financial statements of Arrowhead Pharmaceuticals, Inc. and Subsidiaries and our report dated December 14, 2016 expressed an unqualified opinion thereon.
Rose, Snyder & Jacobs LLP
Encino, California
December 14, 2016
F-3
PART I. FINANCIAL INFORMATION
ITEM 1. | FINANCIAL STATEMENTS |
Arrowhead Pharmaceuticals, Inc.
Consolidated Balance Sheets
|
|
|
|
|
|
|
|
| September 30, 2016 |
|
| September 30, 2015 |
| ||
ASSETS |
|
|
|
|
|
|
|
CURRENT ASSETS |
|
|
|
|
|
|
|
Cash and cash equivalents | $ | 85,366,448 |
|
| $ | 81,214,354 |
|
Trade receivable |
| 75,000 |
|
|
| - |
|
Prepaid expenses |
| 1,289,923 |
|
|
| 3,293,285 |
|
Other current assets |
| 3,771,172 |
|
|
| 823,620 |
|
Short term investments |
| - |
|
|
| 17,539,902 |
|
TOTAL CURRENT ASSETS |
| 90,502,543 |
|
|
| 102,871,161 |
|
Property and equipment, net |
| 15,386,761 |
|
|
| 4,526,848 |
|
Intangible assets, net |
| 22,164,868 |
|
|
| 24,824,116 |
|
Other assets |
| 122,333 |
|
|
| 45,789 |
|
TOTAL ASSETS | $ | 128,176,505 |
|
| $ | 132,267,914 |
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
CURRENT LIABILITIES |
|
|
|
|
|
|
|
Accounts payable | $ | 12,232,906 |
|
| $ | 5,031,706 |
|
Accrued expenses |
| 4,587,467 |
|
|
| 5,376,119 |
|
Accrued payroll and benefits |
| 3,969,706 |
|
|
| 3,824,062 |
|
Deferred rent |
| 440,580 |
|
|
| - |
|
Deferred revenue |
| 2,569,792 |
|
|
| 103,125 |
|
Derivative liabilities |
| 1,602,626 |
|
|
| 1,301,604 |
|
Capital lease obligation |
| - |
|
|
| 217,548 |
|
Note Payable |
| 194,310 |
|
|
| - |
|
Other current liabilities |
| 46,407 |
|
|
| 46,407 |
|
TOTAL CURRENT LIABILITIES |
| 25,643,794 |
|
|
| 15,900,571 |
|
LONG-TERM LIABILITIES |
|
|
|
|
|
|
|
Capital lease obligation, net of current portion |
| - |
|
|
| 540,792 |
|
Contingent consideration obligations |
| - |
|
|
| 5,862,464 |
|
Deferred rent, net of current portion |
| 2,274,997 |
|
|
| 142,453 |
|
Deferred revenue, net of current portion |
| 2,500,000 |
|
|
| - |
|
Note Payable, net of current portion |
| 2,533,455 |
|
|
| - |
|
Other non-current liabilities |
| 200,000 |
|
|
| 200,000 |
|
TOTAL LONG-TERM LIABILITIES |
| 7,508,452 |
|
|
| 6,745,709 |
|
Commitments and contingencies (Note 7) |
|
|
|
|
|
|
|
STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
|
Arrowhead Pharmaceuticals, Inc. stockholders' equity: |
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 5,000,000 shares authorized; 15,652 shares issued and outstanding as of September 30, 2016 and September 30, 2015 |
| 16 |
|
|
| 16 |
|
Common stock, $0.001 par value; 145,000,000 shares authorized; 69,746,685 and 59,544,677 shares issued and outstanding as of September 30, 2016 and September 30, 2015, respectively |
| 162,116 |
|
|
| 151,914 |
|
Additional paid-in capital |
| 493,844,909 |
|
|
| 426,873,358 |
|
Accumulated other comprehensive income (loss) |
| 7,449 |
|
|
| (136,425 | ) |
Accumulated deficit |
| (398,435,043 | ) |
|
| (316,712,041 | ) |
Total Arrowhead Pharmaceuticals, Inc. stockholders' equity |
| 95,579,447 |
|
|
| 110,176,822 |
|
Noncontrolling interest |
| (555,188 | ) |
|
| (555,188 | ) |
TOTAL STOCKHOLDERS' EQUITY |
| 95,024,259 |
|
|
| 109,621,634 |
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | $ | 128,176,505 |
|
| $ | 132,267,914 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Arrowhead Pharmaceuticals, Inc.
Consolidated Statements of Operations
|
| Year Ended September 30, |
|
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| |||
REVENUE |
| $ | 158,333 |
|
| $ | 382,000 |
|
| $ | 175,000 |
|
|
OPERATING EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 41,454,452 |
|
|
| 47,267,361 |
|
|
| 23,138,050 |
|
|
Acquired in-process research and development |
|
| - |
|
|
| 10,142,786 |
|
|
| - |
|
|
Salaries and payroll-related costs |
|
| 19,461,656 |
|
|
| 16,554,008 |
|
|
| 12,829,355 |
|
|
General and administrative expenses |
|
| 9,940,737 |
|
|
| 7,931,184 |
|
|
| 5,894,008 |
|
|
Stock-based compensation |
|
| 11,595,816 |
|
|
| 10,232,897 |
|
|
| 5,696,173 |
|
|
Depreciation and amortization |
|
| 3,260,045 |
|
|
| 2,336,207 |
|
|
| 1,345,655 |
|
|
Impairment expense |
|
| 2,050,817 |
|
|
| - |
|
|
| 2,172,387 |
|
|
Contingent consideration - fair value adjustments |
|
| (5,862,464 | ) |
|
| 1,891,533 |
|
|
| 2,375,658 |
|
|
TOTAL OPERATING EXPENSES |
|
| 81,901,059 |
|
|
| 96,355,976 |
|
|
| 53,451,286 |
|
|
OPERATING LOSS |
|
| (81,742,726 | ) |
|
| (95,973,976 | ) |
|
| (53,276,286 | ) |
|
OTHER INCOME (EXPENSE) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity in income (loss) of unconsolidated affiliates |
|
| - |
|
|
| - |
|
|
| (78,874 | ) |
|
Gain (loss) on sale of fixed assets, net |
|
| - |
|
|
| 19,195 |
|
|
| (58,878 | ) |
|
Interest income (expense), net |
|
| 265,794 |
|
|
| 729,158 |
|
|
| 645,493 |
|
|
Change in value of derivatives |
|
| (301,022 | ) |
|
| 2,869,267 |
|
|
| (6,033,659 | ) |
|
Other income (expense) |
|
| 57,352 |
|
|
| 417,874 |
|
|
| 82,092 |
|
|
TOTAL OTHER INCOME (EXPENSE) |
|
| 22,124 |
|
|
| 4,035,494 |
|
|
| (5,443,826 | ) |
|
LOSS BEFORE INCOME TAXES |
|
| (81,720,602 | ) |
|
| (91,938,482 | ) |
|
| (58,720,112 | ) |
|
Provision for income taxes |
|
| (2,400 | ) |
|
| (2,400 | ) |
|
| (5,300 | ) |
|
NET LOSS |
|
| (81,723,002 | ) |
|
| (91,940,882 | ) |
|
| (58,725,412 | ) |
|
Net loss attributable to non-controlling interests |
|
| - |
|
|
| - |
|
|
| 95,222 |
|
|
NET LOSS ATTRIBUTABLE TO ARROWHEAD |
| $ | (81,723,002 | ) |
| $ | (91,940,882 | ) |
| $ | (58,630,190 | ) |
|
NET LOSS PER SHARE ATTRIBUTABLE TO ARROWHEAD SHAREHOLDERS - BASIC & DILUTED |
| $ | (1.34 | ) |
| $ | (1.60 | ) |
| $ | (1.25 | ) |
|
Weighted average shares outstanding - basic and diluted |
|
| 61,050,880 |
|
|
| 57,358,442 |
|
|
| 46,933,030 |
|
|
OTHER COMPREHENSIVE INCOME (LOSS), NET OF TAX: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign Currency Translation Adjustments |
|
| 143,874 |
|
|
| (136,425 | ) |
|
| - |
|
|
COMPREHENSIVE LOSS
|
| $ | (81,579,128 | ) |
| $ | (92,077,307 | ) |
| $ | (58,630,190 | ) |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Arrowhead Pharmaceuticals, Inc.
Consolidated Statement of Stockholders' Equity
| Preferred Stock |
|
| Amount ($) |
|
| Common Stock |
|
| Amount ($) |
|
| Additional Paid-In Capital |
|
| Accumulated Other Comprehensive Income (loss) |
|
| Accumulated Deficit |
|
| Non-controlling Interest |
|
| Totals |
| |||||||||
Balance at September 30, 2013 |
| 9,900 |
|
| $ | 10 |
|
|
| 32,489,444 |
|
| $ | 124,859 |
|
| $ | 193,514,766 |
|
| $ | - |
|
| $ | (166,140,969 | ) |
| $ | (1,763,877 | ) |
| $ | 25,734,789 |
|
Exercise of warrants |
| - |
|
|
| - |
|
|
| 2,911,919 |
|
|
| 2,911 |
|
|
| 10,145,133 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 10,148,044 |
|
Exercise of stock options |
| - |
|
|
| - |
|
|
| 454,863 |
|
|
| 455 |
|
|
| 2,729,545 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 2,730,000 |
|
Stock-based compensation |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 5,696,173 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 5,696,173 |
|
Common stock issued for cash at $5.86, net of offering costs |
| - |
|
|
| - |
|
|
| 3,071,672 |
|
|
| 3,072 |
|
|
| 14,057,040 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 14,060,112 |
|
Common stock issued for cash at $18.95, net of offering costs |
| - |
|
|
| - |
|
|
| 6,325,000 |
|
|
| 6,325 |
|
|
| 112,575,234 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 112,581,559 |
|
Preferred stock issued for cash at $1,000 per share |
| 46,000 |
|
|
| 46 |
|
|
| - |
|
|
| - |
|
|
| 45,999,954 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 46,000,000 |
|
Common stock issued to Galloway |
| - |
|
|
| - |
|
|
| 131,579 |
|
|
| 132 |
|
|
| 499,868 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 500,000 |
|
Settlements related to derivative liability |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 5,956,079 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 5,956,079 |
|
Preferred stock converted to common stock |
| (37,600 | ) |
|
| (38 | ) |
|
| 9,272,459 |
|
|
| 9,272 |
|
|
| (9,234 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
Deconsolidation of Calando Pharmaceuticals, Inc. |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 1,303,911 |
|
|
| 1,303,911 |
|
Net loss for the year ended September 30, 2014 |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (58,630,190 | ) |
|
| (95,222 | ) |
|
| (58,725,412 | ) |
Balance at September 30, 2014 |
| 18,300 |
|
| $ | 18 |
|
|
| 54,656,936 |
|
| $ | 147,026 |
|
| $ | 391,164,558 |
|
| $ | - |
|
| $ | (224,771,159 | ) |
| $ | (555,188 | ) |
| $ | 165,985,255 |
|
Exercise of warrants |
| - |
|
|
| - |
|
|
| 79,828 |
|
|
| 81 |
|
|
| 401,795 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 401,876 |
|
Exercise of stock options |
| - |
|
|
| - |
|
|
| 28,758 |
|
|
| 29 |
|
|
| 101,841 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 101,870 |
|
Stock-based compensation |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 10,232,897 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 10,232,897 |
|
Exercise of exchange rights |
| - |
|
|
| - |
|
|
| 5,250 |
|
|
| 5 |
|
|
| 3,067 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 3,072 |
|
Preferred stock converted to common stock |
| (2,648 | ) |
|
| (2 | ) |
|
| 1,316,215 |
|
|
| 1,316 |
|
|
| (1,314 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
Common stock- Restricted Stock Units vesting |
| - |
|
|
| - |
|
|
| 136,307 |
|
|
| 136 |
|
|
| (26,165 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (26,029 | ) |
Common stock issued to Novartis at $7.53 |
| - |
|
|
| - |
|
|
| 3,321,383 |
|
|
| 3,321 |
|
|
| 24,996,679 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 25,000,000 |
|
Foreign currency translation adjustments |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (136,425 | ) |
|
| - |
|
|
| - |
|
|
| (136,425 | ) |
Net loss for the year ended September 30, 2015 |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (91,940,882 | ) |
|
| - |
|
|
| (91,940,882 | ) |
Balance at September 30, 2015 |
| 15,652 |
|
| $ | 16 |
|
|
| 59,544,677 |
|
| $ | 151,914 |
|
| $ | 426,873,358 |
|
| $ | (136,425 | ) |
| $ | (316,712,041 | ) |
| $ | (555,188 | ) |
| $ | 109,621,634 |
|
Exercise of warrants |
| - |
|
|
| - |
|
|
| 852,532 |
|
|
| 853 |
|
|
| 3,690,545 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 3,691,398 |
|
Exercise of stock options |
| - |
|
|
| - |
|
|
| 37,187 |
|
|
| 37 |
|
|
| 133,832 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 133,869 |
|
Stock-based compensation |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 11,595,816 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 11,595,816 |
|
Common stock- Restricted Stock Units vesting |
| - |
|
|
| - |
|
|
| 428,187 |
|
|
| 428 |
|
|
| (671,193 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (670,765 | ) |
Common stock issued for cash at $5.90 per share, net of offering costs |
| - |
|
|
| - |
|
|
| 7,627,119 |
|
|
| 7,627 |
|
|
| 43,223,808 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 43,231,435 |
|
Common stock issued to Amgen at $7.16 per share |
| - |
|
|
| - |
|
|
| 1,256,983 |
|
|
| 1,257 |
|
|
| 8,998,743 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 9,000,000 |
|
Foreign currency translation adjustments |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 143,874 |
|
|
| - |
|
|
| - |
|
|
| 143,874 |
|
Net loss for the year ended September 30, 2016 |
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (81,723,002 | ) |
|
| - |
|
|
| (81,723,002 | ) |
Balance at September 30, 2016 |
| 15,652 |
|
| $ | 16 |
|
|
| 69,746,685 |
|
| $ | 162,116 |
|
| $ | 493,844,909 |
|
| $ | 7,449 |
|
| $ | (398,435,043 | ) |
| $ | (555,188 | ) |
| $ | 95,024,259 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Arrowhead Pharmaceuticals, Inc.
Consolidated Statements of Cash Flows
| Year ended September 30, |
| |||||||||
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
Net loss | $ | (81,723,002 | ) |
| $ | (91,940,882 | ) |
| $ | (58,725,412 | ) |
Net loss attributable to non-controlling interests |
| - |
|
|
| - |
|
|
| 95,222 |
|
Net loss attributable to Arrowhead |
| (81,723,002 | ) |
|
| (91,940,882 | ) |
|
| (58,630,190 | ) |
(Gain) loss on disposal of fixed assets |
| - |
|
|
| (19,195 | ) |
|
| 58,878 |
|
Change in value of derivatives |
| 301,022 |
|
|
| (2,869,267 | ) |
|
| 6,033,659 |
|
Contingent consideration - fair value adjustments |
| (5,862,464 | ) |
|
| 1,891,533 |
|
|
| 2,375,658 |
|
Noncash impairment expense |
| 2,050,817 |
|
|
| - |
|
|
| 2,172,387 |
|
Acquired-in-process research and development |
| - |
|
|
| 10,142,786 |
|
|
| - |
|
Stock-based compensation |
| 11,595,816 |
|
|
| 10,232,897 |
|
|
| 5,696,173 |
|
Depreciation and amortization |
| 3,260,045 |
|
|
| 2,336,207 |
|
|
| 1,345,655 |
|
Amortization of note premiums |
| 231,902 |
|
|
| 1,110,524 |
|
|
| 793,887 |
|
Gain on debt extinguishment |
| - |
|
|
| - |
|
|
| (84,721 | ) |
Noncash gain on equity investment |
| - |
|
|
| - |
|
|
| (87,197 | ) |
Non-controlling interest |
| - |
|
|
| - |
|
|
| (95,222 | ) |
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
| (75,000 | ) |
|
| - |
|
|
| 75,000 |
|
Prepaid expenses and Other Current Assets |
| (1,020,734 | ) |
|
| (3,485,421 | ) |
|
| (54,966 | ) |
Deferred revenue |
| 5,000,000 |
|
|
| - |
|
|
| - |
|
Accounts payable |
| 2,554,802 |
|
|
| 2,497,804 |
|
|
| 1,412,275 |
|
Accrued expenses |
| (871,833 | ) |
|
| 4,435,784 |
|
|
| 3,478,094 |
|
Other |
| 131,143 |
|
|
| (40,385 | ) |
|
| 94,257 |
|
NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES |
| (64,427,486 | ) |
|
| (65,707,615 | ) |
|
| (35,416,373 | ) |
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
| (3,860,237 | ) |
|
| (1,970,612 | ) |
|
| (1,717,362 | ) |
Proceeds from sale of fixed assets |
| - |
|
|
| 500 |
|
|
| 10,000 |
|
Purchase of marketable securities |
| - |
|
|
| - |
|
|
| (46,365,528 | ) |
Proceeds from sale of marketable securities |
| 17,308,000 |
|
|
| 26,090,950 |
|
|
| 11,591,120 |
|
Cash paid for acquisitions |
| - |
|
|
| (10,000,000 | ) |
|
| - |
|
NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES |
| 13,447,763 |
|
|
| 14,120,838 |
|
|
| (36,481,770 | ) |
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
Principal payments on capital leases and notes payable |
| (217,549 | ) |
|
| (213,991 | ) |
|
| (225,406 | ) |
Payments of taxes for net share settled restricted stock unit issuances |
| (634,187 | ) |
|
| - |
|
|
| - |
|
Proceeds from the issuance of common stock |
| 52,231,433 |
|
|
| - |
|
|
| 172,641,671 |
|
Proceeds from the exercise of warrants and stock options |
| 3,752,120 |
|
|
| 504,512 |
|
|
| 12,878,044 |
|
NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES |
| 55,131,817 |
|
|
| 290,521 |
|
|
| 185,294,309 |
|
NET INCREASE (DECREASE) IN CASH |
| 4,152,094 |
|
|
| (51,296,256 | ) |
|
| 113,396,166 |
|
CASH AT BEGINNING OF PERIOD |
| 81,214,354 |
|
|
| 132,510,610 |
|
|
| 19,114,444 |
|
CASH AT END OF PERIOD | $ | 85,366,448 |
|
| $ | 81,214,354 |
|
| $ | 132,510,610 |
|
Supplementary disclosures: |
|
|
|
|
|
|
|
|
|
|
|
Interest paid | $ | (11,287 | ) |
| $ | (14,429 | ) |
| $ | (25,635 | ) |
Property and Equipment purchased through tenant improvement allowance financing | $ | (4,849,360 | ) |
| $ | - |
|
| $ | - |
|
Property and Equipment expenditures included in accounts payable and accrued expenses | $ | (4,801,930 | ) |
| $ | - |
|
| $ | - |
|
Income Tax Credits Refunded | $ | 1,365,288 |
|
| $ | - |
|
| $ | - |
|
Income Taxes Paid | $ | (2,400 | ) |
| $ | (2,400 | ) |
| $ | (5,862 | ) |
Common stock issued to Galloway Limited in settlement of services agreement | $ | - |
|
| $ | - |
|
| $ | (500,000 | ) |
Common Stock issued to Novartis for asset acquisition | $ | - |
|
| $ | (25,000,000 | ) |
| $ | - |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Arrowhead Pharmaceuticals, Inc.
Notes to Consolidated Financial Statements
Unless otherwise noted, (1) the term "Arrowhead" refers to Arrowhead Pharmaceuticals, Inc., a Delaware corporation formerly known as Arrowhead Research Corporation and its Subsidiaries, (2) the terms "Company," "we," "us," and "our," refer to the ongoing business operations of Arrowhead and its Subsidiaries, whether conducted through Arrowhead or a subsidiary of Arrowhead, (3) the term "Subsidiaries" refers collectively to Arrowhead Madison Inc. ("Arrowhead Madison"), Arrowhead Australia Pty Ltd ("Arrowhead Australia") and Ablaris Therapeutics, Inc. ("Ablaris"), (4) the term "Common Stock" refers to Arrowhead's Common Stock, (5) the term "Preferred Stock" refers to Arrowhead's Preferred Stock and (6) the term "Stockholder(s)" refers to the holders of Arrowhead Common Stock.
NOTE 1. ORGANIZATION AND SIGNIFICANT ACCOUNTING POLICIES
Nature of Business and Recent Developments
Arrowhead develops novel drugs to treat intractable diseases by silencing the genes that cause them. Using a broad portfolio of RNA chemistries and efficient modes of delivery, Arrowhead therapies trigger the RNA interference mechanism to induce rapid, deep and durable knockdown of target genes. RNA interference (RNAi) is a mechanism present in living cells that inhibits the expression of a specific gene, thereby affecting the production of a specific protein. Arrowhead's RNAi-based therapeutics leverage this natural pathway of gene silencing. The company's pipeline includes ARO-HBV for chronic hepatitis B virus, ARO-AAT for liver disease associated with alpha-1 antitrypsin deficiency (AATD), ARO-F12 for hereditary angioedema and thromboembolic disorders, ARO-HIF2 for renal cell carcinoma, and ARO-AMG1 for an undisclosed genetically validate cardiovascular target under a license and collaboration agreement with Amgen, Inc., a Delaware corporation ("Amgen"). ARO-LPA for cardiovascular disease was recently out-licensed to Amgen.
In April 2016, the Company changed its name from Arrowhead Research Corporation to Arrowhead Pharmaceuticals, Inc., to better reflect the Company's focus on advancing products through clinical development to bring innovative new medicines to patients.
During fiscal year 2016, the Company continued to develop its clinical candidates, ARC-520 and ARC-521, for the treatment of chronic hepatitis B infection as well as its second clinical candidate, ARC-AAT, an RNAi therapeutic designed to treat liver disease associated with AATD. However, in November 2016, the Company announced that it would be discontinuing these clinical programs, and redeploying its resources and focus toward utilizing the Company's new proprietary subcutaneous and extra-hepatic delivery systems. Each of these clinical candidates utilized the intravenously administered DPC iv , or EX1, delivery vehicle. The decision to discontinue development of EX1-containing programs was based primarily on two factors. First, during ongoing discussions with regulatory agencies and outside experts, it became apparent that there would be substantial delays in all clinical programs that utilize EX1, while the Company further explored the cause of deaths in a non-clinical toxicology study in non-human primates. Second, the Company has made substantial advances in RNA chemistry and targeting resulting in large potency gains for subcutaneous administered and extra-hepatic RNAi-based development programs. In preclinical studies with the subcutaneous platform, the Company has obtained depth and duration of target gene knockdown approaching that of intravenously administered EX1-containing candidates, at lower doses and with good safety margins. ARO-HBV and ARO-AAT are the Company's subcutaneous administered preclinical candidates for chronic hepatitis B virus and liver disease associated with AATD, respectively. Because of the discontinuation of its existing clinical programs, the Company has also reduced its workforce by approximately 30%, while maintaining resourcing necessary to support current and potential future partner-based programs and the Company's pipeline.
Liquidity
The Consolidated Financial Statements have been prepared in conformity with the accounting principles generally accepted in the United States of America, which contemplate the continuation of the Company as a going concern. Historically, the Company's primary source of financing has been through the sale of its securities. Research and development activities have required significant capital investment since the Company's inception. The Company expects its operations to continue to require cash investment to pursue its research and development goals, including clinical trials and related drug manufacturing.
At September 30, 2016, the Company had $85.4 million in cash to fund operations. During the year ended September 30, 2016, the Company's cash position increased by $ 4.2 million, which was primarily the result of net proceeds of $43.2 million related to the issuance of Common Stock as part of its August 2016 equity financing, $17.3 million of maturities of fixed income investments, and $9 million related to the issuance of Common Stock as part of the Company's Common Stock Purchase Agreement with Amgen. The August 2016 equity financing and the Common Stock Purchase Agreement with Amgen, Inc. are discussed in further detail in Note 6 and Note 2, respectively. These inflows were partially offset by cash outflows related to operating activities of $64.4 million.
F-8
On November 18, 2016, the Company and Amgen received Hart-Scott-Rodino clearance with regard to the Second Collaboration and Lic ense Agreement discussed in Note 2 below. Based on the terms of this agreement, and the Common Stock Purchase Agreement, the Company issued 1,745,810 shares of Common Stock to Amgen, and received proceeds of approximately $12.5 million. Additionally, the Company received a $30 million upfront payment due under the Second Collaboration and License Agreement discussed above.
Summary of Significant Accounting Policies
Principles of Consolidation-The consolidated financial statements include the accounts of Arrowhead and its Subsidiaries. Arrowhead's primary operating subsidiary is Arrowhead Madison, which is located in Madison, Wisconsin, where the Company's research and development facility is located. All significant intercompany accounts and transactions are eliminated in consolidation.
Basis of Presentation and Use of Estimates-The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could materially differ from those estimates. Additionally, certain reclassifications have been made to prior period financial statements to conform to the current period presentation.
Cash and Cash Equivalents-The Company considers all liquid debt instruments purchased with a maturity of three months or less to be cash equivalents. The Company had no restricted cash at September 30, 2016 and September 30, 2015.
Concentration of Credit Risk-The Company maintains several bank accounts at two financial institutions for its operations. These accounts are insured by the Federal Deposit Insurance Corporation (FDIC) for up to $250,000 per institution. Management believes the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which these deposits are held.
Investments-The Company may invest excess cash balances in short-term and long-term marketable debt securities. Investments may consist of certificates of deposits, money market accounts, government-sponsored enterprise securities, corporate bonds and/or commercial paper. The Company accounts for its investment in marketable securities in accordance with FASB ASC 320, Investments – Debt and Equity Securities. This statement requires certain securities to be classified into three categories:
Held-to-maturity-Debt securities that the entity has the positive intent and ability to hold to maturity are reported at amortized cost.
Trading Securities-Debt and equity securities that are bought and held primarily for the purpose of selling in the near term are reported at fair value, with unrealized gains and losses included in earnings.
Available-for-Sale-Debt and equity securities not classified as either securities held-to-maturity or trading securities are reported at fair value with unrealized gains or losses excluded from earnings and reported as a separate component of shareholders' equity.
The Company classifies its investments in marketable debt securities based on the facts and circumstances present at the time of purchase of the securities. During the year ended September 30, 2016, all of the Company's investments were classified as held-to-maturity, and they all matured during the period.
Held-to-maturity investments are measured and recorded at amortized cost on the Company's Consolidated Balance Sheet. Discounts and premiums to par value of the debt securities are amortized to interest income/expense over the term of the security. No gains or losses on investment securities are realized until they are sold or a decline in fair value is determined to be other-than-temporary.
Property and Equipment-Property and equipment are recorded at cost, which may equal fair market value in the case of property and equipment acquired in conjunction with a business acquisition. Depreciation of property and equipment is recorded using the straight-line method over the respective useful lives of the assets ranging from three to seven years. Leasehold improvements are amortized over the lesser of the expected useful life or the remaining lease term. Long-lived assets, including property and equipment are reviewed for impairment whenever events or circumstances indicate that the carrying amount of these assets may not be recoverable.
Intangible Assets Subject to Amortization-Intangible assets subject to amortization include certain patents and license agreements. Intangible assets subject to amortization are reviewed for impairment whenever events or circumstances indicate that the carrying amount of these assets may not be recoverable.
F-9
In-Process Research & Development (IPR&D)-IPR&D assets represent capitalized on-going research projects that were acquired through business combinations. Such assets are initi ally measured at their acquisition date fair values. The amounts capitalized are being accounted for as indefinite-lived intangible assets, subject to impairment testing until completion or abandonment of R&D efforts associated with the project. Upon succe ssful completion of a project, Arrowhead will make a determination as to the then remaining useful life of the intangible asset and begin amortization. Arrowhead tests its indefinite-lived assets for impairment at least annually, through a two-step process . The first step is a qualitative assessment to determine if it is more likely than not that the indefinite lived assets are impaired. Arrowhead considers relevant events and circumstances that could affect the inputs used to determine the fair value of th e intangible assets. If the qualitative assessment indicates that it is more likely than not that the intangible assets are impaired, a second step is performed which is a quantitative test to determine the fair value of the intangible asset. If the carryi ng amount of the intangible assets exceeds its fair value, an impairment loss is recorded in the amount of that excess. If circumstances determine that it is appropriate, the Company may also elect to bypass step one, and proceed directly to the second ste p.
Contingent Consideration - The consideration for the Company's acquisitions often includes future payments that are contingent upon the occurrence of a particular event. For example, milestone payments might be based on the achievement of various regulatory approvals or future sales milestones, and royalty payments might be based on drug product sales levels. The Company records a contingent consideration obligation for such contingent payments at fair value on the acquisition date. The Company estimates the fair value of contingent consideration obligations through valuation models designed to estimate the probability of such contingent payments based on various assumptions and incorporating estimated success rates. Estimated payments are discounted using present value techniques to arrive at an estimated fair value at the balance sheet date. Changes in the fair value of the contingent consideration obligations are recognized within the Company's Consolidated Statements of Operations and Comprehensive Loss. Changes in the fair value of the contingent consideration obligations can result from changes to one or multiple inputs, including adjustments to the discount rates, changes in the amount or timing of expected expenditures associated with product development, changes in the amount or timing of cash flows from products upon commercialization, changes in the assumed achievement or timing of any development milestones, changes in the probability of certain clinical events and changes in the assumed probability associated with regulatory approval. These fair value measurements are based on significant inputs not observable in the market. Substantial judgment is employed in determining the appropriateness of these assumptions as of the acquisition date and for each subsequent period. Accordingly, changes in assumptions could have a material impact on the amount of contingent consideration expense the Company records in any given period.
Revenue Recognition- Revenue from product sales is recorded when persuasive evidence of an arrangement exists, title has passed and delivery has occurred, a price is fixed and determinable, and collection is reasonably assured.
The Company may generate revenue from technology licenses, collaborative research and development arrangements, research grants and product sales. Revenue under technology licenses and collaborative agreements typically consists of nonrefundable and/or guaranteed technology license fees, collaborative research funding, manufacturing and development services and various milestone and future product royalty or profit-sharing payments. These agreements are generally referred to as multiple element arrangements.
The Company applies the accounting standard on revenue recognition for multiple element arrangements. The fair value of deliverables under the arrangement may be derived using a best estimate of selling price if vendor specific objective evidence and third-party evidence is not available. Deliverables under the arrangement will be separate units of accounting if a delivered item has value to the customer on a standalone basis, if the arrangement includes a general right of return for the delivered item, and if delivery or performance of the undelivered item is considered probable and substantially in the control of the vendor.
The Company recognizes upfront license payments as revenue upon delivery of the license only if the license has standalone value from any undelivered performance obligations and that value can be determined. The undelivered performance obligations typically include manufacturing or development services or research and/or steering committee services. If the fair value of the undelivered performance obligations can be determined, then these obligations would be accounted for separately. If the license is not considered to have standalone value, then the license and other undelivered performance obligations would be accounted for as a single unit of accounting. In this case, the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed or deferred indefinitely until the undelivered performance obligation is determined.
Whenever the Company determines that an arrangement should be accounted for as a single unit of accounting, the Company determines the period over which the performance obligations will be performed and revenue will be recognized. Revenue is recognized using a proportional performance or straight-line method. The proportional performance method is used when the level of effort required to complete performance obligations under an arrangement can be reasonably estimated. The amount of revenue recognized under the proportional performance method is determined by multiplying the total payments under the contract, excluding royalties and payments contingent upon achievement of milestones, by the ratio of the level of effort performed to date to the estimated total level of effort required to complete performance obligations under the arrangement. If the Company cannot reasonably
F-10
estimate the level of effort to complete performance obligations under an arrangement, the Comp any recognizes revenue under the arrangement on a straight-line basis over the period the Company is expected to complete its performance obligations. Under either method, r evenue is limited to the lesser of the cumulative amount of payments received or th e cumul ative amount of revenue earned , as calculated under either method, as of the period ending date. Significant management judgment is required in determining the level of effort required under an arrangement and the period over which the Company is ex pected to complete its performance obligations under an arrangement.
Many of the Company's collaboration agreements entitle the Company to additional payments upon the achievement of development, regulatory and sales performance-based milestones. If the achievement of a milestone is considered probable at the inception of the collaboration, the related milestone payment is included with other collaboration consideration, such as upfront fees and research funding, in the Company's revenue calculation. Typically these milestones are not considered probable at the inception of the collaboration. As such, milestones will typically be recognized in one of two ways depending on the timing of when the milestone is achieved. If the milestone is achieved during the performance period, the Company will only recognize revenue to the extent of the proportional performance achieved at that date, or the proportion of the straight-line basis achieved at that date, and the remainder will be recorded as deferred revenue to be amortized over the remaining performance period. If the milestone is achieved after the performance period has completed and all performance obligations have been delivered, the Company will recognize the milestone payment as revenue in its entirety in the period the milestone was achieved.
Deferred revenue will be classified as part of Current or Long-Term Liabilities in the accompanying Consolidated Balance Sheets based on the Company's estimate of the portion of the performance obligations regarding that revenue will be completed within the next 12 months divided by the total performance period estimate. This estimate is based on the Company's current operating plan and, if the Company's operating plan should change in the future, the Company may recognize a different amount of deferred revenue over the next 12-month period.
Allowance for Doubtful Accounts-The Company accrues an allowance for doubtful accounts based on estimates of uncollectible revenues by analyzing historical collections, accounts receivable aging and other factors. Accounts receivable are written off when all collection attempts have failed.
Research and Development-Costs and expenses that can be clearly identified as research and development are charged to expense as incurred in accordance with FASB ASC 730-10. Included in research and development costs are operating costs, facilities, supplies, external services, clinical trial and manufacturing costs, overhead directly related to the Company's research and development operations, and costs to acquire technology licenses.
Earnings (Loss) per Share-Basic earnings (loss) per share is computed using the weighted-average number of common shares outstanding during the period. Diluted earnings (loss) per share are computed using the weighted-average number of common shares and dilutive potential common shares outstanding during the period. Dilutive potential common shares primarily consist of stock options and restricted stock units issued to employees and warrants to purchase Common Stock of the Company. All outstanding stock options, restricted stock units and warrants for the years ended September 30, 2016 and 2015 have been excluded from the calculation of Diluted earnings (loss) per share due to their anti-dilutive effect.
Stock-Based Compensation-The Company accounts for share-based compensation arrangements in accordance with FASB ASC 718, which requires the measurement and recognition of compensation expense for all share-based payment awards to be based on estimated fair values. The Company uses the Black-Scholes option valuation model to estimate the fair value of its stock options at the date of grant. The Black-Scholes option valuation model requires the input of subjective assumptions to calculate the value of stock options. For restricted stock units, the value of the award is based on the Company's stock price at the grant date. For performance-based restricted stock unit awards, the value of the award is based on the Company's stock price at the grant date, with consideration given to the probability of the performance condition being achieved. The Company uses historical data and other information to estimate the expected price volatility for stock option awards and the expected forfeiture rate for all awards. Expense is recognized over the vesting period for all awards, and commences at the grant date for time-based awards and upon the Company's determination that the achievement of such performance conditions is probable for performance-based awards. This determination requires significant judgment by management.
F-11
Derivative Assets and Liabilities – The Company accounts for war rants and other derivative financial instruments as either equity or assets/liabilities based upon the characteristics and provisions of each instrument. Warrants classi fied as equity are recorded as Additional Paid-In C apital on the Company's Consolidated Balance Sheet. Some of the Company's warrants were determined to be ineligible for equity classification due to provisions that may result in an adjustment to their exercise price. Warrants classified as derivative liabilities and other derivative financi al instruments that require separate accounting as assets or liabilities are recorded on the Company's Consolidated Balance Sheet at their fair value on the date of issuance and are revalued on each subsequent balance sheet date until such instruments are exercised or expire, with any changes in the fair value between reporting periods recorded as Other Income or E xpense. The Company estimates the fair value of these assets/liabilities using option pricing models that are based on the individual characteris tics of the warrants or instruments on the valuation date, as well as assumptions for expected volatility, expected life and risk-free interest rate.
Income Taxes-The Company accounts for income taxes under the liability method, which requires the recognition of deferred income tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred income tax assets to the amount expected to be realized. The provision for income taxes, if any, represents the tax payable for the period and the change in deferred income tax assets and liabilities during the period.
Recent Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09 Revenue from Contracts with Customers (Topic 606), which will supersede nearly all existing revenue recognition guidance under GAAP. ASU No. 2014-09 provides that an entity recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This update also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments, and assets recognized from costs incurred to obtain or fulfill a contract. ASU No. 2014-09 allows for either full retrospective or modified retrospective adoption and will become effective for the Company in the first quarter of 2018. In April 2016, the FASB issued an amendment to ASU No. 2014-09 with update ASU 2016-10 which provided more specific guidance around the idenitification of performance obligations and licensing arrangements. The Company is evaluating the potential effects of the adoption of this update on its financial statements.
In March 2016, the FASB issued ASU No. 2016-02, Leases. Under ASU 2016-02, lessees will be required to recognize a right-of-use asset and a lease liability for virtually all of their leases (other than leases that meet the definition of a short-term lease). For income statement purposes, a dual model was retained, requiring leases to be classified as either operating or finance. Operating leases will result in straight-line expense (similar to current operating leases) while finance leases will result in a front-loaded expense pattern (similar to current capital leases). ASU 2016-02 becomes effective for the Company in the first quarter of fiscal 2020. The Company expects the adoption of this update to have a material effect on the classification and disclosure of its leased facilities in Madison, Wisconsin.
In March 2016, the FASB issued ASU No. 2016-09, Compensation - Stock Compensation: Improvements to Employee Share-Based Payment Accounting. ASU 2016-09 eliminates additional paid in capital ("APIC") pools and requires excess tax benefits and tax deficiencies to be recorded in the income statement when the awards vest or are settled. The accounting for an employee's use of shares to satisfy the employer's statutory income tax withholding obligation and the accounting for forfeitures is also changing. ASU 2016-09 becomes effective for the Company in the first quarter of 2018. The Company early adopted ASU 2016-09 during the three months ended March 31, 2016, and the adoption of this update is not expected to have a material effect on its Consolidated Financial Statements.
In August 2016, the FASB issued ASU No. 2016-15 , Classification of Certain Cash Receipts and Cash Payments. This ASU amends Accounting Standards Codification ("ASC") 230 and is intended to provide guidance and clarification in regards to the classification of eight types of receipts and payments in the statement of cash flows, including debt repayment or extinguishment costs, settlement of zero-coupon bonds, proceeds from the settlement of insurance claims, distributions received from equity method investees and cash receipts from beneficial interest in securitization transactions. The guidance will be effective for the Company as of October 1, 2018, and the adoption of this update is not expected to have a material effect on its Consolidated Financial Statements.
F-12
NOTE 2. COLLABORATION AND LICENSE AGREEMENTS – AMGEN, INC.
On September 28, 2016, the Company entered into two Collaboration and License agreements, and a Common Stock Purchase Agreement with Amgen Inc., a Delaware corporation ("Amgen"). Under one of the license agreements (the "Second Collaboration and License Agreement"), Amgen will receive a worldwide, exclusive license to Arrowhead's novel, RNAi ARO-LPA program. These RNAi molecules are designed to reduce elevated lipoprotein(a), which is a genetically validated, independent risk factor for atherosclerotic cardiovascular disease. Under the other license agreement (the "First Collaboration and License Agreement"), Amgen received an option to a worldwide, exclusive license for ARO-AMG1, an RNAi therapy for an undisclosed genetically validated cardiovascular target. In both agreements, Amgen will be wholly responsible for clinical development and commercialization.
Under the Common Stock Purchase Agreement, the Company has sold 3,002,793 shares of Common Stock to Amgen at a price of $7.16 per share, which represents the 30-day volume-weighted average price of the Common Stock on the NASDAQ stock market over the 30 trading days preceding execution. The Common Stock was delivered in two closings per the terms of the agreement. The first tranche of 1,256,983 shares of Common Stock was issued on September 29, 2016, and the Company received proceeds of approximately $9 million. The second tranche of 1,745,810 shares was subject to Hart-Scott-Rodino clearance which was reached on November 18, 2016. The Company received proceeds of approximately $12.5 million from this tranche of shares. Subject to Amgen's exercise of the Option, as defined in the First Collaboration and License Agreement, Amgen has agreed to purchase, and the Company has agreed to sell, an additional $5 million worth of shares of Common Stock based on a 30 trading day formula surrounding the date of the Option exercise.
Under the terms of the agreements taken together, the Company will receive $35 million in upfront payments, $21.5 million in the form of an equity investment by Amgen in the Company's Common Stock, and up to $617 million in option payments, and development, regulatory and sales milestone payments. The Company is further eligible to receive single-digit royalties for sales of products under the ARO-AMG1 agreement and up to low double-digit royalties for sales of products under the ARO-LPA agreement.
Under the terms of the First Collaboration and License Agreement, the Company is granting a worldwide, exclusive license to ARO-AMG1, an undisclosed genetically validated cardiovascular target. The collaboration between the Company and Amgen is governed by a joint steering committee comprised of an equal number of representatives from each party. The Company is also responsible for developing, optimizing and manufacturing the candidate through certain preclinical efficacy and toxicology studies to determine whether the candidate the Company has developed meets the required criteria as defined in the agreement (the "Arrowhead Deliverable"). If this is achieved, Amgen will then have the option to an exclusive license for the intellectual property generated through the Company's development efforts, and will likely assume all development, regulatory and commercialization efforts for the candidate upon the option exercise. The Company has determined that the significant deliverables under the First Collaboration and License Agreement include the license, the joint research committee and the development and manufacturing activities toward achieving the Arrowhead Deliverable. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and collective undelivered activities and services do not have standalone value due to the specialized nature of the activities and services to be provided by the Company. Therefore, the deliverables are not separable and, accordingly, the license and undelivered services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, the Company bases its revenue recognition pattern on the final deliverable. The Company will recognize revenue on a straight-line basis from October 1, 2016, through September 30, 2018. The due date for achieving the Arrowhead Deliverable, as defined in the agreement, is September 28, 2018. The Company received the upfront payment of $5 million due under this agreement in September 2016. It has been be initially recorded as deferred revenue in the Company's Consolidated Balance Sheets, and will be amortized over the period discussed above.
Under the terms of the Second Collaboration and License Agreement, the Company is granting a worldwide, exclusive license to ARO-LPA. The collaboration between the Company and Amgen is governed by a joint research committee comprised of an equal number of representatives from each party, however Amgen has the final decision making authority regarding ARO-LPA in this committee. The Company is also responsible for assisting Amgen in the oversight of certain development and manufacturing activities, most of which are to be covered at Amgen's cost. The Company has determined that the significant deliverables under the Second Collaboration and License Agreement include the license and the oversight of certain of the development and manufacturing activities. The Company also determined that, pursuant to the accounting guidance governing revenue recognition on multiple element arrangements, the license and collective undelivered activities and services do not have standalone value due to the specialized nature of the activities and services to be provided by the Company. Therefore, the deliverables are not separable and, accordingly, the license and undelivered services are being treated as a single unit of accounting. When multiple deliverables are accounted for as a single unit of accounting, the Company bases its revenue recognition pattern on the final deliverable. The Company will recognize revenue on a straight-line basis from November 18, 2016 (the Hart-Scott-Rodino clearance date), through October 31, 2017, which is the date where the significant development and manufacturing related deliverables are anticipated to be completed. The Company received the upfront payment of $30 million due under this agreement in November 2016. It will be initially recorded as deferred revenue, and amortized over the period discussed above.
F-13
NOTE 3. PROPERTY AND EQUIPMENT
The following table summarizes the Company's major classes of property and equipment:
|
|
| |||||
| September 30, 2016 |
|
| September 30, 2015 |
| ||
Computers, office equipment and furniture | $ | 442,915 |
|
| $ | 404,964 |
|
Research equipment |
| 7,490,400 |
|
|
| 6,354,584 |
|
Software |
| 80,841 |
|
|
| 110,428 |
|
Leasehold improvements |
| 11,885,365 |
|
|
| 3,117,537 |
|
Total gross fixed assets |
| 19,899,521 |
|
|
| 9,987,513 |
|
Less: Accumulated depreciation and amortization |
| (4,512,760 | ) |
|
| (5,460,665 | ) |
Property and equipment, net | $ | 15,386,761 |
|
| $ | 4,526,848 |
|
During the year ended September 30, 2016, the Company's leasehold improvements increased as the Company has moved into a larger research facility in Madison, Wisconsin. The lease terms of this facility are discussed in Note 7 – Commitments and Contingencies . Additionally, during the year ended September 30, 2016, the Company recognized a $1.1 million impairment expense related to leasehold improvements at its previous research facility in Madison, Wisconsin. This amount represented the entire net book value remaining for the leasehold improvements associated with the previous facility, and was recognized during the year ended September 30, 2016.
NOTE 4. INVESTMENTS
The Company had invested a portion of its excess cash balances in short-term and long-term debt securities. During the year ended September 30, 2016, the Company's investments all matured, and the Company has not invested any excess cash at September 30, 2016. The Company may also invest excess cash balances in certificates of deposit, money market accounts, U.S. Treasuries, U.S. government agency obligations, corporate debt securities, and/or commercial paper. The Company has historically accounted for its investments in accordance with FASB ASC 320, Investments – Debt and Equity Securities. All investments historically have been classified as held-to-maturity securities.
The following tables summarize the Company's short-term investments as of September 30, 2016, and September 30, 2015.
| As of September 30, 2016 |
| |||||||||||||
| Amortized Cost |
|
| Gross Unrealized Gains |
|
| Gross Unrealized Losses |
|
| Fair Value |
| ||||
Commercial notes (due within one year) | $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
| As of September 30, 2015 |
| |||||||||||||
| Amortized Cost |
|
| Gross Unrealized Gains |
|
| Gross Unrealized Losses |
|
| Fair Value |
| ||||
Commercial notes (due within one year) | $ | 17,539,902 |
|
| $ | - |
|
| $ | (304,942 | ) |
| $ | 17,234,960 |
|
NOTE 5. INTANGIBLE ASSETS
Intangible assets consist of in-process research and development ("IPR&D") not subject to amortization, and patents and license agreements subject to amortization, which were capitalized as a part of an asset acquisition.
IPR&D represents projects that have not yet received regulatory approval and are required to be classified as indefinite assets until the successful completion or the abandonment of the associated R&D efforts. These assets include IPR&D capitalized as part of a business combination from the acquisition of the Roche RNAi business in 2011. In November 2016, the Company announced the discontinuation of its clinical trial efforts for ARC-520, ARC-AAT and ARC-521. Given this development, the Company has impaired the IPR&D asset previously recorded during the year ended September 30, 2016.
F-14
Intangible assets subject to amortization include patents and a license agreement capitalized as part of the Novartis RNAi asse t acquisition in March 2015 and license agreements capitalized from the acquisition of the Roche RNAi business in 2011. The license agreement associated with the Novartis RNAi asset acquisition is being amortized over the estimated life remaining at the ti me of acquisition, which was 21 years, and the accumulated amortization of the asset is approximately $ 234,975 . The license agreements associated with the acquisition of the Roche RNAi business were amortized over the estimated life remaining at the time of acquisition, which was 4 years, and the accumulated amortization of the assets is approximately $230,000. These assets have been fully amortized as of September 30 , 2016. The patents associated with the Novartis RNAi asset acquisition are being amortiz ed over the estimated life remaining at the time of acquisition, which was 14 years, and the accumulated amortization of the assets is approximately $2, 457,371 . Amortization expense for the years ended September 30, 2016, 2015 and 2014 was $ 1,714,313, $1, 046,571 and $ 54,653, respectively . Amortization expense is expected to be approximately $ 1,700,429 for fiscal year 201 7 , $1,700,429 in 2018 , $1,700,429 in 2019 , $1,700,429 in 2020 , $1,700,429 in 2021 , $1,700,429 in 2022 , and $1 1,962,294 thereafter.
The following table provides details on the Company's intangible asset balances:
| Intangible assets not subject to amortization |
|
| Intangible assets subject to amortization |
|
| Total Intangible assets |
| |||
Balance at September 30, 2015 | $ | 944,935 |
|
| $ | 23,879,181 |
|
| $ | 24,824,116 |
|
Impairment |
| (944,935 | ) |
|
| - |
|
|
| (944,935 | ) |
Amortization |
| - |
|
|
| (1,714,313 | ) |
|
| (1,714,313 | ) |
Balance at September 30, 2016 | $ | - |
|
| $ | 22,164,868 |
|
| $ | 22,164,868 |
|
NOTE 6. STOCKHOLDERS' EQUITY
At September 30, 2016, the Company had a total of 150,000,000 shares of capital stock authorized for issuance, consisting of 145,000,000 shares of Common Stock, par value $0.001 per share, and 5,000,000 shares of Preferred Stock, par value $0.001 per share.
At September 30, 2016, 69,746,685 shares of Common Stock were outstanding. Additionally, 15,652 shares of Series C Preferred Stock were outstanding, which are convertible into 2,670,990 shares of Common Stock. At September 30, 2016, 8,696,623 shares of Common Stock were reserved for issuance upon exercise of options and vesting of restricted stock units granted or available for grant under Arrowhead's 2004 Equity Incentive Plan and 2013 Incentive Plan, as well as for inducement grants made to new employees.
The Preferred Stock is convertible to Common Stock by its holder at its stated conversion price, though it is not convertible to the extent the holder would beneficially own more than 9.99% of the number of shares of outstanding Common Stock immediately after the conversion. The holders of Preferred Stock are eligible to vote with the Common Stock of the Company on an as-converted basis, but only to the extent they are eligible for conversion without exceeding the 9.99% ownership limitation. The Preferred Stock does not carry a coupon, but it is entitled to receive dividends on a pari passu basis with Common Stock, when and if declared. In any liquidation or dissolution of the Company, the holders of Preferred Stock are entitled to participate in the distribution of the assets, to the extent legally available for distribution, on a pari passu basis with the Common Stock.
On March 3, 2015, the Company issued 3,321,383 shares of Common Stock as part of the Company's entry into an Asset Purchase and Exclusive License Agreement (the "RNAi Purchase Agreement") with Novartis Institutes for BioMedical Research, Inc., a Delaware corporation ("Novartis"), pursuant to which the Company acquired Novartis' RNAi assets and rights thereunder.
On August 8, 2016 the Company sold 7,627,119 shares of Common Stock to certain institutional investors at a price of $5.90 per share. The aggregate purchase price paid by the investors for the Common Stock was $45.0 million, and the Company received net proceeds of approximately $43.2 million, after advisory fees and offering expenses.
On September 28, 2016 the Company sold 1,256,983 shares of Common Stock to Amgen, Inc. at a price of $7.16 per share as part of the Common Stock Purchase Agreement executed with Amgen and discussed further in Note 2 – Collaboration and License Agreements – Amgen, Inc . The Company received proceeds of $9 million. On November 18, 2016, a second tranche of 1,745,810 shares were also sold to Amgen at a price of $7.16 per share as part of the Common Stock Purchase Agreement. The Company received proceeds of $12.5 million in November 2016.
F-15
The following table summarizes information about warrants outstanding at September 30 , 2016:
Exercise prices |
| Number of Warrants |
|
| Remaining |
| ||||
$ | 70.60 |
|
|
| 94,897 |
|
|
| 0.6 |
|
$ | 4.16 |
|
|
| 1,000 |
|
|
| 0.2 |
|
$ | 2.12 |
|
|
| 75,000 |
|
|
| 1.4 |
|
$ | 1.83 |
|
|
| 277,284 |
|
|
| 1.2 |
|
$ | 7.14 |
|
|
| 80,000 |
|
|
| 1.7 |
|
Total warrants outstanding |
|
| 528,181 |
|
|
|
|
| ||
NOTE 7. COMMITMENTS AND CONTINGENCIES
Leases
The Company leases approximately 8,500 square feet of office space for its corporate headquarters in Pasadena, California. The lease will expire in September 2019. Rental costs are approximately $26,000 per month, increasing approximately 3% annually.
On January 8, 2016, the Company entered into a new lease for a Madison, Wisconsin research facility. The 10-year lease between with University Research Park, Inc. is for approximately 60,000 square feet of office and laboratory space located at 502 South Rosa Road, Madison, Wisconsin. This lease will replace the Company's research facility lease, also with University Research Park, Inc. for property located at 465 Science Drive, Madison Wisconsin. The larger facility is designed to accommodate increased research and development space needed for the Company's pipeline of current and future drug candidates.
The initial term of the lease commenced on January 1, 2016, with occupancy occurring October 2016. The lease payments and payments against a note payable for a tenant improvement allowance, which begin on October 1, 2016, will total approximately $15.2 million over the initial 10-year term. The Company also estimates payments for the Company's pro rata share of certain real estate taxes, operating expenses and common area maintenance expenses to be approximately $0.6 million for the first year of the lease, and these payments will continue throughout the initial 10-year term. The Company has paid or accrued for approximately $7.0 million for leasehold improvements at September 30, 2016, net of tenant improvement allowances, and the work on the facility has been substantially completed in October 2016. The primary tenant improvement allowance of $2.1 million is accounted for as deferred rent and the secondary tenant improvement allowance of $2.7 million is accounted for as a note payable on the Company's Consolidated Balance Sheet. Pursuant to the lease, within six months of the expiration of the initial 10-year term, the Company has the option to extend the lease for up to two additional five-year terms, with certain annual increases in base rent.
Additionally, on January 8, 2016 and in conjunction with signing the new lease agreement as discussed above, the Company entered into an amendment to the Company's research facility lease for property located at 465 Science Drive, Madison, Wisconsin with University Research Park, Inc. to terminate the lease agreement for this property, effective on October 31, 2016.
Monthly rental expense under the previous facility which was in effect through September 30, 2016 was approximately $26,000. Other monthly rental expenses include common area maintenance and real estate taxes totaling approximately $20,000 per month. Utilities costs are approximately $18,000 per month. Total monthly costs are approximately $83,000 per month, including monthly payments recorded under a capital lease of approximately $19,000.
The Company leased additional research facility space in Middleton, Wisconsin, and this space is leased through December 2016. Monthly rental expense for the additional space is approximately $14,000. Other monthly rental expenses include common area maintenance and real estate taxes totaling approximately $4,000 per month.
Facility rent expense for the years ended September 30, 2016, 2015 and 2014 was $839,000, $744,000 and $554,000, respectively.
F-16
As of September 30, 2016, future minimum lease payments due in fiscal years under operating leases are as follows:
2017 | $ | 1,559,259 |
|
201 8 |
| 1,531,765 |
|
201 9 |
| 1,435,409 |
|
2020 |
| 1,044,431 |
|
2021 |
| 1,070,496 |
|
2022 and thereafter |
| 5,766,495 |
|
Total | $ | 12,407,855 |
|
Note Payable
As part of the Company's lease for its new research facility in Madison, Wisconsin discussed above, the Company entered into a $2.7 million promissory note payable with its landlord to finance certain tenant improvements made to the new facility. The note will be amortized over the 10-year term of the lease, commencing on October 1, 2016. The note will bear interest at a rate of 7.1% and shall be payable in equal monthly installments of principal and interest.
As of September 30, 2016, future principal payments due in fiscal years under the note payable are as follows:
2017 | $ | 194,310 |
|
201 8 |
| 208,506 |
|
201 9 |
| 223,820 |
|
2020 |
| 240,258 |
|
2021 |
| 257,903 |
|
2022 and thereafter |
| 1,602,968 |
|
Total | $ | 2,727,765 |
|
Litigation
The Company and certain of its officers and directors were named as defendants in a putative consolidated class action in the United States District Court for the Central District of California regarding certain public statements in connection with the Company's hepatitis B drug research. The consolidated class action, initially filed as Wang v. Arrowhead Research Corp., et al. , No. 2:14-cv-07890 (C.D. Cal., filed Oct. 10, 2014), and Eskinazi v. Arrowhead Research Corp., et al. , No. 2:14-cv-07911 (C.D. Cal., filed Oct. 13, 2014), asserted claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and sought damages in an unspecified amount. Additionally, three putative stockholder derivative actions captioned Weisman v. Anzalone et al ., No. 2:14-cv-08982 (C.D. Cal., filed Nov. 20, 2014), Bernstein (Backus) v. Anzalone, et al. , No. 2:14-cv-09247 (C.D. Cal., filed Dec. 2, 2014); and Johnson v. Anzalone, et al. , No. 2:15-cv-00446 (C.D. Cal., filed Jan. 22, 2015), were filed in the United States District Court for the Central District of California, alleging breach of fiduciary duty by the Company's Board of Directors in connection with the facts underlying the securities claims. An additional consolidated derivative action asserting similar claims is pending in Los Angeles County Superior Court, initially filed as Bacchus v. Anzalone, et al. , (L.A. Super., filed Mar. 5, 2015); and Jackson v. Anzalone, et al. (L.A. Super., filed Mar. 16, 2015). Each of these suits seeks damages in unspecified amounts and some seek various forms of injunctive relief. On October 7, 2016, the federal district court dismissed the consolidated class action with prejudice. On October 10, 2016 the plaintiffs appealed the consolidated class action to the United States Court of Appeals for the Ninth Circuit. The Weisman and Johnson derivative actions have been dismissed without prejudice. The Bernstein derivative action remains pending. The Company believes it has meritorious defenses and intends to vigorously defend itself in each of these matters. The Company makes provisions for liabilities when it is both probable that a liability has been incurred and the amount can be reasonably estimated. No such liability has been recorded related to these matters. The Company does not expect these matters to have a material effect on its Consolidated Financial Statements. With regard to legal fees, such as attorney fees related to these matters or any other legal matters, the Company's recognizes such costs as incurred.
F-17
The Company and two of its former exec utives were named as defendants in a complaint filed on November 11, 2014 and captioned William Marsh Rice University vs. Unidym, Inc. and Arrowhead Research Corporation , No. 2014-66088, in the United States District Court for the Southern District of Texa s relating to alleged breaches of a license agreement between Rice University and the Company's former subsidiary, Unidym, Inc. The Company and the plaintiff settled the matter under an agreement in which the Company paid a confidential settlement amount a nd the plaintiff permanently dismissed its claims. The amount of the settlement was recorded during the three months ended March 31, 2016 and did not have a material effect on the Company's Consolidated Financial Statements.
The Company and certain executive officers were named as defendants in related putative securities class actions filed on November 15, 2016 and December 2, 2016 in the Central District of California and respectively captioned Meller v. Arrowhead Pharmaceuticals, Inc., et al. , No. 2:16-cv-08505, and Siegel v. Arrowhead Pharmaceuticals, Inc., et al ., No. 2:16-cv-8954. The plaintiff brings claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 regarding certain public statements in connection with the Company's drug research programs and seek damages in an unspecified amount. The Company believes it has meritorious defenses and intends to vigorously defend itself in these matters. The Company makes provisions for liabilities when it is both probable that a liability has been incurred and the amount can be reasonably estimated. No such liability has been recorded related to these matters. The Company cannot predict the ultimate outcome of this matter and cannot accurately estimate any potential liability the Company may incur or the impact of the results of this matter on the Company. With regard to legal fees, such as attorney fees related to these matters or any other legal matters, the Company's recognizes such costs as incurred.
Purchase Commitments
In the normal course of business, we enter into various purchase commitments for the manufacture of drug components, for toxicology studies, and for clinical studies. As of September 30, 2016, these future commitments were estimated at approximately $10.4 million, of which approximately $10.4 million is expected to be incurred in fiscal 2017, and $0 is expected to be incurred beyond fiscal 2017. The reduction in amount and timing of these commitments is due to the Company's discontinuation of its clinical trials for ARC-520, ARC-AAT and ARC-521.
Technology License Commitments
The Company has licensed from third parties the rights to use certain technologies for its research and development activities, as well as in any products the Company may develop using these licensed technologies. These agreements and other similar agreements often require milestone and royalty payments. Milestone payments, for example, may be required as the research and development process progresses through various stages of development, such as when clinical candidates enter or progress through clinical trials, upon NDA and upon certain sales level milestones. These milestone payments could amount to the mid to upper double-digit millions of dollars. During the year ended September 30, 2016, 2015, and 2014, we reached milestones amounting to $3.0 million, $1.0 million and $1.0 million, respectively, based on progress achieved on our clinical candidates. In certain agreements, the Company may be required to make mid to high single-digit percentage royalty payments based on a percentage of the sales of the relevant products.
NOTE 8. STOCK-BASED COMPENSATION
Arrowhead has two plans that provide for equity-based compensation. Under the 2004 Equity Incentive Plan and 2013 Incentive Plan, as of September 30, 2016, 2,514,518 and 5,604,151 shares, respectively, of Arrowhead's Common Stock are reserved for the grant of stock options, stock appreciation rights, restricted stock awards and performance unit/share award to employees, consultants and others. No further grants may be made under the 2004 Equity Incentive Plan. As of September 30, 2016, there were options granted and outstanding to purchase 2,514,518 and 3,632,060 shares of Common Stock under the 2004 Equity Incentive Plan and the 2013 Incentive Plan, respectively, and there were 1,323,334 restricted stock units granted and outstanding under the 2013 Incentive Plan. Also, as of September 30, 2016, there were 544,622 shares reserved for options and 33,333 restricted stock units issued as inducement grants to new employees outside of equity compensation plans. During the year ended September 30, 2016, no options or restricted stock units were granted under the 2004 Equity Incentive Plan, 1,402,000 options and 838,517 restricted stock units were granted under the 2013 Incentive Plan, and no options or restricted stock units were granted as inducement awards to new employees outside of equity incentive plans.
F-18
The following table summarizes information about stock options:
| Number of Options Outstanding |
|
| Weighted- Average Exercise Price Per Share |
|
| Weighted- Average Remaining Contractual Term |
|
| Aggregate Intrinsic Value |
| ||||
Balance At September 30, 201 5 |
| 5,435,640 |
|
| $ | 6.71 |
|
|
|
|
|
|
|
|
|
Granted |
| 1,402,000 |
|
|
| 6.12 |
|
|
|
|
|
|
|
|
|
Cancelled |
| (109,253) |
|
|
| 9.63 |
|
|
|
|
|
|
|
|
|
Exercised |
| (37,187) |
|
|
| 3.60 |
|
|
|
|
|
|
|
|
|
Balance At September 30, 2016 |
| 6,691,200 |
|
| $ | 6.56 |
|
|
| 7.3 years |
|
| $ | 12,794,064 |
|
Exercisable At September 30, 2016 |
| 3,850,419 |
|
| $ | 6.22 |
|
|
| 6.5 years |
|
| $ | 9,265,570 |
|
Stock-based compensation expense related to stock options for the years ended September 30, 2016, 2015 and 2014 was $6,361,396, $4,760,831, and $3,144,776, respectively. The Company does not recognize an income tax benefit as the Company is currently operating at a loss and an actual income tax benefit may not be realized. For non-qualified stock options, the loss creates a timing difference, resulting in a deferred tax asset, which is fully reserved by a valuation allowance.
The grant date fair value of the options granted by the Company for the years ended September 30, 2016, 2015 and 2014 was estimated at $ 6,426,207, $7,338,395 and $9,267,048, respectively.
The intrinsic value of the options exercised during the years ended September 30, 2016, 2015 and 2014 was $142,690, $128,391 and $4,360,850, respectively.
As of September 30, 2016, the pre-tax compensation expense for all outstanding unvested stock options in the amount of approximately $11,915,534 will be recognized in the Company's results of operations over a weighted average period of 2.4 years.
The fair value of each stock option award is estimated on the date of grant using the Black-Scholes option pricing model. The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options, which do not have vesting restrictions and are fully transferable. The determination of the fair value of each stock option is affected by the Company's stock price on the date of grant, as well as assumptions regarding a number of highly complex and subjective variables. Because the Company's employee stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management's opinion, the existing models do not necessarily provide a reliable single measure of the fair value of its employee stock options.
The assumptions used to value stock options are as follows:
|
|
Years ended September 30, | ||||
|
| 201 6 |
| 201 5 |
| 201 4 |
Dividend yield |
| - |
| - |
| - |
Risk-free interest rate |
| 1.05 – 1.89% |
| 1.46 – 1.89% |
| 1.8 – 2.4% |
Volatility |
| 89% |
| 75% |
| 69% |
Expected life (in years) |
| 6.25 |
| 6 - 6.25 |
| 6.25 – 9.47 |
Weighted average grant date fair value per share of options granted |
| $4.58 |
| $4.24 |
| $8.92 |
The dividend yield is zero as the Company currently does not pay a dividend.
The risk-free interest rate is based on that of the U.S. Treasury bond.
Volatility is estimated based on volatility average of the Company's Common Stock price.
F-19
Restricted Stock Units
Restricted stock units (RSUs), including time-based and performance-based awards, were granted under the Company's 2013 Incentive Plan and as inducement grants granted outside of the Plan. During the year ended September 30, 2016, the Company issued 838,517 restricted stock units to certain members of management. Of the restricted stock units granted during the year ended September 30, 2016, 0 were granted outside of the Plan as an inducement grant to a new employee. At vesting, each RSU will be exchanged for one share of the Company's Common Stock. Restricted stock unit awards generally vest subject to the satisfaction of service requirements or the satisfaction of both service requirements and achievement of certain performance targets.
The following table summarizes the activity of the Company's Restricted Stock Units:
| Number of RSUs |
|
| Weighted- Average Grant Date Fair Value |
| ||
Unvested at September 30, 2015 |
| 934,167 |
|
| $ | 9.18 |
|
Granted |
| 838,517 |
|
|
| 6.15 |
|
Vested |
| (416,017 | ) |
|
| 11.08 |
|
Forfeited |
| - |
|
|
| - |
|
Unvested at September 30, 2016 |
| 1,356,667 |
|
| $ | 6.72 |
|
During the years ended September 30, 2016, 2015 and 2014, the Company recorded $5,234,420, $4,489,931 and $2,551,397 of expense, respectively. Such expense is included in stock-based compensation expense in the Company's Consolidated Statement of Operations and Comprehensive Loss.
For restricted stock units, the grant date fair value of the award is based on the Company's closing stock price at the grant date, with consideration given to the probability of achieving performance conditions for performance based awards.
As of September 30, 2016, the pre-tax compensation expense for all unvested restricted stock units in the amount of approximately $2,567,761 will be recognized in the Company's results of operations over a weighted average period of 1.4 years.
NOTE 9. FAIR VALUE MEASUREMENTS
The Company measures its financial assets and liabilities at fair value. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e., exit price) in an orderly transaction between market participants at the measurement date. Additionally, the Company is required to provide disclosure and categorize assets and liabilities measured at fair value into one of three different levels depending on the assumptions (i.e., inputs) used in the valuation. Level 1 provides the most reliable measure of fair value while Level 3 generally requires significant management judgment. Financial assets and liabilities are classified in their entirety based on the lowest level of input significant to the fair value measurement. The fair value hierarchy is defined as follows:
Level 1-Valuations are based on unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2-Valuations are based on quoted prices for similar assets or liabilities in active markets, or quoted prices in markets that are not active for which significant inputs are observable, either directly or indirectly.
Level 3-Valuations are based on prices or valuation techniques that require inputs that are both unobservable and significant to the overall fair value measurement. Inputs reflect management's best estimate of what market participants would use in valuing the asset or liability at the measurement date.
The following table summarizes fair value measurements at September 30, 2016 and September 30, 2015 for assets and liabilities measured at fair value on a recurring basis:
September 30, 2016:
| Level 1 |
|
| Level 2 |
|
| Level 3 |
|
| Total |
| ||||
Cash and cash equivalents | $ | 85,366,448 |
|
| $ | - |
|
| $ | - |
|
| $ | 85,366,448 |
|
Derivative liabilities | $ | - |
|
| $ | - |
|
| $ | 1,602,626 |
|
| $ | 1,602,626 |
|
Acquisition-related contingent consideration obligations | $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
F-20
September 30, 2015 :
| Level 1 |
|
| Level 2 |
|
| Level 3 |
|
| Total |
| ||||
Cash and cash equivalents | $ | 81,214,354 |
|
| $ | - |
|
| $ | - |
|
| $ | 81,214,354 |
|
Derivative liabilities | $ | - |
|
| $ | - |
|
| $ | 1,301,604 |
|
| $ | 1,301,604 |
|
Acquisition-related contingent consideration obligations | $ | - |
|
| $ | - |
|
| $ | 5,862,464 |
|
| $ | 5,862,464 |
|
As part of a financing in December 2012, Arrowhead issued warrants to purchase up to 912,543 shares of Common Stock (the "2012 Warrants") of which 265,161 warrants were outstanding at September 30, 2016. Further, as part of a financing in January 2013, Arrowhead issued warrants to purchase up to 833,530 shares of Common Stock (the "2013 Warrants" and, together with the 2012 Warrants, the "Warrants") of which 12,123 warrants were outstanding at September 30, 2016. Each of the Warrants contains a mechanism to adjust the strike price upon the issuance of certain dilutive equity securities. If during the terms of the Warrants, the Company issues Common Stock at a price lower than the exercise price for the Warrants, the exercise price would be reduced to the amount equal to the issuance price of the Common Stock. As a result of these features, the Warrants are subject to derivative accounting as prescribed under ASC 815. Accordingly, the fair value of the Warrants on the date of issuance was estimated using an option pricing model and recorded on the Company's Consolidated Balance Sheet as a derivative liability. The fair value of the Warrants is estimated at the end of each reporting period and the change in the fair value of the Warrants is recorded as a non-operating gain or loss as change in value of derivatives in the Company's Consolidated Statement of Operations and Comprehensive Loss. During the years ended September 30, 2016, 2015 and 2014 the Company recorded a non-cash gain/(loss) from the change in fair value of the derivative liability of $(293,072), $2,684,712 and $(5,821,796), respectively. Additionally, as part of an equity financing in June 2010, Arrowhead issued warrants to purchase up to 329,649 shares of Common Stock (the "2010 Warrants"), of which warrants to exercise 24,324 shares remained unexercised and were cancelled at their expiration during fiscal 2016.
F-21
The assumptions used in valuing the derivative liability were as follows:
2012 Warrants |
| September 30, 2016 |
|
| September 30, 2015 |
| September 30, 2014 |
|
Risk-free interest rate |
| 0.68% |
|
| 0.6% |
| 1.07% |
|
Expected life |
| 1.2 Years |
|
| 2.2 Years |
| 3.2 Years |
|
Dividend yield |
| - |
|
| - |
| - |
|
Volatility |
| 89% |
|
| 75% |
| 69% |
|
|
|
|
|
|
|
|
|
|
2013 Warrants |
| September 30, 2016 |
|
| September 30, 2015 |
| September 30, 2014 |
|
Risk-free interest rate |
| 0.68% |
|
| 0.6% |
| 1.07% |
|
Expected life |
| 1.3 Years |
|
| 2.3 Years |
| 3.3 Years |
|
Dividend yield |
| - |
|
| - |
| - |
|
Volatility |
| 89% |
|
| 75% |
| 69% |
|
The following is a reconciliation of the derivative liability related to these warrants:
Value at September 30, 2015 | $ | 1,272,802 |
|
Issuance of instruments |
| - |
|
Change in value |
| 293,072 |
|
Net settlements |
| - |
|
Value at September 30, 2016 | $ | 1,565,874 |
|
|
|
|
|
In conjunction with the financing of Ablaris in fiscal 2011, Arrowhead sold exchange rights to certain investors whereby the investors have the right to exchange their shares of Ablaris for a prescribed number of Arrowhead shares of Common Stock based upon a predefined ratio. The exchange rights have a seven-year term and a current exchange ratio of 0.01. Exchange rights for 675,000 Ablaris shares were sold in fiscal 2011, and 500,000 remain outstanding at September 30, 2016. The exchange rights are subject to derivative accounting as prescribed under ASC 815. Accordingly, the fair value of the exchange rights on the date of issuance was estimated using an option pricing model and recorded on the Company's Consolidated Balance Sheet as a derivative liability. The fair value of the exchange rights is estimated at the end of each reporting period and the change in the fair value of the exchange rights is recorded as a non-operating gain or loss in the Company's Consolidated Statement of Operations and Comprehensive Loss. During the years ended September 30, 2016, 2015 and 2014, the Company recorded a non-cash gain/(loss) from the change in fair value of the derivative liability of $(7,950), $184,555 and $(211,860), respectively.
The assumptions used in valuing the derivative liability were as follows:
| September 30, 2016 |
|
| September 30, 2015 |
| September 30, 2014 |
Risk-free interest rate | 0.68% |
|
| 1.00% |
| 1.07% |
Expected life | 1.5 Years |
|
| 2.5 Years |
| 3.3 Years |
Dividend yield | - |
|
| - |
| - |
Volatility | 89% |
|
| 75% |
| 100% |
The following is a reconciliation of the derivative liability related to these exchange rights:
Value at September 30, 201 5 | $ | 28,802 |
|
Issuance of instruments |
| - |
|
Change in value |
| 7,950 |
|
Net settlements |
| - |
|
Value at September 30, 2016 | $ | 36,752 |
|
|
|
|
|
The derivative assets/liabilities are estimated using option pricing models that are based on the individual characteristics of the warrants or instruments on the valuation date, as well as assumptions for expected volatility, expected life and risk-free interest rate. Changes in the assumptions used could have a material impact on the resulting fair value. The primary input affecting the value of the Company's derivatives liabilities is the Company's stock price. Other inputs have a comparatively insignificant effect.
F-22
As of September 30 , 2016, the Company has a liability for contingent consideration related to its acquisition of the Roche RNAi business completed in 2011 . The fair value measurement of the contingent consideration obligations is determined u sing Level 3 inputs. The fair value of contingent consideration obligations is based on a discounted cash flow model using a probability-weighted income approach. The measurement is based upon unobservable inputs supported by little or no market activity b ased on the Company's assumptions and experience. Estimating timing to complete the development and obtain approval of products is difficult, and there are inherent uncertainties in developing a product candidate, such as obtaining U.S. Food and Drug Admin istration (FDA) and other regulatory approvals. In determining the probability of regulatory approval and commercial success, the Company utilizes data regarding similar milestone events from several sources, including industry studies and its own experien ce. These fair value measurements represent Level 3 measurements as they are based on significant inputs not observable in the market. Significant judgment is employed in determining the appropriateness of these assumptions as of the acquisition date and f or each subsequent period. Accordingly, changes in assumptions could have a material impact on the amount of contingent consideration expense the Company records in any given period. Changes in the fair value of the contingent consideration obligations are recorded in the Company's Consolidated Statement of Operations and Comprehensive Loss.
The following is a reconciliation of contingent consideration fair value.
Value at September 30, 2015 | $ | 5,862,464 |
|
Purchase price contingent consideration |
| - |
|
Contingent consideration payments |
| - |
|
Change in fair value of contingent consideration |
| (5,862,464 | ) |
Value at September 30, 2016 | $ | - |
|
The fair value of contingent consideration obligations is estimated through valuation models designed to estimate the probability of such contingent payments based on various assumptions and incorporating estimated success rates. Estimated payments are discounted using present value techniques to arrive at estimated fair value at the balance sheet date. Changes in the fair value of the contingent consideration obligations can result from changes to one or multiple inputs, including adjustments to the discount rates, changes in the amount or timing of expected expenditures associated with product development, changes in the amount or timing of cash flows from products upon commercialization, changes in the assumed achievement or timing of any development milestones, changes in the probability of certain clinical events and changes in the assumed probability associated with regulatory approval. Each of these assumptions can have a significant impact on the calculation of contingent consideration. In November 2016, the Company announced the discontinuation of its clinical trial efforts for ARC-520, ARC-AAT and ARC-521. Given this development, the Company has assessed the fair value of its contingent consideration obligation to be $0 at September 30, 2016.
The carrying amounts of the Company's other financial instruments, which include accounts receivable, accounts payable, and accrued expenses approximate their respective fair values due to the relatively short-term nature of these instruments. The carrying value of the Company's other long-term liabilities approximates fair value based on market interest rates.
NOTE 10. - INCOME TAXES
The Company utilizes the guidance issued by the FASB for accounting for income taxes which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns.
Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each year-end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax payable for the period and the change during the period in deferred tax assets and liabilities.
Components of the net deferred tax asset (liability) at September 30, 2016 and 2015 are as follows:
|
| 2016 |
|
| 2015 |
| |
Deferred tax assets: |
|
|
|
|
|
|
|
Accrued compensation | $ | 1,691,050 |
|
| $ | 1,513,021 |
|
Stock compensation |
| 7,224,958 |
|
|
| 6,571,774 |
|
Capitalized research and development |
| 2,957,528 |
|
|
| - |
|
Fair value adjustments |
| - |
|
|
| 2,850,125 |
|
Net operating losses |
| 125,120,957 |
|
|
| 88,965,968 |
|
F-23
|
| 2016 |
|
| 2015 |
| |
Intangible Assets |
| 5,544,091 |
|
|
| 5,551,705 |
|
Total deferred tax assets |
| 142,538,584 |
|
|
| 105,452,593 |
|
Valuation allowance |
| (128,695,371 | ) |
|
| (95,085,045 | ) |
Deferred tax liabilities: |
|
|
|
|
|
|
|
State taxes |
| (13,804,268 | ) |
|
| (10,282,834 | ) |
Fixed assets |
| (38,945 | ) |
|
| (84,714 | ) |
Total deferred tax liability |
| (13,843,213 | ) |
|
| (10,367,548 | ) |
Net deferred tax assets (liabilities) | $ | - |
|
| $ | - |
|
The Company has concluded, in accordance with the applicable accounting standards, that it is more likely than not that the Company may not realize the benefit of all of its deferred tax assets . Accordingly, management has provided a 100% valuation allowance against its deferred tax assets until such time as management believes that its projections of future profits as well as expected future tax rates make the realization of these deferred tax assets more-likely-than-not. Significant judgment is required in the evaluation of deferred tax benefits and differences in future results from our estimates could result in material differences in the realization of these assets. The Company has recorded a full valuation allowance related to all of its deferred tax assets. The Company has performed an assessment of positive and negative evidence regarding the realization of the net deferred tax asset in accordance with FASB ASC 740-10, "Accounting for Income Taxes." This assessment included the evaluation of scheduled reversals of deferred tax liabilities, the availability of carry forwards and estimates of projected future taxable income. The Company's net deferred tax liability is related to indefinite lived assets which are not considered as a source of future taxable income when determining the need for a valuation allowance.
As of September 30, 2015, the Company had available gross federal net operating loss (NOL) carry forwards of $ 185.1 million and gross state NOL carry forwards of $ 285.4 million. Gross federal NOL carry forwards for 2016 are estimated at $78.7 million, and gross state NOL carry forwards for 2016 are estimated at $133.9 million. The NOLs expire at various dates through 2036.
The provision for income taxes for the years ended September 30, 2016 and 2015 are as follows:
| 2016 |
|
| 2015 |
| ||
Federal: |
|
|
|
|
|
|
|
Current |
| - |
|
|
| - |
|
Deferred |
| - |
|
|
| - |
|
Total Federal |
| - |
|
|
| - |
|
State: |
|
|
|
|
|
|
|
Current | $ | 2,400 |
|
|
| 2,400 |
|
Deferred |
| - |
|
|
| - |
|
Total State | $ | 2,400 |
|
|
| 2,400 |
|
Provision from income taxes | $ | 2,400 |
|
|
| 2,400 |
|
The Company's effective income tax rate differs from the statutory federal income tax rate as follows for the years ended September 30, 2016 and 2015:
|
| 2016 |
|
| 2015 |
| |
At U.S. federal statutory rate |
| 34.0 | % |
|
| 34.0 | % |
State taxes, net of federal effect |
| 9.0 |
|
|
| 9.3 |
|
Stock compensation |
| (1.1 | ) |
|
| (0.7 | ) |
Mark-to-market adjustments |
| (0.1 | ) |
|
| 0.7 |
|
Valuation allowance |
| (41.1 | ) |
|
| (43.4 | ) |
Other |
| (0.7 | ) |
|
| 0.1 |
|
Effective income tax rate |
| 0.0 | % |
|
| 0.0 | % |
The Company has adopted guidance issued by the FASB that clarifies the accounting for uncertainty in income taxes recognized in an enterprise's financial statements and prescribes a recognition threshold of more likely than not and a measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. In making this assessment, a company must determine whether it is more likely than not that a tax position will be sustained upon examination, based solely on the technical merits of the position and must assume that the tax position will be examined by taxing authorities. The Company's policy is to include interest and penalties related to unrecognized tax benefits in income tax expense. The Company has not recognized any unrecognized tax benefits and does not have any interest or penalties related to uncertain tax positions as of September 30, 2016 and 2015.
F-24
The Company files income tax returns with the Internal Revenue Service ("IRS"), the s tate of California and certain other taxing jurisdictions. T he Company is subject to income tax examinations by the IRS and by state tax authoriti es until the net operating losses are settled. During the three months ended September 30, 2016, the IRS comm enced an audit for the tax year ended September 30, 2015.
NOTE 11. EMPLOYEE BENEFIT PLANS
In January 2005, the Company adopted a defined contribution 401(k) retirement savings plan covering substantially all of its employees. The Plan is administered under the "safe harbor" provision of ERISA. Under the terms of the plan, an eligible employee may elect to contribute a portion of their salary on a pre-tax basis, subject to federal statutory limitations. The plan allows for a discretionary match in an amount up to 100% of each participant's first 3% of compensation contributed plus 50% of each participant's next 2% of compensation contributed.
For the years ended September 30, 2016, 2015, and 2014, we recorded expenses under this plan of approximately $476,835, $407,603 and $264,193, respectively.
In addition to the employee benefit plans described above, the Company provides certain employee benefit plans, including those which provide health and life insurance benefits to employees.
NOTE 12. UNAUDITED QUARTERLY FINANCIAL DATA
The following table presents selected unaudited quarterly financial data for each full quarterly period of the years ended September 30, 2016 and 2015:
|
| First |
| Second |
| Third |
| Fourth |
| ||||
Year ended September 30, 2016 |
| Quarter |
| Quarter |
| Quarter |
| Quarter |
| ||||
Revenues |
| $ | 43,750 |
| $ | 43,750 |
| $ | 39,583 |
| $ | 31,250 |
|
Operating Losses |
| $ | (19,341,270) |
| $ | (21,264,855) |
| $ | (19,341,487) |
| $ | (21,795,114) |
|
Net Loss |
| $ | (19,264,414) |
| $ | (20,815,860) |
| $ | (19,420,743) |
| $ | (22,221,985) |
|
Net Loss Attributable to Arrowhead |
| $ | (19,264,414) |
| $ | (20,815,860) |
| $ | (19,420,743) |
| $ | (22,221,985) |
|
Loss per share (Basic and Diluted) |
| $ | (0.32) |
| $ | (0.35) |
| $ | (0.32) |
| $ | (0.34) |
|
|
| First |
| Second |
| Third |
| Fourth |
| ||||
Year ended September 30, 2015 |
| Quarter |
| Quarter |
| Quarter |
| Quarter |
| ||||
Revenues |
| $ | 170,750 |
| $ | 43,750 |
| $ | 123,750 |
| $ | 43,750 |
|
Operating Losses |
| $ | (25,115,276) |
| $ | (29,632,743) |
| $ | (15,993,706) |
| $ | (25,232,251) |
|
Net Loss |
| $ | (22,575,282) |
| $ | (28,683,993) |
| $ | (15,936,053) |
| $ | (24,745,554) |
|
Net Loss Attributable to Arrowhead |
| $ | (22,575,282) |
| $ | (28,683,993) |
| $ | (15,936,053) |
| $ | (24,745,554) |
|
Loss per share (Basic and Diluted) |
| $ | (0.41) |
| $ | (0.51) |
| $ | (0.27) |
| $ | (0.42) |
|
NOTE 13. SUBSEQUENT EVENTS
The Company and certain executive officers were named as defendants in related putative securities class actions filed on November 15, 2016 and December 2, 2016 in the Central District of California and respectively captioned Meller v. Arrowhead Pharmaceuticals, Inc., et al. , No. 2:16-cv-08505, and Siegel v. Arrowhead Pharmaceuticals, Inc., et al ., No. 2:16-cv-8954. The plaintiff brings claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 regarding certain public statements in connection with the Company's drug research programs and seek damages in an unspecified amount. The Company believes it has meritorious defenses and intends to vigorously defend itself in these matters. The Company makes provisions for liabilities when it is both probable that a liability has been incurred and the amount can be reasonably estimated. No such liability has been recorded related to these matters. The Company cannot predict the ultimate outcome of this matter and cannot accurately estimate any potential liability the Company may incur or the impact of the results of this matter on the Company. With regard to legal fees, such as attorney fees related to these matters or any other legal matters, the Company's recognizes such costs as incurred.
On November 18, 2016, the Company and Amgen received Hart-Scott-Rodino clearance with regard to the Second Collaboration and License Agreement discussed in Note 2 above. Based on the terms of this agreement, and the Common Stock Purchase Agreement, the Company issued 1,745,810 shares of Common Stock to Amgen, and received proceeds of approximately $12.5
F-25
million. Additionally, the Co mpany received a $30 million up front payment due under the Second Collaboration and Licen se Agreement discussed above.
On November 29, 2016, the Company announced that it would be discontinuing its clinical programs ARC-520, ARC-AAT and ARC-521, and redeploying its resources and focus toward utilizing the Company's new proprietary subcutaneous and extra-hepatic delivery systems. Each of these clinical candidates utilized the intravenously administered DPC iv , or EX1, delivery vehicle. The decision to discontinue development of EX1-containing programs was based primarily on two factors. First, during ongoing discussions with regulatory agencies and outside experts, it became apparent that there would be substantial delays in all clinical programs that utilize EX1, while the Company further explored the cause of deaths in a non-clinical toxicology study in non-human primates. Second, the Company has made substantial advances in RNA chemistry and targeting resulting in large potency gains for subcutaneous administered and extra-hepatic RNAi-based development programs. In preclinical studies with the subcutaneous platform, the Company has obtained depth and duration of target gene knockdown approaching that of intravenously administered EX1-containing candidates, at lower doses and with good safety margins. ARO-HBV and ARO-AAT are the Company's subcutaneous administered preclinical candidates for chronic hepatitis B virus and liver disease associated with AATD, respectively. Because of the discontinuation of its existing clinical programs, the Company has also reduced its workforce by approximately 30%, while maintaining resourcing necessary to support current and potential future partner-based programs and the Company's pipeline.
F-26
